Abstract
This paper reviews different types of conversational agents used in health care for chronic conditions, examining their underlying communication technology, evaluation measures, and AI methods. A systematic search was performed in February 2021 on PubMed Medline, EMBASE, PsycINFO, CINAHL, Web of Science, and ACM Digital Library. Studies were included if they focused on consumers, caregivers, or healthcare professionals in the prevention, treatment, or rehabilitation of chronic diseases, involved conversational agents, and tested the system with human users. The search retrieved 1087 articles. Twenty-six studies met the inclusion criteria. Out of 26 conversational agents (CAs), 16 were chatbots, seven were embodied conversational agents (ECA), one was a conversational agent in a robot, and another was a relational agent. One agent was not specified. Based on this review, the overall acceptance of CAs by users for the self-management of their chronic conditions is promising. Users’ feedback shows helpfulness, satisfaction, and ease of use in more than half of included studies. Although many users in the studies appear to feel more comfortable with CAs, there is still a lack of reliable and comparable evidence to determine the efficacy of AI-enabled CAs for chronic health conditions due to the insufficient reporting of technical implementation details.
Keywords: conversational agents, dialogue systems, relational agents, chatbot
1. Introduction
The availability and use of conversational agents have been increasing due to advances in technologies such as natural language processing (NLP), voice recognition, and artificial intelligence (AI). Conversational agents (CAs), also known as chatbots or dialogue systems, are computer systems that communicate with users through natural language user interfaces involving images, text, and voice [1,2]. Google Assistance, Apple Siri, Amazon Alexa, and Microsoft Cortana are common CAs with voice-activated interfaces. In the last decade, CAs’ popularity has increased, particularly those that use unconstrained natural language [3,4,5]. For example, consumers can talk to CAs on their smartphones for daily tasks, such as managing their calendars and retrieving information [6,7].
Recently, AI-based CAs have demonstrated multiple benefits in many domains, especially in healthcare. It is used to deliver scalable, less costly medical support solutions that can help at any time via smartphone apps or online [8,9]. For example, support and follow-up for adults after cancer treatment via chatbot reduced the patients’ anxiety without needing a psychiatrist [10,11,12]. Hence, CAs can play an useful role in health care, improving consultations by assisting clinicians and patients, supporting consumers with behavior change, and assisting older people in their living environments [13,14,15]. They can also help in completing specific tasks such as self-monitoring and overcoming obstacles for self-management, which is important in chronic disease management and in the fight against pandemics [6,16].
Chronic conditions and mental health conditions are increasing worldwide. Chronic diseases are one of the biggest healthcare challenges of the 21st century [17,18]. Chronic conditions are “characterized by their long-lasting and persistent effects. Once present, they often persist throughout a person’s life, so there is generally a need for long-term management by individuals and health professionals” [1]. Additionally, chronic conditions reduce one’s quality of life and increase healthcare expenses through disability, repeated hospitalization, and treatment procedures. According to the World Health Organization statistics of 2020, non-communicable diseases (e.g., hypertension, diabetes, and depression) and suicide are still prevalent reasons for death in 2016 [19]. In the US, about 60% of adults have chronic diseases, causing the annual health care expenditure approximately 86.2% of the $2.6 trillion [20]. In 2018, the Australian Institute of Health and Welfare claims that diabetes is one of Australia’s eight common chronic conditions, contributing to 61% of the disease burden, 37% of hospitalizations, and 87% of deaths [21]. There are about 1.13 billion people who had suffered from hypertension in 2015, and the number is still increasing. About 46% of adults do not know that they have hypertension. All statistics about chronic conditions show how serious they are and their effect on people’s lives [19].
Some research studies have shown advantages from the use of AI-enabled CAs in different healthcare settings, such as enabling behavior change, coaching to support a healthy lifestyle, helping breast cancer patients, and self-anamnesis for therapy patients [7,22,23]. Prior systematic literature reviews explored a variety of CAs in general health care [1,6,24] and aspects of the personalization of health care chatbots using AI [25]. However, there is little evidence on the use of AI-based CAs in chronic disease health care. This paper aims to address the gap by reviewing different kinds of CAs used in health care for chronic conditions, different types of communication technology, evaluation measures of CAs, and AI methods used.
Section 2 presents methods explaining the search strategy, eligibility criteria, screening, and data extraction processes. Section 3 addresses the results that include descriptions of included studies, CAs, AI methods, and evaluation measures. Section 4 provides a discussion of findings and outcomes. Section 5 presents the conclusion and future work.
2. Methods
Reporting standards
A systematic literature review has been performed which followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [24]. The review protocol in Appendix A was registered on OSF Preregistration, with DOI 10.17605/OSF.IO/GDWSH.
Search strategy
A systematic search was performed in February 2021, on PubMed Medline, EMBASE, PsycINFO, CINAHL, Web of Science, and ACM Digital Library, not restricted by year or language. Search terms included “conversational agents”, “dialogue systems”, “relational agents”, and “chatbots” (complete search strategy available in Appendix A) [1,6,25,26]. Gray literature that was also identified in those databases (including conference proceedings, theses, dissertations), were included for screening.
Study selection criteria
The criteria included primary research studies that focused on consumers, caregivers, or healthcare professionals in the prevention, treatment, or rehabilitation of chronic diseases using CAs, and tested the system with human users. Reviews, perspectives, opinion papers, or news articles were excluded based on exclusion criteria. In addition, studies that reported on evaluations based on human users interacting with the entire health system were excluded. The studies that evaluated only individual components of natural language understanding and CAs’ automatic speech recognition, dialogue management, response generation, and text-to-speech synthesis were excluded. The last exclusion criteria were studies using “Wizard of Oz” methods, where dialogue generated by a human operator rather than the CAs, were excluded [1,6,9].
Screening, data extraction, and synthesis
All references identified through the searches were downloaded. Then, duplicates were eliminated using reference managers (Endnote and Mendeley). Next, the titles and abstracts for each paper were exported from the reference manager into an Excel spreadsheet.
Before starting the screening process, the procedures of the screening were handled. After that, the first filter used was a screening filter based on the information contained in their titles and abstracts. Two independent reviewers conducted this screening. Two independent reviewers also conducted the full-text screening. The exclusion of an article was resolved with a Zoom meeting between two independent reviewers. Four reviewers extracted the following data for each study: first author, year of publication, study location, chronic condition, study aim, study types and methods, participants’ characteristics, evaluation measures, and main findings (Table 1). Evaluation measures were extracted based on three types: technical performance, user experience, and health-related measures. The technical performance of CAs was considered an objective assessment of the technical properties of the whole system. The technical performance measure is not included in Table 1 because most papers had not reported it, so the information about this measure will be in the next sections. User experience evaluation included the subjective assessment, where users tested the system properties or components based on their perspectives, via quantitative or qualitative methods [27]. Health-related measures were considered, alongside any health outcomes present in the included studies, such as diagnostic accuracy or symptom reduction.
Table 1.
Overview and characteristics of included studies.
| Author, Year | Study Location | Type of Chronic Condition | Study Aim | Study Type and Methods | Participants’ Characteristics | Evaluation Measures and Main Findings | |
|---|---|---|---|---|---|---|---|
| User Experience | Health Related Measures | ||||||
| Azzini et al., 2003 | Italy | Hypertension (patients with essential hypertension) | Data collection, developing a prototype home monitoring system. | Quasi-experimental (150 dialogues; 15 patients with essential hypertension entered the data at home; physicians used interface to store and update patient information). | Fifteen patients with no information about age, gender and duration. | Not reported | Not reported |
| Baptista et al., 2020 | Australia–New South Wales, Queensland, Victoria, and Western Australia | Diabetes–Type 2 (T2D) | Self-management, education, and support. |
Qualitative (six months baseline; 66 of the 93 patients completed a survey). Quantitative (between October 2017 to February 2018; 16 of the total patients had semi-structured interviews). RCT (testing the effectiveness of the T2D self-management smartphone app). |
Ninety-three patients from My Diabetes Coach app. Sixty-six responses in 6 months post baseline. Nineteen of these respondents participated in the interviews. Avg. age: 57; male: 33; female: 33. |
User experience feedback is as the following: helpful and friendly (86%), competent (85%), trustworthy (73%), likable (61%), not real (27%), boring (39%), annoying (30%), more motivated (44%), comfortable (36%), confident (21%), happy (17%), hopeful (12%), frustrated (20%), and feel guilty (17%). |
|
| Beaudry et al., 2019 | America-Vermont | Chronic condition (teenagers with pediatric Inflammatory Bowel Disease, Cardiology, or Type 1 Diabetes) | Learning self-care for teenagers (transition from pediatric to adult) with a chronic condition. | Quasi-experimental (24 weeks; pilot study on 13 teenagers with a chronic medical condition using a text messaging platform (chatbot) with scripted interactions) | Thirteen teenagers from the University of Vermont Children’s Hospital. Age: 14–17; duration: 24 weeks. |
|
Participants suggest this chatbot should be expanded, and that it shows promise to help teenagers attain self-care skills on the transition journey. |
| Bickmore et al., 2010 | America-Boston | Depressive Symptoms | Hospital patients know about their post-discharge self-care regimen through an automated system. | Quasi-experimental (one month; 131 patients interacted with the agent from their hospital beds; two rounds of pilot studies to assess usability, acceptance, and satisfaction with the agent; 347 subjects were enrolled and randomized; only 173 subjects were used into the relational agent of the study; nurses to follow up with patients). | One hundred and thirty patients from Boston Medical Centre. Age: 18; male: 70; female: 60; duration: 30 days. |
|
|
| Bickmore et al., 2010 | America-Pennsylvania | Schizophrenia | Promoting antipsychotic medication adherence for patients with schizophrenia. |
Quasi-experimental (initial range of responses, then modifying the list as needed to pilot testing). RCT (1–2 months; two RCTs. One with young adults (Bickmore et al., 2005 a, b) and another with geriatric patients (Bickmore et al., 2005a); both were conducted on home desktop computers). |
Twenty patients from a mental health outpatient clinic. Age: 19–58; male: 67%; female: 33%; duration: 1–2 months. The nurse visited each participant’s home to explain how to use the computer and to make sure the software worked normally. |
|
|
| Bott et al., 2019 | America-New York | Loneliness, Depression, Delirium, Falls | Supporting nurses and mitigating risks of hospitalization for elders. | Quasi-experimental (2 groups; group 1 (intervention)—41 participants received an avatar for the duration of their hospital stay, group 2—(control) 54 participants received a daily 15 min visit from a nursing student). | Ninety-five elders from an urban community hospital in New York. Age: over 65 years; male: 43; female: 52; the average length of stay for a patient: 3–6 days. |
The mean for patient engagement data was as follows: number of check-ins: 71.30/day; observational and engagement time: 61 min/day; media files used: 11.50/day; completed protocol tasks: 6.5 tasks/day. |
|
| Chaix et al., 2019 | France and Europe | Breast Cancer | Support, education, and improving medication adherence. | Analysis (1 year; 4737 patients, collecting data to analyze the number of conversations between patients and chatbot) + Prospective study (8 months; 958 patients received a weekly survey). | Analysis for the conversations between patients and chatbot (Vik). Patients: 4737; male: 526; female: 4211; avg age: 48; duration: 1 year. Prospective study patients: 958; duration: 8 months; no details about gender. |
|
Not reported |
| Dworkin et al., 2018 | America- Chicago | HIV | Promoting HIV medication adherence and retention in care. | Iterative approach (five months; 16 men; five iterative focus groups to develop the phone app, each group have 3–4 participants; participants were divided based on the questionnaire they filled out). | Sixteen men participated (African American men who have sex with men) recruited from four Universities of Illinois at Chicago. Age: 18–34; duration: January to May 2016. |
|
|
| Easton et al., 2019 | UK | Patients with an Exempla r Long-Term Condition (LTC; Chronic Pulmonary Obstructive Disease (COPD)) | Data collection, support, self-management, and diagnosis. | Co-design workshop (10 patients; 2 co-design workshops including health professionals and patients to fill out questionnaires). | Ten patients were identified through the local British Lung Foundation Breathe Easy support group. Avg. age: 71; male: 5; female: 5. Workshop 1 was run in July 2017 and lasted 5 h. Workshop 2 was run in October 2017 and lasted 5 h. |
|
Not reported |
| Greer et al., 2019 | America | After Cancer Treatment | Support and follow-up | RCT (8 weeks; 45 young adults; 2 groups, group 1 was experimental group (25 young adults), group 2 was control group (20 young adults); all participants filling-out a survey at baseline). | Forty-five young adults from Facebook advertising, survivorship organizations and direct email. Age: 18–29; male: 9; female: 36; duration: 8 weeks. |
|
|
| Hauser-Ulrich et al., 2019 | German and Swiss | Self-Management of Chronic Pain | Pain self-management | RCT (8 weeks; 102 participants were recruited online, 59 of them were in the intervention group (cognitive behaviour therapy), and the rest were in the control group are not related to pain management). | One hundred and two participants from the SELMA app. Avg. age: 43.7 years; male: 14; female: 88; duration: 2 months. |
|
|
| Inkster et al., 2018 | America- Brooklyn and Chicago | Symptoms of Depression | Data collection and self-reported symptoms of depression | Quasi-experimental (11 July 2017, and 5 September 2017; 129 users were divided into two groups (high users and low users); quantitative was to check the impact of the intervention; qualitative was to check the user experience with Wysa app). | One hundred and twenty-nine users from the Wysa app (high users, n = 108; low users, n = 21). No. of female and male: not reported; duration: 11 July 2017 to 5 September 2017. |
|
Not reported |
| Lobo et al., 2017 | Portugal | Heart Failure Care and Pharmacological Information | Managing information about medicines and increasing adherence | Survey (11 adults; participants filled out a questionnaire to assess system’s performance, feasibility, and drawbacks). | Eleven native Portuguese adults. Age: 22–33; no information about gender and duration. |
|
CARMIE has proven the capability of addressing the pharmacological and treatment information for heart failure daily care. |
| Neerincx et al., 2019 | Netherlands and Italy | Diabetes–Type 1 (T1DM) | Support and manage children diabetes | Iterative refinement process (6 months; this process went through three cycles that include knowledge base, interaction, and some functions to achieve an effective partner for diabetes management). | Children from diabetes camps and hospitals in Netherlands and Italy. Age: 7–14; duration: 6 months. |
|
|
| Rehman et al., 2020 | Korea | Glaucoma and Diabetic Conditions | Data collection and diagnosing |
Experimental method (60 min per patient; 11 groups based on availability and feasibility (three patients per group); each patient interacted with the chatbot individually) (119 responses from 11 countries (overseas students) for the questionnaire were sent by email from the university)). |
Thirty-three international students from the University of Kyung Hee. Age: 18–43; male: 20; female: 13; 60 min per patient. |
Using Cronbach’s Alpha Coefficient correlation of items per scale: attractiveness: 0.74; perspicuity: 0.67; efficiency: 0.77; dependability: 0.60; stimulation: 0.67; novelty: 0.48. |
Not reported |
| Stephens et al., 2019 | America- Boston | Obesity and Prediabetes | Self-reported progress, support and follow-up with a clinician | Feasibility study (6 months; 23 youth encouraged to use Tess chatbot to help users to achieve the progress). | Twenty-three youths with obesity symptoms from children’s healthcare system. Age: 9.78–18.54; male: 10; female: 13; duration: 6 months. |
Ninety-six percent of the total patients reported this chatbot is helpful. | Not reported |
| O’Hara et al., 2008 | America | Intellectual Disabilities; Poor Dental Hygiene | Education and self-management | Quasi-experimental (6 months; 36 dental patients used personal assistive devices (PDs) and had their oral health tracked by a dentist). | Thirty-six participants from a single dental practice. No information about age and gender; 9 participants left study partway through; duration: 6 months. | More than half of participants reported PDAs not functioning correctly (mostly problems keeping the battery charged). | Ten participants (40%) achieved improvement in at least three areas of oral health. |
| Philip et al., 2017 | France | Major Depressive Disorders (MDD) | Clinical interview (major depressive disorder diagnosis) | Clinical interviews (179 participants with major depressive disorders; interview 1 with CA, interview 2 with sleep clinic psychiatrist). | One hundred and seventy-nine outpatients from a sleep clinic in Bordeaux University Hospital. Age: 18–65; male: 42.5%; female: 57.5%; duration: November 2014 to June 2015. |
|
Not reported |
| Piau et al., 2019 | France | Cancer (Geriatric Oncology) | Data collection | Quasi-experimental (7 weeks; 9 participants to test semi-automated CA). | Nine participants (undergoing chemotherapy after cancer diagnosis). Age: +65; male: 5; female: 4; duration: 6 months. |
|
Not reported |
| Puskar et al., 2011 | America | Schizophrenia | Treatment, support and education. | Quasi-experimental (1 month; 17 participants from a local outpatient clinic given laptop computers with a relational agent) | Seventeen patients completed the study, but only results from two participants were mentioned in the study. Age: 18–55; the majority is female; duration: 1 month. |
|
Before CA, the participants had an adherence level of 21%, but with the CA, the rate rose to 46%. |
| Richards and Caldwell, 2018 | Australia | Urinary Incontinence | Treatment and education |
Quasi-experimental ((Pilot studies 1,2,3; 62 patients used web-based eADVICE service (without an added ECA) prior to consultation, with a specialist; study 1—10 patients (2012), study 2—25 patients (2013), study 3—27 patients (2014)) (pilot study 4; 13 patients; testing initial reactions to an ECA called “Dr Evie”. (pilot study 5; over 6 months; 29 participants tested usability and usefulness of eADVICE service + Dr Evie and patient adherence)). |
Children with urinary incontinence. Age: 6–16; 79 families enrolled; 74 completed pre-study survey; males: 44; females: 30; duration: not reported. |
|
|
| Ryu et al., 2020 | South Korea | Mental Health (Depression and Anxiety) | Treatment |
Quasi-experimental Initial field study (1 day; 24 older adults; 10 min of use, video recording hands and screen; thematic analysis of interviews to find five features). Beta-testing field study (2 weeks; 25 older participants; 4 excluded from analysis; chat-initiated message three times a day; Epidemiologic Studies Depression and Beck Anxiety Inventory scales used before and after testing; negative polarity analysis of chat). |
Initial testing had 24 older adults. male: 7; female: 17. Beta-testing had 25 older adults; 4 excluded from analysis for missing second interview; 4 lost their chat history, 2 declined chat history collection; no information about age and duration. |
|
|
| Schroeder et al., 2018 | America | Mental Health | Treatment and education | Quasi-experimental (4 weeks; 73 individuals; surveys containing OASIS and PHQ-9 scales for anxiety and depression, and 5-point Likert scale question for user satisfaction). | Seventy-three participants. Female: 65; male: 7; age: 18–63; duration: 4 weeks. |
|
|
| Sebastian & Richards, 2017 | Australia | Mental and Physical Health (Anorexia Nervosa) | Education and increased awareness | RCT (245 participants; 4 min video, variant-time ECA interaction, but same transcript length; 4-way design Mental health literacy (MHL) framework is used to assess stigma amongst participants). | Two hundred and forty-five undergraduate university students. Age: +18; no information about gender and study duration. |
|
|
| Shamekhi & Bickmore, 2018 | America | Various Chronic Conditions; Pain, Anxiety, and Depression | Treatment and coaching |
RCT (Respiration) (2 × 12 min meditations; Mindful Attention Awareness Scale (MAAS) used to assess mindfulness; the control group was given agent treatment without respiratory sensors). RCT (Comparison) (24 participants; 2 × 12 min meditations; the control group was shown Eckhart Tolle video; agent dialogue was modified to match the video). |
Respiratory RCT had 21 participants. Age: +18; male: 38%; female: 62%; duration: 2 sessions; (12 min per session). Comparison RCT had 24 participants; Age: +18; male: 63%; female: 37%; duration: 2 sessions; (12 min per session). |
|
|
| Tielman et al., 2017 | Netherla-nds | Post-Traumatic Stress Disorder (PTSD). | Treatment. | Quasi-experimental (4 participants; 12 sessions with a CA to create a virtual diary, then the PTSD environment was recreated in Worldbuilder. Participants started with self-assessments, and sessions are closed with a questionnaire (5 pt. Likert scale)). | Four participants. Two males were war-veterans; two females experienced childhood sexual abuse; no information about age and study duration. |
|
|
Abbreviations: Avg.: average; OOV: out of vocabulary; RCT: randomized control trial, app: application; p: p-value; Laura: chatbot prototype who blends speech recognition, AI, and realistic 3D animation; SELMA app: A Digital Coach for Self-Management of Pain; Wysa app: AI-powered mental health app; CARMIE: a smartphone-based assistant developed with the aim to deliver information and knowledge-based advice to help chronic disease patients; CA: conversational agents; ECA: Embodied Conversational Agent; PDA: Personal digital assistance; eADVICE: electronic Advice and Diagnosis via the Internet following Computerised Evaluation; Dr. Evie: eVirtual agent for incontinence and enuresis; PHQ-9: Patient Health Questionnaire 9-item scale, measures the frequency and severity of depressive symptoms; DBT: Dialectical Behavior Therapy. OASIS: Development and Validation of an Overall Anxiety Severity and Impairment Scale; SD: standard deviation.
Table 2 has retained the characteristics of the CAs (the categories defined in Box 1) that were evaluated in the included studies. In addition, it shows AI methods used, based on a list of keywords, defined from three systematic literature reviews for CAs in health care [1,6,27].
Table 2.
Characteristics of the conversational agents evaluated in the included studies.
| Author, Year | Type of Communication Technology; Type of Conversational Agent | AI Methods Used | Dialogue Management | Dialogue Initiative | Input | Output | Task-Oriented |
|---|---|---|---|---|---|---|---|
| Azzini et al., 2003 | Smartphone and web-based; spoken dialog system. | Speech recognition and spoken dialog system. | Finite-state | Mixed | Spoken | Spoken, written | Yes |
| Baptista et al., 2020 | Smartphone app; ECA. | Speech recognition, natural language processing. | Finite-state | System | Spoken, visual | Spoken, written, visual | Yes |
| Beaudry et al., 2019 | Text messaging platform; chatbot. | Machine learning, NLU, NLP, deep learning, speech recognition. | Finite-state | System | Written | Written | Yes |
| Bickmore et al., 2010 | Framework; ECA. | Speech recognition, synthetic voice. | Finite-state | System | Spoken, visual | Spoken, written, visual | Yes |
| Bickmore et al., 2010 | Home desktop software; animated agent and interaction dialogues. | Not reported. | Finite-state | System | Visual | Spoken, visual | Yes |
| Bott et al., 2019 | Platform; ECA. | Text-to-speech, NLU. | Frame-based | Mixed | Spoken, visual | Spoken, written, visual | Yes |
| Chaix et al., 2019 | Smartphone and web-based; chatbot. | Machine learning, NLP. | Finite-state | System | Written, visual | Written | Yes |
| Dworkin et al., 2018 | Smartphone app; Avatar-based embodied agent. | Not reported. | Finite-state | Mixed | Spoken, written, visual | Spoken, written, visual | Yes |
| Easton et al., 2019 | Web-based; avatar and chatbot. | NLP, speech recognition. | Frame-based | Mixed | Spoken, written | Spoken, written, visual | Yes |
| Greer et al., 2019 | Facebook messenger; chatbot. | Not reported. | Finite-state | System | Written | Written, visual | Yes |
| Hauser-Ulrich et al., 2019 | Smartphone app; chatbot. | Not reported. | Finite-state | System | Written | Written, visual | No |
| Inkster et al., 2018 | Smartphone app; chatbot. | Machine learning, unsupervised learning. | Finite-state | System | Written | Written, visual | Yes |
| Lobo et al., 2017 | Android app; chatbot. | Speech recognition, speech synthesis, spoken natural language, hidden Markov model, natural language Understanding, natural language dialogue system. |
Frame-based | Mixed | Spoken, written | Spoken, written | Yes |
| Neerincx et al., 2019 | Platform independent app, robot and avatar. | Machine learning, deep learning, speech recognition, speech synthesis. | Finite-state | System | Visual | Spoken, written, visual | Yes |
| Rehman et al., 2020 | Android app; chatbot. | NLU, speech recognition, text to speech synthesis, neural network algorithm, machine learning, natural language processing, deep learning, spoken dialog. | Frame-based | User | Spoken, written | Spoken, written | Yes |
| Stephens et al., 2019 | SMS text messaging; chatbot. | Not reported. | Frame-based | Mixed | Written | Written | Yes |
| O’Hara et al., 2008 | Personal Digital Assistants (PDAs). | Not reported. | Finite-state | System | Written | Written | Yes |
| Philip et al., 2017 | Home desktop software; Virtual human ECA. |
Speech recognition, synthetic voice. |
Finite-state | System | Spoken | Spoken | Yes |
| Piau et al., 2019 | Semi-automated smartphone messaging system; chatbot. |
Speech to text. | Finite-state | System | Written | Written | Yes |
| Puskar et al., 2011 | Home desktop software; Relational Agent. | NLU, facial recognition, speech dialogue system. |
Frame-based | System | Written | Written | Yes |
| Richards and Caldwell, 2018 | Website; Avatar and Empathic ECA a. | Speech to text. | Finite-state | System | Visual | Written; spoken | No |
| Ryu et al., 2020 | Smartphone app; chatbot. | Speech recognition. | Frame-based | System | Visual | Written | No |
| Schroeder et al., 2018 | Smartphone app; chatbot. | Not reported. | Finite-state | System | Visual | Written | Yes |
| Sebastian & Richards, 2017 | Platform independent app; ECA. | Not reported. | Finite-state | System | Visual | Written | Yes |
| Shamekhi & Bickmore, 2018 | Home desktop software; an animated agent with spoken dialogue and sensing. | Spoken dialog system. | Frame-based | System | Respiration sensor | Spoken | Yes |
| Tielman et al., 2017 | Home desktop software; an animated agent with spoken dialogue. | Spoken dialog system. | Finite-state | System | Visual | Spoken; written | Yes |
Abbreviations: app: application; ECA: Embodied Conversational Agent; a Empathic ECA: empathic agent that provides face-to-face conversation in an empathic and caring way, to act as a virtual doctor for the family to interact with.
3. Results
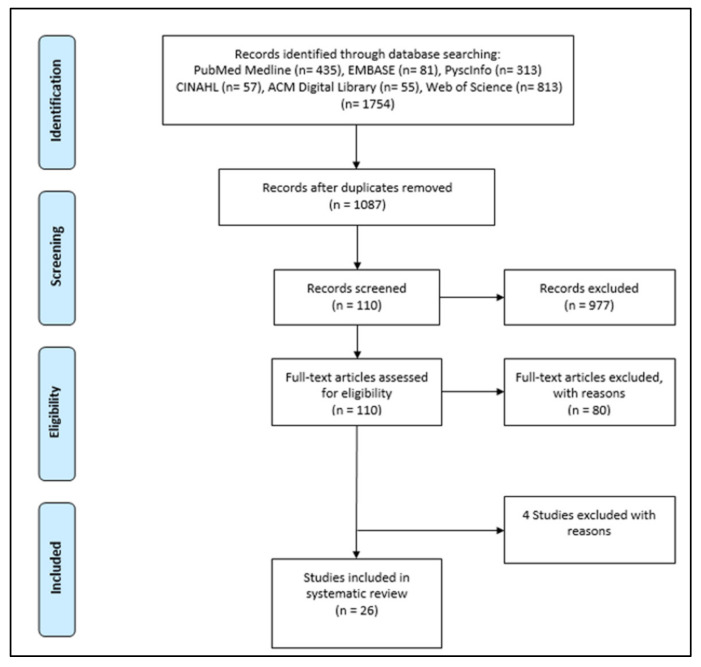
The six databases that were searched retrieved 1754 articles. Then, the duplicates were removed, which resulted in 1087 unique articles. After the abstract and title screening, 110 articles remained. After the full-text screening, 80 of these were excluded. Thirty articles were considered eligible for inclusion in the systematic literature review. Four more papers were excluded during extraction data based on the exclusion criteria. Twenty-six articles were considered eligible for inclusion in the systematic literature review (Figure 1).
Figure 1.
Flow Diagram.
3.1. Description of Included Studies
The complete list of included studies (26 studies) used CAs to support tasks undertaken by patients (n = 14), clinicians (n = 1), and both (n = 11). Fourteen studies focused on patients mostly supporting education and self-care [28,29,30,31,32,33,34,35,36,37,38,39,40,41]. One study focused on clinicians, where the CAs were used to educate and increase awareness of the introductory psychology students in mental and physical health [42]. Eleven further studies supported patients and clinicians, where seven used CAs in treatment, education, and data collection [43,44,45,46,47,48,49]. Two studies helped in following-up [50,51], and two studies promoted medication adherence [52,53]. The most common chronic condition was diabetes (n = 5). Two studies were for type 1 [29,39], one study was for type 2 [28], and one focused on diabetes in general, whether the patients have type 1 or 2 [40]. One study focused on prediabetes with obesity [51]. The other four studies concentrated on different aspects of depression, such as symptoms and disorders [37,45,46,52]. Three studies dealt with cancer (breast cancer, geriatric oncology, after cancer treatment) [34,47,50]. Anxiety that could lead to depression [32,48], mental health [33,42], and schizophrenia [30,44] were the focus of two studies for each condition. Other conditions included hypertension [43], heart failure [38], HIV [53,54,55,56], long-term conditions [35], chronic pain [36], chronic problems in oral health [41], urinary incontinence [31], and post-traumatic stress disorder [49]. In terms of methods, the methods used in most studies were RCT, pilot study, or quasi-experimental. Only two studies had used mixed methods, and the remaining studies used other methods.
3.2. Description of Conversational Agents and AI Methods
Different technologies have supported CAs, including independent platforms, apps delivered via web or mobile device, short message services (SMS), and telephone (Table 2). Out of 26 conversational agents, 16 were chatbots (a computer program that simulates human conversation via voice or text communication). Seven were embodied conversational agents (ECA), a virtual agent that appeared on computer screens and was equipped with a virtual, human-like body that had real-time conversations with humans. One was a conversational agent in a robot, and another was a relational agent explicitly designed to remember history and manage future expectations in their interactions with users. One agent was not specified [43]. The characterisation of conversational agents are as shown in Table 3, and this summarization is adapted from Laranjo et al. 2018 [27].
Table 3.
Characterisation of conversational agents (Laranjo et al. 2018 [27]).
| Dialogue management | Finite-state | The user is taken through a dialogue consisting of a sequence of pre-determined steps or states. |
| Frame-based | The user is asked questions that enable the system to fill slots in a template in order to perform a task. | |
| The dialogue flow is not pre-determined, but it depends on the content of the user’s input and the information that the system has to elicit. | ||
| Agent-based | These systems enable complex communication between the system, the user, and the application. There are many variants of agent-based systems, depending on what aspects of intelligent behavior are designed into the system. In agent-based systems, communication is viewed as the interaction between two agents, each of which is capable of reasoning its own actions and beliefs, and sometimes the actions and beliefs of the other agent. The dialogue model takes the preceding context into account, with the result that the dialogue evolves dynamically as a sequence of related steps that build on each other. | |
| Dialogue initiative | User | The user leads the conversation. |
| System | The system leads the conversation. | |
| Mixed | Both the user and the system can lead the conversation. | |
| Input modality | Spoken | The user uses spoken language to interact with the system. |
| Written | The user uses written language to interact with the system. | |
| Output modality | Spoken, Written, visual (e.g., non-verbal communication like facial expressions or body movements). | |
| Task-oriented | Yes | The system is designed for a particular task and is set up to have short conversations, in order to get the necessary information to achieve the goal (e.g., booking a consultation). |
| No | The system is not directed to the short-term achievement of a specific end-goal or task (e.g., purely conversational chatbots). | |
The CAs in the papers used various AI methods such as speech recognition, facial recognition, and NLP. However, most studies did not provide sufficient information on the implementation details. In order to identify the AI methods, a list of common words (Appendix B) used for building AI CAs [1,6,27] were employed. Several papers reported that AI methods could improve the user’s interaction with the system [1,2,5,6,27]. For example, speech recognition can capture speech much faster than you can type. Half of the included papers utilized speech recognition in many CAs (e.g., chatbot, ECA, or relational agent). Although having speech recognition can capture speech much faster than typing, it could lead to difficulties with some keywords because of misinterpretation of words. Six studies did not report these technical methods.
3.3. Evaluation Measures
Evaluation measures were identified based on three types: technical performance (six studies), user experience (25 studies), and health-related measures (18 studies). The most common technical performance measures were accuracy (89–99.2% for five CAs) [31,37,40,43,48] and specificity (93–99.7% for three CAs) [37,40,46]. One study for hypertension identified that the rate of the achieved goal for the CAs was 96%. In addition, the authors clarified that the accuracy of the spoken dialogue system in cough and compliance were 81% and 41%, respectively [43]. Another study in glaucoma and diabetic conditions used Cohen’s D to calculate the task completion (k = 0.848), and the accuracy of the CAs was 89% [40]. Two studies were on depression (symptoms and major depressive disorder) and used finite-state dialogue management. The study for symptoms of depression noted that the written chatbot showed an accuracy of 99.2% and a specificity of 99.7% [52]. The spoken system for a major depressive disorder used embodied CAs, and showed sensitivity (49%) and specificity (93%) [46]. Two studies were about treating urinary incontinence and various chronic conditions such as pain and anxiety. The urinary incontinence article mentioned accuracy, but without clarifying the percentage or rate of accuracy [31]. Another paper for various chronic conditions (pain, anxiety, and depression) showed almost 92% accuracy in the breathing rate for patients [48].
Almost all studies reported on user experience except one study [43]. Helpfulness, satisfaction, and ease of use were the common features in more than half of the included studies. Three studies mentioned that users were unsatisfied. In two studies, the participants found the CAs hard to use. Regarding diabetes–type 2 [28], a study reported the feedback from patients through various measures, such as competency (85%), helpfulness and friendliness (86%). On the other hand, some patients described the embodied CAs as annoying (39%) and boring (30%). Another study for diabetes [40] illustrated the user experience through attractiveness (0.74), perspicuity (0.67), and efficiency (0.77), by using the scale of Cronbach’s Alpha Coefficient correlation. In mental health, a study for treatment and education reported that some users felt the chatbot was hard to engage with and had no availability to ask questions [33]. A study after cancer treatment clarified that the users found the chatbot nonjudgmental and helpful. Additionally, users supported recommending it to a friend (69%).
Regarding health-related measures, 18 out of the 26 studies included the health-related measures. The most common method that has been used is quasi-experimental, where it was used in 12 out of 26 studies. The second most common method used was RCT, used in six studies. One of the quasi-experimental studies evaluated the medication adherence system of interaction dialogue, finding decreased delirium (p < 0.001) and loneliness (p = 0.01) [45]. Another study showed a reduction in depression and anxiety by p = 0.053 and 0.029, respectively [32]. One RCT measured the outcomes using a 5-point Likert-type scale, finding improved self-management for older people with the chatbot (p = 0.001) [28]. One study reported quasi-experimental and RCT which evaluated a medication adherence intervention, finding that system use, medication adherence, physical activity, and satisfaction measures were high (84–89%) [44].
4. Discussion
The most commonly used method in the included studies was quasi-experimental, which was used in almost half of the included papers. This is aligned with the findings of the previous systematic reviews of CAs in healthcare [1,27]. Quasi-experimental demonstrates the involvement of real-world interventions, instead of artificial laboratory settings. It allows the research to move with higher internal validity than other non-experimental types of research. In addition, quasi-experimental design requires fewer resources and is less expensive compared with RCT. This systematic review introduced a list of AI CAs in healthcare for chronic disease. It reflects the efficiency, acceptability, and usability of the AI CAs in the daily education of, and support for, chronic disease patients. Our review reflected this as most of the included studies were published after 2016 (21 papers). Most included studies evaluated task-oriented AI CAs (23 studies out of 26) that are used to assist patients and clinicians through specific processes. The majority of the included studies were focused entirely on designing, developing, or evaluating AI CAs that are specific to one chronic condition. This finding implies that AI CAs evolve to provide tailored support for specific chronic conditions, rather than general interventions for a broad range of chronic conditions.
The outcomes of the included studies were assessed on three measures: technical performance, user experience, and a health-related measure. There were only six studies that mentioned some technical details. Due to the lack of details reported on the technical implementation of AI methods, it was not possible to establish consistent relationships with the intervention used, disease areas, and measured outcomes. The evaluation measures of the identified AI-based CAs and their effects on the targeted chronic conditions were not unified and broad. This inconsistency shows the complexity of contrasting and comparing the current AI CAs. Regardless of some studies that showed the complexity in use and chatbot constraints (four studies), most studies reported satisfaction with agents and feeling more comfortable than continuous follow-ups with a doctor in the hospitals. User experience was the most commonly reported measure (25 studies). It reflects the positive effect and enhancement of the quality of life in most studies through AI CAs that help patients who suffer from a chronic condition. This systematic review found that most included studies focused on designing, developing, or evaluating AI CAs for a specific chronic condition. That resulted in more accuracy, a tailored interaction with patients, and enhanced interventions for a wide range of conditions. In dialogue management, nine studies used a mixed initiative, whereas most applied system initiatives. No study in the included studies targeted the dialogue management of agent-based interactions. Moreover, these studies do not contain CAs that can be used across other populations. No analysis is applied on a broad scale, especially for communities or countries that suffer a lot from managing chronic conditions due to the high demand on hospitals or the cost and effort of following up with doctors. Targeting this area will help many people deal with chronic conditions and live their lives, especially as the CAs’ supporting preventive measures can prove very effective.
Compared to prior reviews focused on AI CAs for healthcare, we found only two review studies that targeted AI CAs for chronic conditions, where one of them focused on voice-based CAs only. Those reviews did not differentiate between the type of CAs used besides the AI methods used in each study, so this review focused on investigating the different types of dialogue management with the AI method used in each study. This review also focused on technical descriptions of the CAs used. Clarifying the technical features of the AI CAs will help to choose the appropriate type of AI CAs. Regarding limitations, most studies did not include technical performance details, which makes replicability of the studies reviewed problematic. Another limitation of the reviewed literature is the heterogeneity and the prevalence of quasi-experimental studies. This suggests that this is still a nascent field.
5. Conclusions
Many studies in this review showed some positive evidence for the usefulness and usability of AI CAs to support the management of different chronic diseases. The overall acceptance of CAs by users for the self-management of their chronic conditions is promising. Users’ feedback shows helpfulness, satisfaction, and ease of use in more than half of the included studies. Although the users in many studies appear to feel more comfortable with CAs, there is still a lack of reliable and comparable evidence to determine the efficacy of AI-enabled CAs for chronic health conditions. This is mainly due to the insufficient reporting of technical implementation details. Future research studies should provide more detailed accounts of the technical aspects of the CAs used. This includes developing a comprehensive and clear taxonomy for the CAs in healthcare. More RCT studies are required to evaluate the efficacy of using AI CAs to manage chronic conditions. Safety aspects of CAs is still a neglected area, and needs to be included as part of core design considerations.
Appendix A
Study Protocol
Adopted from PRISMA-P (Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols) and PROSPERO.
| Topic | Content | |
| Title | Conversational Healthcare Agent for Chronic Diseases: A Systematic Review | |
| Authors | Abdullah Bin Sawad, Baki Kocaballi, Mukesh Prasad, Bhuva Narayan, Ahlam Alnefaie, Ashwaq Maqbool, Indra Mckie, Jemma Smith, Berkan Yuksel | |
| Review team members and their organisational affiliations | Abdullah Bin Sawad 1 | (PhD Student) |
| Dr Baki Kocaballi 1 | (Lecturer) | |
| Dr Mukesh Prasad 1 | (Senior Lecturer) | |
| Dr Bhuva Narayan 2 | (Associate Professor) | |
| Ahlam Alnefaie 1 | (PhD Student) | |
| Dr Ashwaq Maqbool 3 | (Master Student) | |
| Indra Mckie 1 | (PhD Student) | |
| Jemma Smith 4 | (Bachelor Student) | |
| Berkan Yuksel 1 Deepak Puthal 5 |
(Bachelor Student) (Assistant Professor) |
|
| Contact details of the corresponding author | Abdullah Bin Sawad abdullahhatima.binsawad-1@student.uts.edu.au |
|
| Organisational affiliation of the review | University of Technology Sydney | |
| Type and method of review | Systematic literature review | |
| Contributions | Study design: AS; Search strategy: AS; Screening: AS, AA, IM Data extraction and Data analysis: AS, AM, JS, BY; First draft: AS; Revisions and subsequent drafts: BK, MP, BN, DP; Critical feedback for the final draft: BK, MP, BN, DP | |
| Sources/Sponsors | NA | |
| Conflict of interest | None | |
| Rationale | What kinds of conversational agents are used for chronic conditions, what type of communication technology, what AI methods are used, what are the outcomes, the research gaps, and who are the target users/population group. | |
| Eligibility criteria |
Inclusion Criteria
Exclusion Criteria
|
|
| Information sources | A database search will be conducted by accessing PubMed Medline, EMBASE, PsycINFO, CINAHL, ACM Digital Library, and Web of Science databases. Search terms include synonyms, acronyms, and commonly known terms of the constructs “conversational agent” and “healthcare”. Grey literature will be excluded, such as posters, reviews, and presentations. | |
| Search strategy | The following search strategy will be used in the whole six databases. Filters: none Conduct started in February 2021 “Conversational agent” OR “conversational agents” OR “conversational system” OR “conversational systems” OR “dialog system” OR “dialog systems” OR “dialogue systems” OR “dialogue system” OR “assistance technology” OR “assistance technologies” OR “relational agent” OR “relational agents” OR “chatbot” OR “chatbots” OR “digital agent” OR “digital agents” OR “digital assistant” OR “digital assistants” OR “virtual assistant” OR “virtual assistants” AND “healthcare” OR “digital healthcare” OR “digital health” OR “health” OR “mobile health” OR “mHealth” OR “mobile healthcare”. |
|
| Type of included study | Any primary research | |
| Studied domain | Chronic health conditions | |
| Population/Participants | Any population and any participants (caregivers, healthcare professionals, clinical/non-clinical, patients) | |
| Data collection and selection process | AS and AA will conduct the initial screening of the obtained studies based on titles and abstracts. Then, AS and IM will conduct full-text screening based on the eligibility/inclusion criteria. AS, AM, JS, and BY will extract data from eligible papers. Any disagreement will be discussed in the zoom meeting. Dr. Kocaballi and Dr. Prasad will supervise all these processes to ensure the measures are on the right path. | |
| Data items for coding | The following data items will be extracted from each included study: first author, year of publication, study location, study design/type, study aim, conversational agent evaluation measures, main reported outcomes and findings, type of chronic condition, type of study participants, type of the conversational agent, the goal of the conversational agent, communication channel, interaction modality, technique, system development. AS, AM, JS and BY will conduct the data extraction, and it will be discussed with Kocaballi and Dr Prasad. | |
| Outcomes and prioritisation | Main outcomes: Any healthcare related intervention outcomes (e.g., type of chronic condition, health goal, intervention targets), any architecture related outcomes (e.g., technique type, system development). Additional outcomes: Any conversational agent related outcomes (e.g., feasibility, accuracy, acceptability, functionality) and design features. |
|
| Risk of bias in individual studies | AS and IM will review the included papers to appraise their quality. Disagreement will be discussed to reach a consensus. Any disagreement will be resolved with Dr Kocaballi and Dr. Prasad. | |
| Data synthesis | The PRISMA guidelines will be used for data synthesis. A narrative synthesis of the included studies will be performed. | |
| Language | English | |
| Country | Australia | |
| Anticipated or actual start date | February 2021 | |
| Anticipated or actual end date | September 2021 | |
| 1 School of Computer Science, Faculty of Engineering and IT, University of Technology Sydney. 2 School of Communication, Faculty of Arts and Social Sciences, University of Technology Sydney. 3 School of Public Health, Faculty of Medicine and Health, The University of Sydney. 4 School of Biomedical Engineering, Faculty of Engineering and IT, University of Technology Sydney. 5 Department of Electrical Engineering and Computer Science, Khalifa University. | ||
Appendix B
Keywords for AI Methods Used
| Artificial Intelligence or AI |
| Natural Language Understanding or NLU |
| Natural Language Processing or NLP |
| NR |
| neural networks |
| deep learning |
| machine learning |
| clustering/classification |
| unsupervised/supervised learning |
| CNN or convolutional neural network |
| Markov chain |
| hidden Markov chain |
| reinforcement learning |
| facial recognition |
| speech recognition |
| text analysis |
| sentiment analysis |
| natural language generation |
| text-to-speech or TTS |
| speech-to-text or STT |
| synthetic speech |
| spoken dialog system |
Author Contributions
Conceptualization, A.B.S., M.P. and A.B.K.; methodology, A.B.S., B.N., M.P., D.P. and A.B.K.; software, A.B.S., A.A., A.M., I.M., J.S. and B.Y.; validation, A.B.S., A.A., A.M., I.M., J.S. and B.Y.; formal analysis A.B.S., A.A., A.M., I.M., J.S. and B.Y., investigation, A.B.S., B.N., D.P., M.P. and A.B.K.; resources, A.B.S., M.P. and A.B.K.; data curation, A.B.S., A.A. and I.M.; writing—original draft preparation, A.B.S.; writing—review and editing, A.B.S., B.N., A.A., A.M., I.M., J.S., B.Y., D.P., M.P. and A.B.K.; visualization, I.M., J.S. and B.Y.; supervision, B.N., M.P. and A.B.K.; project administration, A.A., A.M. and I.M.; funding acquisition, B.N., M.P., D.P. and A.B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schachner T., Keller R., Wangenheim F.V. Artificial Intelligence-Based Conversational Agents for Chronic Conditions: Systematic Literature Review. J. Med. Internet Res. 2020;22:e20701. doi: 10.2196/20701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kramer L.L., Ter Stal S., Mulder B., De Vet E., Van Velsen L. Developing Embodied Conversational Agents for Coaching People in a Healthy Lifestyle: Scoping Review. J. Med. Internet Res. 2020;22:e14058. doi: 10.2196/14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrand J., Hockensmith R., Houghton R.F., Walsh-Buhi E.R. Evaluating Smart Assistant Responses for Accuracy and Misinformation Regarding Human Papillomavirus Vaccination: Content Analysis Study. J. Med. Internet Res. 2020;22:e19018. doi: 10.2196/19018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sezgin E., Militello L.K., Huang Y., Lin S. A scoping review of patient-facing, behavioral health interventions with voice assistant technology targeting self-management and healthy lifestyle behaviors. Transl. Behav. Med. 2020;10:606–628. doi: 10.1093/tbm/ibz141. [DOI] [PubMed] [Google Scholar]
- 5.Safi Z., Abd-Alrazaq A., Khalifa M., Househ M. Technical Aspects of Developing Chatbots for Medical Applications: Scoping Review. J. Med. Internet Res. 2020;22:e19127. doi: 10.2196/19127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffin A.C., Xing Z., Khairat S., Wang Y., Bailey S., Arguello J., Chung A.E. AMIA Annual Symposium Proceedings. American Medical Informatics Association; Bethesda, MD, USA: 2021. Conversational Agents for Chronic Disease Self-Management: A Systematic Review; pp. 504–513. [PMC free article] [PubMed] [Google Scholar]
- 7.McGreevey J.D., 3rd, Hanson C.W., 3rd, Koppel R. Clinical, Legal, and Ethical Aspects of Artificial Intelligence–Assisted Conversational Agents in Health Care. JAMA J. Am. Med. Assoc. 2020;324:552. doi: 10.1001/jama.2020.2724. [DOI] [PubMed] [Google Scholar]
- 8.Bickmore T.W., Kimani E., Trinh H., Pusateri A., Paasche-Orlow M.K., Magnani J.W. Managing Chronic Conditions with a Smartphone-based Conversational Virtual Agent; Proceedings of the 18th International Conference on Intelligent Virtual Agents, IVA 2018; Sydney, NSW, Australia. 5–8 November 2018; [DOI] [Google Scholar]
- 9.Pereira J., Díaz Ó. Using Health Chatbots for Behavior Change: A Mapping Study. J. Med. Syst. 2019;43:135. doi: 10.1007/s10916-019-1237-1. [DOI] [PubMed] [Google Scholar]
- 10.Greer S., Ramo D., Chang Y.-J., Fu M., Moskowitz J., Haritatos J. Use of the Chatbot “Vivibot” to Deliver Positive Psychology Skills and Promote Well-Being Among Young People After Cancer Treatment: Randomized Controlled Feasibility Trial. JMIR mHealth uHealth. 2019;7:e15018. doi: 10.2196/15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hebbar A. Augmented intelligence: Enhancing human capabilities; Proceedings of the 2017 Third International Conference on Research in Computational Intelligence and Communication Networks (ICRCICN); Kolkata, India. 3–5 November 2017; pp. 251–254. [DOI] [Google Scholar]
- 12.Miner A.S., Shah N., Bullock K.D., Arnow B.A., Bailenson J., Hancock J. Key Considerations for Incorporating Conversational AI in Psychotherapy. Front. Psychiatry. 2019;10:746. doi: 10.3389/fpsyt.2019.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coiera E., Kocaballi A.B., Halamka J., Laranjo L. The digital scribe. NPJ Digit. Med. 2018;1:58. doi: 10.1038/s41746-018-0066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coffey S., Vanderlip E., Sarvet B. The Use of Health Information Technology Within Collaborative and Integrated Models of Child Psychiatry Practice. Child Adolesc. Psychiatr. Clin. N. Am. 2017;26:105–115. doi: 10.1016/j.chc.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Ly K.H., Ly A.-M., Andersson G. A fully automated conversational agent for promoting mental well-being: A pilot RCT using mixed methods. Internet Interv. 2017;10:39–46. doi: 10.1016/j.invent.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miner A.S., Laranjo L., Kocaballi A.B. Chatbots in the fight against the COVID-19 pandemic. NPJ Digit. Med. 2020;3:1–4. doi: 10.1038/s41746-020-0280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kvedar J.C., Fogel A.L., Elenko E., Zohar D. Digital medicine’s march on chronic disease. Nat. Biotechnol. 2016;34:239–246. doi: 10.1038/nbt.3495. [DOI] [PubMed] [Google Scholar]
- 18.Fadhil A., Wang Y., Reiterer H. Assistive Conversational Agent for Health Coaching: A Validation Study. Methods Inf. Med. 2019;58:009–023. doi: 10.1055/s-0039-1688757. [DOI] [PubMed] [Google Scholar]
- 19.McGreevey J.D., Hanson C.W., Koppel R., Darcy A., Robinson A., Wicks P. Conversational Agents in Health Care-Reply. JAMA. 2020;324:2444. doi: 10.1001/jama.2020.21518. [DOI] [PubMed] [Google Scholar]
- 20.Stephens T.N., Joerin A., Rauws M., Werk L.N. Feasibility of pediatric obesity and prediabetes treatment support through Tess, the AI behavioral coaching chatbot. Transl. Behav. Med. 2019;9:440–447. doi: 10.1093/tbm/ibz043. [DOI] [PubMed] [Google Scholar]
- 21.Australian Institute of Health and Welfare . Australia’s Health 2018. Volume 15. Australian Institute of Health and Welfare; Canberra, Australia: 2018. [(accessed on 1 January 2022)]. 3.3 Chronic Conditions, Chapter 3 Causes of Ill Health. Available online: https://www.aihw.gov.au/getmedia/6bc8a4f7-c251-4ac4-9c05-140a473efd7b/aihw-aus-221-chapter-3-3.pdf.aspx. [Google Scholar]
- 22.Dunkel-Jackson S.M., Dixon M.R., Szekely S. Portable data assistants: Potential in evidence-based practice autism treatment. Res. Autism Spectr. Disord. 2012;6:65–72. doi: 10.1016/j.rasd.2011.06.004. [DOI] [Google Scholar]
- 23.Kang J., Thompson R.F., Aneja S., Lehman C., Trister A., Zou J., Obcemea C., El Naqa I. National Cancer Institute Workshop on Artificial Intelligence in Radiation Oncology: Training the Next Generation. Pract. Radiat. Oncol. 2020;11:74–83. doi: 10.1016/j.prro.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Montenegro J.L.Z., da Costa C.A., da Rosa Righi R. Survey of conversational agents in health. Expert Syst. Appl. 2019;129:56–67. doi: 10.1016/j.eswa.2019.03.054. [DOI] [Google Scholar]
- 26.Kocaballi A.B., Quiroz J.C., Rezazadegan D., Berkovsky S., Magrabi F., Coiera E., Laranjo L. Responses of Conversational Agents to Health and Lifestyle Prompts: Investigation of Appropriateness and Presentation Structures. J. Med. Internet Res. 2020;22:e15823. doi: 10.2196/15823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laranjo L., Dunn A., Tong H.L., Kocaballi A.B., Chen J., Bashir R., Surian D., Gallego B., Magrabi F., Lau A.Y., et al. Conversational agents in healthcare: A systematic review. J. Am. Med. Inform. Assoc. 2018;25:1248–1258. doi: 10.1093/jamia/ocy072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baptista S., Wadley G., Bird D., Oldenburg B., Speight J. The My Diabetes Coach Research Group Acceptability of an Embodied Conversational Agent for Type 2 Diabetes Self-Management Education and Support via a Smartphone App: Mixed Methods Study. JMIR mHealth uHealth. 2020;8:e17038. doi: 10.2196/17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beaudry J., Consigli A., Clark C., Robinson K.J. Getting Ready for Adult Healthcare: Designing a Chatbot to Coach Adolescents with Special Health Needs Through the Transitions of Care. J. Pediatr. Nurs. 2019;49:85–91. doi: 10.1016/j.pedn.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Puskar K., Schlenk E., Callan J., Bickmore T., Sereika S. Relational Agents as an Adjunct in Schizophrenia Treatment. J. Psychosoc. Nurs. Ment. Health Serv. 2011;49:22–29. doi: 10.3928/02793695-20110705-01. [DOI] [PubMed] [Google Scholar]
- 31.Richards D., Caldwell P. Improving Health Outcomes Sooner Rather Than Later via an Interactive Website and Virtual Specialist. IEEE J. Biomed. Health Inform. 2017;22:1699–1706. doi: 10.1109/JBHI.2017.2782210. [DOI] [PubMed] [Google Scholar]
- 32.Ryu H., Kim S., Kim D., Han S., Lee K., Kang Y. Simple and Steady Interactions Win the Healthy Mentality: Designing a Chatbot Service for the Elderly. Proc. ACM Hum.-Comput. Interact. 2020;4:125. doi: 10.1145/3415223. [DOI] [Google Scholar]
- 33.Schroeder J., Wilkes C., Rowan K., Toledo A., Paradiso A., Czerwinski M., Mark G., Linehan M.M. Pocket Skills: A conversational Mobile web app to support dialectical behavioral therapy; Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems; Montreal, QC, Canada. 21–26 April 2018; p. 398. [DOI] [Google Scholar]
- 34.Chaix B., Bibault J.-E., Pienkowski A., Delamon G., Guillemassé A., Nectoux P., Brouard B. When Chatbots Meet Patients: One-Year Prospective Study of Conversations between Patients with Breast Cancer and a Chatbot. JMIR Cancer. 2019;5:e12856. doi: 10.2196/12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Easton K., Potter S., Bec R., Bennion M., Christensen H., Grindell C., Mirheidari B., Weich S., De Witte L., Wolstenholme D., et al. A Virtual Agent to Support Individuals Living with Physical and Mental Comorbidities: Co-Design and Acceptability Testing. J. Med. Internet Res. 2019;21:e12996. doi: 10.2196/12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hauser-Ulrich S., Künzli H., Meier-Peterhans D., Kowatsch T. A Smartphone-Based Health Care Chatbot to Promote Self-Management of Chronic Pain (SELMA): Pilot Randomized Controlled Trial. JMIR mHealth uHealth. 2020;8:e15806. doi: 10.2196/15806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inkster B., Sarda S., Subramanian V. An Empathy-Driven, Conversational Artificial Intelligence Agent (Wysa) for Digital Mental Well-Being: Real-World Data Evaluation Mixed-Methods Study. JMIR mHealth uHealth. 2018;6:e12106. doi: 10.2196/12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lobo J., Ferreira L., Ferreira A.J. CARMIE: A conversational medication assistant for heart failure. Int. J. E-Health Med. Commun. 2017;8:21–37. doi: 10.4018/IJEHMC.2017100102. [DOI] [Google Scholar]
- 39.Neerincx M.A., Van Vught W., Henkemans O.B., Oleari E., Broekens J., Peters R., Kaptein F., Demiris Y., Kiefer B., Fumagalli D., et al. Socio-Cognitive Engineering of a Robotic Partner for Child’s Diabetes Self-Management. Front. Robot. AI. 2019;6:118. doi: 10.3389/frobt.2019.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rehman U.U., Chang D.J., Jung Y., Akhtar U., Razzaq M.A., Lee S. Medical Instructed Real-Time Assistant for Patient with Glaucoma and Diabetic Conditions. Appl. Sci. 2020;10:2216. doi: 10.3390/app10072216. [DOI] [Google Scholar]
- 41.O’Hara D.M., Seagriff-Curtin P., Levitz M., Davies D., Stock S. Using Personal Digital Assistants to improve self-care in oral health. J. Telemed. Telecare. 2008;14:150–151. doi: 10.1258/jtt.2008.003016. [DOI] [PubMed] [Google Scholar]
- 42.Sebastian J., Richards D. Changing stigmatizing attitudes to mental health via education and contact with embodied conversational agents. Comput. Hum. Behav. 2017;73:479–488. doi: 10.1016/j.chb.2017.03.071. [DOI] [Google Scholar]
- 43.Azzini I., Falavigna D., Giorgino T., Gretter R., Quaglini S., Rognoni C., Stefanelli M. Automated Spoken Dialog System for Home Care and Data Acquisition from Chronic Patients. Stud. Health Technol. Inform. 2003;95:146–151. doi: 10.3233/978-1-60750-939-4-146. [DOI] [PubMed] [Google Scholar]
- 44.Bickmore T.W., Puskar K., Schlenk E.A., Pfeifer L.M., Sereika S.M. Maintaining reality: Relational agents for antipsychotic medication adherence. Interact. Comput. 2010;22:276–288. doi: 10.1016/j.intcom.2010.02.001. [DOI] [Google Scholar]
- 45.Bott N., Wexler S., Drury L., Pollak C., Wang V., Scher K., Narducci S. A Protocol-Driven, Bedside Digital Conversational Agent to Support Nurse Teams and Mitigate Risks of Hospitalization in Older Adults: Case Control Pre-Post Study. J. Med. Internet Res. 2019;21:e13440. doi: 10.2196/13440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Philip P., Micoulaud-Franchi J.-A., Sagaspe P., de Sevin E., Olive J., Bioulac S., Sauteraud A. Virtual human as a new diagnostic tool, a proof of concept study in the field of major depressive disorders. Sci. Rep. 2017;7:42656. doi: 10.1038/srep42656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piau A., Crissey R., Brechemier D., Balardy L., Nourhashemi F. A smartphone Chatbot application to optimize monitoring of older patients with cancer. Int. J. Med. Inform. 2019;128:18–23. doi: 10.1016/j.ijmedinf.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 48.Shamekhi A., Bickmore T. Proceedings of the ACM International Conference Proceeding Series. Association for Computing Machinery; New York, NY, USA: 2018. Breathe Deep: A breath-sensitive interactive meditation coach; pp. 108–117. [DOI] [Google Scholar]
- 49.Tielman M.L., Neerincx M.A., Bidarra R., Kybartas B.A., Brinkman W.-P. A Therapy System for Post-Traumatic Stress Disorder Using a Virtual Agent and Virtual Storytelling to Reconstruct Traumatic Memories. J. Med. Syst. 2017;41:125. doi: 10.1007/s10916-017-0771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puthal D., Mir Z.H., Filali F., Menouar H. Cross-layer architecture for congestion control in Vehicular Ad-hoc Networks; Proceedings of the 2013 International Conference on Connected Vehicles and Expo (ICCVE); Las Vegas, NV, USA. 2–6 December 2013; pp. 887–892. [DOI] [Google Scholar]
- 51.Sahu A.K., Sharma S., Puthal D. Lightweight Multi-party Authentication and Key Agreement Protocol in IoT-based E-Healthcare Service. ACM Trans. Multimed. Comput. Commun. Appl. (TOMM) 2021;17:1–20. doi: 10.1145/3398039. [DOI] [Google Scholar]
- 52.Bickmore T.W., Mitchell S., Jack B.W., Paasche-Orlow M., Pfeifer L.M., O’Donnell J. Response to a relational agent by hospital patients with depressive symptoms. Interact. Comput. 2010;22:289–298. doi: 10.1016/j.intcom.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dworkin M., Chakraborty A., Lee S., Monahan C., Hightow-Weidman L., Garofalo R., Qato D., Jimenez A. A Realistic Talking Human Embodied Agent Mobile Phone Intervention to Promote HIV Medication Adherence and Retention in Care in Young HIV-Positive African American Men Who Have Sex With Men: Qualitative Study. JMIR mHealth uHealth. 2018;6:e10211. doi: 10.2196/10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puthal D., Ranjan R., Nanda A., Nanda P., Jayaraman P.P., Zomaya A. Secure authentication and load balancing of distributed edge datacenters. J. Parallel Distrib. Comput. 2019;124:60–69. doi: 10.1016/j.jpdc.2018.10.007. [DOI] [Google Scholar]
- 55.Sahoo B.P.S., Rath S., Puthal D. Energy Efficient Protocols for Wireless Sensor Networks: A Survey and Approach. Int. J. Comput. Appl. 2012;44:43–48. doi: 10.5120/6367-8773. [DOI] [Google Scholar]
- 56.Puthal D., Nepal S., Ranjan R., Chen J. A secure big data stream analytics framework for disaster management on the cloud; Proceedings of the 2016 IEEE 18th International Conference on High Performance Computing and Communications; IEEE 14th International Conference on Smart City; IEEE 2nd International Conference on Data Science and Systems (HPCC/SmartCity/DSS); Sydney, NSW, Australia. 12–14 December 2016; pp. 1218–1225. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.