This systematic review and meta-analysis examines the risks of neurocognitive and/or psychological impairment and other disorders in young children after antenatal corticosteroid administration.
Key Points
Question
What are the long-term outcomes for children exposed to antenatal corticosteroids?
Findings
In this systematic review and meta-analysis of 30 studies involving more than 1.25 million children, exposure to a single course of antenatal corticosteroids was associated with a significant decrease in the adjusted odds of neurodevelopmental impairment in children with extremely preterm birth. In children with late-preterm and full-term birth, antenatal corticosteroid exposure was associated with increased adjusted risks of neurocognitive and/or psychological impairment.
Meaning
Findings of this study suggest that caution may be required in administering antenatal corticosteroids given the associated neurocognitive and/or psychological harms for children with late-preterm and full-term birth.
Abstract
Importance
Animal studies have found that antenatal corticosteroids affect many organs across multiple stages of life. However, the long-term outcomes in human children are not well understood.
Objective
To conduct a systematic review and meta-analysis of long-term outcomes associated with preterm exposure to antenatal corticosteroids compared with no exposure in all children as well as children with preterm and full-term birth.
Data Sources
Academic databases were searched for articles published from January 1, 2000, to October 29, 2021, including Ovid MEDLINE, Ovid Embase, PsycInfo, CINAHL (Cumulative Index of Nursing and Allied Health Literature), Web of Science, ClinicalTrials.gov, and Google Scholar. References of articles were also searched for relevant studies.
Study Selection
Randomized clinical trials (RCTs), quasi-RCTs, and cohort studies that assessed long-term neurodevelopmental, psychological, or other outcomes at 1 year or older in those who had preterm exposure to antenatal corticosteroids were included. No language restrictions were set.
Data Extraction and Synthesis
Two reviewers independently extracted data using a piloted data extraction form. Data on study population, pregnancy characteristics, exposure to antenatal corticosteroids, and outcomes were collected. Preferred Reporting Items for Systematic Reviews and Meta-analyses reporting guidelines were followed, and random-effects models were used for the meta-analysis.
Main Outcomes and Measures
The primary outcome was an author-defined composite of any adverse neurodevelopmental and/or psychological disorder. The secondary outcomes included specific measures of psychological disorders; neurodevelopmental delay; and anthropometric, metabolic, and cardiorespiratory outcomes.
Results
A total of 30 studies met the inclusion criteria, and involved more than 1.25 million children who were at least 1 year of age when the outcomes were assessed. Exposure to a single course of antenatal corticosteroids for children with extremely preterm birth was associated with a significant reduction in risk of neurodevelopmental impairment (adjusted odds ratio, 0.69 [95% CI, 0.57-0.84]; I2 = 0%; low certainty). For children with late-preterm birth, exposure to antenatal corticosteroids was associated with a higher risk of investigation for neurocognitive disorders (n = 25 668 children; adjusted hazard ratio [aHR], 1.12 [95% CI, 1.05-1.20]; low certainty). For children with full-term birth, exposure to antenatal corticosteroids was associated with a higher risk of mental or behavioral disorders (n = 641 487 children; aHR, 1.47 [95% CI, 1.36-1.60]; low certainty) as well as proven or suspected neurocognitive disorders (n = 529 205 children; aHR, 1.16 [95% CI, 1.10-1.21]; low certainty).
Conclusions and Relevance
Results of this study showed that exposure to a single course of antenatal corticosteroids was associated with a significantly lower risk of neurodevelopmental impairment in children with extremely preterm birth but a significantly higher risk of adverse neurocognitive and/or psychological outcomes in children with late-preterm and full-term birth, who made up approximately half of those with exposure to antenatal corticosteroids. The findings suggest a need for caution in administering antenatal corticosteroids.
Introduction
Antenatal corticosteroids administered in pregnancies at risk for preterm birth decrease the risk of mortality and morbidity in preterm newborns.1,2 Given these benefits, numerous clinical guidelines advocate the use of antenatal corticosteroids in pregnancies that are at risk for preterm birth.3,4 Studies in animals have found an association between exposure to antenatal corticosteroids at doses similar to those given to humans and harmful neurological outcomes, including alterations to the hypothalamic-pituitary-adrenal axis,5,6 diminished cortical surface,7 loss of essential synaptic proteins,8 and decreased blood flow in areas of the brain.9 In full-term animals, preterm exposure to antenatal corticosteroids was associated with harmful neural outcomes, such as decreased hippocampal development.10 Moreover, in both preterm and full-term animals, there were implications for other organs,11 including reduced glomerular filtration rate12; in full-term animals, the use of antenatal corticosteroids was associated with an increased insulin to glucose ratio.13 Given these reported harmful outcomes in animals, understanding the long-term implications of preterm exposure to antenatal corticosteroids in children with both preterm and full-term birth is a critical research priority.
We conducted a systematic review and meta-analysis of long-term outcomes associated with (1) preterm exposure to a single course of antenatal corticosteroids (either 24 mg of betamethasone or dexamethasone), or (2) preterm exposure to an unspecified number of courses (although likely 1 course) vs no exposure. Given that approximately half of children who were exposed to antenatal corticosteroids exceeded expectations and were born at or after 35 weeks of gestation,14 we aimed to conduct a systematic review and meta-analysis of long-term outcomes in all children as well as children with preterm (ie, born before 37 weeks of gestation) and full-term (ie, born at or after 37 weeks of gestation) birth.
Methods
We followed the Cochrane Handbook for Systematic Reviews of Intervention15 and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.16 The protocol was registered on PROSPERO (CRD42020194167) and updated on December 8, 2021.
Data Sources and Study Eligibility
With the guidance of an information specialist, we searched the following 6 academic databases for articles published from January 1, 2000, through October 29, 2021: Ovid MEDLINE, Ovid Embase, PsycInfo, CINAHL (Cumulative Index of Nursing and Allied Health Literature), Web of Science, and ClinicalTrials.gov; the first 400 results were retrieved from Google Scholar (eAppendix 1 in the Supplement). We also screened the references in articles for relevant studies.
Having set no language restrictions, we considered randomized clinical trials (RCTs), quasi-RCTs, and observational studies (ie, follow-up of RCTs, cohort studies, and case-control studies) that assessed long-term neurodevelopmental, psychological, or other outcomes at 1 year or older in those who had preterm exposure to antenatal corticosteroids. Other types of publications were excluded. Studies involving births occurring in or after 2000 were considered given the implications of recent advances in neonatal care for short-term and long-term outcomes.17,18
The primary outcome was an author-defined composite of any adverse neurodevelopmental and/or psychological disorder. Prespecified secondary outcomes included specific measures of visual impairment; auditory impairment; psychological developmental disorders (eg, disorders of speech and language as well as scholastic skills); autism spectrum disorders; attention-deficit/hyperactivity disorder or conduct disorders; mixed disorders of conduct and emotions; emotional, social functioning, or tic disorders; other behavioral and emotional disorders; psychotic, mood, neurotic, stress-related, or somatization disorders; eating disorders; sleep disorders; measures of anxiety-related symptoms or clinical measures of anxiety; measures of anxiety-related symptoms or clinical measures of depression; special educational needs; cerebral palsy; Bayley Scales of Infant and Toddler Development (BSID)-II Mental Developmental Index score less than 70 points; BSID-II Psychomotor Developmental Index score less than 70 points; BSID-III cognitive score less than 85 points; BSID-III language score less than 85 points; IQ scores; intellectual impairment (defined as an IQ or developmental quotient at least 2 SDs below the mean); mild, moderate, or unspecified intellectual disability; and other neurodevelopmental and/or psychological outcomes included in the literature. For the secondary outcomes, we considered long-term anthropometric (ie, weight, height, and head circumference), cardiorespiratory, endocrine, and metabolic outcomes as well as survival in childhood and adulthood.
Furthermore, we reported the proportion of children who were born full term with preterm exposure to antenatal corticosteroids. Because there was not a specific core outcome set for long-term follow-up, all outcomes that were reported at 1 year or older were included.
Data Screening and Extraction
Two of us (K.N. and S.K.L.) independently screened the titles and abstracts for a full-text review. The full-text articles were assessed, and data were extracted using a piloted data extraction form. Data were collected on pregnancy characteristics, study population, exposure to antenatal corticosteroids, and outcomes included in the review. Discrepancies were resolved through discussion between the 2 reviewers, and although consultation with a third reviewer (S.D.M.) was available for resolving discrepancies, it proved unnecessary.
In addition, 2 of us (K.N. and S.K.L.) planned to independently appraise the quality of RCTs and their follow-up studies using version 2 of the Cochrane risk-of-bias tool.15 For observational studies, we used the modified Newcastle-Ottawa Scale (NOS) to assess risk of bias across 8 domains, awarding a minimum of one-half star or one-half point (indicating the lowest obtainable score) to a maximum of 9 stars or 9 points (indicating the highest obtainable score).19 For the comparability domain of the modified NOS, one-half star was awarded for each confounder and 2 stars were awarded if at least 4 of the following 6 key confounders were addressed: use of postnatal steroids, gestational age at birth, intrauterine growth restriction, family or maternal history of neurodevelopmental and psychological problems, socioeconomic status, and maternal substance use. We identified these key confounders on the basis of clinical expertise and review of the literature.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool was used to rate the certainty of included outcomes in 6 domains (risk of bias, inconsistency, imprecision, indirectness, publication bias, and other considerations). The GRADE ratings were high, moderate, low, or very low certainty.
Data Analysis and Synthesis
Data for all children and the preterm and full-term subgroups were analyzed. Data on the race and ethnicity of children were collected when available.
A random-effects meta-analysis was performed for outcomes that could be pooled. Risk ratio (RR), odds ratio (OR), and 95% CIs were identified for binary summary effect sizes, whereas mean differences and 95% CIs were reported for continuous summary effect sizes. The I2 statistic was used to report heterogeneity. The meta-analysis was performed using Review Manager, version 5.4.1 (Cochrane Community).20 For studies with overlapping populations, results for each study were presented. However, the largest study was considered for the meta-analysis. Single-study outcomes were also reported.
A 2-sided P < .05 was considered to be statistically significant. A priori, we planned to conduct sensitivity analyses limited to higher-quality studies (ie, those rated with at least 6 of 9 stars on the NOS) as well as subgroup analyses to compare outcomes by treatment (betamethasone vs dexamethasone), by sex (male vs female), and by gestational age at administration of antenatal corticosteroids (<28 weeks, 28-33 weeks, or 34-36 weeks).
Results
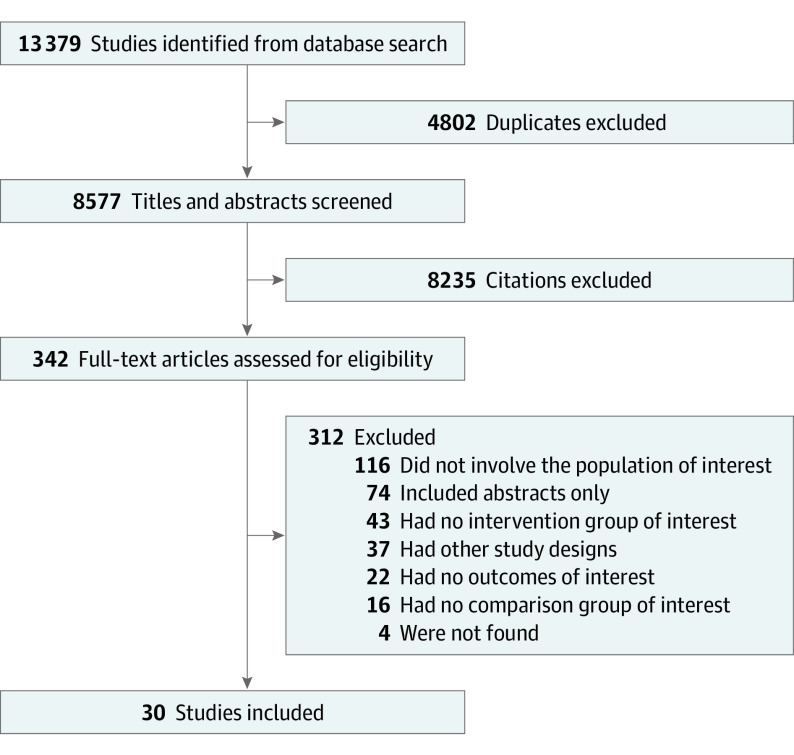
A total of 13 379 records were retrieved (Figure 1). After excluding 4802 duplicate records, we screened 8577 titles and abstracts and assessed 342 full-text articles. A total of 30 cohort studies21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50 met the final inclusion criteria that, together, involved more than 1.25 million children who were at least 1 year of age when the outcomes were assessed (Table 1; eTable 1 in the Supplement); the justifications for excluding other important studies are provided in eAppendix 2 in the Supplement. Race and ethnicity categories were reported in 6 studies22,27,35,38,40,42 (eTable 1 in the Supplement). A few studies adjusted for race and ethnicity but did not report the adjusted effect sizes of race and ethnicity on long-term outcomes.22,38,42
Figure 1. PRISMA Diagram of Study Selection .
Table 1. Characteristics of Included Studies.
| Source and country | Risk-of-bias ratinga | Recruitment period (timing of follow-up) | Study design | Treatment (dose pattern) |
|---|---|---|---|---|
| Single antenatal corticosteroid course vs nonexposure | ||||
| Children with preterm birth | ||||
| Chawla et al,40 2013, US | ★★★★★★★★ | 2005-2008 (18-22 mo corrected age) | Retrospective cohort | Betamethasone, 2 doses (NS) |
| Gover et al,46 2012, Canada | ★★★★★★★½ | 2001-2004 (18 mo corrected age) | Prospective cohort | Dexamethasone (two 12 mg once every 12-24 h) |
| Chawla et al,38 2016, US | ★★★★★★★ | 2006-2011 (18-22 mo corrected age) | Prospective cohort study | Betamethasone (two 12 mg once every 24 h) or dexamethasone (four 6 mg once every 12 h) |
| Lee et al,42 2008, US | ★★★★★★½ | 2002-2003 (18-22 mo corrected age) | Retrospective cohort | Betamethasone (two 12 mg once every 24 h) or dexamethasone (four 6 mg once every 12 h) |
| Agarwal et al,35 2018, Singapore | ★★★★★★ | 2010-2011 (mean [SD]: 24 [4] mo corrected age) | Prospective cohort | Dexamethasone (two 12 mg once every 24 h) |
| Kim et al,36 2018, Korea | ★★★★★½ | 2001-2016 (18-22 mo after birth) | Retrospective cohort | Betamethasone (two 12 mg once every 24 h) or dexamethasone (four 6 mg once every 12 h) |
| Laughon et al,43 2009, unspecified | ★★★★★½ | 2002-2004 (24 mo corrected age) | Prospective cohort | Betamethasone (two 12 mg once every 24 h) or dexamethasone (four 6 mg once every 12 h) |
| McElrath et al,41 2009, US | ★★★★★½ | 2002-2004 (approximately 24 mo corrected age) | Prospective cohort | Betamethasone (dose NS; two once every 24 h) or dexamethasone (dose NS; four once every 12 h) administered at least 48 h after the first dose |
| Lardón et al,37 2017, Spain | ★★★★½ | 2008-2013 (during first 2 y of corrected age) | Prospective cohort | Betamethasone (two 12 mg once every 24 h) |
| Tseng et al,39 2016, Taiwan | ★★★★½ | 2007-2010 (2-5 y) | Prospective cohort | Betamethasone (two 12 mg once every 24 h) or dexamethasone (four 6 mg once every 12 h) |
| Unspecified No. of antenatal corticosteroid courses vs nonexposure | ||||
| Children with preterm or full-term birth | ||||
| Lamminmäki et al,45 2021, Finland | ★★★★★★★½ | 2003-2008 (1.5-8 y) | Retrospective cohort | Betamethasone (two 12 mg once every 24 h)b |
| Räikkönen et al,21 2020, Finland | ★★★★★★★½ | 2006-2017 (1-11 y) | Retrospective cohort | Betamethasone (two 12 mg once every 24 h)b |
| Wolford et al,24 2020, Finland | ★★★★★★★ | 2006-2010 (6-10 y) | Prospective cohort | Betamethasone (two 12 mg once every 24 h, IM)b |
| Children with preterm birth | ||||
| Haslam et al,27 2018, Canada | ★★★★★★★★½ | 2009-2011 (18-21 mo corrected age) | Retrospective cohort | Betamethasone (two 12 mg once every 24 h, IM)b |
| Aviram et al,50 2021, Canada | ★★★★★★★★ | 2006-2011 (at least 5 y) | Retrospective cohort | Betamethasone (two 12 mg once every 24 h, IM)b |
| Räikkönen et al,21 2020, Finland | ★★★★★★★½ | 2006-2017 (1-11 y) | Retrospective cohort | Betamethasone (two 12 mg once every 24 h)b |
| Hutcheon et al,49 2020 Canada | ★★★★★★★ | 2000-2013 (5-6 y) | Regression discontinuity | Betamethasone (two 12 mg once every 24 h, IM)b |
| Gentle et al,22 2020, US | ★★★★★★★ | 2011-2014 (18-26 mo corrected age) | Prospective cohort | Betamethasone (two 12 mg once every 24 h, IM)b |
| Bulbul et al,47 2020, Turkey | ★★★★★★½ | 2011-2014 (18-24 mo corrected age) | Prospective cohort | Antenatal corticosteroids (NS) |
| Miyazaki et al,30 2015, Japan | ★★★★★★½ | 2003-2007 (3 y or 36-42 mo chronological age) | Retrospective cohort | Antenatal corticosteroids (NS) |
| Ushida et al,48 2020, Japan | ★★★★★★½ | 2003-2015 (3 y) | Retrospective cohort | Betamethasone (two 12 mg once every 24 h, IM)b |
| Ushida et al,23 2020, Japan | ★★★★★★½ | 2003-2016 (3 y) | Retrospective cohort | Betamethasone (two 12 mg once every 24 h, IM)b |
| Basset et al,26 2018, France | ★★★★★★ | 2003-2013 (2 y corrected age) | Prospective cohort | Antenatal corticosteroids (NS) |
| Ishikawa et al,29 2015, Japan | ★★★★★½ | 2003-2007 (3 y or 36-42 mo) | Retrospective cohort | Betamethasone (NS)b |
| Li et al,25 2019, China | ★★★★★½ | 2010-2016 (18-24 mo) | Prospective cohort | Betamethasone (12 mg once every 24 h, IM) or dexamethasone (6 mg once every 12 h for 48 h, IM)b |
| Ochiai et al,33 2014, Japan | ★★★★★½ | 2000-2009 (3 y) | Retrospective cohort | Betamethasone or dexamethasone (NS) |
| Young et al,28 2016, Canada | ★★★★★½ | 2008-2010 (at 2 y and 4 y) | Prospective cohort | Betamethasone (two 12 mg once every 24 h, IM)b |
| Källén et al,31 2015, Sweden | ★★★★½ | 2004-2007 (2.5 y corrected age) | Prospective cohort | Betamethasone (NS) |
| Kiechl-Kohlendorfer et al,34 2009, Austria | ★★★★½ | 2003-2006 (1 y corrected age) | Prospective cohort | Antenatal corticosteroids (NS) |
| Sun et al,32 2015, China | ★★★★½ | 2006-2010 (18 mo corrected age) | Retrospective cohort | Antenatal corticosteroids (NS) |
| Children with full-term birth | ||||
| Melamed et al,44 2019, Canada | ★★★★★★★★ | 2006-2011 (5 y) | Retrospective cohort | Betamethasone (two 12 mg once every 24 h, IM) or dexamethasone (four 6 mg once every 12 h, IM)b |
| Räikkönen et al,21 2020, Finland | ★★★★★★★½ | 2006-2017 (1-11 y) | Retrospective cohort | Betamethasone (two 12 mg once every 24 h)b |
Abbreviations: IM, intramuscular; NS, not specified.
Each star represents 1 point and half of a star represents one-half point in the modified Newcastle-Ottawa Scale for assessing risk of bias across 8 domains.
Antenatal corticosteroid dose was not stated but assumed on the basis of available guideline recommendations at the time of recruitment and was still classified as unspecified.
Of the 30 studies included, 26 focused on neurodevelopmental and/or psychological outcomes21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,41,42,43,44,45,47,48,49,50 (eTables 2-6 in the Supplement), 3 had data on both neurodevelopmental and/or psychological outcomes and other outcomes38,39,40 (eTables 2-7 in the Supplement), and 1 included data on other outcomes46 (eTable 7 in the Supplement). The duration of participant follow-up ranged from 1 to 10 years (Table 1).
Scores for the cohort studies on the modified NOS ranged from 4.5 to 8.5 points out of a maximum of 9.0 points, with a median NOS score of 5.75 points for studies that compared a single course of antenatal corticosteroids with nonexposure (eTable 8 in the Supplement) and 6.5 points for studies that compared an unspecified number of courses of antenatal corticosteroids with nonexposure. One study24 addressed at least 4 of the 6 predetermined confounders for scoring the comparability domain of the NOS (eTable 8, eAppendix 3 in the Supplement).
Single Course vs Nonexposure
Ten of 30 studies measured long-term outcomes for children with preterm birth who were exposed to a single course of antenatal corticosteroids compared with those who were unexposed (Table 1).35,36,37,38,39,40,41,42,43,46 The use of a single course of antenatal corticosteroids vs nonexposure was not associated with significant reductions in odds of visual impairment (3 studies37,38,42; adjusted OR [aOR], 1.42 [95% CI, 0.57-3.54]; I2 = 0%; very low certainty), auditory impairment (3 studies37,38,42; aOR, 0.58 [95% CI, 0.33-1.01]; I2 = 9%; very low certainty), or moderate or severe cerebral palsy (2 studies38,42; aOR, 0.82 [95% CI, 0.56-1.19]; I2 = 0%; low certainty) (Figure 2; eTable 6 in the Supplement). For children with extremely premature birth, exposure vs nonexposure was associated with a significantly decreased odds of neurodevelopmental impairment (2 studies38,42; aOR, 0.69 [95% CI, 0.57-0.84]; I2 = 0%; low certainty), cerebral palsy (2 studies38,42; aOR, 0.60 [95% CI, 0.43-0.83]; I2 = 22%; low certainty), and other adjusted adverse neurodevelopmental and/or psychological outcomes (Table 2, Figure 2; eTables 2 and 6, eFigure 1 in the Supplement).38
Figure 2. Primary and Adjusted Long-term Neurodevelopmental and Psychological Outcomes After Exposure to a Single Course of Antenatal Corticosteroids.
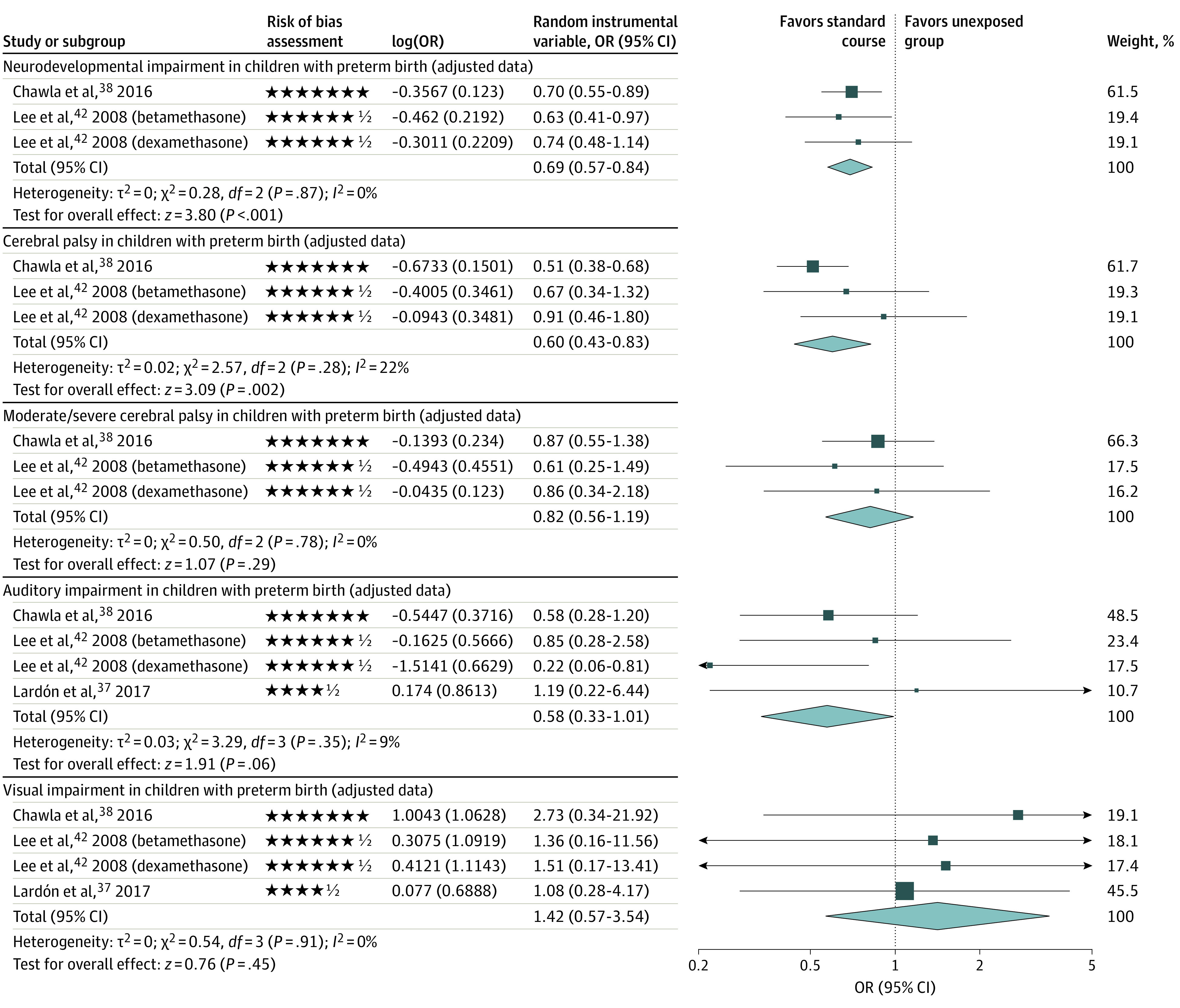
The forest plots show the comparison between a single course of antenatal corticosteriods and no exposure. Each star represents 1 point and half of a star represents one-half point in the modified Newcastle-Ottawa Scale for assessing risk of bias across domains. Squares represent effect size estimates and the whiskers correspond to the 95% CIs. The diamonds represent the overall effect based on pooled data from all included studies for each outcome. OR indicates odds ratio.
Table 2. Summary of Findings for the Primary Outcome in Included Studies .
| Certainty assessment | No./total No. of patients (%) | Effect size (95% CI) | Certaintya | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Unspecified No. of antenatal corticosteroid courses | Nonexposure | Relative | Absolute | ||
| Single antenatal corticosteroid course vs nonexposure | ||||||||||||
| Children with preterm birth | ||||||||||||
| Neurodevelopmental impairment | ||||||||||||
| 2 Studies38,42 | Observational studies | Not serious | Not serious | Not serious | Not serious | None | 1009/3376 (29.9) | 235/572 (41.4) | aOR, 0.69 (0.57-0.84b | <9 per 100 (from <13 to <4) | Low | Important |
| Unspecified No. of antenatal corticosteroid courses vs nonexposure | ||||||||||||
| Children with preterm or full-term birth | ||||||||||||
| Any mental or behavioral disorder | ||||||||||||
| 1 Study21 | Observational study | Not serious | Not serious | Not serious | Not serious | None | 1785/14 868 (12.0) | 42 243/655229 (6.4) | aHR, 1.33 (1.26-1.41)b | >2 per 100 (from >2 to >3) | Low | Important |
| Any mental or behavioral disorder | ||||||||||||
| 1 Study24 | Observational study | Not serious | Not serious | Not serious | Seriousc | None | 24/117 (20.5) | 386/4591 (8.4) | aOR, 2.58 (1.50-4.42)b | >11 per 100 (from >4 to >20) | Very low | Important |
| Children with preterm birth | ||||||||||||
| Any mental or behavioral disorder | ||||||||||||
| 1 Study21 | Observational study | Not serious | Not serious | Not serious | Not serious | None | 1187/8138 (14.6) | 2192/20 472 (10.7) | aHR, 1.00 (0.92-1.09) | <0 per 100 (from <1 to >1) | Low | Important |
| Neurodevelopmental impairment | ||||||||||||
| 5 Studies22,27,30,31,34 | Observational studies | Not serious | Not serious | Not serious | Not serious | None | NA | NA | aOR, 0.78 (0.57-1.06) | <1 per 100 (from <1 to <1) | Low | Important |
| Children with full-term birth | ||||||||||||
| Any mental or behavioral disorder | ||||||||||||
| 1 Study21 | Observational study | Not serious | Not serious | Not serious | Not serious | None | 598/6730 (8.9) | 40 051/634 757 (6.3) | aHR, 1.47 (1.36-1.60)b | >3 per 100 (from <2 to <4) | Low | Important |
Abbreviations: aHR, adjusted hazard ratio; aOR, adjusted odds ratio; NA, not available.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool was used to rate the certainty of included outcomes as high, moderate, low, or very low.
Statistically significant.
Small number of events that was disproportionate between groups. Wide 95% CIs.
Furthermore, Lee et al42 found that exposure to a single course of betamethasone vs nonexposure was significantly associated with a higher adjusted odds of nonimpairment, which was defined as the absence of cerebral palsy, blindness, deafness, or neurodevelopmental delay (aOR, 2.42 [95% CI, 1.49-3.91]; low certainty) (eTable 2 in the Supplement). Sensitivity analyses did not change the conclusion of these findings (eFigure 2 in the Supplement). Many secondary neurodevelopmental and/or psychological outcomes associated with exposure to a single course of antenatal corticosteroids vs nonexposure were nonsignificant or unadjusted and had few low or very low certainty GRADE ratings (eTables 2 and 3, eFigures 1-3 in the Supplement).
In analyses of other long-term outcomes, a single course of antenatal corticosteroids vs nonexposure was not associated with substantial differences in body weight or head circumference (very low certainty) (eTable 7, eFigure 2 in the Supplement). Tseng et al39 (cohort study involving 40 children) found a significantly higher unadjusted proportion of children with asthma and allergic disease among those who were exposed to a single course of antenatal corticosteroids (very low certainty) (eTable 7 in the Supplement). Other unadjusted adverse long-term outcomes for the secondary outcome were rated as very low certainty or had nonsignificant associations (eTable 7 in the Supplement).
Unspecified Number of Courses of Antenatal Corticosteroids vs Nonexposure
Twenty of the 30 included studies had long-term outcomes for children who were exposed to an unspecified number of courses of antenatal corticosteroids vs those who were unexposed; however, most of these studies likely included a single course of antenatal corticosteroids because that was the most common clinical scenario during the study period (Table 1; eTables 4 and 5, eFigures 1-3 in the Supplement).21,22,23,24,25,26,27,28,29,30,31,32,33,34,44,45,47,48,49,50 Two studies (Räikkönen et al21 and Wolford et al24) addressed the primary outcome of an author-defined composite of any adverse neurodevelopmental and/or psychological disorder (Figure 3), whereas another study (Lamminmäki et al45) addressed individual psychological outcomes (eTable 4 in the Supplement). Räikkönen et al21 reported a significantly elevated risk of any mental or behavioral disorder in children with preterm and full-term birth that was associated with exposure to an unspecified number of courses of antenatal corticosteroids vs nonexposure (670 097 children; adjusted hazard ratio [aHR], 1.33 [95% CI, 1.26-1.41]; low certainty) (Table 2). Furthermore, a subgroup analysis of consecutive sibling pairs who were discordant for treatment exposure demonstrated that the use of antenatal corticosteroids was associated with an increased adjusted risk of any mental or behavioral disorder (241 447 children; aHR, 1.38 [95% CI, 1.21-1.58]; low certainty) (eTable 4 in the Supplement).21 An unspecified number of courses of antenatal corticosteroids vs nonexposure was associated with increases in risk for 8 of 12 adverse adjusted secondary neurodevelopmental and/or psychological outcomes in children with preterm and full-term birth (eTable 4 in the Supplement).
Figure 3. Primary and Adjusted Long-term Neurodevelopmental and Psychological Outcomes After Exposure to an Unspecified Number of Courses of Antenatal Corticosteroids.
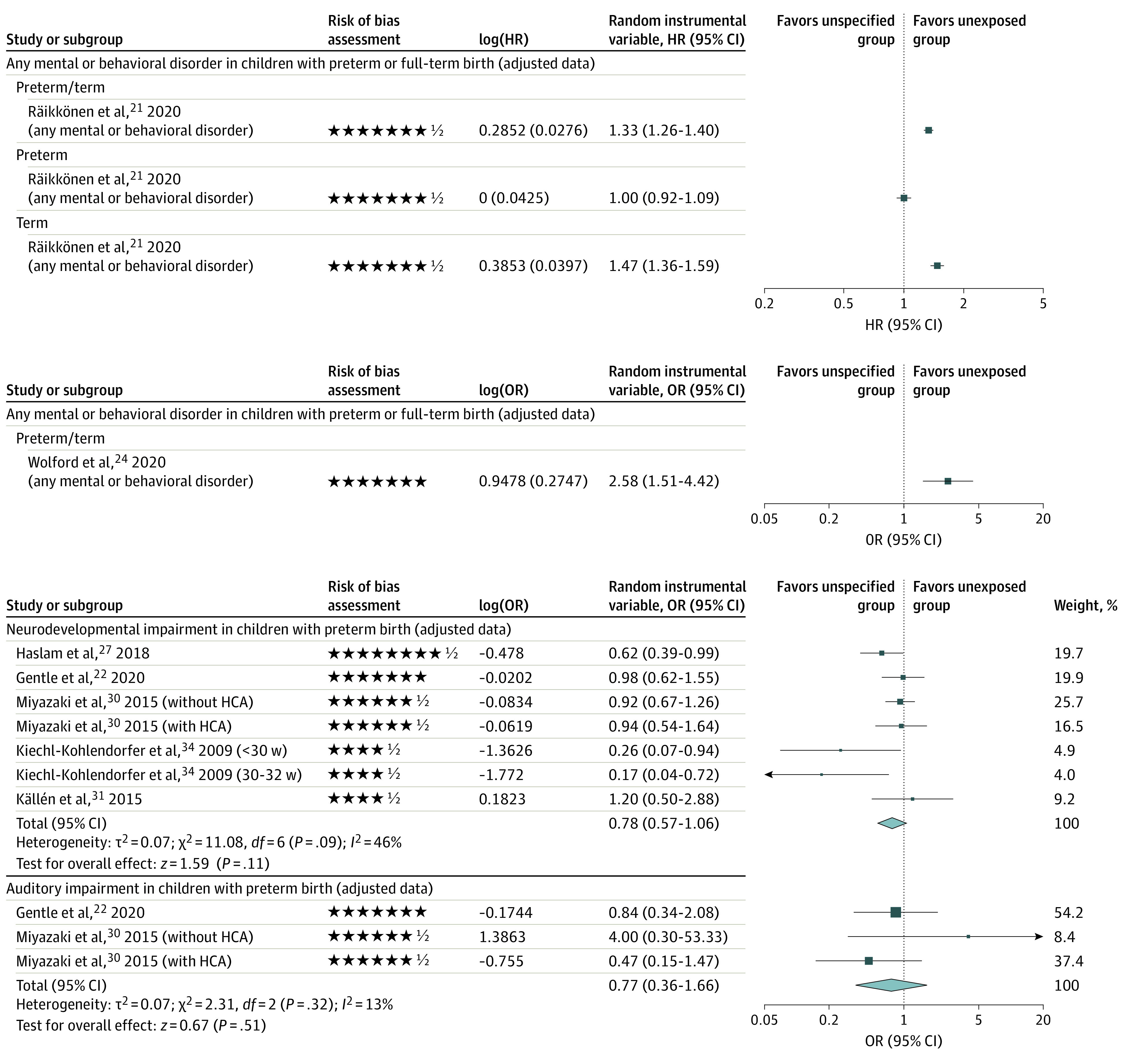
The forest plots show the comparison between an unspecified number of antenatal corticosteriod courses and no exposure. Each star represents 1 point and half of a star represents one-half point in the modified Newcastle-Ottawa Scale for assessing risk of bias across domains. On each forest plot, squares represent effect size estimates and the whiskers correspond to the 95% CIs. The diamonds represent the overall effect based on pooled data from all included studies for each outcome. HR indicates hazard ratio; OR, odds ratio.
Sixteen studies22,23,25,26,27,28,29,30,31,32,33,34,47,48,49,50 reported on adverse or beneficial neurodevelopmental and/or psychological outcomes specifically in children with preterm birth. In addition, Räikkönen et al21 reported on outcomes in children with full-term birth and a combination of children with preterm and full-term birth (eTables 4-6 in the Supplement). For children with preterm birth, exposure to an unspecified number of courses of antenatal corticosteroids vs nonexposure was not associated with significant reductions in the risk of neurodevelopmental impairment (5 studies22,27,30,31,34; aOR, 0.78 [95% CI, 0.57-1.06]; I2 = 46%; low certainty) or hearing impairment (2 studies22,30; aOR, 0.77 [95% CI, 0.36-1.66]; I2 = 13%; low certainty) (Figure 3). Young et al28 reported that exposure to antenatal corticosteroids was associated with decreased cognitive and behavior scores (eTable 4 in the Supplement). Meanwhile, Räikkönen et al21 reported that exposure was associated with an increased adjusted risk of sleeping disorders (low certainty) and decreased adjusted risk of mild, moderate, or unspecified intellectual disabilities (low certainty) (eTable 4, eFigure 1 in the Supplement). Aviram et al50 found that exposure was associated with an increased adjusted risk of investigation for neurocognitive disorders in children with late-preterm birth (ie, 34-36 weeks of gestation) (25 668 children; aHR, 1.12 [95% CI, 1.05-1.20]; low certainty) and the use of visual or audiometry testing. No significant associations were observed in other adjusted adverse or beneficial neurodevelopmental and/or psychological outcomes (eTable 4, eFigure 1 in the Supplement). Sensitivity analyses did not change the conclusion of these findings (eFigure 2 in the Supplement).
Two studies reported the proportion of children with full-term birth after preterm exposure to antenatal corticosteroids: 45.3% (6730 of 14 868) in Räikkönen et al21 and 47.9% (56 of 117) in Wolford et al.24 Exposure to an unspecified number of courses of antenatal corticosteroids was associated with higher risks of any mental or behavioral disorder (641 487 children; aHR, 1.47 [95% CI, 1.36-1.60]; low certainty)21 (Figure 3) and with a composite outcome of audiometry testing, visual testing, or proven or suspected neurocognitive disorder (n = 529 205 children; aHR, 1.12 [95% CI, 1.08-1.16]; low certainty) as well as with individual components of that composite outcome, including proven or suspected neurocognitive disorder (aHR, 1.16 [95% CI, 1.10-1.21]; low certainty)44 (eTable 4, eFigures 1 and 4 in the Supplement). For children with full-term birth, exposure vs no exposure was associated with significant increases in risk for 5 other adjusted adverse neurodevelopmental and/or psychological outcomes (eTable 4 in the Supplement).21 For the secondary outcomes, no studies reported on outcomes of comparing an unspecified number of courses with no exposure.
Discussion
In this systematic review and meta-analysis of 30 studies on long-term neurodevelopmental, psychological, or other outcomes in more than 1.25 million children, a single course of antenatal corticosteroids vs nonexposure was associated with significantly reduced risk of neurodevelopmental impairment and cerebral palsy in children with extremely preterm birth (low certainty). For those with late-preterm and full-term birth (the latter group composed approximately half of those with exposure), there were significantly higher risks of adverse neurocognitive and/or psychological outcomes that were associated with exposure to likely 1 course of antenatal corticosteroids compared with children who were unexposed (low certainty).21,44
Overall Completeness and Applicability of Evidence
We hypothesized that the difference in findings across gestation (with benefits seen in children with preterm birth and harms seen in those with late-preterm and full-term birth) may be explained by the immature vasculature in children with earlier preterm birth that renders the developing brain susceptible to hemorrhage51 and, as shown in animal studies, by exposure to antenatal corticosteroids that increases cerebrovascular resistance.52 This explanation, along with potential blood pressure stabilization,53 can decrease the risk of intraventricular hemorrhage and potential neurodevelopmental impairment.38 Furthermore, fetuses approaching term are exposed to maternal54 and fetal increases55 in cortisol. This additional exposure to antenatal corticosteroids at this gestational age results in high corticosteroid exposure, which may be associated with altered programming in the developing brain and the hypothalamic-pituitary-adrenal axis.54 Animal research has suggested that doses of betamethasone that are supraphysiological are at least several times higher and potentially 10 times higher than what is needed.56 Thus, further study of long-term outcomes after exposure to antenatal corticosteroids is important.
There are concerns about the use of antenatal corticosteroids in later-preterm gestation because of diminishing benefits over the preterm period.55,57 Future research is warranted on the long-term impacts of the use of antenatal corticosteroids across various gestational age strata and the timing of administration to delivery intervals in the preterm period (which were not well reported in the included studies).
We could not conduct planned subgroup analyses by type of corticosteroid treatment and by sex because of the lack of data in included studies. Lee et al42 focused on the impact of betamethasone and dexamethasone separately in comparing exposure to a single course of antenatal corticosteroids vs nonexposure in children with preterm birth; these investigators found an association between a significant decrease in neurodevelopmental impairment and use of only betamethasone compared with nonexposure. Further studies comparing the long-term outcomes of betamethasone and dexamethasone use are needed. Robust evidence on the long-term impact of antenatal corticosteroids is important because of the imprecise art of predicting preterm birth58 and the decrease in the benefits of antenatal corticosteroids in a fetus who remains undelivered 7 days after administration.59
Relation to Other Published Reviews on the Topic
The adjusted and unadjusted findings were similar to those reported in another systematic review, which included data before 2000 and found that a single course of antenatal corticosteroids was associated with unadjusted reductions in cerebral palsy compared with nonexposure.60 The literature search for the previous review concluded in August 2014, and since this time a substantial amount of research has emerged, including 5 studies35,36,37,38,39 that we included in the present work. In addition, the previous review excluded 12 of 42 studies because their data could not undergo a meta-analysis,60 rather than considering the studies narratively as performed in the present study.
Strengths and Limitations
This study has some strengths. We included a comprehensive search of multiple academic sources to provide a thorough synthesis of the long-term outcome of antenatal corticosteroids in children with preterm and full-term birth. For relevance to current neonatal care practice, we included studies involving births occurring in or after 2000. To provide a comprehensive view of the long-term impacts of antenatal corticosteroids, we considered both a single course of antenatal corticosteroids and an unspecified number of antenatal corticosteroid courses, which was most likely 1 course as it is the recommended treatment option in many settings.3,4,61
This study also has some limitations. Randomized follow-up data were scarce. The use of observational data may involve some degree of bias that affects the quality of the data62 and may lead to a lower level of certainty with our conclusions. However, when possible, we focused on adjusted data that addressed issues with confounding. Given the paucity of data, the role of race and ethnicity in long-term outcomes after antenatal corticosteroid exposure should be an area for future study. Furthermore, child development scores have different meanings depending on the age at assessment.63,64 Most pooled studies in the present analysis had similar follow-up ages of assessment (ie, ranging from age 1 to 2 years).
Conclusions
This systematic review and meta-analysis found an association between exposure to a single course of antenatal corticosteroids and a significantly lower risk of neurodevelopmental impairment in extremely preterm birth as well as a significantly higher risk of adverse neurocognitive and/or psychological outcomes in late-preterm and full-term birth. Given that approximately 50% of children who had preterm exposure to antenatal corticosteroids exceeded expectations and were born full term, the timing and dose of antenatal corticosteroid administration should be carefully considered.
eTable 1. Inclusion and Exclusion Criteria for a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eTable 2. Individual Adjusted Neurodevelopmental/Psychological Outcomes of Included Studies on a Single Course of Antenatal Corticosteroids vs Non-Exposure in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eTable 3. Individual Unadjusted Neurodevelopmental/Psychological Outcomes of Included Studies on a Single Course of Antenatal Corticosteroids vs Non-Exposure in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eTable 4. Individual Adjusted Neurodevelopmental/Psychological Outcomes of Included Studies on an Unspecified Number of Courses of Antenatal Corticosteroids vs Non-Exposure in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eTable 5. Individual Unadjusted Neurodevelopmental/Psychological Outcomes of Included Studies on an Unspecified Number of Courses of Antenatal Corticosteroids vs Non-Exposure in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eTable 6. Summary of Findings Table of Secondary Meta-analyzed Outcomes in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eTable 7. Other Long-Term Outcomes of Included Studies in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eTable 8. Newcastle-Ottawa Scale Quality Assessment Scores for Non-Randomized Studies Included in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eFigure 1. Visual Representation of Available Individual Adjusted Neurodevelopmental/Psychological Outcomes in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eFigure 2. Forest Plots of Sensitivity Analyses and Meta-analyzed Unadjusted Neurodevelopmental/Psychological and Other Outcomes in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eFigure 3. Visual Representation of Available Individual Unadjusted Neurodevelopmental/Psychological Outcomes in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eFigure 4. Infographic Abstract of a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eAppendix 1. Electronic Search Strategies for a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eAppendix 2. Excluded Key Articles from a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eAppendix 3. Confounding Factors Addressed in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eReferences
References
- 1.McGoldrick E, Stewart F, Parker R, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020;12(12):CD004454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3(3):CD004454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skoll A, Boutin A, Bujold E, et al. No. 364-Antenatal corticosteroid therapy for improving neonatal outcomes. J Obstet Gynaecol Can. 2018;40(9):1219-1239. doi: 10.1016/j.jogc.2018.04.018 [DOI] [PubMed] [Google Scholar]
- 4.Committee on Obstetric Practice . Committee opinion no. 713: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2017;130(2):e102-e109. doi: 10.1097/AOG.0000000000002237 [DOI] [PubMed] [Google Scholar]
- 5.van der Merwe JL, Sacco A, Toelen J, Deprest J. Long-term neuropathological and/or neurobehavioral effects of antenatal corticosteroid therapy in animal models: a systematic review. Pediatr Res. 2020;87(7):1157-1170. doi: 10.1038/s41390-019-0712-1 [DOI] [PubMed] [Google Scholar]
- 6.Jobe AH, Moss TJ, Nitsos I, Ikegami M, Kallapur SG, Newnham JP. Betamethasone for lung maturation: testing dose and formulation in fetal sheep. Am J Obstet Gynecol. 2007;197(5):523.e1-523.e6. doi: 10.1016/j.ajog.2007.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsiarli MA, Rudine A, Kendall N, et al. Antenatal dexamethasone exposure differentially affects distinct cortical neural progenitor cells and triggers long-term changes in murine cerebral architecture and behavior. Transl Psychiatry. 2017;7(6):e1153. doi: 10.1038/tp.2017.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonow-Schlorke I, Schwab M, Li C, Nathanielsz PW. Glucocorticoid exposure at the dose used clinically alters cytoskeletal proteins and presynaptic terminals in the fetal baboon brain. J Physiol. 2003;547(Pt 1):117-123. doi: 10.1113/jphysiol.2002.025700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwab M, Roedel M, Anwar MA, et al. Effects of betamethasone administration to the fetal sheep in late gestation on fetal cerebral blood flow. J Physiol. 2000;528(Pt 3):619-632. doi: 10.1111/j.1469-7793.2000.00619.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noorlander CW, Visser GH, Ramakers GM, Nikkels PG, de Graan PN. Prenatal corticosteroid exposure affects hippocampal plasticity and reduces lifespan. Dev Neurobiol. 2008;68(2):237-246. doi: 10.1002/dneu.20583 [DOI] [PubMed] [Google Scholar]
- 11.Jobe AH, Goldenberg RL. Antenatal corticosteroids: an assessment of anticipated benefits and potential risks. Am J Obstet Gynecol. 2018;219(1):62-74. doi: 10.1016/j.ajog.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Massmann GA, Rose JC, Figueroa JP. Differential effects of clinical doses of antenatal betamethasone on nephron endowment and glomerular filtration rate in adult sheep. Reprod Sci. 2010;17(2):186-195. doi: 10.1177/1933719109351098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sloboda DM, Challis JR, Moss TJ, Newnham JP. Synthetic glucocorticoids: antenatal administration and long-term implications. Curr Pharm Des. 2005;11(11):1459-1472. doi: 10.2174/1381612053507873 [DOI] [PubMed] [Google Scholar]
- 14.Razaz N, Skoll A, Fahey J, Allen VM, Joseph KS. Trends in optimal, suboptimal, and questionably appropriate receipt of antenatal corticosteroid prophylaxis. Obstet Gynecol. 2015;125(2):288-296. doi: 10.1097/AOG.0000000000000629 [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. John Wiley & Sons; 2019. [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah V, Warre R, Lee SK. Quality improvement initiatives in neonatal intensive care unit networks: achievements and challenges. Acad Pediatr. 2013;13(6 suppl):S75-S83. doi: 10.1016/j.acap.2013.04.014 [DOI] [PubMed] [Google Scholar]
- 18.Horbar JD, Edwards EM, Greenberg LT, et al. Variation in performance of neonatal intensive care units in the United States. JAMA Pediatr. 2017;171(3):e164396. doi: 10.1001/jamapediatrics.2016.4396 [DOI] [PubMed] [Google Scholar]
- 19.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed June 25, 2020. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 20.Review Manager (RevMan). Version 5.4. The Cochrane Collaboration; 2020. Accessed March 2, 2022. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman [Google Scholar]
- 21.Räikkönen K, Gissler M, Kajantie E. Associations between maternal antenatal corticosteroid treatment and mental and behavioral disorders in children. JAMA. 2020;323(19):1924-1933. doi: 10.1001/jama.2020.3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gentle SJ, Carlo WA, Tan S, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network . Association of antenatal corticosteroids and magnesium sulfate therapy with neurodevelopmental outcome in extremely preterm children. Obstet Gynecol. 2020;135(6):1377-1386. doi: 10.1097/AOG.0000000000003882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ushida T, Kotani T, Hayakawa M, et al. Antenatal corticosteroids and preterm offspring outcomes in hypertensive disorders of pregnancy: a Japanese cohort study. Sci Rep. 2020;10(1):9312. doi: 10.1038/s41598-020-66242-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolford E, Lahti-Pulkkinen M, Girchenko P, et al. Associations of antenatal glucocorticoid exposure with mental health in children. Psychol Med. 2020;50(2):247-257. doi: 10.1017/S0033291718004129 [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Meng DH, Wei QF, et al. ; GuangXi Cooperative Research Group for Extremely Preterm Infants. . Neurodevelopmental outcomes of extremely preterm infants in southern China: a multicenter study. Early Hum Dev. 2019;133:5-10. doi: 10.1016/j.earlhumdev.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 26.Basset H, Nusinovici S, Huetz N, et al. Efficacy of antenatal corticosteroid treatment on neurodevelopmental outcome according to head circumference at birth. Neonatology. 2018;113(1):55-62. doi: 10.1159/000479675 [DOI] [PubMed] [Google Scholar]
- 27.Haslam MD, Lisonkova S, Creighton D, et al. ; Canadian Neonatal Network and the Canadian Neonatal Follow-Up Network . Severe neurodevelopmental impairment in neonates born preterm: impact of varying definitions in a Canadian cohort. J Pediatr. 2018;197:75-81.e4. doi: 10.1016/j.jpeds.2017.12.020 [DOI] [PubMed] [Google Scholar]
- 28.Young JM, Morgan BR, Powell TL, et al. Associations of perinatal clinical and magnetic resonance imaging measures with developmental outcomes in children born very preterm. J Pediatr. 2016;170:90-96. doi: 10.1016/j.jpeds.2015.11.044 [DOI] [PubMed] [Google Scholar]
- 29.Ishikawa H, Miyazaki K, Ikeda T, et al. ; Neonatal Research Network of Japan . The effects of antenatal corticosteroids on short-and long-term outcomes in small-for-gestational-age infants. Int J Med Sci. 2015;12(4):295-300. doi: 10.7150/ijms.11523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyazaki K, Furuhashi M, Ishikawa K, et al. Long-term outcomes of antenatal corticosteroids treatment in very preterm infants after chorioamnionitis. Arch Gynecol Obstet. 2015;292(6):1239-1246. doi: 10.1007/s00404-015-3762-6 [DOI] [PubMed] [Google Scholar]
- 31.Källén K, Serenius F, Westgren M, Maršál K; EXPRESS Group . Impact of obstetric factors on outcome of extremely preterm births in Sweden: prospective population-based observational study (EXPRESS). Acta Obstet Gynecol Scand. 2015;94(11):1203-1214. doi: 10.1111/aogs.12726 [DOI] [PubMed] [Google Scholar]
- 32.Sun H, Zhou Y, Xiong H, et al. Prognosis of very preterm infants with severe respiratory distress syndrome receiving mechanical ventilation. Lung. 2015;193(2):249-254. doi: 10.1007/s00408-014-9683-5 [DOI] [PubMed] [Google Scholar]
- 33.Ochiai M, Kinjo T, Takahata Y, et al. Survival and neurodevelopmental outcome of preterm infants born at 22-24 weeks of gestational age. Neonatology. 2014;105(2):79-84. doi: 10.1159/000355818 [DOI] [PubMed] [Google Scholar]
- 34.Kiechl-Kohlendorfer U, Ralser E, Pupp Peglow U, Reiter G, Trawöger R. Adverse neurodevelopmental outcome in preterm infants: risk factor profiles for different gestational ages. Acta Paediatr. 2009;98(5):792-796. doi: 10.1111/j.1651-2227.2009.01219.x [DOI] [PubMed] [Google Scholar]
- 35.Agarwal PK, Shi L, Rajadurai VS, et al. Factors affecting neurodevelopmental outcome at 2 years in very preterm infants below 1250 grams: a prospective study. J Perinatol. 2018;38(8):1093-1100. doi: 10.1038/s41372-018-0138-3 [DOI] [PubMed] [Google Scholar]
- 36.Kim S-M, Sung J-H, Kuk J-Y, et al. Short- and long-term neonatal outcomes according to differential exposure to antenatal corticosteroid therapy in preterm births prior to 24 weeks of gestation. PLoS One. 2018;13(6):e0198471. doi: 10.1371/journal.pone.0198471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lardón M, Uberos J, Narbona E. Does corticosteroid treatment during the pre and postnatal periods affect the neurodevelopmental outcome of premature newborns? Article in Spanish. Biomedica. 2017;37(0):104-111. doi: 10.7705/biomedica.v37i3.3394 [DOI] [PubMed] [Google Scholar]
- 38.Chawla S, Natarajan G, Shankaran S, et al. ; National Institute of Child Health and Human Development Neonatal Research Network . Association of neurodevelopmental outcomes and neonatal morbidities of extremely premature infants with differential exposure to antenatal steroids. JAMA Pediatr. 2016;170(12):1164-1172. doi: 10.1001/jamapediatrics.2016.1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tseng W-N, Chen C-C, Yu H-R, Huang L-T, Kuo H-C. Antenatal dexamethasone exposure in preterm infants is associated with allergic diseases and the mental development index in children. Int J Environ Res Public Health. 2016;13(12):1206. doi: 10.3390/ijerph13121206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chawla S, Bapat R, Pappas A, Bara R, Zidan M, Natarajan G. Neurodevelopmental outcome of extremely premature infants exposed to incomplete, no or complete antenatal steroids. J Matern Fetal Neonatal Med. 2013;26(15):1542-1547. doi: 10.3109/14767058.2013.791273 [DOI] [PubMed] [Google Scholar]
- 41.McElrath TF, Allred EN, Boggess KA, et al. ; ELGAN Study Investigators . Maternal antenatal complications and the risk of neonatal cerebral white matter damage and later cerebral palsy in children born at an extremely low gestational age. Am J Epidemiol. 2009;170(7):819-828. doi: 10.1093/aje/kwp206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee BH, Stoll BJ, McDonald SA, Higgins RD; National Institute of Child Health and Human Development Neonatal Research Network . Neurodevelopmental outcomes of extremely low birth weight infants exposed prenatally to dexamethasone versus betamethasone. Pediatrics. 2008;121(2):289-296. doi: 10.1542/peds.2007-1103 [DOI] [PubMed] [Google Scholar]
- 43.Laughon M, O’Shea MT, Allred EN, et al. ; ELGAN Study Investigators . Chronic lung disease and developmental delay at 2 years of age in children born before 28 weeks’ gestation. Pediatrics. 2009;124(2):637-648. doi: 10.1542/peds.2008-2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melamed N, Asztalos E, Murphy K, et al. Neurodevelopmental disorders among term infants exposed to antenatal corticosteroids during pregnancy: a population-based study. BMJ Open. 2019;9(9):e031197. doi: 10.1136/bmjopen-2019-031197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamminmäki A, Kuiri-Hänninen T, Sankilampi U. Sex-typical behavior in children born preterm at very low birth weight. Pediatr Res. 2021;89(7):1765-1770. doi: 10.1038/s41390-020-01133-7 [DOI] [PubMed] [Google Scholar]
- 46.Gover A, Brummelte S, Synnes AR, et al. Single course of antenatal steroids did not alter cortisol in preterm infants up to 18 months. Acta Paediatr. 2012;101(6):604-608. doi: 10.1111/j.1651-2227.2012.02629.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bulbul L, Elitok GK, Ayyıldız E, et al. Neuromotor development evaluation of preterm babies less than 34 weeks of gestation with Bayley III at 18-24 months. Biomed Res Int. 2020;2020:5480450. doi: 10.1155/2020/5480450.eCollection2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ushida T, Kotani T, Sadachi R, et al. ; Neonatal Research Network of Japan . Antenatal corticosteroids and outcomes in preterm twins. Obstet Gynecol. 2020;135(6):1387-1397. doi: 10.1097/AOG.0000000000003881 [DOI] [PubMed] [Google Scholar]
- 49.Hutcheon JA, Harper S, Liauw J, Skoll MA, Srour M, Strumpf EC. Antenatal corticosteroid administration and early school age child development: a regression discontinuity study in British Columbia, Canada. PLoS Med. 2020;17(12):e1003435. doi: 10.1371/journal.pmed.1003435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aviram A, Murphy K, McDonald S, et al. Antenatal corticosteroids and neurodevelopmental outcomes in late preterm births. Arch Dis Child Fetal Neonatal Ed. 2021;fetalneonatal-2021-322152. doi: 10.1136/archdischild-2021-322152 [DOI] [PubMed] [Google Scholar]
- 51.Tsuji M, Saul JP, du Plessis A, et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics. 2000;106(4):625-632. doi: 10.1542/peds.106.4.625 [DOI] [PubMed] [Google Scholar]
- 52.Löhle M, Müller T, Wicher C, et al. Betamethasone effects on fetal sheep cerebral blood flow are not dependent on maturation of cerebrovascular system and pituitary-adrenal axis. J Physiol. 2005;564(Pt 2):575-588. doi: 10.1113/jphysiol.2004.077537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carson R, Monaghan-Nichols AP, DeFranco DB, Rudine AC. Effects of antenatal glucocorticoids on the developing brain. Steroids. 2016;114:25-32. doi: 10.1016/j.steroids.2016.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006;572(Pt 1):31-44. doi: 10.1113/jphysiol.2006.105254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asztalos EV, Murphy KE, Matthews SG. A growing dilemma: antenatal corticosteroids and long-term consequences. Am J Perinatol. 2020. doi: 10.1055/s-0040-1718573 [DOI] [PubMed] [Google Scholar]
- 56.Jobe AH, Kemp M, Schmidt A, Takahashi T, Newnham J, Milad M. Antenatal corticosteroids: a reappraisal of the drug formulation and dose. Pediatr Res. 2021;89(2):318-325. doi: 10.1038/s41390-020-01249-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jobe AH. Antenatal corticosteroids—a concern for lifelong outcomes. J Pediatr. 2020;217:184-188. doi: 10.1016/j.jpeds.2019.09.015 [DOI] [PubMed] [Google Scholar]
- 58.Shanks AL, Grasch JL, Quinney SK, Haas DM. Controversies in antenatal corticosteroids. Semin Fetal Neonatal Med. 2019;24(3):182-188. doi: 10.1016/j.siny.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 59.McLaughlin KJ, Crowther CA, Walker N, Harding JE. Effects of a single course of corticosteroids given more than 7 days before birth: a systematic review. Aust N Z J Obstet Gynaecol. 2003;43(2):101-106. doi: 10.1046/j.0004-8666.2003.00052.x [DOI] [PubMed] [Google Scholar]
- 60.Sotiriadis A, Tsiami A, Papatheodorou S, Baschat AA, Sarafidis K, Makrydimas G. Neurodevelopmental outcome after a single course of antenatal steroids in children born preterm: a systematic review and meta-analysis. Obstet Gynecol. 2015;125(6):1385-1396. doi: 10.1097/AOG.0000000000000748 [DOI] [PubMed] [Google Scholar]
- 61.World Health Organization . WHO Recommendations on Interventions to Improve Preterm Birth Outcomes. World Health Organization; 2015. [PubMed] [Google Scholar]
- 62.Booth CM, Tannock IF. Randomised controlled trials and population-based observational research: partners in the evolution of medical evidence. Br J Cancer. 2014;110(3):551-555. doi: 10.1038/bjc.2013.725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jobe AH. Predictors of outcomes in preterm infants: which ones and when? J Pediatr. 2001;138(2):153-156. doi: 10.1067/mpd.2001.112760 [DOI] [PubMed] [Google Scholar]
- 64.Patel RM. Short- and long-term outcomes for extremely preterm infants. Am J Perinatol. 2016;33(3):318-328. doi: 10.1055/s-0035-1571202 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Inclusion and Exclusion Criteria for a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eTable 2. Individual Adjusted Neurodevelopmental/Psychological Outcomes of Included Studies on a Single Course of Antenatal Corticosteroids vs Non-Exposure in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eTable 3. Individual Unadjusted Neurodevelopmental/Psychological Outcomes of Included Studies on a Single Course of Antenatal Corticosteroids vs Non-Exposure in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eTable 4. Individual Adjusted Neurodevelopmental/Psychological Outcomes of Included Studies on an Unspecified Number of Courses of Antenatal Corticosteroids vs Non-Exposure in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eTable 5. Individual Unadjusted Neurodevelopmental/Psychological Outcomes of Included Studies on an Unspecified Number of Courses of Antenatal Corticosteroids vs Non-Exposure in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eTable 6. Summary of Findings Table of Secondary Meta-analyzed Outcomes in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eTable 7. Other Long-Term Outcomes of Included Studies in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eTable 8. Newcastle-Ottawa Scale Quality Assessment Scores for Non-Randomized Studies Included in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eFigure 1. Visual Representation of Available Individual Adjusted Neurodevelopmental/Psychological Outcomes in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eFigure 2. Forest Plots of Sensitivity Analyses and Meta-analyzed Unadjusted Neurodevelopmental/Psychological and Other Outcomes in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eFigure 3. Visual Representation of Available Individual Unadjusted Neurodevelopmental/Psychological Outcomes in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eFigure 4. Infographic Abstract of a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eAppendix 1. Electronic Search Strategies for a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eAppendix 2. Excluded Key Articles from a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eAppendix 3. Confounding Factors Addressed in a Systematic Review and Meta-analysis of Long-Term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids
eReferences