Abstract
This study uses the National Cancer Database to examine treatment patterns and survival outcomes in women age ≥80 with invasive breast cancer including molecular subtype analysis. There is improved overall survival in those who received surgery, demonstrating the importance of surgical resection even with increasing age.
Background:
There are no established treatment guidelines for women with breast cancer aged ≥80 despite increasing representation in the US population. Here we identify national treatment patterns and survival outcomes in women with stage I-III invasive breast cancer.
Patients and Methods:
Women age ≥80 diagnosed with stage I-III invasive breast cancer (IBC) were identified from 2005–2014 in the National Cancer Database. χ2, Fisher’s exact test, and logistic regression models were used to identify factors influencing receipt of breast surgery, and Cox proportional hazard models were used to evaluate overall survival (OS).
Results:
A total of 62,575 women with IBC met inclusion criteria, of which the majority received surgery (94%). Receipt of surgery was associated with White race, age < 90, lower stage, and fewer comorbidities. OS was higher for those who received surgery compared to those who did not (HR 3.3 [3.18–3.46] P < .001). Molecular subtype analysis demonstrated improved survival with receipt of surgery or radiation for all subtypes, as well as improved survival with chemotherapy for those with triple negative breast cancer.
Conclusion:
The vast majority of breast cancer patients aged ≥80 in the National Cancer Database with IBC received primary surgical management, which was associated with a significant OS benefit. Due to this finding, surgical resection should be considered for all patients ≥80 who are suitable operative candidates.
Keywords: Geriatric oncology, Breast surgery, Invasive breast cancer, Triple negative breast cancer, Survival, Treatment patterns
Introduction
While guidelines exist for the treatment of women with breast cancer, they may not be universally applicable to older women with differing disease characteristics and clinical considerations. Older women are often excluded from clinical trials and other study cohorts1–3 and there is a paucity of clinical data regarding treatment patterns and outcomes in women age ≥80, resulting in a lack of management guidelines for this population.4 The incidence of breast cancer rises with age, and the risk of death from breast cancer increases significantly after age 80.2 With a growing aged population, there is a rising number of older women diagnosed with breast cancer and therefore a need for greater insight into appropriate disease management in this population.
Key differences exist in tumor biology and presentation between older breast cancer patients and those who present during the time frame indicated for screening mammography. Compared with the cohort typically diagnosed by screening mammography, older women are more likely to be diagnosed on physical exam and present with larger tumors. This finding is likely due to the fact that screening mammograms are often not recommended for women over the age of 70.5–8 However, tumors in older women are more often lower grade and hormone-sensitive,9,10 and therefore are typically more indolent and respond well to endocrine therapy. Yet, this population continues to be both under and over-treated and there is limited evidence on treatment patterns and survival outcomes.
Using the National Cancer Database (NCDB), we investigated treatment patterns including receipt of surgery, radiation therapy, and systemic therapy in women age ≥80 with stage I-III invasive breast cancer (IBC). This study aims to analyze and describe the nationwide treatment patterns and outcomes in women age ≥80 with invasive breast cancer in a contemporary cohort, examine overall survival (OS) outcomes, and assess if a survival advantage exists for those patients who underwent surgical resection.
Methods
A retrospective review of the NCDB was performed. The NCDB is a nation-wide, facility-based, comprehensive clinical oncology data set and a joint project of the American Cancer Society and the Commission on Cancer of the American College of Surgeons, which captures approximately 70% of all newly diagnosed malignancies in the US annually.11 NCDB data is de-identified and this investigation was deemed exempt by our Institutional Review Board.
Women age ≥80 with stages I-III breast cancer diagnosed from 2005 to 2014 were identified. Pathological stage group was used; however, clinical stage group was used when surgery was not performed. The cohort was limited to women for whom breast cancer was their only documented malignancy and included only patients who received most, or all, of their treatment at the reporting facility (Figure 1). Women with evidence of metastatic disease were excluded, including clinical or pathologic stage M1, and those who received radiation directed outside of the breast, chest wall, or regional lymph nodes. Additionally, patients with International Classification of Disease for Oncology histology codes that did not match IBC histology were excluded. Patients with inflammatory breast carcinoma, phyllodes tumors, and Paget’s disease without underlying carcinoma were also excluded.
Figure 1.
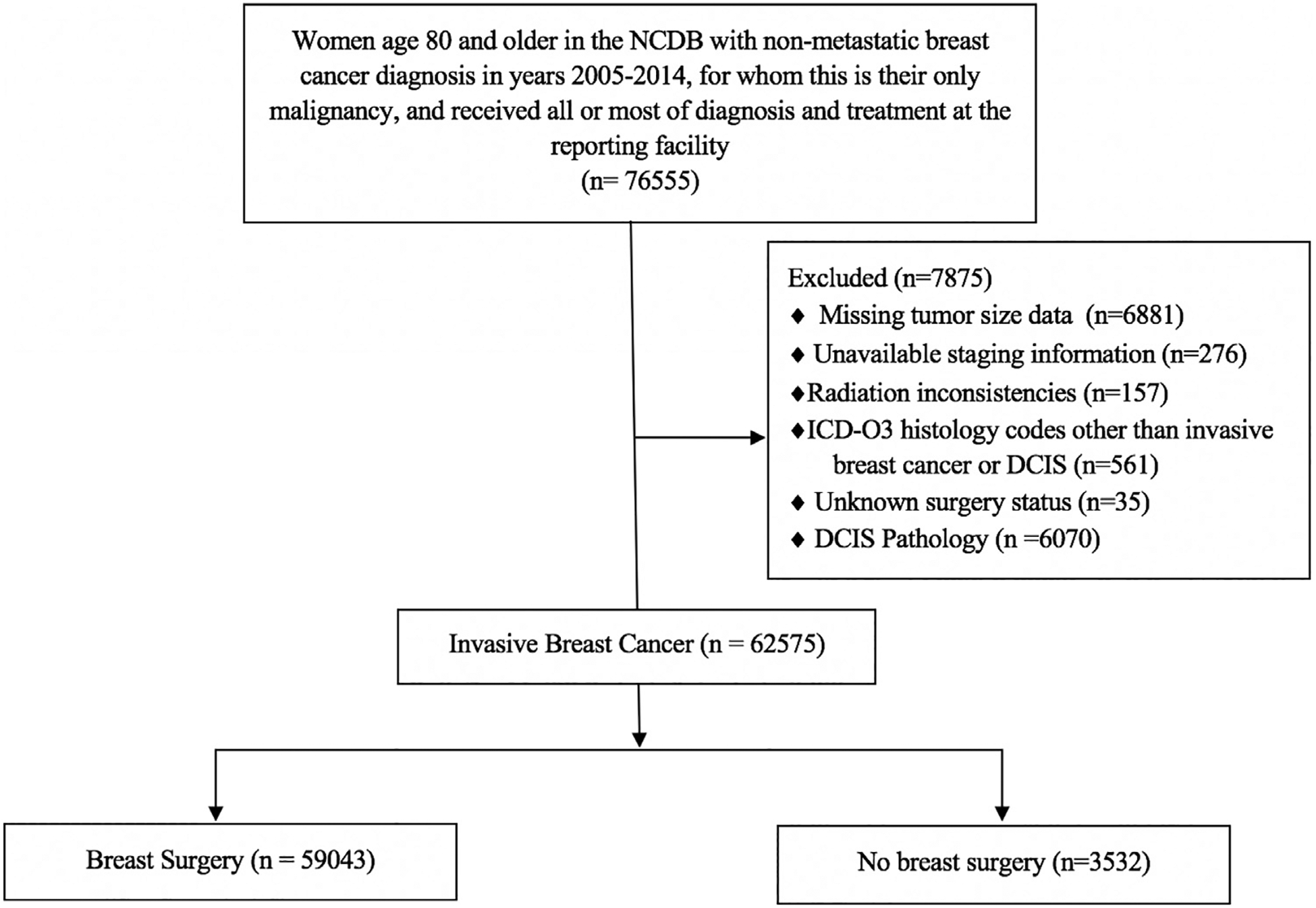
Flow chart of cohort inclusion and exclusion criteria for evaluation of women with breast cancer aged 80 and older in the National Cancer Database.
Demographic information was collected including race/ethnicity, age, Charlson-Deyo comorbidity score, and care facility type.11 Tumor characteristics examined included tumor grade, estrogen receptor (ER) and progesterone receptor status (PR), human epidermal growth factor receptor 2 (HER2) status, tumor size, and lymph node status. Treatment variables included type of surgery and receipt of chemotherapy, radiation therapy, endocrine therapy, and/or HER2-directed therapy (trastuzumab).
Tumor molecular subtype was determined using receptor status, given that tumor-genomic analysis was not available for hormone receptor (HR)+ patients in this dataset. Patients were subdivided into four groups: Luminal A (HR+, HER2−) (n = 17,728), Luminal B (HR+, HER2+) (n = 1,274), HER2+ (HR−, HER2+) (n = 702), and triple negative breast cancer (TNBC) (HR−, HER2−) (n = 2,664).12 Of note, HER2 status only became available in the NCDB in 2010; therefore, it was only available for 45% of patients in our IBC cohort. HER2 status was available for 95% of invasive patients in years 2010 to 2014 but included 768 (2.7%) coded as “borderline” and thus were excluded from subtype analysis. Similarly, hormone-receptor status was available for 95.9% of patients from 2005 to 2014, but only those from 2010 to 2014 were included in this sub-analysis.
Additionally, we gathered data regarding axillary lymph node analysis in this cohort. Specific data regarding type of axillary surgery was not available for all patients in our cohort, however it has been previously published using the NCDB that those with ≥10 nodes pathologically examined likely underwent axillary dissection while it may be unclear for those with 1 to 9 nodes examined. However, this likely indicates sentinel node biopsy or unspecified axillary surgery.13 Documentation of zero nodes examined indicates omission of axillary evaluation or failed axillary surgery, including possible failed axillary mapping or aborted surgery for other reasons.
To identify factors influencing receipt of surgery, χ2 test, Fisher’s exact test, and logistic regression models were used. Cox proportional hazards models were used to evaluate OS.
Results
The final cohort included 62,575 patients (Table 1). The majority (81%) were White, age 80 to 84 at diagnosis (58%), and were healthy, defined as a Charlson-Deyo score of 0 or 1 (95%). Over half had stage I disease (51%), and most tumors were <2 cm (59%), grades 1 and 2 (69%), and ER-positive (83%). Twenty-one percent (21%) had nodal involvement. Most patients (94%) received breast surgery, of which 63% underwent lumpectomy. Of those who reported a reason for not undergoing surgery, most (48%) cited patient refusal. Median follow-up was 48 months for those who received surgery and 24 months for those who did not. Those who received breast surgery were more likely to be ER-positive (P = .001), HER2-negative (P < .001), and healthier, with a Charlson-Deyo comorbidity score of 0 or 1 (P < .001, Table 1).
Table 1.
Characteristics of Women Aged ≥80 With IBC by Receipt of Surgery
| Characteristic | Total n = 62575 (%) | Surgery n = 59043 (%) (94.4%) | No Surgery n = 3532 (%) (5.6%) | P value |
|---|---|---|---|---|
| Survival/Follow-up | ||||
| Median Survival (y) | 6.4 | 6.7 | 2.4 | |
| Average follow-up (y) | ||||
| Mean | 4.4 | 4.4 | 2.5 | |
| Median | 3.9 | 4.0 | 2.0 | |
| Demographic | ||||
| Race/Ethnicity | < .0001 | |||
| NH White | 50838 (81.2) | 48233 (81.7) | 2605 (73.8) | |
| NH Black | 4377 (7.0) | 3914 (6.6) | 463 (13.1) | |
| Hispanic | 1742 (2.8) | 1612 (2.7) | 130 (3.7) | |
| Other | 5618 (9.0) | 5284 (8.9) | 334 (9.5) | |
| Age | < .0001 | |||
| 80–84 | 36244 (57.9) | 35057 (59.4) | 1187 (33.6) | |
| 85–89 | 19065 (30.5) | 17823 (30.2) | 1242 (35.2) | |
| 90+ | 7266 (11.6) | 6163 (10.4) | 1103 (31.2) | |
| Cancer characteristics | ||||
| Tumor size (cm) | < .0001 | |||
| No mass found | 54 (0.1) | 19 (0.03) | 35 (1.0) | |
| Microinvasion | 341 (0.5) | 335 (5.7) | 6 (0.2) | |
| ≤ 2 cm | 36876 (58.9) | 35677 (60.4) | 1199 (33.9) | |
| > 2–5 cm | 21040 (33.6) | 19385 (32.8) | 1655 (46.9) | |
| > 5 cm | 4197 (6.7) | 3593 (6.1) | 604 (17.1) | |
| Node status | < .0001 | |||
| Positive | 13333 (21.3) | 13010 (22.0) | 323 (9.1) | |
| Negative | 32491 (51.9) | 32402 (54.9) | 89 (2.5) | |
| Unknown/Not examined | 16751 (26.8) | 13631 (23.1) | 3120 (88.3) | |
| Stage | < .0001 | |||
| I | 32174 (51.4) | 31264 (53.0) | 910 (25.8) | |
| II | 21414 (34.2) | 19977 (33.8) | 1437 (40.7) | |
| III | 6704 (10.7) | 5950 (10.1) | 754 (21.3) | |
| Grade | < .0001 | |||
| 1 | 15441 (24.7) | 14835 (25.1) | 606 (17.2) | |
| 2 | 27698 (44.3) | 26329 (44.6) | 1369 (38.8) | |
| 3 | 15091 (24.1) | 14422 (24.4) | 669 (18.9) | |
| Unknown | 4345 (6.9) | 3457 (5.9) | 888 (25.1) | |
| Estrogen receptor | .001 | |||
| Positive | 51861 (82.9) | 49149 (83.2) | 2712 (76.8) | |
| Negative | 8563 (13.7) | 8196 (13.9) | 367 (10.4) | |
| Borderline | 54 (0.1) | 52 (0.1) | 2 (0.1) | |
| Missing/unknown | 2097 (3.4) | 1646 (2.8) | 451 (12.8) | |
| Progesterone receptor | .0658 | |||
| Positive | 43907 (70.2) | 41625 (70.5) | 2282 (64.6) | |
| Negative | 16156 (25.8) | 15387 (26.1) | 769 (21.8) | |
| Borderline | 290 (0.5) | 272 (0.5) | 18 (0.5) | |
| Missing/unknown | 2222 (3.6) | 1759 (3.0) | 463 (13.1) | |
| HER2 | < .0001 | |||
| Positive | 2766 (4.4) | 2603 (4.4) | 163 (4.6) | |
| Negative | 24748 (39.5) | 23608 (40.0) | 1140 (32.3) | |
| Borderline | 768 (1.2) | 701 (1.2) | 67 (1.9) | |
| Missing/unknown | 34293 (54.8) | 32131 (54.4) | 2162 (61.2) | |
| Diagnosis/Treatment | ||||
| Diagnosis method | < .0001 | |||
| Biopsy | 49742 (79.5) | 46626 (79.0) | 3116 (88.2) | |
| No biopsy | 12477 (19.9) | 12078 (20.5) | 399 (11.3) | |
| Unknown | 356 (0.6) | 339 (0.6) | 17 (0.5) | |
| Breast surgery | ||||
| Lumpectomy | 36877 (58.9) | 36877 (62.5) | - | |
| Mastectomy | 22150 (35.4) | 22150 (37.5) | - | |
| No surgery | 3532 (5.6) | 0 (0) | - | |
| Unknown type | 16 (0.0) | 16 (0.03) | - | |
| Axillary surgery | < .0001 | |||
| Yes | 45542 (72.8) | 45336 (76.8) | 206 (5.8) | |
| No | 16946 (27.1) | 13632 (23.1) | 3314 (93.8) | |
| Unknown | 87 (0.1) | 75 (0.1) | 12 (0.3) | |
| Nodes examined | < .0001 | |||
| 0 | 16556 (26.5) | 13497 (22.9) | 3059 (86.6) | |
| 1–9 | 35373 (56.5) | 35218 (59.6) | 155 (4.4) | |
| 10+ | 9628 (15.4) | 9610 (16.3) | 18 (0.5) | |
| Unknown | 1018 (1.6) | 718 (1.2) | 300 (8.5) | |
| Reason no surgerya | ||||
| Contraindicated | - | 570 (29.1) | ||
| Died prior to treatment | - | 49 (2.5) | ||
| Recommended, not given | - | 57 (2.9) | ||
| Patient refusal | - | 948 (48.3) | ||
| Other | - | 338 (17.2) | ||
| Chemotherapy | .0009 | |||
| Yes | 3600 (5.8) | 3440 (5.8) | 160 (4.5) | |
| No | 56661 (90.5) | 53390 (90.4) | 3271 (92.6) | |
| Unknown | 2314 (3.7) | 2213 (3.7) | 101 (2.9) | |
| Reason No Chemotherapya | < .0001 | |||
| Contraindicated | 4836 (41.0) | 4522 (41.4) | 314 (36.4) | |
| Died prior to treatment | 77 (0.7) | 56 (0.5) | 21 (2.4) | |
| Recommended, not given | 754 (6.4) | 705 (6.5) | 49 (5.7) | |
| Patient refusal | 6114 (51.9) | 5636 (51.6) | 478 (55.5) | |
| Endocrine therapy | .0022 | |||
| Yes | 33033 (52.8) | 31059 (52.6) | 1974 (55.9) | |
| No | 27307 (43.6) | 25834 (43.8) | 1473 (41.7) | |
| Unknown | 2235 (3.6) | 2150 (3.6) | 85 (2.4) | |
| Reason No Endocrine Therapya | < .0001 | |||
| Contraindicated | 2769 (31.1) | 2708 (31.7) | 61 (17.0) | |
| Died prior to treatment | 158 (1.8) | 129 (1.5) | 29 (8.1) | |
| Recommended, not given | 690 (7.8) | 667 (7.8) | 23 (6.4) | |
| Patient refusal | 5279 (59.3) | 5034 (59.0) | 245 (68.4) | |
| Radiation therapy | < .0001 | |||
| Yes | 21616 (34.5) | 21439 (36.3) | 177 (5.0) | |
| No | 40122 (64.1) | 36806 (62.3) | 3316 (93.9) | |
| Unknown | 837 (1.3) | 798 (1.4) | 39 (1.1) | |
| Reason no Radiation Therapya | < .0001 | |||
| Contraindicated | 2328 (27.5) | 2108 (27.1) | 220 (31.9) | |
| Died prior to treatment | 0 (0) | 0 (0) | 0 (0) | |
| Recommended, not given | 532 (6.3) | 503 (6.5) | 29 (4.2) | |
| Patient refusal | 5620 (66.3) | 5179 (66.5) | 441 (63.9) | |
| Socioeconomic | ||||
| Comorbidity score | < .0001 | |||
| 0 | 47937 (76.6) | 45276 (76.7) | 2661 (75.3) | |
| 1 | 11280 (18.0) | 10726 (18.2) | 554 (15.7) | |
| 2 | 2597 (4.2) | 2373 (4.0) | 224 (6.3) | |
| 3+ | 761 (1.2) | 668 (1.1) | 93 (2.6) | |
| Facility typeb | < .0001 | |||
| CCP | 8454 (13.5) | 8046 (13.6) | 408 (11.6) | |
| CCCP | 32777 (52.4) | 31136 (52.7) | 1641 (46.5) | |
| ARP | 14687 (23.5) | 13546 (22.9) | 1141 (32.3) | |
| INCP | 6657 (10.6) | 6315 (10.7) | 342 (9.7) |
Reported percentages are percent of total available answers in scarcely reported variables
Abbreviations : ARP = academic/research program; CCP = community cancer program; CCCP = comprehensive community cancer program; INCP = integrated network cancer program
When compared to academic programs, surgical management of IBC was more likely to be performed in community cancer centers (P < .001, Table 1). Receipt of breast surgery was significantly associated with receipt of surgery to the axilla (P < .001) – of those who underwent breast surgery, 77% underwent surgical lymph node evaluation. The majority of patients who had breast surgery (60%) had 1 to 9 nodes examined, likely indicating sentinel node biopsy or unspecified axillary surgery; 16% had ≥10 nodes examined and likely underwent axillary dissection, and 23% reported 0 nodes examined, indicating omission of axillary evaluation or failed axillary surgery13, including possible failed axillary mapping or aborted surgery for other reasons (Table 1).
Although chemotherapy was omitted for the vast majority (90%) of patients, those who received surgery were also more likely to receive chemotherapy (P = .0009). Patient refusal (52%) and contraindication (41%) were the most commonly cited reasons for chemotherapy omission. Of patients with hormone receptor-positive tumors, 62% received endocrine therapy. When a reason was cited for omission of endocrine treatment, “patient refusal” was the most common reason (59%, Table 1). Similar proportions of White and Black women received chemotherapy (5.5% vs. 7.7% of White/Black women) and radiation (35% vs. 32%), and for those with hormone receptor-positive tumors, a slightly larger proportion of Black women (65%) received endocrine therapy compared to White women (62%).
The majority (62%) of patients who received surgery did not undergo radiation therapy. However, 36% (n = 21,439) of the patients who underwent surgery also received radiation. Fourteen percent (14%; n = 3118) of the patients who underwent a mastectomy received adjuvant radiation and 50% (n = 18,316) of patients who underwent a lumpectomy received adjuvant radiation. Patient refusal was the most often cited reason for forgoing radiation (66%, Table 1). On multivariate analysis, those patients who were White, age <90, lower stage, ER-negative, and had fewer comorbidities were more likely to have surgery (all P < .001, Table 2). Black women were approximately half as likely to receive surgery compared with White women (P < .001, OR 0.54, 95% CI: [0.47–0.62] Table 2).
Table 2.
Multivariate Analysis of Factors That are Associated With Receipt of Surgery in Women Aged ≥80 With IBC
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Race/Ethnicity | |||
| NH White | 1 (Ref) | ||
| Black | 0.542 | 0.472–0.622 | < .0001 |
| Hispanic | 0.653 | 0.517–0.825 | .0004 |
| Other | 0.903 | 0.777–1.050 | .1853 |
| Age | |||
| 90+ | 1 (Ref) | ||
| 85–89 | 2.263 | 2.029–2.524 | < .0001 |
| 80–84 | 4.567 | 4.809–5.100 | < .0001 |
| Stage | |||
| III | 1 (Ref) | ||
| II | 1.536 | 1.354–1.741 | < .0001 |
| I | 2.161 | 1.775–2.630 | < .0001 |
| Estrogen receptor | |||
| Positive | 1 (Ref) | ||
| Negative | 1.516 | 1.309–1.756 | < .0001 |
| Borderline | 2.729 | 0.358–20.754 | .3321 |
| Tumor size | |||
| No mass found | 1(Ref) | ||
| Microinvasive | 61.091 | 10.375–359.722 | < .0001 |
| ≤2cm | 26.770 | 9.005–79.577 | < .0001 |
| 2cm–5cm | 16.894 | 5.740–49.721 | < .0001 |
| >5cm | 11.191 | 3.791–33.042 | < .0001 |
| Comorbidity Score | |||
| 3+ | 1 (Ref) | ||
| 2 | 1.997 | 1.495–2.668 | .0792 |
| 1 | 2.437 | 1.796–3.307 | < .0001 |
| 0 | 1.997 | 1.495–2.668 | < .0001 |
| Facility typea | |||
| INCP | 1 (Ref) | ||
| ARP | 0.555 | 0.473–0.652 | < .0001 |
| CCP | 1.143 | 0.940–1.390 | .1813 |
| CCCP | 0.925 | 0.792–0.802 | .3266 |
| Grade | |||
| 3 | 1 (Ref) | ||
| 2 | 0.689 | 0.615–0.772 | < .0001 |
| 1 | 0.698 | 0.609–0.802 | < .0001 |
Abbreviations : ARP = academic/research program; CCP = community cancer program; CCCP = comprehensive community cancer program; INCP = integrated network cancer program.
Median survival was 6.7 years for those who received surgery and 2.4 years for those who did not. Surgery was an independent predictor of OS on multivariate analysis (P < .001). While age ≥85, a higher comorbidity score, tumor size >2 cm, and ER-negative status were all predictors of worse OS (P < .001), those who received chemotherapy or adjuvant radiation had an improved OS (P < .001). Additionally, Black women were slightly less likely to die compared with White women, with these results approaching significance (P = .005, Table 3).
Table 3.
Multivariate Analysis of Factors That are Associated With Mortality in Women Aged ≥80 with IBC
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Surgery | |||
| No | 1 (Ref) | ||
| Yes | 0.490 | 0.467–0.515 | < .0001 |
| Race | |||
| NH White | 1 (Ref) | ||
| Black | 0.929 | 0.883–0.977 | .0045 |
| Hispanic | 0.720 | 0.657–0.789 | < .0001 |
| Other | 1.010 | 0.967–1.054 | .6624 |
| Age | |||
| 80–84 | 1 (Ref) | ||
| 85–89 | 1.550 | 1.507–1.595 | < .0001 |
| 90+ | 2.350 | 2.264–2.440 | < .0001 |
| Stage | |||
| I | 1 (Ref) | ||
| II | 1.205 | 1.149–1.264 | < .0001 |
| III | 2.016 | 1.905–2.135 | < .0001 |
| Estrogen receptor | |||
| Positive | 1 (Ref) | ||
| Negative | 1.425 | 1.365–1.487 | < .0001 |
| Borderline | 1.126 | 0.782–1.620 | .5244 |
| Progesterone Receptor | |||
| Positive | 1 (Ref) | ||
| Negative | 1.139 | 1.100–1.180 | < .0001 |
| Borderline | 1.017 | 0.836–1.197 | .8438 |
| Chemotherapy | |||
| No | 1 (Ref) | ||
| Yes | 0.760 | 0.718–0.805 | < .0001 |
| Radiation | |||
| No | 1 (Ref) | ||
| Yes | 0.667 | 0.647–0.686 | < .0001 |
| Comorbidity score | |||
| 0 | 1 (Ref) | ||
| 1 | 1.366 | 1.324–1.410 | < .0001 |
| 2 | 1.900 | 1.798–2.008 | < .0001 |
| 3+ | 2.581 | 2.354–2.828 | < .0001 |
| Tumor Size | |||
| ≤ 2cm | 1 (Ref) | ||
| 2–5 cm | 1.282 | 1.222–1.344 | < .0001 |
| ≥ 5 cm | 1.484 | 1.395–1.579 | < .0001 |
Invasive Breast Cancer by Molecular Subtype
To perform a more contemporary analysis, a subset of invasive breast cancer patients was further divided based on molecular subtype from 2010 to 2014. Multivariate Cox regression for each group by molecular subtype revealed that for all subtypes, there was improved OS in those who received surgery (P < .0001) and radiation (P < .01, Table 4). Additionally, for all subtypes, the risk of mortality increased with age (P < .01, Table 4). In the Luminal A group, there was a significant survival benefit in patients with lower stage, lower comorbidities, smaller tumor size, and for those who received endocrine therapy (P < .01 for all). In the Luminal B group, survival was significantly associated with lower stage, receipt of chemotherapy, and receipt of endocrine therapy (P = .004). However, receipt of HER2-directed therapy (trastuzumab) was not significantly associated with survival in this group (P = .64). In the HR−/HER2+ group, survival was significantly improved in those with lower comorbidity scores (P < .05) and those who underwent chemotherapy (P = .012). Receipt of trastuzumab (HR 0.54, 95% CI 0.31–0.94, P = .03) was also significantly associated with survival. In patients with TNBC, lower stage, receipt of chemotherapy, lower comorbidity score, and smaller tumor size were all predictive of survival (P < .005).
Table 4.
Multivariate Survival Analysis of Invasive Breast Cancer by Molecular Subtype in Women Aged ≥80
| 1 | 2 | 3 | 4 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Luminal A (ER+, PR+, HER2−) (n = 17728) | Luminal B (ER+, PR−, HER2+) (n = 1274) | HER2+ (ER−, PR−, HER2+) (n = 702) | TNBC (ER−, PR−, HER2−) (n = 2664) | ||||||||||
| Variable | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Surgery | No | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | ||||||||
| Yes | 0.42 | 0.38–0.47 | < .0001 | 0.46 | 0.33–.063 | < .0001 | 0.19 | 0.12–0.32 | < .0001 | 0.47 | 0.35–0.62 | < .0001 | |
| Race | White | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | ||||||||
| Black | 0.98 | 0.87–1.11 | .747 | 0.67 | 0.42–1.06 | .084 | 1.02 | 0.68–1.54 | .924 | 0.78 | 0.64–0.95 | .013 | |
| Hispanic | 0.66 | 0.53–0.82 | .0002 | 0.35 | 0.16–0.79 | .012 | 0.76 | 0.37–1.55 | .45 | 0.75 | 0.5–1.12 | .16 | |
| Other | 1.02 | 0.90–1.15 | .789 | 0.85 | 0.55–1.32 | .479 | 0.71 | 0.38–1.31 | .269 | 1.21 | 0.94–1.55 | .137 | |
| Age | 80–84 | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | ||||||||
| 85–89 | 1.51 | 1.40–1.62 | < .0001 | 1.82 | 1.43–2.32 | < .0001 | 1.44 | 1.08–1.93 | .0140 | 1.28 | 1.11–1.47 | .0005 | |
| ≥90 | 2.29 | 2.10–2.51 | < .0001 | 2.78 | 2.09–3.70 | < .0001 | 2.01 | 1.41–2.85 | < .0001 | 1.83 | 1.53–2.18 | < .0001 | |
| Stage | I | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | ||||||||
| II | 1.25 | 1.10–1.42 | .0005 | 1.74 | 1.20–2.55 | .004 | 1.26 | 0.72–2.21 | .409 | 1.83 | 1.42–2.35 | < .0001 | |
| III | 2.00 | 1.72–2.33 | < .0001 | 2.88 | 1.92–4.33 | < .0001 | 2.05 | 1.16–3.62 | .0133 | 3.72 | 2.87–4.83 | < .0001 | |
| Chemotherapy | No | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | ||||||||
| Yes | 0.93 | 0.75–1.16 | .537 | 0.62 | 0.45–0.85 | .003 | 0.68 | 0.5–0.93 | .0157 | 0.68 | 0.57–0.81 | < .0001 | |
| Radiation | No | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | ||||||||
| Yes | 0.62 | 0.57–0.67 | < .0001 | 0.75 | 0.58–0.95 | .019 | 0.63 | 0.47–0.85 | .003 | 0.66 | 0.58–0.76 | < .0001 | |
| Comorbidity Score | 0 | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | ||||||||
| 1 | 1.45 | 1.35–1.56 | < .0001 | 1.55 | 1.22–1.98 | .0004 | 1.38 | 1.01–1.9 | .047 | 1.38 | 1.19–1.61 | < .0001 | |
| 2 | 2.06 | 1.82–2.32 | < .0001 | 1.51 | 0.96–2.38 | .075 | 2.59 | 1.58–4.25 | .0002 | 2.05 | 1.63–2.58 | < .0001 | |
| ≥3 | 2.66 | 2.23–3.19 | < .0001 | 5.65 | 2.94–10.86 | < .0001 | 1.51 | 0.69–3.29 | .305 | 2.23 | 1.52–3.28 | < .0001 | |
| Tumor size | 0–2 cm | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | ||||||||
| >2–5 cm | 1.38 | 1.22–1.57 | < .0001 | 1.13 | 0.80–1.60 | .494 | 1.66 | 0.99–2.78 | .054 | 1.4 | 1.12–1.76 | .004 | |
| >5 cm | 1.77 | 1.5–2.08 | < .0001 | 1.07 | 0.69–1.66 | .755 | 1.87 | 1.04–3.36 | .037 | 1.63 | 1.26–2.12 | .0002 | |
| Endocrine therapy | No | 1 (Ref) | 1 (Ref) | - | - | - | - | - | - | ||||
| Yes | 0.64 | 0.6–0.68 | < .0001 | 0.71 | 0.57–0.90 | .004 | - | - | - | - | - | - | |
| trastuzumab therapy | No | - | - | - | 1 (Ref) | 1 (Ref) | - | - | - | ||||
| Yes | - | - | - | 0.90 | 0.56–1.42 | .64 | 0.54 | 0.31–0.94 | .030 | - | - | - | |
Discussion
Differences in tumor characteristics and treatment preferences exist between younger and older women with breast cancer, including larger tumors at diagnosis, lower grade tumors, omission of part or all of treatment due to differences in functional status, treatment preference, and comorbidities. However, treatment patterns and outcomes for breast cancer in the older population are not well understood. Using the NCDB, we have described these patterns on a nationwide scale. The vast majority of NCDB IBC patients aged ≥80 received primary surgical management, which was associated with a significant increase in OS. There was an over 4-year increase in median survival in those who received surgery compared with those who did not. However, those who received surgery were more likely to be younger, healthier, and have lower stage disease. These findings are expected and reflect a selection bias, as healthier patients make better surgical candidates. Those who received breast surgery were also more likely to receive additional treatment, including radiation, chemotherapy, endocrine therapy if patients had ER-positive disease, or axillary surgery.
While some studies have demonstrated that older women are less likely than younger patients to undergo breast cancer surgery,4 many analyses, including this report, show the vast majority of older patients do undergo surgery,14–16 with a clear survival benefit.17 In our analysis, lumpectomy was performed more often than mastectomy. This finding is in concordance with prior studies demonstrating a preference for lumpectomy in older women16 given the fact that breast conserving surgery is less morbid than a mastectomy.
Additionally, of those who received breast surgery, 77% underwent axillary surgery which is much higher than anticipated given that 68% of patients who underwent breast surgery had a clinical nodal status of N0. In 2016, the Choosing Wisely guidelines were updated to include the safe omission of routine sentinel node biopsy in women ≥70 with early stage, hormone-receptor (HR) positive, HER2-negative and node negative IBC.18 Our analysis included the years prior to the release of this guideline and indicates this omission was not common practice during these years. In addition, the initial results of the ACOSOG Z0011 trial were published in 2011, during the time period of the study, which demonstrated that in patients with limited sentinel lymph node metastatic breast cancer who were treated with breast conservation and systemic therapy, the use of sentinel node biopsy alone compared with axillary dissection did not result in inferior survival.19,20 However, there is evidence that even as recent as a survey from 2018, nearly half of surgeons surveyed were not guideline concordant for patients who met inclusion criteria for the Z0011 trial.21
Clinical trials have found no risk of decreased survival with omission of therapies, especially in the older population. In 2004, the Cancer and Leukemia Group B (CALGB) 9343 clinical trial concluded there was no negative impact on survival with omission of radiation in women aged ≥70 with clinical stage I, ER-positive, clinically node-negative breast cancer who received a lumpectomy and 5 years of endocrine therapy.22,23 There is emerging evidence showing slow integration of these results into clinical practice24,25; however, debate exists as to whether all women in this population benefit from omission of therapies or whether some benefit from standard treatment paradigms.4,26 Our current analysis includes the years immediately following the publication of the CALGB 9343 study’s five-year results,22 and our data demonstrates that the majority of surgical patients did not undergo radiation (62%), which perhaps reflects the influence of these guidelines, although it is not possible to know the exact indications for radiation treatment in this dataset.
While the majority of our patients did undergo breast surgery, omission of surgery in exchange for primary endocrine therapy has been shown to be increasing recently.27,28 Primary endocrine therapy has been endorsed as an option for hormone receptor-positive tumors by the International Society of Geriatric Oncology.29 A recent multicenter, prospective, observational study in the UK of over 3000 women over age 70 with operable breast cancer from 2013 to 2018 found that those who were fit benefitted from surgery, whereas those who were frail benefitted from endocrine therapy alone.30 Omission of surgery for primary endocrine therapy has been shown to be most popular among those with higher age, more comorbidities, and patient preference to forgo surgery,31 with no impact on OS.32 In our analysis, the majority of reasons given for omission of surgery was patient refusal, identifying a patient preference to avoid a potentially morbid procedure in the context of advanced age and decreased fitness.
In the entire cohort, we found borderline significance in mortality risk between White and Black women with Black women slightly less likely to die (Table 3). However, we found that Black and White women received similar proportions of chemotherapy and radiation, and a slightly larger proportion of Black women with hormone receptor-positive tumors (65%) received endocrine therapy compared to White women with hormone receptor-positive tumors (62%). Primary endocrine therapy with surgical omission for these patients may explain the large differences in receipt of surgery without corresponding differences in survival. There has historically been a higher mortality rate for breast cancer in Black women compared with White women.33 However, the American Cancer Society recently reported a narrowing of the Black-White cancer death disparity in women ≥70, including a 3% lower mortality in Black women aged 80 to 89 vs. White women in the same age group.34 Though Black women were less likely to have surgery, which our data show is associated with an improved survival, Black women are also less likely to die according to our data. This finding cannot be completely explained with our limited data alone, although increased use of primary endocrine therapy in Black women in our cohort may partially explain the narrowing of this disparity gap.
Our data reveal the importance of shared decision-making with a multidisciplinary team in patients with older age, carefully weighing the individual patient’s preferences, tumor characteristics, and likelihood of survival with or without certain therapies. One study was performed allowing patients to use a tool centered around decision-making: whether to have surgery plus endocrine therapy or endocrine therapy alone. The authors found that the decision-making tool led to more older women opting out of surgery.35 In addition to shared decision-making, patient candidacy for treatment can be analyzed through proper assessment prior to beginning treatment. The Comprehensive Geriatric Assessment can be useful in predicting outcomes for patients undergoing cancer treatment,36 and a valuable pre-treatment evaluation in older breast cancer patients. The American Society of Clinical Oncology published clinical practice guidelines in 2018 recommending the use of geriatric assessment in cancer patients ≥65, specifically those receiving chemotherapy, to identify vulnerabilities beyond those in standard assessments37.
In the subset analysis of IBC by molecular subtype, we found improved OS for those who received surgery and radiation across all subtypes (Table 4). While randomized studies such as CALGB 934322,23 and NSABP-0638,39 did not demonstrate survival benefits in those randomized to radiation, it is possible that the patients in this study who received radiation had a more favorable performance status or other factors that are not captured in the NCDB database variables. Factors could include the ability to travel to daily radiation sessions, to receive the daily radiation treatments (which may be difficult for some with mobility or other musculoskeletal problems), and better support to complete radiation treatments. These factors would not be expected to change across different biologic subtypes, which may be why the difference in OS is evident across subtypes. We also showed improved OS with receipt of chemotherapy for Luminal B, TNBC and HER2-positive subtypes which is consistent with established treatment guidelines. Treatment with trastuzumab for HER2-positive patients was found to improve survival; however, this finding was only significant for the HR−/HER2+ group and not in the HR+/HER2-positive group. This finding could be that due to the age and shorter follow-up time of this patient cohort, it is difficult to demonstrate a clear survival difference. In addition, from neoadjuvant data, it has been established in a large pooled analysis that those who are HR−/HER2+ and HR−/HER2− have the strongest association between pathologic complete response and long-term outcomes which translates into improved survival.40 While it is unknown what percentage of these patients received neoadjuvant therapy, one may extrapolate this survival benefit to even those who were HR−/HER2+ who receive trastuzumab in the adjuvant setting.
Finally, we found that surgical management of IBC was performed less often at academic centers when compared with community hospitals. Unlike pancreatic cancer, for example, breast cancer care is often decentralized from urban areas due to its increased incidence and lower procedural morbidity,41 which is supported by our findings. In addition, Medicare patients, who comprise this study group, are less likely to travel for their medical care at a tertiary care center and are more likely to seek care from a medical center closer to home.42,43
Limitations of this study include the retrospective nature of the data and the dependence on the accuracy of data input with such a large database. In addition, the NCDB does not record disease-specific survival and does not accurately describe nodal surgery (sentinel node biopsy vs. axillary lymph node dissection) until after 2012, and many patients had limited follow-up in this data set. However, given the indolent nature of many breast cancers in older patients and the lifespan of this population, this limitation is less significant than with younger breast cancer patients as these patients are more likely to die of competing comorbidities. Severe comorbidity is a significant factor in survival in older women with breast cancer.44 Older breast cancer patients are also less likely to die from their disease compared with their younger counterparts, and this risk decreases as age increases.17,33,45 Median follow-up was 48 months for those who received surgery and 24 months for those who did not. The latter may represent a group who was deemed unfit for surgery and died prior to receiving treatment, indicating a potential difference in health status unrelated to comorbidity score that may have impacted treatment choice and/or survival. In addition, analysis of comorbidity status using Charlson-Deyo scores does not provide specific comorbidities for each patient, and importantly the score does not include frailty, which is of large importance in this population. Finally, the rationale behind specific treatment omissions is not readily apparent within the NCDB.22,23 Despite these limitations, however, we were able to capture a large and diverse data set and demonstrate current trends in the characteristics and treatment patterns in this population on a nationwide scale.
The management of breast cancer patients ≥80 years old is complex and nuanced. No set guidelines exist and many of the existing studies looking at older patients start with patients in their seventh decade. Larger studies, such as CALGB 9343 and the Choosing Wisely guidelines, as well as smaller studies provide conflicting data regarding de-escalating therapy in the older breast cancer population. Our cohort of relatively healthy women in this age group demonstrates that treatments should be carefully considered and not automatically omitted based on age alone as there are certain individuals who will derive a survival benefit from standard treatment. Those patients who have a life expectancy that is >5 years and who are relatively healthy will likely benefit from surgery, particularly because adherence to endocrine therapy may be poor.46 With a clinically negative axilla, in HR+/HER2-negative patients, sentinel node staging may be omitted and thus, surgery may be limited to the breast.18 We also found that systemic therapy is significant with respect to survival based on molecular subtype and decisions on pursuing adjuvant systemic therapies must be weighed against the risk of toxicities in this older population. While approximately two-thirds of our study population did not receive radiation, combining this finding with the results of the CALGB 9343 study, radiation should be considered for those with higher stage disease, those patients who do not have hormone receptor-positive tumors, or those unable to receive endocrine therapy.
Conclusion
In this analysis, we report treatment patterns and outcomes in women age ≥80 with IBC using the NCDB. The vast majority of patients received surgery, of which most underwent breast conserving surgery. Those who received breast surgery had an improved OS compared to those who did not, including across all molecular subtypes. Overall, the results suggest that surgical intervention should be considered in patients with few comorbidities and favorable tumor characteristics. The choice to include surgery as part of breast cancer management in older women should not be based on age alone but should include a careful assessment of surgical risks and benefits, an individual fitness assessment, and discussion of therapy goals with patients, their families, and the multidisciplinary breast cancer team. Continued evaluation of treatment patterns will be needed as evidence-based guidelines are updated and become widely integrated into clinical practice.
Clinical Practice Points
Women age ≥80 comprise a growing subset of breast cancer patients, however there is a paucity of data on national treatment patterns and survival outcomes despite an understanding that older women have differences in tumor characteristics and treatment goals compared with younger women. Using the NCDB, we describe an OS benefit to receipt of surgery in patients with all molecular subtypes of invasive breast cancer. This study shows an overall healthy population of women ≥80 with breast cancer, and increased utilization of surgical treatment in those with younger age and fewer comorbidities. With improved OS to receiving surgery, as well as improved OS with chemotherapy or radiotherapy in most groups, emphasis should be placed on the importance of considering interventions in a shared decision-making manner and not based on age alone.
References
- 1.Schonberg MA, Marcantonio ER, Li D, Silliman RA, Ngo L, McCarthy EP. Breast cancer among the oldest old: tumor characteristics, treatment choices, and survival. J Clin Oncol. 2010;28:2038–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67:439–448. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. Breast Cancer (Version 1.2019). https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed January 8, 2019.
- 4.Bouchardy C, Rapiti E, Fioretta G, et al. Undertreatment strongly decreases prognosis of breast cancer in elderly women. J Clin Oncol. 2003;21:3580–3587. [DOI] [PubMed] [Google Scholar]
- 5.Biganzoli L, Wildiers H, Oakman C, et al. Management of eldelry patients with breast cancer: updated recommendations of the Internal Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol. 2012;13(4):143–160. [DOI] [PubMed] [Google Scholar]
- 6.Sardanelli F, Aase HS, Alvarez M, Azavedo Eea. Position paper on screening for breast cancer by the European Society of Breast Imaging (EUSOBI) and 30 national breast radiology bodies from Austria, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finald, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Israel, Lithuania, Moldova, The Netherlands, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Spain, Sweden, Switzerland and Turkey. Eur Radiol. 2017;27:2737–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qaseem A, Lin JS, Mustafa RA, Wilt TJ. Screening for breast cancer in average-risk women: a guidance statement from the American College of Physicians. Ann Intern Med. 2019(8):547–560. [DOI] [PubMed] [Google Scholar]
- 8.Siu AL. Screening for breast cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2016;164:279–296. [DOI] [PubMed] [Google Scholar]
- 9.Lodi M, Scheer L, Reix N, et al. Breast cancer in elderly women and altered clinico-pathological characteristics: a systematic review. Breast Cancer Res Treat. 2017;166:657–668. [DOI] [PubMed] [Google Scholar]
- 10.Grumpelt AM, Ignatov A, Tchaikovski SN, Burger E, Costa SD, Eggemann H. Tumor characteristics and therapy of elderly patients with breast cancer. J Cancer Res Clin Oncol. 2016;142:1109–1116. [DOI] [PubMed] [Google Scholar]
- 11.American College of Surgeons. 2020. About the national cancer database. https://www.facs.org/quality-programs/cancer/ncdb/about2020. Accessed February 2020.
- 12.NIH National Cancer Institute: surveillance epidemiology and end results program. cancer stat facts: female breast cancer subtypes. seer.cancer.gov/statfacts/html/breast-subtypes. Accessed May 2021.
- 13.Dominici LS, Sineshaw HM, Jemal A, Lin CC, King TA, Freedman RA. Patterns of axillary evaluation in older patients with breast cancer and associations with adjuvant therapy receipt. Breast Cancer Res Treat. 2018;167:555–566. [DOI] [PubMed] [Google Scholar]
- 14.Cyr A, Gillanders WE, Aft RL, Eberlein TJ, Margenthaler JA. Breast cancer in elderly women (≥ 80 Years): variation in standard of care? J Surg Oncol. 2011(3):201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrigni E, Bergom C, Yin Z, Szabo A, Kong AL. Breast cancer in women aged 80 years or older: an analysis of treatment patterns and disease outcomes. Clin Breast Cancer. 2019;19(3):157–164. [DOI] [PubMed] [Google Scholar]
- 16.Mogal HD, Clark C, Dodson R, Fino NF, Howard-McNatt M. Outcomes after mastectomy and lumpectomy in elderly patients with early-stage breast cancer. Ann Surg Oncol. 2017;24:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong AL, Nattinger AB, McGinley E, Pezzin LE. The relationship between patient and tumor characteristics, patterns of breast cancer care, and 5-year survival among elderly women with incident breast cancer. Breast Cancer Res Treat. 2018;171(2):477–488. [DOI] [PubMed] [Google Scholar]
- 18.Society of Surgical Oncology. Choosing Wisely. 2016. http://www.choosingwisely.org/clinician-lists/sso-sentinel-node-biopsy-in-node-negative-women-70-and-over/. Accessed December 18, 2018.
- 19.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011(6):569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary disseciton vs no axillary dissection on 10-year overall survival among women wiht invasive breast cancer and sentinel node metastasis. The ACOSOG Z0011 (Alliance) Randomized Clinical Trial JAMA. 2017(10):918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrow M, Jagsi R, McLeod MC, Shumway D, Katz SJ. Surgeon attitudes toward the omission of axillary dissection in early breast cancer. JAMA Oncol. 2018;4(11):1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy Plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31:2382–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with elderly breast cancer. N Engl J Med. 2004(10):971–977. [DOI] [PubMed] [Google Scholar]
- 24.Wallace AS, Keene KS, Williams CP, et al. Radiation therapy utilization in Medicare beneficiaries with early-stage breast cancer. Cancer. 2018;124:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soulos PR, Yu JB, Roberts KB, et al. Assessing the impact of a cooperative group trial on breast cancer care in the medicare population. J Clin Oncol. 2012;30:1601–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanz J, Zhao M, Rodriguez N, et al. Once-weekly hypofractionated radiotherapy for breast cancer in elderly patients: efficacy and tolerance in 486 patients. Biomed Res Int. 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kantor O, Pesce C, Liederbach E, Wang C-H, Winchester DJ, Yao K. Surgery and hormone therapy trends in octogenarians with invasive breast cancer. Am J Surg. 2016;211:541–545. [DOI] [PubMed] [Google Scholar]
- 28.Fietz T, Zahn MO, Kohler A, et al. Routine treatment and outcome of breast cancer in younger versus elderly patients: results from the SENORA project of the prospective German TMK cohort study. Breast Cancer Res Treat. 2018;167:567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biganzoli L, Wildiers H, Oakman C, et al. Management of eldelry patients with breast cancer: updated recommendations of the Internal Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol. 2012;13(4):148–160. [DOI] [PubMed] [Google Scholar]
- 30.Wyld L, Reed M, Collins K, al e. Impacts of omission of breast cancer surger in older women with ER+ early breast cancer. 12th European Breast Cancer Conference. Abstract 8A. [Google Scholar]
- 31.Wink CJ, Woensdregt K, Nieuwenhuijzen GA, et al. Hormone treatment without surgery for patients aged 75 years or older with operable breast cancer. Ann Surg Oncol. 2012;19:1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hind D, Wyld L, Reed MW. Surgery, with or without tamoxifen, vs tamoxifen alone for older women with operable breast cancer: cochrane review. Br J Cancer. 2007;96:1025–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson LC, Henley SJ, Miller J, Massetti G, Thomas CC. Patterns and trends in age-specific black-white differences in breast cancer incidence and mortality - United States, 1999–2014. MMWR Morb Mortal Wkly Rep. 2016;65(40):1093–1098. [DOI] [PubMed] [Google Scholar]
- 34.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019(69):211–233. [DOI] [PubMed] [Google Scholar]
- 35.Wyld L, Reed M, Collins K. 12th European Breast Cancer Conference. Abstract 8B. al e. Cluster randomized trial to evaluate the clincial benefits of decision support interventions for older women with operable breast cancer; 2020. [Google Scholar]
- 36.Clough-Gorr KM, Stuck AE, Thwin SS, Silliman RA. Older breast cancer survivors: geriatric assessment domains are associated with poor tolerance of treatment adverse effects and predict mortality over 7 years of follow-up. J Clin Oncol. 2010;28:380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohile S, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy. J Clin Oncol. 2018;36(22):2326–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312(11):665–673. [DOI] [PubMed] [Google Scholar]
- 39.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. [DOI] [PubMed] [Google Scholar]
- 40.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. [DOI] [PubMed] [Google Scholar]
- 41.Neuner JM, Gilligan MA, Sparapani R, Laud PW, Haggstrom D, Nattinger AB. Decentralization of breast cancer surgery in the United States. Cancer. 2004;101:1323–1330. [DOI] [PubMed] [Google Scholar]
- 42.Kong AL, Yen TWF, Pezzin LE, et al. Socioeconomic and racial differences in treatment for breast cancer at a low-volume hospital. Ann Surg Oncol. 2011;18(11):3220–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouche G, Migeot V, Mathoulin-Pélissier S, Salamon R, Ingrand P. Breast cancer surgery: do all patients want to go to high-volume hospitals? Surgery. 2008;143(6):699–705. [DOI] [PubMed] [Google Scholar]
- 44.Kimmick G, Li X, Fleming ST, et al. Risk of cancer death by comorbidity severity and use of adjuvant chemotherapy among women with locoregional breast cancer. J Geriatr Oncol. 2017;9:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diab SG, Elledge RM, Clark GM. Tumor characteristics and clinical outcome of elderly women with breast cancer. J Nat Cancer Inst. 2000;92(7):550–556. [DOI] [PubMed] [Google Scholar]
- 46.Cortina CS, Agarwal S, Mulder LL, et al. Are Providers and patients following hormonal therapy guidelines for patients over the age of 70? the influence of CALGB 9343. Clin Breast Cancer. 2018;18:e1289–e1292. [DOI] [PubMed] [Google Scholar]


