Abstract
Objective:
We investigated real-world vaccine effectiveness for Oxford-AstraZeneca (ChAdOx1) and CoronaVac against laboratory-confirmed severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infection among healthcare workers (HCWs).
Methods:
We conducted a retrospective cohort study among HCWs (aged ≥18 years) working in a private healthcare system in Brazil between January 1, 2021 and August 3, 2021, to assess vaccine effectiveness. We calculated vaccine effectiveness as 1 − rate ratio (RR), with RR determined by adjusting Poisson models with the occurrence of SARS-CoV-2 infection as the outcome and the vaccination status as the main variable. We used the logarithmic link function and simple models adjusting for sex, age, and job types.
Results:
In total, 13,813 HCWs met the inclusion criteria for this analysis. Among them, 6,385 (46.2%) received the CoronaVac vaccine, 5,916 (42.8%) received the ChAdOx1 vaccine, and 1,512 (11.0%) were not vaccinated. Overall, COVID-19 occurred in 6% of unvaccinated HCWs, 3% of HCWs who received 2 doses of CoronaVac vaccine, and 0.7% of HCWs who received 2 doses of ChAdOx1 vaccine (P < .001). In the adjusted analyses, the estimated vaccine effectiveness rates were 51.3% for CoronaVac, and 88.1% for ChAdOx1 vaccine. Both vaccines reduced the number of hospitalizations, the length of hospital stay, and the need for mechanical ventilation. In addition, 19 SARS-CoV-2 samples from 19 HCWs were screened for mutations of interest. Of 19 samples, 18 were the γ (gamma) variant.
Conclusions:
Although both COVID-19 vaccines (viral vector and inactivated virus) can significantly prevent COVID-19 among HCWs, CoronaVac was much less effective. The COVID-19 vaccines were also effective against the dominant γ variant.
Healthcare workers (HCWs) are at risk of coronavirus disease 2019 (COVID-19) due to high levels of exposure. When compared to the general population, frontline HCWs have >10 times the risk of testing positive for severe acute respiratory coronavirus virus 2 (SARS-CoV-2), and those reporting inadequate access to personal protective equipment (PPE) have a 23% higher risk. 1,2 In addition, when compared to HCWs reporting adequate access to PPE and who were not caring for patients with COVID-19, HCWs caring for patients with documented COVID-19 had a nearly 5-times higher risk of testing positive if they had adequate access to PPE and a nearly 6-times higher risk if they had inadequate access to PPE. 2 These reports emphasize the need for effective vaccines, especially among frontline HCWs.
Over the last few months, multiple studies have yielded a large amount of data from different institutions that provided real-world data on short-term vaccine effectiveness. 3,4 The great majority of these studies examined COVID-19 mRNA vaccines that significantly prevented symptomatic and asymptomatic severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infection among HCWs. 5 However, data regarding the vaccine effectiveness of other COVID-19 vaccines (eg, viral vector or inactivated virus) are limited.
We investigated real-world vaccine effectiveness for Oxford-AstraZeneca (ChAdOx1), and CoronaVac against laboratory-confirmed SARS-CoV-2 infection among HCWs.
Methods
Population and setting
This retrospective cohort study was conducted between January 1, 2021, and August 3, 2021, in Brazil. We included all adult HCWs (aged ≥18 years) working at the Hospital Israelita Albert Einstein (HIAE). The HIAE is a Brazilian, nonprofit, healthcare, education, and research organization, with its headquarters in the city of São Paulo. HIAE manages a diverse healthcare system, including primary healthcare to tertiary-care services, in the public and private healthcare sectors. This hospital operates 40 healthcare units, mainly in the state of São Paulo, and in 2020 it had ∼700,000 emergency department visits, 900,000 outpatient visits, and 70,000 hospital discharges overall. Since the beginning of the COVID-19 pandemic, HCWs with COVID-19 symptoms had access to free-of-charge SARS-CoV-2 RT-PCR testing conducted by the institution’s laboratory. Vaccination with 2 doses of CoronaVac vaccine, 21 days apart, and with 2 doses of the ChAdOx1 vaccine, 12 weeks apart, were evaluated for COVID-19 vaccine effectiveness. HCWs were considered unvaccinated if no COVID-19 vaccine doses were received. Individuals who tested positive for SARS-COV-2 prior to the first vaccine dose, between vaccine doses, or before 14 days after the second vaccine dose, and individuals who had been vaccinated before the study period, were excluded from the study. We also excluded HCWs who received the Pfizer COVID-19 vaccine because the sample size (∼130 HCWs) was too small to obtain an estimate of vaccine effectiveness (Supplementary Appendix 1 online).
Real-time polymerase chain reaction (RT-PCR) methodologies for SARS-CoV-2 detection
The diagnostic confirmation of COVID-19 was performed using RT-PCR on specimens obtained via nasopharyngeal swab, according to the protocol instituted at the hospital. The following RT-PCR kits were utilized: XGEN MASTER COVID-19 (Mobius, Pinhais, Paraná, Brazil); cobas SARS-CoV-2 Test (Roche Molecular Systems, Branchburg, NJ); Xpert Xpress SARS-CoV-2 (Cepheid, Sunnyvale, CA); and Abbott RealTime SARS-C0V-2 (Abbott Molecular, Des Plaines, IL).
Next-generation sequencing of viral full-length genome
We extracted total nucleic acid from the naso-oropharyngeal (NOP) swab samples with QIAamp Viral RNA Mini kit (QIAGEN, Hilden, Germany). After purification and concentration, DNAse I treatment, and depletion of human ribosomal RNA, the samples were subjected to random amplification. 6 The preparation of sequencing libraries for the Illumina platform was carried out with DNA Prep (Illumina, San Diego, CA) using the random 2-step PCR amplification product as input. The libraries were quantified with the Qubit instrument (Thermo Fisher Scientific, Waltham, MA) and were loaded on the NextSeq 550 equipment (Illumina) for sequencing with MID 300 paired-end reads (Illumina).
Outcome measures and statistical analyses
Laboratory-confirmed COVID-19 was considered the primary outcome to calculate vaccination effectiveness after 2 doses of a COVID-19 vaccine (CoronaVac or ChAdOx1). RT-PCR testing for the diagnosis of COVID-19 was performed only on symptomatic HCWs. Hospitalization related to COVID-19, length of stay, ICU admission, necessity of mechanical ventilation and death were secondary outcomes. Vaccination status and SARS-CoV-2 RT-PCR results of all study participants were obtained from institutional electronic records. We excluded those with a positive COVID-19 diagnosis before January 15, 2021, because it corresponded to data from the first vaccine (January 1, 2021) plus 14 days. For those vaccinated, the initial follow-up date was 14 days after the second vaccine dose. The last date was defined as the date COVID-19 was diagnosed, or up to August 3, 2021, for the censored cases without a positive diagnosis of COVID-19.
The qualitative variables were characterized using absolute and relative frequencies in general and by interest groups; for comparisons, we used the χ 2 or the Fisher exact test. The quantitative variables have been reported as medians, interquartile range (IQR, first and third quartiles), minimum and maximum values due to the asymmetry observed in the variables, 7 and comparisons were performed via nonparametric Kruskal-Wallis tests. Vaccine effectiveness was calculated as 1 − rate ratio (RR), 8 with RR obtained by adjusting Poisson models with SARS-CoV-2 infection confirmed by RT-PCR as the outcome and vaccination status as the main exploratory variable, in addition to 95% confidence intervals. We used the logarithmic link function to estimate unadjusted models and models adjusted for sex, age, and HCW job type (ie, direct patient contact vs no direct patient contact). The cumulative incidence curves of COVID-19 for the vaccinated and unvaccinated groups were estimated using the Kaplan-Meier method 9 and the cumulative incidence estimated at 30 and 90 days with unadjusted models. All analyses were performed with the R software environment for statistical computing and graphics version 4.1.0. 10 All reported tests were 2-sided, and P < .05 was considered significant. The study was approved by the Hospital Israelita Albert Einstein Ethics Committee (no. CAAE 47110421.7.0000.0071), which waived the need for informed consent.
Results
By the end of the study period, 18,359 individuals were screened for eligibility to evaluate the COVID-19 vaccination effectiveness after the second vaccine dose. Overall, 13,813 HCWs met inclusion criteria (Supplementary Appendix 1 online). Among this cohort, 6,385 (46.2%) received the CoronaVac vaccine, 5,916 (42.8%) received the ChAdOx1 vaccine, and 1,512 (11.0%) were unvaccinated. Most were female (71.0%), and the median age of the entire study population was 35 years. Unvaccinated workers were younger, and women more frequently received 2 doses of CoronaVac (Table 1). The proportions of HCWs with direct patient contact were ∼20% among the unvaccinated, 80% among those with CorovaVac, and ∼20% among those with ChAdOx1. Of 13,813 HCWs, past medical history was available for 10,786 HCWs (78.1%). Among them, 2,783 (25.8%) had at least 1 comorbidity: obesity (n = 1,009, 9.4%), hypertension (n = 843, 7.8%), dyslipidemia (n = 592, 5.5%), asthma (n = 478, 4.4%), and diabetes mellitus (n = 250, 2.3%).
Table 1.
Baseline Characteristics of Study Participants, Hospital Israelita Albert Einstein, São Paulo, Brazil, from January 1, 2021, to August 3, 2021
| Charaacterstics | Unvaccinated (n = 1,512) |
2 Doses of CoronaVac Vaccine (n = 6,385) |
2 Doses of ChAdOx1 Vaccine (n = 5,916) |
Total (13,813) |
P
Value |
|---|---|---|---|---|---|
| Sex, no. (%) | <.0001 a | ||||
| Female | 1,043 (69.2) | 4,672 (73.2) | 4,090 (69.1) | 9,805 (71.0) | |
| Male | 465 (30.8) | 1,710 (26.8) | 1,826 (30.9) | 4,001 (29.0) | |
| Missing | 4 (0.26) | 3 (0.05) | 0 (0) | 7 (0.05) | |
| Age, median y | <.0001 b | ||||
| Median (IQR) | 32 (26–38) | 36 (30–42) | 35 (28–42) | 35 (29–42) | |
| Minimum–Maximum | 18–84 | 18–82 | 18–83 | 18–84 | |
| Job type | <.0001 a | ||||
| No direct patient contact | 1,174 (77.6) | 1,333 (20.9) | 4,603 (77.8) | 7,110 (51.5) | |
| Direct patient facing | 338 (22.4) | 5,052 (79.1) | 1,313 (22.2) | 6,703 (48.5) | |
| Comorbidity c | <.0001 a | ||||
| No | 752 (79.7) | 3,568 (75.1) | 3,683 (72.3) | 8,003 (74.2) | |
| Yes | 191 (20.3) | 1,181 (24.9) | 1,411 (27.7) | 2,783 (25.8) | |
| Hypertension c | <.0001 a | ||||
| No | 901 (95.5) | 4,391 (92.5) | 4,651 (91.3) | 9,943 (92.2) | |
| Yes | 42 (4.5) | 358 (7.5) | 443 (8.7) | 843 (7.8) | |
| Diabetes mellitus c | .0856 a | ||||
| No | 929 (98.5) | 4,645 (97.8) | 4,962 (97.4) | 10,536 (97.7) | |
| Yes | 14 (1.5) | 104 (2.2) | 132 (2.6) | 250 (2.3) | |
| Obesity c | .0102 a | ||||
| No | 871 (92.4) | 4,331 (91.2) | 4,575 (89.8) | 9,777 (90.6) | |
| Yes | 72 (7.6) | 418 (8.8) | 519 (10.2) | 1,009 (9.4) | |
| Dyslipidemia c | .0126 a | ||||
| No | 911 (96.6) | 4,479 (94.3) | 4,804 (94.3) | 10,194 (94.5) | |
| Yes | 32 (3.4) | 270 (5.7) | 290 (5.7) | 592 (5.5) | |
| Asthma c | .0984 a | ||||
| No | 908 (96.3) | 4,554 (95.9) | 4,846 (95.1) | 10,308 (95.6) | |
| Yes | 35 (3.7) | 195 (4.1) | 248 (4.9) | 478 (4.4) | |
| Follow-up between COVID-19 vaccine doses, d | <.0001 d | ||||
| Median (IQR) | … | 25 (22–27) | 84 (80–88) | 35 (25–84) | |
| Minimum–Maximum | … | 15–172 | 50–164 | 15–172 | |
| Follow-up period, d e | <.0001 b | ||||
| Median (IQR) | 214 (214–214) | 151 (144–154) | 78 (73–83) | 138 (78–153) | |
| Minimum–Maximum | 17–214 | 1–176 | 1–118 | 1–214 | |
| SARS-COV-2 infection (by PCR) | <.0001 a | ||||
| No | 1,421 (94.0) | 6,194 (97.0) | 5,873 (99.3) | 13,488 (97.6) | |
| Yes | 91 (6.0) | 191 (3.0) | 43 (0.7) | 325 (2.4) | |
| No. of hospitalizations | .0048 f | ||||
| 0 | 1,501 (99.3) | 6,371 (99.8) | 5,904 (99.8) | 13,776 (99.7) | |
| 1 | 11 (0.7) | 14 (0.2) | 11 (0.2) | 36 (0.3) | |
| 2 | 0 (0.0) | 0 (0.0) | 1 (0.0) | 1 (0.0) | |
| Length of hospital stay, d | .0154 b | ||||
| Median (IQR) | 10 (7–21) | 4 (3–6) | 6 (3–9) | 6 (3–10) | |
| Minimum–Maximum | 1–40 | 1–7 | 2–20 | 1–40 | |
| ICU | .3392 f | ||||
| No | 6 (54.5) | 11 (78.6) | 10 (83.3) | 27 (73.0) | |
| Yes | 5 (45.5) | 3 (21.4) | 2 (16.7) | 10 (27.0) | |
| Mechanical ventilation | .0050 f | ||||
| No | 7 (63.6) | 14 (100.0) | 12 (100.0) | 33 (89.2) | |
| Yes | 4 (36.4) | 0 (0.0) | 0 (0.0) | 4 (10.8) | |
Note. ChAdOx1 vaccine, Oxford-AstraZeneca vaccine; IQR, interquartile range; ICU, intensive care unit.
χ 2 test.
Kruskal-Wallis test.
Information available for 10,786 participants, 943 unvaccinated, 4,749 with 2 doses of CoronaVac vaccine and 5,094 with 2 doses of ChAdOx1 vaccine.
Mann-Whitney test.
Follow-up was initiated 15 days after the second dose for those vaccinated.
Fisher exact test.
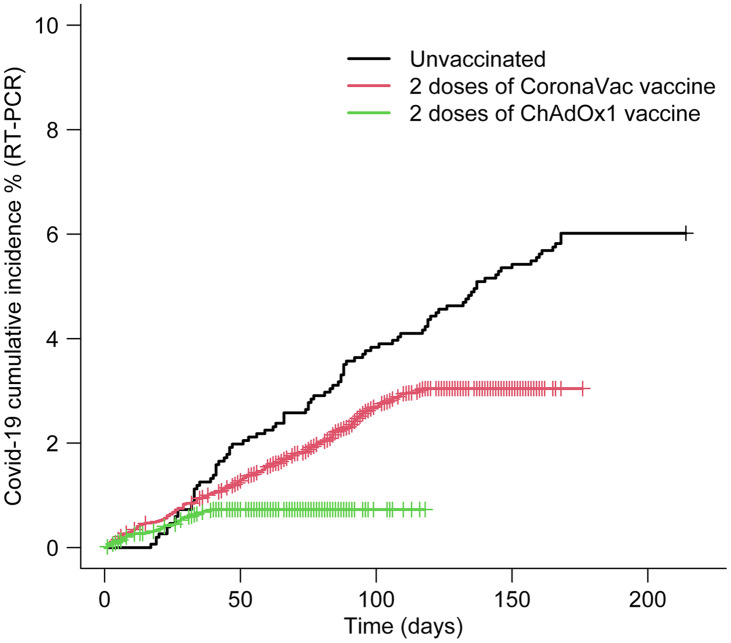
During the study period, 325 HCWs (2.4%) were diagnosed with COVID-19. For the unvaccinated HCWs, the cumulative incidences of COVID-19 were 0.73% at 30 days of follow-up and 3.57% at 90 days of follow-up. The cumulative incidences were 0.85% at 30 days and 2.32% at 90 days for those vaccinated with the CoronaVac vaccine. The cumulative incidences were 0.58% in 30 days and 0.73% in 90 days for those vaccinated with the ChAdOx1 vaccine (Fig. 1). None of the HCWs (vaccinated or unvaccinated) died during the study period.
Fig. 1.
Cumulative incidence of COVID-19 infection (by RT-PCR) among vaccinated (2 doses of CoronaVac vaccine, and 2 doses of ChAdOx1 [Oxford-AstraZeneca] vaccine) and unvaccinated healthcare workers.
The estimated vaccine effectiveness for CoronaVac after 2 doses was 50.3% (95% CI, 36.2%–61.3%), and the vaccine effectiveness for ChAdOx1 vaccine after 2 doses was 87.9% (95% CI, 82.6%–91.6%). After controlling for sex, age, and professional category, the estimated vaccine effectiveness rates were 51.3% (95% CI, 34.6%–63.7%) for CoronaVac after 2 doses and 88.1% (95% CI, 82.8%–91.7%) for ChAdOx1 vaccine after 2 doses (Table 2).
Table 2.
Observed Rate Ratios and Vaccine Effectiveness Among Healthcare Workers after COVID-19 Vaccine Second Dose and COVID-19 Infection by RT-PCR, Hospital Israelita Albert Einstein, São Paulo, Brazil, from January 1, 2021, to August 3, 2021
| Variable | RR (95% CI) | P Value | Vaccine Effectiveness (95% CI) |
|---|---|---|---|
| COVID-19 infection | |||
| Unvaccinated | 1.0 (Reference) | ||
| CoronaVac | 0.497 (0.387–0.638) | <.001 | 50.3% (36.2%–61.3%) |
| ChAdOx1 | 0.121 (0.084–0.174) | <.001 | 87.9% (82.6%–91.6%) |
| COVID-19 infection adjusted for covariates | |||
| Unvaccinated | 1.0 (Reference) | ||
| CoronaVac | 0.487 (0.363–0.654) | <.001 | 51.3% (34.6%–63.7%) |
| ChAdOx1 | 0.119 (0.083–0.172) | <.001 | 88.1% (82.8%–91.7%) |
| Sex, male | 0.859 (0.669–1.105) | .237 | |
| Age, y | 0.996 (0.984–1.008) | .540 | |
| HCW job type (direct patient exposure) | 1.020 (0.782–1.331) | .885 | |
Note. RT-PCR, real-time polymerase chain reaction; RR, rate ratio; CI, confidence interval; ChAdOx1, Oxford-AstraZeneca vaccine; HCW, healthcare worker.
Whole-genome sequencing analysis
From March to June 2021, 19 SARS-CoV-2 samples from 19 HCWs were screened for mutations of interest. Of those, 18 were the P1 strain (γ variant), and 1 B.1.1.7 strain (ie, the α [alpha] variant) was identified (Table 3).
Table 3.
Characteristics of Participants With SARS-CoV-2 Variants of Concern (n=19), Hospital Israelita Albert Einstein, São Paulo, Brazil, from January 1, 2021, to August 3, 2021 a
| SARS-CoV-2 Variant of Concern |
Date | Age, y | Sex | Job Type | COVID-19 Vaccine |
Hospitalization | ICU | Mechanical Ventilation | Death |
|---|---|---|---|---|---|---|---|---|---|
| P1 | 3/20/2021 | 37 | Female | DPF | CoronaVac | No | … | … | No |
| P1 | 3/21/2021 | 33 | Female | DPF | CoronaVac | No | … | … | No |
| B.1.1.7 | 3/25/2021 | 26 | Female | DPF | CoronaVac | No | … | … | No |
| P1 | 3/25/2021 | 46 | Male | DPF | CoronaVac | No | … | … | No |
| P1 | 3/26/2021 | 30 | Female | DPF | CoronaVac | No | … | … | No |
| P1 | 4/5/2021 | 48 | Female | DPF | CoronaVac | No | … | … | No |
| P1 | 4/26/2021 | 43 | Female | DPF | CoronaVac | No | … | … | No |
| P1 | 4/27/2021 | 42 | Female | DPF | CoronaVac | No | … | … | No |
| P1 | 5/4/2021 | 42 | Female | NDPC | CoronaVac | No | … | … | No |
| P1 | 5/10/2021 | 35 | Female | DPF | CoronaVac | No | … | … | No |
| P1 | 5/12/2021 | 51 | Female | DPF | CoronaVac | No | … | … | No |
| P1 | 5/17/2021 | 39 | Male | DPF | CoronaVac | Yes | No | No | No |
| P1 | 5/31/2021 | 37 | Female | NDPC | ChAdOx1 | No | … | … | No |
| P1 | 6/2/2021 | 42 | Female | DPF | CoronaVac | No | … | … | No |
| P1 | 6/3/2021 | 37 | Female | DPF | CoronaVac | No | … | … | No |
| P1 | 6/4/2021 | 44 | Female | DPF | CoronaVac | No | … | … | No |
| P1 | 6/4/2021 | 31 | Female | DPF | CoronaVac | No | … | … | No |
| P1 | 6/5/2021 | 44 | Female | DPF | ChAdOx1 | No | … | … | No |
| P1 | 6/7/2021 | 27 | Female | DPF | CoronaVac | No | … | … | No |
Note. P1, γ (gamma) variant; B.1.1.7, α variant; ChAdOx1, Oxford-AstraZeneca vaccine; DPC, direct patient facing; NDPC, no direct patient contact.
The whole-genome sequencing was performed from March to June 20
Discussion
This retrospective study revealed that the estimated vaccination effectiveness among HCWs against symptomatic COVID-19 were 51.3% for CoronaVac and 88.1% for AstraZeneca after adjusting for age, sex, and job type. Both a viral vector vaccine (ChAdOx1) and an inactivated viral vaccine (CoronaVac) reduced the number of COVID-19 cases, the number of hospitalizations, the length of hospital stay, and the need for mechanical ventilation. These vaccines were even effective against a new variant of concern in Brazil, the γ (gamma) variant.
Based on a recent published systematic literature review evaluating short-term vaccination effectiveness between December 2020 and April 2021, COVID-19 vaccines (primarily the mRNA vaccines) decrease symptomatic COVID-19 infection with vaccine effectiveness of 92.8%. 5 Our study showed that the estimated vaccine effectiveness rates after 2 doses of CoronaVac and ChAdOx1 among HCWs were lower than the vaccine effectiveness rates of mRNA COVID-19 vaccines among the general population reported in the randomized trials 11,12 and also in a noncontrolled setting. 3 A randomized clinical trial evaluating vaccine effectiveness of CoronaVac among HCWs in Brazil reported vaccine effectiveness after 2 doses of 50.7%. 13 Another randomized clinical trial of CoronaVac in Turkey reported an estimated vaccine effectiveness after 2 doses of 83.5%. 14 Both clinical trials were conducted in 2020, prior to the emergence of the variants of concern. More recent observational studies evaluating the vaccine effectiveness of the CoronaVac vaccine did not include genomic surveillance for SARS-CoV-2 virus but reported the circulation of at least 2 viral lineages considered to be variants of concern: B.1.1.7 (α variant) 15 and P.1 (γ variant). 15,16 Results from these studies after 2 doses, in a prospective national cohort study from Chile, demonstrated that the estimated vaccine effectiveness of CoronaVac in a general population was 65.9%. 15 Results from a test-negative case–control study of the vaccine effectiveness of CoronaVac vaccine among HCWs in Manaus, Brazil, where the γ variant was also predominant, showed that the estimated vaccine effectiveness after 2 doses was low (36.8%) against COVID-19. 16 In terms of the ChAdOx1 vaccine, a randomized clinical trial conducted in Brazil, South Africa, and the United Kingdom showed that the vaccine effectiveness of ChAdOx1 after 2 doses was 62% against COVID-19. 17 Results from a test-negative case–control study evaluating the vaccination effectiveness of ChAdOx1 vaccine after 2 doses for the B.1.1.7 (α variant) and the δ (delta) variant were 74.5% and 67.5%, respectively. 18 Although it is not clear why the vaccine effectiveness rates in our present study were higher compared to vaccine effectiveness rates in previously published studies. 17,18 Possible explanations are the strict infection control policies in our institution and adequate PPE throughout the pandemic while many other institutions suffered critical shortages of PPE. 1,2 Although a peak of COVID-19 was observed in March–June 2021, the community incidence of COVID-19 was relatively stable and the γ (gamma) variant was dominant during the study period.
ChAdOx1 (Oxford-AstraZeneca) and CoronaVac were the first COVID-19 vaccines authorized by the Brazilian Heath Surveillance Agency, 19 and HCWs were considered the priority group to receive them as of January 2021. 20 We observed that HCWs who received 2 doses of CoronaVac were more likely to provide direct patient care in comparison to HCWs that received 2 doses of the ChAdOx1 vaccine. This difference can be explained by the CoronaVac vaccine being the first available COVID-19 vaccine in our institution, and for that reason, the frontline HCWs were prioritized to receive the COVID-19 vaccine. Later in the pandemic, our institution started using ChAdOx1 vaccine, which was mainly given to nonclinical persons. The duration of our study (8 months) among HCWs is justified, particularly to understand the short-term vaccination effectiveness in the context of a global pandemic with a novel pathogen. 21 We collected data during a rapid vaccination campaign during a period with one of the highest community transmission rates of the pandemic, which allowed for a relatively short follow-up period and the estimation of the prevention of COVID-19 cases, related hospitalization, necessity of mechanical ventilation, and ICU stay. Both CoronaVac and ChAdOx1 vaccines were effective at preventing COVID-19 and serious illness (hospitalizations, necessity of mechanical ventilation and ICU care).
During the HCW COVID-19 vaccine campaign, the dominant variant in circulation was P.1 (γ variant), and both COVID-19 vaccines (CoronaVac and ChAdOx1) showed effectiveness against this variant. More studies are needed regarding the SARS-CoV-2 variants of concerns (VOC) that have multiple spike-protein mutations and appear to be more infectious or cause more disease than other circulating SARS-CoV-2 variants. 22 Some deletions in the spike-protein mutations can alter the shape of the spike and may help it evade some antibodies. 23 No COVID-19 vaccine is 100% effective against SARS-CoV-2 infection, consistent with COVID-19 breakthrough infections reported in HCWs after COVID-19 vaccination. 24,25 We detected a clear effect of the vaccines against the new variants (mainly P.1).
Our study had several limitations. First, this was an observational study, subject to multiple biases 26 ; however, this is the most common study design in the infection prevention literature. 26 Second, we estimated vaccine effectiveness based on short-term duration, and longer-term observational studies are needed to assess sustained immune response and vaccine effectiveness. Third, due to the uncertainty related to the number of days required to develop immunity postvaccination, we decided to adopt the CDC definition for the CoronaVac vaccine and for the ChAdOx1 vaccine, which defines people fully vaccinated as being ≥14 days after the second dose in a 2-dose series (Pfizer/BioNTech or Moderna), or ≥14 days after a single-dose vaccine (Johnson & Johnson/Janssen). 27 Other studies adopted different definitions of a fully vaccinated person. 5 Currently, no postvaccination time limit on fully vaccinated status has been established. In addition, the CDC defines unvaccinated people as individuals of all ages including children who have not completed a vaccination series or have not received a single-dose vaccine. 27 Fourth, we have not reported nonneutralizing viral antigen-binding antibody levels in our HCW cohort study. However, the US Food and Drug Administration (FDA) does not recommend antibody testing for SARS-CoV-2 to determine immunity or protection from COVID-19, especially among those who are vaccinated. 28 Lastly, since our study focused only the short-term vaccination effectiveness among HCWs, we could not evaluate the need for a third dose. Consolidated knowledge indicates that each HCW needs to get 2 doses of CoronaVac or 2 doses of ChAdOx1 vaccine; thus, we decided to not report the analysis of vaccine effectiveness after 1 dose only. Considering our data regarding vaccine effectiveness for both COVID-19 vaccines, our institution began administering a third dose to HCWs in October 2021, after authorization from the Ministry of Health.
In conclusion, both COVID-19 vaccines (viral vector and inactivated virus) can significantly prevent COVID-19 among HCWs. The 2 COVID-19 vaccines were also effective among HCWs even after an emergence of a new variant (ie, the γ variant). More observational studies are needed to evaluate vaccination effectiveness of other COVID-19 vaccines (eg, other types of viral vector or inactivated virus). Studies are also needed to evaluate the impact of COVID-19 vaccines on personal protective equipment among HCWs, on vaccine effectiveness, and on COVID-19 breakthrough infection. Also, the vaccine effectiveness of unmatched COVID-19 vaccines as a third dose should be evaluated. Further genomic surveillance is needed for better understanding of vaccine effectiveness against the new SARS-CoV-2 variants.
Acknowledgments
We thank all the participants for their contributions to this study.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/ice.2022.50.
click here to view supplementary material
Financial support
The sequencing reactions carried out to characterize the circulating SARS-CoV-2 described in this study were supported by Chamada MCTIC/CNPq/FNDCT/MS/SCTIE/Decit 07/2020 in Brazil (grant no. 402669/2020-7).
Conflicts of interest
All authors report no conflict of interest relevant to this article.
References
- 1. Mutambudzi M, Niedwiedz C, Macdonald EB, et al. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK Biobank participants. Occupat Environ Med 2020;78:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID-19 among frontline healthcare workers and the general community: a prospective cohort study. Lancet Public Health 2020;5:e475–e483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. NEJM 2021;384:1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tenforde MW, Olson SM, Self WH, et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥65 years—United States, January–March 2021. Morb Mortal Wkly Rep 2021;70:674–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marra AR, Kobayashi T, Suzuki H, et al. The short-term effectiveness of coronavirus disease 2019 (COVID-19) vaccines among healthcare workers: a systematic literature review and meta-analysis. ASHE 2021;1:E33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greninger AL, Naccache SN, Federman S, et al. Rapid metagenomic identification of viral pathogens in clinical samples by real-time nanopore sequencing analysis. Genome Med 2015;7:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ashby DG. Practical Statistics for Medical Research. Boca Raton, FL: CRC Press; 1991. [Google Scholar]
- 8. Nauta J. Statistics in Clinical Vaccine Trials. Berlin: Springer Science & Business Media; 2010. [Google Scholar]
- 9. Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. Berlin: Springer Science & Business Media; 2006. [Google Scholar]
- 10. RC Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 11. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 2021;384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palacios R, Batista AP, Albuquerque CSN, et al. Efficacy and safety of a COVID-19 inactivated vaccine in healthcare professionals in Brazil: the PROFISCOV Study. Soc Sci Res Netw 2021. doi: 10.2139/ssrn.3822780.
- 14. Tanriover MD, Doğanay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021;398:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med 2021;385:875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hitchings MDT, Ranzani OT, Torres MSS, et al. Effectiveness of CoronaVac among healthcare workers in the setting of high SARS-CoV-2 gamma-variant transmission in Manaus, Brazil: a test-negative case-control study. Lancet Reg Health Am 2021;1:100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med 2021;385:585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plano Nacional de Operacionalização da Vacinação contra a COVID-19. 2021. Ministério da Saúde website. https://www.gov.br/saude/pt-br/coronavirus/vacinas/plano-nacional-de-operacionalizacao-da-vacina-contra-a-covid-19. Published December 16, 2020. Accessed November 2, 2021.
- 20. Mehrotra DV, Janes HE, Fleming TR, et al. Clinical endpoints for evaluating efficacy in COVID-19 vaccine trials. Ann Intern Med 2021;174:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW, Pollard AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis 2021;21:e26–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ 2021;372:n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021;593:130–135. [DOI] [PubMed] [Google Scholar]
- 24. Hacisuleyman E, Hale C, Saito Y, et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med 2021;384:2212–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.COVID-19 vaccine breakthrough infections reported to CDC—United States, January 1–April 30, 2021. Morb Mortal Wkly Rep 2021;70:792–793. [DOI] [PMC free article] [PubMed]
- 26. Harris AD, Lautenbach E, Perencevich E. A systematic review of quasi-experimental study designs in the fields of infection control and antibiotic resistance. Clin Infect Dis 2005;41:77–82. [DOI] [PubMed] [Google Scholar]
- 27. Interim Public Health recommendations for fully vaccinated people. Centers for Disease Control and Prevention website. https://stacks.cdc.gov/view/cdc/105629. Updated October 15, 2021. Accessed November 2, 2021.
- 28.FDA Safety Communication. Antibody testing is not currently recommended to assess immunity after COVID-19 vaccination. US Food and Drug Administration website. https://www.fda.gov/medical-devices/safety-communications/antibody-testing-not-currently-recommended-assess-immunity-after-covid-19-vaccination-fda-safety Published May 19, 2021. Accessed November 2, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/ice.2022.50.
click here to view supplementary material