Abstract
Ten human immunodeficiency virus-infected patients were given rifabutin in addition to fluconazole and clarithromycin. There was a 76% increase in the area under the concentration-time curve of rifabutin when either fluconazole or clarithromycin was given alone and a 152% increase when both drugs were given together with rifabutin. Patients should be monitored for adverse effects of rifabutin administered concomitantly with clarithromycin and/or fluconazole.
The potential for drug interactions is high in human immunodeficiency virus (HIV)-infected patients treated with multiple medications. Rifabutin has been well documented to cause uveitis and arthralgias when high doses are prescribed or when high levels in plasma are attained (8). The objective of this study was to characterize the pharmacokinetics of rifabutin in the presence of fluconazole, clarithromycin, or both agents in combination.
Data were obtained from stored samples collected during an earlier study that evaluated the pharmacokinetics of stavudine in the presence of multiple medications for opportunistic infections (7). The study was an open-label, sequential, randomized trial that enrolled 10 HIV-infected patients with a CD4 count of less than 200 cells/mm3, who were at least 18 years old and had no clinically significant renal or hepatic impairment. The study was reviewed by the National Institute of Allergy and Infectious Diseases Institutional Review Board, and all patients provided written informed consent. Patients received Infectious Diseases Society of America-Centers for Disease Control and Prevention-recommended doses of either trimethoprim-sulfamethoxazole or aerosolized pentamidine for prophylaxis of Pneumocystis carinii pneumonia (10). Exclusion criteria included a history of allergies or adverse reactions to the study medications, a history of peripheral neuropathy or uveitis, laboratory values outside of protocol guidelines, persistent diarrhea or malabsorption, the use of agents within 10 days of the study known to influence renal or hepatic metabolism, or the use of drugs and alcohol which could impair safety or compliance.
Four drug regimens were given at constant doses: (i) rifabutin (300 mg once a day [q.d.]) alone, (ii) rifabutin and fluconazole (200 mg q.d.), (iii) rifabutin and clarithromycin (500 mg q.d.), and (iv) rifabutin, fluconazole, and clarithromycin. After receiving rifabutin alone, patients were randomly assigned to take each of the two-drug combinations for 7 days. The triple combination was always administered last. On the seventh day of each regimen, serial blood samples were collected over 8 h with sampling times of 0 h (predose) and 0.5, 1, 2, 4, 6, and 8 h postdose. Each regimen was followed by a 1-week wash-out period. All patients received each of the four regimens during the course of the study. The patients were allowed to take all medications with food.
Rifabutin and 25-O-desacetylrifabutin concentrations in plasma were determined at Pharmacia & Upjohn laboratories (Kalamazoo, Mich.) using a validated high-performance liquid chromatography method (5). The interday and intraday coefficients of variance were less than 10%, and the lower limit of quantification was 5 ng/ml for both compounds.
Compartmental and noncompartmental analyses were performed on rifabutin concentration-time data. Data were fit by a two-compartment model that included a lag time for oral absorption. Prior literature was used to aid in model construction and parameter estimation (6). Due to the limited number of concentration-time points and short duration of sampling, the data were fit using Bayesian estimation with the ADAPT II pharmacokinetic program (3). The AUC0–24 represents area under the fitted concentration-versus-time curve from 0 to 24 h at steady state. The maximum concentration of drug in serum (Cmax), the minimum concentration of drug in serum (Cmin), and the time to Cmax (Tmax) were determined using standard noncompartmental methods.
Differences in pharmacokinetic parameters of rifabutin and 25-O-desacetylrifabutin were determined by a two-way analysis of variance with SYSTAT, version 6.0 (SPSS Inc., Chicago, Ill.). Data were log transformed for statistical analysis. Post-hoc pairwise comparisons were performed using Tukey's honestly significant difference test. A P value of <0.05 was considered significant.
Ten patients (9 male and 1 female), with an average age of 39 years (range, 30 to 49), were enrolled in and completed the study. The mean baseline CD4 cell count, percent CD4 cells, and viral load were 62 cells/mm3 (range, 9 to 143), 6% (range, 1 to 14), and 233,727 HIV RNA copies/ml (range, <10,000 to 608,000), respectively. No significant alterations were observed in hepatic or renal function during the study.
Since rifabutin is usually dosed as a once-daily regimen, our 8-h sampling would not yield concentrations that are reflective of levels in plasma for the entire dosing interval. A Bayesian approach was applied to simulate a 24-h dosing interval, using a model with parameters for rifabutin from a previous study. Using this model and the previous data (6) as Bayesian priors, we could achieve excellent fits of our data and accurately estimate a 24-h AUC. Rifabutin disposition was well described by a two-compartment model with a lag time for absorption. The median r2 value was 0.94 for the individual fits, and the plot of observed versus fitted concentrations had a slope of 1.05 and an intercept not significantly different from zero.
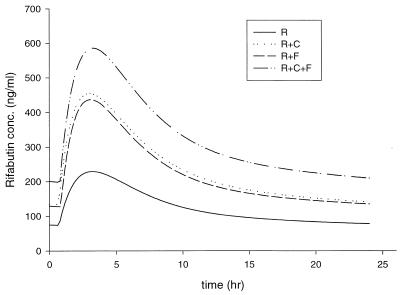
Pharmacokinetic parameters of rifabutin are shown in Table 1. Figure 1 illustrates the mean fitted concentration in plasma as a function of time for each regimen of rifabutin administered. When rifabutin was given as a two-drug combination with either fluconazole or clarithromycin, the AUC increased 76% compared to that of rifabutin alone (P = nonsignificant). The AUC of rifabutin with fluconazole and clarithromycin significantly increased, 152%, from baseline (P = 0.002).
TABLE 1.
Pharmacokinetics of rifabutin in combination with clarithromycin and fluconazole
| Drug regimena | Cmax (ng/ml) | Cmin (ng/ml) | AUC0–24 h (ng · h/ml) | Tmax (h) |
|---|---|---|---|---|
| R | 244.9 ± 91 | 53.3 ± 49 | 3,109 ± 1,402 | 4.3 ± 1.8 |
| R + F | 468.6 ± 206 | 138.9 ± 101 | 5,463 ± 2,750 | 3.2 ± 1.4 |
| R + C | 452.2 ± 201 | 140.6 ± 82 | 5,476 ± 2,852 | 3.6 ± 2.1 |
| R + F + C | 609.5 ± 284 | 214.8 ± 146 | 7,852 ± 3,907 | 4.0 ± 1.9 |
R, rifabutin; F, fluconazole; C, clarithromycin.
FIG. 1.
Fitted concentration-time profiles of rifabutin (R) in combination with clarithromycin (C) and/or fluconazole (F).
Other pharmacokinetic parameters of rifabutin (Cmax, Cmin, and tmax) were determined using noncompartmental methods. The Cmax of rifabutin increased 91% when given with fluconazole, 85% when given with clarithromycin, and 149% when both fluconazole and clarithromycin were given. There were no significant changes demonstrated in Tmax between regimens.
The limited sampling scheme and prolonged half-life did not allow for calculation of a steady-state AUC0–24 for 25-O-desacetylrifabutin. Peak concentrations and troughs (collected at time zero on days of pharmacokinetics measurements) were evaluated. Compared to that of with rifabutin alone, the Cmax of 25-O-deacetylrifabutin combined with other drugs was significantly increased, i.e., 3.7-fold with clarithromycin, 3.6-fold with fluconazole, and 4.9-fold with the combination of all three agents (P < 0.005). Cmins significantly increased, 5.3- and 8.1-fold, with clarithromycin and the combination, respectively (P < 0.05). A nonsignificant (2.3-fold) increase was observed with fluconazole.
Drug interaction studies examining two-drug combinations may not reflect the current clinical practice of HIV therapy, and such studies cannot necessarily be extrapolated to patients on multiple medications. Three drugs that are often used in the management of opportunistic infections are rifabutin, fluconazole, and clarithromycin. Rifabutin is both an inducer and a substrate of the cytochrome P450 drug metabolism system, specifically the CYP3A4 isoform. Fluconazole and clarithromycin are both inhibitors of CYP3A4. Given the shared metabolic pathways, there is great potential for clinically significant interactions of these three agents.
Our data showing a 76% increase in the rifabutin AUC when given with fluconazole and clarithromycin are consistent with previous studies (4, 9). The evaluation of the concurrent use of three agents is rare, and it is difficult to predict whether there will be an additive, synergistic, or saturable effect on concentrations in plasma. In this study, we have demonstrated that the administration of fluconazole and clarithromycin together appears to have additive effects on rifabutin concentrations.
Sun and colleagues reported that increased rifabutin concentrations due to concomitant administration with the CYP3A4 inhibitor ritonavir resulted in a significant increase in arthralgias, joint stiffness, and uveitis compared to the appearance of these conditions in a group of patients receiving rifabutin alone (E. Sun, M. Health-Chiozzi, D. W. Cameron, A. Hsu, R. G. Granneman, C. J. Maurath, and J. M. Leonard, XI Int. Conf. AIDS, abstr. Mo. B. 171, 1996). The incidence of leukopenia was 38% in those receiving the combination and 19% in patients receiving only rifabutin. The mean AUC0–24 of rifabutin when combined with ritonavir has been reported to be similar to that in our study for rifabutin combined with clarithromycin and fluconazole (8,362 ± 2,229 ng · h/ml versus 7,852 ± 3,907 ng · h/ml), suggesting that administration of rifabutin with clarithromycin and fluconazole would be associated with significant adverse effects (2).
The 25-O-desacetyl metabolite was also markedly increased in this study, with peak concentrations fivefold higher with the triple-drug combination than with rifabutin alone. This metabolite has been reported to have antimicrobial activity similar to that of rifabutin, but it normally contributes approximately 10% of total activity because of its lower AUC (1). Although its formation is not dependent upon CYP3A4, its metabolism may be CYP3A4-mediated, resulting in the large concentrations observed here and with other CYP3A4 inhibitors (2).
Our results demonstrate that rifabutin concentrations may be increased with concomitant administration of fluconazole or clarithromycin and that even higher concentrations are achieved with the three-drug combination. Thus, the likelihood of adverse effects such as uveitis, rash, bone marrow toxicity, or increased values in liver function tests may be increased. Clinicians should be aware of the possibility of an additive drug interaction when these agents are given concurrently, and they should monitor patients for adverse effects.
Acknowledgments
We acknowledge the contributions of Keith Rodvold.
REFERENCES
- 1.Blaschke T F, Skinner M H. The clinical pharmacokinetics of rifabutin. Clin Infect Dis. 1996;22(Suppl. 1):S15–S22. [PubMed] [Google Scholar]
- 2.Cato A, Cavanaugh J, Shi H, Hsu A, Leonard J, Granneman R. The effect of multiple doses of ritonavir on the pharmacokinetics of rifabutin. Clin Pharmacol Ther. 1998;63:414–421. doi: 10.1016/S0009-9236(98)90036-4. [DOI] [PubMed] [Google Scholar]
- 3.D'Argenio D Z, Schumitzky A. ADAPTII user's guide. Los Angeles: Biomedical Simulations Resource, University of Southern California; 1990. [Google Scholar]
- 4.Hafner R, Bethel J, Power M, Landry B, Banach M, Mole L, Standiford H C, Follansbee S, Kumar P, Raasch R, Cohn D, Mushatt D, Drusano G. Tolerance and pharmacokinetic interactions of rifabutin and clarithromycin in human immunodeficiency virus-infected volunteers. Antimicrob Agents Chemother. 1998;42:631–639. doi: 10.1128/aac.42.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau Y Y, Hanson G D, Carel B J. Determination of rifabutin in human plasma by high-performance liquid chromatography with ultraviolet detection. J Chromatogr B. 1996;676:125–130. doi: 10.1016/0378-4347(95)00423-8. [DOI] [PubMed] [Google Scholar]
- 6.Li R C, Narang P K, Poggesi I, Strolin-Benedetti M. A model based assessment of redistribution dependent elimination and bioavailability of rifabutin. Biopharm Drug Dispos. 1996;17:223–236. doi: 10.1002/(SICI)1099-081X(199604)17:3<223::AID-BDD954>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 7.Piscitelli S C, Kelly G, Walker R E, Kovacs J, Falloon J, Davey R T, Jr, et al. A multiple drug interaction study of stavudine with agents for opportunistic infections in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 1999;43:647–650. doi: 10.1128/aac.43.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shafran S D, Singer J, Zarowny D P, Phillips P, Salit I, Walmsley S L, Fong I W, Gill M J, Rachlis A R, Lalonde R G, Fanning M M, Tsoukas C M. A comparison of two regimens for the treatment of Mycobacterium avium complex bacteremia in AIDS: rifabutin, ethambutol, and clarithromycin versus rifampin, ethambutol, clofazimine, and ciprofloxacin. N Engl J Med. 1996;335:377–383. doi: 10.1056/NEJM199608083350602. [DOI] [PubMed] [Google Scholar]
- 9.Trapnell C B, Narang P K, Li R, Lavelle J P. Increased plasma rifabutin levels with concomitant fluconazole therapy in HIV-infected patients. Ann Intern Med. 1996;124:573–576. doi: 10.7326/0003-4819-124-6-199603150-00006. [DOI] [PubMed] [Google Scholar]
- 10.USPHS/IDSA Prevention of Opportunistic Infections Working Group. USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. Morbid Mortal Wkly Rep. 1999;48:1–66. [PubMed] [Google Scholar]