Abstract
Background
Lung cancer survivors have a high risk of developing second primary lung cancer (SPLC), but little is known about the survival impact of SPLC diagnosis.
Methods
We analyzed data from 138 969 patients in the Surveillance, Epidemiology, and End Results (SEER), who were surgically treated for initial primary lung cancer (IPLC) in 1988-2013. Each patient was followed from the date of IPLC diagnosis to SPLC diagnosis (for those with SPLC) and last vital status through 2016. We performed multivariable Cox regression to evaluate the association between overall survival and SPLC diagnosis as a time-varying predictor. To investigate potential effect modification, we tested interaction between SPLC and IPLC stage. Using data from the Multiethnic Cohort Study (MEC) (n = 1540 IPLC patients with surgery), we evaluated the survival impact of SPLC by smoking status. All statistical tests were 2-sided.
Results
A total of 12 115 (8.7%) patients developed SPLC in SEER over 700 421 person-years of follow-up. Compared with patients with single primary lung cancer, those with SPLC had statistically significantly reduced overall survival (hazard ratio [HR] = 2.12, 95% confidence interval [CI] = 2.06 to 2.17; P < .001). The effect of SPLC on reduced survival was more pronounced among patients with early stage IPLC vs advanced-stage IPLC (HR = 2.14, 95% CI = 2.08 to 2.20, vs HR = 1.43, 95% CI = 1.21 to 1.70, respectively; Pinteraction < .001). Analysis using MEC data showed that the effect of SPLC on reduced survival was statistically significantly larger among persons who actively smoked at initial diagnosis vs those who formerly or never smoked (HR = 2.31, 95% CI = 1.48 to 3.61, vs HR = 1.41, 95% CI = 0.98 to 2.03, respectively; Pinteraction = .04).
Conclusions
SPLC diagnosis is statistically significantly associated with decreased survival in SEER and MEC. Intensive surveillance targeting patients with early stage IPLC and active smoking at IPLC diagnosis may lead to a larger survival benefit.
With recent advances in therapeutics and early detection technologies, the 5-year survival rate among lung cancer patients has doubled over the past 4 decades (1,2). As of 2019, there are 571 340 survivors of lung cancer in the United States, which is expected to rapidly increase (3). Lung cancer survivors are known to have a high risk of second primary lung cancer (SPLC), with approximately 1%-2% of SPLC risk per patient-year after resection (4). While several guidelines have been proposed for SPLC surveillance (5-7), evidence-based screening strategies for SPLC are still lacking among lung cancer survivors.
Numerous studies have examined potential risk factors and predictive models for SPLC in an effort to establish efficient screening strategies for SPLC (8-12). Radiotherapy for initial primary lung cancer (IPLC) is associated with an increased risk of SPLC (11), and smoking is a statistically significant risk factor for SPLC based on an international multicohort study (8). Prediction models for SPLC risk have been developed to identify high-risk lung cancer survivors for surveillance and screening for SPLC (9,12,13). The underlying assumption of these prior studies is that an early detection of SPLC can help reduce the overall mortality burden among lung cancer survivors. However, few studies have used large population-based data to examine whether patients who develop SPLC vs those with a single primary lung cancer have a higher risk of mortality, and, therefore, whether such efforts for early detection in SPLC are justified in reducing overall mortality among lung cancer survivors.
In this study, we aimed to compare survival between patients with SPLC vs single primary lung cancer, who were surgically treated for IPLC and have a potentially curable disease. We investigated the potential effect modification of SPLC diagnosis on survival by IPLC stage, histology, and age at initial diagnosis in the population-based data from the Surveillance, Epidemiology, and End Results Program (SEER). We use an external epidemiological cohort, the Multiethnic Cohort Study (MEC), to evaluate the survival impact of SPLC by smoking history in lung cancer survivors.
Methods
SEER Study Population
We obtained cancer data from the SEER database, covering approximately 35% of the US population. SEER-18 was used to identify all patients (n = 850 341) who were diagnosed with IPLC in 1988-2013 and were followed for SPLC and survival through 2016. Patients were excluded if they had histologic subtypes that did not belong to lung cancer (n = 92) (14) or if they had no information for survival (n = 19 110). We also excluded patients who did not undergo surgery (n = 686 000) because the majority of second malignancies develop among surgically treated patients (9,10), who are potential curable patients (15), and SPLC surveillance will therefore be most beneficial for these patients. Given that advanced-staged patients do not usually undergo surgery, we checked their surgical procedure and further excluded if they underwent limited resection (n = 6027) because these procedures might have been conducted for the sake of diagnostic resection or palliation purpose. We preserved advanced-staged patients with larger operations in the primary analysis, and we also conducted sensitivity analyses excluding all advanced-stage patients. Lastly, patients with missing information in race were excluded (n = 143). The final study population included 138 969 patients (Supplementary Figure 1, A, available online).
SPLC was defined by the well-established Martini and Melamed criteria: if the new tumor is diagnosed 2 years after the IPLC diagnosis, or the histology of new tumor developed within 2 years is different from the histology of IPLC (16). The following data were extracted in the SEER: age at IPLC diagnosis, sex, race, IPLC stage (early for localized and regional, and advanced for distant stage based on information given in the SEER summary stage), IPLC histology, and first course treatment for IPLC. We derived a variable for personal history of cancer (other than lung cancer) before IPLC diagnosis using a record number that sequentially numbers a person’s tumors, which was previously identified as a risk factor of second malignancies (13).
Study Outcomes
The primary outcome of the study was the time from IPLC diagnosis to all-cause mortality (ie, overall survival), and the secondary outcome was the time from IPLC diagnosis to lung cancer–specific mortality (ie, lung cancer–specific survival). Causes of death due to lung cancer or other causes were ascertained from death certificates by the National Center for Health Statistics. Patients were censored at the end of follow-up (December 31, 2016) or at the date of last living contact.
Statistical Analysis
Multivariable Cox regression was used to assess survival differences between patients with a single primary lung cancer and SPLC during the follow-up period. In evaluating the association between survival and SPLC, we considered SPLC diagnosis as a time-varying predictor to account for patients whose SPLC status changed from a single primary lung cancer to SPLC during the study period. The model was adjusted for known prognostic factors and risk factors for SPLC based on prior literature (8-10,13,17): sex, race, age at IPLC diagnosis, IPLC stage, and IPLC histology.
We evaluated potential interactions between SPLC diagnosis and key variables selected a priori that are known to be associated with SPLC risk (8-10) as well as mortality (17). In particular, we fitted a set of models with interaction terms between SPLC and IPLC stage, IPLC histology, and age at IPLC diagnosis—one at a time to the main model. We conducted a likelihood ratio test by comparing a full model with interaction terms vs a null model without interaction terms. In addition, we performed a stratified analysis to examine the effect of SPLC diagnosis on survival in each subgroup stratified by the variables used for interaction analysis. Given the multiplicity involved in the interaction analysis, we applied the Bonferroni method to account for multiple comparisons and used the nominal significance level of P = 0.05 is divided by the number of tests, 3 to adjust for multiple testing. In all other tests in this study including the analyses for the MEC, the statistical significance level of 0.05 was used. All tests were 2-sided, and statistical analyses were performed using R version 4.0.3 (https://cran.r-project.org).
Secondary Epidemiological Cohort: The MEC
Given that SEER lacks important covariates relating to smoking—a key factor affecting both SPLC and mortality—we used data from the MEC to conduct a more detailed analysis that addresses the survival impact of SPLC by smoking status.
The MEC is a prospective population-based cohort comprised of adults aged 45-75 years at enrollment (1993-1996) from 5 racial and ethnic groups, followed through 2017 for this study. Our study population from the MEC consisted of 1540 patients, who were diagnosed with IPLC in 1993-2017, had undergone surgery for IPLC, and had complete information in key variables including smoking status and IPLC stage (Supplementary Figure 1, B, available online).
Epidemiologic data were collected through a baseline questionnaire at enrollment. Smoking-related variables were updated via a 10-year follow-up survey (2003-2008). If the 10-year follow-up data was collected before the time at IPLC diagnosis (28.5%), we updated smoking information to accurately measure the smoking effect among lung cancer patients. Tumor characteristics and treatment information were collected at diagnosis (1993-2017), as specified by SEER registries.
Using the study population derived from the MEC, we tested for effect modification by smoking status on SPLC-survival associations. As a sensitivity analysis, we examined the interaction between SPLC diagnosis and an extended set of smoking variables.
Results
In SEER (n = 138 969), 12 115 (8.7%) patients were diagnosed with SPLC, and 91.3% (n = 126 854) patients remained with a single primary lung cancer (Table 1). Among SPLC patients, 54.6% (n = 6619) patients were diagnosed with SPLC within the first 5 years after IPLC diagnosis, and 7.4% (n = 895) patients developed SPLC more than 10 years after initial diagnosis (Supplementary Figure 2, available online). The study cohort was comprised of 8.3% African Americans, 5.2% Asian and Pacific Islanders, 4.5% Latinos, and 81.6% Whites. Of the study cohort, 96.0% (n = 133 445) patients had an early stage IPLC diagnosis, and the major type of surgical procedures (77.5%) was lobectomy (Supplementary Table 1, available online).
Table 1.
All patients with surgery for IPLC in the SEER from 1998 to 2013, stratified by SPLC vs single primary lung cancer
| Characteristics | Total (n = 138 969) | Patients with SPLC (n = 12 115) | Patients with single primary lung cancer (n = 126 854) |
|---|---|---|---|
| Age at IPLC diagnosis | |||
| Mean (SD) | 67.1 (10.6) | 66.8 (8.9) | 67.2 (10.7) |
| Age at IPLC diagnosis in groups, No. (%) | |||
| 0-49 y | 7986 (5.7) | 447 (3.7) | 7539 (5.9) |
| 50-59 y | 22 608 (16.3) | 1962 (16.2) | 20 646 (16.3) |
| 60-69 y | 45 396 (32.7) | 4804 (39.7) | 40 592 (32.0) |
| 70-79 y | 48 391 (34.8) | 4109 (33.9) | 44 282 (34.7) |
| ≥80 y | 14 588 (10.5) | 793 (6.5) | 13 795 (10.9) |
| Sex, No. (%) | |||
| Male | 69 017 (49.7) | 5571 (46.0) | 63 446 (50.1) |
| Female | 69 952 (50.3) | 6544 (54.0) | 63 408 (49.9) |
| Race, No. (%) | |||
| African American | 11 520 (8.3) | 962 (7.9) | 10 558 (8.3) |
| Asian and Pacific Islander | 7287 (5.2) | 518 (4.3) | 6769 (5.3) |
| Hispanic | 6201 (4.5) | 422 (3.5) | 5779 (4.6) |
| Other | 521 (0.4) | 31 (0.3) | 490 (0.4) |
| White | 113 440 (81.6) | 10 182 (84.0) | 103 258 (81.4) |
| Personal history of cancer, No. (%) | |||
| Yes | 28 364 (20.4) | 2480 (20.5) | 25 884 (20.4) |
| No | 110 605 (79.6) | 9635 (79.5) | 100 970 (79.6) |
| Stage at IPLCa, No. (%) | |||
| Early | 133 445 (96.0) | 11 861 (97.9) | 121 584 (95.8) |
| Advanced | 5524 (4.0) | 254 (2.1) | 5270 (4.2) |
| Histology of IPLC, No. (%) | |||
| Adenocarcinoma | 75 738 (54.5) | 6631 (54.7) | 69 107 (54.5) |
| Large cell | 4911 (3.5) | 467 (3.9) | 4444 (3.5) |
| Squamous cell | 35 666 (25.7) | 3488 (28.8) | 32 178 (25.4) |
| Small cell | 1970 (1.4) | 133 (1.1) | 1837 (1.4) |
| Non-small cell carcinoma/NOS | 5721 (4.1) | 535 (4.4) | 5186 (4.1) |
| Others | 14 963 (10.8) | 861 (7.1) | 14 102 (11.1) |
| Radiotherapy for IPLC, No. (%) | |||
| Yes | 20 269 (14.6) | 1216 (10.0) | 19053 (15.0) |
| No | 117 868 (84.8) | 10 851 (89.6) | 107 017 (84.4) |
| Missing | 832 (0.6) | 48 (0.4) | 784 (0.6) |
| Chemotherapy for IPLC, No. (%) | |||
| Yes | 31 268 (22.5) | 2322 (19.2) | 28 946 (22.8) |
| No/Unknown | 107 701 (77.5) | 9793 (80.8) | 97 908(77.2) |
| Follow-up status/cause of death, No. (%) | |||
| Alive | 48 202 (34.7) | 3936 (32.5) | 44 266 (34.9) |
| Dead/Lung cancer | 53 916 (38.8) | 6033 (49.8) | 47 883 (37.7) |
| Dead/Other causes | 36 851 (26.5) | 2146 (17.7) | 34 705 (27.4) |
Stage of IPLC was defined using SEER summary stage: “localized” and “regional” for early stage and “distant” for advanced stage. IPLC = initial primary lung cancer; NOS = not otherwise specified; SEER = Surveillance, Epidemiology, and End Results Program; SPLC = second primary lung cancer.
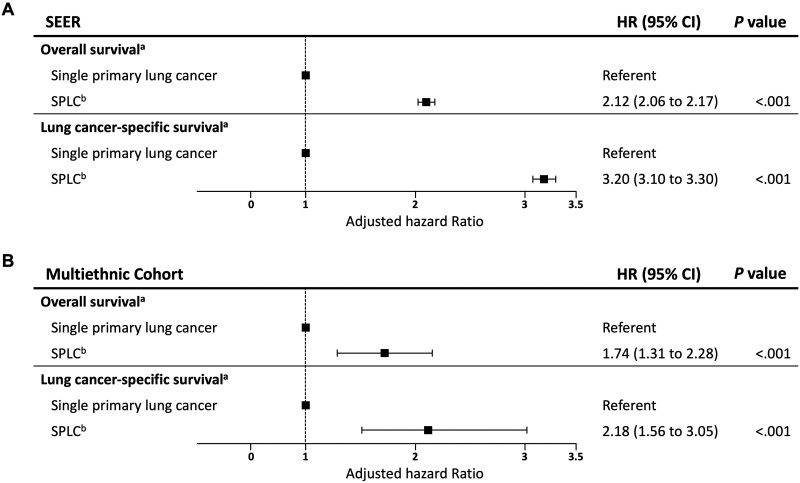
Patients diagnosed with SPLC had statistically significantly reduced overall survival compared with patients with a single primary lung cancer (hazard ratio [HR] = 2.12, 95% confidence interval [CI] = 2.06 to 2.17; P < .001) (Figure 1, A; Supplementary Table 2, available online). Adjusted survival curves by SPLC status are shown in Supplementary Figure 3, A (available online). Similar results were observed for lung cancer–specific survival (Supplementary Table 3, available online), with a more pronounced effect of SPLC observed on the reduction of lung cancer–specific survival (HR = 3.20, 95% CI = 3.10 to 3.30; P < .001) (Figure 1, A; Supplementary Figure 3, B, available online).
Figure 1.
Forest plot of association between survival and SPLC diagnosis (vs single primary lung cancer) in multivariable Cox regression in (A) SEER and (B) Multiethnic Cohort (MEC). aOverall and lung cancer–specific mortality was estimated using multivariable Cox regression adjusting for sex, race, age, stage, and histology at IPLC. Square symbols indicate the estimates of hazard ratio. Error bars indicate the 95% confidence intervals (CIs). The hazard ratios (HR) for the covariates in the Cox models are shown in Supplementary Tables 2 and 3 (available online) for SEER and Supplementary Tables 7 and 8 (available online) for MEC. bTime-varying predictor for overall and lung cancer–specific survival. SEER = Surveillance, Epidemiology, and End Results; SPLC = second primary lung cancer.
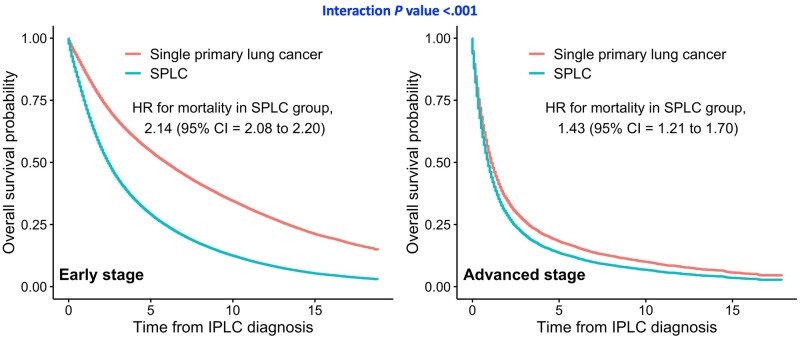
Stratified analysis by IPLC stage showed that the effect of SPLC on decreased overall survival was more evident among patients with early stage IPLC vs advanced-stage IPLC (HR = 2.14, 95% CI = 2.08 to 2.20, vs HR = 1.43, 95% CI = 1.21 to 1.70, respectively; Pinteraction < .001) (Table 2); the difference between the 2 survival curves for SPLC vs single primary lung cancer was larger among early stage IPLC patients (Figure 2) vs advanced-stage IPLC patients, indicating that patients with advanced disease have poor prognosis, and an additional diagnosis of SPLC would not make a large impact on overall survival.
Table 2.
Evaluation of an association between SPLC and overall survival stratified by subgroups for assessing effect modification (ie, interaction)
| Data source and subgroup | Case | aHR of SPLCa (95% CI) | P interaction b |
|---|---|---|---|
| SEER | |||
| Stage at IPLCc | |||
| Early | 133 445 | 2.14 (2.08 to 2.20) | <.001 |
| Advanced | 5524 | 1.43 (1.21 to 1.70) | |
| Age at IPLC diagnosis, y | |||
| 0-49 | 7 986 | 3.10 (2.67 to 3.60) | |
| 50-59 | 22 608 | 2.79 (2.60 to 2.99) | <.001 |
| 60-69 | 45 396 | 2.31 (2.22 to 2.41) | |
| 70-79 | 48 391 | 1.93 (1.85 to 2.01) | |
| ≥80 | 14 588 | 1.50 (1.37 to 1.64) | |
| Histology of IPLC | |||
| Small cell | 1970 | 1.43 (1.14 to 1.79) | |
| Large cell | 4911 | 1.87 (1.65 to 2.12) | |
| Adenocarcinoma | 75 738 | 2.06 (1.99 to 2.14) | <.001 |
| Squamous cell | 35 666 | 2.16 (2.07 to 2.27) | |
| Non-small cell carcinoma/NOS | 5721 | 2.04 (1.81 to 2.28) | |
| Others | 14 963 | 2.40 (2.17 to 2.65) | |
| MEC | |||
| Smoking statusd | |||
| Former/Never | 983 | 1.41 (0.98 to 2.03) | .04 |
| Current | 557 | 2.31 (1.48 to 3.61) |
Time-varying predictor for overall survival. aHR = adjusted hazard ratio; CI = confidence interval; IPLC = initial primary lung cancer; MEC = Multiethnic Cohort Study; NOS = not otherwise specified; SEER = Surveillance, Epidemiology, and End Results Program; SPLC = second primary lung cancer.
2-sided likelihood ratio test comparing models with and without interaction with SPLC.
Stage of IPLC was defined using SEER summary stage: “localized” and “regional” for early stage and “distant” for advanced stage.
Assessment of smoking status was collected at baseline and updated using 10-year follow-up, if prior to IPLC diagnosis.
Figure 2.
Stratified analysis by IPLC stage: adjusted survival curves for overall mortality among patients with second primary lung cancer vs single primary lung cancer in SEER. Subgroup of patients with early stage of IPLC (n = 133 445) and patients with advanced stage of IPLC (n = 11 551). Overall mortality was estimated using multivariable Cox regression with time-varying SPLC adjusting for sex, race, age, stage, and histology at IPLC. Interaction was assessed between IPLC stage and SPLC diagnosis on overall survival using a 2-sided likelihood ratio test. CI = confidence interval; HR = adjusted hazard ratio; IPLC = initial primary lung cancer; SEER = Surveillance, Epidemiology, and End Results; SPLC = second primary lung cancer.
Similar results were observed when the cohort was stratified by the age of IPLC diagnosis (Table 2; Supplementary Figure 4, available online); compared with patients diagnosed with IPLC at ages 80 years and older (HR = 1.50, 95% CI = 1.37 to 1.64) the effect of SPLC on reduced overall survival was greater among younger patients (HR = 3.10, 95% CI = 2.67 to 3.60; Pinteraction < .001). Stratified analysis by IPLC histology showed that the impact of SPLC on overall survival was most pronounced among patients with adenocarcinoma IPLC (HR = 2.06, 95% CI = 1.99 to 2.14) and squamous cell IPLC (HR = 2.16, 95% CI = 2.07 to 2.27) (Table 2; Supplementary Figure 5, available online) vs patients with small cell IPLC (HR = 1.43, 95% CI = 1.14 to 1.79) who have poor prognosis, thus, SPLC diagnosis making a smaller difference on overall survival. The results for lung cancer–specific survival showed similar patterns overall (Supplementary Table 4, available online). Sensitivity analysis that excludes all patients with advanced-stage IPLC also showed consistent results for effect modification (Supplementary Table 5, available online) and for the main effects of SPLC on survival outcomes (Supplementary Figure 6, A, available online).
The MEC cohort was used to evaluate the impact of SPLC on survival using detailed smoking information. Of 1540 IPLC patients who underwent surgery for IPLC in the MEC, 8.0% of the patients were diagnosed with an SPLC over 6922 person-years (Supplementary Table 6, available online). The associations between SPLC diagnosis and overall survival and lung cancer–specific survival in the MEC (Figure 1, B; Supplementary Figure 7 and Supplementary Tables 7-8, available online) were similar to those under SEER (Figure 1, A; Supplementary Figure 3, available online).
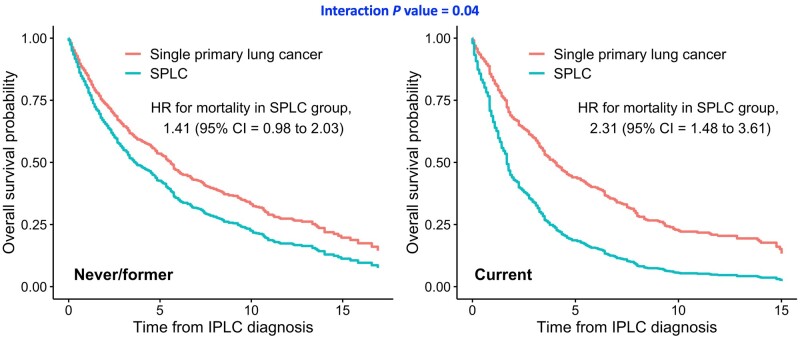
Analysis stratified by smoking status in the MEC showed that the effect of SPLC on decreased overall survival was greater among actively smoking patients at initial diagnosis (HR = 2.32, 95% CI = 1.49 to 3.61) than patients who had quit smoking (HR = 1.45, 95% CI = 0.99 to 2.14) or never smoked (HR = 1.28, 95% CI = 0.48 to 3.35); the difference between the 2 survival curves among patients with SPLC vs single primary lung cancer was largest in active smokers compared with never or former smokers (Pinteraction = .04; Table 2 and Figure 3). Sensitivity analysis for evaluating interactions between SPLC and other smoking variables and detailed smoking status (never, former, current) did not show statistical significance (Supplementary Table 9 and Supplementary Figure 8, available online). The results for lung cancer–specific survival showed similar patterns compared with overall survival (Supplementary Table 4, available online). In a sensitivity analysis that excludes all advanced-stage IPLC patients in the MEC, a consistent effect modification by smoking status was observed as in the primary analysis in SEER, but it did not achieve statistical significance potentially because of the decreased sample size especially among active smokers (Supplementary Table 5, available online).
Figure 3.
Stratified analysis by smoking status at initial diagnosis: adjusted survival curves for overall mortality among patients with second primary lung cancer vs single primary lung cancer in the Multiethnic Cohort Study (MEC). Subgroup of patients who were former or never smoked (n = 983) and who were actively smoking (n = 557) at diagnosis of IPLC. Overall mortality was estimated using multivariable Cox regression with time-varying SPLC adjusting for sex, race, age, stage, and histology at IPLC. Interaction was assessed between smoking status and SPLC diagnosis on overall survival using a 2-sided likelihood ratio test. CI = confidence interval; HR = adjusted hazard ratio; IPLC = initial primary lung cancer; SPLC = second primary lung cancer.
Discussion
In this study, we used large population-based data in SEER to show that patients who developed SPLC after an initial diagnosis of lung cancer had statistically significantly reduced survival compared with those who remained with a single primary lung cancer. Notably, the impact of SPLC diagnosis on poor prognosis was more pronounced among patients who were diagnosed with early stage IPLC vs advanced-stage IPLC. Analysis using the fully characterized cohort MEC demonstrated that the effect of SPLC diagnosis on reduced survival was larger among persons who actively smoked at initial diagnosis vs those who formerly or never smoked, suggesting that the potential impact of prevention or early detection of SPLC could be higher among patients with early stage IPLC and active smoking at initial diagnosis.
We recently demonstrated that smoking is a risk factor for SPLC among lung cancer survivors (8). Other factors associated with SPLC risk included IPLC stage; patients with early-stage IPLC had a statistically significantly increased risk of SPLC vs those with advanced-stage IPLC (8,9). Taken together, our current findings suggest that targeting high-risk patients for SPLC surveillance and prevention—specifically active smokers at initial diagnosis and early stage patients with IPLC—may lead to a statistically significant mortality reduction among lung cancer survivors. Potential prevention strategies for SPLC include smoking cessation programs for lung cancer patients. Recent evidence showed that participation in computed tomography screening increased smoking cessation rates in the general population (18-20). Moreover, evidence-based cessation support programs, such as counseling or pharmacotherapy, are available for cancer patients (21). Although the clinical implementation of cessation support for patients undergoing computed tomography screening is still limited, effective programs to convince lung cancer patients to stop smoking after an initial diagnosis will be a key factor in reducing SPLC risk as well as improving survival.
Prior studies examined overall survival among SPLC patients in comparison to patients with a single primary lung cancer (15,22,23). However, their limitations included a small sample size from a single institute (22,23) or different follow-up time frames used for comparing SPLC patients vs patients with a single primary lung cancer (15,22), which led to inconsistent results. In comparing survival between SPLC and single primary lung cancer in these studies, SPLC patients were followed from the time of SPLC diagnosis through death or loss to follow-up, whereas patients with a single primary lung cancer were followed from the time of initial diagnosis (15,22). Thus, clinical implications obtained from this comparison are limited with regards to evaluating surveillance strategies for SPLC, as SPLC patients were followed from the time of SPLC diagnosis. Furthermore, none of the existing studies examined potential modifications of the effect of SPLC on survival by key factors, such as smoking or stage. In our study, we performed a thorough analysis by following all patients from the time of initial diagnosis to evaluate survival by SPLC diagnosis status and by conducting a set of interaction analyses that helped identify potential areas of focused interventions.
Notably, our study found that the impact of SPLC diagnosis on reduced survival was pronounced among patients with adenocarcinoma and squamous cell IPLC vs those with small cell or large cell IPLC histology. Given that patients with small cell or large cell IPLC are associated with having poor prognosis, the potential effect of intensive SPLC surveillance would be smaller for these patients. Similarly, SPLC was found to have a greater impact on decreasing survival among younger patients who are expected to live longer, implying that follow-up strategies targeting younger patients will be more impactful. We also explored other potential effect modifiers (ie, sex or race), but we did not detect any effect modification (data not shown), in line with previous studies not detecting their association with SPLC (8).
To the best of our knowledge, the present study provides the first effort that evaluates the survival impact of SPLC in lung cancer patients using large population-based data by following patients from the time of initial diagnosis to death. A set of rigorous statistical methods has been applied to assess survival after IPLC diagnosis by treating SPLC as a time-varying predictor, thus preventing a potential time-related bias in estimating survival. We used an epidemiological cohort MEC to examine whether smoking exposure, a modifiable risk factor, may potentially interact with the effects of SPLC on survival. Testing for effect modification on SPLC-survival association identified specific subgroups of patients (eg, patients with active smoking at initial diagnosis) for whom tailored screening and surveillance could provide a potentially larger survival benefit.
This study has several limitations. The SEER data include limited epidemiologic data such as smoking information. Therefore, we used the MEC as an additional source of data to further examine effect modification by smoking history. However, the MEC cohort consists of a relatively healthy and light-smoking lung cancer patient population, and the median age at IPLC diagnosis is higher than that from other cohorts (24). Although the MEC is not as representative of the general patient population with lung cancer, we found that the results between the MEC and nationally representative SEER were consistent in our analysis, which ensured generalizability of our main finding. The absolute number of SPLC cases in the MEC was relatively small to examine effect modification. We did not account for smoking behavioral changes after initial diagnosis because of limited data availability. Among the limited number of patients whose follow-up smoking data were available after IPLC diagnosis in the MEC, we found a large proportion changed their smoking behaviors (data not shown). Given this observation, our current results on SPLC by smoking interaction based on the patients whose smoking behaviors were measured at IPLC diagnosis may change when complete data for longitudinal smoking information are used. We focused on patients who underwent surgery for IPLC, which reduced the sample size by 83% and 79% in SEER and MEC, respectively. However, analysis using the entire cohort that included nonsurgical patients showed a consistent effect of SPLC on mortality (data not shown), indicating that our findings are still generalizable across all patients with lung cancer. Lastly, our data included advanced-stage IPLC patients who underwent surgery. Although not as common, patients with advanced-stage lung cancer could undergo resection for curative intent if they present with oligometastatic disease (25,26). Prior single-institution studies reported that approximately 7% of patients with metastatic non-small cell lung cancer is oligometastatic (27-29). We confirmed that a smaller rate, 4% of all advanced-stage non-small cell lung cancer patients, underwent surgery in SEER.
To conclude, based on large population-based registry data and an epidemiological cohort study, SPLC diagnosis is statistically significantly associated with reduced survival among lung cancer patients. More intensive surveillance and follow-up strategies are warranted for patients with a high risk of developing SPLC to reduce overall mortality among lung cancer survivors. In particular, efforts targeting patients actively smoking at IPLC diagnosis and patients with early stage IPLC may lead to a larger survival benefit.
Funding
This study is supported by grant from the National Institutes of Health (1R37CA226081).
Notes
Role of the funder: The funder had no role in study design, the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Disclosures: Dr Backhus reports personal fees from Johnson & Johnson, outside the submitted work. Ms Su reports participation in summer internship at ZS Associates, Incorporated, in 2021. Dr Neal reports grants from Genetech/Roche, grants from Merck, grants from Boehringer Ingelheim, grants from Exelixis, grants from Nektar Therapeutics, grants from Takeda Pharmaceuticals, grants from Adaptimmune, grants from GlaxoSmithKline, grants from Janssen, grants from AbbVie, other from continuing medical education (CME) Matters, other from Clinical Care Options, other from Research to Practice, other from Medscape, other from Biomedical Learning Institute, other from MLI Peerview, other from Prime Oncology, other from Projects in Knowledge, other from Rockpointe, other from MJH Life Science, other from AstraZeneca, other from Genetech/Roche, other from Exelixis, other from Jounce Therapeutics, other from Takeda Pharmaceuticals, other from Eli Lilly and Company, other from Calithera BIosciences, other from Amgen, other from Iovance Biotherapeutics, other from Blueprint Pharmaceuticals, other from Regeneron Pharmaceuticals, other from Natera, outside the submitted work. Dr Wakelee reports grants from ACEA Biosciences, grants from Arrys Therapeutics, grants from Bristol Myers Squibb, grants from Celgene, grants from Clovis Oncology, grants from Exelixis, grants from Genetech/Roche, grants from Gilead, grants from Merck, grants from Novartis, grants from Pharmacyclics, grants from Sea Gen, grants from Xcovery, other from AstraZeneca, other from Xcovery, other from Janssen, other from Daiichi Sankyo, other from Blueprint, other from Mirati, other from Helsinn, other from Merch—not compensated, other from Genetech/Roche—not compensated, other from the International Association for the Study of Lung Cancer (IASLC), other from the Eastern Cooperative Oncology Group (ECOG)-the American College of Radiology Imaging Network (ACRIN), outside the submitted work. All remaining authors report no other disclosures.
Author contributions: Conceptualization, SSH, EC; Methodology, SSH, EC, SJL; Data curation, EC, LRW; Formal analysis, EC, SJL; Writing—original draft, EC, SJL; Writing—review and editing, all authors; Supervision, SSH.
Data Availability
The data underlying this article are available in SEER 18 Database: Incidence-Based Mortality—SEER Research Data, 18 Registries, Nov 2020 Sub (2000-2018) at https://seer.cancer.gov/data/access.html, and can be accessed with Surveillance Research Program, National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat). The data underlying this analysis were provided by the Multiethnic Cohort Study (MEC) under data use agreement. Researchers interested in the MEC data may submit an inquiry online: https://www.uhcancercenter.org/for-researchers/mec-data-sharing.
Supplementary Material
Contributor Information
Eunji Choi, Quantitative Sciences Unit, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA.
Sophia J Luo, Quantitative Sciences Unit, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA.
Jacqueline V Aredo, Stanford University School of Medicine, Stanford, CA, USA.
Leah M Backhus, Division of Thoracic Surgery, Department of Cardiothoracic Surgery, Stanford University School of Medicine, Stanford, CA, USA.
Lynne R Wilkens, Cancer Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI, USA.
Chloe C Su, Quantitative Sciences Unit, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA.
Joel W Neal, Division of Oncology, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA; Stanford Cancer Institute, Stanford University School of Medicine, Stanford, CA, USA.
Loïc Le Marchand, Cancer Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI, USA.
Iona Cheng, Department of Epidemiology and Biostatistics, University of California, San Francisco, CA, USA.
Heather A Wakelee, Division of Oncology, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA; Stanford Cancer Institute, Stanford University School of Medicine, Stanford, CA, USA.
Summer S Han, Quantitative Sciences Unit, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA; Stanford Cancer Institute, Stanford University School of Medicine, Stanford, CA, USA; Department of Neurosurgery, Stanford University School of Medicine, Stanford, CA, USA.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. SEER Cancer Stat Facts: Lung and Bronchus Cancer. Bethesda, MD: National Cancer Institute, 2021. [Google Scholar]
- 3. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA A Cancer J Clin. 2019;69(5):363–385. [DOI] [PubMed] [Google Scholar]
- 4. Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst. 1998;90(18):1335–1345. [DOI] [PubMed] [Google Scholar]
- 5.NCCN Clinical Practice Guidelines in Oncology. Non-small cell lung cancer. Version 8 Edition; 2020. https://www.nccn.org/guidelines/category_1.
- 6. Schneider BJ, Ismaila N, Aerts J, et al. Lung cancer surveillance after definitive curative-intent therapy: ASCO guideline. J Clin Oncol. 2020;38(7):753–766. [DOI] [PubMed] [Google Scholar]
- 7. Postmus PE, Kerr KM, Oudkerk M, et al. ; for the ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv1–iv21. [DOI] [PubMed] [Google Scholar]
- 8. Aredo JV, Luo SJ, Gardner RM, et al. Tobacco smoking and risk of second primary lung cancer. J Thorac Oncol. 2021;16(6):968–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han SS, Rivera GA, Tammemagi MC, et al. Risk stratification for second primary lung cancer. J Clin Oncol. 2017;35(25):2893–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thakur MK, Ruterbusch JJ, Schwartz AG, Gadgeel SM, Beebe-Dimmer JL, Wozniak AJ. Risk of second lung cancer in patients with previously treated lung cancer: analysis of Surveillance, Epidemiology, and End Results (SEER) data. J Thorac Oncol. 2018;13(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leroy T, Monnet E, Guerzider S, et al. Let us not underestimate the long-term risk of SPLC after surgical resection of NSCLC. Lung Cancer. 2019;137:23–30. [DOI] [PubMed] [Google Scholar]
- 12. Hu ZG, Li WX, Ruan YS, Zeng FJ. Incidence trends and risk prediction nomogram of metachronous second primary lung cancer in lung cancer survivors. PLoS One. 2018;13(12):e0209002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi E, Sanyal N, Ding VY, et al. Development and validation of a risk prediction tool for second primary lung cancer. J Natl Cancer Inst. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howlader NN, Krapcho M, Miller D, et al. SEER Cancer Statistics Review, 1975-2017. Bethesda, MD: National Cancer Institute, 2020. [Google Scholar]
- 15. Varlotto JM, Voland R, DeCamp MM, et al. The rates of second lung cancers and the survival of surgically-resected second primary lung cancers in patients undergoing resection of an initial primary lung cancer. Lung Cancer. 2020;147:115–122. [DOI] [PubMed] [Google Scholar]
- 16. Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70(4):606–612. [PubMed] [Google Scholar]
- 17. Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med. 2013;369(3):245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tammemägi MC, Berg CD, Riley TL, Cunningham CR, Taylor KL. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst. 2014;106(6):dju084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fucito LM, Czabafy S, Hendricks PS, Kotsen C, Richardson D, Toll BA; for the Association for the Treatment of Tobacco Use and Dependence/Society for Research on Nicotine and Tobacco Synergy Committee. Pairing smoking-cessation services with lung cancer screening: a clinical guideline from the association for the treatment of tobacco use and dependence and the society for research on nicotine and tobacco. Cancer. 2016;122(8):1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Piñeiro B, Simmons VN, Palmer AM, Correa JB, Brandon TH. Smoking cessation interventions within the context of low-dose computed tomography lung cancer screening: a systematic review. Lung Cancer. 2016;98:91–98. [DOI] [PubMed] [Google Scholar]
- 21. Gallaway MS, Huang B, Chen Q, et al. Smoking and smoking cessation among persons with tobacco- and non-tobacco-associated cancers. J Community Health. 2019;44(3):552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ha D, Choi H, Chevalier C, Zell K, Wang XF, Mazzone PJ. Survival in patients with metachronous second primary lung cancer. Ann Am Thorac Soc. 2015;12(1):79–84. [DOI] [PubMed] [Google Scholar]
- 23. Fisher A, Kim S, Farhat D, et al. Risk factors associated with a second primary lung cancer in patients with an initial primary lung cancer. Clin Lung Cancer. 2021;22(6):e842-e850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ. 2010;340:b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Couñago F, Luna J, Guerrero LL, et al. Management of oligometastatic non-small cell lung cancer patients: current controversies and future directions. World J Clin Oncol. 2019;10(10):318–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ashworth A, Rodrigues G, Boldt G, Palma D. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer. 2013;82(2):197–203. [DOI] [PubMed] [Google Scholar]
- 27. Pfannschmidt J, Dienemann H. Surgical treatment of oligometastatic non-small cell lung cancer. Lung Cancer. 2010;69(3):251–258. [DOI] [PubMed] [Google Scholar]
- 28. Luketich JD, Martini N, Ginsberg RJ, Rigberg D, Burt ME. Successful treatment of solitary extracranial metastases from non-small cell lung cancer. Ann Thorac Surg. 1995;60(6):1609–1611. [DOI] [PubMed] [Google Scholar]
- 29. Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol. 1991;9(9):1618–1626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in SEER 18 Database: Incidence-Based Mortality—SEER Research Data, 18 Registries, Nov 2020 Sub (2000-2018) at https://seer.cancer.gov/data/access.html, and can be accessed with Surveillance Research Program, National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat). The data underlying this analysis were provided by the Multiethnic Cohort Study (MEC) under data use agreement. Researchers interested in the MEC data may submit an inquiry online: https://www.uhcancercenter.org/for-researchers/mec-data-sharing.