Abstract
Background:
H7 influenza viruses have emerged as potential pandemic threat. We evaluated the safety and immunogenicity of two candidate H7 pandemic live attenuated influenza vaccines (pLAIV) and their ability to prime for responses to an unadjuvanted H7 pandemic inactivated influenza vaccine (pIIV).
Methods:
Healthy seronegative adults received two doses of A/Netherlands1219/03 (H7N7) or one dose of Alchicken/British Columbia/CN-6/04 (H7N3) PLAIV all given as 107.5 50% tissue culture infective doses (TCID50) intranasally. A subset of subjects received one 45 μg dose of H7N7 pIIV containing the A/Mallard/Netherlands/12/2000 HA intramuscularly 18–24 months after pLAIV. Viral shedding was assessed by culture and real-time polymerase chain reaction (rRT-PCR), B cell responses following PLAIV were evaluated by ELISPOT and flow cytometry. Serum antibody was assessed by hemagglutinationinhibition (HAI), microneutralization (MN) and ELISA assays after each vaccine.
Results:
Serum HAI or MN responses were not detected in any subject following one or two doses of either H7 pLAIV, although some subjects had detectable H7 specific B cells after vaccination. However, 10/13 subjects primed with two doses of H7N7 PLAIV responded to a subsequent dose of the homologous H7N7 pIIV with high titer HAI and MN antibody that cross-reacted with both North American and Eurasian lineage H7 viruses, including H7N9. In contrast, naïve subjects and recipients of a single dose of the mismatched H7N3 PLAIV did not develop HAI or MN antibody after pIIV.
Conclusions:
While pLAIVs did not elicit detectable serum MN or HAI antibody, strain-specific PLAIV priming established long term immune memory that was cross-reactive with other H7 influenza strains. Understanding the mechanisms underlying priming by PLAIV may aid in pandemic vaccine development.
Keywords: Influenza, H7N7, Pandemic, Priming, Live attenuated vaccine
1. Introduction
Avian influenza viruses of the H7 hemagglutinin (HA) subtype have been recognized as potential sources of pandemic influenza in humans for many years. Sporadic cases of H7 influenza infection in humans have been identified since 1980, including H7N3 cases associated with poultry exposure in Canada [1–4], and multiple cases of H7N7 infection associated with a large poultry outbreak in the Netherlands [3,4]. Currently, there is a large outbreak of H7N9 associated with live poultry markets in eastern China [5,6], with a high case fatality rate. This recent outbreak has proven difficult to contain as the virus has low pathogenicity in poultry and affected flocks are not easy to identify.
Development of effective vaccines against H7 viruses is an important component of pandemic preparedness. However, results of studies conducted to date have suggested that generating strong immune responses to H7 viruses will be challenging. Previous studies have evaluated pandemic inactivated influenza vaccines (pIIV) against the H7N7 A/Netherlands/03 virus prepared in cell culture [7] or in eggs [8], and in both cases have demonstrated infrequent serum antibody responses even after two doses. Specifically, in a previous study of the egg-grown split product H7N7 pIIV, a serum hemagglutination inhibition (HAI) titer of >1 :40 was seen in only 1 of 25 subjects who received two doses of 90 mcg, the highest dose evaluated [8].
Live attenuated vaccine candidates for pandemic influenza (pLAIV) have also been developed. Seasonal LAIV has demonstrated high levels of immunogenicity and protective efficacy in immunologically naïve young children [9–11], and these advantages could also apply to naïve populations in the event of a pandemic. However, H7 pLAIV candidates have also demonstrated low levels of immunogenicity in previous trials. In a previous study of an A/British Columbia/CN-6/2004 (H7N3) PLAIV candidate based on the cold-adapted A/Ann Arbor/6/60 master donor virus, low titer serum HAI antibody responses were seen in 43% of the vaccines [12]. A second study evaluating an A/ 17/mallard/Netherlands/00/95 (H7N3) PLAIV on the background of the cold-adapted A/Leningrad/66 virus reported HAI responses in 31% of subjects after two doses [13].
Although pLAIV candidates have not generated strong antibody responses, it is possible that they would prime the immune system for responses to subsequent booster doses of a matched or related vaccine. In a study evaluating administration of inactivated H5N1 vaccine to subjects who had previously received a variety of H5 pLAIVs, significant HAI responses were seen after a single dose of inactivated H5 vaccine in 13/21 previous recipients of LAIV, and in only 2/20 vaccine naïve subjects [14].
In the current study, we evaluated the A/British Columbia/CN-6/2004 (H7N3) pLAIV as a single dose, and an A/Netherlands/ 219/2003 (H7N7) PLAIV in a two dose schedule. Eighteen to 24 months later, we administered a single dose of pIIV containing the HA of A/Mallard/Netherlands/12/2000 (antigenically similar to A/Netherlands/219/2003) to a subset of these subjects, and to H7N7 naïve subjects. While the PLAIV were not detectably immunogenic, two doses of H7N7 pLAIV, but not a single dose of H7N3 pLAIV, primed for a strong response to the H7N7 pIIV, with a broadly cross reactive response among H7 viruses, including the A/Anhui/13 (H7N9) virus associated with an ongoing outbreak in China.
2. Methods
2.1. Vaccines
The live attenuated vaccines used in this study were influenza A/Netherlands/219/2003 (H7N7) and A/chicken/British Columbia/CN-6/2004 (H7N3) reassortants with the influenza A/Ann Arbor/6/60 cold-adapted (ca) Master donor virus (MDV). The vaccines were generated by reverse genetics as 6–2 reassor-tants deriving the HA and NA genes from the wild-type parent and all other gene segments from the ca MDV. In addition, the protease cleavage site of the A/Netherlands/03 H7 HA was modified to a monobasic sequence [15]. Each of the live attenuated viruses was confirmed to be ca, temperature sensitive, and attenuated in ferrets, as well as trypsin dependent in cell culture, nonpathogenic in chickens, and sensitive to the antiviral drug oseltamivir.
The inactivated vaccine used in this study was an inactivated subvirion H7N7 vaccine used in previous studies [8]. This vaccine was derived from a reassortant virus containing the H7 gene of the A/mallard/Netherlands/12/2000 (H7N3) and the N7 of A/mallard/Netherlands/2/2000 (H10N7), with the internal protein genes from a PR8-based vaccine strain [16], and is antigenically similar to the A/Netherlands/219/2003 H7N7 virus. The potency of the vaccine was confirmed by single radial immunodiffusion (SRID) prior to use.
2.2. Study design
Evaluation of pLAIV candidates was performed using previously published methods [12,17,18] Briefly, subjects were screened for absence of detectable antibody to the vaccine viruses, and for general good health. Eligible subjects were admitted to an isolation facility and observed for two days, and then received 107.5 50% tissue culture infective doses (TCID50) of the vaccine virus by intranasal spray in open label fashion. Physical exam and assessment of reactogenicity events was performed daily after inoculation until discharge. The presence of influenza virus in nasal washings was detected by inoculation of Madin-Darby Canine Kidney (MDCK) cells at 33 °c and by real-time reverse transcriptase polymerase chain reaction (rRT-PCR) as previously described [19].
Subjects were discharged from the facility on day 9 after inoculation if they had at least two consecutive negative rRT-PCR tests for virus. Serum for assessment of antibody responses was obtained prior to inoculation and on days 14, 28, and 56 post-inoculation. Recipients of the H7N7 pLAIV returned to the isolation facility on day 26, and received a second dose of vaccine on day 28. Follow-up of these subjects was identical to that after the first dose. Because a previous study had suggested that a single dose of the H7N3 pLAIV was possibly as immunogenic as two doses [12], subjects received only one dose of the H7N3 pLAIV.
Subjects were then invited back approximately 18–24 months following receipt of pLAIV to receive a single booster does of 45 ug ofH7N7 pIIV by intramuscular injection. Their responses were compared to those of an additional cohort of H7 PLAIV naïve subjects who were administered a single dose of the H7N7 pIIV. Serum samples were obtained prior to vaccination, and day 3, day 7, day 14, day 28, day 56, and day 180 post-vaccination. Some subjects also returned at 1 year following pIIV.
2.3. Serology
Sera were tested by hemagglutination-inhibition (HAI) and microneutralization (MN) assays against the vaccine virus and other H7 viruses. HAI assays were performed on sera after treatment with receptor destroying enzyme (RDE, Denka Senken) using .75% or 1% horse erythrocytes [12] with four hemagglutination units of virus. MN assays were performed on MDCI< cells as previously described [20], except that studies using the pLAIV viruses were performed at 33 ° C. Assays using wild-type H7 viruses were conducted under enhanced biosafety level 3 (eBSL-3) conditions at the Centers for Disease Control, Atlanta GA. For serum HAI and MN antibody assays, subjects were defined as responders if they achieved a 4-fold or greater increase in antibody compared to baseline at any time point after vaccination. Samples with a titer of <1 by MN were assigned a value of 5, and samples with an HAI titer of <1:4 were assigned a value of 2 for calculation.
Sera were also tested for HA-specific antibody by enzyme-linked immunosorbent assay (ELISA). 96-well Nunc Maxisorb plates (Thermal Scientific) were coated with purified baculovirus-expressed H7 HA protein from A/Netherlands/219/2003 (BEI Resources) at .25 μg/well and blocked with 5% non-fat dry milk. Sera were tested at a starting dilution of 1/100, and binding was detected with alkaline-phosphatase-conjugated isotype-specific goat anti-human IgG, IgM or IgA antibody (Invitrogen, Frederick MD). The endpoint titer was the highest dilution giving an optical density at least twice that of background. A four-fold increase in titer over baseline was considered a response.
2.4. Analysis of circulating Ab-secreting cells
On days 6, 7, or 8 after each dose of H7N7 PLAIV in the first cohort (15 subjects), circulating IgG Antibody-secreting cells (ASCs) specific for the vaccine and the H7 HA were enumerated by ELISpot assay. The ELISpot assay was performed largely as described previously [21]. Immobilon P membrane-based 96-well plates (Millipore, Billerica, MA) were coated wi th β-propiolactone-inactivated vaccine virus diluted in PBS to 5000 HAU/ml or with recombinant H7 from A/Netherlands/219/2003 (H7N7) (BEI Resources) at 1 μg/ml. PBS only was added to negative control wells. Enriched B cells were resuspended in complete medium containing alkaline phosphatase-conjugated goat anti-human IgG (H + L) (KPL, Gaithersburg, MD) at .2 μg/ml. Serial 2-fold dilutions of the cell suspensions were prepared in coated and blocked plates and incubated overnight. After washing and spot development, spots were counted using a CTL ImmunoSpot plate reader and counting software (Cellular Technology Limited, Cleveland, OH). Spot counts are expressed as a frequency of input CDI 9+ cells. The enriched B cells were also characterized by flow cytometry as described previously [22].
2.5. Statistical analysis
Geometric mean titers (GMTs) for serum antibody tests were determined as the antilog of the average of the logarithmically transformed titers. Mann-Whitney rank sum tests were used to compare the duration of viral shedding between groups, and Fisher Exact Test to compare rates. Because PCR positivity on the day after vaccination could represent input virus, infection was defined as the presence of positive PCR on any day after day 1 [18].
3. Results
A total of 20 subjects were enrolled in the single dose study of A/British Columbia/CN-6/2004 H7N3 pLAlV, and all of these subjects completed the study. The evaluation of the A/Netherlands/219/2003 H7N7 pLAIV was done in two cohorts. In the first cohort, 15 subjects received the first dose, and 13 of these subjects returned for the second dose. In the second cohort, an additional nine subjects received both doses of H7N7 pLAIV.
Approximately 18–24 months after receipt of pLAIV, subjects were contacted and invited to return to participate in the evaluation of the response to a single dose of H7N7 pIIV. In total, five subjects who had previously received a single dose ofH7N3 pLAIV returned and received H7N7 pIIV 22 months after pLAIV. A total of eight subjects from the first cohort of H7N7 pLAIV, including the two subjects who had only received one dose of H7N7 pLAIV, returned and received H7N7 pIIV 18 months after their last dose of pLAIV, while six subjects from the second H7N7 cohort also returned and received pIIV 24 months after their last dose ofpLAIV. The responses of these subjects were compared to those in an additional cohort of 20 H7 naïve subjects who received a single dose of H7N7 pIIV. There were no substantial differences between the demographics of the entire group receiving pLAIV and the subset who were boosted with pIIV, or between primed and unprimed recipients of pIIV (Supplemental Table 1).
3.1. Response to pLAIVs
The frequency and duration of vaccine viral shedding following both pLAIV candidates is shown in Table 1. Shedding of the vaccine virus was primarily detected by rRT-PCR and was of relatively short duration. The frequency of infection, defined as isolation of virus in culture or detection by rRT-PCR after day 1, was significantly higher after the first dose of H7N7 pLAIV compared to H7N3 pLAIV (P = .045, Fisher exact test). The frequency of vaccine virus shedding and evidence of infection was also significantly lower following the second dose of H7N7 vaccine compared to the first dose of H7N7 PLAIV .(P=01 ).
Table 1.
Detection of virus by rRT-PCR or cell culture in subjects following receipt of H7 pLAIVs.
| pLAIV administered | Dose | N | Cell culture | RT-PCR | No. infectedb (%) | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| No. positive (%) | Mean days durationa (SD) | No. positive (%) | Mean days durationa (SD) | ||||
|
| |||||||
| H7N3 | Dose 1 | 20 | 1.(5) | 1.0 | 13(65) | 1.4 (1.0)c | 2 (10)d |
| H7N7 | Dose 1 | 24 | 5.(21) | 3.2 (1.5) | 14 (58)e | 3.8 (3.2)c | 9 (38)d,f |
| Dose 2 | 22 | 0 | 0 | 5 (23)e | 1.2 (.4) | 1 (5)f | |
SD, standard deviation.
Among those with detectable virus on any day. Duration is calculated as the duration in days between virus inoculation and the last day of test positivity. Note that all culture positive samples were also PCR positive.
Infection was defined as shedding of vaccine virus by culture and/or rRT-PCR at any time other than day 1 post vaccination. Since no subjects manifested detectable HAI responses to vaccination, this was not considered in the definition of infection.
P = .024, Mann-Whitney test.
P = .045.
P = .012.
P = .010, Fisher Exact Test.
Serum antibody responses were not detected by HAI and MN following either dose of H7N7 or H7N3 pLAIVs, even at day 56. IgG ELISA responses to the A/Netherlands/03 HA were seen in one of 20 subjects following H7N7 pLAIV and in none of the subjects after H7N3 pLAIV. IgA antibody responses were not detected in any subjects following pLAIV. Sera following pLAIV were not tested for IgM responses.
3.2. Cellular response to H7N7 PLAIV
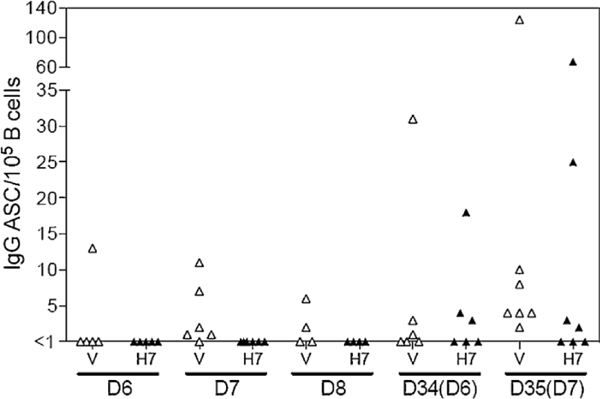
Circulating ASC following H7N7 pLAIV administration were analyzed by ELISpot assay and flow cytometry. On days 6–8 after the first vaccine dose, IgG ASCs specific for vaccine components were present in 8 of 15 subjects, but IgG ASCs specific for the viral H7 HA were not detected (Fig. 1 ). The second dose resulted in vaccine-specific IgG ASCs in most subjects, as well as H7 HA-specific IgG ASCs in 7 of 13 subjects. H7-specific ASC frequencies were relatively high in three of the subjects. Frequencies of circulating plasmablasts (CDI 9+ CD27+ CD38+ cells) measured by flow cytometry were not significantly increased above baseline levels after either dose of H7N7 pLAIV (data not shown).
Fig. 1.
Vaccine virus-specific (V) and H 7 HA-specific (H7) IgG ASC frequencies in peripheral blood following administration of H7N7 pLAIV. Enriched B cells were analyzed by ELISpot assay on days 6, 7, or 8 after the first dose of H7N7 pLAIV, A second dose was administered on day 28 and cells were analyzed on days 34 or 35 (days 6 or 7, respectively, after the second dose). IgG ASC frequencies are expressed as a proportion of CD19+ B cells.
3.3. Response to inactivated H7N7 vaccine
The serum HAI and MN antibody responses following H7N7 pIIV in these subjects are shown in Table 2. One subject in the H7N7 pLAIV primed group is not included in this analysis because he did not return for follow-up visits beyond day 7 following pIIV, although he did not manifest antibody responses up to that time. Despite the lack of detectable response to primary vaccination, strong serum antibody responses to H7N7 were detected by both MN and HAI in 9 of 13 individuals previously primed with H7N7 pLAIV, including the two subjects who had received only one dose of H7N7 pLAIV, with peak titers of equal to or greater than 1:40. A tenth subject had a lower level response detected by MN only. In contrast, none of the subjects primed with a single dose of H7N3 pLAIV, and none of the naïve individuals had a detectable response to the inactivated vaccine by either HAI or MN.
Table 2.
Serum antibody response to the A/Netherlands/219/2003 following administration of a single dose of H7N7 pIIV in naïve subjects, or subjects who previously received two doses of H7N7 PLAIV or one dose of H7N3 pLAlV.
| Priming pLAIV | N | MN | HAI | Serum HA-specific antibody response* by ELISA | ||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Responding* | Titer ≥ 1:40 | Responding* | Titer ≥ 1:40 | IgG ELISA | IgA ELISA | IgM ELISA* | ||
|
| ||||||||
| Number (%) of subjects who responded, and who achieved the indicated post-vaccination titer by the following assays | ||||||||
| H7N7 | 13 | 10.(77)** | 9.(69)** | 9.(69)** | 9.(69)** | 12.(92)** | 8(62) | 0 |
| H7N3 | 5 | 0 | 0 | 0 | 0 | 1 (20) | NT | NT |
| None | 20 | 0 | 0 | 0 | 0 | 11 (55) | NT | NT |
NT, not tested.
Response defined as a four fold increase in titer above baseline at any time point after receipt of H7N7 pIIV.
P < .05 compared to either H7N7 primed or naïve subjects, Fisher Exact Test.
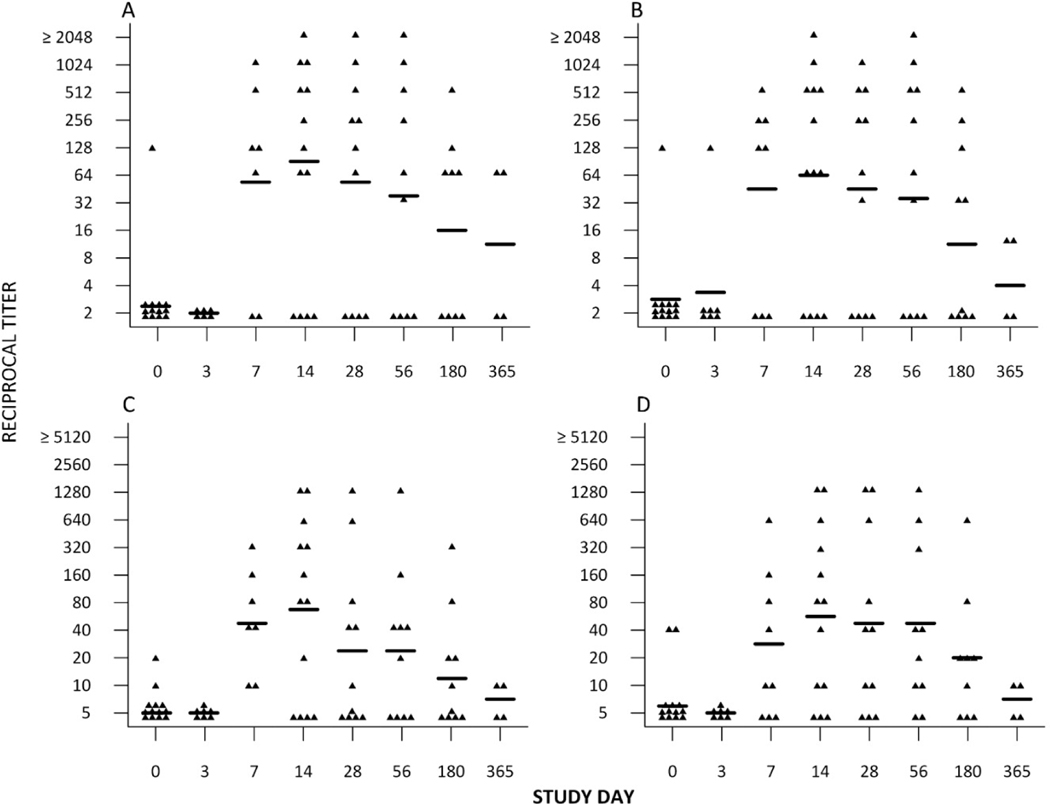
The kinetics of the serum antibody responses in H7N7 PLAIV primed subjects are shown in Fig. 2. The antibody response was rapid, reaching peak titers on day 14 following pIIV, and then declined over time. One year after revaccination with pIIV two of the four subjects tested still had detectable titers against H7N7, although the levels of antibody were low.
Fig. 2.
Kinetics of the (A) hemagglutination-inhibition (HAI) against H7N7 (B) hemagglutination-inhibition (HAI) against H7N3 (C) microneutralization (MN) antibody response against H7N7 (D) microneutralization (MN) antibody response against H7N3 for each subject following inactivated H7N7 vaccine in subjects previously primed with H7N7 pLAIV. GMTs are displayed by the horizontal line. In each graph the assays were done using the H7N7 PLAIV virus or H7N3 PLAIV as the test antigen (see Section 2). Samples with a titer of <4 by HAI are assigned a value of 2, and samples with a titer of <10 by MN are assigned a value of 5
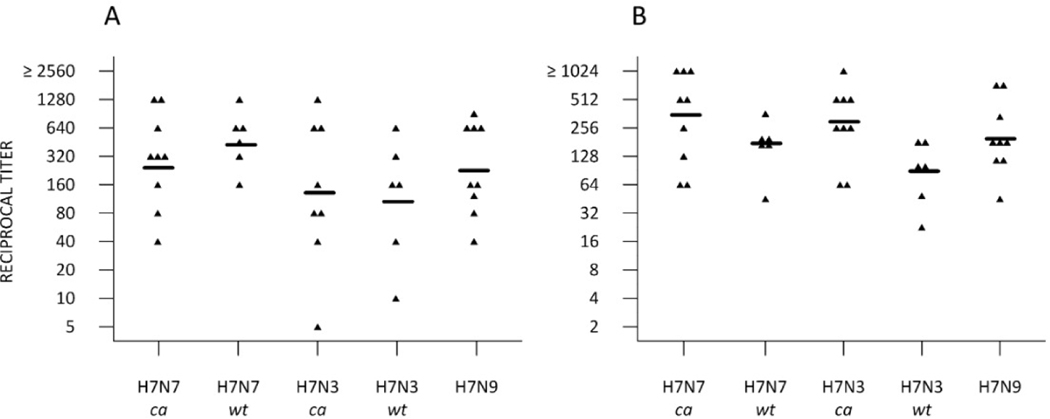
Sera were also tested for reactivity against other H7 variant viruses, including the A/chicken/British Columbia/CN6/2006 wild-type virus (H7N3, North American lineage), the A/mallard/Netherlands/12/2000 wild type virus (H7N3, Eurasian lineage and donor ofthe HA gene for the pIIV) and the human isolate A/Anhui/112013 (H7N9, Eurasian lineage), shown in Fig. 3. Revaccination with H7N7 pIIV resulted in a broadly cross reactive antibody response in H7N7 PLAIV primed subjects that recognized antigenically distinct viruses in both the North American and Eurasian lineages, including the A/Anhui/0112013 (H7N9) virus. Of note, the antibody response to pIIV in subjects previously primed with H7N7 PLAIV also recognized the H7N3 virus, but subjects primed with H7N3 PLAIV did not generate antibody to H7N3 following H7N7 pIIV boosting (data not shown).
Fig. 3.
Cross reactivity of the sera from H7N7 PLAIV primed subjects who received pIIV 18 months following pLAIV. The graph shows the individual titers and the geometric mean titer (GMT) of antibody at day 14 among individuals who responded to the booster vaccine as assessed by microneutralization (A) or hemagglutination Inhibition (B) of sera collected on day 14 post pIIV against the following test antigens: A/Netherlands/219/2003 PLAIV (H7N7 ca), A/Netherlands/219/2003 wild-type virus (H7N7 wt), A/chicken/British Columbia/CN-6/2004 PLAIV (H7N3 ca), Almallard/Netherlands/12/2000 (H7N3 wt), and A/Anhui/1/2013 (H7N9 wt). Tests using the wild-type viruses were performed at the Centers for Disease Control and Prevention.
Serum antibody responses to the A/Netherlands/03 HA were also detected by IgG and IgA ELISA in most H7N7 primed subjects after pIIV boost (Table 2). However, IgM responses were not detected. In addition, HA-specific IgG responses were detected in some naïve recipients of pIIV, although the titers were low. Only H7N7 primed subjects were tested for IgA and IgM responses.
3.4. Relationships between priming and boosting
There was no discernable relationship between the response to H7N7 pLAIV and the subsequent response to H7N7 pIIV. Of the 13 subjects primed with H7N7 pLAIV and subsequently boosted, 7 had shed virus by PCR on one or more days post dose 1, and 6 subjects did not. Of the seven shedders, boosted responses to IIV were seen in four, and in the non-shedders, boosting was seen in five. ASC were detected in 1 of 3 shedders tested and in 4 of 4 non-shedders. Among those in whom B cells were assessed and who were subsequently given pIIV, one of the two subjects that did not manifest an ASC response to PLAIV had a very vigorous response to pIIV and the other did not respond at all. Of the five subjects with ASC detected, there was no relationship between the number of ASC detected after pLAIV and magnitude of the subsequent response to pIIV.
3.5. Safety
Both of the pLAIV and the pIIV were well tolerated (Supplemental Tables 2A and 2B) with a safety profile similar to that of seasonal LAIV. The most commonly reported reactogenicity events following receipt of pLAIV were headache, stuffy nose, and sore throat, all at mild or moderate severity. There were no differences in the reactogenicity of the three pLAIV candidates evaluated in this study, and all of the noted events had resolved by the time the subjects were discharged from the isolation unit. Upper respiratory symptoms were not associated with vaccine virus shedding (data not shown). There were no vaccine-related serious or severe adverse events following any pLAIV candidate.
Inactivated vaccine was associated with mild to moderate pain at the site of administration. All reactogenicity events resolved and there were no serious or severe adverse events noted following pIIV that were related to receipt of vaccine. There were no differences in the rates of reactogenicity events following pIIV in the naïve subjects compared to previously PLAIV vaccinated subjects.
4. Discussion
The ongoing outbreak of severe respiratory disease in China associated with a novel avian influenza H7N9 virus has underscored the pandemic threat of H7 viruses and the need for continued efforts to develop effective H7 subtype vaccines. Therefore, we evaluated two candidate H7 pLAIV vaccines in serologically susceptible adults with no known exposure to avian influenza viruses, under carefully controlled conditions designed to prevent transmission of the vaccine viruses to others.
We found that both of the vaccine viruses could be recovered in the nasal secretions of vaccine recipients following inoculation. However, virus could only be detected at low levels by rRT-PCR and was infrequently isolated in cell culture. In addition, we were unable to detect serum antibody to the respective vaccine viruses by either HAI or MN, even after administration of two doses. These results are generally consistent with the findings of clinical trials of other PLAIV candidates based on the ca A/Ann Arbor/6/60 virus, which have shown low levels of viral replication and modest immunogenicity as measured by HAI and MN assays [12,17,23]. The reasons for the highly restricted replication of these vaccine viruses in humans are not clear, as the PLAIV candidates evaluated in these studies replicate well in cell culture and in animal models [15,24,25]
We found that despite the lack of a detectable serum antibody response to primary immunization, recipients of two doses of H7N7 pLAIV responded vigorously to a subsequent dose of H7N7 pIIV. This response was broad, and included recognition of H7 viruses from both the North American and Eurasian lineages, including the recent H7N9 virus associated with severe disease in humans. The finding of a broad immune response to the booster dose has also been seen in other studies evaluating priming and boosting regimens for pandemic influenza vaccines [26], and would be potentially advantageous for pandemic preparedness because the actual strain that may cause a pandemic cannot be predicted.
In contrast, HAI or MN responses were not seen following a single dose of pIIV in naïve subjects, although some low level IgG responses to the HA were detected by ELISA. The poor immunogenicity of unadjuvanted H7N7 pIIV in naïve subjects is consistent with a previous report, in which less than 10% of naïve subjects responded after two doses of 90 mcg of vaccine [8]. The reasons for this poor immunogenicity are not known, although it has been noted that the vaccine does not form rosettes by electron microscopy and that it does not have hemagglutinating activity, factors which are known to affect the immunogenicity of inactivated influenza vaccines [27]. However, poor immune responses were also noted using a whole virion H7N1 vaccine manufactured in mammalian cell culture [7].
Surprisingly, H7N3 PLAIV primed subjects also did not respond to H7N7 pIIV. The reasons for this are unclear, although the study is limited by the small numbers of subjects in this group. H7N3 primed subjects only received a single dose of pLAIV, but two subjects in the H7N7 group who responded well to boosting also had received only a single dose of pLAIV. The pattern of viral shedding was somewhat less vigorous after the H7N3 pLAIV, although generally there was a poor correlation between vaccine virus shedding and antibody response. Alternatively, the differences could be related to antigenic differences between the Netherlands and British Columbia H7 HAS, which are approximately 86% identical on an amino acid level. In a previous study, post infection ferret antisera following infection with A/British Columbia/CN-7104 (H7N3) virus recognized only North American lineage viruses in neutralization tests, while post infection sera from ferrets infected with the A/Netherlands/2003 (H7N7) virus recognized both North American and Eurasian lineage viruses equally [28].
The results of this study are consistent with those of other studies evaluating prime-boost strategies for pandemic influenza vaccination, including studies in which recipients of unadjuvanted inactivated H5 vaccines have been boosted with subsequent doses of inactivated H5 vaccines [29–31], studies in which subjects who received a DNA vaccine for H5 influenza were boosted with a subsequent dose of inactivated H5N1 vaccine [32,33], and a recent study demonstrating the ability of a two distinct H5 PLAIV candidates to prime for boosting with an inactivated H5 vaccine [14].
The interval between priming and boosting may have an important effect on the boosting response. In studies evaluating two doses of inactivated H5 vaccine, separating the doses by 180 days generated stronger responses than two doses separated by 28 days [31]. With DNA vaccines, separation of the priming and boosting doses by 16 to 24 weeks resulted in stronger responses than intervals of 4 or 8 weeks. In addition, in studies where subjects were primed with two doses of inactivated H5 vaccine separated by 28 days, a booster dose at 6 months resulted in antibody titers that were similar to those achieved with the primary series [34] while a third dose given as late as 8 years after the primary series generated stronger responses [30]. Future studies of the ability of pLAIV to prime for pIIV response will evaluate the effect of time between doses.
We were not able to study the mechanism of the priming effect of PLAIV or determine markers that would predict which subjects would respond well to boosting with pIIV. While ASCs were detected following the second dose of H7N7 PLAIV in most subjects, there was no correlation between the presence or number of H7 specific ASC in peripheral blood following pLAIV and the subsequent response to pIIV. There was also no correlation between the duration or magnitude of vaccine virus shedding and the subsequent B cell response or with the subsequent response to boosting. Overall, the results suggest that pLAIV induced memory responses that are not detected in the assays that were used to assess immunity in these studies. Further studies to evaluate the mechanism by which exposure to pLAIV may prime the immune system and to evaluate the optimal timing and sequence of vaccination should provide useful insights into the immune response to PLAIV and their potential use during an emerging pandemic.
4.1. Study oversight
This open label Phase 1 study (NCT01534468) was performed under an investigational new drug application (BB-IND #14944) reviewed by the US Food and Drug Administration and approved by the Western Institutional Review Board. Informed, witnessed, written consent was obtained from each subject.
Evaluation of the pandemic live attenuated influenza vaccines was performed under Cooperative Research and Development Agreement (CRADA) No. Al-0155 between the NIH Laboratory of Infectious Diseases, and Medlmmune. This study was supported in part by the Division of Intramural Research, NIAID, NIH and by the Biomedical Advanced Research and Development Authority, US Department of Health and Human Services (under contract # HHSN272200900026C).
Supplementary Material
Acknowledgements
The authors acknowledge the efforts of our study nurses, led by study coordinators Doreen Francis and Diane O’Brien, and of Ken Schnabel in the laboratory of Dr. Caren Hall for performance of rtRT-PCR assays. We wish to thank Dr. Feng Liu, Mr. David Wang, and Mrs Bonnie-Dighero-kemp from Influenza Division, Centers for Disease Control and Prevention for conducting the microneutralization and hemagglutination inhibition assays using wild type viruses, and Dr. Diane Post of NIAID for support and helpful suggestions. Monitoring of the clinical studies was performed by the Regulatory Compliance and Human Subjects Protection Branch, NIAID, NIH. Clinical Trials Registration NCT01534468.
This study was supported in part by the Division of Intramural Research, NIAID, NIH and by the Biomedical Advanced Research and Development Authority, US Department of Health and Human Services (under contract # HHSN272200900026C). Preliminary results of this study were presented at the Options for Control of Influenza meeting in Cape Town, South Africa September 2013.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi: 10.1016/j.vaccine.2014.09.070.
Conflict of interest: The authors of the manuscript have no conflict of interest.
References
- [1].Hirst M, Astell CR, Griffith M, Coughlin SM, Moksa M, Zeng T, et al. Novel avian influenza H7N3 strain outbreak. British Columbia Emerg Infect Dis 2004; 10:2192–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tweed SA, Skowronski DM, David ST, Larder A, Petric M, Lees W, et al. Human illness from avian influenza H7N3. British Columbia Emerg Infect Dis 2004; 10:2196–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fouchier RAM, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SAG, Munster V, et al. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A 2004;101 : 1356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koopmans M, Wilbrink B, Conyn M, Natrop G, van der Nat H, Vennema H, et al. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet 2004;363:587–93 [DOI] [PubMed] [Google Scholar]
- 5.Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med 2013;368:1888–97. [DOI] [PubMed] [Google Scholar]
- 6.Li Q Zhou L, Zhou M, Chen Z, Li F, Wu H, et al. Epidemiology of the avian influenza A (H7N9) outbreak in China. N Engl J Med 2014;370:520–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox RJ, Madhun AS, Hauge S, Sjursen H, Major D, Kuhne M, et al. A phase I clinical trial of a PER.C6 cell grown influenza H7 virus vaccine. Vaccine 2009;27:1889–97 [DOI] [PubMed] [Google Scholar]
- 8.Couch RB, Patel SM, Wade-Bowers CL, Nino D. A randomized clinical trial of an inactivated avian influenza A (H7N7) vaccine. PLOS ONE 2012;7:e49704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashkenazi S, Vertruyen A, Aristegui J, Esposito S, McKeith DD, Klemola T, et al. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J 2006;25:870–9. [DOI] [PubMed] [Google Scholar]
- 10.Fleming DM, Crovari P, Wahn U, Klemola T, Schlesinger Y, Langussis A, et al. Comparison ofthe efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect DisJ 2006;25:860–9. [DOI] [PubMed] [Google Scholar]
- 11.Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med 2007;356:685–96 [DOI] [PubMed] [Google Scholar]
- 12.Talaat KR, Karron RA, Callahan KA, Luke CJ, DiLorenzo SC, Chen GL, et al. A live attenuated H7N3 influenza virus vaccine is well tolerated and immunogenic in a Phase I trial in healthy adults. Vaccine 2009;27:3744–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudenko L, Kiseleva I, Naykhin AN, Erofeeva M, Stukova M, Sonina S, et al. Assessment of human immune responses to H 7 avian influenza virus of pandemic potential: results from a placebo-controlled, randomized double-blind phase I study of live attenuated H7N3 influenza vaccine. PLOS ONE 2014;e87962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talaat KR, Luke CJ, Khurana S, Manischewitz J, King LR, McMahon BA, et al. A live attenuated influenza A (H5N1 ) vaccine induces long-term immunity in the absence of a primary antibody response. J Infect Dis 2014;209: 1860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min J-Y, Vogel L, Matsuoka Y, Lu B, Swayne DE, Jin H, et al. A live attenuated H7N7 candidate vaccine virus induces neutralizing antibody that confers protection from challenge in mice, ferrets, and monkeys. J Virol 2010;84: 11950–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jadhao SJ, Achenback J, Swayne DE, Donis R, Cox N, Matsuoka Y. Development of Eurasian H7N7 /PR8 high growth reassortant virus for clinical evaluation as an inactivated pandemic influenza vaccine. Vaccine 2008;26: 1742–50. [DOI] [PubMed] [Google Scholar]
- 17.Karron Ruth A, Callahan K, Luke C, Thumar B, McAuliffe J, Schappell E, et al. A live attenuated H9N2 influenza vaccine is well tolerated and immunogenic in healthy adults. J Infect Dis 2009; 199: 711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karron RA, Talaat KR, Luke CJ, Callahan KA, Thumar B, diLorenzo SC, et al. Evaluation of two live attenuated cold-adapted H5N1 influenza virus vaccines in healthy adults. Vaccine 2009;27:4953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shu B, Wu KH, Emery S, Villanueva J, Johnson R, Guthrie E, et al. Design and performance of the CDC real-time reverse transcriptase PCR swine flu panel for detection of 2009 A (HINI) pandemic influenza virus. J Clin Microbiol 2011;49:2614–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, et al. Detection of antibody to avian influenza A (H5N1 ) virus in human serum by using a combination of serologic assays. J Clin Microbiol 1999;37:937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He XS, Sasaki S, Narvaez CF, Zhang C, Liu HW, Woo JC, et al. Plasmablast-derived polyclonal antibody response after influenza vaccination. J Immunol Methods 2011;365:67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sangster MY, Baer J, Santiago FW, Fitzgerald T, Ilyushina NA, Sundararajan A, et al. The B cell response and hemagglutinin stalk-reactive an tibody production in different age cohorts following 2009 HI NI influenza vaccination. Clin Vac Immunol 2013:867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karron RA, Talaat K, Luke C, Callahan K, Thumar B, DiLorenzo S, et al. Evaluation of two live attenuated cold-adapted H5N1 influenza virus vaccines in healthy adults. Vaccine 2009;27:4953–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suguitan AL Jr, McAuliffe J, Mills KL, Jin H, Duke G, Lu B, et al. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med 2006;3:1541–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Joseph T, McAuliffe J, Lu B, Vogel L, Swayne D, Jin H, et al. A live attenuated cold-adapted influenza A H7N3 virus vaccine provides protection against homologous and heterologous H7 viruses in mice and ferrets. Virology 2008;378:123–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Khurana S, Wu J, Dimitrova M, King LR, Manischewitz J, Graham BS, et al. DNA priming prior to inactivated influenza A (H5N1) vaccination expands the antibody epitope repertoire and increases affinity maturation in a boost-interval-dependent manner in adults. J Infect Dis 2013;208:413–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Couch RB, Decker WK, Utama B, Atmar RL, Nino D, Feng JQ, et al. Evaluations for in vitro correlates of immunogenicity of inactivated influenza A H5, H7, and H9 vaccines in humans. PLoS ONE 2013;7:e50830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Joseph T, McAuliffe J, Lu B, Jin H, Kemble G, Subbarao K. Evaluation of replication and pathogenicity of avian influenza A H7 subtype viruses in a mouse model. J Virol 2007;81 : 10558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephenson I, Nicholson KG, Colegate AE, Podda A, Wood JM, Yprna E, et al. Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. Vaccine 2003;21:1687–93 [DOI] [PubMed] [Google Scholar]
- 30.Goji NA, Nolan C, Hill H, Wolff M, Rowe T, Treanor JJ. Immune responses of healthy subjects to a single dose of intramuscular inactivated influenza A/Vietnam/1203/04 (H5N1) vaccine after priming with an antigenic variant. J Infect Dis 2008;198:635–41 [DOI] [PubMed] [Google Scholar]
- 31.Belshe RB, Frey SE, Graham I, Mulligan MJ, Edupuganti S, Jackson LA, et al. Safety and immunogenicity of infl uenza A H5 subunit vaccines: effect of vaccine schedule and antigenic variant. J Infect Dis 2011. ;203:666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ledgerwood JE, Wei C-J, Hu Z, Gordon IJ, Enama ME, Hendel CS, et al. DNA priming and influenza vaccine immunogenicity: two phase I open label randomised clinical trials. Lancet Infect Dis 2011. ; 11 :916–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ledgerwood JE, Zephir K, Hu Z, Wei C-J, Chang L, Enama ME, et al. Prime-boost interval matters: a randomized phase I study to identify the minimum interval necessary to observe the H5 DNA influenza vaccine priming effect. J Infect Dis 2013;354:418–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zangwill KM, Treanor JJ, Campbell JD, Noah DL, Ryea J. Evaluation of the safety and immunogenicity of a booster (third) dose of inactivated subvirion H5N1 influenza vaccine in humans. J Infect Dis 2008;197:580–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.