Abstract
Overweight and obesity are now considered a worldwide pandemic and a growing public health problem with severe economic and social consequences. Adipose tissue is an organ with neuroimmune-endocrine functions, which participates in homeostasis. So, adipocyte hypertrophy and hyperplasia induce a state of chronic inflammation that causes changes in the brain and induce neuroinflammation. Studies with obese animal models and obese patients have shown a relationship between diet and cognitive decline, especially working memory and learning deficiencies. Here we analyze how obesity-related peripheral inflammation can affect central nervous system physiology, generating neuroinflammation. Given that the blood-brain barrier is an interface between the periphery and the central nervous system, its altered physiology in obesity may mediate the consequences on various cognitive processes. Finally, several interventions, and the use of natural compounds and exercise to prevent the adverse effects of obesity in the brain are also discussed.
Keywords: obesity, cognitive decline, inflammation, oxidative stress, natural products, exercise, blood-brain barrier
Introduction
Obesity is a chronic and stigmatized disease that affects children, adolescents, adults, and elderly people (The Lancet Diabetes Endocrinology, 2017; Goisser et al., 2020; Smith et al., 2020; Bray and Ryan, 2021). Some authors do not consider obesity a disease but a state; the problem with this definition is that it has led to neglecting the importance of this sickness and serious measures have not been taken to counteract it. This has led to obesity becoming a growing public health problem with severe economic consequences (Hruby and Hu, 2015; Berthoud et al., 2020).
According to the World Health Organization (WHO), more than 1.9 trillion adults in the world are overweight, and 650 million are obese; besides, this prevalence has also dramatically increased in children and adolescents. Around 2.8 million people die each year because of this pandemic (World Health Organization [WHO], 2021).
Obesity arises due to an energy imbalance between calories consumed and calories expended, creating an excessive energy balance state that increases body weight. The WHO has defined obesity as an excessive accumulation of body fat mass that can affect health and is diagnosed in adults with a body mass index (BMI) ≥ 30 kg/m2 (Whitlock et al., 2009). However, the pathogenesis of obesity is much more complex than a simple imbalance between energy intake and expenditure that leads to the passive accumulation of excessive weight. The etiologies associated with obesity include diverse aspects, such as genetic and epigenetic factors, and psychological, social, and cultural features that make obesity and overweight a multifactorial disease (Heindel and Blumberg, 2019; Genario et al., 2020).
It is well known that obesity substantially increases the risk of metabolic and chronic diseases such as type 2 diabetes mellitus (DM2), some types of cancer, cardiovascular and musculoskeletal diseases (Castro et al., 2017; Blüher, 2019), along with other disorders such as depression and neurodegenerative diseases (Gainey et al., 2016; Buie et al., 2019). Interestingly obesity has been accepted as an important risk factor for cognitive impairment (Castanon et al., 2015; Dye et al., 2017). During obesity, adipose tissue produces cytokines such as IL1ß, IL6, IFNγ, TNFα, MCP1, promoting chronic inflammation (Guillemot-Legris and Muccioli, 2017). Chronic low-grade inflammation disrupts the blood-brain barrier (BBB) due to endothelial dysfunction, generating neuroinflammation and increasing oxidative stress, leading to cognitive decline (Tucsek et al., 2014; Castanon et al., 2015). It is essential to mention that, although there are few studies, obesity has been shown to impact men and women differently, so the outcomes related to cognitive decline, dementia, and other diseases might be different (Censin et al., 2019).
For all these reasons, in this review, we will focus on analyzing how obesity-related peripheral inflammation can affect the central nervous system (CNS), generating neuroinflammation. Since the BBB is the interface between the periphery and the central nervous system, it may be the link between peripheral inflammation and the consequences that obesity may have on various cognitive processes. Furthermore, since the WHO has outlined different measures to prevent the adverse effects of obesity, several of those interventions will be discussed.
Obesity and Inflammation
Changes in Adipose Tissue During Obesity
The adipose tissue is a specialized connective tissue classified into brown (BAT) and white adipose tissue (WAT) (Saely et al., 2012). BAT predominates in the newborn’s in the interscapular, perirenal, and inguinal regions. In adults, BAT is present in the neck, interscapular, and supraclavicular regions but is absent in the elderly and obese (Mittal, 2019). Sympathetic endings innervate BAT to mediate lipolysis since it specializes in heat generation by oxidating fatty acids through the dissipation of the proton gradient in the inner mitochondrial membrane. The generation of heat aims to thermoregulate body temperature (Wang et al., 2021).
Nowadays, WAT is considered an organ with relevant neuroimmune-endocrine functions, which participates in the organism’s homeostasis. One of the main functions of the WAT is the storage of fatty acids to provide these substrates to other tissues such as muscle during fasting or in periods of high energy demand. WAT also has a relevant role in appetite regulation, insulin resistance, cytokine secretion, mechanical protection, among others (Morigny et al., 2021). WAT is the most abundant adipose tissue distributed subcutaneously, perivascularly, and viscerally, the latter participating in metabolic dysregulations during obesity (Morigny et al., 2021; Porro et al., 2021). This adipose tissue is primarily composed of adipocytes, cells specialized in accumulating lipids. The WAT is also formed by the stromal cells, including pre-adipocytes, stem cells, endothelial cells, and immune cells such as macrophages, lymphocytes, and neutrophils (Ràfols, 2014). Macrophages and T lymphocytes have generated significant interest for their participation in the chronic low-grade inflammatory process present in obesity (Maurizi et al., 2018).
Macrophages can be classically (M1) or alternatively (M2) activated. Polarization into the M1 profile happens after pro-inflammatory tumor necrosis factor α (TNFα) and interferon γ (IFNγ) or toll-like receptor 4 (TLR4) signaling. In consequence, M1 macrophages express TNFα, interleukin (IL) 1α, IL1β, IL6, monocyte chemoattractant protein 1 (MCP1, also known as CCL2), chemokine (C-X-C motif) ligand 9 (CXCL9), and CXCL10, among other molecules. In turn, these factors attract unpolarized macrophages and induce the differentiation into the M1 state (Murray, 2017). Cytokines such as IL4 and IL13 induce M2 polarization. In turn, M2 macrophages secrete IL10, transforming growth factor β (TGFβ), chemokine (C-C motif) ligand 1 (CCL1), CCL17, CCL18, CCL22, and CCL24, favoring the differentiation of unpolarized macrophages into the M2 profile (Murray, 2017).
Under physiological conditions, the sensitivity toward insulin is maintained by releasing anti-inflammatory cytokines such as TGFβ and IL10 by the resident or M2 macrophages, favoring insulin-mediated glucose uptake (Li et al., 2020). Adipocytes release IL4 and IL13 (Table 1), promoting the polarization of macrophages to an M2 profile, thus favoring lipid metabolism and the secretion of TGFβ and IL10, ensuring a reduction in inflammation and resistance to insulin (Tsao et al., 2014; Akash et al., 2018).
TABLE 1.
Main cytokines and their effects in obesity.
| Cytokines | Cytokine source | Levels in obesity | Cytokine mechanisms in obesity | References |
| IL1β | • Subcutaneous adipose tissue • Visceral adipose tissue |
↑ adipose tissue ↑ serum |
• Induces Pre-adipocyte differentiation. • Reduction of insulin-induced glucose transport. • Inhibition of glucose uptake by adipocytes via ERK signaling. • Acts synergistically with TNFα and IL6, altering the lipase activity, leading to lipid accumulation in the liver and muscle. • Contribution to hepatic lipogenesis, triglyceride accumulation, and development of hepatic steatosis. • IL6 production. • T cell and macrophage activation. |
Jager et al., 2007; Um et al., 2011; McArdle et al., 2013; Negrin et al., 2014; Wang et al., 2021 |
| IL2 | • Visceral adipose tissue • CD4 + and CD8 + T cells • Dendritic cells • Macrophages |
↑ adipose tissue ↑ serum |
• T cell activation. • Induction of inflammatory molecules like IL8, IL12A, CCL5, CCL19, CCR2, and CCR5. • Contribution to increased insulin resistance secondary to TLR2, TLR4, and TLR10 interaction. |
Liu and Nikolajczyk, 2019; Kochumon et al., 2020 |
| IL4 | • TH2 cells • Visceral adipose tissue • M2 macrophages |
↓adipose tissue ↓serum |
• Inhibits lipid deposits. • Inhibits adipogenesis through the expression of peroxisome proliferator-activated receptor γ (PPARγ). • Promotes lipolysis due to binding to hormone-sensitive lipase (HSL). |
Tsao et al., 2014; Lu et al., 2015; Shiau et al., 2019 |
| IL6 | • Subcutaneous adipose tissue • Visceral adipose tissue • Monocytes • M1 macrophages |
↑ adipose tissue ↓ hypothalamus |
• Promotes energy consumption by stimulating the hypothalamus. • Correlation with high TNFα levels and insulin resistance. • Chemotaxis and monocyte infiltration in adipose tissue by the expression of CD11b and CD163. |
Sindhu et al., 2015; Kern et al., 2019; El-Mikkawy et al., 2020; Wang et al., 2021 |
| IL10 | • TH2 cells • Regulatory T cells • B cells • M2 macrophages |
↓ adipose tissue ↓ serum |
• Inhibition of pro-inflammatory cytokine synthesis by suppressing NF-kB in macrophages. • Association with hypertriglyceridemia by the affection of the JAK-STAT 3 signaling pathway. |
Azizian et al., 2016; Kondo et al., 2018; Liu et al., 2018 |
| IL13 | • TH2 cells | ↑ serum | • Polarization of macrophages into an M2 profile through the IL-13Rα1/IL-4R receptor. • Decrease insulin resistance. • Involved in increasing inflammation via the NLRP3 inflammasome. • Increases fatty acid oxidation in muscle. |
Duffen et al., 2018; Martínez-Reyes et al., 2018; Knudsen et al., 2020 |
| IL17 | • Th17 cells in visceral adipose tissue • M1 macrophages • Neutrophils |
↑ adipose tissue | • Inhibition of adipocyte differentiation. • Increase of inflammatory molecules like COX2 and PEG2. • Induction of IL6 synthesis by adipocytes. • CDK5-dependent phosphorylation of PPARγ in adipocytes, favoring gene expression related to diabetes. |
Ahmed and Gaffen, 2010; Liu and Nikolajczyk, 2019; Teijeiro et al., 2021 |
| IFNγ | • TH1 cells | ↑ adipose tissue ↑ serum |
• Macrophage regulation switching to the M1 profile. • Increase of insulin resistance. • Increase of adipocyte cell size. |
Wada et al., 2011; O’Rourke et al., 2012; Wang et al., 2014; Surendar et al., 2019 |
| MCP1 (CCL2) | • M1 macrophages | ↑ adipose tissue ↑ serum |
• Participation in adipogenesis promoting adipocyte growth. • Facilitation of insulin resistance and glucose intolerance. • Recruitment of immune cells. |
Rocha et al., 2008; Cranford et al., 2016 |
| TGFβ | • Regulatory T cells • M2 macrophages • Platelets |
↑ serum | • Increase insulin resistance through TGFβ/Smad3 signaling via the repression of the insulin promoter and suppression of insulin level and secretion. • Inhibition of adipocyte differentiation. • Correlation with high levels of serum glucose. |
Yadav et al., 2011; Zamani and Brown, 2011; Hong et al., 2016; Lee, 2018 |
| TNFα | • TH1 cells • Subcutaneous adipose tissue • Visceral adipose tissue • M1 macrophages |
↑ adipose tissue ↑ serum |
• Inhibition of GLUT4 membrane translocation. • Induction of the serine phosphorylation of insulin substrate-1, leading to insulin resistance. • Suppression of the lipoprotein lipase activity. • Inhibitor of adipocyte differentiation. • Suppression of genes involved in uptake and storage of non-esterified fatty acids and glucose. |
Bennet et al., 2006; Tzanavari et al., 2010; Kern et al., 2019; Liu and Nikolajczyk, 2019; Alzamil, 2020; Wang et al., 2021 |
During obesity, excessive energy coming from the diet and the lack of physical activity promotes lipid storage in adipocytes (Trayhurn, 2013; Maurizi et al., 2018). If that persists, adipocytes broaden in a phenomenon called hypertrophy. The pre-adipocyte differentiation into adipocytes complements this process to compensate for the growth of existing ones to maintain a balance in the storage capacity, in a phenomenon called hyperplasia (Choe et al., 2016).
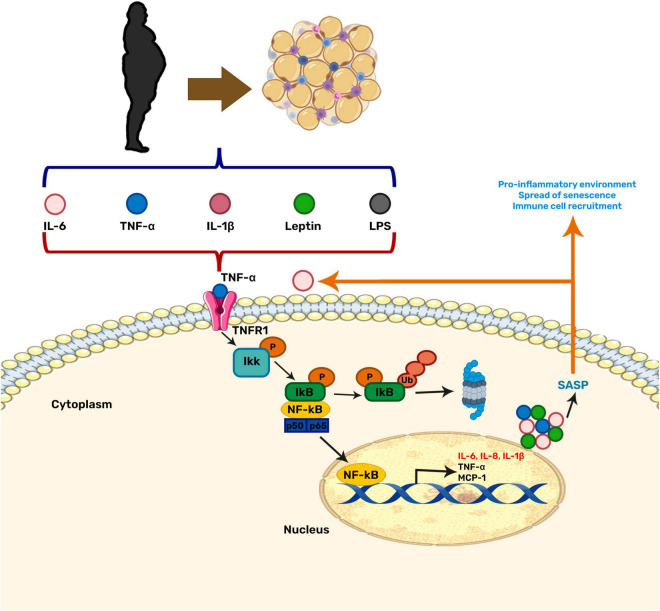
Because of cytokine release, especially TNFα, a high number of classically activated M1 macrophages infiltrate the adipose tissue, which is associated with insulin resistance (Akash et al., 2018). Those changes alter the WAT microenvironment, favoring the expression of pro-inflammatory adipokines and the activation of adipose tissue macrophages (ATMs) to a pro-inflammatory or M1 profile, promoting the secretion of more pro-inflammatory molecules, such as TNFα, IL1β, IL6, and MCP1 (Table 1), generating an increased accumulation of ATMs, which in turn secrete a higher amount of pro-inflammatory cytokines (Sam and Mazzone, 2014). A marker of damage caused by ATMs infiltration is the formation of crown-like structures characterized by M1 ATMs expressing CD11c around dead adipocytes. These structures are increased in obesity and are related to inflammation and insulin resistance (Lumeng et al., 2007; Sam and Mazzone, 2014).
In normal conditions, WAT is highly irrigated, ensuring adequate transport of nutrients and oxygen from the diet; nevertheless, in obesity, adipocyte hypertrophy hinders the diffusion of oxygen and nutrients, causing a hypoxic state (Sam and Mazzone, 2014). The cellular environment with low oxygen tension elicits hypoxia-sensitive genes that activate major hypoxia-inducible molecules (HIF) and inflammatory transcription factors such as NF-κB (Trayhurn, 2014), triggering a change in the adipokine secretion profile to enter into cellular stress, both at the mitochondria and the endoplasmic reticulum (Deng and Scherer, 2010). This first change alters the local microenvironment, as pro-inflammatory cytokines increase and the ATMs change to an M1 phenotype producing TNFα, IL1β, IL6, and MCP1, culminating in a local inflammatory process and a significant number of infiltrating macrophages (Tsao et al., 2014). In obese mice, the lack of oxygen in WAT generates changes related to the dysfunction of adipocytes, where it is worth highlighting the increase in adipokines related to inflammation (IL6, leptin, Angptl4, and VEGF), in addition to an increase in lactate production and the induction of fibrosis and insulin resistance (de Oliveira and Mafra, 2013; Trayhurn, 2014). Also, elevated adipose HIF1A protein and RNA levels are present in patients with obesity class 3, confirming hypoxia in WAT (Todorćević et al., 2021).
Among the various bioactive molecules produced by adipocytes are adipokines such as TNFα, leptin, resistin, and plasminogen activator inhibitor type 1 (PAI-1) (Deng and Scherer, 2010). The loss of their regulation during obesity is related to the pathophysiology of metabolic diseases; for example, decreased adiponectin levels are associated with DM2 (Frankenberg et al., 2017). Its main functions include glucose and lipid metabolism, and the prevention of inflammation. The mechanism through which it exerts these functions has not been explicitly explained.
Inflammatory Mediators in Obesity
As mentioned before, the alteration of adipocytes causes ATMs to polarize to an M1 profile, synthesizing and secreting pro-inflammatory cytokines, which are considered obesity inflammatory mediators and have diverse effects (Sam and Mazzone, 2014). Ym1, arginase 1, and IL10 gene expression is observed in lean mice, stimulating an M2 activation on ATMs as an anti-inflammatory mechanism. While in obese mice, the transcription of TNFα and iNOS genes increase, contributing to TNFα-induced insulin resistance (Lumeng et al., 2007; Sam and Mazzone, 2014).
Among the mentioned cytokines, IL6 regulates multiple aspects of metabolism like the regulation of adipose tissue, lipolysis, oxidative metabolism, and energy expenditure (Wueest and Konrad, 2020). Adipose tissue, endothelial cells (vascular stroma), fibroblasts, macrophages, monocytes, and lymphocytes secrete IL6 contributing to acute phase reactions, chronic inflammatory processes, and homeostatic energy regulation, influencing obesity and insulin resistance (Stȩpień et al., 2014; Han et al., 2020). During obesity, adipose tissue increases leptin secretion and suppresses satiety, promoting gluconeogenesis and hepatic insulin resistance (Han et al., 2020). Furthermore, one-third of total IL6 circulating levels is produced in adipose tissue (Makki et al., 2013).
Tumor Necrosis Factor α is a peptide secreted by different cells types like monocytes, macrophages, and microglia. In adipose tissue, pre-adipocytes, stromal vascular cells, and infiltrating macrophages also secrete TNFα, where it suppresses the genes involved with the internalization and storage of non-esterified fatty acids, glucose, and transcription factors involved in adipogenesis and lipogenesis (Tzanavari et al., 2010). MCP1 is a chemokine produced by adipocytes and M1 macrophages; it recruits monocytes/macrophages for their infiltration into adipose tissue, and its concentration increases in response to IL1, TNFα, and TLR4 signaling (Surmi and Hasty, 2008; Rajasekaran et al., 2019). MCP1 increases lipolysis and leptin secretion while lowering insulin-stimulated glucose uptake, increasing plasma levels during obesity, contributing to impaired insulin sensitivity (Makki et al., 2013).
Interleukin 1 is a family of cytokines in which IL1α and IL1ß stand out. These cytokines are crucial in innate inflammatory responses, being responsible for fever; however, in recent decades, they have been recognized for their participation in the progression of insulin resistance induced by obesity due to their elevated plasma levels and increased inflammasome NLRP3 activity (Di Renzo et al., 2007; Ballak et al., 2015).
Leptin, the product of the LEP gene, is a 16 kDa peptide hormone secreted mainly by adipose tissue (Pérez-Pérez et al., 2020). Its main function is the homeostatic regulation of appetite and body weight through the induction of anorectic factors and the expression of orexigenic neuropeptides in the hypothalamus (Vera et al., 2018). Circulating leptin levels correlate with body weight. Therefore, obese people tend to produce more leptin than slimmer people. Mice and humans with leptin deficiency attain intense hyperphagia and develop severe obesity and various metabolic and endocrine disorders (Paz-Filho et al., 2012). Leptin may participate in the activation and maintenance of the inflammatory response due to its ability to regulate innate and adaptive immune responses (Bernotiene et al., 2006). In innate immunity, leptin increases the cytotoxicity of natural killer (NK) cells, and induces the activation of a wide range of cells such as granulocytes, dendritic cells and macrophages. In the adaptive immune response, leptin increases the proliferation of naive T lymphocytes and B lymphocytes and decreases regulatory T lymphocytes (Treg). Leptin can polarize T helper cells (Th) toward a pro-inflammatory (Th1) rather than an anti-inflammatory (Th2) phenotype (Abella et al., 2017). In addition, pro-inflammatory cytokines increase leptin synthesis and release, which perpetuates the chronic inflammatory state characteristic of obesity.
Another mediator of inflammation during obesity is the oxidative stress (OS) generated in fat tissue. OS is defined as the imbalance between the oxidant molecules generated by the cells and the antioxidant systems that neutralize them (Thannickal and Fanburg, 2000). It is well recognized that OS and inflammation are damaging events that enhance each other. During obesity, the pro-inflammatory adipokines activate signaling cascades that can stimulate enzymes that generate reactive oxygen species (ROS), such as NADPH oxidase (NOX), which mainly produce superoxide radicals and hydrogen peroxide (Hsu et al., 2019). ROS, especially the hydroxyl radical, can oxidize proteins, damage membrane lipids and DNA, increasing the risk of degenerative diseases. In the CNS, the nitric oxide synthase (NOS) is also activated, generating nitric oxide, which produces the peroxynitrite anion that nitrates proteins, damaging them. Increased NOS activity is associated with increased calcium and excitotoxicity (Brown, 2010; Yuste et al., 2015). Inflammation has been related to mitochondrial dysfunction that causes a decrease in ATP levels and an increased ROS generation, thus enhancing OS. The changes toward a more oxidized state activate the NLRP3 inflammasome and transcription factors such as NF-κB, which in turn induce the synthesis of more pro-inflammatory cytokines that activate immune cells, thus perpetuating the damage of the OS and inflammation (Morgan and Liu, 2011; Sandhir et al., 2017). The chronic low-grade inflammation produced by adipocytes generates OS and creating a vicious cycle that alters the functions of the immune, endocrine, and nervous systems and has been associated with the establishment of metabolic, cardiovascular, and degenerative diseases.
Moreover, inflammation and OS can induce cellular senescence (Burton and Faragher, 2018). Senescence is a stress response state in which cells lose their ability to proliferate and secrete a set of pro-inflammatory and growth factors, proteases, among other proteins, known as the senescence-associated secretory phenotype (SASP). The SASP attracts immune cells aiming to remove damaged cells, hence promoting the restoration of cell homeostasis (Burton and Faragher, 2018). Senescent cells can be beneficial when they contribute to tumor suppression, but, in the long term, they promote tissue deterioration during aging. During obesity, the inflammation and OS generated in the brain increases the amount and accumulation of senescent cells, thus contributing to the neuroinflammation due to the SASP secretion. The neuroinflammation induced by the senescent cells creates a vicious cycle that escalates inflammation and OS, and has been linked to age-related diseases (Maciel-Barón et al., 2017; Ogrodnik et al., 2019; Figure 1).
FIGURE 1.
Relationship between obesity-related inflammation and senescence. NF-κB is the central regulator in the stress response and may be activated by various stimuli. Among them, pro-inflammatory cytokines secreted by adipose tissue during obesity. This factor has also been related to the aging process by contributing to cellular senescence through the senescence-associated secretory phenotype (SASP).
High-fat diets (HFD) in experimental rodent models cause OS, increased circulating pro-inflammatory cytokines, and the appearance of senescent markers (Minamino et al., 2009; Cavaliere et al., 2018). The KK-Ay mouse model with ectopic expression of the Agouti-related protein (AgRP) is hyperphagic and develops severe obesity. These animals increased ROS generation though they were fed with a standard diet. The KK-Ay mice adipose tissue exhibited a senescent phenotype, characterized by enhanced β-galactosidase activity, high p53 protein, and elevated expression of CDK1 mRNA compared to wild-type mice. Increased expression of the pro-inflammatory cytokines TNFα and MCP1, and macrophage markers in the adipose tissue of KK-Ay mice were also observed, suggesting that excessive calorie intake may induce senescence-like changes in adipose tissue (Minamino et al., 2009).
Chronic Obesity and Neuroinflammation
Systemic inflammation, particularly low grade chronic inflammation, such as the one generated during obesity, has been reported to cause changes in the brain and induce neuroinflammation (Ellulu et al., 2017; Ugalde-Muñiz et al., 2020). The neuroinflammatory response during obesity occurs in different structures of the CNS, such as the cerebellum, amygdala, cerebral cortex, and hypothalamus (Guillemot-Legris and Muccioli, 2017; Jais and Brüning, 2017; Van Dyken and Lacoste, 2018). Of particular interest in the context of obesity-induced cognitive decline is the neuroinflammation generated in the hippocampus, since experimental animal models subjected to diets rich in fat and carbohydrates have shown learning and memory deficits. This neuroinflammation has also been associated with changes in the integrity of the blood-brain barrier (BBB), so these structures will be discussed next (Bruce-Keller et al., 2009).
Blood-Brain Barrier as an Interface Between the Periphery and the Central Nervous System
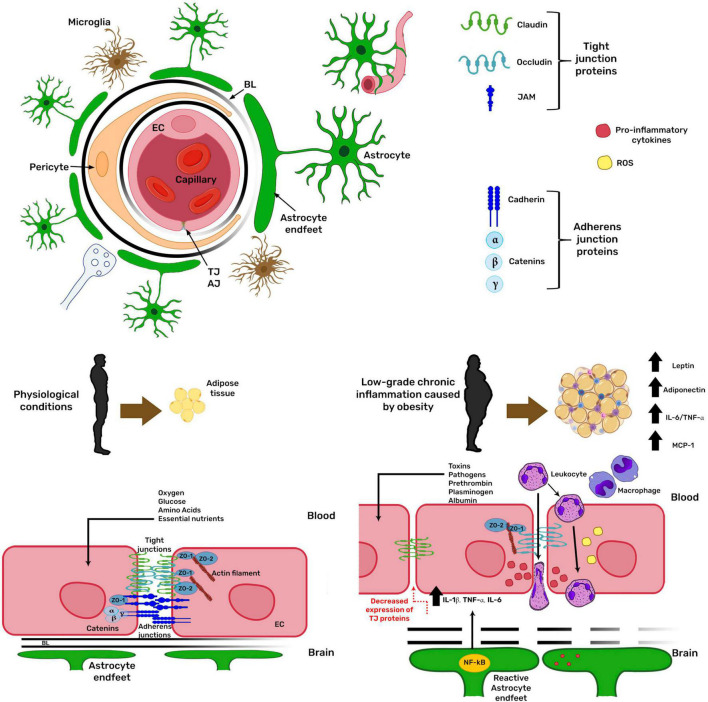
The communication between the peripheral tissues capable of acquiring, detecting, and storing nutrients with the specialized nuclei in the CNS is essential to regulate nutrition and metabolism. The BBB is the interface for these communications because several signals are transferred through the blood (Rhea et al., 2017). The BBB is located at 99% of brain capillaries (Pachter et al., 2003) and is formed by brain endothelial cells, which acquire its barrier phenotype by their cellular interactions with mural cells, such as pericytes, and by the soluble factors released by the astroglia (Daneman and Prat, 2015). The BBB ensures that the composition of the interstitial fluid is adequate for the physiological properties of each brain region (Fletcher and Callanan, 2012). It protects the brain from toxic substances present in the circulation, conferring a chemical and physical barrier to the CNS (Fletcher and Callanan, 2012) as it restricts the unregulated diffusion of macromolecules between the blood and the CNS and selectively regulates the transport of circulating nutrients and hormonal signals from the blood to the brain and vice versa (Rhea et al., 2017). The BBB structure involves the establishment of inter-endothelial tight junctions and the expression of specialized carrier systems. Inter-endothelial tight junctions are formed by the transmembrane proteins claudin, occludin, and junctional adhesion molecule (JAM), which are anchored to the cytoskeleton through their interaction with adaptor proteins of the MAGUK family, such as zonula occludens-1 (ZO-1) and ZO-2 (Daneman and Prat, 2015; Figure 2). Another junction type at the BBB is the adherens junction, formed mainly by the transmembrane protein cadherin docked to the cytoskeleton through catenins (α, β, and γ). Adherens junctions are a prerequisite for tight junction assembly and maintenance (Kadry et al., 2020). When the BBB function is lost, potentially neurotoxic molecules within the bloodstream, such as prothrombin, plasminogen, and albumin, can freely enter the brain (Fletcher and Callanan, 2012). The inflammation caused by obesity has been related to changes in BBB permeability (reviewed in Hurtado-Alvarado et al., 2016), inducing leukocyte extravasation along with the potential entry of pathogens and toxins into the CNS, which in turn stimulate more inflammatory responses, causing a vicious cycle (Van Dyken and Lacoste, 2018). All these mechanisms are regulated by the NF-κB pathway increasing the expression of pro-inflammatory proteins such as IL1β, TNFα, and IL6. The increase in pro-inflammatory cytokines is related to the decrease in tight junction protein expression and disturbed BBB integrity (Van Dyken and Lacoste, 2018; Figure 2).
FIGURE 2.
Obesity modifies blood-brain barrier physiology. The blood-brain barrier (BBB) is formed by brain endothelial cells (EC), which acquire its barrier phenotype by their cellular interactions with pericytes and the soluble factors released by astroglia. The barrier restricts the unregulated diffusion of macromolecules between blood and the brain. In obesity, low-grade chronic inflammation increases BBB permeability. Obesity-related inflammation depends on the NF-κB pathway, increasing the expression of pro-inflammatory proteins, related to decreased tight junction protein expression, deranging BBB integrity. AJ, adherens junction; TJ, tight junction; BL, basal lamina; ZO, zonula occludens; IL, interleukin; TNFα, tumor necrosis factor α; MCP1, monocyte chemoattractant protein 1.
The Role of Diet and Aging in the Blood-Brain Barrier Function
As mentioned above, changes in the BBB structure and function during obesity may cause further pathologies in the CNS, increasing neuroinflammation and cognitive decline (Rhea et al., 2017). Astrocytes and microglia are essential in maintaining the BBB integrity supporting neuronal metabolism, and preventing/responding to local tissue injury, and both cell types are activated in the brain of rodents and humans with HFD consumption (Rhea et al., 2017).
A high fat and glucose diet administrated for 90 days to juvenile male Sprague Dawley rats increased BBB permeability to sodium-fluorescein, a low molecular weight exogenous tracer, in the hippocampus. This effect was related to a lower expression in the mRNA of the tight junction proteins claudin-5, claudin-12, and occludin, and deficits in hippocampal-related learning and memory (Kanoski et al., 2010). Likely, a Mediterranean diet, rich in saturated fat and dextrose, administrated to male Sprague Dawley rats for 10, 40, and 90 days increased the BBB permeability to sodium-fluorescein at day 90 in restricted regions of the hippocampus and the dorsal striatum; indicating that the loss of barrier function is gradual. This study also associated the increase in the BBB permeability with the deficits in the hippocampus-dependent learning and memory (Hargrave et al., 2016).
Another diet rich in cholesterol administered to male C57 BL/6 mice during ten weeks potentiated the effect of ischemia on BBB permeability by increasing the extravasation of immunoglobulin (IgG) in the frontal cortex as compared to ischemic mice fed with a standard diet (ElAli et al., 2011). Another study showed that obesity at old age increases cognitive decline, particularly in the hippocampus. Young (7 months) and old (24 months) male C57 BL/6 mice received either a standard diet or a HFD. Cognitive impairment in obese and aged mice was associated with decreased microvascular density and pericyte coverage in the hippocampus and cerebral cortex; in addition, reduced blood flow in the cerebral cortex was related to memory problems (Tucsek et al., 2014).
Under pathological conditions, such as DM2, increased BBB permeability has also been reported. A rodent model of streptozotocin-induced DM2 increased BBB permeability to low-molecular-weight tracers earlier in the midbrain (at 28 days post-induction) and later in the hippocampus, basal nuclei, and cerebral cortex (at 56- and 90-days post-induction). However, for large molecules (e.g., Evans blue), increased BBB permeability in diabetic animals was observed until later times (more than 56 days post-induction) and only in the midbrain and basal nuclei (Huber et al., 2006).
Glial Activation in Obesity
This loss of BBB permeability during the chronic low-grade inflammatory state associated with obesity facilitates that pro-inflammatory molecules access to the brain parenchyma, thus allowing them to interact with the microglia (Ouyang et al., 2014; Gianfrancesco et al., 2019; González-Olmo et al., 2021). Furthermore, it has been reported that there is increased activation and proliferation of microglia and astrocytes in both obese humans and rodent models of obesity (Castanon et al., 2015).
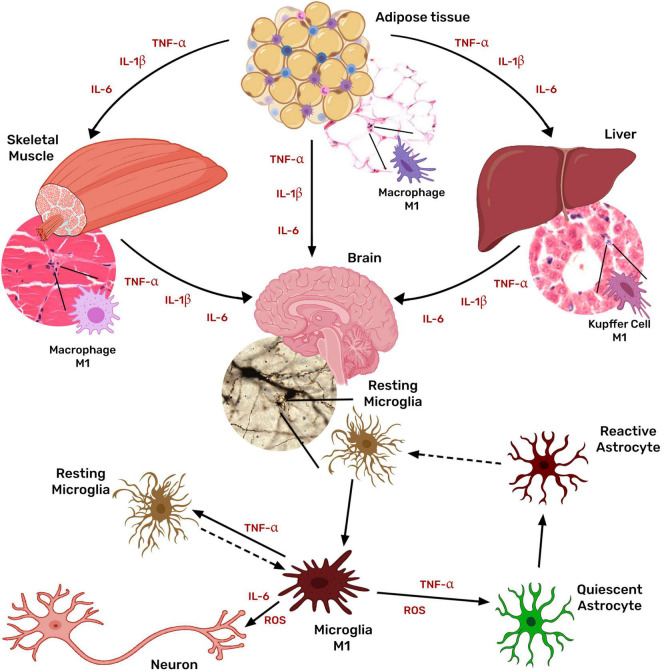
Microglia, the brain-resident macrophage, responds to peripheral inflammatory signals by its activation and thus the secretion of more inflammatory cytokines, perpetuating the neuroinflammatory condition and leading to neuronal damage (Thaler et al., 2012; Figure 3).
FIGURE 3.
Systemic inflammation associated with obesity activates microglia and astrocytes. Obesity chronic low-grade inflammation is mediated mainly by tissue macrophages in the adipose tissue through the secretion of TNF, IL1β, IL6, and MCP1. Cytokines originated in the adipose tissue stimulate macrophages of other tissues (liver, muscle, and even brain) further to produce TNF, IL1β, IL6, and MCP1, inducing a generalized inflammatory state. Peripheral inflammatory signals also activate microglia, which then secretes more inflammatory cytokines, activating astrocytes and favoring a chronic neuroinflammatory condition that leads to neuronal damage.
After stimulation, the microglial cells are activated to a state of “priming or pre-activation” (Perry and Holmes, 2014). This state makes the microglia more sensitive to pro-inflammatory stimuli. Microglial priming occurs during aging, neurodegenerative diseases, and traumatic brain injury (Perry and Holmes, 2014; Li J. W. et al., 2018). However, evidence has begun to emerge that systemic inflammation, either induced with peripheral LPS or the administration of one or more of the pro-inflammatory cytokines such as TNFα, IL1β, IL6, IL33, is involved in microglial priming (Perry and Holmes, 2014; Li J. W. et al., 2018). Due to the heterogeneity of microglial density depending on the brain region, brain structures are differentially affected; the main affected brain regions are the hypothalamus, hippocampus, cerebral cortex, and striatum (Milanova et al., 2021). Another example is the NLRP3 protein of the inflammasome in visceral adipose tissue, which directly affects IL1ß levels in the brain, activating microglia through the IL1R1 receptor and thus affecting memory in obese animals (Guo et al., 2020).
Microglial cells rapidly react to HFD, inducing morphological changes in the hypothalamus (Thaler et al., 2012). HFD increases Iba-1 expression in the arcuate nucleus (ARC), paraventricular nucleus (PVN), and hippocampus. No specific microglial changes are observed in the cerebral cortex and striatum (Thaler et al., 2012; Milanova et al., 2021). In the medio -basal hypothalamus (MBH), microgliosis is mediated by activating pathways such as NF-κB, favoring cell infiltration, increased food intake, and local inflammation (Valdearcos et al., 2017). As mentioned above, the activation of these inflammatory pathways is crucial because the activation of IKKβ/NF-κB influences leptin and insulin metabolism, affecting even the processes of glucose intolerance in obesity (Zhang et al., 2005).
The hypothalamic microglia has been one of the most studied for its importance in metabolic regulation; this microglia acts as a sensor that regulates the function of the hypothalamus and is very sensitive to changes; for example, males are more susceptible to neuroinflammation in this brain area (Dorfman et al., 2017; Rosin and Kurrasch, 2019). CX3CL1-CX3CR1 signaling seems to be relevant in microglial regulation, metabolic homeostasis, and obesity susceptibility. Male mice fed with HFD presented lower expression of CX3CL1-CX3CR1 in the hypothalamus, while HFD-female preserved both ligand and receptor normal expression (Dorfman et al., 2017).
In the hypothalamus, microglial activation is related to alterations in the organelles responsible for energy metabolism, i.e., the mitochondria. HFD increases the mitochondrial number and the mRNA expression of the uncoupling protein 2 (UCP2); the selective deletion of UCP2 in microglia prevents diet-induced obesity (Kim et al., 2019).
However, microglia cells are not the only component in neuroinflammation secondary to obesity; other glia, specifically astrocytes, have been extensively studied in an inflammatory setting. The glial-vascular mechanism in which astrocytes and endothelial cells are involved modulates microglial activation and, therefore, inflammation. In in vitro experiments, the activation of the endothelium favored microglia differentiation into the amoeboid forms and increased the release of TNFα, IL1β, and IL10, while IGF1 levels decreased. In contrast, microglia exposed to conditioned medium from activated astrocytes showed a M2 phenotype and higher levels of IGF1 secretion; a promotion of phagocytosis was also observed (Xing et al., 2018).
Multiple studies show astrocyte importance in obesity. Hypothalamic astrocytes accumulate lipid droplets in an obese environment, favoring astrogliosis and inflammatory markers such as TNFα, IL1β, IL6, MCP1, stimulating microglia, and other astrocytes, enhancing the inflammatory response (Kwon et al., 2017).
The selective isolation of microglia and astrocytes has made it possible to differentiate and identify the molecules involved in neuroinflammation and their changes over time. In a HFD animal model, on day 3 of diet administration, the microglia TNFα expression was elevated while astrocytic IL10 increased; after 28 days of the diet, both astrocytes and microglia became clearly inflammatory with high expression levels of TNFα (Sugiyama et al., 2020).
Microglial activation also correlates with deficits in hippocampal function in obesity models. Hippocampal dysfunction was secondary to increased synaptic phagocytosis and neuronal elimination after 3 months of HFD; regular diet reversed this effect and normalized hippocampal function (Hao et al., 2016). Likely, blockade of specific microglial receptors, such as fractalkine-receptor, prevents the loss of dendritic spines and cognitive decline in obese mice (Cope et al., 2018).
Inflammatory Molecules in the Obese Brain
The inflammatory molecules in the obese brain have been studied extensively by testing different diets in animal models. For example, high-sugar diets, which promote cognitive decline in young animals, are associated with high IL6 and IL1β levels in the dorsal hippocampus (Hsu et al., 2015). The cafeteria diet model increased Iba-1 expression in the obese brain (de Oliveira et al., 2021). Chronic HFD feeding for 12 weeks enhanced TNFα, IL6, and leptin levels in the hippocampus and also promoted microglial activation in the prefrontal cortex and hippocampus (Gomes et al., 2020).
Dietary changes may reverse or protect against obesity-induced neuroinflammation. Modifying the Western diet, high in fat and low in fiber, decreased cognitive deterioration by adding β-glucans, prominent soluble fibers. Also, obese animals treated with β-glucans diminished microglial activation, TNFα, IL1β, and IL6, favoring hippocampal synaptogenesis markers (Shi et al., 2020).
In the obese brain, multiple pathways and molecules are affected (Table 2). TNFα is a crucial cytokine in neuroinflammation secondary to obesity, even critical in glucose metabolism by attenuating insulin signaling pathways and increasing levels of IL6, activating a neuroinflammatory state (Clemenzi et al., 2019). In addition, TNFα is associated with anxiety secondary to obesity since its pharmacological blockade improves anxiolytic triggers in obese animals (Fourrier et al., 2019). Labban et al. (2020) fed rats with HFD for 4 weeks and found an increase in pro-inflammatory cytokines, such as IL6 and IL12 in serum, an increase in brain OS markers, a decrease in brain serotonin levels, and an increase in brain dopamine and glutamate levels.
TABLE 2.
Main cytokines in the CNS during obesity and their effects in neuroinflammation.
| Cytokines | Cytokine source in the CNS | Expression in CNS cells in obesity | Cytokine mechanisms in CNS in obesity | References |
| IL1β | • Microglia • Neurons • Astrocytes • Oligodendrocytes |
↑ Astrocytes ↑ Microglia |
• Leukocyte recruitment to the CNS. • Rapid cellular infiltration to the brain parenchyma. • Increased MCP1(CCL2) expression by astrocytes and ICAM1 on vascular endothelial cells. • Impairment of hippocampal-dependent memory processing. • Regulation of food intake. • Increased neuronal cell death. |
Shaftel et al., 2008; Dorfman et al., 2017; Ding et al., 2018; Lainez and Coss, 2019; Guo et al., 2020 |
| IL2 | • Neurons | ↓ Neurons | • T cell proliferation. • Inhibition of the development of Th17 cells. • Reduction of neutrophil infiltration. • Diminishment of tight junction proteins degradation. • Expression of CD206. |
Hoyer et al., 2008; Gao et al., 2017 |
| IL4 | • M2 microglia • TH2 cells |
↓ Neurons | • M2 microglial phenotype differentiation. • Increased microgliosis and astrogliosis. • Expression of CD206. • Decreased production of inflammatory cytokines such as TNFα. |
Gadani et al., 2012; Luzina et al., 2012; Latta et al., 2015; Rossi et al., 2018; Daseke et al., 2020 |
| IL6 | • Microglia • Astrocytes • Neurons • Endotelial cells |
↑ M1 microglia ↑ Astrocytes ↑ Neurons |
• Differentiation of oligodendrocytes. • Modulation of microglial activation. • Induction of nerve injury. • Bodyweight loss induced by enhanced leptin signaling through the STAT-3 pathway. |
Szelényi, 2001; Thaler et al., 2012; Le Foll et al., 2015; Rothaug et al., 2016; Bobbo et al., 2019; Hu et al., 2020; Recasens et al., 2021 |
| IL10 | • Regulatory T cells • B cells • Neurons • Microglia • Epithelial cells |
↓ Neurons ↓ Microglia |
• Vascular remodeling. • Reduction of leukocyte adhesion and extravasation. • Regulation of the NFκB signaling. • Improvement of neurogenesis. |
Pereira et al., 2015; Azizian et al., 2016; Garcia et al., 2017; Kondo et al., 2018; Liu et al., 2018 |
| IL17 | • Th17 cells • T CD4 + cells • T CD8 + cells |
↑ Th17 cells ↑ T CD4 + cells ↑ T CD8 + cells |
• Induction of the NFκB pathway. • Contribution to the BBB permeability. • M1 polarization of microglia. • Activation of glial cells to produce inflammatory mediators, matrix metalloproteinases, chemokines, and free radicals. |
Basu et al., 2015; Yang and Yuan, 2018; Qiu et al., 2021; Chen et al., 2022 |
| TGFβ | • Regulatory T cells • Oligodendrocytes • M2 Microglia • Astrocytes |
↑ Astrocytes | • Free radical production through NOX1. • Cytotoxicity and neurodegenerative changes through the SMAD3 pathway. • Expression of inflammatory genes in pericytes like NOX4, COX2, IL6, and MMP2. |
Von Bernhardi et al., 2015; Rustenhoven et al., 2016; Patel et al., 2017 |
| TNFα | • Astrocytes • M1 Microglia |
↑ Astrocytes ↑ M1 Microglia |
• Increase of the anorexigenic POMC activity. • Potentiation of glutamate-mediated cytotoxicity. • Induction of the NFκB pathway and secretion of IL1β. • Affection of the spatial learning and memory function. • M1 phenotype polarization. |
Belarbi et al., 2012; Thaler et al., 2012; Olmos and Lladó, 2014; Lainez and Coss, 2019; Rodrigues et al., 2020 |
BBB, blood brain barrier; CNS, central nervous system; COX2, Cyclooxygenase 2; ICAM1, intercellular adhesion molecule,1; MCP1, monocyte chemoattractant protein 1; MMP2, matrix metalloproteinase 2; NADPH, nicotinamide adenine dinucleotide phosphate; NFκB, Nuclear factor kappa B; NOX1, NADPH oxidase 1; NOX4, NADPH Oxidase 4; POMC, proopiomelanocortin.
Consequences of Obesity: Cognitive Decline
The obesity-related neuroinflammation, the BBB integrity loss, and the microglial activation induce synaptic remodeling, neuronal apoptosis, and decreases neurogenesis, which have been associated with cognitive decline (Miller and Spencer, 2014; Li J. W. et al., 2018; Zhou et al., 2020).
Studies performed with obese animal models have shown a relationship between diet and cognition (Castanon et al., 2015; Duffy et al., 2019). Rodents fed with HFD have shown deficiencies in working memory and learning (Nguyen et al., 2014; Gainey et al., 2016). Moreover, OS and inflammation promoted by obesity contribute to neuronal damage and cognitive failure. Zhang et al. (2005) reported that HFD administration to male Sprague-Dawley rats for 5 months activated NF-κB pathway, increased ROS production and NOX expression in the cerebral cortex, as well as prostaglandin E2 (PGE2), cyclooxygenase 1 (COX1), and COX2 levels, contributing to neuronal damage and cognitive deterioration. It has been reported that feeding C57B1/6 mice with HFD for 16 weeks modified the redox state and decreased Nrf2 activation, contributing to cognitive impairment evaluated by 14-Unit Stone Maze (Morrison et al., 2010). Something similar was observed in Wistar rats fed a high-calorie diet (HCD) for 13 weeks; these rats presented memory loss evaluated with the Morris water maze, in addition to increased OS (Treviño et al., 2015).
On the other hand, there are studies in mice where memory deficits were quickly reversed by switching the animals from an HFD to a low-fat diet (McLean et al., 2018). The above was seen even after prolonged exposure to HFD-feeding (24 weeks), where after returning to a regular diet, the animals did not present learning deficits or spatial memory impairment (Leyh et al., 2021), suggesting that these impairments might be reversible, at least at some point.
A relationship between abdominal adiposity and cognitive decline has been reported regarding human studies where cognitive behavior was analyzed in obese patients. A negative association between anthropometric measurements, such as BMI and waist circumference, and the detriment in some cognitive tasks was proposed (Dye et al., 2017). Nevertheless, Ntlholang et al. (2018) found that in older adults, central adiposity was a stronger predictor of poor cognitive performance than BMI in older adults. In that study, the neuropsychological assessment determinations included the Mini-Mental State Examination (MMSE), Frontal Assessment Battery (FAB), and Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). This was confirmed by Gardener et al. (2021) where a neuropsychological battery evaluating cognitive domains (episodic memory, processing speed, semantic memory, and executive function) was evaluated in obese patients over 65 years of age, and a detrimental effect of mid-life rather than later life was found. Interestingly, abdominal adiposity was an important factor related to cognitive impairment and decline; however, overall adiposity (determined as BMI) was not a risk factor. Something similar was reported by Morys et al. (2021), where obesity was associated with cognitive impairment cerebrovascular disease.
Moreover, magnetic resonance imaging (MRI) has been used to assess neuroinflammation and axonal integrity to determine if there are similar effects in obese humans as observed in rodents. The results show increased cell density related to neuroinflammation and decreased axonal density in obese humans positively correlating with BMI, but not with age (Kullmann et al., 2020; Samara et al., 2020).
These studies support the relationship between obesity and cognitive health and attract the researchers’ attention because obesity may have an immediate and long-term detrimental impact on cognitive functions. The problem is that the molecular mechanisms that participate in obesity-related cognitive decline are diverse, including OS, metabolic dysfunction, cardiovascular disease, and systemic inflammation (Ajayi et al., 2021), highlighting those that are related to the impairment of vascular components where the integrity of the BBB is lost and the microglia are activated (Buie et al., 2019). Therefore, it is of paramount importance to continue researching the mechanisms of action and the interventions to reverse obesity’s detrimental effects. Mainly because in both, humans and rodents, the effectiveness of weight loss (through restrictive diets or other procedures) has been observed to rescue some aspects of neuroinflammation and defects in cognition and behavior (Guillemot-Legris and Muccioli, 2017).
Differential Inflammatory Response Between Sexes During Obesity
There is a differential response in adiposity and the prevalence of obesity-associated diseases between males and females, particularly in mammals. In obese men, the occurrence of heart disease and myocardial infarction is higher, while in women, obesity is associated with ischemic stroke (Chen et al., 2021). When comparing men and women with the same BMI, it has been observed that women have a 10% higher body fat content. In addition, women show a greater subcutaneous fat volume than men, while men have a larger volume of intra-abdominal or visceral fat (Griffin et al., 2016).
Still, there are limited data to explain the origin of these differences, but several studies have proposed estrogen’s role in the sex-dependent differential responses in metabolism. The decrease in estrogen levels in menopausal women is associated with the loss of subcutaneous fat and the abdominal fat accumulation (Lizcano and Guzmán, 2014). In support of estrogen importance, increased adiposity after oophorectomy and ovarian estrogen clearance were observed in rodents and monkeys (Chen et al., 2021). In the case of men, low testosterone levels have also been proposed as a risk factor for pathophysiology, including insulin sensitivity and DM2 (Terrazas et al., 2019).
On the other hand, the association between sex and OS is significant because OS is involved in many diseases that occur differentially in men and women (Kander et al., 2017). Previous studies revealed that oxidative and nitrosative stress markers are higher in obese men compared to obese women of the same age. Although it is difficult to determine whether these interactions are additive or synergistic, most redox biomarkers depend not only on age and sex but also on age-sex or age-obesity interactions (Choromańska et al., 2021).
Recently, several reports comparing the cognitive impairment by sex associated with obesity have been published. Wang et al. (2021) linked blood lipid levels and obesity using and index named lipid accumulation product (LAP). They reported that high LAP is associated with cognitive decline in females with normal blood pressure but not in those with high blood pressure or males. Suggesting that there is a relationship between obesity and cognitive decline that is differentially affected by blood pressure and sex. Hu et al. (2021) informed that in an older Chinese population, BMI and hip circumference are positively related to cognitive function in women, while no association was found in men. Conversely, another study was performed by Espeland et al. (2021) where older women and men (mean age 68 years old) with DM2 and overweight or obesity were evaluated. Cognitive advantages for women with DM2 and overweight/obesity over men during aging were observed. These differences given by sex are yet uncertain, but their understanding is important since the therapeutic targets and treatments may present variations and should be specifically directed toward men or women.
Interventions to Reduce the Effects of Obesity
Pharmacological Treatments
Most weight control medications act in the brain to stimulate satiety signals, motivationally helping the patients adhere to their dietary interventions, with the primary goal of weight loss. Medical guidelines recommend seven drug treatments for weight control, including orlistat, liraglutide, phentermine, phentermine/topiramate, lorcaserin, and naltrexone/bupropion (Lei et al., 2021).
Phentermine/topiramate therapy is known to significantly decrease body weight compared to placebo, and the amount of weight loss has been related to the used dose. Another beneficial effect of phentermine/topiramate treatment was the waist-circumference reduction, blood pressure, blood sugar levels, and lipid levels decrease. However, this drug combination risks adverse events related to the nervous system (Lei et al., 2021).
Orlistat has been well studied in different obese populations, including DM2 and patients with impaired glucose tolerance. Overall, a modest but significant weight loss was observed in all the groups with favorable effects on obesity comorbidities. Orlistat has not been associated with severe adverse events and only mild gastrointestinal effects have been reported in some patients. In obese patients who do not have diabetes, weight loss is achieved and maintained for 2 years. Orlistat, together with a hypocaloric diet, was shown to be effective in preventing DM2 in patients with glucose intolerance and significantly lowers glycated hemoglobin levels (Hollander, 2003).
The possible benefits of using liraglutide for long periods of time have been investigated in people with a BMI greater than 30 or 27 kg/m2 associated with dyslipidemia or hypertension. Subjects treated with liraglutide achieved significant weight loss vs. the placebo group. Moreover, when the drug was combined with physical activity, it significantly increased weight loss compared to liraglutide alone or physical exercise alone. These results reinforce the benefits of liraglutide in weight loss and emphasize the fundamental role of physical activity in chronic weight control (Lundgren et al., 2021; Tilinca et al., 2021).
Treatment with phentermine 37.5 mg/day for 3 months to reduce obesity showed a percentage of total weight loss of 7.65% and a more significant reduction in BMI –3.16 kg/m2 compared to Lorcaserin 10 mg/2 times a day with a total weight loss of 2.99% and a BMI reduction of –1.15 kg/m2. In this same study, the administration of phentermine was performed in a group of patients who had received bariatric surgery but had regained weight and who were subsequently treated with pharmacotherapy, patients using Lorcaserin had a 1.86% total weight loss vs. at 7.62% for phentermine and a smaller BMI reduction of –0.74 vs. –3.06 kg/m2 for phentermine. Lorcaserin treatment showed a significant decrease in total cholesterol and low density lipoprotein (LDL) only among surgical patients with a significant weight reduction (≥5% total weight). Both drugs were not associated with glycemic improvements, and no differences were observed between the surgical and non-surgical groups (Elhag et al., 2019).
Naltrexone/bupropion (NB) has also been used as an interesting combination therapy to treat weight and risk factors related to overweight and obesity. A double-blind, placebo-controlled study with 1496 obese patients with BMI 30–45 kg/m2 or overweight 27–45 kg/m2 with dyslipidemia and/or hypertension was conducted. A significant weight loss was observed with NB (–6.5%) vs. placebo (–1.9%) at week 28 of treatment, and at week 56 a reduction of –6.4% in NB vs. –1.2% in the placebo. NB enhanced different markers related to cardiometabolic risk, and the participants reported improvement in the quality of life. The most common adverse event with NB was nausea, which was generally mild to moderate and transient. NB was not associated with increased depression events or suicidal tendencies compared to placebo (Apovian et al., 2013).
These drugs have beneficial effects; however, numerous medications have been withdrawn due to potentially dangerous or undesirable side effects. In the face of the adverse side effects of synthetic drugs, natural products have been explored, as they are considered non-toxic and healthy. Different dietary, herbal, and natural products, and their active components have been analyzed for their potential anti-obesity effects (Sun et al., 2016).
Natural Products Against Obesity
There is a long list of natural compounds that have been used to control obesity. Examples of those molecules are alkaloids (capsaicin, caffeine, nicotine), terpenoids (lycopene, lutein, carotene), phytosterols (diosgenin, guggulsterone), organosulfur compounds (allyl sulfide, allicin, allixin), phenolic acids (ferulic, chlorogenic, and caffeic acids), curcuminoids (curcumin), chalcones (naringenin), lignans (matairesinol), flavonoids (kaempferol, quercetin, catechins, cyanidin), isoflavones (genistein), and stilbenes (resveratrol). Anti-obesity effects of these products include energy expenditure stimulants, appetite suppressants, α-amylase, α-glucosidase, lipase inhibitors, adipocyte differentiation inhibitors (decreased adipogenesis), increased lipolysis, or a combination of these effects (Mohamed et al., 2014; Sun et al., 2016).
In this review, we have discussed that obesity-induced inflammation is considered a potential mechanism that links this disease to neuroinflammation and cognitive decline, so targeting obesity-related inflammatory components is proposed as a valuable strategy to prevent or ameliorate the development of such CNS detrimental effects (Hirai et al., 2010).
Effect of Dietary Products on Obesity-Associated Neuroinflammation
Momordica charantia (bitter melon) has been reported to reduce brain OS and FoxO, as well as normalize neuroinflammatory markers (NFκB, IL16, IL22, and IL17R) in the brain of female mice fed with HFD (Nerurkar et al., 2011). Green tea extract ameliorates HFD-induced hypothalamic inflammation reducing the increase in TLR4, IκB-α, NF-κB p50, and IL6 in mice (Okuda et al., 2014). Epigallocatechin gallate, the major polyphenol in green tea, inhibited HFD-induced obesity by enhancing BAT thermogenesis and diminishing the hypothalamic inflammation and microglia overactivation through NF-κB and STAT3 pathway regulation (Zhou et al., 2018). In another study, this green tea compound was found to attenuate hypothalamic inflammation inhibiting the JAK2/STAT3 signaling pathway in HFD-induced obese mice (Mao et al., 2019).
Anthocyanin-rich blackberry extract counteracted HFD-induced dysbiosis, and modifications in gut microbiota were linked to its anti-neuroinflammatory effect (Marques et al., 2018). Purple sweet potato anthocyanin pigment diminished neuroinflammation induced by HFD in mice by inhibiting MAPK and NF-κB activation, downregulating the expression of iNOS, COX2, IL1β, IL6, and TNFα, and raising IL10 expression (Li J. et al., 2018). Several studies support the use of dietary anthocyanins coming from fruits, vegetables, and beans against DM2-mediated Alzheimer’s disease (Khan et al., 2021).
Peel extract of pineapple fruit protects against HFD-induced behavioral disturbances by decreasing the risk of atherogenicity due to anti-inflammatory, and antioxidant effects. The extract improves brain antioxidant status by increasing reduced glutathione (GSH) and catalase and decreasing IL6 and malondialdehyde (MDA) levels (Ajayi et al., 2021).
Effect of Herbal Products on Obesity-Associated Neuroinflammation
Dry leaf powder of Withania somnifera used in ayurvedic formulations ameliorated HFD-induced neuroinflammation, suppressing the expression of inflammatory markers (PPARγ, iNOS, MCP1, TNFα, IL1β, and IL6) (Kaur and Kaur, 2017). Furthermore, Xuefu Zhuyu decoction, a traditional Chinese medicine, reduced insulin and leptin levels, neuroinflammation, astrocyte and microglia activation, and amyloid deposition in an animal model of Alzheimer’s disease (Yeh et al., 2017). In this model, an ethyl acetate extract of leaves of Ugni molinae Turcz containing tannins, flavonoid derivatives, phenolic acids, and pentacyclic triterpenoids exhibited neuroprotective, anti-inflammatory, and anti-oxidative properties (Jara-Moreno et al., 2018).
Malva parviflora used in traditional medicine in Africa and America has anti-inflammatory, antioxidant, and hypoglycemic effects. In a recent study, the anti-inflammatory effect of a hydroalcoholic leaf extract was found to ameliorate HFD effects in an obese transgenic 5XFAD mouse model of Alzheimer’s disease. This extract, which contains oleanolic and scopoletin as active compounds, suppresses neuroinflammation by inhibiting microglia pro-inflammatory M1 phenotype and rescuing microglia phagocytosis via a PPAR-γ/CD36 dependent mechanism (Medrano-Jiménez et al., 2019).
A Mucuna pruriens (L.) extract rich in oligosaccharide (1-kestose and levodopa) and phenolic compounds (catechins, chlorogenic acid, trans-resveratrol, and kaempferol 3-glucoside), reduced food intake, neuroinflammation, and hippocampal IL6 levels of obese rats (Tavares et al., 2020). Moreover, Ghaddar et al. (2020) reported that Antirhea borbonica herbal tea prevents BBB leakage, cerebral OS, and partly improves neurogenesis in a diet-induced overweight zebrafish model.
Tinospora cordifolia extract supplemented in HDF-fed female rats reduced anxiety-like behavior and improved locomotor behavior by decreasing the expression of inflammatory cytokines, modulating apoptosis, and synaptic plasticity (Singh et al., 2021).
Finally, new approaches such as molecular docking studies targeting microglia-specific proteins support using some natural products (like curcumin, cannabidiol, and resveratrol) as possible candidates to regulate redox imbalance, OS, and neuroinflammation (Maurya et al., 2021).
Diets and Exercise
The primary strategy for treating obesity is diet supplemented with physical exercise and cognitive-behavioral therapy. Low-calorie diets are the most recommended to start reducing body weight. However, these dietary regimens must be supplemented with macronutrients, vitamins, and minerals. The 2015–2020 Dietary Guideline for Americans recommends that carbohydrates comprise 45–65% of calories, fat 25–35% of calories, and protein 10–30% of calories. Once the desired body weight has been reached, the number of calories consumed in the diet can be gradually increased to balance the calories consumed and calories expended.
Regular physical exercise improves the balance between energy consumed and expended, thereby gradually improving the diet’s effectiveness and maintaining diet-induced weight loss. There is a weight loss of 5–8.5 kg in 6 months after the intervention through calorie restriction and exercise. After 48 months, an average of 3–6 kg of the weight loss was maintained (Fock and Khoo, 2013; Bales and Porter-Starr, 2018). Likewise, combining a hypocaloric diet with supervised aerobic exercise 2 days a week offers an optimal non-pharmacological tool in managing blood pressure, cardiorespiratory conditions, and body composition in overweight/obese and sedentary people with hypertension (Gorostegi-Anduaga et al., 2018).
In regards to neuroinflammation, exercise has also shown very promising results. Exercise on a treadmill reduced the levels of inflammation markers such as TNFα, IL1β, and COX2 in the hippocampus of 8-month-old Sprague–Dawley rats on an HFD diet (Kang et al., 2016). Additionally, exercise decreased the activation of microglia and astrocytes in the cerebral cortex and hippocampus compared to sedentary rats fed with HFD (Koga et al., 2014). In HFD obese mice, treadmill exercise enhanced cognitive function by improving neuroplasticity and brain-derived neurotrophic factor (BDNF) expression (Kim et al., 2016). In another study, voluntary physical activity (wheel running) increased hippocampal neurogenesis and spatial learning in female C57BL/6 mice fed with HFD (Klein et al., 2016). Likewise, C57BL/6J (B6) mice fed with a western diet from 2 to 12 months of age, prevented cerebrovascular and white matter damage by free access to running saucer wheels exercise (Graham et al., 2019).
Conclusion and Perspectives
As discussed throughout this paper, obesity is a severe health problem associated with many diseases, including neuroinflammation and cognitive decline. To date, multiple interventions have been proposed to minimize or prevent neuroinflammation and cognitive impairment. However, the most important would be to develop prevention programs to teach people to eat healthily and perform an adequate exercise regimen.
Author Contributions
VS-V and MK collaborated in writing of the manuscript, integrated the information, and revised the final version. RF-T, YR-C, DR-R, RR-C, LC-C, LP-F, and AA-A collaborated in writing of the manuscript. NL-D, BG-G, and AC collaborated in writing of the manuscript and revised the final version. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
This work was supported by the Consejo Nacional de Ciencia y Tecnología (CONACyT) grant FORDECYT-PRONACES/263957/2020, PRODEP UAM-PTC-695, and CONACYT Ciencia de Frontera 2019 (1783), as well as Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México (UNAM), PAPIIT (IN214821). VS-V, RF-T, and YR-C are CONACyT scholarship holders.
References
- Abella V., Scotece M., Conde J., Pino J., Gonzalez-Gay M. A., Gomez-Reino J. J., et al. (2017). Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 13 100–109. 10.1038/nrrheum.2016.209 [DOI] [PubMed] [Google Scholar]
- Ahmed M., Gaffen S. L. (2010). IL-17 in obesity and adipogenesis. Cytokine Growth Factor Rev. 21 449–453. 10.1016/j.cytogfr.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajayi A. M., John K. A., Emmanuel I. B., Chidebe E. O., Adedapo A. D. (2021). High-fat diet-induced memory impairment and anxiety-like behavior in rats attenuated by peel extract of Ananas comosus fruit via atheroprotective, antioxidant and anti-inflammatory actions. Metabol. Open 9:100077. 10.1016/j.metop.2021.100077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akash M. S. H., Rehman K., Liaqat A. (2018). Tumor necrosis factor-alpha: role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J. Cell. Biochem. 119 105–110. 10.1002/jcb.26174 [DOI] [PubMed] [Google Scholar]
- Alzamil H. (2020). Elevated serum TNF-α is related to obesity in Type 2 diabetes mellitus and is associated with glycemic control and insulin resistance. J. Obesity 2020:5076858. 10.1155/2020/5076858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apovian C. M., Aronne L., Rubino D., Still C., Wyatt H., Burns C. (2013). A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity 21 935–943. 10.1002/oby.20309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizian M., Mahdipour E., Mirhafez S. R., Shoeibi S., Nematy M., Esmaily H., et al. (2016). Cytokine profiles in overweight and obese subjects and normal weight individuals matched for age and gender. Ann. Clin. Biochem. 53 663–668. 10.1177/0004563216629997 [DOI] [PubMed] [Google Scholar]
- Bales C. W., Porter-Starr K. N. (2018). Obesity interventions for older adults: diet as a determinant of physical function. Adv. Nutr. 9 151–159. 10.1093/advances/nmx016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballak D. B., Stienstra R., Tack C. J., Dinarello C. A., van-Diepen J. A. (2015). IL-1 family members in the pathogenesis and treatment of metabolic disease: focus on adipose tissue inflammation and insulin resistance. Cytokine 75 280–290. 10.1016/j.cyto.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R., Whitley S. K., Bhaumik S., Zindl C. L., Schoeb T. R., Benveniste E. N., et al. (2015). IL-1 signaling modulates activation of STAT transcription factors to antagonize retinoic acid signaling and control the TH 17 cell–iT reg cell balance. Nat. Immunol. 16 286–295. 10.1038/ni.3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belarbi K., Jopson T., Tweedie D., Arellano C., Luo W., Greig N. H., et al. (2012). TNF-α protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J. Neuroinflamm. 9 1–13. 10.1186/1742-2094-9-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet A. M., Van Maarle M. C., Hallqvist J., Morgenstern R., Frostegård J., Wiman B., et al. (2006). Association of TNF-α serum levels and TNFA promoter polymorphisms with risk of myocardial infarction. Atherosclerosis 187 408–414. 10.1016/j.atherosclerosis.2005.09.022 [DOI] [PubMed] [Google Scholar]
- Bernotiene E., Palmer G., Gabay C. (2006). The role of leptin in innate and adaptive immune responses. Arthritis Res. Ther. 8 1–10. 10.1186/ar2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud H. R., Morrison C. D., Münzberg H. (2020). The obesity epidemic in the face of homeostatic body weight regulation: what went wrong and how can it be fixed? Physiol. Behav. 222:112959. 10.1016/j.physbeh.2020.112959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blüher M. (2019). Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 15 288–298. 10.1038/s41574-019-0176-8 [DOI] [PubMed] [Google Scholar]
- Bobbo V. C., Jara C. P., Mendes N. F., Morari J., Velloso L. A., Araujo E. P. (2019). Interleukin-6 expression by hypothalamic microglia in multiple inflammatory contexts: a systematic review. BioMed Res. Int. 2019:1365210. 10.1155/2019/1365210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray G. A., Ryan D. H. (2021). Evidence-based weight loss interventions: individualized treatment options to maximize patient outcomes. Diabetes Obesity Metabol. 23 50–62. 10.1111/dom.14200 [DOI] [PubMed] [Google Scholar]
- Brown G. C. (2010). Nitric oxide and neuronal death. Nitric Oxide 23 153–165. 10.1016/j.niox.2010.06.001 [DOI] [PubMed] [Google Scholar]
- Bruce-Keller A. J., Keller J. N., Morrison C. D. (2009). Obesity and vulnerability of the CNS. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 1792 395–400. 10.1016/j.bbadis.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buie J. J., Watson L. S., Smith C. J., Sims-Robinson C. (2019). Obesity-related cognitive impairment: the role of endothelial dysfunction. Neurobiol. Dis. 132:104580. 10.1016/j.nbd.2019.104580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D. G., Faragher R. G. (2018). Obesity and type-2 diabetes as inducers of premature cellular senescence and ageing. Biogerontology 19 447–459. 10.1007/s10522-018-9763-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon N., Luheshi G., Layé S. (2015). Role of neuroinflammation in the emotional and cognitive alterations displayed by animal models of obesity. Front. Neurosci. 9:229. 10.3389/fnins.2015.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro A. M., Macedo-De la Concha L. E., Pantoja-Meléndez C. A. (2017). Low-grade inflammation and its relation to obesity and chronic degenerative diseases. Revista Méd. Hospital Gen. México 80 101–105. 10.1016/j.hgmx.2016.06.011 [DOI] [Google Scholar]
- Cavaliere G., Viggiano E., Trinchese G., De Filippo C., Messina A., Monda V., et al. (2018). Long feeding high-fat diet induces hypothalamic oxidative stress and inflammation, and prolonged hypothalamic AMPK activation in rat animal model. Front. Physiol. 9:818. 10.3389/fphys.2018.00818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censin J. C., Peters S. A., Bovijn J., Ferreira T., Pulit S. L., Mägi R., et al. (2019). Causal relationships between obesity and the leading causes of death in women and men. PLoS Genet. 15:e1008405. 10.1371/journal.pgen.1008405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Tang X., Li J., Hu B., Yang W., Zhan M., et al. (2022). IL-17 crosses the blood–brain barrier to trigger neuroinflammation: a novel mechanism in nitroglycerin-induced chronic migraine. J. Headache Pain 23 1–18. 10.1186/s10194-021-01374-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. H. E., Lainez N. M., Coss D. (2021). Sex differences in macrophage responses to obesity-mediated changes determine migratory and inflammatory traits. J. Immunol. 206 141–153. 10.4049/jimmunol.2000490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S. S., Huh J. Y., Hwang I. J., Kim J. I., Kim J. B. (2016). Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front. Endocrinol. 7:30. 10.3389/fendo.2016.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choromańska B., Myśliwiec P., Dadan J., Maleckas A., Zalewska A., Maciejczyk M. (2021). Effects of age and gender on the redox homeostasis of morbidly obese people. Free Radical Biol. Med. 175 108–120. 10.1016/j.freeradbiomed.2021.08.009 [DOI] [PubMed] [Google Scholar]
- Clemenzi M. N., Wellhauser L., Aljghami M. E., Belsham D. D. (2019). Tumour necrosis factor α induces neuroinflammation and insulin resistance in immortalised hypothalamic neurones through independent pathways. J. Neuroendocrinol. 31:e12678. 10.1111/jne.12678 [DOI] [PubMed] [Google Scholar]
- Cope E. C., LaMarca E. A., Monari P. K., Olson L. B., Martinez S., Zych A. D., et al. (2018). Microglia play an active role in obesity-associated cognitive decline. J. Neurosci. 38 8889–8904. 10.1523/JNEUROSCI.0789-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranford T. L., Enos R. T., Velázquez K. T., McClellan J. L., Davis J. M., Singh U. P., et al. (2016). Role of MCP-1 on inflammatory processes and metabolic dysfunction following high-fat feedings in the FVB/N strain. Int. J. Obesity 40 844–851. 10.1038/ijo.2015.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R., Prat A. (2015). The blood–brain barrier. Cold Spring Harb. Perspect. Biol. 7:a020412. 10.1101/cshperspect.a020412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daseke M. J., II, Tenkorang-Impraim M. A., Ma Y., Chalise U., Konfrst S. R., Garrett M. R., et al. (2020). Exogenous IL-4 shuts off pro-inflammation in neutrophils while stimulating anti-inflammation in macrophages to induce neutrophil phagocytosis following myocardial infarction. J. Mol. Cell. Cardiol. 145 112–121. 10.1016/j.yjmcc.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira S., Feijo G. D. S., Neto J., Jantsch J., Braga M. F., Castro L. F. D. S., et al. (2021). Zinc supplementation decreases obesity-related neuroinflammation and improves metabolic function and memory in rats. Obesity 29 116–124. 10.1002/oby.23024 [DOI] [PubMed] [Google Scholar]
- de Oliveira V., Mafra D. (2013). Adipokines in obesity. Clin. Chim. Acta 419 87–94. 10.1016/j.cca.2013.02.003 [DOI] [PubMed] [Google Scholar]
- Deng Y., Scherer P. E. (2010). Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann. N. Y. Acad. Sci. 1212:E1. 10.1111/j.1749-6632.2010.05875.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Renzo L., Bigioni M., Del Gobbo V., Premrov M. G., Barbini U., Di Lorenzo N., et al. (2007). Interleukin-1 (IL-1) receptor antagonist gene polymorphism in normal weight obese syndrome: relationship to body composition and IL-1 α and β plasma levels. Pharmacol. Res. 55 131–138. 10.1016/j.phrs.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Ding H. G., Deng Y. Y., Yang R. Q., Wang Q. S., Jiang W. Q., Han Y. L., et al. (2018). Hypercapnia induces IL-1β overproduction via activation of NLRP3 inflammasome: implication in cognitive impairment in hypoxemic adult rats. J. Neuroinflamm. 15 1–16. 10.1186/s12974-017-1051-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman M. D., Krull J. E., Douglass J. D., Fasnacht R., Lara-Lince F., Meek T. H., et al. (2017). Sex differences in microglial CX3CR1 signalling determine obesity susceptibility in mice. Nat. Commun. 8:14556. 10.1038/ncomms14556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffen J., Zhang M., Masek-Hammerman K., Nunez A., Brennan A., Jones J. E., et al. (2018). Modulation of the IL-33/IL-13 axis in obesity by IL-13Rα2. J. Immunol. 200 1347–1359. 10.4049/jimmunol.1701256 [DOI] [PubMed] [Google Scholar]
- Duffy C. M., Hofmeister J. J., Nixon J. P., Butterick T. A. (2019). High fat diet increases cognitive decline and neuroinflammation in a model of orexin loss. Neurobiol. Learn. Mem. 157 41–47. 10.1016/j.nlm.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye L., Boyle N., Champ C., Lawton C. (2017). The relationship between obesity and cognitive health and decline. Proc. Nutr. Soc. 76 443–454. 10.1017/S0029665117002014 [DOI] [PubMed] [Google Scholar]
- ElAli A., Doeppner T. R., Zechariah A., Hermann D. M. (2011). Increased blood–brain barrier permeability and brain edema after focal cerebral ischemia induced by hyperlipidemia: role of lipid peroxidation and calpain-1/2, matrix metalloproteinase-2/9, and RhoA overactivation. Stroke 42 3238–3244. 10.1161/STROKEAHA.111.615559 [DOI] [PubMed] [Google Scholar]
- Elhag W., El Ansari W., Razaq S., Elsherif M., Mustafa I. (2019). Lorcaserin vs. Phentermine among non-surgical and surgical obese patients: anthropometric, glycemic, lipid, safety and cost outcomes. Ann. Med. Surg. 45 75–81. 10.1016/j.amsu.2019.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellulu M. S., Patimah I., Khaza’ai H., Rahmat A., Abed Y. (2017). Obesity and inflammation: the linking mechanism and the complications. Arch. Med. Sci.: AMS 13:851. 10.5114/aoms.2016.58928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mikkawy D. M., El-Sadek M. A., El-Badawy M. A., Samaha D. (2020). Circulating level of interleukin-6 in relation to body mass indices and lipid profile in Egyptian adults with overweight and obesity. Egyptian Rheumatol. Rehabil. 47 1–7. 10.1186/s43166-020-00003-8 [DOI] [Google Scholar]
- Espeland M. A., Yassine H., Hayden K. D., Hugenschmidt C., Bennett W. L., Chao A., et al. (2021). Sex-related differences in cognitive trajectories in older individuals with type 2 diabetes and overweight or obesity. Alzheimer’s Dementia 7:e12160. 10.1002/trc2.12160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher N. F., Callanan J. J. (2012). Chapter VIII. Cell culture models of the blood -brain barrier?: new research. Annu. Rev. Pharmacol. Toxicol. 43 629–656. [Google Scholar]
- Fock K. M., Khoo J. (2013). Diet and exercise in management of obesity and overweight. J. Gastroenterol. Hepatol. 28 59–63. 10.1111/jgh.12407 [DOI] [PubMed] [Google Scholar]
- Fourrier C., Bosch-Bouju C., Boursereau R., Sauvant J., Aubert A., Capuron L., et al. (2019). Brain tumor necrosis factor-α mediates anxiety-like behavior in a mouse model of severe obesity. Brain Behav. Immunol. 77 25–36. 10.1016/j.bbi.2018.11.316 [DOI] [PubMed] [Google Scholar]
- Frankenberg A. D. V., Reis A. F., Gerchman F. (2017). Relationships between adiponectin levels, the metabolic syndrome, and type 2 diabetes: a literature review. Arch. Endocrinol. Metabol. 61 614–622. 10.1590/2359-3997000000316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani S. P., Cronk J. C., Norris G. T., Kipnis J. (2012). IL-4 in the brain: a cytokine to remember. J. Immunol. 189 4213–4219. 10.4049/jimmunol.1202246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainey S. J., Kwakwa K. A., Bray J. K., Pillote M. M., Tir V. L., Towers A. E., et al. (2016). Short-term high-fat diet induced anxiety-like behaviors and cognitive impairment are improved with treatment by glyburide. Front. Behav. Neurosci. 10:156. 10.3389/fnbeh.2016.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Li F., Zhou Z., Xu X., Wu Y., Zhou S., et al. (2017). IL-2/Anti-IL-2 complex attenuates inflammation and BBB disruption in mice subjected to traumatic brain injury. Front. Neurol. 8:281. 10.3389/fneur.2017.00281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J. M., Stillings S. A., Leclerc J. L., Phillips H., Edwards N. J., Robicsek S. A., et al. (2017). Role of interleukin-10 in acute brain injuries. Front. Neurol. 8:244. 10.3389/fneur.2017.00244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardener S. L., Rainey-Smith S. R., Bondonno C. P., Martins R. N. (2021). Intake of products containing anthocyanins, flavanols, and flavanones, and cognitive function: a review. Front. Aging Neurosci. 13:640381. 10.3389/fnagi.2021.640381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genario R., Cipolla-Neto J., Bueno A. A., Santos H. O. (2020). Melatonin supplementation in the management of obesity and obesity-associated disorders: a review of physiological mechanisms and clinical applications. Pharmacol. Res. 163:105254. 10.1016/j.phrs.2020.105254 [DOI] [PubMed] [Google Scholar]
- Ghaddar B., Veeren B., Rondeau P., Bringart M., d’Hellencourt C. L., Meilhac O., et al. (2020). Impaired brain homeostasis and neurogenesis in diet-induced overweight zebrafish: a preventive role from A. borbonica extract. Sci. Rep. 10:14496. 10.1038/s41598-020-71402-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfrancesco M. A., Dehairs J., L’homme L., Herinckx G., Esser N., Jansen O., et al. (2019). Saturated fatty acids induce NLRP3 activation in human macrophages through K+ efflux resulting from phospholipid saturation and Na, K-ATPase disruption. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 1864 1017–1030. 10.1016/j.bbalip.2019.04.001 [DOI] [PubMed] [Google Scholar]
- Goisser S., Kiesswetter E., Schoene D., Torbahn G., Bauer J. M. (2020). Dietary weight-loss interventions for the management of obesity in older adults. Rev. Endocrine Metabol. Disord. 21 355–368. 10.1007/s11154-020-09577-2 [DOI] [PubMed] [Google Scholar]
- Gomes J. A., Silva J. F., Marçal A. P., Silva G. C., Gomes G. F., de Oliveira A. C., et al. (2020). High-refined carbohydrate diet consumption induces neuroinflammation and anxiety-like behavior in mice. J. Nutr. Biochem. 77:108317. 10.1016/j.jnutbio.2019.108317 [DOI] [PubMed] [Google Scholar]
- González-Olmo B. M., Butler M. J., Barrientos R. M. (2021). Evolution of the human diet and its impact on gut microbiota, immune responses, and brain health. Nutrients 13:196. 10.3390/nu13010196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorostegi-Anduaga I., Corres P., MartinezAguirre-Betolaza A., Pérez-Asenjo J., Aispuru G. R., Fryer S. M., et al. (2018). Effects of different aerobic exercise programs with nutritional intervention in sedentary adults with overweight/obesity and hypertension: EXERDIET-HTA study. Eur. J. Prevent. Cardiol. 25 343–353. 10.1177/2047487317749956 [DOI] [PubMed] [Google Scholar]
- Graham L. C., Grabowska W. A., Chun Y., Risacher S. L., Philip V. M., Saykin A. J. (2019). Exercise prevents obesity-induced cognitive decline and white matter damage in mice. Neurobiol. Aging 80 154–172. 10.1016/j.neurobiolaging.2019.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin C., Lanzetta N., Eter L., Singer K. (2016). Sexually dimorphic myeloid inflammatory and metabolic responses to diet-induced obesity. Am. J. Physiol.-Regul. Integrat. Comparat. Physiol. 311 R211–R216. 10.1152/ajpregu.00136.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot-Legris O., Muccioli G. G. (2017). Obesity-induced neuroinflammation: beyond the hypothalamus. Trends Neurosci. 40 237–253. 10.1016/j.tins.2017.02.005 [DOI] [PubMed] [Google Scholar]
- Guo D. H., Yamamoto M., Hernandez C. M., Khodadadi H., Baban B., Stranahan A. M. (2020). Visceral adipose NLRP3 impairs cognition in obesity via IL-1R1 on CX3CR1+ cells. J. Clin. Investigat. 130 1961–1976. 10.1172/JCI126078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M. S., White A., Perry R. J., Camporez J. P., Hidalgo J., Shulman G. I., et al. (2020). Regulation of adipose tissue inflammation by interleukin 6. Proc. Natl. Acad. Sci. U.S.A. 117 2751–2760. 10.1073/pnas.1920004117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S., Dey A., Yu X., Stranahan A. M. (2016). Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav. Immun. 51 230–239. 10.1016/j.bbi.2015.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrave S. L., Davidson T. L., Zheng W., Kinzig K. P. (2016). Western diets induce blood-brain barrier leakage and alter spatial strategies in rats. Behav. Neurosci. 130:123. 10.1037/bne0000110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel J. J., Blumberg B. (2019). Environmental obesogens: mechanisms and controversies. Annu. Rev. Pharmacol. Toxicol. 59 89–106. 10.1146/annurev-pharmtox-010818-021304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai S., Takahashi N., Goto T., Lin S., Uemura T., Yu R., et al. (2010). Functional food targeting the regulation of obesity-induced inflammatory responses and pathologies. Mediators Inflamm. 2010:367838. 10.1155/2010/367838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander P. (2003). Orlistat in the treatment of obesity. Prim. Care: Clin. Off. Pract. 30 427–440. 10.1016/s0095-4543(03)00042-3 [DOI] [PubMed] [Google Scholar]
- Hong S. H., Kang M., Lee K. S., Yu K. (2016). High fat diet-induced TGF-β/Gbb signaling provokes insulin resistance through the tribbles expression. Sci. Rep. 6:30265. 10.1038/srep30265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer K. K., Dooms H., Barron L., Abbas A. K. (2008). Interleukin-2 in the development and control of inflammatory disease. Immunol. Rev. 226 19–28. 10.1111/j.1600-065X.2008.00697.x [DOI] [PubMed] [Google Scholar]
- Hruby A., Hu F. B. (2015). The epidemiology of obesity: a big picture. Pharmacoeconomics 33 673–689. 10.1007/s40273-014-0243-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P. S., Lin C. M., Chang J. F., Wu C. S., Sia K. C., Lee I. T., et al. (2019). Participation of NADPH oxidase-related reactive oxygen species in leptin-promoted pulmonary inflammation: regulation of cPLA2α and COX-2 expression. Int. J. Mol. Sci. 20:1078. 10.3390/ijms20051078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T. M., Konanur V. R., Taing L., Usui R., Kayser B. D., Goran M. I., et al. (2015). Effects of sucrose and high fructose corn syrup consumption on spatial memory function and hippocampal neuroinflammation in adolescent rats. Hippocampus 25 227–239. 10.1002/hipo.22368 [DOI] [PubMed] [Google Scholar]
- Hu Y., Xia C., Chen H., Song W., Zhou Q., Yang X., et al. (2021). Sex differences in the association between different obesity parameters and cognitive function in older adults: a cross-sectional study in rural China. Gerontology 1–9. 10.1159/000520081 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Hu Z., Deng N., Liu K., Zhou N., Sun Y., Zeng W. (2020). CNTF-STAT3-IL-6 Axis mediates neuroinflammatory cascade across Schwann cell-neuron-microglia. Cell Rep. 31:107657. 10.1016/j.celrep.2020.107657 [DOI] [PubMed] [Google Scholar]
- Huber J. D., VanGilder R. L., Houser K. A. (2006). Streptozotocin-induced diabetes progressively increases blood-brain barrier permeability in specific brain regions in rats. Am. J. Physiol.-Heart Circulat. Physiol. 291 H2660–H2668. 10.1152/ajpheart.00489.2006 [DOI] [PubMed] [Google Scholar]
- Hurtado-Alvarado G., Domínguez-Salazar E., Pavon L., Velazquez-Moctezuma J., Gomez-Gonzalez B. (2016). Blood-brain barrier disruption induced by chronic sleep loss: low-grade inflammation may be the link. J. Immunol. Res. 2016:4576012. 10.1155/2016/4576012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager J., Gremeaux T., Cormont M., Le Marchand-Brustel Y., Tanti J. F. (2007). Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 148 241–251. 10.1210/en.2006-0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jais A., Brüning J. C. (2017). Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Investigat. 127 24–32. 10.1172/JCI88878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jara-Moreno D., Castro-Torres R. D., Ettcheto M., Auladell C., Kogan M. J., Folch J., et al. (2018). The ethyl acetate extract of leaves of Ugni molinae Turcz. improves neuropathological hallmarks of Alzheimer’s disease in female APPswe/PS1dE9 mice fed with a high fat diet. J. Alzheimer’s Dis. 66 1175–1191. 10.3233/JAD-180174 [DOI] [PubMed] [Google Scholar]
- Kadry H., Noorani B., Cucullo L. (2020). A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 17:69. 10.1186/s12987-020-00230-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kander M. C., Cui Y., Liu Z. (2017). Gender difference in oxidative stress: a new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 21 1024–1032. 10.1111/jcmm.13038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang E. B., Koo J. H., Jang Y. C., Yang C. H., Lee Y., Cosio-Lima L. M., et al. (2016). Neuroprotective effects of endurance exercise against high-fat diet-induced hippocampal neuroinflammation. J. Neuroendocrinol. 28 10.1111/jne.12385 [DOI] [PubMed] [Google Scholar]
- Kanoski S. E., Zhang Y., Zheng W., Davidson T. L. (2010). The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J. Alzheimer’s Dis. 21 207–219. 10.3233/JAD-2010-091414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T., Kaur G. (2017). Withania somnifera as a potential candidate to ameliorate high fat diet-induced anxiety and neuroinflammation. J. Neuroinflamm. 14 1–18. 10.1186/s12974-017-0975-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern L., Mittenbühler M. J., Vesting A. J., Ostermann A. L., Wunderlich C. M., Wunderlich F. T. (2019). Obesity-induced TNFα and IL-6 signaling: the missing link between obesity and inflammation—driven liver and colorectal cancers. Cancers 11:24. 10.3390/cancers11010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. S., Ikram M., Park T. J., Kim M. O. (2021). Pathology, risk factors, and oxidative damage related to Type 2 diabetes-mediated Alzheimer’s disease and the rescuing effects of the potent antioxidant anthocyanin. Oxidative Med. Cell. Longevity 2021:4051207. 10.1155/2021/4051207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. D., Yoon N. A., Jin S., Diano S. (2019). Microglial UCP2 mediates inflammation and obesity induced by high-fat feeding. Cell Metabol. 30 952–962. 10.1016/j.cmet.2019.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T. W., Choi H. H., Chung Y. R. (2016). Treadmill exercise alleviates impairment of cognitive function by enhancing hippocampal neuroplasticity in the high-fat diet-induced obese mice. J. Exerc. Rehabil. 12:156. 10.12965/jer.1632644.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C., Jonas W., Iggena D., Empl L., Rivalan M., Wiedmer P., et al. (2016). Exercise prevents high-fat diet-induced impairment of flexible memory expression in the water maze and modulates adult hippocampal neurogenesis in mice. Neurobiol. Learn. Mem. 131, 26–35. 10.1016/j.nlm.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Knudsen N. H., Stanya K. J., Hyde A. L., Chalom M. M., Alexander R. K., Liou Y. H., et al. (2020). Interleukin-13 drives metabolic conditioning of muscle to endurance exercise. Science 368:eaat3987. 10.1126/science.aat3987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochumon S., Al Madhoun A., Al-Rashed F., Thomas R., Sindhu S., Al-Ozairi E., et al. (2020). Elevated adipose tissue associated IL-2 expression in obesity correlates with metabolic inflammation and insulin resistance. Sci. Rep. 10:16364. 10.1038/s41598-020-73347-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga S., Kojima A., Ishikawa C., Kuwabara S., Arai K., Yoshiyama Y. (2014). Effects of diet-induced obesity and voluntary exercise in a tauopathy mouse model: implications of persistent hyperleptinemia and enhanced astrocytic leptin receptor expression. Neurobiol. Dis. 71 180–192. 10.1016/j.nbd.2014.08.015 [DOI] [PubMed] [Google Scholar]
- Kondo H., Abe I., Gotoh K., Fukui A., Takanari H., Ishii Y., et al. (2018). Interleukin 10 treatment ameliorates high-fat diet–induced inflammatory atrial remodeling and fibrillation. Circulat.: Arrhythmia Electrophysiol. 11:e006040. 10.1161/CIRCEP.117.006040 [DOI] [PubMed] [Google Scholar]
- Kullmann S., Abbas Z., Machann J., Shah N. J., Scheffler K., Birkenfeld A. L., et al. (2020). Investigating obesity-associated brain inflammation using quantitative water content mapping. J. Neuroendocrinol. 32:e12907. 10.1111/jne.12907 [DOI] [PubMed] [Google Scholar]
- Kwon Y. H., Kim J., Kim C. S., Tu T. H., Kim M. S., Suk K., et al. (2017). Hypothalamic lipid-laden astrocytes induce microglia migration and activation. FEBS Lett. 591 1742–1751. 10.1002/1873-3468.12691 [DOI] [PubMed] [Google Scholar]
- Labban R. S. M., Alfawaz H., Almnaizel A. T., Hassan W. M., Bhat R. S., Moubayed N. M., et al. (2020). High-fat diet-induced obesity and impairment of brain neurotransmitter pool. Transl. Neurosci. 11 147–160. 10.1515/tnsci-2020-0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainez N. M., Coss D. (2019). Obesity, neuroinflammation, and reproductive function. Endocrinology 160 2719–2736. 10.1210/en.2019-00487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latta C. H., Sudduth T. L., Weekman E. M., Brothers H. M., Abner E. L., Popa G. J., et al. (2015). Determining the role of IL-4 induced neuroinflammation in microglial activity and amyloid-β using BV2 microglial cells and APP/PS1 transgenic mice. J. Neuroinflammat. 12 1–13. 10.1186/s12974-015-0243-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll C., Johnson M. D., Dunn-Meynell A. A., Boyle C. N., Lutz T. A., Levin B. E. (2015). Amylin-induced central IL-6 production enhances ventromedial hypothalamic leptin signaling. Diabetes 64 1621–1631. 10.2337/db14-0645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. J. (2018). Transforming growth factor beta superfamily regulation of adipose tissue biology in obesity. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 1864 1160–1171. 10.1016/j.bbadis.2018.01.025 [DOI] [PubMed] [Google Scholar]
- Lei X. G., Ruan J. Q., Lai C., Sun Z., Yang X. (2021). Efficacy and safety of phentermine/topiramate in adults with overweight or obesity: a systematic review and meta-analysis. Obesity 29 985–994. 10.1002/oby.23152 [DOI] [PubMed] [Google Scholar]
- Leyh J., Winter K., Reinicke M., Ceglarek U., Bechmann I., Landmann J. (2021). Long-term diet-induced obesity does not lead to learning and memory impairment in adult mice. PLoS One 16:e0257921. 10.1371/journal.pone.0257921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Shi Z., Mi Y. (2018). Purple sweet potato color attenuates high fat-induced neuroinflammation in mouse brain by inhibiting MAPK and NF-κB activation. Mol. Med. Rep. 17 4823–4831. 10.3892/mmr.2018.8440 [DOI] [PubMed] [Google Scholar]
- Li J. W., Zong Y., Cao X. P., Tan L., Tan L. (2018). Microglial priming in Alzheimer’s disease. Ann. Transl. Med. 6:176. 10.21037/atm.2018.04.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Yun K., Mu R. (2020). A review on the biology and properties of adipose tissue macrophages involved in adipose tissue physiological and pathophysiological processes. Lipids Health Dis. 19 1–9. 10.1186/s12944-020-01342-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Nikolajczyk B. S. (2019). Tissue immune cells fuel obesity-associated inflammation in adipose tissue and beyond. Front. Immunol. 10:1587. 10.3389/fimmu.2019.01587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Xu D., Yin C., Wang S., Wang M., Xiao Y. (2018). IL-10/STAT3 is reduced in childhood obesity with hypertriglyceridemia and is related to triglyceride level in diet-induced obese rats. BMC Endocr. Disord. 18:39. 10.1186/s12902-018-0265-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizcano F., Guzmán G. (2014). Estrogen deficiency and the origin of obesity during menopause. BioMed Res. Int. 2014:757461. 10.1155/2014/757461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Van Bever H. P., Lim T. K., Kuan W. S., Goh D. Y., Mahadevan M., et al. (2015). Obesity, asthma prevalence and IL-4: roles of inflammatory cytokines, adiponectin, and neuropeptide Y. Pediatr. Allergy Immunol. 26, 530–536. 10.1111/pai.12428 [DOI] [PubMed] [Google Scholar]
- Lumeng C. N., Bodzin J. L., Saltiel A. R. (2007). Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investigat. 117 175–184. 10.1172/JCI29881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren J. R., Janus C., Jensen S. B., Juhl C. R., Olsen L. M., Christensen R. M., et al. (2021). Healthy weight loss maintenance with exercise, liraglutide, or both combined. N. Engl. J. Med. 384 1719–1730. 10.1056/NEJMoa2028198 [DOI] [PubMed] [Google Scholar]
- Luzina I. G., Keegan A. D., Heller N. M., Rook G. A., Shea-Donohue T., Atamas S. P. (2012). Regulation of inflammation by interleukin-4: a review of “alternatives”. J. Leukocyte Biol. 92 753–764. 10.1189/jlb.0412214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel-Barón L. A., Pérez V., I, Torres C., González-Puertos V. Y., Konigsberg M., López-Diazguerrero N. E. (2017). La senescencia celular como denominador común de enfermedades asociadas a la edad. Revista Méd. Del Instituto Mexicano Del Seguro Soc. 55 490–497. [PubMed] [Google Scholar]
- Makki K., Froguel P., Wolowczuk I. (2013). Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. Int. Scholarly Res. Notices 2013:139239. 10.1155/2013/139239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Hochstetter D., Yao L., Zhao Y., Zhou J., Wang Y., et al. (2019). Green tea polyphenol (-)-epigallocatechin gallate (EGCG) attenuates neuroinflammation in palmitic acid-stimulated BV-2 microglia and high-fat diet-induced obese mice. Int. J. Mol. Sci. 20:5081. 10.3390/ijms20205081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques C., Fernandes I., Meireles M., Faria A., Spencer J. P., Mateus N., et al. (2018). Gut microbiota modulation accounts for the neuroprotective properties of anthocyanins. Sci. Rep. 8:11341. 10.1038/s41598-018-29744-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Reyes C. P., Gómez-Arauz A. Y., Torres-Castro I., Manjarrez-Reyna A. N., Palomera L. F., Olivos-García A., et al. (2018). Serum levels of interleukin-13 increase in subjects with insulin resistance but do not correlate with markers of low-grade systemic inflammation. J. Diabetes Res. 2018:7209872. 10.1155/2018/7209872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizi G., Della Guardia L., Maurizi A., Poloni A. (2018). Adipocytes properties and crosstalk with immune system in obesity-related inflammation. J. Cell. Physiol. 233 88–97. 10.1002/jcp.25855 [DOI] [PubMed] [Google Scholar]
- Maurya S. K., Bhattacharya N., Mishra S., Bhattacharya A., Banerjee P., Senapati S., et al. (2021). Microglia specific drug targeting using natural products for the regulation of redox imbalance in neurodegeneration. Front. Pharmacol. 12:654489. 10.3389/fphar.2021.654489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle M. A., Finucane O. M., Connaughton R. M., McMorrow A. M., Roche H. M. (2013). Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front. Endocrinol. (Lausanne) 10:52. 10.3389/fendo.2013.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean F. H., Grant C., Morris A. C., Horgan G. W., Polanski A. J., Allan K., et al. (2018). Rapid and reversible impairment of episodic memory by a high-fat diet in mice. Sci. Rep. 8:11976. 10.1038/s41598-018-30265-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano-Jiménez E., Carrillo I. J. F., Pedraza-Escalona M., Ramírez-Serrano C. E., Álvarez-Arellano L., Cortés-Mendoza J., et al. (2019). Malva parviflora extract ameliorates the deleterious effects of a high fat diet on the cognitive deficit in a mouse model of Alzheimer’s disease by restoring microglial function via a PPAR-γ-dependent mechanism. J. Neuroinflamm. 16 1–26. 10.1186/s12974-019-1515-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanova I. V., Correa-da-Silva F., Kalsbeek A., Yi C. X. (2021). Mapping of microglial brain region, sex and age heterogeneity in obesity. Int. J. Mol. Sci. 22:3141. 10.3390/ijms22063141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. A., Spencer S. J. (2014). Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav. Immun. 42 10–21. 10.1016/j.bbi.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Minamino T., Orimo M., Shimizu I., Kunieda T., Yokoyama M., Ito T., et al. (2009). A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 15 1082–1087. 10.1038/nm.2014 [DOI] [PubMed] [Google Scholar]
- Mittal B. (2019). Subcutaneous adipose tissue & visceral adipose tissue. Ind. J. Med. Res. 149:571. 10.4103/ijmr.IJMR_1910_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed G. A., Ibrahim S. R., Elkhayat E. S., El Dine R. S. (2014). Natural anti-obesity agents. Bull. Faculty Pharm. Cairo Univ. 52 269–284. 10.1016/j.bfopcu.2014.05.001 [DOI] [Google Scholar]
- Morgan M. J., Liu Z. G. (2011). Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 21 103–115. 10.1038/cr.2010.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morigny P., Boucher J., Arner P., Langin D. (2021). Lipid and glucose metabolism in white adipocytes: pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 17 276–295. 10.1038/s41574-021-00471-8 [DOI] [PubMed] [Google Scholar]
- Morrison C. D., Pistell P. J., Ingram D. K., Johnson W. D., Liu Y., Fernandez-Kim S. O., et al. (2010). High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J. Neurochem. 114 1581–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morys F., Dadar M., Dagher A. (2021). Association between mid-life obesity, its metabolic consequences, cerebrovascular disease and cognitive decline. J. Clin. Endocrinol. Metabol. 106 e4260–e4274. 10.1210/clinem/dgab135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. (2017). Macrophage polarization. Annu. Rev. Physiol. 79 541–566. 10.1146/annurev-physiol-022516-034339 [DOI] [PubMed] [Google Scholar]
- Negrin K. A., Roth Flach R. J., DiStefano M. T., Matevossian A., Friedline R. H., Jung D., et al. (2014). IL-1 signaling in obesity-induced hepatic lipogenesis and steatosis. PLoS One 9:e107265. 10.1371/journal.pone.0107265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerurkar P. V., Johns L. M., Buesa L. M., Kipyakwai G., Volper E., Sato R., et al. (2011). Momordica charantia (bitter melon) attenuates high-fat diet-associated oxidative stress and neuroinflammation. J. Neuroinflamm. 8 1–19. 10.1186/1742-2094-8-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen J. C., Killcross A. S., Jenkins T. A. (2014). Obesity and cognitive decline: role of inflammation and vascular changes. Front. Neurosci. 8:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntlholang O., McCarroll K., Laird E., Molloy A. M., Ward M., McNulty H., et al. (2018). The relationship between adiposity and cognitive function in a large community-dwelling population: data from the Trinity Ulster Department of Agriculture (TUDA) ageing cohort study. Br. J. Nutr. 120 517–527. 10.1017/S0007114518001848 [DOI] [PubMed] [Google Scholar]
- Ogrodnik M., Zhu Y. I., Langhi L. G., Tchkonia T., Krüger P., Fielder E., et al. (2019). Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab. 29 1061–1077. 10.1016/j.cmet.2018.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda M. H., Zemdegs J. C., de Santana A. A., Santamarina A. B., Moreno M. F., Hachul A. C., et al. (2014). Green tea extract improves high fat diet-induced hypothalamic inflammation, without affecting the serotoninergic system. J. Nutr. Biochem. 25 1084–1089. 10.1016/j.jnutbio.2014.05.012 [DOI] [PubMed] [Google Scholar]
- Olmos G., Lladó J. (2014). Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm. 2014:861231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke R. W., White A. E., Metcalf M. D., Winters B. R., Diggs B. S., Zhu X., et al. (2012). Systemic inflammation and insulin sensitivity in obese IFN-γ knockout mice. Metabolism 61 1152–1161. 10.1016/j.metabol.2012.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang S., Hsuchou H., Kastin A. J., Wang Y., Yu C., Pan W. (2014). Diet-induced obesity suppresses expression of many proteins at the blood–brain barrier. J. Cerebral Blood Flow Metabol. 34 43–51. 10.1038/jcbfm.2013.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachter J. S., de Vries H. E., Fabry Z. (2003). The blood-brain barrier and its role in immune privilege in the central nervous system. J. Neuropathol. Exp. Neurol. 62 593–604. 10.1093/jnen/62.6.593 [DOI] [PubMed] [Google Scholar]
- Patel R. K., Prasad N., Kuwar R., Haldar D., Abdul-Muneer P. M. (2017). Transforming growth factor-beta 1 signaling regulates neuroinflammation and apoptosis in mild traumatic brain injury. Brain Behav. Immun. 64 244–258. 10.1016/j.bbi.2017.04.012 [DOI] [PubMed] [Google Scholar]
- Paz-Filho G., Mastronardi C., Franco C. B., Wang K. B., Wong M. L., Licinio J. (2012). Leptin: molecular mechanisms, systemic pro-inflammatory effects, and clinical implications. Arquivos Brasileiros Endocrinol. Metabol. 56 597–607. 10.1590/s0004-27302012000900001 [DOI] [PubMed] [Google Scholar]
- Pereira L., Font-Nieves M., Van den Haute C., Baekelandt V., Planas A. M., Pozas E. (2015). IL-10 regulates adult neurogenesis by modulating ERK and STAT3 activity. Front. Cell. Neurosci. 9:57. 10.3389/fncel.2015.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Pérez A., Sánchez-Jiménez F., Vilariño-García T., Sánchez-Margalet V. (2020). Role of leptin in inflammation and vice versa. Int. J. Mol. Sci. 21:5887. 10.3390/ijms21165887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry V. H., Holmes C. (2014). Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 10 217–224. 10.1038/nrneurol.2014.38 [DOI] [PubMed] [Google Scholar]
- Porro S., Genchi V. A., Cignarelli A., Natalicchio A., Laviola L., Giorgino F., et al. (2021). Dysmetabolic adipose tissue in obesity: morphological and functional characteristics of adipose stem cells and mature adipocytes in healthy and unhealthy obese subjects. J. Endocrinol. Investigat. 44 921–941. 10.1007/s40618-020-01446-8 [DOI] [PubMed] [Google Scholar]
- Qiu A. W., Huang D. R., Li B., Fang Y., Zhang W. W., Liu Q. H. (2021). IL-17A injury to retinal ganglion cells is mediated by retinal Müller cells in diabetic retinopathy. Cell Death Dis. 12 1–10. 10.1038/s41419-021-04350-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ràfols M. E. (2014). Adipose tissue: cell heterogeneity and functional diversity. Endocrinol. Nutr. (English Edition) 61 100–112. 10.1016/j.endonu.2013.03.011 [DOI] [PubMed] [Google Scholar]
- Rajasekaran M., Sul O. J., Choi E. K., Kim J. E., Suh J. H., Choi H. S. (2019). MCP-1 deficiency enhances browning of adipose tissue via increased M2 polarization. J. Endocrinol. 242 91–101. 10.1530/JOE-19-0190 [DOI] [PubMed] [Google Scholar]
- Recasens M., Almolda B., Pérez-Clausell J., Campbell I. L., González B., Castellano B. (2021). Chronic exposure to IL-6 induces a desensitized phenotype of the microglia. J. Neuroinflamm. 18 1–22. 10.1186/s12974-020-02063-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea E. M., Salameh T. S., Logsdon A. F., Hanson A. J., Erickson M. A., Banks W. A. (2017). Blood-brain barriers in obesity. AAPS J. 19 921–930. 10.1208/s12248-017-0079-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha V. Z., Folco E. J., Sukhova G., Shimizu K., Gotsman I., Vernon A. H., et al. (2008). Interferon-γ, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circulat. Res. 103 467–476. 10.1161/CIRCRESAHA.108.177105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M. E. D. S., Houser M. C., Walker D. I., Jones D. P., Chang J., Barnum C. J., et al. (2020). Targeting soluble tumor necrosis factor as a potential intervention to lower risk for late-onset Alzheimer’s disease associated with obesity, metabolic syndrome, and type 2 diabetes. Alzheimer’s Res. Ther. 12 1–16. 10.1186/s13195-019-0546-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothaug M., Becker-Pauly C., Rose-John S. (2016). The role of interleukin-6 signaling in nervous tissue. Biochim. Biophys. Acta 1863(6 Pt A), 1218–1227. 10.1016/j.bbamcr.2016.03.018 [DOI] [PubMed] [Google Scholar]
- Rosin J. M., Kurrasch D. M. (2019). Emerging roles for hypothalamic microglia as regulators of physiological homeostasis. Front. Neuroendocrinol. 54:100748. 10.1016/j.yfrne.2019.100748 [DOI] [PubMed] [Google Scholar]
- Rossi C., Cusimano M., Zambito M., Finardi A., Capotondo A., Garcia-Manteiga J. M., et al. (2018). Interleukin 4 modulates microglia homeostasis and attenuates the early slowly progressive phase of amyotrophic lateral sclerosis. Cell Death Dis. 9 1–16. 10.1038/s41419-018-0288-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustenhoven J., Aalderink M., Scotter E. L., Oldfield R. L., Bergin P. S., Mee E. W., et al. (2016). TGF-beta1 regulates human brain pericyte inflammatory processes involved in neurovasculature function. J. Neuroinflamm. 13 1–15. 10.1186/s12974-016-0503-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saely C. H., Geiger K., Drexel H. (2012). Brown versus white adipose tissue: a mini-review. Gerontology 58 15–23. 10.1159/000321319 [DOI] [PubMed] [Google Scholar]
- Sam S., Mazzone T. (2014). Adipose tissue changes in obesity and the impact on metabolic function. Transl. Res. 164 284–292. 10.1016/j.trsl.2014.05.008 [DOI] [PubMed] [Google Scholar]
- Samara A., Murphy T., Strain J., Rutlin J., Sun P., Neyman O., et al. (2020). Neuroinflammation and white matter alterations in obesity assessed by diffusion basis spectrum imaging. Front. Hum. Neurosci. 13:464. 10.3389/fnhum.2019.00464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhir R., Halder A., Sunkaria A. (2017). Mitochondria as a centrally positioned hub in the innate immune response. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 1863 1090–1097. 10.1016/j.bbadis.2016.10.020 [DOI] [PubMed] [Google Scholar]
- Shaftel S. S., Griffin W. S. T., O’Banion M. K. (2008). The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J. Neuroinflamm. 5 1–12. 10.1186/1742-2094-5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Yu Y., Lin D., Zheng P., Zhang P., Hu M., et al. (2020). β-glucan attenuates cognitive impairment via the gut-brain axis in diet-induced obese mice. Microbiome 8 1–21. 10.1186/s40168-020-00920-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau M. Y., Chuang P. H., Yang C. P., Hsiao C. W., Chang S. W., Chang K. Y., et al. (2019). Mechanism of interleukin-4 reducing lipid deposit by regulating hormone-sensitive lipase. Sci. Rep. 9:11974. 10.1038/s41598-019-47908-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhu S., Thomas R., Shihab P., Sriraman D., Behbehani K., Ahmad R. (2015). Obesity is a positive modulator of IL-6R and IL-6 expression in the subcutaneous adipose tissue: significance for metabolic inflammation. PLoS One 10:e0133494. 10.1371/journal.pone.0133494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Bajaj P., Kalotra S., Bhandari A., Kaur T., Singh A. P., et al. (2021). Tinospora cordifolia ameliorates brain functions impairments associated with high fat diet induced obesity. Neurochem. Int. 143:104937. 10.1016/j.neuint.2020.104937 [DOI] [PubMed] [Google Scholar]
- Smith C. J., Perfetti T. A., Hayes A. W., Berry S. C. (2020). Obesity as a source of endogenous compounds associated with chronic disease: a review. Toxicol. Sci. 175 149–155. 10.1093/toxsci/kfaa042 [DOI] [PubMed] [Google Scholar]
- Stȩpień M., Stȩpień A., Wlazeł R. N., Paradowski M., Banach M., Rysz J. (2014). Obesity indices and inflammatory markers in obese non-diabetic normo-and hypertensive patients: a comparative pilot study. Lipids Health Dis. 13 1–10. 10.1186/1476-511X-13-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama M., Banno R., Yaginuma H., Taki K., Mizoguchi A., Tsunekawa T., et al. (2020). Hypothalamic glial cells isolated by MACS reveal that microglia and astrocytes induce hypothalamic inflammation via different processes under high-fat diet conditions. Neurochem. Int. 136:104733. 10.1016/j.neuint.2020.104733 [DOI] [PubMed] [Google Scholar]
- Sun N. N., Wu T. Y., Chau C. F. (2016). Natural dietary and herbal products in anti-obesity treatment. Molecules 21:1351. 10.3390/molecules21101351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendar J., Frohberger S. J., Karunakaran I., Schmitt V., Stamminger W., Neumann A. L., et al. (2019). Adiponectin limits IFN-γ and IL-17 producing CD4 T cells in obesity by restraining cell intrinsic glycolysis. Front. Immunol. 10:2555. 10.3389/fimmu.2019.02555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmi B., Hasty A. (2008). Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future Lipidol. 3 545–556. 10.2217/17460875.3.5.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szelényi J. (2001). Cytokines and the central nervous system. Brain Res. Bull. 54 329–338. 10.1016/s0361-9230(01)00428-2 [DOI] [PubMed] [Google Scholar]
- Tavares R. L., Vasconcelos M., Dutra M., D’Oliveira A. B., Lima M., Salvadori M., et al. (2020). Mucuna pruriens administration minimizes neuroinflammation and shows anxiolytic, antidepressant and slimming effects in obese rats. Molecules (Basel, Switzerland) 25:5559. 10.3390/molecules25235559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijeiro A., Garrido A., Ferre A., Perna C., Djouder N. (2021). Inhibition of the IL-17A axis in adipocytes suppresses diet-induced obesity and metabolic disorders in mice. Nat. Metabol. 3 496–512. 10.1038/s42255-021-00371-1 [DOI] [PubMed] [Google Scholar]
- Terrazas S., Brashear L., Escoto A. K., Lynch S., Slaughter D., Xavier N., et al. (2019). “Sex differences in obesity-induced inflammation,” in Translational Studies on Inflammation, ed. Nunes A. (London: IntechOpen; ). 10.5772/intechopen.78112 [DOI] [Google Scholar]
- Thaler J. P., Yi C. X., Schur E. A., Guyenet S. J., Hwang B. H., Dietrich M. O., et al. (2012). Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investigat. 122 153–162. 10.1172/JCI59660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal V. J., Fanburg B. L. (2000). Reactive oxygen species in cell signaling. Am. J. Physiol.-Lung Cellular Mol. Physiol. 279 L1005–L1028. 10.1152/ajplung.2000.279.6.L1005 [DOI] [PubMed] [Google Scholar]
- The Lancet Diabetes Endocrinology (2017). Should we officially recognize obesity as a disease? Lancet Diabetes Endocrinol. 5:483. 10.1016/S2213-8587(17)30191-2 [DOI] [PubMed] [Google Scholar]
- Tilinca M. C., Tiuca R. A., Burlacu A., Varga A. (2021). A 2021 update on the use of liraglutide in the modern treatment of ‘Diabesity’: a narrative review. Medicina 57:669. 10.3390/medicina57070669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorćević M., Manuel A. R., Austen L., Michailidou Z., Hazlehurst J. M., Neville M., et al. (2021). Markers of adipose tissue hypoxia are elevated in subcutaneous adipose tissue of severely obese patients with obesity hypoventilation syndrome but not in the moderately obese. Int. J. Obesity (Lond.) 45 1618–1622. 10.1038/s41366-021-00793-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P. (2013). Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 93 1–21. 10.1152/physrev.00017.2012 [DOI] [PubMed] [Google Scholar]
- Trayhurn P. (2014). Hypoxia and adipocyte physiology: implications for adipose tissue dysfunction in obesity. Annu. Rev. Nutr. 34 207–236. 10.1146/annurev-nutr-071812-161156 [DOI] [PubMed] [Google Scholar]
- Treviño S., Aguilar-Alonso P., Flores Hernandez J. A., Brambila E., Guevara J., Flores G., et al. (2015). A high calorie diet causes memory loss, metabolic syndrome and oxidative stress into hippocampus and temporal cortex of rats. Synapse 69 421–433. 10.1002/syn.21832 [DOI] [PubMed] [Google Scholar]
- Tsao C. H., Shiau M. Y., Chuang P. H., Chang Y. H., Hwang J. (2014). Interleukin-4 regulates lipid metabolism by inhibiting adipogenesis and promoting lipolysis. J. Lipid Res. 55 385–397. 10.1194/jlr.M041392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z., Toth P., Tarantini S., Sosnowska D., Gautam T., Warrington J. P., et al. (2014). Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J. Gerontol. - Ser. A Biol. Sci. Med. Sci. 69 1339–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanavari T., Giannogonas P., Karalis K. (2010). TNF-alpha and obesity. Curr. Dir Autoimmun. 11 145–156. 10.1159/000289203 [DOI] [PubMed] [Google Scholar]
- Ugalde-Muñiz P., Fetter-Pruneda I., Navarro L., García E., Chavarría A. (2020). Chronic systemic inflammation exacerbates neurotoxicity in a Parkinson’s disease model. Oxidat. Med. Cell. Longevity 2020:4807179. 10.1155/2020/4807179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um J. Y., Rim H. K., Kim S. J., Kim H. L., Hong S. H. (2011). Functional polymorphism of IL-1 alpha and its potential role in obesity in humans and mice. PLoS One 6:e29524. 10.1371/journal.pone.0029524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdearcos M., Douglass J. D., Robblee M. M., Dorfman M. D., Stifler D. R., Bennett M. L., et al. (2017). Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metabol. 26 185–197. 10.1016/j.cmet.2017.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyken P., Lacoste B. (2018). Impact of metabolic syndrome on neuroinflammation and the blood–brain barrier. Front. Neurosci. 12:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera F., Pino J., Campos-Cabaleiro V., Ruiz-Fernández C., Mera A., Gonzalez-Gay M. A., et al. (2018). Obesity, fat mass and immune system: role for leptin. Front. Physiol. 9:640. 10.3389/fphys.2018.00640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bernhardi R., Cornejo F., Parada G., Eugenín J. (2015). Role of TGFβ signaling in the pathogenesis of Alzheimer’s disease. Front. Cell. Neurosci. 9:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T., Hoshino M., Kimura Y., Ojima M., Nakano T., Koya D., et al. (2011). Both type I and II IFN induce insulin resistance by inducing different isoforms of SOCS expression in 3T3-L1 adipocytes. Am. J. Physiol.-Endocrinol. Metabol. 300 E1112–E1123. 10.1152/ajpendo.00370.2010 [DOI] [PubMed] [Google Scholar]
- Wang G. X., Cho K. W., Uhm M., Hu C. R., Li S., Cozacov Z., et al. (2014). Otopetrin 1 protects mice from obesity-associated metabolic dysfunction through attenuating adipose tissue inflammation. Diabetes 63 1340–1352. 10.2337/db13-1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wang Q. A., Liu Y., Jiang L. (2021). Energy metabolism in brown adipose tissue. FEBS J. 288 3647–3662. 10.1111/febs.16015 [DOI] [PubMed] [Google Scholar]
- Whitlock G., Lewington S., Sherliker P., Clarke R., Emberson J., Halsey J., et al. (2009). Prospective Studies Collaboration: body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 373 1083–1096. 10.1016/S0140-6736(09)60318-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization [WHO] (2021). Obesity and Overweight. Geneva: WHO. [Google Scholar]
- Wueest S., Konrad D. (2020). The controversial role of IL-6 in adipose tissue on obesity-induced dysregulation of glucose metabolism. Am. J. Physiol.-Endocrinol. Metabol. 319 E607–E613. 10.1152/ajpendo.00306.2020 [DOI] [PubMed] [Google Scholar]
- Xing C., Li W., Deng W., Ning M., Lo E. H. (2018). A potential gliovascular mechanism for microglial activation: differential phenotypic switching of microglia by endothelium versus astrocytes. J. Neuroinflamm. 15:143. 10.1186/s12974-018-1189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav H., Quijano C., Kamaraju A. K., Gavrilova O., Malek R., Chen W., et al. (2011). Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell Metab. 14 67–79. 10.1016/j.cmet.2011.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. Y., Yuan C. X. (2018). IL-17A promotes the neuroinflammation and cognitive function in sevoflurane anesthetized aged rats via activation of NF-κB signaling pathway. BMC Anesthesiol. 18:147. 10.1186/s12871-018-0607-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh C. W., Liu H. K., Lin L. C., Liou K. T., Huang Y. C., Lin C. H., et al. (2017). Xuefu Zhuyu decoction ameliorates obesity, hepatic steatosis, neuroinflammation, amyloid deposition and cognition impairment in metabolically stressed APPswe/PS1dE9 mice. J. Ethnopharmacol. 209 50–61. 10.1016/j.jep.2017.07.036 [DOI] [PubMed] [Google Scholar]
- Yuste J. E., Tarragon E., Campuzano C. M., Ros-Bernal F. (2015). Implications of glial nitric oxide in neurodegenerative diseases. Front. Cell. Neurosci. 9:322. 10.3389/fncel.2015.00322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani N., Brown C. W. (2011). Emerging roles for the transforming growth factor-β superfamily in regulating adiposity and energy expenditure. Endocrine Rev. 32 387–403. 10.1210/er.2010-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Dong F., Ren J., Driscoll M. J., Culver B. (2005). High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp. Neurol. 191 318–325. 10.1016/j.expneurol.2004.10.011 [DOI] [PubMed] [Google Scholar]
- Zhou J., Mao L., Xu P., Wang Y. (2018). Effects of (-)-epigallocatechin gallate (EGCG) on energy expenditure and microglia-mediated hypothalamic inflammation in mice fed a high-fat diet. Nutrients 10:1681. 10.3390/nu10111681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Mareljic N., Michaelsen M., Parhizkar S., Heindl S., Nuscher B., et al. (2020). Active poly-GA vaccination prevents microglia activation and motor deficits in a C9orf72 mouse model. EMBO Mol. Med. 12:e10919. 10.15252/emmm.201910919 [DOI] [PMC free article] [PubMed] [Google Scholar]