Abstract
MIC end point determination for the most commonly prescribed azole antifungal drug, fluconazole, can be complicated by “trailing” growth of the organism during susceptibility testing by the National Committee for Clinical Laboratory Standards approved M27-A broth macrodilution method and its modified broth microdilution format. To address this problem, we previously developed the sterol quantitation method (SQM) for in vitro determination of fluconazole susceptibility, which measures cellular ergosterol content rather than growth inhibition after exposure to fluconazole. To determine if SQM MICs of fluconazole correlated better with in vivo outcome than M27-A MICs, we used a murine model of invasive candidiasis and analyzed the capacity of fluconazole to treat infections caused by C. albicans isolates which were trailers (M27-A MICs at 24 and 48 h, ≤1.0 and ≥64 μg/ml, respectively; SQM MIC, ≤1.0 μg/ml), as well as those which were fluconazole sensitive (M27-A and SQM MIC, ≤1.0 μg/ml) and fluconazole resistant (M27-A MIC, ≥64 μg/ml; SQM MIC, 54 μg/ml). Compared with the untreated controls, fluconazole therapy increased the survival of mice infected with a sensitive isolate and both trailing isolates but did not increase the survival of mice infected with a resistant isolate. These results indicate that the SQM is more predictive of in vivo outcome than the M27-A method for isolates that give unclear MIC end points due to trailing growth in fluconazole.
Antifungal susceptibility testing has become more important in recent years due to the increase in serious fungal infections and the concomitant emergence of antifungal drug resistance (15, 20). Significant advances have been made by the National Committee for Clinical Laboratory Standards (NCCLS) to improve the reproducibility of antifungal susceptibility testing by publishing a reference macrobroth dilution method (M27-A) for in vitro testing of the susceptibilities of Cryptococcus neoformans and Candida spp. to amphotericin B, flucytosine, fluconazole, itraconazole, and ketoconazole (11). Whereas this reference method has significantly improved the reproducibility of antifungal susceptibility testing of most isolates, interpretation of the NCCLS M27-A broth dilution method can be complicated because some isolates do not have a clear-cut end point and exhibit a “trailing” growth effect (16). In our laboratory, we have observed that approximately 5% of all C. albicans isolates display trailing growth when tested against the azole antifungals (unpublished observations). For isolates with trailing end points, MICs of less than 1 μg/ml at 24 h and of 64 μg/ml or greater at 48 h are usually observed (16). Therefore, these isolates would be considered resistant by NCCLS M27-A methodologies, which recommend reading results after 48 h of growth (11). Reports of clinical outcome in human immunodeficiency virus-infected patients with oropharyngeal candidiasis have demonstrated that infections caused by organisms which produce trailing growth in fluconazole typically respond to low doses of fluconazole, suggesting that the lower, 24-h MICs better reflect host responsiveness to therapy (16). Results reported by Rex et al. demonstrated that M27-A fluconazole MICs determined visually after 24 h of incubation and/or with a less restrictive 50% growth inhibition end point (determined spectrophotometrically) better matched the in vivo response pattern to fluconazole treatment in a murine model of candidiasis (17). Therefore, in an attempt to address the discrepancy between in vitro NCCLS results and in vivo outcome for trailing isolates, we developed the sterol quantitation method (SQM). This method measures the sensitivities of Candida albicans isolates to the effect of fluconazole on ergosterol biosynthesis by quantitation of steady-state amounts of ergosterol following growth of the organism in various concentrations of fluconazole (3). The ergosterol content determined by the SQM is a physical measurement, eliminating the bias of subjectively determining 80% growth inhibition, as required for broth-based susceptibility testing methods. Therefore, in this regard, this method was more objective and reproducible than standard NCCLS methods and enabled us to assign unequivocal fluconazole MIC end points to organisms which exhibit trailing growth (3). However, an in vitro determination of drug susceptibility is meaningless unless it correlates with in vivo outcome. To examine the value of an SQM MIC, compared with that of the standard NCCLS MIC, to predict in vivo outcome, we examined the in vitro-in vivo correlation of test results using a murine model of candidiasis.
MATERIALS AND METHODS
Isolates.
Four C. albicans isolates, spanning different M27-A MIC susceptibility categories for fluconazole, were tested in an animal model of disseminated candidiasis (isolate 1, CDC accession no. B5956, fluconazole susceptible; isolate 2, B5957, fluconazole resistant; isolate 3, CDC accession no. B5958, fluconazole resistant with trailing; isolate 4, B5959, fluconazole resistant with trailing). Isolates were obtained from David A. Stevens (Stanford University, Palo Alto, Calif., and Santa Clara Valley Medical Center, San Jose, Calif.) and Dora Warren (Division of Reproductive Health, Centers for Disease Control and Prevention, Atlanta, Ga.). Isolates were identified to the species level by the API 20C (Analytab Products, Plainview, N.Y.) yeast identification system.
Isolates were retrieved from storage at −70°C and were subcultured twice on Sabouraud dextrose agar plates (BBL, Cockeysville, Md.) to ensure optimal growth. Prior to testing, subcultures on Sabouraud dextrose agar plates were incubated at 35°C for 24 h.
Antifungal susceptibility testing. (i) Broth microdilution method.
The broth microdilution modification of the NCCLS M27-A method was performed (11). Analytical-grade powder of fluconazole was obtained as a gift from Pfizer (Groton, Conn.). A stock solution of fluconazole was prepared in sterile distilled water, diluted with RPMI 1640 medium (with l-glutamine but without bicarbonate) (Sigma Chemical Co., St. Louis, Mo.), and buffered to pH 7.0 with 0.165 M morpholinopropanesulfonic acid (MOPS; Sigma). The final concentration range used for fluconazole was 0.125 to 64 μg/ml.
Testing was performed in 96-well round-bottom microtitration plates. Cell suspensions were prepared in RPMI 1640 medium and were adjusted to give a final inoculum concentration of 0.5 × 103 to 2.5 × 103 cells/ml. The plates were incubated at 35°C and were read visually after 48 h. The MIC of fluconazole was defined as the lowest concentration at which there was 80% inhibition of growth compared with that in the drug-free control well. Fluconazole resistance was defined as an MIC of ≥64 μg/ml, and fluconazole susceptibility was defined as an MIC of ≤8 μg/ml (11).
(ii) SOM.
Total intracellular sterols were extracted as reported by Breivik and Owades (4) with slight modification. Briefly, a single C. albicans colony from an overnight Sabouraud dextrose agar plate culture was used to inoculate 50 ml of Sabouraud dextrose broth (Difco, Detroit, Mich.) containing 0, 1, 4, 16, or 64 μg of fluconazole per ml. The cultures were incubated at 35°C for 18 h with shaking at 120 rpm. The stationary-phase cells were harvested by centrifugation at 2,700 rpm (model TJ-6 centrifuge; Beckman Instruments, Palo Alto, Calif.) for 5 min and washed once with sterile distilled water. The net weight of the cell pellet was determined. Three milliliters of 25% alcoholic potassium hydroxide solution (25 g of KOH and 36 ml of sterile distilled water, brought to 100 ml with 100% ethanol) was added to each pellet and mixed by vortex for 1 min. Cell suspensions were then incubated in an 80°C water bath for 1 h. Following incubation, tubes were allowed to cool to room temperature. Sterols were then extracted by addition of a mixture of 1 ml of sterile distilled water and 3 ml of n-heptane, followed by vigorous vortex mixing for 3 min. The heptane layer was transferred to a clean borosilicate glass screw-cap tube and stored at −20°C for up to 24 h. A 20-ml aliquot of sterol extract was diluted fivefold in 100% ethanol and scanned spectrophotometrically between 200 and 300 nm with a Gilford response spectrophotometer (Ciba Corning Diagnostics Corp., Gilford Systems, Oberlin, Ohio). The presence of ergosterol and the late sterol intermediate 24(28) dehydroergosterol (DHE) in the extracted sample resulted in a characteristic four-peaked curve (3). The absence of detectable ergosterol in extracts was indicated by a flat line. A dose-dependent decrease in the height of the absorbance peaks was evident and corresponded to decreased ergosterol concentration in the sample.
Ergosterol content was calculated as a percentage of the wet weight of the cells by the following equations: percent ergosterol + percent 24(28) DHE = [(A281.5/290) × F]/pellet weight, percent 24(28) DHE = [(A230/518) × F]/pellet weight, and percent ergosterol = [percent ergosterol + percent 24(28) DHE] − percent 24(28) DHE, where F is the factor for dilution in ethanol and 290 and 518 are the E values (in percent per centimeter) determined for crystalline ergosterol and 24(28) DHE, respectively (4). The wet weight of the cell pellet ranged from 1.03 ± 0.04 g for organisms grown without fluconazole to 0.98 ± 0.02 g for organisms grown in 64 μg of fluconazole per ml. The MIC of fluconazole was defined as the concentration of fluconazole which caused an 80% reduction in the total cellular ergosterol content compared to that in the drug-free control. MICs which fell between two fluconazole concentrations (i.e., less than 80% reduction at one concentration but more than 80% reduction at the next-higher concentration) were mathematically extrapolated based on the amount of reduction at the fluconazole concentration which gave results closest to an 80% reduction end point.
Animal model.
ND4 female mice (Harlan Sprague-Dawley, Indianapolis, Ind.) weighing approximately 25 g each were used. Upon arrival at our animal facility, 120 to 200 mice were separated into groups of 10 and allowed to rest for 1 week before experimentation to reduce stress and behavior disturbances among the animals. Mice were given food and water ad libitum. To test the efficacy of fluconazole to therapeutically treat infected mice, randomly selected groups of 10 mice were intravenously infected with each C. albicans isolate using an inoculum size resulting in a mean percent survival without treatment of 29% by day 14 (3.9 × 106, 4 × 105, 3 × 106, and 1.1 × 106 CFU/mouse for isolates 1, 2, 3, and 4, respectively). Fluconazole (prepared in sterile saline) was given by the intraperitoneal route daily at 1 or 5 mg per kg of body weight in 0.2 ml of saline for 5 consecutive days beginning 1 h after infection as described by Rex et al. (17). Separate controls were used for each experimental group. Mice were sacrificed if signs of morbidity were observed, and death was recorded as occurring on the following day. Observation for survival was conducted through day 14.
To study renal tissue burden, mice were infected intravenously with the standardized inoculum used for survival studies. Mice were then treated intraperitoneally (0.2 ml) with fluconazole (1 or 5 mg/kg per day) for 5 consecutive days beginning 1 h after infection. In all cases, control animals received intraperitoneal injections of sterile saline only. Four animals from each group were sacrificed on day 3 and on day 6 postinfection, and both kidneys were aseptically removed and homogenized in 2 ml of sterile saline using a stomacher homogenizer (Seward Medical, London, United Kingdom). Tissue burden was determined by serial dilution of homogenates in physiological saline followed by plating 0.1-ml aliquots onto Sabouraud dextrose agar. Plates were then incubated at 35°C for 48 h before enumeration of colonies formed.
Analysis.
Organ clearance data were compared using Student's t test and values of mean recoverable CFU ± standard deviation. Correlation of SQM and M27-A MICs with response to fluconazole therapy was calculated using Pearson's r.
RESULTS
In vitro susceptibility testing results.
The results of fluconazole MIC testing of the isolates used to infect mice in this study are presented in Table 1. Each isolate was tested by the NCCLS M27-A microbroth dilution method and by the SQM. For isolates which gave unequivocal end points (isolates 1 and 2), the M27-A and the SQM results were similar. For the isolates which exhibited heavy trailing (isolates 3 and 4), the M27-A MIC at 48 h was significantly higher than the SQM MIC and resulted in an interpretation of resistant by the former method and susceptible by the latter method (Table 1).
TABLE 1.
Comparison of fluconazole susceptibilities of isolates by the NCCLS M27-A method and the SQM
| Isolate no. | M27-A method
|
SQM
|
|||
|---|---|---|---|---|---|
| MIC (μg/ml) at:
|
Interpretationa | MIC (μg/ml) | Interpretation | ||
| 24 h | 48 h | ||||
| 1 | 0.125 | 0.125 | S | ≤1.0 | S |
| 2 | 32 | 64 | R | 54 | R |
| 3 | 0.25 | ≥64 | R (tr) | ≤1.0 | S |
| 4 | 0.25 | ≥64 | R (tr) | ≤1.0 | S |
S, susceptible; R (tr), resistant with trailing growth.
Response to therapy in vivo: survival studies.
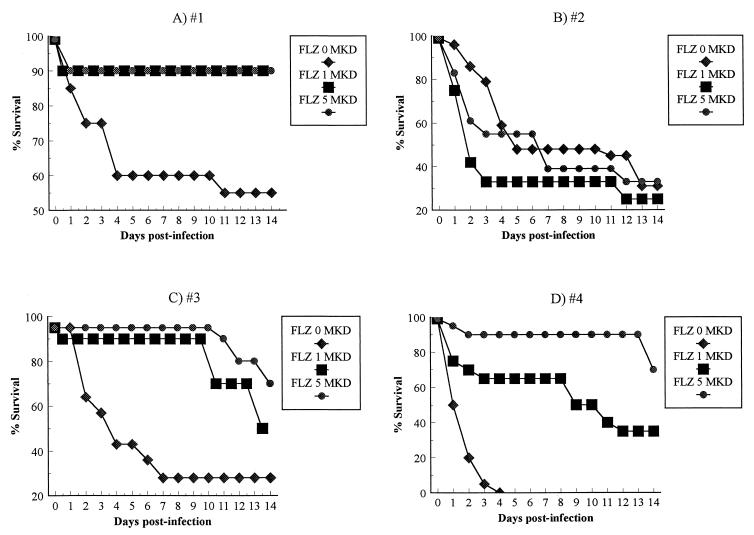
The results of survival studies for mice infected with susceptible (Fig. 1A), resistant (Fig. 1B), and trailing (Fig. 1C and D) C. albicans isolates are shown. Two treatment doses were administered to mice infected with each isolate. At 1 mg/kg/day, fluconazole treatment improved animal survival compared to no treatment by as much as 42% (35 to 42%) by day 13 for all isolates (Fig. 1A, C, and D) except the C. albicans isolate which was resistant by both M27-A and SQM methods (Fig. 1B). Fluconazole doses of 5 mg/kg/day further increased survival of mice infected with trailing isolates by as much as 90% (50 to 90%) above control survival and were as effective as 1 mg/kg/day for mice infected with the susceptible isolate. Either dose (1 or 5 mg/kg/day) not only did not significantly increase survival of mice infected with the fluconazole-resistant isolate but actually reduced survival somewhat in these animals (Fig. 1B).
FIG. 1.
Survival curves for mice infected with C. albicans isolates which were, by the M27-A method, susceptible (A), resistant (B), or resistant with trailing (C and D) to fluconazole. The results represent the means of at least two separate experiments. There were 10 mice per group in each experiment. FLZ, fluconazole; MKD, milligrams per kilogram per day.
Response to therapy in vivo: kidney burden studies.
Data on the reduction in the number of CFU per gram of kidney tissue by fluconazole therapy showed a pattern which paralleled that obtained in the survival studies (Table 2). Fluconazole therapy at 1 and 5 mg/kg/day dramatically decreased the number of CFU in the kidneys of animals infected with the fluconazole-susceptible and trailing isolates but did not reduce kidney burden for animals infected with the fluconazole-resistant isolate. An increase in CFU per gram of kidney tissue for animals infected with the resistant isolate and treated with 5 mg/kg/day fluconazole was observed relative to either the control animals or those treated with the lower dose of fluconazole (1 mg/kg/day).
TABLE 2.
Effect of fluconazole on recovery of CFU from kidney tissue of C. albicans-infected mice
| Isolate no.b | Fluconazole dose (mg/kg/day) | CFU per g of kidney tissue (104) at day 6 sacrifice
|
Pa for indicated pairs of doses (mg/kg/day)
|
|||
|---|---|---|---|---|---|---|
| Median | Range | 0 vs 1 | 0 vs 5 | 1 vs 5 | ||
| 1 (S) | 0 | 57 | 41–73 | |||
| 1 | 1 | 0.9–1.4 | <0.005 | |||
| 5 | 0.53 | 0.32–0.74 | <0.005 | n.s. | ||
| 2 (R) | 0 | 337 | 40–630 | |||
| 1 | 177 | 120–235 | n.s. | |||
| 5 | 583 | 300–1,100 | n.s. | n.s. | ||
| 3 (Rtr) | 0 | 45 | 3.5–78 | |||
| 1 | 5 | 3.5–7.1 | <0.05 | |||
| 5 | 1.5 | 0.7–2.4 | <0.05 | <0.01 | ||
| 4 (Rtr) | 0 | 127 | 63–192 | |||
| 1 | 0.44 | 0.3–0.6 | <0.025 | |||
| 5 | 0.08 | 0.05–0.11 | <0.025 | <0.005 | ||
P values were obtained using Student's t test; n.s., not significant (P > 0.05).
Interpretation of fluconazole susceptibilities as determined by the M27-A broth microdilution method are in parentheses. S, susceptible; Rtr, resistant with trailing growth; R, resistant.
Correlation between SQM MIC and in vivo response to therapy.
The clinical relevance of an MIC value is determined by its capacity to predict response to therapy by that drug in vivo. To evaluate how predictive an SQM MIC was for treatment response in our murine model, we calculated the correlation coefficient between the SQM MIC and survival after fluconazole treatment as well as that between the SQM MIC and fungal burden in the kidneys of infected mice after fluconazole treatment. We also conducted the same analysis using the M27-A MIC results. The data in Table 3 demonstrates that there is a strong inverse correlation between the SQM MICs and survival (Pearson's r = 0.9 or 1.0) and an equally strong positive correlation between the SQM MICs and fungal burden in the kidneys for all isolates tested (Pearson's r = 1.0) except for fungal burden at day 3 postinfection in animals treated with fluconazole at 1 mg/kg/day. The reason for the decreased correlation between SQM MIC and kidney fungal burden at 3 days postinfection may be that the lower dosage of fluconazole may be less effective than the higher dosage to significantly decrease fungal burden in the kidney early during infection. Two additional doses of 1 mg/kg/day were required to result in a reduction of kidney burden evident at day 6 in mice infected with fluconazole-susceptible or trailing isolates (Table 3). While there was good correlation between M27-A MICs and in vivo response for the isolates which were fluconazole susceptible or resistant by both susceptibility test methods, (isolates 1 and 2 only; Pearson's r = 1.0; P < 0.01), there was no significant correlation between the M27-A MICs and survival or kidney burden in animals infected with the two trailing isolates (Table 3). These results indicate that the SQM improves the predictive value of in vitro MIC results for in vivo fluconazole efficacy in our murine model compared to the M27-A method for isolates which display trailing growth.
TABLE 3.
Correlation between the SQM MICs and treatment response compared with the M27-A MICs and treatment response
| Isolate | MIC
|
Day 3
|
Day 6
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SQM | M27-A method | % Survivala
|
Fungal burdenb
|
% Survival
|
Fungal burden
|
|||||
| 1 mg/kg/day [0.9, 0.5]c | 5 mg/kg/day [1.0, 0.3] | 1 mg/kg/day [0.6, 0.8] | 5 mg/kg/day [1.0, 0.2] | 1 mg/kg/day [0.9, 0.5] | 5 mg/kg/day [1.0, 0.3] | 1 mg/kg/day [1.0, 0.3] | 5 mg/kg/day [1.0, 0.2] | |||
| 1 | 1.0 | 0.125 | 90 | 90 | 13 | 19 | 90 | 90 | 1.9 | 9.3 |
| 2 | 54 | >64 | 33 | 55 | 106 | 123 | 33 | 55 | 85 | 86 |
| 3 | 1.0 | >64 (trd) | 90 | 95 | 51 | 0.02 | 90 | 95 | 0.15 | 0.06 |
| 4 | 1.0 | >64 (tr) | 65 | 90 | 93 | 11 | 65 | 90 | 0.67 | 0.13 |
Number of animals alive at the indicated day/total number of animals infected × 100.
104 CFU/g of kidney tissue.
Pearson's coefficients of correlation between the SQM and M27-A MICs (in that order) and therapeutic outcome are given in brackets.
tr, trailing growth.
DISCUSSION
The increased incidence of invasive fungal diseases and the growing problem of antifungal drug resistance in recent years have magnified the need for improved antifungal susceptibility tests which can reliably predict in vivo response to therapy. Fluconazole has become a desirable treatment choice for C. albicans infections because it offers the advantages of both oral and parenteral formulations, excellent bioavailability, and a low level of toxicity (7). Because fluconazole is a fungistatic rather than a fungicidal drug, in vitro MIC end point determinations using standard broth-based methods can be complicated by excessive trailing growth (16). The NCCLS has made substantial progress in standardizing antifungal susceptibility testing, resulting in the publication of the M27-A guidelines and the acceptance of a standard broth macrodilution format (11). This method and the simplified microdilution adaptation of it have greatly improved the reproducibility of MIC results among different laboratories, but it still falls short in accurately determining the fluconazole susceptibilities of Candida isolates which display excessive trailing growth (16, 17). When the NCCLS M27-A guidelines for end point determination are followed, with visual readings after 48 h of incubation, trailing isolates are often interpreted as resistant (11).
Once the standardized M27-A method was in place, interpretative breakpoints for Candida spp. tested against fluconazole were established and studies to evaluate the correlation between in vitro MICs and in vivo outcome were initiated (1, 17). While good correlation has been demonstrated in cases involving AIDS patients with oropharyngeal candidiasis (2, 16), the results have emphasized that an MIC determination is very method dependent and that minor variations in the M27-A method or the breakpoints used to define resistance can significantly alter the resulting MIC and subsequent susceptibility interpretation and therefore the correlation to in vivo outcome (13, 14).
Three general mechanisms of azole resistance have been described for Candida spp. The first is alteration in the target enzyme, lanosterol 14α-demethylase, leading to its overexpression and/or reduced susceptibility to azole inhibition (9, 18, 21). Decreased drug accumulation, mediated by either diminished uptake or increased efflux of the drug, is a second mechanism (12, 19). A third is deficiency in C5(6) sterol desaturase, which suppresses the accumulation of toxic sterol intermediates, as a result of azole-mediated inhibition of lanosterol 14α-demethylase activity (6, 8). The SQM of in vitro susceptibility testing is capable of detecting increased resistance due to any of the above mechanisms based on its ability to determine intracellular ergosterol content following exposure of the organism to fluconazole (3). The SQM is an absolute measurement of ergosterol concentration and not a subjective determination of growth inhibition. Therefore, it removes the subjectivity of end point determination, which can be complicated by trailing growth, and eliminates the problem of falsely high fluconazole resistance rates for organisms which do not give a clear-cut end point due to trailing growth (16, 17).
The ability of an MIC to predict a clinical response to therapy is the ultimate goal of any drug susceptibility test method. Therefore, to evaluate the utility of the SQM to predict fluconazole treatment outcome, we used a murine model of invasive candidiasis and determined the in vivo correlation to the in vitro SQM MIC. The C. albicans isolates used to infect mice in this study represented three distinct fluconazole susceptibility groups, i.e., fluconazole susceptible, resistant, and resistant with trailing. Results showed excellent correlation between the in vitro SQM MIC and the in vivo response to fluconazole in infected animals. Furthermore, isolates which were determined to be fluconazole resistant with trailing by the M27-A method were found to be susceptible by the SQM. The SQM results correlated better with therapeutic outcome in the murine model than did results obtained by the M27-A method for trailing isolates. Interestingly, we also found that the survival of mice infected with a resistant isolate and treated with fluconazole was reduced compared to that of untreated control mice particularly at the lowest dose tested. This is consistent with data reported by Wu et al. (T. Wu, K. Wright, S. F. Hurst, and C. J. Morrison, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. F81, 1999), who also showed that animals infected with fluconazole-resistant isolates and treated with low doses of fluconazole fared worse than the untreated control animals. Data from these studies suggested that this phenomenon was due to increased secretion of aspartyl proteinase, a recognized virulence factor of C. albicans, in response to low doses of fluconazole. Survival could be improved however, when a higher dose (10 mg/kg/day) of fluconazole was administered (Wu et al., Abstr. 99th Gen. Meet. Am. Soc. Microbiol.).
Other studies have found that isolates which give trailing growth are predominately susceptible to fluconazole in vivo even though they would be interpreted as resistant using the strict end point definition of 80% inhibition after 48 h of incubation as recommended by the NCCLS (16, 17). Revankar et al. demonstrated that episodes of oral candidiasis caused by trailing C. albicans isolates (MIC ≥ 64 at 48 h) in patients with human immunodeficiency virus infection responded to low doses of fluconazole equivalent to the level necessary to treat a susceptible infection (16). Rex et al. studied six Candida isolates which exhibited trailing growth when tested against fluconazole using the M27-A method in a murine model of candidiasis and found that the MICs determined at 48 h did not always correlate well with in vivo response to fluconazole (17). In fact, the M27-A MICs significantly overestimated the resistance of three of the six isolates tested (17). While a 24-h MIC end point was found to correlate better with outcome, there was an isolate which had a high MIC at both 24 and 48 h but which responded well to fluconazole treatment in vivo (17). In this case, the authors suggested a less restrictive end point of 50% reduction of growth (determined spectrophotometrically) relative to the control to better correlate the MIC with in vivo outcome (17). It would be interesting to test this isolate to determine how well the SQM susceptibility end point determination correlated with clinical outcome.
Another approach to improve the correlation of M27-A MICs and in vivo outcome for trailing isolates has been to adjust the pH of the RPMI 1640 medium to 4.5 (10). However, it has yet to be determined whether or not the MICs obtained using acidic media correlate with in vivo outcome. Because the SQM is a direct measurement of total intracellular ergosterol content, MIC end point determination using this method is unequivocal even for isolates which exhibit trailing growth. Thus, the dilemma of whether to use a 24-h endpoint, a 50% inhibition cutoff, and/or a low-pH medium is eliminated. Furthermore, preliminary data collected by our laboratory have shown that the SQM may be equally useful for the determination of antifungal drug susceptibilities of filamentous fungi, where determination of visual or spectrophotometric end points may be problematic (5). The correlation between the in vitro drug susceptibilities determined by the SQM versus those determined by the NCCLS M27-A method and the in vivo outcome for filamentous fungal infections remains to be determined.
In summary, the SQM demonstrated good agreement with the NCCLS broth dilution method for isolates which do not exhibit trailing growth and these MICs correlated well with in vivo outcome in the murine model of invasive Candida infection. For isolates with trailing growth, the SQM MIC was more predictive of therapeutic response and may offer increased clinical correlation along with the potential for better therapeutic decision making compared with standard antifungal susceptibility testing methods.
REFERENCES
- 1.Anaissie E J, Karyotakis N C, Hachem R, Dignani M C, Rex J H, Paetznick V. Correlation between in vitro and in vivo activity of antifungal agents against Candida species. J Infect Dis. 1994;170:384–389. doi: 10.1093/infdis/170.2.384. [DOI] [PubMed] [Google Scholar]
- 2.Arikan S, Akova M, Hayran M, Azdemir O, Erman M, Gur D, Unal S. Correlation of in vitro fluconazole susceptibility with clinical outcome for severely ill patients with oropharyngeal candidiasis. Clin Infect Dis. 1998;26:903–908. doi: 10.1086/513927. [DOI] [PubMed] [Google Scholar]
- 3.Arthington-Skaggs B A, Jradi H, Desai T, Morrison C J. Quantitation of ergosterol content: novel method for determination of fluconazole susceptibility of Candida albicans. J Clin Microbiol. 1999;37:3332–3337. doi: 10.1128/jcm.37.10.3332-3337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breivik O N, Owades J L. Spectrophotometric semi-microdetermination of ergosterol in yeast. Agric Food Chem. 1957;5:360–363. [Google Scholar]
- 5.Espinel-Ingroff A, Bartlett M S, Bowden R, Chin N X, Cooper J C R, Fothergill A, McGinnis M R, Menezes P, Messer S A, Nelson P W, Odds F C, Pasarell L, Peter J, Pfaller M A, Rex J H, Rinaldi M G, Shankland G S, Walsh T J, Weitzman I. Multicenter evaluation of proposed standardized procedures for antifungal susceptibility testing of filamentous fungi. J Clin Microbiol. 1997;35:139–143. doi: 10.1128/jcm.35.1.139-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geber A, Hitchcock C A, Swartz J E, Pullen F S, Marsden K E, Kwon-Chung K J, Bennett J E. Deletion of the Candida glabrata ERG3 and ERG11 genes: effect on cell viability, cell growth, sterol composition, and antifungal susceptibility. Antimicrob Agents Chemother. 1995;39:2708–2727. doi: 10.1128/aac.39.12.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graybill J R. Fluconazole and itraconazole: a primer for the professional—part 1. Infect Dis Clin Prac. 2000;9:43–50. [Google Scholar]
- 8.Kelly S L, Lamb D C, Kelly D E, Manning N J, Loeffler J, Hebart H, Schunacher U, Einsele E. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol delta 5,6-desaturation. FEBS Lett. 1997;400:80–82. doi: 10.1016/s0014-5793(96)01360-9. [DOI] [PubMed] [Google Scholar]
- 9.Lamb D C, Kelly D E, Schunck W H, Shyadehi A Z, Akhtar M, Lowe D J, Baldwin B C, Kelly S L. The mutation T315A in Candida albicans sterol 14 alpha-demethylase causes reduced enzyme activity and fluconazole resistance. J Biol Chem. 1997;272:5682–5688. doi: 10.1074/jbc.272.9.5682. [DOI] [PubMed] [Google Scholar]
- 10.Marr K A, Rustad T R, Rex J H, White T C. The trailing end point phenotype in antifungal susceptibility testing is pH dependent. Antimicrob Agents Chemother. 1999;43:1383–1386. doi: 10.1128/aac.43.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 12.Parkinson T, Falconer D J, Hitchcock C A. Fluconazole resistance due to energy-dependent drug efflux in Candida glabrata. Antimicrob Agents Chemother. 1995;39:1696–1699. doi: 10.1128/aac.39.8.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller M A, Rinaldi M G. Antifungal susceptibility testing: current state of technology, limitations and standardization. Infect Dis Clin N Am. 1993;7:435–444. [PubMed] [Google Scholar]
- 14.Pfaller M A, Rinaldi M G, Galgiani J N, Bartlett M S, Body B A, Espinel-Ingroff A, Fromtling R A, Hall G S, Hughes C E, Odds F C, Sugar A M. Collaborative investigations of variables in susceptibility testing of yeasts. Antimicrob Agents Chemother. 1990;34:1648–1654. doi: 10.1128/aac.34.9.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rees J R, Pinner R W, Hajjeh R A, Brandt M E, Reingold A L. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992–1993: results of population-based laboratory active surveillance. Clin Infect Dis. 1998;27:1138–1147. [PubMed] [Google Scholar]
- 16.Revankar S G, Kirkpatrick W R, McAtee R K, Fothergill A W, Redding S W, Rinaldi M G, Patterson T F. Interpretation of trailing endpoints in antifungal susceptibility testing by the National Committee for Clinical Laboratory Standards method. J Clin Microbiol. 1998;36:153–156. doi: 10.1128/jcm.36.1.153-156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rex J H, Nelson P W, Paetznick V L, Lozano-Chiu M, Espinel-Ingroff A, Anaissie E J. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob Agents Chemother. 1998;42:129–134. doi: 10.1128/aac.42.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanglard D, Ischer F, Koymans L, Bille J. Amino acid substitutions in the cytochrome P-450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob Agents Chemother. 1998;42:241–253. doi: 10.1128/aac.42.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanglard D, Kuchler K, Ischer F, Pagani J-L, Monod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanden Bossche H, Dromer F, Improvisi I, Lozano-Chiu M, Rex J H, Sanglard D. Antifungal drug resistance in pathogenic fungi. Med Mycol. 1998;36(Suppl. 1):119–121. [PubMed] [Google Scholar]
- 21.White T C. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14α demethylase in Candida albicans. Antimicrob Agents Chemother. 1997;41:1488–1494. doi: 10.1128/aac.41.7.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]