Summary
The Mycobacterium bovis BCG vaccine was first used in 1921, but has not controlled the global spread of tuberculosis (TB). There are still no new licensed tuberculosis vaccines, although there much active research and a vaccine development pipeline, with vaccines designed to prevent infection, prevent disease, or accelerate TB treatment. These vaccines are of different types, and designed to replace BCG, or to boost immunity following BCG vaccination. This viewpoint discusses why, when it has been possible to develop new vaccines for SARS-CoV-2 so quickly, it is taking so long to develop new tuberculosis vaccines.
Keywords: Tuberculosis, Vaccines, SARS-CoV-2
The Mycobacterium bovis BCG vaccine was first used in 1921 and so has now been in use for more than 100 years.1,2 This attenuated mycobacterial vaccine lost significant regions of the M. bovis genome during the 231 successive subcultures that led to its attenuation. Further genetic deletions occurred as the new BCG was shared around the world and then cultured locally, leading to the BCG strains known as Japan, Pasteur, etc.3 First delivered orally, it was a lifesaver as tuberculosis killed many infants and children as well as adults. Administered at birth, BCG delivers good protection against the disseminated forms of tuberculosis disease in childhood, such as tuberculous meningitis, and also miliary tuberculosis.4 Tuberculosis can affect a range of organs including the bone, kidney, etc, but the most common form is the pulmonary disease seen in adolescents and young adults, and it is this clinical manifestation that is responsible for most infections and disease transmission, spread by coughing but also even breathing. However, the track record for BCG's ability to protect against pulmonary disease is more variable, with clear evidence that although it can protect against the disseminated forms of disease in childhood,4 it can either protect, or fail to protect against pulmonary disease in adolescents and adults, in different settings and trials.5
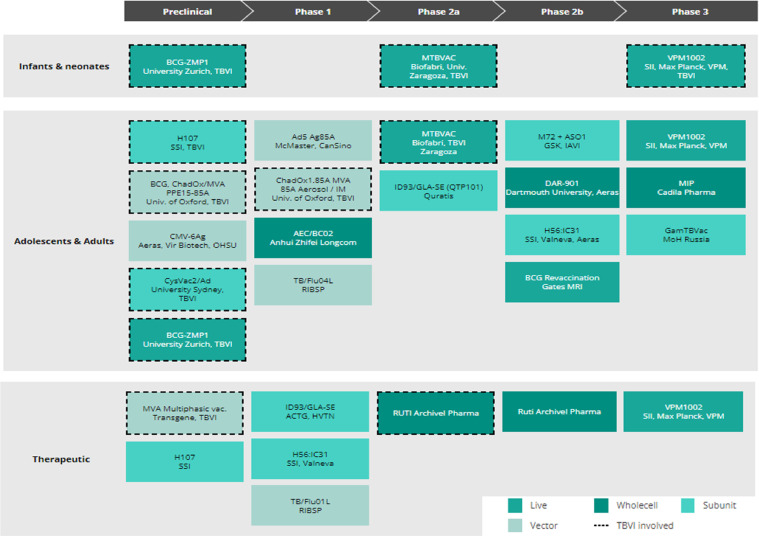
As we approached the Millennium in 2000, although the BCG vaccine itself was still in widespread use globally, the BCG vaccine had not controlled the continued spread of M. tuberculosis or removed tuberculosis as a global public health threat. No new tuberculosis vaccines were licensed or even in early clinical trials. Over twenty years later, despite small improvements, tuberculosis remains a major global public health challenge and we still do not have any new licensed tuberculosis vaccines other than BCG. We now have a pipeline of tuberculosis vaccine candidates6 - with a number of candidates showing good promise or evidence of efficacy in animal studies and human trials. This pipeline spans early pre-clinical development through to clinical trials -phase 1 to phase 2b/3 trials (Figure 1). These vaccine candidates are varied in type and intended use.7, 8, 9 They range from recombinant antigens to be delivered with adjuvant, antigens to be delivered by viral vectors, and genetically modified live bacterial vaccines – recombinant BCG vaccines designed to improve on our existing BCG vaccines such as VPM1002,10 and a live attenuated M. tuberculosis vaccine, MTBVAC.11 There are vaccines that could replace BCG immediately after birth or be given to boost immunity in a prime boost strategy, as well as vaccines that might be given to adolescents to prevent the peak of tuberculosis seen in young adults.12 Some vaccines are also designed to be given as an adjunct to drug therapy, to accelerate cure and shorten treatment, prevent subsequent relapse, or help treat drug-resistant tuberculosis.
Figure 1.
The TB vaccine pipeline. The live, whole cell, subunit and viral-vectored vaccine candidates in the TB vaccine development pipeline as at October 2021 are shown, together with their intended target population.6 Reproduced with permission from TBVI.
But given the dramatic progress in developing vaccines to protect us against SARS-CoV-2, with new SARS-CoV-2 vaccines licensed within 12 months of the genome of the virus being sequenced, the question is why progress developing a more effective tuberculosis vaccine has been so slow? And what is now required to accelerate the introduction of new, more effective vaccines for tuberculosis? We now know how quickly airborne pathogens can spread worldwide, and although most tuberculosis patients can be successfully treated, albeit with a long course of antibiotics, we still have the spectre of increasing numbers of multi and extensively drug resistant strains of M. tuberculosis, that are resistant to most if not all of our available antibiotics.13 Thus, we urgently need to prioritise the development of new tuberculosis vaccines, to prevent a pandemic of drug-resistant tuberculosis.
Even compared to the COVID pandemic, the numbers dying from tuberculosis make sobering reading.14 By mid-January 2022, there had been over 5.5 million deaths from COVID, but there are ∼1.4M deaths from tuberculosis each year – which had until the arrival of SARS-CoV-2 been the leading infectious killer. And this is before the deterioration in tuberculosis control programmes because of the COVID pandemic are taken into account –estimates are that COVID itself could lead to a 36% increase in deaths from tuberculosis over the next 5 years.15 So why it is taking so long to make a vaccine that is better than the 100-year-old BCG vaccine?
A number of M. tuberculosis animal challenge models have been developed to expedite vaccine development. Candidate vaccines are usually first evaluated in mouse models, which are often criticised as not being fully representative of the pathology of human disease. Guinea pigs are considered a better model and are more sensitive to M. tuberculosis infection, but their use is limited by a lack of immunological reagents. BCG vaccination is highly effective in both mice and guinea pigs and is used as a gold standard positive control in challenge studies. Candidate vaccines have to be at least as good as BCG, and usually better, to progress. There are new murine models that better represent human pathology16 and ultra-low dose infection models look promising17 but non-human primates (NHP) are undoubtedly the best model18 even if the cost of experiments and restrictions on their use limits their availability. The lack of good small animal models, and the costs of using non-human primates has undoubtedly slowed successful vaccine development, as has uncertainty as to which animal model, if any, best represents the human situation.
A further challenge to establishing representative animal models for tuberculosis vaccine development is that exposure to, and co-infection with, other pathogens may alter both susceptibility to M. tuberculosis and BCG vaccine efficacy, in humans. Exposure to non-tuberculous mycobacteria is the most likely explanation for the varying immunogenicity of BCG closer to the equator,19 co-infection with HIV increases susceptibility to tuberculosis even in the presence of anti-retroviral therapy,20 and more recently co-infection with CMV can increase susceptibility to tuberculosis.21 Exposure to these pathogens varies across TB endemic countries and modelling these co-infections in animal models is extremely difficult.22
Another common reason given for the slow progress in tuberculosis vaccine R&D is the slow growth of mycobacteria. Whereas many extracellular bacteria can divide every 20 minutes, the intracellular mycobacteria are slow growing. This means it takes mycobacteria about 24 hours or longer to divide, and so tuberculosis develops slowly. Animal models of tuberculosis are time-consuming, with timelines of 3-6 months for many experiments. What such protracted experimental work needs is longer term funding, and here the slow growing mycobacteria can contribute to a lack of rapid progress. Even clinical efficacy trials require a timescale of at least 3 years if progression to disease (POD) is the primary endpoint.
Despite these problems, as noted above there has been recent progress with a number of vaccine candidates of different types reaching phase 2 /3 trials, and more at earlier stages of development6 (Figure 1).
If COVID vaccines can be developed so quickly, why are new tuberculosis vaccines different? Here there are a number of important factors (Table 1), although they are not insurmountable. Firstly, despite tuberculosis being declared a global emergency by the World Health Organisation in 1994, tuberculosis research has lacked the urgency that the COVID pandemic brought about. The situation with tuberculosis has been so bad for so long, that either people think that tuberculosis is a historic disease that no longer poses a threat to human health - or being a chronic problem with higher endemicity in low- and middle- income countries (LMIC), there has been no sense of urgency in terms of funding priorities. Funding is limited and there has been no sense of a crisis, or that we might only have 1-2 years to deliver an effective vaccine, as there was with SARS-CoV-2.
Table 1.
Comparison of key issues for development of vaccines for SARS-CoV-2 and Mycobacterium tuberculosis infection.
| SARS-CoV-2 | Tuberculosis | Notes | |
|---|---|---|---|
| Key protective antigen | Spike protein | Number of key specific and cross-reactive antigens identified | M. tuberculosis genome encodes ∼4000 genes |
| Antigenic variation | Mutations common | Antigenic variation limited | Both SARS-CoV-2 and M. tuberculosis show strain variation in transmissibility |
| Immune correlates of protection | Neutralising antibodies considered key although T cells likely to play a role | Cell-mediated immunity critical although precise definition unclear. Role of humoral immunity also unclear. | Was not needed for the development of effective SARS-CoV-2 vaccines but would be game-changing in the development of a new TB vaccine |
| Categories of infection | Subclinical, clinical | Latent (incipient/subclinical) and clinical | In both infections, transmission may occur from subclinical infection |
| Time to develop disease | 1-2 weeks | Can be years/decades/lifespan | |
| Identification of pathogen | 2019 with resulting pandemic | 1882 | WHO declared TB a global health emergency in 1994 |
| Licensed vaccines by January 2022 | 4 (UK) | 1 (BCG) | BCG first used in 1921 |
| Vaccine candidates in Preclinical and clinical development | 195 pre-clinical 146 clinical |
Unknown but <50 14 clinical |
SARS-CoV-2: WHO as at 02/2022; TB: TBVI as at 11/2021. |
Secondly, there are critical differences between a virus such as SARS-CoV-2 and M. tuberculosis. SARS-CoV-2 has a 29.9kB genome with 12 expressed genes, open reading frames encoding ∼30,000 nucleotides and with a single Spike protein that is required for cell invasion.23 M. tuberculosis has a genome with ∼4.4 million base pairs encoding ∼3906 protein genes,24 and although a number of proteins play a role in its pathogenicity, there is no consensus on the best single antigen or even antigens that would be most protective in a vaccine. The tuberculosis vaccines currently in the development pipeline use both M. tuberculosis specific or cross-reactive antigens shared with other mycobacteria. Some candidate vaccines have aimed to improve the existing BCG vaccine (or vaccines as there are a number of BCG vaccine strains in current use, which differ in the genes and antigens they express). However, M. tuberculosis does have a major advantage for vaccine developers in that although the M. tuberculosis strains in circulation and causing human tuberculosis vary genetically, there is very limited evidence of antigenic variation, and moreover some evidence that it is the human T cell epitopes that are most highly conserved.25 This means that there is no issue with immune evasion resulting from mutations, as seen with SARS-CoV-2.
Differences in the clinical course of infection also help explain why progress with tuberculosis vaccine research and development is slower than for SARS-CoV-2. The natural history of infection with M. tuberculosis is complex. Most people who are infected with M. tuberculosis do not develop primary disease but mount a sufficient immune response to control, but not eradicate infection, called latent tuberculosis infection (LTBI).26,27 When host immunity breaks down, because of co-infection with HIV, treatment with biological agents such as monoclonal antibodies against Th1 cytokines such as TNFa28 or simply when immune senescence occurs with aging, this latent infection can reactivate and cause disease. We now consider there is a continuous spectrum of tuberculosis, rather than discrete binary outcomes of infection or disease, and new intermediate states of incipient (without detectable live mycobacteria) and subclinical disease in which asymptomatic transmission may occur, as well as “resistors” who may clear infections without induction of a persistent detectable immune response have been proposed.29,30 It is clear that the outcome of M. tuberculosis infection is a complex interplay between the pathogen and the host immune response. For SARS-CoV-2, as with other Corona viruses, there is no latent and reactivation stage. The incubation period is relatively short. Not everyone develops disease, and asymptomatic transmission can occur.31
Another challenge for tuberculosis vaccine design is that M. tuberculosis remains latent in many people in part because of an ability to evade and subvert the host immune response, and this complicates vaccine design.32 From delaying phagolysosomal fusion and preventing acidification of the vacuole, through to downregulating Class II MHC presentation on antigen presenting cells, there are a variety of mechanisms by which M. tuberculosis can persist in the human host. Whilst some of the long-term sequelae and serious disease caused by SARS–CoV-2 may be in part mediated by the host immune response, there is no evidence of immune evasion caused by the virus manipulating its intracellular milieu, although mutant strains can escape pre-existing immunity. The ability to suppress the development of protective immune responses is one of the reasons why M. tuberculosis has been such a successful pathogen over centuries.
Controlled human infection models, whereby small numbers of healthy volunteers are deliberately exposed to the pathogen in question, have been used very successfully for other globally important but complex pathogens such as Plasmodium falciparum.33 Malaria human challenge models have expedited both vaccine development and the identification of immune correlates of protection. Developing a controlled human infection model for tuberculosis raises issues including which pathogen to use, whether it is acceptable to deliberately infect with a fully virulent strain, and which route of infection to use in such models.34,35 Whilst various attempts have been made to develop such models, these are not yet routinely used for vaccine evaluation and prioritisation. Interestingly there are now studies underway to establish a controlled human infection model for SARS-CoV-2.36
Those designing the new Covid vaccines have had another advantage. Not only has it been clear that the Spike protein would be an obvious target, but the ability to measure antibody concentrations to the Spike protein provides a straightforward correlate of protection,37 even though there is evidence that T cell-mediated protection may also be important.38 This is something the tuberculosis community has struggled with over decades, despite the involvement of many leading immunologists worldwide. Being an intracellular pathogen, the assumption has always been that T cell immunity to M. tuberculosis, and likely a Th1 T cell response was the key to protection. T cells, and Th1 cytokines such as IFN-γ are necessary for protection, but may or may not correlate with protection.39,40 Measuring T cell responses to M. tuberculosis is more complex than measuring antibody titres and as yet there is no confirmed correlate of protection. Recent work with multi-omics, single cell analyses, multiplex bead arrays, CYToF and more have confirmed the complexity of T cell responses and their plasticity. There may even be a role for antibodies.41 But more work is still needed, and this will include identifying biomarkers that predict vaccine efficacy in the lung.42 Developing an effective vaccine will be accelerated once a vaccine-specific correlate of protection or protective biosignature is identified.
All 4 UK-licensed vaccines against SARS-CoV-2 have demonstrated high levels of efficacy in phase III studies, less than one year after first being tested in human clinical trials. Although this success was only possible because of significant prior investment in R&D into the platform technologies for adenovirus vectored vaccines and mRNA vaccines, it remains the case that to develop new vaccines against COVID disease so quickly was extraordinary. In contrast, the most promising candidate tuberculosis vaccine, M72, a fusion protein of two M. tuberculosis antigens administered together with a potent adjuvant, AS01, was first tested in clinical trials in 200443; it took until 2019 for the final results of a phase IIb trial which showed this vaccine provided 50% protection against tuberculosis disease in young, M. tuberculosis latently infected adults.44 This result needs confirming in a larger phase III trial which has yet to start, despite the phase IIb trial reporting in 2019. Key questions remain about this vaccine candidate including whether it would also confer protection in those with prior BCG but uninfected with M. tuberculosis. Ideally, we would have a tuberculosis vaccine that demonstrated the kinds of levels of efficacy seen with the SARS-CoV-2 vaccines. Progress with complex pathogens such as M. tuberculosis is slower and more iterative than vaccines for respiratory viruses such as SARS-CoV-2, but can be accelerated; for example, the mRNA technologies that have proved so effective for SARS-CoV-2 are now being applied to M. tuberculosis, and would make it possible for new vaccines to be produced in Africa. It is worth noting here that the first report of a RNA vaccine for tuberculosis was published in 2004.45
Innovation in clinical trial design has also led to improvements in our understanding in tuberculosis vaccine development. Phase IIb clinical trials where the primary endpoint is clinical disease require extended periods of follow up and large numbers of subjects. Unlike malaria, where incidence of infection can be as high as 60% in a malaria season, incidence of tuberculosis disease in even the highest burden settings is often only 1-2%. Using Prevention of Infection (POI), rather than Prevention of Disease (POD) as an endpoint means smaller trials with shorter periods of follow up are possible,46 but requires vaccination pre-exposure, either in neonates or in adolescents who are pre-screened for infection. The first reported POI trial demonstrated that BCG revaccination conferred 45% protection against sustained infection, as measured by sustained Quantiferon conversion.47 Whilst there are caveats with this approach including the fact that the definition of infection is indirect, and the relationship between POI and POD is unclear, as a way to demonstrate a biological signal of efficacy in the target species, humans, such an approach has merit. Another efficacy endpoint, Prevention of Recurrence of tuberculosis disease (POR), is also being explored in some trials, for example with the subunit vaccine candidate H56:IC31.48 Further work to explore alternative clinical endpoints along the continuous spectrum of tuberculosis is needed, in parallel with detailed interrogation of potential biomarkers as surrogate endpoints to improve the feasibility of tuberculosis vaccine efficacy trials.
There are now two candidate vaccines designed to replace BCG currently in late-stage testing. The first and most advanced, VPM1002, is a recombinant strain of BCG designed to induce a broader immune response than BCG.10 After extensive evaluation in preclinical and clinical studies,49,50 this vaccine candidate is currently being tested in a POI trial in infants across several sites in Africa.51 The second, MTBVAC, works on the premise that M. tuberculosis is a better starting point for a rationally attenuated human tuberculosis vaccine than M. bovis.11 MTBVAC has also been evaluated extensively in preclinical and clinical studies52,53 and will be evaluated in a Phase IIb efficacy trial in infants commencing in 2022.54 Whilst it is clear that progress is being made, it will be several years before the next tuberculosis vaccine efficacy trial reports, and longer still before a new tuberculosis vaccine is licensed and deployed throughout the world.
There are other candidate vaccines in late-stage preclinical development, for example a recombinant CMV-vectored tuberculosis vaccine which has demonstrated efficacy against infectious challenge in non-human primates.55 Animal models also allow alternative routes of vaccination to be evaluated. There is much recent interest in delivery of BCG and other vaccines by the mucosal route.56 A recent study in non-human primates has demonstrated that mucosally delivered BCG is more protective than the licensed intradermal route.57 Two independent studies have demonstrated that intravenous (iv) BCG is much more protective than any other route; giving BCG iv to NHPs reduced lung pathology assessed by imaging and at necropsy as well as bacterial load.58,59 Whilst intravenous BCG may not be an easily deployable route of vaccination, particularly in infants in LMIC countries, such studies provide important proof-of-concept of what is possible. Interrogation of the immune response to such routes of immunisation can then allow more easily deployable vaccines that induce the same immune response to be developed.
So what does the tuberculosis vaccine field need to make more rapid progress and deliver a tuberculosis vaccine that would give better protection that our current BCG vaccines? More funding, including a long-term commitment from research funders. Despite the commitment made in the declaration from the 2018 United Nations General Assembly to mobilise 2 billion dollars/year funding for tuberculosis research,60 there is still a significant funding gap. An analysis of the 2019 funding data suggests that less than 50% of this figure was available, and post-COVID, the funding available for tuberculosis vaccine research is likely to reduce still further.61 The ready availability of significant funding, together with the use of pull mechanisms such as Advance Market Commitments was one of the many factors that expedited the development of vaccines for SARS-CoV-2. There is often talk of vaccine development or control of a pandemic such as that with SARS-CoV-2 being “a marathon not a sprint”. Developing a more effective tuberculosis vaccine feels more like a long-distance footpath. The tuberculosis field, together with international agencies and other donors, needs to understand that there is an urgency, not only to save many lives and livelihoods, given that tuberculosis mostly affects those adults in their most productive years, but because a pandemic of almost untreatable drug-resistant tuberculosis is a real threat. The economic burden created by the ongoing tuberculosis epidemic provides a significant impediment to economic growth in many LMIC countries.
Secondly, progress requires coordination of the research effort. Vaccinology is a multi-disciplinary team science and it is critical that academic and industrial partners with complementary areas of expertise work together on this endeavour. The global tuberculosis community is well networked, and efforts have been accelerated through coordination provided by the Collaboration for TB Vaccine Discovery (CTVD) initiated in 2015 that links >375 members in 86 institutions,62 and in Europe, the TuBerculosis Vaccine Initiative, TBVI, that has coordinated the European tuberculosis vaccine effort.63 A succession of research consortia funded by the European Community’s Framework Programmes have helped, both by linking researchers, but TBVI has also provided supportive mechanisms such as contributing to the development of a pipeline tool,6,64 Product Development Teams, and direct head-to-head testing of vaccine candidates in animal models.
Here the COVID pandemic has had a negative impact on progress. Not only is there a research funding gap until the new European Community Horizon calls are awarded, but the lack of face-to-face interactions and too many meetings moved online have reduced opportunities for this mutually supportive community to do what it does best – discuss, challenge, and innovate. We need to work together to encourage early career researchers into this field and this will be facilitated by better funding opportunities and tuberculosis specific calls.
However, the COVID pandemic has shown the tuberculosis community the way. There is much to do and a number of priority areas to address (Table 2). With positivity, commitment and funding, improved vaccines for tuberculosis will be found and moved through clinical trials into global use. The tuberculosis community just needs to make it happen.
Table 2.
Recommendations to accelerate the development of new TB vaccines.
| Recommendation | Comments |
|---|---|
| Improved definition of trial endpoints for POI and POD trial designs and harmonisation across efficacy trials to allow direct comparison between vaccine candidates | Recognition of continuous spectrum of M. tuberculosis infection/disease including incipient/subclinical disease should replace simplistic categorisation into LTBI and clinical disease |
| Head-to-head testing of vaccine candidates in murine and NHP models in independent laboratories | Requires coordination and funding |
| Identification of biomarkers for use in vaccine trials | Must be quantifiable, and include exploration of new platform technologies including single cell analyses |
| Better definition of protective immunity within the lung | Most human immunology performed on peripheral blood |
| Evaluate BCG replacement vaccine candidates for induction of non-specific protection against non-mycobacterial infections that is at least equivalent to BCG | Non-specific protection or innate training should be at least equivalent to that given by BCG |
| Acceleration of vaccine trials | Requires funding for trials and site infrastructure |
| Source more financial support for both laboratory-based research and for clinical trials | Should provide funding for at least 5 years; coordination mechanisms required |
Contributors
Both authors contributed to conceptualization, writing the original draft, and editing and approving the final text.
Declaration of interests
HMD and HMcS both report no conflicts of interest.
Acknowledgements
No specific funding was used for the writing of this viewpoint. HMD and HMcS were partners in the TBVAC2020 Consortium which received funding from the European Union Horizon2020 Programme under grant agreement 643381. HMcS is a member of CTVD.
References
- 1.Lange C, Aaby P, Behr MA, et al. 100 years of Mycobacterium bovis bacille Calmette-Guérin. Lancet Infect Dis. 2022;22:e2–e12. doi: 10.1016/S1473-3099(21)00403-5. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann SHE. Vaccine development against tuberculosis over the last 140 years: failure as part of success. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.750124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brosch R, Gordon SV, Garnier T, et al. Genome plasticity of BCG and impact on vaccine efficacy. Proc Natl Acad Sci USA. 2007;104:5596–5601. doi: 10.1073/pnas.0700869104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 5.Mangtani P, Abubakar I, Ariti C, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58:470–480. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- 6.TB vaccine pipeline, 2021. https://www.tbvi.eu/what-we-do/pipeline-of-vaccines. Accessed 20 January 2022.
- 7.Schrager LK, Vekemens J, Drager N, Lewinsohn DM, Olesen OF. The status of tuberculosis vaccine development. Lancet Infect Dis. 2020;20:e28–e37. doi: 10.1016/S1473-3099(19)30625-5. [DOI] [PubMed] [Google Scholar]
- 8.Brazier B, McShane H. Towards new TB vaccines. Semin Immunopathol. 2020;42:315–331. doi: 10.1007/s00281-020-00794-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saramago S, Magalhães J, Pinheiro M. Tuberculosis vaccines: an update of recent and ongoing clinical trials. Appl Sci. 2021;11:9250. [Google Scholar]
- 10.Nieuwenhuizen NE, Kulkarni PS, Shaligram U, et al. The recombinant bacille Calmette-Guérin vaccine VPM1002: ready for clinical efficacy testing. Front Immunol. 2017;8:1147. doi: 10.3389/fimmu.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martín C, Marinova D, Aguiló N, Gonzalo-Asensio J. MTBVAC, a live TB vaccine poised to initiate efficacy trials 100 years after BCG. Vaccine. 2021;39:7277–7285. doi: 10.1016/j.vaccine.2021.06.049. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann S, Weiner J, von Reyn C. Novel approaches to tuberculosis vaccine development. Int J Infect Dis. 2017;56:263–267. doi: 10.1016/j.ijid.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Migliori GB, Tiberi S, Zumla A, et al. MDR/XDR-TB management of patients and contacts: challenges facing the new decade. The 2020 clinical update by the Global Tuberculosis Network. Int J Infect Dis. 2020;92S:S15–S25. doi: 10.1016/j.ijid.2020.01.042. [DOI] [PubMed] [Google Scholar]
- 14.WHO Global TB Report 2021. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021. Accessed 20 January 2022.
- 15.Hogan AB, Jewell BL, Sherrard-Smith E, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8:e1132–e1141. doi: 10.1016/S2214-109X(20)30288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basaraba RJ, Hunter RL. Pathology of tuberculosis: how the pathology of human tuberculosis informs and directs animal models. Microbiol Spectr. 2017;5 doi: 10.1128/microbiolspec.TBTB2-0029-2016. 10.1128. [DOI] [PubMed] [Google Scholar]
- 17.Plumlee CR, Duffy FJ, Gern BH, et al. Ultra-low dose aerosol infection of mice with mycobacterium tuberculosis more closely models human tuberculosis. Cell Host Microbe. 2021;29:68–82. doi: 10.1016/j.chom.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardona PJ, Williams A. Experimental animal modelling for TB vaccine development. Int J Infect Dis. 2017;56:268–273. doi: 10.1016/j.ijid.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 19.Black GF, Weir RE, Floyd S, et al. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet. 2002;359:1393–1401. doi: 10.1016/S0140-6736(02)08353-8. [DOI] [PubMed] [Google Scholar]
- 20.Cohen K, Meintjes G. Management of individuals requiring antiretroviral therapy and TB treatment. Curr Opin HIV AIDS. 2010;5:61–69. doi: 10.1097/COH.0b013e3283339309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller J, Tanner R, Matsumiya M, et al. Cytomegalovirus infection is a risk factor for tuberculosis disease in infants. JCI Insight. 2019;4:e130090. doi: 10.1172/jci.insight.130090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poyntz HC, Stylianou E, Griffiths KL, Marsay L, Checkley AM, McShane H. Non-tuberculous mycobacteria have diverse effects on BCG efficacy against Mycobacterium tuberculosis. Tuberculosis (Edinb) 2014;94:226–237. doi: 10.1016/j.tube.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu A, Peng Y, Huang B, et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole ST, Brosch R, Parkhill J, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 25.Comas I, Chakravartti J, Small PM, et al. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat Genet. 2010;42:498–503. doi: 10.1038/ng.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barry CE, 3rd, Boshoff HI, Dartois V, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7:845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esmail H, Barry CE, 3rd, Young DB, Wilkinson RJ. The ongoing challenge of latent tuberculosis. Philos Trans R Soc Lond B Biol Sci. 2014;369 doi: 10.1098/rstb.2013.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 29.Drain PK, Bajema KL, Dowdy D, et al. Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin Microbiol Rev. 2018;31:e00021. doi: 10.1128/CMR.00021-18. -e00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simmons JD, Stein CM, Seshadri C, et al. Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nat Rev Immunol. 2018;18:575–589. doi: 10.1038/s41577-018-0025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ernst JD. Mechanisms of M. tuberculosis immune evasion as challenges to TB vaccine design. Cell Host Microbe. 2018;24:34–42. doi: 10.1016/j.chom.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ewer KJ, O'Hara GA, Duncan CJ, et al. Protective CD8+ T-cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunisation. Nat Commun. 2013;4:2836. doi: 10.1038/ncomms3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McShane H. Controlled human infection models: is it really feasible to give people tuberculosis? Am J Respir Crit Care Med. 2020;201:1180–1181. doi: 10.1164/rccm.201912-2408ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davids M, Pooran A, Hermann C, et al. A human lung challenge model to evaluate the safety and immunogenicity of PPD and live bacillus Calmette-Guérin. Am J Respir Crit Care Med. 2020;201:1277–1291. doi: 10.1164/rccm.201908-1580OC. [DOI] [PubMed] [Google Scholar]
- 36.Clinicaltrials.gov NCT04865237; NCT04864548
- 37.McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng Y, Mentzer AJ, Liu G, et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fletcher HA, Snowden MA, Landry B, et al. T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat Commun. 2016;7:11290. doi: 10.1038/ncomms11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kagina BM, Abel B, Scriba TJ, et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guérin vaccination of newborns. Am J Respir Crit Care Med. 2010;182:1073–1079. doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu LL, Chung AW, Rosebrock TR, et al. A Functional Role for Antibodies in Tuberculosis. Cell. 2016;167:433–443. doi: 10.1016/j.cell.2016.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morrison H, McShane H. Local pulmonary immunological biomarkers in tuberculosis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.640916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Von Eschen K, Morrison R, Braun M, et al. The candidate tuberculosis vaccine Mtb72F/AS02A: tolerability and immunogenicity in humans. Hum Vaccin. 2009;5:475–482. doi: 10.4161/hv.8570. [DOI] [PubMed] [Google Scholar]
- 44.Tait DR, Hatherill M, Van Der Meeren O, et al. Final analysis of a trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med. 2019;381:2429–2439. doi: 10.1056/NEJMoa1909953. [DOI] [PubMed] [Google Scholar]
- 45.Xue T, Stavropoulos E, Yang M, et al. RNA encoding the MPT83 antigen induces protective immune responses against Mycobacterium tuberculosis infection. Infect Immun. 2004;72(11):6324–6329. doi: 10.1128/IAI.72.11.6324-6329.2004. doi: 10.1128/IAI.72.11.6324-6329.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hawn TR, Day TA, Scriba TJ, et al. Tuberculosis vaccines and prevention of infection. Microbiol Mol Biol Rev. 2014;78:650–671. doi: 10.1128/MMBR.00021-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nemes E, Geldenhuys H, Rozot V, et al. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med. 2018;379:138–149. doi: 10.1056/NEJMoa1714021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.clinicaltrials.gov NCT03512249
- 49.Grode L, Seiler P, Baumann S, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guérin mutants that secrete listeriolysin. J Clin Invest. 2005;115:2472–2479. doi: 10.1172/JCI24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grode L, Ganoza CA, Brohm C, Weiner J, 3rd, Eisele B, Kaufmann SH. Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine. 2013;31:1340–1348. doi: 10.1016/j.vaccine.2012.12.053. [DOI] [PubMed] [Google Scholar]
- 51.Clinicaltrials.gov NCT04351685
- 52.Verreck FA, Vervenne RA, Kondova I, et al. MVA.85A boosting of BCG and an attenuated, phoP deficient M. tuberculosis vaccine both show protective efficacy against tuberculosis in rhesus macaques. PLoS One. 2009;4:e5264. doi: 10.1371/journal.pone.0005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spertini F, Audran R, Chakour R, et al. Safety of human immunisation with a live-attenuated Mycobacterium tuberculosis vaccine: a randomised, double-blind, controlled phase I trial. Lancet Respir Med. 2015;3:953–962. doi: 10.1016/S2213-2600(15)00435-X. [DOI] [PubMed] [Google Scholar]
- 54.Clinicaltrials.gov NCT04975178
- 55.Hansen SG, Zak DE, Xu G, et al. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat Med. 2018;24:130–143. doi: 10.1038/nm.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stylianou E, Paul MJ, Reljic R, McShane H. Mucosal delivery of tuberculosis vaccines: a review of current approaches and challenges. Expert Rev Vaccines. 2019;18:1271–1284. doi: 10.1080/14760584.2019.1692657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dijkman K, Sombroek CC, Vervenne RAW, et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat Med. 2019;25:255–262. doi: 10.1038/s41591-018-0319-9. [DOI] [PubMed] [Google Scholar]
- 58.Sharpe S, White A, Sarfas C, et al. Alternative BCG delivery strategies improve protection against Mycobacterium tuberculosis in non-human primates: protection associated with mycobacterial antigen-specific CD4 effector memory T-cell populations. Tuberculosis (Edinb) 2016;101:174–190. doi: 10.1016/j.tube.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Darrah PA, Zeppa JJ, Maiello P, et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature. 2020;577:95–102. doi: 10.1038/s41586-019-1817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Resolution from United Nations General Assembly high-level meeting on tuberculosis, 26 September 2018, Resolution document A/RES/73/3 accessed 28 February 2022.
- 61.Tuberculosis Research Funding Trends 2005-2019. Stop TB Partnership and Treatment Action Group. https://www.treatmentactiongroup.org/wp-content/uploads/2020/12/tbrd_2020_final_web.pdf. Accessed 7 March 2022.
- 62.Collaboration for TB Vaccine Discovery. https://www.ctvd.org/Accessed 23 January 2022.
- 63.TuBerculosis Vaccine Initiative. https://www.tbvi.eu Accessed 20 January 2022.
- 64.TB Vaccine Development Pathway. https://www.tbvacpathway.org Accessed 5 April 2022.