Abstract
In order to investigate structure-activity relationships between antimycobacterial activities and basic substituents at the C-10 position of levofloxacin (LVFX), we synthesized a series of pyridobenzoxazine derivatives by replacement of the N-methylpiperazinyl group of LVFX with various basic substituents. A compound with a 3-aminopyrrolidinyl group had one-half the activity of LVFX against Mycobacterium avium, M. intracellulare, and M. tuberculosis. Mono- and dimethylation of the 3-amino moiety of the pyrrolidinyl group increased the activities against M. avium and M. intracellulare but not those against M. tuberculosis. On the other hand, dialkylation at the C-4 position of the 3-aminopyrrolidinyl group enhanced the activities against M. avium, M. intracellulare, and M. tuberculosis. Thus, introduction of an N-alkyl or a C-alkyl group(s) into the 3-aminopyrrolidinyl group may contribute to an increase in potency against M. avium, M. intracellulare, and/or M. tuberculosis, probably through elevation of the lipophilicity. However, among the compounds synthesized, compound VII, which was a 2,8-diazabicyclo[4.3.0]nonanyl derivative with relatively low lipophilicity, showed the most potent activity against mycobacterial species: the activity was 4- to 32-fold more potent than that of LVFX and two to four times as potent as that of gatifloxacin. These results suggested that an increase in the lipophilicity of LVFX analogues in part contributed to enhancement of antimycobacterial activities but that lipophilicity of the compound was not a critical factor affecting the potency.
During the past decade, an increase in the number of patients with tuberculosis has been one of the most serious health problems in many countries (2, 27). In particular, the now pandemic combination of tuberculosis with human immunodeficiency syndrome (2, 3, 20) and the appearance of multidrug-resistant Mycobacterium tuberculosis (6, 10, 32) have aggravated attempts to treat these patients. In addition, the number of patients infected with Mycobacterium avium-M. intracellulare complex (MAC) is on the increase (5, 9). However, an effective therapy for MAC infection has not yet been established. Given these observations, the development of effective drugs for the mycobacterial infections described above has been keenly desired.
Recently, new quinolone antibacterial agents have been developed and marketed and are widely used clinically. They have potent and broad activities against both gram-negative and gram-positive pathogens. These agents also have been evaluated and shown to have potent activities against certain types of mycobacterial species in in vitro tests and in experimental animals (ofloxacin [26, 29, 31], levofloxacin [LVFX] [18, 22, 33], ciprofloxacin [4, 34], sparfloxacin [12, 21, 30], gatifloxacin [GFLX, formerly AM-1155] [28], and sitafloxacin [formerly DU-6859a] [25]).
LVFX is a representative new quinolone which is characterized by its potency, safety, and good pharmacokinetic profiles in humans. This agent has a unique pyridobenzoxazine structure. In the previous paper, members of our group reported the synthesis of pyridobenzoxazines bearing a series of 3-aminopyrrolidinyl substituents at the C-10 positon and evaluated their activities agaisnt gram-negative and -positive bacteria (13). In this paper, we report the in vitro activities of novel pyridobenzoxazine derivatives having various basic substituents against M. avium, M. intracellulare, and M. tuberculosis and the structure-activity relationships (SARs) between basic substituents and antimycobacterial activities.
MATERIALS AND METHODS
Organisms.
M. avium (four strains), M. intracellulare (four strains), and M. tuberculosis (12 strains) were grown in MYCOBACTERIA 7H11 agar medium (Difco Laboratories, Detroit, Mich.) supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC) (Difco Laboratories).
Drugs.
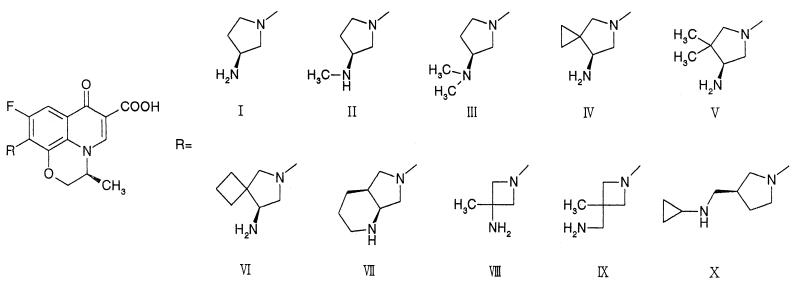
Rifampin (RFP; Sigma-Aldrich Japan, Tokyo, Japan) and isoniazide (INH; Sigma-Aldrich Japan) were obtained commercially and were used as potent drugs. LVFX was synthesized at New Product Research Laboratories I, Daiichi Pharmaceutical Co., Ltd., Tokyo, Japan, as was GFLX. The synthesis of pyridobenzoxazine derivatives I, IV, V, and VI has been reported previously (13); other compounds were newly prepared, and brief descriptions of the synthetic method as well as the physical properties of the compounds are given below. The structures of all the compounds synthesized are shown in Fig. 1.
FIG. 1.
Structures of pyridobenzoxazine derivatives.
All melting points (mp) were taken on a micro-mp apparatus (MP-500D; Yanagimoto Co., Kyoto, Japan) and are uncorrected. Proton nuclear magnetic resonance spectra (1H-NMR) were recorded at 400 MHz with a JNM-EX400 spectrometer (JEOL, Tokyo, Japan) in 0.1 N NaOD. Chemical shifts are expressed in ppm (δ) with sodium 2,2-dimethyl-2-silapentane-5-sulfonate as an internal standard. Elemental analyses were indicated only by the symbols of the elements; analytical results were within ±0.4% of the theoretical values unless otherwise noted. Optical rotation ([α]D) was measured at 589 nm with a SEPA-300 polarimeter (Horiba Co., Kyoto, Japan).
Representative procedure: 10-[(S,S)-2,8-diazabicyclo[4.3.0]nonan-8-yl]-9-fluoro-2,3-dihydro-3(S)-methyl-7-oxo-7H-pyrido[1,2,3-de][1,4]benzoxazine-6- carboxylic acid (VII).
A solution of 9,10-difluoro-2,3-dihydro-3(S)-methyl-7-oxo-7H-pyrido[1,2,3-de][1,4]benzoxazine-6-carboxylic acid difluoroborate chelete (329 mg, 1.0 mmol), (S,S)-2-tert-butoxycarbonyl-2,8-diazabicyclo[4.3.0]nonan (452 mg, 2.0 mmol), and triethylamine (Et3N) (0.5 ml) in dimethyl sulfoxide (4.0 ml) was stirred at room temperature for 30 min. After evaporation of the Et3N, water was added to the residue with ice-water cooling, and then the mixture was stirred at room temperature for 30 min. The precipitate was washed with water, collected by filtration, and then dissolved in 80% aqueous methanol (20 ml). Et3N (5.0 ml) was added to the solution, and the mixture was refluxed for 5 h. After concentration, the residue was dissolved in chloroform (CHCl3), which was washed with 10% aqueous citric acid and brine, dried over anhydrous sodium sulfate (Na2SO4), and then evaporated to dryness. The residue was dissolved in concentrated HCl (10 ml) with ice-water cooling and stirred for 5 min at room temperature. The mixture was adjusted to pH 11 with 20% aqueous NaOH with ice-water cooling and then was neutralized with 10% aqueous HCl to pH 7.4, which was extracted with CHCl3. The extract was dried over Na2SO4 and evaporated to dryness to yield a crude VII, which was recrystallized from ethanol–28% NH4OH to yield VII (260 mg, 67%) as slightly yellow needles. mp, 296 to 299°C (decomposition). 1H-NMR δ: 1.40 to 1.78 (4H, m), 1.46 (3H, d, J = 6.35 Hz), 2.12 to 2.22 (1H, m), 2.48 to 2.59 (1H, m), 2.84 to 2.91 (1H, m), 3.23 to 3.28 (1H, m), 3.30 to 3.39 (2H, m), 3.76 to 3.90 (2H, m), 4.19 and 4.39 (each 1H, d, J = 11.72 Hz), 4.46 to 4.45 (1H, m), 7.39 (1H, d, J = 14.65 Hz), 8.34 (1H, s). Elemental analysis results were as follows. Calculated for C20H22FN3O4: C, 62.01; h, 5.72; N, 10.85. Found: C, 62.00; H, 5.93; N, 10.82. [α]D, −221.81° (concentration, 0.550 in 1N NaOH). Analogous procedures were used to obtain other compounds, for which physical, analytical, and 1H-NMR spectral data are described below.
9-Fluoro-2,3-dihydro-3(S)-methyl-10-[3(S)-N-methylamino-1-pyrrolidinyl]- 7-oxo-7H-pyrido[1,2,3-de][1,4]benzoxazine-6-carboxylic acid (II).
mp, 147 to 156°C (decomposition). 1H-NMR δ: 1.46 (3H, d, J = 6.83 Hz), 1.70 to 1.77 (1H, m), 2.13 to 2.20 (1H, m), 2.34 (3H, s), 3.24 to 3.37 (2H, m), 3.54 to 3.60 (2H, m), 3.67 to 3.73 (1H, m), 4.30 and 4.45 (each 1H, d, J = 11.23 Hz), 4.52 to 4.60 (1H, m), 7.44 (1H, d, J = 14.16 Hz), 8.31 (1H, s). Elemental analysis results were as follows. Calculated for C18H20FN3O4 · 1.0H2O: C, 56.99; H, 5.84; N, 11.08. Found: C, 57.20; H, 5.88; N, 11.37. [α]D, −32.62° (concentration, 0.802 in 1 N NaOH).
9-Fluoro-2,3-dihydro-3(S)-methyl-10-[3(S)-N,N′-dimethylamino-1-pyrrolidinyl]-7-oxo-7H-pyrido[1,2,3-de][1,4]benzoxazine-6-carboxylic acid (III).
mp, 220 to 224°C (decomposition). 1H-NMR δ: 1.48 (3H, d, J = 6.35 Hz), 1.72 to 1.82 (1H, m), 2.16 to 2.21 (1H, m), 2.26 (6H, s), 2.86 to 2.94 (1H, m), 3.45 to 3.60 (3H, m), 3.67 to 3.73 (1H, m), 4.30 and 4.45 (each 1H, d, J = 11.23 Hz), 4.51 to 4.58 (1H, m), 7.43 (1H, d, J = 14.16 Hz), 8.30 (1H, s). Elemental analysis results were as follows. Calculated for C19H22FN3O4: C, 60.79; H, 5.91; N, 11.19. Found: C, 60.08; H, 5.97; N, 11.16. [α]D: +40.51° (concentration, 0.669 in 1 N NaOH).
10-(3-Amino-3-methyl-1-azetidinyl)-9-fluoro-2,3-dihydro-3(S)-methyl-7-oxo-7H-pyrido[1,2,3-de][1,4]benzoxazine-6-carboxylic acid (VIII).
mp, 300 to 302°C (decomposition). 1H-NMR δ: 1.47 (3H, s), 1.50 (3H, d, J = 7.00 Hz), 4.07 to 4.11 (2H, m), 4.21 to 4.23 (2H, m), 4.27 and 4.43 (each 1H, d, J = 11.00 Hz), 4.56 to 4.58 (1H, m), 7.49 (1H, d, J = 13.00 Hz), 8.30 (1H, s). Elemental analysis results were as follows. Calculated for C17H18FN3O4 · 0.5H2O: C, 57.29; H, 5.37; N, 11.79. Found: C, 57.48; H, 5.41; N, 11.73. [α]D, −74.48° (concentration, 0.827 in 1 N NaOH).
10-(3-Aminomethyl-3-methyl-1-azetidinyl)-9-fluoro-2,3-dihydro-3(S)-methyl-7-oxo-7H-pyrido[1,2,3-de][1,4]benzoxazine-6-carboxylic acid (IX).
mp, 276 to 278°C (decomposition). 1H-NMR δ: 1.29 (3H, s), 1.49 (3H, d, J = 7.00 Hz), 2.81 (2H, s), 4.00 to 4.02 (2H, m), 4.11 to 4.12 (2H, m), 4.26 to 4.29 (2H, m), 4.39 to 4.44 (2H, m), 4.56 (1H, broads), 7.44 to 7.52 (1H, m), 8.32 (1H, s). Elemental analysis results were as follows. Calculated for C18H20FN3O4 · 0.75H2O: C, 57.67; H, 5.78; N, 11.21. Found: C, 57.38; H, 5.75; N, 11.23. [α]D, −68.28° (concentration, 0.700 in 1 N NaOH).
10-[3-(R)-N-Cyclopropylaminomethyl-3-pyrrolidinyl]-9-fluoro-2,3-dihydro- 3(S)-methyl-7-oxo-7H-pyrido[1,2,3-de][1,4]benzoxazine-6-carboxylic acid (X).
mp, 173 to 176°C (decomposition). 1H-NMR δ: 0.35 to 0.45 (2H, m), 0.47 to 0.56 (2H, m), 1.48 (3H, d, J = 6.83 Hz), 1.48 to 1.58 (1H, m), 2.05 to 2.24 (2H, m), 2.37 to 2.46 (1H, m), 2.69 to 2.76 (2H, m), 3.22 to 3.34 (1H, m), 3.38 to 3.70 (3H, m), 4.27 and 4.44 (each 1H, d, J = 9.28 Hz), 4.54 to 4.61 (1H, m), 7.44 (1H, d, J = 14.16 Hz), 8.36 (1H, s). Elemental analysis results were as follows. Calculated for C21H24FN3O4 · 0.25H2O: C, 62.14; H, 6.08; N, 10.35. Found: C, 62.13; H, 6.01; N, 10.16. [α]D, −114.70° (concentration, 0.544 in 1 N NaOH).
Determination of apparent partition coefficients (P′).
The apparent partition coefficients of the compounds synthesized were measured according to the method reported previously (1).
Susceptibility testing.
The MICs of the drugs for mycobacteria were measured by the twofold agar dilution method reported by Saito et al. with MYCOBACTERIA 7H11 agar supplemented with 10% OADC (25). The MICs were determined after 14 days (M. avium and M. intracellulare) or 21 days (M. tuberculosis) of incubation at 37°C.
RESULTS
The MICs for M. avium, M. intracellulare, and M. tuberculosis are shown in Table 1. The data for RFP and INH are included for comparison. Among the compounds synthesized, compound VII, bearing a 2,8-diazabicyclo[4.3.0]nonanyl group at the C-10 position, showed the most potent activity against both MAC and M. tuberculosis. Compound VII was four to eight times as potent as LVFX and two to four times as potent as GFLX, which showed more potent activity than standard antituberculosis agents RFP and INH against RFP-susceptible and -resistant M. tuberculosis. Compound I, having a 3-aminopyrrolidinyl group, had one-half the activity of LVFX against both MAC and M. tuberculosis. Compound II, with a 3-methylaminopyrrolidinyl group, was two times more active than I against MAC. Compound III, with a 3-dimethylaminopyrrolidinyl group, was furthermore two times more active than II against MAC, and its potency was higher than that of INH. However, compounds II and III were not superior to RFP, INH, and compound I in activities against some M. tuberculosis strains. Compounds IV to VI, bearing 4,4-dialkylated 3-aminopyrrolidinyl moieties, showed more potent activities than nonalkylated compound I against both MAC and M. tuberculosis; in particular, compound VI was two to eight times as potent as LVFX, and its potency was comparable to that of GFLX. Compound X, with a 3-aminomethylpyrrolidinyl moiety, was two times as potent as LVFX in activity against both MAC and M. tuberculosis. Compound VIII, with a 3-aminoazetidinyl moiety substituted, showed activity equipotent to that of LVFX, and 3-aminomethylazetidinyl derivative IX was less active than LVFX against MAC.
TABLE 1.
MICs of pyridobenzoxazine derivatives and other drugs for MAC and M. tuberculosis strains
| Drug | P′a | MIC (μg/ml)b
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
M. avium
|
M. intracellulare
|
RFP-s M. tuberculosisc
|
RFP-r M. tuberculosisd
|
||||||
| Range | Mean | Range | Mean | Range | Mean | Range | Mean | ||
| Ie | 0.3 | 6.25–25 | 15.6 | 6.25–50 | 39.1 | 0.39–12.5 | 3.5 | 1.56–25 | 9.4 |
| II | 0.7 | 3.13–50 | 17.2 | 6.25–25 | 14.1 | 0.78–6.25 | 3.2 | 1.56–25 | 9.7 |
| III | 17.7 | 1.56–25 | 9.0 | 3.13–12.5 | 7.0 | 0.39–6.25 | 3.0 | 0.78–25 | 9.5 |
| IV | 1.8 | 1.56–6.25 | 3.1 | 0.78–6.25 | 4.1 | 0.10–1.56 | 1.0 | 0.39–6.25 | 2.4 |
| V | 10.7 | 1.56–12.5 | 5.5 | 1.56–6.25 | 4.3 | 0.10–3.13 | 1.4 | 0.78–6.25 | 3.1 |
| VI | 16.6 | 0.78–6.25 | 2.3 | 0.78–3.13 | 2.2 | 0.05–1.56 | 0.6 | 0.20–1.56 | 1.1 |
| VII | 2.7 | 0.20–1.56 | 0.7 | 0.39–3.13 | 1.7 | 0.05–0.39 | 0.2 | 0.10–0.78 | 0.4 |
| VIII | NTf | 3.13–50 | 20.3 | 6.25–25 | 17.2 | NT | NT | ||
| IX | NT | 25–100 | 50.0 | 25–100 | 62.5 | NT | NT | ||
| X | 14.7 | 1.56–25 | 7.8 | 3.13–6.25 | 7.0 | 0.10–3.13 | 0.9 | 0.20–3.13 | 1.7 |
| LVFX | 5.1 | 1.56–50 | 16.8 | 6.25–25 | 14.1 | 0.20–3.13 | 1.3 | 0.39–6.25 | 3.4 |
| GFLX | 5.9 | 0.78–6.25 | 2.3 | 0.78–6.25 | 3.3 | 0.10–0.39 | 0.2 | 0.20–3.13 | 1.1 |
| RFP | NT | 6.25–100 | 45.3 | 0.78–3.13 | 1.8 | 0.05–12.5 | 4.3 | >100 | >100 |
| INH | NT | 3.13–6.25 | 4.7 | 6.25–12.5 | 10.9 | 0.05–>100 | >33 | 0.78–100 | 22.0 |
P′, apparent partition coefficient, chloroform (CHCl3)/0.1 mole/liter of phosphate buffer (pH 7.4).
The numbers of strains tested for the species listed were as follows: M. avium, 4; M. intracellulare, 4; RFP-s M. tuberculosis, 7; RFP-r M. tuberculosis, 5.
RFP-s, RFP-susceptible (RFP MIC, ≤12.5 μg/ml).
RFP-r, RFP resistant (RFP MIC, >100 μg/ml).
See Fig. 1 and Materials and Methods.
NT, not tested.
DISCUSSION
In order to investigate SARs between antimycobacterial activities and basic substituents at the C-10 position of the pyridobenzoxazine nucleus, we modified LVFX by replacement of the N-methylpiperazine with various basic substituents. As shown in Table 1, the activity order of pyridobenzoxazine derivatives was M. tuberculosis > M. avium > M. intracellulare; e.g., the most potent compound, VII, had MIC ranges of 0.05 to 0.78, 0.20 to 1.56 and 0.39 to 3.13 μg/ml for M. tuberculosis, M. avium, and M. intracellulare, respectively. In addition, these pyridobenzoxazines had potent activities against RFP-resistant M. tuberculosis, demonstrating no cross-resistance to RFP.
At first, we synthesized pyridobenzoxazines substituted with 3-aminopyrrolidinyl groups at the C-10 position and evaluated the effects of these groups on the activities against mycobacteria. Compound I, having a 3-aminopyrrolidinyl group, was less active than LVFX against both MAC and M. tuberculosis. Methylation of the 3-amino moiety on the pyrrolidine ring of compound I enhanced the activity against MAC (most of all for compound III, then for compound II, and finally for compound I). This result substantiated the reports suggesting that alkylation of a terminal amino group enhanced the activities against MAC (8, 15, 16). However, N-methylation did not elevate the activities against M. tuberculosis. On the other hand, dialkylation of the fourth position on the 3-aminopyrrolidine ring yielded compounds IV to VI, having potent activities against both MAC and M. tuberculosis (IV to VI > LVFX > I to III). Compound VII showed the most potent activity against both MAC and M. tuberculosis. This compound had a 2,8-diazabicyclo[4.3.0]nonanyl group, which could be considered a hybrid structure of the N- and C-alkylation products of the original 3-aminopyrrolidinyl group, suggesting that ring formation by connecting the N-alkyl and C-alkyl terminals on the 3-aminopyrrolidinyl group enhances the activity against both MAC and M. tuberculosis. This result is consistent with the previous reports concerning the potent antimycobacterial activities of quinolones with this bicyclo substituent (17, 19).
It is well known that the mycobacterial cell wall contains unique lipophilic substances such as mycolic acid. This distinctive cell wall formation may play an important role in hindering drug penetration. Based on this assumption, it is expected that the more lipophilic compounds would have the advantage for penetration through the cell wall and exhibit potent antimycobacterial activities. Actually, some previous reports demonstrated that higher lipophilicity played an important role in the antimycobacterial activity (8). In comparing the activities of compounds I, II, and III, an increase in the lipophilicity of the compounds contributed to enhancement of the activities against MAC, but not M. tuberculosis. In a series of compounds, I and IV to VI, the more lipophilic compounds had more potent activities against both MAC and M. tuberculosis. However, the most potent compound, VII, did not have the highest lipophilicity compared to compounds III, IV, V, and VI. These results suggested that an increase in lipophilicity by introduction of an N-alkyl or a C-alkyl group(s) into the 3-aminopyrrolidinyl group in part contributed to an increase in activities against MAC and/or M. tuberculosis, but the lipophilicity of the compound was not the critical factor affecting their potency. In this study, dealing with pyridobenzoxazines, the order of potency of basic substituents against mycobacteria (from the highest to the lowest) was 2,8-diazabicyclo[4.3.0]nonane > 3-aminomethylpyrrolidines > 3-aminopyrrolidines ≧ piperazines ≧ 3-aminoazetidine > 3-aminomethylazetidine.
In conclusion, we have found that pyridobenzoxazine derivatives VI, VII, and X exhibited enhanced activities against mycobacteria compared with LVFX. These results suggested that 2,8-diazabicyclo[4.3.0]nonanyl, 3-aminomethylpyrrolidinyl, and 4,4-dialkyl-3-aminopyrrolidinyl groups were more effective for the activities against both MAC and M. tuberculosis than the piperazinyl group. There have been several reports demonstrating the SARs between antimycobacterial activity and the substituents of the N-1 and C-8 position of the 4-quinolone nucleus (7, 11, 14, 23, 24). In practice, a combination of the basic substituents and variations of the 4-quinolone nucleus leads to subtle changes in the intrinsic antibacterial activity. Consequently, the introduction of the basic substituents described above to the appropriate 4-quinolone nucleus could contribute to obtaining novel compounds possessing excellent antimycobacterial activities.
ACKNOWLEDGMENTS
We are grateful to Haruaki Tomioka and Hajime Saito for providing us with four M. avium strains, four M. intracellulare strains, and 12 M. tuberculosis strains.
REFERENCES
- 1.Atarashi S, Imamura M, Kimura Y, Yoshida A, Hayakawa I. Fluorocyclopropyl quinolones. 1. Synthesis and structure-activity relationships of 1-(2-fluorocyclopropyl)-3-pyridonecarboxylic acid antibacterial agents. J Med Chem. 1993;36:3444–3448. doi: 10.1021/jm00074a027. [DOI] [PubMed] [Google Scholar]
- 2.Bruwen D R, Bloch A B, Griffin L D, Ciesielski C A, Stern H A, Onorato I M. National trends in the occurrence of tuberculosis and acquired immunodeficiency syndrome. Arch Intern Med. 1995;155:1281–1286. [PubMed] [Google Scholar]
- 3.Cantwell M F, Snider D E, Jr, Cauthen G M, Onorato I M. Epidemiology of tuberculosis in the United States, 1985–1992. JAMA. 1994;272:535–539. [PubMed] [Google Scholar]
- 4.Collins C H, Uttley A H C. In vitro susceptibility of mycobacteria to ciprofloxacin. J Antimicrob Chemother. 1985;16:575–580. doi: 10.1093/jac/16.5.575. [DOI] [PubMed] [Google Scholar]
- 5.Collins F M. AIDS-related mycobacterial disease. Springer Semin Immunopathol. 1988;10:375–391. doi: 10.1007/BF02053847. [DOI] [PubMed] [Google Scholar]
- 6.Dooley S W, Jarvis W R, Martone W J, Snider D E. Multidrug-resistant tuberculosis. Ann Intern Med. 1992;117:257–259. doi: 10.7326/0003-4819-117-3-257. [DOI] [PubMed] [Google Scholar]
- 7.Franzblau S G, White K E. Comparative in vitro activities of 20 fluoroquinolones against Mycobacterium leprae. Antimicrob Agents Chemother. 1990;34:229–231. doi: 10.1128/aac.34.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haemers A, Leysen D C, Bollaert W, Zhang M, Pattyn S R. Influence of N substitution on antimycobacterial activity of ciprofloxacin. Antimicrob Agents Chemother. 1990;34:496–497. doi: 10.1128/aac.34.3.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horsburg C R, Jr, Cohn D L, Roberts R B, Miller H, Masur R A, Tsang A Y, Tseman M D. Mycobacterium avium-M. intracellulare isolates from patients with or without acquired immunodeficiency syndrome. Antimicrob Agents Chemother. 1986;30:955–957. doi: 10.1128/aac.30.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iseman M D. Treatment of multidrug-resistant tuberculosis. N Engl J Med. 1993;329:784–791. doi: 10.1056/NEJM199309093291108. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs M R. Activity of quinolones against mycobacteria. Drugs. 1995;49:67–75. doi: 10.2165/00003495-199500492-00011. [DOI] [PubMed] [Google Scholar]
- 12.Ji B, Truffot-Pernot C, Grosset J. In vitro and in vivo activities of sparfloxacin (AT-4140) against Mycobacterium tuberculosis. Tubercle. 1991;72:181–186. doi: 10.1016/0041-3879(91)90004-c. [DOI] [PubMed] [Google Scholar]
- 13.Kawakami K, Atarashi S, Kimura Y, Takemura M, Hayakawa I. Synthesis and antibacterial activity of novel pyridobenzoxazine analogues. Chem Pharm Bull. 1998;46:1710–1715. doi: 10.1248/cpb.46.1710. [DOI] [PubMed] [Google Scholar]
- 14.Klopman G, Fercu D, Renau T E, Jacobs M R. N-1-tert-Butyl-substituted quinolones: in vitro anti-Mycobacterium avium activities and structure-activity relationship studies. Antimicrob Agents Chemother. 1996;40:2637–2643. doi: 10.1128/aac.40.11.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klopman G, Wang S, Jacobs M R, Ellner J J. Anti-Mycobacterium avium activity of quinolones: structure-activity relationship studies. Antimicrob Agents Chemother. 1993;37:1807–1815. doi: 10.1128/aac.37.9.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klopman G, Wang S, Jacobs M R, Bajaksouzian S, Edmonds K, Ellner J J. Anti-Mycobacterium avium activities of quinolones: in vitro activities. Antimicrob Agents Chemother. 1993;37:1799–1806. doi: 10.1128/aac.37.9.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyazaki E, Miyazaki M, Chen J M, Chaisson R E, Bishai W R. Moxifloxacin (BAY12-8039), a new 8-methoxyquinolone, is active in a mouse model of tuberculosis. Antimicrob Agents Chemother. 1999;43:85–89. doi: 10.1128/aac.43.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mor N, Vanderkolk J, Heifets L. Inhibitory and bactericidal activities of levofloxacin against Mycobacterium tuberculosis in vitro and in human macrophages. Antimicrob Agents Chemother. 1994;38:1161–1164. doi: 10.1128/aac.38.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oleksijew A, Meulbroek J, Ewing P, Jarvis K, Mitten M, Paige L, Tovcimak A, Nukkula M, Chu D, Alder J D. In vitro efficiency of ABT-255 against drug-sensitive and -resistant Mycobacterium tuberculosis strains. Antimicrob Agents Chemother. 1998;42:2674–2677. doi: 10.1128/aac.42.10.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onorato I M, McCray E the Field Services Branch. Prevalence of human immunodeficiency virus infection among patients attending tuberculosis clinics in the United States. J Infect Dis. 1992;165:87–92. doi: 10.1093/infdis/165.1.87. [DOI] [PubMed] [Google Scholar]
- 21.Rastogi N, Goh K S. In vitro activity of the new difluorinated quinolone sparfloxacin (AT-4140) against Mycobacterium tuberculosis compared with activities of ofloxacin and ciprofloxacin. Antimicrob Agents Chemother. 1991;35:1933–1936. doi: 10.1128/aac.35.9.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rastogi N, Goh K S, Bryskier A, Devallois A. In vitro activities of levofloxacin used alone and in combination with first- and second-line antituberculosis drugs against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1996;40:1610–1616. doi: 10.1128/aac.40.7.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renau T E, Sanchez J P, Gage J W, Dever J A, Shapiro M A, Grachek S J, Domagala J M. Structure-activity relationships of quinolone antibacterials against mycobacteria: effect of structural changes at N-1 and C-7. J Med Chem. 1996;39:729–735. doi: 10.1021/jm9507082. [DOI] [PubMed] [Google Scholar]
- 24.Renau T E, Sanchez J P, Shapiro M A, Dever J A, Grachek S J, Domagala J M. Effect of lipophilicity at N-1 on activity of fluoroquinolones against mycobacteria. J Med Chem. 1995;38:2974–2977. doi: 10.1021/jm00015a021. [DOI] [PubMed] [Google Scholar]
- 25.Saito H, Tomioka H, Sato K, Dekio S. In vitro and in vivo antimycobacterial activities of a new quinolone, DU-6859a. Antimicrob Agents Chemother. 1994;38:2877–2882. doi: 10.1128/aac.38.12.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito H, Watanabe T, Tomioka H, Sato K. Susceptibility of various mycobacteria to quinolones. Rev Infect Dis. 1988;10(Suppl. 1):52. [Google Scholar]
- 27.Sudre P, ten-Dam G, Kochi A. Tuberculosis: a global overview of the situation today. Bull W H O. 1992;70:149–159. [PMC free article] [PubMed] [Google Scholar]
- 28.Tomioka H, Saito H, Sato K. Comparative antimycobacterial activities of the newly synthesized quinolone AM-1155, sparfloxacin, and ofloxacin. Antimicrob Agents Chemother. 1993;37:1259–1263. doi: 10.1128/aac.37.6.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomioka H, Sato K, Saito H. Comparative in vitro and in vivo activity of fleroxacin and ofloxacin against various mycobacteria. Tubercle. 1991;72:176–180. doi: 10.1016/0041-3879(91)90003-b. [DOI] [PubMed] [Google Scholar]
- 30.Tomioka H, Sato K, Saito H. Antimycobacterial activities of new quinolone, sparfloxacin. Kekkaku. 1991;66:643–649. [PubMed] [Google Scholar]
- 31.Tsukamura M. In vitro antimycobacterial activity of a new antibacterial substance DL-8280 differentiation between some species of mycobacteria and related organisms by the DL-8280 susceptibility test. Microbiol Immunol. 1983;27:1129–1132. doi: 10.1111/j.1348-0421.1983.tb02933.x. [DOI] [PubMed] [Google Scholar]
- 32.Weltman A C, Rose D N. Tuberculosis susceptibility patterns, predictors of multidrug resistance, and implications for initial therapeutic regimens at a New York city hospital. Arch Intern Med. 1994;154:2161–2167. [PubMed] [Google Scholar]
- 33.Yew W W, Piddock L J V, Li M S K, Lyon D, Chan C Y, Cheng A F B. In-vitro activity of quinolones and macrolides against mycobacteria. J Antimicrob Chemother. 1994;34:343–351. doi: 10.1093/jac/34.3.343. [DOI] [PubMed] [Google Scholar]
- 34.Young L S, Berlin G W, Inderlied C B. Activity of ciprofloxacin and other fluorinated quinolones against mycobacteria. Am J Med. 1987;82(Suppl. 4A):23–26. [PubMed] [Google Scholar]