Abstract
Tetragonal barium titanate nanoparticles (BTNPs) have been exploited as nanotransducers owing to their piezoelectric properties, in order to provide indirect electrical stimulation to SH-SY5Y neuron-like cells. Following application of ultrasounds to cells treated with BTNPs, fluorescence imaging of ion dynamics revealed that the synergic stimulation is able to elicit a significant cellular response in terms of calcium and sodium fluxes; moreover, tests with appropriate blockers demonstrated that voltage-gated membrane channels are activated. The hypothesis of piezoelectric stimulation of neuron-like cells was supported by lack of cellular response in the presence of cubic nonpiezoelectric BTNPs, and further corroborated by a simple electroelastic model of a BTNP subjected to ultrasounds, according to which the generated voltage is compatible with the values required for the activation of voltage-sensitive channels.
Keywords: barium titanate nanoparticles, ultrasounds, piezoelectricity, SH-SY5Y cells, calcium imaging
The possibility to stimulate/modulate the neural activity is often limited by the restricted accessibility of the nervous system,1 and several innovative techniques are being explored in order to address this challenge. Among these, the rapid development of genetically encoded actuators/sensors, i.e., optogenetics,2 is allowing for the detailed investigation of the mechanisms of various diseases, especially those presenting a neuronal etiology, in different animal models, thanks to a fine cell activity modulation/monitoring.3−7 However, the exploitation of optogenetics is currently limited by several complications, including the phototoxicity8 and the scarce penetration of the light through the tissues due to their opacity at the suitable wavelengths.9 Other well-characterized neural stimulation approaches are represented by the deep brain stimulation (DBS),10 the trans-cranial direct current stimulation (tDCS),11,12 and the trans-cranial magnetic stimulation (TMS).13 Drawbacks of DBS are the necessity of an invasive surgical operation, followed by inflammation and gliosis at the implant site, while the main disadvantage of the tDCS and TMS is the low spatial resolution (a brain volume of about 1 cm in diameter).
Ultrasounds (US) can be instead exploited for trans-cranial stimulation without the requirement of surgical processes, and with a resolution of about 3 mm,14 which can be further improved by the use of hyperlenses and acoustic metamaterials.15,16 However, the heterogeneous results obtained with the US stimulation, that in certain conditions may induce neural silencing rather than excitation, are influenced by the physical parameters of the US, including frequency and power. Recently, Tufail et al. reported an exhaustive summary of the US-mediated neuromodulation effects observed by using different US parameters and different neural models.17
US can be exploited in combination with piezoelectric materials in order to generate direct-current output:18,19 indeed, piezoelectric materials are able to efficiently generate electricity in response to the US mechanical stimulations. Taking advantage of the piezoelectricity of different nanomaterials, such as ZnO nanowires and BaTiO3 nanoparticles (BTNPs), it is possible to obtain US-driven nanogenerators able to generate output currents inside biological liquid for energy harvesting and for self-powered electronics.20−22 In a previous work of our group, the US-driven piezoelectric stimulation of PC12 neural-like cells was performed for the first time by using boron nitride nanotubes (BNNTs).23 In this work, a significant enhancement of the neurite outgrowth was observed in response to the combined US + BNNT stimulus, with respect to the US stimulus without the presence of the piezoelectric nanoparticles; moreover, the significant increase of the neurite length dependent by the US + BNNT stimulation was suggested to be mediated by a Ca2+ influx. However, the impossibility to perform electrophysiological recordings in conjunction with the US stimulation has not allowed the mechanisms giving rise the Ca2+ influx (i.e., piezoelectric-mediated plasma membrane depolarization) to be investigated, and consequently the different ion currents involved to be elucidated.
In this work, we carried out neural stimulation with US and piezoelectric BTNPs characterized by a tetragonal crystalline configuration, and the effects of both US and US + BTNP stimulations on SH-SY5Y-derived neurons were deeply investigated, by exploiting imaging techniques for detecting the Ca2+/Na+ fluxes and temperature levels. Our results show as the stimulation with US + piezoelectric BTNPs was able to induce tetrodotoxin (TTX) and cadmium (Cd2+) sensitive high-amplitude Ca2+ transients, and that the observed transients are specifically evoked thanks to the piezoelectric properties of these nanoparticles with single cell resolution. Our findings thus strongly support the hypothesis that the piezoelectric stimulation is able to induce a Ca2+ influx, which likely mediates the enhancement of the neurite outgrowth and neural differentiation,23,24 and suggest the possible use of this approach as a wireless tool to modulate neuronal activity even deeply in vivo, by a combination of piezoelectric nanoparticles and a pulse of ultrasounds.
Results
Tetragonal Barium Titanate Nanoparticle Characterization
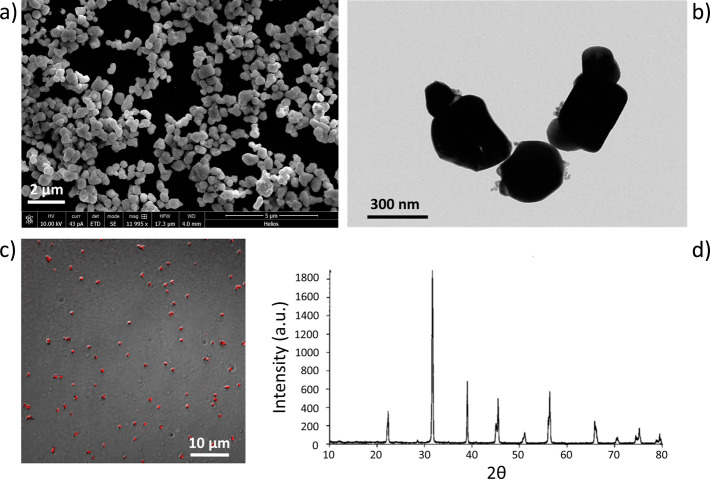
Images of the BTNPs used in this work, wrapped with gum Arabic, are provided in Figure 1a (scanning electron microscopy, SEM) and Figure 1b (transmission electron microscopy, TEM). These observations revealed quite well-dispersed structures, with some aggregates of a few nanoparticles. The hydrodynamic size of the BTNPs, measured through dynamic light scattering (DLS), resulted of 479.0 ± 145.3 nm, with a polydispersity index of 0.180. The Z-potential was −40.4 ± 5.2 mV, highlighting an excellent stability of the dispersion. Hydrodynamic size and Z-potential were evaluated also in experimental conditions (50 μg/mL of BTNPs in artificial cerebrospinal fluid) and resulted 573.3 ± 173.8 nm and −17.2 ± 1.2 mV, respectively. Most useful for tracking in cell experiments, BTNPs resulted visible through confocal fluorescence imaging (Figure 1c), by using an excitation at 633 nm and a collection from 645 to 745 nm. From XRD analysis (Figure 1d), BTNPs resulted to have a perovskite-like crystallographic structure. Tetragonal phase of BaTiO3, typical of piezoelectric nanoparticles, was detected, with two close peaks at 2θ = 44.85° and 2θ = 45.38°.25
Figure 1.
Barium titanate nanoparticles (BTNPs) characterized by a tetragonal crystalline structure. SEM (a), TEM (b) and confocal fluorescence (c) imaging of BTNPs. In (c), confocal fluorescence image (red) is merged with the transmitted light image (gray) of the same field of view. The crystallographic structure revealed thanks to the XRD analysis shows two close peaks at 2θ = 44.85° and 2θ = 45.38°, specific of the tetragonal configuration of the crystal (d).
US-Driven BTNP Stimulation Induces Ca2+ Transients
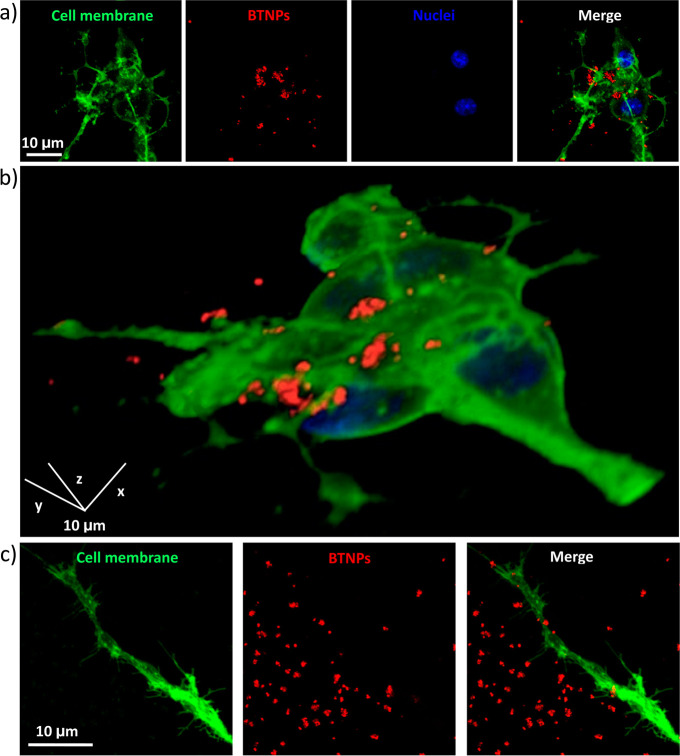
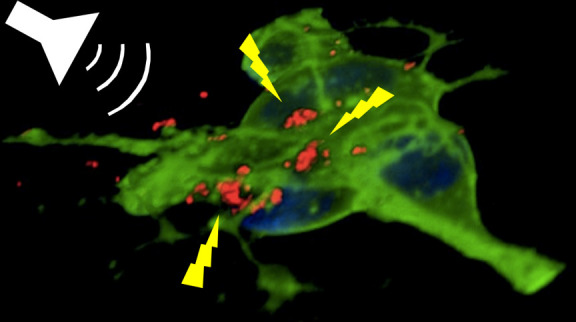
In order to evaluate the BTNPs/neurons interaction, confocal fluorescence microscopy was carried out (Figure 2). Particularly, high amount of BTNPs (in red) was observed associated with the plasma membrane (in green) of the SH-SY5Y-derived neurons after 24 h from the BTNP treatment (Figure 2a); Figure 2b depicts the 3D rendering of a confocal z-stack acquisition (see also Video S1, provided as Supporting Information). From all these observations, we can conclude as nanoparticles are mainly associated with the plasma membrane without a significant cellular internalization. Finally, BTNPs were observed associated not only to the membrane of the SH-SY5Y cell bodies, but also to their neurites, as shown by Figure 2c.
Figure 2.
Confocal fluorescence microscopy of BTNPs associating to the neuronal plasma membranes. (a) Characteristic confocal z-stack of BTNP-treated SH-SY5Y-derived neurons (neuronal plasma membranes in green, BTNPs in red and nuclei in blue). (b) 3D rendering of the confocal z-stacks of the same field as in (a). BTNPs were also detected associating to the neurite membranes (c).
Cellular viability following the experimental procedures was confirmed by WST-1 metabolic activity assay and propidium iodide staining, the latter evaluating the integrity of the cellular membrane after the stimulation. Four experimental groups were considered: control cultures, cultures treated with BTNPs (50 μg/mL), cultures treated with US (0.8 W/cm2), and cultures treated with both BTNPs (50 μg/mL) and US (0.8 W/cm2). Results are provided in Figure S1 as Supporting Information, and highlight no statistically significant differences among the different treatments in terms of both metabolic activity (Figure S1a, evaluated after 24 h since the stimulation) and membrane integrity (Figure S1b, evaluated immediately after the stimulation in order to highlight also temporarily phenomena of membrane permeabilization).
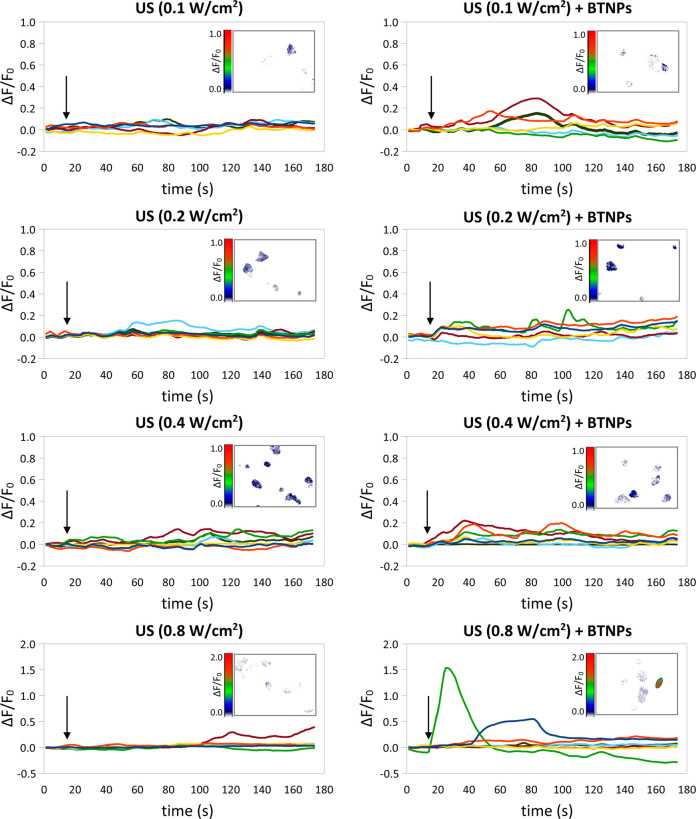
After the evaluation of the BTNP/cell interactions at the level of the plasma membrane, we monitored the intracellular Ca2+ dynamics in response to the US stimulation performed at different intensities (0.1, 0.2, 0.4 and 0.8 W/cm2), with or without BNTPs (Figure 3). Interestingly, we observed that, by stimulating SH-SY5Y-derived neurons with BTNPs + US at 0.8 W/cm2, it was possible to activate high-amplitude Ca2+ transients (ΔF/F0 peak = 0.62 ± 0.12), significantly higher with respect to the low-amplitude Ca2+ transients detected in all the other conditions (p < 0.05), including the treatment with US at 0.8 W/cm2 but without BTNPs (ΔF/F0 peak = 0.15 ± 0.04; please refer to the Table 1 for the comparison of all of the peak amplitudes). Concerning the tests performed with US intensities < 0.8 W/cm2, no significant differences in terms of calcium transients amplitude evoked by US + BTNPs were found with respect to the plain US stimulation (p > 0.05). In the following results, relative to the investigation of the mechanisms at the base of the observed phenomenon, just the stimulation at the highest intensity (0.8 W/cm2) will be thus considered. Video S2, provided as Supporting Information, shows the Ca2+ imaging time-lapse course (18X accelerated) performed on cultures stimulated with US (0.8 W/cm2) in the presence of BTNPs. The US stimulation was applied at tvideo = 1 s.
Figure 3.
Calcium imaging of SH-SY5Y-derived neurons in response to the US stimulation performed at different intensities (0.1, 0.2, 0.4 and 0.8 W/cm2), with or without BNTPs: time courses of the ΔF/F0 traces. Arrows indicate the moment when the 5-s US pulse was initiated; in the inlet of each graph a representative calcium imaging time-lapse frame is reported (at t = 30).
Table 1. Calcium/Sodium Transient Amplitude (In Terms of ΔF/F0 Peak Average ± Standard Error) Measured in Response to the Different Stimulations and Treatments.
| calcium imaging | |||
|---|---|---|---|
| treatment | US | US + BTNPs | |
| 0.1 W/cm2 | No transients | 0.14 ± 0.03 | |
| 0.2 W/cm2 | 0.09 ± 0.02 | 0.15 ± 0.02 | |
| 0.4 W/cm2 | 0.16 ± 0.02 | 0.22 ± 0.04 | |
| 0.8 W/cm2 | 0.15 ± 0.04 | 0.62 ± 0.12 | |
| 0.8 W/cm2 | Cd2+ | N/A | 0.16 ± 0.02 |
| TTX | N/A | 0.14 ± 0.01 | |
| EGTA | 0.16 ± 0.04 | 0.12 ± 0.03 | |
| thapsigargin + EGTA | no transients | no transients | |
| gentamicin | 0.15 ± 0.05 | 0.78 ± 0.24 | |
| cubic crystal | 0.15 ± 0.04 | 0.12 ± 0.01 | |
| sodium imaging | |||
|---|---|---|---|
| treatment | US | US + BTNPs | |
| 0.8 W/cm2 | no transients | 0.032 ± 0.001 | |
| 0.8 W/cm2 | Cd2+ | N/A | 0.011 ± 0.001 |
| TTX | N/A | no transients | |
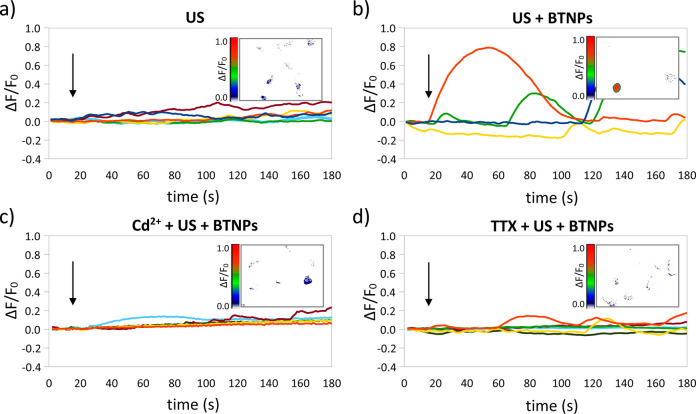
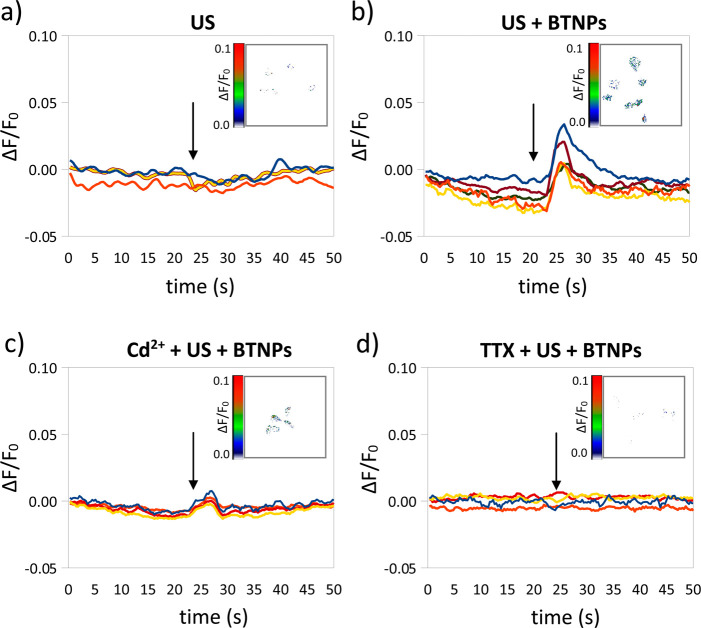
In order to investigate the ion channels involved in the observed Ca2+ transients, Ca2+ imaging experiments were performed in the presence of blockers either of the voltage-gated Ca2+ channels (Cd2+) or of the voltage-gated Na+ channels (TTX) (Figure 4).26,27Figure 4a–d are representative ΔF/F0 traces relative to Ca2+ imaging time-lapses of neurons stimulated by US, US + BTNPs, Cd2+ + US + BTNPs, and TTX + US + BTNPs, respectively; in the inlet of each graph a representative Ca2+ imaging time-lapse frame is reported (more time-lapse images are reported in Figure S2 as Supporting Information). In Figure 4a we can appreciate low-amplitude Ca2+ transients (ΔF/F0 peak = 0.15 ± 0.04) induced by the US stimulus. In Figure 4b, the US in the presence of BTNPs induce significantly higher Ca2+ peaks (ΔF/F0 peak = 0.62 ± 0.12; p < 0.05) compared to the US stimulation without nanoparticles. These high-amplitude Ca2+ transients were suppressed by both the Cd2+ (Figure 4c) and the TTX (Figure 4d) treatments. In both conditions (Cd2+ + US + BTNPs and TTX + US + BTNPs) we detected low-amplitude Ca2+ peaks (ΔF/F0 peak = 0.16 ± 0.02 in the presence of Cd2+ and ΔF/F0 peak = 0.14 ± 0.01 in the presence of TTX), similar to those observed by stimulating with US without BTNPs (ΔF/F0 peak = 0.15 ± 0.04; p > 0.05; Table 1), thus suggesting that the induction of the high-amplitude Ca2+ transients by the US + BTNPs stimulation is mediated by both Ca2+ and Na+ voltage-gated channels.
Figure 4.
US + BTNP stimulation (0.8 W/cm2) evokes Cd2+ and TTX-sensitive calcium transients. Representative ΔF/F0 traces relative to calcium imaging time-lapses of SH-SY5Y-derived neurons stimulated by US (a), US + BTNPs (b), US + BTNPs in the presence of Cd2+ (c) or TTX (d). Arrows indicate the moment when the 5-s US pulse was initiated; in the inlet of each graph a representative calcium imaging time-lapse frame is reported (at t = 50).
US-Driven BTNP Stimulation Induces TTX-Sensitive Na+ Transients
Possible effects of the stimulation on the Na+ fluxes were evaluated by performing Na+ imaging experiments on SH-SY5Y-derived neurons treated with US, US + BTNPs, Cd2+ + US + BTNPs, and TTX + US + BTNPs. Representative ΔF/F0 traces relative to Na+ imaging time-lapses are shown in Figure 5a–d; in the inlet of each graph a representative Na+ imaging time-lapse frame is reported (more time-lapse images are reported in Figure S3 as Supporting Information). The US stimulation without BTNPs was not able to induce any appreciable Na+ peak (Figure 5a), while a Na+ transient was clearly detected in response to the US + BTNP activation (ΔF/F0 peak = 0.032 ± 0.001; Figure 5). Na+ peaks of lower amplitude were revealed by stimulating SH-SY5Y-derived neurons with US + BTNPs in the presence of the voltage-gated Ca2+ channel blocker Cd2+ (ΔF/F0 peaks =0.011 ± 0.001; Figure 5c), while no peaks were detected in the TTX treatment (Figure 5d).
Figure 5.
US + BTNP stimulation (0.8 W/cm2) induces TTX-sensitive sodium transients. Representative ΔF/F0 traces relative to sodium imaging time-lapses of SH-SY5Y-derived neurons stimulated by US (a), US + BTNPs (b), US + BTNPs in the presence of Cd2+ (c) or TTX (d). Arrows indicate the moment when the 5-s US pulse was initiated; in the inlet of each graph a representative sodium imaging time-lapse frame is reported (at t = 27).
Investigation of Ca2+ Sources Involved in the US-Driven BTNP Stimulation
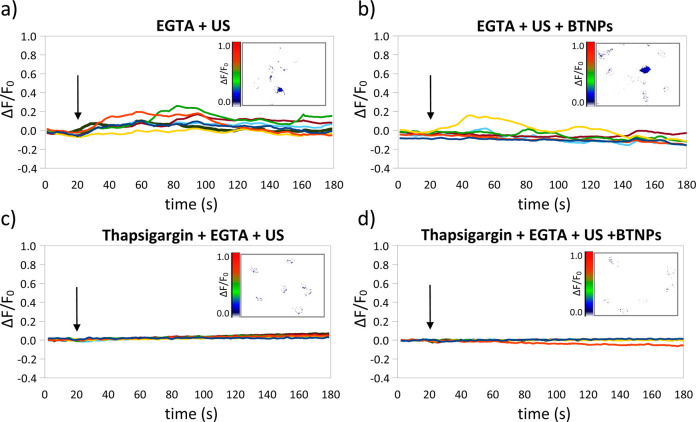
The Ca2+ sources involved in the stimulations, e.g., intracellular stores and/or extracellular environment, were investigated as reported in Figure 6 (in the inlet of each graph a representative Ca2+ imaging time-lapse frame is reported; more time-lapse images are reported in Figure S4 as Supporting Information). The US stimulation in Ca2+-free conditions (and in the presence of 5 mM of the Ca2+ chelator ethylene glycol tetraacetic acid, EGTA) induced low-amplitude Ca2+ transients (ΔF/F0 peak = 0.16 ± 0.04; Figure 6a), nonsignificantly different from those observed in standard conditions, i.e., in the presence of 2 mM extracellular Ca2+ (ΔF/F0 peak = 0.15 ± 0.04; p > 0.05; Figure 4a). This result suggests that the intracellular Ca2+ stores are implicated in the low-amplitude Ca2+ transients observed during the stimulations with US, but not in the presence of BTNPs. Interestingly, we also observed low-amplitude Ca2+ transients when stimulating with BTNPs and US in the EGTA-supplemented Ca2+-free medium (ΔF/F0 peak = 0.12 ± 0.03; Figure 6b). This low-amplitude peak is significantly lower compared to the high amplitude peak measured in standard conditions (i.e., 2 mM of extracellular Ca2+; ΔF/F0 peak = 0.62 ± 0.12; p < 0.05; Table1), suggesting that the US + BTNP stimulation, conversely to the plain US stimulation, is able to activate the Ca2+ influx through the plasma membrane. This result is in agreement with the previous observation highlighting that the high-amplitude peaks are inhibited by the treatments with TTX and Cd2+, which are blockers of the cell membrane voltage-gated channels.26,27 Further study suggests that the source of these low-amplitude Ca2+ transients is the endoplasmic reticulum (ER): both the low-amplitude peaks reported after the plain US (Figure 6c) and the US + BTNP (Figure 6d) stimulation in extracellular Ca2+-free conditions completely disappear by depleting the Ca2+ flux from ER with thapsigargin, suggesting that the ER represents the involved Ca2+ store.
Figure 6.
Calcium sources involved during US and US + BTNP stimulations (0.8 W/cm2). Representative ΔF/F0 traces relative to calcium imaging time-lapses of SH-SY5Y-derived neurons in calcium-free conditions stimulated by US (a) and US + BTNPs (b) show in both case low-amplitude calcium transients. Observed transients were completely hindered by depleting the Ca2+ flux from the endoplasmic reticulum with thapsigargin before both the US (c) and US + BTNP (d) stimulations. Arrows indicate the moment when the 5-s US pulse was initiated; in the inlet of each graph a representative calcium imaging time-lapse frame is reported (at t = 50).
Thermal Effects of the US-Driven BTNP Stimulation
US stimulations higher than 0.5 W/cm2 are known to locally increase the temperature,17,28,29 and the ER is prone to release Ca2+ in response to a heat pulse.30,31 We therefore investigated whether the US and US + BTNP stimuli are able to induce a temperature increment at ER level (Figure S5, supplied as Supporting Information). In order to monitor the ER temperature during the stimulations, we took advantage of a thermosensitive fluorescent dye able to specifically target the ER, the ER thermo yellow.32 In Figure S5a we can appreciate the colocalization of the fluorescence signal stemming from the ER thermo yellow with that one of the ER tracker, demonstrating the high specificity of the ER thermo yellow in SH-SY5Y cells. The sensitivity of the ER thermo yellow in SH-SY5Y cells was determined to be −2.0%/°C by linearly fitting the F/F0 data measured at different ER temperature increment (ΔT, °C) starting from the room temperature (Figure S5b). Figure S5c and S5d show representative time courses of the ER thermometer fluorescence signal during the stimulation with US (0.8 W/cm2) and with US + BTNPs, respectively. The relative ΔT (°C) traces are shown in Figure S5e and S5f, showing that the US stimulation is able to increase the ER temperature both in presence (1.66 ± 0.30 °C) and in absence (1.68 ± 0.31 °C) of BTNPs, but without any significant difference between the two conditions (p > 0.05).
Further Evaluations of the Mechanisms at the Base of the US-Driven BTNP Stimulation
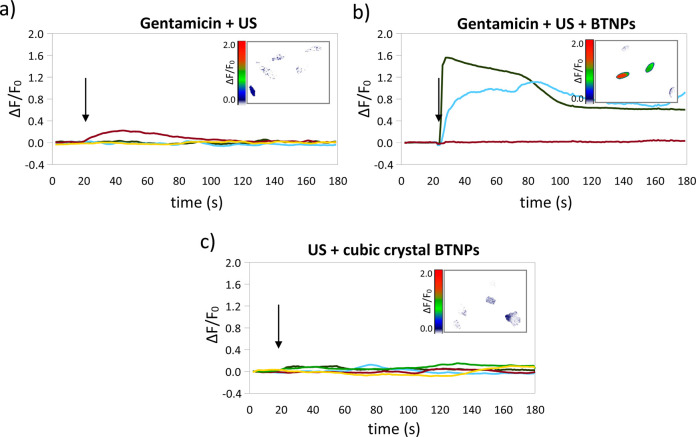
The role of mechanosensitive channels in the low-amplitude and high-amplitude Ca2+ transients respectively observed after the US and US + BTNP stimulations was investigated by perfoming Ca2+ imaging experiments in the presence of 200 μM gentamicin, a blocker of mechano-sensitive cation channels that does not affect the voltage-gated Ca2+ currents.33−35 Even in the presence of gentamicin, low-amplitude (ΔF/F0 peak = 0.15 ± 0.05) and high-amplitude (ΔF/F0 peak = 0.78 ± 0.24; p < 0.05) Ca2+ peaks were respectively detected after the US (Figure 7a) and the US + BTNP stimulations (Figure 7b), suggesting that the mechano-sensitive cation channels are not at the base of the mechanism describing the effects observed during the stimulations.
Figure 7.
Calcium transients induced by US + BTNP stimulation (0.8 W/cm2) are mediated by the piezoelectricity of the nanoparticles. Representative ΔF/F0 traces relative to calcium imaging time-lapses of SH-SY5Y-derived neurons stimulated by US (a) and US + BTNPs (b) in the presence of gentamicin, a blocker of mechano-sensitive cation channels which does not affect the voltage-gated Ca2+ currents. In (c) the Ca2+ time course of neurons stimulated by US and nonpiezoelectric BTNPs, characterized by a cubic crystalline configuration. Arrows indicate the moment when the 5-s US pulse was initiated; in the inlet of each graph a representative calcium imaging time-lapse frame is reported (at t = 50).
Finally, in order to investigate whether the high-amplitude Ca2+ transients observed in the US + BTNP stimulation are indeed due to a piezoelectric effect, we performed stimulation in the presence of BTNPs characterized by a cubic crystalline configuration (Figure 7c), and thus not piezoelectric.25 Interestingly, we observed that in this case the US + BTNP stimulation was able to induce only low-amplitude Ca2+ transients (ΔF/F0 peak = 0.12 ± 0.01), not significantly different to those observed after the plain US stimulation (0.15 ± 0.04; p < 0.05), and significantly lower compared to the high-amplitude peaks induced by the US stimulation in the presence of BTNPs characterized by a tetragonal crystalline structure (p < 0.05).
A representative Ca2+ imaging time-lapse frame is reported in the inlet of each graph of Figure 7, while more time-lapse images are provided in Figure S6 as Supporting Information. The XRD analysis of BTNPs characterized by a cubic crystalline structure is also reported in Supporting Information, as Figure S7.
Discussion
Several in vitro studies have focused on the behavior of different cell types interacting with piezoelectric substrates/scaffolds. Detailed analysis of cytocompatibility and adhesion of human skin fibroblasts on electruspun poly(vinylidene fluoride-trifluoroethylene) (PVDF-TrFE) scaffolds have been for example carried out by the Arinzeh group.36 In another work, Martins et al. investigated the biological effects of piezoelectric PVDF fiber polarization and alignment on the myoblast cell adhesion and morphology for muscles tissue engineering purposes.37 Concerning neuron-piezoelectric substrate interactions, an interesting work of Lee et al. demonstrated the potential of micron-sized aligned PVDF-TrFE substrates in promoting and guiding the neurite outgrowth of dorsal root ganglion neurons.38 These results are particularly interesting in view of exploiting piezoelectric substrates for promoting the neural tissue repair. Furthermore, in another work of the same group, the neural differentiation of human neural stem/progenitor cells (hNS/PCs) resulted significantly enhanced in terms of β-III tubulin positive cells and average neurite length when carried out on annealed (and thus characterized by improved piezoelectric properties) vs as-cast PVDF-TrFE scaffolds.39 Obtained results were observed without making use of any mechanical stimulation, and authors hypothesized that fiber piezoelectricity was likely triggered by the forces exerted by the cells throughout adhesion and migration.40 In other approaches, instead, piezoelectric PVDF scaffolds were able to generate an alternating electric field on their surfaces when mechanically deformed by a custom-made vibration base. A neurite length increase of rat spinal cord neurons was reported on stimulated piezoelectric PVDF substrates compared to nonpiezoelectric stimulated control scaffolds.24 Alternatively, a dedicated device allowed to apply specific tension to the substrate by regulating the vacuum pressure in order to piezoelectrically stimulate fibroblasts cultured on polyurethane/PVDF scaffolds.41 Finally, Inaoka et al. demonstrated as acoustic vibrations were also able to excite a piezoelectric membrane and, consequently, to generate electrical signals. The amplified signals were than transferred to the cochlea of deafened guinea pigs, so artificially mimicking the functions of the cochlear epithelium.42
In this work we successfully exploited for the first time piezoelectric BTNPs, characterized by a tetragonal crystalline configuration, for an US-driven piezoelectric stimulation of SH-SY5Y-derived neurons. Primarily, we observed as BTNPs functionalized with gum Arabic are associated with the plasma membrane of cells after 24 h of incubation. The observed low internalization of BTNPs is likely due to their negative external charge, which is known to limit the nanoparticle up-take.43 However, the localization of the BTNPs at the neural plasma membrane, which is enriched of voltage-gated channels and it is electrically excitable, is optimal with the perspective of a piezoelectric stimulation.44 The US + BTNP stimulation was able to activate high-amplitude Ca2+ transients, while the simple US stimulation, without BTNPs, induce low-amplitude Ca2+ transients. The nonstereotyped amplitude and the duration (in the order of minute) of the transients observed after the US + BTNP stimulation suggest that these transient are Ca2+ waves.45 These phenomena are known to be dependent on extracellular Ca2+ and mediated by voltage-gated Na+ and Ca2+ channels.46,47 Furthermore, it is well-known from the literature as the Ca2+ waves, both spontaneous and stimuli-induced, intercellularly propagate through adjacent neurons thanks to gap junctions.46 Interestingly, we found as some of the observed high-amplitude calcium transients do not occur simultaneously to the US + BTNP stimulation: a possible explanation of this phenomenon is related to the possibility that other activated neurons propagate the excitation to adjacent neurons. Calcium waves are known to play a key role in promoting the maturation of the neural network, especially by regulating the neurite outgrowth:45 at this regard, it is worth to mention as we previously demonstrated how the US-driven stimulation of piezoelectric BNNTs was able to significantly promote the neurite elongation of PC12 neural-like cells, compared to the US control stimulation without BNNTs. In that work, it was hypothesized that Ca2+ influx was involved in the increase of the neurite outgrowth, since this was sensible to the nonspecific Ca2+ influx blockers. The findings presented in the current work are consistent with that hypothesis, having observed how an US-driven piezoelectric stimulation is able to induce calcium influx.
The high-amplitude Ca2+ transients recorded following the US + BTNP stimulation are sensitive to TTX and Cd2+ treatment, suggesting that both Na+ and Ca2+ voltage-gated channels are involved. In particular, it is possible to argue that the opening of voltage-gated Na+ channels induces a depolarization of the plasma membrane, thus activates the voltage-gated Ca2+ channels, and, finally, increases the cytoplasmic Ca2+ concentration:48 indeed, the TTX + US + BTNP experiments revealed as the voltage-gated Ca2+ channels were not sufficient, alone, to evoke high-amplitude Ca2+ transients. The opening of these voltage-gated channels in response to the US + BTNP stimulus is supposed to induce, as final outcome, a depolarization of the neuronal plasma membrane.49
Since the voltage-gated channels are expressed on the plasma membrane, the extracellular Ca2+ influx is likely involved in the generation of the observed high-amplitude Ca2+ transients. The US + BTNP stimulation in Ca2+-free conditions reveals a significant decrease of the Ca2+ transient amplitude, confirming the role of the extracellular Ca2+ influx. However, in these conditions, it is possible to detect low-amplitude transients, similar to those measured in response to the US stimulation in standard conditions (with 2 mM of extracellular Ca2+), suggesting that a small component of the cytoplasmic Ca2+ increase is due to a release from intracellular Ca2+ stores. Moreover, it is also possible to detect similar low-amplitude Ca2+ transients in response to the simple US stimulation in Ca2+-free conditions. These results suggest that the US stimulation without BTNPs is able to induce low-amplitude Ca2+ transients triggering a release from intracellular Ca2+ stores. By depleting the Ca2+ from ER and in extracellular Ca2+-free conditions, it is possible to completely eliminate the Ca2+ transients in response to both US and to US + BTNPs. This finding confirms the contribution of the ER in the US-dependent increase of the cytoplasmic Ca2+ concentration.
In this context, it is well-known from the literature that US stimulations higher than 0.5 W/cm2 can induce a temperature increase,28,17,29 which results into Ca2+ transients upon Ca2+ release from ER.30 Particularly, the temperature-dependent Ca2+ release from the ER is likely evoked by an imbalance between an increased SERCA pump activity30,31,50 and a decreased open probability of IP3R.30,31,51,52 Interestingly, we noticed as the US stimulation was able to increase the ER temperature independently by the presence of BTNPs, suggesting a possible role of the temperature in the US-induced Ca2+ release from ER. Since there is not a significantly different temperature increment following the US and the US + BTNP stimulations, it is possible to confirm that the high-amplitude Ca2+ transients evoked by US + BTNPs are not caused by a temperature increment, but instead induced by the voltage-gated channel opening.
In order to assess whether the observed phenomena were actually ascribable to the piezoelectric properties of the nanoparticles, that act as nanotransducers by inducing the opening of the voltage-gated channels in response to the US stimulation, we performed analogue experiments with US + BTNPs characterized by a cubic crystalline configuration, thus not piezoelectric. Indeed, this stimulation was not able to induce high-amplitude Ca2+ transients, confirming that the piezoelectricity of the tetragonal BTNPs is a necessary requirement for eliciting the observed high-amplitude Ca2+ transients.
This hypothesis was corroborated by a simple electroelastic model of a BTNP subjected to US stimulation. In particular, the model provides the following expression for the maximum electric potential increment φR generated by the US on the BTNP surface (r = R) with respect to the stress-free condition:
| 1 |
In eq 1, R denotes the radius, err and erθ the piezoelectric coefficients, and εrr the dielectric constant of the BTNP. Moreover, α, γ and s are known expressions also depending on the elastic properties of the BTNP (namely the Young modulus and the Poisson ratio). Finally, pUS denotes the maximum pressure associated with the US wave. The model derivation is fully detailed in the Section S1 of Supporting Information, and relevant parameter values are discussed/referenced therein. On the basis of eq 1, when stimulating with 0.1 W/cm2 the maximum voltage is around 0.07 mV, yet it rises to 0.19 mV when operating with 0.8 W/cm2. In light of the BTNP clusters on the cell membrane (clusters of about 10 BTNPs in average have been quantified from confocal images), when using 0.8 W/cm2 the induced voltage of a few mV can locally affect channel open probability,49 by superposition. Furthermore, this induced voltage can virtually redistribute the charges of the bivalent ions in correspondence of the external surface of the plasma membrane and, consequently, enhance the voltage sensitivity of the voltage-gated channels through a shift of the channel activation curves.53 Differently, when using 0.1 W/cm2, it is more difficult for the voltage to affect channel activation, even where the BTNPs cluster. The above model predictions are fully compatible with the experimental observations, and they quantitatively foster the hypothesis of BTNP-mediated, piezoelectric cell stimulation.
Conclusion
Successfully US-driven piezoelectric neural stimulation was performed by exploiting BTNPs, and the involved ion fluxes at the base of this phenomenon were fully described. The US + BTNP stimulation can be considered a novel tool for a wireless neural stimulation. Future works will be devoted to the functionalization of the BTNPs with specific molecules, in order to target nanoparticles to the membranes of specific cell types: cell type selectivity will be in fact fundamental envisaging in vivo wireless stimulations of different parts of the brain, and in order to foster peculiar cellular functions. Obtained results, collectively, open new intriguing perspectives not only in the field of neural prosthetics, but also in tissue engineering and biorobotics.
Methods
BTNP Characterization
Barium titanate nanoparticles were purchased by Nanostructured & Amorphous Materials, Inc., Houston, TX (1144DY). Details of sample purity and composition, as provided by the supplier, include the following: BaO/TiO2 0.999–1.001, purity 99.9%; APS 300 nm; SSA 3.5–3.7 m2/g. X-ray diffraction (XRD) patterns were recorded using an X-ray powder diffractometer (Kristalloflex 810, Siemens) using Cu Kα radiation (λ = 1.5406 A) at a scanning rate of 0.016° s–1 with 2θ ranging in 10°–80° at a temperature of 25 °C.
For use in biological experiments, BTNPs were dispersed in aqueous environment through a noncovalent wrapping with gum Arabic (G9752 from Sigma-Aldrich). Briefly, 10 mg of nanoparticles and 10 mg of gum Arabic were mixed in 10 mL of phosphate buffered saline (PBS) solution. The samples were sonicated for 12 h with a Bransonic sonicator 2510, by using an output power of 20 W. The final product is a stable 1 mg/mL nanoparticle dispersion, that was appropriately diluted in cell culture medium for biological experiments. Obtained dispersion was characterized through scanning electron microscopy (SEM, Helios NanoLab 600i FIB/SEM, FEI) and transmission electron microscopy (TEM, Zeiss 902). Moreover, particle size distribution and Z-potential were analyzed with a Nano Z-Sizer 90 (Malvern Instrument), both in water and in experimental conditions (50 μg/mL of BTNPs in artificial cerebrospinal fluid, see in the following for details).
For a control experiment, analogous nanoparticles but with cubic crystal structure (1143DY, from Nanostructured & Amorphous Materials) were used following the same preparation procedures.
Cell Culture, Differentiation and BTNP Treatment
Human neuroblastoma-derived cells (SH-SY5Y, ATCC CRL-2266) were cultured in DMEM/F12 with 10% fetal bovine serum (FBS, Gibco), 100 U/mL penicillin, and 100 μg/mL streptomycin on 35 mm diameter μ-dishes (Ibidi) at a density of 20000 cell/cm2, and subsequently differentiated toward neurons in DMEM with 1% FBS, 10 μM all-trans-retinoic acid, 100 U/mL penicillin, and 100 μg/mL streptomycin. After 4 days of differentiation, SH-SY5Y-derived neurons were treated for 24 h with BTNPs at the final concentration of 50 μg/mL in the differentiation medium. This nanoparticle concentration was previously tested on the SH-SY5Y cell line and was demonstrated to be not toxic by performing several independent biocompatibility investigations in terms of proliferation, metabolic activity, apoptosis detection and reactive oxygen species detection.54 Control neurons were instead treated for 24 h with the differentiation medium and vehicle of the nanoparticles (50 μg/mL gum Arabic).
Further cell viability evaluations in the present experimental conditions were performed with WST-1 assay ((2-(4-iodophenyl)-3-(4-nitophenyl)-5-(2,4disulfophenyl)-2H-tetrazoilium monosodium salt, provided in a premix electrocoupling solution, BioVision) and propidium iodide staining, in order to respectively assess metabolic activity and membrane integrity after the stimulation procedures. More in details, cultures undergone the stimulation protocol (described in the next paragraph) were incubated for further 24 h, thereafter culture medium was replaced with a 1:11 premix:medium solution, and incubation performed for further 2 h. Finally, the absorbance was read at 450 nm with a microplate reader (Victor3, PerkinElmer). Membrane integrity evaluation following the stimulation procedure was performed by adding 1 μg/mL of propidium iodide (PI, Molecular Probes) during the application of the ultrasounds, and assessing the number of PI-positive cells over the total number of cells through fluorescence microscopy (TE2000U, Nikon).
The analysis of the nanoparticle localization was performed by confocal fluorescence microscopy (FluoView FV1000, PLAPON 60XO, NA1.42, Olympus). Neuronal plasma membranes and nuclei were stained with CellMask Green Plasma Membrane Stain (1:1000, Invitrogen) and Hoechst 33342 (1 μg/mL, Invitrogen), respectively. The ER staining in live cells was performed by using ER tracker Green (500 nM, Invitrogen) and ER thermo yellow32 (300 nM). BTNPs, CellMask Green, Hoechst 33342, ER tracker Green and ER thermo yellow were excited by 633, 488, 405, 488, and 543 nm lasers, and the emission lights were collected at 645–745, 500–555, 425–525, 500–555 and 555–655 nm, respectively.
Ca2+, Na+ and Temperature Imaging during US Stimulation
Before the stimulation experiments, Fluo-4 AM (1 μM, Invitrogen), CoroNa Green AM (1 μM, Invitrogen) or the ER thermo yellow32 (300 nM) were incubated in serum-free DMEM for 30 min at 37 °C. After the reagent incubation, samples were washed, supplied with artificial cerebrospinal fluid (aCSF, composition in mM: NaCl 140, KCl 5, CaCl2 2, MgCl2 2, HEPES 10, d-glucose 10; pH 7.4) and positioned on an inverted fluorescence microscope. Fluo-4 and ER thermo yellow were imaged with a microscope IX81 (Olympus) equipped with an objective UPLFLN 40XO, NA 1.3, and a cooled CCD camera (Cool SNAP HQ2, Photometrics) by using 460–480HQ and 535–555HQ as excitation filters, DM485 and DM565HQ as dichroic mirrors, and 495–540HQ and 570–625HQ as emission filters, respectively (all from Olympus). CoroNa Green was imaged with a microscope IX83 (Olympus) equipped with an objective UPLFLN 40XO, NA 1.3, and an electron multiplying charge-coupled device camera (iXon3, Andor Technology) by using BP470–495, DM505, and BP510–550 as an excitation filter, a dichroic mirror and an emission filter, respectively (all from Olympus).
Concerning the temperature imaging experiments, calibration was performed by using a near-infrared laser (1064 nm) as previously described,32 and a sensitivity of −2.0%/°C relative to room temperature (24 °C) was measured (please refer to Supporting Information, Figure S5, for the calibration curve).
Stimulation was carried out after waiting 20 min for the stabilization of the cell conditions after the medium change, and for allowing the full de-esterification of the AM groups. Ultrasounds were applied with a Sonitron GTS Sonoporation System (ST-GTS, Nepagene) equipped with a plane wave transducer module (PW-1.0–6 mm, 6 mm diameter tip, 1 MHz). The probe was vertically fixed at a distance of 5 mm from the cells, and the US stimulations were carried out for 5 s at different intensities (from 0.1 W/cm2 to 0.8 W/cm2).
During the stimulation experiments, cadmium chloride (CdCl2 100 μM, Sigma), tetrodotoxin (TTX, 100 nM, Tocris), ethylene glycol tetraacetic acid (EGTA, 5 mM, Dojindo Laboratories), thapsigargin (2 μM, Sigma) or gentamicin (200 μM, Invitrogen) were added to the aCSF (Ca2+-free aCSF was used in the case of the EGTA experiments). Concerning the thapsigargin experiments, this reagent was also applied during the dye internalization process, in order to allow the complete release of Ca2+ from the ER, as previously reported.55
Image and Statistical Analysis
The fluorescence images acquired during the time lapses were analyzed with ImageJ software (http://rsbweb.nih.gov/ij/). Images were thresholded in order to define the region of interest (ROI) to analyze; subsequently, they were converted in ΔF/F0 by using the divide and subtract functions of the Math process. After a double smoothing, the average values of the pixels inside the ROIs were measured by using the multimeasure function of the ROI manager and, finally, plotted on the ΔF/F0 graphs. Transient amplitudes (ΔF/F0 peaks) 3-fold higher than the standard deviation of the noise were reported in terms of peak average ± standard error.
All the described experiments were carried out in triplicate (at least the response of 20 cells for all the conditions was analyzed), the normality of all the data distributions was tested with the Shapiro normality test and, subsequently, the ANOVA parametric test followed by Tukey’s HSD post-hoc test was performed in order to compare the different distributions.
Acknowledgments
The authors gratefully thank Mr. Piero Narducci (Department of Chemical Engineering, University of Pisa, Pisa, Italy) for XRD technical assistance. This research was partially supported by the JSPS KAKENHI Grant Number 26107717 (to M.S.), by the JSPS Core-to-Core Program, A. Advanced Research Networks (to M.S.), and by the Italian Ministry of Health Grant Number RF-2011-02350464 (to G.C.).
Supporting Information Available
Section S1 contains an electroelastic model of the voltage generated by a piezoelectric BTNP subjected to ultrasounds. Figure S1 reports cell viability data following treatment with BTNPs and US. Figures S2, S3, S4 and S6 report time-lapse frames of the ΔF/F0 traces reported in Figures 4, 5, 6 and 7, respectively. Figure S5 shows the ER temperature imaging performed during the US (0.8 W/cm2) and BTNP + US (0.8 W/cm2) stimulations. Figure S7 reports the XRD analysis of BTNPs characterized by a cubic crystalline structure. Video S1 shows the confocal z-stack 3D rendering of a cluster of cells (plasma membrane in green, nuclei in blue) and BTNPs (in red) associated with their plasma membrane. Video S2 shows a Ca2+ imaging time-lapse course (18X accelerated) performed on cultures stimulated with US (0.8 W/cm2) in the presence of BTNPs. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsnano.5b03162.
The authors declare no competing financial interest.
Supplementary Material
References
- Chang J.-Y. Brain Stimulation for Neurological and Psychiatric Disorders, Current Status and Future Direction. J. Pharmacol. Exp. Ther. 2004, 309, 1–7. 10.1124/jpet.103.049718. [DOI] [PubMed] [Google Scholar]
- Deisseroth K. Optogenetics. Nat. Methods 2011, 8, 26–29. 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V.; Mogri M.; Thompson K. R.; Henderson J. M.; Deisseroth K. Optical Deconstruction of Parkinsonian Neural Circuitry. Science 2009, 324, 354–359. 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye K. M.; Prakash R.; Kim S.-Y.; Fenno L. E.; Grosenick L.; Zarabi H.; Thompson K. R.; Gradinaru V.; Ramakrishnan C.; Deisseroth K. Amygdala Circuitry Mediating Reversible and Bidirectional Control of Anxiety. Nature 2011, 471, 358–362. 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten I. B.; Lin S.-C.; Brodsky M.; Prakash R.; Diester I.; Anikeeva P.; Gradinaru V.; Ramakrishnan C.; Deisseroth K. Cholinergic Interneurons Control Local Circuit Activity and Cocaine Conditioning. Science 2010, 330, 1677–1681. 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D.; Walsh J. J.; Friedman A. K.; Juarez B.; Ku S. M.; Koo J. W.; Ferguson D.; Tsai H.-C.; Pomeranz L.; Christoffel D. J.; et al. Rapid Regulation of Depression-Related Behaviours by Control of Midbrain Dopamine Neurons. Nature 2013, 493, 532–536. 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöpfel T.; Lin M. Z.; Levskaya A.; Tian L.; Lin J. Y.; Boyden E. S. Toward the Second Generation of Optogenetic Tools. J. Neurosci. 2010, 30, 14998–15004. 10.1523/JNEUROSCI.4190-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diester I.; Kaufman M. T.; Mogri M.; Pashaie R.; Goo W.; Yizhar O.; Ramakrishnan C.; Deisseroth K.; Shenoy K. V. An Optogenetic Toolbox Designed for Primates. Nat. Neurosci. 2011, 14, 387–397. 10.1038/nn.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley S. A.; Gagner J. E.; Damanpour S.; Yoshida M.; Dordick J. S.; Friedman J. M. Radio-Wave Heating of Iron Oxide Nanoparticles Can Regulate Plasma Glucose in Mice. Science 2012, 336, 604–608. 10.1126/science.1216753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter J. S.; Mink J. W. Deep Brain Stimulation. Annu. Rev. Neurosci. 2006, 29, 229–257. 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni A. R.; Nitsche M. A.; Bolognini N.; Bikson M.; Wagner T.; Merabet L.; Edwards D. J.; Valero-Cabre A.; Rotenberg A.; Pascual-Leone A.; et al. Clinical Research with Transcranial Direct Current Stimulation (tDCS): Challenges and Future Directions. Brain Stimulat. 2012, 5, 175–195. 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C. J.; Nitsche M. A. Physiological Basis of Transcranial Direct Current Stimulation. Neuroscientist 2011, 17, 37–53. 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial Magnetic Stimulation and the Human Brain. Nature 2000, 406, 147–150. 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- Tufail Y.; Matyushov A.; Baldwin N.; Tauchmann M. L.; Georges J.; Yoshihiro A.; Tillery S. I. H.; Tyler W. J. Transcranial Pulsed Ultrasound Stimulates Intact Brain Circuits. Neuron 2010, 66, 681–694. 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Li J.; Fok L.; Yin X.; Bartal G.; Zhang X. Experimental Demonstration of an Acoustic Magnifying Hyperlens. Nat. Mater. 2009, 8, 931–934. 10.1038/nmat2561. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Yin L.; Fang N. Focusing Ultrasound with an Acoustic Metamaterial Network. Phys. Rev. Lett. 2009, 102, 194301. 10.1103/PhysRevLett.102.194301. [DOI] [PubMed] [Google Scholar]
- Tufail Y.; Yoshihiro A.; Pati S.; Li M. M.; Tyler W. J. Ultrasonic Neuromodulation by Brain Stimulation with Transcranial Ultrasound. Nat. Protoc. 2011, 6, 1453–1470. 10.1038/nprot.2011.371. [DOI] [PubMed] [Google Scholar]
- Wang X.; Song J.; Liu J.; Wang Z. L. Direct-Current Nanogenerator Driven by Ultrasonic Waves. Science 2007, 316, 102–105. 10.1126/science.1139366. [DOI] [PubMed] [Google Scholar]
- Qin Y.; Wang X.; Wang Z. L. Microfibre–nanowire Hybrid Structure for Energy Scavenging. Nature 2008, 451, 809–813. 10.1038/nature06601. [DOI] [PubMed] [Google Scholar]
- Wang X.; Liu J.; Song J.; Wang Z. L. Integrated Nanogenerators in Biofluid. Nano Lett. 2007, 7, 2475–2479. 10.1021/nl0712567. [DOI] [PubMed] [Google Scholar]
- Kim K.-H.; Kumar B.; Lee K. Y.; Park H.-K.; Lee J.-H.; Lee H. H.; Jun H.; Lee D.; Kim S.-W. Piezoelectric Two-Dimensional Nanosheets/anionic Layer Heterojunction for Efficient Direct Current Power Generation. Sci. Rep. 2013, 10.1038/srep02017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Liao Q.; Zhang G.; Zhang Z.; Liang Q.; Liao X.; Zhang Y. High Output Piezoelectric Nanocomposite Generators Composed of Oriented BaTiO3 NPs@PVDF. Nano Energy 2015, 11, 719–727. 10.1016/j.nanoen.2014.11.061. [DOI] [Google Scholar]
- Ciofani G.; Danti S.; D’Alessandro D.; Ricotti L.; Moscato S.; Bertoni G.; Falqui A.; Berrettini S.; Petrini M.; Mattoli V.; et al. Enhancement of Neurite Outgrowth in Neuronal-Like Cells Following Boron Nitride Nanotube-Mediated Stimulation. ACS Nano 2010, 4, 6267–6277. 10.1021/nn101985a. [DOI] [PubMed] [Google Scholar]
- Royo-Gascon N.; Wininger M.; Scheinbeim J. I.; Firestein B. L.; Craelius W. Piezoelectric Substrates Promote Neurite Growth in Rat Spinal Cord Neurons. Ann. Biomed. Eng. 2013, 41, 112–122. 10.1007/s10439-012-0628-y. [DOI] [PubMed] [Google Scholar]
- Tao J.; Ma J.; Wang Y.; Zhu X.; Liu J.; Jiang X.; Lin B.; Ren Y. Synthesis of Barium Titanate Nanoparticles via a Novel Electrochemical Route. Mater. Res. Bull. 2008, 43, 639–644. 10.1016/j.materresbull.2007.04.007. [DOI] [Google Scholar]
- Lee C. H.; Ruben P. C. Interaction between Voltage-Gated Sodium Channels and the Neurotoxin, Tetrodotoxin. Channels 2008, 2, 407–412. 10.4161/chan.2.6.7429. [DOI] [PubMed] [Google Scholar]
- Lacinová L. Voltage-Dependent Calcium Channels. Gen. Physiol. Biophys. 2005, 24 (Suppl 1), 1–78. [PubMed] [Google Scholar]
- Ter Haar G. Therapeutic Applications of Ultrasound. Prog. Biophys. Mol. Biol. 2007, 93, 111–129. 10.1016/j.pbiomolbio.2006.07.005. [DOI] [PubMed] [Google Scholar]
- O’Brien W. D. Ultrasound—biophysics Mechanisms. Prog. Biophys. Mol. Biol. 2007, 93, 212–255. 10.1016/j.pbiomolbio.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H.; Oyama K.; Suzuki M.; Ishiwata S. Microscopic Heat Pulse-Induced Calcium Dynamics in Single WI-38 Fibroblasts. Biophysics 2014, 10, 109–119. 10.2142/biophysics.10.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseeb V.; Suzuki M.; Oyama K.; Iwai K.; Ishiwata S. Highly Thermosensitive Ca2+ Dynamics in a HeLa Cell through IP3 Receptors. HFSP J. 2009, 3, 117–123. 10.2976/1.3073779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S.; Lee S.-C.; Zhai D.; Suzuki M.; Chang Y. T. A Molecular Fluorescent Probe for Targeted Visualization of Temperature at the Endoplasmic Reticulum. Sci. Rep. 2014, 4, 6701. 10.1038/srep06701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese B.; Manzi S.; Pellegrini M.; Pellegrino M. Stretch-Activated Cation Channels of Leech Neurons: Characterization and Role in Neurite Outgrowth. Eur. J. Neurosci. 1999, 11, 2275–2284. 10.1046/j.1460-9568.1999.00648.x. [DOI] [PubMed] [Google Scholar]
- Barsanti C.; Pellegrini M.; Ricci D.; Pellegrino M. Effects of Intracellular pH and Ca2+ on the Activity of Stretch-Sensitive Cation Channels in Leech Neurons. Pfluegers Arch. 2006, 452, 435–443. 10.1007/s00424-006-0056-7. [DOI] [PubMed] [Google Scholar]
- Jacques-Fricke B. T.; Seow Y.; Gottlieb P. A.; Sachs F.; Gomez T. M. Ca2+ Influx through Mechanosensitive Channels Inhibits Neurite Outgrowth in Opposition to Other Influx Pathways and Release from Intracellular Stores. J. Neurosci. 2006, 26, 5656–5664. 10.1523/JNEUROSCI.0675-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber N.; Lee Y.-S.; Shanmugasundaram S.; Jaffe M.; Arinzeh T. L. Characterization and in Vitro Cytocompatibility of Piezoelectric Electrospun Scaffolds. Acta Biomater. 2010, 6, 3550–3556. 10.1016/j.actbio.2010.03.035. [DOI] [PubMed] [Google Scholar]
- Martins P. M.; Ribeiro S.; Ribeiro C.; Sencadas V.; Gomes A. C.; Gama F. M.; Lanceros-Méndez S. Effect of Poling State and Morphology of Piezoelectric Poly(vinylidene Fluoride) Membranes for Skeletal Muscle Tissue Engineering. RSC Adv. 2013, 3, 17938–17944. 10.1039/c3ra43499k. [DOI] [Google Scholar]
- Lee Y.-S.; Collins G.; Arinzeh T. L. Neurite Extension of Primary Neurons on Electrospun Piezoelectric Scaffolds. Acta Biomater. 2011, 7, 3877–3886. 10.1016/j.actbio.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Lee Y.-S.; Arinzeh T. L. The Influence of Piezoelectric Scaffolds on Neural Differentiation of Human Neural Stem/progenitor Cells. Tissue Eng., Part A 2012, 18, 2063–2072. 10.1089/ten.tea.2011.0540. [DOI] [PubMed] [Google Scholar]
- Wang N.; Tolić-Nørrelykke I. M.; Chen J.; Mijailovich S. M.; Butler J. P.; Fredberg J. J.; Stamenović D.; Cell Prestress I. Stiffness and Prestress Are Closely Associated in Adherent Contractile Cells. Am. J. Physiol. Cell Physiol. 2002, 282, C606–C616. 10.1152/ajpcell.00269.2001. [DOI] [PubMed] [Google Scholar]
- Guo H.-F.; Li Z.-S.; Dong S.-W.; Chen W.-J.; Deng L.; Wang Y.-F.; Ying D.-J. Piezoelectric PU/PVDF Electrospun Scaffolds for Wound Healing Applications. Colloids Surf., B 2012, 96, 29–36. 10.1016/j.colsurfb.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Inaoka T.; Shintaku H.; Nakagawa T.; Kawano S.; Ogita H.; Sakamoto T.; Hamanishi S.; Wada H.; Ito J. Piezoelectric Materials Mimic the Function of the Cochlear Sensory Epithelium. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 18390–18395. 10.1073/pnas.1110036108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M.; Hille B. Voltage-Gated Ion Channels and Electrical Excitability. Neuron 1998, 20, 371–380. 10.1016/S0896-6273(00)80981-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. S.; Spitzer N. C. Calcium Signaling in Neuronal Development. Cold Spring Harbor Perspect. Biol. 2011, 3, a004259. 10.1101/cshperspect.a004259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles A. C.; Kodali S. K.; Tyndale R. F. Intercellular Calcium Waves in Neurons. Mol. Cell. Neurosci. 1996, 7, 337–353. 10.1006/mcne.1996.0025. [DOI] [PubMed] [Google Scholar]
- Gu X.; Olson E. C.; Spitzer N. C. Spontaneous Neuronal Calcium Spikes and Waves during Early Differentiation. J. Neurosci. 1994, 14, 6325–6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J.; Bootman M. D.; Roderick H. L. Calcium Signalling: Dynamics, Homeostasis and Remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Structure and Function of Voltage-Gated Ion Channels. Annu. Rev. Biochem. 1995, 64, 493–531. 10.1146/annurev.bi.64.070195.002425. [DOI] [PubMed] [Google Scholar]
- Dode L.; Van Baelen K.; Wuytack F.; Dean W. L. Low Temperature Molecular Adaptation of the Skeletal Muscle Sarco(endo)plasmic Reticulum Ca2+-ATPase 1 (SERCA 1) in the Wood Frog (Rana Sylvatica). J. Biol. Chem. 2001, 276, 3911–3919. 10.1074/jbc.M007719200. [DOI] [PubMed] [Google Scholar]
- Stavermann M.; Buddrus K.; St John J. A.; Ekberg J. A. K.; Nilius B.; Deitmer J. W.; Lohr C. Temperature-Dependent Calcium-Induced Calcium Release via InsP3 Receptors in Mouse Olfactory Ensheathing Glial Cells. Cell Calcium 2012, 52, 113–123. 10.1016/j.ceca.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Dickinson G. D.; Parker I. Temperature Dependence of IP3-Mediated Local and Global Ca2+ Signals. Biophys. J. 2013, 104, 386–395. 10.1016/j.bpj.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B.Modifiers of Gating; Sinauer Associates Inc.: Sunderland, MA, 1992; Ionic Channels of Excitable Membranes, Vol. 17. [Google Scholar]
- Ciofani G.; Danti S.; D’Alessandro D.; Moscato S.; Petrini M.; Menciassi A. Barium Titanate Nanoparticles: Highly Cytocompatible Dispersions in Glycol-Chitosan and Doxorubicin Complexes for Cancer Therapy. Nanoscale Res. Lett. 2010, 5, 1093. 10.1007/s11671-010-9607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen W.; Doutheil J.; Gissel C.; Treiman M. Depletion of Neuronal Endoplasmic Reticulum Calcium Stores by Thapsigargin: Effect on Protein Synthesis. J. Neurochem. 1996, 67, 1735–1743. 10.1046/j.1471-4159.1996.67041735.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.