Abstract
Background
The COVID-19 pandemic has led to delays in patients seeking care for life-threatening conditions; however, its impact on treatment patterns for patients with metastatic cancer is unknown. We assessed the COVID-19 pandemic’s impact on time to treatment initiation (TTI) and treatment selection for patients newly diagnosed with metastatic solid cancer.
Methods
We used an electronic health record–derived longitudinal database curated via technology-enabled abstraction to identify 14 136 US patients newly diagnosed with de novo or recurrent metastatic solid cancer between January 1 and July 31 in 2019 or 2020. Patients received care at approximately 280 predominantly community-based oncology practices. Controlled interrupted time series analyses assessed the impact of the COVID-19 pandemic period (April-July 2020) on TTI, defined as the number of days from metastatic diagnosis to receipt of first-line systemic therapy, and use of myelosuppressive therapy.
Results
The adjusted probability of treatment within 30 days of diagnosis was similar across periods (January-March 2019 = 41.7%, 95% confidence interval [CI] = 32.2% to 51.1%; April-July 2019 = 42.6%, 95% CI = 32.4% to 52.7%; January-March 2020 = 44.5%, 95% CI = 30.4% to 58.6%; April-July 2020 = 46.8%, 95% CI= 34.6% to 59.0%; adjusted percentage-point difference-in-differences = 1.4%, 95% CI = −2.7% to 5.5%). Among 5962 patients who received first-line systemic therapy, there was no association between the pandemic period and use of myelosuppressive therapy (adjusted percentage-point difference-in-differences = 1.6%, 95% CI = −2.6% to 5.8%). There was no meaningful effect modification by cancer type, race, or age.
Conclusions
Despite known pandemic-related delays in surveillance and diagnosis, the COVID-19 pandemic did not affect TTI or treatment selection for patients with metastatic solid cancers.
The COVID-19 pandemic has led to declines in patients seeking care for life-threatening conditions, such as acute myocardial infarction and stroke, as well as care delays for screening and management of chronic medical conditions (1-5). For patients with cancer, who may be particularly vulnerable to COVID-19 infection (6–8), early research suggested changes in practice patterns leading to care delays and treatment modifications (9–17). Some of these changes were supported by guidelines issued during the pandemic (18), which encouraged consideration of nonmyelosuppressive regimens despite mixed evidence linking the risk and severity of COVID-19 infection to immunosuppression from cancer therapy (8,19–21). These care disruptions may have been particularly prominent for patients with metastatic cancer for whom treatments are palliative rather than curative. A recent systematic review identified 62 studies evaluating pandemic-related delays across the cancer care continuum; however, the majority of these studies used single-institution data and did not focus on patients with metastatic cancer (22). Thus, little is known about the impact of the pandemic on changes in treatment patterns for patients with metastatic cancer.
Because treatment delays cause patient distress and are associated with increased mortality for patients with cancer (23–27), time to treatment initiation (TTI) is a patient-centered quality metric and outcome that has been used to evaluate the impact of health policies on cancer care (9,28,29). TTI may also serve as a barometer of capacity limitation and care delivery disruption during the COVID-19 pandemic (30–34). Moreover, pandemic-related delays or changes in cancer treatment may have disproportionately affected minority groups, including African American patients, who even before the pandemic were less likely to receive guideline-concordant systemic therapy for metastatic cancer than White patients (35–40). It is thus critical to identify whether the COVID-19 pandemic resulted in changes in treatment patterns for patients with metastatic cancer, with potential downstream consequences that could adversely affect patient outcomes and equitable cancer care.
The objective of this study was to evaluate the impact of the COVID-19 pandemic on TTI and treatment selection for patients newly diagnosed with metastatic solid cancer, with attention to race- and age-based disparities. We hypothesized that the pandemic would be associated with delays in initiation of systemic therapy and increased use of nonmyelosuppressive therapies.
Methods
Study Design
We applied a retrospective controlled interrupted time series approach to evaluate associations between the COVID-19 pandemic period and changes in TTI and use of myelosuppressive therapy. The study adhered to Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines and was exempted by the University of Pennsylvania and WCG Institutional Review Boards before study conduct due to use of deidentified data only.
Data Source
This study used the nationwide Flatiron Health database, an electronic health record (EHR)-derived, longitudinal database comprising deidentified patient-level structured and unstructured data curated via technology-enabled abstraction (41,42). During the study period, data originated from approximately 280 US cancer clinics (approximately 800 sites of care). The majority of patients in the database originated from community oncology settings. The data were deidentified and subject to obligations to prevent reidentification and protect patient confidentiality.
Participants
The main study sample included adult patients (aged ≥18 years) with a new diagnosis of metastatic solid cancer from January 1 to July 31, 2019, or January 1 to July 31, 2020. Metastatic status was determined using both structured data and abstracted unstructured data from clinical, imaging, and pathology notes and included de novo (defined as stage M1 at initial diagnosis) or recurrent (M0 at initial diagnosis) diagnoses. Eligible cancer types were breast, colorectal, non-small cell lung carcinoma (NSCLC), pancreas, prostate, renal cell, or urothelial cancer. Patients were included regardless of treatment status, including those who did not receive systemic therapy during the study period. We excluded patients with incomplete historical treatment data (defined as 90 days or more) between diagnosis and the earliest date of structured activity (defined as a clinical visit, laboratory check, or treatment receipt documented in the EHR [n = 1631]), fewer than 2 documented clinical visits after metastatic cancer diagnosis (n = 1275), multiple metastatic malignancies (n = 66), first-line treatment starting before recorded metastatic diagnosis date (n = 682), or who were receiving therapy that was not part of National Comprehensive Cancer Network guidelines (n = 344). We also excluded patients diagnosed during a 30-day “washout” period (March 8 to April 7) encompassing the start of most state stay-at-home orders in 2020 (Supplementary Table 1, available online) and historical control patients the comparable period in 2019 (n = 2127). Supplementary Figure 1 (available online) illustrates our cohort selection.
We evaluated changes in treatment selection in a subsample of patients diagnosed with metastatic breast, NSCLC, prostate, or urothelial cancer during the study period who received a systemic therapy within 60 days of metastatic diagnosis (n = 6721). We selected these 4 cancers because they have guideline-based myelosuppressive and nonmyelosuppressive options for frontline therapy. Furthermore, frontline treatment guidelines (43–46) for these metastatic cancers did not change substantially during the study period, allowing for comparisons with historical controls. In addition to exclusions applied to the main study sample, patients were excluded if they received first-line treatment directed at a targetable mutation (EGFR, ALK, ROS-1, or BRAF for NSCLC; HER-2 for breast) or microsatellite instability (n = 759). These patients were excluded because their treatment decisions were likely influenced by the presence of an actionable genetic or molecular aberration rather than by factors related to the pandemic.
Main Outcomes and Measures
The primary outcome was TTI, defined as the number of days from metastatic diagnosis to receipt of first-line systemic therapy. Patients were censored at their last structured activity within the Flatiron Health network or 90 days after diagnosis, whichever occurred first. The secondary outcome was receipt of myelosuppressive treatment. Myelosuppressive treatment was defined as any regimen containing cytotoxic chemotherapy or a cyclin-dependent kinase inhibitor. Checkpoint inhibitors (NSCLC, urothelial) and hormone therapies (breast, prostate) without concurrent myelosuppressive therapy were considered nonmyelosuppressive (see Supplementary Table 2, available online for treatment categorizations).
The primary exposure was time period (April 8-July 31 vs January 1-March 8) and year (2020 vs 2019) of metastatic cancer diagnosis. These intervals corresponded with time periods in 2020 when the COVID-19 pandemic would be more vs less likely to influence patient treatment based on the date of most states’ stay-at-home orders. In our controlled interrupted time series approach, the comparison of interest was defined as the change in TTI (or receipt of myelosuppressive therapy) across time periods in 2020 compared with the change across time periods in 2019.
Covariates included age, sex, race (Hispanic, non-Hispanic Black, non-Hispanic White, or other [includes Asian American, American Indian or Alaska Native, Hawaiian or Pacific Islander, and multiracial]), insurance type (commercial, government, or other), Eastern Cooperative Oncology Group performance status (<2 or ≥2), documented opioid medication order (yes or no), calendar day of metastatic cancer diagnosis, and cancer type. All covariates were ascertained at the time of metastatic cancer diagnosis.
Missing baseline covariate data were accounted for using multiple imputation via chained equations with 10 imputations. Continuous variables were imputed using an approach that allowed for heterogeneous within-group variance by practice (47). Categorical and dichotomous variables were imputed using multinomial logistic regression and logistic regression, respectively.
Statistical Methods
Frequencies and proportions of baseline characteristics were summarized by time period. Standardized mean differences were used to describe differences in baseline characteristics across the 4 time periods; a standardized mean difference greater than 0.1 was considered a meaningful difference (48). The Kaplan-Meier estimator was used to estimate unadjusted median TTI within each time period. We conducted adjusted analyses of TTI using Cox proportional hazards regression. The primary exposure was an interaction between period (April-July vs January-March) and year (2020 vs 2019) of metastatic cancer diagnosis. All models were adjusted for age, sex, race, insurance, Eastern Cooperative Oncology Group, opioid prescription, a linear time trend for calendar day of metastatic cancer diagnosis, and cancer type and used robust standard errors to allow for within-practice correlation. Our primary analysis included an additional 3-way interaction between period, year, and cancer type to investigate effect modification by cancer type. Exploratory analyses excluded the cancer type interaction and included 3-way interactions between period, year, and race or, in a separate model, period, year, and age group, to investigate effect modification by race or age group, respectively. After fitting the Cox models, we used marginal standardization to estimate the predicted probabilities of treatment within 30 days of metastatic cancer diagnosis within each time period. Estimates across the 10 imputations were combined using Rubin’s rules (47,49).
Analyses of the subsample of patients who initiated treatment within 60 days of diagnosis used a similar approach using logistic regression rather than Cox regression to model use of myelosuppressive therapy (vs not). Marginal standardization was applied to logistic regression estimates to obtain adjusted probabilities of receiving myelosuppressive therapy.
Sensitivity Analyses
We performed a sensitivity analysis to verify the robustness of our findings to an alternate definition of pandemic period exposure. Rather than defining 1 exposure period for all study participants that encompassed most state stay-at-home orders, we varied the exposure period for individual participants, defining the start of the 30-day washout period using the stay-at-home order date of a patient’s state of residence (see Supplementary Table 1, available online for dates).
Data analyses were conducted between November 2020 and April 2021 using R, version 4.0.4. The statistical significance of interaction terms was tested by comparing the full model (with interaction terms) with the corresponding nested model (without interaction terms) using the Wald-like tests for multiple parameters for use with multiply imputed data (50). All hypothesis tests were 2-tailed with alpha = 0.05. Missing data were imputed using the mice package, version 3.13.0 (51). Cox proportional hazards models were fit using the survival package, version 3.2.11 (52), and regression standardization conducted using stdReg, version 3.4.1 (53). The functional form of continuous variables (age and calendar day of diagnosis) in Cox models was assessed using Martingale Residuals, and the proportional hazards assumption was evaluated for all variables using Schoenfeld Residuals. All analytic code is available at https://github.com/PRACTICE-research-group/COVID19-treatment-patterns.
Results
Baseline Characteristics
Table 1 shows the distribution of patient characteristics in the main study sample by time period and year. Of 14 136 patients with documented newly diagnosed metastatic solid cancer during the study period, 2954 (20.9%) were diagnosed from January to March 2019, 4745 (33.6%) from April to July 2019, 2640 (18.7%) from January to March 2020, and 3797 (26.9%) from April to July 2020. There were no meaningful differences in the distributions of age, sex, race, insurance, practice setting, and performance status by time period within each year (standardized mean differences <0.1). The most common cancers were NSCLC (41.3%), colorectal (18.4%), and breast (11.6%); there were no differences in the distribution of cancers by time period. Overall, 62.9% of patients were diagnosed with de novo metastatic disease; as a proportion of overall new metastatic cancer diagnoses, de novo metastatic diagnoses were more common in the COVID-19 period (April-July 2020 67.0%) than in the pre-COVID-19 periods (January-March 2019 = 61.2%; April-July 2019 = 61.5%; January-March 2020 = 61.5%; standardized mean difference = 0.11). Supplementary Table 3 (available online) describes the subsample of patients (n = 5962) who were diagnosed with metastatic NSCLC, breast, prostate, or urothelial cancer and treated within 60 days of diagnosis. The distribution of baseline characteristics in this subsample was similar to the full cohort.
Table 1.
Population characteristicsa
| Variable | 2019 |
2020 |
Total | ||||
|---|---|---|---|---|---|---|---|
| January 1-March 8 | April 8-July 31 | SMD | January 1-March 8 | April 8-July 31 | SMD | (N = 14 136) | |
| (n = 2954) | (n = 4745) | (n = 2640) | (n = 3797) | ||||
| Cancer, No. (%) | |||||||
| Breast | 382 (12.9) | 589 (12.4) | 0.089 | 282 (10.7) | 393 (10.4) | 0.066 | 1646 (11.6) |
| Colorectal | 553 (18.7) | 862 (18.2) | 466 (17.7) | 722 (19.0) | 2603 (18.4) | ||
| NSCLC | 1170 (39.6) | 1987 (41.9) | 1122 (42.5) | 1562 (41.1) | 5841 (41.3) | ||
| Pancreatic | 261 (8.8) | 388 (8.2) | 251 (9.5) | 368 (9.7) | 1268 (9.0) | ||
| Prostate | 282 (9.5) | 383 (8.1) | 214 (8.1) | 347 (9.1) | 1226 (8.7) | ||
| RCC | 133 (4.5) | 274 (5.8) | 147 (5.6) | 178 (4.7) | 732 (5.2) | ||
| UCC | 173 (5.9) | 262 (5.5) | 158 (6.0) | 227 (6.0) | 820 (5.8) | ||
| Median age (IQR) | 70 (61-77) | 70 (61-77) | 0.013 | 69 (61-77) | 69 (62-77) | 0.008 | 70 (61-77) |
| Sex, No. (%) | |||||||
| Female | 1330 (45.0) | 2295 (48.4) | 0.067 | 1250 (47.3) | 1784 (47.0) | 0.007 | 6659 (47.1) |
| Male | 1624 (55.0) | 2450 (51.6) | 1390 (52.7) | 2013 (53.0) | 7477 (52.9) | ||
| Race, No. (%) | |||||||
| Hispanic | 164 (6.3) | 240 (5.7) | 131 (5.6) | 197 (5.9) | 732 (5.9) | ||
| Non-Hispanic Black | 270 (10.3) | 458 (10.9) | 242 (10.4) | 355 (10.6) | 1325 (10.6) | ||
| Non-Hispanic White | 1801 (68.7) | 2912 (69.1) | 0.031 | 1554 (66.8) | 2211 (66.3) | 0.015 | 8478 (67.8) |
| Other | 386 (14.7) | 606 (14.4) | 400 (17.2) | 571 (17.1) | 1963 (15.7) | ||
| Missing | 333 | 529 | 313 | 463 | 1638 | ||
| Insurance, No. (%) | |||||||
| Commercial | 1435 (48.6) | 2315 (48.8) | 0.048 | 1343 (50.9) | 1915 (50.4) | 0.026 | 7008 (49.6) |
| Government | 585 (19.8) | 1015 (21.4) | 556 (21.1) | 775 (20.4) | 2931 (20.7) | ||
| Unknown/not documented/self-pay | 934 (31.6) | 1415 (29.8) | 741 (28.1) | 1107 (29.2) | 4197 (29.7) | ||
| Practice type, No. (%) | |||||||
| Academic | 279 (9.4) | 471 (9.9) | 0.016 | 235 (8.9) | 341 (9.0) | 0.003 | 1326 (9.4) |
| Community | 2675 (90.6) | 4274 (90.1) | 2405 (91.1) | 3456 (91.0) | 12 810 (90.6) | ||
| ECOG performance status, No. (%) | |||||||
| 0-1 | 1176 (82.1) | 1816 (82.1) | 0.001 | 1086 (83.5) | 1540 (81.7) | 0.047 | 5618 (82.3) |
| ≥2 | 257 (17.9) | 396 (17.9) | 214 (16.5) | 344 (18.3) | 0.047 | 1211 (17.7) | |
| Missing | 1521 | 2533 | 1340 | 1913 | 7307 | ||
| Opioid prescription, No. (%) | 220 (7.4) | 362 (7.6) | 0.007 | 179 (6.8) | 274 (7.2) | 0.017 | 1035 (7.3) |
| De novo metastatic, No. (%) | 1647 (61.2) | 2676 (61.5) | 0.006 | 1491 (61.5) | 2329 (67.0) | 0.113 | 8143 (62.9) |
| Missing | 261 | 391 | 217 | 319 | 1188 | ||
ECOG = Eastern Cooperative Oncology Group; IQR = interquartile range; NSCLC = non-small cell lung carcinoma; RCC = renal cell carcinoma; SMD = standardized mean difference; UCC = urothelial cell carcinoma.
Time to Treatment Initiation
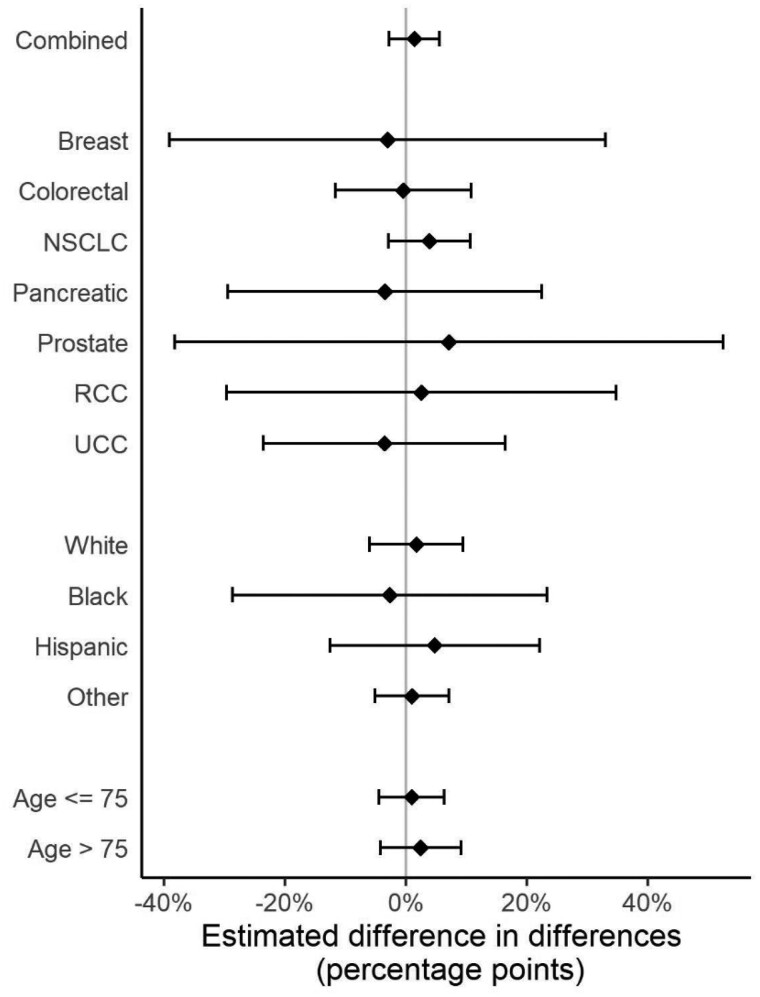
Across all periods, the median time to systemic treatment initiation was 35 days, with 44.0% (95% confidence interval [CI] = 43.2% to 44.8%) of patients initiating treatment within 30 days of metastatic diagnosis. Unadjusted and adjusted probabilities of treatment initiation within 30 days are shown in Table 2. In our primary analysis, the difference in the proportion of patients initiating treatment within 30 days in April-July compared with January-March was similar in 2019 and 2020 (adjusted probability of treatment within 30 days: January-March 2019 = 41.7%, 95% CI = 32.2% to 51.1%; April-July 2019 = 42.6%, 95% CI = 32.4% to 52.7%; January-March 2020 = 44.5%, 95% CI = 30.4% to 58.6%; April-July 2020 = 46.8%, 95% CI = 34.6% to 59.0%; adjusted percentage-point difference-in-differences = 1.4%, 95% CI = −2.7% to 5.5%) (Table 2). There was no evidence of effect modification by cancer type (Pinteraction = .25) (Figure 1), race (Supplementary Table 4, available online; P = .10), or age (Supplementary Table 5, available online; P = .65).
Table 2.
Adjusted probability of treatment within 30 daysa
| Model and category | 2019 |
2020 |
Difference in differences |
||||
|---|---|---|---|---|---|---|---|
| January 1-March 8 |
April 8-July 31 |
Difference |
January 1-March 8 |
April 8-July 31 |
Difference |
2020-2019 |
|
| Est (95% CI) | Est (95% CI) | Est (95% CI) | Est (95% CI) | Est (95% CI) | Est (95% CI) | Est (95% CI) | |
| Unadjusted | 0.429 (0.407 to 0.452) | 0.415 (0.383 to 0.447) | −0.014 (−0.032 to 0.003) | 0.453 (0.433 to 0.472) | 0.453 (0.426 to 0.487) | 0.004 (−0.014 to 0.075) | 0.018 (0.000 to 0.036) |
| Adjusted | |||||||
| Combined | 0.417 (0.322 to 0.511) | 0.426 (0.324 to 0.527) | 0.009 (−0.044 to 0.061) | 0.445 (0.304 to 0.586) | 0.468 (0.346 to 0.590) | 0.023 (−0.029 to 0.075) | 0.014 (−0.027 to 0.055) |
| Breast | 0.546 (0.363 to 0.728) | 0.573 (0.337 to 0.809) | 0.027 (−0.223 to 0.277) | 0.637 (0.360 to 0.914) | 0.635 (0.298 to 0.972) | −0.002 (−0.190 to 0.185) | −0.029 (−0.385 to 0.326) |
| Colorectal | 0.402 (0.259 to 0.545) | 0.410 (0.296 to 0.524) | 0.008 (−0.089 to 0.106) | 0.430 (0.233 to 0.628) | 0.434 (0.307 to 0.561) | 0.004 (−0.112 to 0.120) | −0.005 (−0.115 to 0.106) |
| NSCLC | 0.397 (0.308 to 0.486) | 0.390 (0.301 to 0.480) | −0.007 (−0.075 to 0.062) | 0.404 (0.284 to 0.524) | 0.436 (0.300 to 0.572) | 0.032 (−0.007 to 0.071) | 0.039 (−0.031 to 0.108) |
| Pancreatic | 0.508 (0.290 to 0.727) | 0.549 (0.228 to 0.869) | 0.040 (−0.188 to 0.268) | 0.541 (0.327 to 0.755) | 0.546 (0.293 to 0.798) | 0.005 (−0.293 to 0.302) | −0.036 (−0.299 to 0.227) |
| Prostate | 0.369 (0.212 to 0.526) | 0.363 (0.182 to 0.545) | −0.006 (−0.232 to 0.221) | 0.371 (0.197 to 0.545) | 0.438 (0.262 to 0.614) | 0.067 (−0.184 to 0.317) | 0.072 (−0.385 to 0.530) |
| RCC | 0.343 (0.176 to 0.510) | 0.361 (0.006 to 0.716) | 0.018 (−0.365 to 0.402) | 0.396 (0.068 to 0.724) | 0.440 (0.132 to 0.748) | 0.044 (−0.283 to 0.371) | 0.025 (−0.293 to 0.344) |
| UCC | 0.345 (0.195 to 0.495) | 0.390 (0.224 to 0.556) | 0.045 (−0.170 to 0.260) | 0.408 (0.237 to 0.579) | 0.418 (0.264 to 0.572) | 0.010 (−0.205 to 0.225) | −0.035 (−0.236 to 0.166) |
Results are from cancer type: period interaction model. Est = estimate; CI = confidence interval; NSCLC = non-small cell lung carcinoma; RCC = renal cell carcinoma; UCC = urothelial cell carcinoma.
Figure 1.
Changes in the adjusted probability of treatment initiation within 30 days of metastatic diagnosis between COVID-19 and pre–COVID-19 periods. This figure displays the differential effect of the COVID-19 period on the probability of 30-day treatment initiation by cancer type, race, and age (years) among patients with newly diagnosed de novo or recurrent metastatic solid cancer. The error bars represent the 95% confidence intervals. NSCLC = non-small cell lung carcinoma; RCC = renal cell carcinoma; UCC = urothelial cell carcinoma.
Treatment Selection
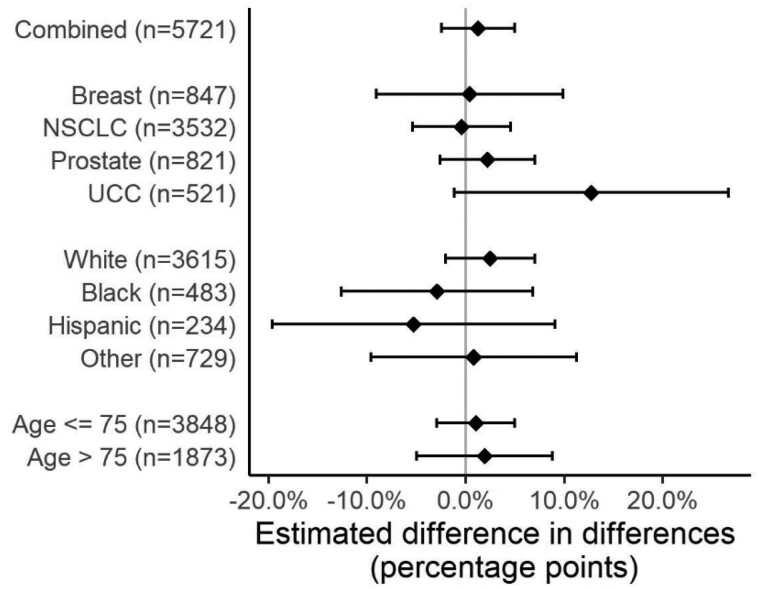
Among the 5962 patients who received first-line systemic therapy within 60 days of diagnosis, 67.2% received myelosuppressive therapy (range = 3.2% for prostate cancer to 81.0% for breast cancer). The difference in the adjusted probability of receiving myelosuppressive therapy in April-July compared with January-March was similar in 2019 and 2020 (January-March 2019 = 69.8%, 95% CI = 65.1% to 74.4%; April-July 2019 = 66.7%, 95% CI = 60.9% to 72.5%; January-March 2020 = 68.3%, 95% CI = 65.1% to 71.4%; April-July 2020 = 66.8%, 95% CI = 63.3% to 70.2%; adjusted percentage-point difference-in-differences = 1.6%, 95% CI = −2.6% to 5.8%) (Table 3). There was no evidence of effect modification by cancer type (P = .21) (Figure 2), race (Supplementary Table 6, available online; P = .13), or age (Supplementary Table 7, available online; P = .48).
Table 3.
Adjusted probabilities of receipt of myelosuppressive therapya
| Model and category | 2019 |
2020 |
Difference in differences |
||||
|---|---|---|---|---|---|---|---|
| January 1-March 8 |
April 8-July 31 |
Difference |
January 1-March 8 |
April 8-July 31 |
Difference |
2020-2019 |
|
| Est (95% CI) | Est (95% CI) | Est (95% CI) | Est (95% CI) | Est (95% CI) | Est (95% CI) | Est (95% CI) | |
| Unadjusted | 0.687 | 0.676 | −0.012 | 0.691 | 0.658 | −0.033 | −0.022 |
| (0.651 to 0.723) | (0.622 to 0.729) | (−0.059 to 0.036) | (0.656 to 0.727) | (0.626 to 0.690) | (−0.062 to −0.004) | (−0.081 to 0.038) | |
| Adjusted | |||||||
| Combined | 0.698 | 0.667 | −0.031 | 0.683 | 0.668 | −0.015 | 0.016 |
| (0.651 to 0.744) | (0.609 to 0.725) | (−0.065 to 0.002) | (0.651 to 0.714) | (0.633 to 0.702) | (−0.038 to 0.008) | (−0.026 to 0.058) | |
| Breast | 0.815 | 0.776 | −0.039 | 0.827 | 0.807 | −0.019 | 0.020 |
| (0.746 to 0.883) | (0.701 to 0.851) | (−0.122 to 0.044) | (0.783 to 0.870) | (0.767 to 0.848) | (−0.073 to 0.034) | (−0.077 to 0.116) | |
| NSCLC | 0.835 | 0.808 | −0.026 | 0.807 | 0.783 | −0.024 | 0.003 |
| (0.797 to 0.872) | (0.761 to 0.856) | (−0.071 to 0.018) | (0.773 to 0.841) | (0.749 to 0.817) | (−0.059 to 0.012) | (−0.056 to 0.061) | |
| Prostate | 0.046 | 0.025 | −0.022 | 0.025 | 0.041 | 0.016 | 0.037 |
| (0.000 to 0.101) | (0.004 to 0.045) | (−0.080 to 0.036) | (0.008 to 0.042) | (0.012 to 0.071) | (−0.015 to 0.047) | (−0.025 to 0.100) | |
| UCC | 0.558 | 0.497 | −0.061 | 0.583 | 0.589 | 0.007 | 0.068 |
| (0.452 to 0.664) | (0.402 to 0.593) | (−0.190 to 0.068) | (0.505 to 0.660) | (0.518 to 0.661) | (−0.081 to 0.095) | (−0.086 to 0.221) | |
Results are from cancer type: period interaction model. Est = estimate; CI = 95% confidence interval; NSCLC = non-small cell lung carcinoma; UCC = urothelial cell carcinoma.
Figure 2.
Changes in the adjusted probability of receiving myelosuppressive therapy after metastatic diagnosis between COVID-19 and pre–COVID-19 periods. This figure displays the differential effect of the COVID-19 period on the probability of receiving myelosuppressive therapy by cancer type, race, and age (years) among patients with newly diagnosed de novo or recurrent metastatic solid cancer. The error bars represent the 95% confidence intervals. NSCLC = non-small cell lung carcinoma; UCC = urothelial cell carcinoma.
Sensitivity Analyses
Results from a sensitivity analysis using a state-specific exposure definition based on dates of state stay-at-home orders were consistent with results from the primary analysis (Supplementary Tables 8 and 9, available online).
Discussion
In this large, multi-site cohort of patients with metastatic solid cancer, we assessed the impact of the COVID-19 pandemic on TTI and treatment selection using a quasi-experimental approach. We did not find evidence that the pandemic period was associated with delayed systemic therapy or increased use of nonmyelosuppressive therapy. We did observe changes in disease presentation during the COVID-19 period—most notably, an increased proportion of patients presenting with de novo metastatic disease. Our analysis suggests that previously reported pandemic-associated diagnostic delays may have resulted in more acute presentations of metastatic disease but not delays in systemic treatment initiation or preference against use of myelosuppressive therapies.
Our findings stand in contrast to earlier studies evaluating COVID-19 pandemic–related disruptions in cancer care, which found evidence of care delays across the cancer continuum (11,13,17). Several factors may account for this discrepancy. First, previously reported declines in cancer screening and diagnoses may have contributed to greater available capacity in outpatient clinics and infusion suites for those needing prompt treatment (16). Second, we observed a 5-6 percentage-point increase in the proportion of de novo metastatic diagnoses in the COVID-19 period compared with pre-COVID periods. Relative to recurrent metastatic diagnoses, which are often detected via routine surveillance imaging or laboratory testing when patients may not be symptomatic, de novo metastatic diagnoses are associated with greater symptomatic burden and worse overall mortality (54). It is possible that known pandemic-related decreases in routine imaging and laboratory surveillance contributed to the observed relative increase in presentation of potentially more symptomatic de novo metastatic diagnoses, which has been suggested in prior single-institution studies (52). Consequently, any pandemic-related delays in treatment initiation may have been balanced by the need for quicker treatment initiation for more symptomatic cases. Our findings of COVID-related impacts on de novo metastatic presentation are hypothesis-generating, and this study was not well powered to assess this. Future studies with longer follow-up will be necessary to evaluate whether the relative increase of de novo presentations will persist and what the consequences of this potential shift will be on future cancer-related outcomes. Nevertheless, delays in detection and diagnosis of recurrent metastatic disease during the early phase of the COVID-19 pandemic may be a harbinger for increased rates of symptomatic metastatic disease and cancer-associated mortality in later stages of the pandemic.
We did not find evidence of changes in the type of treatment selected despite early professional society guidance in some cases cautioning against use of myelosuppressive therapy (18). The mechanisms behind this finding are unclear. An increased proportion of de novo metastatic diagnoses presenting with symptomatic disease may have led more physicians and patients than expected to prefer chemotherapy to achieve rapid debulking and disease control (55). Additionally, evidence emerged during the pandemic suggesting that myelosuppressive therapies might not, as initially suspected, be associated with increased COVID-19 severity or mortality among patients with cancer (8). Oncologists may have thus grown more comfortable with using myelosuppressive therapy during the pandemic period.
Our study has several advantages compared with prior studies examining pandemic-related treatment delays. First, we studied a large national cohort using EHR–derived data with minimal data lag, allowing for broad geographic coverage that accounted for state-specific stay-at-home orders, strong representation of community oncology practices, and greater data recency compared with other administrative databases. Second, we used a real-world dataset that harnesses technology-enabled chart abstraction to ascertain diagnoses and treatments rather than relying solely on administrative claims from the COVID-19 pandemic period, which may be subject to measurement error and data lag (56,57). Finally, we used a quasi-experimental design to account for temporal confounding, such as known seasonal patterns in diagnoses and treatment-seeking behavior (58).
Our study has several limitations. First, it is a retrospective study of a sample of predominantly community-based US oncology practices, and therefore our findings may not be reflective of all oncology practice. However, this database has been shown to be broadly representative of US oncology practices and patients (41). Second, outpatient EHR data may incompletely capture important variables that contribute to treatment patterns, such as patient preference or comorbidities, thus raising the possibility of unmeasured confounding. However, our quasi-experimental approach should account for these unmeasured confounders, assuming such confounders were consistent across time periods. Third, although we used the most up-to-date data available, there may be COVID-related delays in data capture affecting completeness of data from more recent time periods. In particular, the pandemic could affect capture of metastatic cancer diagnoses. Although this remains a hypothetical concern, future analyses should address this possibility. Fourth, our cohort was limited by a relatively small proportion of racial minorities and those with noncommercial insurance. This may have resulted in limited power for analyses of race- or age-based interactions, though notably there was some non-statistically significant evidence of delayed treatment among African American patients. Given the disproportionate impact of the pandemic on care for minority groups, future analyses with larger, more diverse cohorts are needed. Finally, although we did not find any delays in systemic therapy initiation, our study was not designed to evaluate possible changes in rates of systemic therapy initiation (or lack thereof) over time or to assess changes in systemic therapy dosing or schedules that may have occurred during the pandemic.
In this large, nationwide study of patients newly diagnosed with metastatic solid cancer, we did not find evidence of treatment delays or preferential use of nonmyelosuppressive therapies associated with the COVID-19 pandemic. An increased proportion of patients presenting with de novo metastatic cancers during the pandemic may portend a backlog of recurrent metastatic diagnoses stemming from pandemic-related delays in surveillance and diagnosis. Future studies with longer follow-up should assess whether COVID-related delays in presentation affect cancer-related outcomes among patients with metastatic cancers.
Funding
This study was supported by the National Cancer Institute K08-CA-263541–01 (to RBP).
Notes
Role of the funder: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Author disclosures: Ravi Parikh and Ronac Mamtani have received reimbursement from Flatiron, Inc for travel and speaking. Rebecca Miksad and Gregory Calip report employment at Flatiron Health, Inc. There are no other conflicts of interest relevant to this submitted work.
Author contributions: All authors have directly participated in the planning, execution, or analysis of the study, and have approved the final version of this manuscript. RBP, SUT, DV, RM, RH: Conception and design. DV, EPW: Data Curation, Formal Analysis, Software. WF and CH: Project Administration. All authors: Methodology. RBP and SUT: Writing—Original Draft. All authors: Writing—Review and Editing.
Disclaimers: The views expressed in this article are those of the authors, and no official endorsement by the National Cancer Institute, National Institutes for Health, or the Department of Health and Human Services is intended or should be inferred.
Prior presentations: This was previously presented as a Poster Presentation at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting (Virtual).
Data Availability
All deidentified data generated or analyzed during this study is available upon request to Ravi Parikh, MD, MPP, ravi.parikh@pennmedicine.upenn.edu.
Supplementary Material
Contributor Information
Ravi B Parikh, Department of Medical Ethics and Health Policy, University of Pennsylvania, Philadelphia, PA, USA; Division of Hematology and Oncology, Department of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Samuel U Takvorian, Division of Hematology and Oncology, Department of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Daniel Vader, Department of Biostatistics, Epidemiology, & Informatics, University of Pennsylvania, Philadelphia, PA, USA.
E Paul Wileyto, Department of Biostatistics, Epidemiology, & Informatics, University of Pennsylvania, Philadelphia, PA, USA.
Amy S Clark, Division of Hematology and Oncology, Department of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Daniel J Lee, Division of Urology, Department of Surgery, University of Pennsylvania, Philadelphia, PA, USA.
Gaurav Goyal, Division of Hematology and Oncology, Department of Medicine, University of Alabama at Birmingham, Birmingham, AL, USA.
Gabrielle B Rocque, Division of Hematology and Oncology, Department of Medicine, University of Alabama at Birmingham, Birmingham, AL, USA.
Efrat Dotan, Department of Medical Oncology, Fox Chase Cancer Center, Philadelphia, PA, USA.
Daniel M Geynisman, Department of Medical Oncology, Fox Chase Cancer Center, Philadelphia, PA, USA.
Pooja Phull, Department of Medical Oncology, Fox Chase Cancer Center, Philadelphia, PA, USA.
Philippe E Spiess, Department of Genitourinary Oncology, Moffitt Cancer Center, Tampa, FL, USA.
Roger Y Kim, Division of Pulmonary, Allergy, and Critical Care, Department of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Amy J Davidoff, Healthcare Delivery Research Program, National Cancer Institute, Bethesda, MD, USA.
Cary P Gross, Cancer Outcomes Public Policy and Effectiveness Research, Yale School of Medicine, New Haven, CT, USA.
Natalia Neparidze, Cancer Outcomes Public Policy and Effectiveness Research, Yale School of Medicine, New Haven, CT, USA.
Rebecca A Miksad, Flatiron Health, New York, NY, USA.
Gregory S Calip, Flatiron Health, New York, NY, USA.
Caleb M Hearn, Department of Medical Ethics and Health Policy, University of Pennsylvania, Philadelphia, PA, USA.
Will Ferrell, Department of Medical Ethics and Health Policy, University of Pennsylvania, Philadelphia, PA, USA.
Lawrence N Shulman, Division of Hematology and Oncology, Department of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Ronac Mamtani, Division of Hematology and Oncology, Department of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Rebecca A Hubbard, Department of Biostatistics, Epidemiology, & Informatics, University of Pennsylvania, Philadelphia, PA, USA.
References
- 1. Baum A, Schwartz MD. Admissions to Veterans Affairs Hospitals for emergency conditions during the COVID-19 pandemic. JAMA. 2020;324(1):96–99. doi: 10.1001/jama.2020.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kansagra AP, Goyal MS, Hamilton S, Albers GW. Collateral effect of Covid-19 on stroke evaluation in the United States. N Engl J Med. 2020;383(4):400–401. doi: 10.1056/NEJMc2014816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Czeisler ME, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19-related concerns--United States. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75(22):2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Solomon MD, McNulty EJ, Rana JS, et al. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383(7):691–693. doi: 10.1056/NEJMc2015630. [DOI] [PubMed] [Google Scholar]
- 6. Gupta S, Hayek SS, Wang W, et al. ; STOP-COVID Investigators. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1436–1446. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee LY, Cazier J-B, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet Lond Engl. 2020;395(10241):1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hawrot K, Shulman LN, Bleiweiss IJ, et al. Time to treatment initiation for breast cancer during the 2020 COVID-19 pandemic. J Clin Oncol Oncol Pract. 2021;17(9):534-540. doi: 10.1200/OP.20.00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perkons N, Kim C, Boedec C, et al. Quantifying the impact of the COVID-19 pandemic on gastrointestinal cancer care delivery. J Clin Oncol. 2021;39(3_suppl):30. doi:10.1200/J Clin Oncol.2021.39.3_suppl.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Papautsky EL, Hamlish T. Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res Treat. 2020;184(1):249–254. doi: 10.1007/s10549-020-05828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schrag D, Hershman DL, Basch E. Oncology practice during the COVID-19 pandemic. JAMA. 2020;323(20):2005–2006. doi: 10.1001/jama.2020.6236. [DOI] [PubMed] [Google Scholar]
- 13. Patt D, Gordan L, Diaz M, et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. J Clin Oncol Clin Cancer Inform. 2020;4:1059–1071. doi: 10.1200/CCI.20.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elkrief A, Kazandjian S, Bouganim N. Changes in lung cancer treatment as a result of the coronavirus disease 2019 pandemic. JAMA Oncol. 2020;6(11):1805–1806. doi: 10.1001/jamaoncol.2020.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Satish T, Raghunathan R, Prigoff JG, et al. Care delivery impact of the COVID-19 pandemic on breast cancer care. J Clin Oncol Oncol Pract. 2021;17(8):e1215-e1224. doi: 10.1200/OP.20.01062. [DOI] [PubMed] [Google Scholar]
- 16. Bakouny Z, Paciotti M, Schmidt AL, Lipsitz SR, Choueiri TK, Trinh Q-D. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol. 2021;7(3):458–460. doi: 10.1001/jamaoncol.2020.7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu J, Bobo S, Henry S, Mills M, Kurian A, Dirbas F. Abstract PS6-32: impact of COVID-19 on breast cancer care at a Bay Area academic center. Cancer Res. 2021;81(4 Supplement):PS6-32. doi: 10.1158/1538-7445.SABCS20-PS6-32.34421401 [DOI] [Google Scholar]
- 18. Burki TK. Cancer guidelines during the COVID-19 pandemic. Lancet Oncol. 2020;21(5):629–630. doi: 10.1016/S1470-2045(20)30217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grivas P, Khaki AR, Wise-Draper TM, et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol. 2021;32(6):787-800. doi: 10.1016/j.annonc.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tassone D, Thompson A, Connell W, et al. Immunosuppression as a risk factor for COVID-19: a meta-analysis. Intern Med J. 2021;51(2):199–205. doi: 10.1111/imj.15142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen MF, Coronel MT, Pan S, et al. Abstract S11-02: factors associated with developing COVID-19 among cancer patients in New York City. Clin Cancer Res. 2021;27(6 Supplement):S11-02. doi: 10.1158/1557-3265.COVID-19-21-S11-02. [DOI] [Google Scholar]
- 22. Riera R, Bagattini ÂM, Pacheco RL, Pachito DV, Roitberg F, Ilbawi A. Delays and disruptions in cancer health care due to COVID-19 pandemic: systematic review. J Clin Oncol Glob Oncol. 2021;7:311–323. doi: 10.1200/go.20.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cone EB, Marchese M, Paciotti M, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open. 2020;3(12):e2030072. doi: 10.1001/jamanetworkopen.2020.30072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087. doi: 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khorana AA, Tullio K, Elson P, et al. Time to initial cancer treatment in the United States and association with survival over time: an observational study. PLoS One. 2019;14(3):e0213209. doi: 10.1371/journal.pone.0213209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bleicher RJ, Ruth K, Sigurdson ER, et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2(3):330–339. doi: 10.1001/jamaoncol.2015.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jung SY, Sereika SM, Linkov F, Brufsky A, Weissfeld JL, Rosenzweig M. The effect of delays in treatment for breast cancer metastasis on survival. Breast Cancer Res Treat. 2011;130(3):953–965. doi: 10.1007/s10549-011-1662-4. [DOI] [PubMed] [Google Scholar]
- 28. Takvorian SU, Oganisian A, Mamtani R, et al. Association of Medicaid Expansion under the Affordable Care Act with insurance status, cancer stage, and timely treatment among patients with breast, colon, and lung cancer. JAMA Netw Open. 2020;3(2):e1921653. doi: 10.1001/jamanetworkopen.2019.21653. [DOI] [PubMed] [Google Scholar]
- 29. Adamson BJS, Cohen AB, Estevez M, et al. Affordable Care Act (ACA) Medicaid expansion impact on racial disparities in time to cancer treatment. J Clin Oncol. 2019;37(18_suppl):LBA1. doi:10.1200/J Clin Oncol.2019.37.18_suppl.LBA1. [Google Scholar]
- 30. Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yancy CW. COVID-19 and African Americans. JAMA. 2020;323(19):1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 32. Esai Selvan M. Risk factors for death from COVID-19. Nat Rev Immunol. 2020;20(7):407–407. doi: 10.1038/s41577-020-0351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mueller AL, McNamara MS, Sinclair DA. Why does COVID-19 disproportionately affect older people? Aging (Albany NY). 2020;12(10):9959–9981. doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021;7(2):220–227. doi: 10.1001/jamaoncol.2020.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Holmes JA, Chen RC. Racial disparities in time from diagnosis to treatment for stage I non–small cell lung cancer. JNCI Cancer Spectr. 2018;2:pky007. doi: 10.1093/jncics/pky007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ailawadhi S, Parikh K, Abouzaid S, et al. Racial disparities in treatment patterns and outcomes among patients with multiple myeloma: a SEER-Medicare analysis. Blood Adv. 2019;3(20):2986–2994. doi: 10.1182/bloodadvances.2019000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Frankenfeld CL, Menon N, Leslie TF. Racial disparities in colorectal cancer time-to-treatment and survival time in relation to diagnosing hospital cancer-related diagnostic and treatment capabilities. Cancer Epidemiol. 2020;65:101684. doi: 10.1016/j.canep.2020.101684. [DOI] [PubMed] [Google Scholar]
- 38. Blom EF, ten Haaf K, Arenberg DA, de Koning HJ. Disparities in receiving guideline-concordant treatment for lung cancer in the United States. Ann Am Thorac Soc. 2020;17(2):186–194. doi: 10.1513/AnnalsATS.201901-094OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *39. Earle CC, Venditti LN, Neumann PJ, et al. Who gets chemotherapy for metastatic lung cancer? Chest. 2000;117(5):1239–1239. [DOI] [PubMed] [Google Scholar]
- 40. Khanal N, Upadhyay S, Dahal S, Bhatt VR, Silberstein PT. Systemic therapy in stage IV pancreatic cancer: a population-based analysis using the National Cancer Data Base. Ther Adv Med Oncol. 2015;7(4):198–205. doi: 10.1177/1758834015579313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ma X, Long L, Moon S, Adamson BJS, Baxi SS. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. medRxiv. 2020. doi: 10.1101/2020.03.16.20037143. [DOI] [Google Scholar]
- 42. Birnbaum B, Nussbaum N, Seidl-Rathkopf K, et al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv. 2020. https://arxiv.org/abs/2001.09765. Accessed April 23, 2021.
- 43. Ettinger DS, Wood DE, Aggarwal C, et al. OCN. NCCN guidelines insights: non–small cell lung cancer, version 1.2020: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2019;17(12):1464–1472. doi: 10.6004/jnccn.2019.0059. [DOI] [PubMed] [Google Scholar]
- 44. Flaig TW, Spiess PE, Agarwal N, et al. Bladder cancer, Version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(3):329–354. doi: 10.6004/jnccn.2020.0011. [DOI] [PubMed] [Google Scholar]
- 45. Gradishar WJ, Anderson BO, Abraham J, et al. Breast cancer, Version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(4):452–478. doi: 10.6004/jnccn.2020.0016. [DOI] [PubMed] [Google Scholar]
- 46. Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate cancer, Version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(5):479–505. doi: 10.6004/jnccn.2019.0023. [DOI] [PubMed] [Google Scholar]
- 47. Kasim RM, Raudenbush SW. Application of Gibbs sampling to nested variance components models with heterogeneous within-group variance. J Educ Behav Stat. 1998;23(2):93–116. doi: 10.2307/1165316. [DOI] [Google Scholar]
- 48. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Little RJA, Rubin DB, Statistical Analysis with Missing Data, 3rd Edition. Hoboken, NJ: Wiley, 2019. https://www.wiley.com/en-us/Statistical+Analysis+with+Missing+Data%2C+3rd+Edition-p-9780470526798. Accessed April 23, 2021. [Google Scholar]
- 50. Li KH, Raghunathan TE, Rubin DB. Large-sample significance levels from multiply imputed data using moment-based statistics and an F reference distribution. J Am Stat Assoc. 1991;86(416):1065–1073. doi: 10.2307/2290525. [DOI] [Google Scholar]
- 51. Buuren S, van Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(1):1–67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 52. Borgan Ø. Modeling survival data: extending the Cox Model. Stat Med. 2001;20(13):2053–2054. doi: 10.1002/sim.956. [DOI] [Google Scholar]
- 53. Sjölander A. Regression standardization with the R package stdReg. Eur J Epidemiol. 2016;31(6):563–574. doi: 10.1007/s10654-016-0157-3. [DOI] [PubMed] [Google Scholar]
- 54. Hassett MJ, Uno H, Cronin AM, Carroll NM, Hornbrook MC, Ritzwoller DP. Comparing survival after recurrent vs de novo stage IV advanced breast, lung, and colorectal cancer. JNCI Cancer Spectr. 2018;2(2):pky024. doi: 10.1093/jncics/pky024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yardley DA, Kaufman PA, Brufsky A, et al. Treatment patterns and clinical outcomes for patients with de novo versus recurrent HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2014;145(3):725–734. doi: 10.1007/s10549-014-2916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pottegård A, Kurz X, Moore N, Christiansen CF, Klungel O. Considerations for pharmacoepidemiological analyses in the SARS-CoV-2 pandemic. Pharmacoepidemiol Drug Saf. 2020;29(8):825–831. doi: 10.1002/pds.5029. [DOI] [PubMed] [Google Scholar]
- 57. Webster‐Clark M. Ways COVID-19 may impact unrelated pharmacoepidemiologic research using routinely collected data. Pharmacoepidemiol Drug Saf. 2021;30(3):400–401. doi: 10.1002/pds.5182. [DOI] [PubMed] [Google Scholar]
- 58. Lambe M, Blomqvist P, Bellocco R. Seasonal variation in the diagnosis of cancer: a study based on national cancer registration in Sweden. Br J Cancer. 2003;88(9):1358–1360. doi: 10.1038/sj.bjc.6600901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All deidentified data generated or analyzed during this study is available upon request to Ravi Parikh, MD, MPP, ravi.parikh@pennmedicine.upenn.edu.