Abstract
Rice (Oryza sativa) is an important food crop and has two subspecies, indica and japonica. Since the last century, four generations of rice varieties have been applied to rice production. Semi-dwarf rice, intra-subspecific hybrid rice, and inter-subspecific introgression rice were developed successively by genetic modification based on the first generation of tall rice. Each generation of rice has greater yield potential than the previous generation. Due to the stronger heterosis of indica-japonica hybrids, utilization of the inter-subspecific heterosis has long been of interest. However, indica-japonica hybrid sterility hinders the utilization of heterosis. In the past decades, indica-japonica hybrid sterility has been well understood. It is found that indica-japonica hybrid sterility is mainly controlled by six loci, S5, Sa, Sb, Sc, Sd, and Se. The indica-japonica hybrid sterility can be overcome by developing indica-compatible japonica lines (ICJLs) or wide-compatible indica lines (WCILs) using genes at the loci. With the understanding of the genetic and molecular basis of indica-japonica hybrid sterility and the development of molecular breeding technology, the development of indica-japonica hybrid rice has become possible. Recently, great progress has been made in breeding indica-japonica hybrid rice. Therefore, the indica-japonica hybrid rice will be the next generation of rice. It is expected that the indica-japonica hybrid rice will be widely applied in rice production in the near future.
Keywords: heterosis, hybrid sterility, subspecies, rice generation, hybrid rice
Introduction
Rice (O. sativa) is the best-known cultivated crop, providing staple food for more than half of the world’s population (Fukagawa and Ziska, 2019). The domestication of cultivated rice began about 10,000 years ago. During domestication, the cultivated rice differentiated into two varietal groups. Since the Han dynasty, the Chinese have recognized two rice varietal groups, Hsien (Xian) and Keng (Geng) (Ting, 1949; Wang et al., 2018). In the last century, Kato et al. (1928) divided O. sativa into two subspecies, indica and japonica. Ting (1949, 1957) named the two subspecies as hsien and keng. Recently, the two subspecies are considered to be Xian/Indica (XI) and Geng/Japonica (GJ) (Wang et al., 2018). The two subspecies of cultivated rice are two different ecological types, where indica rice is suitable for growing in tropical and subtropical areas at low latitudes and low altitudes, while japonica rice is suitable for growing in temperate areas at high latitudes or high altitudes (Chang, 1976; Khush, 1997).
Rice is a self-pollinating plant. The heterosis of self-pollinating plants is usually considered to be weak. In the 1970s, indica hybrid rice was successfully applied in China. Since the 1980s, indica hybrid rice has been widely planted in China, accounting for more than 50% of the rice planting area (Cheng et al., 2007). The indica hybrid rice shows strong heterosis, which plays an important role in increasing rice yield (Yuan and Virmani, 1988; Zhang et al., 2021). At the same time, japonica hybrid rice has also been successfully developed, and a large number of japonica hybrid varieties have been released (Li and Wu, 1991; Zheng et al., 2020). It is believed that inter-subspecific hybrids have stronger heterosis than intra-subspecific hybrids (Fu et al., 2014; Birchler, 2015). Rice inter-subspecific heterosis has long been attempted, but the sterility of inter-subspecific hybrids has hindered the utilization of heterosis (Cheng et al., 2007; Zhang, 2020). Therefore, the key to utilization of inter-subspecific heterosis is to overcome the hybrid sterility.
The Five Generations of Rice
Although domestication of the cultivated rice started about 10,000 years ago, purposeful genetic improvement of varieties began in the last century. According to the genetic basis of varieties, the cultivated rice can be divided into five generations (Zhang, 2019, 2020).
The first generation (1G) of rice is tall rice. Since rice was cultivated about 10,000 years ago, the cultivated rice had been tall rice. Tall rice with high stalk was easy to cover the weeds in the field. Before the 1960s, tall rice was suitable for cultivating without chemical fertilizer. Tall rice had many landraces, which were the result of local intuitive selection by farmers for a long time (Zeven, 1998). As the first generation of rice, tall varieties have become an important genetic resource for rice breeding.
The second generation (2G) of rice is semi-dwarf rice. Around the 1950s, farmers began using chemical fertilizer. The yield of tall rice was greatly improved, but with it came the lodging. From lodging tall varieties, semi-dwarf mutants were selected as new varieties. In 1956, for example, two farmers of Guangdong province of China selected a semi-dwarf mutant from the field of lodging tall rice variety Nan-te 16. This semi-dwarf mutant became a new semi-dwarf variety Ai-jiao-nan-te, which was soon widely planted in southern China (Hu, 1965). At the same time, some semi-dwarf germplasm resources were selected to develop new semi-dwarf varieties by hybridization breeding. In 1956, for example, a breeding team of Guangdong Academy of Agricultural Sciences of China selected Ai-zi-zhan as a semi-dwarf parent and developed new semi-dwarf varieties Guang-chang-ai in 1959, Zhen-zhu-ai in 1961, and Guang-liu-ai 4 in 1966 (Guangdong Academy of Agricultural Sciences, 1966). Meanwhile, a semi-dwarf variety with high yield potential, IR8, was released by International Rice Research Institute (IRRI) in 1966 (Peng et al., 1994; Khush, 2001). The semi-dwarf varieties were bred by incorporating a recessive dwarf gene, sd1, to reduce plant height (Suh and Hue, 1978; Khush, 2001).
The third generation (3G) of rice is intra-subspecific hybrid rice, including indica hybrid rice and japonica hybrid rice. In the 1970s, indica hybrid rice was developed in China by a group of scientists led by Longping Yuan. Since then, the indica hybrid rice has been rapidly applied to production because of its strong heterosis (Yuan and Virmani, 1988). Meanwhile, japonica hybrid rice has also been developed and applied in rice production (Shiniyo, 1969; Li and Wu, 1991; Zheng et al., 2020).
The fourth generation (4G) of rice is inter-subspecific introgression rice, including japonica-introgressive indica rice and indica-introgressive japonica rice. Since the 1970s, restorer genes have been transferred from indica to japonica to develop japonica restorer lines for japonica hybrid rice. In the 1980s, the finding of S5-n gene (Ikehashi and Araki, 1986) and the “new plant type” program of IRRI (Peng et al., 2008) promoted the hybridization breeding between indica and japonica rice. Since then, more and more inter-subspecific introgression varieties have been developed and applied in rice production (Cheng et al., 2007; Ma et al., 2007; Lin et al., 2016).
The fifth generation (5G) of rice is inter-subspecific indica-japonica hybrid rice. The next generation rice has heterosis between indica and japonica subspecies, which will greatly improve the yield potential (Zhang, 2020). It is worth noting that many so-called indica-japonica hybrid varieties released are actually inter-subspecific introgression varieties that are 4G rice (Ma et al., 2007; Lin et al., 2016; Zhu et al., 2020).
Since the second half of the last century, rice breeding has developed rapidly. With the utilization of new genetic resources and new breeding techniques, the genetic basis of varieties has changed more and more. The yield potential of each generation of varieties has been greatly improved.
Genetic Basis of Indica-Japonica Hybrid Sterility
Reproductive isolation usually appears in inter-specific and inter-subspecific hybrids of plants. Reproductive isolation can occur at the prezygotic and postzygotic stages. Postzygotic reproductive isolation usually shows hybrid lethality, hybrid necrosis/weakness and hybrid sterility (Baack et al., 2015; Ouyang and Zhang, 2018). In the past decades, about 50 loci related to reproductive isolation of the genus Oryza have been identified (Ouyang and Zhang, 2013, 2018; Guo et al., 2016; Li et al., 2020). In indica-japonica crosses, reproductive isolation usually shows hybrid sterility. Among the loci for reproductive isolation of Oryza, only some of the loci are responsible for the hybrid sterility of indica-japonica crosses (Zhang, 2020).
In indica-japonica hybrids, female or embryo sac sterility is controlled by the S5 locus. At the locus, indica varieties usually have S5-i allele, while japonica varieties usually have S5-j allele. The interaction of S5-i and S5-j in indica-japonica hybrids causes the abortion of female gametes with the S5-j allele (Ikehashi and Araki, 1986). By genetic mapping, the S5 locus was located on chromosome 6 (Ikehashi and Araki, 1986; Yanagihara et al., 1995). Furthermore, the S5 gene was cloned and functionally analyzed (Chen et al., 2008; Yang et al., 2012). In the hybrids of wide indica-japonica crosses, female sterility is usually under the control of the S5 locus (Ikehashi and Araki, 1986; Song et al., 2005).
For male or pollen sterility of indica-japonica hybrids, five loci, Sa, Sb, Sc, Sd, and Se, were identified in wide indica-japonica crosses. At the loci, indica varieties usually have S-i allele, while japonica varieties usually have S-j allele. In indica-japonica hybrids, the interaction between S-i and S-j at the loci leads to the abortion of male gametes with the S-j allele. The male sterility shows two types of abortive pollens. The empty abortive pollen is caused by the Sa locus, while the stained abortive pollen is caused by the Sb, Sc, Sd, and Se loci. The degree of pollen sterility in indica-japonica hybrids depends on the number of heterozygous loci (Zhang and Lu, 1989, 1993, 1996; Zhang et al., 1993, 1994). By molecular mapping, the Sa, Sb, Sc, Sd, and Se loci were located on chromosomes 1, 5, 3, 1, and 12, respectively (Zhuang et al., 1999, 2002; Zhang and Zhang, 2001; Su and Liu, 2003; Yang et al., 2004; Li et al., 2006, 2008; Zhu et al., 2008). Furthermore, the Sa and Sc genes have been cloned and functionally analyzed (Long et al., 2008; Shen et al., 2017; Xie et al., 2017). The genes for hybrid sterility S24, S35, and S25 (S36) are found to be located in the same chromosomal regions as Sb, Sd, and Se, respectively, which may be the same loci (Kubo and Yoshimura, 2001; Wen et al., 2007; Kubo et al., 2008; Zhao et al., 2011). Therefore, the male sterility of indica-japonica hybrids is usually under the control of the five loci (Zhang, 2020).
Neutral (n) allele is usually found at the loci for hybrid sterility in plants. When two alleles of a locus interact to cause sterility, there may be a third allele, n allele, at the locus, whose interaction with other two alleles can’t cause sterility (Rich, 1966). At S5 locus, some tropical japonica accessions carry S5-n allele except indica varieties having S5-i and japonica varieties having S5-j (Ikehashi and Araki, 1986). At the Sa, Sb, Sc, Sd, and Se loci, not only S-i, S-j, and S-n alleles can be divided, but the effects of alleles from different donors also vary quantitatively, resulting in the continuous variation of pollen sterility at a single locus (Zhang et al., 1993). The molecular basis of neutral alleles has been revealed by the cloned genes of S5 (Chen et al., 2008; Yang et al., 2012), Sa (Long et al., 2008; Xie et al., 2017), and Sc (Shen et al., 2017).
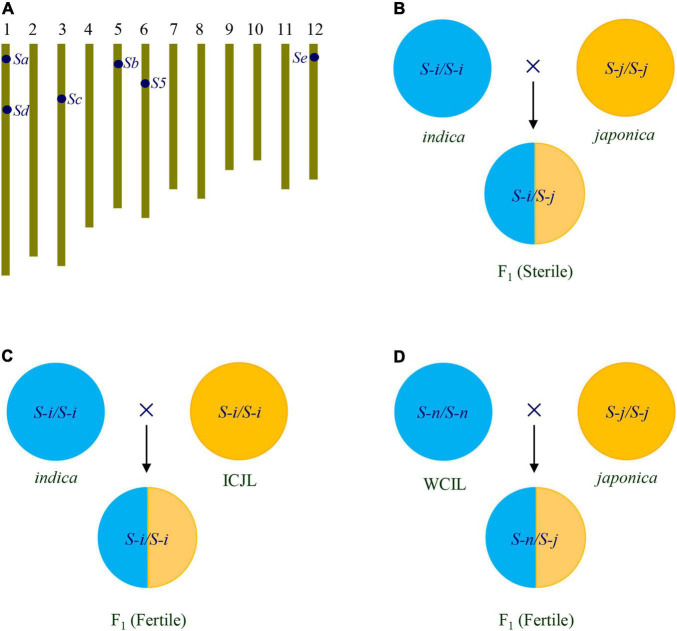
Summarily, six loci of hybrid sterility are usually found in indica-japonica crosses, S5 for female sterility, and Sa, Sb, Sc, Sd, and Se for male sterility (Table 1 and Figure 1A). Generally, indica varieties have S-i allele, japonica varieties have S-j allele, while some accessions have S-n allele at these loci. The genic model of the loci is the one-locus sporo-gametophytic interaction model. In indica-japonica hybrids, the allelic interaction of S-i and S-j causes the abortion of female gametes carrying the S-j allele of S5 locus, and the abortion of male gametes carrying the S-j allele of Sa, Sb, Sc, Sd, and Se loci, resulting in hybrid sterility (Figure 1B). In contrast, the interaction of S-n with S-i or S-j can’t cause the abortion of any gametes (Ikehashi and Araki, 1986; Zhang, 2020). The understanding of the genetic basis of indica-japonica hybrid sterility has laid the foundation for overcoming the hybrid sterility.
TABLE 1.
The loci for indica-japonica hybrid sterility.
| Sterility | Locus | Chr. | Molecular mechanism | References |
||
| Identification | Molecular mapping | Cloning and functional analysis | ||||
| Female | S5 | 6 | A killer-protector system encoded by three tightly linked genes | Ikehashi and Araki, 1986 | Yanagihara et al., 1995; Ji et al., 2005; Qiu et al., 2005 | Chen et al., 2008; Yang et al., 2012 |
| Male | Sa | 1 | A two-gene/three component interaction model | Zhang and Lu, 1989, 1993; Zhang et al., 1993, 1994 | Zhuang et al., 1999; Su and Liu, 2003 | Long et al., 2008; Xie et al., 2017 |
| Sb | 5 | Zhuang et al., 2002; Li et al., 2006 | ||||
| Sc | 3 | A model of gene dosage- dependent hybrid male sterility |
Zhang and Zhang, 2001; Yang et al., 2004 | Shen et al., 2017 | ||
| Sd | 1 | Li et al., 2008 | ||||
| Se | 12 | Zhu et al., 2008 | ||||
FIGURE 1.
Strategies for overcoming indica-japonica hybrid sterility. (A) Chromosomal location of the S5, Sa, Sb, Sc, Sd, and Se loci for indica-japonica hybrid sterility. (B) Hybrid of indica/japonica is sterile, which is caused by the interaction between S-i and S-j at the S5, Sa, Sb, Sc, Sd, and Se loci. (C) Hybrid of indica/ICJL is fertile due to ICJL carrying S-i at the S5, Sa, Sb, Sc, Sd, and Se loci. (D) Hybrid of WCIL/japonica is fertile due to WCIL carrying S-n at the S5, Sa, Sb, Sc, Sd, and Se loci. ICJL, indica-compatible japonica line; WCIL, wide-compatible indica line.
Strategies for Overcoming Indica-Japonica Hybrid Sterility
Based on the genetic basis of indica-japonica hybrid sterility, two types of breeding lines can be developed to overcome the hybrid sterility. They are indica-compatible japonica lines (ICJLs) (Zhang et al., 1994; Zhang and Lu, 1999) and wide-compatibility lines (WCLs). The breeding lines can be used to develop indica-japonica hybrid rice without hybrid sterility (Zhang, 2020).
The ICJLs can be developed by transferring S-i alleles at the S5, Sa, Sb, Sc, Sd, and Se loci from indica to japonica by backcrossing (Figure 1C). For example, a set of Taichung 65 (T65) isogenic F1-sterile lines (TISLs) having S-i alleles at the Sa, Sb, Sc, Sd, and Se loci were developed using a set of indica varieties as S-i donors in the genetic background of T65, a japonica variety. Then, the S-i alleles of these loci were pyramided together by crossing the TISLs. The hybrid pollen fertility of pyramiding lines with different genotypes at these loci were tested with indica and japonica testers. The results showed that as the number of S-i alleles at these loci in the pyramiding lines increased, the pollen fertility of hybrids with indica testers increased, while that of hybrids with japonica testers decreased (Zhang and Lu, 1996; Guo et al., 2016). Furthermore, by pyramiding the S5-n allele in the pyramiding lines with the S-i alleles of the Sb, Sc, Sd, and Se loci, several ICJLs with Sb-i, Sc-i, Sd-i, Se-i, and S5-n alleles in japonica genetic background were developed. The ICJLs showed normal or near normal pollen fertility and spikelet fertility in their hybrids with indica testers, but serious pollen sterility and spikelet sterility in the hybrids with japonica testers. Therefore, the indica-japonica hybrid sterility can be overcome in the crosses of ICJLs with indica varieties (Guo et al., 2016).
The WCLs can be developed by using the S-n alleles of six loci. WCLs with indica genetic background are wide-compatible indica lines (WCILs), and WCLs with japonica genetic background are wide-compatible japonica lines (WCJLs). The indica-japonica hybrid rice can be developed by using WCILs crossed with japonica lines, or by using WCJLs crossed with indica lines (Figure 1D).
Discussion
Since the 1970s, the intra-subspecific hybrid rice, including indica hybrid rice and japonica hybrid rice, has been developed. The cytoplasmic male sterility (CMS) system is a three-line system including CMS line, maintainer line and restorer line (Yuan and Virmani, 1988; Chen and Liu, 2014). The photoperiod/thermosensitive genic male-sterility (PTGMS) system is a two-line system including PTGMS line and restorer line (Shi, 1985; Ding et al., 2012; Zhou et al., 2012). The CMS system and the PTGMS system have been widely utilized in hybrid rice production. The application of genic male-sterility (GMS) materials in hybrid rice is the third-generation hybrid rice breeding technology (Deng et al., 2013; Wang and Deng, 2018; Song et al., 2021). A large number of breeding lines have been developed by the three generations of hybrid rice breeding technology. These techniques and breeding lines can be used to develop not only intra-subspecific hybrid rice but also inter-subspecific hybrid rice. For examples, the CMS lines, PTGMS lines, and GMS lines are male sterility lines (MSLs) that can also be used for the breeding of inter-subspecific hybrid rice. The restorer lines of CMS system, PTGMS system and GMS system can be used for the breeding of inter-subspecific hybrid rice after improving their compatibility. Thus, the breeding of intra-subspecific hybrid rice provides available breeding lines for the development of inter-subspecific hybrid rice.
Compared with intra-subspecific hybrid rice and inter-subspecific introgression rice, the development of inter-subspecific hybrid rice will face greater challenges. Firstly, the overcoming of indica-japonica hybrid sterility requires to pyramid multiple genes for compatibility. Secondly, male sterility and fertility restoration should be considered in hybrid rice breeding. Third, there may be some problems caused by the remote genetic backgrounds of the two subspecies (Lin et al., 2016). To meet the challenge, it is necessary to develop molecular breeding techniques. For the past two decades, we have been building a library of chromosome single-segment substitution lines (SSSLs) to construct a target chromosome-segment substitution platform for rice design (Zhang, 2021). The SSSL library was constructed by using forty-three accessions from seven species with AA genome as donors of chromosome segments in the genetic background of Huajingxian 74 (HJX74), an elite indica variety in South China. The HJX74-SSSL library consists of 2,360 SSSLs, which collects rich gene resources from donors with genetic diversity (Zhang et al., 2004; Xi et al., 2006; He et al., 2017; Zhao et al., 2019; Zhang, 2021). The HJX74-SSSLs have been used to detect QTLs for complex traits (Zhang et al., 2012; Yang et al., 2016, 2021a,2021b; Zhou et al., 2017; Tan et al., 2020, 2021, 2022; Pan et al., 2021), to clone genes of agronomic importance and to assess allelic variation (Wang et al., 2008, 2012, 2015; Teng et al., 2012; Sui et al., 2019; Zhang et al., 2020; Gao et al., 2021). Using the HJX74-SSSL library as platform for rice breeding by design, several CMS, maintainer and restorer lines have been developed (Dai et al., 2015, 2016; Luan et al., 2019). These results suggest that the target chromosome-segment substitution is an effective way to rice breeding by design (Zhang, 2021). Recently, the HJX74-SSSL library was used to develop WCILs. Through the restorer gene pyramiding, the WCILs will be developed into wide-compatible indica restorer lines (WCIRLs) as restorer lines of indica-japonica hybrid rice. Therefore, the target chromosome-segment substitution based on HJX74-SSSL platform provides technical support for the development of indica-japonica hybrid rice.
For a century, four generations of rice have provided a large number of elite varieties for rice production. With the changes of genetic basis, each generation of rice has greater yield potential than the previous generation. The application of new generation rice has greatly improved the productivity of modern rice. However, the intra-subspecific hybrid rice can only have intra-subspecific heterosis, and the inter-subspecific introgression rice can only utilize partial inter-subspecific heterosis. In comparison, the inter-subspecific indica-japonica hybrid rice can take advantage of complete inter-subspecific heterosis. Therefore, the utilization of heterosis between indica and japonica subspecies has been expected (Cheng et al., 2007; Zhang, 2020). With the understanding of the genetic and molecular basis of indica-japonica hybrid sterility and the development of molecular breeding techniques, it is now possible to develop indica-japonica hybrid rice. We are developing indica-japonica hybrid rice by crossing WCIRLs developed on the HJX74-SSSL platform with existing japonica CMS lines collected from japonica planting areas. Many indica-japonica hybrid rice combinations have been extensively tested in various rice planting areas of China. Our results showed that indica-japonica hybrid rice had stronger heterosis and higher yield potential. Therefore, indica-japonica hybrid rice will become the next generation of rice and will be widely applied in rice production in the near future.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
GZ wrote the manuscript independently.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
This research was funded by grants from the National Natural Science Foundation of China (91435207 and 91735304) and the Major Program of Transgenic New Variety Breeding of China (2014ZX08009-037B).
References
- Baack E., Melo M. C., Rieseberg L. H., Ortiz-Barrientos D. (2015). The origins of reproductive isolation in plants. New Phytol. 207 968–984. 10.1111/nph.13424 [DOI] [PubMed] [Google Scholar]
- Birchler J. A. (2015). Heterosis: the genetic basis of hybrid vigour. Nat. Plants. 1:15020. 10.1038/nplants.2015.20 [DOI] [PubMed] [Google Scholar]
- Chang T. T. (1976). The origin, evolution, cultivation, dissemination, and diversification of Asian and African rices. Euphytica 25 425–441. 10.1007/bf00041576 [DOI] [Google Scholar]
- Chen J., Ding J., Ouyang Y., Du H., Yang J., Cheng K., et al. (2008). A triallelic system of S5 is a major regulator of the reproductive barrier and compatibility of indica-japonica hybrids in rice. Proc. Natl. Acad. Sci. U.S.A. 105 11436–11441. 10.1073/pnas.0804761105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Liu Y. (2014). Male sterility and fertility restoration in crops. Annu. Rev. Plant Biol. 65 579–606. 10.1146/annurev-arplant-050213-040119 [DOI] [PubMed] [Google Scholar]
- Cheng S., Cao L., Zhuang J., Chen S., Zhan X., Fan Y., et al. (2007). Super hybrid rice breeding in China: Achievements and prospects. J. Integr. Plant Biol. 49 805–810. 10.16288/j.yczz.18-213 [DOI] [PubMed] [Google Scholar]
- Dai Z., Lu Q., Luan X., Cai J., Zhu H., Liu Z., et al. (2015). Development of a platform for breeding by design of CMS lines based on an SSSL library in rice (Oryza sativa L.). Euphytica 205 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z., Lu Q., Luan X., Ouyang L., Guo J., Liang J., et al. (2016). Development of a platform for breeding by design of CMS restorer lines based on an SSSL library in rice (Oryza sativa L.). Breed. Sci. 66 768–775. 10.1270/jsbbs.16044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., Wang H., Tang X., Zhou J., Chen H., He G., et al. (2013). Hybrid rice breeding welcomes a new era of molecular crop design. Sci. Sin. Vitae. 43 864–868. 10.1360/052013-299 [DOI] [Google Scholar]
- Ding J., Lu Q., Ouyang Y., Mao H., Zhang P., Yao J., et al. (2012). A long noncoding RNA regulates photoperiod-sensitive male sterility, an essential component of hybrid rice. Proc. Natl. Acad. Sci. U.S.A. 109 2654–2659. 10.1073/pnas.1121374109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D., Xiao M., Hayward A., Fu Y., Liu G., Jiang G., et al. (2014). Utilization of crop heterosis: a review. Euphytica 197 161–173. 10.1007/s10681-014-1103-7 [DOI] [Google Scholar]
- Fukagawa N. K., Ziska L. H. (2019). Rice: Importance for global nutrition. J. Nutr. Sci. Vitaminol. 65 S2–S3. 10.3177/jnsv.65.S2 [DOI] [PubMed] [Google Scholar]
- Gao M., He Y., Yin X., Zhong X., Yan B., Wu Y., et al. (2021). Ca2+ sensor-mediated ROS scavenging suppresses rice immunity and is exploited by a fungal effector. Cell 184 5391–5404. 10.1016/j.cell.2021.09.009 [DOI] [PubMed] [Google Scholar]
- Guangdong Academy of Agricultural Sciences. (1966). Preliminary summary of rice dwarf breeding in Guangdong Province. Acta Agron. Sin. 5 33–40. [Google Scholar]
- Guo J., Xu X., Li W., Zhu W., Zhu H., Liu Z., et al. (2016). Overcoming inter-subspecific hybrid sterility in rice by developing indica-compatible japonica lines. Sci. Rep. 6:26878. 10.1038/srep26878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N., Wu R., Pan X., Peng L., Sun K., Zou T., et al. (2017). Development and trait evaluation of chromosome single-segment substitution lines of O. meridionalis in the background of O. sativa. Euphytica 213:281. [Google Scholar]
- Hu Q. (1965). Experience of Ai-jiao-nan-te in producing 500 kilogram per mu. Hubei Agri. Sci. 1965 24–29. [Google Scholar]
- Ikehashi H., Araki H. (1986). Genetics of F1 sterility in remote crosses in rice. In Rice Genetics: Proceedings of the First Rice Genetics Symposium. Philippines: International Rice Research Institute, 119–130. [Google Scholar]
- Ji Q., Lu J., Chao Q., Gu M., Xu M. (2005). Delimiting a rice wide-compatibility gene S5n to a 50 kb region. Theor. Appl. Genet. 111 1495–1503. 10.1007/s00122-005-0078-0 [DOI] [PubMed] [Google Scholar]
- Kato S., Kosaka H., Hara S. (1928). On the affinity of rice varieties as shown by fertility of hybrid plants. Sci. Bull. Faculty Agric. Kyushu Univ. 3 132–147. [Google Scholar]
- Khush G. S. (1997). Origin, dispersal, cultivation and variation of rice. Plant Mol. Biol. 35 25–34. 10.1007/978-94-011-5794-0_3 [DOI] [PubMed] [Google Scholar]
- Khush G. S. (2001). Green revolution: The way forward. Nat. Rev. Genet. 2 815–822. 10.1038/35093585 [DOI] [PubMed] [Google Scholar]
- Kubo T., Yamagata Y., Eguchi M., Yoshimura A. (2008). A novel epistatic interaction at two loci causing hybrid male sterility in an inter-subspecific cross of rice (Oryza sativa L.). Genes Genet. Syst. 83 443–453. 10.1266/ggs.83.443 [DOI] [PubMed] [Google Scholar]
- Kubo T., Yoshimura A. (2001). Linkage analysis of an F1 sterility gene in Japonica/Indica cross of rice. Rice Genet. Newslett. 18 52–54. [Google Scholar]
- Li J., Zhou J., Zhang Y., Yang Y., Pu Q., Tao D. (2020). New insights into the nature of interspecific hybrid sterility in rice. Front. Plant Sci. 11:555572. 10.3389/fpls.2020.555572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Zeng R., Zhang Z., Ding X., Zhang G. (2006). Fine mapping of locus S-b for F1 pollen sterility in rice (Oryza sativa L.). Chinese Sci. Bull. 51 675–680. 10.1007/s11434-006-0675-6 [DOI] [Google Scholar]
- Li W., Zeng R., Zhang Z., Ding X., Zhang G. (2008). Identification and fine mapping of S-d, a new locus conferring the partial pollen sterility of intersubspecific F1 hybrids in rice (Oryza sativa L.). Theor. Appl. Genet. 116 915–922. 10.1007/s00122-008-0723-5 [DOI] [PubMed] [Google Scholar]
- Li Z., Wu J. (1991). The present situation and prospect of three-line hybrid japonica rice breeding in China. Hybrid Rice 15 13–16. [Google Scholar]
- Lin J.-R., Song X.-W., Wu M.-G., Cheng S.-H. (2016). Breeding technology innovation of indica–japonica super hybrid rice and varietal breeding. Sci. Agric. Sin. 49 207–218. [Google Scholar]
- Long Y., Zhao L., Niu B., Su J., Wu H., Chen Y., et al. (2008). Hybrid male sterility in rice controlled by interaction between divergent alleles of two adjacent genes. Proc. Natl. Acad. Sci. U.S.A. 105 18871–18876. 10.1073/pnas.0810108105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan X., Dai Z., Yang W., Tan Q., Lu Q., Guo J., et al. (2019). Breeding by design of CMS lines on the platform of SSSL library in rice. Mol. Breed 39:126. [Google Scholar]
- Ma R. R., Xu D. H., Wang X. Y., Yu S. M., Jin Q. Y., Ouyang Y. N., et al. (2007). Heterosis on plant morphology of Yongyou 6, an indica-japonica inter-subspecific super high-yielding hybrid rice. Chinese J. Rice Sci. 21 281–286. [Google Scholar]
- Ouyang Y., Zhang Q. (2013). Understanding reproductive isolation based on the rice model. Annu. Rev. Plant Biol. 64 111–135. 10.1146/annurev-arplant-050312-120205 [DOI] [PubMed] [Google Scholar]
- Ouyang Y., Zhang Q. (2018). The molecular and evolutionary basis of reproductive isolation in plants. J. Genet. Genomics. 45 613–620. 10.1016/j.jgg.2018.10.004 [DOI] [PubMed] [Google Scholar]
- Pan Z., Tan B., Cao G., Zheng R., Liu M., Zeng R., et al. (2021). Integrative QTL identification, fine mapping and candidate gene analysis of a major locus qLTG3a for seed low-temperature germinability in rice. Rice 14:103. 10.1186/s12284-021-00544-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S., Khush G. S., Cassman K. G. (1994). “Evolution of the new plant ideotype for increased yield potential,” in Breaking the Yield Barrier: Proceedings of a Workshop on Rice Yield Potential in Favorable Environments, ed. Cassman K. G. (Philippines: International Rice Research Institulte; ), 5–20. [Google Scholar]
- Peng S., Khush G. S., Virk P., Tang Q., Zou Y. (2008). Progress in ideotype breeding to increase rice yield potential. Field Crops Res. 108 32–38. 10.1016/j.fcr.2008.04.001 [DOI] [Google Scholar]
- Qiu S. Q., Liu K., Jiang J. X., Song X., Xu C. G., Li X. H., et al. (2005). Delimitation of the rice wide compatibility gene S5n to a 40-kb DNA fragment. Theor. Appl. Genet. 111 1080–1086. 10.1007/s00122-005-0033-0 [DOI] [PubMed] [Google Scholar]
- Rich C. M. (1966). Abortion of male and female gametes in the tomato determined by allelic interaction. Genetics 53 85–96. 10.1093/genetics/53.1.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R., Wang L., Liu X., Wu J., Jin W., Zhao X., et al. (2017). Genomic structural variation-mediated allelic suppression causes hybrid male sterility in rice. Nat. Commun. 8:1310. 10.1038/s41467-017-01400-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M. (1985). The discovery and preliminary studies of the photoperiod-sensitive recessive male sterile rice (Oryza sativa L. subsp. japonica). Sci. Agric. Sin. 2 44–48. [Google Scholar]
- Shiniyo C. (1969). Cytoplasmic-genetic male sterility in cultivated rice. Oryza sativa L. J. Genet. 44 149–156. 10.1266/jjg.44.149 [DOI] [Google Scholar]
- Song S., Wang T., Li Y., Hu J., Kan R., Qiu M., et al. (2021). A novel strategy for creating a new system of third-generation hybrid rice technology using a cytoplasmic sterility gene and a genic male-sterile gene. Plant Biotechnol. J. 19 251–260. 10.1111/pbi.13457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Qiu S., Xu C., Li X., Zhang Q. (2005). Genetic dissection of embryo sac fertility, pollen fertility, and their contributions to spikelet fertility of intersubspecific hybrids in rice. Theor. Appl. Genet. 110 205–211. 10.1007/s00122-004-1798-2 [DOI] [PubMed] [Google Scholar]
- Su J., Liu Y. (2003). Fine mapping and cloning of the gene S-a for F1 pollen sterility in cultivated rice (Oryza sativa L.). Mol. Plant Breed. 1 757–758. [Google Scholar]
- Suh H. S., Hue M. H. (1978). The segregation mode of plant height in the cross of rice varieties. XI. Linkage analysis of the semi-dwarfness of the rice variety ‘Tongil’. Korean J. Breed. 10 1–6. 10.1270/jsbbs1951.10.1 26081539 [DOI] [Google Scholar]
- Sui F., Zhao D., Zhu H., Gong Y., Tang Z., Huang X., et al. (2019). Map-based cloning of a new total loss-of-function allele of OsHMA3 causes high cadmium accumulation in rice grain. J. Exp. Bot. 70 2857–2871. 10.1093/jxb/erz093 [DOI] [PubMed] [Google Scholar]
- Tan Q., Wang C., Luan X., Zheng L., Ni Y., Yang W., et al. (2021). Dissection of closely linked QTLs controlling stigma exsertion rate in rice by substitution mapping. Theor. Appl. Genet. 134 1253–1262. 10.1007/s00122-021-03771-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Q., Zhu H., Liu H., Ni Y., Wu S., Luan X., et al. (2022). Fine mapping of QTLs for stigma exsertion rate from Oryza glaberrima by chromosome segment substitution. Rice Sci. 29 55–66. 10.1016/j.rsci.2021.12.005 [DOI] [Google Scholar]
- Tan Q., Zou T., Zheng M., Ni Y., Luan X., Li X., et al. (2020). Substitution mapping of the major quantitative trait loci controlling stigma exsertion rate from Oryza glumaepatula. Rice 13:37. 10.1186/s12284-020-00397-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng B., Zeng R., Wang Y., Liu Z., Zhang Z., Zhu H., et al. (2012). Detection of allelic variation at the Wx locus with single-segment substitution lines in rice (Oryza sativa L.). Mol. Breed. 30 583–595. 10.1007/s11032-011-9647-x [DOI] [Google Scholar]
- Ting Y. (1949). Origination of the rice cultivation in China. J. College Agric. Sun Yat-sen Univ. 7 11–24. [Google Scholar]
- Ting Y. (1957). The origin and evolution of cultivated rice in China. Acta Bot. Sin. 8 243–260. [Google Scholar]
- Wang E., Wang J., Zhu X., Hao W., Wang L., Li O., et al. (2008). Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nat. Genet. 40 1370–1374. 10.1038/ng.220 [DOI] [PubMed] [Google Scholar]
- Wang H., Deng X. W. (2018). Development of the “third-generation” hybrid rice in China. Genom. Proteom. Bioinf. 16 393–396. 10.1016/j.gpb.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Li S., Liu Q., Wu K., Zhang J., Wang S., et al. (2015). The OsSPL16-GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat. Genet. 47 949–955. 10.1038/ng.3352 [DOI] [PubMed] [Google Scholar]
- Wang S., Wu K., Yuan Q., Liu X., Liu Z., Lin X., et al. (2012). Control of grain size, shape and quality by OsSPL16 in rice. Nat. Genet. 44 950–954. 10.1038/ng.2327 [DOI] [PubMed] [Google Scholar]
- Wang W., Mauleon R., Hu Z., Chebotarov D., Tai S., Wu Z., et al. (2018). Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature 557 43–49. 10.1038/s41586-018-0063-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J., Zhang W., Jiang L., Chen L., Zhai H., Wan J. (2007). Two novel loci for pollen sterility in hybrids between the weedy strain Ludao and the japonica variety Akihikari of rice (Oryza sativa L.). Theor. Appl. Genet. 114 915–925. 10.1007/s00122-006-0489-6 [DOI] [PubMed] [Google Scholar]
- Xi Z., He F., Zeng R., Zhang Z., Ding X., Li W., et al. (2006). Development of a wide population of chromosome single-segment substitution lines in the genetic background of an elite cultivar of rice (Oryza sativa L.). Genome 49 476–484. 10.1139/g06-005 [DOI] [PubMed] [Google Scholar]
- Xie Y., Niu B., Long Y., Li G., Tang J., Zhang Y., et al. (2017). Suppression or knockout of SaF/SaM overcomes the Sa-mediated hybrid male sterility in rice. J. Integr. Plant Biol. 59 669–679. 10.1111/jipb.12564 [DOI] [PubMed] [Google Scholar]
- Yanagihara S., Mccouch S. R., Ishikawa K., Ogi Y., Maruyama K., Ikehashi H. (1995). Molecular analysis of the inheritance of the S-5 locus, conferring wide compatibility in Indica/Japonica hybrids of rice (Oryza sativa L.). Theor. Appl. Genet. 90 182–188. 10.1007/bf00222200 [DOI] [PubMed] [Google Scholar]
- Yang C., Chen Z., Zhuang C., Mei M., Liu Y. (2004). Genetic and physical fine-mapping of the Sc locus conferring indica-japonica hybrid sterility in rice (Oryza sativa L.). Chinese Sci. Bull. 49 1718–1721. 10.1360/04wc0197 [DOI] [Google Scholar]
- Yang J., Zhao X., Cheng K., Du H., Ouyang Y., Chen J., et al. (2012). A killer-protector system regulates both hybrid sterility and segregation distortion in rice. Science 337 1336–1340. 10.1126/science.1223702 [DOI] [PubMed] [Google Scholar]
- Yang T., Zhang S., Zhao J., Liu Q., Huang Z., Mao X., et al. (2016). Identification and pyramiding of QTLs for cold tolerance at the bud bursting and the seedling stages by use of single segment substitution lines in rice (Oryza sativa L.). Mol. Breed. 36:96. [Google Scholar]
- Yang W., Liang J., Hao Q., Luan X., Tan Q., Lin S., et al. (2021a). Fine mapping of two grain chalkiness QTLs sensitive to high temperature in rice. Rice 14:33. 10.1186/s12284-021-00476-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Xiong L., Liang J., Hao Q., Luan X., Tan Q., et al. (2021b). Substitution mapping of two closely linked QTLs controlling grain shape on rice chromosome 8. Rice 14:85. 10.1186/s12284-021-00526-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Virmani S. (1988). “Status of hybrid rice research and development,” in Hybrid rice, (Manila: Internationa Rice Research Institute; ), 7–24. [Google Scholar]
- Zeven A. C. (1998). Landraces: a review of definitions and classifications. Euphytica 104 127–139. [Google Scholar]
- Zhang G. (2019). Evolution and development of five generations of rice. J. South China Agri. Univ. 40 211–216. 10.1007/s11103-020-01037-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G. (2020). Prospects of utilization of inter-subspecific heterosis between indica and japonica rice. J. Integr. Agr. 19 1–10. 10.1016/s2095-3119(19)62843-1 [DOI] [Google Scholar]
- Zhang G. (2021). Target chromosome-segment substitution: A way to breeding by design in rice. Crop J. 9 658–668. 10.1016/j.cj.2021.03.001 [DOI] [Google Scholar]
- Zhang G., Lu Y. (1989). Genetic studies of the hybrid sterility in cultivated rice (Oryza sativa). I. Diallel analysis of the hybrid sterility among isogenic F1 sterile lines. Chinese J. Rice Sci. 3 97–101. [Google Scholar]
- Zhang G., Lu Y. (1993). Genetic studies of the hybrid sterility in cultivated rice (Oryza sativa). II. A genic model for F1 pollen sterility. Acta Genet. Sin. 20 222–228. [Google Scholar]
- Zhang G., Lu Y. (1996). “Genetics of F1 pollen sterility in Oryza sativa,” in Rice Genetics III, ed. Khush G. (Los Banos: International Rice Research Institute; ), 418–422. [Google Scholar]
- Zhang G., Lu Y. (1999). Breeding of the indica-compatible japonica lines and their use in the breeding of super-high-yield hybrid rice. Hybrid Rice 14 3–5. [Google Scholar]
- Zhang G., Lu Y., Liu G., Yang J., Zhang H. (1993). Genetic studies on the hybrid sterility in cultivated rice (Oryza sativa). III. Allele Differentiation of F1 pollen sterility in different types of varieties. Acta Genet. Sin. 20 541–551. [Google Scholar]
- Zhang G., Lu Y., Zhang H., Yang J., Liu G. (1994). Genetic studies on the hybrid sterility in cultivated rice (Oryza sativa). IV. Genotypes for F1 pollen sterility. Acta Genet. Sin. 21 34–41. [Google Scholar]
- Zhang G., Zeng R., Zhang Z., Ding X., Li W., Liu G., et al. (2004). The construction of a library of single segment substitution lines in rice (Oryza sativa L.). Rice Genet. Newsl. 21 85–87. [Google Scholar]
- Zhang S., Huang X., Han B. (2021). Understanding the genetic basis of rice heterosis: Advances and prospects. Crop J. 9 688–692. 10.1016/j.cj.2021.03.011 [DOI] [Google Scholar]
- Zhang S., Zhu L., Shen C., Ji Z., Zhang H., Zhang T., et al. (2020). Natural allelic variation in a modulator of auxin homeostasis improves grain yield and nitrogen use efficiency in rice. Plant Cell. 33 566–580. 10.1093/plcell/koaa037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yang J., Shan Z., Chen S., Qiao W., Zhu X., et al. (2012). Substitution mapping of QTLs for blast resistance with SSSLs in rice (Oryza sativa L.). Euphytica 184 141–150. 10.1007/s10681-011-0601-0 [DOI] [Google Scholar]
- Zhang Z., Zhang G. (2001). Fine mapping of the S-c locus and marker-assisted selection using PCR markers in rice. Acta Agron. Sin. 27 704–709. [Google Scholar]
- Zhao H., Sun L., Xiong T., Wang Z., Liao Y., Zou T., et al. (2019). Genetic characterization of the chromosome single-segment substitution lines of O. glumaepatula and O. barthii and identification of QTLs for yield-related traits. Mol. Breed. 39:51. [Google Scholar]
- Zhao Z., Zhu S., Zhang Y., Bian X., Wang Y., Jiang L., et al. (2011). Molecular analysis of an additional case of hybrid sterility in rice (Oryza sativa L.). Planta 233 485–494. 10.1007/s00425-010-1313-8 [DOI] [PubMed] [Google Scholar]
- Zheng W., Ma Z., Zhao M., Xiao M., Zhao J., Wang C., et al. (2020). Research and development strategies for hybrid japonica rice. Rice 13:36. 10.1186/s12284-020-00398-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Liu Q., Li J., Jiang D., Zhou L., Wu P., et al. (2012). Photoperiod- and thermo-sensitive genic male sterility in rice are caused by a point mutation in a novel noncoding RNA that produces a small RNA. Cell Res. 22 649–660. 10.1038/cr.2012.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Xie Y., Cai J., Liu C., Zhu H., Jiang R., et al. (2017). Substitution mapping of QTLs controlling seed dormancy using single segment substitution lines derived from multiple cultivated rice donors in seven cropping seasons. Theor. Appl. Genet. 130 1191–1205. 10.1007/s00122-017-2881-9 [DOI] [PubMed] [Google Scholar]
- Zhu K., Zhou Q., Shen Y., Yan J., Xu Y., Wang Z., et al. (2020). Agronomic and physiological performance of an indica–japonica rice variety with a high yield and high nitrogen use efficiency. Crop Sci. 60 1556–1568. 10.1002/csc2.20150 [DOI] [Google Scholar]
- Zhu W., Li W., Ding X., Zhang Z., Zeng R., Zhu H., et al. (2008). Preliminary identification of F1 pollen sterility gene S-e in Oryza sativa. J. South China Agri. Univ. 29 1–5. 10.15625/2615-9023/15888 [DOI] [Google Scholar]
- Zhuang C., Mei M., Zhang G., Lu Y. (2002). Chromosome mapping of the S-b locus for F1 pollen sterility in cultivated rice (Oryza sativa L.) with RAPD markers. Acta Genet. Sin. 29 700–705. [PubMed] [Google Scholar]
- Zhuang C., Zhang G., Mei M., Lu Y. (1999). Molecular mapping of the S-a locus for F1 pollen sterility in cultivated rice (Oryza sativa L.). Acta Genet. Sin. 26 213–218. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.