Abstract
Metronidazole (Mtz) is a critical ingredient of modern multidrug therapies for Helicobacter pylori infection. Mtz resistance reduces the effectiveness of these combinations. Although null mutations in a rdxA gene that encodes oxygen-insensitive NAD(P)H nitroreductase was reported in Mtz-resistant H. pylori, an intact rdxA gene has also been reported in Mtz-resistant H. pylori, suggesting that additional Mtz resistance mechanisms exist in H. pylori. We explored the nature of Mtz resistance among 544 clinical H. pylori isolates to clarify the role of rdxA inactivation in Mtz resistance and to identify another gene(s) responsible for Mtz resistance in H. pylori. Mtz resistance was present in 33% (181 of 544) of the clinical isolates. There was marked heterogeneity of resistance, with Mtz MICs ranging from 8 to ≥256 μg/ml. rdxA inactivation resulted in Mtz MICs of up to 32 μg/ml for 6 Mtz-sensitive H. pylori strains and 128 μg/ml for one Mtz-sensitive strain. Single or dual (with rdxA) inactivation of genes that encode ferredoxin-like protein (designated fdxB) and NAD(P)H flavin oxidoreductase (frxA) also increased the MICs of Mtz for sensitive and resistant strains with low to moderate levels of Mtz resistance. fdxB inactivation resulted in a lower level of resistance than that from rdxA inactivation, whereas frxA inactivation resulted in MICs similar to those seen with rdxA inactivation. Further evidence for involvement of the frxA gene in Mtz resistance included the finding of a naturally inactivated frxA but an intact rdxA in an Mtz-resistant strain, complementation of Mtz sensitivity from an Mtz-sensitive strain to an Mtz-resistant strain or vice versa by use of naturally inactivated or functional frxA genes, respectively, and transformation of an Mtz-resistant Escherichia coli strain to an Mtz sensitive strain by a naturally functional frxA gene but not an inactivated frxA gene. These results are consistent with the hypothesis that null mutations in fdxB, frxA, or rdxA may be involved in Mtz resistance.
Helicobacter pylori is recognized as the major cause of peptic ulcer disease and a risk factor for gastric adenocarcinoma and primary gastric lymphoma (4, 34, 35). H. pylori infection is one of the most common infections worldwide and accounts for tremendous morbidity and mortality. Clinical experience has demonstrated that H. pylori infection is not easy to cure. The primary impediments to successful treatment are lack of compliance with the drug regimens and development of antibiotic-resistant H. pylori (15). Metronidazole (Mtz) was a critical ingredient of the first successful therapy for H. pylori infection and remains a major component of newer triple and quadruple therapies (16, 21). Monotherapy with Mtz results in the acquisition of Mtz resistance by more than 50% of H. pylori isolates (31), and Mtz-containing therapies are being undermined by the development of resistance (36, 40).
Mtz has action against a wide variety of prokaryotic and eukaryotic pathogens including H. pylori and is a mainstay of therapy for infections with organisms such as Bacteroides species, Clostridium species, Trichomonas vaginalis, Giardia lamblia, and Entamoeba histolytica (8, 33). Understanding of the antimicrobial action and resistance to Mtz has come from studies with anaerobic microorganisms such as Bacteroides, Trichomonas, and Clostridium species (8, 9, 30). The cytotoxicity of Mtz is not directly due to the final products of Mtz reduction but to the unstable and/or less reduced intermediates that damage DNA, resulting in strand breakage, helix destabilization, unwinding, and cell death (5, 6). Reductive activation of Mtz depends on the redox system of the target cell. Any redox system that possesses a reduction potential more negative than that of Mtz will donate its electrons preferentially to Mtz (27). The direct donors of electrons in anaerobic bacteria are thought to be ferredoxin-like Fe-S electron transport proteins such as ferredoxin (10, 29). In anaerobic organisms, the redox potential is −430 to −460 mV, the typical value for ferredoxin-like Fe-S proteins, whereas Mtz has a reduction potential of −415 mV, making Mtz an efficient electron acceptor. The lowest redox potentials obtainable by aerobic organisms (e.g., Escherichia coli) are those of NAD or NADH (−320 mV) and NADP or NADPH (−324 mV), such that these organisms are intrinsically Mtz resistant as they are unable to reduce Mtz. However, under aerobic conditions, one electron step can result in reoxidization by oxygen to the original compound, producing inactive Mtz (8, 33). As noted above, the most important step in the antimicrobial action of Mtz in bacteria is the reductive activation of the nitro group of Mtz (which makes Mtz toxic), which is controlled by the redox system of the target cell.
It has been proposed that the mode of action of Mtz in H. pylori is similar to that in anaerobic bacteria, although the optimal in vitro culture conditions for this pathogen are microaerophilic (2, 25). In addition, ferredoxin and ferredoxin-like proteins have been identified from two complete H. pylori genomic sequences (1, 39). Putative Mtz nitroreductases include ferredoxin (HP0277; FdxA), flavodoxin (HP1161; FldA), three ferredoxin-like proteins (HP1508 [which we named FdxB], HP0588 [δ subunit of 2-oxoglutarate oxidoreductase; OorDABC], and HP1109 [δ subunit of pyruvate ferredoxin oxidoreductase; PorCDAB]), NAD(P)H flavin oxidoreductase (HP0642; FrxA), and oxygen-insensitive NAD(P)H nitroreductase (HP0954; RdxA). OorDABC, PorCDAB, and FldA have been purified from H. pylori, and the possible involvement of those proteins in Mtz resistance has been described (20, 22, 23, 26). Furthermore, Mtz resistance in H. pylori has also been suggested to be related to efficient DNA repair exerted by the recA protein (3) and the decreasing oxygen-scavenging capability at the site of Mtz reduction in resistant H. pylori strains (38). The most convincing data regarding Mtz resistance in H. pylori relate to inactivation of the rdxA gene, which inactivates Mtz nitroreductase activity (14). However, other pathways that lead to Mtz resistance exist because Mtz resistance has been described in H. pylori strains with an intact rdxA gene (24). In addition, the inactivation of rdxA alone is insufficient to explain the heterogeneity of Mtz resistance among clinical H. pylori isolates (7, 42).
In this study, we present the rate of incidence and the heterogeneity of Mtz resistance among 544 clinical H. pylori isolates from the United States with the full range of Mtz resistance (Mtz MICs, 8 to ≥256 μg/ml). Putative H. pylori Mtz nitroreductases (e.g., FdxA, FdxB, FldA, FrxA, RdxA, OorD, and PorD) were inactivated to explore which gene or gene combinations are involved in Mtz resistance. We found that only the fdxB, frxA, and rdxA genes could be inactivated without causing a lethal effect on H. pylori. Mtz resistance was conferred by inactivation of fdxB, frxA, or rdxA.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
H. pylori ATCC 700392 (which is the same as H. pylori 26695 [39]) was obtained from American Type Culture Collection (Rockville, Md.), and all other H. pylori isolates (n = 544) were obtained from the Veterans Affairs Medical Center, Houston, Tex. The isolation of H. pylori strains from gastric biopsy specimens was performed as described previously (17). The isolated H. pylori strains including ATCC 700392 were routinely cultured on brain heart infusion (BHI; Difco, Detroit, Mich.) agar plates containing 7% horse blood in a microaerobic atmosphere (10% CO2 and 5% O2) at 37°C for 2 to 3 days. Rifampin-resistant H. pylori 1857 for conjugation was generated by selection of spontaneously resistant colonies on 7% horse serum–BHI agar plates supplemented with 100 μg of rifampin per ml. When needed, selection for chloramphenicol- or kanamycin-resistant H. pylori was performed by adding 10 μg of chloramphenicol per ml or 15 μg of kanamycin per ml to the 7% horse blood–BHI agar plate. E. coli cells (XL-Blue [Stratagene] or DH5α [Bethesda Research Laboratories, Inc.]) were cultured in Luria-Bertani (37) broth or agar plates for the amplification of plasmids.
Determination of Mtz MICs.
The MICs for 544 H. pylori isolates were determined by twofold agar dilution. Agar dilution plates were prepared with Mueller-Hinton (MH) agar as the base medium. Aged sheep blood (2 weeks old) was added to the MH base medium at a concentration of 5%. An Mtz solution (Sigma Chemical Co., St. Louis, Mo.) was prepared in sterile distilled water and was added to the 5% sheep blood–MH base medium to achieve serial twofold concentrations of between 0.015 and 256 μg of Mtz per ml. Fresh H. pylori isolates (2 to 3 days culture) were prepared in saline to an optical density at 625 nm of between 0.38 and 0.4. With a Steers-type replicating device (Cathra [no longer in business]), 1 to 5 μl of the adjusted inoculum was delivered to the agar plates. All plates were incubated under CampyPak Plus conditions (Becton Dickinson BBL, Cockeysville, Md.) for 3 days. The MIC was defined as the lowest concentration of Mtz that completely inhibited the growth of the inoculum. Mtz-resistant H. pylori ATCC 43504 was used as a quality control organism. Any test in which the Mtz MIC for the quality control organism was outside the approved range (64 to 256 μg/ml) was completely discarded. The MICs for all H. pylori strains with inactivated fdxB, frxA, and rdxA genes were determined with 7% horse blood–BHI agar or 5% sheep blood–MH agar plates supplemented with 0.5, 1, 2, 4, 8, 16, 32, 64, 128, or 256 μg of Mtz per ml and incubated for 3 to 4 days. The measurement was repeated twice to confirm the results by using the same 7% horse blood–BHI medium supplemented with Mtz. The MIC for E. coli DH5α harboring frxA and/or rdxA genes was determined by growing cells on LB agar plates supplemented with 10, 20, 40, 80, 160, or 320 μg of Mtz per ml.
PCR amplification of portions of fdxA, fdxB, fldA, frxA, rdxA, oorD, porD, and ureB (as a control) from H. pylori and their in vitro inactivation mutagenesis.
Portions of the genes that encode FdxA, FdxB, FldA, FrxA, RdxA, OorD, PorD, and UreB were amplified by PCR with PCR primer pairs, as shown in Table 1. The identity of each PCR-amplified fragment was confirmed by DNA sequencing, and the confirmed DNA fragments were inserted into the EcoRV restriction enzyme site of pBluescript SK(+) (Stratagene, La Jolla, Calif.). The insert DNA was digested with an appropriate restriction enzyme to inactivate the genes in vitro. A chloramphenicol resistance gene cassette (cat) (41) was inserted into the MunI and BalI sites of the insert DNAs for PorD and OorD, respectively. The cat cassette was also inserted into the BamHI, NsiI, and HindIII sites of the insert DNAs for FdxA, FrxA, and FldA, respectively, and into the Eco47III sites of the insert DNAs for FdxB and RdxA. The resulting plasmids were named pGH67 for the plasmid with fdxA::cat, pGH58 for that with fdxB::cat, pGH64 for that with fldA::cat, pGH130 for that with frxA::cat, pGH55 for that with rdxA::cat, pGH46 for that with porD::cat, and pGH60 for that with oorD::cat. A kanamycin resistance gene cassette from pHel3 (km) (19) was also inserted into the Eco47III site of insert DNA for RdxA, and the resulting plasmid (pGH87) was used for dual inactivation by selection on a plate with chloramphenicol and kanamycin. All the resulting plasmids (1 to 2 μg) were used for inactivation of chromosomal genes by natural transformation as described previously (19).
TABLE 1.
PCR primers used to amplify portions of H. pylori genes
| Primer pair | Encoded protein (gene) | Nucleotide sequencesa | Size (bp) of PCR fragment |
|---|---|---|---|
| FX-Ab | Ferredoxin (fdxA; HP0277d) | 5′-CATGTCATTATTGGTGAATG-3′ | 441 |
| FX-Bc | 5′-GGCTCGTTGCATGGGGATTT-3′ | ||
| FXLK-A | Ferredoxin-like protein (fdxB; HP1508) | 5′-ATGCTTGAAACTTCTAGCCA-3′ | 475 |
| FXLK-B | 5′-CTGGGGCGATGAAATAAAAG-3′ | ||
| FLD-A | Flavodoxin (fldA; HP1161) | 5′-ATTGGTATTTTTTTGGGAC-3′ | 583 |
| FLD-B | 5′-AAAAGTCTGATTCTAGCGGGG-3′ | ||
| FLVN-A | NAD(P)H flavin oxidoreductase (frxA: HP0642) | 5′-ACAAGTGGTTGCTTTACAGC-3′ | 450 |
| FLVN-B | 5′-GCCGCTGCCATCATCATGTT-3′ | ||
| OXI-A | Oxygen-insensitive nitroductase (rdxA; HP0954) | 5′-GACAATTATTAAACGAGCGC-3′ | 460 |
| OXI-B | 5′-CCTCCAATAATGCAACTATC-3′ | ||
| OOR-A | 2-Oxoglutarate oxidoreductase (oorD; HP0588) | 5′-ATGGCTAAAATGAGCGCTCC-3′ | 480 |
| OOR-B | 5′-CGCATTTGGGTAAAGCCACG-3′ | ||
| POR-A | Pyruvate oxidoreductase (porD; HP1109) | 5′-ATGAAAGATTGGAACGAATT-3′ | 364 |
| POR-B | 5′-GCCATTGAGTGAGAGCGGTA-3′ | ||
| URE-A | UreaseB (ureB; HP0072) | 5′-CTTCTGCAATCAATCATGCG-3′ | 717 |
| URE-B | 5′-ATAGAAGCGTTCGCGTCACC-3′ |
Nucleotide sequences were obtained from the complete H. pylori genome sequence (39).
Forward primer.
Backward primer.
Designation in the complete H. pylori genome sequence.
Cloning of frxA and rdxA genes from H. pylori and DNA sequence analysis.
To isolate the frxA and rdxA genes from H. pylori ATCC 700392, 2600, 6013, 1857, and 1700, lambda phage genomic libraries were constructed with genomic DNAs from the strains as described previously (12). The frxA- and rdxA-positive phage clones from each genomic library were screened by plaque hybridization with the frxA- and rdxA-specific PCR clones described above. The appropriate restriction fragments from the screened phage clones carrying the frxA and rdxA genes were identified by hybridization with the same probes and inserted into pBluescript SK(+) or H. pylori-E. coli shuttle vector pHel2 (19). The cloned frxA and rdxA genes from each H. pylori strain were used for DNA sequencing or complementation of Mtz sensitivity. The DNA sequences of both DNA strands of the cloned genes were determined at the Molecular Genetics Facility at the Baylor College of Medicine. DNA sequence analysis was accomplished by the BLAST network service of the National Center for Biotechnology Information.
Complementation of Mtz sensitivity.
For complementation of an Mtz-sensitive strain to an Mtz-resistant strain, plasmid DNA [1 to 2 μg; the cloning vector was pBluescript SK(+), which is not replicated in H. pylori] that carried naturally inactivated frxA or rdxA genes was introduced into Mtz-sensitive H. pylori 2600 by natural transformation (19). Transformed H. pylori 2600 was screened on a 7% horse blood–BHI agar plate supplemented with 4 μg of Mtz per ml. For the complementation of an Mtz-resistant strain to an Mtz-sensitive strain, the functional frxA and/or rdxA gene from an Mtz-sensitive H. pylori 2600 isolate was cloned into H. pylori-E. coli shuttle vector pHel2. The cloned frxA and/or rdxA gene in pHel2 was introduced into rifampin-resistant H. pylori strain 1857 (Mtz MIC, 128 μg/ml) by triparental conjugation (19), and the conjugated H. pylori colonies (10 of each) were used to measure Mtz sensitivity.
Mtz nitroreductase enzyme assay.
E. coli XL-1 Blue carrying the cloned frxA and rdxA genes was aerobically cultured to the late log phase in LB broth to measure Mtz nitroreductase activity. The cells were harvested in phosphate-buffered saline containing 1 mM dithiothreitol (4°C) to protect oxygen-sensitive enzymes and subjected to French pressure (600 lb; Aminco, Urbana, Ill.). The cell-free crude extracts were centrifuged at 15,000 × g for 15 min at 4°C to remove the cell debris, and the supernatant was immediately used as the enzyme source. The Mtz nitroreductase activity from the cells was measured by the method of Goodwin et al. (14). All enzyme reactions were performed at 25°C in 1-ml volumes in triplicate, and enzymatic activities were calculated as nanomoles per minute per milligram of protein. The protein concentrations of the crude cell extracts were determined by the Bradford procedure (Bio-Rad) with bovine serum albumin as the standard.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the frxA genes are AF183174 for strain 2600, AF1833992 for strain 6013, AF183176 for strain 1857, and AF183175 for strain 1700. The GenBank accession numbers for the rdxA genes are AF184266 for strain 2600, AF184268 for strain 6013, AF184269 for strain 1857, and AF184267 for strain 1700.
RESULTS
Analysis of Mtz resistance among clinical H. pylori isolates.
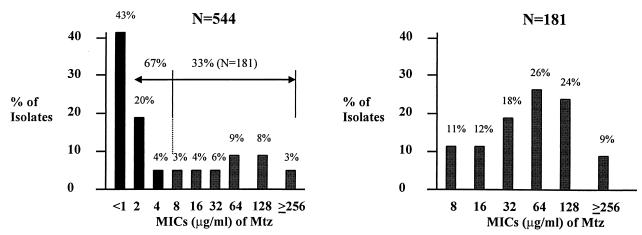
The heterogeneity of Mtz resistance in a single or multiple colony expansion was demonstrated with 12 H. pylori strains isolated from duodenal ulcer patients (7). To understand the variations in the MICs for clinical H. pylori isolates, we determined the MICs for 544 H. pylori strains from the Veterans Affairs Medical Center, Houston, Tex., using the agar dilution method as described above. Since strain ATCC 700392 was considered Mtz sensitive (14), we carefully repeated the MIC measurement using both the agar dilution method and the E-test as described previously (18). The Mtz MIC for H. pylori ATCC 700392 was repeatedly 8 μg/ml by the agar dilution method and 16 μg/ml by the E-test. The MICs for 544 clinical H. pylori isolates revealed that 33% were Mtz resistant (181 of 544 isolates; Mtz MICs, ≥8 μg/ml) and 67% were Mtz sensitive strains (363 of 544 isolates; Mtz MICs, ≤4 μg/ml), with a wide spectrum in the MICs (8 to ≥256 μg/ml) among Mtz-resistant strains (Fig. 1).
FIG. 1.
Heterogeneity of Mtz resistance among clinical H. pylori isolates. (A) Distribution of Mtz MICs for clinical H. pylori isolates (n = 544). The MICs were determined by twofold agar dilution methods. A total of 181 of 544 isolates were Mtz resistant. (B) Distribution of Mtz MICs for Mtz-resistant H. pylori isolates (n = 181). The MICs were used to analyze the Mtz-resistant H. pylori isolates (181 of 544 isolates).
Inactivation analysis of genes encoding putative Mtz nitroreductases (fdxA, fdxB, fldA, frxA, rdxA, oorD, porD) using genetic transformation of clinical H. pylori.
We identified genes from the complete H. pylori genomic DNA sequence (e.g., fdxA, fdxB, fldA, frxA, rdxA, oorD, and porD) that encode putative Mtz nitroreductases. We evaluated the natural competence and transformation frequencies of 50 strains (25 Mtz-sensitive and 25 Mtz-resistant strains including H. pylori ATCC 700392) selected from among the 544 clinical H. pylori isolates. As a control gene for natural transformation, we chose the ureB gene, which is not essential for H. pylori survival (11). The amino terminus (717 bp) of the ureB gene from H. pylori ATCC 700392 was amplified with a PCR primer pair (URE-A–URE-B; Table 1), and the PCR-amplified ureB gene was inactivated by inserting a chloramphenicol resistance cassette (cat) (41). The plasmid that contained the inactivated ureB gene (pUre1) was used for inactivation of the H. pylori chromosomal ureB gene. Of the 50 clinical H. pylori isolates, 18 strains (7 Mtz-sensitive and 11 Mtz-resistant strains) were able to take up plasmid pUre1, as determined by expression of the chloramphenicol resistance marker in the progeny H. pylori, when it was applied by natural transformation (19). The inactivation of ureB in the chromosomal DNA was confirmed by PCR amplification of the ureB gene from parental and mutant H. pylori strains following Southern blot hybridization as described previously (28) and by the urease-negative activities of the ureB mutant H. pylori strains. The transformation frequencies of the 18 H. pylori isolates ranged from 4 × 10−3 (strain 2600) to 9 × 10−6 (strain 2399) viable cells with plasmid pUre1. Thirty-two of the 50 H. pylori isolates were nontransformable with pUre1. The 32 nontransformable strains were also tested for natural competence by the method of Wang et al. (41), and the antibiotic resistance markers from pUre1 failed to be introduced into these strains. We used the 18 transformable H. pylori strains for the inactivation of the genes that encode putative Mtz nitroreductases. To test whether the genes were inactivated without a lethal effect, in vitro inactivated genes (by the cat gene) that encode putative Mtz nitroreductases (pGH67 for fdxA, pGH58 for fdxB, pGH64 for fldA, pGH130 for frxA, pGH55 for rdxA, pGH60 for oorD, pGH46 for porD) were introduced into H. pylori 2600. The results revealed that only fdxB, frxA, and rdxA were inactivated without a lethal effect on H. pylori 2600. Inactivation of all the other genes (fdxA, fldA, oorD, and porD) to produce viable cells failed when the inactivation was repeated by the transformation method of either Heuermann and Haas (19) or Wang et al. (41), suggesting that the cells were nonviable as a result of the inactivation. The rdxA inactivation and the lethal effect of oorDABC and porCDAB inactivation have been reported previously (14, 22).
Mtz sensitivity analysis of H. pylori strains in which fdxB, frxA, and rdxA are inactivated.
Although the involvement of the null mutation in the rdxA gene in Mtz resistance has been shown (14), inactivation of rdxA results in a narrow range of MICs (e.g., 16 to 32 μg/ml), which is different from the wide range of MICs shown for clinical H. pylori isolates. To assess the roles of the fdxB, frxA, and rdxA genes in Mtz resistance, we inactivated the fdxB, frxA, and rdxA genes using the 18 transformable H. pylori strains (7 Mtz-sensitive and 11 Mtz-resistant strains). In addition, we also inactivated the fdxB or frxA with the rdxA genes (dual inactivation). Inactivation of one or both genes was confirmed by PCR amplification following Southern blot hybridization as described previously (28). To avoid chloramphenicol- or kanamycin-resistant H. pylori mutants that contained a single crossover during homologous recombination (i.e., false-positive recombination), new PCR primer pairs positioned 193 to 960 bp away from the positions of the sequences of the PCR primer pairs for the PCR clones used for inactivation were designed (Table 2). The integrities of the mutant genes were then reconfirmed by PCR amplification with the new PCR primer pairs. The integrity of the mutant phenotype (antibiotic resistance) was also confirmed with 10 colonies isolated from each mutant strain. All the confirmed mutant strains were then analyzed for Mtz sensitivity (Table 3 and Table 4). The MICs for all mutant strains were determined simultaneously, and the results were confirmed twice. The MIC for a strain that had an inactivated fdxB gene and that was constructed from the Mtz-sensitive strains was not different from those for the parental strains. The MICs for strains that had inactivated rdxA genes and that were constructed from seven Mtz-sensitive strains were increased to 32 μg/ml for six strains, to 128 μg/ml for one strain (strain 2600). The MICs for the same seven strains but with inactivated frxA genes were also increased to the same levels as those for the strains with inactivated rdxA genes, irrespective of the slower growth rate of the strains inactivated with the frxA genes. Interestingly, the MICs for the seven Mtz-sensitive strains with the rdxA-fdxB dual inactivation increased to 64 μg/ml (i.e., greater than that for strains with either an inactivated rdxA gene or an inactivated fdxB gene) for six strains and to 128 μg/ml for one strain (strain 2600). These results are consistent with the notion that fdxB inactivation is involved in Mtz resistance but at a lower level than the level of involvement of rdxA inactivation. In addition, the MICs for the seven Mtz-sensitive strains with the frxA-rdxA dual inactivation increased to 128 μg/ml Mtz for six strains; the MIC for one strain (strain 2600) increased to >256 μg/ml (Table 3).
TABLE 2.
PCR primers used to confirm whether the H. pylori genes were interrupted
| Primer pair | Encoded protein (gene) | Nucleotide sequencea | Size (bp) of PCR fragment |
|---|---|---|---|
| FXLK-A1b | Ferredoxin-like protein (fdxB; HP1508d) | 5′-CCTAAAATGCTAGCGATAGC-3′ | 1,850 |
| FXLK-B1c | 5′-ATCAAACAAGGCTTGCCTTA-3′ | ||
| FLVN-A1 | NAD(P)H flavin oxidoreductase (frxA: HP0642) | 5′-CAAAGCTTGGGTTACCAGCACC-3′ | 1,254 |
| FLVN-B1 | 5′-CCGCTTCCGCGTTTTGCTTCGTA-3′ | ||
| OXI-A1 | Oxygen-insensitive nitroreductase (rdxA; HP0954) | 5′-AAGCTTTTGATTTATTTGGA-3′ | 1,130 |
| OXI-B1 | 5′-CTTTAATTTAGGTTTGATTA-3′ | ||
| URE-A1 | Urease B (ureB; HP0072) | 5′-ATGAAAAAGATTAGCAGAAA-3′ | 1,710 |
| URE-B1 | 5′-CTAGAAAATGCTAAAGAGTT-3′ |
Nucleotide sequences were obtained from the complete H. pylori genome sequence.
Forward primer.
Backward primer.
Designation in the complete H. pylori genome sequence.
TABLE 3.
Mtz sensitivity analysis of Mtz-sensitive H. pylori strains with inactivated fdxB, frxA, and rdxA genes
| H. pylori strain | MIC (μg/ml) for the clinical isolate or mutant straina:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Clinical isolates | fdxB::catb | frxA::cat | rdxA::cat | ureBc::cat | fdxB::cat rdxA::kmd | frxA::cat rdxA::km | ureB::cat rdxA::km | |
| 2714 | 1 | 1 | 32 | 32 | 1 | 64 | 128 | 32 |
| 2393 | 1 | 4 | 32 | 32 | 1 | 64 | 128 | 32 |
| 2667 | 1 | 2 | 32 | 32 | 1 | 64 | 128 | 32 |
| 2600 | 2 | 2 | 128 | 128 | 2 | 128 | >256 | 128 |
| 2201 | 2 | 2 | 32 | 32 | 2 | 64 | 128 | 32 |
| 2399 | 4 | 2 | 32 | 32 | 2 | 64 | 128 | 32 |
| 2418 | 4 | 2 | 32 | 32 | 2 | 64 | 128 | 32 |
MICs were determined by growth on 7% horse blood–BHI agar plates supplemented with 0.5, 1, 2, 4, 8, 16, 32, 64, 128, or 256 μg of Mtz per ml and incubation for 4 days. The results were confirmed twice by measuring all MICs at the same time.
cat, chloramphenicol resistance gene.
ureB::cat was used as a negative control.
km, kanamycin resistant gene.
TABLE 4.
Mtz sensitivity analysis of Mtz-resistant H. pylori strains with inactivated fdxB, frxA, and rdxA genes
| H. pylori strain | MIC (μg/ml) for the clinical isolate or mutant straina:
|
|||
|---|---|---|---|---|
| Clinical isolates | fdxB::cat | frxA::cat | rdxA::cat | |
| ATCC 700392 | 8 | 64 | 32 | 64 |
| 2617 | 8 | 32 | 64 | 64 |
| 2593 | 8 | 32 | 64 | 64 |
| 2620 | 32 | 64 | 128 | 64 |
| 6013 | 32 | 64 | 32 | 128 |
| 2723 | 64 | 128 | 128 | 128 |
| 9004 | 64 | 128 | 128 | 128 |
| 9002 | 128 | NDb | 128 | 128 |
| 1857 | 128 | ND | 128 | 128 |
| 1700 | 256 | ND | 256 | 256 |
| 7200 | 256 | ND | 256 | 256 |
MICs were determined by growth on 7% horse blood–BHI agar plates supplemented with 0.5, 1, 2, 4, 8, 16, 32, 64, 128, 256 μg of Mtz per ml and incubation for 4 days. The results were confirmed twice by measuring all MICs at the same time.
ND, not determined.
We also analyzed the Mtz sensitivities of mutant strains constructed from Mtz-resistant strains for which Mtz MICs were between 8 and 64 μg/ml. The MICs for the strains that had inactivated fdxB genes and that were constructed from these resistant strains increased up to eightfold, which provided additional evidence for the involvement of fdxB inactivation in the Mtz resistance of H. pylori. The MICs for the strains that had inactivated frxA or rdxA genes and that were constructed from strains with low or moderate levels of resistance also increased up to eightfold, suggesting that multiple genes or factors are involved in the wide spectrum of Mtz resistance. Interestingly, the MIC for strain 6013 (32 μg/ml) was not changed when the frxA gene was inactivated, whereas it increased fourfold when the rdxA gene was inactivated, suggesting that strain 6013 contains a nonfunctional frxA gene and a functional rdxA gene. Additionally, the MICs for strains 9002 and 1857 (128 μg/ml) and strains 1700 and 7200 (256 μg/ml) did not change because of either frxA or rdxA inactivation, suggesting that the strains may contain both nonfunctional frxA and nonfunctional rdxA genes in their genomes (Table 4).
Cloning and nucleotide sequence analysis of frxA and rdxA genes from H. pylori strains.
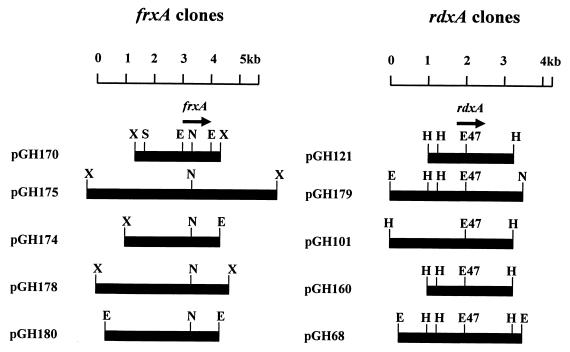
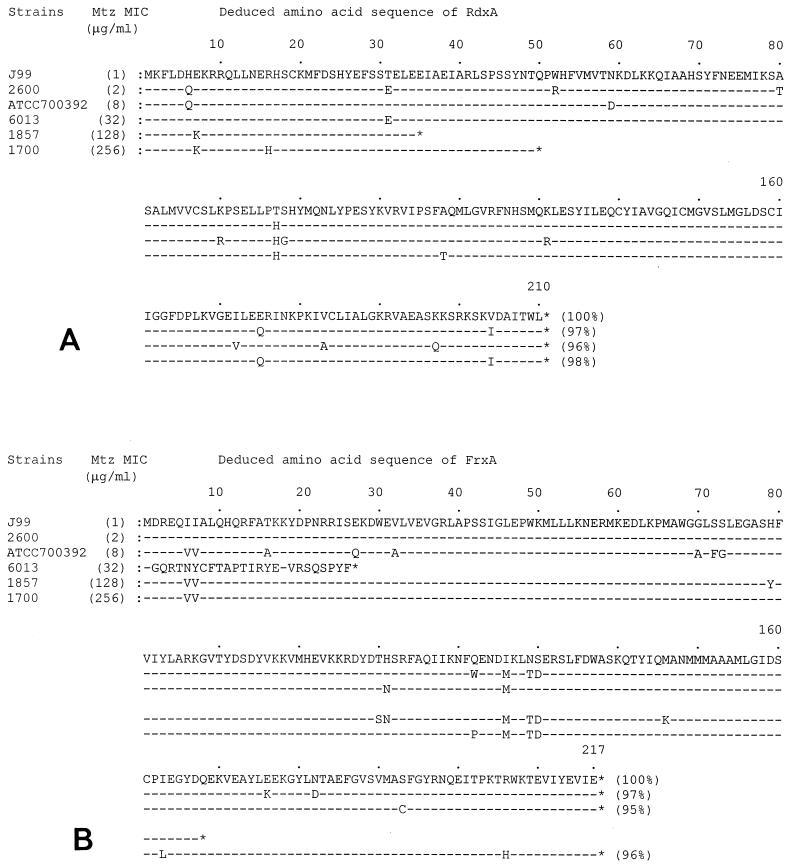
To prove that naturally inactivated frxA genes are present in clinical Mtz-resistant H. pylori isolates, the frxA and rdxA genes were cloned from Mtz-resistant strains and the nucleotide sequences were analyzed. Because the DNA sequences of the PCR clones may not always be identical to the parental DNA sequence, we constructed lambda phage libraries using genomic DNAs purified from Mtz-resistant strains ATCC 700392, 6013, 1857, and 1700 and Mtz-sensitive strain 2600. frxA- and rdxA-positive clones were isolated from each genomic library, and the restriction enzyme maps of the initial clones are shown in Fig. 2. The restriction enzyme sites were highly diverse among the clones except in the frxA- and the rdxA-coding regions, reflecting the genomic DNA sequence diversity in H. pylori strains. The DNA sequences of the frxA and rdxA genes were determined by using subclones of the initial clones, and the deduced amino acid sequences of the genes were in alignment, as shown in Fig. 3. Nucleotide sequence analysis of an ∼1.0-kb EcoRI fragment from pGH170 (cloned from strain 2600) revealed that the 971-bp fragment contained an frxA gene with 97% identity compared to the sequence of frxA from strain J99 (Fig. 3B). Upstream of the frxA gene was the carboxyl terminus (78 of 285 amino acids) of the putative 3-hydroxyacid dehydrogenase gene, with only 2 bp of intervening nucleotides. The FrxA proteins from resistant strains 6013 and 1857 were truncated by insertion of one nucleotide (G) in the FrxA-coding region (between the 1st and 2nd amino acids), which shifted a reading frame of FrxA, and a nonsense mutation (168th amino acid, CAA [Gln] to TAA [stop codon]), respectively (Fig. 3B). However, the FrxA protein from resistant strain 1700 was not truncated and showed 97% identity compared with the sequence of the FrxA protein from Mtz-sensitive strain J99 (Mtz MIC, 1 μg/ml) (1; Richard A. Alm, personal communication). The FrxA protein from low-level Mtz-resistant strain ATCC 700392 showed 95% identity compared with the sequence of the FrxA protein from strain J99. On the other hand, the RdxA proteins from resistant strains 1857 and 1700 were truncated by nonsense mutations. However, the RdxA protein from resistant strain 6013 was not truncated and showed 98% identity compared with the sequence of the RdxA protein from Mtz-sensitive strain J99. The RdxA protein from low-level Mtz resistant strain ATCC 700392 showed 96% identity compared with the sequence of the RdxA protein from strain J99 (Fig. 3A).
FIG. 2.
Restriction endonuclease map of frxA and rdxA clones from the lambda phage library constructed with genomic DNAs from Mtz-sensitive and -resistant H. pylori strains. The phage clones that carried an frxA gene or an rdxA gene were screened by plaque hybridization. Restriction fragments that contained an frxA or an rdxA gene were identified by Southern hybridization. The restriction fragments that contained the frxA or rdxA gene were inserted into pBluescript SK(+) digested with the same or appropriate restriction enzymes. pGH170 and pGH121 were cloned from the genomic DNA of H. pylori 2600 (Mtz MIC, 2 μg/ml), pGH175 and pGH179 were cloned from the genomic DNA of H. pylori ATCC 700392 (Mtz MIC, 8 μg/ml), pGH174 and pGH101 were cloned from the genomic DNA of H. pylori 6013 (Mtz MIC, 32 μg/ml), pGH178 and pGH160 were cloned from the genomic DNA of H. pylori 1857 (Mtz MIC, 128 μg/ml), and pGH180 and pGH68 were cloned from the genomic DNA of H. pylori 1700 (Mtz MIC, 256 μg/ml). E, EcoRI; E47, Eco47III; H, HindIII; N, NsiI; S, SphI; X, XbaI.
FIG. 3.
Alignment of RdxA (A) and FrxA (B) amino acid sequences from Mtz-sensitive and -resistant H. pylori strains. Percentages in parentheses are percent identity.
Complementation analysis of Mtz sensitivity using cloned frxA and rdxA genes in H. pylori.
It has been shown that the Mtz resistance of H. pylori can be transferred from a resistant strain to a sensitive strain by introduction of genomic DNA from a resistant strain into a sensitive strain (20, 41). On the basis of the results presented above, the Mtz resistance acquired by the sensitive strain may be due to replacement of the inactivated rdxA and/or frxA genes from the resistant strain. We examined whether the naturally inactivated frxA genes from Mtz-resistant strains were able to transfer Mtz resistance to Mtz-sensitive strains. As shown in Table 5, plasmid DNA that contained naturally inactivated frxA genes from Mtz-resistant strains 6013 and 1857 (pGH174 and pGH160, respectively) successfully transferred Mtz resistance to Mtz-sensitive strain 2600 with transformation frequencies of 0.5 × 103 and 0.58 × 103 CFU per 1 μg of plasmid DNA, respectively. In the same complementation study, the naturally inactivated rdxA genes from Mtz-resistant strains 1857 and 1700 (pGH178 and pGH68, respectively) also transferred Mtz resistance to Mtz-sensitive strain 2600 with transformation frequencies of 0.52 × 103 and 0.5 × 104 CFU per 1 μg of plasmid DNA, respectively. However, none of the plasmid DNAs that contained functional frxA or rdxA genes (pGH170, pGH121, pGH101, and pGH180) transferred Mtz resistance to Mtz-sensitive strain 2600. We also introduced a functional frxA and/or rdxA gene cloned in the H. pylori-E. coli shuttle vector (pHel2) into one of the Mtz-resistant strains to confirm whether the functional genes were able to restore the Mtz sensitivities of the strains. Mtz resistance in strain 1857 (Mtz MIC, 128 μg/ml) was partially decreased by a functional frxA gene (pGH177) or a functional rdxA gene (pGH127), but it was made completely susceptible (MIC, 1 μg/ml) when both functional frxA and rdxA genes (pGH181) were introduced. These results are consistent with the notion that the frxA inactivation is involved in Mtz resistance.
TABLE 5.
Complementation analysis of Mtz sensitivity with cloned frxA and rdxA genes
| H. pylori strain and clone | Transformation frequencya (CFU/μg of plasmid DNA) |
|---|---|
| No DNA | Noneb |
| H. pylori 2600 (Mtz MIC, 2 μg/ml) | |
| pGH170 (frxA) | None |
| pGH121 (rdxA) | None |
| H. pylori 6013 (Mtz MIC, 32 μg/ml) | |
| pGH174 (frxA)c | 0.5 × 103 |
| pGH101 (rdxA) | None |
| H. pylori 1857 (Mtz MIC, 128 μg/ml) | |
| pGH160 (frxA)d | 0.58 × 103 |
| pGH178 (rdxA)d | 0.52 × 103 |
| H. pylori 1700 (Mtz MIC, 256 μg/ml) | |
| pGH180 (frxA) | None |
| pGH68 (rdxA)d | 0.5 × 104 |
Approximately 3 × 107 cells (optical density at 550 nm = 0.1) of H. pylori 2600 were used for natural transformation by applying plasmid DNA (1 μg) purified from E. coli DH5α, and Mtz-resistant H. pylori 2600 was screened on BHI-horse blood agar plates supplemented with 4 μg of Mtz per ml (the values are the averages of two experiments).
Spontaneous Mtz-resistant cells (two or three colonies) were detected, and these cell numbers were subtracted from the number of transformants.
FrxA was truncated by insertion of one nucleotide (G) in the coding region (see text).
FrxA or RdxA was truncated by nonsense mutations (see text).
Expression of cloned frxA and rdxA genes in E. coli.
We performed the Mtz nitroreductase assay using E. coli strains that harbored cloned frxA genes or an rdxA gene (as a positive control) to verify whether the cloned frxA gene from the Mtz-sensitive H. pylori strain possessed Mtz nitroreductase activity. Mtz nitroreductase activity was always demonstrable in the E. coli strains that harbored a cloned rdxA gene from the Mtz-sensitive strain H. pylori 2600, with the enzyme activity varying between 5.3 and 7.8 nmol/mg/min. By the same assay Mtz nitroreductase activity was not detected in the E. coli strains that harbored a cloned frxA gene from Mtz-sensitive strain H. pylori 2600. The difficulty in measuring Mtz nitroreductase activity in crude extracts may be due to oxidation of key components during the preparation of crude extracts or the interference of endogenous nitroreductase from E. coli as described by Goodwin et al. (14).
An alternative method for detection of Mtz nitroreductase activity in E. coli is an in vivo assay, which measures the MICs for E. coli strains that harbor a cloned frxA or rdxA gene from H. pylori. The in vivo assay is based on expression of a cloned frxA or rdxA gene in E. coli cells and measurement of the Mtz sensitivities of the E. coli cells that harbor the cloned genes. If the cloned gene expresses and produces Mtz nitroreductase in intrinsically Mtz-resistant E. coli, the enzyme should convert nontoxic Mtz to toxic Mtz and the E. coli cells will become sensitive to Mtz (decreased MICs). Since E. coli DH5α is intrinsically Mtz resistant (MIC, >320 μg/ml), we introduced the cloned frxA and rdxA genes from Mtz-sensitive and -resistant H. pylori strains. The E. coli DH5α cells that harbored the genes were cultured aerobically in LB broth and spotted (5 μl of each strain) onto LB agar plates supplemented with 10 to 320 μg of Mtz per ml. The plates were then incubated aerobically at 37°C for 18 h. The E. coli DH5α cells that harbored either the frxA or the rdxA gene cloned from Mtz-sensitive strain H. pylori 2600 (pGH170 or pGH121, respectively) did not grow in the presence of 20 to 40 μg of Mtz per ml. In contrast, only the vector [pBluescript SK(+) or pHel2] or plasmid that contained a part of the frxA or the rdxA gene (pGH172, XbaI-NsiI fragment of pGH170; pGH104, Eco47III-HindIII fragment of pGH121; see Fig. 2) grew on medium with 320 μg of Mtz per ml. Interestingly, E. coli cells that harbored the SphI-XbaI fragment (2.5 kb; pGH173) of pGH170 also grew on medium with 320 μg of Mtz per ml (Table 6). E. coli cells that harbored the same frxA or rdxA gene in the H. pylori-E. coli shuttle vector (pHel2) were also sensitive to Mtz, but the MICs for the strains were slightly higher (MICs, 40 to 80 μg/ml for strains with pGH127 and pGH177) than those obtained when pBluescript SK(+) was used as a cloning vector. In addition, E. coli cells that harbored both genes (frxA and rdxA) in pHel2 (pGH181) did not grow on LB agar plates supplemented with 20 to 40 μg of Mtz per ml. Although the MICs were slightly variable in the in vivo assay, the results were reproducible and consistent. Therefore, we applied the assay to the other cloned frxA and rdxA genes from Mtz-resistant H. pylori strains. E. coli cells that harbored a frxA gene (pGH175) or a rdxA gene (pGH179) from H. pylori ATCC 700392 did not grow on LB agar plates supplemented with 80 to 160 and 20 to 40 μg of Mtz per ml, respectively. Repetition of these assays with pGH175 and pGH179 gave identical results. We also confirmed the involvement of an frxA gene from strain ATCC 700392 in Mtz sensitivity by comparing the expression of a part of the frxA gene (XbaI-NsiI fragments of pGH175; Fig. 2) that, when expressed in E. coli, resulted in loss of Mtz nitroreductase activity of the gene. E. coli cells that harbored frxA (pGH174 and pGH160) or rdxA (pGH178 and pGH68) from Mtz-resistant strains 6013, 1857, and 1700 grew on LB agar plates supplemented with 320 μg of Mtz per ml, while E. coli cells that harbored frxA (pGH180) and rdxA (pGH101) from Mtz-resistant strains 6013 and 1700 did not grow on LB agar plates supplemented with 20 to 40 μg of Mtz per ml (Table 6).
TABLE 6.
MICs for E. coli cells expressing cloned frxA and rdxA genes
| H. pylori strain and clone | Mtz MIC (μg/ml)a |
|---|---|
| pBluescript SK(+)b | >320 |
| pHel2c | >320 |
| H. pylori 2600 (Mtz MIC, 2 μg/ml) | |
| pGH170 (frxA) | 40 |
| pGH121 (rdxA) | 40 |
| pGH177 (frxA) | 80 |
| pGH127 (rdxA) | 80 |
| pGH181 (frxA/rdxA) | 40 |
| pGH172 (ΔfrxA)d | >320 |
| pGH173 (frxA)d | >320 |
| pGH104 (ΔrdxA)e | >320 |
| H. pylori ATCC 700392 (Mtz MIC, 8 μg/ml) | |
| pGH175 (frxA) | 160 |
| pGH179 (rdxA) | 40 |
| H. pylori 6013 (Mtz MIC, 32 μg/ml) | |
| pGH174 (frxA)f | >320 |
| pGH101 (rdxA) | 40 |
| H. pylori 1857 (Mtz MIC, 128 μg/ml) | |
| pGH160 (frxA)g | >320 |
| pGH178 (rdxA)g | >320 |
| H. pylori 1700 (Mtz MIC, 256 μg/ml) | |
| pGH180 (frxA) | 40 |
| pGH68 (rdxA)g | >320 |
Overnight cultures (5 μl) of E. coli DH5α harboring each clone were spotted onto LB agar plates supplemented with 10, 20, 40, 80, 160, or 320 μg of Mtz per ml, and the plates were incubated for 18 h. The measurement was repeated three times with identical results.
pBluescript SK(+) was used as a cloning vector for all clones except pGH127, pGH177, and pGH181.
pHel2 was used as a cloning vector for clone pGH127, pGH177, and pGH181, which carried frxA or rdxA from pGH170 or pGH121, respectively, to introduce the clones into H. pylori 1857.
pGH172 and pGH173 originated from pGH170.
pGH104 originated from pGH121.
FrxA was truncated by insertion of one nucleotide (G) in the coding region (see text).
FrxA or RdxA were truncated by nonsense mutations (see text).
DISCUSSION
H. pylori infection is responsible for most cases of peptic ulcer disease, and successful treatment of the infection results in cure of the disease. In the last decade, a number of regimens for the treatment of H. pylori infection have been introduced. Mtz resistance among H. pylori isolates has been found worldwide and has become an increasing problem for current therapies. The deciphering of the Mtz resistance mechanism may provide critical information for (i) the appropriate antibiotic treatment of this infection, (ii) better therapy for infections caused by Mtz-resistant H. pylori strains and perhaps for those caused by other Mtz-resistant microorganisms, and (iii) the design of new antibiotics. The mechanism of Mtz resistance in H. pylori was initially explained by mutations in an rdxA gene (14). However, as shown here and by Jenks et al. (24), an intact rdxA gene can be found in some Mtz-resistant strains, suggesting that an additional resistance mechanism(s) is involved in Mtz resistance. To investigate whether additional Mtz resistance mechanisms were present in H. pylori, we examined the nature of Mtz resistance among 544 clinical H. pylori isolates, clarified the role of an rdxA gene in a wide range of Mtz-resistant H. pylori isolates, and explored additional genes that might be involved in Mtz resistance. The 33% rate of Mtz resistance found in this study is in agreement with the rates detected by other investigators (13, 32). The proposed breakpoint for Mtz resistance used in this study was an MIC of ≥8 μg/ml, which is based on the finding that inactivation of the rdxA or frxA genes of H. pylori strains for which the MICs are ≤4 μg/ml always increased the MICs to 32 μg/ml but inactivation of strains for which the MICs are ≥8 μg/ml increased the MICs to >32 μg/ml. Inactivation of fdxB in strains for which the MICs are ≥8 μg/ml also increased the MICs compared with those for the parental strains. These results suggest that acquired Mtz resistance begins at an MIC of approximately ≥8 μg/ml. Mtz MICs for Mtz-sensitive strain 2600 fluctuated from <1 to 4 μg/ml, but the strain never grew in the presence of 4 μg of Mtz per ml (MIC, <8 μg/ml).
Inactivation of rdxA in Mtz-sensitive strains always increased the MIC to 32 μg/ml. For the Mtz-sensitive strains with inactivated rdxA genes, the MICs were never lower than 32 μg/ml for any of the isolates, suggesting that complete rdxA inactivation may generally increase the MIC to 32 μg/ml (moderate level of Mtz resistance). For one strain (strain 2600), inactivation of rdxA increased the Mtz MIC to 128 μg/ml (high-level resistance), suggesting that rdxA inactivation may play a role in high-level Mtz resistance, although the possibility that an additional factor(s) or a lack of a factor(s) may also be involved in high-level Mtz resistance. This observation was also shown in high-level Mtz-resistant clinical isolate 1700, in which only rdxA was inactivated.
Theoretically, any protein that produces or inhibits Mtz nitroreductase activity could be involved in Mtz sensitivity. Purified PorCDAB and FldA proteins were tested in vitro and the results suggested that these proteins are putative Mtz nitroreductases (23, 26). Inactivation of PorCDAB was lethal to H. pylori (22), and we also confirmed that inactivation of the porCDAB or fldA gene was lethal to H. pylori. The inactivation of other ferredoxin-like or -linked proteins (FdxA and OorD) that may have Mtz nitroreductase activities was also lethal to H. pylori. Since FdxA, FldA, PorCDAB, and OorDABC appeared to be essential for cell survival, it was difficult to assess the roles of these proteins in Mtz resistance. Although inactivation of a single gene (fdxB) in an Mtz-sensitive strain had no effect on the MIC compared to that for the parental strain, the rdxA-fdxB dual inactivation increased the MIC twofold compared with that for Mtz-sensitive strain in which a single gene (rdxA) was inactivated (from 32 to 64 μg/ml). In addition, the MICs for the low-level Mtz-resistant strains (MICs, 8 μg/ml) in which a single gene (fdxB) was inactivated were also increased fourfold and eightfold, as shown in Table 4. These results indicate that the fdxB inactivation is also involved in increasing the level of Mtz resistance. It is not clear why the MIC was unchanged for an Mtz-sensitive strain with an inactivated fdxB gene. However, it could be possible that the Mtz nitroreductase activities from RdxA and FrxA were much higher than that from FdxB in the Mtz-sensitive strain that contained fully functional rdxA and frxA genes, which might lead to no effect of the single fdxB inactivation.
Expression of the frxA gene cloned from H. pylori 26695 in E. coli resulted in no significant difference in the Mtz sensitivity of Mtz-resistant E. coli (14). However, Mtz sensitivity analysis by frxA inactivation of Mtz-sensitive H. pylori strains and H. pylori strains with low-level or moderate Mtz resistance showed that frxA inactivation conferred Mtz resistance at a level similar to that achieved by rdxA inactivation. In particular, nucleotide sequence analysis of the frxA genes from clinical isolates with moderate and high levels of Mtz resistance provided genetic evidence of the involvement of frxA in Mtz resistance. One moderately Mtz-resistant strain 6013 (Mtz MIC, 32 μg/ml) carried an inactivated frxA gene but a fully functional rdxA gene. Mtz sensitivity analysis of strains with inactivated frxA and/or rdxA genes showed that strain 1857 with both inactivated frxA and inactivated rdxA genes had a high level of Mtz resistance, as shown for strain 2600, in which both frxA and rdxA were inactivated. In addition, Mtz sensitivity analysis of strains with inactivated frxA and/or rdxA genes allowed us to find strain 1700, which contained a single inactivated gene (rdxA) and which had a high level of Mtz resistance, as was also shown for strain 2600, which had a single inactivated gene (rdxA). Comparative analyses of complementation of Mtz sensitivity from either an Mtz-sensitive strain to an Mtz-resistant strain, or vice versa, with inactivated or functional frxA and rdxA genes, respectively, coupled with the expression of cloned inactivated and functional frxA and rdxA genes in E. coli, prove that the frxA gene is involved in Mtz resistance. Comparison of FrxA and RdxA protein sequences from H. pylori ATCC 700392 showed 25% identity and 63% similarity with the absolutely conserved amino acid PW (Pro, Trp) at positions 51 and 52 of classical nitroreductases (14), indicating that the FrxA protein also possesses Mtz nitroreductase activity, as shown for the RdxA protein.
We also confirmed that the frxA gene from H. pylori strain ATCC 700392 was involved in Mtz resistance. The frxA gene cloned from strain ATCC 700392 [5.3-kb XbaI fragment in pBluescript SK(+)] was expressed in E. coli and converted the Mtz MICs for the cells from >320 μg/ml to 80 to 160 μg/ml. Although the difference in the MIC was not very impressive, as shown for the frxA gene from strain 2600 (Mtz MIC, >320 to 20- to 40 μg/ml), cloning of the frxA gene from strain ATCC 700392 significantly decreased the MIC. However, the MIC for strain ATCC 700392 with an inactivated frxA gene did not increase as much as those for strains 2617 and 2593 with inactivated frxA genes (for which the MICs were the same as that for strain ATCC 700392 [MIC, 8 μg/ml]), suggesting that frxA from strain ATCC 700392 may be partially inactivated. Indeed, 8 amino acids in the N terminus (the first 80 amino acids) of FrxA from strain ATCC 700392 were replaced by other amino acids, while none of the amino acids in the same FrxA from Mtz-sensitive strain 2600 was replaced when the sequence was compared with the FrxA sequence of Mtz-sensitive strain J99. The MIC of 8 μg/ml for strain ATCC 700392 was additional supporting evidence for the partial inactivation of the frxA gene. Another feature of frxA was the fact that a 972-bp EcoRI fragment and an ∼2.5-kb SphI-XbaI fragment from pGH170 (which carried the frxA gene from strain 2600) did not change MICs in E. coli (no difference in Mtz sensitivity). These results indicate that at least a 2.0-kb upstream flanking region of the frxA gene is required for appropriate Mtz nitroreductase expression in E. coli. Nucleotide sequence analysis showed that the FrxA protein followed a putative 3-hydroxyacid dehydrogenase at the carboxyl terminus with only two intervening nucleotide sequences, suggesting that the frxA mRNA may be cotranscribed with an upstream gene(s).
In summary, the overall finding of this study is that two genes (fdxB and frxA), in addition to the rdxA gene, are responsible for the Mtz resistance of H. pylori. The inactivation of fdxB, frxA, or rdxA is involved in different levels of Mtz resistance in H. pylori. These results lead us to hypothesize that the wide range of Mtz MICs seen for clinical H. pylori isolates may be due to partial and/or complete inactivation of the fdxB, frxA, and rdxA genes. Indeed, Mtz MICs of 32, 64, 128, and 256 μg/ml were created by inactivation of one or two of the three genes in Mtz-sensitive strains. Additionally, Mtz MICs of 8 and 16 μg/ml are also theoretically achievable by partial inactivation of the genes, as shown for strain ATCC 700392. However, the high-level Mtz resistance of some strains (e.g., strains 2600 and 1700) in which a single frxA or rdxA gene is inactivated suggests that additional Mtz resistance mechanisms exist in H. pylori.
ACKNOWLEDGMENTS
Thanks to Richard A. Alm for information on the Mtz MIC for H. pylori strain J99, to R. Haas for providing H. pylori-E. coli shuttle vectors and E. coli strain GC7 (pRK2013), and to D. E. Taylor for providing the chloramphenicol resistance gene cassette.
This work was supported in part by the U.S. Department of Veterans Affairs.
REFERENCES
- 1.Alm R A, Ling L L, Moir D T, King B L, Brown E D, Doig P C, Smith D R, Noonan B, Guild B C, deJonge B L, Carmel G, Tummino P J, Caruso A, Uria-Nickelsen M, Mills D M, Ives C, Gibson R, Merberg D, Mills S D, Jinag Q, Taylor D E, Vovis G F, Trust T J. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 2.Cederbrant G, Kahlmeter G, Ljungh A. Proposed mechanism for metronidazole resistance in Helicobacter pylori. J Antimicrob Chemother. 1992;29:115–120. doi: 10.1093/jac/29.2.115. [DOI] [PubMed] [Google Scholar]
- 3.Chang K C, Ho S W, Yang J C, Wang J T. Isolation of a genetic locus associated with metronidazole resistance in Helicobacter pylori. Biochem Biophys Res Commun. 1997;236:785–788. doi: 10.1006/bbrc.1997.7050. [DOI] [PubMed] [Google Scholar]
- 4.Crump M, Gospodarowicz M, Shepherd F A. Lymphoma of the gastrointestinal tract. Semin Oncol. 1999;26:324–337. [PubMed] [Google Scholar]
- 5.Declerk P J, Ranter C J. In vitro reductive activation of nitroimidazoles. Biochem Pharmacol. 1986;35:59–61. doi: 10.1016/0006-2952(86)90555-1. [DOI] [PubMed] [Google Scholar]
- 6.Declerk P J, de Ranter C J, Volckaert G. Base specific interaction of reductively activated nitroimidazoles with DNA. FEBS Lett. 1983;164:145–148. doi: 10.1016/0014-5793(83)80038-6. [DOI] [PubMed] [Google Scholar]
- 7.Dore M P, Osato M S, Kwon D H, Graham D Y, El-Zaatari F A K. Demonstration of unexpected antibiotic resistance of genotypically identical Helicobacter pylori isolates. Clin Infect Dis. 1998;27:84–89. doi: 10.1086/514640. [DOI] [PubMed] [Google Scholar]
- 8.Edwards D I. Nitroimidazole drugs—action and resistance mechanisms. I. Mechanisms of action. J Antimicrob Chemother. 1993;31:9–20. doi: 10.1093/jac/31.1.9. [DOI] [PubMed] [Google Scholar]
- 9.Edwards D I. Nitroimidazole drugs—action and resistance mechanisms. II. Mechanisms of resistance. J Antimicrob Chemother. 1993;31:201–210. doi: 10.1093/jac/31.2.201. [DOI] [PubMed] [Google Scholar]
- 10.Edwards D I, Dye M, Carne H. The selective toxicity of antimicrobial nitroheterocyclic drugs. J Gen Microbiol. 1973;76:135–145. doi: 10.1099/00221287-76-1-135. [DOI] [PubMed] [Google Scholar]
- 11.Ferrero R L, Cussac V, Courcoux P, Labigne A. Construction of isogenic urease-negative mutants of Helicobacter pylori by allelic exchange. J Bacteriol. 1992;174:4212–4217. doi: 10.1128/jb.174.13.4212-4217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge Z, Hiratsuka K, Taylor D E. Nucleotide sequence and mutational analysis indicate that two Helicobacter pylori genes encode a P-type ATPase and a cation-binding protein associated with copper transport. Mol Microbiol. 1995;15:97–106. doi: 10.1111/j.1365-2958.1995.tb02224.x. [DOI] [PubMed] [Google Scholar]
- 13.Glupczynski Y. Antimicrobial resistance in Helicobacter pylori: a global overview. Acta Gastro-Enterol Belg. 1998;61:357–366. [PubMed] [Google Scholar]
- 14.Goodwin A, Kersulyte D, Sisson G, Veldhuyzen van Zanten S J O, Berg D E, Hoffman P S. Metronidazole-resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NAD(P)H nitroreductase. Mol Microbiol. 1998;28:383–393. doi: 10.1046/j.1365-2958.1998.00806.x. [DOI] [PubMed] [Google Scholar]
- 15.Graham D Y, de Boer W A, Tytgat G N. Choosing the best anti-Helicobacter pylori therapy: effect of antimicrobial resistance. Am J Gastroenterol. 1996;91:1072–1076. [PubMed] [Google Scholar]
- 16.Graham D Y. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology. 1998;115:1272–1277. doi: 10.1016/s0016-5085(98)70100-3. [DOI] [PubMed] [Google Scholar]
- 17.Hachem C Y, Clarridge J E, Evans D G, Graham D Y. Comparison of agar based media for primary isolation of Helicobacter pylori. J Clin Pathol. 1995;48:714–716. doi: 10.1136/jcp.48.8.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hachem C Y, Clarridge J E, Reddy R, Graham D Y. Antimicrobial susceptibility testing of Helicobacter pylori. Comparison of E-test, broth microdilution, and disk diffusion for ampicillin, clarithromycin, and metronidazole. Diagn Microbiol Infect Dis. 1996;24:37–41. doi: 10.1016/0732-8893(95)00252-9. [DOI] [PubMed] [Google Scholar]
- 19.Heuermann D, Haas R. A stable shuttle vector system for efficient genetic complementation of Helicobacter pylori strains by transformation and conjugation. Mol Gen Genet. 1998;257:519–528. doi: 10.1007/s004380050677. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman P S, Goodwin A, Johnsen J, Magee K, Veldhuygen van Zanten S J. Metabolic activities of metronidazole-sensitive and -resistant strains of Helicobacter pylori: repression of pyruvate oxidoreductase and expression of isocitrate lyase activity correlate with resistance. J Bacteriol. 1996;178:4822–4829. doi: 10.1128/jb.178.16.4822-4829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houben M H, Beek D V, Hensen E F, Craen A J, Rauws E A, Tytgat G N. A systematic review of Helicobacter pylori eradication therapy—the impact of antimicrobial resistance on eradication rates. Aliment Pharmacol Ther. 1999;13:1047–1055. doi: 10.1046/j.1365-2036.1999.00555.x. [DOI] [PubMed] [Google Scholar]
- 22.Hughes N J, Clayton C L, Chalk P A, Kelly D J. Helicobacter pylori porCDAB and oorDABC genes encode distinct pyruvate:flavodoxin and 2-oxoglutarate:acceptor oxidoreductases which mediate electron transport to NADP. J Bacteriol. 1998;180:1119–1128. doi: 10.1128/jb.180.5.1119-1128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes N J, Chalk P A, Clayton C L, Kelly D J. Identification of carboxylation enzymes and characterization of a novel four-subunit pyruvate:flavodoxin oxidoreductase from Helicobacter pylori. J Bacteriol. 1995;177:3953–3959. doi: 10.1128/jb.177.14.3953-3959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenks P J, Ferrero R L, Labigne A. The role of the rdxA gene in the evolution of metronidazole resistance in Helicobacter pylori. J Antimicrob Chemother. 1999;43:753–758. doi: 10.1093/jac/43.6.753. [DOI] [PubMed] [Google Scholar]
- 25.Jorgensen M A, Manos J, Mendz G L, Hazell S L. The mode of action of metronidazole in Helicobacter pylori: futile cycling or reduction? J Antimicrob Chemother. 1998;41:67–75. doi: 10.1093/jac/41.1.67. [DOI] [PubMed] [Google Scholar]
- 26.Kaihovaara P, Hook-Nikanne J, Uusi-Oukari M, Kosunen T U, Salaspuro M. Flavodoxin-dependent pyruvate oxidation, acetate production and metronidazole reduction by Helicobacter pylori. J Antimicrob Chemother. 1998;41:171–177. doi: 10.1093/jac/41.2.171. [DOI] [PubMed] [Google Scholar]
- 27.Kedderis G L, Argenbright L S, Miwa G T. Mechanism of reductive activation of a 5-nitroimidazole by flavoproteins: model studies with dithionite. Arch Biochem Biophys. 1988;262:40–48. doi: 10.1016/0003-9861(88)90166-x. [DOI] [PubMed] [Google Scholar]
- 28.Kwon D H, Woo J S, Perng C L, Go M F, Graham D Y, El-Zaatari F A K. The effect of galE gene inactivation on lipopolysaccharide profile of Helicobacter pylori. Curr Microbiol. 1998;37:144–148. doi: 10.1007/s002849900354. [DOI] [PubMed] [Google Scholar]
- 29.Lindmark D G, Muller M. Antitrichomonad action, mutagenicity, and reduction of metronidazole and other nitroimidazoles. Antimicrob Agents Chemother. 1976;10:476–482. doi: 10.1128/aac.10.3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lockerby L, Rabin H R, Laishley E J. Role of the phosphoroclastic reaction of Clostridium pasteurianum in the reduction of metronidazole. Antimicrob Agents Chemother. 1985;27:863–867. doi: 10.1128/aac.27.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Megraud F. Helicobacter pylori resistance to antibiotics. In: Hunt R H, Tytgat G N J, editors. Helicobacter pylori: basic mechanisms to clinical cure. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 570–583. [Google Scholar]
- 32.Megraud F. Antibiotic resistance in Helicobacter pylori infection. Br Med Bull. 1998;54:207–216. doi: 10.1093/oxfordjournals.bmb.a011671. [DOI] [PubMed] [Google Scholar]
- 33.Muller M. Mode of action of metronidazole on anaerobic bacteria and protozoa. Surgery. 1983;93:165–171. [PubMed] [Google Scholar]
- 34.Parsonnet J. Helicobacter pylori. Infect Dis Clin N Am. 1998;12:185–197. doi: 10.1016/s0891-5520(05)70417-7. [DOI] [PubMed] [Google Scholar]
- 35.Parsonnet J. Helicobacter pylori: the size of the problem. Gut. 1998;43(Suppl. 1):S6–S9. doi: 10.1136/gut.43.2008.s6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Realdi G, Dore M P, Piana A, Atzei A, Carta M, Cugia L, Manca A, Are B M, Massarelli G, Mura I, Maida A, Graham D Y. Pretreatment antibiotic resistance in Helicobacter pylori infection: results of three randomized controlled studies. Helicobacter. 1999;4:106–112. doi: 10.1046/j.1523-5378.1999.99002.x. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Smith M A, Edwards D I. Oxygen scavenging, NADH oxidase and metronidazole resistance in Helicobacter pylori. J Antimicrob Chemother. 1997;39:347–353. doi: 10.1093/jac/39.3.347. [DOI] [PubMed] [Google Scholar]
- 39.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenny K, Fitzegerald L M, Lee N M, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidman J M, Fujii C, Bowman C, Watthey L, Wallin E, Hays W S, Borodovsky M, Karp P D, Smith H O, Fraser C M, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 40.Van der Wouden E J, Thijs J C, van Zwet A A, Sluiter W J, Kleibeuker J H. The influence of in vitro nitroimidazole resistance on the efficacy of nitroimidazole-containing anti-Helicobacter pylori regimens: a meta-analysis. Am J Gastroenterol. 1999;94:1751–1759. doi: 10.1111/j.1572-0241.1999.01202.x. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Roos K P, Taylor D E. Transformation of Helicobacter pylori by chromosomal metronidazole resistance and by a plasmid with a selectable chloramphenicol resistance marker. J Gen Microbiol. 1993;139:2485–2493. doi: 10.1099/00221287-139-10-2485. [DOI] [PubMed] [Google Scholar]
- 42.Weel J F, van der Hulst R W, Gerrits Y, Tytgat G N, van der Ende A, Dankert J. Heterogeneity in susceptibility to metronidazole among Helicobacter pylori isolates from patients with gastritis or peptic ulcer disease. J Clin Microbiol. 1996;34:2158–2162. doi: 10.1128/jcm.34.9.2158-2162.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]