Abstract
Approximately half of all patients with heart failure (HF) have a preserved ejection fraction (HFpEF) and the prevalence is growing rapidly given the aging population in many countries and rising prevalence of obesity, diabetes, and hypertension. Functional capacity and quality of life are severely impaired in HFpEF, with high morbidity and mortality. In striking contrast to HF with reduced ejection fraction, there are few effective treatments currently identified for HFpEF, limited mostly to decongestion by diuretics, promotion of a healthy active lifestyle, and management of comorbidities. Improved phenotyping of subgroups within the overall HFpEF population might promote enhanced individualization of treatment. This review focuses on the current understanding of the pathophysiological mechanisms underlying HFpEF and treatment strategies for this complex syndrome.
Keywords: heart failure, heart failure with preserved ejection fraction, pathophysiology, treatment
INTRODUCTION
Heart failure (HF) is a major public health problem that afflicts millions of adults worldwide (1). HF with preserved ejection fraction (HFpEF) accounts for over one-half of all HF cases, and the incidence and prevalence are growing as the population ages and with an increasing prevalence of metabolic disorders including obesity, diabetes, and hypertension (2–4). Although cardiovascular mortality in HFpEF is lower when compared to HF with reduced ejection fraction (HFrEF), hospital readmissions are frequent and quality of life is poor (5; 6). Moreover, no unequivocally effective treatment for HFpEF has been identified in clinical trials (5; 7; 8). This is believed to relate in part to the pathophysiologic heterogeneity within the clinical syndrome of HFpEF, and it is hoped that through better phenotyping of patients, treatments can be more tailored to the individual. In this review, we focus on the current understanding of the pathophysiological mechanisms underlying HFpEF, and then tie this mechanistic understanding together with current and investigational treatment strategies for this complex syndrome.
PATHPHYSIOLOGY
Although diastolic dysfunction is the lynchpin and fundamental component underlying the pathophysiology in HFpEF, there are multiple cardiac, vascular, and non-cardiac abnormalities that contribute. These include impairments in left ventricular (LV) diastolic and systolic function, left arterial (LA) structure and function (i.e., LA myopathy), pulmonary hypertension and gas exchange abnormalities, right heart dysfunction, autonomic deregulation, vascular stiffening, myocardial ischemia, endothelial dysfunction, kidney disease, and peripheral abnormalities in skeletal muscle and fat (Figure 1). Importantly, not all patients with HFpEF exhibit each of these features, implying the presence of specific phenotypes (3; 8; 9). In this first section, key pathophysiologic mechanisms of HFpEF are individually reviewed from an organ-based perspective. The cellular mechanisms causing these abnormalities are beyond the scope of this review but have recently been reviewed elsewhere (10).
Figure 1:
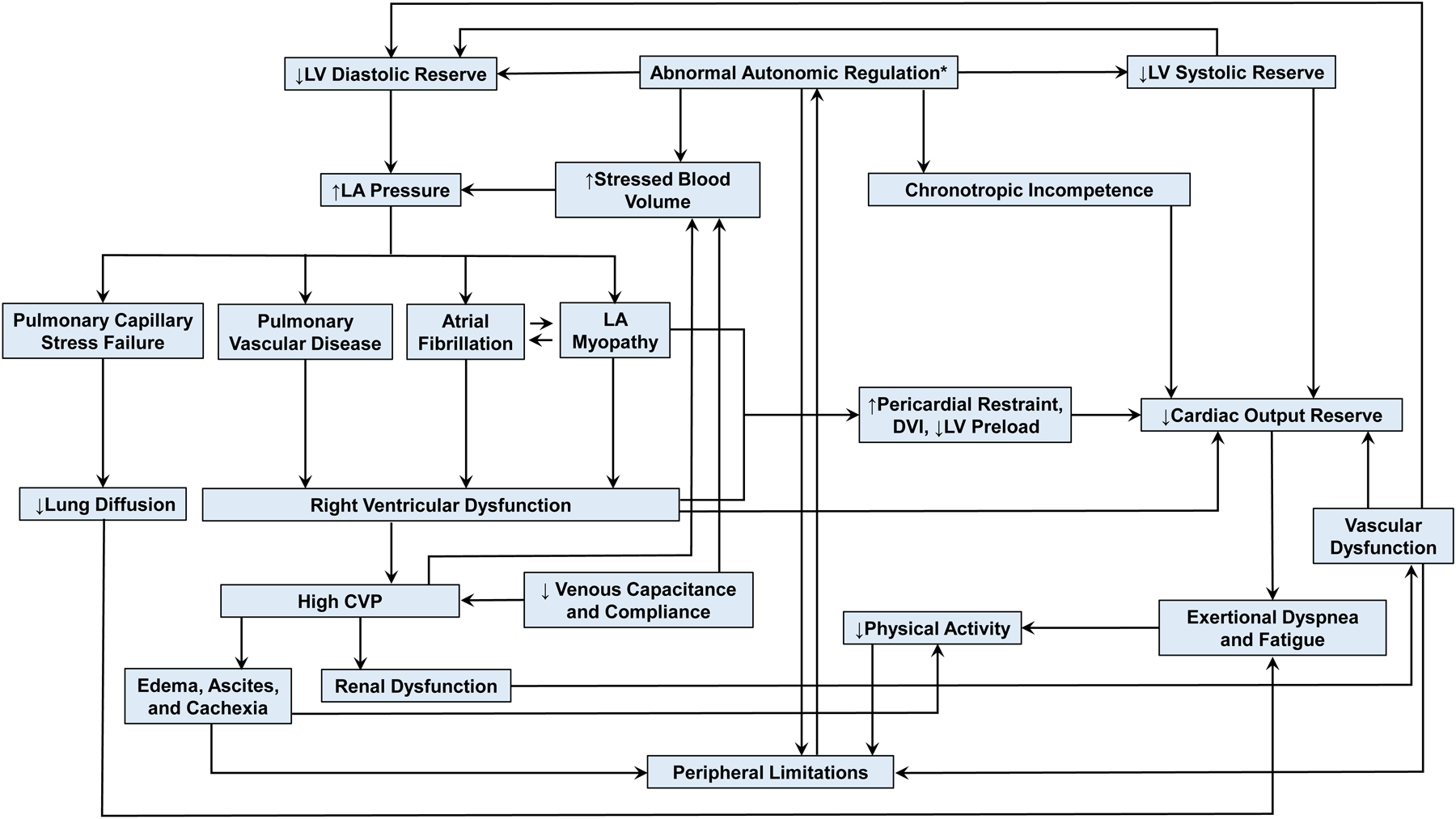
The pathophysiology of heart failure with preserved ejection fraction. Arrows show causal inter-relationships between components. Abbreviations: CVP, central venous pressure; DVI, diastolic ventricular interaction; LA, left atrial; LV, left ventricular. *Since it is uncertain whether autonomic dysfunction can connect to chronotropic, it is titled as “Abnormal Autonomic Regulation*”.
Diastolic Dysfunction
Diastolic dysfunction is broadly defined as an inability to fill the ventricle to an adequate preload volume at normal filling pressures at rest and during activity (8; 9). From a clinical perspective, diastolic dysfunction leads to an increase in left ventricular, left atrial, and pulmonary capillary pressures that promotes pulmonary congestion, dyspnea, and abnormalities in gas exchange and pulmonary vascular function (11–13). Diastolic dysfunction may be characterized by abnormalities in active relaxation or passive chamber stiffening caused by alterations in the myocardium and extracellular matrix. The active process of pressure decay (relaxation) requires adenosine triphosphate to initiate reuptake of calcium into the sarcoplasmic reticulum (14). Abnormalities in diastolic calcium cycling have been revealed in myocardial tissue from individuals with hypertensive left ventricular hypertrophy (15), coupled with increases in T-tubule density and increased cytosolic calcium, particularly in the setting of diabetes (16; 17). Diastolic function is influenced by heightened afterload. With aging and arterial hypertension, the aorta stiffens, resulting in more rapid pressure wave transit, and augmentation of reflected pressure waves that cause late systolic afterload elevation contributing to prolonged relaxation (18; 19). This may lead to elevation in end-diastolic filling pressure when heart rate increases (20).
Increased passive stiffness related to the viscoelastic properties of the myocardium is present in HFpEF, shifting the LV end-diastolic pressure-volume relationship upward and to the left, an effect that is amplified during exercise (21; 22). Such myocardial stiffness is caused by intrinsic cardiomyocyte alterations, particularly involving the giant macromolecule titin, which essentially acts as a bidirectional spring that affects myocyte stiffness (3; 23). Titin’s stiffness properties are dynamically regulated through phosphorylation by cyclic guanosine monophosphate (cGMP)-kinases, providing a mechanism by which impaired nitric oxide bioavailability may contribute to HFpEF (24). Stiffness is also increased in HFpEF through alterations in fibrillar collagen in the extracellular matrix (25). This may be suggested using novel techniques such as T1 mapping by cardiac MRI, which reveals an increase in extracellular volume in such patients (26; 27).
Systolic Dysfunction
Despite a preserved ejection fraction, patients with HFpEF often display subtle abnormalities in LV systolic function as well. This may be driven by abnormalities in calcium handling, beta-adrenergic signaling, myocardial energetics, or tissue perfusion reserve (3; 8). Impaired contractility may be detected by echocardiographic tissue doppler imaging, strain imaging, or other measures of chamber and myocardial contractility (28–31). Patients with LV contractile dysfunction display increased risk of mortality in HFpEF (28; 31). Impaired systolic function is often subtle at rest, but with physiological stress, may worsen dramatically and contribute to a decreased cardiac output reserve and impaired exercise intolerance (30; 32–35). Systolic dysfunction begets diastolic impairments, as the inability to contract to a lower end systolic volume reduces elastic recoil that contributes to diastolic suction of blood into ventricle, further promoting pulmonary capillary hypertension (30; 36). LV systolic dysfunction also contributes to LA dysfunction through atrioventricular coupling, since both chambers sit in continuum with the mitral annulus.
Left Atrial Myopathy and Atrial Fibrillation
The left atrium plays an important role to facilitate LV filling and protect the pulmonary vasculature and right heart from elevation in LV pressures (37–43). In the early stages of HFpEF, the LA is able to compensate for LV diastolic dysfunction, acting as through its reservoir function to store blood without untoward elevation in LA pressure, then facilitating LV filling through its booster function. However, with prolonged or more advanced LV dysfunction, LA dilation and dysfunction progress, which is associated with pulmonary hypertension and right ventricular (RV) dysfunction (9; 39; 44; 45). This progression is strongly tied to the development of atrial fibrillation (AF), which may be considered as an electrical biomarker of LA myopathy (44). In healthy hearts under normal circumstances, LA systole contributes approximately 20% of filling to the left ventricle, but when LV diastolic dysfunction progresses, this contribution increases (46). LA dysfunction and loss of atrioventricular synchrony with atrial fibrillation are associated with dramatic limitations in cardiac output at rest and with activity (44), as well as development of mitral regurgitation due to annular dilatation (47). The combination of LA enlargement and increases in right heart volume accompanying LA dysfunction (due to pulmonary hypertension, below) leads to an increase in total heart volume. This increase in total heart volume amplifies interaction between the epicardial surface of the heart and the pericardium (44; 48), termed enhanced diastolic ventricular interaction (DVI). With an increase in DVI, left heart pressures can be elevated out of proportion to the degree of LV diastolic stiffness due to the right heart and pericardium compressing the left, and LV preload is reduced, resulting in failure to maintain cardiac output by the Frank Starling mechanism (48).
LA strain assessed by speckle tracking globally reflects LA function, remodeling and distensibility components that become progressively more impaired in the setting of chronic LV diastolic dysfunction (39; 41–43). Recent studies have demonstrated that LA reservoir strain and LA compliance allow for discrimination of HFpEF from noncardiac dyspnea with greater accuracy than other echocardiographic indices (39). LA compliance and mechanics progressively decline with increasing atrial burden in HFpEF, increasing the risk for AF (44). Development of AF represents a watershed moment in the natural history of HFpEF, associated with increased risk of right ventricular dysfunction (below), worsening exercise capacity, and increased mortality (49–51).
Pulmonary Abnormalities and Right Ventricular Dysfunction
Between 50–80% of patients with HFpEF have pulmonary hypertension (PH), which is defined by a mean pulmonary artery (PA) pressure exceeding 20 mmHg (12). PH in HFpEF is initially caused by passive transmission of elevated downstream LA pressure (termed isolated post-capillary PH). However, with chronic, sustained exposure to elevated LA pressure, pulmonary vascular remodeling often develops, resulting in an increase in pulmonary vascular resistance (termed combined pre- and postcapillary PH) (12; 52). Patients with this precapillary component to PH display poorer exercise capacity, a unique hemodynamic signature characterized by right heart failure and left heart underfilling, and increased risk of hospitalization and death (53–55). While originally assumed to reflect abnormalities in the arterial vasculature, recent data have revealed that remodeling in the pulmonary veins plays an equal or perhaps even greater role in increasing pulmonary vascular load in chronic HFpEF (56).
The first victim of PH in HFpEF is the RV (50; 57; 58). The thin-walled RV is poorly suited to eject against high pressure, and this heightened sensitivity to afterload (i.e. PA pressures) is further amplified in HFpEF (58). Patients with HFpEF and RV dysfunction (RVD) often display systemic venous congestion leading to edema, ascites, abdominal congestion, gut malabsorption, renal and hepatic dysfunction, atrial fibrillation, tricuspid regurgitation, and cardiac cachexia (45; 50; 57). Obokata et al. showed that new onset RV dysfunction in patients with HFpEF is independently associated with adverse outcome even after adjustment for other established risk factors, including age, body mass index, atrial fibrillation, LV ejection fraction, and E/e’ ratio (50). This study also found that the development of RVD is closely linked to potentially modifiable risk factors, including atrial fibrillation, coronary artery disease, obesity, and abnormal cardiac hemodynamics (50).
In addition to inducing pulmonary arterial and venous remodeling, long-term exposure to LA hypertension has important effects on the fragile pulmonary capillaries, where repeated episodes of capillary stress failure promote ultrastructural remodeling (45). This leads to a reduction in pulmonary capillary blood volume and impaired alveolar-capillary membrane conductance, both of which reduce the diffusion capacity for carbon monoxide (DLCO) (59; 60). Impairment in DLCO in HFpEF is typically associated with normal findings on chest computed tomography, but is strongly associated with increased mortality in this population (61).
Vascular Stiffening and Endothelial Dysfunction
The vast majority (80–90%) of patients with HFpEF are hypertensive, and increases in aortic and conduit vessel stiffening are common, especially among diabetics (62). This vascular stiffening becomes amplified during exertion, contributing to the elevation in LV filling pressures that characteristically develops in HFpEF (18). The combination of increased ventricular and arterial stiffness promotes blood pressure lability, wherein patients frequently oscillate between hypertensive crises and symptomatic hypotension, making treatment challenging (63; 64). In addition to arterial stiffening, recent studies have revealed impairments in venous compliance and capacitance, that importantly contribute to increased filling pressures (32).
In addition to material changes in arterial structure, patients with HFpEF frequently display abnormal endothelium-dependent vasodilation, which is associated with symptom severity, functional limitation, and risk of hospitalization (34; 65). These changes are believed to be related to comorbidity-associated systemic inflammation, which impairs nitric oxide bioavailability, affecting cardiovascular structure and function through a variety of mechanisms (66; 67). Recent studies have revealed abnormalities in coronary microvascular function, which are related to both endothelium-dependent and independent processes (68–70). The presence of coronary microvascular dysfunction in HFpEF is associated with other markers of greater disease severity, including atrial fibrillation, microalbuminuria, RVD, greater exertional hemodynamic abnormalities and worse clinical outcome (68; 70; 71). Together with alterations in myocardial supply-demand relationships, coronary microvascular dysfunction may lead to myocardial ischemia and injury during exertion, which is associated with impairments in myocardial reserve and aerobic capacity (71).
Autonomics and Adrenergic Signaling
Cardiac output is typically normal at rest in patients with HFpEF, but the ability to increase cardiac output with exertion is frequently abnormal (33; 72), leading to impaired aerobic capacity. Cardiac output limitations are related in part to impairments in stroke volume reserve from myocardial dysfunction (above), and to chronotropic incompetence (73). Limitations in heart rate reserve in HFpEF appear to be mediated by depressed adrenergic sensitivity rather than central outflow, as plasma norepinephrine and epinephrine increase similarly in HFpEF and matched controls (73), and beta-receptor sensitivity is reduced (74).
There are conflicting reports on autonomic function in HFpEF. Abnormal arterial baroreflex sensitivity was demonstrated in one study (73), whereas another has shown that both central command (parasympathetic withdrawal) and sympathetic outflow responses with stress are intact in HFpEF (75). In a rat model of HFpEF (76), arterial baroreflex sensitivity was severely depressed, resulting in an inability to tolerate volume loading with greater increases in LA pressure with saline loading. Sympathetic outflow also plays a key role in mediating constriction of large capacitance veins in the splanchnic circulation, to increase venous return to the heart (77). Recent data have shown that this increase in “stressed” blood volume is exaggerated in patients with HFpEF, contributing to the increase in cardiac filling pressures that develops during exertion (32).
Skeletal Muscle and Fat
According to the Fick principal, oxygen consumption (VO2) is equal to the product of cardiac output and arterial–venous oxygen content difference (AVO2diff). Recent studies have reported that many patients with HFpEF display abnormalities in the ability to augment AVO2diff during exertion, suggesting a problem in peripheral O2 transport and utilization in skeletal muscle (78–81). Histologic studies have revealed reductions in capillary density and increases in Type II (fast-twitch) fibers, with reduction in the more aerobic Type I fibers (82). There are also abnormalities in mitochondrial function in skeletal muscle in HFpEF that may contribute to abnormalities in peripheral O2 utilization (83; 84).
HFpEF is strongly tied to excess body fat. In the United States, approximately 75% of patients are obese. Patients with HFpEF and obesity display features consistent with a distinct phenotype, wherein there is greater plasma volume expansion, more RVD and PH, greater epicardial fat, enhanced ventricular interdependence, and more systemic inflammation (85; 86). Increases in visceral fat appear to be particularly important, as elevated abdominal visceral adipose tissue is independently associated with increased risk of HFpEF (87; 88). The relationship between visceral fat and HFpEF is notably stronger in women, where increased abdominal visceral fat is associated with more severe hemodynamic alterations in women with HFpEF compared to men (89).
Masqueraders of HFpEF
There are a number of other cardiovascular diseases that differ from typical ‘garden-variety’ HFpEF, including cardiac amyloidosis, sarcoidosis, infiltrative, restrictive or hypertrophic cardiomyopathy, constrictive pericarditis, valvular heart disease, high-output HF, myocarditis and toxin-mediated cardiomyopathies (Table 1). Discussion of these “secondary” causes of HF is beyond the scope of this review, but it is important to emphasize that they should not be regarded as true “primary” HFpEF. Because the secondary etiologies require specific treatments, every effort should be made to exclude these masqueraders when evaluating the patient with new-onset HF (7).
Table 1.
Masqueraders of HFpEF
| Secondary causes of HF with a normal EF | Typical diagnostic methods | Specific treatment strategies |
|---|---|---|
| Cardiac amyloidosis | Screen for presence of a monoclonal light chain, CMR, EMB, Tc-99m-PYP | Transthyretin tetramer stabilizers for transthyretin amyloid cardiomyopathy, Chemotherapy for light-chain amyloidosis |
| Cardiac sarcoidosis | Blood test (ACE, lysozyme, sIL-2R), EMB, CMR, 67Ga scintigraphy or 18FDG-PET | Corticosteroid or Immunosuppressants |
| Hypertrophic cardiomyopathy | Echocardiogram, CMR | β-blockers, Calcium-channel blockers or Alcohol septal ablation for obstructive cardiomyopathy, Avoid vasodilators |
| Valvular heart disease | Echocardiogram, Invasive hemodynamic measurements | Percutaneous valve interventions or Surgical interventions |
| High-output heart failure | Invasive hemodynamic measurements (including cardiac output as well as mixed venous oxygen saturation), Evaluation for physical signs (hyperthyroidism, cardiac beriberi, etc.) | Treatments for underlying disease causing high-output state such as fistula, hyperthyroidism, vitamin B-1 deficiency, or ligation for shunts |
| Myocarditis | 12-lead ECG and/or Holter (AV block or ST-T wave change), Blood test (troponin), CMR, EMB | Immunosuppressive agents for eosinophilic myocarditis or giant cell myocarditis |
| Constrictive pericarditis | Invasive hemodynamic measurements, CMR or Chest CT, Echocardiogram | Pericardiectomy |
| Toxin-mediated cardiomyopathies | Assessment of clinical and medical history (e.g. chemotherapeutics, illicits), Blood testing, EMB | Removal of toxin |
ACE, angiotensin converting enzyme; AV, atrioventricular, CMR, cardiovascular magnetic resonance; CT, computed tomography; ECG, electrocardiogram; EMB, endomyocardial biopsy; 18FDG-PET, 18F-fluorodeoxyglucose positron emission tomography; HF, heart failure; sIL-2R, soluble interleukin-2 receptor; Tc-99m-PYP, Technetium-99m pyrophosphate.
TREATMENT
To date, no conclusively effective treatment has been identified for HFpEF, and therapies with efficacy for HFrEF have failed to improve outcomes in HFpEF (7; 8). Current treatment recommendations focus on diuretics, including mineralocorticoid receptor antagonists (MRA) to reduce congestion. Lifestyle interventions including exercise and weight loss through caloric restriction have shown promise (90–92). Finally, management of common comorbidities such as coronary artery disease and atrial fibrillation may also improve prognosis. Complete revascularization is associated with preservation of LV function and lower mortality (93), while treatment of atrial fibrillation with catheter ablation may improve outcomes as well (94; 95). Both questions requiring testing in controlled trials.
Pharmacological Therapy
Diuretics
Despite the absence of placebo-controlled trial data, diuretic therapy is a cornerstone therapy in HFpEF that improves outcomes. A post-hoc analysis of the CHAMPION trial indirectly supports the efficacy of aggressive diuresis in patients with HFpEF (96). In the CHAMPION trial (97), individuals with HF and an implantable hemodynamic monitor (CardioMEMS™ Heart Sensor) were randomly assigned to a treatment strategy guided by knowledge of PA pressures or usual care. Subjects with pressure-informed therapy received more frequent diuretic titration and demonstrated a significantly reduced risk of HF readmissions. The number needed to treat to prevent one HF hospitalization over 18 months in patients with normal EF was 2.
Mineralocorticoid receptor antagonists
The MRA spironolactone was evaluated as a treatment for HFpEF in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial (98). As compared to placebo, spironolactone did not reduce the composite primary outcome of cardiovascular-related death, aborted cardiac arrest and HF hospitalization in patients with symptomatic HFpEF (EF ≥45%) (98). However, a reduction in HF readmissions was observed with spironolactone, and a post hoc analysis revealed a significant reduction in the rate of the primary outcome with spironolactone compared with placebo among patients who were enrolled according to elevated natriuretic peptide levels, with important regional variations in the trial (99). Roughly half of the patients were enrolled in the Americas with the other half in eastern Europe. The latter group displayed very low event rates raising questions with the veracity of the diagnosis of HFpEF, and in a post hoc analysis restricted to the Americas, spironolactone reduced the primary endpoint compared with placebo. Other novel MRAs such as finerenone hold promise in HFpEF and are under investigation (NCT04435626). Patients with HF and have an EF ≥ 45% are recommended to be treated with an MRA (class IIb indication), usually in addition to a loop diuretic (100).
Dual angiotensin-neprilysin inhibitor
The dual angiotensin–neprilysin inhibitor sacubitril–valsartan was tested in the Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction (PARAGON-HF) trial (101). In this trial, angiotensin–neprilysin inhibition narrowly-missed showing a reduction in the frequency of the primary composite outcome of death from cardiovascular causes and total HF hospitalizations compared to treatment with valsartan only (rate ratio, 0.87; 95% confidence interval [CI], 0.75 to 1.01; p=0.06). Prespecified subgroup analyses suggest a possible greater benefit among individuals with EF below the median (≤57%) and among women (102; 103). In addition, a post hoc analysis found that patients with more recent HF hospitalization derived more benefit from sacubitril-valsartan, with no benefit among patients with no prior hospitalization (104).
Other Pharmacotherapies
Angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and beta-adrenergic antagonists are frequently used to treat comorbid conditions in HFpEF such as kidney disease and coronary artery disease, but evidence to support their use independent of these comorbidities is scant (7; 8). The data is particularly weak for beta-adrenergic antagonists (105), which have also been associated with adverse outcomes in HFpEF (106). A prespecified subgroup analyses of the SOLOIST-WHF trial (107) including participants with HFpEF revealed that the rate of cardiovascular death or hospitalization for HF was lower with the sodium–glucose cotransporter 2 (SGLT2) inhibitor sotagliflozin as compared with placebo. This effect is believed to be related in large part renal protective effects, and SGLT2 inhibitors are currently being tested in two large pivotal trials in HFpEF, the results of which are eagerly anticipated (NCT03619213 and NCT03057951).
Observational studies suggest benefit from statins in HFpEF (108), and these patients frequently have other indications for this class of drug. Numerous trials have evaluated whether treatments that augment nitric oxide signaling might be effective in HFpEF, including phosphodiesterase inhibitors (109), organic nitrates (110), inorganic nitrite (111), and stimulators of guanylate cyclase (112; 113), with unanimously disappointing results.
Life-style intervention targeting cardiometabolic risk
Exercise training has been shown to improve exercise capacity as well as quality of life in patients with HFpEF (90; 92). There appears to be no difference in moderate intensity continuous and interval training regimens, and beneficial effects are difficult to maintain chronically (92). Intentional weight loss may improve morbidity and mortality in HFpEF given favorable effects on hemodynamics, heart rate and blood pressure (114). A recent randomized clinical trial found that short-term, modest weight loss induced by caloric restriction improved peak oxygen consumption (peak VO2) in obese patients with HFpEF (90). Clinical trials evaluating pharmacologic weight loss as a treatment for HFpEF are currently underway (NCT04788511, NCT04847557).
Investigational Device-based therapies
Devices to reduce LA pressure with a percutaneously implanted intra-atrial septostomy device has been shown to improve exercise hemodynamics, symptoms and exercise capacity in patients with HFpEF (115–118) and are currently being evaluated in ongoing clinical trials (NCT03499236, NCT03088033). The interatrial shunt device may also improve pulmonary vascular function in patients without significant preexisting pulmonary vascular disease (119). As pericardial restraint and enhanced ventricular interdependence contribute to elevation in filling pressures in many patients with HFpEF, percutaneous pericardial resection is another potential treatment that has shown promise in HFpEF (120; 121), and is currently under investigation in HFpEF (NCT03923673). A single-center study is on-going to investigate the efficacy of pacemakers to treat chronotropic incompetence in patients with HFpEF (NCT02145351). Blockade of the greater splanchnic nerve has been shown to effectively reduce cardiac filling pressures in HFrEF (122). A multicenter trial is underway to evaluate the effects of greater splanchnic nerve ablation in HFpEF (NCT04592445).
Phenotype-specific Approach
The failure of clinical trials to identify effective treatments using a “one size fits-all” approach may be at least partly explained by heterogeneity in the underlying pathophysiological mechanisms of HFpEF, as described above (7). Accordingly, it is hoped that better phenotyping of patients based upon their predominant pathophysiologic abnormalities may enable more individually tailored therapy (Figure 2). Several early phase trials have begun to target more specific phenotypes, including weight loss for obese HFpEF (90), levosimendan for PH/RVD (123), nitrite (124; 125), pericardiotomy (120; 121), and atrial septostomy for patients with high LV filling pressures during exercise (115), and inorganic nitrite for peripheral limitations (126). While this approach holds great promise, there is as yet no universally-accepted method by which different HFpEF phenogroups should be categorized for diagnostic and therapeutic purposes, and there is marked overlap between groups shown in Figure 2 (127; 128).
Figure 2:
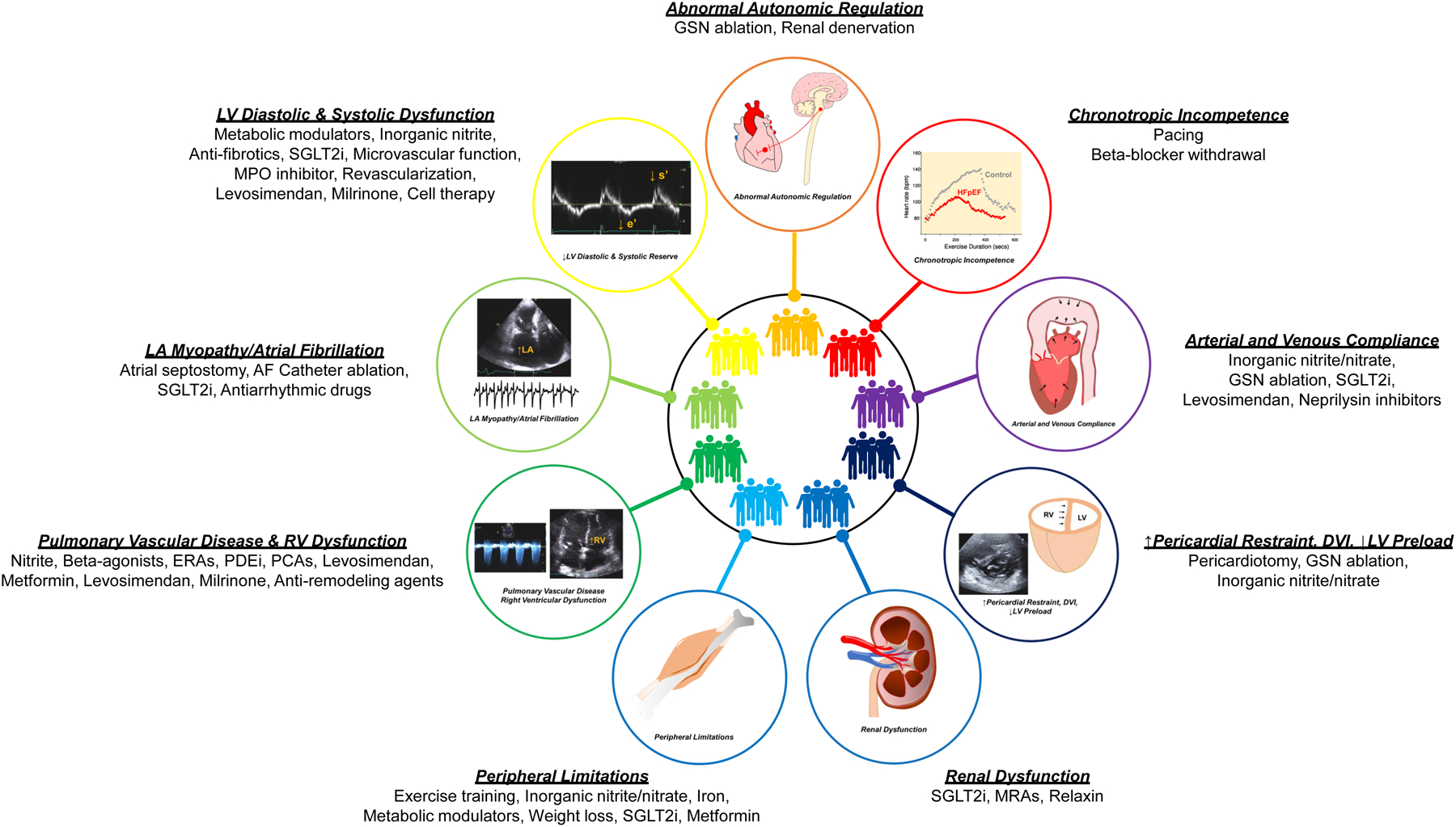
Potential Treatment according to HFpEF phenogroups. Abbreviations: AF, atrial fibrillation; ERAs, endothelin receptor antagonists; GSN, greater splanchnic nerve; HFpEF, heart failure with preserved ejection fraction; MPO, myeloperoxidase; MRA, mineralocorticoid receptor antagonists; PCAs, prostacyclin analogues; PDEi, phosphodiesterase inhibitor; SGLT-2i, sodium-glucose transporter-2 inhibitor; Other abbreviations are as in Figure 1.
CONCLUSION
The prevalence of HFpEF has grown to epidemic proportions and no single effective treatment for HFpEF has yet been identified that clearly improves outcomes. The lack of therapeutic options is largely related to the complexity and heterogeneity within the HFpEF syndrome, limiting “one size fits-all” approaches that have proven so effective in HFrEF. Limitations in ventricular diastolic and systolic function, LA myopathy, ventricular-vascular uncoupling, pulmonary vascular disease and RVD, altered venous capacitance, and abnormalities in the periphery including the vasculature, endothelium, autonomics, fat, and skeletal muscle all play a significant but variable role within the individual patient. Current treatment of HFpEF is aimed at volume control with diuretics, consideration for use of MRA, and management of comorbidities and lifestyle modifications, including exercise training and weight loss. Future study should investigate whether novel therapies aimed at specific HFpEF pathophysiologic phenotypes can improve outcomes and quality of life in adequately powered randomized clinical trials.
FUNDING
BAB is supported by R01 HL128526, from the National Institutes of Health. FHV is supported by the Special Research Fund (BOF) of Hasselt University (BOF19PD04). KO is supported by Japan Heart Fundation / Bayer Yakuhin Research Grant Abroad and the JSPS Overseas Research Fellowships from the Japan Society for the Promotion of Science.
Footnotes
DISCLOSURE STATEMENT
None
LITERATURE CITED
- 1.Virani SS, Alonso A, Benjamin EJ, et al. 2020. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 141:e139–e596 [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, et al. 2006. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355:251–9 [DOI] [PubMed] [Google Scholar]
- 3.Shah SJ, Borlaug BA, Kitzman DW, et al. 2020. Research Priorities for Heart Failure With Preserved Ejection Fraction: National Heart, Lung, and Blood Institute Working Group Summary. Circulation 141:1001–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsao CW, Lyass A, Enserro D, et al. 2018. Temporal Trends in the Incidence of and Mortality Associated With Heart Failure With Preserved and Reduced Ejection Fraction. JACC Heart Fail 6:678–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy YNV, Rikhi A, Obokata M, et al. 2020. Quality of life in heart failure with preserved ejection fraction: importance of obesity, functional capacity, and physical inactivity. Eur J Heart Fail 22:1009–18 [DOI] [PubMed] [Google Scholar]
- 6.Shah KS, Xu H, Matsouaka RA, et al. 2017. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J Am Coll Cardiol 70:2476–86 [DOI] [PubMed] [Google Scholar]
- 7.Borlaug BA. 2020. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol 17:559–73 [DOI] [PubMed] [Google Scholar]
- 8.Pfeffer MA, Shah AM, Borlaug BA. 2019. Heart Failure With Preserved Ejection Fraction In Perspective. Circ Res 124:1598–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borlaug BA. 2014. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 11:507–15 [DOI] [PubMed] [Google Scholar]
- 10.Mishra S, Kass DA. 2021. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat Rev Cardiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obokata M, Olson TP, Reddy YNV, et al. 2018. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J 39:2810–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy YNV, Borlaug BA. 2021. Pulmonary Hypertension in Left Heart Disease. Clin Chest Med 42:39–58 [DOI] [PubMed] [Google Scholar]
- 13.Reddy YNV, Obokata M, Wiley B, et al. 2019. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J 40:3721–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bers DM. 2008. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70:23–49 [DOI] [PubMed] [Google Scholar]
- 15.Selby DE, Palmer BM, LeWinter MM, et al. 2011. Tachycardia-induced diastolic dysfunction and resting tone in myocardium from patients with a normal ejection fraction. J Am Coll Cardiol 58:147–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frisk M, Le C, Shen X, et al. 2021. Etiology-Dependent Impairment of Diastolic Cardiomyocyte Calcium Homeostasis in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 77:405–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilfoil PJ, Lotteau S, Zhang R, et al. 2020. Distinct features of calcium handling and β-adrenergic sensitivity in heart failure with preserved versus reduced ejection fraction. J Physiol 598:5091–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy YNV, Andersen MJ, Obokata M, et al. 2017. Arterial Stiffening With Exercise in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol 70:136–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber T, Chirinos JA. 2018. Pulsatile arterial haemodynamics in heart failure. Eur Heart J 39:3847–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hay I, Rich J, Ferber P, et al. 2005. Role of impaired myocardial relaxation in the production of elevated left ventricular filling pressure. Am J Physiol Heart Circ Physiol 288:H1203–8 [DOI] [PubMed] [Google Scholar]
- 21.Borlaug BA, Jaber WA, Ommen SR, et al. 2011. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart 97:964–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zile MR, Baicu CF, Gaasch WH. 2004. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 350:1953–9 [DOI] [PubMed] [Google Scholar]
- 23.Methawasin M, Strom JG, Slater RE, et al. 2016. Experimentally Increasing the Compliance of Titin Through RNA Binding Motif-20 (RBM20) Inhibition Improves Diastolic Function In a Mouse Model of Heart Failure With Preserved Ejection Fraction. Circulation 134:1085–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Heerebeek L, Hamdani N, Falcão-Pires I, et al. 2012. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 126:830–9 [DOI] [PubMed] [Google Scholar]
- 25.Zile MR, Baicu CF, Ikonomidis JS, et al. 2015. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 131:1247–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng ACT, Delgado V, Borlaug BA, et al. 2021. Diabesity: the combined burden of obesity and diabetes on heart disease and the role of imaging. Nat Rev Cardiol 18:291–304 [DOI] [PubMed] [Google Scholar]
- 27.Rommel KP, von Roeder M, Latuscynski K, et al. 2016. Extracellular Volume Fraction for Characterization of Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol 67:1815–25 [DOI] [PubMed] [Google Scholar]
- 28.Borlaug BA, Lam CS, Roger VL, et al. 2009. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol 54:410–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraigher-Krainer E, Shah AM, Gupta DK, et al. 2014. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol 63:447–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan YT, Wenzelburger F, Lee E, et al. 2009. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol 54:36–46 [DOI] [PubMed] [Google Scholar]
- 31.Shah AM, Claggett B, Sweitzer NK, et al. 2015. Prognostic Importance of Impaired Systolic Function in Heart Failure With Preserved Ejection Fraction and the Impact of Spironolactone. Circulation 132:402–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorimachi H, Burkhoff D, Verbrugge FH, et al. inpress 2021. Obesity, Venous Capacitance, and Venous Compliance in Heart Failure with Preserved Ejection Fraction. Eur J Heart Fail [DOI] [PubMed] [Google Scholar]
- 33.Borlaug BA, Kane GC, Melenovsky V, et al. 2016. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J 37:3293–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borlaug BA, Olson TP, Lam CS, et al. 2010. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol 56:845–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phan TT, Abozguia K, Nallur Shivu G, et al. 2009. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol 54:402–9 [DOI] [PubMed] [Google Scholar]
- 36.Backhaus SJ, Lange T, George EF, et al. 2021. Exercise-Stress Real-time Cardiac Magnetic Resonance Imaging for Non-Invasive Characterisation of Heart Failure with Preserved Ejection Fraction: The HFpEF Stress Trial. Circulation [DOI] [PubMed] [Google Scholar]
- 37.Melenovsky V, Borlaug BA, Rosen B, et al. 2007. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol 49:198–207 [DOI] [PubMed] [Google Scholar]
- 38.Melenovsky V, Hwang SJ, Redfield MM, et al. 2015. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail 8:295–303 [DOI] [PubMed] [Google Scholar]
- 39.Reddy YNV, Obokata M, Egbe A, et al. 2019. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur J Heart Fail 21:891–900 [DOI] [PubMed] [Google Scholar]
- 40.Triposkiadis F, Pieske B, Butler J, et al. 2016. Global left atrial failure in heart failure. Eur J Heart Fail 18:1307–20 [DOI] [PubMed] [Google Scholar]
- 41.Freed BH, Daruwalla V, Cheng JY, et al. 2016. Prognostic Utility and Clinical Significance of Cardiac Mechanics in Heart Failure With Preserved Ejection Fraction: Importance of Left Atrial Strain. Circ Cardiovasc Imaging 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santos AB, Roca GQ, Claggett B, et al. 2016. Prognostic Relevance of Left Atrial Dysfunction in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail 9:e002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Telles F, Nanayakkara S, Evans S, et al. 2019. Impaired left atrial strain predicts abnormal exercise haemodynamics in heart failure with preserved ejection fraction. Eur J Heart Fail 21:495–505 [DOI] [PubMed] [Google Scholar]
- 44.Reddy YNV, Obokata M, Verbrugge FH, et al. 2020. Atrial Dysfunction in Patients With Heart Failure With Preserved Ejection Fraction and Atrial Fibrillation. J Am Coll Cardiol 76:1051–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verbrugge FH, Guazzi M, Testani JM, et al. 2020. Altered Hemodynamics and End-Organ Damage in Heart Failure: Impact on the Lung and Kidney. Circulation 142:998–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phan TT, Abozguia K, Shivu GN, et al. 2009. Increased atrial contribution to left ventricular filling compensates for impaired early filling during exercise in heart failure with preserved ejection fraction. J Card Fail 15:890–7 [DOI] [PubMed] [Google Scholar]
- 47.Tamargo M, Obokata M, Reddy YNV, et al. 2020. Functional mitral regurgitation and left atrial myopathy in heart failure with preserved ejection fraction. Eur J Heart Fail 22:489–98 [DOI] [PubMed] [Google Scholar]
- 48.Borlaug BA, Reddy YNV. 2019. The Role of the Pericardium in Heart Failure: Implications for Pathophysiology and Treatment. JACC Heart Fail 7:574–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lam CS, Rienstra M, Tay WT, et al. 2017. Atrial Fibrillation in Heart Failure With Preserved Ejection Fraction: Association With Exercise Capacity, Left Ventricular Filling Pressures, Natriuretic Peptides, and Left Atrial Volume. JACC Heart Fail 5:92–8 [DOI] [PubMed] [Google Scholar]
- 50.Obokata M, Reddy YNV, Melenovsky V, et al. 2019. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J 40:689–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zakeri R, Chamberlain AM, Roger VL, et al. 2013. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation 128:1085–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guazzi M, Naeije R. 2017. Pulmonary Hypertension in Heart Failure: Pathophysiology, Pathobiology, and Emerging Clinical Perspectives. J Am Coll Cardiol 69:1718–34 [DOI] [PubMed] [Google Scholar]
- 53.Gorter TM, Obokata M, Reddy YNV, et al. 2018. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J 39:2825–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lai YC, Wang L, Gladwin MT. 2019. Insights into the pulmonary vascular complications of heart failure with preserved ejection fraction. J Physiol 597:1143–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vanderpool RR, Saul M, Nouraie M, et al. 2018. Association Between Hemodynamic Markers of Pulmonary Hypertension and Outcomes in Heart Failure With Preserved Ejection Fraction. JAMA Cardiol 3:298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fayyaz AU, Edwards WD, Maleszewski JJ, et al. 2018. Global Pulmonary Vascular Remodeling in Pulmonary Hypertension Associated With Heart Failure and Preserved or Reduced Ejection Fraction. Circulation 137:1796–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gorter TM, Hoendermis ES, van Veldhuisen DJ, et al. 2016. Right ventricular dysfunction in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Heart Fail 18:1472–87 [DOI] [PubMed] [Google Scholar]
- 58.Melenovsky V, Hwang SJ, Lin G, et al. 2014. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 35:3452–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olson TP, Johnson BD, Borlaug BA. 2016. Impaired Pulmonary Diffusion in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 4:490–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fermoyle CC, Stewart GM, Borlaug BA, et al. 2021. Simultaneous Measurement of Lung Diffusing Capacity and Pulmonary Hemodynamics Reveals Exertional Alveolar-Capillary Dysfunction in Heart Failure with Preserved Ejection Fraction. J Am Heart Assoc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoeper MM, Meyer K, Rademacher J, et al. 2016. Diffusion Capacity and Mortality in Patients With Pulmonary Hypertension Due to Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 4:441–9 [DOI] [PubMed] [Google Scholar]
- 62.Chirinos JA, Bhattacharya P, Kumar A, et al. 2019. Impact of Diabetes Mellitus on Ventricular Structure, Arterial Stiffness, and Pulsatile Hemodynamics in Heart Failure With Preserved Ejection Fraction. J Am Heart Assoc 8:e011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawaguchi M, Hay I, Fetics B, et al. 2003. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation 107:714–20 [DOI] [PubMed] [Google Scholar]
- 64.Schwartzenberg S, Redfield MM, From AM, et al. 2012. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol 59:442–51 [DOI] [PubMed] [Google Scholar]
- 65.Akiyama E, Sugiyama S, Matsuzawa Y, et al. 2012. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol 60:1778–86 [DOI] [PubMed] [Google Scholar]
- 66.Paulus WJ, Tschöpe C. 2013. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62:263–71 [DOI] [PubMed] [Google Scholar]
- 67.Sanders-van Wijk S, Tromp J, Beussink-Nelson L, et al. 2020. Proteomic Evaluation of the Comorbidity-Inflammation Paradigm in Heart Failure With Preserved Ejection Fraction: Results From the PROMIS-HFpEF Study. Circulation 142:2029–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahmad A, Corban MT, Toya T, et al. 2020. Coronary microvascular dysfunction is associated with exertional haemodynamic abnormalities in patients with heart failure with preserved ejection fraction. Eur J Heart Fail [DOI] [PubMed] [Google Scholar]
- 69.Shah SJ, Lam CSP, Svedlund S, et al. 2018. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J 39:3439–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang JH, Obokata M, Reddy YNV, et al. 2020. Endothelium-dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur J Heart Fail 22:432–41 [DOI] [PubMed] [Google Scholar]
- 71.Obokata M, Reddy YNV, Melenovsky V, et al. 2018. Myocardial Injury and Cardiac Reserve in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol 72:29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abudiab MM, Redfield MM, Melenovsky V, et al. 2013. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail 15:776–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Borlaug BA, Melenovsky V, Russell SD, et al. 2006. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation 114:2138–47 [DOI] [PubMed] [Google Scholar]
- 74.Sarma S, Stoller D, Hendrix J, et al. 2020. Mechanisms of Chronotropic Incompetence in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail 13:e006331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sarma S, Howden E, Lawley J, et al. 2021. Central Command and the Regulation of Exercise Heart Rate Response in Heart Failure With Preserved Ejection Fraction. Circulation 143:783–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Funakoshi K, Hosokawa K, Kishi T, et al. 2014. Striking volume intolerance is induced by mimicking arterial baroreflex failure in normal left ventricular function. J Card Fail 20:53–9 [DOI] [PubMed] [Google Scholar]
- 77.Fudim M, Sobotka PA, Dunlap ME. 2021. Extracardiac Abnormalities of Preload Reserve: Mechanisms Underlying Exercise Limitation in Heart Failure with Preserved Ejection Fraction, Autonomic Dysfunction, and Liver Disease. Circ Heart Fail 14:e007308. [DOI] [PubMed] [Google Scholar]
- 78.Bhella PS, Prasad A, Heinicke K, et al. 2011. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail 13:1296–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dhakal BP, Malhotra R, Murphy RM, et al. 2015. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail 8:286–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haykowsky MJ, Brubaker PH, John JM, et al. 2011. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol 58:265–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Houstis NE, Eisman AS, Pappagianopoulos PP, et al. 2018. Exercise Intolerance in Heart Failure With Preserved Ejection Fraction: Diagnosing and Ranking Its Causes Using Personalized O(2) Pathway Analysis. Circulation 137:148–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kitzman DW, Nicklas B, Kraus WE, et al. 2014. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol 306:H1364–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kumar AA, Kelly DP, Chirinos JA. 2019. Mitochondrial Dysfunction in Heart Failure With Preserved Ejection Fraction. Circulation 139:1435–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Molina AJ, Bharadwaj MS, Van Horn C, et al. 2016. Skeletal Muscle Mitochondrial Content, Oxidative Capacity, and Mfn2 Expression Are Reduced in Older Patients With Heart Failure and Preserved Ejection Fraction and Are Related to Exercise Intolerance. JACC Heart Fail 4:636–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koepp KE, Obokata M, Reddy YNV, et al. 2020. Hemodynamic and Functional Impact of Epicardial Adipose Tissue in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 8:657–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Obokata M, Reddy YNV, Pislaru SV, et al. 2017. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure With Preserved Ejection Fraction. Circulation 136:6–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Packer M, Lam CSP, Lund LH, et al. 2020. Characterization of the inflammatory-metabolic phenotype of heart failure with a preserved ejection fraction: a hypothesis to explain influence of sex on the evolution and potential treatment of the disease. Eur J Heart Fail 22:1551–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rao VN, Zhao D, Allison MA, et al. 2018. Adiposity and Incident Heart Failure and its Subtypes: MESA (Multi-Ethnic Study of Atherosclerosis). JACC Heart Fail 6:999–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sorimachi H, Obokata M, Takahashi N, et al. 2020. Pathophysiologic importance of visceral adipose tissue in women with heart failure and preserved ejection fraction. Eur Heart J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kitzman DW, Brubaker P, Morgan T, et al. 2016. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA 315:36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pandey A, Parashar A, Kumbhani D, et al. 2015. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail 8:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mueller S, Winzer EB, Duvinage A, et al. 2021. Effect of High-Intensity Interval Training, Moderate Continuous Training, or Guideline-Based Physical Activity Advice on Peak Oxygen Consumption in Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA 325:542–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hwang SJ, Melenovsky V, Borlaug BA. 2014. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol 63:2817–27 [DOI] [PubMed] [Google Scholar]
- 94.Packer DL, Piccini JP, Monahan KH, et al. 2021. Ablation Versus Drug Therapy for Atrial Fibrillation in Heart Failure: Results From the CABANA Trial. Circulation 143:1377–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sugumar H, Nanayakkara S, Vizi D, et al. 2021. A prospective STudy using invAsive haemodynamic measurements foLLowing catheter ablation for AF and early HFpEF: STALL AF-HFpEF. Eur J Heart Fail [DOI] [PubMed] [Google Scholar]
- 96.Adamson PB, Abraham WT, Bourge RC, et al. 2014. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail 7:935–44 [DOI] [PubMed] [Google Scholar]
- 97.Abraham WT, Adamson PB, Bourge RC, et al. 2011. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 377:658–66 [DOI] [PubMed] [Google Scholar]
- 98.Pitt B, Pfeffer MA, Assmann SF, et al. 2014. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 370:1383–92 [DOI] [PubMed] [Google Scholar]
- 99.Pfeffer MA, Claggett B, Assmann SF, et al. 2015. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 131:34–42 [DOI] [PubMed] [Google Scholar]
- 100.Yancy CW, Jessup M, Bozkurt B, et al. 2017. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 136:e137–e61 [DOI] [PubMed] [Google Scholar]
- 101.Solomon SD, McMurray JJV, Anand IS, et al. 2019. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med 381:1609–20 [DOI] [PubMed] [Google Scholar]
- 102.McMurray JJV, Jackson AM, Lam CSP, et al. 2020. Effects of Sacubitril-Valsartan Versus Valsartan in Women Compared With Men With Heart Failure and Preserved Ejection Fraction: Insights From PARAGON-HF. Circulation 141:338–51 [DOI] [PubMed] [Google Scholar]
- 103.Solomon SD, Vaduganathan M, B LC, et al. 2020. Sacubitril/Valsartan Across the Spectrum of Ejection Fraction in Heart Failure. Circulation 141:352–61 [DOI] [PubMed] [Google Scholar]
- 104.Vaduganathan M, Claggett BL, Desai AS, et al. 2020. Prior Heart Failure Hospitalization, Clinical Outcomes, and Response to Sacubitril/Valsartan Compared With Valsartan in HFpEF. J Am Coll Cardiol 75:245–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cleland JGF, Bunting KV, Flather MD, et al. 2018. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J 39:26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Silverman DN, Plante TB, Infeld M, et al. 2019. Association of β-Blocker Use With Heart Failure Hospitalizations and Cardiovascular Disease Mortality Among Patients With Heart Failure With a Preserved Ejection Fraction: A Secondary Analysis of the TOPCAT Trial. JAMA Netw Open 2:e1916598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bhatt DL, Szarek M, Steg PG, et al. 2021. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med 384:117–28 [DOI] [PubMed] [Google Scholar]
- 108.Alehagen U, Benson L, Edner M, et al. 2015. Association Between Use of Statins and Mortality in Patients With Heart Failure and Ejection Fraction of ≥50. Circ Heart Fail 8:862–70 [DOI] [PubMed] [Google Scholar]
- 109.Redfield MM, Chen HH, Borlaug BA, et al. 2013. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 309:1268–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Redfield MM, Anstrom KJ, Levine JA, et al. 2015. Isosorbide Mononitrate in Heart Failure with Preserved Ejection Fraction. N Engl J Med 373:2314–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Borlaug BA, Anstrom KJ, Lewis GD, et al. 2018. Effect of Inorganic Nitrite vs Placebo on Exercise Capacity Among Patients With Heart Failure With Preserved Ejection Fraction: The INDIE-HFpEF Randomized Clinical Trial. JAMA 320:1764–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Armstrong PW, Lam CSP, Anstrom KJ, et al. 2020. Effect of Vericiguat vs Placebo on Quality of Life in Patients With Heart Failure and Preserved Ejection Fraction: The VITALITY-HFpEF Randomized Clinical Trial. JAMA 324:1512–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Udelson JE, Lewis GD, Shah SJ, et al. 2020. Effect of Praliciguat on Peak Rate of Oxygen Consumption in Patients With Heart Failure With Preserved Ejection Fraction: The CAPACITY HFpEF Randomized Clinical Trial. JAMA 324:1522–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reddy YNV, Anantha-Narayanan M, Obokata M, et al. 2019. Hemodynamic Effects of Weight Loss in Obesity: A Systematic Review and Meta-Analysis. JACC Heart Fail 7:678–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Feldman T, Mauri L, Kahwash R, et al. 2018. Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure With Preserved Ejection Fraction (REDUCE LAP-HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]): A Phase 2, Randomized, Sham-Controlled Trial. Circulation 137:364–75 [DOI] [PubMed] [Google Scholar]
- 116.Hasenfuß G, Hayward C, Burkhoff D, et al. 2016. A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): a multicentre, open-label, single-arm, phase 1 trial. Lancet 387:1298–304 [DOI] [PubMed] [Google Scholar]
- 117.Kaye DM, Hasenfuß G, Neuzil P, et al. 2016. One-Year Outcomes After Transcatheter Insertion of an Interatrial Shunt Device for the Management of Heart Failure With Preserved Ejection Fraction. Circ Heart Fail 9:e003662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shah SJ, Feldman T, Ricciardi MJ, et al. 2018. One-Year Safety and Clinical Outcomes of a Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure With Preserved Ejection Fraction in the Reduce Elevated Left Atrial Pressure in Patients With Heart Failure (REDUCE LAP-HF I) Trial: A Randomized Clinical Trial. JAMA Cardiol 3:968–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Obokata M, Reddy YNV, Shah SJ, et al. 2019. Effects of Interatrial Shunt on Pulmonary Vascular Function in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 74:2539–50 [DOI] [PubMed] [Google Scholar]
- 120.Borlaug BA, Carter RE, Melenovsky V, et al. 2017. Percutaneous Pericardial Resection: A Novel Potential Treatment for Heart Failure With Preserved Ejection Fraction. Circ Heart Fail 10:e003612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Borlaug BA, Schaff HV, Pochettino A, et al. 2018. Pericardiotomy Enhances Left Ventricular Diastolic Reserve With Volume Loading in Humans. Circulation 138:2295–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fudim M, Patel MR, Boortz-Marx R, et al. 2021. Splanchnic Nerve Block Mediated Changes in Stressed Blood Volume in Heart Failure. JACC Heart Fail 9:293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Burkhoff D, Borlaug BA, Shah SJ, et al. 2021. Levosimendan Improves Hemodynamics and Exercise Tolerance in PH-HFpEF: Results of the Randomized Placebo-Controlled HELP Trial. JACC Heart Fail [DOI] [PubMed] [Google Scholar]
- 124.Borlaug BA, Koepp KE, Melenovsky V. 2015. Sodium Nitrite Improves Exercise Hemodynamics and Ventricular Performance in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 66:1672–82 [DOI] [PubMed] [Google Scholar]
- 125.Borlaug BA, Melenovsky V, Koepp KE. 2016. Inhaled Sodium Nitrite Improves Rest and Exercise Hemodynamics in Heart Failure With Preserved Ejection Fraction. Circ Res 119:880–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Reddy YNV, Stewart GM, Obokata M, et al. 2021. Peripheral and pulmonary effects of inorganic nitrite during exercise in heart failure with preserved ejection fraction. Eur J Heart Fail [DOI] [PubMed] [Google Scholar]
- 127.Shah SJ, Kitzman DW, Borlaug BA, et al. 2016. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation 134:73–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sorimachi H, Omote K, Borlaug BA. 2021. Clinical Phenogroups in Heart Failure with Preserved Ejection Fraction Heart Fail Clin [DOI] [PubMed] [Google Scholar]


