Abstract
Poultry provides an important protein source consumed globally by human population, and simultaneously, acts as a substantial reservoir of antibiotic resistant bacterial species such as Escherichia coli, Salmonella, Campylobacter, Clostridium perfringens. These bacterial species can include commensal strains with beneficial roles on poultry health and productivity, and pathogenic strains not only to poultry but zoonotically to man. This review paper evaluates the role of phytochemicals as possible alternatives to antibiotics and natural anti-bacterial agents to control antibiotic resistance in poultry. The focus of this paper is on the polyphenolic phytochemicals as they constitute the major group; carvacrol oil (the active ingredient of oregano), thymol oil (the main ingredient of oregano), oregano oil, and tannins oil as feed additives and their mechanism of actions that might enhance avian gut health by controlling antibiotic-resistant bacterial strains spread in poultry.
Keywords: Escherichia coli, Salmonella, Campylobacter, Clostridium perfringens, Phytochemicals, AMR
Introduction
Improvements in white meat productivity have increased significantly in the recent years, to meet the continuous increase in local and global demand for white meat and feed the growing population (Delgado 2005). As a result, huge efforts have been put to achieve higher level of effectiveness in poultry production such as improving diet and husbandry practices (Thornton 2010) which collectively led to improving feed conversion ratio (FCR) to 1.4 (Science 1999). High-quality diet costs around 60% of the production costs, but it increases the standards of poultry production due to its ability to maintain healthy intestines of these birds and avoid developing pathogenic diseases (Porter 1998). These transformations had positive impact on modern poultry industry such as decreasing the time required to get to the commercial weight (Zuidhof et al. 2014), improving food economy and ensuring its sustainability (Flock and Preisinger 2002), and reducing environmental pollution (Gerber et al. 2007).
Antibiotics used as a prophylactic agent have shown to have positive effects on the growth performance of chicken as a presumed result of reduced pathogen load, reduction in competition for nutrients in the small intestine, reduction of inflammation, and improvement of digestion (Thomke and Elwinger 1998). Also, they were used to fight bacterial infections as a therapeutic drug and at sub-therapeutic levels as feed ingredients, because they were shown to enhance growth, but unfortunately this led to the rise of the first incident of resistant Salmonella enterica ser. Typhimurium in 1963 (Dewey et al. 1997). The growth promoting effect of antibiotics was discovered in the 1940s (Hughes and Datta 1983), and later in the 1950s and 1960s, they were authorised with set guidelines by the EU to be used in animal feeds (Castanon 2007), which also made a contribution to an improved poultry productivity (Bunyan et al. 1977), but this resulted in negative consequences of selecting highly resistant bacteria leading to the emergence of global antibiotic resistant bacteria (ARB) carrying antibiotic resistant genes (ARG) which was marked in the 1980s (Aarestrup 2003). This raised worries as these ARG would be transferred through the food chain from animals to man (Greko 2001), and this was proved by a European surveillance study conducted in 2005 that demonstrated the presence of ARB of animal origin among patients admitted to intensive care units (ICU) (Hanberger et al. 2009) which indicates that humans are direct recipient of these ARB and ARG. ARB were the reason behind high numbers of medical illnesses and even death among human (Cosgrove 2006).
As a result of the antimicrobial resistance (AMR) issue, the World Health Organization (WHO) set guidelines and recommendations to stop the use of antibiotics as growth promoters in 1997 (Caron et al. 2009). A year later in 1998, the EU imposed an initial ban on the use of antibiotics as feed additives in poultry feed and water (Dibner and Richards 2005), and this was followed by a complete ban on the use of prophylactic antibiotics in animal feed in 2006 (Millet and Maertens 2011). Later in 2013, the United States (US) Food and Drug Administration (FDA) ordered major medical manufacturers to stop labelling antibiotics as growth promoters. This came as a result of AMR and consumers demand for healthier antibiotic-free poultry products (Food and Administration 2013). Also recently in 2017, the FDA imposed new rules restricting the use of clinical antibiotics for the purpose of growth promotion in animal husbandry (Brüssow 2017). These precautionary measurements are being taken into consideration as they are important for the poultry welfare and its sustainability, and moreover for human (Casewell et al. 2003) as they are at the top of the food chain hierarchy. The emergence of poultry diseases has a negative economic impact and an increase in financial costs due to loss in poultry productivity and emergence of public health problems (Bryan and Doyle 1995). The previously mentioned rising issues in the poultry production has prompted a search for alternatives to control diseases (Si et al. 2006), and therefore looking for antibiotics alternative to serve the purpose of growth promotion and enhancement in the gut microbiota since diet has a direct impact on productivity and animal health (Borda-Molina et al. 2018), but this needs to be achieved without the current issue of AMR. There are many antibiotic alternatives used currently at commercial level such as probiotics, prebiotics, synbiotics, and bacteriophages (Gadde et al. 2017), but the focus of this literature review paper will be on phytochemicals.
Bacterial infections in poultry
Bacterial infections in poultry are inevitable and are the cause of high mortality rate among birds (Porter 1998). These infections can be caused by a variety of Gram-negative and Gram-positive bacteria.
Escherichia coli (E. coli)
E. coli is a facultative anaerobe (Finegold et al. 1983), Gram-negative bacterium (Scheutz and Strockbine 2015) belonging to the phylum Proteobacteria (Marchesi et al. 2016) and the family Enterobacteriaceae (Ewing 1986). E. coli is normally part of the human and animals’ intestinal natural microbiota (Ørskov and Ørskov 1992), being the most dominant aerobic bacterium (Savageau 1983) with 106–109 colony forming unit (CFU) per cm of the poultry (chicken and turkey) intestine (Leitner and Heller 1992), and it is one of the first species to colonise the gut of human (Mitsuoka 1973) and animal (Hudault et al. 2001). Also, it is one of the best studied bacterial species and often used as a model microorganism because of their different commensal and pathogenic types, and with E. coli K12 being the most common reference strain (Hobman et al. 2007). The pathogenic type such as avian pathogenic E. coli (APEC) strains are the causative agent of colibacillosis in poultry (Gross 1994), which studies have shown to be of multi-resistant nature to 10 or more antibiotics (Lima Barbieri et al. 2017). Moreover, E. coli can be easily grown in the laboratory, as it needs simple growth requirements, grows at a fast rate, and extensive information is already provided in literature (Donachie and Begg 1970).
In terms of antibiotic resistance, E. coli is the most common carrier of extended spectrum β-lactamase (ESBL) genes which are located on plasmids that facilitate their transfer (Donachie and Begg 1970), and these genes are widespread among chickens (Machado et al. 2008). Ingestion of food of animals origin containing ARB becomes a source of ARB and their ARG in the human gut, and this might affect antibiotics use or might cause opportunistic diseases in the future (Smith et al. 2002). In human hosts, it is the main causative agent and is responsible for most cases of urinary tract infection (UTI) (Stamm and Hooton 1993). Therefore, the need for alternative control measurement and this can be through using natural phytochemicals. There are many studies that suggest the efficacy of using phytochemicals to control E. coli growth as summarised in Table 1.
Table 1.
A summary of the mechanisms of actions of carvacrol, thymol and oregano (at sub-MIC level) against some of the bacteria responsible for poultry infections
| Phytochemical | Bacteria | Target site | Mode of action | References |
|---|---|---|---|---|
| Thymol/carvacrol | E. coli | Heat and oxidative stress responses and iron transportation | Increased expression of membrane genes (pspD and pspG), heat responses genes (ibpB), oxidative stress responses genes (grxA and soxS) and iron transport gene (feoA) | Yuan et al. (2018) |
| Carvacrol/oregano | E. coli | Survival mechanism and multi-drug efflux system | Missense mutation in cadC and marR | Al-Mnaser and Woodward (2020) |
| Carvacrol | E. coli | Redox sensor system and multi-drug efflux system | Missense mutation in soxR and frameshift in marR | Chueca et al. (2018) |
| Carvacrol/oregano | Salmonella | Stress response | Influence on the rpoS gene | Cariri et al. (2019) |
| Carvacrol | Salmonella | Oxidative stress response | Single nucleotide modification in the transcriptional regulators (yfhP and soxR) | Berdejo et al. (2020) |
| Thymol | E. coli | Multi-drug efflux system | Non-sense mutation in acrR gene encoding for the AcrAB repressor | Al-Kandari et al. (2019) |
| Thymol | Salmonella | Thermal stress response | Upregulation in the expression of the chaperones (GroEl and DnaK) | Di Pasqua et al. (2010) |
| Thymol/carvacrol | Salmonella | Virulence genes | Downregulation in the expression of the main virulence genes (hilA, prgH, invA, sipA, sipC, sipD, sopB, sopE2) | Giovagnoni et al. (2020) |
| Carvacrol | Campylobacter | Motility systems | Downregulation in the expression of genes encoding for motility systems (flaA, flaB and flgA) | Wagle et al. (2019) |
| Carvacrol | Campylobacter | Thermal stress response | Upregulation in the expression of the stress response genes (dnaK, grpE and groEL) | Windiasti et al. (2019) |
Salmonella
Salmonella is a facultative anaerobe, Gram-negative bacilli bacterium belonging to the phylum Proteobacteria and the family Enterobacteriaceae (MacConkey 1905). It usually resides in the intestinal tract of animals and humans and it is the causative agent of Salmonellosis disease, where it infects food such as poultry meat and egg (Hikasa et al. 1982). Salmonellosis as a food-borne disease is known as a public health issue causing concerns in industrial countries (D'Aoust et al. 1992) and responsible for high morbidity and mortality cases among human as indicated by the US Center for Disease Control and Prevention (CDC) (Mead et al. 1999). Among food-borne pathogens in the USA, Salmonella was found to be responsible for the highest percentage of these disease (Gast and Porter 2020). Ingestion of foods of animal origin contaminated with AMR-resistant Salmonella is likely to be responsible for most of Salmonellosis diseases (Angulo et al. 1998). These AMR-resistant Salmonella were first arising from the misuse of antibiotics in poultry industry, and due to its zoonotic nature, it found its way to human (Angulo et al. 2000). In Denmark, it was found out that by decreasing the use of sub-therapeutic in poultry industry, it led to a significant decrease in the prevalence of antibiotic-resistant Salmonella in broilers (Evans and Wegener 2003).
The genus Salmonella includes six sub-species of Salmonella enterica which is found to be responsible for diseases among warm-blooded animals (Gast and Porter 2020). There are more than 2600 serotypes of Salmonella enterica (Achtman et al. 2012), but Salmonella enterica serovars Heidelberg (SH) and Typhimurium (ST) are widespread among human and animal hosts (Zhao et al. 2008; Glenn et al. 2013). These bacterial pathogens are usually found to be responsible for food-borne outbreaks in human due to consumption of food products of animal origin (Authority et al. 2017). The misuse of antibiotics in poultry industry has led to the dissemination of antibiotic resistant Salmonella to ampicillin, chloramphenicol, quinolones and sulphonamide (Su et al. 2004). There are many factors that control the epidemiology of Salmonella infections such as (1) human demography, (2) human lifestyle, (3) human behavior, (4) industrial and technological revolutions, (5) changes in aviation industry, (6) bacterial adaptation, (7) status of public health infrastructure (Oaks et al. 1992), (8) human knowledge in food safety and health practices (Bruhn and Schutz 1999).
Campylobacter
Campylobacter is a microaerophilic, Gram-negative spiral-curved bacilli bacterium (Skirrow 2006) belonging to the phylum Proteobacteria and the family Campylobacteriaceae (Huang et al. 2020). It can be found in the intestinal tract of animals and human oral cavity with the ability to cause diseases in both hosts (Lee et al. 2016). Campylobacter species which inhabit poultry intestine and are associated with poultry diseases are Campylobacter jejuni and Campylobacter coli (Pezzotti et al. 2003) with the former specie being responsible for most of the infection cases. These bacteria species are the causative agents of enteritis and linked to chronic gastritis, gastric ulceration, and gastric cancer in human (Lee and Newell 2006). Campylobacter infection in human results from handling or ingestion of undercooked poultry meat contaminated with this pathogen, which 80% of the raw meat in the UK was found to be contaminated with it (Corry and Atabay 2001). Moreover, practicing low hygienic levels and food safety skills in the kitchen can lead to the spread of Campylobacter contamination with other undercooked food (Lee and Newell 2006).
Campylobacter and Salmonella share similar infection outcome and zoonotic nature, but Campylobacter are fastidious and differ in their metabolic and stress responses as demonstrated by genomic analysis (Lee and Newell 2006). Among zoonotic diseases, Campylobacteriosis has been reported to be the most frequently spread among humans in the EU (Hugas et al. 2009; Westrell et al. 2009), and the major source of infection was fresh broiler chicken meat infected with Campylobacter jejuni (Authority 2005; Wingstrand et al. 2006). Therefore, reducing the occurrence of Campylobacter in poultry meat would decrease its infection cases among human and that would be through various preventative measures: (1) vaccination (Wyszyńska et al. 2004), (2) bacteriophages (Wagenaar et al. 2005), (3) bacteriocins (Line et al. 2008), (4) organic acids (Chaveerach et al. 2004), (5) probiotics (Ghareeb et al. 2012), (6) antibiotics. However, administration of fluroquinolone antibiotic in poultry industry has led to the emergence of resistant Campylobacter and was found to be responsible for 10% of Campylobacter diseases in human (Randall et al. 2003). Hence, the need to look for alternative ways.
Clostridium perfringens
Clostridium is an anaerobic, Gram-positive spore-forming bacterium (Songer and Meer 1996) belonging to the phylum Firmicutes and the family Clostridiaceae (Wiegel 2015). Clostridium perfringens is a bacterium of ubiquitous nature and it is part of animal and human gut microbiota (Miller et al. 2010). At the same time, it is one of the frequent zoonotic bacterial pathogens that causes foodborne diseases outbreak in humans after Campylobacter and Salmonella (Buzby and Roberts 1997). It costs poultry industry economically as it is the main causative agent of necrotic enteritis (Van der Sluis 2000). Necrotic enteritis infections can be in the form of acute or subclinical, with the former being responsible for higher mortality rates among broiler chickens (Kaldhusdal and Lovland 2000). The acute form of the infection leads to the formation of severe necrosis in the mucosal layer of the small intestine, thereby increasing the rate of death (Gazdzinski and Julian 1992). While, the subclinical form of the infection causes reduced absorption and digestion, decrease in the weight gain and an increase in the FCR due to the damage in the intestinal mucosa (Kaldhusdal and Hofshagen 1992). At the level of bacterial gut microbiota, this infection leads to its disturbance which can be reversed by feeding a monoculture of Lactobacillus acidophilus or Streptococcus faecalis (Fukata et al. 1991). The classification of Clostridium perfringens puts them into five toxinotypes (A, B, C, D and E) according to the ability to produce four major toxins (α, β, ε and ι). The subclinical form of the infection is caused mainly by Clostridium perfringens type A producing alpha toxin and to a less degree by Clostridium perfringens type C producing alpha and beta toxin (Songer and Meer 1996). The European ban on the use of antibiotics as a growth promoter has been associated with the wide spread of necrotic enteritis (Immerseel et al. 2004). Hence, the need to find alternatives to control this pathogen.
Phytochemicals
Phytochemicals are natural plant products produced as secondary metabolites of which some possess antimicrobial effects (Metabolhtes 2004), and natural sources of feed additives and have been proven to be generally recognised as safe (GRAS) (Hashemi et al. 2008). These secondary metabolites can be non-digestible carbohydrates and compounds such as lignin, resistant protein, polyphenols and carotenoids, some of which are considered anti-oxidants (Saura-Calixto et al. 2000) and display anti-microbial activities (Wink 2004). They differ in chemical structure, biological activity, plant source, and method of production. In other words, they are natural sources of growth promoters coming from plants, herbs, or spices (Hashemi and Davoodi 2010). They come in different materialistic form: solid in the form of dried leaves or ground powder, or liquid in the form of an essential oil (Gadde et al. 2017). They differ in their composition, extraction method, storage conditions, growth stage, the source of plant and its geographical location (Dhami and Mishra 2015). Generally, phytochemicals are categorised into five main groups; terpenoids, polyphenols, organosulfur compounds, phytosterols, and alkaloids (Somani et al. 2015). However, polyphenols represent the major and main compounds of these phytochemicals (Gadde et al. 2017), hence the focus of this current paper.
Presently, plant-based natural therapies are of increased popularity, because consumers are becoming aware of concerns regarding synthetic additives (Hammer et al. 1999) as well as the dangers of antibiotic use as discussed earlier. Scientific research changed the perception of food including phytochemicals from being an energy source to that of health promoting supplements because of their bioactive roles (Berner and O’Donnell 1998). Therefore, it is crucial to understand the scientific background behind the beneficial roles of phytochemicals as anti-microbial agents (Mitscher et al. 1987), and the process of using them as an alternative to antibiotics. The scientific interests in phytochemicals are due to the rising problem of ARB in poultry industry, consumers demand, and the EU ban on the usage of antibiotics for growth promotion. The biological mechanism of action of these phytochemicals is not very well-understood, but it depends on their chemical structure (Hashemi and Davoodi 2010).
Phytochemicals used as poultry feed additives can improve animal’s health and performance because of their anti-microbial, anti-stress (Wang et al. 1998) and anti-oxidant properties (Valenzuela 1995), and their ability to modulate gut microbiota (Hashemi et al. 2009) and enhance immune responses (Chowdhury et al. 2018). The improvements in the animal health performance can be observed in the form of an increased body weight, feed intake and FCR. Physically, the positive effects included an improved carcass quality, meat quality and nutritional values (Valenzuela-Grijalva et al. 2017). The efficiency of these phytochemicals is determined by intrinsic and extrinsic factors such as animal’s nutrition and health, type of diet and environment (Giannenas et al. 2003). They can act as prebiotics by enhancing the growth of beneficial bacteria and suppressing the growth of pathogenic bacteria (Cencic and Chingwaru 2010) which leads to the enhancement in the gut microbiota (Hashemi and Davoodi 2010). Thus, they reward the host by shaping gut microbiota in a beneficial way (Laparra and Sanz 2010). On the bacterial level, previous research has demonstrated that using phytochemicals as feed additives results in a decrease in the population of E. coli and also an increase in the activity of specific digestive enzymes (Jang et al. 2007) such as amylase in the intestinal system of female broiler chickens (Lee et al. 2003) and maltase in the intestinal system of male broiler chickens (Xu et al. 2003).
Carvacrol, thymol and oregano
Thymol (2-isopropyl-5-methylphenol) (Fig. 1) and carvacrol (5-Isopropyl-2-methylphenol) (Fig. 2) are phenolic compounds (Kim et al. 2016) and they are the main constituents of the essential oils of oregano (Fig. 3). They are structural l isomers, sharing the same chemical structure in the form of a phenolic ring but differing in the location of hydroxyl groups (Ultee et al. 2002). Moreover, carvacrol is the key ingredient of oregano essential oil that is extracted from plants of the genus Origanum (Kintzios 2002), but its abundance in plants differs from one species to another (Gounaris et al. 2002). Thymol, carvacrol, and oregano share the same chief components which are monoterpenic phenols consisting of two main ingredients of γ-terpinene and p-cymeme (Kokkini 1996). Carvacrol and oregano exhibit anti-microbial activities against pathogenic microorganisms whether from plant, animal or human sources, and these microorganisms include bacteria and fungi (Baricevic and Bartol 2002).
Fig. 1.
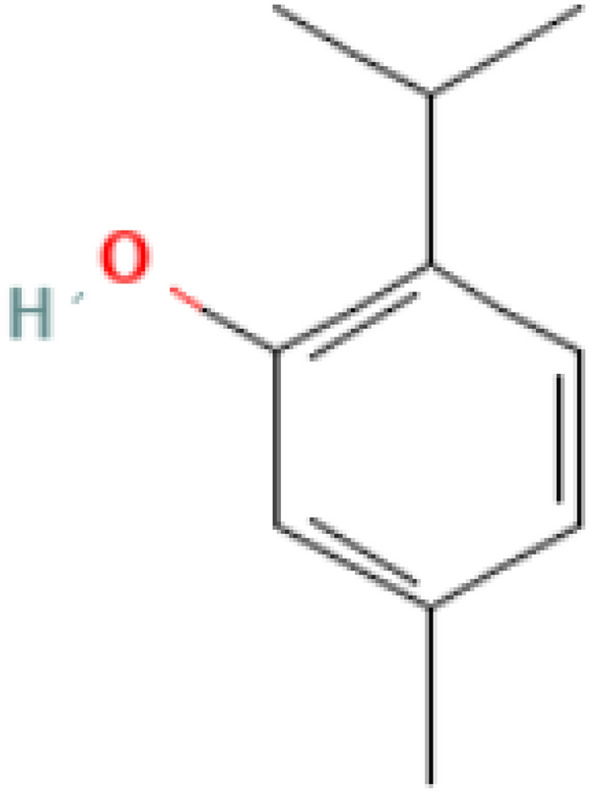
Chemical structure of thymol (Kim et al. 2016)
Fig. 2.
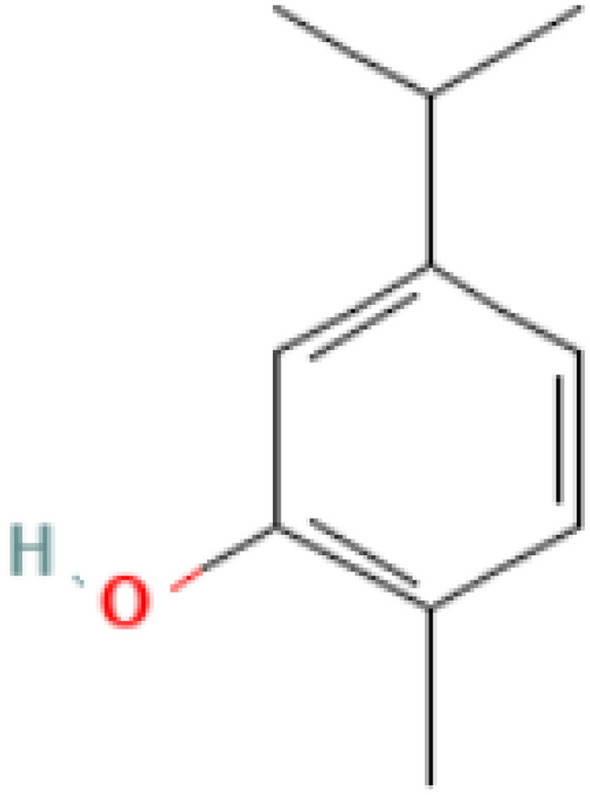
Chemical structure of carvacrol (Kim et al. 2016)
Fig. 3.
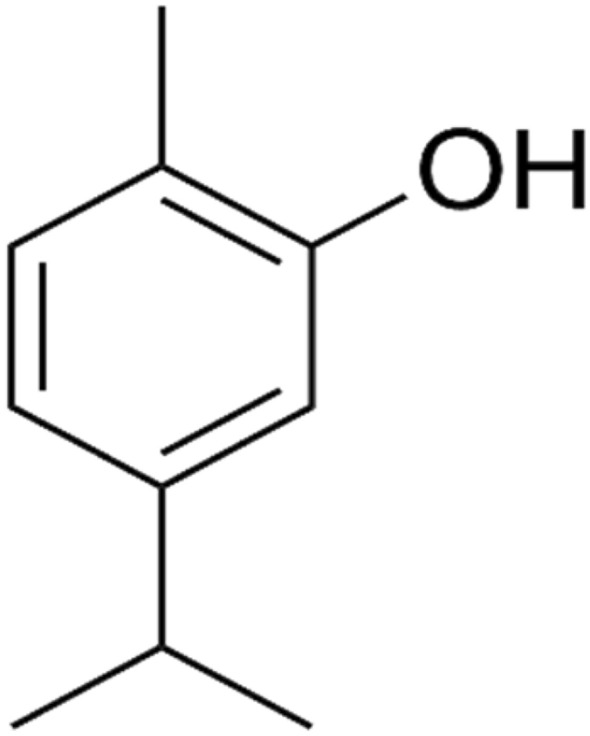
Chemical structure of oregano (Kim et al. 2016)
Carvacrol and thymol as feed additives showed enhanced growth promoting effects on anti-oxidant enzyme activities, immune responses, digestive enzyme activities among broiler chickens (Hashemipour et al. 2013). Oregano oil containing carvacrol and thymol is effective against E. coli in a dosage-dependent manner (Friedman et al. 2002; Al-Mnaser 2019; Alvarez et al. 2019). Generally, the phytochemicals (e.g. carvacrol) with a high percentage of other phenolic compounds display potent anti-bacterial properties (Guynot et al. 2003). As anti-bacterial agents, the main mechanism of action appears to be disruption of the integrity and functionality of the cell wall and cell membrane structures (Sikkema et al. 1995). At minimum inhibitory concentration (MIC) level, they disrupt the outer membrane structure of Gram-negative bacteria, increasing the permeability of cell membrane, leading to leakage of cellular energy sources in the form of adenosine tri-phosphate (ATP) (Gill and Holley 2006) and may also result in the bursting of the bacterial cell (Sikkema et al. 1995). These essential oils are highly hydrophobic and thus can readily integrate into and transition across the bacterial cell membrane (Sikkema et al. 1995). Interestingly, exposing bacteria to sub-lethal concentrations of these phytochemicals leads to changes in the ratio of unsaturated and saturated fatty acid component of the cell membrane (Di Pasqua et al. 2006) suggesting that bacteria develop an adaptive response upon exposure. Furthermore, oregano oil exhibits high biological activities resulting in growth promotion when used as feed additives in poultry (Giannenas et al. 2005). Another study showed that oregano extract (Origanum vulgare) contains a high phenolic content that exhibits anti-oxidant properties (Gómez-Estaca et al. 2009). More recent studies showed that broiler chickens fed diet supplemented with oregano resulted in the following: (1) significant increase in the digestive enzyme chymotrypsin and enhanced protein digestion (Basmacioğlu Malayoğlu et al. 2010), (2) significant increase in body weight, higher anti-oxidant activity of serum, significant decrease in cecal E. coli population resulting in an increased growth performance (Roofchaee et al. 2011), (3) significant increase in body weight and significant decrease in FCR among broilers chickens infected with Eimeria species (Pajić et al. 2019). Moreover, oregano and other herb extracts can suppress the growth of harmful coliform bacteria, but do not affect the growth of beneficial bacteria (Namkung et al. 2004).
In vitro study demonstrated the anti-bacterial activity of carvacrol and oregano by decreasing the number of Salmonella and Campylobacter jejuni in chicken cecal content (Johny et al. 2010), which was further supported by an in vivo study suggesting the efficacy of using these phytochemicals as feed additives in 10 days old broiler chickens due to the significant reduction in the number of Campylobacter in chicken ceca (Arsi et al. 2014). The mode of action of the carvacrol treatment against Campylobacter jejuni can be due to its ability to act as membrane destabilization agent and therefore increasing the susceptibility and cell membrane damage of this bacteria (Windiasti et al. 2019). A longer period study covered 35 days showed that the absence of Campylobacter sp. at day 21 was due to the increase in the number of Lactobacillus sp. with probiotic beneficial effects and thereby improving chicken health by preventing Campylobacter infection (Kelly et al. 2017). As for the spore-forming bacteria Clostridium perfringens, carvacrol, oregano and thymol have showed their ability in preventing their sporulation and controlling their numbers in meat (Juneja and Friedman 2007). Another study further supported the anti-bacterial efficacy of thymol and carvacrol when used as feed additives in broiler chickens challenged with Clostridium perfringens leading to an improve in the chicken gut health supported by the presence of Lactobacillus strains with probiotic beneficial properties (Du et al. 2015). Table 1 provides a summary of the proposed mode of actions of these phytochemicals in further details and at the genetic level of the bacterial cell.
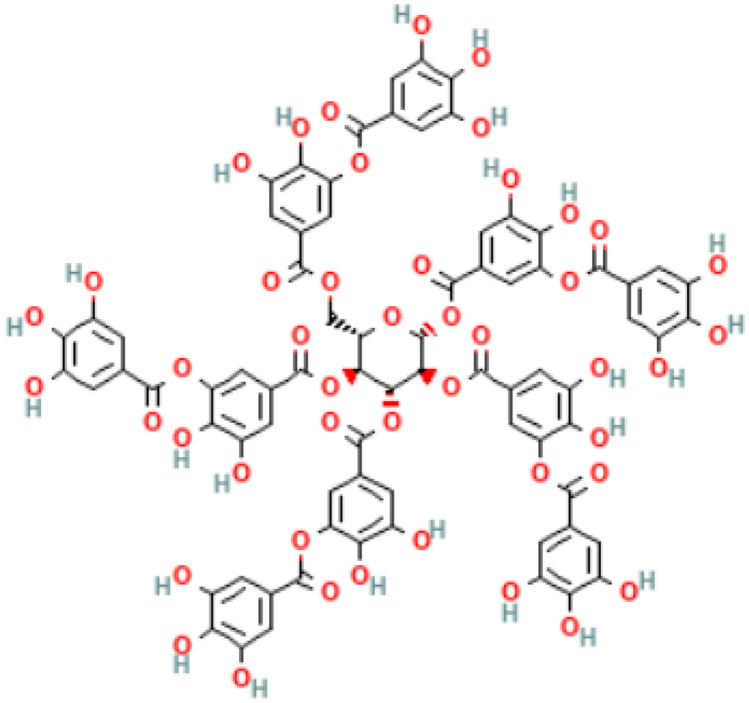
Tannins
Tannins are polyphenolic compounds [2,3-dihydroxy-5-[[(2R,3R,4S,5R,6S)-3,4,5,6-tetrakis[[3,4-dihydroxy-5-(3,4,5-trihydroxybenzoyl)oxybenzoyl]oxy]oxan-2-yl]methoxycarbonyl]phenyl] 3,4,5-trihydroxybenzoate] (Fig. 4) (Kim et al. 2016) categorised into four groups: (1) condensed tannins or proanthocyanidins, (2) hydrolysable tannins, (3) phlorotannins from brown algae, and (4) complex tannins (Suvanto et al. 2017; Brus et al. 2018), and come in different chemical structures (Lillehoj et al. 2018). The presence of different hydroxyl groups at different positions in its structure believes to be behind its ability to bind with the carboxyl groups of the proteins (Wang et al. 2016). Moreover, different chemical composition and structure of tannins makes it bacterial species-specific (Huang et al. 2018). In nature, tannins originate in several plant species, specifically in the inedible parts (i.e. bark or wood) (Brus et al. 2018). Some of these tannins are responsible for defending the plants and others give the plants their odor or color (Redondo et al. 2014). Tannins have been known for their ability to promote growth and their anti-microbial properties, making them a broiler feed additive of choice in South America (Lee et al. 2021). Though, the exact mechanism of action of tannins is still poorly understood (Smith and Mackie 2004), the proposed inhibitory activities involve its interaction with proteins, bacterial cell membrane (Hemingway and Laks 2012), and chelation of iron metals (Scalbert 1991). Other intracellular activities include inhibiting enzymatic and metabolic activities that result in bacterial cell morphological changes (Liu et al. 2013). A recent study showed the immunomodulation effect of tannins when used as a feed additive, and this was due to a significant increased expression of cytokines (IL-6 and IL-10) in the cecal cells. This resulted in an altered metabolism, enhanced gut health and bird growth, and feed efficiency (Lee et al. 2021).
Fig. 4.
Chemical structure of tannins (Kim et al. 2016)
On a larger scale, inclusion of tannins in the poultry diet had health promoting effects on growth and intestinal effects (Schiavone et al. 2008), and increased feed efficiency (Redondo et al. 2014). The health promoting effects on growth can include a decrease in lipid oxidation and cholesterol level, an increase in the content of beneficial fatty acids, and an increase in body weight (Starčević et al. 2015). On the bacterial level, it can lead to changes in the gut morphology and type of bacteria and thereby increasing their biodiversity in the gut of broiler chickens (Viveros et al. 2011). Also, it can act as an anti-oxidant and provides a source of vitamin E in animal nutrition (Brenes et al. 2008). On the physiological level of the broiler, inclusion of tannins leads to the following: (1) red blood cell growth and maturation of the small intestine (Iji et al. 2001), (2) elongation of the villi of the small intestine, and (3) increased number of cell mitosis of the duodenum (Khambualai et al. 2009). Collectively, these result in increased weight gain on a daily basis and in total, and enhanced feed utilization (Lee et al. 2021). On the broiler production level, tannins increase the quality and nutritional values of meat (Mannelli et al. 2019), and eggs (Minieri et al. 2016).
Tannins as an anti-bacterial agent can reduce the occurrence of avian diseases and transmission of zoonotic pathogens (Hassan et al. 2020). Examples on this can include the following: (1) inhibit the growth of E. coli by acting as anti-biofilm and anti-motility agents (Dakheel et al. 2020), (2) inhibit the growth of Salmonella by acting as anti-quorum sensing and anti-virulence agent (Sivasankar et al. 2020), (3) inhibit the growth of Campylobacter sp. which may be resorted to their ability to bind to the proteins and enzymes within the bacterial cell (Nagayama et al. 2002), (4) inhibit the growth of Clostridium perfringens by acting as a membrane destabilization agent (Kaimudin and Manduapessy 2020) and as an anti-toxin agent (Elizondo et al. 2010), (5) chelates iron that is crucial for the growth of most pathogenic bacteria (Chung et al. 1998). Table 2 provides a summary of the proposed mode of actions of tannins in further details and at the genetic level of the bacterial cell.
Table 2.
A summary of the mechanisms of actions of tannins (at sub-MIC level) against some of the bacteria responsible for poultry infections
| Phytochemical | Bacteria | Target site | Mode of action | References |
|---|---|---|---|---|
| Tannins | E. coli | Biofilm formation and motility genes | Repression in the production of curli genes (csgB and csgD) and downregulation in the expression of motility genes (fimA, fimH, flhD, motB, qseB, qseC) | Yang et al. (2016) |
| Tannins | E. coli | Motility genes and quorum sensing | Downregulation in the expression of motility genes (fliA, fliY, fljB, flhC, fimD) and repression in the expression of quorum sensing genes (sdiA and srgE) | Li et al. (2014) |
| Tannins/phenolic compounds | Salmonella | Type III secretion system/pathogenicity genes | Downregulation in the expression of the type III secretion system-related genes (hilA, hilC, invA, invF, sirA and sirB) | Salaheen et al. (2016) |
| Condensed tannins (proanthocyanidins) | Salmonella | Pathogenicity island 1/virulence secretion system | Suppression in the secretion of the pathogenicity island SPI1 | Morita et al. (2016) |
Conclusion
Antibiotic resistance issue made the scientific community shift their perspective and search for antibiotic alternatives. One of the antibiotic alternatives is the use of natural plant products, phytochemicals. This review has focused on four polyphenolic phytochemicals with promising results which make them good candidates to be used as feed additives in the poultry industry instead of antibiotics. However, more studies need to be done to increase our understanding in the long-term used of these phytochemicals and how will they affect us as humans considering that we are at the end of the food chain. This AMR issue will continue to increase in the coming years due to the current overuse of anti-bacterial compounds during this coronavirus pandemic. Therefore, the need to increase our understanding in this area and find effective alternatives is very crucial and of high importance.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and material
Not applicable.
Code of availability
Not applicable.
Declarations
Conflict of interest
The author(s) declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
All authors agreed to participate in this paper.
Consent for publications
All authors agreed for this paper to be published.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aarestrup FM. Effects of termination of AGP use on antimicrobial resistance in food animals. Geneva: World Health Organization; 2003. pp. 6–11. [Google Scholar]
- Achtman M, Wain J, Weill F-X, Nair S, Zhou Z, Sangal V, Krauland MG, Hale JL, Harbottle H, Uesbeck A. Multilocus sequence typing as a replacement for serotyping in Salmonella enterica. PLoS Pathog. 2012;8(6):e1002776. doi: 10.1371/journal.ppat.1002776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kandari F, Al-Temaimi R, van Vliet AHM, Woodward MJ. Thymol tolerance in Escherichia coli induces morphological, metabolic and genetic changes. BMC Microbiol. 2019;19(1):1–11. doi: 10.1186/s12866-019-1663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mnaser A (2019) Phytochemicals (carvacrol and oregano extract) as possible alternatives to antibiotics in poultry feed. Ph.D. in Food and Nutritional Sciences, Department of Food and Nutritional Sciences, University of Reading
- Al-Mnaser AA, Woodward MJ. Sub-lethal concentrations of phytochemicals (carvacrol and oregano) select for reduced susceptibility mutants of Escherichia coli O23: H52. Pol J Microbiol. 2020;69(1):121. doi: 10.33073/pjm-2020-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez MV, Ortega-Ramirez LA, Silva-Espinoza BA, Gonzalez-Aguilar GA, Ayala-Zavala JF. Antimicrobial, antioxidant, and sensorial impacts of oregano and rosemary essential oils over broccoli florets. J Food Process Preserv. 2019;43(3):e13889. doi: 10.1111/jfpp.13889. [DOI] [Google Scholar]
- Angulo FJ, Johnson KR, Tauxe RV, Cohen ML. Origins and consequences of antimicrobial-resistant nontyphoidal Salmonella: implications for the use of fluoroquinolones in food animals. Microbial Drug Resistance (larchmont, N y) 2000;6(1):77. doi: 10.1089/mdr.2000.6.77. [DOI] [PubMed] [Google Scholar]
- Angulo FJ, Tauxe RV, Cohen ML (1998) Significance and sources of antimicrobial-resistant nontyphoidal Salmonella infections in humans in the United States: the need for prudent use of antimicrobial agents, including restricted use of fluoroquinolones, in food animals. In: Proceedings, American Association of Bovine Practitioners 31st meeting
- Arsi K, Donoghue AM, Venkitanarayanan K, Kollanoor-Johny A, Fanatico AC, Blore PJ, Donoghue DJ. The efficacy of the natural plant extracts, thymol and carvacrol against C ampylobacter colonization in broiler chickens. J Food Saf. 2014;34(4):321–325. doi: 10.1111/jfs.12129. [DOI] [Google Scholar]
- Authority, European Food Safety Opinion of the scientific panel on additives and products or substances used in animal feed (FEEDAP) on the updating of the criteria used in the assessment of bacteria for resistance to antibiotics of human or veterinary importance. EFSA J. 2005;3(6):223. doi: 10.2903/j.efsa.2005.223. [DOI] [Google Scholar]
- Authority, European Food Safety, European Centre for Disease Prevention, and Control The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017;15(12):e05077. doi: 10.2903/j.efsa.2017.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri L, Nicolle DW, Nielsen YW, Cavender T, Hussein A, Yan S, Nolan LK, Logue CM. mcr-1 identified in avian pathogenic Escherichia coli (APEC) PLoS ONE. 2017;12(3):e0172997. doi: 10.1371/journal.pone.0172997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baricevic D, Bartol T (2002) The biological/pharmacological activity of the Origanum genus. In: Medicinal and aromatic plants-industrial profiles, Oregano. The genera Origanum and Lippia, vol 25, pp 177–213
- Basmacioğlu Malayoğlu H, Baysal Ş, Misirlioğlu Z, Polat M, Yilmaz H, Turan N. Effects of oregano essential oil with or without feed enzymes on growth performance, digestive enzyme, nutrient digestibility, lipid metabolism and immune response of broilers fed on wheat–soybean meal diets. Br Poult Sci. 2010;51(1):67–80. doi: 10.1080/00071660903573702. [DOI] [PubMed] [Google Scholar]
- Berdejo D, Merino N, Pagán E, García-Gonzalo D, Pagán R. Genetic variants and phenotypic characteristics of Salmonella typhimurium-resistant mutants after exposure to carvacrol. Microorganisms. 2020;8(6):937. doi: 10.3390/microorganisms8060937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner LA, O’Donnell JA. Functional foods and health claims legislation: applications to dairy foods. Int Dairy J. 1998;8(5):355–362. doi: 10.1016/S0958-6946(98)00058-2. [DOI] [Google Scholar]
- Borda-Molina D, Seifert J, Camarinha-Silva A. Current perspectives of the chicken gastrointestinal tract and its microbiome. Comput Struct Biotechnol J. 2018;16:131–139. doi: 10.1016/j.csbj.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenes A, Viveros A, Goñí I, Carmen Centeno SG, Sayago-Ayerdy IA, Saura-Calixto F. Effect of grape pomace concentrate and vitamin E on digestibility of polyphenols and antioxidant activity in chickens. Poult Sci. 2008;87(2):307–316. doi: 10.3382/ps.2007-00297. [DOI] [PubMed] [Google Scholar]
- Bruhn CM, Schutz HG. Consumer food safety knowledge and practices. J Food Saf. 1999;19(1):73–87. doi: 10.1111/j.1745-4565.1999.tb00235.x. [DOI] [Google Scholar]
- Brus M, Gradišnik L, Trapečar M, Škorjanc D, Frangež R. Beneficial effects of water-soluble chestnut (Castanea sativa Mill.) tannin extract on chicken small intestinal epithelial cell culture. Poult Sci. 2018;97(4):1271–1282. doi: 10.3382/ps/pex424. [DOI] [PubMed] [Google Scholar]
- Brüssow H. Adjuncts and alternatives in the time of antibiotic resistance and in-feed antibiotic bans. Microb Biotechnol. 2017;10(4):674. doi: 10.1111/1751-7915.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan FL, Doyle MP. Health risks and consequences of Salmonella and Campylobacter jejuni in raw poultry. J Food Prot. 1995;58(3):326–344. doi: 10.4315/0362-028X-58.3.326. [DOI] [PubMed] [Google Scholar]
- Bunyan JL, Jeffries L, Sayers JR, Gulliver AL, Coleman K. Antimicrobial substances and chick growth promotion: the growth-promoting activities of antimicrobial substances, including fifty-two used either in therapy or as dietary additives. Br Poult Sci. 1977;18(3):283–294. doi: 10.1080/00071667708416364. [DOI] [Google Scholar]
- Buzby JC, Roberts T. Economic costs and trade impacts of microbial foodborne ilness. World Health Stat Q. 1997;50(1/2):57–66. [PubMed] [Google Scholar]
- Cariri ML, de Melo ANF, Mizzi L, Ritter AC, Tondo E, de Souza EL, Valdramidis V, Magnani M. Quantitative assessment of tolerance response to stress after exposure to oregano and rosemary essential oils, carvacrol and 1, 8-cineole in Salmonella Enteritidis 86 and its isogenic deletion mutants∆ dps,∆ rpoS and∆ ompR. Food Res Int. 2019;122:679–687. doi: 10.1016/j.foodres.2019.01.046. [DOI] [PubMed] [Google Scholar]
- Caron P, Craufurd P, Martin A, Mcdonald A, Abedini W, Afiff S, Bakurin N, Bass S, Hilbeck A, Jansen T (2009) Impacts of AKST on development and sustainability goals. Embrapa Pecuária Sudeste-Capítulo em livro científico (ALICE)
- Casewell M, Friis C, Marco E, McMullin P, Phillips I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J Antimicrob Chemother. 2003;52(2):159–161. doi: 10.1093/jac/dkg313. [DOI] [PubMed] [Google Scholar]
- Castanon JIR. History of the use of antibiotic as growth promoters in European poultry feeds. Poult Sci. 2007;86(11):2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- Cencic A, Chingwaru W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients. 2010;2(6):611–625. doi: 10.3390/nu2060611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaveerach P, Keuzenkamp DA, Lipman LJA, Van Knapen F. Effect of organic acids in drinking water for young broilers on Campylobacter infection, volatile fatty acid production, gut microflora and histological cell changes. Poult Sci. 2004;83(3):330–334. doi: 10.1093/ps/83.3.330. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Mandal GP, Patra AK, Kumar P, Samanta I, Pradhan S, Samanta AK. Different essential oils in diets of broiler chickens: 2. Gut microbes and morphology, immune response, and some blood profile and antioxidant enzymes. Anim Feed Sci Technol. 2018;236:39–47. doi: 10.1016/j.anifeedsci.2017.12.003. [DOI] [Google Scholar]
- Chueca B, Renzoni A, Berdejo D, Pagán R, Kelley WL, García-Gonzalo D. Whole-genome sequencing and genetic analysis reveal novel stress responses to individual constituents of essential oils in Escherichia coli. Appl Environ Microbiol. 2018;84(7):e02538-17. doi: 10.1128/AEM.02538-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K-T, Lu Z, Chou MW. Mechanism of inhibition of tannic acid and related compounds on the growth of intestinal bacteria. Food Chem Toxicol. 1998;36(12):1053–1060. doi: 10.1016/S0278-6915(98)00086-6. [DOI] [PubMed] [Google Scholar]
- Corry JEL, Atabay HI. Poultry as a source of Campylobacter and related organisms. J Appl Microbiol. 2001;90(S6):96S–114S. doi: 10.1046/j.1365-2672.2001.01358.x. [DOI] [PubMed] [Google Scholar]
- Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42(Supplement_2):S82–S89. doi: 10.1086/499406. [DOI] [PubMed] [Google Scholar]
- Dakheel MM, Alkandari FAH, Mueller-Harvey I, Woodward MJ, Rymer C. Antimicrobial in vitro activities of condensed tannin extracts on avian pathogenic Escherichia coli. Lett Appl Microbiol. 2020;70(3):165–172. doi: 10.1111/lam.13253. [DOI] [PubMed] [Google Scholar]
- D'Aoust JY, Sewell AM, Daley E, Greco P. Antibiotic resistance of agricultural and foodborne Salmonella isolates in Canada: 1986–1989. J Food Prot. 1992;55(6):428. doi: 10.4315/0362-028X-55.6.428. [DOI] [PubMed] [Google Scholar]
- Delgado C (2005) Rising demand for meat and milk in developing countries: implications for grasslands-based livestock production. In: Grassland: a global resource, pp 29–39
- Dewey CE, Cox BD, Straw BE, Bush EJ, Scott H, Hurd. Associations between off-label feed additives and farm size, veterinary consultant use, and animal age. Prev Vet Med. 1997;31(1):133–146. doi: 10.1016/S0167-5877(96)01077-X. [DOI] [PubMed] [Google Scholar]
- Dhami N, Mishra AD. Phytochemical variation: how to resolve the quality controversies of herbal medicinal products? J Herb Med. 2015;5(2):118–127. doi: 10.1016/j.hermed.2015.04.002. [DOI] [Google Scholar]
- Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci. 2005;84(4):634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Donachie WD, Begg KJ. Growth of the bacterial cell. Nature. 1970;227(5264):1220. doi: 10.1038/2271220a0. [DOI] [PubMed] [Google Scholar]
- Du E, Gan L, Li Z, Wang W, Liu D, Guo Y. In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with Clostridium perfringens. J Anim Sci Biotechnol. 2015;6(1):58. doi: 10.1186/s40104-015-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizondo AM, Mercado EC, Rabinovitz BC, Fernandez-Miyakawa ME. Effect of tannins on the in vitro growth of Clostridium perfringens. Vet Microbiol. 2010;145(3–4):308–314. doi: 10.1016/j.vetmic.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Evans MC, Wegener HC. Antimicrobial growth promoters and Salmonella spp., Campylobacter spp. in poultry and swine, Denmark. Emerg Infect Dis. 2003;9(4):489. doi: 10.3201/eid0904.020325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing WH. Edwards and Ewing's identification of Enterobacteriaceae. Amsterdam: Elsevier Science Publishing Co. Inc.; 1986. [Google Scholar]
- Finegold SM, Sutter VL, Mathisen GE. Human intestinal microflora in health and disease. London: Academic; 1983. p. 3731. [Google Scholar]
- Flock DK, Preisinger R (2002) Breeding plans for poultry with emphasis on sustainability. In: 7th World congress on genetics applied to livestock production
- Food, US, and Drug Administration (2013) Guidance for Industry# 213: new animal drugs and new animal drug combination products administered in or on medicated feed or drinking water of food-producing animals: recommendations for drug sponsors for voluntarily aligning product use conditions with GFI# 209. Center for Veterinary Medicine, New York
- Friedman M, Henika PR, Mandrell RE. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J Food Prot. 2002;65(10):1545–1560. doi: 10.4315/0362-028X-65.10.1545. [DOI] [PubMed] [Google Scholar]
- Fukata T, Hadate Y, Baba E, Arakawa A. Influence of bacteria on Clostridium perfringens infections in young chickens. Avian Dis. 1991;35:224–227. doi: 10.2307/1591319. [DOI] [PubMed] [Google Scholar]
- Gadde U, Kim WH, Oh ST, Lillehoj HS. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim Health Res Rev. 2017;18(1):26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- Gast RK, Porter Jr RE (2020) Salmonella infections. In: Diseases of poultry, pp 717–753
- Gazdzinski P, Julian RJ. Necrotic enteritis in turkeys. Avian Dis. 1992;36:792–798. doi: 10.2307/1591787. [DOI] [PubMed] [Google Scholar]
- Gerber P, Opio C, Steinfeld H. Poultry production and the environment—a review. Rome: Animal Production and Health Division, Food and Agriculture Organization of the United Nations; 2007. [Google Scholar]
- Ghareeb K, Awad WA, Mohnl M, Porta R, Biarnes M, Böhm J, Schatzmayr G. Evaluating the efficacy of an avian-specific probiotic to reduce the colonization of Campylobacter jejuni in broiler chickens. Poult Sci. 2012;91(8):1825–1832. doi: 10.3382/ps.2012-02168. [DOI] [PubMed] [Google Scholar]
- Giannenas I, Florou-Paneri P, Papazahariadou M, Christaki E, Botsoglou NA, Spais AB. Effect of dietary supplementation with oregano essential oil on performance of broilers after experimental infection with Eimeria tenella. Arch Anim Nutr. 2003;57(2):99–106. doi: 10.1080/0003942031000107299. [DOI] [PubMed] [Google Scholar]
- Giannenas IA, Florou-Paneri P, Botsoglou NA, Christaki E, Spais AB. Effect of supplementing feed with oregano and/or alpha-tocopheryl acetate on growth of broiler chickens and oxidative stability of meat. J Anim Feed Sci. 2005;14(3):521. doi: 10.22358/jafs/67120/2005. [DOI] [Google Scholar]
- Gill AO, Holley RA. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int J Food Microbiol. 2006;108(1):1–9. doi: 10.1016/j.ijfoodmicro.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Giovagnoni G, Rossi B, Tugnoli B, Ghiselli F, Bonetti A, Piva A, Grilli E. Thymol and carvacrol downregulate the expression of salmonella typhimurium virulence genes during an in vitro infection on caco-2 cells. Microorganisms. 2020;8(6):862. doi: 10.3390/microorganisms8060862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn LM, Lindsey RL, Folster JP, Pecic G, Boerlin P, Gilmour MW, Harbottle H, Zhao S, McDermott PF, Fedorka-Cray PJ. Antimicrobial resistance genes in multidrug-resistant Salmonella enterica isolated from animals, retail meats, and humans in the United States and Canada. Microb Drug Resist. 2013;19(3):175–184. doi: 10.1089/mdr.2012.0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Estaca J, Laura Bravo MC, Gómez-Guillén AA, Montero P. Antioxidant properties of tuna-skin and bovine-hide gelatin films induced by the addition of oregano and rosemary extracts. Food Chem. 2009;112(1):18–25. doi: 10.1016/j.foodchem.2008.05.034. [DOI] [Google Scholar]
- Gounaris Y, Skoula M, Fournaraki C, Drakakaki G, Makris A. Comparison of essential oils and genetic relationship of Origanum × intercedens to its parental taxa in the island of Crete. Biochem Syst Ecol. 2002;30(3):249–258. doi: 10.1016/S0305-1978(01)00079-5. [DOI] [Google Scholar]
- Greko C. Safety aspects on non-use of antimicrobials as growth promoters. In: Piva A, Bach Knudsen KE, Lindberg JE, editors. Gut environment of pigs. Nottingham: Nottingham University Press; 2001. pp. 219–230. [Google Scholar]
- Gross WB (1994) Diseases due to Escherichia coli in poultry. In: Escherichia coli in domestic animals and man, pp 237–259
- Guynot ME, Ramos AJ, Setó L, Purroy P, Sanchis V, Marín S. Antifungal activity of volatile compounds generated by essential oils against fungi commonly causing deterioration of bakery products. J Appl Microbiol. 2003;94(5):893–899. doi: 10.1046/j.1365-2672.2003.01927.x. [DOI] [PubMed] [Google Scholar]
- Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol. 1999;86(6):985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- Hanberger H, Arman D, Gill H, Jindrák V, Kalenic S, Kurcz A, Licker M, Naaber P, Scicluna EA, Vanis V. Surveillance of microbial resistance in European Intensive Care Units: a first report from the care-ICU programme for improved infection control. Intensive Care Med. 2009;35(1):91–100. doi: 10.1007/s00134-008-1237-y. [DOI] [PubMed] [Google Scholar]
- Hashemi SR, Davoodi H. Phytogenics as new class of feed additive in poultry industry. J Anim Vet Adv. 2010;9(17):2295–2304. doi: 10.3923/javaa.2010.2295.2304. [DOI] [Google Scholar]
- Hashemi SR, Zulkifli I, Hair Bejo M, Farida A, Somchit MN. Acute toxicity study and phytochemical screening of selected herbal aqueous extract in broiler chickens. Int J Pharmacol. 2008;4(5):352–360. doi: 10.3923/ijp.2008.352.360. [DOI] [Google Scholar]
- Hashemi SR, Zulkifli I, Zunita Z, Hair-Bejo M, Loh TC, Somchit MN, Kok PC, Davoodi H (2009) Effects of dietary supplementation with Euphorbia hirta and acidifier on performance and Salmonella colonization in broiler chickens. In: Proceedings of the 30th Malaysia Society of Animal Production annual conference
- Hashemipour H, Kermanshahi H, Golian A, Veldkamp T. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult Sci. 2013;92(8):2059–2069. doi: 10.3382/ps.2012-02685. [DOI] [PubMed] [Google Scholar]
- Hassan ZM, Manyelo TG, Selaledi L, Mabelebele M. The effects of tannins in monogastric animals with special reference to alternative feed ingredients. Molecules. 2020;25(20):4680. doi: 10.3390/molecules25204680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway RW, Laks PE. Plant polyphenols: synthesis, properties, significance. Berlin: Springer Science & Business Media; 2012. [Google Scholar]
- Hikasa Y, Baba E, Fukata T, Arakawa A. The invasion of Salmonella typhimurium into the cecal wall of chickens infected with Eimeria tenella. Zentralblatt Für Bakteriologie, Mikrobiologie Und Hygiene. 1. Abt. Originale. a, Medizinische Mikrobiologie, Infektionskrankheiten Und Parasitologie. 1982;253(3):344–354. doi: 10.1016/S0174-3031(82)80069-3. [DOI] [PubMed] [Google Scholar]
- Hobman JL, Penn CW, Pallen MJ. Laboratory strains of Escherichia coli: model citizens or deceitful delinquents growing old disgracefully? Mol Microbiol. 2007;64(4):881–885. doi: 10.1111/j.1365-2958.2007.05710.x. [DOI] [PubMed] [Google Scholar]
- Huang Q, Liu X, Zhao G, Tianming Hu, Wang Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim Nutr. 2018;4(2):137–150. doi: 10.1016/j.aninu.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Raymond P, Grenier C, Fahey J (2020) Development and improvement of a colony blot immunoassay for the detection of thermotolerant Campylobacter species. In: Methods in microbiology. Elsevier, pp 209–244
- Hudault S, Guignot J, Servin AL. Escherichia coli strains colonising the gastrointestinal tract protect germfree mice against Salmonella typhimurium infection. Gut. 2001;49(1):47–55. doi: 10.1136/gut.49.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugas M, Tsigarida E, Robinson T, Calistri P. The EFSA scientific panel on biological hazards first mandate: May 2003–May 2006. Insight into foodborne zoonoses. Trends Food Sci Technol. 2009;20(5):188–193. doi: 10.1016/j.tifs.2009.03.001. [DOI] [Google Scholar]
- Hughes VM, Datta N. Conjugative plasmids in bacteria of the ‘pre-antibiotic’ era. Nature. 1983;302(5910):725. doi: 10.1038/302725a0. [DOI] [PubMed] [Google Scholar]
- Iji PA, Saki A, Tivey DR. Body and intestinal growth of broiler chicks on a commercial starter diet. 1. Intestinal weight and mucosal development. Br Poult Sci. 2001;42(4):505–513. doi: 10.1080/00071660120073151. [DOI] [PubMed] [Google Scholar]
- Jang IS, Ko YH, Kang SY, Lee CY. Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens. Anim Feed Sci Technol. 2007;134(3):304–315. doi: 10.1016/j.anifeedsci.2006.06.009. [DOI] [Google Scholar]
- Johny AK, Darre MJ, Donoghue AM, Donoghue DJ, Venkitanarayanan K. Antibacterial effect of trans-cinnamaldehyde, eugenol, carvacrol, and thymol on Salmonella enteritidis and Campylobacter jejuni in chicken cecal contents in vitro. J Appl Poult Res. 2010;19(3):237–244. doi: 10.3382/japr.2010-00181. [DOI] [Google Scholar]
- Juneja VK, Friedman M. Carvacrol, cinnamaldehyde, oregano oil, and thymol inhibit Clostridium perfringens spore germination and outgrowth in ground turkey during chilling. J Food Prot. 2007;70(1):218–222. doi: 10.4315/0362-028X-70.1.218. [DOI] [PubMed] [Google Scholar]
- Kaimudin M, Manduapessy KRW (2020) Potential of Seaweed Gracilaria sp. As inhibitors of Escherichia coli, Clostridium perfringens and Stapylococcus aureus. In: IOP conference series: earth and environmental science
- Kaldhusdal M, Lovland A (2000) The economical impact of Clostridium perfringens is greater than anticipated, pp 50–51
- Kaldhusdal M, Hofshagen M. Barley inclusion and avoparcin supplementation in broiler diets. 2. Clinical, pathological, and bacteriological findings in a mild form of necrotic enteritis. Poult Sci. 1992;71(7):1145–1153. doi: 10.3382/ps.0711145. [DOI] [PubMed] [Google Scholar]
- Kelly C, Gundogdu O, Pircalabioru G, Cean A, Scates P, Linton M, Pinkerton L, Magowan E, Stef L, Simiz E. The in vitro and in vivo effect of carvacrol in preventing Campylobacter infection, colonization and in improving productivity of chicken broilers. Foodborne Pathog Dis. 2017;14(6):341–349. doi: 10.1089/fpd.2016.2265. [DOI] [PubMed] [Google Scholar]
- Khambualai O, Yamauchi K, Koge K, Kashimura J. Morphology of the intestinal mucosa and growth performance of chickens fed diets containing sugar cane extract. J Anim Feed Sci. 2009;18(2):322–334. doi: 10.22358/jafs/66397/2009. [DOI] [Google Scholar]
- Kim S, Thiessen PA, Bolton EE, Chen J, Gang Fu, Gindulyte A, Han L, He J, He S, Shoemaker BA. PubChem substance and compound databases. Nucleic Acids Res. 2016;44(D1):D1202–D1213. doi: 10.1093/nar/gkv951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintzios SE. Oregano: the genera Origanum and Lippia. New York: Taylor & Francis; 2002. [Google Scholar]
- Kokkini S (1996) Taxonomy, diversity and distribution of Origanum. In: Oregano: Proceedings of the IPGRI international workshop on oregano
- Laparra JM, Sanz Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol Res. 2010;61(3):219–225. doi: 10.1016/j.phrs.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Lee MD, Newell DG. Campylobacter in poultry: filling an ecological niche. Avian Dis. 2006;50(1):1–9. doi: 10.1637/7474-111605R.1. [DOI] [PubMed] [Google Scholar]
- Lee K-W, Everts H, Kappert HJ, Frehner M, Losa R, Beynen AC. Effects of dietary essential oil components on growth performance, digestive enzymes and lipid metabolism in female broiler chickens. Br Poult Sci. 2003;44(3):450–457. doi: 10.1080/0007166031000085508. [DOI] [PubMed] [Google Scholar]
- Lee S, Lee J, Ha J, Choi Y, Kim S, Lee H, Yoon Y, Choi KH. Clinical relevance of infections with zoonotic and human oral species of Campylobacter. J Microbiol. 2016;54(7):459–467. doi: 10.1007/s12275-016-6254-x. [DOI] [PubMed] [Google Scholar]
- Lee A, Pont GCD, Farnell MB, Jarvis S, Battaglia M, Arsenault RJ, Kogut MH. Supplementing chestnut tannins in the broiler diet mediates a metabolic phenotype of the ceca. Poult Sci. 2021;100(1):47–54. doi: 10.1016/j.psj.2020.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner G, Heller ED. Colonization of Escherichia coli in young turkeys and chickens. Avian Dis. 1992;36:211–220. doi: 10.2307/1591493. [DOI] [PubMed] [Google Scholar]
- Li G, Yan C, Yunfeng Xu, Feng Y, Qian Wu, Lv X, Yang B, Wang X, Xia X. Punicalagin inhibits Salmonella virulence factors and has anti-quorum-sensing potential. Appl Environ Microbiol. 2014;80(19):6204–6211. doi: 10.1128/AEM.01458-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj H, Liu Y, Calsamiglia S, Fernandez-Miyakawa ME, Chi F, Cravens RL, Sungtaek Oh, Gay CG. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet Res. 2018;49(1):1–18. doi: 10.1186/s13567-018-0562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Line JE, Svetoch EA, Eruslanov BV, Perelygin VV, Mitsevich EV, Mitsevich IP, Levchuk VP, Svetoch OE, Seal BS, Siragusa GR. Isolation and purification of enterocin E-760 with broad antimicrobial activity against gram-positive and gram-negative bacteria. Antimicrob Agents Chemother. 2008;52(3):1094–1100. doi: 10.1128/AAC.01569-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X-L, Hao Y-Q, Jin L, Zhong-Jun Xu, McAllister TA, Wang Y. Anti-Escherichia coli O157: H7 properties of purple prairie clover and sainfoin condensed tannins. Molecules. 2013;18(2):2183–2199. doi: 10.3390/molecules18022183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacConkey A. Lactose-fermenting bacteria in faeces. Epidemiol Infect. 1905;5(3):333–379. doi: 10.1017/s002217240000259x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado E, Coque TM, Canton R, Sousa JC, Peixe L. Antibiotic resistance integrons and extended-spectrum β-lactamases among Enterobacteriaceae isolates recovered from chickens and swine in Portugal. J Antimicrob Chemother. 2008;62(2):296–302. doi: 10.1093/jac/dkn179. [DOI] [PubMed] [Google Scholar]
- Mannelli F, Minieri S, Tosi G, Secci G, Daghio M, Massi P, Fiorentini L, Galigani I, Lancini S, Rapaccini S. Effect of chestnut tannins and short chain fatty acids as anti-microbials and as feeding supplements in broilers rearing and meat quality. Animals. 2019;9(9):659. doi: 10.3390/ani9090659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi JR, Adams DH, Fava F, Hermes GDA, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65(2):330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead PS, Slutsker L, Griffin PM, Tauxe RV. Food-related illness and death in the United States reply to Dr. Hedberg. Emerg Infect Dis. 1999;5(6):841. doi: 10.3201/eid0506.990625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metabolhtes, Nhtrogen-Containing Secondary (2004) Phytochemical diversity of secondary metabolites
- Miller RW, Skinner J, Sulakvelidze A, Mathis GF, Hofacre CL. Bacteriophage therapy for control of necrotic enteritis of broiler chickens experimentally infected with Clostridium perfringens. Avian Dis. 2010;54(1):33–40. doi: 10.1637/8953-060509-Reg.1. [DOI] [PubMed] [Google Scholar]
- Millet S, Maertens L. The European ban on antibiotic growth promoters in animal feed: from challenges to opportunities. Vet J (lond Engl 1997) 2011;187(2):143–144. doi: 10.1016/j.tvjl.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Minieri S, Buccioni A, Serra A, Galigani I, Pezzati A, Rapaccini S, Antongiovanni M. Nutritional characteristics and quality of eggs from laying hens fed on a diet supplemented with chestnut tannin extract (Castanea sativa Miller) Br Poult Sci. 2016;57(6):824–832. doi: 10.1080/00071668.2016.1216944. [DOI] [PubMed] [Google Scholar]
- Mitscher LA, Drake S, Gollapudi SR, Okwute SK. A modern look at folkloric use of anti-infective agents. J Nat Prod. 1987;50(6):1025–1040. doi: 10.1021/np50054a003. [DOI] [PubMed] [Google Scholar]
- Mitsuoka T. The fecal flora in man. I. Composition of the fecal flora of various age group. Zbl Bakt Hyg i Abt Orig. 1973;223:333–342. [PubMed] [Google Scholar]
- Morita Ai, Tai A, Ito H, Ganeko N, Aizawa S-I. Proanthocyanidins in an astringent persimmon inhibit Salmonella pathogenicity island 1 (SPI1) secretion. J Sci Food Agric. 2016;96(5):1798–1802. doi: 10.1002/jsfa.7289. [DOI] [PubMed] [Google Scholar]
- Nagayama K, Iwamura Y, Shibata T, Hirayama I, Nakamura T. Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J Antimicrob Chemother. 2002;50(6):889–893. doi: 10.1093/jac/dkf222. [DOI] [PubMed] [Google Scholar]
- Namkung H, Li M, Gong J, Yu H, Cottrill M, de Lange CFM. Impact of feeding blends of organic acids and herbal extracts on growth performance, gut microbiota and digestive function in newly weaned pigs. Can J Anim Sci. 2004;84(4):697–704. doi: 10.4141/A04-005. [DOI] [Google Scholar]
- Oaks J, Stanley C, Shope RE, Lederberg J. Emerging infections: microbial threats to health in the United States. Washington: National Academies Press; 1992. [PubMed] [Google Scholar]
- Ørskov F, Ørskov I. Escherichia coli serotyping and disease in man and animals. Can J Microbiol. 1992;38(7):699–704. doi: 10.1139/m92-115. [DOI] [PubMed] [Google Scholar]
- Pajić M, Aleksić N, Vejnović B, Polaček V, Novakov N, Ostojić-Andrić D, Stanimirović Z. Influence of anticoccidials on oxidative stress, production performance and faecal oocyst counts in broiler chickens infected with Eimeria species. Kafkas Üniversitesi Veteriner Fakültesi Dergisi. 2019;25(3):379–385. [Google Scholar]
- Pasqua Di, Rosangela NH, Betts G, Mauriello G. Changes in membrane fatty acids composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J Agric Food Chem. 2006;54(7):2745–2749. doi: 10.1021/jf052722l. [DOI] [PubMed] [Google Scholar]
- Pasqua Di, Rosangela GM, Ferranti P, Ercolini D, Mauriello G. Changes in the proteome of Salmonella enterica serovar Thompson as stress adaptation to sublethal concentrations of thymol. Proteomics. 2010;10(5):1040–1049. doi: 10.1002/pmic.200900568. [DOI] [PubMed] [Google Scholar]
- Pezzotti G, Serafin A, Luzzi I, Mioni R, Milan M, Perin R. Occurrence and resistance to antibiotics of Campylobacter jejuni and Campylobacter coli in animals and meat in northeastern Italy. Int J Food Microbiol. 2003;82(3):281–287. doi: 10.1016/S0168-1605(02)00314-8. [DOI] [PubMed] [Google Scholar]
- Porter R., Jr Bacterial enteritides of poultry. Poult Sci. 1998;77(8):1159–1165. doi: 10.1093/ps/77.8.1159. [DOI] [PubMed] [Google Scholar]
- Randall LP, Ridley AM, Cooles SW, Sharma M, Sayers AR, Pumbwe L, Newell DG, Piddock LJV, Woodward MJ. Prevalence of multiple antibiotic resistance in 443 Campylobacter spp. isolated from humans and animals. J Antimicrob Chemother. 2003;52(3):507–510. doi: 10.1093/jac/dkg379. [DOI] [PubMed] [Google Scholar]
- Redondo LM, Chacana PA, Dominguez JE, Miyakawa MEDF. Perspectives in the use of tannins as alternative to antimicrobial growth promoter factors in poultry. Front Microbiol. 2014;5:118. doi: 10.3389/fmicb.2014.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roofchaee A, Irani M, Ebrahimzadeh MA, Akbari MR. Effect of dietary oregano (Origanum vulgare L.) essential oil on growth performance, cecal microflora and serum antioxidant activity of broiler chickens. Afr J Biotechnol. 2011;10(32):6177–6183. [Google Scholar]
- Salaheen S, Jaiswal E, Joo J, Peng M, Ho R, OConnor D, Adlerz K, Aranda-Espinoza JH, Biswas D. Bioactive extracts from berry byproducts on the pathogenicity of Salmonella typhimurium. Int J Food Microbiol. 2016;237:128–135. doi: 10.1016/j.ijfoodmicro.2016.08.027. [DOI] [PubMed] [Google Scholar]
- Saura-Calixto F, García-Alonso A, Goni I, Bravo L. In vitro determination of the indigestible fraction in foods: an alternative to dietary fiber analysis. J Agric Food Chem. 2000;48(8):3342–3347. doi: 10.1021/jf0000373. [DOI] [PubMed] [Google Scholar]
- Savageau MA. Escherichia coli habitats, cell types, and molecular mechanisms of gene control. Am Nat. 1983;122(6):732–744. doi: 10.1086/284168. [DOI] [Google Scholar]
- Scalbert A. Antimicrobial properties of tannins. Phytochemistry. 1991;30(12):3875–3883. doi: 10.1016/0031-9422(91)83426-L. [DOI] [Google Scholar]
- Scheutz F, Strockbine NA (2015) Escherichia. In: Bergey’s manual of systematics of archaea and bacteria, pp 1–49
- Schiavone A, Guo K, Tassone S, Gasco L, Hernandez E, Denti R, Zoccarato I. Effects of a natural extract of chestnut wood on digestibility, performance traits, and nitrogen balance of broiler chicks. Poult Sci. 2008;87(3):521–527. doi: 10.3382/ps.2007-00113. [DOI] [PubMed] [Google Scholar]
- Science, Council for Agricultural . Animal agriculture and global food supply. New Delhi: Council for Agricultural; 1999. [Google Scholar]
- Si W, Gong J, Tsao R, Zhou T, Yu H, Poppe C, Johnson R, Du Z. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J Appl Microbiol. 2006;100(2):296–305. doi: 10.1111/j.1365-2672.2005.02789.x. [DOI] [PubMed] [Google Scholar]
- Sikkema J, De Bont JA, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59(2):201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasankar C, Jha NK, Ghosh R, Shetty PH. Anti quorum sensing and anti virulence activity of tannic acid and it’s potential to breach resistance in Salmonella enterica Typhi/Paratyphi a clinical isolates. Microb Pathog. 2020;138:103813. doi: 10.1016/j.micpath.2019.103813. [DOI] [PubMed] [Google Scholar]
- Skirrow MB. John McFadyean and the centenary of the first isolation of Campylobacter species. Clin Infect Dis. 2006;43(9):1213–1217. doi: 10.1086/508201. [DOI] [PubMed] [Google Scholar]
- Smith AH, Mackie RI. Effect of condensed tannins on bacterial diversity and metabolic activity in the rat gastrointestinal tract. Appl Environ Microbiol. 2004;70(2):1104–1115. doi: 10.1128/AEM.70.2.1104-1115.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Harris AD, Johnson JA, Silbergeld EK, Glenn J, Morris. Animal antibiotic use has an early but important impact on the emergence of antibiotic resistance in human commensal bacteria. Proc Natl Acad Sci. 2002;99(9):6434–6439. doi: 10.1073/pnas.082188899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somani SJ, Modi KP, Majumdar AS, Sadarani BN. Phytochemicals and their potential usefulness in inflammatory bowel disease. Phytother Res. 2015;29(3):339–350. doi: 10.1002/ptr.5271. [DOI] [PubMed] [Google Scholar]
- Songer JG, Meer RR. Genotyping of Clostridium perfringens by polymerase chain reaction is a useful adjunct to diagnosis of clostridial enteric disease in animals. Anaerobe. 1996;2(4):197–203. doi: 10.1006/anae.1996.0027. [DOI] [Google Scholar]
- Stamm WE, Hooton TM. Management of urinary tract infections in adults. N Engl J Med. 1993;329(18):1328–1334. doi: 10.1056/NEJM199310283291808. [DOI] [PubMed] [Google Scholar]
- Starčević K, Krstulović L, Brozić D, Maurić M, Stojević Z, Mikulec Ž, Bajić M, Mašek T. Production performance, meat composition and oxidative susceptibility in broiler chicken fed with different phenolic compounds. J Sci Food Agric. 2015;95(6):1172–1178. doi: 10.1002/jsfa.6805. [DOI] [PubMed] [Google Scholar]
- Su L-H, Chiu C-H, Chu C, Ou JT. Antimicrobial resistance in nontyphoid Salmonella serotypes: a global challenge. Clin Infect Dis. 2004;39(4):546–551. doi: 10.1086/422726. [DOI] [PubMed] [Google Scholar]
- Suvanto J, Nohynek L, Seppänen-Laakso T, Rischer H, Salminen J-P, Puupponen-Pimiä R. Variability in the production of tannins and other polyphenols in cell cultures of 12 Nordic plant species. Planta. 2017;246(2):227–241. doi: 10.1007/s00425-017-2686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomke S, Elwinger K. Growth promotants in feeding pigs and poultry. I. Growth and feed efficiency responses to antibiotic growth promotants. Ann Zootech. 1998;47:85–97. doi: 10.1051/animres:19980201. [DOI] [Google Scholar]
- Thornton PK. Livestock production: recent trends, future prospects. Philos Trans R Soc Lond B Biol Sci. 2010;365(1554):2853–2867. doi: 10.1098/rstb.2010.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ultee A, Bennik MHJ, Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 2002;68:1561–1568. doi: 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela A (1995) Natural antioxidants: a new perspective for the problem of oxidative rancidity of lipids. In: Proceedings of 11th annual symposium of biotechnology in the feed industry. Nottingham University Press, Loughborough, Leicester, RU
- Valenzuela-Grijalva NV, Pinelli-Saavedra A, Muhlia-Almazan A, Domínguez-Díaz D, González-Ríos H. Dietary inclusion effects of phytochemicals as growth promoters in animal production. J Anim Sci Technol. 2017;59(1):1–17. doi: 10.1186/s40781-017-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Sluis W. Clostridial enteritis—a syndrome emerging world wide. World Poult. 2000;16(5):56–57. [Google Scholar]
- Van Immerseel F, De Buck J, Pasmans F, Huyghebaert G, Haesebrouck F, Ducatelle R. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 2004;33(6):537–549. doi: 10.1080/03079450400013162. [DOI] [PubMed] [Google Scholar]
- Viveros A, Susana Chamorro M, Pizarro IA, Centeno C, Brenes A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult Sci. 2011;90(3):566–578. doi: 10.3382/ps.2010-00889. [DOI] [PubMed] [Google Scholar]
- Wagenaar JA, Van Bergen MAP, Mueller MA, Wassenaar TM, Carlton RM. Phage therapy reduces Campylobacter jejuni colonization in broilers. Vet Microbiol. 2005;109(3–4):275–283. doi: 10.1016/j.vetmic.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Wagle BR, Upadhyay A, Upadhyaya I, Shrestha S, Arsi K, Liyanage R, Venkitanarayanan K, Donoghue DJ, Donoghue AM. Trans-cinnamaldehyde, eugenol and carvacrol reduce Campylobacter jejuni biofilms and modulate expression of select genes and proteins. Front Microbiol. 2019;10:1837. doi: 10.3389/fmicb.2019.01837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-H, Wan Z-L, Yang X-Q, Wang J-M, Guo J, Lin Y. Colloidal complexation of zein hydrolysate with tannic acid: constructing peptides-based nanoemulsions for alga oil delivery. Food Hydrocolloids. 2016;54:40–48. doi: 10.1016/j.foodhyd.2015.09.020. [DOI] [Google Scholar]
- Wang R, Li D, Bourne S (1998) Can 2000 years of herbal medicine history help us solve problems in the year 2000. In: Alltechs annual symposium
- Westrell T, Ciampa N, Boelaert F, Helwigh B, Korsgaard H, Mariann Chriél A, Ammon, and P Mäkelä. Zoonotic infections in Europe in 2007: a summary of the EFSA-ECDC annual report. Eurosurveillance. 2009;14(3):19100. doi: 10.2807/ese.14.03.19100-en. [DOI] [PubMed] [Google Scholar]
- Wiegel J (2015) Clostridiaceae. In: Bergey’s manual of systematics of archaea and bacteria, pp 1–5
- Windiasti G, Feng J, Ma L, Yaxi Hu, Hakeem MJ, Amoako K, Delaquis P, Xiaonan Lu. Investigating the synergistic antimicrobial effect of carvacrol and zinc oxide nanoparticles against Campylobacter jejuni. Food Control. 2019;96:39–46. doi: 10.1016/j.foodcont.2018.08.028. [DOI] [Google Scholar]
- Wingstrand A, Neimann J, Engberg J, Nielsen EM, Gerner-Smidt P, Wegener HC, Mølbak K. Fresh chicken as main risk factor for campylobacteriosis, Denmark. Emerg Infect Dis. 2006;12(2):280. doi: 10.3201/eid1202.050936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink M (2004) Phytochemical diversity of secondary metabolites. In: Encyclopedia of plant and crop science (print). Routledge, pp 915–919
- Wyszyńska A, Raczko A, Lis M, Jagusztyn-Krynicka EK. Oral immunization of chickens with avirulent Salmonella vaccine strain carrying C. jejuni 72Dz/92 cjaA gene elicits specific humoral immune response associated with protection against challenge with wild-type Campylobacter. Vaccine. 2004;22(11–12):1379–1389. doi: 10.1016/j.vaccine.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Xu ZR, Hu CH, Xia MS, Zhan XA, Wang MQ. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult Sci. 2003;82(6):1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- Yang Q, Wang L, Gao J, Liu X, Feng Y, Qian Wu, Baloch AB, Cui Lu, Xia X. Tannin-rich fraction from pomegranate rind inhibits quorum sensing in Chromobacterium violaceum and biofilm formation in Escherichia coli. Foodborne Pathog Dis. 2016;13(1):28–35. doi: 10.1089/fpd.2015.2027. [DOI] [PubMed] [Google Scholar]
- Yuan W, Seng ZJ, Kohli GS, Yang L, Yuk H-G. Stress resistance development and genome-wide transcriptional response of Escherichia coli O157: H7 adapted to sublethal thymol, carvacrol, and trans-cinnamaldehyde. Appl Environ Microbiol. 2018;84(22):e01616-18. doi: 10.1128/AEM.01616-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, White DG, Friedman SL, Glenn A, Blickenstaff K, Ayers SL, Abbott JW, Hall-Robinson E, McDermott PF. Antimicrobial resistance in Salmonella enterica serovar Heidelberg isolates from retail meats, including poultry, from 2002 to 2006. Appl Environ Microbiol. 2008;74(21):6656–6662. doi: 10.1128/AEM.01249-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuidhof MJ, Schneider BL, Carney VL, Korver DR, Robinson FE. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 20051. Poult Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.