Abstract
Omega-3 long-chain polyunsaturated fatty acids (n-3 LCPUFA) are critical for cell membrane structure and function. Human beings have a limited ability to synthesise docosahexaenoic acid (DHA), the main n-3 LCPUFA required for neurological development. Inadequate levels of n-3 LCPUFA can affect the dopaminergic system in the brain and, when combined with genetic and other factors, increase the risk of developing aggression, inattention and impulse-control disorders. In this study, male prisoners were administered questionnaires assessing aggressive behaviour and executive functions. Participants also produced blood sampling for the measurement of the Omega-3 Index and the genotyping of dopaminergic genetic variants. Significant associations were found between functional genetic polymorphism in DBH rs1611115 and verbal aggression and between DRD2 rs4274224 and executive functions. However, the Omega-3 Index was not significantly associated with the tested dopaminergic polymorphisms. Although previous interactions between specific genotypes and n-3 LCPUFA were previously reported, they remain limited and poorly understood. We did not find any association between n-3 LCPUFA and dopaminergic polymorphisms in adult male prisoners; however, we confirmed the importance of genetic predisposition for dopaminergic genes (DBH and DRD2) in aggressive behaviour, memory dysfunction and attention-deficit disorder.
Keywords: Omega-3 Index, dopaminergic receptors, dopaminergic enzymes, aggression, attention, hyperactivity, genetic polymorphisms, prisoners
1. Introduction
During the last decade, there has been an increased focus on the importance of diet on cognitive ability and behaviour, including aggressive behaviour, impulsivity and attention disorders [1,2,3,4]. It has been suggested that dysfunction in dopaminergic signalling pathways may underlie the development of many of these disorders [5,6,7]. In studies on both adults and children, dopamine (DA) hyperactivity in brain regions linked to reward-related motivation (such as the limbic system and prefrontal cortex) has been associated with increases in impulsive and aggressive behaviours [7,8]. Levels of extracellular DA present in the synaptic cleft can act on dopaminergic receptors, such as dopamine receptors 1 (DRD1) and 2 (DRD2), which are involved in the development of aggressive behaviour [9,10]. Furthermore, pharmacological blockage of the DRD2 receptors by antipsychotic treatment revealed an anti-aggression effect in individuals [11]. Animal studies have shown that the selected stimulation of DRD2 receptors with bromocriptine substantially increased the aggressive behaviour of low-aggression genetically modified mice, whereas the blockade of DRD2 receptors with sulpiride decreased or prevented the manifestation of aggressivity in high-aggression genetically modified mice, confirming the critical role of DRD2 receptors in the development of aggressive behaviour [12]. Taking into account the results of a meta-analysis of 24 genetic studies on aggression reporting that heritability accounts for approximately 50% of the variance in aggression, it is not surprising that genes coding for dopaminergic receptors were largely implicated in the genetic vulnerability for aggressive behaviour and impulsivity in several studies [13,14,15,16,17,18]. Both single-nucleotide polymorphisms (SNPs) rs4648317 and rs12364283 in the DRD2 gene (DRD2) were previously associated with impulsive behaviour in a population of healthy volunteers [19]. Functional polymorphism in the DRD1 gene (encoding for DRD1) rs 686 was also reported to be associated with heroin-use and impulsive behaviour [20].
Additional dopaminergic genes encoding for enzymes modulating dopaminergic neurotransmission, such as monoamine oxidase (MAO) and catechol O methyl transferase (COMT) enzymes, play a significant role in the individual response to aggressive behaviour, hyperactivity and attention disorders [13,15,21,22]. Functional SNP in COMT (rs 4680), resulting in a valine (Val) to methionine (Met) substitution in the amino-acid protein sequence, leads to differential levels of activity of the COMT enzyme [23,24]. COMT rs4680 has been previously associated with aggressive behaviour in both psychiatric and healthy populations [21,25,26]). Similarly, SNP (rs1799836) in the MAO B gene was previously reported to be significantly involved in the genetic vulnerability to develop attention-deficit/hyperactivity disorder (ADHD) and negative personality traits in both Han Chinese and Caucasian case-control cohorts [27,28].
Another isoform of the MAO enzyme encoded by MAO A has earned the nickname “warrior gene” due to its link to aggression in observational and survey-based studies [29,30]. Metabolic dopamine beta hydroxylase (DBH) is a synaptic enzyme capable of reducing extracellular DA levels. One functional SNP in the promoter (rs1611115) was described as responsible for more than half of DBH enzymatic activity [31]. The minor allele T, leading to a reduction in half of the DBH activity of this genetic variant, was previously found to represent a risk factor for impulsiveness, aggression and adult ADHD [32]. Altogether, dopaminergic gene variants seem to play a critical role in the individual response for impulsive and aggressive behaviours as well as attention and hyperactivity disorders.
Omega-3 long-chain polyunsaturated fatty acids (n-3 LCPUFA) comprise eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA) and docosahexaenoic acid (DHA). The main n-3 LCPUFA involved in neurological development is DHA, particularly at the time of the neural tube closure during very early pregnancy [33] and during the second trimester when the fetus accrues its brain matter [34] but also throughout the lifespan [35]. The Omega-3 Index [36], measured as EPA plus DHA expressed as a percent of total erythrocyte membrane fatty acids, is a good biomarker for the heart tissue levels of n-3 LCPUFA [37]. The Omega-3 Index has been postulated as a new risk factor for death from coronary heart disease [36] and, more recently, for mental health [38]. DHA is critical for all cell membrane structures and functions with particular importance for neuronal cells in the frontal lobes [39]. Evidence suggests that inadequate levels of n-3 LCPUFA can affect the dopaminergic system in the brain and, when combined with genetic and other factors, increase the risk of developing aggression, attention and impulse-control disorders [40].
Taking into account that impulsive aggression is significantly involved in the manifestation of violent and criminal behaviour [5] and the results of our pilot study showing a correlation between high aggressive scoring and a lower Omega-3 Index in adult male prisoners [41], the aim of this study was to investigate the relevant polymorphisms in dopaminergic genes in adult male prisoners in relation to their Omega-3 Index. We hypothesise here that dopaminergic polymorphisms will be associated with aggressive and metacognitive traits and with individual variation with respective Omega-3 Index.
2. Materials and Methods
2.1. Subjects
One hundred and thirty-six male adult participants were recruited from the South Coast Correctional Centre (SCCC) in Nowra, NSW, Australia, as previously described [41]. Ethics approvals were granted by the University of Wollongong and the Corrective Services (Wollongong, NSW, Australia) ethics committees (11/93185). Of the 136 subjects in this study, 74 (54%) were of Caucasian origin, 14 (10%) of Australian Aboriginal origin, 11 (8%) of Middle Eastern origin, 13 (9.5%) from the Pacific Islands, 12 (8.8%) from the Asian region and 5 (3.6%) of Hispanic origin [41].
2.2. Blood Samples and Omega-3 Index
Blood samples were collected in EDTA tubes and subjected to centrifugation at 4 °C for 10 min, and plasma was separated from the packed erythrocytes, which were stored at 80 °C degrees until further use. Levels of EPA and DHA were assessed as previously described [41], and omega-3 levels in erythrocytes were expressed as the Omega-3 Index (calculated as the sum of EPA and DHA expressed as a percent of total erythrocyte fatty acids).
2.3. Genotyping
Genomic DNA was isolated from whole blood tubes using a QIAsymphony DSP DNA Midi Kit (96) (Qiagen, Hilden, Germany) following the manufacturer’s recommendations. The tested SNPs (see Table 1) were selected because of the evidence of significant genetic associations with aggressive disorders, memory and/or the relevance of genes in the dopaminergic system. SNP genotyping was performed using the Multiplex MassARRAY® genotyping assay (Sequenom, Inc., San Diego, CA, USA), with the analysis performed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS, Sequenom, Inc., San Diego, CA, USA).
Table 1.
The Hardy–Weinberg equilibrium (HWE) p-values and distribution of genotype frequencies of selected single-nucleotide polymorphisms (SNPs).
| Genes | Chromosomic Location |
SNPs | Alleles | MAF 1, (%) | HWE, p |
|---|---|---|---|---|---|
| DRD1 | 5 (3′UTR Variant) | rs686 * | G > A | 29.3 | 0.66 |
| DRD2 | 11 (Intron Variant) | rs4648317 | G > A | 89.6 | 0.02 |
| DRD2 | 11 (Intron Variant) | rs4274224 | G > A | 54.1 | 0.42 |
| DRD2 | 11 (Intron Variant) | rs4581480 | C > T | 87 | 0.03 |
| DBH | 9 (3′UTR Variant) | rs1611115 * | C > T | 77.7 | 0.76 |
| MAO B | X (Intron Variant) | rs1799836 | T > C | 53.4 | <0.001 |
| MAO A | X (Downstream variant 3′) | rs660925 | T > C | 60.4 | <0.001 |
| COMT | 22 (Missense Variant in exon 4 Val158Met) | rs4680 * | G > A | 86.2 | <0.001 |
DRD1: dopaminergic receptor D1; DRD2: dopaminergic receptor D2; DBH: dopaminergic beta hydroxylase; MAO B: monoamine oxidase B; MAO A: monoamine oxidase A; COMT: catechol O methyl transferase; SNPs: single-nucleotide polymorphisms; HWE: Hardy–Weinberg equilibrium, G: Guanine; A: Adenine; T: Thymine; C: Cytosine. 1 Major/Reference Allelic Frequency. * Functional polymorphism.
2.4. Psychological Assessments
Aggressive behaviours and cognitions were assessed using the Aggression Questionnaire (AQ) [42] and a 7-point observation scale (IBOS: Inmate Behavioural Observation Scale) as rated by prison officers for a duration of four weeks as previously described [41]. Briefly, case notes detailing significant observations and interactions with and between prisoners are routinely digitally recorded by custodial and non-custodial staff. The IBOS is a 7-point scale which classifies these observations of inmate behaviour, creating a numerical rating of frequency and severity of aggression. A weekly total score is derived, enabling longitudinal assessment of behaviour across weeks. A score of −1 is applied to all instances of pro-social behaviour recorded in that week, whereas a score of 0 is given if there were no behaviour of significance recorded. Thereafter, instances of hostile/aggressive behaviour are scored from 1 (non-compliant) to 5 (physically aggressive), with each level of hostility/aggression operationally defined and illustrated by examples [41]. IBOS assessments correlated with the AQ results [41]. The AQ reports five subscales categorised into Physical Aggression, Verbal Aggression, Anger, Hostility and Indirect Aggression.
Since attention-deficit/hyperactivity disorder is one of the most disrupted metacognitive traits in prisoners [43], Brown Attention-Deficit Disorder Scales (BADDS) [44] were administered to this cohort by one of the authors (Byrne, a clinical and forensic psychologist) to assess executive function via measurement of Activation (organising/prioritising), Attention (focussing, sustaining and shifting attention to tasks), Effort (alertness, maintaining effort and speed for processing), Emotion/Affect (managing emotions and frustrations) and Memory (using working memory and recall testing) as previously described [41].
2.5. Statistical Analysis
Analyses were performed using SPSS (version 22.0, SPSS Inc., Chicago, IL, USA). The genotypic distribution for the eight tested SNPs was first assessed for deviation from the Hardy–Weinberg equilibrium (HWE) and linkage disequilibrium (LD) using Graphical Overview of Linkage Disequilibrium (GOLD) software [45]. Due to a limited number of participants and, for some SNPs, lower minor allelic frequency, two SNPs in DRD2 and SNPs in COMT and MAO A and B genes showed genotypic distribution which did deviate from the HWE. Therefore, only rs686 in DRD1, rs42744224 in DRD2 and rs1611115 in DBH were further analysed for their association with genotype and Omega-3 Index and AQ and BADDS results using the Kruskal–Wallis test (non-parametric test leading to H value testing analysis of variance between genotypes and assessed behavioural parameters/ Omega-3 Index). When an association was reported as significant between genetic variants and neuropsychological assessments, odd ratios were calculated for the individual genotypes to estimate the risk of returning high scores on the AQ and BADDS for higher/lower total aggression level groups (using a cut-off score of 55 assessed in the AQ questionnaire) and for high/low ADD groups (using a cut off of 40 reported in BADDS). Significance was corrected using the Bonferroni test and set to p ≤ 0.025.
3. Results
3.1. Description and Relation between the Tested Genetic Polymorphisms
Genetic information regarding the SNPs originally considered in this study, as well as the results for the HWE are reported in Table 1. Major allelic frequencies (MAFs) for the tested SNPs were found within the 0–2% range from global MAFs reported in the genetic database, except for the DRD1 variant being within 13% of the reference allelic frequency [46].
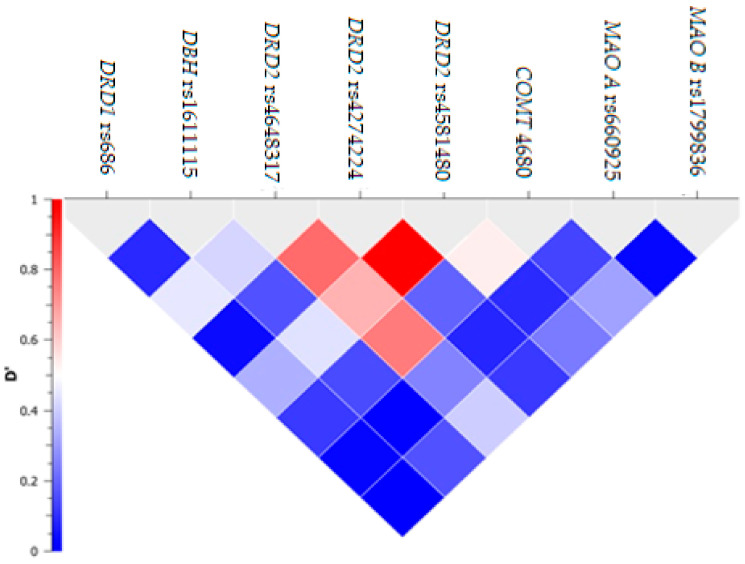
Albeit a strong LD between DRD2 rs4274224 and rs4581480 (D’ = 0.85) and a moderate LD (ranging between 0.6 and 0.8) between rs4648317 and two other SNPs within the DRD2 gene, no haplotype block was considered in this study due to the exclusion of genetic markers for violation of the HWE (Figure 1). The demographic features (age and ethnicity) did not show any significant difference when analysed for each of the three SNPs (i.e., rs686, rs4274224 and rs161115) included in this study (p > 0.025).
Figure 1.
Linkage disequilibrium between selected single-nucleotide polymorphisms (SNPs). On y axis, D’ represents the coefficient of linkage disequilibrium between the tested SNPs (represented on x axis). DRD1: dopaminergic receptor D1; DRD2: dopaminergic receptor D2; DBH: dopaminergic beta hydroxylase; MAO B: monoamine oxidase B; MAO A: monoamine oxidase A; COMT: catechol O methyl transferase.
3.2. Omega-3 Index, Dopaminergic Variants and Neuropsychological Testing
The levels of Omega-3 Index measured in blood were not associated with any of the DRD1, DRD2 and DBH gene variants (p > 0.025; Table 2, Table 3 and Table 4). The DRD1 variant did not show any genetic association with aggression and metacognitive traits per the AQ (p > 0.025; Table 2).
Table 2.
DRD1 genotypes analysis according to Omega-3 Index and neuropsychological testing.
| DRD1 (rs686) | AA (n = 65) | AG (n = 51) | GG (n = 12) | p |
|---|---|---|---|---|
| Omega-3 Index |
5.2 (4.3, 6.0) | 5.4 (4.1, 6.7) | 5.3 (3.9, 7.0) | 0.970 |
| PhysAgg | 21.3 (13.0, 26.0) | 21.9 (15.0, 29.0) | 24.1 (18.2, 27.5) | 0.572 |
| VerbAgg | 12.9 (9.0, 15.0) | 13.3 (11.0, 17.0) | 13.6 (11.0, 16.7) | 0.875 |
| Anger | 16.6 (11.0, 20.0) | 17.2 (13.0, 23.0) | 17.6 (12.2, 20.5) | 0.866 |
| Hostility | 19.5 (14.0, 24.0) | 18.5 (13.0, 23.0) | 21.0 (12.5, 26.5) | 0.620 |
| IndirAgg | 13.7 (9.0, 17.0) | 13.6 (10.0, 17.0) | 15.7 (13.2, 18.7) | 0.303 |
| Total ADD | 47.5 (23.0, 59.0) | 42.6 (18.0, 65.0) | 52.5 (24.0, 66.2) | 0.631 |
| Activation | 10.9 (6.0, 16.0) | 9.2 (3.0, 14.0) | 13.0 (6.2, 16.0) | 0.212 |
| Attention | 12.2 (4.0, 17.0) | 11.6 (5.0, 18.0) | 13.1 (7.0, 15.7) | 0.873 |
| Effort | 8.9 (4.0, 11.0) | 7.3 (3.0, 11.0) | 10.1 (4.0, 13.0) | 0.432 |
| Affect | 7.9 (4.0, 11.0) | 7.5 (3.0, 12.0) | 8.2 (2.0, 12.0) | 0.904 |
| Memory | 7.3 (3.0, 11.0) | 6.6 (2.0, 11.0) | 8.1 (4.0, 11.5) | 0.731 |
PhysAgg: Physical Aggression; VerbAgg: Verbal Aggression; IndirAgg: Indirect Aggression; Total ADD: total attention-deficit disorder. AA, AG and GG are the genotypes of alleles.
Table 3.
DRD2 genotypes analysis according to Omega-3 Index and neuropsychological testing.
|
DRD2 (rs4274224) |
AA (n = 41) | AG (n = 31) | GG (n = 30) | p |
|---|---|---|---|---|
| Omega-3 Index |
5.2 (4.0, 6.0) | 5.1 (4.0, 6.0) | 5.6 (4.2, 7.2) | 0.591 |
| PhysAgg | 22.7 (17.0, 28.0) | 20.6 (13.0, 27.5) | 22.0 (13.7, 29.2) | 0.513 |
| VerbAgg | 12.7 (9.0, 15.0) | 13.5 (11.0, 17.5) | 12.8 (9.5, 16.0) | 0.635 |
| Anger | 18.0 (14.0, 20.0) | 16.7 (11.0, 22.5) | 15.2 (10.7, 20.0) | 0.256 |
| Hostility | 20.1 (14.0; 26.0) | 19.8 (13.5, 26.0) | 17.0 (12.5, 19.0) | 0.220 |
| IndirAgg | 14.5 (11.0, 18.0) | 14.3 (11.0, 18.5) | 12.0 (8.7, 14.0) | 0.113 |
| Total ADD | 48.9 (32.0, 71.0) | 48.0 (23.0, 70.0) | 28.9 (13.0, 38.5) | 0.004 |
| Activation | 11.4 (7.0, 17.0) | 10.5 (5.0, 16.5) | 7.0 (3.0, 10.0) | 0.008 |
| Attention | 12.9 (7.0, 18.0) | 12.2 (6.0, 19.0) | 8.0 (3.0, 12.7) | 0.022 |
| Effort | 9.0 (4.0, 15.0) | 8.8 (4.0, 12.5) | 4.5 (1.7, 6.0) | 0.002 |
| Affect | 7.9 (4.0, 12.0) | 8.2 (3.0, 13.0) | 5.0 (1.0, 6.0) | 0.026 |
| Memory | 7.5 (5.0, 11.0) | 7.7 (4.0, 12.0) | 4.3 (0.7, 6.0) | 0.009 |
Table 4.
DBH genotypes analysis according to Omega-3 Index and neuropsychological testing.
|
DBH (rs1611115) |
CC (n = 79) | CT (n = 47) | TT (n = 6) | p |
|---|---|---|---|---|
| Omega-3 Index | 5.1 (3.9, 6.0) | 5.6 (4.3, 7.4) | 5.0 (4.3, 5.5) | 0.275 |
| PhysAgg | 20.6 (13.0, 27.0) | 23.8 (17.0, 29.0) | 17.5 (8.0, 8.0) | 0.120 |
| VerbAgg | 13.0 (9.0, 16.0) | 14.0 (11.0, 17.7) | 8.3 (5.0, 7.0) | 0.022 |
| Anger | 16.5 (11.0, 20.0) | 17.5 (13.0, 22.0) | 14.6 (7.0, 12.0) | 0.521 |
| Hostility | 19.5 (14.0, 24.0) | 19.4 (13.0, 24.7) | 15.5 (8, 18.0) | 0.453 |
| IndirAgg | 13.9 (10.0, 18.0) | 14.0 (12.0, 17.5) | 12.0 (10.0, 10.0) | 0.589 |
| Total ADD | 42.8 (23.0, 65.0) | 43.9 (21.2, 62) | 65.0 (10.0, 87.0) | 0.576 |
| Activation | 9.5 (5.0, 14.0) | 10.5 (6.0, 15.0) | 10.0 (5.0, 22.0) | 0.615 |
| Attention | 10.9 (6.0, 18.0) | 11.9 (5.51, 17.0) | 18.3 (3.0, 25.0) | 0.427 |
| Effort | 7.8 (3.0, 11.0) | 7.6 (4.0, 11.0) | 12.0 (2.0, 11.0) | 0.749 |
| Affect | 7.4 (4.0, 12.0) | 7.1 (2.2, 11.7) | 10.7 (0.0, 15.0) | 0.489 |
| Memory | 6.9 (3.0, 11.0) | 6.7 (2.2, 11.0) | 6.7 (0.0, 7.0) | 0.983 |
CC, CT and TT are the genotypes of alleles.
However, DRD2 rs4274224 was strongly associated with attention-deficit disorder (ADD) (including all subscales of BADDS testing) (H(2)= 11.249, p = 0.004) and also with the following individual subscales of BADDS: Activation (H(2) = 9.640, p = 0.008), Attention (H (2) = 7.661; 0.022), Effort (H(2) = 12.771, p = 0.002) and Memory (H(2) = 9.414, p = 0.009; Table 3). The odd ratio (OR) for participants with an AA genotype to return a high score in executive function as assessed by BADDS was 3.12 times more frequent than prisoners with a GG genotype. Only the Affect subscale of BADDS was not found to be significantly associated with the DRD2 rs4274224 variant, albeit being borderline (H(2) = 7.313, P = 0.026). When considering the genetic association of DRD2 rs4274224 with the results from the AQ questionnaire, no significant result was reported for both Total Aggression and/or any of the individual subscales on the test (p > 0.025, Table 3).
In contrast, functional polymorphism DBHrs1611115 showed a significant association with the Verbal Aggression subscale from the AQ (H(2) = 7.609, p = 0.022; Table 4), with TT genotypes showing an odds ratio (OR) = 4.18 to develop high scores in aggressive behaviour. No association was reported between DBHrs1611115 and any other subscale from both the AQ and BADDS tests (p > 0.025; Table 4).
4. Discussion
Functional polymorphisms in both DRD1 and DBH genes, along with an intronic variant of DRD2, were studied in relation to blood Omega-3 Index and aggressive behavioural and metacognitive assessment in adult male prisoners. None of the tested polymorphisms showed an association with blood Omega-3 Index. However, functional DBH polymorphism was significantly associated with Verbal Aggression, whereas DRD2 polymorphism showed a strong association with total ADD and every subscale of BADDS tests except for Affect (reported borderline to corrected significance set value). In this cohort, no association was observed between functional DRD1 polymorphism and neuropsychological tested parameters. Interestingly, DRD1 allelic distribution showed a higher level of A allele in the tested inmate cohort compared with the global minor allelic frequency [46]. Considering that rs686 is a functional SNP located in the promoter region of the DRD1 gene and its A allele is linked with an increased transcriptional gene activity compared with the G allele [47], a difference in the DRD1 density number may occur in the tested cohort compared with the general population. Since these receptors mediate aggressive behaviour [9,10], this hypothesis is supported by a previous study reporting higher levels of aggressive behaviour in this cohort of prisoners when compared with university students [43]. The DRD2 genetic variant did not show any genetic association with a measurement of aggression through the AQ in accordance with a previous study looking at DRD2 rs1800479’s (located in the downstream of the gene) association with criminal behaviour and self-reported aggression in violent prison inmates [48]. However, significant associations were found in this study between DRD2 rs4274224 and assessment of executive function through BADDS and total ADD phenotypes. This finding confirms the critical role of intronic DRD2 rs4274224 on inter-individual variations in personality traits such as negative emotional processing and openness to experience previously reported [49]. The functionality of DRD2 rs4274224 regulates the neural responses to reward and emotion processing through modulation of dopaminergic neurotransmission in the dorsolateral prefrontal cortex (DLPFC, Brodmann Area BA46/9) and the subgenual cortical area (BA25/32), respectively [49]. Higher-order cognitive and executive functions such as working memory and attention rely on activation of mesocortical dopaminergic pathways, particularly towards the DLPFC [50,51], whereas the dopaminergic neurons in the anterior region of the cingulate cortex (BA25) appear to play a causal role in behavioural changes such as emotion, negative affect and anhedonia [52]. Dysfunction of dopaminergic neurotransmission in these cerebral areas was associated with impaired social and cognitive functions typically seen in neurodevelopmental disorders such as schizophrenia, autism spectrum disorder and ADHD, as well as in depression, impulsivity and substance abuse disorders [53]. The strong association between total ADD, attention and DRD2 rs4274224 also confirms findings from a previous study investigating the association of this genetic marker with executive functions in an obesity context [54] and from meta-analyses reporting significant associations between some DRD2 polymorphisms and ADHD [55,56,57].
Another way of modulating dopaminergic neurotransmission without the direct implication of neuronal receptors signalling is to act on the level of extracellular DA in the brain. DBH transforms DA in noradrenaline, allowing modulation of the synaptic levels of DA [31]. Functional polymorphism DBH was significantly associated with verbal aggression in the tested cohort of prisoners. Individuals with low enzymatic activity genotypes (CT and TT) are expected to display high levels of DA in the synapse [31]. High levels of DA in the synapse can activate dopaminergic receptors, such as DRD1 and DRD2 involved in aggressive behaviour, impulsivity and attention [5,6,7,8]. Functional DBH rs1611115 was previously reported to be associated with impulsive personality styles but not with affective disorders [32], suggesting a strong involvement of meso-cortical dopaminergic pathways in this personality trait. This finding is also in line with previous studies reporting that low activity of DBH would relate to impulsive behaviours and with higher sensation seeking in adults (consistent with impulsiveness) [58,59]. Verbal aggression involves the activation of higher brain areas (temporal and frontal cortices), whereas physical aggression seems to emerge primarily from the activation of more primitive brain structures such as the limbic system [60]. Consequently, we can hypothesise that the tested DA genetic polymorphisms in this cohort appear to preferentially affect meso-cortical dopaminergic pathways (supporting higher connective function) than mesolimbic dopamine pathways (which involve pleasure and reward) [7]. Further imaging work in the tested cohort will be necessary to validate this hypothesis. Interestingly, differential genetic regulation of DA genes was previously reported through compensatory gene–gene interactions between COMT and DRD2, with genotypes conferring either elevated prefrontal dopamine or diminished striatal dopamine directly affecting the activation of dopaminergic receptors in the respective brain region [61]. In addition, two mRNA splice variants for DRD2 were previously reported as the D2 receptor short (DRD2-S) variant mainly found in the pre-synaptic space with relatively greater abundance in the prefrontal cortex compared with the D2 receptor long (DRD2-L) variant located mainly postsynaptically and which is relatively more abundant in the striatum [62,63]. Previous studies reported that genetic differences in the proportion of these two DRD2 isoforms within the corticostriatal system contribute to symptom variability in schizophrenic patients including working memory disturbances [64,65]. Due to previous associations of DRD2 splice variants with schizophrenia and its addiction-like phenotypes [62,64,65] and tissue-specific expression [62], it will be interesting to explore this dopaminergic variant in the context of the present study.
Studies have shown a regulation of expression of dopaminergic genes by n-3 LCPUFA [40,66]. DHA, which is an essential structure of the neuronal membrane, can also bind the nuclear receptor retinoid X receptor (RXR). This ligand/receptor complex results in the modulation of gene transcription of dopaminergic genes essential for neuronal development [40]. Knockout RXR mice were found to have abnormalities in synaptic plasticity and learning [67], both neuronal processes underlying memory. A positive association was found between plasma levels of DHA and memory in healthy adults [68], whereas an inverse association between DHA levels and risk of dementia was identified through a meta-analysis [69]. In this study, no significant association was found between Omega-3 Index and dopaminergic polymorphisms (p < 0.025). This result may be due to the omega-3 deficiency in this cohort compared with the levels of Omega-3 Index assessed in a university cohort [43] and/or to potential differences in individual metabolic genotypes.
Although there is a growing body of literature showing the beneficial effect of fish-oil consumption (such as a higher level of n-3 LCPUFA in the blood associated with a lower risk of heart disease), high blood levels of n-3 LCPUFA can be detrimental for some individuals depending on their genotype in the context of lipid profiles [70]. A recent study reported that omega-3 supplementation can either aid in reducing blood triglycerides or increase blood triglycerides depending on the genotypes of four variants for the GJB2, SLC12A3, ABCA6, and MLXIPL genes in individuals [70]. For instance, individuals with minor allele (G) for GJB2 rs112803755 polymorphism who received fish oil supplementation showed a decreased level of triglycerides, whereas individuals with the AA genotype who also received the same fish oil supplementation had their triglycerides levels slightly increased [70]. Individual genotypes are critical for the modulation of EPA and DHA in the blood due to their effects on encoding factors and metabolic enzymes (such as fatty acid desaturases and elongases) essential for lipid metabolism and bioactivities [71,72]. Consequently, this genetic variability may contribute to explaining the difference existing between the inmates’ incorporation of EPA and DHA as well as the activation/inhibition of cellular pathways, potentially including dopaminergic pathways [73].
A better understanding of the effects of the Omega-3 Index and genetic variants for dopaminergic genes on aggressive behavioural and executive function phenotypes in adult male prisoners, as well in the general population, will improve prediction of, and response to, aggressive behaviour. Our finding adds to our knowledge regarding the genetic effects on neurobiological mechanisms (such as dopaminergic neurotransmission) and attention and on the aggressive behavioural, attention and memory phenotypes in adult male prisoners. Studies with larger sample sizes should be conducted to confirm these findings and explore the associations from additional genetic polymorphisms in the dopaminergic system and metabolic enzymes with the Omega-3 Index.
5. Conclusions
Although n-3 LCPUFA is essential to brain development and maintenance, Omega-3 Index was not significantly associated with genotypes from relevant dopaminergic genes in adult male prisoners. However, as we reported here, the genetic predisposition for dopaminergic genes (DBH and DRD2) in aggressive behaviour, memory dysfunction and attention deficit disorder has been confirmed.
Acknowledgments
Correctional Officer Carole Collier is acknowledged for her role as the clinical trial coordinator at South Coast Correctional Centre, Nowra, NSW, Australia. The study volunteers are acknowledged for their participation.
Author Contributions
Conceptualization, F.F., B.J.M., M.K.B., L.G. and M.B.; Methodology, B.J.M. and M.K.B.; Formal Analysis, F.F. and M.B.; Investigation, B.J.M. and M.K.B.; Resources, B.J.M., M.K.B. and L.G.; Data Curation, B.J.M. and M.K.B.; Writing—Original Draft Preparation, F.F.; Writing—Review and Editing, F.F., B.J.M., M.K.B., L.G. and M.B.; Project Administration, B.J.M., M.K.B. and L.G.; Funding Acquisition, B.J.M., M.K.B., L.G. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a URC partnership grant, with Corrective Services NSW as our partner, and was obtained by Meyer B.J., Winberg P., Sinn N., Byrne M., Grant L., Batterham M. “Effect of fish oil and micronutrient supplementation in young adult offenders: a randomised controlled trial”.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Human Ethics Committee of the University of Wollongong and the Department of Corrective Services (NSW, Australia) ethics committees (11/93185; October 2011) for studies involving humans.
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data may be made available upon a reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bellisle F. Effects of diet on behaviour and cognition in children. Br. J. Nutr. 2004;92((Suppl. 2)):S227–S232. doi: 10.1079/BJN20041171. [DOI] [PubMed] [Google Scholar]
- 2.Khalid S., Williams C., Reynolds S.A. Is there an association between diet and depression in children and adolescents? A systematic review. Br. J. Nutr. 2016;116:2097–2108. doi: 10.1017/S0007114516004359. [DOI] [PubMed] [Google Scholar]
- 3.Han C.S., Dingemanse N. You are what you eat: Diet shapes body composition, personality and behavioural stability. BMC Evol. Biol. 2017;17:8. doi: 10.1186/s12862-016-0852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kidd P.M. Omega-3 DHA and EPA for cognition, behavior, and mood: Clinical findings and structural-functional synergies with cell membrane phospholipids. Altern. Med. Rev. 2007;12:207. [PubMed] [Google Scholar]
- 5.Seo D., Patrick C.J., Kennealy P.J. Role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggress. Violent Behav. 2008;13:383–395. doi: 10.1016/j.avb.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belfry K.D., Kolla N.J. Cold-Blooded and on Purpose: A Review of the Biology of Proactive Aggression. Brain Sci. 2021;11:1412. doi: 10.3390/brainsci11111412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein M.O., Battagello D.S., Cardoso A.R., Hauser D.N., Bittencourt J.C., Correa R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell Mol. Neurobiol. 2019;39:31–59. doi: 10.1007/s10571-018-0632-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berridge K.C., Kringelbach M. Pleasure Systems in the Brain. Neuron. 2015;86:646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plavén-Sigray P., Matheson G.J., Gustavsson P., Stenkrona P., Halldin C., Farde L., Cervenka S. Is dopamine D1 receptor availability related to social behavior? A positron emission tomography replication study. PLoS ONE. 2018;13:e0193770. doi: 10.1371/journal.pone.0193770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felippe R.M., Oliveira G.M., Barbosa R.S., Esteves B.D., Gonzaga B.M.S., Horita S.I.M., Garzoni L.R., Beghini D.G., Araújo-Jorge T.C., Fragoso V.M.S. Experimental Social Stress: Dopaminergic Receptors, Oxidative Stress, and c-Fos Protein Are Involved in Highly Aggressive Behavior. Front. Cell. Neurosci. 2021;15:696834. doi: 10.3389/fncel.2021.696834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Mallakh R.S., McKenzie C. The dopamine D4/D2 receptor antagonist affinity ratio as a predictor of anti-aggression medi-cation efficacy. Med. Hypotheses. 2013;80:530–533. doi: 10.1016/j.mehy.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Nikulina E.M., Kapralova N.S. Role of dopamine receptors in the regulation of aggression in mice; relationship to genotype. Neurosci. Behav. Physiol. 1992;22:364–369. doi: 10.1007/BF01186627. [DOI] [PubMed] [Google Scholar]
- 13.Forbes E., Brown S.M., Kimak M., Ferrell R.E., Manuck S.B., Hariri A.R. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol. Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colzato L., Wildenberg W.V.D., Van der Does W., Hommel B. Genetic markers of striatal dopamine predict individual differences in dysfunctional, but not functional impulsivity. Neuroscience. 2010;170:782–788. doi: 10.1016/j.neuroscience.2010.07.050. [DOI] [PubMed] [Google Scholar]
- 15.Congdon E., Canli T. A Neurogenetic Approach to Impulsivity. J. Personal. 2008;76:1447–1484. doi: 10.1111/j.1467-6494.2008.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimm O., Weber H., Kittel-Schneider S., Kranz T.M., Jacob C.P., Lesch K.-P., Reif A. Impulsivity and Venturesomeness in an Adult ADHD Sample: Relation to Personality, Comorbidity, and Polygenic Risk. Front. Psychiatry. 2020;11:557160. doi: 10.3389/fpsyt.2020.557160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhee S.H., Waldman I.D. Genetic and environmental influences on antisocial behavior: A meta-analysis of twin and adoption studies. Psychol. Bull. 2002;128:490–529. doi: 10.1037/0033-2909.128.3.490. [DOI] [PubMed] [Google Scholar]
- 18.Miles D.R., Carey G. Genetic and environmental architecture on human aggression. J. Personal. Soc. Psychol. 1997;72:207–217. doi: 10.1037/0022-3514.72.1.207. [DOI] [PubMed] [Google Scholar]
- 19.Hamidovic A., Dlugos A., Skol A., Palmer A.A., de Wit H. Evaluation of genetic variability in the dopamine receptor D2 in relation to behavioral inhibition and impulsivity/sensation seeking: An exploratory study with d-amphetamine in healthy par-ticipants. Exp. Clin. Psychopharmacol. 2009;17:374–383. doi: 10.1037/a0017840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moses T.E., Burmeister M., Greenwald M.K. Heroin delay discounting and impulsivity: Modulation by DRD1 genetic var-iation. Addict. Biol. 2020;25:e12777. doi: 10.1111/adb.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halleland H., Lundervold A., Halmøy A., Haavik J., Johansson S. Association between Catechol O-methyltransferase (COMT) haplotypes and severity of hyperactivity symptoms in Adults. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2009;150B:403–410. doi: 10.1002/ajmg.b.30831. [DOI] [PubMed] [Google Scholar]
- 22.Liu J., Zhang T., Mo Y., Gong J. The COMT gene rs4680 polymorphism moderates the relationship between adult ADHD symptoms and executive dysfunction. Asian J. Psychiatry. 2021;56:102546. doi: 10.1016/j.ajp.2021.102546. [DOI] [PubMed] [Google Scholar]
- 23.Lotta T., Vidgren J., Tilgmann C., Ulmanen I., Melen K., Julkunen I., Taskinen J. Kinetics of human soluble and mem-brane-bound catechol O-methyltransferase: A revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- 24.Chen J., Lipska B.K., Halim N., Ma Q.D., Matsumoto M., Melhem S., Kolachana B.S., Hyde T.M., Herman M.M., Apud J., et al. Functional Analysis of Genetic Variation in Catechol-O-Methyltransferase (COMT): Effects on mRNA, Protein, and Enzyme Activity in Postmortem Human Brain. Am. J. Hum. Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volavka J., Bilder R., Nolan K. Catecholamines and Aggression: The Role of COMT and MAO Polymorphisms. Ann. N. Y. Acad. Sci. 2004;1036:393–398. doi: 10.1196/annals.1330.023. [DOI] [PubMed] [Google Scholar]
- 26.Hirata Y., Zai C.C., Nowrouzi B., Beitchman J.H., Kennedy J.L. Study of the Catechol-O-Methyltransferase (COMT) Gene with High Aggression in Children. Aggress. Behav. 2013;39:45–51. doi: 10.1002/ab.21448. [DOI] [PubMed] [Google Scholar]
- 27.Li J., Wang Y., Hu S., Zhou R., Yu X., Wang B., Guan L., Yang L., Zhang F., Faraone S. The monoamine oxidase B gene exhibits significant association to ADHD. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2008;147B:370–374. doi: 10.1002/ajmg.b.30606. [DOI] [PubMed] [Google Scholar]
- 28.Antypa N., Giegling I., Calati R., Schneider B., Hartmann A.M., Friedl M., Konte B., Lia L., De Ronchi D., Serretti A., et al. MAOA and MAOB polymorphisms and anger-related traits in suicidal participants and controls. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263:393–403. doi: 10.1007/s00406-012-0378-8. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y.-Y., Cate S.P., Battistuzzi C., Oquendo M.A., Brent D., Mann J.J. An Association between a Functional Polymorphism in the Monoamine Oxidase A Gene Promoter, Impulsive Traits and Early Abuse Experiences. Neuropsychopharmacology. 2004;29:1498–1505. doi: 10.1038/sj.npp.1300455. [DOI] [PubMed] [Google Scholar]
- 30.McDermott R., Tingley D., Cowden J., Frazzetto G., Johnson D.D.P. Monoamine oxidase A gene (MAOA) predicts behavioral aggression following provocation. Proc. Natl. Acad. Sci. USA. 2009;106:2118–2123. doi: 10.1073/pnas.0808376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cubells J.F., van Kammen D.P., Kelley M.E., Anderson G.M., O’Connor D.T., Price L.H., Malison R., Rao P.A., Kobayashi K., Nagatsu T., et al. Dopamine beta-hydroxylase: Two polymorphisms in linkage disequilibrium at the structural gene DBH associate with biochemical phenotypic variation. Hum. Genet. 1998;102:533–540. doi: 10.1007/s004390050736. [DOI] [PubMed] [Google Scholar]
- 32.Hess C., Reif A., Strobel A., Boreatti-Hümmer A., Heine M., Lesch K.P., Jacob C.P. A functional dopamine-beta-hydroxylase gene promoter polymorphism is associated with impulsive personality styles, but not with affective disorders. J. Neural. Transm. 2009;116:121–130. doi: 10.1007/s00702-008-0138-0. [DOI] [PubMed] [Google Scholar]
- 33.Meyer B.J., Onyiaodike C.C., Brown E.A., Jordan F., Murray H., Nibbs R.J., Sattar N., Lyall H., Nelson S.M., Freeman D.J. Maternal Plasma DHA Levels Increase Prior to 29 Days Post-LH Surge in Women Undergoing Frozen Embryo Transfer: A Prospective, Observational Study of Human Pregnancy. J. Clin. Endocrinol. Metab. 2016;101:1745–1753. doi: 10.1210/jc.2015-3089. [DOI] [PubMed] [Google Scholar]
- 34.Zamai N., Cortie C.H., Jarvie E.M., Onyiaodike C.C., Alrehaili A., Francois M., Freeman D.J., Meyer B.J. In pregnancy, ma-ternal HDL is specifically enriched in, and carries the highest proportion of, DHA in plasma. Prostaglandins Leukot. Essent. Fat. Acids. 2020;163:102209. doi: 10.1016/j.plefa.2020.102209. [DOI] [PubMed] [Google Scholar]
- 35.Weiser M.J., Butt C.M., Mohajeri M.H. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients. 2016;8:99. doi: 10.3390/nu8020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris W.S., von Schacky C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004;39:212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 37.Harris W.S., Sands S.A., Windsor S.L., Ali H.A., Stevens T.L., Magalski A., Porter C.B., Borkon A.M. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: Correlation with erythrocytes and response to supplementation. Circulation. 2004;110:1645–1649. doi: 10.1161/01.CIR.0000142292.10048.B2. [DOI] [PubMed] [Google Scholar]
- 38.Bozzatello P., De Rosa M., Rocca P., Bellino S. Effects of Omega 3 Fatty Acids on Main Dimensions of Psychopathology. Int. J. Mol. Sci. 2020;21:6042. doi: 10.3390/ijms21176042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goustard-Langelier B., Guesnet P., Durand G., Antoine J.-M., Alessandri J.-M. n−3 and n−6 fatty acid enrichment by dietary fish oil and phospholipid sources in brain cortical areas and nonneural tissues of formula-fed piglets. Lipids. 1999;34:5–16. doi: 10.1007/s11745-999-331-6. [DOI] [PubMed] [Google Scholar]
- 40.Healy-Stoffel M., Levant B. N-3 (Omega-3) Fatty Acids: Effects on Brain Dopamine Systems and Potential Role in the Etiology and Treatment of Neuropsychiatric Disorders. CNS Neurol. Disord. Drug Targets. 2018;17:216–232. doi: 10.2174/1871527317666180412153612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer B.J., Byrne M.K., Collier C., Parletta N., Crawford D., Winberg P.C., Webster D., Chapman K., Thomas G., Dally J., et al. Baseline omega-3 index correlates with aggressive and attention deficit disorder behaviours in adult prisoners. PLoS ONE. 2015;10:e0120220. doi: 10.1371/journal.pone.0120220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buss A.H., Perry M. The aggression questionnaire. J. Personal. Soc. Psychol. 1992;63:452–459. doi: 10.1037/0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- 43.Byrne M.K., Parletta N., Webster D.G., Batterham M., Meyer B.J. Adult Attention Deficit Disorder and Aggressive Behaviour: An Exploration of Relationships between Brown Attention-Deficit Disorder Scales and the Aggression Questionnaire. Psychiatry Psychol. Law. 2015;22:407–416. doi: 10.1080/13218719.2014.960027. [DOI] [Google Scholar]
- 44.Brown T.E. Brown Attention Deficit Disorder Scales for Adolescents and Adults. [(accessed on 27 February 2022)]. Available online: https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/referencespapers.aspx?referenceid=285903.
- 45.Abecasis G.R., Cookson W.O.C. GOLD—Graphical Overview of Linkage Disequilibrium. Bioinformatics. 2000;16:182–183. doi: 10.1093/bioinformatics/16.2.182. [DOI] [PubMed] [Google Scholar]
- 46.Sherry S.T., Ward M.-H., Kholodov M., Baker J., Phan L., Smigielski E.M., Sirotkin K. dbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 2001;29:308–311. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang W., Ma J.Z., Payne T., Beuten J., Dupont R.T., Li M.D. Significant association of DRD1 with nicotine dependence. Qual. Life Res. 2008;123:133–140. doi: 10.1007/s00439-007-0453-9. [DOI] [PubMed] [Google Scholar]
- 48.Qadeer M.I., Amar A., Mann J.J., Hasnain S. Polymorphisms in dopaminergic system genes; association with criminal behavior and self-reported aggression in violent prison inmates from Pakistan. PLoS ONE. 2017;12:e0173571. doi: 10.1371/journal.pone.0173571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peciña M., Mickey B.J., Love T., Wang H., Langenecker S., Hodgkinson C., Shen P.-H., Villafuerte S., Hsu D., Weisenbach S.L., et al. DRD2 polymorphisms modulate reward and emotion processing, dopamine neurotransmission and openness to experience. Cortex. 2013;49:877–890. doi: 10.1016/j.cortex.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldman-Rakic P.S. The Cortical Dopamine System: Role in Memory and Cognition. Adv. Pharmacol. 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- 51.Fuster J.M. The Prefrontal Cortex—An Update: Time Is of the Essence. Neuron. 2001;30:319–333. doi: 10.1016/S0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 52.Alexander L., Clarke H.F., Roberts A.C. A Focus on the Functions of Area 25. Brain Sci. 2019;9:129. doi: 10.3390/brainsci9060129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Islam K.U.S., Meli N., Blaess S. The Development of the Mesoprefrontal Dopaminergic System in Health and Disease. Front. Neural Circuits. 2021;15:746582. doi: 10.3389/fncir.2021.746582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ariza M., Garolera M., Jurado M.A., Garcia-Garcia I., Hernan I., Sanchez-Garre C., Vernet-Vernet M., Sender-Palacios M.J., Marques-Iturria I., Pueyo R., et al. Dopamine genes (DRD2/ANKK1-TaqA1 and DRD4-7R) and executive function: Their interaction with obesity. PLoS ONE. 2012;7:e41482. doi: 10.1371/journal.pone.0041482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu J., Xiao H., Sun H., Zou L., Zhu L.-Q. Role of Dopamine Receptors in ADHD: A Systematic Meta-analysis. Mol. Neurobiol. 2012;45:605–620. doi: 10.1007/s12035-012-8278-5. [DOI] [PubMed] [Google Scholar]
- 56.Sullivan P.F., Daly M.J., O’Donovan M. Genetic architectures of psychiatric disorders: The emerging picture and its implications. Nat. Rev. Genet. 2012;13:537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pan Y.-Q., Qiao L., Xue X.-D., Fu J.-H. Association between ANKK1 (rs1800497) polymorphism of DRD2 gene and attention deficit hyperactivity disorder: A meta-analysis. Neurosci. Lett. 2015;590:101–105. doi: 10.1016/j.neulet.2015.01.076. [DOI] [PubMed] [Google Scholar]
- 58.Zuckerman M. P-Impulsive Sensation Seeking and Its Behavioral, Psychophysiological and Biochemical Correlates. Neuropsychobiology. 1993;28:30–36. doi: 10.1159/000118996. [DOI] [PubMed] [Google Scholar]
- 59.Rogeness G.A., Hernandez J.M., Macedo C.A., Mitchell E.L. Biochemical differences in children with conduct disorder so-cialized and undersocialized. Am. J. Psychiatry. 1982;139:307–311. doi: 10.1176/ajp.139.3.307. [DOI] [PubMed] [Google Scholar]
- 60.Progovac L., Benítez-Burraco A. From Physical Aggression to Verbal Behavior: Language Evolution and Self-Domestication Feedback Loop. Front. Psychol. 2019;10:2807. doi: 10.3389/fpsyg.2019.02807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zmigrod L., Robbins T.W. Dopamine, Cognitive Flexibility, and IQ: Epistatic Catechol-O-MethylTransferase:DRD2 Gene-Gene Interactions Modulate Mental Rigidity. J. Cogn. Neurosci. 2021;34:153–179. doi: 10.1162/jocn_a_01784. [DOI] [PubMed] [Google Scholar]
- 62.Colelli V., Fiorenza M.T., Conversi D., Orsini C., Cabib S. Strain-specific proportion of the two isoforms of the dopamine D2 receptor in the mouse striatum: Associated neural and behavioral phenotypes. Genes Brain Behav. 2010;9:703–711. doi: 10.1111/j.1601-183X.2010.00604.x. [DOI] [PubMed] [Google Scholar]
- 63.Giros B., Sokoloff P., Martres M.P., Riou J.-F., Emorine L.J., Schwartz J.-C. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature. 1989;342:923–926. doi: 10.1038/342923a0. [DOI] [PubMed] [Google Scholar]
- 64.Bertolino A., Fazio L., Caforio G., Blasi G., Rampino A., Romano R., Di Giorgio A., Taurisano P., Papp A., Pinsonneault J., et al. Functional variants of the dopamine receptor D2 gene modulate prefronto-striatal phenotypes in schizophrenia. Pt 2Brain. 2009;132:417–425. doi: 10.1093/brain/awn248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamamoto K., Kuriu T., Matsumura K., Nagayasu K., Tsurusaki Y., Miyake N., Yamamori H., Yasuda Y., Fujimoto M., Fujiwara M., et al. Multiple alterations in glu-tamatergic transmission and dopamine D2 receptor splicing in induced pluripotent stem cell-derived neurons from patients with familial schizophrenia. Transl. Psychiatry. 2021;11:548. doi: 10.1038/s41398-021-01676-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kitajka K., Sinclair A.J., Weisinger R.S., Weisinger H.S., Mathai M., Jayasooriya A.P., Halver J.E., Puskás L.G. Effects of dietary omega-3 polyunsaturated fatty acids on brain gene expression. Proc. Natl. Acad. Sci. USA. 2004;101:10931–10936. doi: 10.1073/pnas.0402342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Szántó A., Narkar V., Shen Q., Uray I.P., Davies P.J.A., Nagy L. Retinoid X receptors: X-ploring their (patho)physiological functions. Cell Death Differ. 2004;11((Suppl. 2)):S126–S143. doi: 10.1038/sj.cdd.4401533. [DOI] [PubMed] [Google Scholar]
- 68.Yurko-Mauro K., Alexander D.D., Van Elswyk M.E. Docosahexaenoic Acid and Adult Memory: A Systematic Review and Meta-Analysis. PLoS ONE. 2015;10:e0120391. doi: 10.1371/journal.pone.0120391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Djuricic I., Calder P. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients. 2021;13:2421. doi: 10.3390/nu13072421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Francis M., Li C., Sun Y., Zhou J., Li X., Brenna J.T., Ye K. Genome-wide association study of fish oil supplementation on lipid traits in 81,246 individuals reveals new gene-diet interaction loci. PLoS Genet. 2021;17:e1009431. doi: 10.1371/journal.pgen.1009431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O’Neill C.M., Minihane A.-M. The impact of fatty acid desaturase genotype on fatty acid status and cardiovascular health in adults. Proc. Nutr. Soc. 2017;76:64–75. doi: 10.1017/S0029665116000732. [DOI] [PubMed] [Google Scholar]
- 72.Minihane A.M. Impact of Genotype on EPA and DHA Status and Responsiveness to Increased Intakes. Nutrients. 2016;8:123. doi: 10.3390/nu8030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Serini S., Calviello G. Omega-3 PUFA Responders and Non-Responders and the Prevention of Lipid Dysmetabolism and Related Diseases. Nutrients. 2020;12:1363. doi: 10.3390/nu12051363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be made available upon a reasonable request to the corresponding author.