Abstract
Herbivore-induced plant volatiles (HIPVs), chemicals produced by plants infested by herbivorous insects, can act as kairomones that recruit natural enemies of the pest herbivore. Agrotis segetum (Denis and Schiffermüller) is a common, important pest of seedling cotton in Xinjiang Province, China, and the braconid Microplitis mediator (Haliday) is an important mortality factor of this pest’s larvae. In olfactometer tests, which included healthy foliage, infested foliage, or infested roots, M. mediator preferred A. segetum-infested cotton plants to healthy cotton plants. In GC-MS analyses of plant-emitted volatiles, we found that compounds emitted increased 14.9- and 13.3- fold after leaf infestation and root infestation, respectively, compared to healthy control plants. The volatiles were mainly p-xylene, nonanal, tetradecane, decanal, benzaldehyde, β-caryophyllene, and humulene, while linalool was only present in the leaf-infestation treatment. In addition, principal component analysis indicated that all 18 compounds were associated with the infested plants, especially β-caryophyllene, p-xylene, and decanal. Based on the above studies and previous functional evaluations of the volatile compounds, it can be demonstrated that these compounds play a crucial role in modulating the interactions between A. segetum and M. mediator and regulating parasitoid behavior. It may be possible to enhance the biological control of A. segetum by M. mediator through the application of HIPVs.
Keywords: turnip moth, parasitoid wasp, herbivore-induced plant volatiles, behavioral response, biological control
1. Introduction
Volatiles emitted from leaves, fruits, or flowers not only provide herbivorous insects with cues useful in foraging for nutritional resources, but also play an important role in herbivore oviposition behavior, host orientation, mate location, and mating behaviors [1,2,3]. For example, the preference of Plutella xylostella for cruciferous plants is due to the presence of isothiocyanates (ITCs), and these compounds also stimulate P. xylostella oviposition [4]. Fragrant volatiles emitted by flowers mediate the mirid bug Apolygus lucorum’s preference for flowering host plants [5], and temporal shifts in plant volatiles may regulate the host plant foraging behavior of Ap. lucorum adults [6]. Such flower-emitted volatile organic compounds (VOCs), when applied to fields, act as attractants for several mirids, including Adelphocoris suturalis, Ad. lineolatus, and Ad. fasciaticollis [7].
Plants damaged by herbivores also release herbivore-induced plant volatiles (HIPVs), such as terpenoids, green leaf volatiles (GLVs), and benzenoids [8]. HIPVs help the natural enemies of herbivores find their prey (or hosts) and mates, and they can also influence the foraging behavior of herbivores. For instance, adults of the predaceous lady beetle Harmonia axyridis showed a preference for aphid-infested plants over un-infested plants or aphids alone [9]. In addition, females of the parasitic wasp Peristenus spretus use Ap. lucorum-induced volatiles to locate hosts, and the parasitoid preference for flowering host plants was consistent with host preferences of Ap. lucorum [10]. The ability of HIPVs to increase the recruitment of natural enemies allows HIPVs to be used as natural enemy attractants in crop fields.
The cutworm known as the turnip moth, Agrotis segetum (Denis and Schiffermüller) (Lepidoptera: Noctuidae), is a significant underground pest that is widely distributed in China. It has a wide host range, including wheat, corn, cotton, and sugar beets. In the Xinjiang Province of China, A. segetum is a common, serious pest of seedling cotton [11] that can feed on the leaves, petioles, branches, and main stems of plants [12]. The larvae feed mainly near the ground on the stems of seedlings, causing severe crop loss and even the death of plants [12,13]. HIPVs emitted from cotton plants infested by A. segetum larvae significantly deter the oviposition of conspecific females [14].
Microplitis mediator (Haliday) (Hymenoptera: Braconidae) is a solitary endoparasitoid of noctuid and geometrid caterpillars in Europe and Asia [15]. Because of its biological and ecological traits, M. mediator has a broad potential for application in agricultural fields for pest control. Studies have shown that parasitism by M. mediator on the larvae of A. segetum can reach 37.8% under laboratory conditions [16]. Compounds such as (3E)-4,8-dimethyl-1,3,7-nonatriene and (E,E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene, which are emitted by cotton seedlings damaged by chewing caterpillars or sucking bugs, are attractive to M. mediator [17]. M. mediator uses volatiles emitted by Helicoverpa armigera-damaged cotton for host location and foraging [15]. Therefore, it is likely that HIPVs also play a significant role in the biological control of A. segetum by M. mediator [15]. However, the tritrophic interactions among A. segetum, M. mediator, and cotton plants have not been studied.
In this study, we used a Y-tube olfactometer and GC-MS to assess the behavioral responses of M. mediator females to A. segetum-infested cotton and to the HIPVs produced by such feeding, with a view to further exploring the interactions among cotton, A. segetum, and M. mediator.
2. Results
2.1. Behavioral Responses to Larvae-Infested Cotton Plants
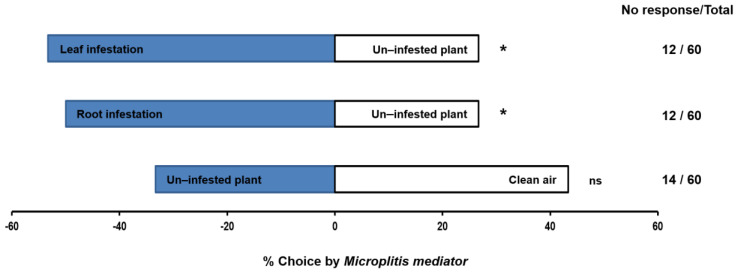
There were no significant differences between un-infested cotton plants and clean air, although a numerically greater proportion of female M. mediator (43.3%) chose clean air (χ2 = 0.78, df = 1, p = 0.3763). However, female parasitoids did prefer the odor from either A. segetum-infested leaves (χ2 = 5.33, df = 1, p = 0.0209) or A. segetum-infested roots (χ2 = 4.26, df = 1, p = 0.0390) over healthy, undamaged cotton plants (Figure 1).
Figure 1.
Behavioral responses of female Microplitis mediator adults to leaf and root volatiles induced by Agrotis segetum larval feeding. Female parasitoids had a choice between: (i) leaves infested by A. segetum larvae versus un-infested cotton plants, (ii) roots infested by A. segetum larvae versus un-infested cotton plants, and (iii) un-infested cotton plants versus clean air. “*” means a significant difference at the p < 0.05 level, while “ns” indicates no significant difference.
2.2. Analysis of Cotton Volatiles
The cotton volatiles emitted from the three treatments (healthy leaves, infested leaves, and infested roots) differed (Table 1). Compounds often associated with air (e.g., toluene and benzene) or laboratory equipment (e.g., siloxanes or phthalates) were not included in our list of putative plant volatiles [18]. Compared to healthy plants, total emissions increased 14.9- and 13.3- fold after leaf and root infestation by A. segetum larvae, respectively. Of the 18 compounds detected, all but (1) β-caryophyllene, (2) γ-chlorobutyrophenone, (3) humulene, and (4) 5,9-undecadien-2-one, 6,10-dimethyl-, (E)- were emitted in significantly higher amounts from infested plants than from un-infested plants. The compound p-xylene had the greatest concentration, followed by nonanal, decanal, benzaldehyde, β-caryophyllene, and humulene. The concentrations of p-xylene, nonanal, and decanal were significantly higher from plants with pest-infested leaves, while tetradecane, β-caryophyllene, and humulene were released mainly by plants with pest-infested roots. Linalool was only present in plants with pest-infested leaves.
Table 1.
Concentration of volatile compounds collected from cotton plants after infestation by A. segetum larvae.
| Volatile Compound | Un-Infested Plants | Leaf Infestation | Root Infestation |
|---|---|---|---|
| p-Xylene | 1.34 ± 0.22 c | 10.59 ± 0.53 a | 5.79 ± 0.35 b |
| Nonanal | 0.22 ± 0.02 c | 2.79 ± 0.07 a | 1.84 ± 0.33 b |
| Tetradecane | 0.14 ± 0.02 c | 1.26 ± 0.08 b | 2.90 ± 0.44 a |
| Hexyl butyrate | 0.07 ± 0.02 b | 1.13 ± 0.06 a | 1.04 ± 0.13 a |
| 1-Hexanol, 2-ethyl- | 0.08 ± 0.01 b | 1.53 ± 0.10 a | 1.47 ± 0.25 a |
| Decanal | 0.18 ± 0.05 c | 8.14 ± 0.48 a | 3.28 ± 0.66 b |
| Pentadecane | 0.11 ± 0.03 b | 1.07 ± 0.13 a | 1.11 ± 0.20 a |
| Benzaldehyde | 0.21 ± 0.003 b | 4.29 ± 0.38 a | 4.15 ± 0.25 a |
| Linalool | ND | 1.23 ± 0.13 | ND |
| β-caryophyllene | 0.15 ± 0.02 a | 3.79 ± 0.05 a | 7.25 ± 3.34 a |
| Hexadecane | 0.09 ± 0.02 b | 1.11 ± 0.21 a | 1.72 ± 0.32 a |
| γ-chlorobutyrophenone | 0.07 ± 0.02 a | 0.67 ± 0.05 a | 0.66 ± 0.32 a |
| Humulene | 0.07 ± 0.01 a | 1.91 ± 0.08 a | 3.10 ± 1.25 a |
| Heptadecane | 0.09 ± 0.03 c | 0.81 ± 0.16 b | 1.49 ± 0.11 a |
| Naphthalene | 0.06 ± 0.01 c | 1.14 ± 0.10 a | 0.88 ± 0.08 b |
| 2-Methylnaphthalene | 0.02 ± 0.01 c | 0.33 ± 0.08 b | 0.58 ± 0.07 a |
| 5,9-Undecadien-2-one, 6,10-dimethyl-, (E)- | 0.03 ± 0.00 a | 1.41 ± 0.35 a | 1.45 ± 0.62 a |
| 1, 2-Hexanediol | 0.04 ± 0.01 b | 1.03 ± 0.05 a | 0.83 ± 0.23 a |
| Total | 2.97 ± 0.35 | 44.23 ± 1.62 | 39.54 ± 6.47 |
ND = not detected. Data are Mean ± SE (μg/mL); the same lowercase letters within rows indicate no significant difference based on ANOVA followed by Duncan’s MRT, α = 0.05.
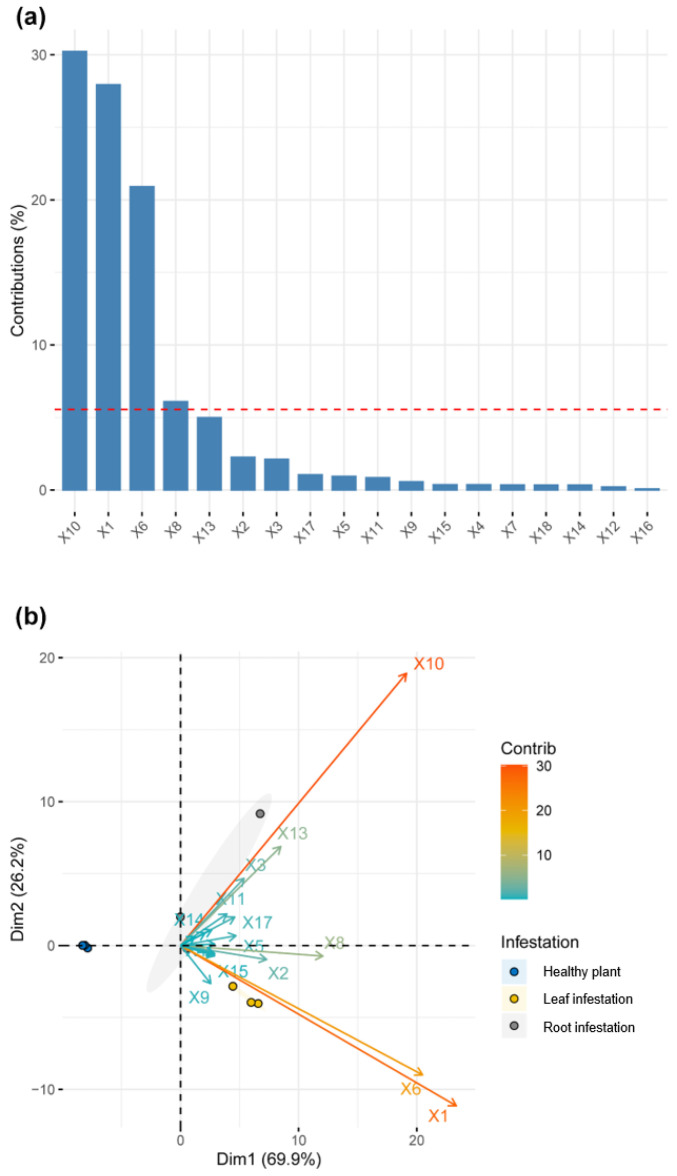
2.3. Principal Component Analysis
PCA identified which volatile compounds dominated the volatile blends from healthy cotton plants or ones whose leaves or roots were infested by A. segetum larvae. Of the 18 components in the volatile blend, the major volatiles were β-caryophyllene, decanal, and p-xylene (Figure 2a), and all 18 compounds emitted from cotton plants contributed to the infestation process (Figure 2b).
Figure 2.
Principal component analysis (PCA) of volatile compounds induced by feeding of Agrotis segetum larvae on cotton plants. (a) Contribution of each volatile compound to the first two principal components (PC1 + PC2), which together explained >99% of all variation. The horizontal red dashed line represents the mean contribution (5.3%) of all 18 volatiles. (b) PCA biplot for assessing each volatile compound for three different treatments. The points with different colors represent samples from un-infested plants (blue), plants with larval feeding on leaves (yellow), and on roots (gray), respectively. Black lines with arrows indicate volatile compounds that were the top seven contributors to the first two PC-axes. X1: p-xylene; X2: nonanal; X3: tetradecane; X4: hexyl butyrate; X5: 1-hexanol, 2-ethyl-; X6: decanal; X7: pentadecane; X8: benzaldehyde; X9: linalool; X10: β-caryophyllene; X11: hexadecane; X12: γ-chlorobutyrophenone; X13: humulene; X14: heptadecane; X15: naphthalene; X16: 2-methylnaphthalene; X17: 5,9-undecadien-2-one, 6,10-dimethyl-, (E)-; X18: 1, 2-hexanediol.
3. Discussion
Volatile compounds play indispensable roles in the interactions among trophic levels in native food webs. HIPVs, as key information chemicals, provide herbivore-specific cues to parasitoids and predators [19]. We found that M. mediator was attracted by A. segetum-induced HIPVs. Li [14] showed that A. segetum females preferred to lay eggs on healthy (versus previously infested) cotton plants, and that the HIPVs induced by conspecific larvae on cotton plants had significant repellent effects on oviposition. Therefore, from an integrated pest management (IPM) perspective, leaf HIPVs in this system should both reduce A. segetum attacks and increase M. mediator parasitism of the pest larvae [14].
HIPVs can both repel herbivores and recruit natural enemies of pests [20]. However, comparative GC-MS analyses of headspace volatiles comparing compounds from healthy versus infested plants showed mostly quantitative, not qualitative effects. Linalool was the only compound that was present only in the VOCs of infested leaves. This compound is significantly repellent at high doses to the foraging and oviposition of several pest herbivores [14]. After infestation of a plant, the amount of p-xylene, nonanal, tetradecane, decanal, benzaldehyde, β-caryophyllene, and humulene all increased significantly. However, field and greenhouse experiments are necessary to confirm parasitoid attraction.
Insects can perceive chemical signals related to feeding, mating, and oviposition through diverse chemoreceptor families, including odorant receptors (ORs), and ionotropic receptors (IRs) [21,22,23]. For example, decanal was reported to be involved in the olfactory recognition process of M. mediator by binding strongly to MmedOBP18, which is mainly involved in the short-distance recognition of chemical information from hosts or host habitats [24]. Our results showed that the concentration of decanal emitted was significantly higher from plants with infested leaves (8.14 ± 0.48 μg/mL) compared to plants with infested roots (3.28 ± 0.66 μg/mL) or healthy (un-infested) plants (0.18 ± 0.05 μg/mL), suggesting that decanal plays an important role in the location of hosts and their habitats by M. mediator. In conclusion, plant volatiles induced by A. segetum can bind to both ORs and IRs, affecting both herbivores and their parasitoids (Table 2).
Table 2.
Known effects of host-plant volatiles on the behavioral activity and EAG activity of Microplitis mediator and their corresponding binding or recognition protein. The “+” indicates a substance inducing behavioral attraction or electrophysiological (EAG) activity in the antennae of M. mediator adults.
| Volatile Compound | Behavioral Activity | EAG Activity | Corresponding Binding or Recognition Protein | Reference |
|---|---|---|---|---|
| p-Xylene | + | NT | MmedOBP8 | [25,26] |
| Nonanal | + | + | MmedOBP8, MmedOBP9, MmedOBP10 MmedIR64a1, MmedIR64a2 |
[16,23,27,28] |
| Tetradecane | NT | NT | MmedIR64a1 | [23] |
| Decanal | NT | + | MmedOBP18, MmedIR64a1, MmedIR64a2 | [16,23,24,28] |
| Benzaldehyde | + | + | MmedOBP2, MmedCSP2, MmedNPC2a, MmedIR64a1 | [16,23,25,29,30,31,32,33,34] |
| β-Caryophyllene | NT | + | MmedIR64a1 | [23,32,33] |
| Humulene | NT | NT | MmedOBP4, MmedOBP6 | [30] |
Currently, control of A. segetum in China relies on the application of chemical insecticides [12]. HIPVs play important roles in pest control in agriculture systems [35,36]. In our present study, female M. mediator wasps significantly preferred plants damaged by A. segetum, especially after foliar infestation. This preference may be related to changes in the release of HIPVs by the host plant. The amount of various volatile compounds in cotton increased after herbivore infestation, especially p-xylene, nonanal, tetradecane, decanal, benzaldehyde, β-caryophyllene, and humulene. Our results emphasize the important role of HIPVs in host selection by M. mediator and provide insights that may help improve the biological control of A. segetum through the combined application of HIPVs and the release of parasitic wasps. Future studies may explore parasitoid efficiency under laboratory and field conditions and investigate the effects of co-infestation of H. armigera and A. segetum on M. mediator in cotton.
4. Materials and Methods
4.1. Plants
Cotton (CCRI49) seeds were obtained from the Institute of Cotton Research of the Chinese Academy of Agricultural Sciences (CAAS) and sown in a greenhouse at Langfang Experimental Station, CAAS, under the following conditions: 26 ± 1 °C, 60 ± 10% RH, 14:10 h (L:D) photoperiod. Plants used for these tests were at the 3-true leaf growth stage.
4.2. Insects
Agrotis segetum larvae were reared continuously in a climate chamber under the same conditions described for plant production at the Langfang Experimental Station of CAAS. Second or third instar A. segetum larvae were used for our experiments.
The colony of M. mediator was established from diapausing cocoons provided by the Plant Protection Institute, Hebei Academy of Agriculture and Forestry Sciences. M. mediator larvae were reared in an incubator at 25 ± 1 °C, 60 ± 10% RH, 14:10 h (L:D) photoperiod at the Langfang Experimental Station, CAAS. All female parasitoids used in olfactometer tests were 3–6 d old, mated, and fed with 10% honey solution after emergence. Wasps had no previous oviposition experience or contact with plants before experiments.
4.3. Olfactometer Tests
A Y-tube olfactometer was used to evaluate the behavioral responses of 3–6 d old active, mated M. mediator adults (n = 60 females), when offered choices between the odors of (1) cotton leaves infested by four A. segetum larvae for 12 h, (2) cotton roots infested by four A. segetum larvae for 12 h, (3) un-infested plants, and (4) clean air. To ensure that A. segetum fed only on cotton leaves, we made a net bag (20 × 30 cm) with 120 mesh gauze to cover the above-ground parts of the cotton along with four individuals. In order to make A. segetum larvae feed only on cotton roots, we cut circular rings of blow molding paper (diam: 8 cm) to cover the cotton cotyledon stalks and fixed them with plastic rods, so that they could not climb higher. All parts of the equipment were connected with Teflon tubes, and the direction of air flow was from the atmospheric air intake, through activated charcoal, a distilled water humidification device, a gas flow control meter, a glass odor source vessel, and then into the Y-tube test arena, with similar parameters as those in previous studies [37].
One M. mediator adult was introduced to the initial test chamber after the airflow of both arms had been adjusted to 400 mL/min. Wasps that moved 1/3 of the way down a test arm within 5 min and stayed there for more than 10 s were counted as having made a ‘choice’, while wasps that did not respond as such were discarded and recorded as making ‘no choice’. After testing five parasitic wasps, the two arms of the Y-tube were reversed (with respect to their odor source), and after testing 10 wasps a clean Y-tube (washed with 95% ethanol and soaked and rinsed with distilled water and dried naturally at room temperature) was used.
4.4. Collection and Analysis of Cotton Volatiles
For volatile collection and identification of blends associated with our treatments, 3-true leaf cotton plants were separated into three groups: (1) leaves infested by four A. segetum larvae for 12 h; (2) roots infested by four A. segetum larvae for 12 h; (3) healthy, undamaged plants. To create infested plant foliage or roots, four larvae (second or third instar) of A. segetum (starved for 4 h) were placed on the whole plant and allowed to feed on foliage or roots for 12 h. Larvae were then removed, and the plants were immediately processed to collect headspace volatiles, which were then analyzed for their components.
Cotton headspace volatiles were collected from 1300 h to 1700 h every day using a dynamic headspace collection method. Cotton plants at the 3-true leaf stage (n = 3 for each treatment) were placed individually in a custom glass chamber (diam: 20 cm; height: 66 cm; Yuansu Glassware Supply Station, Shenzhen, China) and the soil was covered with aluminum foil before cotton volatile collection. An airflow at 500 mL/min passed over the plant, and volatiles were absorbed by 50 mg of Porapak® Type Q adsorbent (Altech Assoc, Chicago, IL, USA). Then, cotton volatile samples were extracted into 1.5 mL sample bottles using 400 µL HPLC-grade n-Hexane (Aladdin, Shanghai, China) and, finally, were stored at −20 °C until GC-MS analyses.
GC-MS (GC: Agilent 7890A, equipped with a DB-WAX chromatographic column [30 m × 0.25 mm × 0.25 µm]; MS: Agilent 5975C) was used to analyze cotton volatiles, with similar parameters as those in previous studies [37]. The injector temperature for GC analysis was 230 °C, the oven temperature was kept at 50 °C for 1 min, and then raised by 5 °C/min to 180 °C for 2 min, and then increased by 10 °C/min to 230 °C, and held for 2 min. Helium was the carrier gas, at an average flow rate of 1 mL/min. The ion source temperature was 230 °C. The volatile compounds obtained were first identified by NIST 14 and were then compared with standard compounds to carry out qualitative-quantitative analyses.
4.5. Statistical Analysis
Chi-square tests were used to analyze the Y-tube olfactometer data to detect differences between the pairs of treatments. χ2 and P values were calculated, and non-responsive adults were excluded from the analysis. The amounts of each volatile compound emitted under different treatments were compared using one-way ANOVA, followed by Duncan’s new multiple range tests. Principal component analysis (PCA) was performed to analyze the patterns of volatiles from different treatments given its ability to reduce the complexity of the data while identifying the features in the dataset that contribute the most to the treatment effects. Chi-square tests and one-way ANOVAs were conducted using SPSS 25.0, while PCA analysis was performed using R 4.0.2 with a 0.05 level of significance.
Acknowledgments
We thank Bing Liu from the Institute of Plant Protection, Chinese Academy of Agricultural Sciences for assistance with the data analysis.
Author Contributions
M.L.: data curation and analysis, investigation, writing—original draft; S.X.: data curation, formal analysis, investigation, writing—original draft; T.Z.: methodology, supervision, validation; L.W.III: conceptualization, writing—review and editing; H.X.: supervision, writing—review and editing; Y.L.: conceptualization, data curation and analysis, funding acquisition, methodology, project administration, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (U2003212), China Agriculture Research System (CARS-15-21).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xu H., Turlings T.C.J. Plant volatiles as mate-finding cues for insects. Trends Plant Sci. 2018;23:100–111. doi: 10.1016/j.tplants.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Kafle B.D., Morawo T., Fadamiro H. Host-induced plant volatiles mediate ability of the parasitoid Microplitis croceipes to discriminate between unparasitized and parasitized Heliothis virescens larvae and avoid superparasitism. J. Chem. Ecol. 2020;46:967–977. doi: 10.1007/s10886-020-01218-x. [DOI] [PubMed] [Google Scholar]
- 3.Gontijo L., Cascone P., Giorgini M., Michelozzi M., Rodrigues H.S., Spiezia G., Iodice L., Guerrieri E. Relative importance of host and plant semiochemicals in the foraging behavior of Trichogramma achaeae, an egg parasitoid of Tuta absoluta. J. Pest Sci. 2019;92:1479–1488. doi: 10.1007/s10340-019-01091-y. [DOI] [Google Scholar]
- 4.Liu X.L., Zhang J., Yan Q., Miao C.L., Han W.K., Hou W., Yang K., Hansson B.S., Peng Y.C., Guo J.M., et al. The molecular basis of host selection in a crucifer-specialized moth. Curr. Biol. 2020;30:4476–4482.e5. doi: 10.1016/j.cub.2020.08.047. [DOI] [PubMed] [Google Scholar]
- 5.Pan H.S., Lu Y.H., Xiu C.L., Geng H.H., Cai X.M., Sun X.L., Zhang Y.J., Williams L., III, Wyckhuys K.A.G., Wu K.M. Volatile fragrances associated with flowers mediate host plant alternation of a polyphagous mirid bug. Sci. Rep. 2015;5:14805. doi: 10.1038/srep14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan H.S., Xiu C.L., Williams L., III, Lu Y.H. Plant volatiles modulate seasonal dynamics between hosts of the polyphagous mirid bug Apolygus lucorum. J. Chem. Ecol. 2021;47:87–98. doi: 10.1007/s10886-020-01236-9. [DOI] [PubMed] [Google Scholar]
- 7.Xiu C.L., Pan H.S., Liu B., Luo Z.X., Williams L., III, Yang Y.Z., Lu Y.H. Perception of and behavioral responses to host plant volatiles for three Adelphocoris species. J. Chem. Ecol. 2019;45:779–788. doi: 10.1007/s10886-019-01102-3. [DOI] [PubMed] [Google Scholar]
- 8.Hao Y., Lou Y.G. Advances in the study of plant volatiles induced by insect pests. J. Yangtze Univ. 2013;10:12–15. doi: 10.16772/j.cnki.1673-1409.2013.11.013. [DOI] [Google Scholar]
- 9.Xiu C.L., Zhang W., Xu B., Wyckhuys K.A.G., Cai X.M., Su H.H., Lu Y.H. Volatiles from aphid-infested plants attract adults of the multicolored Asian lady beetle Harmonia axyridis. Biol. Control. 2019;129:1–11. doi: 10.1016/j.biocontrol.2018.11.008. [DOI] [Google Scholar]
- 10.Dai W.J., Pan H.S., Xiu C.L., Luo S.P., Yang Y.Z., Lu Y.H. Behavioral selection of endoparasitoid Peristenus spretus to green mirid bug Apolygus lucorum and its damaged host plants. J. Plant Prot. 2018;45:194–200. doi: 10.13802/j.cnki.zwbhxb.2018.2016086. [DOI] [Google Scholar]
- 11.Chen J., Liu R., Liang H.J., Luo S.K., Luo F.J. Population monitoring and occurrence characteristics of Agrotis segetum Schiff. in Aral Reclamation Area of Xinjiang. China Cotton. 2021;48:26–28. doi: 10.11963/cc20210066. [DOI] [Google Scholar]
- 12.Guo J.L., Fu X.W., Zhao X.C., Wu K.M. Preliminary study on the flight capacity of Agrotis segetum (Lepidoptera: Noctuidae) J. Environ. Entomol. 2016;38:888–895. doi: 10.3969/j.issn.1674-0858.2016.05.3. [DOI] [Google Scholar]
- 13.Esbjerg P., Sigsgaard L. Temperature dependent growth and mortality of Agrotis segetum. Insects. 2019;10:7. doi: 10.3390/insects10010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li M. Master’s Thesis. Jiangxi Agricultural University; Nanchang, China: 2021. Analysis on the Chemical Ecology Effect of Cotton Volatiles Induced by Feeding of Agrotis segetum Larvae. [Google Scholar]
- 15.Li J.C., Yan F.M., Coudron T.A., Pan W.L., Zhang X.F., Liu X.X., Zhang Q.W. Field release of the parasitoid Microplitis mediator (Hymenoptera: Braconidae) for control of Helicoverpa armigera (Lepidoptera: Noctuidae) in cotton fields in northwestern China’s Xinjiang province. Environ. Entomol. 2006;35:694–699. doi: 10.1603/0046-225X-35.3.694. [DOI] [Google Scholar]
- 16.Khan S.M. Effectiveness of Microplitis mediator (HYM.: Braconidae) against its hosts Agrotis segetum and A. ipsilon (Lepidoptera:noctuidae) Pak. J. Biol. Sci. 1999;2:344–346. doi: 10.3923/pjbs.1999.344.346. [DOI] [Google Scholar]
- 17.Xu M.L., Jiang Y.F., Chen S.M., Chen F.D., Chen F. Herbivory-induced emission of volatile terpenes in Chrysanthemum morifolium functions as an indirect defense against Spodoptera litura larvae by attracting natural enemies. J. Agric. Food Chem. 2021;69:9743–9753. doi: 10.1021/acs.jafc.1c02637. [DOI] [PubMed] [Google Scholar]
- 18.Mohammed K., Agarwal M., Du X.B., Newman J., Ren Y. Behavioural responses of the parasitoid Aphytis melinus to volatiles organic compounds (VOCs) from Aonidiella aurantii on its host fruit Tahitian lime fruit Citrus latifolia. Biol. Control. 2019;133:103–109. doi: 10.1016/j.biocontrol.2019.03.015. [DOI] [Google Scholar]
- 19.Wang B., Li H.M., Cao H.Q., Wang G.R. Mechanisms and applications of plant-herbivore-natural enemy tritrophic interactions mediated by volatile organic compounds. Sci. Agric. Sin. 2021;54:1653–1672. doi: 10.3864/j.issn.0578-1752.2021.08.007. [DOI] [Google Scholar]
- 20.War A.R., Sharma H.C., Paulraj M.G., War M.Y., Ignacimuthu S. Herbivore induced plant volatiles: Their role in plant defense for pest management. Plant Signal. Behav. 2011;6:1973–1978. doi: 10.4161/psb.6.12.18053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H.W., Yang W., Yang H., Yang C.P., Zhu X.Q., Liu F.J., Jiang L.H., Shan C.Y. Electroantennogram and behavioral responses of Tomicus minor (Hartig) (Coleoptera: Scolytidae) to plant volatiles. Chin. J. Ecol. 2014;33:1260–1266. doi: 10.13292/j.1000-4890.20140327.048. [DOI] [Google Scholar]
- 22.Yang Y.Q., Wang S.N., Peng Y., Shan S., Zheng Y., Li R.J., Zhang Y.J., Guo Y.Y. Expression of the odor binding protein MmedOBP19 in the legs of Microplitis mediator (Hymenoptera: Braconidae) and its ligand binding characteristics. Acta Entomol. Sin. 2017;60:613–620. doi: 10.16380/j.kcxb.2017.06.001. [DOI] [Google Scholar]
- 23.Shan S., Wang S.N., Song X., Khashaveh A., Lu Z.Y., Dhiloo K.H., Li R.J., Gao X.W., Zhang Y.J. Antennal ionotropic receptors IR64a1 and IR64a2 of the parasitoid wasp Microplitis mediator (Hymenoptera: Braconidate) collaboratively perceive habitat and host cues. Insect Biochem. Mol. Biol. 2019;114:103204. doi: 10.1016/j.ibmb.2019.103204. [DOI] [PubMed] [Google Scholar]
- 24.Song X., Shan S., Wang S.N., Tao Y.X., Li R.J., Zhang Y.J. Prokaryotic expression and ligand binding characteristics of odorant binding protein MmedOBP18 of the parasitoid wasp Microplitis mediator (Hymenoptera: Bracondae) Acta Entomol. Sin. 2019;62:150–159. doi: 10.16380/j.kcxb.2019.02.002. [DOI] [Google Scholar]
- 25.Zhang K., Ren L.Y., Li K.M., Wang M., Zhang Y.J., Guo Y.Y. Effect of volatile odors on taxis response of Microplitis mediator and its efficiency in field trapping test. Chin. J. Biol. Control. 2011;27:157–164. doi: 10.16409/j.cnki. [DOI] [Google Scholar]
- 26.Zhang K. Master’s Thesis. Huazhong Agricultural University; Wuhan, China: 2011. Binding Characterization of Odorant-Binding Protein MmedOBP8 in the Microplitis mediator and the Taxis Response Authentication. [Google Scholar]
- 27.Li K.M., Wang S.N., Zhang K., Ren L.Y., Ali A., Zhang Y.J., Zhou J.J., Guo Y.Y. Odorant binding characteristics of three recombinant odorant binding proteins in Microplitis mediator (Hymenoptera: Braconidae) J. Chem. Ecol. 2014;40:541–548. doi: 10.1007/s10886-014-0458-5. [DOI] [PubMed] [Google Scholar]
- 28.Yu H.L., Zhang Y.J., Wyckhuys K.A.G., Wu K., Gao X.W., Guo Y.Y. Electrophysiological and behavioral responses of Microplitis mediator (Hymenoptera: Braconidae) to caterpillar-induced volatiles from cotton. Environ. Entomol. 2010;39:600–609. doi: 10.1603/EN09162. [DOI] [PubMed] [Google Scholar]
- 29.Dong W.X., Zhang Z.N., Fang Y.L., Zang F., Kan W. Response of parasitoid Microplitis mediator to plant volatiles in an olfactometer. Insect Sci. 2000;7:344–350. doi: 10.1111/j.1744-7917.2000.tb00233.x. [DOI] [Google Scholar]
- 30.Zhang S., Chen L.Z., Gu S.H., Cui J.J., Gao X.W., Zhang Y.J., Guo Y.Y. Binding characterization of recombinant odorant-binding proteins from the parasitic wasp, Microplitis mediator (Hymenoptera: Braconidae) J. Chem. Ecol. 2011;37:189–194. doi: 10.1007/s10886-010-9902-3. [DOI] [PubMed] [Google Scholar]
- 31.Zheng Y., Wang S.N., Peng Y., Lu Z.Y., Shan S., Yang Y.Q., Li R.J., Zhang Y.J., Guo Y.Y. Functional characterization of a Niemann-Pick type C2 protein in the parasitoid wasp Microplitis mediator. Insect Sci. 2018;25:765–777. doi: 10.1111/1744-7917.12473. [DOI] [PubMed] [Google Scholar]
- 32.Dong W.X., Wang R., Zhang Z.N. Antennae potential response of parasitoid Braconidae to cotton volatiles. Acta Entomol. Sin. 2000;43:119–125. doi: 10.16380/j.kcxb.2000.s1.019. [DOI] [Google Scholar]
- 33.Dong W.X., Hu B.J., Zhang Z.N., Han B.Y. Electrophysiological and behavioral responses of Microplitis mediator to tobacco plant volatiles. Acta Ecol. Sin. 2004;10:2252–2256. doi: 10.3321/j.issn:1000-0933.2004.10.024. [DOI] [Google Scholar]
- 34.Peng Y. Master’s Thesis. Hebei Agricultural University; Baoding, China: 2017. Identification and Functional Characterization of Chemosensory Proteinsin Microplitis mediator. [Google Scholar]
- 35.Cusumano A., Weldegergis B.T., Colazza S., Dicke M., Fatouros N.E. Attraction of egg-killing parasitoids toward induced plant volatiles in a multi-herbivore context. Oecologia. 2015;179:163–174. doi: 10.1007/s00442-015-3325-3. [DOI] [PubMed] [Google Scholar]
- 36.Yu H.L., Khashaveh A., Li Y.H., Li X.J., Zhang Y.J. Field trapping of predaceous insects with synthetic herbivore-induced plant volatiles in cotton fields. Environ. Entomol. 2018;47:114–120. doi: 10.1093/ee/nvx201. [DOI] [PubMed] [Google Scholar]
- 37.Xiu C.L., Dai W.J., Pan H.S., Zhang W., Luo S.P., Wyckhuys K.A.G., Yang Y.Z., Lu Y.H. Herbivore-induced plant volatiles enhance field-level parasitism of the mirid bug Apolygus lucorum. Biol. Control. 2019;135:41–47. doi: 10.1016/j.biocontrol.2019.05.004. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.