Abstract
BACKGROUND
Left ventricular hypertrophy (LVH) is prevalent in obese individuals. Besides, both of LVH and obesity is associated with subclinical LV dysfunction. The study aims to investigate the interplay between body fat and LVH in relation to all-cause death in patients with coronary artery disease (CAD).
METHODS
In this retrospective cohort study, a total of 2243 patients with angiographically proven CAD were included. Body fat and LV mass were calculated using established formulas. Patients were grouped according to body fat percentage and presence or absence of LVH. Cox-proportional hazard models were used to observe the interaction effect of body fat and LVH on all-cause death.
RESULTS
Of 2243 patients enrolled, 560 (25%) had a higher body fat percentage, and 1045 (46.6%) had LVH. After a median follow-up of 2.2 years, the cumulative mortality rate was 8.2% in the group with higher body fat and LVH, 2.5% in those with lower body fat and no LVH, 5.4% in those with higher body fat and no LVH, and 7.8% in those with lower body fat and LVH (log-rank P < 0.001). There was a statistically significant interaction between body fat percentage and LVH ( P interaction was 0.003). After correcting for confounding factors, patients with higher body fat and LVH had the highest risk of all-cause death (HR = 3.49, 95% CI: 1.40–8.69, P = 0.007) compared with those with lower body fat and no LVH; in contrast, patients with higher body fat and no LVH had no statistically significant difference in risk of death compared with those with lower body fat and no LVH (HR = 2.03, 95% CI: 0.70–5.92, P = 0.195).
CONCLUSION
A higher body fat percentage was associated with a different risk of all-cause death in patients with CAD, stratified by coexistence of LVH or not. Higher body fat was significantly associated with a greater risk of mortality among patients with LVH but not among those without LVH.
Last 40 years have witnessed sharply rise of obesity prevalence at any age and gender around the world. In the concept of obesity transition, China has been classified into stage 2, which is characterized by a significant increase of obesity prevalence in adults surpassing that among children, and a narrowing distinction between sexes.[1] In 2012, obesity prevalence in Chinese residents aged over 18 years old is 11.9%. About 13.5% of cardiovascular deaths were attributable to high body mass index in 2017; furthermore, the direct economic burden of overweight and obesity rose to 90.768 billion yuan (RMB), which is accounted for 4.5% of total health expenditure of China in 2010.[2] Obesity, itself or concomitant with other comorbidities, contribute to a higher risk of incident coronary artery disease (CAD).[3] Cardiac remodeling and functional changes are also prevailing phenomena in obese individuals. A meta-analysis showed that the prevalence of left ventricular hypertrophy (LVH) in obese individuals was 56%, ranging from 20% to 85% according to different criteria of LVH.[4] In patients with coronary artery stenosis, LVH was associated with the presence of myocardial ischemia and the extent of involvement independent of traditional cardiovascular risk factors.[5]
With in-depth research on the pathogenesis heart failure with preserved ejection fraction, increasing weight has been given to the impact of obesity and LVH on cardiovascular system.[6] Although we are well aware of the adverse effects of body fat on cardiovascular metabolism, for patients who have been diagnosed with CAD, the role of body fat may be heterogeneous.[7,8] In other words, for those patients, some studies have reported the phenomenon of the obesity paradox, while other studies have observed the U-shaped curve between obesity and prognosis, and some studies have not observed the relationship between obesity and better clinical endpoints.[8,9] It is very important to clarify the association between body fat and the prognosis among patients with CAD because it will determine how to guide them on weight management. Actually, physical activity can improve the cardiopulmonary function status of patients with cardiovascular disease. Therefore, identifying which patients with obesity have a worse prognosis and need to be focused on as target population to improve management and reduce the burden of disease is worthy of further observation. Since LVH is very closely related to adipose tissue and adverse cardiovascular events, it may play an important role in the risk stratification of patients with obesity concomitant with CAD.[10] Previous studies demonstrated that the reaction of ventricular sarcomere contractility to increased calcium stimulation was substantially depressed in severely obese patients; furthermore, LVH and obesity had a superposition effect on regional LV dysfunction.[11,12] In this study, we investigated the interplay relationship of body fat and LVH with the risk of all-cause death in patients with CAD.
METHODS
Study Population
The study population was from the CAD database of West China Hospital, Sichuan University. In brief, West China Hospital CAD database is a large prospective registry study designed to explore risk factors, early warnings, risk stratification and management of patients with CAD (ChiCTR-OOC-17010433). The database was created in July 2008 and is ongoing to enroll all patients who have undergone invasive coronary angiography at West China Hospital. The information collected includes demographic data, cardiovascular risk factors, laboratory data, echocardiographic indicators, angiographic results, medication, revascularization and clinical outcomes. Informed consent was given to all enrolled patients in this study, and ethical approval was obtained from the local institution. The primary inclusion criteria for this study were angiographically proven of stenosis greater than 50% in at least one major epicardial coronary artery. The exclusion criteria included patients who had contraindications to coronary angiography, patients who did not receive echocardiographic examination during hospitalization, patients who were lost during follow-up, patients who died within the first month of discharge, or patients who were followed up for less than 1 month.
Measurement of Major Exposure Factors
The main exposure factors of interest in this study were percentage of body fat and left ventricular mass index. A formula based on body mass index, sex and age (Clínica Universidad de Navarra-Body Adiposity Estimator) was used to calculate body fat percentage, which has been validated in Asian populations.[13,14,15] Height and weight are measured by a trained nurse at admission. Body mass index (BMI) was the ratio of body weight to the square of height. The study defined higher body fat as the percentage of body fat greater than the 75th percentile of included patients for different gender groups. During the hospital stay, the patient underwent a comprehensive transthoracic echocardiographic examination in accordance with guideline recommendations.[16] From the parasternal long axis view, the left ventricular diameter was measured using M-type or 2D guidance. Left ventricular volume and left ventricular ejection fraction (LVEF) were measured using the two-plane Simpsons method. The left ventricular myocardial mass was calculated using the formula recommended by the American Society of Echocardiography, and it was normalized using the height of 2.7 to obtain the left ventricular myocardial mass index (LVMI).[16] According to the guideline definition, LVMI greater than 44 g/m2 in women and 48 g/m2 in men is defined as LVH. According to body fat percentage and LVMI, patients were divided into four groups: lower body fat and no LVH; lower body fat and LVH; higher body fat and no LVH; higher body fat and LVH.
Data on Potential Confounders and Modifiers
Demographic characteristics, cardiovascular risk factors, comorbidities, and medical history were collected by a questionnaire interview at admission or search in medical record. Data of blood pressure, heart rate, laboratory data, angiographic results, medications, and revascularization therapy were obtained from medical records.
Follow-up and Study Endpoint
The endpoint of the study was all-cause death. Telephone consultation, review of medical records, and outpatient visits were the mainly adopted follow-up method. The events were confirmed by death certificates or close relatives. Each patient was followed up from the discharge until either death occurrence or the last follow-up, whichever came first.
Statistical Analysis
Continuous data were presented as median with interquartile range, and categorical data were presented as number and percentage. For continuous variables, the Kruskal–Wallis test was used to analyze the overall distribution differences among groups. The pairwise comparisons were then conducted, and the Bonferroni method was used to adjust α levels. For categorical variables, we used the chi-square test to test the difference in the overall sample, and then the difference between two proportions was compared using z-test, using the Bonferroni method to adjust theP value. We used Cox proportional risk models to examine the interplay associations between body fat percentage and left ventricular hypertrophy for the risk of all-cause death. Different models were used to examine the effect of covariates on prognosis. Model 1 was the result of no correction. Model 2 was adjusted for age and sex. Model 3 was adjusted for age, sex, LVEF, and creatinine. Model 4 corrected age, sex, LVEF, serum creatinine, hypertension, diabetes, current smoker, systolic blood pressure, diastolic blood pressure, heart rate, triglyceride, glucose, hemoglobin, angiotensin converting enzyme inhibitors/angiotensin II receptor blockers, beta-blockers, left main of three-vessel disease, and stent numbers. All analyses were performed using IBM SPSS 20.0 software or and R ( http://www.R-project.org). P value < 0.05 on both sides indicated statistical difference.
RESULTS
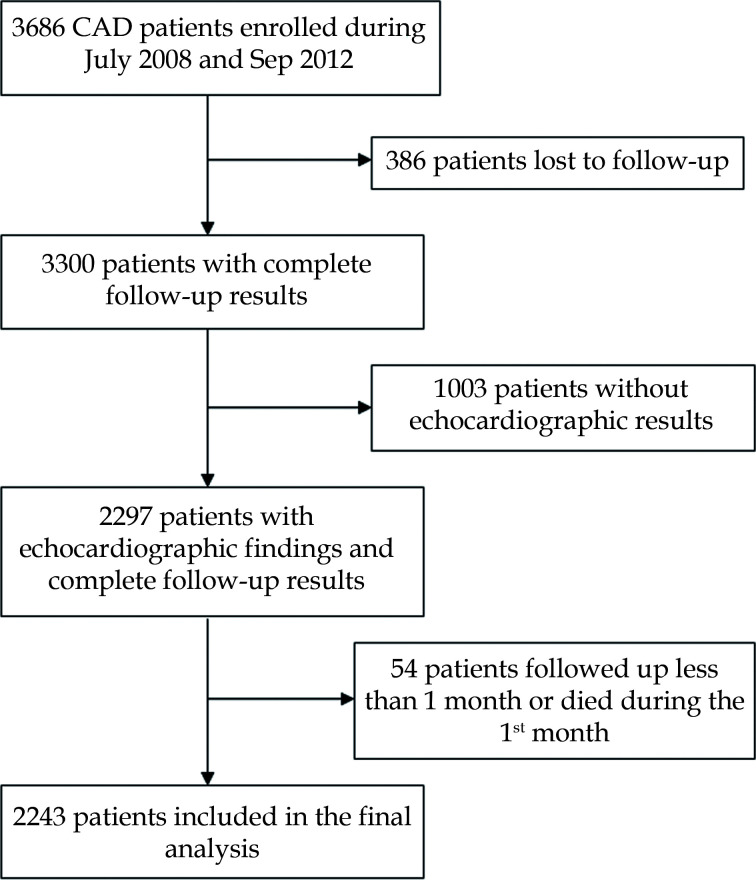
Figure 1 showed the flow chart of patient inclusion. Of the 3686 patients enrolled during July 2008 and Sep. 2012, 386 patients lost to follow-up, 1003 without echocardiographic results, 54 followed up less than one month or died during the first month were excluded. Finally, our analyses were limited to 2243 patients.
Figure 1.
Flow chart of the screening of patients.
This figure shows the flow chart of the screening of patients included in this study. A total of 3686 patients with coronary heart disease were included in the database at the beginning of the study. After excluding those who were lost to follow-up, with incomplete echocardiographic data, or followed up for less than 1 month/died within 1 month after discharge, a total of 2243 patients were included in the final analysis.
Of the 2243 patients enrolled, the median age was 66 years, 79% were male, 560 (25%) had a higher body fat percentage, and 1045 (46.6%) had LVH. Among subjects with a higher body fat percentage, 43.2% did not have LVH, while 56.8% did. The baseline characteristics of the patients in the different groups are shown in Table 1. Patients with higher body fat and LVH had a higher proportion of females, higher rates of hypertension, higher systolic blood pressure and relative ventricular wall thickness, faster heart rate, and lower hemoglobin and LVEF (all P values < 0.05) than patients with lower body fat and no LVH and those with higher body fat but no LVH. In addition, among patients without LVH, patients with higher body fat were older, had higher rates of hypertension, and had higher serum glucose and creatinine values ( P < 0.05 for all relevant variables) compared with patients with lower body fat.
Table 1. Baseline characteristics by body fat percentage and presence or absence of LVH.
| Characteristics | All patients
(n = 2243) |
Lower body fat,
no LVH (n = 956) |
Lower body fat, LVH
(n = 727) |
Higher body fat,
no LVH (n = 242) |
Higher body fat, LVH
(n = 318) |
P value |
| Data are presented as median(IQR) or n (%). *P < 0.05 vs. lower body fat and no LVH; †P < 0.05 vs. lower body fat and LVH; ‡P < 0.05 vs. higher body fat and no LVH. The table shows the baseline comparisons of each group of subjects. Compared with patients with lower body fat percentage and no LVH or those with higher body fat percentage but no LVH, patients with higher body fat and LVH had a higher proportion of females and hypertension, higher systolic blood pressure and relative ventricular wall thickness, faster heart rate, and lower serum hemoglobin and LVEF (all P values < 0.05). Furthermore, among patients without LVH, those with higher body fat percentages were older, had higher rates of hypertension, and had higher serum glucose and creatinine values ( P < 0.05 for all relevant variables) than patients with lower body fat percentages. ACE: angiotensin converting enzyme; ARBs: angiotensin II receptor blockers; BMI: body mass index; CAD: coronary artery disease; IQR: interquartile range; LDL: low-density lipoprotein; LVEF: left ventricular ejection fraction; LVMI: left ventricular mass index; LVH:left ventricular hypertrophy; NSTEMI: non-ST segment elevation myocardial infarction; STEMI: ST-Segment Elevation Myocardial Infarction; PCI: percutaneous coronary intervention. | ||||||
| Demograohics and history | ||||||
| Age, yrs | 66.0 (57.0−73.0) | 64.0 (55.0−72.0) | 65.0 (58.0−72.0)* | 68.0 (61.0−73.0)*† | 69.0 (62.0−75.0)*† | < 0.001 |
| Male | 1778 (79.3%) | 820 (85.8%) | 514 (70.7%)* | 217 (89.7%)† | 227 (71.4%)*‡ | < 0.001 |
| Hypertension | 1265 (56.4%) | 430 (45.0%) | 455 (62.6%)* | 146 (60.3%)* | 234 (73.6%)*†‡ | < 0.001 |
| Diabetes | 506 (22.6%) | 168 (17.6%) | 187 (25.7%)* | 58 (24.0%) | 93 (29.2%)* | < 0.001 |
| Current smoker | 640 (28.5%) | 325 (34.0%) | 195 (26.8%)* | 61 (25.2%) | 59 (18.6%)*† | < 0.001 |
| CAD subtypes | 0.344 | |||||
| STEMI | 254 (11.3%) | 100 (10.5%) | 95 (13.1%) | 27 (11.2%) | 32 (10.1%) | |
| NSTEMI | 143 (6.4%) | 53 ( 5.5%) | 52 ( 7.2%) | 13 ( 5.4%) | 25 ( 7.9%) | |
| Unstable angina | 1178 (52.5%) | 498 (52.1%) | 371 (51.0%) | 134 (55.4%) | 175 (55.0%) | |
| Stable CAD | 668 (29.8%) | 305 (31.9%) | 209 (28.7%) | 68 (28.1%) | 86 (27.0%) | |
| Clinical measurements | ||||||
| BMI, kg/m2 | 24.0 (22.3−25.8) | 23.0 (21.5−24.2) | 23.4 (22.2−24.6)* | 27.2 (26.1−28.3)*† | 27.4 (26.1−29.0)*† | < 0.001 |
| Body fat percentage, % | 26.7 (23.9−31.1) | 24.6 (22.5−26.7) | 26.1 (24.0−34.5)* | 29.5 (28.8−31.7)*† | 31.0 (29.2−40.4)*† | < 0.001 |
| Systolic blood pressure, mmHg | 130.0 (118.0−144.0) | 126.0 (113.0−140.0) | 132.0 (119.5−150.0)* | 130.0 (118.0−142.0) | 136.5 (121.0−150.0)*†‡ | < 0.001 |
| Diastolic blood pressure, mmHg | 76.0 (70.0−84.0) | 74.0 (68.0−80.0) | 78.0 (70.0−86.0)* | 76.0 (68.0−83.8) | 78.0 (70.0−85.0)* | < 0.001 |
| Heart rate, beats/min | 72.0 (64.0−80.0) | 71.0 (64.0−80.0) | 74.0 (66.0−82.0)* | 70.0 (63.0−78.0)† | 74.0 (64.0−82.0)*‡ | < 0.001 |
| Laboratory test | ||||||
| Total cholesterol, mmol/L | 3.9 (3.3−4.7) | 3.9 (3.3−4.6) | 4.0 (3.4−4.7) | 3.9 (3.2−4.6) | 4.0 (3.4−4.7) | 0.107 |
| LDL, mmol/L | 2.2 (1.7−2.9) | 2.2 (1.6−2.8) | 2.3 (1.8−2.9) | 2.2 (1.7−2.9) | 2.3 (1.8−2.9) | 0.161 |
| Triglyceride, mmol/L | 1.4 (1.0−2.0) | 1.3 (1.0−2.0) | 1.4 (1.1−2.0) | 1.5 (1.1−2.0) | 1.6 (1.2−2.3)*† | < 0.001 |
| Glucose, mmol/L | 5.9 (5.1−7.6) | 5.6 (5.0−7.1) | 6.1 (5.1−8.3)* | 6.1 (5.1−8.0)* | 6.4 (5.3−8.0)* | < 0.001 |
| Serum creatinine, μmol/L | 85.3 (74.4−100.3) | 84.0 (74.2−96.0) | 85.0 (72.4−103.0) | 88.8 (80.7−103.3)*† | 88.2 (77.8−105.9)*† | < 0.001 |
| Hemoglobin, g/L | 135.0 (123.0−146.0) | 137.0 (126.0−147.0) | 133.0 (120.0−144.0)* | 139.0 (128.0−147.0)† | 133.0 (121.0−145.0)*‡ | < 0.001 |
| Echocardiographic parameters | ||||||
| LVMI, g/m2.7 | 46.3 (38.7−56.2) | 38.8 (34.6−43.0) | 56.1 (51.1−64.6)* | 41.2 (36.7−44.5)† | 58.5 (53.6−67.7)*‡ | < 0.001 |
| Relative wall thickness | 0.40 (0.36−0.45) | 0.40 (0.36−0.44) | 0.41 (0.35−0.46) | 0.39 (0.36−0.43) | 0.42 (0.38−0.46)*‡ | < 0.001 |
| LVEF, % | 64.0 (56.0−69.0) | 65.0 (60.0−70.0) | 60.0 (48.0−66.0)* | 66.0 (60.0−70.0)† | 63.0 (53.0−68.0)*†‡ | < 0.001 |
| Medication and angiographic results | ||||||
| Aspirin | 2111 (94.7%) | 899 (95.0%) | 684 (94.3%) | 227 (93.8%) | 301 (95.0%) | 0.848 |
| Clopidogrel | 2051 (92.0%) | 876 (92.6%) | 661 (91.2%) | 220 (90.9%) | 294 (92.7%) | 0.624 |
| Statins | 2049 (91.9%) | 870 (92.0%) | 658 (90.8%) | 228 (94.2%) | 293 (92.4%) | 0.372 |
| ACE inhibitors/ARBs | 1304 (58.5%) | 491 (51.9%) | 463 (63.9%)* | 142 (58.7%) | 208 (65.6%)* | < 0.001 |
| Beta-blockers | 1527 (68.5%) | 619 (65.4%) | 514 (70.9%) | 176 (72.7%) | 218 (68.8%) | 0.045 |
| Left main and three-vessel disease | 666 (29.7%) | 232 (24.3%) | 242 (33.3%)* | 69 (28.5%) | 123 (38.7%)* | < 0.001 |
| PCI | 1558 (69.5%) | 675 (70.6%) | 493 (67.8%) | 169 (69.8%) | 221 (69.5%) | 0.673 |
| Stent numbers | 2.0 (1.0−2.0) | 1.0 (1.0−2.0) | 2.0 (1.0−2.8)* | 2.0 (1.0−2.0) | 2.0 (1.0−2.0)* | 0.002 |
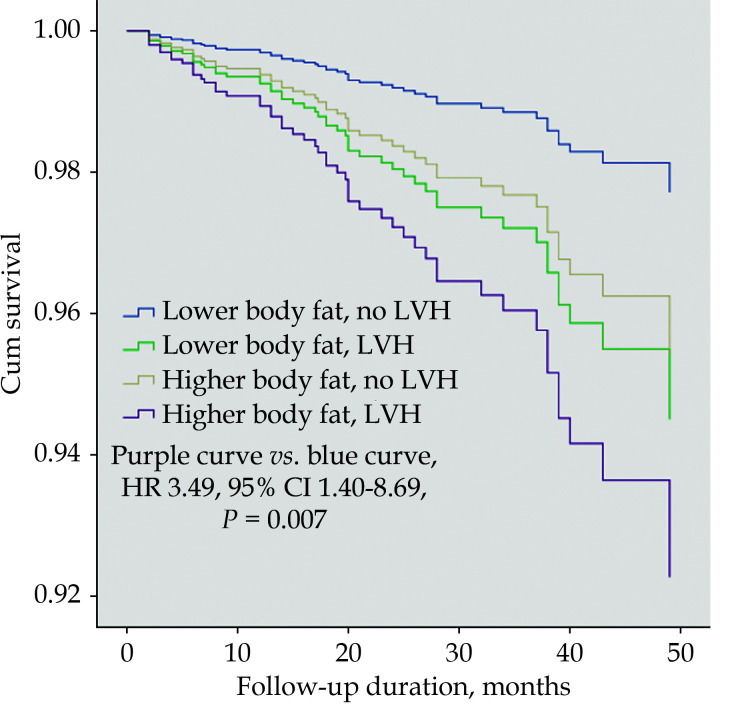
After a median follow-up of 2.2 (interquartile range 1.3 to 3.2) years, 120 (5.3%) patients had died. Compared with 2.5% (low body fat percentage and no LVH), 5.4% (high body fat percentage and no LVH), 7.8% (low body fat percentage and LVH), the cumulative event rate of all-cause death in the group of higher body fat and LVH was 8.2%, and the log-rank P value was < 0.001. Although patients with a higher body fat percentage and LVH accounted for only 14% of the total number of subjects in this study, deaths in this group accounted for 22% of the total population. Table 2 presents the results of the Cox proportional hazards model. The crude HR and 95% CI for all-cause death was 3.16 (95% CI: 1.81−5.50,P < 0.001) in patients with higher body fat and LVH compared with patients with lower body fat and no LVH. The results of the interaction test showed that there was a statistically significant interaction between body fat percentage and LVH ( P for interaction was 0.003). After adjusting for age, sex, cardiovascular risk factors, heart failure related parameters, cardiovascular metabolic indicators, angiographic lesions, number of stent implanted, cardiovascular secondary preventive drug treatment and other confounding factors, the results showed that compared with patients with a lower body fat percentage and no LVH, patients with a higher body fat percentage and LVH had the highest risk of all-cause death (HR = 3.49, 95% CI: 1.40−8.69, P = 0.007), while patients with a higher body fat percentage and no LVH had no statistically significant difference in risk of death compared with the control group (HR = 2.03, 95% CI: 0.70−5.92, P = 0.195) (Figure 2).
Table 2. Number of death events across groups and association of body fat, LVH and all-cause death with different adjustment model.
| Outcome, models | Outcome number and event rates by subgroups, HR (95%CI), P value | ||||
| Lower body fat, no LVH
(n = 956) |
Lower body fat, LVH
(n = 727) |
Higher body fat, no LVH
(n = 242) |
Higher body fat, LVH
(n = 318) |
P for interaction | |
| This table shows the unadjusted and multivariate analysis of relationship between body fat percentage, LVH, and all-cause death. After adjusting for age, sex, cardiovascular risk factors, heart failure related parameters, cardiovascular metabolic indicators, angiographic lesions, number of stent implanted, cardiovascular secondary preventive drug treatment and other confounding
factors, patients with a high body fat percentage had different prognoses stratified by LVH existence status. Compared with patients with a lower body fat percentage and no LVH, patients with a higher body fat rate and LVH had the highest risk of all-cause death (HR = 3.49; 95% CI: 1.40−8.69; P = 0.007), while patients with a higher body fat percentage but no LVH had no statistically significant difference in risk of death compared with the control group (HR = 2.03, 95% CI: 0.70−5.92; P= 0.195). There was statistically significant interaction between body fat percentage and LVH (P for interaction = 0.003). ACE: angiotensin converting enzyme; ARBs: angiotensin II receptor blockers; LVEF: left ventricular ejection fraction;LVH: left ventricular hypertrophy. | |||||
| All-cause death | 24 (2.5%) | 57 (7.8%) | 13 (5.4%) | 26 (8.2%) | |
| Unadjusted hazard ratio | Reference | 3.19 (1.98−5.15), P < 0.001 | 2.10 (1.07−4.13), P = 0.031 | 3.16 (1.81−5.50), P < 0.001 | 0.003 |
| Adjusted for age and sex | Reference | 3.04 (1.88−4.93), P < 0.001 | 1.57 (0.80−3.10), P = 0.191 | 2.44 (1.39−4.27), P = 0.002 | |
| Adjustd for age, sex, LVEF and serum creatinine | Reference | 2.08 (1.26−3.43), P = 0.004 | 1.56 (0.79−3.08), P = 0.198 | 1.86 (1.05−3.30), P = 0.033 | |
| Adjusted for age, sex, LVEF, serum creatinine, hypertension, diabetes, current smoker, systolic blood pressure, diastolic blood pressure, heart rate, triglyceride, glucose, hemoglobin, ACE inhibitors/ARBs, beta-blockers, left main of three-vessel disease, stent numbers | Reference | 2.45 (1.06−5.62), P = 0.035 | 2.03 (0.70−5.92), P = 0.195 | 3.49 (1.40−8.69), P = 0.007 | |
Figure 2.
Cumulative survival according to body fat percentage and presence of LVH.
The figure shows the survival curve according to the body fat percentage and whether it is combined with LVH. Compared with patients with a lower body fat percentage and no LVH, patients with a higher body fat percentage and LVH had the highest risk of death (adjusted HR = 3.49, 95% CI: 1.40-8.69, P= 0.007). LVH: left ventricular hypertrophy.
DISCUSSION
The primary findings of the present study are as follows: first, patients with higher body fat but without LVH have a similar risk of all-cause death when compared to those with lower body fat and no LVH; second, regardless of high or low level of body fat percentage, existence of LVH is associated with an increased risk of all-cause death; third, LVH modifies the relationship between body fat percentage and all-cause death. Among patients with CAD, those with higher body fat and LVH have the highest risk of all-cause death.
Individuals with excess body fat were exposed to greater cardiometabolic risks. However, in patients with CAD, the interrelationship between body fat and the presence of LVH for mortality risk is not well known. Our study demonstrated that patients with higher body fat and no LVH were more likely to suffer from death than their counterparts with lower body fat and no LVH; nevertheless, the possible negative association disappeared after adjusting for age and other confounders. Many previous studies have observed the obesity paradox in patients with cardiovascular disease. In a Chinese cohort of 8943 patients with angiographically confirmed triple-vessel disease, overweight and mild obesity (adjusted HR = 0.83, 95% CI: 0.69−1.00) were correlated with a lower odds of mortality with a median follow-up of over seven years.[17] In a prospective multicenter study, patients who had undergone percutaneous coronary intervention were divided into three groups according to the changes in BMI. During a follow-up of four years, patients with reduced BMI have a significantly higher risk of incident major adverse cardiac events than those with maintained or increased BMI, even after adjusting for confounders.[18] BMI fluctuation and decrease were also associated with cardiovascular death in patients with myocardial infarction and left ventricular dysfunction or heart failure.[19] The U-shaped relationship between obesity and cardiovascular outcomes in patients with CAD has been confirmed in meta-analyses.[20] Our results not only suggest the view that moderate body fat does not increase the risk of death in patients with CAD but also explore the interaction between body fat and LVH in more depth, indicating that LVH modifies the relationship between body fat and death. In patients with CAD and LVH, higher body fat percentage is associated with an increased risk of all-cause death, but this relationship does not hold for patients without LVH. Our research results suggest that the interpretation of the results of the obesity paradox needs to be considered separately according to the changes in the left ventricular configuration. Once the patient has a change in the heart configuration, the obesity paradox may no longer exist.
LVH can manifest as cardiomyocyte hypertrophy, heart chamber dilation, or a combination of the two. A wealth of supporting evidence has confirmed that LVH is associated with adverse outcomes in different populations.[21] The mechanical stretch stress caused by changes in hemodynamics or the response to neurohormonal regulation, such as catecholamines, angiotensin II and growth factors, interact in a complex manner, which together lead to left ventricular remodeling and myocardial hypertrophy.[22] Subjects with obesity or higher BMI are more prone to have an increased left ventricular mass. In addition, it has been demonstrated that peri-coronary adipose tissue attenuation was independently correlated with LVMI.[23] Previous studies have shown that when patients with metabolic syndrome have LVH, their risk of fatal or nonfatal cardiovascular events is higher than that of patients without LVH. The findings indicated that LVH largely mediates the cardiovascular risk associated with metabolic syndrome.[24] In addition, other studies have shown that the relationship between metabolic syndrome and LVH is mainly mediated by the body’s fat content.[25] Our results show that depending on whether LVH is coexistence or not, the relationship between body fat percentage and all-cause death in patients with CAD is different. Although there was no significant statistical relationship between a higher body fat percentage and all-cause death in patients without LVH, patients with both LVH and a higher body fat percentage had the highest risk of death. This result shows that body fat, especially visceral fat, at least partly influences the patient’s prognosis by affecting the configuration of the left ventricle.
Although LVH is one of the strongest prognostic factors in patients with CAD and has an equivalent effect as LVEF, effective therapies targeting the regression of LVH are numbered and have yielded limited effects.[26] Considering that hemodynamics or the regulation of neurohormones or inflammatory factors in the body will affect the left ventricular configuration, the treatment of obese patients with CAD whose left ventricular is hypertrophic should be a composite therapeutic strategy, with the target of regression of LVH.
Understanding the relationship of obesity, LVH, and death is very important for the long-term management of patients with CAD. Our results show that the relationship between obesity and death is different when the association was stratified by LVH status. This result is not difficult to understand. First, the BMI of patients with higher body fat in our study was approximately 27−28, which is intermediate between the traditional definition of overweight and obesity; however, the body fat range may be ideal in patients with CAD when considering age, ethnicity and the presence of established disease. With many biological benefits, appropriate increases in weight and body fat are essential in aging and disease states.[27] Second, a higher body fat percentage is associated with the highest risk of death in patients who had LVH, while no association was observed among those did not, suggesting that in patients with CAD and increased body fat, LVH modifies the risk of death. This is because the coexistence of LVH suggests that the superposition of multiple mechanisms, such as disorders of water and sodium metabolism in these patients with obesity, together with the regulation dysfunction of the pre- and afterload of the heart, neurohormones, and the balance of metabolic state, have led to maladaptation of cardiac remodeling.[10] Our results suggest that, as a modifiable risk factor for chronic diseases, it is necessary to strengthen the management of obese patients with LVH to avoid the progression from LVH to abnormal left ventricular function and adverse clinical outcomes.
LIMITATIONS
First of all, body fat distribution was unavailable in the present study. Visceral adipose tissue (VAT) may have a superior predictive effect for progression of CAD than subcutaneous adipose tissue, while the assessment of demands more advanced equipment such as computerized tomography scan.[28] Secondly, body fat value in the study was calculated by formulas but not measured directly by dual-energy X-ray measurements or bioelectrical impedance techniques. Thirdly, because there was lack of generally acknowledged cutoff values for define obesity using body fat value, we used 75 quantile arbitrarily to divided investigated subjects into different groups. However, the optimal value of body fat may be varied on the basis of age, sex, and energy demand. Lastly, we acknowledge that the primary effects of body fat or obesity and LVH on patients with CAD are related to abnormal cardiovascular metabolism. Focusing on the causes of death may help us understand the mechanism of the combined effect of body fat and LVH on prognosis. However, it is worth noting that the interaction effects of obesity and LVH occur through multiple pathways, such as hemodynamics, inflammation, and metabolism. Therefore, these two risk factors are related not only to cardiovascular prognosis but also to non-cardiovascular outcomes. Due to the complex relationship between obesity, LVH, CAD and prognosis, there is a competitive risk relationship between the different causes of death (events) of these patients; that is, when analyzing the time of an event, another event may occur before it and may hinder the occurrence of the former, which may result in the occurrence of the former not being observed. For this reason and the limitations of our follow-up conditions, we chose the hard endpoint of all-cause death as the observation endpoint to more reliably illustrate the combined effect of obesity and LVH on the clinical prognosis of patients with CAD. In addition, improving the overall prognosis of patients and extending the life span of patients with CAD is also the ultimate goal of our study series.
CONCLUSIONS
Depending on whether LVH was present, a higher body fat percentage was associated with a different risk of all-cause death in patients with CAD. Higher body fat was significantly associated with a greater risk of mortality among patients with LVH but not those without LVH.
AUTHORS’ CONTRIBUTIONS
HBT and YL designed the study, collected the data, and drafted the article. YBS designed the study, analyzed the data, and drafted the article. HFY, XQF and PXB collected the data and revised the article. PY and CM designed the study, drafted the article, and revised it. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
Dr Huang reported a grant from the Key Research and Development Projects of Science & Technology Department of Sichuan Province (2019YFS0351).
References
- 1.Jaacks LM, Vandevijvere S, Pan A, et al The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 2019;7:231–240. doi: 10.1016/S2213-8587(19)30026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Writing Committee of the Report on Cardiovascular Health and Diseases in China Report on cardiovascular health and diseases in China 2019: an Updated Summary. Chinese Circulation Journal. 2020;35:833–854. [Google Scholar]
- 3.Mandviwala T, Khalid U, Deswal A Obesity and Cardiovascular Disease: a Risk Factor or a Risk Marker? Curr Atheroscler Rep. 2016;18:21. doi: 10.1007/s11883-016-0575-4. [DOI] [PubMed] [Google Scholar]
- 4.Cuspidi C, Rescaldani M, Sala C, Grassi G Left-ventricular hypertrophy and obesity: a systematic review and meta-analysis of echocardiographic studies. J Hypertens. 2014;32:16–25. doi: 10.1097/HJH.0b013e328364fb58. [DOI] [PubMed] [Google Scholar]
- 5.Eskerud I, Gerdts E, Larsen TH, Lønnebakken MT Left ventricular hypertrophy contributes to Myocardial Ischemia in Non-obstructive Coronary Artery Disease (the MicroCAD study) Int J Cardiol. 2019;286:1–6. doi: 10.1016/j.ijcard.2019.03.059. [DOI] [PubMed] [Google Scholar]
- 6.Rao VN, Fudim M, Mentz RJ, et al Regional adiposity and heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22:1540–1550. doi: 10.1002/ejhf.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khafagy R, Dash S Obesity and cardiovascular disease: The emerging role of inflammation. Front Cardiovasc Med. 2021;8:768119. doi: 10.3389/fcvm.2021.768119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katta N, Loethen T, Lavie CJ, Alpert MA Obesity and coronary heart disease: epidemiology, pathology, and coronary artery imaging. Curr Probl Cardiol. 2021;46:100655. doi: 10.1016/j.cpcardiol.2020.100655. [DOI] [PubMed] [Google Scholar]
- 9.Csige I, Ujvárosy D, Szabó Z The Impact of Obesity on the Cardiovascular System. J Diabetes Res. 2018;2018:3407306. doi: 10.1155/2018/3407306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.daSilva-deAbreu A, Alhafez BA, Lavie CJ, et al Interactions of hypertension, obesity, left ventricular hypertrophy, and heart failure. Curr Opin Cardiol. 2021;36:453–460. doi: 10.1097/HCO.0000000000000868. [DOI] [PubMed] [Google Scholar]
- 11.Aslam MI, Hahn VS, Jani V, et al Reduced right ventricular sarcomere contractility in heart failure with preserved ejection fraction and severe obesity. Circulation. 2021;143:965–967. doi: 10.1161/CIRCULATIONAHA.120.052414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos JL, Salemi VM, Picard MH, et al Subclinical regional left ventricular dysfunction in obese patients with and without hypertension or hypertrophy. Obesity (Silver Spring) 2011;19:1296–1303. doi: 10.1038/oby.2010.253. [DOI] [PubMed] [Google Scholar]
- 13.Gómez-Ambrosi J, Silva C, Catalán V, et al Clinical usefulness of a new equation for estimating body fat. Diabetes Care. 2012;35:383–388. doi: 10.2337/dc11-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong Y, Wangsheng F, Wang S, et al Positive association between body fat percentage and hyperuricemia in patients with hypertension: The China H-type hypertension registry study. Nutr Metab Cardiovasc Dis. 2021;31:3076–3084. doi: 10.1016/j.numecd.2021.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Guo X, Ding Q, Liang M Evaluation of eight anthropometric indices for identification of metabolic syndrome in adults with diabetes. Diabetes Metab Syndr Obes. 2021;14:1431–1443. doi: 10.2147/DMSO.S294244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, et al Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Feng X, Zhang C, Jiang L, et al Body mass index and mortality in patients with severe coronary artery diseases: A cohort study from China. Nutr Metab Cardiovasc Dis. 2021;31:448–454. doi: 10.1016/j.numecd.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Yui H, Ebisawa S, Miura T, et al Impact of changes in body mass index after percutaneous coronary intervention on long-term outcomes in patients with coronary artery disease. Heart Vessels. 2020;35:1657–1663. doi: 10.1007/s00380-020-01648-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stienen S, Ferreira JP, Girerd N, et al Mean BMI, visit-to-visit BMI variability and BMI changes during follow-up in patients with acute myocardial infarction with systolic dysfunction and/or heart failure: insights from the High-Risk Myocardial Infarction Initiative. Clin Res Cardiol. 2019;108:1215–1225. doi: 10.1007/s00392-019-01453-7. [DOI] [PubMed] [Google Scholar]
- 20.Dwivedi AK, Dubey P, Cistola DP, Reddy SY Association between obesity and cardiovascular outcomes: updated evidence from meta-analysis studies. Curr Cardiol Rep. 2020;22:25. doi: 10.1007/s11886-020-1273-y. [DOI] [PubMed] [Google Scholar]
- 21.Miller RJH, Mikami Y, Heydari B, et al Sex-specific relationships between patterns of ventricular remodelling and clinical outcomes. Eur Heart J Cardiovasc Imaging. 2020;21:983–990. doi: 10.1093/ehjci/jeaa164. [DOI] [PubMed] [Google Scholar]
- 22.Yildiz M, Oktay AA, Stewart MH, et al Left ventricular hypertrophy and hypertension. Prog Cardiovasc Dis. 2020;63:10–21. doi: 10.1016/j.pcad.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Hirano H, Kanaji Y, Sugiyama T, et al Impact of pericoronary adipose tissue inflammation on left ventricular hypertrophy and regional physiological indices in stable coronary artery disease patients with preserved systolic function. Heart Vessels. 2021;36:24–37. doi: 10.1007/s00380-020-01658-1. [DOI] [PubMed] [Google Scholar]
- 24.de Simone G, Devereux RB, Chinali M, et al Metabolic syndrome and left ventricular hypertrophy in the prediction of cardiovascular events: the Strong Heart Study. Nutr Metab Cardiovasc Dis. 2009;19:98–104. doi: 10.1016/j.numecd.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerra F, Mancinelli L, Angelini L, et al The association of left ventricular hypertrophy with metabolic syndrome is dependent on body mass index in hypertensive overweight or obese patients. PLoS One. 2011;6:e16630. doi: 10.1371/journal.pone.0016630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohan M, Al-Talabany S, McKinnie A, et al A randomized controlled trial of metformin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: the MET-REMODEL trial. Eur Heart J. 2019;40:3409–3417. doi: 10.1093/eurheartj/ehz203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dixon JB, Egger GJ, Finkelstein EA, et al ‘Obesity paradox’ misunderstands the biology of optimal weight throughout the life cycle. Int J Obes (Lond) 2015;39:82–84. doi: 10.1038/ijo.2014.59. [DOI] [PubMed] [Google Scholar]
- 28.Lee H, Park HE, Yoon JW, Choi SY Clinical significance of body fat distribution in coronary artery calcification progression in Korean population. Diabetes Metab J. 2021;45:219–230. doi: 10.4093/dmj.2019.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]