Abstract
Background
Nuts are in the spotlight because of their association with improved health outcomes. We aimed to summarize the findings of previous studies to evaluate the impact of nuts consumption on glycaemic and lipid profile, inflammation, and oxidative stress.
Methods
Electronic searches for observational and intervention studies were undertaken in PubMed, Embase, Web of Science, and Science Direct until 2022 for searching the studies aiming the application of different types of nuts and the beneficial effects of nuts in improving glycemia, dyslipidemia, inflammation, and oxidative stress.
Results
Results from 56 interventional, 9 narrative and 3 systematic reviews, and 12 meta-analysis studies, aiming at the evaluating beneficial effects of different types of nuts on metabolic markers, showed that nut consumption could improve metabolic markers, including glycaemic factors, lipid profile, and inflammatory and oxidative stress parameters in both healthy and individuals with metabolic disorders in a type-, dose- and duration-dependent manner. According to their unique nutrient components, nuts can be known as a part of a healthy diet, resulting in improved metabolic biomarkers.
Conclusion
Considering the efficacy of nuts in improving metabolic markers, incorporation of, incorporating nuts the effectiveness of nuts in improving metabolic markers, incorporating nuts in the diet may prevent the incidence or aggravation of chronic metabolic diseases. Considering the health benefits of the nuts' components, including essential micronutrients, if consumed in the appropriate dose and duration to provide the necessary amount of effective micronutrients to improve health, we will see an improvement in metabolic factors. At the same time, more research is required to determine the optimal type, dose, and duration of nut intervention with regards to metabolic control and reducing the risk of developing metabolic disorders.
Keywords: lipid profile, oxidative stress, glycemic control (A1C), metabolic biomarkers, inflammation
Introduction
Nuts are known as healthy foods in the Mediterranean diet (MeDiet) because of their unique nutritional contents, and their consumption has been recommended to populations worldwide (1). Tree nuts, such as cashew nuts, hazelnuts, Brazil nuts, walnuts, almonds, pistachios, macadamias, and peanuts, are nutrient-dense foods, each with a unique combination of nutrients. Generally, these foods contain healthy fatty acid profiles including monounsaturated (MUFA) and polyunsaturated (PUFA) fatty acids, soluble and insoluble fibers, protein, vitamin K, vitamin E, thiamine, folate, minerals such as copper, magnesium, selenium (Se), and potassium, and substances such as antioxidants, phytosterols compounds, and xanthophyll carotenoids, with known health benefits for humans (2, 3). Due to their low levels of saturated fatty acids and high levels of unsaturated fatty acids and bioactive compounds, nuts have been reported to decrease the risk of cardio-metabolic disorders (4). The beneficial effects of nuts consumption on chronic disorders have been studied in previous research. Prior trials have recommended that regular nut consumption can provide beneficial effects on health outcomes and cardio-metabolic disorders, such as hypertension (5), cardiovascular diseases (2), obesity (6), and diabetes mellitus (7), with a reduction in chronic diseases mediators such as inflammation, oxidative stress, hyper-glycemia, visceral adiposity, endothelial dysfunction, and insulin resistance (8). Moreover, several meta-analyses of clinical trials and observational studies support the beneficial effects of nuts consumption on several cardio-metabolic disorders (9–13).
Nuts consumption offers a wide range of health benefits on humans; however, the present review will provide an update for giving an overview of recent findings of focus on metabolic benefits of nuts on glycaemic control, lipid profile, oxidative stress, and inflammatory status, and the appropriate dose and duration of nuts consumption to achieve metabolic benefits.
Nuts and Metabolic Biomarkers
Evidence of epidemiological and interventional studies suggest that nuts consumption is associated with in reducing the incidence and aggravation of some metabolic diseases (14). Nuts are good sources of fiber, healthy fats, and other beneficial nutrients (Figure 1) (15), and each type of nut offers unique nutritional benefits.
Figure 1.
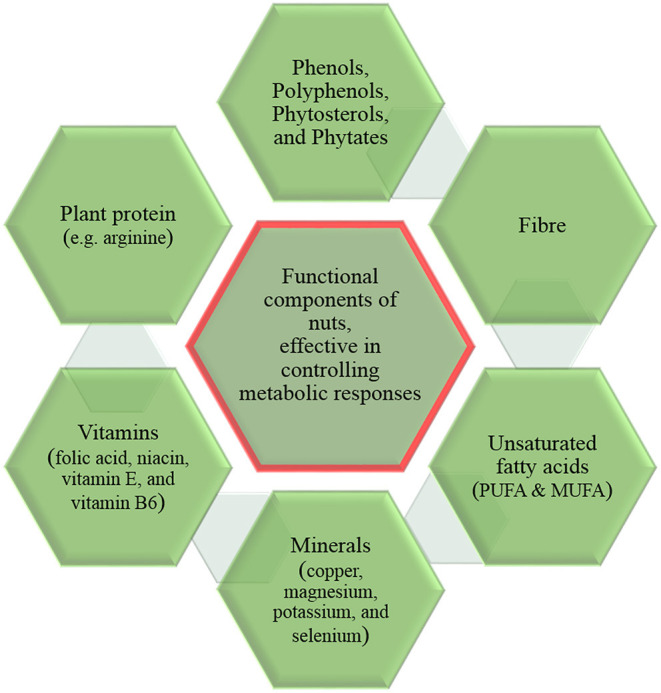
Beneficial components of nuts offering health benefits.
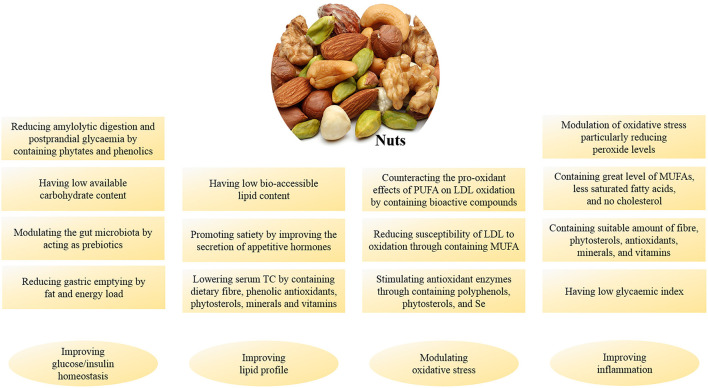
The ability of nuts to reduce the risk of chronic diseases and improving the level of metabolic markers is now well recognized (Figure 2) (16). Previous studies have shown that nuts consumption is associated with a reduced risk of cardiovascular disease (CVD), diabetes, and other chronic metabolic disorders (Table 1).
Figure 2.
A summary of nuts mechanisms of action in controlling metabolic responses.
Table 1.
A summary of studies evaluating the effect of nuts supplementation on metabolic markers.
| N | ID | Type of study | Study population | Dose | Duration | Findings |
|---|---|---|---|---|---|---|
| Almond | ||||||
| 1 | Li et al. (17) | Randomized crossover clinical trial | Chinese patients with T2DM | 60 g/day | 4 weeks | Those in the almond diet had lower levels of fasting insulin, FBS, and HOMA.IR. |
| 2 | Abazarfard et al. (18) | Randomized controlled trial (RCT) | Overweight and obese women | 50 g/day | 12 weeks | TC, TG, LDL-C, and FBS decreased significantly in the almond group compared to the nut-free group. |
| 3 | Ashley et al. (19) | Randomized crossover trial | Healthy individuals and individuals with T2DM | One serving per meal | 12 weeks | A standard serving of almonds reduced postprandial glycaemia significantly in participants with diabetes but did not influence glycaemia in participants without diabetes. |
| 4 | Jung et al. (20) | Randomized, crossover trial | Overweight and obese Korean adults | 56 g/day | 4 weeks | Almond consumption decreased TC, LDL-C, and non-HDL-C, compared to the control. Of serum inflammatory markers, IL-10 was decreased by almond intake, and IL-1β and IL-6 tended to be lower with almonds, compared to the control. |
| 5 | Gulati et al. (21) | Free-living pre-post intervention study | Asian Indians in North India with T2DM | 20% of energy intake | 24 weeks | TC, TG, LDL-C, HbA1c, and hs-CRP significant improved after intervention. |
| 6 | Chen et al. (22) | RCT | Patients with T2DM | 12 weeks | Almond decreased post-interventional FBS and HbA1c as compared to that of control. | |
| 7 | Foster et al. (23) | RCT | Overweight and obese individuals | 24 almonds per day | 24 weeks | The almond-enriched diet, compared with the hypo-caloric nut-free diet, was associated with greater reductions in TC, total:HDL-C, and TG. |
| 8 | Liu et al. (24) | RCT | Healthy adults | 56 g/day | 20 weeks | Participants in the almond group showed favorable significant changes in including levels of TG, TC, LDL-C, and non-HDL-C after consuming of almond compared with those at baseline. |
| 9 | Sabaté et al. (25) | Randomized crossover design | Healthy subjects | 10%, and 20% of total energy | 4 weeks | Compared with the Step I diet, the high-almond diet reduced TC, LDL-C, Apo B, and ratio of LDL to HDL-C, and increased HDL-C. |
| 10 | Berryman et al. (26) | Randomized, crossover, controlled-feeding study | Individuals with elevated LDL-C | 1.5 oz./day | 6 weeks | The almond diet, compared with the control diet, decreased non-HDL-C and LDL-C |
| 11 | Liu et al. (27) | RCT | Young Korean adults | 56 g/day | 16 weeks | Consuming almonds as a daily snack reduced the levels of TC and LDL-C. |
| 12 | Liu et al. (28) | Randomized crossover controlled feeding trial | Chinese patients with T2DM | 56 g/day | 4 weeks | Compared to the control diet, the almond diet decreased IL-6, CRP, and TNF-α. The almond diet also enhanced the resistance of LDL against Cu2+-induced oxidation compared to the control diet. |
| 13 | Jia et al. (29) | Pilot study | Healthy adult male regular smokers | 84 g/day | 4 weeks | MDA levels in the almond-treated groups were lower than the controls. Almond consumption has preventive effects on oxidative stress caused by smoking. |
| 14 | Li et al. (30) | Randomized, crossover clinical trial | Healthy smoker male soldiers | 84 g/day | 4 weeks | After the almond intervention, serum α-tocopherol, SOD, and GPX increased and MDA decreased significantly in smokers. |
| 15 | Sweazea et al. (31) | Randomized, parallel-arm controlled study | Individuals with T2DM | 1.5 oz/day | 12 weeks | The inflammatory biomarker CRP was significantly reduced in the almond-treated group vs. controls. |
| Pistachios | ||||||
| 16 | Sauder et al. (32) | Randomized, crossover, controlled feeding study | Adults with well-controlled T2DM | 20% of total energy intake | 4 weeks | TC, the ratio of total to HDL-C, and TG were significantly lower following the pistachio diet compared to the control diet. |
| 17 | Hern'andez-Alonso et al. (33) | RCT | Prediabetic subjects | 57 g/day | 16 weeks | FBS, insulin, and HOMA.IR decreased significantly after the intervention period compared with the control group. |
| 18 | Parham et al. (34) | double-blind, randomized, placebo-controlled, crossover trial | Patients with T2DM | 50g/day | 12 weeks | There was a marked decrease in HbA1c, FBS, and CRP in the pistachio group compared with the control group. |
| 19 | Gulati et al. (35) | RCT | Individuals with the MetS | 20% energy | 24 weeks | FBG, TC, LDL-C, hs-CRP, TNF-α, TBARS, and adiponectin levels were significant improved in the intervention group compared with control group. |
| 20 | Sari et al. (36) | RCT | Healthy young men | 20% of daily caloric intake | 4 weeks | Compared with the MeDiet, the pistachio diet decreased FBS, LDL-C, TC, TC/HDL-C and LDL-C/HDL-C ratios, and TG significantly. The pistachio diet significantly decreased serum IL-6, total oxidant status, lipid hydroperoxide, and MDA and increased SOD. |
| 21 | Canudas et al. (37) | Randomized crossover clinical trial | Prediabetic subjects | 57 g/day | 16 weeks | Compared with the control diet, the pistachio diet reduced oxidative damage to DNA and improved FBS and HOMA.IR. |
| Walnut | ||||||
| 22 | Wu et al. (38) | Randomized, controlled, cross-over study | Healthy Caucasian men and post-menopausal women | 43 g/day | 8 weeks | Walnut supplementation significantly decreased fasting non-HDL-C and Apo B in healthy senior individuals. |
| 23 | Hwang et al. (39) | Two-arm, randomized, controlled crossover study | Korean adults with MetS | 45 g/day | 16 weeks | Significant improvements after walnut intake, compared to control intervention, in HDL-C, FBS, and HbA1c were observed. |
| 24 | Ros et al. (40) | Randomized crossover trial | Hypercholesterolemic men and women | 32% of the energy from MUFA | 4 weeks | The walnut diet significantly reduced TC and LDL-C. |
| 25 | Ashraf et al. (41) | Experimental study | Individuals with hyperlipidemia | 25 g and 50 g/day | 8 weeks | Consumption of walnut showed significant improvements in lipid profile of hyperlipidemic individuals. |
| 26 | Zambo'n et al. (42) | Randomized, crossover feeding trial | Men and women with polygenic hypercholesterolemia | 35% of the energy obtained from MUFA | 6 weeks | Compared with the MeDiet, the walnut diet produced significant changes in level of TC, LDL-C, and lipoprotein (a). |
| 27 | Bashan et al. (43) | RCT | Patients with dyslipidemia | 40–50 g/day | 12 weeks | TC, LDL-C, VLDL-C, and TG levels significantly decreased and HDL-C levels significantly increased in the walnut group at the end of the trial. |
| 28 | Torabian et al. (44) | Randomized crossover trial | Subjects with normal to moderate high plasma total cholesterol | 12% of total daily energy intake | 24 weeks | Significant changes in serum concentrations of TC and TG were seen and nearly significant changes in LDL-C were found by supplementing a habitual diet with walnuts. |
| 29 | Bamberger et al. (45) | Randomized, controlled, prospective, cross-over study | Healthy subjects | 43 g/day | 8 weeks | The walnut diet resulted in a significant reduction in fasting cholesterol, non-HDL-C, LDL-C, TG, and Apo B levels. |
| 30 | Rock et al. (46) | RCT | Overweight and obese men and women | 15% of energy | 24 weeks | The walnut-enriched diet group reduced TC and LDL-C. |
| 31 | Alibabaie et al. (47) | RCT | Female Undergraduate Students | 40 g/day | 4 weeks | A significant reduction was observed in the serum levels of LDL-C and TG after the consumption of walnuts. |
| Peanuts | ||||||
| 32 | Liu et al. (48) | Randomized, controlled, crossover postprandial study | Healthy overweight or obese men | 85 g | Acute peanut consumption blunted the serum TG. | |
| Hazelnuts | ||||||
| 33 | Damavandi et al. (49) | Controlled randomized parallel study | Patients with type 2 Diabetes | 29 g/day | 8 weeks | Hazelnut consumption non-significantly reduced TG, FBS, TC, and LDL-C levels. |
| 34 | Renzo et al. (50) | Prospective pilot clinical trial |
Healthy volunteers | 40 g/day | 6 weeks | Significant up-regulation was detected for SOD, CAT, PPAR-γ, and ACE at the end of the study. |
| Brazilian nut | ||||||
| 35 | Cominetti et al. (51) | RCT | Obese women | One nut per day | 8 weeks | Obese people who implement daily consumption of Brazilian nuts could improve lipid profile, especially HDL-C levels |
| 36 | Colpo et al. (52) | Randomized crossover study | Healthy individuals | 20 or 50 g | A single intake of Brazil nuts caused a significant decrease in serum IL-1, IL-6, TNF-α, and IFN-γ levels. | |
| 37 | Maranhão et al. (53) | RCT | Obese female adolescents | 15–25 g/day | 16 weeks | Compared to placebo group, Brazil nuts intake reduced TC, TG, and LDL-ox |
| 38 | Macan et al. (54) | RCT | Patients with T2DM | One nut per day | 24 weeks | Supplementation with Brazil nuts significantly increased serum Se levels. Furthermore, it was found that the cells were more resistant to H2O2-induced DNA damage after the supplementation. |
| 39 | Stockler-Pinto et al. (55) | RCT | Hemodialysis patients | One nut per day | 12 weeks | The plasma Se and GPx activity increased; moreover, HDL-C levels increased and LDL-C levels decreased significantly after supplementation. |
| 40 | Watanabe et al. (56) | RCT | Patients in regular use of statins | One nut per day | 12 weeks | Brazil nut decreased levels of CK activity in serum, MDA, and SOD and increased levels of GPX activity. Moreover, the supplementation caused significantly positive changes in plasma and erythrocyte Se concentrations. |
| Cashew | ||||||
| 41 | Mah et al. (57) | Randomized, crossover, isocaloric, controlled-feeding study | Normally active men and women | 28-64 g/day (11% of total energy intake) | 4 weeks | Consumption of the cashew diet resulted in a significant change from baseline (compared with the control) in TC, LDL-C, non-HDL-C, and the TC:HDL-C ratio. |
| 42 | Damavandi et al. (58) | Randomized, isocaloric, controlled-feeding study | Patients with T2DM | 10% of total calorie intake | 8 weeks | Serum insulin, HOMA-IR, and LDL-C/HDL-C ratio significantly decreased in the cashew group compared with those of the controls. |
| 43 | Shidfar et al. (59) | Randomized parallel clinical trial | Patients with T2DM | 10% of total daily calorie intake | 8 weeks | Mean HDL-C and insulin concentration were significantly improved in intervention group compared with control group. |
| 44 | Mohan et al. (60) | Parallel-arm, randomized controlled trial | Patients with T2DM | 30 g/day | 12 weeks | Participants in the intervention group had a greater increase in plasma HDL-C compared with controls. |
| Pecan | ||||||
| 45 | McKay et al. (61) | Randomized, controlled feeding trial |
Patients with T2DM | 15% of total calories | 4 weeks | Changes in serum insulin, HOMA-IR, and HOMA-β were significantly greater in intervention group than those in control group. |
| 46 | Campos et al. (62) | RCT | Patients with stable coronary artery disease | 30 g/day | 12 weeks | The pecan nut consumption exhibited a significant reduction in non-HDL-C levels and in the TC/HDL-C ratio compared to the control group. |
| Soy nut | ||||||
| 47 | Sedaghat et al. (63) | Case-control study | Patients with T2DM | 60 g/day | 8 weeks | Soy consumption significantly lowered FPG, HbA1c, plasma insulin levels, insulin-resistance, TC, and LDL-C. |
| 48 | Sedaghat et al. (64) | RCT | Patients with T2DM | 60 g/day | 8 weeks | Consuming soy nut significantly decreased the FBS, TC, and LDL-C and increased the capacity of serum total antioxidants. |
| 49 | Bakhtiary et al. (65) | RCT | Women with MetS | 35 g/day | 12 weeks | The soy-nut improved FBG, insulin, HOMA-IR, MDA, and TAC significantly after intervention. |
| 50 | Bakhtiari et al. (66) | RCT | Old women with MetS | 35 g/day | 12 weeks | Soy-nut significantly decreased TC, LDL-C, VLDL-C, Apo B100, FBS, serum insulin, HOMA-IR, and MDA levels. Moreover, the intervention significantly increased TAC compared with the control group. |
| 51 | Karamali et al. (67) | RCT | Women with polycystic ovary syndrome | 35% daily protein intake | 8 weeks | Consumption of soy-nut, compared with the control group, resulted in significant decreases in FBS, insulin, and insulin resistance, as well as a significant increase in quantitative insulin sensitivity check index. In addition, significant decreases in TC, TG, and MDA and significant increases in NO and GSH were seen in the test group compared to the control. |
| 52 | Azadbakht et al. (68) | Randomized crossover clinical trial | Postmenopausal women with the MetS | 30 g/day | 8 weeks | The soy-nut regimen significantly decreased HOMA.IR, FBS, and LDL-C compared with the soy-protein or control. |
| 53 | Hematdar et al. (69) | RCT | Subjects with T2DM | a cup of cooked soy beans three days a week | 8 weeks | A significant decrease was observed in serum CRP of soy bean group which was significantly more than the controls. |
| Baru almond | ||||||
| 54 | Bento et al. (70) | Randomized, crossover, placebo-controlled study | Mildly hypercholesterolemic subjects | 20 g/day | 6 weeks | Compared to placebo, supplementation of baru almonds reduced TC, LDL-C, and non-HDL-C. |
| 55 | Souza et al. (71) | RCT | Overweight and obese women | 20 g/day | 8 weeks | The consumption of baru almonds increased HDL-C level compared to baru almond-free diet. |
| 56 | Souza et al. (72) | Parallel-arm, randomized placebo-controlled trial | Overweight and obese women | 20 g/day | 8 weeks | The baru almond group increased the activity of GPx and plasma copper concentration when compared to the placebo group. |
FBS, fasting blood sugar; HOMA.IR, homeostatic model assessment for insulin resistance; HOMA-β, homeostasis model assessment of β-cell function; HbA1c, glycated hemoglobin; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; IL, interleukin; hs-CRP, high-sensitivity C-reactive protein; TNF-α, tumor necrosis factor alpha; MDA, Malondialdehyde; TAC, total antioxidan capacity; SOD, superoxide dismutase; GPX, glutathione peroxidase; TBARS, thiobarbituric acid reactive substances; CAT, catalase; ACE, angiotensin-converting enzyme; PPAR-γ, peroxisome-proliferator activator receptor γ; FPG, form-amido-pyrimidine glycosylase; NO, nitric oxide; Apo B, apolipoprotein B; T2DM, type 2 diabetes mellitus; MetS, metabolic syndrome.
Glycaemic Control
A significant number of studies showed a link between regular nuts consumption and a reduction in risk of heart and metabolic disorders (73–76). Moreover, studies have shown that nuts consumption can improve glycemic responses in healthy and diabetic individuals (73). Nuts affect glycaemic response in a dose-dependent manner (77). According to the previous investigations, nuts help decrease glycaemic excursions; moreover, consuming nuts with carbohydrate-rich foods could reduce the postprandial impact on the insulin demand (77–80). The dose and duration of supplementation in studies that observed a significant effect of nuts consumption on glycaemic factors ranged from 30–60 g/day and 4–24 weeks, respectively (17, 18, 33, 35, 49, 52). The dose-dependent improvement in the glycaemic response to the meal has been revealed in previous investigations. In a study conducted on 10 healthy volunteers, it has been shown that the addition of 28 g of pistachios to white bread could improve glycaemic response, and this improvement was greater with the addition of 84 g of pistachios (79). In another study conducted on normo-glycaemic and individuals with T2D, adding 30, 60, and 90 g nuts to white bread reduced the glycaemic response of the meal by 11.2 ± 11.6% (P = 0.354), 29.7 ± 12.2% (P = 0.031), and 53.5 ± 8.5% (P < 0.001) (80).
It has been shown that people who eat more nuts in their diet have been shown to have a higher cardioprotective profile of glucose/insulin homeostasis (81). Findings from previous studies indicate a significant reduction in fasting insulin levels or an improvement in insulin resistance following the nuts consumption in both healthy and diabetic individuals (82, 83). Results from the Nurses' Health Study cohort indicated that eating more than 5 servings/week of nuts reduces the risk of diabetes compared to rare or no consumption (RR = 0.73, 95% CI = 0.60–0.89; P < .001) (84). Beneficial evidence is also supported by randomized controlled trials (RCTs). A meta-analysis of 12 RCTs with more than a 3-week follow-up period showed that consumption of a median dose of 56 g/day tree nuts could improve glycemic control in individuals with T2D compared to isocaloric diet without tree nuts (7). Additionally, the results of the study, conducted by Kendall et al. (80), showed that nuts eaten alone or with a high glycaemic index (GI) diet can lower postprandial blood sugar (PBG).
In addition to fat and protein, nuts have been reported to be rich sources of phenolics and phytates, both of which can decrease amylolytic digestion and postprandial blood sugar (85). In nuts, abundant fiber and polyphenols (flavonoids and non-flavonoids) may have a prebiotic effect and affect glucose metabolism (86). Several researchers have revealed that prebiotics' modulation of gut microbiota can improve glycemic control in healthy and diabetic subjects (87–89). Moreover, the blood sugar controlling effect of nuts can be attributed in part to the low carbohydrate content of nuts. Additionally, it has been shown that gastric emptying can be reduced by energy and fat load. Therefore, increasing the energy and fat load by increasing the dose of consumed nuts can partly explain the dose-dependent decrease in blood sugar in response to nuts consumption (80). In addition, it has also been shown that nuts can reduce the glycaemic response to a meal compared to a balanced meal in terms of energy and macronutrient contents (78). This can be due to nutsnuts' high unsaturated fat content, unsaturated fat content,and their unique physical structure. Unsaturated fatty acids in nuts in place of saturated fat and carbohydrate appear to, improve fasting, improve fasting and 2-h glucose levels significantly, but further studies should be performed with careful consideration of different types and amounts of nuts (90, 91).
In summary, overall nut intake has been revealed to be inversely associated with glycaemic factors. It may delay the development and progression of chronic metabolic diseases related to impaired glucose tolerance or glycaemic response.
Lipid Profile
The beneficial effects of nuts consumption on lipid profiles, especially total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C), have been reported in clinical studies in both healthy and high-cholesterolemic individuals from various geographical areas (92). However, the effects varied based on the type and amount of nut, consumption duration, characteristics of the studied individuals, and study design (93–98). Most studies in this field that have shown a significant effect of nuts consumption on lipid factors have reported a dose of 20–64 g/day and a duration of 4–24 weeks (18, 20, 21, 53, 57, 70).
A Systematic Review conducted by Altamimi et al. (99) evaluated several dietary intervention studies that examined the effect of eating nuts on blood lipid levels. Analyses were performed on different types of nuts. Most of the studies showed improvement in lipid profile, including TC, LDL-C, high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), and total cholesterol/high-density lipoprotein cholesterol (TC/HDL-C) after nut consumption. Tapsell et al. (95) found that following a healthy diet enriched with 30 g of walnuts for 6 months caused a significant reduction in LDL-C and increased HDL-C in subjects with diabetes. A recent study also showed beneficial changes in lipid profile after a MeDiet enriched with 30 g of mixed nuts compared to the control diet in high-risk CVD patients, about half of whom with T2D (96). Compared to the low-fat diet, the average changes in the MeDiet enriched with the nuts group were −6.20 (P = 0.040) for TC level, −13.0 (p = 0.022) for TG level, and −0.26 (P = 0.002) for the TC/HDL-C ratio (96). Lee et al. (97) showed that supplementing 30 g/day of mixed nuts (walnuts, peanuts, and pine nuts) with a usual diet for 6 weeks had beneficial effects on lipid profile in women with MetS. Compared to those in the control group, TC and non-HDL-C levels significantly decreased in the nut group (P = 0.023 and P = 0.016, respectively). A cross-sectional study conducted to examine the relationship between nuts intake and lipid profile among Iranians showed that regular consumption of pistachios, almonds, hazelnuts, and walnuts is associated with a lower incidence of hyperlipidemia (98). Results of this study revealed that nuts consumption is significantly associated with decreased LDL-C, TG, and Apo B/Apo A in both men and women and decreased TC only in women. In a systematic review, Mukuddem-Petersen et al. (92) found a 2–16% reduction in TC and 2–19% in LDL-C among people who consumed nuts compared to those on controlled diets. In addition, a prospective cohort study with positive quality showed the same results of nuts consumption in reducing TC, non-HDL-C, LDL-C, and Apo B-100 concentrations in women with diabetes (100). Sabaté et al. (25) compared the effects of two different amounts of almonds with the effects of the National Cholesterol Education Program Step I on serum lipids and lipoproteins in healthy adults and those with mildly high cholesterol (Low and High almond diets for replaceing 10 and 20%, respectively, of energy of the Step I diet with almonds). They found that in addition to lowering LDL-C, the high-almond diet significantly reduced Apo B concentrations. Apo B is a component of serum LDL and VLDL and consequently reflects the concentration of atherogenic lipoprotein particles that confer risk of CVD (101). Sabaté et al. (25) observed a decreasing trend in Apo B concentration with increasing amounts of almonds in the diet, indicating a decrease in LDL-cholesterol concentration and the number of LDL particles.
Several claimed reasons explain the biological rationale of consuming nuts and improving the lipid profile. First, the bioavailability of fat content of nuts is low, which means that large amounts of this fat are excreted in the feces (102). Second, the crunchy texture of nuts promotes satiety because the mechanical action of chewing leads to the release of appetite suppressant hormones such as cholecystokinin, which ultimately leads to lower calorie and fat intake (103). Third, it is suggested that nut components other than fatty acids, such as vitamins (e.g., vitamin E, vitamin B6, niacin, and folic acid), minerals (e.g., magnesium, potassium, and copper), dietary fiber, plant protein (e.g., arginine), phytosterols, and phenolic antioxidants are also bioactive in lowering serum TC level (104). Recent evidence suggests that the phytosterols in nuts which are more hydrophobic than cholesterol, impair cholesterol absorption because their hydrocarbon molecule is larger and has a greater affinity for micelles than cholesterol (105). As a result, cholesterol is displaced from the micelles, and the amount available for absorption becomes more limited (106). Nuts are also a rich source of protein (nearly 25% of energy), especially high in L-arginine (107). L-arginine supplementation is usually recommended for people with dyslipidemia. It has been suggested that L-arginine ihelpmproves the lipid profile because of its potential in increasing nitric oxide (NO) production. Production of NO increases the activity of lipoprotein lipase and finally, improving the hydrolysis of TGs reduces its plasma concentration (108). A meta-analysis, conducted by Hadi et al. (109), concluded that L-arginine supplementation could significantly reduce blood TG levels; however, more studies are needed to prove its hypocholesterolemic effects.
Due to their unique nutrient profile, enriching a healthy diet with nuts may positively affecting lipid profile. Lipid-lowering effects of nuts should be considered in future food-based dietary strategies for improving plasma lipid levels.
Inflammation
Inflammation plays a key role in the development of cardio-metabolic diseases, such as CVD and T2D and nuts may moderate inflammation and the development of endothelial dysfunction and cardio-metabolic disorders through their bioactive components such as L-arginine, alpha-linolenic acid (MUFA), polyphenols, and fiber (110). Cross-sectional studies in this field showed that people with regular nut consumption had lower serum concentrations of proinflammatory cytokines or endothelial cell adhesion molecules. Effective interventional periods ranged from 4 to 24 weeks, with doses ranging from 20 to 56 g/day (20, 21, 34, 52).
It has been shown that α-linolenic acid [18:3(n-3)], extracted from nut is inversely associated with interleukin-6 (IL-6), soluble tumor necrosis factor (TNF) receptors 1 and 2, fibrinogen, and C-reactive protein (CRP) levels in both healthy subjects and individuals with coronary artery disease (111, 112). Furthermore, subjects who ate more nuts showed lower levels of intercellular adhesion molecule 1 (ICAM-1)-1, vascular cell adhesion protein 1 (VCAM-1), CRP, and IL-6 (113). In a cross-sectional analysis of the Multi-Ethnic Study of Atherosclerosis, consumption of seed and nut was inversely associated with IL-6, CRP, and fibrinogen (114). Yu et al. examined the association of common consumption of nuts with inflammatory biomarkers in two large groups of American men and women (115). They showed that nuts consumption was inversely associated with the concentration of IL-6 and CRP. The health effects of nuts on inflammatory markers have also been investigated in clinical trials. In a randomized trial, patients with MetS were advised to follow a healthy diet with 30 g of daily supplementation of raw nuts for 12 weeks (7.5 g hazelnuts, 15 g walnuts, and 7.5 g almonds) (82). Among inflammatory markers, the diet of nuts significantly resulted in changes in plasma IL-6. In another clinical trial, almond diet (56 g/day) for 4 weeks reduced CRP by a median 10.3 % (95% CI: −24.1, 40.5), TNF-α by a median 15.7 % (95% CI: −0.3, 29.9), and IL-6 by a median 10.3 % (95% CI: 5.2, 12.6 %) in comparison with the control diet (28). Gulati et al. (35) showed that supplementation of pistachio (20% energy) for 24-wk significantly improved hs-CRP (P < 0.05) and TNF-α (P < 0.03) in individuals with MetS. A meta-analysis of 32 RCTs showed that consumption of nuts resulted in small and non-significant differences in CRP levels (10).
The beneficial effects of nuts on inflammatory markers are attributed to their composition, which is recognized by lesser saturated fatty acids, a greater level of mono-unsaturated fatty acids (MUFAs), no cholesterol, and a suitable amount of phytosterols, fiber, protein, antioxidants, and numerous vitamins and minerals (116, 117). An increasing number of studies have examined the effect of these nutrients on inflammation. As suggested in cross-sectional studies, the high content of phenolic compounds in nuts may anticipate the anti-inflammatory effect of regular consumption of nuts (100). Furthermore, nuts are rich sources of fiber and therefore have a low glycaemic index (GI). It has been reported that diets with low GI can reduce CRP levels (118). Nuts are an important source of antioxidants essential for human health (8). Modulation of oxidative stress (particularly reducing peroxide levels) can decrease inflammation. The fatty acid composition of nuts is beneficial because the content of saturated fatty acids (SFAs) is low (4–16%), and almost half of the total fat content is composed of unsaturated fats. In most nuts MUFA (oleic acid) and different amounts of PUFA (119). Earlier data indicate that MUFA and PUFAs can exhibit anti-inflammatory properties in various experimental models of inflammation (120). The mechanisms by which dietary fatty acids inhibit cytokine production are unknown but may be related to inhibition of the inflammatory cascade at the level of lipoxygenase (LOX) and cyclooxygenase (COX) (121). Regarding the protective effect of nuts, it has been reported that the MeDiet enriched with nuts can lower the incidence of major cardiovascular events among people at high risk of cardiovascular diseases (122).
Considering existing evidence regular consumption of nuts can be associated with a nutritional profile of inflammatory biomarkers. However, further studies are needed to definitively address the important question of the anti-inflammatory effects of nuts.
Oxidative Stress
Consumption of nuts as food sources of antioxidants can prevent pro-oxidation and excessive oxidation of LDL (123). Consumption of antioxidant foods as components of the diet playss an important role in managing cardio-metabolic disorders (124). Vitamin E and phenolic compounds are among the nutrients with antioxidant activities that nuts are a rich source of both. (125). In vitro studies (126, 127), animal models (128, 129), observational studies (130), and randomized trials (35, 72) suggest the potential benefits of including various nuts in the diet with regards to oxidative stress biomarkers. Most of the studies that have reported the potential beneficial effects of eating nuts on oxidative stress have focused on pistachios, almonds, peanuts, or walnuts, all of which are rich sources of MUFA. For instance, a sub-analysis of the PREDIMED (Prevención con Dieta Mediterránea) study, which assessed biomarkers associated with oxidative stress, identified higher superoxide dismutase (SOD) and catalase activity and lower plasma xanthine oxidase activity in the intervention group (the MeDiet + extra-virgin olive oil and the MeDiet + nuts) (131). The effective dose and duration observed in clinical studies were 20–84 g/day and 4–16 weeks, respectively (28, 30, 50, 53, 65, 72).
The nutritional intervention of 1 unit/d (20 g/day) of Brazil nuts for 3 months in hemodialysis patients resulted in improved plasma glutathione peroxidase (GPx) levels and a reduction in 8-isoprostane and 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels (p < 0.001) (55). A clinical trial on 46 overweight and obese women found that after 8 weeks of intervention, the Baru nuts group (20 g/day) showed a significant increase in GPx activity compared to the placebo group (72). Jenkins et al. (132) showed that a full dose of almond supplement (73 ± 3 g/day, 22% of energy) or half a dose over 4 weeks reduced oxidized LDL in subjects with dyslipidemia. Liu et al. (28) found similar results after 4 weeks of supplementation with almond (~ 6 g/day) in individuals with dyslipidemia and T2D. Pistachio supplementation (10 or 20% of energy) for 4 weeks was also effective in reducing oxidized LDL in people with hyperlipidemia (133).
It has been revealed that bioactive compounds (phytosterols, polyphenols), MUFA, Se, and tocopherols are the main factors in the beneficial effects of nuts in modulating oxidative stress. These agents can also reduce the pro-oxidant effects of PUFA on LDL oxidation and DNA damages (134). The main fat compounds in hazelnuts and almonds are MUFA and are associated with decreased LDL sensitivity to oxidation (119). Therefore, differences in the fat content of different nuts may partly explain why pistachios, walnuts, almonds, and other MUFA-rich nuts can reduce oxidative stress, whereas walnuts (PUFA-rich) Do not have. Se, phytosterols, and polyphenols can activate the Nrf2 (nuclear factor erythroid 2-related factor 2) pathway (134). Nrf2 stimulates the antioxidant response element (ARE) genes transcription and encodes the antioxidant (e.g., GPx) and detoxifying enzymes (55). The Nfr2 pathway also activates NQO1, stabilizing proteins and protecting them against oxidative degradation (135).
Consumption of antioxidant food sources and/or antioxidant supplements seems to contribute to the prevention and/or treatment of metabolic disorders through widely known mechanisms; nuts are a good example of such food sources because of their good taste and dose-effect that allows them to be included in diets.
Based on the findings of evaluated studies, depending on the dose and duration of consumption (Table 2), mixed nuts can have health effects on metabolic markers, including glycaemic response, lipid factors, oxidative stress, and inflammation. In contrast, no adverse effects of nuts intake on metabolic biomarkers have been found in clinical trials (136).
Table 2.
Appropriate dose and duration of mixed nut consumption to insert metabolic efficacy.
| Metabolic response | Optimal dose (g/day) | Optimal duration (weeks) |
|---|---|---|
| Glycaemic response | 28–60 | 4–24 |
| Lipid profile | 20–64 | 4–24 |
| Inflammatory response | 20–56 | 4–24 |
| Oxidative stress markers | 20–84 | 4–16 |
Conclusions
As ready-to-eat snack foods, nuts have healthy lipid components and are excellent sources of fiber and some micronutrients. The US FDA has approved a qualified health claim for nuts suggesting that daily consumption of nuts may help to reduce the risk of some chronic disorders purportedly through the improvement of metabolic markers. However, the intervention's selected type, dose, and duration should be based on the therapeutic goals. For example, the effectiveness of different types of nuts including, Almond, Pistachios, Walnut, Hazelnuts, Brazilian nut, Peanuts, Cashew, Pecan, and Soy nut on glycaemic response and lipid factors has been shown in several clinical trials. But, in the case of inflammation, the effectiveness of walnuts has been more supported due to their high ALA and phenolic compounds. Additionally, in the case of oxidative stress, the effectiveness of almonds, pistachios, pecans, or peanuts due to their rich sources of MUFA has been supported. Further research is needed to establish the intakes of different varieties of nuts to deliver optimal metabolic benefits.
Author Contributions
All authors conceived the idea, participated in the study design, drafted the manuscript, reviewed, edited, and approved the final manuscript.
Funding
This work is funded by RMC-UTM with industrial Grant Nos. RJ130000.7609.4C187 and RJ130000.7609.4C284.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to acknowledge the help of Almanac Life Science India Pvt. Ltd., New Delhi, India for data analysis and valuable inputs.
References
- 1.Ros E, Martínez-González MA, Estruch R, Salas-Salvadó J, Fitó M, Martínez JA, et al. Mediterranean diet and cardiovascular health: teachings of the PREDIMED study. Advances in nutrition. (2014) 5:330S−6S. 10.3945/an.113.005389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Souza RG, Gomes AC, Naves MM, Mota JF. Nuts and legume seeds for cardiovascular risk reduction: scientific evidence and mechanisms of action. Nutr Rev. (2015) 73:335–47. 10.1093/nutrit/nuu008 [DOI] [PubMed] [Google Scholar]
- 3.Taş NG, Gökmen V. Phenolic compounds in natural and roasted nuts and their skins: a brief review. Current Opinion in Food Science. (2017) 14:103–9. 10.1016/j.cofs.2017.03.001 [DOI] [Google Scholar]
- 4.Barbour JA, Howe PR, Buckley JD, Bryan J, Coates AM. Effect of 12 weeks high oleic peanut consumption on cardio-metabolic risk factors and body composition. Nutrients. (2015) 7:7381–98. 10.3390/nu7095343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammadifard N, Salehi-Abargouei A, Salas-Salvadó J, Guasch-Ferré M, Humphries K, Sarrafzadegan N. The effect of tree nut, peanut, and soy nut consumption on blood pressure: a systematic review and meta-analysis of randomized controlled clinical trials. Am J Clin Nutr. (2015) 101:966–82. 10.3945/ajcn.114.091595 [DOI] [PubMed] [Google Scholar]
- 6.Jackson CL, Hu FB. Long-term associations of nut consumption with body weight and obesity. Am J Clin Nutri. (2014) 100(suppl_1):408S−11S. 10.3945/ajcn.113.071332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viguiliouk E, Kendall CW, Mejia SB, Cozma AI, Ha V, Mirrahimi A, et al. Effect of tree nuts on glycemic control in diabetes: a systematic review and meta-analysis of randomized controlled dietary trials. PLoS ONE. (2014) 9:e103376. 10.1371/journal.pone.0103376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mejia SB, Kendall CW, Viguiliouk E, Augustin LS, Ha V, Cozma AI, et al. Effect of tree nuts on metabolic syndrome criteria: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. (2014) 4:4660. 10.1136/bmjopen-2013-004660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tindall AM, Johnston EA, Kris-Etherton PM, Petersen KS. The effect of nuts on markers of glycemic control: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. (2019) 109:297–314. 10.1093/ajcn/nqy236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neale EP, Tapsell LC, Guan V, Batterham MJ. The effect of nut consumption on markers of inflammation and endothelial function: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. (2017) 7:e016863. 10.1136/bmjopen-2017-016863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao Y, Xia J, Ke Y, Cheng J, Yuan J, Wu S, et al. Effects of nut consumption on selected inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Nutrition. (2018) 54:129–43. 10.1016/j.nut.2018.02.017 [DOI] [PubMed] [Google Scholar]
- 12.Liu K, Hui S, Wang B, Kaliannan K, Guo X, Liang L. Comparative effects of different types of tree nut consumption on blood lipids: a network meta-analysis of clinical trials. Am J Clin Nutr. (2020) 111:219–27. 10.1093/ajcn/nqz280 [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Zhang D-Z. Relationship between nut consumption and metabolic syndrome: a meta-analysis of observational studies. J Am Coll Nutr. (2019) 38:499–505. 10.1080/07315724.2018.1561341 [DOI] [PubMed] [Google Scholar]
- 14.Kim Y, Keogh J, Clifton PM. Nuts and cardio-metabolic disease: a review of meta-analyses. Nutrients. (2018) 10:1935. 10.3390/nu10121935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dheir IM, Mettleq ASA, Elsharif AA, Abu-Naser SS. Classifying nuts types using convolutional neural network. Int J Acad Inf Syst Res (IJAISR). (2020) 3:12–8.34577429 [Google Scholar]
- 16.De Souza RGM, Schincaglia RM, Pimentel GD, Mota JF. Nuts and human health outcomes: a systematic review. Nutrients. (2017) 9:1311. 10.3390/nu9121311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li SC, Liu YH, Liu JF, Chang WH, Chen CM, Chen CYO. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism. (2011) 60:474–9. 10.1016/j.metabol.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 18.Abazarfard Z, Salehi M, Keshavarzi S. The effect of almonds on anthropometric measurements and lipid profile in overweight and obese females in a weight reduction program: a randomized controlled clinical trial. J Res Med Sci: Off J Isfahan Uni Med Sci. (2014) 19:457. [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen AE, Johnston CS. Almond ingestion at mealtime reduces postprandial glycemia and chronic ingestion reduces hemoglobin A1c in individuals with well-controlled type 2 diabetes mellitus. Metabolism. (2011) 60:1312–7. 10.1016/j.metabol.2011.01.017 [DOI] [PubMed] [Google Scholar]
- 20.Jung H, Chen C-YO, Blumberg JB, Kwak H-K. The effect of almonds on vitamin E status and cardiovascular risk factors in Korean adults: a randomized clinical trial. Eur J Nutr. (2018) 57:2069–79. 10.1007/s00394-017-1480-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gulati S, Misra A, Pandey RM. Effect of almond supplementation on Glycemia and cardiovascular risk factors in Asian Indians in North India with type 2 diabetes mellitus: a 24–week study. Metab Syndr Relat Disord. (2017) 15:98–105. 10.1089/met.2016.0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen CM, Liu JF, Li SC, Huang CL, Hsirh AT, Weng SF, et al. Almonds ameliorate glycemic control in Chinese patients with better controlled type 2 diabetes: a randomized, crossover, controlled feeding trial. Nutr Metab. (2017) 14:1–12. 10.1186/s12986-017-0205-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster GD, Shantz KL, Vander Veur SS, Oliver TL, Lent MR, Virus A, et al. A randomized trial of the effects of an almond-enriched, hypocaloric diet in the treatment of obesity. Am J Clin Nutr. (2012) 96:249–54. 10.3945/ajcn.112.037895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Hwang H-J, Kim H-S, Park H. Time and intervention effects of daily almond intake on the changes of lipid profile and body composition among free-living healthy adults. J Med Food. (2018) 21:340–7. 10.1089/jmf.2017.3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabaté J, Haddad E, Tanzman JS, Jambazian P, Rajaram S. Serum lipid response to the graduated enrichment of a Step I diet with almonds: a randomized feeding trial. Am J Clin Nutr. (2003) 77:1379–84. 10.1093/ajcn/77.6.1379 [DOI] [PubMed] [Google Scholar]
- 26.Berryman CE, West SG, Fleming JA, Bordi PL, Kris-Etherton PM. Effects of daily almond consumption on cardiometabolic risk and abdominal adiposity in healthy adults with elevated LDL-cholesterol: a randomized controlled trial. J Am Heart Assoc. (2015) 4:e000993. 10.1161/JAHA.114.000993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Hwang H-J, Ryu H, Lee Y-S, Kim H-S, Park H. The effects of daily intake timing of almond on the body composition and blood lipid profile of healthy adults. Nutr Res Pract. (2017) 11:479. 10.4162/nrp.2017.11.6.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu JF, Liu YH, Chen CM, Chang WH, Chen CO. The effect of almonds on inflammation and oxidative stress in Chinese patients with type 2 diabetes mellitus: a randomized crossover controlled feeding trial. Eur J Nutr. (2013) 52:927–35. 10.1007/s00394-012-0400-y [DOI] [PubMed] [Google Scholar]
- 29.Jia X, Li N, Zhang W, Zhang X, Lapsley K, Huang G, et al. A pilot study on the effects of almond consumption on DNA damage and oxidative stress in smokers. Nutr Cancer. (2006) 54:179–83. 10.1207/s15327914nc5402_4 [DOI] [PubMed] [Google Scholar]
- 30.Li N, Jia X, Chen C-YO, Blumberg JB, Song Y, Zhang W, et al. Almond consumption reduces oxidative DNA damage and lipid peroxidation in male smokers. J Nutr. (2007) 137:2717–22. 10.1093/jn/137.12.2717 [DOI] [PubMed] [Google Scholar]
- 31.Sweazea KL, Johnston CS, Ricklefs KD, Petersen KN. Almond supplementation in the absence of dietary advice significantly reduces C-reactive protein in subjects with type 2 diabetes. J Funct Foods. (2014) 10:252–9. 10.1016/j.jff.2014.06.024 [DOI] [Google Scholar]
- 32.Sauder KA, McCrea CE, Ulbrecht JS, Kris-Etherton PM, West SG. Effects of pistachios on the lipid/lipoprotein profile, glycemic control, inflammation, and endothelial function in type 2 diabetes: a randomized trial. Metabolism. (2015) 64:1521–9. 10.1016/j.metabol.2015.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernández-Alonso P, Salas-Salvadó J, Baldrich-Mora M, Juanola-Falgarona M, Bulló M. Beneficial effect of pistachio consumption on glucose metabolism, insulin resistance, inflammation, and related metabolic risk markers: a randomized clinical trial. Diabetes Care. (2014) 37:3098–105. 10.2337/dc14-1431 [DOI] [PubMed] [Google Scholar]
- 34.Parham M, Heidari S, Khorramirad A, Hozoori M, Hosseinzadeh F, Bakhtyari L, et al. Effects of pistachio nut supplementation on blood glucose in patients with type 2 diabetes: a randomized crossover trial. Rev Diabetic Stu: RDS. (2014) 11:190. 10.1900/RDS.2014.11.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gulati S, Misra A, Pandey RM, Bhatt SP, Saluja S. Effects of pistachio nuts on body composition, metabolic, inflammatory and oxidative stress parameters in Asian Indians with metabolic syndrome: a 24-wk, randomized control trial. Nutrition. (2014) 30:192–7. 10.1016/j.nut.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 36.Sari I, Baltaci Y, Bagci C, Davutoglu V, Erel O, Celik H, et al. Effect of pistachio diet on lipid parameters, endothelial function, inflammation, and oxidative status: a prospective study. Nutrition. (2010) 26:399–404. 10.1016/j.nut.2009.05.023 [DOI] [PubMed] [Google Scholar]
- 37.Canudas S, Hernández-Alonso P, Galié S, Muralidharan J, Morell-Azanza L, Zalba G, et al. Pistachio consumption modulates DNA oxidation and genes related to telomere maintenance: a crossover randomized clinical trial. Am J Clin Nutr. (2019) 109:1738–45. 10.1093/ajcn/nqz048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu L, Piotrowski K, Rau T, Waldmann E, Broedl UC, Demmelmair H, et al. Walnut-enriched diet reduces fasting non-HDL-cholesterol and apolipoprotein B in healthy Caucasian subjects: a randomized controlled cross-over clinical trial. Metabolism. (2014) 63:382–91. 10.1016/j.metabol.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 39.Hwang HJ, Liu Y, Kim HS, Lee H, Lim Y, Park H. Daily walnut intake improves metabolic syndrome status and increases circulating adiponectin levels: randomized controlled crossover trial. Nutr Res Pract. (2019) 13:105. 10.4162/nrp.2019.13.2.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ros E, Núñez I, Pérez-Heras A, Serra M, Gilabert R, Casals E, et al. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation. (2004) 109:1609–14. 10.1161/01.CIR.0000124477.91474.FF [DOI] [PubMed] [Google Scholar]
- 41.Ashraf S, Arfeen A, Amjad S, Ahmed Z. Effect of walnut (Juglans Regia) consumption on hyperlipidemic adults. Food Sci Technol. (2020) 8:432–8. 10.1590/fst.2972022959886 [DOI] [Google Scholar]
- 42.Zambón D, Sabaté J, Munoz S, Campero B, Casals E, Merlos M, et al. Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women: a randomized crossover trial. Ann Intern Med. (2000) 132:538–46. 10.7326/0003-4819-132-7-200004040-00005 [DOI] [PubMed] [Google Scholar]
- 43.Bashan I, Bakman M. The effect of daily walnut consumption on dyslipidemia. J Food Qual. (2018) 2018:1–6. 10.1155/2018/473182626396054 [DOI] [Google Scholar]
- 44.Torabian S, Haddad E, Cordero-MacIntyre Z, Tanzman J, Fernandez M, Sabate J. Long-term walnut supplementation without dietary advice induces favorable serum lipid changes in free-living individuals. Eur J Clin Nutr. (2010) 64:274–9. 10.1038/ejcn.2009.152 [DOI] [PubMed] [Google Scholar]
- 45.Bamberger C, Rossmeier A, Lechner K, Wu L, Waldmann E, Stark RG, et al. A walnut-enriched diet reduces lipids in healthy Caucasian subjects, independent of recommended macronutrient replacement and time point of consumption: a prospective, randomized, controlled trial. Nutrients. (2017) 9:1097. 10.3390/nu9101097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rock CL, Flatt SW, Barkai H-S, Pakiz B, Heath DD. Walnut consumption in a weight reduction intervention: effects on body weight, biological measures, blood pressure and satiety. Nutr J. (2017) 16:1–10. 10.1186/s12937-017-0304-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ali Babaei A, Tavakoli Ghouchani H, Khoshghamat N, Sabermoghaddam M, Khosravi M. Effects of Walnut Consumption on Lipid Profile of Female Undergraduate Students. J Nutri, Fasting Health. (2019) 7:92–6. 10.22038/JNFH.2019.38643.1176 [DOI] [Google Scholar]
- 48.Liu X, Hill AM, West SG, Gabauer RM, McCrea CE, Fleming JA, et al. Acute peanut consumption alters postprandial lipids and vascular responses in healthy overweight or obese men. J Nutr. (2017) 147:835–40. 10.3945/jn.116.246785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Damavandi RD, Eghtesadi S, Shidfar F, Heydari I, Foroushani AR. Effects of hazelnuts consumption on fasting blood sugar and lipoproteins in patients with type 2 diabetes. Jf Res Med Sci: Off J Isfahan University Med Sci. (2013) 18:314. [PMC free article] [PubMed] [Google Scholar]
- 50.Di Renzo L, Cioccoloni G, Bernardini S, Abenavoli L, Aiello V, Marchetti M, et al. A hazelnut-enriched diet modulates oxidative stress and inflammation gene expression without weight gain. Oxid Med Cell Longev. (2019) 2019:1–11. 10.1155/2019/4683723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cominetti C, de Bortoli MC, Garrido Jr AB, Cozzolino SM. Brazilian nut consumption improves selenium status and glutathione peroxidase activity and reduces atherogenic risk in obese women. Nutrition Research. (2012) 32:403–7. 10.1016/j.nutres.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 52.Colpo E, Vilanova CDD, Reetz LGB, Duarte MM, Farias ILG, Meinerz DF, et al. Brazilian nut consumption by healthy volunteers improves inflammatory parameters. Nutrition. (2014) 30:459–65. 10.1016/j.nut.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 53.Maranhão PA, Kraemer-Aguiar LG, de Oliveira CL, Kuschnir MC, Vieira YR, Souza MG, et al. Brazil nuts intake improves lipid profile, oxidative stress and microvascular function in obese adolescents: a randomized controlled trial. Nutr Metab. (2011) 8:1–8. 10.1186/1743-7075-8-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Macan TP, de Amorim TA, Damiani AP, Beretta ÂCdL, Magenis ML, Vilela TC, et al. Brazil nut prevents oxidative DNA damage in type 2 diabetes patients. Drug Cheml Toxicol. (2020) 8:1–7. 10.1080/01480545.2020.1808667 [DOI] [PubMed] [Google Scholar]
- 55.Stockler-Pinto MB, Mafra D, Moraes C, Lobo J, Boaventura GT, Farage NE, et al. Brazil nut (Bertholletia excelsa, HBK) improves oxidative stress and inflammation biomarkers in hemodialysis patients. Biol Trace Elem Res. (2014) 158:105–12. 10.1007/s12011-014-9904-z [DOI] [PubMed] [Google Scholar]
- 56.Watanabe LM, de Lima LF, Ferraz-Bannitz R, Takaara D, Romano BC, Costa TMB, et al. Association between creatine kinase activity, oxidative stress and selenoproteins mRNA expression changes after Brazil nut consumption of patients using statins. Clin Nutri. (2020) 39:3175–81. 10.1016/j.clnu.2020.02.012 [DOI] [PubMed] [Google Scholar]
- 57.Mah E, Schulz JA, Kaden VN, Lawless AL, Rotor J, Mantilla LB, et al. Cashew consumption reduces total and LDL cholesterol: a randomized, crossover, controlled-feeding trial. Am J Clin Nutr. (2017) 105:1070–8. 10.3945/ajcn.116.150037 [DOI] [PubMed] [Google Scholar]
- 58.Damavandi RD, Mousavi SN, Shidfar F, Mohammadi V, Rajab A, Hosseini S, et al. Effects of daily consumption of cashews on oxidative stress and Atherogenic indices in patients with type 2 diabetes: a randomized, Controlled-Feeding Trial. Int J Endocrinol Metabol. (2019) 17:70744. 10.5812/ijem.70744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Damavandi RD, Shidfar F, Rajab A, Mohammadi V, Hosseini S. The effects of cashew consumption on serum glucose, insulin and lipoprotein in type 2 diabetic patients. Iranian J Endocrinol Metabol. (2012) 14:325–34. [Google Scholar]
- 60.Mohan V, Gayathri R, Jaacks LM, Lakshmipriya N, Anjana RM, Spiegelman D, et al. Cashew nut consumption increases HDL cholesterol and reduces systolic blood pressure in Asian Indians with type 2 diabetes: a 12-week randomized controlled trial. J Nutr. (2018) 148:63–9. 10.1093/jn/nxx001 [DOI] [PubMed] [Google Scholar]
- 61.McKay DL, Eliasziw M, Chen C, Blumberg JBA. pecan-rich diet improves cardiometabolic risk factors in overweight and obese adults: a randomized controlled trial. Nutrients. (2018) 10:339. 10.3390/nu10030339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campos V, Portal V, Markoski M, Quadros A, Bersch-Ferreira Â, Garavaglia J, et al. Effects of a healthy diet enriched or not with pecan nuts or extra-virgin olive oil on the lipid profile of patients with stable coronary artery disease: a randomised clinical trial. J Human Nutri Dietetics. (2020) 33:439–50. 10.1111/jhn.12727 [DOI] [PubMed] [Google Scholar]
- 63.Sedaghat A, Shahbazian H, Haidari F, Payami SP, Jahanshahi A, Latifi SM. The effect of soy nuts on glycemic control, lipid profile and insulin-resistance in type 2 diabetic patients. Open J Endo Metabol Dis. (2015) 5:1–6. 10.4236/ojemd.2015.51001 [DOI] [Google Scholar]
- 64.Sedaghat A, Shahbazian H, Rezazadeh A, Haidari F, Jahanshahi A, Latifi SM, et al. The effect of soy nut on serum total antioxidant, endothelial function and cardiovascular risk factors in patients with type 2 diabetes. Diabetes Metabol Syn: Clin Res Rev. (2019) 13:1387–91. 10.1016/j.dsx.2019.01.057 [DOI] [PubMed] [Google Scholar]
- 65.Bakhtiary A, Yassin Z, Hanachi P, Rahmat A, Ahmad Z, Halalkhor S, et al. Evaluation of the oxidative stress and glycemic control status in response to soy in older women with the metabolic syndrome. Iran Red Crescent Med J. (2011) 13:795–804. [Google Scholar]
- 66.Bakhtiari A, Hajian-Tilaki K, Omidvar S, Nasiri-Amiri F. Clinical and metabolic response to soy administration in older women with metabolic syndrome: a randomized controlled trial. Diabetol Metab Syndr. (2019) 11:1–12. 10.1186/s13098-019-0441-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karamali M, Kashanian M, Alaeinasab S, Asemi Z. The effect of dietary soy intake on weight loss, glycaemic control, lipid profiles and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: a randomised clinical trial. J Human Nutri Dietetics. (2018) 31:533–43. 10.1111/jhn.12545 [DOI] [PubMed] [Google Scholar]
- 68.Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Padyab M, Hu FB, et al. Soy inclusion in the diet improves features of the metabolic syndrome: a randomized crossover study in postmenopausal women. Am J Clin Nutr. (2007) 85:735–41. 10.1093/ajcn/85.3.735 [DOI] [PubMed] [Google Scholar]
- 69.Hematdar Z, Ghasemifard N, Phishdad G, Faghih S. Substitution of red meat with soybean but not non-soy legumes improves inflammation in patients with type 2 diabetes; a randomized clinical trial. J Diabetes Metabol Dis. (2018) 17:111–6. 10.1007/s40200-018-0346-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bento A, Cominetti C, Simões Filho A, Naves M. Baru almond improves lipid profile in mildly hypercholesterolemic subjects: a randomized, controlled, crossover study. Nutri, Metabol Cardiovasc Dis. (2014) 24:1330–6. 10.1016/j.numecd.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 71.de Souza RGM, Gomes AC, de Castro IA, Mota JFA. baru almond–enriched diet reduces abdominal adiposity and improves high-density lipoprotein concentrations: a randomized, placebo-controlled trial. Nutrition. (2018) 55:154–60. 10.1016/j.nut.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 72.de Souza RGM, Gomes AC, Navarro AM. Cunha LCd, Silva MAC, Junior FB, et al. Baru almonds increase the activity of glutathione peroxidase in overweight and obese women: a randomized, placebo-controlled trial. Nutrients. (2019) 11:1750. 10.3390/nu11081750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kendall CW, Josse AR, Esfahani A, Jenkins DJ. Nuts, metabolic syndrome and diabetes. Br J Nutr. (2010) 104:465–73. 10.1017/S0007114510001546 [DOI] [PubMed] [Google Scholar]
- 74.Kendall CW, Esfahani A, Truan J, Srichaikul K, Jenkins DJ. Health benefits of nuts in prevention and management of diabetes. Asia Pac J Clin Nutr. (2010) 19:110–6. [PubMed] [Google Scholar]
- 75.Payahoo L, Khajebishak Y, Alivand MR, Soleimanzade H, Alipour S, Barzegari A, et al. Investigation the effect of oleoylethanolamide supplementation on the abundance of Akkermansia muciniphila bacterium and the dietary intakes in people with obesity: a randomized clinical trial. Appetite. (2019) 141:104301. 10.1016/j.appet.2019.05.032 [DOI] [PubMed] [Google Scholar]
- 76.Hassanalilou T, Payahoo L, Shahabi P, Abbasi MM. Jafar-abadi MA, Bishak YK, et al. The protective effects of Morus nigra L leaves on the kidney function tests and histological structures in streptozotocin-induced diabetic rats. Biomedical Res. (2017) 28:6113–18. [Google Scholar]
- 77.Josse AR, Kendall CW, Augustin LS, Ellis PR, Jenkins DJ. Almonds and postprandial glycemia—a dose-response study. Metabolism. (2007) 56:400–4. 10.1016/j.metabol.2006.10.024 [DOI] [PubMed] [Google Scholar]
- 78.Jenkins DJ, Kendall CW, Josse AR, Salvatore S, Brighenti F, Augustin LS, et al. Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. J Nutr. (2006) 136:2987–92. 10.1093/jn/136.12.2987 [DOI] [PubMed] [Google Scholar]
- 79.Kendall C, Josse A, Esfahani A, Jenkins D. The impact of pistachio intake alone or in combination with high-carbohydrate foods on post-prandial glycemia. Eur J Clin Nutr. (2011) 65:696–702. 10.1038/ejcn.2011.12 [DOI] [PubMed] [Google Scholar]
- 80.Kendall C, Esfahani A, Josse A, Augustin L, Vidgen E, Jenkins D. The glycemic effect of nut-enriched meals in healthy and diabetic subjects. Nutr, Metabol Cardiovas Dis. (2011) 21:S34–9. 10.1016/j.numecd.2011.03.013 [DOI] [PubMed] [Google Scholar]
- 81.Mazidi M, Vatanparast H, Katsiki N, Banach M. The impact of nuts consumption on glucose/insulin homeostasis and inflammation markers mediated by adiposity factors among American adults. Oncotarget. (2018) 9:31173. 10.18632/oncotarget.25168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Casas-Agustench P, López-Uriarte P, Bulló M, Ros E, Cabré-Vila J, Salas-Salvadó J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr, Metabol Cardiovas Dis. (2011) 21:126–35. 10.1016/j.numecd.2009.08.005 [DOI] [PubMed] [Google Scholar]
- 83.Tapsell LC, Batterham M, Teuss G, Tan SY, Dalton S, Quick CJ, et al. Long-term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur J Clin Nutr. (2009) 63:1008–15. 10.1038/ejcn.2009.19 [DOI] [PubMed] [Google Scholar]
- 84.Jiang R, Manson JE, Stampfer MJ, Liu S, Willett WC, Hu FB. Nut and peanut butter consumption and risk of type 2 diabetes in women. Jama. (2002) 288:2554–60. 10.1001/jama.288.20.2554 [DOI] [PubMed] [Google Scholar]
- 85.Thompson LU, Button CL, Jenkins D. Phytic acid and calcium affect the in vitro rate of navy bean starch digestion and blood glucose response in humans. Am J Clin Nutr. (1987) 46:467–73. 10.1093/ajcn/46.3.467 [DOI] [PubMed] [Google Scholar]
- 86.Kim Y, Keogh JB, Clifton PM. Polyphenols and glycemic control. Nutrients. (2016) 8:17. 10.3390/nu8010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jafarabadi MA, Dehghani A, Khalili L, Barzegar A, Mesrizad M, Hassanalilou T, et al. Meta-analysis of randomized controlled trials of the effect of probiotic food or supplement on glycemic response and body mass index in patients with type 2 diabetes, updating the evidence. Curr Diabetes Rev. (2021) 17:356–64. 10.2174/1573399816666200812151029 [DOI] [PubMed] [Google Scholar]
- 88.Khalili L, Alipour B, Jafarabadi MA, Hassanalilou T, Abbasi MM, Faraji I. Probiotic assisted weight management as a main factor for glycemic control in patients with type 2 diabetes: a randomized controlled trial. Diabetol Metab Syndr. (2019) 11:1–9. 10.1186/s13098-019-0400-7 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Zepeda-Hernández A, Garcia-Amezquita LE, Requena T, García-Cayuela T. Probiotics, prebiotics, and synbiotics added to dairy products: uses and applications to manage type 2 diabetes. Food Res Int. (2021) 142:110208. 10.1016/j.foodres.2021.110208 [DOI] [PubMed] [Google Scholar]
- 90.Kim Y, Keogh JB, Clifton PM. Benefits of nut consumption on insulin resistance and cardiovascular risk factors: multiple potential mechanisms of actions. Nutrients. (2017) 9:1271. 10.3390/nu9111271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lovejoy JC, Most MM, Lefevre M, Greenway FL, Rood JC. Effect of diets enriched in almonds on insulin action and serum lipids in adults with normal glucose tolerance or type 2 diabetes. Am J Clin Nutr. (2002) 76:1000–6. 10.1093/ajcn/76.5.1000 [DOI] [PubMed] [Google Scholar]
- 92.Mukuddem-Petersen J, Oosthuizen W, Jerling JCA. systematic review of the effects of nuts on blood lipid profiles in humans. J Nutr. (2005) 135:2082–9. 10.1093/jn/135.9.2082 [DOI] [PubMed] [Google Scholar]
- 93.Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Ruiz-Gutiérrez V, Covas MI, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. (2006) 145:1–11. 10.7326/0003-4819-145-1-200607040-00004 [DOI] [PubMed] [Google Scholar]
- 94.Sabaté J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med. (2010) 170:821–7. 10.1001/archinternmed.2010.79 [DOI] [PubMed] [Google Scholar]
- 95.Tapsell LC, Gillen LJ, Patch CS, Batterham M, Owen A, Baré M, et al. Including walnuts in a low-fat/modified-fat diet improves HDL cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabetes Care. (2004) 27:2777–83. 10.2337/diacare.27.12.2777 [DOI] [PubMed] [Google Scholar]
- 96.Samaha F. Effects of a Mediterranean-style diet on cardiovascular risk factors. Ann Intern Med. (2007) 146:73. 10.7326/0003-4819-146-1-200701020-00018 [DOI] [PubMed] [Google Scholar]
- 97.Lee YJ, Nam GE, Seo JA, Yoon T, Seo I, Lee JH, et al. Nut consumption has favorable effects on lipid profiles of Korean women with metabolic syndrome. Nut Res. (2014) 34:814–20. 10.1016/j.nutres.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 98.Askari G, Yazdekhasti N, Mohammadifard N, Sarrafzadegan N, Bahonar A, Badiei M, et al. The relationship between nut consumption and lipid profile among the Iranian adult population; Isfahan healthy heart program. Eur J Clin Nutr. (2013) 67:385–9. 10.1038/ejcn.2013.21 [DOI] [PubMed] [Google Scholar]
- 99.Altamimi M, Zidan S, Badrasawi M. Effect of tree nuts consumption on serum lipid profile in hyperlipidemic individuals: a systematic review. Nutr Metab Insights. (2020) 13:1178638820926521. 10.1177/1178638820926521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li TY, Brennan AM, Wedick NM, Mantzoros C, Rifai N, Hu FB. Regular consumption of nuts is associated with a lower risk of cardiovascular disease in women with type 2 diabetes. J Nutr. (2009) 139:1333–8. 10.3945/jn.108.103622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sniderman AD, Thanassoulis G, Glavinovic T, Navar AM, Pencina M, Catapano A, et al. Apolipoprotein B particles and cardiovascular disease: a narrative review. JAMA Cardiol. (2019) 4:1287–95. 10.1001/jamacardio.2019.3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mandalari G, Faulks RM, Rich GT, Lo Turco V, Picout DR, Lo Curto RB, et al. Release of protein, lipid, and vitamin E from almond seeds during digestion. J Agric Food Chem. (2008) 56:3409–16. 10.1021/jf073393v [DOI] [PubMed] [Google Scholar]
- 103.Cassady BA, Hollis JH, Fulford AD, Considine RV, Mattes RD. Mastication of almonds: effects of lipid bioaccessibility, appetite, and hormone response. Am J Clin Nutr. (2009) 89:794–800. 10.3945/ajcn.2008.26669 [DOI] [PubMed] [Google Scholar]
- 104.Tey S, Brown R, Chisholm A, Delahunty C, Gray A, Williams S. Effects of different forms of hazelnuts on blood lipids and α-tocopherol concentrations in mildly hypercholesterolemic individuals. Eur J Clin Nutr. (2011) 65:117–24. 10.1038/ejcn.2010.200 [DOI] [PubMed] [Google Scholar]
- 105.Garrido I, Monagas M, Gómez-Cordovés C, Bartolomé B. Polyphenols and antioxidant properties of almond skins: influence of industrial processing. J Food Sci. (2008) 73:C106–15. 10.1111/j.1750-3841.2007.00637.x [DOI] [PubMed] [Google Scholar]
- 106.Escurriol V, Cofán M, Serra M, Bulló M, Basora J, Salas-Salvadó J, et al. Serum sterol responses to increasing plant sterol intake from natural foods in the Mediterranean diet. Eur J Nutr. (2009) 48:373–82. 10.1007/s00394-009-0024-z [DOI] [PubMed] [Google Scholar]
- 107.Segura R, Javierre C, Lizarraga MA, Ros E. Other relevant components of nuts: phytosterols, folate and minerals. Br J Nut. (2006) 96:S36–44. 10.1017/BJN20061862 [DOI] [PubMed] [Google Scholar]
- 108.Pahlavani N, Jafari M, Sadeghi O, Rezaei M, Rasad H, Rahdar HA, et al. L-arginine supplementation and risk factors of cardiovascular diseases in healthy men: a double-blind randomized clinical trial. F1000Research. (2014) 3:306. 10.12688/f1000research.5877.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hadi A, Arab A, Moradi S, Pantovic A, Clark CC, Ghaedi E. The effect of l-arginine supplementation on lipid profile: a systematic review and meta-analysis of randomised controlled trials. Br J Nut. (2019) 122:1021–32. 10.1017/S0007114519001855 [DOI] [PubMed] [Google Scholar]
- 110.Casas-Agustench P, Bulló M, Salas-Salvadó J. Nuts, inflammation and insulin resistance. Asia Pac J Clin Nutr. (2010) 19:124. [PubMed] [Google Scholar]
- 111.Lopez-Garcia E, Schulze MB, Manson JE, Meigs JB, Albert CM, Rifai N, et al. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr. (2004) 134:1806–11. 10.1093/jn/134.7.1806 [DOI] [PubMed] [Google Scholar]
- 112.Pischon T, Hankinson SE. Hotamisligil GkS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. (2003) 108:155–60. 10.1161/01.CIR.0000079224.46084.C2 [DOI] [PubMed] [Google Scholar]
- 113.Salas-Salvadó J, Garcia-Arellano A, Estruch R, Marquez-Sandoval F, Corella D, Fiol M, et al. Components of the mediterranean-type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. Eur J Clin Nutr. (2008) 62:651–9. 10.1038/sj.ejcn.1602762 [DOI] [PubMed] [Google Scholar]
- 114.Jiang R, Jacobs Jr DR, Mayer-Davis E, Szklo M, Herrington D, Jenny NS, et al. Nut and seed consumption and inflammatory markers in the multi-ethnic study of atherosclerosis. Am J Epidemiol. (2006) 163:222–31. 10.1093/aje/kwj033 [DOI] [PubMed] [Google Scholar]
- 115.Yu Z, Malik VS, Keum N, Hu FB, Giovannucci EL, Stampfer MJ, et al. Associations between nut consumption and inflammatory biomarkers. Am J Clin Nutr. (2016) 104:722–8. 10.3945/ajcn.116.134205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ros E. Health benefits of nut consumption. Nutrients. (2010) 2:652–82. 10.3390/nu2070652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ros E. Nuts and novel biomarkers of cardiovascular disease. Am J Clin Nut. (2009) 89:1649S−56S. 10.3945/ajcn.2009.26736R [DOI] [PubMed] [Google Scholar]
- 118.Wolever T, Chiasson J, Josse R, Leiter L, Maheux P, Rabasa-Lhoret R, et al. Effect of modifying source or amount of carbohydrate glucose and lipid control in type 2 diabetes. Can J Diab. (2004) 48. [Google Scholar]
- 119.Ros E, Mataix J. Fatty acid composition of nuts–implications for cardiovascular health. Br J Nut. (2006) 96:S29–35. 10.1017/BJN20061861 [DOI] [PubMed] [Google Scholar]
- 120.Rocha DM, Bressan J, Hermsdorff HH. The role of dietary fatty acid intake in inflammatory gene expression: a critical review. São Paulo Med J. (2017) 135:157–68. 10.1590/1516-3180.2016.008607072016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Rad Biol Med. (2014) 72:76–90. 10.1016/j.freeradbiomed.2014.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Eng J Med. (2013) 368:1279–90. 10.1056/NEJMoa1200303 [DOI] [PubMed] [Google Scholar]
- 123.Davis L, Stonehouse W, Loots DT, Mukuddem-Petersen J, van der Westhuizen FH, Hanekom SM, et al. The effects of high walnut and cashew nut diets on the antioxidant status of subjects with metabolic syndrome. European J Nutr. (2007) 46:155–64. 10.1007/s00394-007-0647-x [DOI] [PubMed] [Google Scholar]
- 124.Soory M. Nutritional antioxidants and their applications in cardiometabolic diseases. Infect Disord Drug Targets. (2012) 12:388–401. 10.2174/187152612804142233 [DOI] [PubMed] [Google Scholar]
- 125.Chaalal M, Ouchemoukh S, Mehenni C, Salhi N, Soufi O, Ydjedd S, et al. Phenolic contents and in vitro antioxidant activity of four commonly consumed nuts in algeria. Acta Alimentaria. (2019) 48:125–31. 10.1556/066.2018.0009 [DOI] [Google Scholar]
- 126.Yang L, Xian D, Xiong X, Lai R, Song J, Zhong J. Proanthocyanidins against oxidative stress: from molecular mechanisms to clinical applications. BioMed Res Int. (2018) 2018:8584136. 10.1155/2018/8584136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sheng J, Yang X, Chen J, Peng T, Yin X, Liu W, et al. Antioxidative effects and mechanism study of bioactive peptides from defatted walnut (Juglans regia L.) meal hydrolysate J Agricul Food Chem. (2019) 67:3305–12. 10.1021/acs.jafc.8b05722 [DOI] [PubMed] [Google Scholar]
- 128.Anselmo NA, Paskakulis LC, Garcias RC, Botelho FFR, Toledo GQ, Cury MFR, et al. Prior intake of Brazil nuts attenuates renal injury induced by ischemia and reperfusion. Braz J Nephrol. (2018) 40:10–7. 10.1590/1678-46a85-jbn-3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hong MY, Groven S, Marx A, Rasmussen C, Beidler J. Anti-inflammatory, antioxidant, and hypolipidemic effects of mixed nuts in atherogenic diet-fed rats. Molecules. (2018) 23:3126. 10.3390/molecules23123126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bitok E, Sabaté J. Nuts and cardiovascular disease. Prog Cardiovasc Dis. (2018) 61:33–7. 10.1016/j.pcad.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 131.Sureda A, Bibiloni MDM, Martorell M, Buil-Cosiales P, Marti A, Pons A, et al. Mediterranean diets supplemented with virgin olive oil and nuts enhance plasmatic antioxidant capabilities and decrease xanthine oxidase activity in people with metabolic syndrome: the PREDIMED study. Mol Nut Food Res. (2016) 60:2654–64. 10.1002/mnfr.201600450 [DOI] [PubMed] [Google Scholar]
- 132.Jenkins DJ, Kendall CW, Marchie A, Parker TL, Connelly PW, Qian W, et al. Dose response of almonds on coronary heart disease risk factors: blood lipids, oxidized low-density lipoproteins, lipoprotein (a), homocysteine, and pulmonary nitric oxide: a randomized, controlled, crossover trial. Circulation. (2002) 106:1327–32. 10.1161/01.CIR.0000028421.91733.20 [DOI] [PubMed] [Google Scholar]
- 133.Kay CD, Gebauer SK, West SG, Kris-Etherton PM. Pistachios increase serum antioxidants and lower serum oxidized-LDL in hypercholesterolemic adults. J Nutr. (2010) 140:1093–8. 10.3945/jn.109.117366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Silveira BKS, da Silva A, Hermsdorff HHM, Bressan J. Effect of chronic consumption of nuts on oxidative stress: a systematic review of clinical trials. Crit Rev Food Sci Nut. (2020) 62:1–12. 10.1080/10408398.2020.1828262 [DOI] [PubMed] [Google Scholar]
- 135.Ross D, Siegel D. Functions of NQO1 in cellular protection and CoQ10 metabolism and its potential role as a redox sensitive molecular switch. Front Physiol. (2017) 8:595. 10.3389/fphys.2017.00595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Salas-Salvado J, Guasch-Ferre M, Bullo M, Sabate J. Nuts in the prevention and treatment of metabolic syndrome. Am J Clin Nut. (2014) 100(suppl_1):399S–407S. 10.3945/ajcn.113.071530 [DOI] [PubMed] [Google Scholar]