Abstract
Introduction:
This study compared the relative efficacy of aerobic training to resistance training on physical functioning in older breast cancer survivors and determined whether benefits could be maintained by transitioning to unsupervised home-based training.
Materials and Methods:
Early-stage, post-treatment, older (≥65 years) breast cancer survivors (n=114; mean age 72 years) were randomized to 12 months of supervised aerobic (n=37), resistance (n=39) or stretching (active control; n=38) training followed by 6 months of unsupervised home-based training. Outcomes included aerobic capacity by 6-minute walk distance (6MWD; m), maximal upper and lower body strength (1-repetition maximum; kg); physical function by short physical performance battery (SPPB), SF-36 and Late Life Function and Disability Instruments.
Results:
Over 12-months of supervised exercise, all groups improved in muscle strength and SPPB scores, but resistance trained women also improved 6MWD. Improvements in upper and lower body strength in the resistance group were significantly greater than those in the stretching control (+2.5 kg vs. +1.8 kg; p=0.05) and aerobic groups (+8.3 kg vs +2.7 kg; p=0.047), respectively, with trends for greater improvements in 6MWD (+57.9m vs. +22.5m; p=0.057) and self-report physical function (+4.8 vs. −4.4; 0.066) in resistance trained women versus controls. Compared to values at 12 months, there were no changes during unsupervised training in any measure within or between groups, except for self-reported advanced lower extremity function which improved in the resistance group and fell in the aerobic group (+1.3 vs. −3.1; p=0.043).
Discussion:
Supervised exercise can improve strength and physical functioning among older breast cancer survivors. Resistance training may lead to better improvements compared to aerobic or flexibility training, whether in a supervised or unsupervised setting.
Keywords: physical activity, exercise, neoplasm, physical fitness, gerontology, ADLs, neoplasm
Introduction
Older women (65 years+) constitute the largest group of breast cancer survivors in the U.S.1, 2 By 2040 the proportion of cancer survivors who are older will rise to 73% and most will survive for 5 years or longer.3 Cancer treatment may accelerate aging, which could hasten the trajectory toward dependence and disabilty among older women.4 Older cancer survivors are more likely to report difficulty with daily activities requiring endurance, strength, and mobility than their older peers without cancer.5, 6 Poor physical functioning could impact overall survival as persistent declines in physical functioning following a breast cancer diagnosis predicts shorter survival time in older women.7
Despite these projections, studies of older women cancer survivors comprise only a small proportion of research, especially exercise research, on reducing symptoms, maintaining function, and improving quality of life.8, 9 Reissued in 2018, the DHHS Physical Activity Guidelines (PAG) for Americans recommend that all adults, regardless of age, aim for 150 minutes of moderate intensity aerobic exercise and 2–3 sessions of resistance exercise every week.10 Older women cancer survivors report falling short of recommended aerobic activity levels and virtually none participate in resistance training.11 Recently updated exercise guidelines for cancer survivors from an American College of Sports Medicine (ACSM) roundtable recommend lower levels of exercise (i.e., 30 minutes of moderate-intensity exercise 3 times per week) than those in the PAG may be sufficient to improve cancer-related health outcomes, including physical functioning. However, due to the lack of trials in older survivors and of trials that directly compared two or more training modalities, the ACSM recommendations could not include age-specific guidelines for older survivors nor recommend an optimum training modality for specific outcomes. While the ACSM guidelines also suggest that supervised exercise may lead to greater improvements in outcomes and be safer for higher risk populations than unsupervised training, these recommendations were not empirically derived from studies including both supervised and unsupervised training periods.
The purpose of our study was to directly compare supervised aerobic and resistance exercise to each other and to an active control group on physical functioning in older women breast cancer survivors. We felt a head-to-head trial comparing single modalities in older survivors was warranted first, since adding a combined aerobic + resistance training group creates confounding across groups with regard to delivered dose of aerobic and resistance training. We also included a transition period to unsupervised exercise to determine whether or not benefits could be maintained when exercising at home. We hypothesized that supervised aerobic and resistance training would significantly improve physical functioning compared to a control condition and that improvements from aerobic and resistance training would be equivalent and maintained with unsupervised exercise. In a separate publication we reported the effects of supervised training on biomarkers associated with breast cancer recurrence.12
METHODS
Study Design and Setting
The study was a single-blind, randomized controlled trial comparing three parallel groups with an allocation ratio of 1:1:1 to the following progressive low-moderate intensity exercise programs: 1) aerobic (AET), 2) resistance (RET) or 3) flexibility (FLEX; active control) training. Training was supervised for 12 months, then unsupervised for another 6 months. Outcomes were measured at baseline, 3, 6, 12 and 18 months by trained technicians blinded to group assignment. The primary outcome was physical function, measured objectively and by self-report. Secondary outcomes of physical fitness were included to assess fidelity of each training modality. A statistician used a computer-generated random numbers table to allocate participant ID numbers to intervention groups. Randomization was stratified by current use of anti-estrogen therapy (yes/no) in order to reduce potential confounding that could occur due to associated symptoms which might differentially affect exercise tolerance (e.g, arthralgia, myalgia). Individual assignments were placed into sealed envelopes prior to enrollment and were sequentially opened by each participant after completion of baseline testing.
The study was approved by the Oregon Health & Science University (OHSU) Institutional Review Board and each participant gave written informed consent prior to baseline testing. Testing took place at OHSU, while exercise training occurred there and at two community sites in the Portland metro area.
Sample
Participants were recruited through the Oregon State Cancer Registry, physician referrals, support groups and cancer-related community events. To be eligible women had to meet the following inclusion criteria: stage I-III breast cancer; ≥65 years old; ≥2 years post-chemotherapy and/or radiation therapy, to avoid exercise limitations due to treatment-related symptoms such as fatigue; insufficiently physically active (<60 minutes / week of planned moderate-vigorous intensity aerobic and/or resistance exercise)13; and, physician clearance to exercise. Current anti-estrogen therapy with a selective estrogen receptor modulator or aromatase inhibitor was permitted. Enrollment was open from 2011–2014.
Supervised Exercise Training
Women participated in three supervised 60-minute (including 5-minute warm-up and cool-down periods) group classes per week led by exercise physiologists or certified fitness instructors. To maintain quality control over intervention delivery, the same set of instructors taught across all three study exercise sites and followed a written protocol. The volume of resistance and aerobic exercise gradually and progressively increased from low to moderate intensity over the first 9 months of training to provide continuous overload and then remained steady for the last 3 months (Table 1). Low to moderate intensity exercise should provide an adequate stimulus for adaptation in older adults14 yet ensure safety in novice exercisers, but is a lower intensity than that recommended in current guidelines for cancer survivors. Thus, we included measures of aerobic capacity, muscle strength and flexibility to evaluate fidelity of training protocols. Increases in intensity were modified when a participant showed signs of limited tolerance, such as an inability to use proper form. On a weekly basis the exercise trainer verbally queried participants about adverse events related to study exercise programs. Adverse events were subsequently documented by the trainer and severity was then assigned by the project director according to institutional criteria.
Table 1.
Progression of resistance and aerobic training over 12 months
| Month | Resistance Exercise | Aerobic Exercise | ||||||
|---|---|---|---|---|---|---|---|---|
| Lower Body | Upper Body | |||||||
| Intensity | Sets | Reps | Intensity | Sets | Reps | Intensity (%HRR) | Duration (minutes) | |
| 1 | 0–1% BW | 1–3 | 14–15 | 14–15 RM | 1–3 | 14–15 | 35–40% | 20–35 |
| 2 | 2–3% BW | 1–3 | 14–15 | 14–15 RM | 1–3 | 14–15 | 40–45% | 25–30 |
| 3 | 4–5% BW | 1–3 | 12–13 | 12–13 RM | 1–3 | 12–13 | 45–50% | 30–35 |
| 4–6 | 6–8% BW | 1–3 | 11–13 | 11–13 RM | 1–3 | 11–13 | 50–55% | 35–40 |
| 7–9 | 8–10% BW | 1–3 | 10–12 | 10–12 RM | 1–3 | 10–12 | 55–65% | 40–45 |
| 10–12 | 10% BW | 1–3 | 10 | 10 RM | 1–3 | 10 | 65% | 45 |
BW: Body Weight; RM: Repetition Maximum; HRR: Heart Rate Reserve
AET:
The aerobic exercises consisted of low-impact dance aerobic exercises designed to increase heart rate (HR) by working large muscle groups of upper and lower body. Exercises including a variety of marching exercises, side steps, knee lifts, upper arm movements, and dance movements. Over 9 months the duration of aerobic exercise progressed from 20 to 45 minutes of active training and intensity progressed from 35% to 65% of estimated heart rate reserve (HRR).15 Participants used the Borg Perceived Exertion Scale16 and HR monitors (Polar RS400; Kempere, Finland) to adjust their effort to the prescribed intensity. Data from HR monitors were downloaded weekly so that compliance to training could be subsequently calculated.
RET:
Resistance exercises consisted of 5 upper body and 5 lower body exercises designed to utilize major muscle groups and employ functional movements (chair stands, lunges (front, backward, lateral), calf raises, one-arm row, chest press, front/lateral shoulder raise, and push-ups). Based on ACSM resistance training guidelines for older adults, participants completed 2–3 sets of 10–15 repetition maximum (RM) of each exercise.14 Upper body exercises were performed with body weight (e.g., pushups) or dumbbells and weight was progressively increased to maintain a 10–15 RM. The lower body exercises were performed with participants wearing a weighted vest that was gradually increased from 1% of a participant’s body weight up to 10%, or as tolerated.
FLEX:
The active control group performed supervised stretching and relaxation exercise. Stretching exercises targeted the whole body and followed the ACSM recommendations for static stretching, where 2–3 repetitions of each stretch were held to the point of tension for 15–60 seconds.15 Stretches were performed in either a seated or lying position to provide a contrast to the weight-bearing aerobic and resistance training groups. Relaxation techniques (guided imagery, progressive neuromuscular relaxation, focused breathing) were included during the last 10 minutes of each session.
Unsupervised Training
After the first 12-months of the study, supervised exercise classes ended and participants were encouraged to follow a DVD-based version of their program, 3x/week. The exercise DVD was provided at no cost and was led by their same in-class exercise instructors using exercises consistent with those in supervised sessions. Participants were provided some equipment for home training (i.e., weight vest, resistance bands, HR monitors, stretching straps). Adherence, as well as any exercise-related AEs, were tracked on calendars mailed back monthly to the research team or collected by phone.
Measures
Participant Characteristics
Demographic and clinical characteristics were obtained by self-report. Women also self-reported chronic medical conditions by the Charlson Comorbidity Index,17 physical activity (kcal/week) by the Community Health Activity Model Program for Seniors (CHAMPS) physical activity questionnaire,18 which was also used to monitor for changes in physical activity across the intervention period.
Primary Outcomes: Physical Functioning
Objective physical function was assessed by the Physical Performance Battery (SPPB) using the standard protocol.19 The SPPB consists of 3 timed performance tests: 5 repeated chair stands, standing balance, and usual gait speed over 4 meters. Each test is scored 0 (unable) to 4 and then scores are summed. Higher scores indicate better physical function and low scores on the SPPB (≤ 9) predict disability, hospitalization, nursing home admission, and mortality.19–22 Improvements of ≥1 points indicate substantial meaningful change.23 We also reported times for the chair stand (sec) and 4m walk (m/sec) separately. As mentioned below, we also considered 6MWD as an objective measure of physical functioning.
Self-report physical function was determined from both the SF-36 physical function subscale and the Late-Life Function and Disability Instrument (LLFDI). Both the SF-36 physical function subscale and LLFDI are valid and reliable instruments, though only the LLFDI contains separate subscales of basic and advanced lower extremity function and upper extremity function.24–26 For both instruments, scales are scored 0–100, with higher scores indicating better function. Minimally clinically important differences (MCID) in older adults for LLFDI lower extremity, advanced lower extremity and upper extremity function are 3, 4, and 4 points, respectively. 27
Secondary Outcomes of Intervention Fidelity: Physical Fitness
Submaximal aerobic capacity was measured with a valid and reliable field measure of distance (m) walked on a treadmill at a pace that can be held for six minutes (6MWD).28 6MWD predicts mortality and morbidity in older adults and clinical populations29–32 and a change in walk distance of 54m represents a clinically significant change in functional status among older adults,33 and individuals with chronic lung disease.34
Maximal muscle strength of the upper and lower body was evaluated by a 1-repetition maximum leg press and chest press (1-RM; kg) where the maximum weight that can be pressed once is determined. We followed standard protocols35 that we have used in our prior studies.36, 37
Flexibility, or range of motion, in the upper and lower extremities was measured by the back scratch and chair sit-and-reach tests conducted according to standard protocols38 and recorded as distance (cm) between fingertips of both hands and between fingertips and toes, respectively.
Statistical Analysis
Standard descriptive statistics of frequency, central tendency, and dispersion were used to describe the sample. Standard ANOVAs and chi-square tests of proportion were used to compare baseline values across groups. Study aims were tested using piecewise linear mixed-effects regression models with a break point at 12 months for all outcome measures implemented using the nlme package in R.39 This strategy allowed estimation of mean baseline values and slopes representing within group changes over both the supervised (0–12 months) and unsupervised (12–18 months) periods using one model for each outcome. Group x time interaction terms for both time periods assessed differences in changes over time between groups. Fidelity measures were only assessed from 0–12 months and were tested with standard non-piecewise models. Age, baseline BMI, and Charlson comorbidity index were included as covariates to account for any influence of these variables on exercise tolerance and adaptations. The mixed-effects approach accounts for the correlation between measurements from the same individual without the requirement of complete data as needed for methods like repeated measures ANOVA.40 Using piecewise models ensured that parameter estimates at 12 months were consistent between the intervention and follow-up periods. P-values for the main outcomes were presented as unadjusted and then adjusted using the Benjamini-Hochberg (BH) method under the assumption that the main outcome models have positive dependency.41,42 Fidelity measures were also adjusted using BH as a separate hypothesis family. Calculations were performed using the formula provided by Benjamini, Heller, and Yekutieli43 in R’s p.adjust function. Both the original, unadjusted p-values and the BH adjusted p-values were reported as suggested by White, van der Ende, and Nichols.44
A priori power analyses were conducted assuming a mixed between-within ANOVA model with alpha=.05 and power=.80. The effect of interest was the group x time (0–12 month) interaction on the primary outcomes of physical function. A priori power analyses estimated a final sample size of n=33 participants per group to detect a 5-point group x time difference in self-report physical function between experimental and control groups.45 Based on prior trials in older adults this sample size was more than sufficient to also detect a 1.5 second difference in chair stand time, an objective measure of physical functioning, between groups.46 To protect against ~20% attrition we aimed to randomize 38 women per group.
RESULTS
Sample
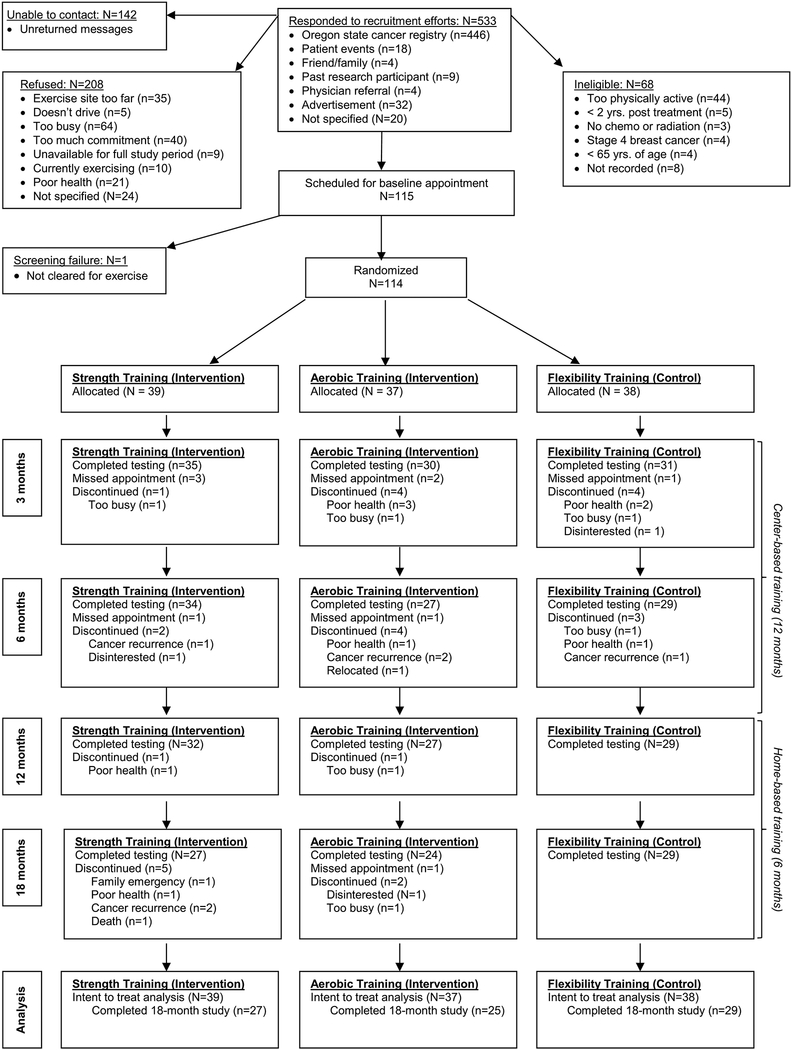
Of 533 women who contacted the study team about the trial, 114 women (21%) were eligible and enrolled in the trial. The majority of refusals (69%) were related to inconvenience (time and travel), while the main reason for ineligibility was being too active (65%). Women were randomized to AET (n=37), RET (n=39) or FLEX (n=38) groups. On average, participants were 72 years of age, inactive, overweight/obese based on BMI and had an additional comorbidity other than cancer (Table 2). Thirty-five women (31% of the sample) had SPPB scores ≤ 9 at baseline, 39% had a history of falls in the year prior to enrollment, but as a whole the sample reported functioning independently. There were no differences in baseline characteristics across study groups.
Table 2.
Demographic and clinical characteristics of the full sample and by study arm.
| Full Sample (n = 114) |
RET (n = 39) |
AET(n = 37) |
FLEX (n = 38) |
||
|---|---|---|---|---|---|
| Characteristic | Mean (SD) or % of sample | Range | Mean (SD) or % of sample | Mean (SD) or % of sample | Mean (SD) or % of sample |
| Age (yrs) | 70.9(5.1) | 64–87 | 70.6 (5.4) | 71.1 (4.6) | 70.9 (5.4) |
| White/Caucasian | 97% | 100% | 100% | 95% | |
| Non-Hispanic | 100% | 100% | 100% | 100% | |
| Charlson Comorbidity lndexA | 2.0 (1.7) | 0–8 | 1.9 (1.7) | 1.8 (1.3) | 2.4 (1.9) |
| BMI (kg/m2) | 29.2 (5.9) | 17.4–51.8 | 27.5 (4.6) | 29.9 (7.1) | 30.2 (5.4) |
| Time since diagnosis (months) | 87.1 (45.7) | 22–233 | 84.1 (45.9) | 87.1 (472) | 90.4 (44.9) |
| Stage | |||||
| Stage 0 (%) | 12% | 8% | 17% | 14% | |
| Stage I (%) | 47% | 55% | 49% | 41% | |
| Stage II (%) | 30% | 26% | 31% | 35% | |
| Stage III (%) | 8% | 11% | 3% | 11% | |
| Received chemotherapy (%) | 46% | 46% | 41% | 50% | |
| Received radiation therapy (%) | 84% | 87% | 84% | 84% | |
| Currently on hormone treatment (%)B | 68% | 69% | 75% | 64% | |
| Energy expenditure - all exercise (kcal/day)C | 327 (246) | 0–1604 | 289.6(192.4) | 349.6(285.5) | 344.0(255.7) |
| Energy expenditure - moderate intensity exercise (kcal/day)C | 135(166) | 0–896 | 131.2(128.3) | 154.6(198.4) | 117.8(116.8) |
| Self-report disabilityD | 81.9(14.9) | 38.6–100 | 82.1 (15.0) | 81.6(14.0) | 82.0(15.3) |
| Self-report fall history (1 + falls in past year) | 39% | 41% | 37% | 38% | |
RET: Resistance Exercise Training group; AET: Aerobic Exercise Training group; FLEX: Flexibility control group
Charlson Comorbidity index includes non-metastatic cancer if treatment was within 5 years and/or metastatic cancer.
Includes selective estrogen receptor modulator (SERM) and aromatase inhibitor (AI) use
Self-reported using the Community Health Activity Model Program for Seniors (CHAMPS) physical activity survey.
Self-reported disability subscale from the Late Life Function and Disability Instrument (scale 0–100) where higher scores indicate greater ability to perform daily activities independently
Retention, Adherence and Compliance
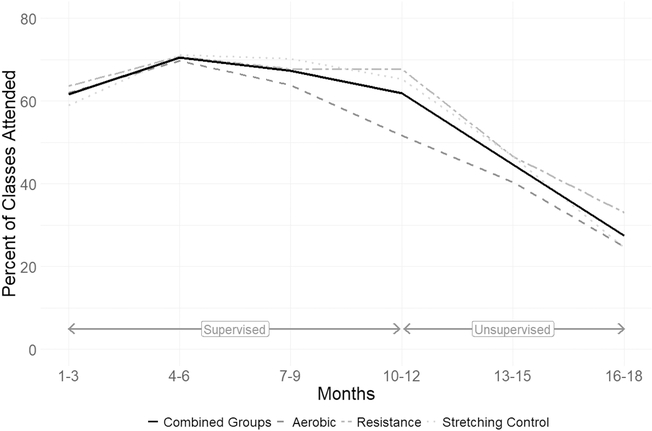
Participant retention over the 12-month supervised intervention was 77% while retention during unsupervised exercise averaged 91% (Fig 1). The majority of women dropped out for health-related reasons and dropouts did not differ across groups. Compared to women who remained in the study, dropouts had a significantly higher BMI and were further from diagnosis at enrollment. Adherence (number of sessions completed, expressed as a % of sessions prescribed) to supervised training averaged 72% ± 24% over 12 months and dropped to 43% ± 40% during the six months of unsupervised training (Fig 2), but was similar across groups. Compliance to the prescribed intensity of lower and upper body resistance training was tracked by vest weight and push-up RM and to aerobic training by the % of the prescribed target HRR reached during training, sampled every three months. For RET, the median final %BW in weight vests was 10% and the median RM for pushups was 12. For AET, the median % of target HRR reached was 97%. Three women in RET and 1 woman in FLEX could not fully comply with the prescribed program due to pre-existing orthopedic limitations that restricted movement and were provided alternative exercises within each modality. No AEs were reported from participation in either study program.
Figure 1.
CONSORT diagram.
Figure 2.
Session attendance by study group and combined for all groups across supervised and unsupervised phases of training.
Supervised Training
When examining within group changes over one year of supervised exercise, improvements occurred in the following outcomes (Table 3 and 5): physical function (SPPB) in all groups, aerobic capacity (6MWD) in RET and AET, muscle strength (1-RM bench press) in all groups, lower body flexibility (chair sit-and-reach) in RET and FLEX. For physical functioning (Table 3), only RET achieved improvements in SPPB score and 6MWD that corresponded to clinically meaningful change. Self-report physical function improving more in AET or RET compared to FLEX using the SF-36 subscale (both, p=0.066) and RET improved lower extremity function more than FLEX using the LLFDI (0=0.064). For fidelity outcomes (Table 5), upper body muscle strength increased more in RET (+2.5 kg) versus FLEX (+1.1 kg; p=0.048), lower body muscle strength increased more in RET versus AET (8.2 kg vs 2.7 kg, respectively, p=0.037), while aerobic capacity increased more in RET (+57.3m) compared to FLEX (+20.4m; p=0.048). After BH adjustment, though, differences were no longer significant.
Table 3.
Baseline physical functioning and within group change over supervised exercise (0–12 months) presented as point estimate with 95% confidence intervals and p-values for group by time interactions.
| Outcome A | RET (n=39) | AET (n=37) | FLEX (n=38) | p-value for interactions B | |||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | (95% CI) | Estimate | (95% CI) | Estimate | (95% CI) | AET vs. FLEX | RET vs. FLEX | AET vs. RET | |
| Chair Stand (sec) | |||||||||
| Baseline | 13.1 | (11.9, 14.3) | 12.4 | (11.2, 13.6) | 13.1 | (11.8, 14.3) | |||
| Within group change (0–12 mos.) | −2.3 | (−3.2, −1.4) | −1.2 | (−2.3, −0.2) | −2.1 | (−3.1, −1.1) | 0.235/0.658 | 0.799/0.866 | 0.235/0.658 |
| Walk Speed (m/s) | |||||||||
| Baseline | 1.02 | 0.98, 1.07) | 1.06 | (1.01, 1.11) | 1.04 | (0.99, 1.09) | |||
| Within group change (0–12 mos.) | 0.04 | (0.00, 0.08) | 0.04 | (−0.01, 0.08) | 0.06 | (0.01, 0.10) | 0.526/0.736 | 0.525/0.736 | 0.976/0.978 |
| SPPB Sum C | |||||||||
| Baseline | 10.3 | (9.8, 10.7) | 10.5 | (10.0, 11.0) | 10.3 | (9.8, 10.8) | |||
| Within group change (0–12 mos.) | 1.0 | (0.6, 1.4) | 0.6 | (0.1, 1.1) | 0.8 | (0.3, 1.2) | 0.602/0.745 | 0.471/0.736 | 0.211/0.658 |
| SF-36 Physical Function D | |||||||||
| Baseline | 74.0 | (68.0, 79.9) | 75.8 | (69.7, 81.9) | 76.4 | (70.3, 82.5) | |||
| Within group change (0–12 mos.) | 4.8 | (−2.0, 11.7) | 5.3 | (−2.1, 12.6) | −4.4 | (−11.6, 2.7) | 0.066/0.554 | 0.066/0.554 | 0.144/0.658 |
| LLFDI Upper Extremity Function E | |||||||||
| Baseline | 82.9 | (79.3, 86.6) | 81.5 | (77.8, 85.3) | 82.7 | (78.9, 86.4) | |||
| Within group change (0–12 mos.) | −0.4 | (−3.5, 2.8) | 1.2 | (−2.2, 4.6) | 1.2 | (−2.0, 4.5) | 0.978/0.978 | 0.490/0.736 | 0.519/0.736 |
| LLFDI Lower Extremity Function E | |||||||||
| Baseline | 81.8 | (77.7, 86.0) | 83.5 | (79.3, 87.7) | 82.5 | (78.3, 86.7) | |||
| Within group change (0–12 mos.) | 1.0 | (−2.2, 4.2) | 0.0 | (−3.5, 3.5) | −3.4 | (−6.7, 0.0) | 0.170/0.658 | 0.064/0.554 | 0.685/0.799 |
| LLFDI Advance Lower Extremity FunctionE | |||||||||
| Baseline | 59.4 | (55.1, 63.7) | 60.4 | (56.0, 64.8) | 61.5 | (57.1, 65.9) | |||
| Within group change (0–12 mos.) | −0.3 | (−3.1, (−3.1, | 2.0 | (−1.1, 5.1) | −1.2 | (−4.2, 1.8) | 0.155/0.58 | 0.670/0.799 | 0.296/0.736 |
RET: Resistance Exercise Training group; AET: Aerobic Exercise Training group; FLEX: Flexibility control group
All models controlled for age, baseline body mass index, and Charlson Comorbidity Index.
Original unadjusted p-values are provided followed by Bejamini-Hochberg adjusted p-values.
Short Physical Performance Battery
SF-36 Physical Function = physical function subscale from the MOS 36-Item Short Form Health Survey
Subscales from the Late Life Function and Disability Instrument (LLFDI)
Table 5.
Physical fitness measures of program fidelity including baseline and within group change over supervised exercise (0–12 months) using point estimate with 95% confidence intervals and p-values for group by time interactions.
| Measure A | RET (n=39) | AET (n=37) | FLEX (n=38) | p-value B | |||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | (95% CI) | Estimate | (95% CI) | Estimate | (95% CI) | AET vs. FLEX | RET vs. FLEX | AET vs. RET | |
| 1 RM Bench Press (kg) | |||||||||
| Baseline | 21.0 | (19.4, 22.5) | 21.9 | (20.3, 23.5) | 22.3 | (20.7, 23.9) | |||
| Change from 0 - 12 months | 2.5 | (1.6, 3.5) | 1.7 | (0.6, 2.8) | 1.1 | (0.0, 2.1) | 0.396/0.580 | 0.048/0.192 | 0.288/0.533 |
| 1 RM Leg Press (kg) | |||||||||
| Baseline | 65.1 | (59.7, 70.5) | 66.7 | (61.2, 72.3) | 69.9 | (64.4, 75.5) | |||
| Change from 0 – 12 months | 8.2 | (4.8, 11.7) | 2.7 | (−1.2, 6.5) | 5.4 | (1.7, 9.15) | 0.311/0.533 | 0.280/0.533 | 0.037/0.192 |
| 6 Minute Walk (m) | |||||||||
| Baseline | 475.3 | (441.2, 509.4) | 501.7 | (467.1, 536.3) | 483.1 | (448.5, 517.7) | |||
| Change from 0 – 12 months | 57.3 | (32.1, 82.5) | 31.1 | (4.0, 58.2) | 20.4 | (−5.7, 46.5) | 0.580/0.696 | 0.048/0.192 | 0.167/0.501 |
| Chair Sit and Reach (cm)C | |||||||||
| Baseline | −1.7 | (−5.4, 2.1) | −0.2 | (−4.1, 3.6) | −1.9 | (−5.7, 2.0) | |||
| Change from 0 – 12 months | 3.2 | (0.9, 5.5) | 1.9 | (−0.6, 4.4) | 2.5 | (0.1, 4.9) | 0.738/0.738 | 0.679/0.738 | 0.435/0.580 |
RET: Resistance Exercise Training group; AET: Aerobic Exercise Training group; FLEX: Flexibility control group
All models controlled for age, baseline body mass index, and Charlson Comorbidity Index.
Original unadjusted p-values are provided followed by Bejamini-Hochberg adjusted p-values.
Negative values for chair sit and reach indicate distance between fingers and toes during reach whereas positive values indicate degree of overlap between fingers and toes. Values moving from negative to positive indicate increased range of motion
Unsupervised Training (Table 4)
Table 4.
Within group changes during unsupervised exercise (months 12–18) presented as point estimate with 95% confidence intervals and p-values for group by time interactions.
| RET (n=39) | AET (n=37) | FLEX (n=38) | p-value for interactions B | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome A | Estimate | (95% CI) | Estimate | (95% CI) | Estimate | (95% CI) | AET vs. FLEX | RET vs. FLEX | AET vs. RET |
| Chair Stand (sec) | 0.0 | (−0.9, 0.9) | 0.2 | (−0.8, 1.1) | −0.2 | (−1.2, 0.7) | 0.549/0.744 | 0.707/0.803 | 0.804/0.866 |
| Walk Speed (m/s) | 0.04 | (0.00, 0.08) | 0.01 | (−0.04, 0.05) | −0.02 | (−0.06, 0.02) | 0.424/0.736 | 0.057/0.554 | 0.306/0.736 |
| SPPB Sum C | −0.3 | (0.6, 0.1) | −0.1 | (−0.5, 0.3) | 0.2 | (−0.2, 0.6) | 0.404/0.736 | 0.098/0.658 | 0.451/0.736 |
| SF-36 Physical Function D | −1.9 | (−8.6, 4.8) | −2.9 | (−10.0, 4.1) | 1.8 | (−4.8, 8.3) | 0.343/0.736 | 0.444/0.736 | 0.840/0.882 |
| LLFDI Upper Extremity Function E | −2.1 | (−5.6, 1.3) | −3.5 | (−7.1, 0.1) | 0.0 | (−3.4, 3.4) | 0.177/0.658 | 0.389/0.736 | 0.603/0.745 |
| LLFDI Lower Extremity Function E | −1.0 | (−4.4, 2.3) | 0.9 | (−2.6, 4.5) | 2.3 | (−1.0, 5.6) | 0.573/0.745 | 0.161/0.658 | 0.430/0.736 |
| LLFDI Advance Extremity Function E | 1.3 | (−1.6, 4.2) | −3.1 | (−6.2, −0.5) | −1.3 | (−4.1, 1.6) | 0.381/0.736 | 0.222/0.658 | 0.043/0.554E |
RET: Resistance Exercise Training group; AET: Aerobic Exercise Training group; FLEX: Flexibility control group
All models controlled for age, baseline body mass index, and Charlson Comorbidity Index.
Original unadjusted p-values are provided followed by Bejamini-Hochberg adjusted p-values.
Short Physical Performance Battery
SF-36 Physical Function = physical function subscale from the MOS 36-Item Short Form Health Survey
Subscales from the Late Life Function and Disability Instrument (LLFDI)
Women in RET reported increases in advanced lower extremity function over 6 months of unsupervised training at home, which differed from declines in AET (p=0.043), but not from FLEX (p=0.22), who did not change; however, significance was lost after applying a BH adjustment. There were no other significant within or between group differences in rates of change during unsupervised training, suggesting that most outcomes remained steady over time across all groups.
DISCUSSION
Our trial is the first exercise intervention specific to older (65+ years) breast cancer survivors using supervised exercise, directly comparing two recommended exercise modalities, and including both supervised and unsupervised settings. The lack of program modification, good compliance to prescribed training protocols, and absence of study-related injuries suggest that low-moderate intensity resistance, aerobic, or stretching exercise is safe and feasible in older women with breast cancer. Across modalities, supervised training improved several objective measures of physical fitness and function, though resistance training produced the most gains in muscle strength and aerobic capacity. Both aerobic and resistance training led to modest self-reported increases in physical functioning. After transitioning to unsupervised exercise at home for another six months most outcomes remained unchanged, except that advanced lower body physical functioning slightly improved with resistance training at home.
Our findings are consistent with the most recent ACSM exercise recommendations for cancer survivors, which suggest that lower amounts of exercise than recommended in the PAG for all adults is enough to improve many cancer-related health outcomes.47 New recommendations suggest that 30 minutes of moderate-vigorous intensity aerobic and/or resistance exercise performed 2–3 times per week for at least 12 weeks is sufficient to improve self-report physical functioning in most cancer survivors. However, age-specific recommendations were not made due to an insufficient number of trials in older survivors. Our findings suggest that the new recommendations might extend to older women with breast cancer, though more studies are needed to refine an exact prescription. Flexibility exercise was not included in the ACSM guidelines for cancer survivors due to heterogeneity of studies for this modality, but meta-analyses of yoga interventions that include stretching in cancer survivors,48 and some controlled trials of flexibility training in older adults without cancer49 suggest that this modality can improve mobility and range of motion, both of which contribute to physical functioning. Only women who did aerobic or resistance exercise reported improvements in self-report physical function that differed from declines in controls, though improvements did not reach those considered as clinically relevant raising a question of whether changes were enough to be meaningful to women. Aerobic and resistance training also improved 6MWD which is correlated with self-report physical function in older women.50
While we found benefits from any of the three types of exercise, resistance exercise improved more outcomes than aerobic or flexibility exercise, including upper and lower body muscle strength, aerobic capacity, and self-report lower-extremity function. Only improvements in SPPB scores in the resistance training group reached the level for meaningful change, though the absence of significant group differences means that we cannot imply resistance exercise is superior than other modalities at improving SPPB scores.23 Likewise, the resistance group was also the only study arm to achieve a clinically meaningful improvement in 6MWD. Muscle strength and mass have been positively associated with 6MWD in older adults33, while trials of healthy older adults49 or those in cardiac rehabilitation51 show that resistance training can improve 6MWD. Collectively, these improvements from RET could translate to a longer delay in disability52 in turn reducing the excess morbidity and mortality associated with cancer treatment. Contrary to our expectations, aerobic training did not improve 6MWD more than RET nor by a clinically meaningful amount. Perhaps the prescribed dose of aerobic exercise and/or the achieved frequency of training were insufficient to change this outcome.
Current ACSM guidelines suggest that supervised exercise may yield greater benefits than unsupervised training and may be safer in higher risk populations, such as older adults. However, most older cancer survivors prefer to exercise at home.53 We designed our study to first deliver exercise in a supervised setting where safety and efficacy could be maximized, but then transition women to home-based training so women could maintain benefits long term. To reduce confounding, we did not add a behavioral support intervention during the transition from supervised to unsupervised training. Retention and adherence across supervised training was good for a yearlong facility-based scheduled program in older adults, but adherence dropped considerably when women transitioned to home-based training. Though older survivors cite a preference for home-based training, women may have lost motivation to train as often due to the loss of social support and instructor attention. The lower frequency of training, though, seemed sufficient to maintain benefits gained from supervised exercise - a pattern consistent with recommendations for the maintenance phase of exercise training where exercise volume can be less than that needed to produce initial adaptations.15 On the other hand, if women continued to train more often they may have continued to improve. Future studies should consider implementing behavioral strategies to sustain exercise adherence to home-based training or possibly online delivery of exercise which has evolved since the COVID-19 pandemic.
Strengths of our study included the direct comparison of aerobic to resistance training, inclusion of both objective and self-report measures of physical functioning, and the inclusion of both supervised and unsupervised settings. We also included women with comorbidities and physical limitations as long as they did not contraindicate exercise to ensure our sample was representative of older breast cancer survivors. One-third of our sample had low SPPB scores, which could further explain why even lower intensity exercise, such as stretching, improved this outcome. Our study also had notable limitations. Though we have used a stretching control group in previous studies focused on bone outcomes37, 54, 55, this group may not have been an appropriate control group for a study focused on physical functioning. Since exercise is currently recommended for all cancer survivors, we felt it was unethical to withhold exercise altogether – a tradeoff all controlled exercise trials must struggle with. In studies of older adults without cancer, flexibility exercise can improve mobility and physical activity.49, 56 Thus, we may be underestimating the full benefit of aerobic or resistance exercise, possibly even stretching exercise, in older breast cancer survivors. While our trial was appropriately powered, those estimates were derived from samples in older adults without cancer. Several of our outcomes nearly reached significance and trends were consistent between subjective and objective measures. However, after statistical adjustment for multiple testing significance was lost thus, it is possible we were underpowered and needed a large sample to account for variability in effort and responsiveness across older breast cancer survivors. It is also possible, though, that adaptations took longer to occur given our lower starting intensity, as evidenced by improvements in functioning that emerged at 18 months. Though we prescribed exercise cautiously in this first study in older women, their high tolerance to training suggests that future trials might progress intensity more quickly or start at a higher level. Additional limitations include an inability to generalize our findings to older breast cancer survivors living far from an academic health center nor to those who are not white or non-Hispanic, and modest attrition over the 18-month study. Attrition was mostly due to health-related reasons that prevented women from resuming supervised exercise, thus future studies should consider whether alternate conditions could be implemented when participants have a health setback.
Older women, with and without cancer, are the most inactive and overweight segment of the U.S. population.57, 58 In older adults, inactivity is associated with reduced endurance and weakness and decreased physical functioning,59–62 a pattern that may have more dire implications in breast cancer survivors than in the general population. These limitations may begin a downward spiral of even less activity, loss of independence, and life-threatening falls and fractures.63–66 Our study showed that breast cancer survivors over the age of 65 can safely participate in supervised and unsupervised low-moderate intensity exercise and that any of the recommended modalities may have benefits, but efforts to boost and sustain adherence to long-term exercise need to be studied. Engaging older women in resistance training may lead to the broadest array and degree of benefits and future efforts should be directed at implementation strategies in practice, both at the clinic and community level.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to thank Jessica Sitemba for her contributions to the intervention delivery and data management for this study and Pablo Herrera-Fuentes for his contributions to the management and analysis of heart rate monitoring data.
Funding:
R01CA120123 to Dr. Winters-Stone. Dr. Winters-Stone is also funded in part by Cancer Center Support Grant 5P30CA069533-23.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts to declare
Disclosures: This report does not reflect the opinions of the Veterans Administration nor those of the US Government
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.National Cancer Institute. SEER: Surveillance, Epidemiology, and End Results: National Cancer Institute, 2018. [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2018. Atlanta: American Cancer Society, 2018. [Google Scholar]
- 3.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiology Biomarkers & Prevention. 2016;25: 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: a cause of early onset frailty? Med Hypotheses. 2006;67: 212–215. [DOI] [PubMed] [Google Scholar]
- 5.Sweeney C, Schmitz KH, Lazovich D, Virnig BA, Wallace RB, Folsom AR. Functional limitations in elderly female cancer survivors. Journal of the National Caner Institute. 2006;98: 521–529. [DOI] [PubMed] [Google Scholar]
- 6.Keating NL, Norredam M, Landrum MB, Huskamp HA, Meara E. Physical and mental health status of older long-term cancer survivors. J Am Geriatr Soc. 2005;53: 2145–2152. [DOI] [PubMed] [Google Scholar]
- 7.Sehl M, Lu X, Silliman R, Ganz PA. Decline in physical functioning in first 2 years after breast cancer diagnosis predicts 10-year survival in older women. J Cancer Surviv. 2013;7: 20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courneya KS, Vallance JK, McNeely ML, Karvinen KH, Peddle CJ, Mackey JR. Exercise issues in older cancer survivors. Crit Rev Oncol Hematol. 2004;51: 249–261. [DOI] [PubMed] [Google Scholar]
- 9.Mandelblatt J, Figueiredo M, Cullen J. Outcomes and quality of life following breast cancer treatment in older women: When, why, how much, and what do women want? Health Qual Life Outcomes. 2003;1: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320: 2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ottenbacher A, Yu M, Moser RP, Phillips SM, Alfano C, Perna FM. Population Estimates of Meeting Strength Training and Aerobic Guidelines, by Gender and Cancer Survivorship Status: Findings From the Health Information National Trends Survey (HINTS). J Phys Act Health. 2015;12: 675–679. [DOI] [PubMed] [Google Scholar]
- 12.Winters-Stone KM, Wood LJ, Stoyles S, Dieckmann NF. The Effects of Resistance Exercise on Biomarkers of Breast Cancer Prognosis: A Pooled Analysis of Three Randomized Trials. Cancer Epidemiol Biomarkers Prev. 2018;27: 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett JA, Winters-Stone K, Nail LM, Scherer J. Definitions of sedentary in physical-activity-intervention trials: a summary of the literature. J Aging Phys Act. 2006;14: 456–477. [DOI] [PubMed] [Google Scholar]
- 14.Chodzko-Zajko WJPD, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS. American College of Sports Medicine Position Stand: Exercise and Physical Activity for Older Adults. Med. Sci. Sports Exerc. 2009;41: 1510–1530. [DOI] [PubMed] [Google Scholar]
- 15.Riebe D, Ehrman JK, Liguori G, & Magal M ACSM’s guidelines for exercise testing and prescription. 10th ed: Philadelphia: Wolters Kluwer, 2018. [Google Scholar]
- 16.Dunbar CC, Kalinski MI. Using RPE to regulate exercise intensity during a 20-week training program for postmenopausal women: a pilot study. Percept Mot Skills. 2004;99: 688–690. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhry S, Jin L, Meltzer D. Use of a self-report-generated Charlson Comorbidity Index for predicting mortality. Med Care. 2005;43: 607–615. [DOI] [PubMed] [Google Scholar]
- 18.Stewart A, Mills K, King A, Haskell W, Gillis D, Ritter P. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33: 1126–1141. [DOI] [PubMed] [Google Scholar]
- 19.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55: M221–231. [DOI] [PubMed] [Google Scholar]
- 20.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49: M85–94. [DOI] [PubMed] [Google Scholar]
- 21.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332: 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2000;55: M691–697. [DOI] [PubMed] [Google Scholar]
- 23.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54: 743–749. [DOI] [PubMed] [Google Scholar]
- 24.Jette AM, Haley SM, Coster WJ, et al. Late life function and disability instrument: I. Development and evaluation of the disability component. J Gerontol A Biol Sci Med Sci. 2002;57: M209–216. [DOI] [PubMed] [Google Scholar]
- 25.Haley SM, Jette AM, Coster WJ, et al. Late Life Function and Disability Instrument: II. Development and evaluation of the function component. J Gerontol A Biol Sci Med Sci. 2002;57: M217–222. [DOI] [PubMed] [Google Scholar]
- 26.McHorney CA, Ware JE, Raczek AE. The MOS 36-item short form health survey (SF-36): Psychometric and clinical tests of validity in measuring physical and mental health construct. Medical Care. 1993;3: 247–263. [DOI] [PubMed] [Google Scholar]
- 27.Beauchamp MK, Ward RE, Jette AM, Bean JF. Meaningful Change Estimates for the Late-Life Function and Disability Instrument in Older Adults. The Journals of Gerontology: Series A. 2018;74: 556–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enright PL, McBurnie MA, Bittner V, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123: 387–398. [DOI] [PubMed] [Google Scholar]
- 29.Zugck C, Kruger C, Durr S, et al. Is the 6-minute walk test a reliable substitute for peak oxygen uptake in patients with dilated cardiomyopathy? Eur Heart J. 2000;21: 540–549. [DOI] [PubMed] [Google Scholar]
- 30.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: A new measure of exercise capacity in patients with chronic heart failure. Canadian Medical Association Journal. 1985;132: 919–923. [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer K, Schwaibold M, Westbrook S, et al. Effects of exercise training and activity restriction on 6-minute walking test performance in patients with chronic heart failure. Am Heart J. 1997;133: 447–453. [DOI] [PubMed] [Google Scholar]
- 32.Lipkin DP, Scriven AJ, Crake T, Poole-Wilson PA. Six minute walking test for assessing exercise capacity in chronic heart failure. Br Med J (Clin Res Ed). 1986;292: 653–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grosicki GJ, Englund DA, Price L, et al. Lower-Extremity Torque Capacity and Physical Function in Mobility-Limited Older Adults. J Nutr Health Aging. 2019;23: 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997;155: 1278–1282. [DOI] [PubMed] [Google Scholar]
- 35.American College of Sports Medicine. ACSM’s guildelines for exercise testing and prescription. 7th ed. Philadelphia: Lippincott Williams & Wilkins, 2006. [Google Scholar]
- 36.Winters-Stone KM, Dobek J, Bennett JA, Nail LM, Leo MC, Schwartz A. The effect of resistance training on muscle strength and physical function in older, postmenopausal breast cancer survivors: a randomized controlled trial. J Cancer Surviv. 2012;6: 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winters-Stone KM, Dobek J, Nail LM, et al. Impact + resistance training improves bone health and body composition in prematurely menopausal breast cancer survivors: a randomized controlled trial. Osteoporos Int. 2013;24: 1637–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rikli R, Jones J. Senior Fitness Testing Manual. Champaign, IL: Human Kinetics, 2001. [Google Scholar]
- 39.Pinheiro JBD, DebRoy S, Sarkar D and R Core Team nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–128, 2016. [Google Scholar]
- 40.Schlomer GL, Bauman S, Card N. Best practices for missing data management in counseling psychology. Journal of Counseling Psychology. 2010;57: 1–10. [DOI] [PubMed] [Google Scholar]
- 41.Benjamini Y, Heller R, & Yekutieli D Selective inference in complex research. Philosophical Transactions of the Royal Society. 2009; 367, 4255–4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benjamini Y & Hochberg Y Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological). 1995; 57(1), pp. 289–300. [Google Scholar]
- 43.Benjamini Y & Yekutieli D The control of the false discovery rate in multiple testing under dependency. The Annals of Statistics. 2001; 29(4): 1165–1188. [Google Scholar]
- 44.White T, van der Ende J, & Nichols T Beyond Bonferroni revisited: concerns over inflated false positive research findings in the fields of conservation genetics, biology, and medicine. Conservation Genetics. 2019; 20:927–937. [Google Scholar]
- 45.Ware JE KM, Keller SD. SF-36 physical and mental health summary scores: a user’s manual.: Boston: The Health Institute, New England Medical Center, 1994. [Google Scholar]
- 46.Singh NA, Clements KM, Fiatarone MA. A randomized controlled trial of progressive resistance training in depressed elders. J Gerontol A Biol Sci Med Sci. 1997;52: M27–35. [DOI] [PubMed] [Google Scholar]
- 47.Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51: 2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Danhauer SC, Addington EL, Cohen L, et al. Yoga for symptom management in oncology: A review of the evidence base and future directions for research. Cancer. 2019;125: 1979–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Locks RR, Costa TC, Koppe S, Yamaguti AM, Garcia MC, Gomes AR. Effects of strength and flexibility training on functional performance of healthy older people. Rev Bras Fisioter. 2012;16: 184–190. [DOI] [PubMed] [Google Scholar]
- 50.Serra AJ, de Carvalho Pde T, Lanza F, et al. Correlation of six-minute walking performance with quality of life is domain- and gender-specific in healthy older adults. PLoS One. 2015;10: e0117359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Busch JC, Lillou D, Wittig G, et al. Resistance and balance training improves functional capacity in very old participants attending cardiac rehabilitation after coronary bypass surgery. J Am Geriatr Soc. 2012;60: 2270–2276. [DOI] [PubMed] [Google Scholar]
- 52.Puthoff ML, Nielsen DH. Relationships Among Impairments in Lower-Extremity Strength and Power, Functional Limitations, and Disability in Older Adults. Physical Therapy. 2007;87: 1334–1347. [DOI] [PubMed] [Google Scholar]
- 53.Wong JN, McAuley E, Trinh L. Physical activity programming and counseling preferences among cancer survivors: a systematic review. Int J Behav Nutr Phys Act. 2018;15: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winters-Stone K, Dobek J, Nail L, Bennett JA, Naik A, Schwartz A. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2011;27: 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winters-Stone KM, Dobek JC, Bennett JA, Maddalozzo GF, Ryan CW, Beer TM. Skeletal response to resistance and impact training in prostate cancer survivors. Med Sci Sports Exerc. 2014;46: 1482–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bird ML, Hill K, Ball M, Williams AD. Effects of resistance- and flexibility-exercise interventions on balance and related measures in older adults. J Aging Phys Act. 2009;17: 444–454. [DOI] [PubMed] [Google Scholar]
- 57.Centers for Disease Control and Prevention. Physical Activity Among Adults: United States, 2000. Washington DC: U.S. Department of Health and Human Services, May 14, 2003. [Google Scholar]
- 58.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of Overweight and Obesity Among US Children, Adolescents, and Adults, 1999–2002. JAMA. 2004;291: 2847–2850. [DOI] [PubMed] [Google Scholar]
- 59.Borst SE. Interventions for sarcopenia and muscle weakness in older people. Age Ageing. 2004;33: 548–555. [DOI] [PubMed] [Google Scholar]
- 60.Courneya KS, Mackey JR, McKenzie DC. Exercise for breast cancer survivors. The Physician and Sportsmedicine [serial online] 2002; 30(8):33–42. [DOI] [PubMed] [Google Scholar]
- 61.Demark-Wahnefried W, Clipp EC, Morey MC, et al. Physical function and associations with diet and exercise: Results of a cross-sectional survey among elders with breast or prostate cancer. Int J Behav Nutr Phys Act. 2004;1: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McTiernan A Physical activity after cancer: physiologic outcomes. Cancer Invest. 2004;22: 68–81. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X, Sun M, Liu S, et al. Risk factors for falls in older patients with cancer. BMJ Supportive & Palliative Care. 2017; 8(1):34–37. [DOI] [PubMed] [Google Scholar]
- 64.Pamoukdjian F, Lévy V, Sebbane G, et al. Slow gait speed is an independent predictor of early death in older cancer outpatients: Results from a prospective cohort study. J Nutr Health Aging. 2017;21: 202–206. [DOI] [PubMed] [Google Scholar]
- 65.Leach CR, Bellizzi KM, Hurria A, Reeve BB. Is it my cancer or am i just getting older?: Impact of cancer on age-related health conditions of older cancer survivors. Cancer. 2016;122: 1946–1953. [DOI] [PubMed] [Google Scholar]
- 66.Derks MGM, de Glas NA, Bastiaannet E, et al. Physical Functioning in Older Patients With Breast Cancer: A Prospective Cohort Study in the TEAM Trial. The Oncologist. 2016;21: 946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.