Abstract
BMS-232632 is an azapeptide human immunodeficiency virus type 1 (HIV-1) protease (Prt) inhibitor that exhibits potent anti-HIV activity with a 50% effective concentration (EC50) of 2.6 to 5.3 nM and an EC90 of 9 to 15 nM in cell culture. Proof-of-principle studies indicate that BMS-232632 blocks the cleavage of viral precursor proteins in HIV-infected cells, proving that it functions as an HIV Prt inhibitor. Comparative studies showed that BMS-232632 is generally more potent than the five currently approved HIV-1 Prt inhibitors. Furthermore, BMS-232632 is highly selective for HIV-1 Prt and exhibits cytotoxicity only at concentrations 6,500- to 23,000-fold higher than that required for anti-HIV activity. To assess the potential of this inhibitor when used in combination with other antiretrovirals, BMS-232632 was evaluated for anti-HIV activity in two-drug combination studies. Combinations of BMS-232632 with either stavudine, didanosine, lamivudine, zidovudine, nelfinavir, indinavir, ritonavir, saquinavir, or amprenavir in HIV-infected peripheral blood mononuclear cells yielded additive to moderately synergistic antiviral effects. Importantly, combinations of drug pairs did not result in antagonistic anti-HIV activity or enhanced cytotoxic effects at the highest concentrations used for antiviral evaluation. Our results suggest that BMS-232632 may be an effective HIV-1 inhibitor that may be utilized in a variety of different drug combinations.
Human immunodeficiency virus type 1 (HIV-1) protease (Prt) specifically processes gag (p55) and gag-pol (p160) viral polyproteins to yield the viral structural proteins (p17, p24, p7, and p6), as well as the viral enzymes reverse transcriptase (RT), integrase, and Prt (23, 24, 34). Both RT and Prt are essential for virus replication, thus providing effective targets for antiviral intervention. The currently approved HIV drugs include six nucleoside RT inhibitors (zidovudine [AZT], didanosine [ddI], stavudine [d4T], lamivudine [3TC], zalcitabine [ddC], and abacavir), three nonnucleoside RT inhibitors (nevirapine, delavirdine, and efavirenz), and five Prt inhibitors (saquinavir [SQV], indinavir [IDV], ritonavir [RTV], nelfinavir [NFV], and amprenavir [APV]) (8, 9, 11, 12, 13, 19, 20, 30, 31, 38–41, 43). However, use of any of these drugs as monotherapy offers only a short-term benefit due to insufficient potency and/or resistance development (3, 25, 31). Combination drug strategies consisting of RT and Prt inhibitors have proven to be highly effective in suppressing viral replication to unquantifiable levels for a sustained period of time (5, 6, 10, 13, 14, 21, 31–33; F. DeWolf, V. V. Lukashov, S. A. Danner, J. Goudsmit, and J. M. A. Lange, Abstr. 5th Conf. Retroviruses Opportunistic Infect., abstr. 384, 1998; K. Gallicano, J. Sahai, S. Kravcik, J. Seguin, N. Bristow, and D. W. Cameron, Abstr. 5th Conf. Retroviruses Opportunistic Infect., abstr. 353, 1998; M. Markowitz, Y. Cao, A. Hurley, R. Schluger, S. Monard, R. Kost, B. Kerr, R. Anderson, S. Eastman, and D. D. Ho, Abstr. 5th Conf. Retroviruses Opportunistic Infect., abstr. 371, 1998). Unfortunately, it is now estimated that 30 to 50% of patients are failing combination therapy. Such nonresponsiveness is presumably due to the development of drug-resistant HIV-1 strains and/or patient noncompliance with demanding dosing regimens. Therefore, the development of new HIV-1 inhibitors that exhibit distinct resistance profiles, with fewer side effects and superior bioavailability, is necessary to provide patients with more alternatives in combination therapy.
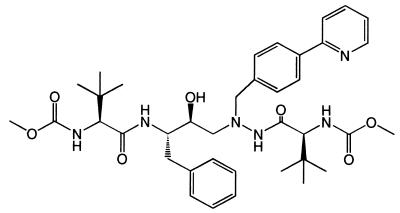
BMS-232632 is an azapeptide HIV-1 Prt inhibitor (Fig. 1) currently in development and undergoing clinical evaluation. The present study characterizes the biochemical and virological aspects of this novel inhibitor in regard to its in vitro and cell culture activity. The potency of BMS-232632 against a variety of HIV-1 strains was evaluated by using different host cells and was shown to be generally greater than those of the other marketed Prt inhibitors. Studies involving two-drug combinations of BMS-232632 with each of nine other anti-HIV drugs suggest that BMS-232632 may be utilized in a variety of potential combinations.
FIG. 1.
Structure for BMS-232632.
MATERIALS AND METHODS
Cells and virus.
H9 cells chronically infected with HIV-1 RF, MT-2 cells, HIV-1 RF, HIV-1 BRU, and the HIV-1 NL4-3 proviral clone were obtained through the AIDS Research and Reference Reagent Program, National Institutes of Health. MT-2 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and 10 mM HEPES buffer. Peripheral blood mononuclear cells (PBMCs) were isolated from healthy seronegative donors by Ficoll-Hypaque density gradient centrifugation. The cells were stimulated for 3 days with phytohemagglutinin-P (2 μg/ml) in the presence of interleukin 2 (10 U/ml) before infection. NL4-3 viruses were generated by transfecting the proviral DNA into 293 cells using the calcium phosphate precipitation method (Promega). The RF, BRU, and NL4-3 HIV-1 strains were amplified in MT-2 cells and titrated in the same cells using a virus yield assay (17). The HIV-1 clinical isolate 006 (26) was expanded and titrated in PBMCs.
Compounds.
BMS-232632, IDV, RTV, NFV, SQV, APV, 3TC, d4T, and ddI were prepared at Bristol-Myers Squibb. AZT was purchased from Sigma.
Prt assay.
HIV-1 RF Prt was expressed in Escherichia coli strain BL21 (DE3) plysS using the pET24 vector from Novagen, and the enzyme was purified using Q-Sepharose and fast performance liquid chromatography Mono S columns as described previously (16, 27). To determine the inhibition constants (Ki) for each Prt inhibitor, purified HIV-1 RF wild-type Prt (2.5 nM) was incubated at 37°C with 1 to 15 μM fluorogenic substrate (DABCYL-δ-Abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS; Bachem no. M-1865) in reaction buffer (1 M NaCl, 1 mM EDTA, 0.1 M sodium acetate [pH 5.5], 0.1% polyethylene glycol 8000) in the presence or absence of inhibitor. Cleavage of the substrate was quantified by measuring an increase in fluorescent emission at 490 nM after excitation at 340 nM (29) using a Cytofluor 4000 (PerSeptive Biosystems). Reactions were carried out using 1.36, 1.66, 2.1, 3.0, 5.0, or 15 μM substrate in the presence of five concentrations of inhibitor (1.25 to 25 nM). Substrate cleavage was monitored at 5-min intervals for 30 min. Cleavage rates were then determined for each sample at early time points in the reaction, and Ki values were determined from the slopes of the resulting Michaelis-Menten plots.
The aspartyl proteases human cathepsin D (Sigma Chemical Co.) and porcine pepsin (Boehringer Mannheim) were assayed using a fluorescein-conjugated casein substrate (Molecular Probes). Cathepsin D (1 U/ml) was assayed at 37°C in 20 mM sodium citrate (pH 5) with 25 μg of fluoresceinated casein/ml. The fluorescence output at 530 nm (excitation at 485 nm) was continuously monitored in a PerSeptive Biosystems Cytofluor 4000. The rate of increase in fluorescence was determined at BMS-232632 concentrations up to 100 μM (final dimethyl sulfoxide concentration, 10%). Pepsin (0.5 μg/ml) was assayed in 20 mM sodium formate (pH 3.5) with 25 μg of fluoresceinated casein/ml and monitored at 37°C as described for cathepsin D.
Immunoblot analysis of gag processing.
An actively growing culture of H9 (107) cells chronically infected with HIV RF was pelleted and washed four times with phosphate-buffered saline. After resuspension in RPM1 1640 medium supplemented with 10% fetal bovine serum, the cultures (105 cells/ml) were treated for 5 days with various concentrations (0, 10, 30, 100, 300, 1,000 nM) of BMS-232632 or 100 nM SQV. The protease cleavage products in mature virions released from the treated cells were quantified by Western blotting. Briefly, the culture supernatants were spun at 47,000 rpm for 2 h in an SW50.1 rotor, and the virus pellets were then resuspended in lysis buffer (50 mM Tris [pH 6.8], 0.5% Triton X-100, 5% glycerol, 0.8 M NaCl), diluted with sample buffer (350 mM Tris [pH 6.8], 10% sodium dodecyl sulfate [SDS], 30% glycerol, 600 mM dithiothreitol, 0.02% bromophenol blue), and boiled for 5 min. The resulting proteins were separated by SDS-polyacrylamide gel electrophoresis using 10% polyacrylamide gels, transferred to nitrocellulose membranes, and immunostained with a mouse monoclonal antibody specific for p24 and p55 (NEA-9306; NEN) (1). The levels of p24 were quantitiated by densitometer analysis (Personal densitometer; SI Molecular Dynamics), and the concentration of compound required to reduce the levels of p24 cleavage product by 50%, relative to the value for the untreated controls, was determined using the Prism computer program (GraphPad).
Drug susceptibility and cytotoxicity assays.
In general, host cells were infected with HIV-1 at a multiplicity of infection (MOI) of 0.005 50% tissue culture infective doses (TCID50)/cell followed by incubation in the presence of serially diluted inhibitors for 4 to 7 days. Virus yields were quantitated using an RT assay (35) or a p24 enzyme-linked immunosorbent assay (ELISA) (NEN). The results from at least three experiments were used to calculate the 50% effective concentrations (EC50s). The EC50s of IDV, SQV, RTV, and NFV were compared to that of BMS-232632 using Dunnett's test. These comparisons were made separately within each assay system. Dunnett's test is used to reduce the probability of false-positive results when a number of treatments are being compared to a control (22) or, in this case, when a number of treatments are being compared to the same alternative treatment. Confidence bounds for the fold increases in EC50s observed when the same drug was tested in two different assay systems were computed using Fieller's theorem. The use of this theorem was necessary because ratios of parameters (in this case, EC50s) are known not to follow a standard probability distribution, such as the normal distribution. Numbers within the confidence interval are not significantly different from the observed fold increase at the 95% level (15).
To determine cytotoxicity, host cells were incubated in the presence of serially diluted inhibitors for 6 days and cell viability was quantitated using an XTT [2,3-bis(2-methoxy-4-nitro-5-sulfophenyl-2H-tetrazolium-5-carboxanilide] assay to calculate the 50% cytotoxic concentrations (CC50s) (42). To assess the effect of human serum proteins on antiviral activity, the 10% fetal calf serum normally used for assays was replaced with 40% adult human serum (Sigma) or 1 mg of α1-acid glycoprotein (Sigma)/ml.
HIV assays for drug combination studies.
PBMCs were infected with a clinical isolate of HIV (006) at an MOI of 0.001 TCID50/cell and subsequently seeded into 96-well microtiter plates in the presence or absence of drug. The drugs were diluted in twofold steps in a 6 by 8 matrix with BMS-232632 diluted in six concentration data points. On day 4 postinfection, one-half of the medium in each well was replenished with fresh medium containing the drug. Supernatants were harvested on day 7 for quantitation of p24 by ELISA. Cytotoxicity was assessed in PBMCs by an XTT assay (42).
Analysis of drug combination effects.
To assess the antiviral effects of different combination drug treatments, combination indices (CIs) were calculated as described by Chou and Talalay (7) and volumes of synergy or antagonism were assessed as described by Prichard et al. (36, 37). For calculation of CIs, drugs were diluted in a fixed ratio and more than one ratio was analyzed. The drug serial dilutions span a range of concentrations near each compound's EC50 so that equivalent antiviral activities were compared. Dose-response curves were determined for each individual drug and each combination using the median effect equation. The equation was fit using the nonlinear regression routine (Proc Nlin) in PC SAS, version 6.08.
The extent of synergy or antagonism was also determined using the MacSynergy program (36). For this analysis, drugs were diluted twofold in a 6 by 8 matrix with BMS-232632 prepared as the sixth dilution. The theoretical additive interactions from the monotherapies were determined using the independent-effect equation and plotted as a plane in a three-dimensional graph. The data from the experimental drug combination assay were then compared with the predicted additive interaction. Points above the additive plane represent synergistic interactions, while points below the plane represent antagonism. The extent of the synergy or antagonism was indicated by the volume of the area above (positive volume) or below (negative volume) the additive plane. According to Prichard and Shipman, a positive volume (micromolar squared times percent) indicates synergy while a negative volume indicates antagonism. Values between +25 and −25 μM2% are considered additive. Values between 25 and 50 μM2% indicate minor but significant synergy, whereas values between +50 and +100 or −50 and −100 μM2% are interpreted as moderately synergistic or antagonistic, respectively. In general, volumes greater than +100 μM2% or less than −100 μM2% would indicate strong drug interactions. Data shown were obtained at the 99.9% confidence level and were plotted using DeltaGraph.
RESULTS
Activity of BMS-232632 against HIV-1 gag processing.
The Ki of BMS-232632 was determined to be 2.66 nM in a fluorogenic Prt assay (Table 1), a value which was comparable to those observed for APV (2.61 nM), IDV (2.91 nM), NFV (3.27 nM), RTV (4.18 nM), and SQV (1.40 nM). The potent inhibition exhibited by BMS-232632 was also selective, since 100 μM BMS-232632 only inhibited porcine pepsin 5% and human cathepsin D 14%.
TABLE 1.
Inhibition of HIV-1 Prt by BMS-232632 versus the marketed Prt inhibitors
| Inhibitor | Kia ± SEM (nM) | nb |
|---|---|---|
| BMS-232632 | 2.66 ± 0.28 | 8 |
| APV | 2.61 ± 0.59 | 4 |
| IDV | 2.91 ± 0.43 | 4 |
| NFV | 3.27 ± 0.36 | 4 |
| RTV | 4.18 ± 0.51 | 5 |
| SQV | 1.40 ± 0.08 | 3 |
Ki values were determined from Michaelis-Menten plots.
n, numbers of experiments that the data are based on.
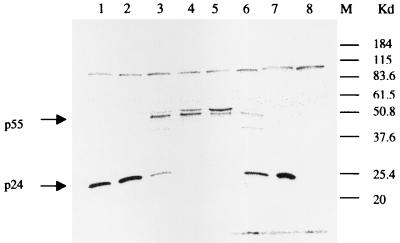
To determine whether BMS-232632 blocks HIV-1 Prt function in virus-infected cells, H9 cells chronically infected with RF were treated with the compound. Inhibition of HIV-1 proteolytic cleavage in the secreted virions should result in the accumulation of the p55 gag precursor and a corresponding reduction in the relative levels of the p24 cleavage product. As illustrated in Fig. 2, BMS-232632 inhibited the proteolytic cleavage of the viral gag precursor p55 polyprotein in a dose-dependent manner, with a 50% inhibitory concentration of approximately 47 nM. These results demonstrate that BMS-232632 inhibits virus-specific proteolytic processing in HIV-infected cells and therefore may serve as an effective inhibitor of viral replication.
FIG. 2.
Inhibition of HIV-1 gag processing by BMS-232632. H9 cells chronically infected with HIV-1 RF were treated with BMS-232632 at 10 (lane 1), 30 (lane 2), 100 (lane 3), 300 (lane 4), or 1,000 nM (lane 5), with 100 nM saquinavir (lane 6), or with no drug (lane 7) for 5 days. Cell supernatants were pelleted, solubilized in lysis buffer, and analyzed for p24 and p55 by Western blotting. Supernatant from uninfected H9 cells was also included as a control (lane 8).
Anti-HIV-1 activity.
The antiviral activity of BMS-232632 was evaluated using a variety of HIV-1 strains (the clinical isolate 006, laboratory strains RF and BRU, and the macrophage-tropic virus Bal) and several host cell types (PBMCs, CEM-SS, and MT-2). BMS-232632 exhibited potent antiviral activity, with EC50s between 2.62 and 5.28 nM (Table 2) and EC90s ranging from 9 to 15 nM (data not shown). Comparative studies using the same viral assay systems revealed that BMS-232632 is generally more potent than other HIV-1 Prt inhibitors, including IDV, SQV, RTV, NFV, and APV. Cytotoxicity determinations showed that BMS-232632 had CC50s of 28, 46, 47, and 50 μM in MT-2 and CEM-SS cells, monocytes/macrophages, and PBMCs, respectively, yielding corresponding selective indices of 6,500 to 23,800 (data not shown).
TABLE 2.
Comparative anti-HIV activity of BMS-232632
| HIV-1 isolate/cell type | Mean EC50c (nM) ± SEM (no. of expts) for:
|
|||||
|---|---|---|---|---|---|---|
| BMS-232632 | IDV | NFV | RTV | SQV | APV | |
| RF/MT-2a | 3.89 ± 0.35 (18) | 16.2* ± 2.22 (17) | 25.9* ± 2.33 (17) | 69.8* ± 10.8 (12) | 13.4* ± 1.19 (19) | 53.4* ± 5.35 (11) |
| RF/CEM-SSa | 4.85 ± 1.17 (6) | 13.3 ± 2.54 (6) | 17.9 ± 5.02 (6) | 35.5 ± 9.75 (4) | 5.28 ± 1.21 (4) | 16.6 ± 3.92 (4) |
| BRU/MT-2a | 5.28 ± 0.77 (6) | 28.7* ± 4.28 (6) | 23.4* ± 5.46 (4) | 129* ± 25.5 (4) | 12.1 ± 2.18 (4) | 39.8* ± 5.96 (4) |
| Bal/PBMCb | 4.38 ± 1.49 (4) | 26.1 ± 7.21 (4) | 25.1* ± 5.58 (4) | 191 ± 83.85 (4) | 17.2 ± 3.41 (4) | 48.3 ± 13.5 (4) |
| 006/PBMCb | 2.62 ± 0.45 (14) | 5.00* ± 0.65 (3) | 6.14* ± 0.21 (3) | 43.1* ± 13.4 (3) | 24.8* ± 4.23 (3) | 54.3* ± 1.14 (2) |
Antiviral end point, RT.
Antiviral end point, p24.
∗, significantly greater than EC50 for BMS-232632 at the 0.05 level by Dunnett's test. All results are based on more than three replicates.
Effect of human serum proteins.
Factors that affect compound potency in vivo include cell permeability and affinity for cellular proteins. It has been shown that most HIV Prt inhibitors have significant protein binding activity (2, 18, 28). For example, serum α1-acid glycoprotein greatly reduced the antiviral activity of SC-52151 (4), so development of this compound was terminated. We therefore evaluated the effect of 40% human serum and 1 mg of α1-acid glycoprotein/ml on the activity of BMS-232632 in the HIV-1 RF infection of MT-4 cells. EC50s were determined 4 days postinfection using an RT assay. As shown in Table 3, the addition of human serum reduced the anti-HIV activity of BMS-232632 by 2.7-fold, similar to what was seen for IDV (3.8-fold), RTV (1.8-fold), SQV (4.3-fold), and APV (2.5-fold). However, a greater reduction in antiviral activity was observed for NFV (10.8-fold). The effects of α1-acid glycoprotein on the anti-HIV activity of protease inhibitors are similar to those of 40% human serum, with decreases in EC50s of 3.6-, 2.3-, 3.6-, 2.2-, 2.2-, and 10.6-fold for BMS-232632, IDV, SQV, RTV, APV, and NFV, respectively.
TABLE 3.
Effect of human serum proteins on anti-HIV activity of Prt inhibitorsa
| Inhibitor | EC50 (nM) ± SEM in:
|
Fold increase in EC50 in HS (bounds) | EC50 (nM) ± SEM in AGP at 1 mg/ml | Fold increase in EC50 in AGP (bounds) | |
|---|---|---|---|---|---|
| 10% FCS | 40% HS | ||||
| BMS-232632 | 1.6 ± 0.30 | 4.4 ± 1.03 | 2.74* (1.08, 5.89) | 5.8 ± 0.65 | 3.64* (2.22, 7.06) |
| IDV | 10.1 ± 3.52 | 38.4 ± 10.17 | 3.79* (1.15, 26.54) | 23.3 ± 10.05 | 2.31 (−0.13, 6.96) |
| SQV | 8.7 ± 2.48 | 37.7 ± 5.35 | 4.31* (2.11, 16.34) | 31.3 ± 10.22 | 3.59 (0.69, 13.17) |
| RTV | 55.8* ± 13.06 | 100.8* ± 10.74 | 1.81* (1.02, 4.64) | 120.6* ± 23.21 | 2.16 (0.95, 5.82) |
| APV | 30.1* ± 5.58 | 75.2* ± 11.46 | 2.50* (1.38, 4.93) | 67.3* ± 4.99 | 2.24* (1.46, 4.18) |
| NFV | 11.1 ± 2.57 | 120.4* ± 18.99 | 10.82* (5.58, 26.19) | 117.6* ± 24.76 | 10.57* (4.55, 6.49) |
All results are based on four replicates, except those for SQV and RTV in human serum (HS) and RTV in α1-acid glycoprotein (AGP), which are based on three replicates. The fold EC50 increases in HS or AGP compared to the EC50s for the fetal calf serum (FCS) control were computed at the 95% confidence bounds by using Fieller's theorem. For EC50s, asterisks indicate values significantly greater than that for BMS-236632 at the 0.05 level by Dunnett's test; for fold increases, asterisks indicate values significantly greater than 1.0 at the 0.05 level (15).
Two-drug combinations of BMS-232632 and RT inhibitors.
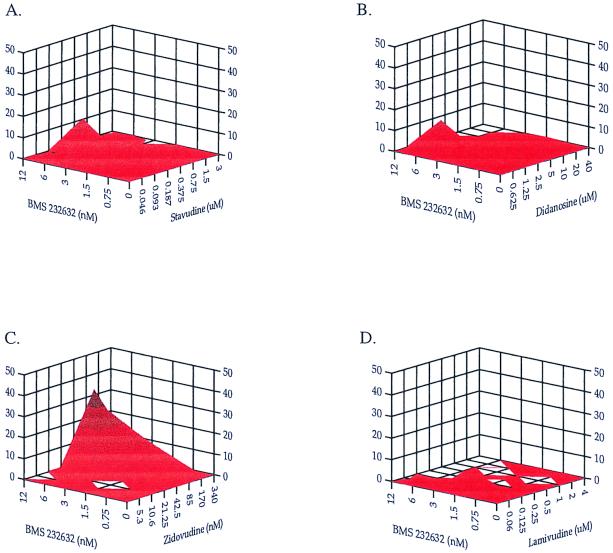
The anti-HIV effects of combining different RT inhibitors with the Prt inhibitor BMS-232632 were evaluated in PBMCs infected by a clinical HIV-1 isolate as previously described (10). The potential cytotoxicities of these combined agents were also analyzed in parallel. Four nucleoside analogs, d4T, ddI, AZT, and 3TC, were combined with BMS-232632, volumes of synergy and antagonism were calculated over a range of drug ratios, and the results were analyzed by the MacSynergy program (Fig. 3 and Table 4). The EC50s of these drugs in monotherapy are 2 nM for BMS-232632, 0.28 μM for d4T, 2.3 μM for ddI, 15 nM for AZT, and 100 nM for 3TC in agreement with published values. When d4T, ddI, and 3TC were individually combined with BMS-232632, the volumes of antagonism were extremely low, −5.4, −1.2, and 0 μM2%, respectively. The volumes of synergy were also small (0.78, 16.6, and 11.2 μM2% for the d4T, ddI, and 3TC combinations, respectively [Figure 3; Table 4]), indicating an additive effect for all three drug combinations. A higher positive volume of synergy was observed when BMS-232632 was combined with AZT (44.3 μM2%), indicating a modest synergistic effect.
FIG. 3.
Analysis of two-drug combinations using the MacSynergy program to compare drug interactions of BMS-232632 with RT inhibitors d4T (A), ddI (B), AZT (C), and 3TC (D). The x- and y-axis values are drug concentrations, and the z-axis values are percent drug interactions.
TABLE 4.
Two-drug combinations using BMS-232632 and RT and Prt inhibitorsa
| Drug combined with BMS-232632 | MacSynergy vol (μM2%)
|
Molar ratio | CI at HIV inhibition of:
|
Overall result | ||
|---|---|---|---|---|---|---|
| Synergy | Antagonism | 50% | 70% | |||
| d4T | +0.78 | −5.4 | 1:5 | 0.90 ± 0.11 | 0.82 ± 0.12 | Additive |
| 1:40 | 0.98 ± 0.10 | 0.80 ± 0.09 | ||||
| ddI | +16.6 | −1.2 | 1:150 | 0.93 ± 0.12 | 0.82 ± 0.16 | Additive |
| AZT | +44.3 | 0 | 2:1 | 0.73 ± 0.14 | 0.76 ± 0.18 | Minor synergy |
| 1:2 | 0.87 ± 0.18 | 0.72 ± 0.16 | ||||
| 3TC | +11.2 | 0 | 1:1.25 | 0.95 ± 0.13 | 0.97 ± 0.16 | Additive |
| 1:5 | 0.91 ± 0.09 | 0.91 ± 0.12 | ||||
| RTV | +31.4 | −36.7 | 1:42 | 1.21 ± 0.19 | 1.12 ± 0.23 | Additive |
| 1:83 | 1.05 ± 0.11 | 1.08 ± 0.13 | ||||
| IDV | +75.5 | −41.7 | 1:2.5 | 0.87 ± 0.14 | 1.08 ± 0.22 | Additive |
| 1:8.3 | 1.00 ± 0.13 | 0.94 ± 0.16 | ||||
| SQV | +42.1 | −2.7 | 1.3:1 | 1.02 ± 0.05 | 1.13 ± 0.06 | Additive |
| 1:3 | 0.86 ± 0.02 | 0.89 ± 0.03 | ||||
| NFV | +5.7 | −0.78 | 1:3.1 | 1.43 ± 0.17 | 0.96 ± 0.13 | Additive |
| 1:12.5 | 1.07 ± 0.09 | 0.94 ± 0.10 | ||||
| APV | 0 | −10.8 | 1:3.75 | 1.34 ± 0.23 | 1.08 ± 0.23 | Additive |
| 1:15 | 1.37 ± 0.18 | 0.95 ± 0.15 | ||||
Results are based on three experiments.
Since the drugs were diluted in a 6 by 8 matrix, CIs at two different constant drug ratios could be obtained for each combination using the same data set. CIs near 1 (0.9 to 1.1) indicate additive effects, while values near 0.8 or 1.2 suggest low levels of synergy or antagonism, respectively. Combining 3TC with BMS-232632 yielded an additive response and a CI near 1 with each drug ratio at both the 50 and 70% effective levels (Table 4). The d4T, ddI, and AZT combinations showed some synergy, with some CIs near 0.8 at 70% effective doses. Taking all the CIs and the MacSynergy analysis into account, the overall effect of combining d4T, ddI, AZT, or 3TC with BMS-232632 is essentially additive to weakly synergistic. Most importantly, no significant drug antagonism was observed when BMS-232632 was combined with any of the four nucleoside analogs. Moreover, we did not detect any enhanced cytotoxicity with these combinations as measured by XTT reduction assay (data not shown).
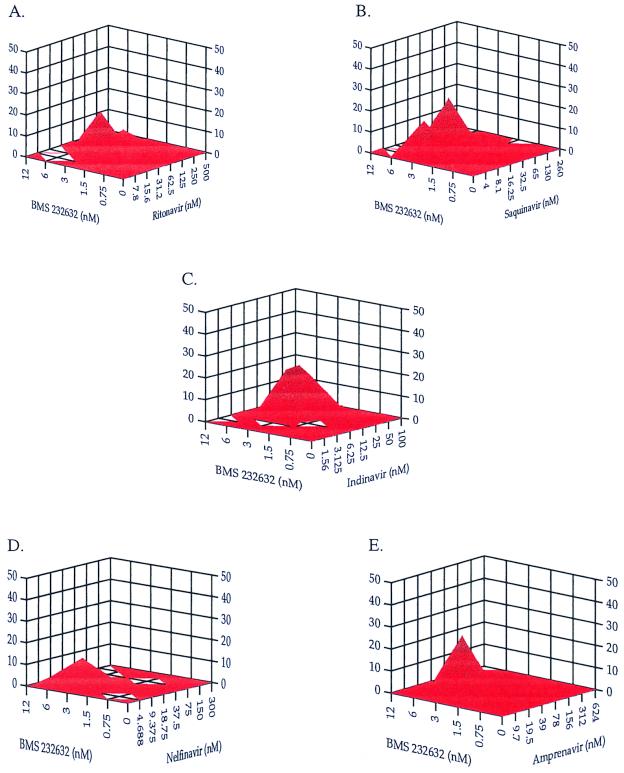
Two-drug combinations of BMS-232632 and other HIV Prt inhibitors.
BMS-232632 was also combined with each of the five approved Prt inhibitors, and anti-HIV activity was determined. The EC50s of the Prt inhibitors in the PBMCs infected with clinical isolate 006 are 2 nM for BMS-232632, 5 nM for IDV, 6 nM for NFV, 45 nM for RTV, 27 nM for SQV, and 21 nM for APV. Results of drug combination effects analyzed by the MacSynergy program are shown in Fig. 4 and are summarized in Table 4. The combination of BMS-232632 with SQV showed modest synergy (42.1 μM2%). When BMS-232632 was combined with NFV or APV, only small volumes of synergy (5.7 and 0 μM2%, respectively) and antagonism (−0.78 and −10.8 μM2%, respectively) were observed, suggesting an additive response. Combinations involving RTV and IDV gave greater positive (+31.4, and +75.5 μM2%, respectively) and negative volumes (−36.7 and −41.7 μM2%, respectively), suggesting an overall additive response. In these two cases, the negative values were scattered throughout the MacSynergy plot and none of the peaks were greater than −9 μM2%, suggesting that there was some variation within the assay systems and that no significant antagonism exists with these combinations.
FIG. 4.
Analysis of two-drug combinations using the MacSynergy program to compare drug interactions of BMS-232632 with Prt inhibitors RTV (A), SQV (B), IDV (C), NFV (D), and APV (E). The x- and y-axis values are drug concentrations, and the z-axis values are percent drug interactions.
The CIs for each Prt combination were also determined at two different constant drug ratios using the same set of experiments as for the MacSynergy analysis. The results (Table 4) show that combinations with any of the five approved Prt inhibitors resulted in an additive anti-HIV effect, with most CIs near 1 at both drug ratios and different effective levels. Finally, no enhanced cytotoxicity was observed in evaluating the combinations containing two Prt inhibitors at the highest concentrations used in antiviral assays (data not shown).
DISCUSSION
The current standard of care for those infected with HIV involves the use of triple drug combinations that frequently include an HIV-1 Prt inhibitor. However, the long-term side effects, inconvenient dosing schedule, and dietary restrictions lead to patient noncompliance, viral resistance, and ultimately treatment failure. Therefore, a Prt inhibitor having high potency, bioavailability supportive of once-daily dosing, and decreased toxicity remains in demand. BMS-232632 has the potential to satisfy these criteria (Y. F. Gong, B. Robinson, R. Rose, C. Deminie, T. Spicer, R. Colonno, and P.-Y. Lin, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. I-79, 1998; E. M. O'Mara, J. Smith, S. J. Olsen, T. Tanner, A. E. Schuster, and S. Kaul, Abstr. 6th Conf. Retroviruses Opportunistic Infect., abstr. 604, 1999). It is a novel potent azapeptide Prt inhibitor with a Ki of 2.66 nM. Proof-of-principle studies demonstrated that BMS-232632 inhibits HIV replication in cells by blocking the cleavage of viral precursor proteins, resulting in the secretion of immature virions consisting of unprocessed gag proteins (Fig. 2). The potent inhibitory effect of BMS-232632 on HIV-1 Prt in vitro and the specific inhibition of gag processing in cells suggest that the compound may possess strong activity against acute viral infection. Indeed, an anti-HIV evaluation showed that the compound possesses generally greater potency (EC50 = 2.6 to 5.3 nM) than the five currently approved HIV Prt inhibitors. Problems associated with protein binding are not expected in the clinic, since the addition of 40% human serum or 1 mg of α1-acid glycoprotein/ml increased the EC50 of BMS-232632 by only 2.7- to 3.6-fold (Table 3). Furthermore, cytotoxicity testing indicated that the compound exhibits favorable selective indices (6,500 to 23,800) in a range of different cell types.
To investigate the potential use of BMS-232632 in combination therapies, we investigated the in vitro antiviral effect of combining BMS-232632 with each of nine available antiretroviral agents. The studies were performed with PBMCs infected with clinical isolate 006 to maximize the in vivo relevance of these experiments. The data presented in Fig. 3 and 4 and Table 4 showed that the interactions of BMS-232632 with the RT inhibitors d4T, ddI, 3TC, and AZT were additive to slightly synergistic. Similarly, combinations of BMS-232632 with each of the other five Prt inhibitors yielded additive results. Most importantly, no antagonistic antiviral effects or enhanced cytotoxic effects resulted from any of the drug combinations.
Of primary importance is whether the desired potency of BMS-232632 can be delivered orally and whether BMS-232632 would be effective against the variants resistant to other Prt inhibitors. Initial indications from first-in-man clinical studies suggest high exposure levels when BMS-232632 is administered orally (O'Mara et al., Abstr. 6th Conf. Retroviruses Opportunistic Infect., abstr. 604). In fact, it is likely that trough levels may exceed EC90s following once-daily dosing (O'Mara et al., Abstr. 6th Conf. Retroviruses Opportunistic Infect., abstr. 604). Hence, BMS-232632 appears to have excellent potential and could be a significant addition to the current antiretroviral armamentarium.
ACKNOWLEDGMENT
We thank D. Morse for preparation of the manuscript.
REFERENCES
- 1.Bechtold C M, Patick A K, Alam M, Greytok J, Tino J A, Chen P, Gordon E, Ahmad S, Barish J C, Zahler R, Lin P-F, Colonno R. Antiviral properties of aminodiol inhibitors against human immunodeficiency virus and Prt. Antimicrob Agents Chemother. 1995;39:374–379. doi: 10.1128/aac.39.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilello J A, Bilello P A, Stellrecht K, Leonard J, Norbeck D W, Kempf D J, Robins T, Drusano G L. Human serum alpha 1 acid glycoprotein reduces uptake, intracellular concentration, and antiviral activity of A-80987, an inhibitor of the human immunodeficiency virus type 1 protease. Antimicrob Agents Chemother. 1996;40:1491–1497. doi: 10.1128/aac.40.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boden D, Markowitz M. Resistance to human immunodeficiency virus type 1 protease inhibitors. Antimicrob Agents Chemother. 1998;42:2775–2783. doi: 10.1128/aac.42.11.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryant M, Getman D, Smidt M, Marr J, Clare M, Dillard R, Lansky D, DeCrescenzo G, Heintz R, Houseman K, Reed K, Stolzenbach J, Talley J, Vazquez M, Mueller R. SC-52151, a novel inhibitor of the human immunodeficiency virus protease. Antimicrob Agents Chemother. 1995;39:2229–2234. doi: 10.1128/aac.39.10.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron D W, Japour A J, Xu Y, Hsu A, Mellors J, Farthing C, Cohen C, Poretz D, Markowitz M, Follansbee S, Angel J B, McMahon D, Ho D, Devanarayan V, Rode R, Salgo M, Kempf D J, Granneman R, Leonard J M, Sun E. Ritonavir and saquinavir combination therapy for the treatment of HIV infection. AIDS. 1999;13:213–224. doi: 10.1097/00002030-199902040-00009. [DOI] [PubMed] [Google Scholar]
- 6.Chong K-T, Pagano P J. In vitro combination of PNU-140690, a human immunodeficiency virus type 1 protease inhibitor, with ritonavir against ritonavir-sensitive and -resistant clinical isolates. Antimicrob Agents Chemother. 1997;41:2367–2373. doi: 10.1128/aac.41.11.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou T-C, Talalay P. Quantitative analysis of dose effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 8.Condra J H, Holder D J, Schleif W A, Blahy O M, Danovich R M, Gabryelski L J, Grahman D J, Laird D, Quintero J C, Rhodes A, Robbins H L, Roth E, Shivaprakash M, Yang T, Chodarkewitz J A, Deutsch P J, Leavitt R Y, Massari F E, Mellors J W, Squires K E, Steigbigel R T, Teppler H, Emini E A. Genetic correlates of in vivo viral resistance to indinavir, a human immunodeficiency virus type 1 protease inhibitor. J Virol. 1996;70:8270–8276. doi: 10.1128/jvi.70.12.8270-8276.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daluge S A, Good S S, Faletto M B, Miller W H, St. Clair M H, Boone L R, Tisdale M, Parry N R, Reardon J E, Dornsife R E, Averett D R, Krenitsky T A. 1592U89, a novel carbocyclic nucleoside analog with potent, selective anti-human immunodeficiency virus activity. Antimicrob Agents Chemother. 1997;41:1082–1093. doi: 10.1128/aac.41.5.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deminie C, Bechtold C M, Stock D, Alam M, Djang F, Balch A H, Chou T-C, Prichard M, Colonno R J, Lin P-F. Evaluation of reverse transcriptase and protease inhibitors in two-drug combinations against human immunodeficiency virus replication. Antimicrob Agents Chemother. 1996;40:1346–1351. doi: 10.1128/aac.40.6.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faletto M B, Miller W H, Garvey E P, St. Clair M H, Daluge S M, Good S S. Unique intracellular activation of the potent anti-human immunodeficiency virus agent 1592U89. Antimicrob Agents Chemother. 1997;41:1099–1107. doi: 10.1128/aac.41.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flexner D. HIV-protease inhibitors. Drug Ther. 1998;338:1281–1292. doi: 10.1056/NEJM199804303381808. [DOI] [PubMed] [Google Scholar]
- 13.Gulick R M. Current antiretroviral therapy: an overview. Quality Life Res. 1997;6:471–474. doi: 10.1023/a:1018447829842. [DOI] [PubMed] [Google Scholar]
- 14.Hammer S M, Squires K E, Hughes M D, Grimes J M, Demeter L M, Currier J S, Eron J J, Jr, Feinberg J E, Balfour H H, Jr, Deyton L R, Chodakewitz J A, Fischl M A. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 15.Hubert J J. Bioassay. Dubuque, Iowa: Kendall/Hunt Publishing Co.; 1992. [Google Scholar]
- 16.Ido E, Han H, Kezdy F J, Tang J. Kinetic studies of human immunodeficiency virus type 1 protease and its active-site hydrogen bond mutant A28S. J Biol Chem. 1991;266:24359–24366. [PubMed] [Google Scholar]
- 17.Johnson V A, Byington R E. Infectivity assay (virus yield assay) In: Aldovini A, Walker B D, editors. Techniques in HIV research. New York, N.Y: Stockton Press; 1990. pp. 71–76. [Google Scholar]
- 18.Kageyama S, Anderson B D, Hoesterey B L, Hayashi H, Kiso Y, Flora K P, Mitsuya H. Protein binding of human immunodeficiency virus protease inhibitor KNI-272 and alteration of its in vitro antiretroviral activity in the presence of high concentrations of proteins. Antimicrob Agents Chemother. 1994;38:1107–1111. doi: 10.1128/aac.38.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalish V, Kaldor S, Shetty B, Tatlock J, Davies J, Hammond M, Dressman B, Fritz J, Appelt K, Reich S, Musick L, Wu B, Su K. Iterative protein structure-based drug design and synthesis of HIV protease inhibitors. Eur J Med Chem. 1995;30:202S–214S. [Google Scholar]
- 20.Kempf D J, Marsh K C, Denissen J F, McDonald E, Vasavanonda S, Flentge C A, Green B E, Fino L, Park C H, Kong X-P, Wideburg N E, Saldivar A, Ruiz L, Kati W M, Sham H L, Robins T, Stewart K D, Hsu A, Plattner J J, Leonard J M, Norbeck D W. ABT-538 is a potent inhibitor of human immunodeficiency virus protease and has high oral bioavailability in humans. Proc Natl Acad Sci USA. 1995;92:2484–2488. doi: 10.1073/pnas.92.7.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kempf D J, Marsh K C, Kumar G, Rodrigues A D, Denissen J F, McDonald E, Kukulka M J, Hsu A, Granneman G R, Baroldi P A, Sun E, Pizzuti D, Plattner J J, Norbeck D W, Leonard J M. Pharmacokinetic enhancement of inhibitors of the human immunodeficiency virus protease by coadministration with ritonavir. Antimicrob Agents Chemother. 1997;41:654–660. doi: 10.1128/aac.41.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keppel G. Design and analysis: a researchers handbook. 2nd ed. Englewood Cliffs, N.J: Prentice-Hall, Inc.; 1982. [Google Scholar]
- 23.Kohl N E, Diehl R E, Rands E, Davis L J, Hanobik M G, Wolanski B, Dixon R A. Expression of active human immunodeficiency virus type 1 protease by noninfectious chimeric virus particles. J Virol. 1991;65:3007–3014. doi: 10.1128/jvi.65.6.3007-3014.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer R A, Schaber M D, Skalka A M, Ganguly K, Wong-Staal F, Reddy E P. HTLV-III gag protein is processed in yeast cells by the virus pol-protease. Science. 1986;231:1580–1584. doi: 10.1126/science.2420008. [DOI] [PubMed] [Google Scholar]
- 25.Kuritzkes D R. HIV resistance to current therapies. Antiviral Ther. 1997;2:61–67. [Google Scholar]
- 26.Lin P F, Samanta H, Rose R E, Patick A K, Trimble J, Bechtold C M, Revie D R, Narayan C K, Federici M E, Li H, Lee A, Anderson R E, Colonno R J. Genotypic and phenotypic analysis of human immunodeficiency virus type 1 isolates from patients on prolonged stavudine therapy. J Infect Dis. 1994;170:1157–1164. doi: 10.1093/infdis/170.5.1157. [DOI] [PubMed] [Google Scholar]
- 27.Lin Y, Lin X, Hong L, Foundling S, Heinrikson R L, Thaisrivongs S, Leelamanit W, Raterman D, Shah M, Dunn B M, Tang J. Effect of point mutations on the kinetics and the inhibition of human immunodeficiency virus type 1 protease: relationship to drug resistance. Biochemistry. 1995;34:1143–1152. doi: 10.1021/bi00004a007. [DOI] [PubMed] [Google Scholar]
- 28.Livingston D J, Pazhanisamy S, Porter D J T, Partaledis J A, Tung R D, Painter G R. Weak binding of VX-478 to human plasma proteins and implications for anti-human immunodeficiency virus therapy. J Infect Dis. 1998;172:1238–1245. doi: 10.1093/infdis/172.5.1238. [DOI] [PubMed] [Google Scholar]
- 29.Matayoshi E D, Wang G T, Krafft G A, Erickson J. Novel fluorogenic substrates for assaying retroviral protease by resonance energy transfer. Science. 1990;247:954–958. doi: 10.1126/science.2106161. [DOI] [PubMed] [Google Scholar]
- 30.Molla A, Granneman G R, Sun E, Kempf D J. Recent developments in HIV protease inhibitor therapy. Antiviral Res. 1998;39:1–23. doi: 10.1016/s0166-3542(98)00011-4. [DOI] [PubMed] [Google Scholar]
- 31.Morris-Jones S, Moyle G, Easterbrook P J. Antiretroviral therapies in HIV-1 infection. Expert Opin Investig Drugs. 1997;6:1049–1061. doi: 10.1517/13543784.6.8.1049. [DOI] [PubMed] [Google Scholar]
- 32.Murphy R L, Gulick R M, DeGruttola V, D'Aquila R T, Eron J J, Sommadossi J P, Currier J S, Smeaton L, Frank I, Caliendo A M, Gerber J G, Tung R, Kuritzkes D R. Treatment with amprenavir alone or amprenavir with zidovudine and lamivudine in adults with human immunodeficiency virus infections. AIDS Clinical Trials Group 347 Study Team. J Infect Dis. 1999;179:808–816. doi: 10.1086/314668. [DOI] [PubMed] [Google Scholar]
- 33.Patick A K, Boritzki T J, Bloom L A. Activities of the human immunodeficiency virus type 1 (HIV-1) protease inhibitor nelfinavir mesylate in combination with reverse transcriptase and protease inhibitors against acute HIV-1 infection in vitro. Antimicrob Agents Chemother. 1997;41:2159–2164. doi: 10.1128/aac.41.10.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pettit S C, Michael S F, Swanstrom R. The specificity of the HIV-1 protease. Perspect Drug Discov Des. 1993;1:69–83. [Google Scholar]
- 35.Potts B J. “Mini” reverse transcriptase (RT) assay. In: Aldovini A, Walker B D, editors. Techniques in HIV research. New York, N.Y: Stockton Press; 1990. pp. 103–106. [Google Scholar]
- 36.Prichard M N, Aseltine K R, Shipman C., Jr . MacSynergy II version 1.0 user's manual. Ann Arbor, Mich: University of Michigan; 1992. [Google Scholar]
- 37.Prichard M N, Prichard L E, Shipman C., Jr Strategic design and three-dimensional analysis of antiviral drug combinations. Antimicrob Agents Chemother. 1993;37:540–545. doi: 10.1128/aac.37.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts N A, Martin J A, Kinchington D, Broadhurst A V, Craig J C, Duncan I B, Galpin S A, Handa B K, Kay J, Krohn A, Lambert R W, Merrett J H, Mills J S, Parkes K E B, Redshaw S, Ritchie A J, Taylor D L, Thomas G J, Machin P J. Rational design of peptide-based HIV proteinase inhibitors. Science. 1990;248:358–361. doi: 10.1126/science.2183354. [DOI] [PubMed] [Google Scholar]
- 39.St. Clair M H S, Millard J, Rooney J, Tisdale M, Parry N, Sadler B M, Blum M R, Painter G. In vitro activity of 141W94 (VX-478) in combination with other antiretroviral agents. Antiviral Res. 1996;29:53–56. doi: 10.1016/0166-3542(95)00916-7. [DOI] [PubMed] [Google Scholar]
- 40.Vacca J P, Condra J H. Clinically effective HIV-1 protease inhibitors. Drug Discov Today. 1997;2:261–272. [Google Scholar]
- 41.Vacca J P, Dorsey B D, Schleif W A, Levin R B, McDaniel S L, Darke P L, Zugay J, Quintero J C, Blahy O M, Roth E, Sardana V V, Schlabach A J, Graham P I, Condra J H, Gotlib L, Holloway M K, Lin J, Chen I-W, Vastag K, Ostovic D, Anderson P S, Emini E A, Huff J R. L-735,524: an orally bioavailable human immunodeficiency virus type 1 protease inhibitor. Proc Natl Acad Sci USA. 1994;91:4096–4100. doi: 10.1073/pnas.91.9.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weislow O S, Kiser R, Fine D L, Bader J, Shoemaker R H, Boyd M R. New soluble-formazan assay for HIV-1 cytopathic effects: application to high-flux screening of synthetic and natural products for AIDS-antiviral activity. J Natl Cancer Inst. 1989;81:577–586. doi: 10.1093/jnci/81.8.577. [DOI] [PubMed] [Google Scholar]
- 43.Young S D, Britcher S F, Tran L O, Payne L S, Lumma W C, Lyle T A, Huff J R, Anderson P S, Olsen D B, Carroll S S, Pettibone D J, O'Brien J A, Ball R G, Balani S K, Lin J H, Chen I-W, Schleif W A, Sardana V V, Long W J, Byrnes V W, Emini E A. L-743,726 (DMP-266): a novel, highly potent nonnucleoside inhibitor of the human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother. 1995;39:2602–2605. doi: 10.1128/aac.39.12.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]