Abstract
Some studies suggest that a higher phytochemical index (PI) is associated with a lower risk of overweight/obesity. This meta-analysis is performed to summarize published studies on the relationship of PI and the risk of overweight/obesity. We searched on PubMed, Cochrane Library and Web of Science from the inception dates to February 2022. The random-effect model was used based on heterogeneity. Meta-regression was used to explore potential sources of between-study heterogeneity. Publication bias was evaluated using Begg’s and Egger’s tests. The dose–response relationship was assessed using a restricted cubic spline model. Nine studies were included in the meta-analysis, with a total of 100,753 participants. The meta-analysis showed that the phytochemical index was associated with a decreased risk of overweight/obesity. The pooled OR (95% CI) was 0.81 (0.74–0.90). The findings from dose–response analysis showed a nonlinear association between the phytochemical index and the risk of overweight/obesity. The results of the meta-regression showed that gender and area were significant covariates influencing the heterogeneity between studies. There was no publication bias in the meta-analysis of this study. In conclusion, although this meta-analysis indicates that a high phytochemical index is associated with a reduced risk of overweight/obesity, all the studies included in this meta-analysis were cross-sectional studies with high heterogeneity. As such, more data from randomized controlled trials are required to confirm the efficacy of PI in evaluating the risk of overweight/obesity.
Keywords: phytochemical index, overweight, obesity, meta-analysis, dose–response relationship
1. Introduction
Overweight and obesity are characterized by abnormal or excessive fat accumulation that can damage health [1]. Recent statistics indicate that overweight/obesity continues to increase worldwide, with more than 2 billion overweight people, representing approximately 30% of the world’s population [2]. The Global Burden of Disease Group reported in 2017 that the prevalence of obesity has doubled in more than 70 countries since 1980 and continues to increase in most other countries [3]. Obesity is mainly caused by lifestyle, genetic and environmental factors [4]. As a modifiable behavior, eating behavior plays an important role in the etiology of obesity [5].
Several epidemiological and cross-sectional studies have shown that vegetarian diets can reduce body weight and body mass index (BMI) [6,7,8,9]. In addition, there is evidence that plant-based diets are associated with the prevention and treatment of chronic diseases [10,11]. The phytochemical index/dietary phytochemical index (PI/DPI) is the percentage of total dietary calories derived from foods rich in phytochemicals, such as fruits, vegetables (except potatoes with few phytochemicals), legumes, nuts, seeds and whole grains. PI is a simple way of assessing the intake of phytochemicals to effectively assess the health effects of foods rich in phytochemicals [12]. Phytochemicals, the physiologically active substances commonly found in plant-based foods such as fruits, vegetables, grains and legumes [13], can help improve chronic diseases such as diabetes, cardiovascular disease and some cancers [14]. Phytochemicals, mainly including polyphenols, alkaloids, terpenoids, flavonoids, saponins, steroids and so on, can prevent obesity by regulating carbohydrate and lipid metabolism [15].
Some studies have suggested that a higher phytochemical index (PI) is associated with a lower risk of overweight/obesity [5,16,17,18,19,20]. However, no study has comprehensively analyzed the relationship between PI and overweight/obesity. Therefore, this meta-analysis was performed to summarize published studies on the relationship between PI and the risk of overweight/obesity.
2. Materials and Methods
2.1. Sources and Methods of Data Retrieval
We searched PubMed, the Cochrane Library and Web of Science from the inception dates to February 2022. The following keywords were used to identify published literature which examined the relationship between PI and the incidence of overweight or obesity: phytochemicals, dietary phytochemicals, plant bioactive compounds, plant biologically active compounds, plant-derived chemicals, phytonutrients, non-nutrient bioactive substances, bioactive food components, index, overweight, and obesity.
2.2. Inclusion Criteria and Exclusion Criteria
The included articles needed to meet the following five inclusion conditions: (1) The exposure variable was PI/DPI; (2) the outcome variables were overweight/obesity or centripetal obesity; (3) the literature needed to provide an odds ratio/relative risk/hazard ratio (OR/RR/HR) and a 95% confidence interval (CI) between PI and overweight/obesity; (4) the literature used for dose–response analysis must provide the dose, number of cases, number of person-years in each group, or the data can be obtained by the conversion of missing values; and (5) the language of the original article was English. The following two exclusion criteria were applied: (1) the study subjects were not humans and (2) review.
2.3. Data Extraction and Quality Assessment
Data were independently extracted and cross-checked by two researchers according to uniform standards, and experts could be consulted in case of disagreement. The main contents of data extraction included: first author, publication year, area, dose, cases per year and OR/RR/HR in each exposure–dose range.
The quality of the included literature was assessed using the 11-item checklist recommended by the Healthcare Research and Quality Authority (AHRQ). Article quality was assessed as follows: low quality = 0–3; moderate quality = 4–7; and high quality = 8–11 [21].
2.4. Statistical Analysis
Stata12.0 software was used for statistical analysis, the Q test was used to test the heterogeneity of the included studies, I2 was used to evaluate the heterogeneity, and the test level was α = 0.05. If I2 < 50% [22], there was no statistical heterogeneity between studies. A fixed-effect model was used to calculate the combined effect OR and 95% CI. If I2 ≥ 50% [23], the random-effects model was used. Meta-regression was used to explore the potential sources of inter-study heterogeneity. Sensitivity analysis was used to test the stability of the results. The Begg and Egger methods [24,25] were used to evaluate publication bias. The dose–response relationship was evaluated using the restricted cubic spline model [26].
3. Results
3.1. Study Characteristics
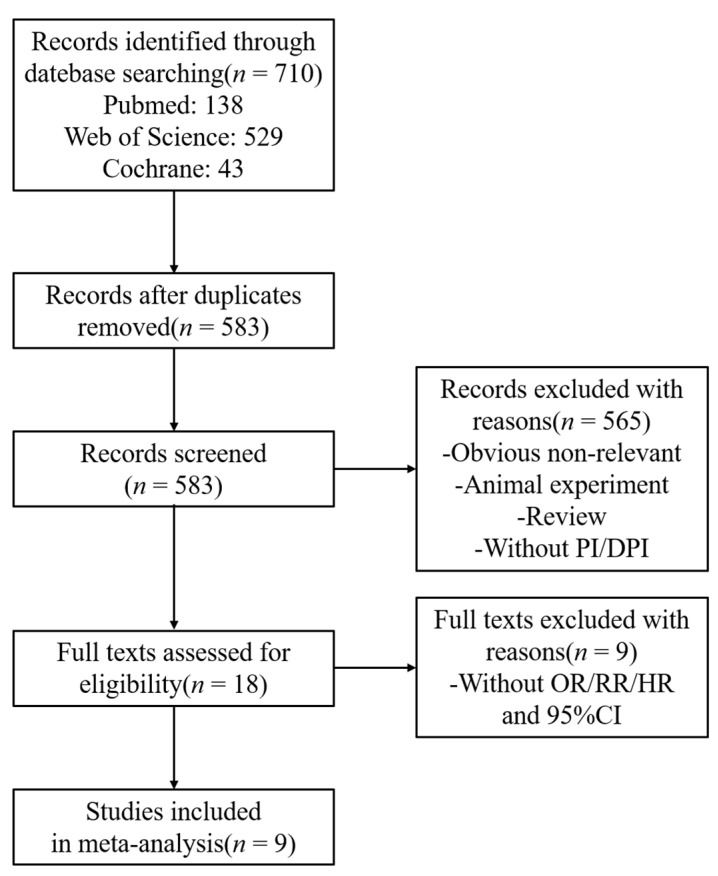
The literature-screening process is shown in Figure 1. We initially screened out 710 studies and, after the removal of duplicates, according to the inclusion and exclusion criteria formulated in this study, nine studies [5,16,17,18,19,20,27,28,29] including 22 groups of data were finally included, with a total of 100,753 participants. The included studies were scored by the AHRQ scale and all were of moderate quality. The characteristics and quality scores of the selected studies are presented in Table 1.
Figure 1.
Flow chart of study selection. Abbreviations: PI: phytochemical index; DPI: dietary phytochemical index; OR: odds ratio; RR: relative risk; HR: hazard ratio; CI: confidence interval.
Table 1.
Characteristics of the studies included.
| Study | Country | Age | Subjects | Outcome Variable | Diagnostic Criteria | Score |
|---|---|---|---|---|---|---|
| Bahadoran Z, 2013 [16] | Iran | 19–70 | 2567 | Abdominal obesity | WC ≥ 95 cm | 7 |
| Im J, 2020 [17] | Korea | ≥19 | 57,940 | Obesity, abdominal obesity | BMI ≥ 25 kg/m2, WC ≥ 90 and ≥85 cm for men and women | 7 |
| Kim M, 2020 [18] | Korea | ≥19 | 31,319 | Abdominal obesity | WC ≥ 90 cm in men and ≥80 cm in women | 6 |
| Eslami O, 2020 [5] | Iran | 7–10 | 356 | Overweight and obesity | overweight: BMI percentile ≥ 85 and <95, obese: ≥95 | 6 |
| Dehghani Firouzabadi F, 2021 [19] | Iran | 18–65 | 844 | Central obesity | WC ≥ 102 cm for men and 88 cm for women | 7 |
| Vasmehjani AA, 2021 [27] | Iran | 20–70 | 2326 | Abdominal obesity | WC ≥ 102 cm for men and >88 cm for women | 6 |
| Azizi-Soleiman F, 2021 [28] | China | 6–18 | 4296 | Obesity or overweight, abdominal obesity |
BMI > 85th percentile, WHtR ≥ 0.5 | 7 |
| Delshad Aghdam S, 2021 [29] | Iran | 18–35 | 261 | Overweight or obesity, abdominal obesity |
BMI > 24.9 kg/m2, WC ≥ 80 cm in women and ≥94 cm in men | 6 |
| Asgari E, 2021 [20] | Iran | 18–59 | 844 | Central obesity, general obesity | BMI ≥ 30 kg/m2, central obesity: WHtR ≥ 0.5; WHR ≥ 0.8 for women and ≥1 for men; WC ≥ 102 cm for men and ≥88 cm for women |
7 |
Abbreviations: BMI: body mass index; WC: waist circumference; WHR: waist-to-hip ratio; WHtR: waist-to-height ratio. Score was rated using an 11-item checklist that was recommended by the Agency for Healthcare Research and Quality.
3.2. Meta-Analysis
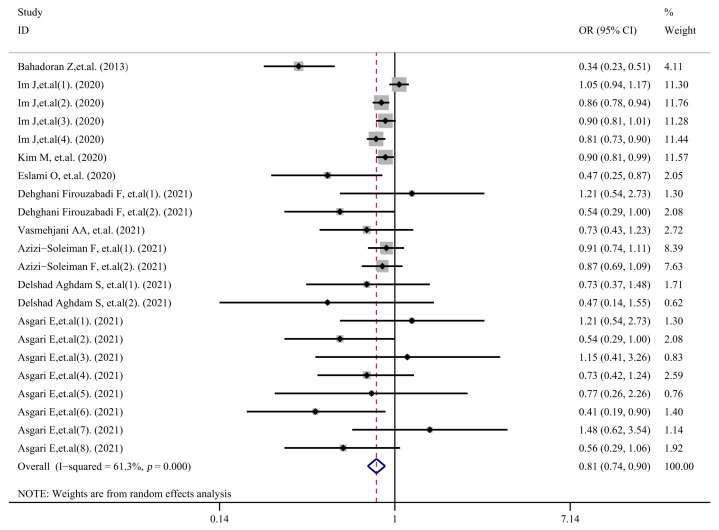
Figure 2 shows the multivariable-adjusted ORs for the highest versus lowest categories of PI. The meta-analysis shows that subjects in the highest category of PI had a significantly decreased risk for overweight/obesity, compared with those in the lowest category. The pooled OR was 0.81 (95% CI: 0.74–0.90) and high heterogeneity was observed (I2 = 61.3%; p < 0.001).
Figure 2.
Meta-analysis of PI (comparing the highest with the lowest PI categories) and risk of overweight/obesity [5,16,17,18,19,20,27,28,29].
3.3. Subgroup Analysis
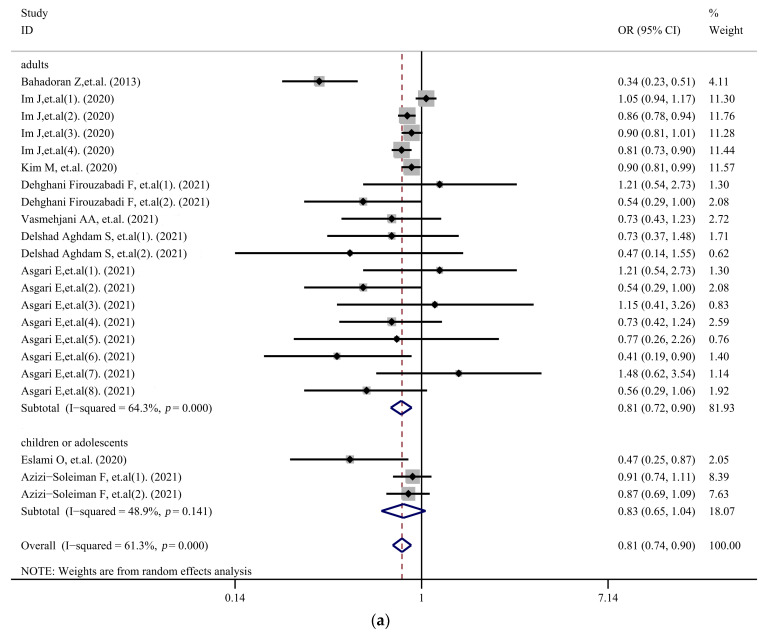
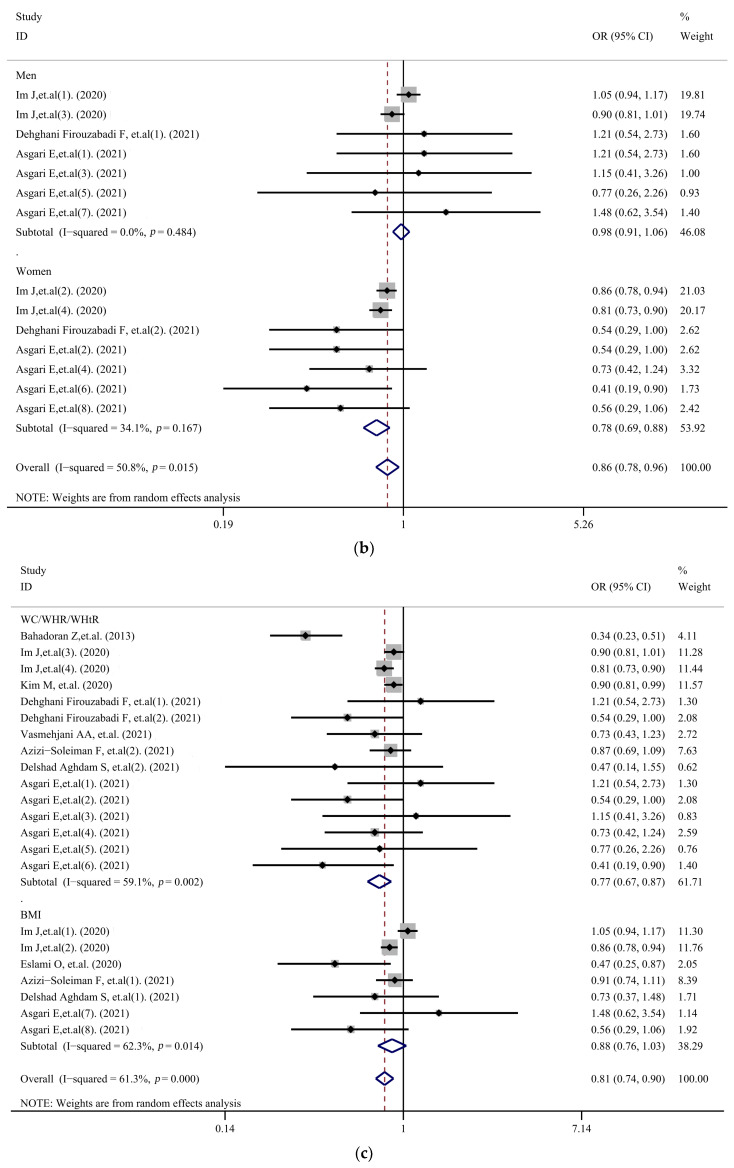
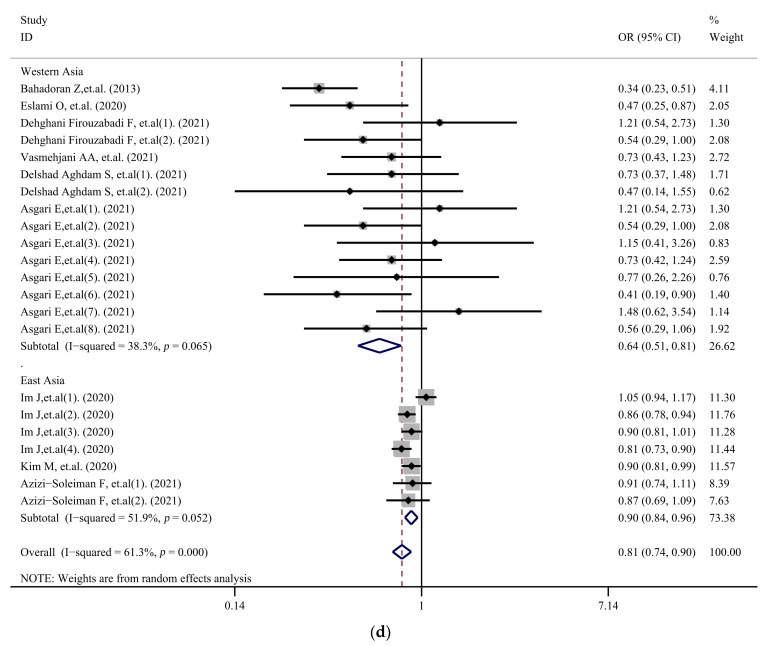
Figure 3 shows the results of the subgroup analysis. The subgroup analysis of age showed that the highest PI was a protective factor in adults (OR, 0.81; 95% CI, 0.72–0.90; I2 = 64.3%, p < 0.001); however, there was no statistically significant relationship between the highest PI and overweight/obesity in children or adolescents (OR, 0.83; 95% CI, 0.65–1.04; I2 = 48.9%, p = 0.141). In addition, a total of three studies [17,19,20] including 14 sets of data were used to study the relationship between PI and obesity incidence for different genders. Therefore, subgroup analysis by gender was performed on these data, which showed that PI was associated with the prevalence of overweight/obesity in women (OR, 0.78; 95%CI, 0.69–0.88; I2 = 34.1%, p = 0.167), but not in men (OR, 0.98; 95%CI, 0.91–1.06; I2 = 0, p = 0.484). The subgroup analysis of diagnostic criteria of overweight/obesity showed that PI was related to the prevalence of overweight/obesity when waist circumference/waist-to-hip ratio/waist-to-height ratio (WC/WHR/WHtR) was used to determine obesity (OR, 0.77; 95%CI, 0.67–0.87; I2 = 59.1%, p = 0.002), but this was not found when BMI was used (OR, 0.88; 95%CI, 0.76–1.03; I2 = 62.3%, p = 0.014). A subgroup analysis of area showed that the highest PI was associated with overweight/obesity in both West Asia (OR, 0.64; 95%CI, 0.51–0.81; I2 = 38.3%, p = 0.065) and East Asia (OR, 0.90; 95%CI, 0.84–0.96; I2 = 51.9%, p = 0.052).
Figure 3.
Subgroup analysis stratified by (a) age; (b) gender; (c) diagnostic criteria of overweight/obesity; and (d) area [5,16,17,18,19,20,27,28,29].
3.4. Sensitivity Analysis
The included studies were removed one by one, and the remaining studies were meta-analyzed. The results showed that the combined effect value changed greatly and heterogeneity decreased (I2 = 38.4%) after the first article [16] was removed, indicating that this article might be the source of heterogeneity (Table 2).
Table 2.
Sensitivity analysis by removing one by one the included studies.
| Study | OR (95%CI) | I2 | p |
|---|---|---|---|
| Bahadoran Z, et al. (2013) [16] | 0.87 (0.80–0.93) | 38.4% | 0.039 |
| Im J, et al. (1). (2020) [17] | 0.79 (0.72–0.87) | 51.9% | 0.003 |
| Im J, et al. (2). (2020) [17] | 0.80 (0.71–0.89) | 63.1% | 0.001 |
| Im J, et al. (3). (2020) [17] | 0.80 (0.71–0.89) | 63.0% | 0.001 |
| Im J, et al. (4). (2020) [17] | 0.81 (0.72–0.90) | 61.4% | 0.001 |
| Kim M, et al. (2020) [18] | 0.79 (0.71–0.89) | 62.9% | 0.001 |
| Eslami O, et al. (2020) [5] | 0.83 (0.75–0.91) | 60.4% | 0.001 |
| Dehghani Firouzabadi F, et al. (1). (2021) [19] | 0.81 (0.73–0.89) | 62.8% | 0.001 |
| Dehghani Firouzabadi F, et al. (2). (2021) [19] | 0.82 (0.75–0.91) | 61.5% | 0.001 |
| Vasmehjani AA, et al. (2021) [27] | 0.82 (0.74–0.90) | 62.9% | 0.001 |
| Azizi-Soleiman F, et al. (1). (2021) [28] | 0.80 (0.72–0.89) | 63.1% | 0.001 |
| Azizi-Soleiman F, et al. (2). (2021) [28] | 0.81 (0.73–0.89) | 63.2% | 0.001 |
| Delshad Aghdam S, et al. (1). (2021) [29] | 0.81 (0.74–0.90) | 63.0% | 0.001 |
| Delshad Aghdam S, et al. (2). (2021) [29] | 0.82 (0.74–0.90) | 62.5% | 0.001 |
| Asgari E, et al. (1). (2021) [20] | 0.81 (0.73–0.89) | 62.8% | 0.001 |
| Asgari E, et al. (2). (2021) [20] | 0.82 (0.75–0.91) | 61.5% | 0.001 |
| Asgari E, et al. (3). (2021) [20] | 0.81 (0.73–0.89) | 63.0% | 0.001 |
| Asgari E, et al. (4). (2021) [20] | 0.82 (0.74–0.90) | 62.9% | 0.001 |
| Asgari E, et al. (5). (2021) [20] | 0.81 (0.74–0.90) | 63.1% | 0.001 |
| Asgari E, et al. (6). (2021) [20] | 0.82 (0.75–0.91) | 60.5% | 0.001 |
| Asgari E, et al. (7). (2021) [20] | 0.81 (0.73–0.89) | 62.2% | 0.001 |
| Asgari E, et al. (8). (2021) [20] | 0.82 (0.74–0.90) | 61.9% | 0.001 |
| Combined | 0.81(0.74–0.90) | 61.3% | 0.001 |
3.5. Meta-Regression
In order to explore the source of heterogeneity, we conducted a meta-regression analysis with age, gender, diagnostic criteria of obesity and area (West or East Asia) as covariates (Table 3). The results showed that gender and area were significant covariates influencing the heterogeneity between studies. Other covariables were not shown to have a significant effect on inter-study heterogeneity.
Table 3.
Meta-regressions by age, gender, diagnostic criteria of obesity and area.
| Covariate | p |
|---|---|
| Age | 0.895 |
| Gender | 0.023 |
| Diagnostic criteria of obesity | 0.360 |
| Area | 0.002 |
3.6. Publication Bias
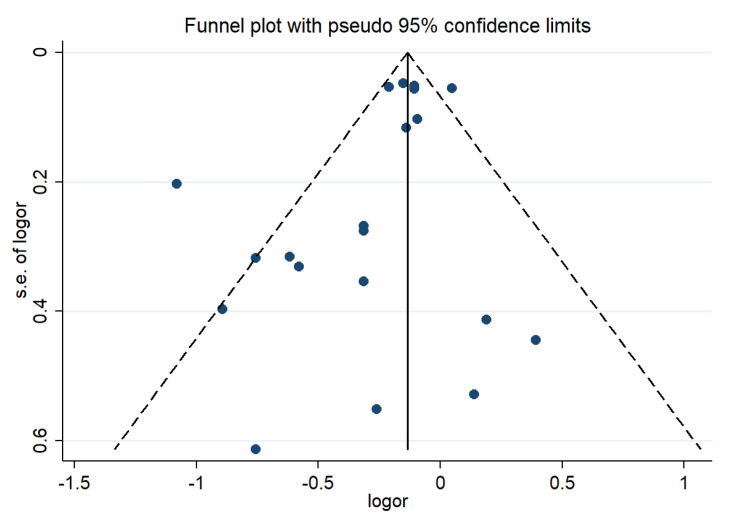
A funnel plot (Figure 4), Begg’s test and Egger’s test were used to detect publication bias, and no publication bias was found (p for Begg’s test = 0.955; p for Egger’s test = 0.059).
Figure 4.
Funnel plot of the associations between PI and overweight/obesity. Abbreviations: s.e.: standard error; logor: the logarithm of OR.
3.7. Dose–Response Analysis
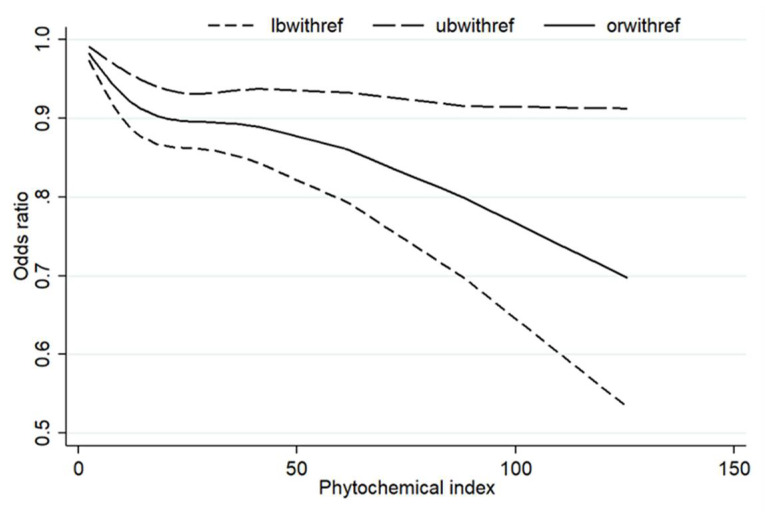
We included four articles including 13 sets of data to study the dose–response relationship (Figure 5). The results showed that there was a nonlinear dose–response relationship between PI and the risk of overweight/obesity (p = 0.0447).
Figure 5.
The dose–response analysis between PI and the risk of overweight/obesity. Abbreviations: lbwithref: lower bound of 95% confidence interval with reference; ubwithref: upper bound of 95% confidence interval with reference; orwithref: odds ratio with reference.
4. Discussion
A growing number of studies have investigated the effects of the phytochemical index on chronic diseases such as metabolic syndrome [18,19], diabetes [30], cardiovascular disease [29] and breast-related diseases [31,32,33]. Phytochemicals also have anti-obesity properties. However, relevant studies reported controversial results for the relationship between the phytochemical index and obesity risk. We conducted a meta-analysis to quantify previous studies. This meta-analysis showed a possible inverse association between a higher PI and the risk of overweight/obesity, which was consistent with a longitudinal study on adults, reporting that increasing energy intake from phytochemical-rich foods can prevent weight gain and aid weight loss in adults [34].
From the results of the subgroup analysis, there are several factors worth considering. First, the results varied according to the age of the participants in the original study. A subgroup analysis of age showed that a higher PI was a protective factor in adults. However, the results were not statistically significant in children or adolescents, which may be due to the lack of corresponding literature. To date, only a few studies have assessed the link between PI and obesity, and most of the research was conducted on adults.
Subgroup analysis by gender found that women with a higher PI had a lower risk of obesity, but no association between PI and obesity prevalence was observed in men. In fact, a study of adults in Korea found that a higher intake of total flavones was associated with a lower risk of obesity; however, this association was not found in men [35]. In addition, an epidemiological study found an association between serum carotenoid levels and abdominal obesity in women, but no corresponding significant association was found in men [36]. This may be due to the interaction between phytochemicals and sex hormones. Certain types of phytochemicals have structures similar to estrogen and can mimic or influence the effects of estrogen in the body. Therefore, the intake of these phytochemicals may improve diseases caused by estrogen deficiency [37,38,39,40]. That is, the intake of phytochemical-rich foods may reduce the obesity rate by helping the female body improve hormone levels.
A subgroup analysis using diagnostic criteria of overweight/obesity found that when obesity was defined by WC/WHR/WHtR, the incidence of overweight/obesity was lower in people with a high PI. A cross-sectional survey of 54 adults showed a negative correlation between DPI and WC. Phytochemicals may inhibit preadipocyte proliferation through partial polyphenols, reduce adipogenesis and promote lipid decomposition to maintain WC normality [15,41]. A subgroup analysis by area showed a negative association between high PI and obesity incidence, but the association was more obvious in the West Asian group, possibly because most of the studies were conducted in Iran. In addition, there are no studies on PI and obesity outside of Asia, which may be due to dietary differences among regions. Given these differences, further studies on the role of phytochemicals in obesity are needed to clarify the causal relationship.
In addition, the dose–response relationship showed a decreased risk of overweight/obesity with increased PI. Diets with more phytochemicals are generally lower in calories, so they are more likely to reduce obesity risk. Some studies have shown that a diet rich in phytochemicals can improve obesity by reducing oxidative stress, inducing the production of pro-inflammatory cytokines, promoting thermogenesis, inhibiting adipocyte differentiation and reducing adipogenesis [42,43].
Inter-study heterogeneity is a key issue in meta-analysis, which directly affects the interpretation of meta-analysis results. Therefore, it is an important aspect of this study to explore the potential sources of heterogeneity between studies. The results of our meta-regression showed that gender and area were the significant covariables affecting inter-study heterogeneity. The sensitivity analysis results showed that after removing Bahadoran Z’s study [16], the heterogeneity was reduced to 38.2%, suggesting that this study may be the source of the heterogeneity. This is thought to be because it is based on survey data from 2006 to 2008, which is early compared with other studies. In addition, there were more elderly people with high PI scores in this study. The above may be the cause of the heterogeneity. Begg’s and Egger’s tests showed that there was no publication bias in the meta-analysis of this study.
5. Conclusions
Our meta-analysis showed that PI was inversely associated with the risk of overweight/obesity. With the increase in PI, the prevalence of overweight/obesity decreased gradually. However, these findings were limited because the studies included in this meta-analysis were all cross-sectional studies. Therefore, no definite conclusions can be drawn at present. Due to the high heterogeneity of cross-sectional studies, this evidence needs further validation.
Author Contributions
Conceiving the research, W.C.; data analysis and manuscript writing, C.W.; data analysis, L.L.; screening of studies, R.L. and W.D.; manuscript review, D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Poletto J.E., Rizzo D.T., Almeida A.M.N., Cândido E.C., Cazzo E., Chaim É.A. Evolution of anthropometric data and quality of life in active bariatric individuals. Rev. Assoc. Med. Bras. 2021;67:1274–1278. doi: 10.1590/1806-9282.20210511. [DOI] [PubMed] [Google Scholar]
- 2.Caballero B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019;10:S4–S9. doi: 10.1093/advances/nmy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afshin A., Forouzanfar M.H., Reitsma M.B., Sur P., Estep K., Lee A., Marczak L., Mokdad A.H., Moradi-Lakeh M., Naghavi M., et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Racette S.B., Deusinger S.S., Deusinger R.H. Obesity: Overview of prevalence, etiology, and treatment. Phys. Ther. 2003;83:276–288. doi: 10.1093/ptj/83.3.276. [DOI] [PubMed] [Google Scholar]
- 5.Eslami O., Khoshgoo M., Shidfar F. Dietary phytochemical index and overweight/obesity in children: A cross-sectional study. BMC Res. Notes. 2020;13:132. doi: 10.1186/s13104-020-04979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabaté J., Wien M. Vegetarian diets and childhood obesity prevention. Am. J. Clin. Nutr. 2010;91:1525s–1529s. doi: 10.3945/ajcn.2010.28701F. [DOI] [PubMed] [Google Scholar]
- 7.Newby P.K., Tucker K.L., Wolk A. Risk of overweight and obesity among semivegetarian, lactovegetarian, and vegan women. Am. J. Clin. Nutr. 2005;81:1267–1274. doi: 10.1093/ajcn/81.6.1267. [DOI] [PubMed] [Google Scholar]
- 8.Brathwaite N., Fraser H.S., Modeste N., Broome H., King R. Obesity, diabetes, hypertension, and vegetarian status among Seventh-Day Adventists in Barbados: Preliminary results. Ethn. Dis. 2003;13:34–39. [PubMed] [Google Scholar]
- 9.Thedford K., Raj S. A vegetarian diet for weight management. J. Am. Diet. Assoc. 2011;111:816–818. doi: 10.1016/j.jada.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Roman D.D.L., Aller R., Castano O. Vegetarian diets; effect on health. Rev. Clin. Esp. 2007;207:141–143. doi: 10.1157/13100230. [DOI] [PubMed] [Google Scholar]
- 11.Fraser G.E. Vegetarian diets: What do we know of their effects on common chronic diseases? Am. J. Clin. Nutr. 2009;89:1607s–1612s. doi: 10.3945/ajcn.2009.26736K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarty M.F. Proposal for a dietary “phytochemical index”. Med. Hypotheses. 2004;63:813–817. doi: 10.1016/j.mehy.2002.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Dillard C.J., German J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000;80:1744–1756. doi: 10.1002/1097-0010(20000915)80:12<1744::AID-JSFA725>3.0.CO;2-W. [DOI] [Google Scholar]
- 14.Firdous S.M. Phytochemicals for treatment of diabetes. EXCLI J. 2014;13:451–453. [PMC free article] [PubMed] [Google Scholar]
- 15.Tucci S.A. Phytochemicals in the Control of Human Appetite and Body Weight. Pharmaceuticals. 2010;3:748–763. doi: 10.3390/ph3030748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bahadoran Z., Golzarand M., Mirmiran P., Saadati N., Azizi F. The association of dietary phytochemical index and cardiometabolic risk factors in adults: Tehran Lipid and Glucose Study. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2013;26((Suppl. 1)):145–153. doi: 10.1111/jhn.12048. [DOI] [PubMed] [Google Scholar]
- 17.Im J., Kim M., Park K. Association between the Phytochemical Index and Lower Prevalence of Obesity/Abdominal Obesity in Korean Adults. Nutrients. 2020;12:2312. doi: 10.3390/nu12082312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim M., Park K. Association between phytochemical index and metabolic syndrome. Nutr. Res. Pract. 2020;14:252–261. doi: 10.4162/nrp.2020.14.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dehghani Firouzabadi F., Jayedi A., Asgari E., Farazi M., Noruzi Z., Djafarian K., Shab-Bidar S. The Association of Dietary Phytochemical Index with Metabolic Syndrome in Adults. Clin. Nutr. Res. 2021;10:161–171. doi: 10.7762/cnr.2021.10.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asgari E., Jayedi A., Dehghani Firouzabadi F., Noruzi Z., Farazi M., Djafarian K., Shab-Bidar S. Association of the dietary phytochemical index with general and central obesity in a sample of Iranian adults. J. Funct. Foods. 2021;83:104546. doi: 10.1016/j.jff.2021.104546. [DOI] [Google Scholar]
- 21.Chen J.P., Chen G.C., Wang X.P., Qin L., Bai Y. Dietary Fiber and Metabolic Syndrome: A Meta-Analysis and Review of Related Mechanisms. Nutrients. 2017;10:24. doi: 10.3390/nu10010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 25.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orsini N., Li R., Wolk A., Khudyakov P., Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: Examples, an evaluation of approximations, and software. Am. J. Epidemiol. 2012;175:66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasmehjani A.A., Darabi Z., Nadjarzadeh A., Mirzaei M., Hosseinzadeh M. The relation between dietary phytochemical index and metabolic syndrome and its components in a large sample of Iranian adults: A population-based study. BMC Public Health. 2021;21:1587. doi: 10.1186/s12889-021-11590-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azizi-Soleiman F., Khoshhali M., Heidari-Beni M., Qorbani M., Ali Pourmirzaei M., Kelishadi R. Higher dietary phytochemical index is associated with anthropometric indices in children and adolescents: The weight disorders survey of the CASPIAN-IV study. Int. J. Vitam. Nutr. Res. 2021;91:531–538. doi: 10.1024/0300-9831/a000657. [DOI] [PubMed] [Google Scholar]
- 29.Delshad Aghdam S., Siassi F., Nasli Esfahani E., Qorbani M., Rajab A., Sajjadpour Z., Bashiri A., Aghayan M., Sotoudeh G. Dietary phytochemical index associated with cardiovascular risk factor in patients with type 1 diabetes mellitus. BMC Cardiovasc. Disord. 2021;21:293. doi: 10.1186/s12872-021-02106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abshirini M., Mahaki B., Bagheri F., Siassi F., Koohdani F., Sotoudeh G. Higher Intake of Phytochemical-Rich Foods is Inversely Related to Prediabetes: A Case-Control Study. Int. J. Prev. Med. 2018;9:64. doi: 10.4103/ijpvm.IJPVM_145_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahadoran Z., Karimi Z., Houshiar-rad A., Mirzayi H.R., Rashidkhani B. Dietary phytochemical index and the risk of breast cancer: A case control study in a population of Iranian women. Asian Pac. J. Cancer Prev. 2013;14:2747–2751. doi: 10.7314/APJCP.2013.14.5.2747. [DOI] [PubMed] [Google Scholar]
- 32.Aghababayan S., Sheikhi Mobarakeh Z., Qorbani M., Abbasvandi F., Tiznobeyk Z., Aminianfar A., Sotoudeh G. Dietary Phytochemical Index and Benign Breast Diseases: A Case-Control Study. Nutr. Cancer. 2020;72:1067–1073. doi: 10.1080/01635581.2019.1658795. [DOI] [PubMed] [Google Scholar]
- 33.Ghoreishy S.M., Aminianfar A., Benisi-Kohansal S., Azadbakht L., Esmaillzadeh A. Association between dietary phytochemical index and breast cancer: A case-control study. Breast Cancer. 2021;28:1283–1291. doi: 10.1007/s12282-021-01265-6. [DOI] [PubMed] [Google Scholar]
- 34.Mirmiran P., Bahadoran Z., Golzarand M., Shiva N., Azizi F. Association between dietary phytochemical index and 3-year changes in weight, waist circumference and body adiposity index in adults: Tehran Lipid and Glucose study. Nutr. Metab. 2012;9:108. doi: 10.1186/1743-7075-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dongwoo H., Seong-Ah K., Jun S., Kang M.S., Joung H. Association between antioxidant vitamin intake and obesity among Korean women: Using the Korea National Health and Nutrition Examination Survey 2007–2016. J. Nutr. Health. 2018;51:400–413. [Google Scholar]
- 36.Suzuki K., Inoue T., Hioki R., Ochiai J., Kusuhara Y., Ichino N., Osakabe K., Hamajima N., Ito Y. Association of abdominal obesity with decreased serum levels of carotenoids in a healthy Japanese population. Clin. Nutr. 2006;25:780–789. doi: 10.1016/j.clnu.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 37.Kim M., Choi M.-K., Ja S.C. The study of pytoestrogen intake and bone mineral density of vegetarian and nonvegetarian postmenopausal women. Korean J. Community Nutr. 2004;9:66–72. [Google Scholar]
- 38.Lee J., Heo J., Park Y., Park H. Survey on the Consumption of the Phytoestrogen Isoflavone in Postmenopausal Korean Women. J. Menopausal Med. 2012;18:163–173. doi: 10.6118/jksm.2012.18.3.163. [DOI] [Google Scholar]
- 39.Kim B.-J. Obesity and Sex Hormones. J. Obes. Metab. Syndr. 2010;19:113–118. [Google Scholar]
- 40.Holubková A., Penesová A., Šturdík E., Mošovská S., Mikušová L. Phytochemicals with potential effects in metabolic syndrome prevention and therapy %J Acta Chimica Slovaca. Acta Chim. Slovaca. 2012;5:186–199. doi: 10.2478/v10188-012-0029-8. [DOI] [Google Scholar]
- 41.Golzarand M., Mirmiran P., Bahadoran Z., Alamdari S., Azizi F. Dietary phytochemical index and subsequent changes of lipid profile: A 3-year follow-up in Tehran Lipid and Glucose Study in Iran. ARYA Atheroscler. 2014;10:203–210. [PMC free article] [PubMed] [Google Scholar]
- 42.Vincent H.K., Bourguignon C.M., Taylor A.G. Relationship of the dietary phytochemical index to weight gain, oxidative stress and inflammation in overweight young adults. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2010;23:20–29. doi: 10.1111/j.1365-277X.2009.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carnauba R.A., Chaves D.F., Baptistella A.B., Paschoal V., Naves A., Buehler A.M. Association between high consumption of phytochemical-rich foods and anthropometric measures: A systematic review. Int. J. Food Sci. Nutr. 2017;68:158–166. doi: 10.1080/09637486.2016.1229761. [DOI] [PubMed] [Google Scholar]