Cucurbitaceae is an important family of flowering plants containing multiple species of important food plants, such as melons, cucumbers, squashes, and pumpkins. However, a highly efficient genetic transformation system has not been established for most of these species (Nanasato and Tabei, 2020). Watermelon (Citrullus lanatus), an economically important and globally cultivated fruit crop, is a model species for fruit quality research due to its rich diversity of fruit size, shape, flavor, aroma, texture, peel and flesh color, and nutritional composition (Guo et al., 2019). Through pan-genome sequencing, many candidate loci associated with fruit quality traits have been identified (Guo et al., 2019). However, few of these loci have been validated. The major barrier is the low transformation efficiency of the species, with only few successful cases of genetic transformation reported so far (Tian et al., 2017; Feng et al., 2021; Wang JF et al., 2021; Wang YP et al., 2021). For example, Tian et al. (2017) obtained only 16 transgenic lines from about 960 cotyledon fragments, yielding a transformation efficiency of 1.67%. Therefore, efficient genetic transformation could not only facilitate the functional genomic studies in watermelon as well as other horticultural species, but also speed up the transgenic and genome-editing breeding.
Reprograming the cell fate of explants in tissue culture is regulated by genes related to plant development. Previous studies have identified numerous genes that can promote or reprogram cell fate, which are known as developmental regulators (DRs) (Méndez-Hernández1 et al., 2019). Several DRs have been reported to improve the regeneration efficiency of various plant species in tissue culture (Méndez-Hernández1 et al., 2019; Zhang et al., 2021). For example, the overexpression of APETALA2/ethylene responsive element-binding factor (AP2/ERF) family transcription factor BABY BOOM (BBM) can promote cell proliferation and ectopic embryo formation in the cotyledons and leaves of Arabidopsis. The co-expression of BBM with the shoot apical meristem identity regulator WUSCHEL (WUS) could greatly improve the in vitro transformation efficiency of various monocot species, including maize inbred lines, rice, and sorghum (Lowe et al., 2016). However, the constitutive overexpression of both BBM and WUS causes severe growth defects, such as the abnormal development of vegetative and reproductive organs and infertility. Therefore, the expression of these DRs needs to be restricted or the genes need to be eliminated during or after tissue culture (Lowe et al., 2016). Plant-specific transcription factor proteins, called growth-regulating factors (GRFs), have also been reported to boost the regeneration and genetic transformation efficiency of various crop plants. Stably expressed GRF5 from Arabidopsis accelerates shoot organogenesis and increases the genetic transformation efficiency of soybean, canola, and sunflower (Kong et al., 2020). Similarly, the overexpression of a chimeric protein consisting of the wheat GRF4 and rice GRF-interacting factor 1 (GIF1) improves the regeneration efficiency and regeneration speed in wheat, triticale, and rice, and also increases the number of transformable wheat genotypes (Debernardi et al., 2020). Therefore, we tested the effects of these DRs on watermelon genetic transformation.
To improve the transformation efficiency of watermelon, we first established a fast and simple detection system for the genetic transformation. Both the neomycin phosphotransferase II (nptII) gene, which confers kanamycin (Kan) resistance to transgenic plants, and the hygromycin (Hyg) phosphotransferase II (hptII) gene, which confers Hyg resistance, have been used as the selectable markers in watermelon tissue culture. Hyg causes more severe damage than Kan during watermelon tissue culture (Park et al., 2005), and the time required for tissue culture in Hyg medium is usually longer than that required for Kan; therefore, nptII was chosen as the selectable marker. In addition, given the high frequency of false positive plants obtained after Kan selection (Park et al., 2005), the red fluorescent protein DsRed2, which can be easily observed using the LUYOR-3415RG hand-held lamp (Luyor Corporation, Shanghai, China), was introduced into the binary vector as another marker to visually identify stable transgenic events. The reporter vector was named as pW501 (Fig. 1a).
Fig. 1. Effects of developmental regulators (DRs) on watermelon transformation. (a) Schematic diagrams of constructs with DRs used in this study. (b) Genetic transformation of the watermelon cultivar WW150. b1, watermelon seeds after sterilization; b2, 4-d-old watermelon seedlings; b3, cotyledon fragments after co-cultivation; b4, callus formed on the selection medium after two weeks; b5, adventitious buds on the callus; b6, adventitious root formed on the regenerated plants; b7, seedlings with roots on the rooting medium (left is a transgenic plant; right is a non-transgenic plant); b8, the same plants as in b6 with the picture taken under a hand-held florescence detection device. Scale bars=0.5 cm. (c) The transformation efficiencies of watermelon cultivar WW150 obtained using the indicated constructs. Positive transformation events were defined as calli showing at least one regenerated adventitious bud expressing the DsRed fluorescent signal. (d) Representative images of adventitious shoots generated by the indicated vectors (upper panels). The fluorescent DsRed2 signals are shown in the lower panels. Scale bars=0.5 cm. (e) The growth phenotype of a watermelon plant transformed with the pW502 vector two weeks after being transferred to soil. Scale bar=4 cm. RB: right border; LB: left border.
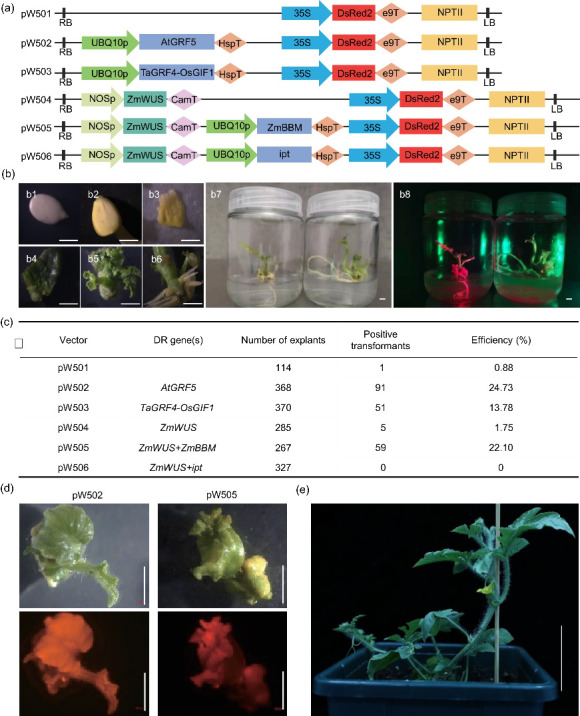
We first tested the genetic transformation of the watermelon cultivar WWl50, the female parental line of the widely grown cultivar "Xinong No. 8," which is relatively recalcitrant to transformation. The vectors were introduced into three widely used laboratory Agrobacterium strains, EHA105, GV3101, and LBA4404, and then delivered to cotyledonary fragments using the established Agrobacterium-mediated transformation method (Tian et al., 2017). Calli were induced after co-cultivation and growth on selection medium containing 50 mg/L Kan for two weeks (Fig. 1b). After about 70 d of selection, adventitious shoots and roots were regenerated, and positive transformation events could be easily detected by a hand-held fluorescent lamp (Fig. 1b). The transgene-specific polymerase chain reaction (PCR) result with primers specific to DsRed also demonstrated that the transfer DNA (T-DNA) fragment was integrated into the watermelon genome in DsRed fluorescent positive lines (Fig. S1a).
The transformation frequencies were determined by counting the number of calli showing at least one regenerated adventitious bud with a DsRed fluorescent signal out of the total number of inoculated cotyledonary fragments. Among the three tested Agrobacterium strains, EHA105 had the highest transformation efficiency of 0.92%, which was comparable with the efficiency reported in previous studies (Tian et al., 2017), while the efficiency was 0.88% for GV3101 and no positive transformation events were detected using LBA4404 (Fig. S1b).
We assumed that the low transformation efficiency might be mainly due to the low regeneration efficiency of transgenic cells. Several DRs were reported to boost the regeneration efficiency or transgenic efficiency of various plant species in tissue culture (Lowe et al., 2016; Méndez-Hernández1 et al., 2019; Debernardi et al., 2020; Kong et al., 2020; Zhang et al., 2021). To test the effects of DRs on watermelon genetic transformation, we added several DR genes individually and in combination to the binary vector pW501. The expression of all DR genes was driven by the Arabidopsis UBQ10 promoter, except for WUS, whose expression was driven by the Nos promoter as previously described (Lowe et al., 2016) (Fig. 1a).
All of the vectors were introduced into the Agrobacterium strain GV3101 and then transformed into the watermelon cultivar WWl50. Some of the DR genes successfully boosted the transformation efficiency of WWl50 (Fig. 1c). Among the vectors carrying DR genes, pW502, which overexpresses Arabidopsis thaliana GRF5 (AtGRF5), resulted in the highest transformation efficiency (24.73%). pW503, which overexpresses the chimeric TaGRF4-OsGIF1 gene (GRF4 from wheat and GIF1 from rice), and pW505, which overexpresses ZmWUS plus ZmBBM (WUS and BBM from maize), also dramatically enhanced the transformation with efficiencies of 13.78% and 22.10%, respectively. The ZmWUS gene alone (pW504) or ZmWUS plus the isopentenyl transferase from Agrobacterium (ipt) (pW506) resulted in very weak or no increases in the transformation efficiency (Fig. 1c). We observed numerous growth abnormalities in plants overexpressing ZmWUS plus ZmBBM, including abnormal leaf development, expansion of the stem, and the lack of an obvious stem apical meristem (pW505); however, we did not detect obvious growth defects when overexpressing AtGRF5 (pW502) on the tissue culture plates and in the seedling stages in the soil (Figs. 1d and 1e). Thus, we concluded that AtGRF5 is a suitable gene for increasing the transformation efficiency in watermelon.
Agrobacterium strain dependence is a key factor affecting transformation efficiency. To identify the most suitable Agrobacterium strain, we introduced the pW502 vector into three Agrobacterium strains, GV3101, EHA105, and LBA4404. Compared with the transformation efficiency obtained with GV3101, which was used in the experiments described above (about 25.00%), that obtained using strain EHA105 was much lower (6.90%). Moreover, we found that LBA4404 failed to generate any positive transformation events (Fig. S1c). These results indicated that Agrobacterium strain selection is an important consideration for watermelon transformation, and that, among the three strains we tested, GV3101 was the most suitable one to deliver pW502 (AtGRF5) for watermelon genetic transformation.
Many Agrobacterium-mediated plant transformation systems exhibit genotype dependence (Zhang et al., 2021). Thus, we evaluated the effects of the preferred design on transformation in another watermelon cultivar, 83166, which is the female parental line of the hybrid "Jingxin." The Agrobacterium strains GV3101, EHA105, and LBA4404 were separately used to deliver pW501 (control vector) and pW502 (AtGRF5) to cotyledon fragments. Different from the watermelon cultivar WWl50, the transformation efficiencies without the help of a DR gene were 7.56% and 9.90% when pW501 was delivered by strains GV3101 and EHA105, respectively, suggesting that 83 166 is not highly recalcitrant to transformation (Fig. S1d). Compared with the other combinations, GV3101 carrying pW502 gave the highest transformation efficiency (20.72%). We also observed that LBA4404 failed to generate any positive transformation events in cultivar 83166 (Fig. S1d). This result confirmed our prior conclusions that the AtGRF5 gene is a robust tool that facilitates watermelon transformation, and that GV3101 is the preferred Agrobacterium strain to deliver these vectors.
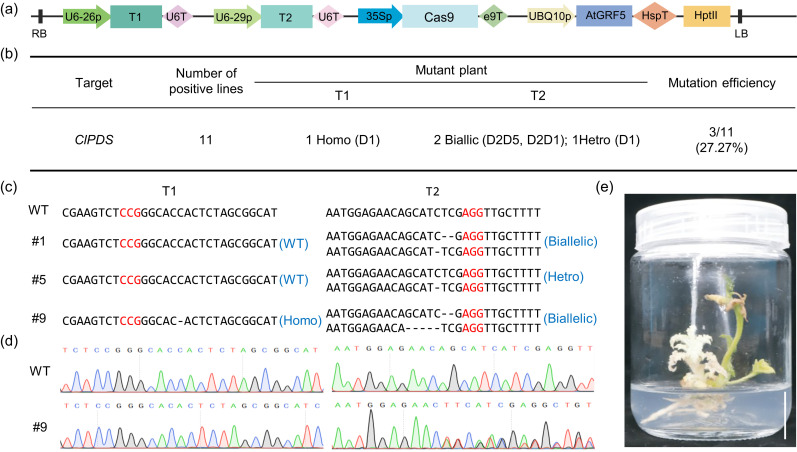
Targeted mutagenesis using genome-editing tools such as the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) system is a new breeding technology that can efficiently produce the desired mutations in the target gene, and it has been applied in multiple crop species including watermelon (Tan et al., 2020; Tian et al., 2017; Wang JF et al., 2021; Wang YP et al., 2021). Therefore, we tested the compatibility of AtGRF5-mediated transformation using genome-editing tools in watermelon. The existence of two BsaI restriction enzyme recognition sites in the original AtGRF5 gene hampered its application in CRISPR/Cas9-mediated genome editing, since many CRISPR/Cas9 genome-editing vectors are constructed using Golden Gate Assembly that requires the use of the type IIS BsaI enzyme. Thus, we added the codon-optimized version of AtGRF5, which lacks the BsaI recognition sites (Table S1), to the CRISPR/Cas9 genome-editing plasmid pHSE401 (Xing et al., 2014), thus generating the pZHW512 vector (Fig. 2a). Two previously designed spacers (T1 and T2) targeting the watermelon phytoene desaturase gene C. lanatus phytoene desaturase (PDS) (ClPDS) (Cla97C07G142100) were chosen for this step (Tian et al., 2017). PDS is widely used to quickly demonstrate the feasibility of CRISPR/Cas9, since its mutation causes photobleaching or an albino phenotype (Tian et al., 2017). The resulting genome-editing plasmid was transformed into WW150 cotyledon fragments. All 11 positive transgenic plants recovered were verified by Sanger sequencing. Among the 11 plants, three had mutations (#1, #5, and #9), giving a mutation efficiency of 27.27% (Fig. 2b). Only one mutation type, a 1-bp deletion (D1), was detected at the T1 site in #9. Meanwhile, at the T2 site, mutations were detected in three plants (#1, #5, and #9), with one heterozygous mutation and two biallelic mutations (Figs. 2c and 2d). As expected, the biallelic mutant exhibited an evident pure albino phenotype (Fig. 2e). These results suggested that the DR gene AtGRF5 could facilitate the generation of desired mutations via the CRISPR/Cas9 system.
Fig. 2. AtGRF5 facilitates the CRISPR/Cas9-mediated genome editing of ClPDS. (a) Schematic diagrams of the genome-editing vector pZHW512. (b) Summary of the ClPDS genome-editing results. D indicates deletion. (c) Summary of the mutation types at two target sites in the ClPDS gene. The PAM sequences for the two target sites are highlighted in red. (d) Results from Sanger sequencing of the two target sites in the wild type (WT) and editing line #9. (e) Phenotype of the genome-edited ClPDS mutant plant. Scale bar=2 cm. AtGRF5: Arabidopsis thaliana growth-regulating factor 5; CRISPR: clustered regularly interspaced short palindromic repeats; Cas9: CRISPR-associated protein 9; ClPDS: Citrullus lanatus phytoene desaturase; PAM: protospacer adjacent motif. RB: right border; LB: left border.
Recently, another research group reported the improvement of watermelon transformation by utilizing the DR gene ClGRF4-ClGIF1 in eight watermelon genotypes, including non-transformable watermelon varieties (Feng et al., 2021). Considering this study and our research, both proved the values of DRs in watermelon transformation, such as GRF4-GIF1. Also, both experiments successfully generated watermelon mutants through the CRISPR/Cas9 genome-editing system with the assistance of DR gene. Feng et al. (2021) reached an efficiency of 47.02% using the watermelon GRF4-GIF1 in the cultivar TC, which is much higher than that in our experiments. This may be caused by the different GRF4-GIF1 gene from different plant species, which may cause variation in the activity and stability of our desired genes in watermelon. The cultivar we used also plays vital roles in determining transformation efficiency. Both experiments achieved different transformation efficiencies in different cultivars. In addition, it is well known that plant transformation efficiency varies between different labs.
Furthermore, we tested five different DRs in watermelon, and found out that AtGRF5 stands out from other genes in watermelon transformation. Also, we revealed that the Agrobacterium strain GV3101 is more suitable to deliver the DR genes compared with EHA105, while EHA105 gives a slightly higher transformation efficiency in transforming a traditional vector without DRs (the pW501 vector). The EHA105 strain is commonly used in watermelon transformation, including the recently published high-efficiency watermelon transformation system (Tian et al., 2017; Feng et al., 2021). Thus, it can be expected that the combination of our strategies and these transformation systems could reach higher transformation efficiencies.
In summary, we have conclusively shown that the expression of DR genes, particularly AtGRF5, significantly improves the transformation efficiency of watermelon without obvious negative effects on plant growth. Among the three tested Agrobacterium strains, GV3101 was found to be the most suitable one for the genetic transformation of watermelon. Using GV3101 to deliver the pW502 (AtGRF5) vector, we successfully achieved high transformation efficiencies of >20% for two watermelon cultivars. We believe that this strategy will facilitate the delivery of CRISPR/Cas9-based genome-editing tools in watermelon, advancing gene functional studies and molecular breeding in this crop species. We also conjecture that similar strategies using DRs could be employed to overcome the transformation barriers in many other Cucurbitaceae species.
Materials and methods
Detailed methods are provided in the electronic supplementary materials of this paper.
Supplementary information
Acknowledgments
This work was supported by the Excellent Youth Foundation of Shandong Scientific Committee (No. ZR202103010168), the Shandong Science and Technology Innovation Funds, and the China Postdoctoral Science Foundation (No. 2021T140017). We thank Dr. Xinping ZHANG (Peking University Institute of Advanced Agricultural Sciences, Weifang, China) for providing watermelon seeds. We also thank Dr. Yun DENG (Peking University Institute of Advanced Agricultural Sciences, Weifang, China) for the help in transgenic plant culture.
Author contributions
Huawei ZHANG conceived this study. Huawei ZHANG and Wanggen ZHANG supervised the research. Wenbo PAN, Zhentao CHENG, Zhiguo HAN, and Hong YANG performed all experiments and analyzed the data. Huawei ZHANG and Wenbo PAN wrote the manuscript with input from all authors. Huawei ZHANG and Wanggen ZHANG agree to serve as the author responsible for contact and ensure communication. All authors have read and approved the final manuscript, and therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines
Wenbo PAN, Zhentao CHENG, Zhiguo HAN, Hong YANG, Wanggen ZHANG, and Huawei ZHANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Debernardi JM, Tricoli DM, Ercoli MF, et al. , 2020. A GRF-GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat Biotechnol, 38(11): 1274-1279. 10.1038/s41587-020-0703-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q, Xiao L, He YZ, et al. , 2021. Highly efficient, genotype-independent transformation and gene editing in watermelon (Citrullus lanatus) using a chimeric CLGRF4-GIF1 gene. J Integr Plant Biol, 63(12): 2038-2042. 10.1111/jipb.13199 [DOI] [PubMed] [Google Scholar]
- Guo SG, Zhao SJ, Sun HH, et al. , 2019. Resequencing of 414 cultivated and wild watermelon accessions identifies selection for fruit quality traits. Nat Genet, 51(11): 1616-1623. 10.1038/s41588-019-0518-4 [DOI] [PubMed] [Google Scholar]
- Kong JX, Martin-Ortigosa S, Finer J, et al. , 2020. Overexpression of the transcription factor GROWTH-REGULATING FACTOR5 improves transformation of dicot and monocot species. Front Plant Sci, 11: 572319. 10.3389/fpls.2020.572319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe K, Wu E, Wang N, et al. , 2016. Morphogenic regulators Baby boom and Wuschel improve monocot transformation. Plant Cell, 28(9): 1998-2015. 10.1105/tpc.16.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez-Hernández1 HA, Ledezma-Rodríguez M, Avilez-Montalvo RN, et al. , 2019. Signaling overview of plant somatic embryogenesis. Front Plant Sci, 10: 77. 10.3389/fpls.2019.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanasato Y, Tabei Y, 2020. A method of transformation and current progress in transgenic research on cucumbers and Cucurbita species. Plant Biotechnol (Tokyo), 37(2): 141-146. 10.5511/plantbiotechnology.20.0225a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, Lee JS, Jegal S, et al. , 2005. Transgenic watermelon rootstock resistant to CGMMV (cucumber green mottle mosaic virus) infection. Plant Cell Rep, 24(6): 350-356. 10.1007/s00299-005-0946-8 [DOI] [PubMed] [Google Scholar]
- Tan YY, Du H, Wu X, et al. , 2020. Gene editing: an instrument for practical application of gene biology to plant breeding. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 21(6): 460-473. 10.1631/jzus.B1900633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian SW, Jiang LJ, Gao Q, et al. , 2017. Efficient CRISPR/Cas9-based gene knockout in watermelon. Plant Cell Rep, 36(3): 399-406. 10.1007/s00299-016-2089-5 [DOI] [PubMed] [Google Scholar]
- Wang JF, Wang YP, Zhang J, et al. , 2021. The NAC transcription factor CLNAC68 positively regulates sugar content and seed development in watermelon by repressing CLINV and CLGH3. 6. Hortic Res, 8: 214. 10.1038/s41438-021-00649-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YP, Wang JF, Guo SG, et al. , 2021. CRISPR/Cas9-mediated mutagenesis of CLBG1 decreased seed size and promoted seed germination in watermelon. Hortic Res, 8: 70. 10.1038/s41438-021-00506-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing HL, Dong L, Wang ZP, et al. , 2014. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol, 14: 327. 10.1186/s12870-014-0327-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Xu GC, Cheng CH, et al. , 2021. Establishment of an Agrobacterium-mediated genetic transformation and CRISPR/Cas9-mediated targeted mutagenesis in hemp (Cannabis sativa L.). Plant Biotechnol J, 19(10): 1979-1987. 10.1111/pbi.13611 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.