Abstract
Acetaminophen, also known as N-acetyl-p-aminophenol (APAP), is commonly used as an antipyretic and analgesic agent. APAP overdose can induce hepatic toxicity, known as acetaminophen-induced liver injury (AILI). However, therapeutic doses of APAP can also induce AILI in patients with excessive alcohol intake or who are fasting. Hence, there is a need to understand the potential pathological mechanisms underlying AILI. In this review, we summarize three main mechanisms involved in the pathogenesis of AILI: hepatocyte necrosis, sterile inflammation, and hepatocyte regeneration. The relevant factors are elucidated and discussed. For instance, N-acetyl-p-benzoquinone imine (NAPQI) protein adducts trigger mitochondrial oxidative/nitrosative stress during hepatocyte necrosis, danger-associated molecular patterns (DAMPs) are released to elicit sterile inflammation, and certain growth factors contribute to liver regeneration. Finally, we describe the current potential treatment options for AILI patients and promising novel strategies available to researchers and pharmacists. This review provides a clearer understanding of AILI-related mechanisms to guide drug screening and selection for the clinical treatment of AILI patients in the future.
Keywords: Acetaminophen, Acetaminophen-induced liver injury, Hepatocyte necrosis, Sterile inflammation, Hepatocyte regeneration
1. Introduction
Acetaminophen, also known as N-acetyl-p-aminophenol (APAP), was approved for clinical application by the US Food and Drug Administration (FDA) in 1950 and is commonly used for the management of fever and pain (Chowdhury et al., 2020). However, APAP overdose can cause hepatic and renal toxicities and even lead to death. The liver is the main organ that metabolizes APAP. Most (85%) APAP can directly combine with glucuronide and sulfate cofactors that are excreted in the form of bile and urine. The remaining fraction (15%) can be metabolized by the cytochrome P450 (CYP450) enzyme system (Chowdhury et al., 2020). In vivo, CYP450 can transform APAP into toxic N-acetyl-p-benzoquinone imine (NAPQI), which may subsequently trigger cell damage in the liver (Dahlin et al., 1984). Under normal circumstances, glutathione (GSH) in liver tissues facilitates the discharge of electrophilic NAPQI by combining thioglycolic acid with cysteine (Lu, 1999). It is well known that NAPQI can bind to key cellular proteins and induce oxidative stress and mitochondrial dysfunction. This may eventually cause liver cell death in the context of uridine diphosphate (UDP)-glucuronic acid and 3'-phosphoadenosine-5'-phosphosulfate saturation and GSH overconsumption (more than 1/3 of the normal level; Fig. 1) (Chiew et al., 2018).
Fig. 1. Schematic diagram of APAP metabolism. Under the action of glucoronosyl-transferase and sulfo-transferase, most monomer APAP forms complexes with sulfate and glucuronic acid, which are excreted in bile or urine. The remaining part of APAP is oxidized by CYP450 to form NAPQI, which combines with GSH. Accompanied with overconsumption of GSH, NAPQI shows accumulation in the liver, eventually causing liver damage. APAP: N-acetyl-p-aminophenol; CYP450: cytochrome P450; NAPQI: N-acetyl-p-benzoquinone imine; GSH: glutathione.
APAP overdose is considered an important factor that contributes to the pathogenesis of acute liver failure (ALF); in particular, a single dose of more than 125 mg/kg can have this effect. In the USA and most western countries, the proportion of acetaminophen-induced liver injury (AILI) reaches up to 46% of ALF cases (Reuben et al., 2016). The risk of AILI appears to be greatly increased in individuals with anorexia, long-term alcohol abuse, and phenytoin sodium. This phenomenon may be related to insufficient reserves of GSH or the increased activity of CYP450 drug-metabolizing enzymes. About 10% of alcohol metabolism is mediated by the CYP450 family (Cederbaum, 2012). Therefore, the relationship between alcohol and APAP/NAPQI is complicated. Hodgman and Garrard (2012) found that chronic alcohol consumers have an increased risk of AILI. In contrast, acute alcohol consumption plays a protective role against APAP because of the competition of alcohol for CYP450 (Schmidt et al., 2002; Waring et al., 2008). The pathological process underlying AILI is divided into three stages: liver cell death, sterile inflammation, and liver cell regeneration and recovery (Bhushan and Apte, 2019). Hepatocyte death is induced mainly by metabolic disorder in hepatocytes. Initially, the accumulation of the NAPQI protein in mitochondria leads to continuous oxidative stress or nitrosative stress and dysfunction, followed by gradual liver cell necrosis (Jaeschke et al., 2012). Subsequently, necrotic hepatocytes release danger-associated molecular patterns (DAMPs). These DAMPs then recruit and activate innate immune cells, such as neutrophils, macrophages, natural killer T (NKT) cells, and natural killer (NK) cells, and participate in necrotic tissue clearance and inflammatory response expansion (Jaeschke et al., 2014). Finally, liver cells proliferate and regenerate in the presence of certain signal mediators and nonparenchymal liver cells (Apte et al., 2009; Bhushan et al., 2017a).
N-acetylcysteine (NAC), the only drug approved for clinical use in the treatment of AILI, contributes to the synthesis of GSH. Moreover, early administration is recommended in clinical settings. In patients with APAP overdose for more than 8 h, the efficiency of NAC treatment is significantly reduced (Smilkstein et al., 1988). Some other drugs, such as 4-methylpyrazole (4MP), methylene blue, and metformin, have been reported to be effective for treating AILI. These drugs are not approved by the authorities, as some obstacles have occurred in the progression of these drugs through clinical trials (Jaeschke et al., 2020). Trials of novel therapies that target the signaling pathways involved in AILI are ongoing. In this review, we summarize the pathogenesis of AILI and elucidate the latest treatment options.
2. Association between hepatocyte necrosis and AILI
2.1. Involvement of NAPQI in hepatocyte necrosis by modulating mitochondrial oxidative/nitrosative stress and endoplasmic reticulum stress
In response to factors that cause physical or chemical damage, the permeability of hepatocyte plasma membranes and organelle membranes increases. This leads to the release of organelles and intracellular contents and the necrosis of hepatocytes. APAP-induced hepatotoxicity is thought to lead mainly to hepatocyte necrosis, but studies on the intracellular signaling mechanism underlying cell death suggest that both apoptosis and necroptosis may be involved (Ramachandran and Jaeschke, 2019). APAP-induced hepatocyte necrosis may overlap with mechanisms of apoptosis and necroptosis, but no intergenerational studies have confirmed that apoptosis or necroptosis is the main form of hepatocyte death.
NAPQI protein adducts are considered the hallmark of hepatocyte necrosis, and subsequent cell death-related signal transduction is closely related to mitochondria. NAPQI protein adducts were first discovered in the 1970s, but their exact function has not yet been well defined (Jollow et al., 1973). Twenty years later, Tirmenstein and Nelson (1989) indicated that in mice treated with APAP, NAPQI protein adducts accumulated in hepatocyte mitochondria, and this accumulation was accompanied by oxidative stress. Moreover, there was remarkable liver damage in these mice. However, these findings were not observed in mice treated with the APAP isomer acetyl-m-aminophenol. This result suggested that mitochondrial protein adducts were associated with oxidative stress and the pathogenesis of AILI (Tirmenstein and Nelson, 1989). In mice with AILI, the formation of NAPQI protein adducts appeared within 30 min after the administration of APAP and reached a peak at about 2‒3 h, while the formation of NAPQI protein adducts was relatively delayed in humans (Xie et al., 2015).
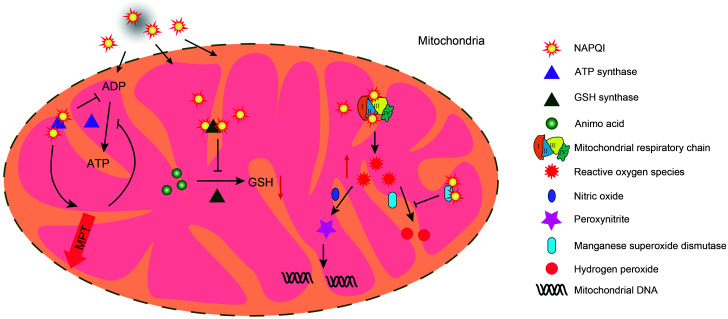
Among a large number of potential pathological factors related to AILI, NAPQI protein adducts can trigger mitochondrial oxidative/nitrosative stress. NAPQI can bind to corresponding proteins in the mitochondrial respiratory chain (MRC), causing the leakage of electrons from the MRC to oxygen. This contributes to the formation of superoxides, which then induce oxidative/nitrosative stress (Lee et al., 2015). Commonly, superoxide is catalyzed into hydrogen peroxide and oxygen by manganese superoxide dismutase or reacts with nitric oxide (NO) to form peroxynitrite. Hydrogen peroxide is scavenged by antioxidant enzymes or directly reacts with GSH (Du et al., 2016). When the mitochondrial GSH content is decreased and the antioxidant enzyme activity is inhibited by protein adducts, aggregated reactive oxygen species (ROS) combine with NO to form peroxynitrite. This results in the formation of protein nitrotyrosine adducts, affecting the function of intracellular proteins. Moreover, the strong polarity of peroxynitrite can also cause mitochondrial DNA (mtDNA) damage (Cover et al., 2005). In addition, NAPQI can inhibit adenosine triphosphate (ATP) synthesis by binding to the α subunit of ATP synthetase. This triggers the opening of mitochondrial membrane permeability transition (MPT) pores, resulting in a decrease in the mitochondrial membrane potential and a termination of energy synthesis via the tricarboxylic acid cycle (Jaeschke et al., 2012). Moreover, NAPQI was reported to function as an inhibitor of GSH synthase by binding to it. This inhibition greatly reduced the rate of NAPQI secretion and contributed to the extent of APAP-mediated damage (Walker et al., 2017). Therefore, in cases of damage to the energy generation of cells, it may affect the energy requirement and induce ROS generation, cellular apoptosis, and calcium imbalance. Based on the latest quantitative protein mass spectrometry analysis, NAPQI can also form adducts after binding to four mitochondrial antioxidant proteins, which greatly reduces the ability of mitochondria to resist oxidative stress (Bruderer et al., 2015) (Fig. 2).
Fig. 2. Mitochondrial oxidative/nitrosative stress and dysfunction. Excessive NAPQI in the mitochondria combines with ATP synthase, GSH synthase, and oxidative respiratory chain enzymes to form protein adducts, which subsequently lead to mitochondrial dysfunction and oxidative stress. NAPQI-ATP synthase hinders ATP synthesis and gradually triggers the opening of MPT pores. The abnormality of MPT pores is exacerbated by the inhibition of ATP synthesis. NAPQI-GSH synthase reduces GSH synthesis, weakens the excretion of NAPQI, and induces the formation of ROS. ROS can be eliminated by the antioxidant enzyme system, but excessive ROS combine with free NO to form peroxynitrite, causing mitochondrial DNA damage. NAPQI: N-acetyl-p-benzoquinone imine; ADP: adenosine diphosphate; ATP: adenosine triphosphate; GSH: glutathione; ROS: reactive oxygen species; MPT: membrane permeability transition; NO: nitric oxide.
Increasing evidence indicates a close relationship between endoplasmic reticulum stress (ERS) and AILI. ERS is associated with NAPQI-mediated damage to the protein folding process and redox environment. Uzi et al. (2013) reported that NAPQI and unfolded proteins in the endoplasmic reticulum (ER) undergo a Michael addition reaction. NAPQI overload induces ERS and stimulates the expression of CCAAT/enhancer-binding protein homologous protein (CHOP), which promotes the expression of apoptotic genes and inhibits liver regeneration. In addition, protein synthesis involves a proper redox environment and GSH homeostasis. After consumption of GSH during ERS, the phosphorylation of α-subunit of eukaryotic initiation factor 2 (eIF2α) and the activation of activating transcription factor 6 (ATF6) and CHOP were observed (Yan et al., 2018). Torres et al. (2019) indicated that valproic acid could increase sensitivity to APAP-mediated liver injury in mice. The pathogenesis is correlated with the fact that ERS upregulates the expression of acute regulatory proteins produced by steroids and promotes the combination of SH3 homology-associated bruton tyrosine kinase-binding (Sab) and phosphorylated c-Jun N-terminal kinase (p-JNK). Specifically, oxidative stress/nitrative stress promotes ERS, but it is not the decisive factor, as the binding of NAPQI and immature protein in the ER triggers ERS.
2.2. Consequence of mitochondrial oxidative/nitrosative stress in the solute
Mitochondrial oxidative/nitrosative stress and dysfunction induce the opening of MPT pores, which contributes to liver cell damage and necrosis (Ramachandran and Jaeschke, 2019). MPT pores located in the inner mitochondrial membrane are immediately opened when a specific cysteine residue of cyclophilin D is attacked by ROS (Ramachandran et al., 2011). In mice treated with low-dose APAP (150 mg/kg), transient JNK activation and reversible opening of MPT pores were observed. In contrast, in the presence of a high dose of APAP (300 mg/kg), irreversible MPT pore opening and hepatocellular death were observed (Hu et al., 2016a). All these results confirm that continuous mitochondrial oxidative/nitrosative stress is the driving force that opens the MPT pores. Upon opening of the MPT pores, the outer mitochondrial membrane ruptures, causing the release of many molecules with molecular weights of less than 1.5 kDa from the mitochondrial solutes into the matrix (Karch and Molkentin, 2014). The leakage of mitochondrial proteins, such as apoptosis-inducing factor (AIF), cytochrome C, and endonuclease G, affects mitochondrial function and induces DNA fragmentation by acting on mitochondria and nuclei. This is consistent with the requirement for liver transplantation for patients with severe hepatitis, which is caused mainly by irreversible hepatocyte necrosis.
Damaged mitochondria and ROS are observed in the matrix of hepatocytes with a large number of NAPQI protein adducts, and then the self-defense reaction in hepatocytes is activated. The resulting lysosomes trigger the degradation of abnormal matrix proteins and organelles. This process is called hepatocyte autophagy (Moore, 2008). The autophagic response is considered a defense mechanism of hepatocytes. Ni et al. (2012) first reported the protective effects of hepatocyte autophagy in mice with AILI, and cultured primary hepatocytes by adding autophagy antagonists and activators. Subsequently, the specific clearance of the autophagic ability in hepatocytes contributed to the generation of ROS and the activation of JNK in a mouse model (Igusa et al., 2012). These results confirm the protective roles of autophagy in AILI. Hepatocyte autophagy has been acknowledged to participate in the clearance of abnormal protein adducts and mitochondria, as well as the reduction of mitochondrial stress and ERS, by regulating the ER transition (Fig. 3).
Fig. 3. Roles of JNK, P38, and ERK activation in AILI. NAPQI in hepatocytes binds to enzymes on the MRC to produce ROS, which are released into the matrix, and phosphorylates JNK through three different pathways. Phosphorylated JNK (p-JNK) is ectopic to the vicinity of mitochondria, and combines with Sab to affect the electron transmission of MRC and induce ROS production, indicating the existence of a feedback loop between ROS and JNK. Persistent mitochondrial oxidative stress and dysfunction contribute to the opening of MPT pores and leakage of mitochondrial solute into the matrix, such as AIF, endonuclease (Endo) G, and cytochrome C. This induces nucleic acid cleavage and necrosis in liver cells. The stress response of the ER aggravates liver cell death. Hepatocytes produce self-defense reactions to deal with the damage, including the production of P53 protein and autophagy. P53 protein reduces mitochondrial damage by inhibiting p-JNK. Autophagosomes can clear up the protein adducts and the damaged mitochondria, to regulate ER transition. P38 and ERK are activated in the process of AILI. The activation of ERK induces hepatocyte apoptosis and pro-inflammatory gene expression of inflammatory cells. JNK: c-Jun N-terminal kinase; ERK: extracellular signal-regulated kinase; AILI: acetaminophen-induced liver injury; NAPQI: N-acetyl-p-benzoquinone imine; MRC: mitochondrial respiratory chain; ROS: reactive oxygen species; MPT: membrane permeability transition; AIF: apoptosis-inducing factor; Sab: SH3 homology-associated bruton tyrosine kinase-binding protein; P: phosphorylated; GSK-3β: glycogen synthase kinase 3β; MLK3: mixed lineage kinase 3; MKK: mitogen-activated protein kinase kinase; ASK: apoptosis signaling-regulating kinase; ULK: unc-51-like kinase; GSH: glutathione; ER: endoplasmic reticulum; CHOP: CCAAT/enhancer-binding protein homologous protein.
2.3. Roles of JNK pathway activation in AILI
As stated above, the formation of mitochondrial NAPQI protein adducts is closely related to mitochondrial oxidative stress and dysfunction. Compared with the powerful antioxidant system inside mitochondria, these changes are minimal and short-lived. Thus, some other potential mechanisms may be involved in the persistent activation of oxidative stress. Lemasters (1998) proposed the "double-hit" theory of mitochondrial damage-related disorders in AILI. The first hit referred to the depletion of mitochondrial GSH by NAPQI, and the second hit related to the activation of the ROS-mediated JNK signaling pathway (Moles et al., 2018).
The activation of JNK continuously amplifies mitochondrial oxidative stress and forms a closed activation loop (Win et al., 2016). The first upstream kinase molecule of interest is apoptosis signaling-regulating kinase-1 (ASK-1), which regulates mainly the late activation of JNK. In the cytoplasm, ASK-1 can combine with thioredoxin to form an inactive complex under non-stressed conditions. Upon oxidative stress in mitochondria, sustained release of mitochondrial ROS into the cytoplasm occurs, followed by the dissociation and activation of ASK-1. Activated ASK-1 phosphorylates mitogen-activated protein kinase kinase 4/7 (MKK4/7) and then triggers the activation of JNK. Recently, extensive studies have been conducted to investigate mixed lineage kinase-3 (MLK-3), an upstream signaling molecule. It has been acknowledged that MLK-3 participates in the early activation of JNK (Sharma et al., 2012). Additionally, in the early stage of mitochondrial oxidative stress, cytoplasmic ROS trigger the phosphorylation of glycogen synthase kinase-3β (GSK-3β), which in turn activates MLK-3 and JNK (Shinohara et al., 2010; Yan et al., 2018). Sun et al. (2018) identified unc-51-like kinase 1/2 (ULK1/2) as the third upstream kinase that participates in the pathogenesis of AILI by phosphorylating MAPK4/7 and JNK. The subsequent processes in the middle and downstream regions include the phosphorylation of JNK and its rapid translocation to the mitochondria, where it binds to the Sab protein on the mitochondrial outer membrane. Finally, these phenomena result in MRC dysfunction and the release of ROS (Win et al., 2016). It has been reported that female mice with a low Sab protein concentration are more resistant to AILI than their male counterparts (Win et al., 2019). Pathological changes result in the activation of the JNK signaling pathway, which leads to the generation of ROS. Then, ROS act on the JNK signaling pathway, indicating that ROS and JNK signaling pathways can form a closed loop.
The P53 protein is a tumor suppressor protein that can be activated in response to moderate cell stimulation. It is involved in regulating glycolysis and oxidative phosphorylation, limits the production of ROS, and promotes cell survival and genetic damage repair (Kruiswijk et al., 2015). Unsurprisingly, P53 was reported to block the JNK signaling pathway in AILI. Huo et al. (2017) found that the P53 protein can protect cells against APAP-induced liver toxicity by inhibiting the activation of JNK. In addition, it participates in the maintenance of metabolic homeostasis and activates cell proliferation signals to promote liver cell regeneration (Borude et al., 2018) (Fig. 3).
2.4. Roles of P38 and extracellular signal-regulated kinase (ERK) activation in AILI
In addition to JNK, many other compensatory signaling pathways, such as P38 and ERK, are activated in AILI. The two members belonging to the MAPK family, P38 and ERK, are involved in many cell functions, such as cell proliferation, migration, death, and oxidative stress (Wu et al., 2018). Several studies found that the activation of P38 in AILI is always accompanied by an increase in inflammation and hepatocyte apoptosis (Ding et al., 2016; Zhang et al., 2017; Fu et al., 2018). Based on these results, we can draw a general conclusion that P38 may be involved in inflammation and hepatocyte apoptosis. However, note that while P38 is activated, JNK and ERK are also activated. Zhang et al. (2017) found that P38 does not contribute to the liver injury induced by APAP. The role of P38 in AILI requires further study. Similarly, previous studies showed that the activation of ERK is associated with oxidative stress, inflammation, and apoptotic events in AILI (Zhang et al., 2017; Liu et al., 2020). Inhibiting the activation of ERK alone via drug intervention and interleukin-17 (IL-17) deficiency can downregulate proinflammatory cytokine production and decrease hepatocyte apoptosis in AILI (Liao et al., 2017; Lee et al., 2018, 2019). Collectively, we conclude that ERK participates in the process of AILI by activating the downstream effectors of hepatocyte apoptosis and inflammatory responses (Fig. 3).
3. Involvement of sterile inflammation in AILI
3.1. Generation and release of DAMPs
Hepatocytes undergoing cellular necrosis release DAMPs, including cytokines and chemotactic factors. Such signaling molecules activate other immunocytes that are then recruited to liver tissues, further inducing sterile inflammation. Sterile inflammation is a form of pathogen-free inflammation that is chronic, but occurs at a low level. Indeed, there is some controversy regarding the role of sterile inflammation, as it can eliminate necrotic cells and promote both tissue repair and injury.
Cellular molecules, including DNA fragments, heat shock proteins (HSPs), high mobility group box 1 (HMGB1), and ATP, passively released after hepatocellular necrosis, are collectively termed DAMPs (Kubes and Mehal, 2012). These molecules can bind to pattern recognition receptors, including Toll-like receptors (TLRs), receptors for advanced glycation end products (RAGEs), and purinergic receptors on liver immune cells, resulting in the activation of immune cells. Then, these immune cells release cytokines to exacerbate the inflammatory response.
Among the DAMPs, extensive attention has been given to HMGB1. HMGB1 is derived mainly from the hypoacetylated form passively released by necrotic hepatocytes and the hyperacetylated form actively secreted by Kupffer cells (KCs). The appearance of KCs suggests that they have entered a period of sterile inflammation (Bonaldi et al., 2003). HMGB1 can bind to TLR4 on KCs and RAGE on neutrophils. These immune cells then produce an immune response. On this basis, we assume that HMGB1 exerts proinflammatory effects. However, HMGB1 alone does not seem to cause a serious proinflammatory response. According to a previous description, anti-HMGB1 antibodies reduce liver neutrophil recruitment, but they cannot reverse liver injury (Yang et al., 2012).
AILI is characterized by the release of nuclear and mitochondrial DNA into the circulation. DNA fragments combine with TLR9 on KCs to induce the production of pro-IL-1β and IL-1α. Pro-IL-1β exerts its biological effects after being hydrolyzed and cleaved into IL-1β by caspase-1 (Imaeda et al., 2009). IL-1 has multiple subtypes, among which IL-1α and IL-1β exert the most predominant proinflammatory effects. Zhang et al. (2018) found that IL-1α can combine with IL-1 receptor (IL-1R)+ cells (mainly neutrophils and monocytes) instead of IL-1β, and participate in the secondary damage of AILI. In particular, IL-1R deficiency exerts protective effects on hepatocytes. Thus, it is reasonable to hypothesize that the IL-1–IL-1R axis plays crucial roles in the pathogenesis of AILI. In addition, the processing of pro-IL-1β by caspase-1 involves the activation of inflammasomes containing nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (Nalp3). Imaeda et al. (2009) reported that administering a TLR9 antibody or aspirin to Tlr9 -/- mice to block the inflammatory effect of Nalp3 can reduce the mortality of mice with AILI. This indicated that TLR9 mediates the pathophysiological process of AILI.
HSPs and exohistones promote APAP-induced liver inflammation by modulating the activity of TLR4 (Xu et al., 2011). It is possible that many different DAMPs may participate in the progression of AILI. Hence, the strategy of targeting only one molecule may not be sufficient to eliminate the danger signals triggered by DAMPs.
3.2. Roles of immune cell function in the pathogenesis of AILI
In response to APAP-induced liver damage, the immune system, including immune cells and cytokines, is mobilized. In addition, innate immunity has a major involvement in the AILI process (Fig. 4).
Fig. 4. Involvement of sterile inflammation in AILI. Liver KCs are activated by different types of DAMPs. Then KCs affect the life cycle of hepatocytes by releasing different inflammatory mediators, and/or induce phenotypic changes in other immune cells. Neutrophils serve as scavengers without aggravating liver damage, and they can promote liver cell recovery and the maturation of MoMFs. MoMFs show crosstalk with neutrophils. Ly-6Chigh MoMF can promote neutrophil chemotaxis, but Ly-6Clow MoMF inhibits neutrophil recruitment and participates in tissue repair. While the expression of MHC-I molecules decreases in necrotic liver cells, NK/NKT cells are activated and the toxic hepatocytes are scavenged. The gut-liver axis has an important function during AILI, and intestinal dysbiosis will aggravate AILI. AILI: acetaminophen-induced liver injury; KCs: Kupffer cells; DAMP: damage-related molecular pattern; MoMF: monocyte-derived macrophage; Ly-6C: lymphocyte antigen 6C; MHC-I: major histocompatibility complex-I; NK: nature killer; NKT: natural killer T; IL: interleukin; MIP1/2: macrophage inflammatory protein 1/2; RAGE: receptor for advanced glycation end products; TLR: Toll-like receptor; MCP-1: monocyte chemoattractant protein-1; ROS: reactive oxygen species; HMGB1: high mobility group box 1 protein; TNF-α: tumor necrosis factor-α; IFN-γ: interferon-γ; GSH: glutathione; CYPE1: cytochrome P450 E1.
3.2.1. Kupffer cells
KCs, also known as liver-resident macrophages, account for 80%‒90% of systemic tissue macrophages and 35% of liver nonparenchymal cells, and play a central role in systemic and regional defense (Bilzer et al., 2006). At the early stage of AILI, KCs are gradually depleted due to increased metabolism and oxidative stress. In the recovery phase, they are either self-renewed or replaced by monocyte-derived macrophages (MoMFs) (Zigmond et al., 2014). Therefore, it is reasonable to hypothesize that KCs participate in AILI.
When KCs are scavenged by the addition of dextran sulfate or gadolinium chloride, the production of ROS in the liver declines, which is accompanied by pathological damage at the early stage. This protects the liver from lobular liver injury (Michael et al., 1999). However, these results are disputed. The ROS generated by KCs around the portal vein are unlikely to induce selective damage at the center of the liver lobules. Additionally, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is not a major source of oxidation for all macrophages. Moreover, there is no evidence that KCs induce liver damage by releasing ROS. Most studies indicate that gadolinium chloride exerts little or no protective effects on liver damage after clearing KCs. In addition, gadolinium chloride may even lead to aggravation of liver damage (Fisher et al., 2013). Therefore, it is still uncertain whether KCs can directly cause liver cell damage by producing ROS.
3.2.2. Monocyte-derived macrophages
Circulating monocytes are recruited to sites of inflammation through the monocyte chemoattractant protein-1 (MCP-1)/chemokine C-C-motif receptor 2 (CCR2) pathway, where infiltrating MoMFs are formed (Antoniades et al., 2012). At the immature stage, the infiltrating MoMFs are characterized by high lymphocyte antigen 6C (Ly-6Chigh) expression. Subsequently, their mature type is designated Ly-6Clow MoMFs in the presence of MCP-1, IL-6, and neutrophil-derived ROS (Yang et al., 2019). Ly-6Clow MoMFs secrete more IL-6 and IL-10, and at the same time, their phagocytotic abilities are enhanced. Ly-6Clow MoMFs inhibit the recruitment and activation of neutrophils and induce the expression of vascular endothelial growth factor (VEGF). Moreover, these cells promote remodeling of the microvascular system during liver recovery, which is crucial for the recovery of hepatocytes (Ehling et al., 2014). Immature Ly-6Chigh MoMFs secrete mainly proinflammatory mediators (e.g., IL-6, tumor necrosis factor-α (TNF-α), and IL-1β) to promote the activation and survival of neutrophils (Jaeschke and Ramachandran, 2020). Studies on MoMFs have focused mainly on blocking their recruitment. It was reported that the number of MoMFs was reduced by 80% in CCR2 -/- or MCP-1 -/- mice and in mice with AILI treated with an anti-CCR2 antibody. These changes exert no effects on APAP-induced liver damage, but they contribute to the delay of liver cell repair and vascular regeneration (Dambach et al., 2002; Jaeschke and Ramachandran, 2020). These results strongly indicate that mature Ly-6Clow MoMFs play protective roles in AILI.
3.2.3. Neutrophils
Neutrophils are recruited to necrotic areas by cytokines or DAMPs secreted by KCs, and they then coordinate with liver macrophages to remove necrotic liver cells and tissues (Krenkel et al., 2014). Subsequently, KCs or MoMFs release macrophage inflammatory protein 1/2 (MIP1/2) and IL-6, which further induce neutrophil aggregation (Woolbright and Jaeschke, 2018). The activated neutrophils are potentially toxic.
Most studies have suggested that neutrophils do not trigger the aggravation of liver damage. First, the quantity of neutrophils is increased at the early stage (Lawson et al., 2000), which suggests that the increase in neutrophil number causes damage in AILI. Then, changes in neutrophil quantity either aggravate or reduce liver damage. However, the current data are controversial. The administration of endotoxin or exogenous IL-1 contributes to the elevation of liver neutrophil numbers in mice without worsening liver damage (Williams et al., 2010). In addition, liver damage is not ameliorated by the addition of anti-lymphocyte antigen 6G antibodies to deplete neutrophils, interference with DAMPs to reduce neutrophil recruitment, or use of granulocyte colony-stimulating factor-/- (G-CSF -/-) mice to decrease neutrophil production (Kono et al., 2010; Yang et al., 2019). The combination of cluster of differentiation 11b (CD11b)/CD18 and intercellular cell adhesion molecule-1 (ICAM-1) is a sign of neutrophil activation, and these molecules are essential for neutrophil migration and tissue damage (Jaeschke and Ramachandran, 2020). Lawson et al. (2000) found that the number of neutrophils in the liver increases within 24 h after APAP overdose, and anti-CD18 antibodies do not trigger liver damage. This indicates that these neutrophils are not activated. Similarly, APAP overdose does not trigger a decrease in liver damage in CD18 -/- or ICAM-1 -/- mice (Cover et al., 2006; Williams et al., 2010). These results indicate that inactive neutrophils have no effect on APAP-induced liver toxicity. Neutrophils can kill target cells through the generation of ROS derived from NADPH oxidase in mice with acute liver injury. However, interventions, including the use of NADPH oxidase inhibitors or gp91 -/- (NADPH oxidase-deficient) mice, cannot protect the liver from APAP overload (Cover et al., 2006; Williams et al., 2014).
Currently, few data support the exacerbation of liver damage by neutrophils. In some studies, specific antibodies were used in advance to deplete neutrophils (Marques et al., 2012). However, inactivated neutrophils feedback to promote the activation of KCs after intervention, leading to activation of hepatocyte genes in the acute phase and increased resistance to APAP toxicity in mice (Jaeschke and Liu, 2007). Therefore, protective effects rather than neutrophil deficiency are induced in the acute phase. Similarly, using antibodies to deplete neutrophils after APAP application does not ameliorate liver damage in AILI mice (Cover et al., 2006; Yang et al., 2019). Recently, the number of activated neutrophils was shown to be increased in mouse models and patients, and that these neutrophils can promote tissue repair during liver recovery (Williams et al., 2014; Yang et al., 2019). Moreover, the ROS released by neutrophils can promote the maturation of Ly-6Chigh MoMFs and coordinate inflammation suppression and tissue repair (Yang et al., 2019).
In summary, the roles of neutrophils in AILI are not well defined, as the process is rather complex. This may be related to the quantity, location, and lifespan of neutrophils.
3.2.4. NK cells and NKT cells
NK cells and NKT cells, which have common biological activitiy, are usually investigated simultaneously in liver diseases (Jaeschke and Ramachandran, 2020). They are the most important cell groups that secrete interferon-γ (IFN-γ), and they can induce hepatocyte apoptosis by mediating leukocyte infiltration and producing NO. In a previous study, the administration of anti-IFN-γ antibodies decreased liver damage and greatly reduced mortality (Ishida et al., 2002). In addition, liver neutrophil aggregation and recombinant factor-related apoptosis ligand (FasL) expression are reduced upon the addition anti-NK/NKT antibodies, which significantly reduces liver damage (Liu et al., 2004). These findings proved the destructive effects of NK/NKT cells in the AILI process.
AILI is more pronounced in CD1d -/- mice with congenital NKT deficiency (Martin-Murphy et al., 2013). These knock-out (KO) mice have elevated levels of cytochrome P2E1 (CYP2E1) enzyme and protein adducts, which indicates that NKT cells play a protective role in interfering with APAP metabolism (Martin-Murphy et al., 2013). However, in Jα18 -/- mice (deficient in innate immune T cells and Vα14iNKT cells), an increase in the GSH levels in KO mice accelerates the secretion of NAPQI and ROS/NO, which contributes to the protection of liver cells (Downs et al., 2012). In summary, more studies are required to confirm the roles of NK/NKT cells in AILI.
3.3. Cytokines secreted by immune cells
Immune cells have variable degrees of influence on the AILI process, and a single cytokine may play a role. These cytokines, including ILs (e.g., IL-1, IL-6, IL-10, and IL-22) and TNF-α, have been shown to aggravate or reduce liver damage by modulating APAP metabolism and sterile inflammation. Most cytokines participate in the process of AILI by affecting the recruitment and activation of neutrophils (Tables 1 and 2). Different cytokines perform various actions, but even the same cytokine may exert opposite effects. Therefore, it is difficult to draw a clear conclusion, as there might be variations even in a single study with different experimental settings.
Table 1.
Well-defined cytokines involved in the pathogenesis of AILI
| Cytokine | Effect | Role in the pathogenesis of AILI | Reference |
|---|---|---|---|
| IL-4 | Protective | Upregulates γ-glutamylcysteine ligase; promotes GSH production; reduces JNK activation. | Ryan et al., 2012 |
| IL-15 | Recruits more neutrophils to the liver in IL-15 -/- mice; increases sensitivity to liver injury by inducing nitric oxide synthase. | Hou et al., 2012 | |
| IL-13 | Aggravates sterile inflammatory response in the presence of an anti-IL-13 antibody or in IL-13 -/- mice. | Yee et al., 2007 | |
| IL-1α | Damaging | Promotes the infiltration of neutrophils and monocytes, which aggravate inflammation; in IL-1R KO mice, liver damage was delayed. | Zhang et al., 2018 |
| IL-17 | Be secreted by γδ T cells; promotes neutrophil infiltration, which then subsequently triggers liver damage; enhances MPO activity and inflammatory response by activating the ERK signaling pathway. | Wang et al., 2013;Lee et al., 2018 |
AILI: acetaminophen-induced liver injury; IL: interleukin; GSH: glutathione; JNK: c-Jun N-terminal kinase; IL-1R: IL-1 receptor; KO: knock-out; MPO: myeloperoxidase; ERK: extracellular signal-regulated kinase.
Table 2.
Cytokines with controversial roles in the pathogenesis of AILI
| Cytokine | Effect | Role in the pathogenesis of AILI | Reference |
|---|---|---|---|
| TNF-α | Protective | In TNFR p55-deficient mice, the expression of antioxidant genes decreases and the regeneration of hepatocytes is impaired. | Chiu et al., 2003 |
| Damaging | Anti-TNF-α antibody delays liver damage in mice. TNF-α enhances the expression of chemokines, IFN-γ and iNOS, which are involved in liver damage. | Blazka et al., 1995;Ishida et al., 2004 | |
| No | In the presence of anti-TNF-α antibody, anti-TNFR antibody, or inTNF-α -/- mice, there was no significant change in liver damage. | Boess et al., 1998 | |
| IL-22 | Protective | Exogenous IL-22 prevents mitochondrial dysfunction, reduces the release of inflammatory factors, and promotes liver cell regeneration by activating STAT3; IL-22 can enhance AMPK-dependent autophagy and prevent liver injury. | Scheiermann et al., 2013;Mo et al., 2018 |
| Damaging | In IL-22 transgenic mice, CYP enzyme and protein adduct levels are increased, and liver damage is exacerbated. | Feng et al., 2014 | |
| No | IL-22 cannot prevent liver damage, but IL-22-binding protein can reduce liver damage. | Kleinschmidt et al., 2017 | |
| IL-10 | Protective | IL-10 can inhibit the release of pro-inflammatory mediators and reduce the expression of iNOS synthase. | Bourdi et al., 2002 |
| Damaging | In AILI patients, higher IL-10 levels indicated a poor prognosis. | Berry et al., 2010 | |
| No | Exogenous IL-10 infusion does not exacerbate liver damage in mice. | Simpson et al., 2000 | |
| IL-6 | Protective | IL-6 upregulates HSPs and activates the STAT3 signaling pathway to prevent liver cell damage. | Gao et al., 2020 |
| Damaging | High IL-6 levels are positively correlated with mortality in AILI patients. | Moore et al., 2013 |
AILI: acetaminophen-induced liver injury; TNF-α: tumor necrosis factor-α; IL: interleukin; TNFR: TNF receptor; IFN-γ: interferon-γ; iNOS: inducible nitric oxide synthase; STAT3: signal transducer and activator of transcription 3; AMPK: adenine monophosphate-activated protein kinase; CYP: cytochrome P450; HSP: heat shock protein.
3.4. Association between the gut‒liver axis and AILI
In recent years, due to the reciprocal interaction between the gut and liver in normal physiology and disease, a new concept has emerged termed the gut–liver axis. Many studies have revealed that the gut–liver axis is involved in various liver diseases, such as liver tumors, liver fibrosis, and alcoholic steatohepatitis (Yan et al., 2011; Seki and Schnabl, 2012; Yoshimoto et al., 2013). Gong et al. (2018) found that gut microbial metabolites are responsible for the diurnal variation in AILI in mice. Due to the depletion of GSH by gut microbial metabolites at night, the liver toxicity caused by APAP in mice increases (Gong et al., 2018). Later, Schneider et al. (2021) found that liver injury is exacerbated in nucleotide-binding oligomerization domain (NOD)-like receptor family pyrin domain-containing 6 (Nlrp6)-/- mice, which is a dysbiotic mouse model, and fecal transplantation from Nlrp6 -/- mice into normal mice resulted in aggravated liver injury and an Ly-6Chigh inflammatory phenotype, suggesting that intestinal dysbiosis affects AILI. This study also showed that long-term antibiotic or proton pump inhibitor intake increased the risk of developing AILI. In summary, these findings show that intestinal dysbiosis aggravates AILI, and that the gut microbiota has an important function during AILI (Fig. 4).
4. Hepatocyte regeneration
Acute liver injury causes compensatory liver regeneration. In most cases, the remaining hepatocytes can proliferate and renew in the presence of signal mediators and nonparenchymal cells (Fig. 5). However, the proliferation of hepatocytes is inhibited in cases of severe liver injury, and cells derived from the bile duct produce bipotent progenitor cells that differentiate into hepatocytes (Michalopoulos, 2007).
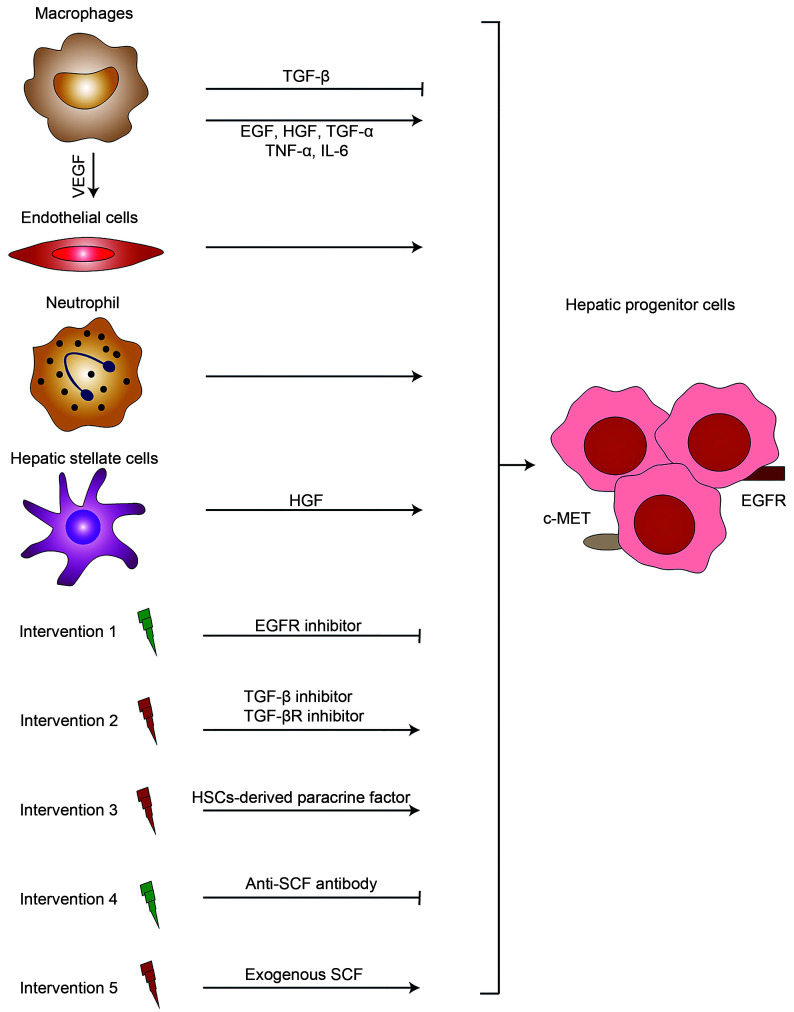
Fig. 5. HPCs regenerated under the action of signal mediators and immune cells. Immune cells (e.g., macrophages and neutrophils) involved in the scavenging of necrotic liver cells and tissues provide space for the survival of HPCs. Meanwhile, the vascular endothelium proliferates under the action of VEGF and participates in liver microangiogenesis. The nonparenchymal cells of the liver can secrete various cytokines, for example, EGF and HGF, and promote the regeneration of hepatic progenitor cells through different signal proliferation pathways. The regeneration process of hepatocytes can be affected through external intervention, for instance, the use of EGFR inhibitors, TGF inhibitors, or HSCs. HPCs: hepatic progenitor cells; TGF: transforming growth factor; TGF-βR: TGF-β receptor; TNF-α: tumor necrosis factor-α; VEGF: vascular endothelial growth factor; EGF: epidermal growth factor; HGF: hepatocytes growth factor; IL: interleukin; EGFR: EGF receptor; HSCs: hepatic stellate cells; SCF: stem cell factor; c-MET: cellular mesenchymal epithelial transition factor.
4.1. Signal mediators
The main mitogens involved in hepatocyte proliferation in liver tissues include transforming growth factor-α (TGF-α), epidermal growth factor (EGF), and hepatocyte growth factor (HGF). Both EGF and TGF-α can bind to EGF receptor (EGFR). Moreover, the binding of HGF to the cellular mesenchymal epithelial transition factor (c-MET) receptor also contributes to hepatocyte regeneration (Michalopoulos, 2007). Increasing evidence indicates that mouse EGFR and c-MET are significantly activated and that EGFR inhibitors can completely inhibit the proliferation of hepatocytes in the recovery phase of AILI (Bhushan et al., 2014, 2017a). However, single mitogen stimulation cannot improve liver regeneration or survival (Bhushan et al., 2014). Indeed, not all cytokines contribute to the regeneration of hepatocytes. In addition, some other growth factors, such as TGF-β, promote hepatocyte senescence and inhibit the regeneration of hepatocytes. An improvement in the regeneration and survival rate of hepatocytes in AILI mice is observed in the presence of TGF-β inhibitors or TGF-β receptor inhibitors (Bird et al., 2018). Thus, we hypothesize that there might be rapid liver regeneration after the escape of hepatocytes from TGF-β inhibition.
In the process of liver regeneration, cytokines function as initiating factors rather than direct mitogens (Michalopoulos, 2007). The TNF-α–TNF receptor p55 axis can attenuate liver damage and promote liver regeneration in AILI mice (James et al., 2005). Elevation of TNF-α triggers the activation of the nuclear factor-κB (NF-κB) pathway and promotes the expression of cyclin D1, a well-known key regulator of the cell cycle (Yang et al., 2011). Low expression of cyclin D1 is associated with a decrease in hepatocyte regeneration (Yang et al., 2009). In addition, activation of the IL-6/signal transducer and activator of transcription 3 (STAT3) signaling pathway contributes to liver regeneration in AILI mice (Bhushan et al., 2014). Liver regeneration is significantly impaired in IL-6 -/- mice, but regeneration recovers upon the administration of exogenous IL-6 to KO mice (James et al., 2003). According to a previous report, a single IL-6/STAT3 signal is not sufficient to enhance liver regeneration (Bhushan et al., 2014). In addition, IL-22 reduces liver damage by activating STAT3 and promotes hepatocyte regeneration in AILI mice (Scheiermann et al., 2013). Similarly, TNF-α participates in the activation of liver regeneration, which is also accompanied by the activation of STAT3 (James et al., 2005). These data show that the interacting network of cytokines involved in liver regeneration is complex and dynamic, and there is a crosstalk with STAT3 activation signals. In addition, soluble stem cell factor (SCF) was found to contribute to the proliferation of hepatocytes and increase the survival of AILI mice (Hu and Colletti, 2008). On this basis, we speculate that SCF plays important roles in liver regeneration.
4.2. Nonparenchymal cells
Clearance of necrotic cells is a prerequisite for the formation of new hepatocytes. Scavenger cells include mainly macrophages and neutrophils. The CCR2 receptor of blood-derived MoMFs receives the MCP-1 signal secreted by KCs, and then the MoMFs migrate to the liver to remove necrotic tissues (Antoniades et al., 2012; Krenkel et al., 2014). In CCR2 -/- AILI mice, there was less accumulation of MoMF in the liver, accompanied by delayed remission of liver injury. These results prove the role of MoMF in liver regeneration (Holt et al., 2008). Macrophages can produce mitogens (e.g., HGF, TGF-α, and TGF-β) and cytokines (e.g., IL-6 and TNF-α), which are crucial for liver regeneration. Additionally, MoMFs can upregulate VEGF expression, which promotes sprouting angiogenesis within the portal vein tract (Ehling et al., 2014). Another component involved in the scavenging of necrotic tissues is neutrophils. Williams et al. (2014) reported that the number of neutrophils increases during the recovery from AILI, and that these cells are in an activated state without ROS-induced toxicity. Then, the ROS released by neutrophils promote the maturation of MoMF and coordinate macrophages to participate in liver tissue repair (Yang et al., 2019).
Hepatic stellate cells (HSCs) and endothelial cells are important parts of the "niche" of liver parenchymal cells, which is crucial for maintaining liver morphology and function. HSCs are the main source of HGF in the liver (Bhushan and Apte, 2019). Many studies have indicated that clearance of activated HSCs triggers hepatocyte apoptosis, which then hinders liver regeneration. In the presence of HSC-derived paracrine factors, liver cell death is reduced to some extent, together with an increase in liver cell regeneration (Chang et al., 2017). However, these studies could not determine the direct effects of HSCs on liver regeneration. Endothelial cells are involved in angiogenesis and microvascular repair (Bhushan and Apte, 2019). VEGF is the mitogen of endothelial cells, and the expression of VEGF and VEGF receptor (VEGFR) is increased in AILI. Blockade of the VEGF–VEGFR axis reduces liver regeneration and the survival rate in AILI mice (Donahower et al., 2006). In contrast, the application of human recombinant VEGF in mice with AILI can reduce liver toxicity and increase liver cell regeneration (Donahower et al., 2010). These results indicate that endothelial cells might be involved in liver regeneration in AILI.
5. Treatment options for AILI
The FDA recommends the oxygen free radical scavenger NAC as the only treatment option for patients with AILI. However, adverse drug reactions and narrow therapeutic windows have limited its use. Therefore, there is a need to develop drugs that are superior to NAC in terms of efficacy and treatment time. In recent years, in-depth studies have provided us with new drugs and therapeutic strategies for the treatment of AILI.
5.1. Traditional NAC treatment
NAC was first approved for treating AILI in clinical practice in the UK. NAC is the only drug approved for the clinical treatment of AILI, and it eliminates NAPQI by supplementing GSH, which protects against liver damage by preventing the formation of NAPQI protein adducts (Corcoran and Wong, 1986). In addition, the latest study demonstrated that NAC can be metabolized into Krebs cycle intermediates, which are involved in the synthesis of mitochondrial ATP and reduce hepatocyte damage (Saito et al., 2010).
5.2. Novel mitochondria-targeted treatment
Over the years, extensive results have highlighted the important role of mitochondrial dysfunction in AILI. Therefore, targeted damaged mitochondrial therapy may be a new treatment strategy.
First, some drugs for the non-targeted treatment of AILI have proven to exert therapeutic effects by regulating mitochondrial dysfunction. For example, fomepizole (4MP), which has been used to treat alcoholism, shows a therapeutic effect on AILI in accidental clinical practice. 4MP prevents JNK activation and reduces GSH consumption and the formation of protein adducts to protect against liver injury (Akakpo et al., 2018). It has shown positive therapeutic effects in preclinical trials (Rasamison et al., 2020), indicating that 4MP may be an important adjuvant therapy for NAC. Metformin, which is used clinically in the treatment of diabetes, has been used to treat AILI in some experiments, as it can inhibit the oxidative stress of complex I in MRC. Moreover, metformin showed protective effects on mitochondrial oxidative stress in AILI models (Saeedi Saravi et al., 2016). Lee et al. (2015) first investigated the treatment efficiency of AILI with methylene blue, which is used to treat methemoglobinemia and is involved in the balance of electrons in the cytoplasm by reducing oxidized substances in cells. Lee et al. (2015) indicated that methylene blue can accept the free electrons leaked from the MRC and is involved in the subsequent transfer of these electrons to cytochrome C, which thereby reduces mitochondrial dysfunction and cellular necrosis. Minocycline, a mitochondrial Ca2+ and Fe2+ uniporter (MCFU) inhibitor, protects primary mouse liver cells treated by APAP by inhibiting Fe2+ from entering mitochondria and protecting mitochondria from further damage. Additionally, since minocycline is a drug approved by the FDA, the protective effect of minocycline on APAP-mediated injury in vivo should be explored in the future (Hu et al., 2016b).
Second, herbal therapy exhibits a beneficial effect. Wuzhi tablets extracted from Schisandra sphenanthera Rehder & E.H. Wilson are widely used for liver support in many Chinese regions. In an AILI mouse model, Wuzhi tablets reduced APAP-induced JNK activation in a dose-dependent manner and inhibited the formation of ROS (Fan et al., 2014). Rezende et al. (2014) found that Baccharis dracunculifolia leaf extract relieves liver damage in mice with AILI by improving 2,2-diphenyl-1-picrylhydrazyl (DPPH) antioxidant activity, showing hepatoprotective properties. Another study found that Glossogyne tenuifolia Cassini increases GSH and inhibits lipid peroxidation, thus showing hepatoprotective capacities in mice with APAP-induced liver damage (Tien et al., 2014). However, some experts think that herbal therapy is not feasible for AILI patients (Akakpo et al., 2020). Because most of these experimental data come from animal experiments, these experts believe that this approach is not realistic for clinical application because it requires long-term treatment and large amounts of herbal medicine. Of course, herbal medicine has been widely used clinically in China and other Asian countries. We believe that through the prospective verification of clinical trials, herbal therapies for liver damage caused by APAP will have certain application prospects (Chang et al., 2020).
Furthermore, some other interesting approaches are emerging. Trnka et al. (2008) first reported a new Mito-Tempo compound formed by the combination of piperidine nitrogen oxide (Tempo) and triphenylphosphonium (TPP+), which exerts mitochondrial-targeted antioxidant effects. Du et al. (2017b) found that Mito-Tempo has a strong ability to remove mitochondrial oxidative stress and reduce damage in a dose-dependent manner. They used the mitochondrial biogenesis inducer N-[2-[3-(piperazin-1-ylmethyl)imidazo[2,1-b][1,3]thiazol-6-yl]phenyl]quinoxaline-2-carboxamide
(SRT1720) to successfully increase peroxisome proliferator-activated receptor γ (PPARγ) coactivator 1-α expression and liver regeneration, thereby preventing advanced liver damage after APAP overdose (Du et al., 2017a). Barbier-Torres et al. (2017) discovered a new type of methylation-controlled J protein (MCJ)-small interfering RNA (siRNA) gene therapy method for treating AILI. MCJ is a mitochondrial oxidation respiratory chain inhibitor. Barbier-Torres et al. (2017) silenced MCJ to protect mice with AILI from liver damage. Of course, exogenous mitochondrial implantation may be another effective strategy for the treatment of AILI. Shi et al. (2018) injected human mitochondria into AILI mice, thereby increasing the energy supply of liver cells, reducing oxidative stress, and reducing tissue damage. In summary, as the hub of the pathogenesis of AILI, mitochondria are an attractive target for the treatment of APAP-mediated liver toxicity.
5.3. Other treatment options
Several novel and specific treatment methods for AILI are being tested in many preclinical studies, including a compensatory survival signal pathway nuclear factor erythroid 2-related factor 2 (Nrf2) activator (Fan et al., 2018; Wang et al., 2018), autophagy inducer (Kang et al., 2019), ERS inhibitor (Uzi et al., 2013), sterile inflammation regulator (Cai et al., 2014; Patel et al., 2016), and liver regeneration repair agent (Soeda et al., 2014; Bhushan et al., 2017b). However, note that there might be opposite effects at different stages for certain cellular events. JNK is involved in hepatocyte necrosis in the early stage, and it also plays an important role in liver regeneration. Therefore, when developing new drugs, researchers should pay attention to the following points: (1) What is the basic functional mechanism by which the drug acts? (2) Which stage of the disease it is suitable for? (3) Is it active for a long time? (4) Does the drug itself have some potential toxicity?
6. Conclusions
The mechanisms underlying AILI are intricate, ranging from hepatocyte necrosis to aseptic inflammation to liver regeneration, and are related to a variety of intracellular and extracellular events. In the initial stage after overdose, APAP is rapidly metabolized to toxic NAPQI, which forms a large number of protein adducts with cellular proteins, leading to mitochondrial and cellular dysfunction followed by hepatocyte necrosis. In the second stage, DAMPs released by necrotic hepatocytes cause the recruitment of inflammatory cells and the production of cytokines, eventually leading to aseptic inflammation. The third stage is liver regeneration. After the initiation of strong liver regeneration compensation, liver damage can be resolved, and liver function gradually recovers. If liver regeneration fails, severe organ failure and death will eventually occur.
Based on the cellular events involved in various stages, several treatment methods have been proposed. In addition to the commonly used NAC, there are many other promising options, such as mitochondria-targeted treatments, Nrf2 activators, and autophagy inducers. However, due to the intricacies of cell signal transduction, it is difficult to achieve positive test results, and much more research will be needed in the future.
Acknowledgments
This work was supported by the National Science and Technology Major Project of China (Nos. 2018ZX10302206 and 2017ZX10202203).
Author contributions
Xiaopeng CAI and Huiqiang CAI wrote the manuscript. Jing WANG, Qin YANG, and Jun GUAN did the data collection. Jingwen DENG and Zhi CHEN were responsible for conceptualization and manuscript revision. All authors have read and approved the final manuscript, and therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines
Xiaopeng CAI, Huiqiang CAI, Jing WANG, Qin YANG, Jun GUAN, Jingwen DENG, and Zhi CHEN declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Akakpo JY, Ramachandran A, Kandel SE, et al. , 2018. 4-Methylpyrazole protects against acetaminophen hepatotoxicity in mice and in primary human hepatocytes. Hum Exp Toxicol, 37(12): 1310-1322. 10.1177/0960327118774902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akakpo JY, Ramachandran A, Jaeschke H, 2020. Novel strategies for the treatment of acetaminophen hepatotoxicity. Expert Opin Drug Metab Toxicol, 16(11): 1039-1050. 10.1080/17425255.2020.1817896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades CG, Quaglia A, Taams LS, et al. , 2012. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology, 56(2): 735-746. 10.1002/hep.25657 [DOI] [PubMed] [Google Scholar]
- Apte U, Singh S, Zeng G, et al. , 2009. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am J Pathol, 175(3): 1056-1065. 10.2353/ajpath.2009.080976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier-Torres L, Iruzubieta P, Fernández-Ramos D, et al. , 2017. The mitochondrial negative regulator MCJ is a therapeutic target for acetaminophen-induced liver injury. Nat Commun, 8: 2068. 10.1038/s41467-017-01970-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry PA, Antoniades CG, Hussain MJ, et al. , 2010. Admission levels and early changes in serum interleukin-10 are predictive of poor outcome in acute liver failure and decompensated cirrhosis. Liver Int, 30(5): 733-740. 10.1111/j.1478-3231.2010.02219.x [DOI] [PubMed] [Google Scholar]
- Bhushan B, Apte U, 2019. Liver regeneration after acetaminophen hepatotoxicity: mechanisms and therapeutic opportunities. Am J Pathol, 189(4): 719-729. 10.1016/j.ajpath.2018.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan B, Walesky C, Manley M, et al. , 2014. Pro-regenerative signaling after acetaminophen-induced acute liver injury in mice identified using a novel incremental dose model. Am J Pathol, 184(11): 3013-3025. 10.1016/j.ajpath.2014.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan B, Chavan H, Borude P, et al. , 2017a. Dual role of epidermal growth factor receptor in liver injury and regeneration after acetaminophen overdose in mice. Toxicol Sci, 155(2): 363-378. 10.1093/toxsci/kfw213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan B, Poudel S, Manley MW, et al. , 2017b. Inhibition of glycogen synthase kinase 3 accelerated liver regeneration after acetaminophen-induced hepatotoxicity in mice. Am J Pathol, 187(3): 543-552. 10.1016/j.ajpath.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilzer M, Roggel F, Gerbes AL, 2006. Role of kupffer cells in host defense and liver disease. Liver Int, 26(10): 1175-1186. 10.1111/j.1478-3231.2006.01342.x [DOI] [PubMed] [Google Scholar]
- Bird TG, Müller M, Boulter L, et al. , 2018. TGFβ inhibition restores a regenerative response in acute liver injury by suppressing paracrine senescence. Sci Transl Med, 10(454): eaan1230. 10.1126/scitranslmed.aan1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazka ME, Wilmer JL, Holladay SD, et al. , 1995. Role of proinflammatory cytokines in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol, 133(1): 43-52. 10.1006/taap.1995.1125 [DOI] [PubMed] [Google Scholar]
- Boess F, Bopst M, Althaus R, et al. , 1998. Acetaminophen hepatotoxicity in tumor necrosis factor/lymphotoxin-α gene knockout mice. Hepatology, 27(4): 1021-1029. 10.1002/hep.510270418 [DOI] [PubMed] [Google Scholar]
- Bonaldi T, Talamo F, Scaffidi P, et al. , 2003. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J, 22(20): 5551-5560. 10.1093/emboj/cdg516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borude P, Bhushan B, Gunewardena S, et al. , 2018. Pleiotropic role of p53 in injury and liver regeneration after acetaminophen overdose. Am J Pathol, 188(6): 1406-1418. 10.1016/j.ajpath.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdi M, Masubuchi Y, Reilly TP, et al. , 2002. Protection against acetaminophen-induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology, 35(2): 289-298. 10.1053/jhep.2002.30956 [DOI] [PubMed] [Google Scholar]
- Bruderer R, Bernhardt OM, Gandhi T, et al. , 2015. Extending the limits of quantitative proteome profiling with data-independent acquisition and application to acetaminophen-treated three-dimensional liver microtissues. Mol Cell Proteomics, 14(5): 1400-1410. 10.1074/mcp.M114.044305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CC, Huang H, Whelan S, et al. , 2014. Benzyl alcohol attenuates acetaminophen-induced acute liver injury in a Toll-like receptor-4-dependent pattern in mice. Hepatology, 60(3): 990-1002. 10.1002/hep.27201 [DOI] [PubMed] [Google Scholar]
- Cederbaum AI, 2012. Alcohol metabolism. Clin Liver Dis, 16(4): 667-685. 10.1016/j.cld.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Xu DW, Zhu JJ, et al. , 2020. Herbal therapy for the treatment of acetaminophen-associated liver injury: recent advances and future perspectives. Front Pharmacol, 11: 313. 10.3389/fphar.2020.00313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WJ, Song LJ, Chang XJ, et al. , 2017. Early activated hepatic stellate cell-derived paracrine molecules modulate acute liver injury and regeneration. Lab Invest, 97(3): 318-328. 10.1038/labinvest.2016.130 [DOI] [PubMed] [Google Scholar]
- Chiew AL, Gluud C, Brok J, et al. , 2018. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst Rev, 2(2): Cd003328. 10.1002/14651858.CD003328.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu H, Gardner CR, Dambach DM, et al. , 2003. Role of p55 tumor necrosis factor receptor 1 in acetaminophen-induced antioxidant defense. Am J Physiol Gastrointest Liver Physiol, 285(5): G959-G966. 10.1152/ajpgi.00219.2003 [DOI] [PubMed] [Google Scholar]
- Chowdhury A, Nabila J, Adelusi Temitope I, et al. , 2020. Current etiological comprehension and therapeutic targets of acetaminophen-induced hepatotoxicity. Pharmacol Res, 161: 105102. 10.1016/j.phrs.2020.105102 [DOI] [PubMed] [Google Scholar]
- Corcoran GB, Wong BK, 1986. Role of glutathione in prevention of acetaminophen-induced hepatotoxicity by N-acetyl-L-cysteine in vivo: studies with N-acetyl-D-cysteine in mice. J Pharmacol Exp Ther, 238(1): 54-61. [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, et al. , 2005. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther, 315(2): 879-887. 10.1124/jpet.105.088898 [DOI] [PubMed] [Google Scholar]
- Cover C, Liu J, Farhood A, et al. , 2006. Pathophysiological role of the acute inflammatory response during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol, 216(1): 98-107. 10.1016/j.taap.2006.04.010 [DOI] [PubMed] [Google Scholar]
- Dahlin DC, Miwa GT, Lu AY, et al. , 1984. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci USA, 81(5): 1327-1331. 10.1073/pnas.81.5.1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambach DM, Watson LM, Gray KR, et al. , 2002. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology, 35(5): 1093-1103. 10.1053/jhep.2002.33162 [DOI] [PubMed] [Google Scholar]
- Ding Y, Li Q, Xu Y, et al. , 2016. Attenuating oxidative stress by paeonol protected against acetaminophen-induced hepatotoxicity in mice. PLoS ONE, 11(5): e0154375. 10.1371/journal.pone.0154375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahower B, McCullough SS, Kurten R, et al. , 2006. Vascular endothelial growth factor and hepatocyte regeneration in acetaminophen toxicity. Am J Physiol Gastrointest Liver Physiol, 291(1): G102-G109. 10.1152/ajpgi.00575.2005 [DOI] [PubMed] [Google Scholar]
- Donahower BC, McCullough SS, Hennings L, et al. , 2010. Human recombinant vascular endothelial growth factor reduces necrosis and enhances hepatocyte regeneration in a mouse model of acetaminophen toxicity. J Pharmacol Exp Ther, 334(1): 33-43. 10.1124/jpet.109.163840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs I, Aw TY, Liu JF, et al. , 2012. Vα14iNKT cell deficiency prevents acetaminophen-induced acute liver failure by enhancing hepatic glutathione and altering APAP metabolism. Biochem Biophys Res Commun, 428(2): 245-251. 10.1016/j.bbrc.2012.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Ramachandran A, Jaeschke H, 2016. Oxidative stress during acetaminophen hepatotoxicity: sources, pathophysiological role and therapeutic potential. Redox Biol, 10: 148-156. 10.1016/j.redox.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Ramachandran A, McGill MR, et al. , 2017a. Induction of mitochondrial biogenesis protects against acetaminophen hepatotoxicity. Food Chem Toxicol, 108: 339-350. 10.1016/j.fct.2017.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Farhood A, Jaeschke H, 2017b. Mitochondria-targeted antioxidant Mito-Tempo protects against acetaminophen hepatotoxicity. Arch Toxicol, 91(2): 761-773. 10.1007/s00204-016-1692-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehling J, Bartneck M, Wei X, et al. , 2014. CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut, 63(12): 1960-1971. 10.1136/gutjnl-2013-306294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan XM, Jiang YM, Wang Y, et al. , 2014. Wuzhi tablet (Schisandra sphenanthera extract) protects against acetaminophen-induced hepatotoxicity by inhibition of CYP-mediated bioactivation and regulation of NRF2-ARE and p53/p21 pathways. Drug Metab Dispos, 42(12): 1982-1990. 10.1124/dmd.114.059535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan XY, Wang LD, Huang JB, et al. , 2018. Pterostilbene reduces acetaminophen-induced liver injury by activating the Nrf2 antioxidative defense system via the AMPK/AKT/GSK3β pathway. Cell Physiol Biochem, 49(5): 1943-1958. 10.1159/000493655 [DOI] [PubMed] [Google Scholar]
- Feng DC, Wang Y, Wang H, et al. , 2014. Acute and chronic effects of IL-22 on acetaminophen-induced liver injury. J Immunol, 193(5): 2512-2518. 10.4049/jimmunol.1400588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JE, Mckenzie TJ, Lillegard JB, et al. , 2013. Role of Kupffer cells and Toll-like receptor 4 in acetaminophen-induced acute liver failure. J Surg Res, 180(1): 147-155. 10.1016/j.jss.2012.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CL, Liu Y, Leng J, et al. , 2018. Platycodin D protects acetaminophen-induced hepatotoxicity by inhibiting hepatocyte MAPK pathway and apoptosis in C57BL/6J mice. Biomed Pharmacother, 107: 867-877. 10.1016/j.biopha.2018.08.082 [DOI] [PubMed] [Google Scholar]
- Gao RY, Wang M, Liu QH, et al. , 2020. Hypoxia-inducible factor-2α reprograms liver macrophages to protect against acute liver injury through the production of interleukin-6. Hepatology, 71(6): 2105-2117. 10.1002/hep.30954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong SH, Lan T, Zeng LY, et al. , 2018. Gut microbiota mediates diurnal variation of acetaminophen induced acute liver injury in mice. J Hepatol, 69(1): 51-59. 10.1016/j.jhep.2018.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgman MJ, Garrard AR, 2012. A review of acetaminophen poisoning. Crit Care Clin, 28(4): 499-516. 10.1016/j.ccc.2012.07.006 [DOI] [PubMed] [Google Scholar]
- Holt MP, Cheng LL, Ju C, 2008. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J Leukoc Biol, 84(6): 1410-1421. 10.1189/jlb.0308173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou HS, Liao CL, Sytwu HK, et al. , 2012. Deficiency of interleukin-15 enhances susceptibility to acetaminophen-induced liver injury in mice. PLoS ONE, 7(9): e44880. 10.1371/journal.pone.0044880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Colletti LM, 2008. Stem cell factor and c-kit are involved in hepatic recovery after acetaminophen-induced liver injury in mice. Am J Physiol Gastrointest Liver Physiol, 295(1): G45-G53. 10.1152/ajpgi.00024.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JT, Ramshesh VK, McGill MR, et al. , 2016a. Low dose acetaminophen induces reversible mitochondrial dysfunction associated with transient c-Jun N-terminal kinase activation in mouse liver. Toxicol Sci, 150(1): 204-215. 10.1093/toxsci/kfv319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JT, Kholmukhamedov A, Lindsey CC, et al. , 2016b. Translocation of iron from lysosomes to mitochondria during acetaminophen-induced hepatocellular injury: protection by starch-desferal and minocycline. Free Radic Biol Med, 97: 418-426. 10.1016/j.freeradbiomed.2016.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y, Yin S, Yan M, et al. , 2017. Protective role of p53 in acetaminophen hepatotoxicity. Free Radic Biol Med, 106: 111-117. 10.1016/j.freeradbiomed.2017.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igusa Y, Yamashina S, Izumi K, et al. , 2012. Loss of autophagy promotes murine acetaminophen hepatotoxicity. J Gastroenterol, 47(4): 433-443. 10.1007/s00535-011-0500-0 [DOI] [PubMed] [Google Scholar]
- Imaeda AB, Watanabe A, Sohail MA, et al. , 2009. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest, 119(2): 305-314. 10.1172/jci35958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Kondo T, Ohshima T, et al. , 2002. A pivotal involvement of IFN-γ in the pathogenesis of acetaminophen-induced acute liver injury. FASEB J, 16(10): 1227-1236. 10.1096/fj.02-0046com [DOI] [PubMed] [Google Scholar]
- Ishida Y, Kondo T, Tsuneyama K, et al. , 2004. The pathogenic roles of tumor necrosis factor receptor p55 in acetaminophen-induced liver injury in mice. J Leukoc Biol, 75(1): 59-67. 10.1189/jlb.0403152 [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Liu J, 2007. Neutrophil depletion protects against murine acetaminophen hepatotoxicity: another perspective. Hepatology, 45(6): 1588-1589. 10.1002/hep.21549 [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Ramachandran A, 2020. Mechanisms and pathophysiological significance of sterile inflammation during acetaminophen hepatotoxicity. Food Chem Toxicol, 138: 111240. 10.1016/j.fct.2020.111240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Ramachandran A, 2012. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev, 44(1): 88-106. 10.3109/03602532.2011.602688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Xie YC, McGill MR, 2014. Acetaminophen-induced liver injury: from animal models to humans. J Clin Transl Hepatol, 2(3): 153-161. 10.14218/jcth.2014.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Akakpo JY, Umbaugh DS, et al. , 2020. Novel therapeutic approaches against acetaminophen-induced liver injury and acute liver failure. Toxicol Sci, 174(2): 159-167. 10.1093/toxsci/kfaa002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LP, Lamps LW, McCullough S, et al. , 2003. Interleukin 6 and hepatocyte regeneration in acetaminophen toxicity in the mouse. Biochem Biophys Res Commun, 309(4): 857-863. 10.1016/j.bbrc.2003.08.085 [DOI] [PubMed] [Google Scholar]
- James LP, Kurten RC, Lamps LW, et al. , 2005. Tumour necrosis factor receptor 1 and hepatocyte regeneration in acetaminophen toxicity: a kinetic study of proliferating cell nuclear antigen and cytokine expression. Basic Clin Pharmacol Toxicol, 97(1): 8-14. 10.1111/j.1742-7843.2005.pto_97102.x [DOI] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Potter WZ, et al. , 1973. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther, 187(1): 195-202. [PubMed] [Google Scholar]
- Kang KY, Shin JK, Lee SM, 2019. Pterostilbene protects against acetaminophen-induced liver injury by restoring impaired autophagic flux. Food Chem Toxicol, 123: 536-545. 10.1016/j.fct.2018.12.012 [DOI] [PubMed] [Google Scholar]
- Karch J, Molkentin JD, 2014. Identifying the components of the elusive mitochondrial permeability transition pore. Proc Natl Acad Sci USA, 111(29): 10396-10397. 10.1073/pnas.1410104111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt D, Giannou AD, McGee HM, et al. , 2017. A protective function of IL-22BP in ischemia reperfusion and acetaminophen-induced liver injury. J Immunol, 199(12): 4078-4090. 10.4049/jimmunol.1700587 [DOI] [PubMed] [Google Scholar]
- Kono H, Chen CJ, Ontiveros F, et al. , 2010. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest, 120(6): 1939-1949. 10.1172/jci40124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenkel O, Mossanen JC, Tacke F, 2014. Immune mechanisms in acetaminophen-induced acute liver failure. Hepatobiliary Surg Nutr, 3(6): 331-343. 10.3978/j.issn.2304-3881.2014.11.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruiswijk F, Labuschagne CF, Vousden KH, 2015. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat Rev Mol Cell Biol, 16(7): 393-405. 10.1038/nrm4007 [DOI] [PubMed] [Google Scholar]
- Kubes P, Mehal WZ, 2012. Sterile inflammation in the liver. Gastroenterology, 143(5): 1158-1172. 10.1053/j.gastro.2012.09.008 [DOI] [PubMed] [Google Scholar]
- Lawson JA, Farhood A, Hopper RD, et al. , 2000. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol Sci, 54(2): 509-516. 10.1093/toxsci/54.2.509 [DOI] [PubMed] [Google Scholar]
- Lee HC, Liao CC, Day YJ, et al. , 2018. IL-17 deficiency attenuates acetaminophen-induced hepatotoxicity in mice. Toxicol Lett, 292: 20-30. 10.1016/j.toxlet.2018.04.021 [DOI] [PubMed] [Google Scholar]
- Lee HC, Yu HP, Liao CC, et al. , 2019. Escin protects against acetaminophen-induced liver injury in mice via attenuating inflammatory response and inhibiting ERK signaling pathway. Am J Transl Res, 11(8): 5170-5182. [PMC free article] [PubMed] [Google Scholar]
- Lee KK, Imaizumi N, Chamberland SR, et al. , 2015. Targeting mitochondria with methylene blue protects mice against acetaminophen-induced liver injury. Hepatology, 61(1): 326-336. 10.1002/hep.27385 [DOI] [PubMed] [Google Scholar]
- Lemasters JJ, 1998. The mitochondrial permeability transition: from biochemical curiosity to pathophysiological mechanism. Gastroenterology, 115(3): 783-786. 10.1016/s0016-5085(98)70160-x [DOI] [PubMed] [Google Scholar]
- Liao CC, Day YJ, Lee HC, et al. , 2017. ERK signaling pathway plays a key role in baicalin protection against acetaminophen-induced liver injury. Am J Chin Med, 45(1): 105-121. 10.1142/s0192415x17500082 [DOI] [PubMed] [Google Scholar]
- Liu FC, Yu HP, Chou AH, et al. , 2020. Corilagin reduces acetaminophen-induced hepatotoxicity through MAPK and NF-κB signaling pathway in a mouse model. Am J Transl Res, 12(9): 5597-5607. [PMC free article] [PubMed] [Google Scholar]
- Liu ZX, Govindarajan S, Kaplowitz N, 2004. Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology, 127(6): 1760-1774. 10.1053/j.gastro.2004.08.053 [DOI] [PubMed] [Google Scholar]
- Lu SC, 1999. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J, 13(10): 1169-1183. 10.1096/fasebj.13.10.1169 [DOI] [PubMed] [Google Scholar]
- Marques PE, Amaral SS, Pires DA, et al. , 2012. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology, 56(5): 1971-1982. 10.1002/hep.25801 [DOI] [PubMed] [Google Scholar]
- Martin-Murphy BV, Kominsky DJ, Orlicky DJ, et al. , 2013. Increased susceptibility of natural killer T-cell-deficient mice to acetaminophen-induced liver injury. Hepatology, 57(4): 1575-1584. 10.1002/hep.26134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael SL, Pumford NR, Mayeux PR, et al. , 1999. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology, 30(1): 186-195. 10.1002/hep.510300104 [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK, 2007. Liver regeneration. J Cell Physiol, 213(2): 286-300. 10.1002/jcp.21172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo RD, Lai RT, Lu J, et al. , 2018. Enhanced autophagy contributes to protective effects of IL-22 against acetaminophen-induced liver injury. Theranostics, 8(15): 4170-4180. 10.7150/thno.25798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A, Torres S, Baulies A, et al. , 2018. Mitochondrial-lysosomal axis in acetaminophen hepatotoxicity. Front Pharmacol, 9: 453. 10.3389/fphar.2018.00453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JK, Craig DG, Pryde EA, et al. , 2013. Persistently elevated troponin I in paracetamol hepatotoxicity: association with liver injury, organ failure, and outcome. Clin Toxicol, 51(7): 532-539. 10.3109/15563650.2013.816853 [DOI] [PubMed] [Google Scholar]
- Moore MN, 2008. Autophagy as a second level protective process in conferring resistance to environmentally-induced oxidative stress. Autophagy, 4(2): 254-256. 10.4161/auto.5528 [DOI] [PubMed] [Google Scholar]
- Ni HM, Bockus A, Boggess N, et al. , 2012. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology, 55(1): 222-232. 10.1002/hep.24690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SJ, Luther J, Bohr S, et al. , 2016. A novel resolvin-based strategy for limiting acetaminophen hepatotoxicity. Clin Transl Gastroenterol, 7(3): e153. 10.1038/ctg.2016.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Jaeschke H, 2019. Acetaminophen hepatotoxicity. Semin Liver Dis, 39(2): 221-234. 10.1055/s-0039-1679919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Baines CP, et al. , 2011. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res, 45(2): 156-164. 10.3109/10715762.2010.520319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasamison R, Besson H, Berleur MP, et al. , 2020. Analysis of fomepizole safety based on a 16-year post-marketing experience in france. Clin Toxicol, 58(7): 742-747. 10.1080/15563650.2019.1676899 [DOI] [PubMed] [Google Scholar]
- Reuben A, Tillman H, Fontana RJ, et al. , 2016. Outcomes in adults with acute liver failure between 1998 and 2013: an observational cohort study. Ann Intern Med, 164(11): 724-732. 10.7326/m15-2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende TP, do A. Corrêa JO, Aarestrup BJV, et al. , 2014. Protective effects of Baccharis dracunculifolia leaves extract against carbon tetrachloride- and acetaminophen-induced hepatotoxicity in experimental animals. Molecules, 19(7): 9257-9272. 10.3390/molecules19079257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PM, Bourdi M, Korrapati MC, et al. , 2012. Endogenous interleukin-4 regulates glutathione synthesis following acetaminophen-induced liver injury in mice. Chem Res Toxicol, 25(1): 83-93. 10.1021/tx2003992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeedi Saravi SS, Hasanvand A, Shahkarami K, et al. , 2016. The protective potential of metformin against acetaminophen-induced hepatotoxicity in BALB/C mice. Pharm Biol, 54(12): 2830-2837. 10.1080/13880209.2016.1185633 [DOI] [PubMed] [Google Scholar]
- Saito C, Zwingmann C, Jaeschke H, 2010. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology, 51(1): 246-254. 10.1002/hep.23267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann P, Bachmann M, Goren I, et al. , 2013. Application of interleukin-22 mediates protection in experimental acetaminophen-induced acute liver injury. Am J Pathol, 182(4): 1107-1113. 10.1016/j.ajpath.2012.12.010 [DOI] [PubMed] [Google Scholar]
- Schmidt LE, Dalhoff K, Poulsen HE, 2002. Acute versus chronic alcohol consumption in acetaminophen-induced hepatotoxicity. Hepatology, 35(4): 876-882. 10.1053/jhep.2002.32148 [DOI] [PubMed] [Google Scholar]
- Schneider KM, Elfers C, Ghallab A, et al. , 2021. Intestinal dysbiosis amplifies acetaminophen-induced acute liver injury. Cell Mol Gastroenterol Hepatol, 11(4): 909-933. 10.1016/j.jcmgh.2020.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki E, Schnabl B, 2012. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol, 590(3): 447-458. 10.1113/jphysiol.2011.219691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Gadang V, Jaeschke A, 2012. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol, 82(5): 1001-1007. 10.1124/mol.112.079863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi XX, Bai HY, Zhao M, et al. , 2018. Treatment of acetaminophen-induced liver injury with exogenous mitochondria in mice. Transl Res, 196: 31-41. 10.1016/j.trsl.2018.02.003 [DOI] [PubMed] [Google Scholar]
- Shinohara M, Ybanez MD, Win S, et al. , 2010. Silencing glycogen synthase kinase-3β inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid cell leukemia sequence 1. J Biol Chem, 285(11): 8244-8255. 10.1074/jbc.M109.054999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson KJ, Lukacs NW, McGregor AH, et al. , 2000. Inhibition of tumour necrosis factor alpha does not prevent experimental paracetamol-induced hepatic necrosis. J Pathol, 190(4): 489-494. [DOI] [PubMed] [Google Scholar]
- Smilkstein MJ, Knapp GL, Kulig KW, et al. , 1988. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. N Engl J Med, 319(24): 1557-1562. 10.1056/nejm198812153192401 [DOI] [PubMed] [Google Scholar]
- Soeda J, Mouralidarane A, Ray S, et al. , 2014. The beta-adrenoceptor agonist isoproterenol rescues acetaminophen-injured livers through increasing progenitor numbers by Wnt in mice. Hepatology, 60(3): 1023-1034. 10.1002/hep.27266 [DOI] [PubMed] [Google Scholar]
- Sun Y, Li TY, Song L, et al. , 2018. Liver-specific deficiency of unc-51 like kinase 1 and 2 protects mice from acetaminophen-induced liver injury. Hepatology, 67(6): 2397-2413. 10.1002/hep.29759 [DOI] [PubMed] [Google Scholar]
- Tien YH, Chen BH, Wang Hsu GS, et al. , 2014. Hepatoprotective and anti-oxidant activities of Glossogyne tenuifolia against acetaminophen-induced hepatotoxicity in mice. Am J Chin Med, 42(6): 1385-1398. 10.1142/s0192415x14500876 [DOI] [PubMed] [Google Scholar]
- Tirmenstein MA, Nelson SD, 1989. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3'-hydroxyacetanilide, in mouse liver. J Biol Chem, 264(17): 9814-9819. 10.1016/S0021-9258(18)81731-8 [DOI] [PubMed] [Google Scholar]
- Torres S, Baulies A, Insausti-Urkia N, et al. , 2019. Endoplasmic reticulum stress-induced upregulation of STARD1 promotes acetaminophen-induced acute liver failure. Gastroenterology, 157(2): 552-568. 10.1053/j.gastro.2019.04.023 [DOI] [PubMed] [Google Scholar]
- Trnka J, Blaikie FH, Smith RAJ, et al. , 2008. A mitochondria-targeted nitroxide is reduced to its hydroxylamine by ubiquinol in mitochondria. Free Radic Biol Med, 44(7): 1406-1419. 10.1016/j.freeradbiomed.2007.12.036 [DOI] [PubMed] [Google Scholar]
- Uzi D, Barda L, Scaiewicz V, et al. , 2013. CHOP is a critical regulator of acetaminophen-induced hepatotoxicity. J Hepatol, 59(3): 495-503. 10.1016/j.jhep.2013.04.024 [DOI] [PubMed] [Google Scholar]
- Walker V, Mills GA, Anderson ME, et al. , 2017. The acetaminophen metabolite N-acetyl-p-benzoquinone imine (NAPQI) inhibits glutathione synthetase in vitro; a clue to the mechanism of 5-oxoprolinuric acidosis? Xenobiotica, 47(2): 164-175. 10.3109/00498254.2016.1166533 [DOI] [PubMed] [Google Scholar]
- Wang XF, Sun R, Wei HM, et al. , 2013. High-mobility group box 1 (HMGB1)-Toll-like receptor (TLR)4-interleukin (IL)-23-IL-17A axis in drug-induced damage-associated lethal hepatitis: interaction of γδ T cells with macrophages. Hepatology, 57(1): 373-384. 10.1002/hep.25982 [DOI] [PubMed] [Google Scholar]
- Wang YQ, Wei JG, Tu MJ, et al. , 2018. Fucoidan alleviates acetaminophen-induced hepatotoxicity via oxidative stress inhibition and Nrf2 translocation. Int J Mol Sci, 19(12): 4050. 10.3390/ijms19124050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring WS, Stephen AF, Malkowska AM, et al. , 2008. Acute ethanol coingestion confers a lower risk of hepatotoxicity after deliberate acetaminophen overdose. Acad Emerg Med, 15(1): 54-58. 10.1111/j.1553-2712.2007.00019.x [DOI] [PubMed] [Google Scholar]
- Williams CD, Bajt ML, Farhood A, et al. , 2010. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int, 30(9): 1280-1292. 10.1111/j.1478-3231.2010.02284.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Bajt ML, Sharpe MR, et al. , 2014. Neutrophil activation during acetaminophen hepatotoxicity and repair in mice and humans. Toxicol Appl Pharmacol, 275(2): 122-133. 10.1016/j.taap.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win S, Than TA, Min RWM, et al. , 2016. c-Jun N-terminal kinase mediates mouse liver injury through a novel Sab (SH3BP5)-dependent pathway leading to inactivation of intramitochondrial Src. Hepatology, 63(6): 1987-2003. 10.1002/hep.28486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win S, Min RWM, Chen CQ, et al. , 2019. Expression of mitochondrial membrane-linked SAB determines severity of sex-dependent acute liver injury. J Clin Invest, 129(12): 5278-5293. 10.1172/jci128289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Jaeschke H, 2018. Mechanisms of inflammatory liver injury and drug-induced hepatotoxicity. Curr Pharmacol Rep, 4(5): 346-357. 10.1007/s40495-018-0147-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KC, Ho YL, Kuo YH, et al. , 2018. Hepatoprotective effect of Ugonin M, a Helminthostachys zeylanica constituent, on acetaminophen-induced acute liver injury in mice. Molecules, 23(10): 2420. 10.3390/molecules23102420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie YC, McGill MR, Cook SF, et al. , 2015. Time course of acetaminophen-protein adducts and acetaminophen metabolites in circulation of overdose patients and in HepaRG cells. Xenobiotica, 45(10): 921-929. 10.3109/00498254.2015.1026426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhang XM, Monestier M, et al. , 2011. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol, 187(5): 2626-2631. 10.4049/jimmunol.1003930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan AW, Fouts DE, Brandl J, et al. , 2011. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology, 53(1): 96-105. 10.1002/hep.24018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan MZ, Huo YZ, Yin ST, et al. , 2018. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol, 17: 274-283. 10.1016/j.redox.2018.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang RK, Miki K, He X, et al. , 2009. Prolonged treatment with N-acetylcystine delays liver recovery from acetaminophen hepatotoxicity. Crit Care, 13(2): R55. 10.1186/cc7782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang RK, Zhang ST, Kajander H, et al. , 2011. Ringer’s lactate improves liver recovery in a murine model of acetaminophen toxicity. BMC Gastroenterol, 11: 125. 10.1186/1471-230x-11-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang RK, Zhang ST, Cotoia A, et al. , 2012. High mobility group B1 impairs hepatocyte regeneration in acetaminophen hepatotoxicity. BMC Gastroenterol, 12: 45. 10.1186/1471-230x-12-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WT, Tao YD, Wu Y, et al. , 2019. Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat Commun, 10: 1076. 10.1038/s41467-019-09046-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee SB, Bourdi M, Masson MJ, et al. , 2007. Hepatoprotective role of endogenous interleukin-13 in a murine model of acetaminophen-induced liver disease. Chem Res Toxicol, 20(5): 734-744. 10.1021/tx600349f [DOI] [PubMed] [Google Scholar]
- Yoshimoto S, Loo TM, Atarashi K, et al. , 2013. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature, 499(7456): 97-101. 10.1038/nature12347 [DOI] [PubMed] [Google Scholar]
- Zhang C, Feng J, Du J, et al. , 2018. Macrophage-derived IL-1α promotes sterile inflammation in a mouse model of acetaminophen hepatotoxicity. Cell Mol Immunol, 15(11): 973-982. 10.1038/cmi.2017.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, Zhang SM, Bi JB, et al. , 2017. Astaxanthin pretreatment attenuates acetaminophen-induced liver injury in mice. Int Immunopharmacol, 45: 26-33. 10.1016/j.intimp.2017.01.028 [DOI] [PubMed] [Google Scholar]
- Zigmond E, Samia-Grinberg S, Pasmanik-Chor M, et al. , 2014. Infiltrating monocyte-derived macrophages and resident Kupffer cells display different ontogeny and functions in acute liver injury. J Immunol, 193(1): 344-353. 10.4049/jimmunol.1400574 [DOI] [PubMed] [Google Scholar]