Abstract
Antimicrobial cationic peptides are ubiquitous in nature and are thought to be a component of the first line of defense against infectious agents. It is widely believed that the killing mechanism of these peptides on bacteria involves an interaction with the cytoplasmic membrane. Cationic peptides from different structural classes were used in experiments with Staphylococcus aureus and other medically important gram-positive bacteria to gain insight into the mechanism of action. The membrane potential-sensitive fluorophore dipropylthiacarbocyanine was used to assess the interactions of selected antimicrobial peptides with the cytoplasmic membrane of S. aureus. Study of the kinetics of killing and membrane depolarization showed that, at early time points, membrane depolarization was incomplete, even when 90% or more of the bacteria had been killed. CP26, a 26-amino-acid α-helical peptide with a high MIC against S. aureus, still had the ability to permeabilize the membrane. Cytoplasmic-membrane permeabilization was a widespread ability and an action that may be necessary for reaching an intracellular target but in itself did not appear to be the killing mechanism. Transmission electron microscopy of S. aureus and Staphylococcus epidermidis treated with CP29 (a 26-amino-acid α-helical peptide), CP11CN (a 13-amino-acid, proline- and tryptophan-rich peptide), and Bac2A-NH2 (a linearized version of the 12-amino-acid loop peptide bactenecin) showed variability in effects on bacterial structure. Mesosome-like structures were seen to develop in S. aureus, whereas cell wall effects and mesosomes were seen with S. epidermidis. Nuclear condensation and abherrent septation were occasionally seen in S. epidermidis. Our experiments indicated that these peptides vary in their mechanisms of action and that the mechanism of action likely does not solely involve cytoplasmic-membrane permeabilization.
Antimicrobial cationic peptides are ubiquitous in nature and are thought to be an important component in innate host defenses against infectious agents (11). There are four structural classes of cationic peptides: the disulfide-bonded β-sheet peptides (including the defensins), the amphipathic α-helical peptides such as the cecropins and melittins, the extended peptides which often have a single amino acid predominating (e.g., indolicidin), and the loop-structured peptides like bactenecin (11). The initial interactions of some cationic peptides with gram-negative bacteria are thought to involve binding to surface lipopolysaccharide (22, 24). The peptides displace divalent cations that are essential for outer membrane integrity and consequently distort the outer membrane bilayer (21). This allows access to the cytoplasmic membrane, where peptide channel formation has been proposed to occur (17). It is increasingly disputed as to whether peptide channel formation leads to dissolution of the proton motive force and leakage of essential molecules (5, 12, 32) or whether it is an intermediate step in the uptake of peptide into the cytoplasm, where it inhibits an essential function by, e.g., binding to polyanionic DNA (19, 35).
Various studies of the effects of cationic peptides on the membranes of gram-positive bacteria have been conducted. Some cationic peptides have been shown to interact with the cytoplasmic membrane in a voltage-dependent manner (7, 16). However, other studies have indicated that the requirements for transmembrane potential vary among cationic peptides. For example, Koo et al. when studying a related pair of Staphylococcus aureus strains differing in membrane potential gradient (Δψ) generation (14) showed that defensins either are transmembrane potential independent or have a low threshold Δψ for activity compared with that of thrombin-induced platelet microbicidal protein-1 (tPMP). Using flow cytometry, Yeaman et al. (34) showed that human neutrophil defensin-1 (HNP-1) depolarized and permeabilized the cytoplasmic membrane of S. aureus in vitro but that tPMP-1 did not depolarize but did permeabilize the membrane. It has also been shown that the properties of the cytoplasmic membrane itself may influence bacterial susceptibility to cationic peptides. Protoplasts from resistant and stationary-phase S. aureus cells were less susceptible to lysis and were more intact after treatment with tPMP-1 than protoplasts from susceptible and log-phase bacteria (15). In addition, ultrastructural studies of S. aureus treated with defensins (27) and platelet microbicidal proteins (34) showed cell membrane damage followed by cell death. The defensins caused mesosome-like structures to appear before the bacteria lost their viability, but no remarkable effects on the cell wall were seen (27). These studies suggested that membrane disruption is an important, but not necessarily lethal, event. Recent studies have indicated that some peptides may indeed have an intracellular target. Xiong et al. (33) found that S. aureus, pretreated with inhibitors of DNA gyrase or protein synthesis, demonstrated decreased or blocked killing by HNP-1 and tPMP-1 and that pretreatment with bacterial cell wall synthesis inhibitors enhanced bacterial killing. Those authors concluded that these cytoplasmic-membrane effects occurred prior to effects on protein and DNA synthesis. Autolysin activation has been implicated as a mode of action of nisin and Pep5 on S. aureus (3) but was not found to be a significant mechanism for HNP-1 and tPMP-1 (33). In addition, a direct correlation was observed between tolerance to antibacterial cationic peptides and the d-Ala content of teichoic acids, a polymer in the peptidoglycan layers of gram-positive bacteria (20). However, another study (25) found no correlation between binding to lipoteichoic acid and MIC.
The 26-amino-acid α-helical peptides CP26 and CP29 (8) are derived from a series of hybrid peptides consisting of the amphipathic, α-helical N-terminal region of cecropin A followed by the hydrophobic N-terminal α-helix of the bee venom peptide melittin (30). The 13-amino-acid peptide indolicidin (26) and its improved derivative CP11CN (6) have unique amino acid compositions consisting of very high percentages of tryptophan and proline and are amidated at their C termini. These peptides have been shown to have an extended structure distinct from that of α-helical and β-structured peptides (7, 23). CP10A is a peptide in which the three proline residues of indolicidin are replaced with alanine (29). Bac2A-NH2 is a linearized, N-terminally amidated version of the 12-amino-acid cyclic peptide bactenecin and has better activity against gram-positive bacteria than that of the native bactenecin (31). In this study we have investigated the effects of these peptides on gram-positive bacteria.
MATERIALS AND METHODS
Materials and bacterial strains.
All peptides (Table 1) were synthesized by N-(9-fluorenyl)methoxy carbonyl chemistry at the Nucleic Acid Protein Service unit at the University of British Columbia. Bovine serum albumin fraction V lyophilizate was purchased from Boehringer Mannheim (Mannheim, Germany). Dipropylthiacarbocyanine [DiSC3(5)] was purchased from Molecular Probes (Eugene, Oreg.). Valinomycin was purchased from Sigma (St. Louis, Mo.).
TABLE 1.
Amino acid sequences of antimicrobial cationic peptides used in this study
| Peptide | Amino acid sequence | Length | Charge | % of amino acids that are hydrophobic |
|---|---|---|---|---|
| CP26 | KWKSFIKKLTSAAKKVVTTAKPLISS | 26 | +7 | 46 |
| CP29 | KWKSFIKKLTTAVKKVLTTGLPALIS | 26 | +6 | 50 |
| CP10CN | ILPWKWPWWPWRR-NH2 | 13 | +3 | 77 |
| CP11CN | ILKKWPWWPWRRK-NH2 | 13 | +5 | 62 |
| CP10A | ILAWKWAWWAWRR-NH2 | 13 | +3 | 77 |
| Bac2A-NH2 | RLARIVVIRVAR-NH2 | 12 | +4 | 67 |
Strains used for determining antimicrobial activity included S. aureus ATCC 25923, S. aureus SAP0017 (methicillin-resistant clinical isolate, a gift from Tony Chow, University of British Columbia) (25), Staphylococcus epidermidis (clinical isolate, a gift from David Speert, University of British Columbia) (25), Enterococcus faecalis ATCC 29212, Listeria monocytogenes NCTC 7973, Streptococcus pyogenes ATCC 19615, Corynebacterium xerosis (from the University of British Columbia Department of Microbiology collection), and Staphylococcus haemolyticus and a vancomycin-resistant mutant (S. S. Lee, E. Bryce, S. Byrne, J. E. Davies, and A. W. Chow, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-140, 1998). The same S. aureus and S. epidermidis strains were used for electron microscopy, and S. aureus ATCC 25923 was also used in the cytoplasmic-membrane depolarization experiments. For most bacteria Luria-Bertani (LB) medium (no salt) (Difco) was used as the growth medium; the exception was S. pyogenes, which was grown in Todd-Hewitt (Difco) broth.
MIC.
The MIC of each peptide was determined using a broth microdilution assay modified from the method of Amsterdam (1). Briefly, serial dilutions of each peptide were made in 0.2% bovine serum albumin–0.01% acetic acid solution in 96-well polypropylene (Costar, Corning Incorporated, Corning, N.Y.) microtiter plates. Each well was inoculated with 100 μl of the test organism in LB (no-salt) broth to a final concentration of approximately 105 CFU/ml. The MIC was taken as the lowest peptide concentration at which growth was inhibited after 24 h of incubation at 37°C.
Bacterial killing assays.
Overnight cultures were diluted 10−2 in LB (no-salt) broth and allowed to grow to exponential phase (optical density at 600 nm of 0.6) and then diluted in fresh medium to give a working concentration of 106 to 107 cells/ml. The peptides or antibiotics were added at 10 times their MICs, and this suspension was incubated at 37°C. At regular intervals after peptide addition, samples were removed, diluted 10−3 or 10−4, and plated onto LB agar plates to obtain a viable count. Kill assays done alongside the depolarization assay differed from the assay described above in that aliquots were removed from samples in the assay buffer, diluted, and plated onto LB agar plates.
Cytoplasmic-membrane depolarization assay.
The depolarization of the cytoplasmic membrane of S. aureus ATCC 25923 by the peptides was determined using the membrane potential-sensitive cyanine dye DiSC3(5) (28) by a modification of the method of Wu et al. (32). Briefly, exponential-phase bacteria were washed and resuspended in 5 mM HEPES–20 mM glucose buffer (pH 7.2) to an optical density of 0.05. This cell suspension was incubated with 100 mM KCl (to equilibrate cytoplasmic and external K+ concentration) and 0.4 μM DiSC3(5) until there was a stable (approximately 90%) reduction in fluorescence due to DiSC3(5) uptake and quenching in the cell in response to an intact membrane potential. A 1-ml aliquot of cell suspension was placed in a cuvette, and the desired concentration of peptide was added. Fluorescence was monitored in a model 650-10S fluorescence spectrometer (Perkin-Elmer Corp., Norwalk, Conn.) at an excitation wavelength of 622 nm and an emission wavelength of 670 nm. Aliquots were removed at time intervals in order to obtain a viable count.
Transmission electron microscopy.
Exponential-phase bacteria were treated with the peptide (at 10 times the MIC) for 30 min at 37°C. This concentration was used in order to see an effect on a greater percentage of cells. After treatment, the bacterial pellets were embedded in 2% Noble agar (Difco) and fixed with 2.5% buffered glutaraldehyde for 1 h. The cells were then postfixed in 1% buffered osmium tetroxide for 1 h, stained en bloc with 1% uranyl acetate, dehydrated in a graded series of ethanol, and embedded in L.R. (London Resin Co. Ltd.) white resin. The buffer used was 0.1 M sodium cacodylate, pH 7.4. Thin sections were prepared on Formvar, carbon-stabilized copper grids and stained with 1% uranyl acetate and lead citrate. The resin and grids were purchased from Marivac (Halifax, Nova Scotia, Canada). Microscopy was performed with a Philips EM300 microscope under standard operating conditions.
RESULTS
Antimicrobial activity.
The amino acid sequences and characteristics of the peptides used in this study are shown in Table 1. The MICs of the peptides for a panel of gram-positive bacteria are shown in Table 2. CP26 was the least effective peptide, with MICs of 16 μg/ml or greater for all bacteria except C. xerosis. CP29, an α-helical peptide closely related to CP26, had MICs two- to eightfold better than those of CP26 against most bacteria. CP11CN was previously shown to be more active than its parent peptide, indolicidin, for gram-negative bacteria (6) but had equivalent or worse activity against gram-positive bacteria. However, replacing the three proline residues of indolicidin with alanines resulted in a peptide (CP10A) that was more active against most gram-positive bacteria and was the only indolicidin variant with appreciable activity against E. faecalis. In general, CP10A and the linearized bactenecin Bac2A-NH2 had the best activity against gram-positive bacteria. The MICs of the peptides for methicillin-resistant S. aureus and vancomycin-resistant S. haemolyticus were not significantly different from those for the nonresistant strains.
TABLE 2.
MICs of cationic peptides for various gram-positive bacteria
| Species | Strain | MIC (μg/ml) ofa:
|
|||||
|---|---|---|---|---|---|---|---|
| CP26 | CP29 | Indolicidin | CP11CN | CP10A | Bac2A-NH2 | ||
| Staphylococcus aureus | ATCC 25923 | 64 | 8 | 8 | 16 | 4 | 4 |
| Staphylococcus aureus | SAP0017 MRSAb | 64 | 8 | 8 | 16 | 4 | 16 |
| Staphylococcus haemolyticus | Clinical isolate | 64 | 8 | 2 | 2 | 2 | 1 |
| Staphylococcus haemolyticus | Vancomycin-resistant isolate | >64 | 16 | 1 | 1 | 1 | 1 |
| Staphylococcus epidermidis | Clinical isolate | 32 | 16 | 4 | 8 | 2 | 1 |
| Enterococcus faecalis | ATCC 29212 | >64 | 64 | >64 | >64 | 8 | 2 |
| Listeria monocytogenes | NCTC 7973 | 16 | 4 | 4 | 16 | 1 | 0.25 |
| Streptococcus pyogenes | ATCC 19615 | 16 | 8 | 4 | 8 | 1 | 2 |
| Corynebacterium xerosis | Lab strain | 1 | 2 | 0.5 | 0.5 | 1 | 0.25 |
These results were taken from three separate experiments, where the MICs never differed by more than 1 twofold dilution.
MRSA, methicillin-resistant S. aureus.
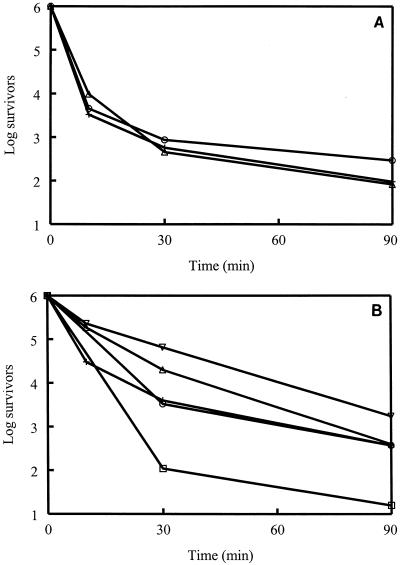
Killing curve data (Fig. 1) revealed 2 to 3 orders of magnitude of killing of S. aureus over 90 min with 10-fold the MICs of indolicidin, CP11CN, Bac2A-NH2, and CP29. CP10A caused between 4 and 5 log orders of killing of S. aureus in the same time frame. CP11CN, CP29, and Bac2A-NH2 also caused a decrease of between 3 and 4 log orders for S. epidermidis. The addition of alginate, a negatively charged antagonist of peptides (8), to the dilution buffer did not have an effect on the killing results (data not shown).
FIG. 1.
Numbers of survivors (in log units) of S. aureus (A) and S. epidermidis (B) in the presence of CP11CN (+), CP29 (○), Bac2A-NH2 (▵) and numbers of survivors of S. aureus (A) in the presence of indolicin (▿) and CP10A (□). All peptides were at concentrations 10 times the MIC. Results are representative of two to three separate experiments.
Cytoplasmic-membrane depolarization.
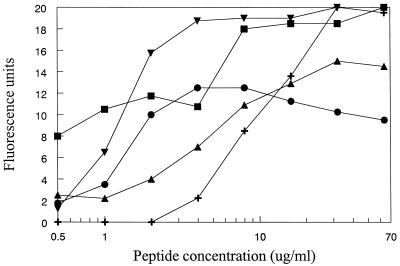
Cytoplasmic-membrane permeabilization has been implicated in the mode of action of peptides against gram-negative bacteria through the use of the ONPG (o-nitrophenyl-β-d-galactopyranoside) assay (6, 8, 17) and, more recently, the DiSC3(5) assay (32). We were interested in determining the effects of the peptides on the membrane of S. aureus and therefore adapted the DiSC3(5) assay used previously by Wu et al. (32). Figure 2 shows membrane depolarization, demonstrated here by an increase in fluorescence units, as a function of peptide concentration. A value of approximately 20 fluorescence units was equivalent to complete depolarization as determined by the use of valinomycin as a positive control, as well as the fluorescence of the dye alone in buffer. All of the peptides studied here had the ability to depolarize the cytoplasmic membrane of S. aureus; however, peptides with different structures had different concentration-activity profiles (Fig. 2). CP26 and CP29 completely depolarized the membrane at lower concentrations than those of the other peptides studied, with 50% depolarization at between 1 and 2 μg/ml. It is important to note that CP26 had very poor MICs against S. aureus but was still able to kill S. aureus in proportion to its concentration. In addition, CP29 had an MIC well above the concentration required for complete depolarization. Conversely, the peptide with the lowest MIC, Bac2A-NH2, did not depolarize the membrane at low concentrations, with 50% depolarization occurring at concentrations between 8 and 16 μg/ml. Indolicidin and CP11CN were depolarizers at relatively low concentrations, but they caused at maximum only 50 to 75% depolarization of the cells.
FIG. 2.
Permeabilization of the cytoplasmic membrane of S. aureus as a function of peptide concentration, indicated by maximum fluorescence reached within 5 min. Shown are results with CP11CN (▴), indolicidin (●), CP29 (▾), CP26 (■), and Bac2A-NH2 (+). Results are representative of two to three separate experiments.
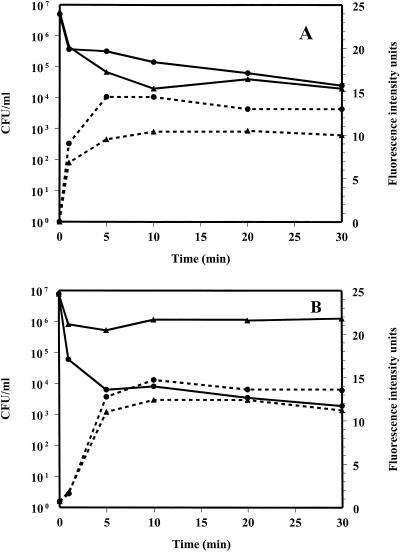
Because the assay conditions included 100 mM KCl in the buffer, MICs were determined in the presence of 100 mM KCl. In general, the addition of 100 mM KCl increased the MIC of the peptides for S. aureus. The MIC of CP29 was increased fourfold from 16 to 64 μg/ml. Indolicidin and CP11CN both demonstrated an 8-fold increase in MIC, while Bac2A-NH2 had a 16-fold increase in MIC. However, the MICs of these peptides in the presence of KCl were all well above the amount needed to cause permeabilization. CP10A affected the fluorescence of the dye and therefore could not be used in this assay. The viabilities of cells taken directly from the assay tube, at one peptide concentration, are shown in Fig. 3. The α-helical peptides (Fig. 3A) and indolicidins (Fig. 3B) were chosen for these experiments. In Fig. 3A, the concentrations of the peptides were chosen to give similar cytoplasmic-membrane permeabilization profiles, resulting in a concentration of CP26 fourfold higher than that of CP29; however, CP26 caused 1 log order of killing of bacteria, whereas CP29 resulted in almost 4 log orders of killing. It also appeared that for all peptides, 90% or more of the killing was complete at a point where either little to no depolarization (Fig. 3A) or less than 50% of complete depolarization (Fig. 3B) has occurred.
FIG. 3.
Permeabilization of the cytoplasmic membrane of S. aureus (dashed lines) as indicated by the kinetics of fluorescence intensity changes in the presence of 8 μg of CP26 (▴) per ml or 2 μg of CP29 (●) per ml (A) and in the presence of 32 μg of indolicidin (▴) or CP11CN (●) per ml (B). Shown are the levels of survival (in CFU per milliliter) of bacteria under the dye assay conditions (solid lines). Results are representative of two to three separate experiments.
Electron microscopy.
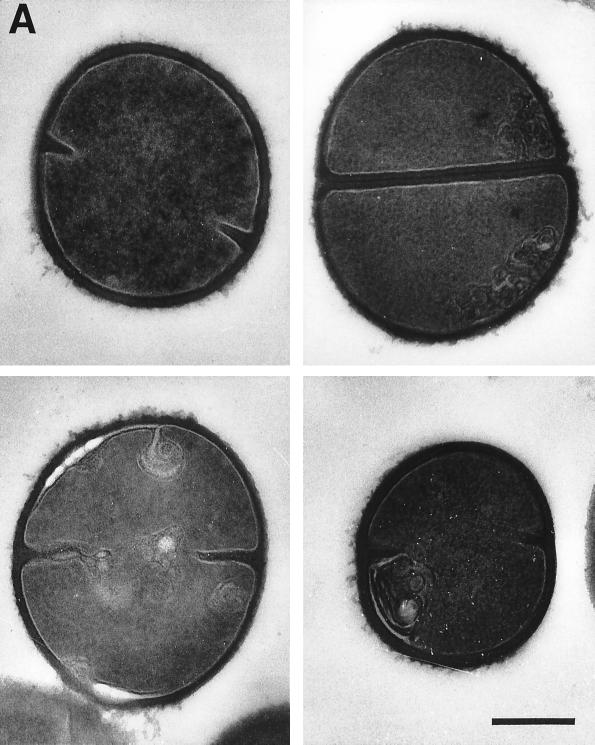
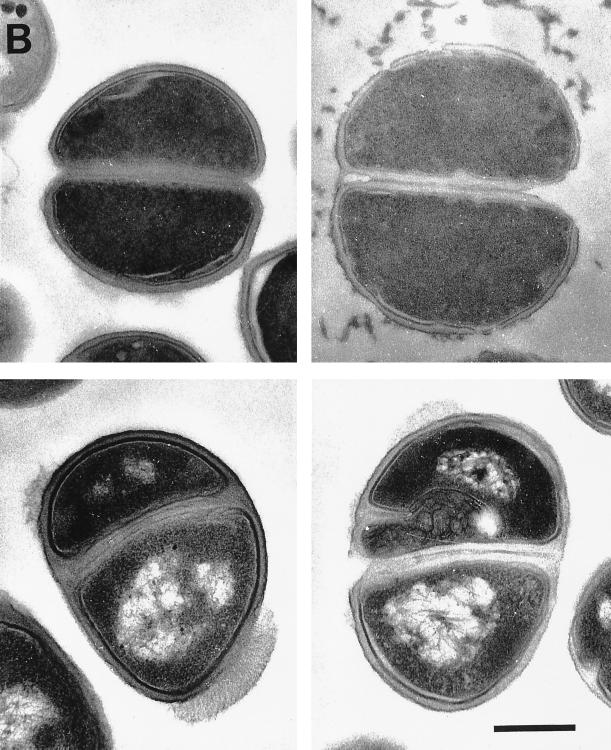
To search for clues to possible alternative mechanisms of action of peptides on gram-positive bacteria, transmission electron microscopy was performed on thin sections of bacteria that had been treated with the peptide for 30 min. CP29, Bac2A-NH2, and CP11CN (Fig. 4A) showed similar effects on S. aureus. Laminar mesosomes were seen arising from the septa and cell wall. Control untreated bacteria did not have detectable mesosome structures. The mesosomal structures caused by Bac2A-NH2 appeared to be not as large as those caused by CP11CN. Some lysis was seen, but with Bac2A-NH2, lysis was seen occurring at the septal site. With S. epidermidis (Fig. 4B) greater effects were seen. All three peptides caused cell wall effects, which included fibers extending from the cell surface. CP11CN appeared to have the most severe effects on the cell wall, including cell wall breaks and variability in wall thickness. CP11CN also caused mesosome structures as well as the condensation of the DNA in S. epidermidis. Bac2A-NH2 showed effects similar to those caused by CP11CN, in addition to abnormal septum formation. The cytoplasmic membrane could be seen separating from the cell wall in some Bac2A-NH2-treated cells and forming mesosomes. CP29 appeared to cause the most lysis compared to the other peptides, but residual intact cells showed no signs of either DNA condensation or mesosome formation. However, CP29, like Bac2A-NH2, caused abnormal septal wall formation as well as cell wall disintegration.
FIG. 4.
Electron micrographs of untreated (top left), CP29-treated (top right), CP11CN-treated (bottom left), and Bac2A-NH2-treated (bottom right) S. aureus (A) and S. epidermidis (B). All peptides were at concentrations 10 times the MIC. The bar is equal to 250 nm.
DISCUSSION
The exact mode of action of cationic antimicrobial peptides on gram-positive bacteria is unknown. However, it has been proposed and is widely believed that the peptides interact with and disrupt the cytoplasmic membrane, leading to the dissolution of the proton motive force and leakage of essential molecules, resulting in cell death. Recently, Castle et al. demonstrated that apidaecins, which are short proline-arginine rich peptides, require a stereospecific interaction in order to enter Escherichia coli cells and kill via protein synthesis inhibition (4). In addition, there has been evidence for an intracellular or alternative target (33) as well as important interactions with the cell walls (20) of gram-positive bacteria. Here, we investigated these issues using peptides with different structures.
The peptides showed various activities against a panel of gram-positive bacteria. CP26, an α-helical peptide with excellent activity against gram-negative bacteria comparable to that of CP29 (8), had little activity against gram-positive bacteria, with the exception of C. xerosis. CP26 is similar to CP29 in that CP26 shares the same N-terminal amino acids as CP29, but the middle hinge region was made more amphipathic and flexible with an added alanine and the C terminus was modified to be more α-helical and hydrophilic. Despite their similar sequences (six amino acids different), CP29 was more active against most gram-positive bacteria. CP11CN, a variant of indolicidin, had improved activity against gram-negative bacteria (6) but was not better against gram-positive bacteria. In contrast, CP10A had the best activity of the indolicidin variants against gram-positive bacteria and its activity against gram-negative bacteria was approximately equal to that of CP11CN (data not shown). Interestingly, CP10A had significant activity against E. faecalis, which is normally resistant to many cationic peptides. All peptides killed methicillin-resistant S. aureus and vancomycin-resistant S. haemolyticus to an extent similar to that of the nonresistant strains, indicating that the mechanisms of methicillin and vancomycin resistance did not affect the peptides, further evidence that the mechanism of action of these peptides is likely very different from that of conventional antibiotics. Thus, the best peptides studied here had activity against a broad range of gram-positive bacteria.
Previously, the DiSC3(5) assay was used to demonstrate disruption of the E. coli cytoplasmic membrane by cationic peptides (32). Here, we further adapted the DiSC3(5) assay for use with S. aureus. The peptides showed considerable differences in this assay, and as shown with E. coli, there was no obvious correlation between cytoplasmic-membrane depolarization and antimicrobial activity (32). For example, CP26, which had the lowest activity of all the peptides, had one of the best abilities to permeabilize at low concentrations, and along with the other α-helical peptide, CP29, caused complete depolarization by 8 μg/ml. CP29, in 100 mM KCl, had MICs well above the concentration needed for complete depolarization, consistent with the results for indolicidin, CP11CN, and Bac2A-NH2. Bac2A-NH2, the peptide with the best MIC in the absence of KCl, did not permeabilize well at low concentrations. It is important to note that depolarization of the cytoplasmic membrane is not, per se, a lethal event, as the depolarizer valinomycin in the presence of 100 mM KCl is in fact bacteriostatic and not bactericidal.
Killing curves done in conjunction with the depolarization assay indicated that similar cytoplasmic-membrane permeabilization profiles did not correspond with similar killing rates, as was shown for the closely related α-helical peptides (Fig. 3A); for example, CP29 killed significantly more bacteria than CP26 without differing significantly from it in permeabilization. In addition, a significant reduction in numbers of bacteria (90 to 99%) appeared to occur within the first minute after addition of the peptide, at which point the permeabilization of the cytoplasmic membrane was not complete by the α-helical peptides (Fig. 3A) and only approximately 50% of the maximum permeabilization possible with indolicidin and CP11CN had occurred (Fig. 3B). This is in contrast to the results with HNP-1 and tPMP-1, with which the cytoplasmic membrane of S. aureus was depolarized within minutes but cell death occurred 1 to 2 h later (34). The passage of the peptide through the membrane in order to reach an intracellular target is expected to cause an increase in membrane permeability, and this might account for the lag time between depolarization and killing. Membrane permeabilization occurring after cell death may be a secondary or subsidiary effect of the peptides. These results are thus consistent with the view that cytoplasmic-membrane permeabilization is not the primary target for bacterial killing but that a certain level of permeabilization is required in order to reach an intracellular target.
Electron microscopy showed that there was frequently an effect on the S. aureus membrane, demonstrated by the appearance of mesosome structures similar to those seen with defensins (27), trimethoprim (18), and rifampin (9). Mesosomes, which are intracytoplasmic membrane inclusions, have been regarded as structural artifacts induced by the chemical fixatives used on the cells prior to plastic embedding and thin sectioning (2). Yet mesosomes must be regarded as being indicative of cytoplasmic membrane alteration, in this case induced by the cationic peptides, since untreated cells did not contain them. Furthermore, since the cytoplasmic membrane is instrumental in cell wall synthesis and turnover, a perturbation of this membrane may also affect cell wall integrity and autolysin regulation (13). Accordingly, the very fact that mesosome-like structures were seen in most treated cells is indicative of cytoplasmic-membrane alteration and (possibly) uncoupling of the synthesis and turnover of cell wall polymers. Clearly, lysis occurred frequently, and with Bac2A-NH2, this lysis was apparently initiated at the septal site. With S. epidermidis, however, more diverse effects were seen. All peptides caused cell wall effects such as cell wall breaks, thinning, and disintegration as well as abnormal septation. Interestingly, trimethoprim also causes defects in cell wall formation and irregular cross-wall formation similar to those seen here (18). CP29, unlike Bac2A-NH2 and CP11CN, did not cause mesosome-like structures or nuclear condensation in S. epidermidis. These various macroscopic effects indicated that these peptides might kill cells in different ways (Fig. 4). We propose that there are multiple possible anionic targets of peptide action against bacteria, including the DNA, RNA, cell wall, cytoplasmic membrane, and various enzymes, and that these targets are differentially accessed depending on the peptide and bacterium in question. Although the exact mechanism of action of peptides on gram-positive bacteria has not been resolved, the evidence reported here is consistent with a multiple-hit model, as has been discussed for polycationic aminoglycosides (10), and with the multimodal model presented for tPMP-1 and HNP-1 (33). Further insight into the mechanism of action will aid in the production of cationic antimicrobial peptides as future therapeutics.
ACKNOWLEDGMENTS
This work was financially supported by the Canadian Bacterial Diseases Network. R.E.W.H. is a recipient of the Medical Research Council of Canada Distinguished Scientist award. The electron microscopy was performed in the Natural Sciences and Engineering Research Council of Canada (NSERC) Guelph Regional Scanning Transmission Electron Microscope (STEM) Facility located in the department of Microbiology of the University of Guelph, which is partially funded by an NSERC Major Facilities Access grant to T.J.B.
REFERENCES
- 1.Amsterdam D. Susceptibility testing of antimicrobials in liquid media. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: Williams and Wilkins; 1996. pp. 52–111. [Google Scholar]
- 2.Beveridge T J. The structure of bacteria. In: Leadbetter E R, Poindexter J S, editors. Bacteria in nature: a treatise on the interaction of bacteria and their habitats. Vol. 3. New York, N.Y: Plenum Publishing Co.; 1989. pp. 1–65. [Google Scholar]
- 3.Bierbaum G, Sahl H G. Induction of autolysis of staphylococci by the basic peptide antibiotics Pep 5 and nisin and their influence on the activity of autolytic enzymes. Arch Microbiol. 1985;141:249–254. doi: 10.1007/BF00408067. [DOI] [PubMed] [Google Scholar]
- 4.Castle M, Nazarian A, Yi S S, Tempst P. Lethal effects of apidaecin on Escherichia coli involve sequential molecular interactions with diverse targets. J Biol Chem. 1999;274:32555–32564. doi: 10.1074/jbc.274.46.32555. [DOI] [PubMed] [Google Scholar]
- 5.Cociancich S, Ghazi A, Hoffman J A, Hetrus C, Letellier C. Insect defensin, an inducible antibacterial peptide, forms voltage-dependent channels in Micrococcus luteus. J Biol Chem. 1993;268:19239–19245. [PubMed] [Google Scholar]
- 6.Falla T J, Hancock R E. Improved activity of a synthetic indolicidin analog. Antimicrob Agents Chemother. 1997;41:771–775. doi: 10.1128/aac.41.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falla T J, Karunaratne D N, Hancock R E W. Mode of action of the antimicrobial peptide indolicidin. J Biol Chem. 1996;271:19298–19303. doi: 10.1074/jbc.271.32.19298. [DOI] [PubMed] [Google Scholar]
- 8.Friedrich C, Scott M G, Karunaratne N, Yan H, Hancock R E. Salt-resistant alpha-helical cationic antimicrobial peptides. Antimicrob Agents Chemother. 1999;43:1542–1548. doi: 10.1128/aac.43.7.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottfredsson M, Erlendsdottir H, Kolka R, Gudmundsson A, Gudmundsson S. Ultrastructural alterations of bacteria during the postantibiotic effect. Chemotherapy. 1993;39:153–162. doi: 10.1159/000239120. [DOI] [PubMed] [Google Scholar]
- 10.Hancock R E. Aminoglycoside uptake and mode of action—with special reference to streptomycin and gentamicin. II. Effects of aminoglycosides on cells. J Antimicrob Chemother. 1981;8:429–445. doi: 10.1093/jac/8.6.429. [DOI] [PubMed] [Google Scholar]
- 11.Hancock R E W, Falla T J, Brown M. Cationic antimicrobial peptides. Adv Microb Physiol. 1995;37:136–175. doi: 10.1016/s0065-2911(08)60145-9. [DOI] [PubMed] [Google Scholar]
- 12.Juretic D, Chan H C, Brown J H, Morell J L, Hendler R W, Westerhoff H. Magainin 2 amide and analogues, antimicrobial activity, membrane depolarization and susceptibility to proteolysis. FEBS Lett. 1989;249:219–223. doi: 10.1016/0014-5793(89)80627-1. [DOI] [PubMed] [Google Scholar]
- 13.Kemper M A, Urrutia M M, Beveridge T J, Koch A L, Doyle R J. Proton motive force may regulate cell wall-associated enzymes of Bacillus subtilis. J Bacteriol. 1993;175:5690–5696. doi: 10.1128/jb.175.17.5690-5696.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koo S P, Bayer A S, Sahl H G, Proctor R A, Yeaman M R. Staphylocidal action of thrombin-induced platelet microbicidal protein is not solely dependent on transmembrane potential. Infect Immun. 1996;64:1070–1074. doi: 10.1128/iai.64.3.1070-1074.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koo S P, Yeaman M R, Nast C C, Bayer A S. The cytoplasmic membrane is a primary target for the staphylocidal action of thrombin-induced platelet microbicidal protein. Infect Immun. 1997;65:4795–4800. doi: 10.1128/iai.65.11.4795-4800.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kordel M, Benz R, Sahl H G. Mode of action of the staphylococcinlike peptide Pep 5: voltage-dependent depolarization of bacterial and artificial membranes. J Bacteriol. 1988;170:84–88. doi: 10.1128/jb.170.1.84-88.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehrer R I, Barton A, Daher K A, Harwig S S, Ganz T, Selsted M E. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J Clin Investig. 1989;84:553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishino T, Wecke J, Kruger D, Giesbrecht P. Trimethoprim-induced structural alterations in Staphylococcus aureus and the recovery of bacteria in drug-free medium. J Antimicrob Chemother. 1987;19:147–159. doi: 10.1093/jac/19.2.147. [DOI] [PubMed] [Google Scholar]
- 19.Park C B, Kim H S, Kim S C. Mechanism of action of the antimicrobial peptide buforin II: buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochem Biophys Res Commun. 1998;244:253–257. doi: 10.1006/bbrc.1998.8159. [DOI] [PubMed] [Google Scholar]
- 20.Peschel A, Otto M, Jack R W, Kalbacher H, Jung G, Gotz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem. 1999;274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 21.Peterson A A, Fesik S W, McGroarty E J. Decreased binding of antibiotics to lipopolysaccharide from polymyxin-resistant strains of Escherichia coli and Salmonella typhimurium. Antimicrob Agents Chemother. 1987;31:230–237. doi: 10.1128/aac.31.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piers K L, Hancock R E W. The interaction of a recombinant cecropin/melittin hybrid peptide with the outer membrane of Pseudomonas aeruginosa. Mol Microbiol. 1994;12:951–958. doi: 10.1111/j.1365-2958.1994.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 23.Rozek, A., C. L. Friedrich, and R. E. W. Hancock. The unique structure of the bovine antimicrobial peptide indolicidin bound to dodecyl phosphocholine micelles. Submitted for publication. [PubMed]
- 24.Sawyer J G, Martin N L, Hancock R E. Interaction of macrophage cationic proteins with the outer membrane of Pseudomonas aeruginosa. Infect Immun. 1988;56:693–698. doi: 10.1128/iai.56.3.693-698.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott M G, Gold M R, Hancock R E W. Interaction of cationic peptides with lipoteichoic acid and gram-positive bacteria. Infect Immun. 1999;67:6445–6453. doi: 10.1128/iai.67.12.6445-6453.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selsted M E, Novotny M J, Morris W L, Tang Y Q, Smith W, Cullor J S. Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J Biol Chem. 1992;267:4292–4295. [PubMed] [Google Scholar]
- 27.Shimoda M, Ohki K, Shimamoto Y, Kohashi O. Morphology of defensin-treated Staphylococcus aureus. Infect Immun. 1995;63:2886–2891. doi: 10.1128/iai.63.8.2886-2891.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sims P J, Waggoner A S, Wang C H, Hoffman J F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974;13:3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- 29.Subbalakshmi C, Krishnakumari V, Nagaraj R, Sitaram N. Requirements for antibacterial and hemolytic activities in the bovine neutrophil derived 13-residue peptide indolicidin. FEBS Lett. 1996;395:48–52. doi: 10.1016/0014-5793(96)00996-9. [DOI] [PubMed] [Google Scholar]
- 30.Wade D, Andreu D, Mitchell S A, Silveira A M, Boman A, Boman H G, Merrifield R B. Antibacterial peptides designed as analogs or hybrids of cecropins and melittin. Int J Pept Protein Res. 1992;40:429–436. doi: 10.1111/j.1399-3011.1992.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 31.Wu M, Hancock R E W. Interaction of the cyclic antimicrobial cationic peptide bactenecin with the outer and cytoplasmic membrane. J Biol Chem. 1999;274:29–35. doi: 10.1074/jbc.274.1.29. [DOI] [PubMed] [Google Scholar]
- 32.Wu M, Maier E, Benz R, Hancock R E W. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry. 1999;38:7235–7242. doi: 10.1021/bi9826299. [DOI] [PubMed] [Google Scholar]
- 33.Xiong Y Q, Yeaman M R, Bayer A S. In vitro antibacterial activities of platelet microbicidal protein and neutrophil defensin against Staphylococcus aureus are influenced by antibiotics differing in mechanism of action. Antimicrob Agents Chemother. 1999;43:1111–1117. doi: 10.1128/aac.43.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeaman M R, Bayer A S, Koo S P, Foss W, Sullam P M. Platelet microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action. J Clin Investig. 1998;101:178–187. doi: 10.1172/JCI562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Benz R, Hancock R E W. Influence of proline residues on the antibacterial and synergistic activities of alpha-helical peptides. Biochemistry. 1999;38:8102–8111. doi: 10.1021/bi9904104. [DOI] [PubMed] [Google Scholar]