This systematic review and meta-analysis investigates the outcomes of various psychosocial interventions for the management of functional abdominal pain disorders in children.
Key Points
Question
What is the certainty in evidence of psychosocial interventions for the management of functional abdominal pain disorders in children?
Findings
In this systematic review and meta-analysis of 33 randomized clinical trials including 2622 children, moderate certainty in evidence is available that receiving cognitive behavior therapy was associated with increased treatment success, lower pain frequency and intensity, and similar withdrawals owing to adverse events compared with no intervention. Hypnotherapy may also be associated with improved outcomes when compared with no intervention.
Meaning
Study results suggest that cognitive behavioral therapy is associated with improved treatment success and lower pain frequency and intensity in the management of functional abdominal pain disorders in children.
Abstract
Importance
Functional abdominal pain disorders (FAPDs) can severely affect the life of children and their families, with symptoms carrying into adulthood. Management of FADP symptoms is also a financial and time burden to clinicians and health care systems.
Objective
To systematically review various randomized clinical trials (RCTs) on the outcomes of cognitive behavioral therapy (CBT), educational support, yoga, hypnotherapy, gut-directed hypnotherapy, guided imagery, and relaxation in the management of FAPDs.
Data Sources
PubMed, MEDLINE, Embase, PsycINFO, and Cochrane Library.
Study Selection
All RCTs that compared psychosocial interventions with any control or no intervention, for children aged 4 to 18 years with FAPDs.
Data Extraction and Synthesis
Pairs of the authors independently extracted data of all included studies, using a predesigned data extraction sheet. One author acted as arbitrator. Risk of bias was assessed using the Cochrane risk of bias tool, and certainty of the evidence for all primary outcomes was analyzed using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework.
Main Outcomes and Measures
Primary outcomes were treatment success, pain frequency, pain intensity, and withdrawal owing to adverse events. Dichotomous outcomes were expressed as risk ratio (RR) with corresponding 95% CIs. Continuous outcomes were expressed as mean difference (MD) or standardized MD with 95% CI.
Results
A total of 33 RCTs with 2657 children (median [range] age, 12 [7-17] years; 1726 girls [67.3%]) were included. Twelve studies compared CBT with no intervention, 5 studies compared CBT with educational support, 3 studiescompared yoga with no intervention, 2 studies compared hypnotherapy with no intervention, 2 studies compared gut-directed hypnotherapy with hypnotherapy, and 2 studies compared guided imagery with relaxation. Seven studies evaluated other unique comparisons (eg, visceral osteopathy vs normal osteopathy). Per the GRADE framework, owing to risk of bias, there was moderate certainty in evidence that CBT was associated with higher treatment success numbers (n = 324 children; RR, 2.37; 95% CI 1.30-4.34; number needed to treat [NNT] = 5), lower pain frequency (n = 446 children; RR, −0.36; 95% CI, −0.63 to −0.09), and lower pain intensity (n = 332 children; RR, −0.58; 95% CI, −0.83 to −0.32) than no intervention. Owing to high imprecision, there was low certainty in evidence that there was no difference between CBT and educational support for pain intensity (n = 127 children; MD, −0.36; 95% CI, −0.87 to 0.15). Owing to risk of bias and imprecision, there was low certainty in evidence that hypnotherapy resulted in higher treatment success compared with no intervention (n = 91 children; RR, 2.86; 95% CI, 1.19-6.83; NNT = 5). Owing to risk of bias and imprecision, there was low certainty in evidence that yoga had similar treatment success to no intervention (n = 99 children; RR, 1.09; 95% CI, 0.58-2.08).
Conclusions and Relevance
Results of this systematic review and meta-analysis suggest that CBT and hypnotherapy may be considered as a treatment for FAPDs in childhood. Future RCTs should address quality issues to enhance the overall certainty of the results, and studies should consider targeting these interventions toward patients who are more likely to respond.
Introduction
According to the Rome IV criteria,1 functional abdominal pain disorders (FAPDs) can be divided into the following 4 subcategories: functional dyspepsia, irritable bowel syndrome, abdominal migraine, and functional abdominal pain not otherwise specified (eAppendix 1 in the Supplement). The causal mechanisms behind FAPDs are not totally understood. The dominant theory suggests that the cause is a dysregulation of the brain-gut communication axis. The interplay and feedback of a variety of genetic, physiological, psychological, and environmental factors are believed to affect the central nervous system and gastrointestinal motility.2,3 These painful conditions can also lead to lower quality of life and affect school/work attendance and performance.4,5,6 Their management can be time-consuming and financially costly for families and health care teams, with many children continuing to present symptoms as adults.7,8
The management of FAPDs is symptom-based and includes dietary and lifestyle changes, pharmacological options, and psychosocial interventions, such as cognitive behavioral therapy (CBT), hypnotherapy, yoga, neurostimulation, guided imagery, and others.9
A Cochrane systematic review10 from 2017 concluded that CBT and hypnotherapy showed promise as treatments for FAPDs; however, the quality of the evidence was poor. Since then, the results of new randomized clinical trials (RCTs) have become available. The aim of this systematic review and meta-analysis was to identify all current psychosocial RCTs for the treatment of FAPDs and examine their quality of evidence using the Cochrane format.11
Methods
Literature Search
In this systematic review and meta-analysis, we searched PubMed, MEDLINE, Embase, PsycINFO, and the Cochrane Library from inception to August 2021 (eAppendix 2 in the Supplement). We also searched the ClinicalTrials.gov register, the World Health Organization International Clinical Trials Registry Platform, and the metaRegister of Controlled Trials for unpublished and ongoing studies. A protocol determining the review outcomes, inclusion criteria, and methods was developed by all researchers, finalized on June 2020, and prospectively registered in PROSPERO.12 This systematic review and meta-analysis used no original or patient data and was therefore exempt from the University of Central Lancashire ethics requirement. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines.
Study Selection
Included in this systematic review and meta-analysis were all published, unpublished, and ongoing RCTs that compared psychosocial interventions to any intervention or control and studies that targeted at children aged 4 to 18 years with FAPDs as defined by the Rome or similar criteria. There were no date or language restrictions. Data on race and ethnicity were not gathered because it was not consistently nor homogeneously reported in the original studies.
The results of the search were imported into systematic review management software (Covidence). Duplicate records were removed. All researchers contributed to title/abstract screening and full-text screening with all titles screened in duplicate. Disagreements were resolved by discussion with first author M. Gordon.
Our primary outcomes included treatment success as defined by the authors, pain frequency, pain intensity, and withdrawal owing to adverse events. Our secondary outcomes included quality of life, anxiety/depression, defecation pattern, adequate relief, school attendance or performance, and serious adverse events.13
Quality Assessment and Data Extraction
Pairs of the authors (R.R. and C.B., M. Gasparetto and T.D., V.S. and H.V.) independently carried out the data extraction of all included studies, using a predesigned data extraction sheet. Disagreements were resolved by reaching consensus with a third author (M. Gordon). The risk of bias for all included studies was assessed using the Cochrane risk of bias tool.13 We judged the studies to be at either low, high, or unclear risk of bias. The assessed domains included sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. We assessed the certainty of the evidence for all primary outcomes using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework and presented these in summary of findings tables.
We chose to include only end-of-intervention outcome data for our meta-analyses as longer follow-up data were not reported consistently enough to allow appropriate homogeneity. When both self-reported data and parent-reported data were presented, we used the self-reported data as it was reported more often.
Statistical Analysis
We performed meta-analyses only where 2 or more intervention studies were available and when patient groups and outcomes were sufficiently similar. For dichotomous outcomes, we expressed treatment outcome as risk ratios (RRs) with corresponding 95% CIs. For continuous outcomes, we expressed the treatment outcome as mean difference (MD) with 95% CIs. If the studies assessed the same continuous outcome in different ways, we estimated the treatment outcome using the standardized MD. Inconsistency was quantified and represented by the I2 statistic using the thresholds indicated by the Cochrane Handbook.11 Statistical analyses were performed using Review Manager, version 5.4 (The Cochrane Collaboration).
Results
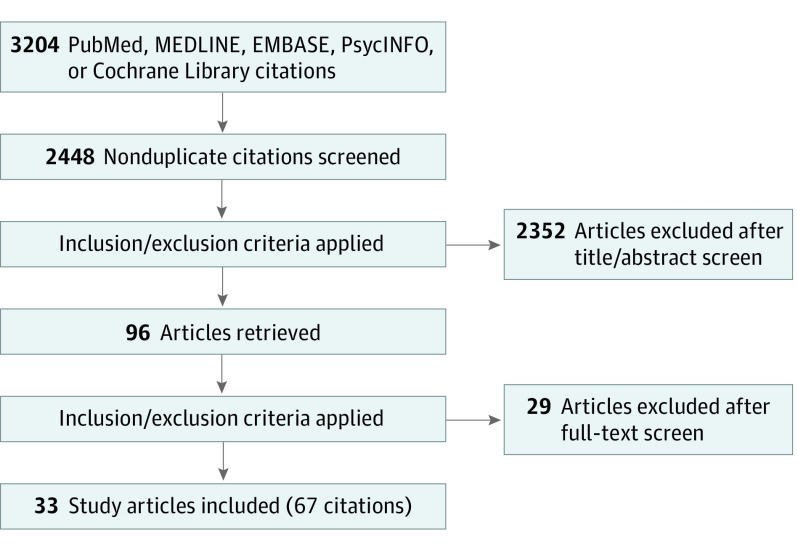
We identified a total of 3313 citations through our search (Figure 1). Of those, 766 records were excluded as duplicates, and 2547 records proceeded to title and abstract screening, of which we excluded 2451. Finally, 96 records were assessed for full-text eligibility. Twenty-seven records were excluded with reasoning. In addition, 3 records of 3 ongoing RCTs were identified. Details on the excluded studies, those awaiting classification, and ongoing studies are provided in eTable 6 in the Supplement.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow Diagram.
Sixty-seven records of 33 RCTs14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46 were included in the review. The included studies had a total sample of 2622 children with an age range of 4 to 18 years (median [range] age, 12 [7-17] years; 838 boys [32.7%]; 1726 girls [67.3%]). Length of follow-up lasted from 5 days to 4 months. Only 1 study14 concentrated on a specific disease subtype, all others considered all subtypes. The studies used a mix of diagnostic criteria.
Twelve studies15,16,17,18,19,20,21,22,23,24,25,26 compared CBT with no intervention, 5 studies27,28,29,30,31 compared CBT with educational support, 3 studies32,33,34 compared yoga with no intervention, 2 studies35,36 compared hypnotherapy with no intervention, 2 studies37,38 compared gut-directed hypnotherapy with hypnotherapy, and 2 studies39,40 compared guided imagery with relaxation.
A total of 7 included RCTs14,41,42,43,44,45,46 had unique intervention comparisons that could not be included in any meta-analysis; these comparisons were visceral osteopathy vs normal osteopathy41; fiber vs fiber, biofeedback vs cognitive-behavioral interventions, biofeedback vs parental support, and cognitive-behavioral interventions, biofeedback, and fiber42; electrical neurostimulation vs placebo43; home-based hypnotherapy vs individual hypnotherapy44; biofeedback-assisted relaxation training vs no intervention14; audio-recorded guided imagery vs no intervention45; and written self-disclosure vs no intervention.46 A summary of the included studies characteristics is provided in eTable 1 in the Supplement.
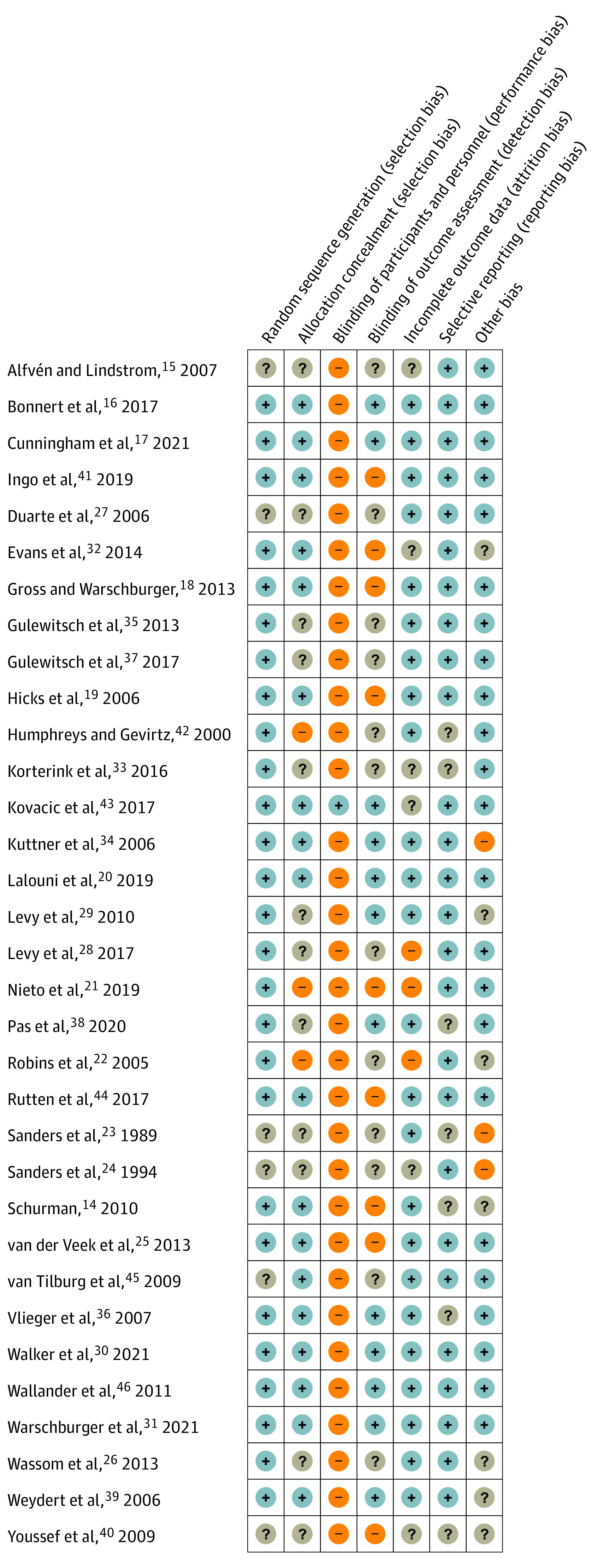
Risk of Bias Assessment
Randomization was properly explained and rated as low risk of bias in 27 of 33 studies (81.8%),14,16,17,18,19,20,21,22,25,26,28,29,30,31,32,33,34,35,36,37,38,39,41,42,43,44,46 whereas 6 studies (18.2%)15,23,24,27,40,45 were rated as unclear for not providing enough information. Allocation concealment was rated as high risk of bias for 3 studies,21,22,42 unclear for 12 studies,15,23,24,26,27,28,29,33,35,37,38,40 and low for 18 studies14,16,17,18,19,20,25,30,31,32,34,36,39,41,43,44,45,46 that provided sufficient information on concealment.
All but 1 study,43 which used a sham control, were rated as high risk of bias for blinding participants and personnel blinding. Blinding the assessors was achieved by 13 of the studies,16,17,18,20,29,30,31,34,36,38,39,43,46 which were rated as low risk of bias in this domain, whereas 12 studies15,22,23,24,26,27,28,33,35,37,42,45 were rated as unclear and 8 studies14,19,21,25,32,40,41,44 as high risk.
Three studies21,22,28 had issues with considerable imbalanced attrition between groups and were rated as high risk of bias for that domain. For 6 studies,15,24,32,33,40,43 the risk was unclear, and 24 studies14,16,17,18,19,20,23,25,26,27,29,30,31,34,35,36,37,38,39,41,42,44,45,46 were rated as low risk of bias. Seven studies14,23,33,36,38,40,42 were rated as unclear for selective reporting bias, and 26 studies15,16,17,18,19,20,21,22,24,25,26,27,28,29,30,31,32,34,35,37,39,41,43,44,45,46 were rated low. Three studies23,24,34 had considerable baseline imbalances between groups and were rated high for other risk of bias; for 7 studies,14,22,26,29,32,39,40 the risk was unclear, and 23 studies15,16,17,18,19,20,21,25,27,28,30,31,33,35,36,37,38,41,42,43,44,45,46 were rated as low.
The details on conflicts of interest and study sponsors are provided on eTable 2 in the Supplement. Full details of the risk of bias assessments for each study are provided on eTable 7 in the Supplement and a summary in Figure 2.
Figure 2. Risk of Bias Summary.
All authors, with the exception of 1 for whom we had no questions,43 were contacted for clarification on unclear risk of bias items and/or requests for their data that were important for the primary outcomes of this meta-analyses. We received responses for 14 of 33 studies (42.4%).
A summary of findings is presented in eTable 8 in the Supplement and primary and secondary outcomes are presented in eTables 3 and 4 in the Supplement.
CBT vs No Intervention
The 12 RCTs15,16,17,18,19,20,21,22,23,24,25,26 that compared CBT to no intervention included a total of 785 participants. Six of the 12 studies (50.0%)17,18,19,23,24,25 provided a dichotomous definition of treatment success. The definitions are provided in eTable 5 in the Supplement. Per the GRADE framework, owing to risk of bias, there was moderate certainty in evidence that CBT was associated with higher treatment success when compared with no intervention, with 62 of 162 successes (38.3%) in the CBT group compared with 25 of 162 (15.4%) in the no intervention group (RR, 2.37; 95% CI, 1.30 to 4.34; number needed to treat [NNT] = 5) (Figure 3A).
Figure 3. Forest Plots of Cognitive Behavioral Therapy (CBT) Compared With No Intervention.
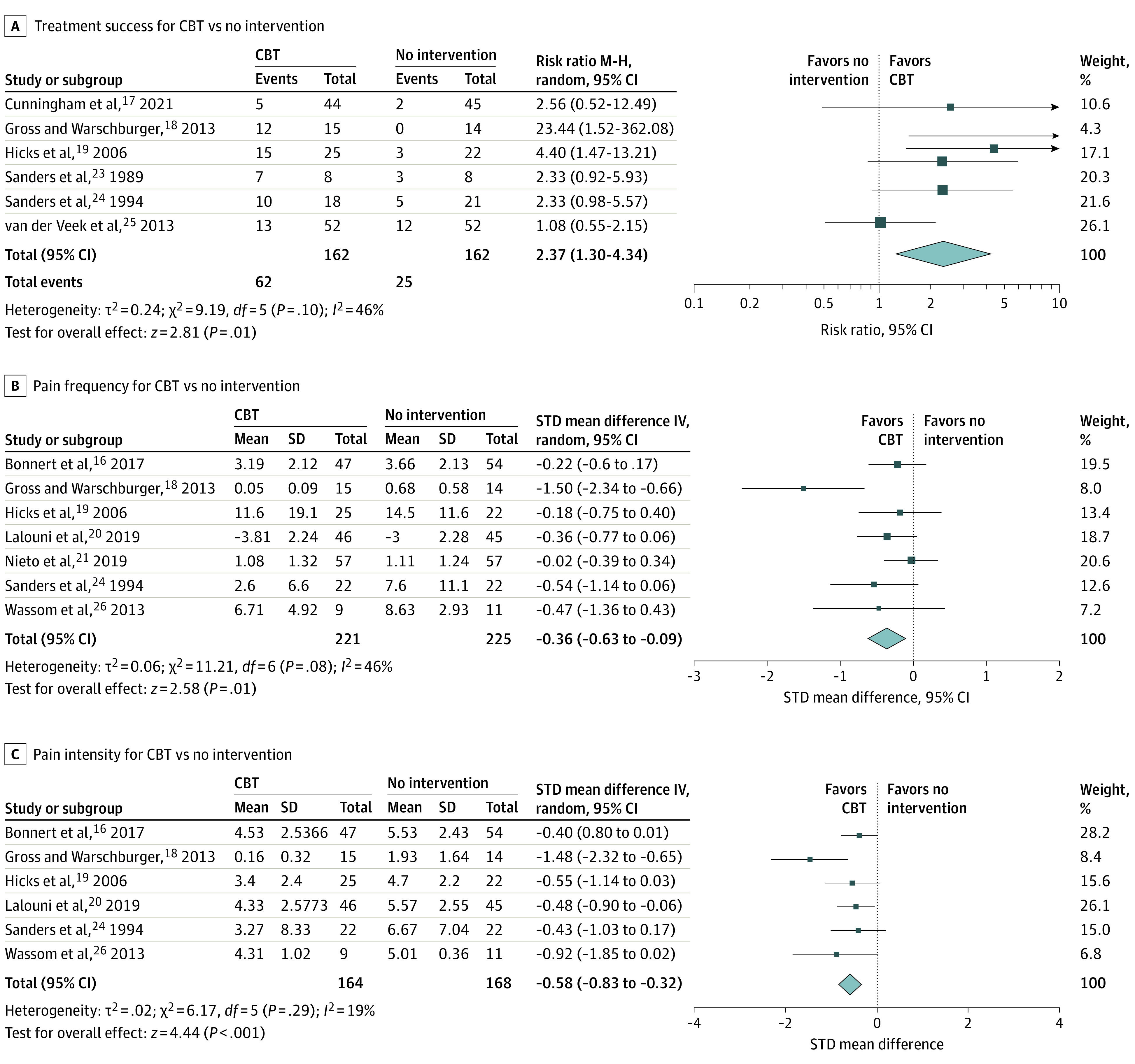
A, Treatment success for CBT vs no intervention; B, pain frequency for CBT vs no intervention; C, pain intensity for CBT vs no intervention. IV indicates inverse variance; M-H, Mantel-Haenszel; random, random effects.
Seven16,18,19,20,21,24,26 of the 12 studies (58.3%) reported on pain frequency. Owing to risk of bias, there was moderate certainty in evidence that CBT (n = 221 children) was associated with lower pain frequency when compared to no intervention (n = 225 children; RR, −0.36; 95% CI, −0.63 to −0.09) (Figure 3B).
Six16,18,19,20,24,26 of the 12 studies (50.0%) reported pain intensity. There was moderate certainty in evidence that CBT (n = 164 children) was associated with lower pain intensity when compared with no intervention (n = 168 children; RR, −0.58; 95% CI, −0.83 to −0.32) (Figure 3C).
There were cases of studies that reported composite pain scores based on pain frequency and intensity. One RCT21 provided mean and variance data that were used in this meta-analysis. The results revealed very low certainty in evidence owing to risk of bias and high imprecision from low participant numbers, therefore, it was decided not to report them.
Withdrawals owing to adverse events were reported by 7 studies.17,18,19,20,22,25,26 They all reported zero events except for one,18 which reported 1 case of hyperthyroidism in the control group, unrelated to the study. Owing to low certainty in evidence attributed to imprecision, when comparing adverse event–related withdrawals, there may be no difference between CBT and no intervention (RR, 0.33; 95% CI, 0.01-31.00).
CBT vs Educational Support
The 5 RCTs27,28,29,30,31 that compared CBT with educational support included a total of 975 participants. No studies provided enough sufficient data for treatment success and pain frequency meta-analyses. One study31 provided enough data for a pain intensity meta-analysis. No difference was observed between CBT (n = 63) and educational support (n = 64; MD, −0.36; 95% CI, −0.87 to 0.15). The results were of low certainty in evidence owing to high imprecision (Figure 4A).
Figure 4. Forest Plots of Cognitive Behavioral Therapy (CBT), Educational Support, Yoga, and Hypnotherapy Compared With No Intervention .
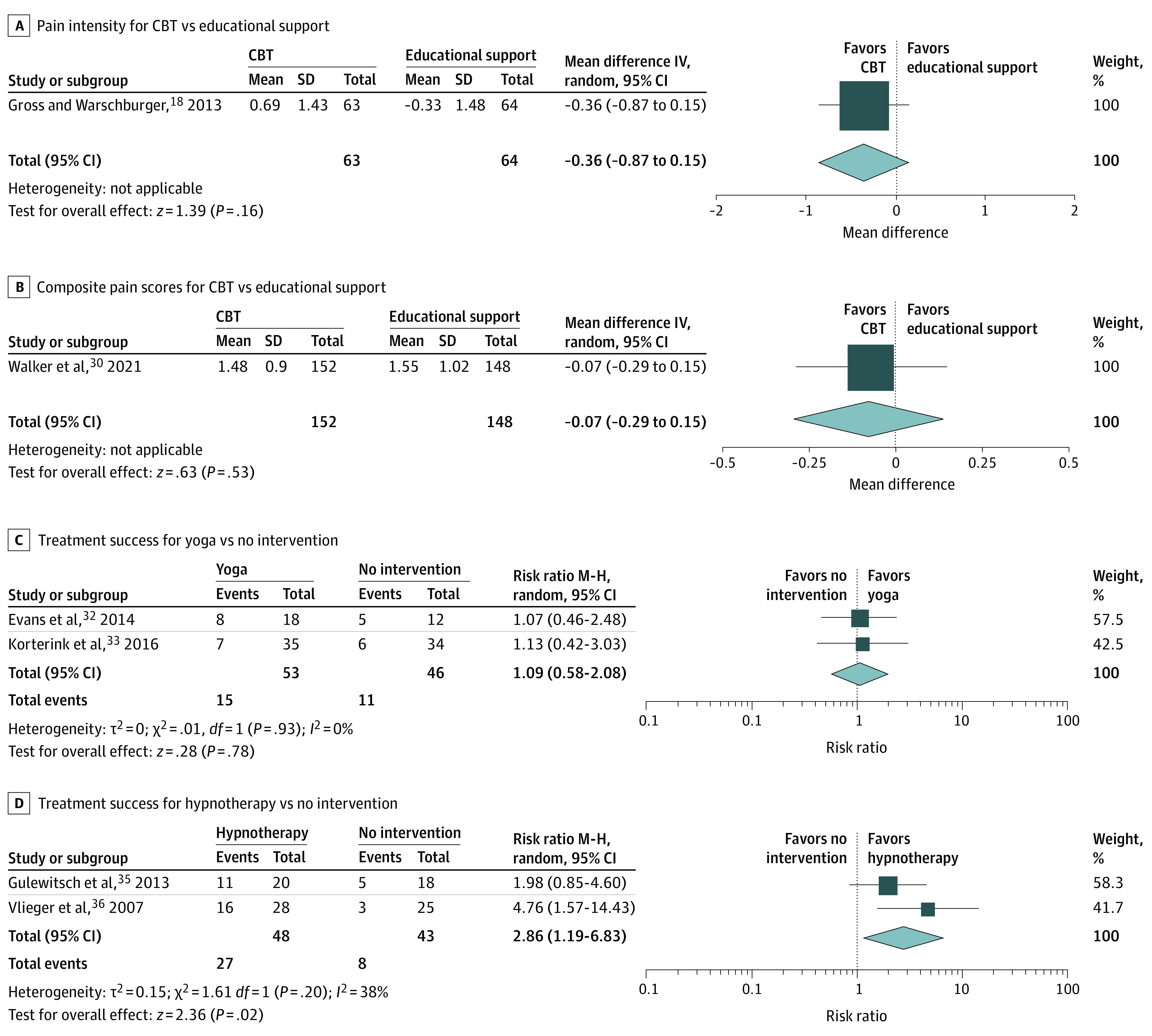
A, Pain intensity for CBT vs educational support; B, composite pain score for CBT vs educational support; C, treatment success for yoga vs no intervention; D, treatment success for hypnotherapy vs no intervention. IV indicates inverse variance; M-H, Mantel-Haenszel; random, random effects.
One study30 provided enough data for a composite pain score meta-analysis. No difference was observed between CBT (n = 152) and educational support (n = 148; MD, −0.07; 95% CI, −0.29 to 0.15). The results were of low certainty in evidence owing to high imprecision (Figure 4B).
Adverse event–related withdrawals were reported by 4 studies.28,29,30,31 They all reported zero events except for one29 which reported 1 case of a child being too ill in the control group. Owing to low certainty in evidence attributed to high imprecision, there may be no difference between CBT and educational support (RR, 0.33; 95% CI, 0.01-8.09).
Yoga vs No Intervention
The 3 RCTs32,33,34 that compared yoga to no intervention included a total of 127 children. Two studies32,33 provided a dichotomous definition of treatment success and data for a meta-analysis. Cumulatively, 15 of 53 children (28.3%) in the yoga group had treatment success compared with 11 of 46 (23.9%) in the control group. Owing to low certainty in evidence attributed to risk of bias and high imprecision, there may be no difference in treatment success when yoga was compared with no intervention (RR, 1.09; 95% CI, 0.58-2.08) (Figure 4C).
One study33 reported pain frequency and intensity and another change in pain frequency.32 However, the results of the meta-analyses were of very low certainty in evidence owing to high imprecision and risk of bias, and no conclusions could be drawn. Adverse event–related withdrawals were reported by 2 studies,32,34 which reported zero events.
Hypnotherapy vs No Intervention
The 2 RCTs35,36 that compared hypnotherapy with no intervention included a total of 91 children. They both provided a dichotomous definition of treatment success. Cumulatively, 27 of 48 children (56.3%) in the hypnotherapy group met the criteria for treatment success compared with 8 of 43 (18.6%) in the no intervention group. There was low certainty in evidence that hypnotherapy may lead to higher treatment success compared with no intervention owing to high risk of bias and imprecision from low participant numbers (RR, 2.86; 95% CI, 1.19-6.83; NNT = 5) (Figure 4D).
Only 1 study36 reported pain frequency, intensity, and a composite pain score. However, the results of the meta-analyses were of very low certainty owing to high imprecision and risk of bias, and no conclusions could be drawn. Withdrawals owing to adverse events were reported by both studies, and they both reported zero events.
Gut-Directed Hypnotherapy vs Hypnotherapy
Two RCTs37,38 compared gut-directed hypnotherapy with hypnotherapy and included a total of 73 children. One study37 provided data on all primary outcomes. Meta-analysis results were of very low certainty in evidence owing to high imprecision and risk of bias. Adverse event–related withdrawals were reported as zero by both studies.
Guided Imagery vs Relaxation
Two RCTs39,40 compared guided imagery with relaxation and included a total of 38 children. One study39 provided data on primary outcomes. The results of the meta-analyses were of very low certainty in evidence owing to high imprecision and risk of bias, and no conclusions could be drawn. Adverse event–related withdrawals were reported as zero by both studies.
Discussion
This systematic review and meta-analysis, to our knowledge, included a considerably larger evidence base compared with the latest Cochrane review on the topic.10 The vast majority of studies evaluated 5 main types of psychosocial interventions: CBT, yoga, hypnotherapy, guided imagery, and bioassisted feedback/neurostimulation. Studies had short-term follow-ups of 5 days to 4 months in duration.
We found moderate certainty in evidence that CBT was associated with higher treatment success numbers than no intervention for children with FAPDs. With a low NNT of 5, this review able to upgrade the quality of evidence and magnitude of outcome.10 Additionally, the evidence was similarly robust for related integral outcomes, including pain frequency and pain intensity reductions, with no evidence of adverse events. This forms a package of findings that are extremely compelling regarding the role of these therapies in this context.
Two studies35,36 compared hypnotherapy with no intervention and the resulting low certainty in evidence indicated that hypnotherapy may be associated with improved treatment success, which is similar to previous findings.10 The certainty of the evidence is mainly affected by imprecision from low study numbers, and it is worth noting that the NNT was identical to CBT at 5 for this analysis. This is interesting for stakeholders and families who may find this option less time consuming, less costly, more readily available, and easily self-commissioned when compared with face-to-face delivered CBT.
It is very difficult to make specific comments on a number of the other therapies owing to issues with low patient numbers and imprecision. Our suggestion would be to gauge children’s, carers’ and professionals’ interest for these therapies and accordingly prioritize future research for expansion of the evidence base.
In terms of safety, all these therapies are generally considered safe, and we found very limited evidence to suggest otherwise. This statement is key, as it is not just core to the issue of safety but underpins the pragmatic role of psychological interventions in general. Psychological mindedness is a construct that recognizes that engagement and acceptance of the role of therapies, such as CBT, are important predictors of future success with their use.47 Similar, but weaker evidence, demonstrates that this is also the case for hypnotherapy.44 Pragmatically, understanding of these principles suggests that identification of differential response predictors could enhance success and ensure that the right patients are offered these treatments and are able to, on average, achieve a better outcome. Future work to apply differential response predictors to the field could be of significant benefit.
Guideline and recommendation bodies should consider the strength and quality of the evidence when making recommendations on these therapies, as well as patient, contextual, cost, and pragmatic factors in line with the GRADE process. This may allow strong recommendations in spite of some weaknesses in evidence or indeed the reverse.
Our recently published review of pharmacological therapies for FAPD in childhood9 recognized the range of therapeutic options that may need to be discussed with families to formulate an individualized plan. We believe the findings of the review further support this viewpoint. The importance of shared decision-making should be emphasized. CBT might be the most appropriate choice of therapy for some, whereas for others it might be hypnotherapy or pharmacological therapies or a combined or hierarchical approach.
Limitations
The only issue or concern that exists with the evidence for the most studied intervention, CBT, was downgrading owing to concerns with risk of bias. This improved after feedback from authors, but blinding was still a concern. Although participant blinding is something that can be applied less stringently in GRADE analyses of such interventions,47 outcome assessor blinding was still a major issue and one that contributed to the downgrading from high certainty. This was the case for the majority of interventions studied in this review. It must be considered by all stakeholders, when the rest of the evidence is so compelling through multiple outcomes, sensitivity analysis, and studies from over almost 30 years, that blinding may not be an issue that can be rectified; clear recommendations can still be made in spite of this. Previous research has discussed the effect of expectation of efficacy in trials that did and did not use double-blinding.48 In this context, some of the well-designed trials tried to take this into account.39 However, it is unclear how reviewers can measure the success or effect of such measures at present and so remains a methodological area of complexity in reviews of behavioral interventions.
The open-label design of the majority of studies may have also influenced the placebo effect in this review. Previous reviews have found that a placebo might be a potential effective treatment of children with FAPDs as well, with placebo success rates of 41% previously identified.49 The true placebo effect comprises conditioning and expectations, as well as other methodological and clinical factors. In our analyses, the placebo response rates stood at between 15% and 25%, which is lower than previously identified and most likely reflects the open-label nature of the majority of studies. It is still remarkable that 1 in 5 patients within the short follow-up time of these studies achieved treatment success with no intervention.
Our primary dichotomous outcome was treatment success, but it was only reported by half of the included studies. Many of the studies used composite pain scores, which we would advise future studies to avoid, as they can increase heterogeneity of the evidence base. In 2016, the Rome Foundation made recommendations for designing trials of FAPDs in children.1 This committee recommends considering abdominal pain as the primary outcome, assessed with daily diaries, which was not the case in many of the studies. Such consistency in outcome measures is important to allow users to compare and contrast findings and for reviewers to make even greater progress in future synthesis.
The potential for meta-analyses was further hampered by the poor reporting of the data in many instances, especially the lack of variance or SD reporting. Allocation concealment was poorly reported but contact with authors allowed much of this information to be obtained and, as such, the overall risk of bias in the data was improved when compared with previous reviews on the topic.9
Most of our outcome results were at high risk of imprecision owing to low numbers of participants per outcome, a common issue in gastroenterological RCTs9 and one that is not only mirrored throughout gastroenterological trials50 but is not improving with time.51
Conclusions
Results of this systematic review and meta-analysis suggest that CBT and hypnotherapy should be considered as a treatment for FAPDs in childhood, and these new findings should be considered by guideline committees internationally. Future RCTs should address sample size and precision issues in integral areas to enhance overall certainty; in addition, studies should also consider the role of targeted interventions for susceptible patients. CBT combined with a pharmacological or microbiome-based therapy may be considered.
eTable 1. Included Studies Characteristics
eTable 2. Study Sponsor Details
eTable 3. Primary Outcomes
eTable 4. Secondary Outcomes
eTable 5. Definitions of Treatment Success in the Included Studies
eTable 6. Excluded Studies
eTable 7. Risk of Bias Details
eTable 8. Summary of Findings
eAppendix 1. ROME IV Criteria for Pediatric FAPDs
eAppendix 2. Search Strategies
References
- 1.Saps M, van Tilburg MA, Lavigne JV, et al. Recommendations for pharmacological clinical trials in children with irritable bowel syndrome: the Rome Foundation pediatric subcommittee on clinical trials. Neurogastroenterol Motil. 2016;28(11):1619-1631. doi: 10.1111/nmo.12896 [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M, Di Lorenzo C. Brain-gut axis: from basic understanding to treatment of IBS and related disorders. J Pediatr Gastroenterol Nutr. 2012;54(4):446-453. doi: 10.1097/MPG.0b013e31823d34c3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med. 2011;62:381-396. doi: 10.1146/annurev-med-012309-103958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chumpitazi BP, Palermo TM, Hollier JM, et al. Multisite pain is highly prevalent in children with functional abdominal pain disorders and is associated with increased morbidity. J Pediatr. 2021;236:131-136. doi: 10.1016/j.jpeds.2021.04.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovacic K, Kapavarapu PK, Sood MR, et al. Nausea exacerbates symptom burden, quality of life, and functioning in adolescents with functional abdominal pain disorders. Neurogastroenterol Motil. 2019;31(7):e13595. doi: 10.1111/nmo.13595 [DOI] [PubMed] [Google Scholar]
- 6.Saps M, Miranda A. Gastrointestinal pharmacology. Handb Exp Pharmacol. 2017;239:147-176. doi: 10.1007/164_2016_119 [DOI] [PubMed] [Google Scholar]
- 7.Hoekman DR, Rutten JMTM, Vlieger AM, Benninga MA, Dijkgraaf MGW. Annual costs of care for pediatric irritable bowel syndrome, functional abdominal pain, and functional abdominal pain syndrome. J Pediatr. 2015;167(5):1103-1108. doi: 10.1016/j.jpeds.2015.07.058 [DOI] [PubMed] [Google Scholar]
- 8.Walker LS, Dengler-Crish CM, Rippel S, Bruehl S. Functional abdominal pain in childhood and adolescence increases risk for chronic pain in adulthood. Pain. 2010;150(3):568-572. doi: 10.1016/j.pain.2010.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rexwinkel R, de Bruijn CMA, Gordon M, Benninga MA, Tabbers MM. Pharmacologic treatment in functional abdominal pain disorders in children: a systematic review. Pediatrics. 2021;147(6):e2020042101. doi: 10.1542/peds.2020-042101 [DOI] [PubMed] [Google Scholar]
- 10.Abbott RA, Martin AE, Newlove-Delgado TV, et al. Psychosocial interventions for recurrent abdominal pain in childhood. Cochrane Database Syst Rev. 2017;1(1):CD010971.doi: 10.1002/14651858.CD010971.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JP, Thomas J, Chandler J, eds. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019. [Google Scholar]
- 12.Functional abdominal pain disorders in children (4-18 years): a systematic review on the effectiveness and safety of nonpharmacological treatment options. PROSPERO identifier:CRD42020159847. Updated April 28, 2020. Accessed March 9, 2022. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=159847
- 13.Zeevenhooven J, Rexwinkel R, Van Berge Henegouwen VWA, et al. ; Consensus Group on Outcome Measures Made in Pediatric Enteral Nutrition Clinical Trials Working Group . A core outcome set for clinical trials in pediatric functional abdominal pain disorders. J Pediatr. 2020;221:115-122.e5. doi: 10.1016/j.jpeds.2020.02.032 [DOI] [PubMed] [Google Scholar]
- 14.Schurman JV, Wu YP, Grayson P, Friesen CA. A pilot study to assess the efficacy of biofeedback-assisted relaxation training as an adjunct treatment for pediatric functional dyspepsia associated with duodenal eosinophilia. J Pediatr Psychol. 2010;35(8):837-847. doi: 10.1093/jpepsy/jsq010 [DOI] [PubMed] [Google Scholar]
- 15.Alfvén G, Lindstrom A. A new method for the treatment of recurrent abdominal pain of prolonged negative stress origin. Acta Paediatr. 2007;96(1):76-81. doi: 10.1111/j.1651-2227.2006.00028.x [DOI] [PubMed] [Google Scholar]
- 16.Bonnert M, Olén O, Lalouni M, et al. Internet-delivered cognitive behavior therapy for adolescents with irritable bowel syndrome: a randomized controlled trial. Am J Gastroenterol. 2017;112(1):152-162. doi: 10.1038/ajg.2016.503 [DOI] [PubMed] [Google Scholar]
- 17.Cunningham NR, Kalomiris A, Peugh J, et al. Cognitive behavior therapy tailored to anxiety symptoms improves pediatric functional abdominal pain outcomes: a randomized clinical trial. J Pediatr. 2021;230:62-70.e3. doi: 10.1016/j.jpeds.2020.10.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross M, Warschburger P. Evaluation of a cognitive-behavioral pain management program for children with chronic abdominal pain: a randomized controlled study. Int J Behav Med. 2013;20(3):434-443. doi: 10.1007/s12529-012-9228-3 [DOI] [PubMed] [Google Scholar]
- 19.Hicks CL, von Baeyer CL, McGrath PJ. Online psychological treatment for pediatric recurrent pain: a randomized evaluation. J Pediatr Psychol. 2006;31(7):724-736. doi: 10.1093/jpepsy/jsj065 [DOI] [PubMed] [Google Scholar]
- 20.Lalouni M, Ljótsson B, Bonnert M, et al. ; Clinical and Cost Effectiveness of Online Cognitive Behavioral Therapy in Children With Functional Abdominal Pain Disorders . Clinical and cost effectiveness of online cognitive behavioral therapy in children with functional abdominal pain disorders. Clin Gastroenterol Hepatol. 2019;17(11):2236-2244.e11. doi: 10.1016/j.cgh.2018.11.043 [DOI] [PubMed] [Google Scholar]
- 21.Nieto R, Boixadós M, Ruiz G, Hernández E, Huguet A. effects and experiences of families following a web-based psychosocial intervention for children with functional abdominal pain and their parents: a mixed-methods pilot randomized controlled trial. J Pain Res. 2019;12:3395-3412. doi: 10.2147/JPR.S221227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robins PM, Smith SM, Glutting JJ, Bishop CT. A randomized controlled trial of a cognitive-behavioral family intervention for pediatric recurrent abdominal pain. J Pediatr Psychol. 2005;30(5):397-408. doi: 10.1093/jpepsy/jsi063 [DOI] [PubMed] [Google Scholar]
- 23.Sanders MR, Rebgetz M, Morrison M, et al. Cognitive-behavioral treatment of recurrent nonspecific abdominal pain in children: an analysis of generalization, maintenance, and side effects. J Consult Clin Psychol. 1989;57(2):294-300. doi: 10.1037/0022-006X.57.2.294 [DOI] [PubMed] [Google Scholar]
- 24.Sanders MR, Shepherd RW, Cleghorn G, Woolford H. The treatment of recurrent abdominal pain in children: a controlled comparison of cognitive-behavioral family intervention and standard pediatric care. J Consult Clin Psychol. 1994;62(2):306-314. doi: 10.1037/0022-006X.62.2.306 [DOI] [PubMed] [Google Scholar]
- 25.van der Veek SM, Derkx BH, Benninga MA, Boer F, de Haan E. Cognitive behavior therapy for pediatric functional abdominal pain: a randomized controlled trial. Pediatrics. 2013;132(5):e1163-e1172. doi: 10.1542/peds.2013-0242 [DOI] [PubMed] [Google Scholar]
- 26.Wassom MC, Shurman JV, Friesen CA, Rapoff MA. A pilot study of “Gutstrong” for adolescents with functional gastrointestinal disorders. Clin Pract Pediatr Psychol. 2013;1(3):201. doi: 10.1037/cpp0000025 [DOI] [Google Scholar]
- 27.Duarte MA, Penna FJ, Andrade EM, Cancela CS, Neto JC, Barbosa TF. Treatment of nonorganic recurrent abdominal pain: cognitive-behavioral family intervention. J Pediatr Gastroenterol Nutr. 2006;43(1):59-64. doi: 10.1097/01.mpg.0000226373.10871.76 [DOI] [PubMed] [Google Scholar]
- 28.Levy RL, Langer SL, van Tilburg MAL, et al. Brief telephone-delivered cognitive behavioral therapy targeted to parents of children with functional abdominal pain: a randomized controlled trial. Pain. 2017;158(4):618-628. doi: 10.1097/j.pain.0000000000000800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy RL, Langer SL, Walker LS, et al. Cognitive-behavioral therapy for children with functional abdominal pain and their parents decreases pain and other symptoms. Am J Gastroenterol. 2010;105(4):946-956. doi: 10.1038/ajg.2010.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker LS, Stone AL, Han GT, et al. Internet-delivered cognitive behavioral therapy for youth with functional abdominal pain: a randomized clinical trial testing differential efficacy by patient subgroup. Pain. 2021;162(12):2945-2955. doi: 10.1097/j.pain.0000000000002288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warschburger P, Calvano C, Becker S, et al. Do children with functional abdominal pain benefit more from a pain-specific cognitive-behavioral intervention than from an unspecific attention-control intervention? results of a randomized controlled trial. Am J Gastroenterol. 2021;116(6):1322-1335. doi: 10.14309/ajg.0000000000001191 [DOI] [PubMed] [Google Scholar]
- 32.Evans S, Lung KC, Seidman LC, Sternlieb B, Zeltzer LK, Tsao JC. Iyengar yoga for adolescents and young adults with irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 2014;59(2):244-253. doi: 10.1097/MPG.0000000000000366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korterink JJ, Ockeloen LE, Hilbink M, Benninga MA, Deckers-Kocken JM. Yoga therapy for abdominal pain-related functional gastrointestinal disorders in children: a randomized controlled trial. J Pediatr Gastroenterol Nutr. 2016;63(5):481-487. doi: 10.1097/MPG.0000000000001230 [DOI] [PubMed] [Google Scholar]
- 34.Kuttner L, Chambers CT, Hardial J, Israel DM, Jacobson K, Evans K. A randomized trial of yoga for adolescents with irritable bowel syndrome. Pain Res Manag. 2006;11(4):217-223. doi: 10.1155/2006/731628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gulewitsch MD, Müller J, Hautzinger M, Schlarb AA. Brief hypnotherapeutic-behavioral intervention for functional abdominal pain and irritable bowel syndrome in childhood: a randomized controlled trial. Eur J Pediatr. 2013;172(8):1043-1051. doi: 10.1007/s00431-013-1990-y [DOI] [PubMed] [Google Scholar]
- 36.Vlieger AM, Menko-Frankenhuis C, Wolfkamp SC, Tromp E, Benninga MA. Hypnotherapy for children with functional abdominal pain or irritable bowel syndrome: a randomized controlled trial. Gastroenterology. 2007;133(5):1430-1436. doi: 10.1053/j.gastro.2007.08.072 [DOI] [PubMed] [Google Scholar]
- 37.Gulewitsch MD, Schlarb AA. Comparison of gut-directed hypnotherapy and unspecific hypnotherapy as self-help format in children and adolescents with functional abdominal pain or irritable bowel syndrome: a randomized pilot study. Eur J Gastroenterol Hepatol. 2017;29(12):1351-1360. doi: 10.1097/MEG.0000000000000984 [DOI] [PubMed] [Google Scholar]
- 38.Pas R, Rheel E, Van Oosterwijck S, et al. Pain neuroscience education for children with functional abdominal pain disorders: a randomized comparative pilot study. J Clin Med. 2020;9(6):1797. doi: 10.3390/jcm9061797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weydert JA, Shapiro DE, Acra SA, Monheim CJ, Chambers AS, Ball TM. Evaluation of guided imagery as treatment for recurrent abdominal pain in children: a randomized controlled trial. BMC Pediatr. 2006;6(1):29. doi: 10.1186/1471-2431-6-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Youssef NN, Van Tilburg MA, Matta EN, Langseder A, Whitehead WE. Feasibility and efficacy of pilot study investigating a school nurse–administered guided-imagery program for childhood functional abdominal pain. Gastroenterology. 2009;136(5):156-157. doi: 10.1016/S0016-5085(09)60705-8 [DOI] [Google Scholar]
- 41.Ingo C. Auswirkungen Osteopathischer Behandlung bei Kindern mit funktionellen Bauchschmerzen: Eine klinische Studie mit 32 Kindern und Jugendlichen im Alter von 6 bis 18 Jahren, in Osteopathie Schule. Dissertation. Dresden International University; 2019. [Google Scholar]
- 42.Humphreys PA, Gevirtz RN. Treatment of recurrent abdominal pain: components analysis of four treatment protocols. J Pediatr Gastroenterol Nutr. 2000;31(1):47-51. doi: 10.1097/00005176-200007000-00011 [DOI] [PubMed] [Google Scholar]
- 43.Kovacic K, Hainsworth K, Sood M, et al. Neurostimulation for abdominal pain-related functional gastrointestinal disorders in adolescents: a randomised, double-blind, sham-controlled trial. Lancet Gastroenterol Hepatol. 2017;2(10):727-737. doi: 10.1016/S2468-1253(17)30253-4 [DOI] [PubMed] [Google Scholar]
- 44.Rutten JMTM, Vlieger AM, Frankenhuis C, et al. Home-based hypnotherapy self-exercises vs individual hypnotherapy with a therapist for treatment of pediatric irritable bowel syndrome, functional abdominal pain, or functional abdominal pain syndrome: a randomized clinical trial. JAMA Pediatr. 2017;171(5):470-477. doi: 10.1001/jamapediatrics.2017.0091 [DOI] [PubMed] [Google Scholar]
- 45.van Tilburg MA, Chitkara DK, Palsson OS, et al. Audio-recorded guided imagery treatment reduces functional abdominal pain in children: a pilot study. Pediatrics. 2009;124(5):e890-e897. doi: 10.1542/peds.2009-0028 [DOI] [PubMed] [Google Scholar]
- 46.Wallander JL, Madan-Swain A, Klapow J, Saeed S. A randomised controlled trial of written self-disclosure for functional recurrent abdominal pain in youth. Psychol Health. 2011;26(4):433-447. doi: 10.1080/08870440903477212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLellan LF, Stapinski LA, Peters L. Pretreatment CBT-mindedness predicts CBT outcome. Cognit Ther Res. 2019;43(2):303-311. doi: 10.1007/s10608-018-9977-7 [DOI] [Google Scholar]
- 48.Colagiuri B. Participant expectancies in double-blind randomized placebo-controlled trials: potential limitations to trial validity. Clin Trials. 2010;7(3):246-255. doi: 10.1177/1740774510367916 [DOI] [PubMed] [Google Scholar]
- 49.Hoekman DR, Zeevenhooven J, van Etten-Jamaludin FS, et al. The placebo response in pediatric abdominal pain–related functional gastrointestinal disorders: a systematic review and meta-analysis. J Pediatr. 2017;182:155-163.e7. doi: 10.1016/j.jpeds.2016.12.022 [DOI] [PubMed] [Google Scholar]
- 50.Iheozor-Ejiofor Z, Lakunina S, Gordon M, Akintelure D, Sinopoulou V, Akobeng A. Sample-size estimation is not reported in 24% of randomised controlled trials of inflammatory bowel disease: a systematic review. United European Gastroenterol J. 2021;9(1):47-53. doi: 10.1177/2050640620967899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korterink JJ, Rutten JMTM, Venmans L, Benninga MA, Tabbers MM. Pharmacologic treatment in pediatric functional abdominal pain disorders: a systematic review. J Pediatr. 2015;166(2):424-431.e6.doi: 10.1016/j.jpeds.2014.09.067 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Included Studies Characteristics
eTable 2. Study Sponsor Details
eTable 3. Primary Outcomes
eTable 4. Secondary Outcomes
eTable 5. Definitions of Treatment Success in the Included Studies
eTable 6. Excluded Studies
eTable 7. Risk of Bias Details
eTable 8. Summary of Findings
eAppendix 1. ROME IV Criteria for Pediatric FAPDs
eAppendix 2. Search Strategies