Abstract
New developments require innovative ecofriendly materials defined by their biocompatibility, biodegradability, and versatility. For that reason, the scientific society is focused on biopolymers such as chitosan, which is the second most abundant in the world after cellulose. These new materials should show good properties in terms of sustainability, circularity, and energy consumption during industrial applications. The idea is to replace traditional raw materials with new ecofriendly materials which contribute to keeping a high production rate but also reducing its environmental impact and the costs. The chitosan shows interesting and unique properties, thus it can be used for different purposes which contributes to the design and development of sustainable novel materials. This helps in promoting sustainability through the use of chitosan and diverse materials based on it. For example, it is a good sustainable alternative for food packaging or it can be used for sustainable agriculture. The chitosan can also reduce the pollution of other industrial processes such as paper production. This mini review collects some of the most important advances for the sustainable use of chitosan for promoting circular economy. Hence, the present review focuses on different aspects of chitosan from its synthesis to multiple applications.
Keywords: chitosan, sustainable development, circular economy, biopolymers
1. Introduction: Necessity of Alternative Materials for a Circular Economy
The new regulations promoted by numerous governments are trying to take care of the environment by protecting actions and behaviors to develop a new sustainable economy. Some of the most important goals of these laws are aimed at the reduction of the excessive consumption of non-renewable raw materials, especially those derived from natural sources. The extraction and cleaning of raw materials are responsible for soil degradation, biodiversity loss, water shortages, and global warming. The use of residues as raw materials is a new concept derived from the circular economy which could definitely contribute to the reduction of the huge amounts of trash accumulated in landfills. The concept of a circular material means that a new product can be obtained from the old one which is acting as a raw material. The new product will exhibit the same properties and qualities as the previous one, i.e., materials will remain in a continuous cycle of life. In general, a huge amount of this waste is composed of plastics whose versatility and wide range of properties makes it difficult to get a competitive alternative in terms of costs. Some biopolymers being investigated by scientists and industry are biodegradable, and specifically, obtained from agricultural and food processing waste. Chitosan is one of the most studied biopolymers due to its biocompatibility, biodegradability, adhesivity, and bioactivity. Chitosan is the second most abundant biopolymer in the world after cellulose; this arouses researchers’ interest in fabricated novel and sustainable materials based on it. On the other hand, its low cost also makes it a good choice of material [1]. The chitosan is used in a wide range of applications and industries, related to agriculture, pharmacy, medicine, food, or textile among others [2,3,4,5,6]. Nonetheless, new developments involve biomedicine, biotechnology, wastewater treatment, catalysis, packaging, or bioimaging which are essential for a new sustainable era where chitosan can provide versatility, recyclability, and low cost. The nature and properties of chitosan lend themselves to sustainability criteria, due to its biodegradability, bioactivity, or the obtaining method, but there are also some specific applications related to sustainability where the chitosan can play an important role, in terms of efficiency, yield, and cost. Probably, the most important applications of chitosan in this field are associated with wastewater treatment, absorption of pollutants, or their uses as a chelation agent, an antiviral agent, or a substitute material in the paper industry [7]. Some of these recent advances involve chitosan for the preparation of composites or functionalized materials, such as aerogels based on chitosan and soot.
Chitosan biopolymer can be functionalized by several function groups. Functionalization can be grafting, addition, coupling, crosslinking, etc. [8]. These were tested for the adsorption of dyes and other pollutants, such as naphthalene, showing interesting results [9]. The combination of chitosan with other materials such as collagen can also increase the range of its features [10]; for instance, the preparation of tailored scaffolds which allows adapting their properties to clinical demand [10].
The preparation of nanoparticles or nanocomposites also contributes to the circular economy, as a lower amount of raw materials is necessary for developing a specific application-based sustainable materials. Nanocomposites with magnesium show great activity against different pathogens developed in many plants, such as Acidovorax oryzae and Rhizoctonia solani which both are rice pathogens [11]. A greater surface area can be obtained through the production of thin films reducing the amount of raw materials and consequently the volume of waste after use, but keeping the same properties of the original films. Some of these developments can be carried out using chitosan, specifically for the food packaging [12]. This mini review collects some of the most relevant points that chitosan can offer for sustainable development. The new trends in science are focused on green chemistry and the circular economy; this manuscript collects brief goals, methods, and applications which are essential for understanding the importance of chitosan for new generations.
1.1. Chitosan as a Renewable Material
1.1.1. Chitosan as a Biomaterial
Chitosan is obtained through the deacetylation of chitin, which is one of the most abundant biomaterials after cellulose. This one is a polysaccharide which can be found in crustaceans, insects, or fungi (Table 1) [13]. Chitin is considered a linear long-chain homopolymer which is composed of N-acetyl glucosamine, and can develop three polymorphic forms known as α-, β-, and γ-chitin [14].
Table 1.
Some of the main chitin sources and percentages [13].
| Source | Percentage (%) |
|---|---|
| Shrimps | 30–40% |
| Squids | 20–40% |
| Krill | 20–30% |
| Crabs | 15–30% |
| Fungi | 10–25% |
| Insects | 5–25% |
| Oysters | 3–6% |
| Clams | 3–6% |
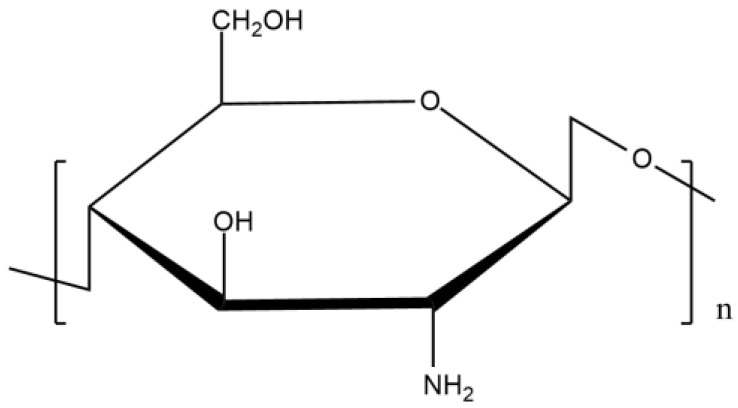
Commercial chitosan (Figure 1) is composed of D-glucosamine and N-acetyl glucosamine and is produced by the partial deacetylation of chitin. This reaction carries out the change of acetamido groups into amino groups. There are three kinds of this biopolymer depending on its molecular weight: low molecular weight, high molecular weight, and oligochitosans [15].
Figure 1.
Chemical structure of chitosan.
1.1.2. General Features and Properties of Chitosan
The main properties which can contribute to a sustainable development that are exhibited by the chitosan are non-toxicity, biodegradability, and biocompatibility. Nevertheless, there are other interesting properties and characteristics which explain its versatility which can be deduced from Table 2.
Table 2.
| Property | Conditions | Use | References |
|---|---|---|---|
| Solubility | Dilute acids (pH < 6). Insoluble in organic solvents and water | Water treatment | [18,19] |
| Activity | Antibacterial, antifungal mucoadhesive analgesic, and hemostatic properties | [20,21,22] | |
| Degradation | Depends on molecular weight and deacetylation degree | [18,23] | |
| Biocompatibility | Physiological medium | Biomedical applications | [7,24] |
| Chelating properties | Capability to bind and adsorb diverse ions | The removal of heavy metals and dyes from wastewater | [25,26] |
| Biodegradability | Biodegradable to normal body constituents | [24,27,28] | |
| Hemostatic | Stop a hemorrhage | [29,30] | |
| Catalyst | Accelerates the formation of osteoblast | [31] | |
| Fungicide | Stopping the development of fungi | [32,33] | |
| Spermicidal | Reduce the mobility of spermatozoa | [34] | |
| Anticholesteremic | Reducing agent cholesterol | [35,36] | |
| Anticancer | Inhibiting the development of cancer cells | [37] | |
| Conductivity | Ionic conductivity | [38,39] | |
| Flocculating agent | Interactions with negatively charged molecules | Water treatment | [40] |
| Thickener | Increase the viscosity | [41] | |
| Polyelectrolytes | Acidic medium | [42] | |
| Adsorption | Separation and filtration | [43,44,45] | |
| Clarifying agent | Immobilization of enzymes | [46] |
From the presentation of Table 2, it can be deduced that chitosan is a sustainable material as it is biodegradable and non-toxicity [47]. Another important reason for using chitosan is the presence of a large number of hydroxyl and amino groups in its structure which are suitable for chemical modifications [48]. This fact and the wide versatility of chitosan makes this material especially interesting for the preparation of suspensions, composites, functionalized materials, or (nano)hybrids for diverse eco-friendly purposes and applications. The interesting polymorphic behavior exhibited by the chitosan [49], together with the molar mass and degree of deacetylation, mainly defines its mechanical properties. The molar mass will also play an important role for other properties such as degradation degree or antibacterial activity as these are strongly affected by the changes in molar mass.
On the other hand, the degree of deacetylation is associated with the content of acetamide groups of polymeric chains. These groups will strongly affect the final features and properties of the chitosan, in particular its capacity to be biodegradable and its immunological activity. The deacetylation degree is defined between 50 and 99%, its content depends on the preparation methods. The deacetylation degree must be higher than 50% for the chitosan; below that value, it is considered chitin [18]. Some of the most important uses of chitosan are associated with biomedical applications. Nevertheless, new developments related to chitosan focus on agriculture, food packaging, textiles, or environmental applications [50]. The solubility of the chitosan depends on the medium being used to dissolve it; in acid mixtures with water, it is soluble, but it is insoluble in common organic solvents [51,52]. The reason for its solubility can be explained due to the presence of amino groups that transforms chitosan into a base, whose protonation produces a polyelectrolyte [53]. The presence of different functional groups is responsible for the reactivity and the flexibility of this polycationic polymer [54]. Chitosan biofilms show a semi-crystalline behavior, together with high hydrophobicity and little flexibility [55].
1.1.3. Chitosan as an Ecofriendly Biopolymer and Its Applications
Chitosan is considered a natural biopolymer; it has received remarkable attention from the scientific community due to the fact that it can be easily biodegraded. Its residues are not toxic and can be easily eliminated and biodegraded by nature [7]. One of the most important problems associated with the raw materials is that these are limited, but chitosan is the most abundant biopolymer after cellulose. Furthermore, chitosan exhibits a great biocompatibility, limited by its low solubility which can be solved through chemical modifications and hydrolysis. Chitosan is a bioactive material which can be modulated and used in many applications [56]. Some of these applications are associated with biomedical purposes such as drug delivery systems, scaffolds, or membranes. Nevertheless, there are other important uses such as in the textile industry, wastewater treatments, agriculture, food, packaging, personal care, and biotechnology, among others. The adsorbent properties of chitosan are very useful for removing different heavy metal ions accumulated in water and derived from industrial processes such as Pb2+, Hg2+, and Cu2+, among others [57]. These can be accumulated inside the body and produce numerous diseases [58]. Chitosan can contribute to the agriculture by improving the harvest and productivity, being an ecofriendly material. It is used as a coating for seeds, enhancing the properties of the plants and the obtained products in terms of shelf life. This use as fertilizer is especially useful for plant protection as it can stimulate the plant defense, but it can also act as an antibacterial and antimicrobial agent [59]. Thus, chitosan acts as a plant growth-promoting agent and plant protector [60]. For that reason, it is considered a pesticide by several countries. The antioxidant properties of chitosan, together with its antimicrobial features, are suitable for the production of films for food packaging. The preparation of hybrid materials with chitosan allows modifying the permeability of those films depending on the requirements [2]. The chitosan can also be used as a food additive, dietary fiber, and functional ingredient [61,62].
2. Sustainable Production
2.1. Chitin Extraction
The extraction of chitin is necessary for the production of chitosan such as it was previously explained. A huge amount of chitin is obtained from crustaceans, but there are multiple advances in its production through insects or fungi and bacteria, thus avoiding the use of animal derivatives [63]. In general, the extraction requires several steps starting with the removal of mineral salts and proteins (Figure 2). It is commonly carried out chemically, using acids and bases, which is not a sustainable process. These processes can destroy some properties of chitosan, reducing its versatility. Currently, there are multiple advances in natural deep eutectic solvents which could replace the hazardous solvents and preserve the features of chitin. There is another option based on the use of microorganisms for the extraction of chitin known as a biological method [64]. In general, these methods are especially indicated for the treatment of fungi and bacteria whilst chemical processes are related to the treatment of crustaceans. After removing the minerals and proteins, chitin requires a depigmentation process which is generally performed using oxidizing agents. The use of the enzymes could be a feasible way for removing the proteins, which can reduce the degree of depolymerization in comparison with traditional methods. That chitin also showed a better solubility in water probably due to a lower crystallinity of the product [65]. The specific use of the trypsin also induces the depigmentation, reducing the steps involved in the extraction of chitin [66]. There is a lot of ground to cover in terms of sustainability around processes for the extraction of chitin associated with environmental pollution, loss of chitin properties, and costs. One of the main consequences of this extraction is the polluted wastewater, which needs to be treated.
Figure 2.
Extraction of chitin. DES: deep eutectic solvents; HBA: hydrogen bond acceptor; HBD: hydrogen bond donor.
2.2. Chitosan Production
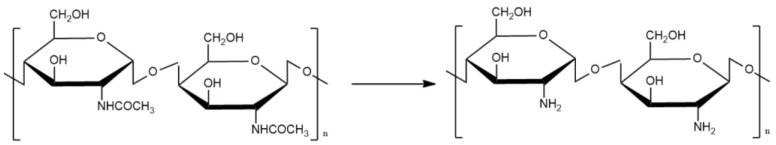
The production of chitosan requires the deacetylation of chitin; this process can be modulated through concentration, temperature, and time [7]. Scheme 1 shows the changes produced in chitin after being transformed into chitosan.
Scheme 1.
Deacetylation of chitin.
The traditional method to obtain chitosan from chitin was reported in 1980, which promotes a high deacetylation due to rapid reaction rates at reduced temperatures [67]. There are different ways to carry out the deacetylation such as alkali treatment, the use of enzymes, or a steam explosion [16,68,69]. The degree of deacetylation will define the spectra of properties of the chitosan in terms of features such as solubility, viscosity, or biodegradability, etc. [70]. There are numerous alternatives where the energy consumption can be reduced, contributing to a green chemistry. Those methods explore the use of microwaves and ultrasonic waves in the deacetylation process. The use of ultrasonic waves leads to enhancing the reactivity of the deacetylation process [71]. Some of the new approaches are displayed in Table 3, showing some of the most interesting advances related to the sustainable production of chitosan.
Table 3.
New methods for the production of chitosan.
| Treatment | Disadvantages | Advantages | Reference |
|---|---|---|---|
| Trypsin (crustaceans) | Only for deproteination step | Depigmentation of treated material | [66] |
| Streptomyces griseus (crustaceans) | Only for deproteinization | Better solubility | [65] |
| Bacillus mojavensis A21 or Balistes capriscus (crustaceans) |
Deproteinization requires NaOH | Optimized process | [72] |
| Rhizopus oryzae (fungi) |
Fermentation | Cheap, low energy consumption, and soft conditions | [73] |
2.3. Circularity in the Chitosan Production
The traditional methods can also be adapted, at least partially, trying to get a sustainable production of chitosan. For that purpose, it is necessary to reduce the energy consumption by reusing the hazardous reagents. The recovery of sodium hydroxide used in the extraction of chitosan was reported in studies. The sodium hydroxide is part of wastewater and could be treated using ultrafiltration and nanofiltration membranes recovering the sodium hydroxide for a new cycle of life [74,75]. The reuse of sodium hydroxide can contribute to a decrease the environmental pollution and reducing the cost of the process, i.e., a lower amount of sodium hydroxide will be required. There were also reports for the preparation of chitosan at ambient temperature, following the general procedure of demineralization, deproteinization, and decolorization [76]. This fact could also be quite interesting, due to the reduced energy consumption. Thus, involving circularity in the production of chitosan can be very beneficial and economically better.
3. Applications of Chitosan for Sustainable Development
Chitosan can contribute to sustainable development through its applications and uses. This review tries to expose some of the most important applications related to the contribution of chitosan to a circular economy and sustainability. Figure 3 depicts the diversified application of chitosan.
Figure 3.
Different uses of chitosan.
3.1. Sustainable Use of Chitosan for Food Packaging and in Agriculture
Many biopolymers are being implemented in different coating materials due to their excellent properties in terms of degradability and compatibility; these biopolymers include gums, starch, proteins cellulose, lipids, and their derivatives [77,78,79,80,81,82,83]. In this sense, chitosan is a promising material for that purpose due to several reasons associated with its biocompatibility and abundance [84,85]. The use of the chitosan in films can also provide other superiorities because of its antibacterial and antioxidant properties [86,87,88,89]. In general, chitosan is used in combination with other polymers due to some of its drawbacks associated with its low mechanical properties. Another important problem associated with chitosan is related to its water sensitivity [90]. The preparation of blends can diminish these problems, thus obtaining films with a wide range of properties. The miscibility problems between the mixtures of polymers can reduce the spectra of possibilities, but in general, the preparation of these films is easy and cheap. The preparation of these systems could be a good alternative regarding traditional films based on oil derivatives [91]. Table 3 displays some of the most promising blends of chitosan, based on the mixtures with other biopolymers. There are other mixtures with synthetic polymer of chitosan that are not included in this review, as those do not fit the sustainability criteria of the present review. Numerous composites of chitosan have been fabricated with graphene, carbon nanotubes, activated carbon, and metal nanoparticles [92,93,94,95]. One study suggests that poly(L-lactic acid)-ZnO multilayered with cationic chitosan and anionic β-cyclodextrin can be used as a promising material in applications for the active packaging of food [96]. A novel bilayer food packing film of Ag-Metal−organic framework loaded p-coumaric acid modified chitosan (P-CS/Ag@MOF) or chitosan nanoparticles (P-CSNPs/Ag@MOF) and polyvinyl alcohol/starch (PVA/ST) was fabricated. The bilayer composite film revealed a relatively smooth surface and higher tensile strength (27.67 MPa). The P-CS/Ag@MOF bilayer films displayed better oil resistance and oxidation resistance, and the bilayer film had good UV-blocking properties and transparency [97]. The diverse blend composites of chitosan have been developed with various natural antimicrobial compounds and have been applied for antimicrobial food packaging; such antimicrobial compounds include thyme oil, spirulina, oregano essential oil, nisin, apple peel polyphenols, bamboo vinegar, cinnamon essential oil, custard apple leaves, plum peel extract, etc. [98,99,100,101,102,103,104]. The antibacterial nanofiber films were fabricated using gelatin, chitosan, and 3-phenyllactic acid (PLA) by electrospinning. Under acidic conditions, chitosan and PLA interacted and formed hydrogen bonds, which decreased the crystallinity of the nanofiber films. The nanofiber film had the best thermal stability, water stability, water vapor permeability, and more effective antibacterial effects against Salmonella enterica Enteritidis and Staphylococcus aureus, suggesting that the nanofiber film mat can be used as an active food packaging [105]. Similarly, Wang et al. discussed various chitosan and gelatin edible films, their synthesis strategies including casting, electrospinning, and thermoplastic method, and their properties in their review, thus highlighting importance of chitosan-based food packing films [106]. In Argentina, chitosan is produced from the waste of the shrimp industry; the synthesized chitosan has similar physicochemical properties to those of analytical grade chitosan. The chitosan coatings applied to processed lettuce at harvest increased nutritional quality and reduced microbiological contaminants in minimal processed lettuce [107]. Panda et al. fabricated ferulic acid-modified water-soluble chitosan and poly(γ-glutamic acid) polyelectrolyte multilayers films. These film surfaces possessed a reduced amount of protein adsorption; thus, these can be used as a potential good biomaterial for biomedical purposes to intensify the bio-active surface [108], thus prompting the concept of circularity and sustainability. Table 4 and Table 5 show the effects of some films over the food due to the use of chitosan which could modify its properties.
Table 4.
Selection of blends of chitosan with other biopolymers for food packaging.
| Biopolymer | Chitosan | Characteristics | Reference |
|---|---|---|---|
| Pectin (2% w/v) | 2% w/v | Good mechanical properties. Antimicrobial activity. |
[109,110] |
| Carboxymethyl cellulose (1–2% w/v) | 1% w/v | Better mechanical properties and permeability. Antioxidant and antimicrobial activity. | [111,112,113] |
| Gum arabic (1.5% w/v) | 1.5% w/v | High elasticity. Antioxidant and antimicrobial activity. | [114,115] |
| Cassava starch (3% w/v) | 0.5% w/v | Antibacterial activity. | [116] |
| Corn starch (5% w/v) | (1, 2, 3, and 4% w/v) | Higher tensile strength and elasticity. Lower permeability. | [117] |
| Rice starch (2% w/v) | Better barrier properties. | [118] |
Table 5.
Effects of films based on chitosan over food.
| Blend | Food | Effects | References |
|---|---|---|---|
| Chitosan-glycerol film (Good mechanical and barrier properties. Stability) |
Strawberry | Better preservation effect than the commercially available PE films. | [119] |
| Gelatin/chitosan film with nanocarriers (FeIII-HMOF-5) (Good results in mechanical properties and permeability) |
Apple cubes | High content of nanocarriers allows the preservation of apple cubes during 5 days. | [120] |
| Chitosan films (modified with mango leaf extract) (Higher hydrophobicity and tensile strength) |
Cashew nuts | High oxidation resistance. | [121] |
| Chitosan/gelatin film with silver nanoparticles (Better hydrophobicity and antibacterial properties) |
Red grapes | Antimicrobial properties and high oxidation resistance. | [122] |
| Polyurethane/chitosan/nano ZnO composite film (Better mechanical properties, low permeability) | Carrot | Better shelf life than polyethylene film | [19] |
| Pullulan/chitosan film (good barrier to O2) | Papayas | Maintained the physiological and nutritional attributes. High shelf life. | [123] |
| Chitosan-TiO2 nanocomposite film (Better tensile strength and barrier properties) | Tomatoes | Delay the ripening process and extend the storage life. | [124] |
| Cellulose/chitosan/polypyrrole film | Cherry tomatoes | Possess good antioxidant, antibacterial, and barrier properties | [125] |
| Baicalin-liposomes loaded polyvinyl alcohol-chitosan electrospinning nanofibrous films | Mushrooms | Possessed effective antibacterial properties, non-cytotoxicity, and preservation performance | [126] |
| Active packaging films based on chitosan and sardinella protein isolate | Shrimps | Good antioxidant and antibacterial activities | [127] |
| ε-polylysine/chitosan nanofibers | Chicken | Inhibiting Salmonella typhimurium and Salmonella enteritidis on chicken |
[128] |
| Chitosan films embedded with Apricot (Prunus armeniaca) oil | Bread | Better antioxidant, mechanical, and antimicrobial properties | [129] |
| Zein active film containing chitosan nanoparticle encapsulated with pomegranate peel extract | Pork | Addition of chitosan nanoparticle can increase the thermal stability of zein active film Film can inhibit the growth of Listeria monocytogenes on pork |
[130] |
| Mahua oil-based polyurethane/chitosan/nano ZnO composite films | Carrot | Excellent anti-bacterial properties against Gram positive and Gram-negative bacteria Increase shelf life of carrot |
[131] |
| Carboxymethyl chitosan (CMCh)-peptide conjugates | Blueberry | Extend the shelf-life of blueberry | [132] |
| Chitosan-based biodegradable bags | Palmer’s mango | Effective in delaying ripening and preserving the quality | [133] |
| Composite films based on chitosan and syringic acid | Quail eggs | Films exhibited higher density, water solubility, good preservation effect | [134] |
| Films based on quaternary ammonium chitosan, polyvinyl alcohol, and betalains-rich cactus pears (Opuntia ficus-indica) extract | Shrimp | Enhanced the UV–vis light barrier, elongation-at-break, and antioxidant, antimicrobial and ammonia-sensitive properties | [135] |
| Chitosan coating with vacuum packaging | Beef | Extend the shelf life of beef Inhibited S. aureus |
[136] |
| Chitosan coatings | Lettuce | Improve quality and extend shelf-life of minimally processed lettuce | [107] |
| Chitosan films incorporating litchi peel extract and titanium dioxide nanoparticles | Watercored apple | Coating treatment significantly inhibited respiration rate, weight loss, and softening | [137] |
| Polylactic acid/chitosan films | Indian white prawn | Antimicrobial properties | [138] |
| Chitosan-Gelatin (CHI-Gel) based edible coating incorporated with longkong pericarp extract (LPE) | Shrimp | Edible coating as a natural antioxidant, antimicrobial activity and inhibiting melanosis, retain the quality and extend the shelf-life | [139] |
| Pink pepper residue extracts incorporated in a chitosan film | Salmon fillets | Shelf-life of the skinless salmon fillet could be extended by 28 days | [140] |
| Chitosan film incorporated with citric acid and glycerol | Green chilies | Improved mechanical, thermal, and antioxidant properties of the film were and increased shelf life | [141] |
The chitosan can act as protector, coating material, stimulator of the growth, nutrient, fertilizer, or pesticide in agriculture. It was also observed that the use of chitosan can increase productivity. Furthermore, the use of chitosan could replace some dangerous chemicals used as compounds of fertilizers in agriculture, protecting soil, aquifers, and ecosystems [142]. It was reported that excellent antimicrobial activity was observed in chitosan against many viruses, bacteria, and fungi. Nevertheless, its activity is higher against fungi than bacteria. In general, the chitosan seems to inactivate the replication of viruses [143]. Moreover, it is considered a potent elicitor which can induce plant defense against diseases [144]. Table 6 shows some of the effects observed of chitosan over some fruits and vegetables.
Table 6.
Effects of chitosan and derivatives over some products.
| Material/Use | Plant | Effects | Reference |
|---|---|---|---|
| Chitosan with copper | Tomato | Plant defense (Enzymatic and anatomical changes). | [145] |
| Seed-priming with chitosan | Cucumber | Disease protection and enhanced plant growth. | [146] |
| Foliar application of chitosan | Sweet pepper | Enhancement of the adverse effects of salinity and improved the growth and yield. | [147] |
| Chitosan solution (using a hand sprayer) | Dracocephalum kotschyi | Increase of antioxidant enzyme. | [148] |
| Chitosan (foliar spray or pre-sowing seed treatments in Cd-stressed plants) | Pea | Improvement in growth, photosynthetic pigments, and reduction in oxidative damage. | [149] |
| Chitosan (protective spray) | Mango (Amrapali and Dashehari) | Reduced malformation of mango. | [150] |
| Chitosan nanoparticles | Durum wheat | Increase the leaf antioxidant pool. | [151] |
| Chitosan oligosaccharide (COS) | Tea plant (Camellia sinensis) | Improved the antioxidant enzyme activities and the content of chlorophyll and soluble sugar. | [152] |
| Chitosan nanoemulsion containing allspice essential oil | Maize | Preserved maize samples from aflatoxin B1 and lipid peroxidation. | [153] |
| Chitosan nanoparticles loaded with garlic essential oil | Wheat, oat, and barley | As a seed dressing agent found to have antifungal activity against Aspergillus versicolor, A. niger, and Fusarium oxysporum. | [154] |
| 1.5% chitosan solution treatment | Berry | Inhibit postharvest berry abscission of the ‘Kyoho’ table grapes. | [155] |
| Preharvest chitosan sprays | Muskmelons | Induced suberin polyphenolic deposition at wound sites during healing thus promoted wound healing and reduced disease development. | [156] |
| Chitosan film containing Akebia trifoliata (Thunb.) Koidz. peel extract/montmorillonite | A. trifoliata fruits | Significant effect on the delaying crack and mature of the fruits. | [157] |
| Chitosan-based nanoencapsulated Foeniculum vulgare Mill. essential oil | Sorghum bicolor | Significantly preserved the nutritional and sensory characteristics of S. bicolor seeds. | [158] |
| Encapsulated peppermint essential oil in chitosan nanoparticles | - | Biological efficacy against stored-grain pest control. | [159] |
3.2. Sustainable Applications of Chitosan in Purification of Water, Paper-Making, and Green Chemistry
The chitosan is a good flocculant for water treatment, especially indicated for organic matter, suspended solids, and ions (metals). Furthermore, the deposition rate is stimulated when chitosan is used [160]. It is used over oil spills as it can preserve the integrity of the oil mass. Its properties are also indicated for anionic waste where the chitosan can remove the metal ions of the acid solutions. Some of the most attractive features of chitosan regarding other flocculants are associated with its biodegradability and its adsorption and flocculating ability, which show excellent results with oils [7]. However, there are many other pollutants where the chitosan shows interesting results as can be observed in Table 7. Chitosan and its composites demonstrate excellent adsorption properties for diversified environmental contaminates ranging from organic pollutants to metal ions [47,161,162,163,164,165]. The mechanism for the adsorption of toxic pollutants by chitosan and its composites involves various types of interactions such as electrostatic, hydrogen bonding, π-π bonding, etc. The chitosan and its composites had several hydroxyls and amino and carboxylic groups which are very helpful for such interactions, thus making it more adsorbent.
Table 7.
Examples of pollutants removed by chitosan and derivatives.
| Pollutant | Adsorbent | Efficiency | References |
|---|---|---|---|
| Tetracycline | Chitosan/poly (vinyl alcohol) nanofibers | 102 mg/g (maximum adsorption capacity) | [166] |
| Ciprofloxacin | Chitosan/biochar hydrogel | 36.72 mg/g (uptake capacity) | [167] |
| Tetracycline | Magnetic polymer nanocomposite was fabricated using chitosan, diphenyl urea, and formaldehyde | 168.24 mg/g (maximum adsorption capacity) | [168] |
| Tetracycline | Nanocomposite of chitosan/thiobarbituric acid/malondialdehyde-Fe3O4 | 215.31 mg/g (highest adsorption capacity) | [169] |
| Antibiotics | Chitosan-grafted SiO2/Fe3O4 nanoparticles | 100.74 mg/g (theoretical adsorption capacity) | [170] |
| Ketoprofen | Chitosan/Zr-MOF (UiO-66) composite | Maximum adsorption capacity of 209.7 mg/g | [171] |
| Tetracycline | Nitrilotriacetic acid modified magnetic chitosan-based microspheres | Adsorption capacity of 373.5 mg g−1 | [172] |
| Congo red | Chitosan nanoparticles | 99.96% | [173] |
| Methylene blue | Chitosan/κ-carrageenan/acid-activated bentonite composite membranes | Maximum adsorption capacity for methylene blue was 18.80 mg/g | [174] |
| Azo dyes | Glass beads coated with chitosan | Maximum adsorption capacity of the column packed with GBCC was 108.7 mg g−1. | [175] |
| Methyl orange | Chitosan-lysozyme biocomposite | Maximum adsorption capacity for MO was 435 mg/g | [176] |
| Methylene blue | Bivinylbenzene cross-linked chitosan/maleic anhydride polymer | Adsorption capacity for MB 503 mg/g | [177] |
| Acid orange 7 (AO7, monovalent), Acid red 13 (AR13, divalent), and Acid red 27 (AR27, trivalent) dyes | Chitosan–magnetite gel microparticles | Acid Orange 7 (AO7, monovalent), Acid Red 13 (AR13, divalent), and Acid Red 27 (AR27, trivalent) dyes with maximum adsorption capacities, Qmax, of 1.71, 1.55, and 1.13 g-dye/g-dry adsorbent, respectively | [178] |
| Methyl orange dye | Fe-loaded chitosan film | Maximum adsorption capacity 205 mg g−1 | [179] |
| Methyl orange dye | Chitosan/carbon/Fe3O4 | Maximum adsorption capacity was 425 mg g−1 | [180] |
| Disperse blue 367 | Magnetic/chitosan/graphene oxide | Adsorption capacity of 298.27 mg/g | [181] |
| Reactive orange 16 dye | Chitosan tripolyphosphate/TiO2 nanocomposite | Adsorption capacity was 618.7 mg/g | [182] |
| Acid red 88 | Phosphorylated chitosan | Adsorption capacity was 230 mg g−1 | [183] |
| Methylene blue | Poly(glycerol sebacate)/chitosan/graphene oxide nanocomposites | Adsorption capacity was 129 mg/g | [184] |
| Methylene blue | Magnetic sodium ferrosilicate/carboxymethyl chitosan composite | Adsorption capacity was 515.0 mg/g | [185] |
| Malachite green (MG), reactive red (RR), and direct yellow (DY) dyes | Chitosan | Adsorption capacities 166 mg/g for dye MG, 1250 mg/g for dye RR and 250 mg/g for dye DY | [186] |
| Methyl orange | Chitosan crosslinked with metal-organic framework (MOF-199)@aminated graphene oxide aerogel | Maximum adsorption capacity for methyl orange 412 mg/g | [187] |
| Reactive orange 16 | Chitosan-polyvinyl alcohol/fly ash (m-Cs-PVA/FA) | Adsorption capacity of m-Cs-PVA/FA for RO16 dye removal was 123.8 mg/g | [188] |
| Methyl orange and methylene blue | Graphene oxide-chitosan composite | Maximum adsorption amounts of MO and MB were 543.4 and 110.9 mg/g | [189] |
| Phenol, BPA, and 2,4-DCP | Chitosan modified nitrogen-doped porous carbon composite | Maximum adsorption capacity for phenol, BPA, and 2,4-DCP was 254.45, 675.68, and 892.86 mg g−1 | [190] |
| Sunset yellow |
Chitosan | Maximum adsorption capacity 1432.98 mg g−1 | [191] |
| Allura red | Luffa-chitosan crosslinked with glutaraldehyde (LCsG) and epichlorohydrin (LCsE) | LCsG and LCsE presented maximum capacities of 89.05 mg/g and 60.91 mg/g. | [192] |
| Brilliant blue | Chitosan | Maximum adsorption capacity 814.27 mg/g | [191] |
| Tartrazine | Chitosan | Maximum adsorption capacity 1065.55 mg/g | [191] |
| Acid blue-25 | Chitosan/porous carbon composite modified in 1-allyl-3-methyl imidazolium bromide ionic liquid | Maximum adsorption capacity 3333.33 mg/g | [193] |
| Morphine, codeine, ephedrine, amphetamine, and benzoylecgonine | Magnetic chitosan-graphene oxide-ionic liquid ternary nanohybrid | Adsorption capacity for morphine, codeine, ephedrine, amphetamine, and benzoylecgonine (7.2, 8.4, 9.2, 5.8, and 11.2 mg g–1, respectively) | [194] |
| Tartrazine | Chitosan/polyaniline composite | Maximum adsorption capacity of 584.0 mg/g | [195] |
| Acetaminophen | Polyaniline with chitosan | Adsorption rate of 385.25 mg.g−1 | [196] |
| Anthocyanins | Chitosan beads | Adsorption capacity was 216 mg g−1 | [197] |
| Tetracycline | Zirconium-loaded chitosan modified by perlite (Zr/Cht/Pt) composites | Maximum adsorption capacity of 104.17 mg/g | [198] |
| Levofloxacin, tetracycline hydrochloride, and sulfamethoxazole | Chitosan | Adsorption capacity of levofloxacin, tetracycline hydrochloride, and sulfamethoxazole were 26, 22, and 67 mg/g | [199] |
| 17α-ethinylestradiol | Graphene oxide, magnetic chitosan, and organophilic clay composite | Maximum adsorption capacity was 50.5 mg/g | [200] |
| Tartrazine | Surfactant-ionic liquid bi-functionalization of chitosan beads | Adsorption capacity was found to be 45.95 mg/g | [201] |
The chitosan also showed good results associated with ions, as it can be observed in Table 8. These are only some examples of the good results that can be achieved.
Table 8.
Examples of chitosan for removing ions.
| Ion | Adsorbent | Efficiency | References |
|---|---|---|---|
| Cr (VI), Cu (II), and Co (II) | Polyethylenimine-grafted chitosan electrospun membrane | 138.96, 69.27, and 68.31 mg/g for Cr(VI), Cu(II), and Co(II), respectively (maximum adsorption capacities) | [202] |
| Cu2+ and Cr6+ | Zeolitic imidazolate framework-67 modified bacterial cellulose/chitosan composite aerogel | 200.6 mg/g and 152.1 mg/g, for Cu2+ and Cr6+, respectively (adsorption capacities) | [203] |
| Cu2+ | Monodispersed chitosan microspheres | 75.52 mg/g (adsorption capacity) | [204] |
| Pb2+, Cu2+, and Cd2+ | Physically crosslinked chitosan/sodium alginate/calcium ion double-network hydrogel | 176.50 mg/g, 70.83 mg/g, and 81.25 mg/g for Pb2+, Cu2+, and Cd2+, respectively (adsorption capacities) | [205] |
| Cu2+, Pb2+, and Cd2+ | Chitosan-coated argillaceous limestone | 64.11 mg/g, 217.4 mg/g, and 52.48 mg/g for Cu2+, Pb2+ and Cd2, respectively (maximum adsorption capacities) | [206] |
| Cr(VI) | Terylene carbon-dots modified chitosan non-woven fabrics | Maximum adsorption capacity was 203 mg/g | [207] |
| Pb2+ | Zeolitic imidazolate framework-8 (ZIF-8) on carboxymethyl chitosan beads | Maximum adsorption capacity of 566.09 mg/g | [208] |
| Cd2+ | Cellulose/chitosan composite spheres loaded with nZVI | Maximum adsorption up to 110.3 mg/g | [209] |
| Cu2+ and Ni2+ | Tripolyphosphate-crosslinked-chitosan-modified montmorillonite | Adsorption capacity for Cu2+ and Ni2+ 0.56 and 0.44 mmol/g | [210] |
| Cr4+ | Chitosan-lysozyme biocomposite | Maximum adsorption 216 mg g−1 | [176] |
| Pb2+ and Cd2+ | Chitosan/Mg-Al-layered double hydroxide nanocomposite | Maximum capacities were 333.3 mg/g for Pb2+ and 140.8 mg/g for Cd2+, respectively. | [211] |
| Arsenic | Silica-stabilized magnetic chitosan Beads | Maximum adsorption capacity 1.699 mg/g | [212] |
| Cr(III) and Cr(VI) | Iron oxide/carbon nanotubes/chitosan magnetic composite film | Maximum adsorption capacity for Cr(III) of 66.25 mg/g and for Cr(VI) of 449.30 mg/g | [213] |
| Cu(II) | Chitosan-coated magnetic nanoparticles | Maximum adsorption capacity was found to be 236.7 mg/g | [214] |
| Cr(VI) | Nano-graphene oxide-assisted hydrotalcite/chitosan biocomposite | Maximum adsorption capacity of 42.64 mg/g | [215] |
| Pb2+ and Hg2+ | Schiff base based on porous chitosan-glutaraldehyde/montmorrilonite nanoparticles modified with 3-aminopropyl triethoxysilane | Maximum adsorption capacity of Pb2+ and Hg2+ were 32.786 and 30.395 mg/g | [216] |
| Re(VII) | Chitosan-silica composite containing Mo-imprinted cavities | Adsorption capacity of 368.8 mg g−1 | [217] |
| Uranium | Chitosan-grafted adenosine 5′-monophosphate foam | Adsorption capacity of 311 mg/g | [218] |
| Li+ | H4Mn5O12/chitosan | Adsorption capacity reached 11.4 mg/g | [219] |
| Fluoride | Zirconium (IV)-impregnated magnetic chitosan graphene oxide | Adsorption capacity was 8.84 mg/g | [220] |
| U(VI) | Chitosan-based aerogel | U(VI) adsorption capacity of 160 mg/g | [221] |
| Au(III) | Chitosan functionalized with N,N-(2-aminoethyl)pyridinedicarboxamide | Maximum adsorption capacity of 659.02 mg/g | [222] |
| Cr(IV) | Chitosan composite | Adsorption capacity was 18 mg/g | [223] |
| Cu(II) | Benzothiazole functionalized chitosan | Maximum copper adsorption capacity of 1439.7 mg/g | [224] |
| Cr(IV) | Chitosan-crosslinked-poly(alginic acid) | Maximum adsorption capacity 26.49 mg/g | [225] |
| Pb(II) | Ninhydrin-functionalized chitosan | Maximum adsorption capacity of 196 mg/g Pb(II) ions | [226] |
| Co2+ and Sr2+ | Fibrous chitosan biosorbent | Adsorption capacity of fibrous chitosan for Co2+ and Sr2+ was 31.3 mg g−1 and 20.0 mg g−1 | [227] |
| Au(III) | Benzothiazole-modified chitosan | Maximum adsorption capacity of 1072.22 mg/g | [228] |
| Cu(II) | Polyacrylamide-modified kaolin enhances adsorption of sodium alginate/carboxymethyl chitosan hydrogel beads | Adsorption capacity of the adsorbent was 5.5157 mg/g | [229] |
| Ag(I) | Chitosan-coated magnetic silica core-shell nanoparticles | 126.74 mg/g | [230] |
| Cu2+, Fe3+ and Pb2+ | Chitosan | Maximum adsorption capacity Cu2+, Fe3+, and Pb2+ were 462 270 mg/g, 934 mg/g | [199] |
| Sr2+ | Carboxymethyl chitosan gel | Maximum adsorption capacity can reach 144.73 mg/g | [231] |
| As(III) | MnO2-strengthened WTRs-chitosan beads | Adsorption capacity of 36.911 mg/g | [232] |
| As(III), Cd(II), Cu(II), and Pb(II) | Chitosan bead-supported MnFe2O4 nanoparticles | As(III), Cd(II), Cu(II), and Pb(II) was achieved maximum adsorption capacities of 9.90, 9.73, 43.94, and 11.98 mg/g | [233] |
Chitosan can be used for paper manufacture due to its mechanical properties which can provide better resistance to recycled paper, reducing the consumption of chemical additives [234]. Table 9 displays the various roles of chitosan in paper production.
Table 9.
Effects of chitosan in paper production.
| Material/Use | Paper Application | Effects | Reference |
|---|---|---|---|
| Nanoparticles with chitosan and starch | Old corrugated containerboard (OCC) | Increase tensile and burst strength Decrease tear resistance |
[235] |
| Chitosan and cellulose nanofibers | Paper recycling (decolorization) | Remove water-based inks | [236] |
| Microparticules with chitosan and bentonite | Paper reinforcement | Chitosan is a good dry strength additive | [237] |
| Chitosan as additive | Papermaking (aging stability of paper) | Increase tensile strength. Decrease the hydrophilicity of paper |
[238] |
| Chitosan with zeolite as filler | Papermaking | Improve the mechanical properties of paper | |
| Chitosan as additive | Paper reinforcement (Kenaf paper (Hibiscus cannabinus)) | Give a good mechanical and dry strength properties | [239] |
| Graphene ink from the exfoliation of graphite in pullulan, chitosan, and alginate | For strain-sensitive paper | Paper-based strain sensor, the chitosan-graphene has the best resistivity value and demonstrates the highest sensitivity towards strain | [240] |
The chitosan can also be used as amino-functionalized structures for CO2 capture. Many industrial processes could reduce their emissions using these systems. Furthermore, there are many other options where chitosan can be used to reduce the greenhouse gas emissions [241]. Table 10 displays the chitosan-based materials used for gas capture.
Table 10.
Chitosan-based materials used for gas capture.
| Adsorbate | Adsorbent | Effects | References |
|---|---|---|---|
| Carbon dioxide | Composite with chitosan and clay | Adsorption capacity of 344.98 mg/g | [242] |
| Carbon dioxide | Arginine-containing chitosan-graphene oxide aerogels | CO2 gas adsorption was equal to 24.15 wt% (5.48 mmol g−1) | [243] |
| Palladium (II) and platinum (IV) | Cross-linked chitosan | 340.3 mg/g and 203.9 mg/g for Pd and Pt, respectively (adsorption capacity) | [244] |
| Carbon dioxide (separation) | Membrane with carboxymethyl chitosan and carbon nanotubes | Good CO2 selectivity and permeability | [245] |
| Carbon dioxide | Acetic acid-mediated chitosan | 368 mg/g adsorption capacity Good CO2 Selectivity |
[246] |
| Carbon dioxide | Chitosan as a porosity agent | 280.5 mg/g adsorption capacity | [247] |
| Formaldehyde gas | Chitosan crosslinked with metal-organic framework (MOF-199)@aminated graphene oxide aerogel | 197.89 mg/g adsorption capacity | [187] |
| Carbon dioxide | Chitosan-grafted multi-walled carbon nanotubes | CO2 uptake capacity was found to be significantly higher (1.92 ccg−1) | [248] |
4. Future Perspectives
It is expected that chitosan uses will increase replacing other traditional materials due to its interesting properties and functionalities, but also due to it being abundant, it can be extracted using green chemistry and easily treated as waste. For these reasons, chitosan is considered a rich renewable resource where some of its shortcomings associated with solubility, mechanical properties, and porosity are being addressed due to the potential of this source.
This article shows some of the most prominent fields where chitosan is an interesting alternative to other conventional materials, but its properties will be reflected soon in other many fields due to its versatility and properties. Some of the most promising applications could be associated with specific areas such as medicine, food packaging, or biotechnology, among others.
There is a lot of room to grow in terms of the production of chitosan, the current goal of which is clearly focused on the removal of hazardous solvents and reducing the energy consumption. On the other hand, chitosan can contribute to sustainability in terms of recycling and waste management due to its degradability.
5. Conclusions
Chitosan shows an interesting range of properties which make it very useful for sustainable development due to it being abundant, biodegradable, biocompatible, and versatile. The production of chitosan is improving in terms of green chemistry, due to the hazardous chemicals being replaced by eutectic solvents, lower energy consumption has been achieved, and circularity can be applied to secondary processes. The use of chitosan in films for food packaging shows better properties than traditional films composed of polyethylene. The edible food packing with enhanced antimicrobial activity can be developed using chitosan. Numerous blends of chitosan have been developed with various essential oils and extracts which are excellent antibacterial and antifungal agents. On the other hand, the chitosan provides interesting and multiple features for a sustainable agriculture, such as a protection for the plant and increasing the production. Finally, the chitosan can contribute to green chemistry in multiple processes such as the paper industry or the treatment of wastewater, reducing the impact and contributing to the circularity of industrial processes. The chitosan-based composites, hydrogels, and membranes can be used for the remediation of diversified pollutants including dyes, antibiotics, phenols, metal ions, etc. Thus, being a second abundant biopolymer in nature, chitosan can be a potential sustainable future material.
Author Contributions
Conceptualization, A.G.-P. and G.S.; methodology, S.M. and M.M.-Z.; investigation, A.G.-P. and G.S.; resources, J.B.; writing—original draft preparation, A.G.-P. and G.S.; writing—review and editing, A.G.-P., G.S., A.K., O.M. and F.J.S.; supervision, A.G.-P. and G.S.; funding acquisition, J.B. All authors have read and agreed to the published version of the manuscript.
Funding
Author wants to thank the Erasmus+ KA107 scholarship.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahmed M.E., Mohamed H.M., Mohamed M.I., Kandile N.G. Sustainable antimicrobial modified chitosan and its nanoparticles hydrogels: Synthesis and characterization. Int. J. Biol. Macromol. 2020;162:1388–1397. doi: 10.1016/j.ijbiomac.2020.08.048. [DOI] [PubMed] [Google Scholar]
- 2.Manigandan V., Karthik R., Ramachandran S., Rajagopal S. Chapter 15-Chitosan Applications in Food Industry. In: Grumezescu A.M., Holban A.M., editors. Biopolymers for Food Design. Academic Press; Cambridge, MA, USA: 2018. pp. 469–491. [Google Scholar]
- 3.Ke C.-L., Deng F.-S., Chuang C.-Y., Lin C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers. 2021;13:904. doi: 10.3390/polym13060904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pathania D., Gupta D., Kothiyal N.C., Sharma G., Eldesoky G.E., Naushad M. Preparation of a novel chitosan-g-poly(acrylamide)/Zn nanocomposite hydrogel and its applications for controlled drug delivery of ofloxacin. Int. J. Biol. Macromol. 2016;84:340–348. doi: 10.1016/j.ijbiomac.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 5.Amirian J., Zeng Y., Shekh M.I., Sharma G., Stadler F.J., Song J., Du B., Zhu Y. In-situ crosslinked hydrogel based on amidated pectin/oxidized chitosan as potential wound dressing for skin repairing. Carbohydr. Polym. 2021;251:117005. doi: 10.1016/j.carbpol.2020.117005. [DOI] [PubMed] [Google Scholar]
- 6.Sharma G., Thakur B., Naushad M., Kumar A., Stadler F.J., Alfadul S.M., Mola G.T. Applications of nanocomposite hydrogels for biomedical engineering and environmental protection. Environ. Chem. Lett. 2018;16:113–146. doi: 10.1007/s10311-017-0671-x. [DOI] [Google Scholar]
- 7.Bakshi P.S., Selvakumar D., Kadirvelu K., Kumar N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020;150:1072–1083. doi: 10.1016/j.ijbiomac.2019.10.113. [DOI] [PubMed] [Google Scholar]
- 8.Negm N.A., Hefni H.H.H., Abd-Elaal A.A.A., Badr E.A., Abou Kana M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020;152:681–702. doi: 10.1016/j.ijbiomac.2020.02.196. [DOI] [PubMed] [Google Scholar]
- 9.Salzano de Luna M., Sirignano M. Upcycling soot particles into chitosan-based aerogels for water purification from organic pollutants. J. Hazard. Mater. 2021;2:100019. doi: 10.1016/j.hazl.2021.100019. [DOI] [Google Scholar]
- 10.Irastorza A., Zarandona I., Andonegi M., Guerrero P., de la Caba K. The versatility of collagen and chitosan: From food to biomedical applications. Food Hydrocoll. 2021;116:106633. doi: 10.1016/j.foodhyd.2021.106633. [DOI] [Google Scholar]
- 11.Ahmed T., Noman M., Luo J., Muhammad S., Shahid M., Ali M.A., Zhang M., Li B. Bioengineered chitosan-magnesium nanocomposite: A novel agricultural antimicrobial agent against Acidovorax oryzae and Rhizoctonia solani for sustainable rice production. Int. J. Biol. Macromol. 2021;168:834–845. doi: 10.1016/j.ijbiomac.2020.11.148. [DOI] [PubMed] [Google Scholar]
- 12.Xavier L.O., Sganzerla W.G., Rosa G.B., da Rosa C.G., Agostinetto L., Veeck A.P.L., Bretanha L.C., Micke G.A., Dalla Costa M., Bertoldi F.C., et al. Chitosan packaging functionalized with Cinnamodendron dinisii essential oil loaded zein: A proposal for meat conservation. Int. J. Biol. Macromol. 2021;169:183–193. doi: 10.1016/j.ijbiomac.2020.12.093. [DOI] [PubMed] [Google Scholar]
- 13.Hamed I., Özogul F., Regenstein J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016;48:40–50. doi: 10.1016/j.tifs.2015.11.007. [DOI] [Google Scholar]
- 14.Morin-Crini N., Lichtfouse E., Torri G., Crini G. Fundamentals and Applications of Chitosan. In: Crini G., Lichtfouse E., editors. Sustainable Agriculture Reviews 35: Chitin and Chitosan: History, Fundamentals and Innovations. Springer International Publishing; Cham, Switzerland: 2019. pp. 49–123. [Google Scholar]
- 15.Tyliszczak B., Drabczyk A., Kudłacik-Kramarczyk S., Sobczak-Kupiec A. Sustainable Production of Chitosan. In: Królczyk G.M., Wzorek M., Król A., Kochan O., Su J., Kacprzyk J., editors. Sustainable Production: Novel Trends in Energy, Environment and Material Systems. Springer International Publishing; Cham, Switzerland: 2020. pp. 45–60. [Google Scholar]
- 16.Venter J.P., Kotze A.F., Auzely-Velty R., Rinaudo M. Synthesis and evaluation of the mucoadhesivity of a CD-chitosan derivative. Int. J. Pharm. 2006;313:36–42. doi: 10.1016/j.ijpharm.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Dutta P.K., Dutta J., Tripathi V.S., Research I. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. 2004;63:20–31. [Google Scholar]
- 18.Priyadarshi R., Rhim J.-W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020;62:102346. doi: 10.1016/j.ifset.2020.102346. [DOI] [Google Scholar]
- 19.Tabriz A., Ur Rehman Alvi M.A., Khan Niazi M.B., Batool M., Bhatti M.F., Khan A.L., Khan A.U., Jamil T., Ahmad N.M. Quaternized trimethyl functionalized chitosan based antifungal membranes for drinking water treatment. Carbohydr. Polym. 2019;207:17–25. doi: 10.1016/j.carbpol.2018.11.066. [DOI] [PubMed] [Google Scholar]
- 20.Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Zabermawi N.M., Arif M., Batiha G.E., Khafaga A.F., Abd El-Hakim Y.M., Al-Sagheer A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020;164:2726–2744. doi: 10.1016/j.ijbiomac.2020.08.153. [DOI] [PubMed] [Google Scholar]
- 21.Li J., Zhuang S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020;138:109984. doi: 10.1016/j.eurpolymj.2020.109984. [DOI] [Google Scholar]
- 22.Yin M., Wang Y., Zhang Y., Ren X., Qiu Y., Huang T.S. Novel quaternarized N-halamine chitosan and polyvinyl alcohol nanofibrous membranes as hemostatic materials with excellent antibacterial properties. Carbohydr. Polym. 2020;232:115823. doi: 10.1016/j.carbpol.2019.115823. [DOI] [PubMed] [Google Scholar]
- 23.Pandit A., Indurkar A., Deshpande C., Jain R., Dandekar P. A systematic review of physical techniques for chitosan degradation. Carbohydr. Polym. Technol. Appl. 2021;2:100033. doi: 10.1016/j.carpta.2021.100033. [DOI] [Google Scholar]
- 24.Ghahremanzadeh F., Alihosseini F., Semnani D. Investigation and comparison of new galactosylation methods on PCL/chitosan scaffolds for enhanced liver tissue engineering. Int. J. Biol. Macromol. 2021;174:278–288. doi: 10.1016/j.ijbiomac.2021.01.158. [DOI] [PubMed] [Google Scholar]
- 25.Gritsch L., Lovell C., Goldmann W.H., Boccaccini A.R. Fabrication and characterization of copper(II)-chitosan complexes as antibiotic-free antibacterial biomaterial. Carbohydr. Polym. 2018;179:370–378. doi: 10.1016/j.carbpol.2017.09.095. [DOI] [PubMed] [Google Scholar]
- 26.Kurita K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006;8:203–226. doi: 10.1007/s10126-005-0097-5. [DOI] [PubMed] [Google Scholar]
- 27.Hoang H.T., Jo S.H., Phan Q.T., Park H., Park S.H., Oh C.W., Lim K.T. Dual pH-/thermo-responsive chitosan-based hydrogels prepared using "click" chemistry for colon-targeted drug delivery applications. Carbohydr. Polym. 2021;260:117812. doi: 10.1016/j.carbpol.2021.117812. [DOI] [PubMed] [Google Scholar]
- 28.Ahsan S.M., Thomas M., Reddy K.K., Sooraparaju S.G., Asthana A., Bhatnagar I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018;110:97–109. doi: 10.1016/j.ijbiomac.2017.08.140. [DOI] [PubMed] [Google Scholar]
- 29.Khan M.A., Mujahid M. A review on recent advances in chitosan based composite for hemostatic dressings. Int. J. Biol. Macromol. 2019;124:138–147. doi: 10.1016/j.ijbiomac.2018.11.045. [DOI] [PubMed] [Google Scholar]
- 30.Du X., Wu L., Yan H., Jiang Z., Li S., Li W., Bai Y., Wang H., Cheng Z., Kong D., et al. Microchannelled alkylated chitosan sponge to treat noncompressible hemorrhages and facilitate wound healing. Nat. Commun. 2021;12:4733. doi: 10.1038/s41467-021-24972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhivya S., Saravanan S., Sastry T.P., Selvamurugan N. Nanohydroxyapatite-reinforced chitosan composite hydrogel for bone tissue repair in vitro and in vivo. J. Nanobiotechnol. 2015;13:40. doi: 10.1186/s12951-015-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torr K.M., Chittenden C., Franich R.A., Kreber B. Advances in understanding bioactivity of chitosan and chitosan oligomers against selected wood-inhabiting fungi. Holzforschung. 2005;59:559–567. doi: 10.1515/HF.2005.092. [DOI] [Google Scholar]
- 33.Pham D.C., Nguyen T.H., Ngoc U.T.P., Le N.T.T., Tran T.V., Nguyen D.H. Preparation, Characterization and Antifungal Properties of Chitosan-Silver Nanoparticles Synergize Fungicide Against Pyricularia oryzae. J. Nanosci. Nanotechnol. 2018;18:5299–5305. doi: 10.1166/jnn.2018.15400. [DOI] [PubMed] [Google Scholar]
- 34.Hong H.-M., Sim G.-Y., Park S.-M., Lee E.-J., Kim D.-Y. Ameliorative Effect of Chitosan Complex on Miniature Pig Sperm Cryopreservation. J. Emb. Trans. 2018;33:337–342. doi: 10.12750/JET.2018.33.4.337. [DOI] [Google Scholar]
- 35.Ahn S.I., Cho S., Choi N.J. Effectiveness of Chitosan as a Dietary Supplement in Lowering Cholesterol in Murine Models: A Meta-Analysis. Mar Drugs. 2021;19:26. doi: 10.3390/md19010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lutjohann D., Marinova M., Wolter K., Willinek W., Bitterlich N., Coenen M., Coch C., Stellaard F. Influence of Chitosan Treatment on Surrogate Serum Markers of Cholesterol Metabolism in Obese Subjects. Nutrients. 2018;10:72. doi: 10.3390/nu10010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moramkar N., Bhatt P. Insight into chitosan derived nanotherapeutics for anticancer drug delivery and imaging. Eur. Polym. J. 2021;154:110540. doi: 10.1016/j.eurpolymj.2021.110540. [DOI] [Google Scholar]
- 38.Hadi J.M., Aziz S.B., Nofal M.M., Hussen S.A., Hamsan M.H., Brza M.A., Abdulwahid R.T., Kadir M.F.Z., Woo H.J. Electrical, Dielectric Property and Electrochemical Performances of Plasticized Silver Ion-Conducting Chitosan-Based Polymer Nanocomposites. Membranes. 2020;10:151. doi: 10.3390/membranes10070151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vorobiov V.K., Smirnov M.A., Bobrova N.V., Sokolova M.P. Chitosan-supported deep eutectic solvent as bio-based electrolyte for flexible supercapacitor. Mater. Lett. 2021;283:128889. doi: 10.1016/j.matlet.2020.128889. [DOI] [Google Scholar]
- 40.Desbrières J., Guibal E. Chitosan for wastewater treatment. Polym. Int. 2018;67:7–14. doi: 10.1002/pi.5464. [DOI] [Google Scholar]
- 41.Dodero A., Brunengo E., Alloisio M., Sionkowska A., Vicini S., Castellano M. Chitosan-based electrospun membranes: Effects of solution viscosity, coagulant and crosslinker. Carbohydr. Polym. 2020;235:115976. doi: 10.1016/j.carbpol.2020.115976. [DOI] [PubMed] [Google Scholar]
- 42.Ferreira L.M.B., Dos Santos A.M., Boni F.I., Dos Santos K.C., Robusti L.M.G., de Souza M.P.C., Ferreira N.N., Carvalho S.G., Cardoso V.M.O., Chorilli M., et al. Design of chitosan-based particle systems: A review of the physicochemical foundations for tailored properties. Carbohydr. Polym. 2020;250:116968. doi: 10.1016/j.carbpol.2020.116968. [DOI] [PubMed] [Google Scholar]
- 43.Kordjazi S., Kamyab K., Hemmatinejad N. Super-hydrophilic/oleophobic chitosan/acrylamide hydrogel: An efficient water/oil separation filter. Adv. Compos. Hybrid Mater. 2020;3:167–176. doi: 10.1007/s42114-020-00150-8. [DOI] [Google Scholar]
- 44.Zhou G., Wang K.P., Liu H.W., Wang L., Xiao X.F., Dou D.D., Fan Y.B. Three-dimensional polylactic acid@graphene oxide/chitosan sponge bionic filter: Highly efficient adsorption of crystal violet dye. Int. J. Biol. Macromol. 2018;113:792–803. doi: 10.1016/j.ijbiomac.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 45.Hui M., Shengyan P., Yaqi H., Rongxin Z., Anatoly Z., Wei C. A highly efficient magnetic chitosan “fluid” adsorbent with a high capacity and fast adsorption kinetics for dyeing wastewater purification. Chem. Eng. J. 2018;345:556–565. doi: 10.1016/j.cej.2018.03.115. [DOI] [Google Scholar]
- 46.Urrutia P., Bernal C., Wilson L., Illanes A. Use of chitosan heterofunctionality for enzyme immobilization: Beta-galactosidase immobilization for galacto-oligosaccharide synthesis. Int. J. Biol. Macromol. 2018;116:182–193. doi: 10.1016/j.ijbiomac.2018.04.112. [DOI] [PubMed] [Google Scholar]
- 47.Pal P., Pal A., Nakashima K., Yadav B.K. Applications of chitosan in environmental remediation: A review. Chemosphere. 2021;266:128934. doi: 10.1016/j.chemosphere.2020.128934. [DOI] [PubMed] [Google Scholar]
- 48.Mohammadzadeh Pakdel P., Peighambardoust S.J. Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohydr. Polym. 2018;201:264–279. doi: 10.1016/j.carbpol.2018.08.070. [DOI] [PubMed] [Google Scholar]
- 49.Rinaudo M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006;31:603–632. doi: 10.1016/j.progpolymsci.2006.06.001. [DOI] [Google Scholar]
- 50.Brigham C. Chitin and Chitosan: Sustainable, Medically Relevant Biomaterials. Int. J. Biotech. Well. Indus. 2017;6:41–47. doi: 10.6000/1927-3037.2017.06.02.1. [DOI] [Google Scholar]
- 51.Lehnert R.J., Kandelbauer A. Comments on “Solubility parameter of chitin and chitosan” Carbohydrate Polymers 36 (1998) 121–127. Carbohydr. Polym. 2017;175:601–602. doi: 10.1016/j.carbpol.2017.07.079. [DOI] [PubMed] [Google Scholar]
- 52.Ravindra R., Krovvidi K.R., Khan A.A. Solubility parameter of chitin and chitosan. Carbohydr. Polym. 1998;36:121–127. doi: 10.1016/S0144-8617(98)00020-4. [DOI] [Google Scholar]
- 53.Pardo-Castaño C., Bolaños G. Solubility of chitosan in aqueous acetic acid and pressurized carbon dioxide-water: Experimental equilibrium and solubilization kinetics. J. Supercrit. Fluids. 2019;151:63–74. doi: 10.1016/j.supflu.2019.05.007. [DOI] [Google Scholar]
- 54.Cunha R.A., Soares T.A., Rusu V.H., Pontes F.J., Franca E.F., Lins R.D. The Complex World of Polysaccharides. BoD–Books on Demand; Norderstedt, Germany: 2012. The Molecular Structure and Conformational Dynamics of Chitosan Polymers: An Integrated Perspective from Experiments and Computational Simulations. [Google Scholar]
- 55.Uragami T.T. Material Science of Chitin and Chitosan. Kodansha; Tokyo, Japan: 2006. [Google Scholar]
- 56.Liu X., Wu Y., Zhao X., Wang Z. Fabrication and applications of bioactive chitosan-based organic-inorganic hybrid materials: A review. Carbohydr. Polym. 2021;267:118179. doi: 10.1016/j.carbpol.2021.118179. [DOI] [PubMed] [Google Scholar]
- 57.Bi J., Huang X., Wang J., Wang T., Wu H., Yang J., Lu H., Hao H. Oil-phase cyclic magnetic adsorption to synthesize Fe3O4@C@TiO2-nanotube composites for simultaneous removal of Pb(II) and Rhodamine B. Chem. Eng. J. 2019;366:50–61. doi: 10.1016/j.cej.2019.02.017. [DOI] [Google Scholar]
- 58.Yan Y., Dong X., Sun X., Sun X., Li J., Shen J., Han W., Liu X., Wang L. Conversion of waste FGD gypsum into hydroxyapatite for removal of Pb2+ and Cd2+ from wastewater. J. Colloid Interface Sci. 2014;429:68–76. doi: 10.1016/j.jcis.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 59.Shahrajabian M.H., Chaski C., Polyzos N., Tzortzakis N., Petropoulos S.A. Sustainable Agriculture Systems in Vegetable Production Using Chitin and Chitosan as Plant Biostimulants. Biomolecules. 2021;11:819. doi: 10.3390/biom11060819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Divya K., Jisha M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018;16:101–112. doi: 10.1007/s10311-017-0670-y. [DOI] [Google Scholar]
- 61.Vidanarachchi J.K., Kim S.K. In: Chitin, Chitosan, Oligosaccharides and Their Derivatives. Biological Activities and Applications. Kim S.-K., editor. CRC Press; Boca Raton, FL, USA: 2010. p. 666. [Google Scholar]
- 62.Gutiérrez T.J. Chapter 8: Chitosan Applications for the Food Industry. In: Ahmed S., editor. Chitosan: Derivatives, Composites and Applications. Wiley Online Library; Hoboken, NJ, USA: 2017. [Google Scholar]
- 63.Song E.H., Shang J., Ratner D.M. 9.08-Polysaccharides. In: Matyjaszewski K., Möller M., editors. Polymer Science: A Comprehensive Reference. Elsevier; Amsterdam, The Netherlands: 2012. pp. 137–155. [Google Scholar]
- 64.Negoi A.-E., Cristea C.-D., Deșliu-Avram M., Trică B., Constantinescu-Aruxandei D., Oancea F. Extraction of Fungal Chitin Using Natural Deep Eutectic Solvents. Proceedings. 2019;29:91. [Google Scholar]
- 65.Hongkulsup C., Khutoryanskiy V.V., Niranjan K. Enzyme assisted extraction of chitin from shrimp shells (Litopenaeus vannamei) J. Chem. Technol. Biotechnol. 2016;91:1250–1256. doi: 10.1002/jctb.4714. [DOI] [Google Scholar]
- 66.Sadighara P., Moghadam H.T., Eskandari S., Salehi A. Optimization of extraction of chitosan and carotenoids from shrimp waste. J. Fish Aquat. Sci. 2015;2:36–38. [Google Scholar]
- 67.Peniston Q.P., Johnson E.L. Process for the Manufacture of Chitosan. No. 4,195,175. U.S. Patent. 1980
- 68.Kurita K., Kaji Y., Mori T., Nishiyama Y. Enzymatic degradation of β-chitin: Susceptibility and the influence of deacetylation. Carbohydr. Polym. 2000;42:19–21. doi: 10.1016/S0144-8617(99)00127-7. [DOI] [Google Scholar]
- 69.Tan T.S., Chin H.Y., Tsai M.L., Liu C.L. Structural alterations, pore generation, and deacetylation of alpha- and beta-chitin submitted to steam e xplosion. Carbohydr. Polym. 2015;122:321–328. doi: 10.1016/j.carbpol.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 70.Anwar M., Anggraeni A.S., Amin M.H.A. Comparison of green method for chitin deacetylation. AIP Conf. Proc. 2017;1823:020071. [Google Scholar]
- 71.Campana-Filho S.P., Signini R., Cardoso M.B. Effects of sonication on the reactivity of chitin toward its heterogeneous deacetylation. Int. J. Polym. Mater. Polym. Biomater. 2002;51:695–700. doi: 10.1080/714975832. [DOI] [Google Scholar]
- 72.Younes I., Ghorbel-Bellaaj O., Nasri R., Chaabouni M., Rinaudo M., Nasri M. Chitin and chitosan preparation from shrimp shells using optimized enzymatic deproteinization. Process Biochem. 2012;47:2032–2039. doi: 10.1016/j.procbio.2012.07.017. [DOI] [Google Scholar]
- 73.Tasar O.C., Erdal S., Taskin M. Chitosan production by psychrotolerant Rhizopus oryzae in non-sterile open fermentation conditions. Int. J. Biol. Macromol. 2016;89:428–433. doi: 10.1016/j.ijbiomac.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 74.Zhao L., Xia W. Stainless steel membrane UF coupled with NF process for the recovery of sodium hydroxide from alkaline wastewater in chitin processing. Desalination. 2009;249:774–780. doi: 10.1016/j.desal.2009.01.036. [DOI] [Google Scholar]
- 75.Zhao L., Xia W., Zhao H. Cost model for chitin production alkali wastewater recovery by couple-membrane filtration. Desalin. Water Treat. 2012;28:202–210. doi: 10.5004/dwt.2011.2433. [DOI] [Google Scholar]
- 76.Jahan M.S., Hossain M.M., Roy S.K., Asaduzzaman M., Masum S.M., Nessa F. A Process for the Preparation of Chitin and Chitosan from Prawn Shell Waste. Bangladesh. J. Sci. Ind. Res. 1970;45:323–330. [Google Scholar]
- 77.Muxika A., Etxabide A., Uranga J., Guerrero P., de la Caba K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017;105:1358–1368. doi: 10.1016/j.ijbiomac.2017.07.087. [DOI] [PubMed] [Google Scholar]
- 78.Deng J., Zhu E.-Q., Xu G.-F., Naik N., Murugadoss V., Ma M.-G., Guo Z., Shi Z.-J. Overview of renewable polysaccharide-based composites for biodegradable food packaging applications. Green Chem. 2022;24:480–492. doi: 10.1039/D1GC03898B. [DOI] [Google Scholar]
- 79.Sharma G., Khosla A., Kumar A., Kaushal N., Sharma S., Naushad M., Vo D.-V.N., Iqbal J., Stadler F.J. A comprehensive review on the removal of noxious pollutants using carrageenan based advanced adsorbents. Chemosphere. 2022;289:133100. doi: 10.1016/j.chemosphere.2021.133100. [DOI] [PubMed] [Google Scholar]
- 80.Sharma G., Kumar A., Ghfar A.A., García-Peñas A., Naushad M., Stadler F.J. Fabrication and Characterization of Xanthan Gum-cl-poly(acrylamide-co-alginic acid) Hydrogel for Adsorption of Cadmium Ions from Aqueous Medium. Gels. 2022;8:23. doi: 10.3390/gels8010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharma G., Kumar A., Chauhan C., Okram A., Sharma S., Pathania D., Kalia S. Pectin-crosslinked-guar gum/SPION nanocomposite hydrogel for adsorption of m-cresol and o-chlorophenol. Sustain. Chem. Pharm. 2017;6:96–106. doi: 10.1016/j.scp.2017.10.003. [DOI] [Google Scholar]
- 82.Elanchezhiyan S.S., Preethi J., Rathinam K., Njaramba L.K., Park C.M. Synthesis of magnetic chitosan biopolymeric spheres and their adsorption performances for PFOA and PFOS from aqueous environment. Carbohydr. Polym. 2021;267:118165. doi: 10.1016/j.carbpol.2021.118165. [DOI] [PubMed] [Google Scholar]
- 83.Sharma G., Kumar A., Naushad M., Thakur B., Vo D.-V.N., Gao B., Al-Kahtani A.A., Stadler F.J. Adsorptional-photocatalytic removal of fast sulphon black dye by using chitin-cl-poly(itaconic acid-co-acrylamide)/zirconium tungstate nanocomposite hydrogel. J. Hazard. Mater. 2021;416:125714. doi: 10.1016/j.jhazmat.2021.125714. [DOI] [PubMed] [Google Scholar]
- 84.Leceta I., Molinaro S., Guerrero P., Kerry J.P., de la Caba K. Quality attributes of map packaged ready-to-eat baby carrots by using chitosan-based coatings. Postharvest Biol. Technol. 2015;100:142–150. doi: 10.1016/j.postharvbio.2014.09.022. [DOI] [Google Scholar]
- 85.Liu S., Gao J., Zhang L., Yang Y., Liu X. Diethylenetriaminepentaacetic acid–thiourea-modified magnetic chitosan for adsorption of hexavalent chromium from aqueous solutions. Carbohydr. Polym. 2021;274:118555. doi: 10.1016/j.carbpol.2021.118555. [DOI] [PubMed] [Google Scholar]
- 86.Kumar S., Mukherjee A., Dutta J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020;97:196–209. doi: 10.1016/j.tifs.2020.01.002. [DOI] [Google Scholar]
- 87.Elmehbad N.Y., Mohamed N.A., Abd El-Ghany N.A. Evaluation of the antimicrobial and anti-biofilm activity of novel salicylhydrazido chitosan derivatives impregnated with titanium dioxide nanoparticles. Int. J. Biol. Macromol. 2022;205:719–730. doi: 10.1016/j.ijbiomac.2022.03.076. [DOI] [PubMed] [Google Scholar]
- 88.Rodrigues P.R., Junior L.M., de Souza W.F.C., Sato H.H., Alves R.M.V., Vieira R.P. O-ATRP synthesized poly(β-pinene) blended with chitosan for antimicrobial and antioxidant bio-based films production. Int. J. Biol. Macromol. 2021;193:425–432. doi: 10.1016/j.ijbiomac.2021.10.156. [DOI] [PubMed] [Google Scholar]
- 89.Nadira P.P., Mujeeb V.M.A., Rahman P.M., Muraleedharan K. Effects of cashew leaf extract on physicochemical, antioxidant, and antimicrobial properties of N, O–Carboxymethyl chitosan films. Carbohydr. Polym. Technol. Appl. 2022;3:100191. doi: 10.1016/j.carpta.2022.100191. [DOI] [Google Scholar]
- 90.Elsabee M.Z., Abdou E.S. Chitosan based edible films and coatings: A review. Mater Sci. Eng. C Mater. Biol. Appl. 2013;33:1819–1841. doi: 10.1016/j.msec.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 91.Haghighi H., Licciardello F., Fava P., Siesler H.W., Pulvirenti A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life. 2020;26:100551–100567. doi: 10.1016/j.fpsl.2020.100551. [DOI] [Google Scholar]
- 92.Panda P.K., Dash P., Yang J.-M., Chang Y.-H. Development of chitosan, graphene oxide, and cerium oxide composite blended films: Structural, physical, and functional properties. Cellulose. 2022;29:2399–2411. doi: 10.1007/s10570-021-04348-x. [DOI] [Google Scholar]
- 93.Kim D.S., Dhand V., Rhee K.Y., Park S.-J. Study on the Effect of Silanization and Improvement in the Tensile Behavior of Graphene-Chitosan-Composite. Polymers. 2015;7:527–551. doi: 10.3390/polym7030527. [DOI] [Google Scholar]
- 94.Sharma G., Naushad M., Kumar A., Kumar A., Ahamad T., Stadler F.J. Facile fabrication of chitosan-cl-poly(AA)/ZrPO4 nanocomposite for remediation of rhodamine B and antimicrobial activity. J. King Saud Univ. Sci. 2020;32:1359–1365. doi: 10.1016/j.jksus.2019.11.028. [DOI] [Google Scholar]
- 95.You J., Liu C., Feng X., Lu B., Xia L., Zhuang X. In situ synthesis of ZnS nanoparticles onto cellulose/chitosan sponge for adsorption–photocatalytic removal of Congo red. Carbohydr. Polym. 2022;288:119332. doi: 10.1016/j.carbpol.2022.119332. [DOI] [PubMed] [Google Scholar]
- 96.Andrade-Del Olmo J., Pérez-Álvarez L., Hernáez E., Ruiz-Rubio L., Vilas-Vilela J.L. Antibacterial multilayer of chitosan and (2-carboxyethyl)- β-cyclodextrin onto polylactic acid (PLLA) Food Hydrocoll. 2019;88:228–236. doi: 10.1016/j.foodhyd.2018.10.014. [DOI] [Google Scholar]
- 97.Zhang M., Zheng Y., Jin Y., Wang D., Wang G., Zhang X., Li Y., Lee S. Ag@MOF-loaded p-coumaric acid modified chitosan/chitosan nanoparticle and polyvinyl alcohol/starch bilayer films for food packing applications. Int. J. Biol. Macromol. 2022;202:80–90. doi: 10.1016/j.ijbiomac.2022.01.074. [DOI] [PubMed] [Google Scholar]
- 98.Zhang H., He P., Kang H., Li X. Antioxidant and antimicrobial effects of edible coating based on chitosan and bamboo vinegar in ready to cook pork chops. LWT. 2018;93:470–476. doi: 10.1016/j.lwt.2018.04.005. [DOI] [Google Scholar]
- 99.Balti R., Mansour M.B., Sayari N., Yacoubi L., Rabaoui L., Brodu N., Massé A. Development and characterization of bioactive edible films from spider crab (Maja crispata) chitosan incorporated with Spirulina extract. Int. J. Biol. Macromol. 2017;105:1464–1472. doi: 10.1016/j.ijbiomac.2017.07.046. [DOI] [PubMed] [Google Scholar]
- 100.Ghaderi-Ghahfarokhi M., Barzegar M., Sahari M.A., Ahmadi Gavlighi H., Gardini F. Chitosan-cinnamon essential oil nano-formulation: Application as a novel additive for controlled release and shelf life extension of beef patties. Int. J. Biol. Macromol. 2017;102:19–28. doi: 10.1016/j.ijbiomac.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 101.He L., Zou L., Yang Q., Xia J., Zhou K., Zhu Y., Han X., Pu B., Hu B., Deng W., et al. Antimicrobial Activities of Nisin, Tea Polyphenols, and Chitosan and their Combinations in Chilled Mutton. J. Food Sci. 2016;81:M1466–M1471. doi: 10.1111/1750-3841.13312. [DOI] [PubMed] [Google Scholar]
- 102.Khanjari A., Karabagias I.K., Kontominas M.G. Combined effect of N,O-carboxymethyl chitosan and oregano essential oil to extend shelf life and control Listeria monocytogenes in raw chicken meat fillets. LWT Food Sci. Technol. 2013;53:94–99. doi: 10.1016/j.lwt.2013.02.012. [DOI] [Google Scholar]
- 103.Bautista-Baños S., Hernández-López M., Bosquez-Molina E., Wilson C.L. Effects of chitosan and plant extracts on growth of Colletotrichum gloeosporioides, anthracnose levels and quality of papaya fruit. Crop Prot. 2003;22:1087–1092. doi: 10.1016/S0261-2194(03)00117-0. [DOI] [Google Scholar]
- 104.Chamanara V., Shabanpour B., Gorgin S., Khomeiri M. An investigation on characteristics of rainbow trout coated using chitosan assisted with thyme essential oil. Int. J. Biol. Macromol. 2012;50:540–544. doi: 10.1016/j.ijbiomac.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 105.Liu Y., Wang D., Sun Z., Liu F., Du L., Wang D. Preparation and characterization of gelatin/chitosan/3-phenylacetic acid food-packaging nanofiber antibacterial films by electrospinning. Int. J. Biol. Macromol. 2021;169:161–170. doi: 10.1016/j.ijbiomac.2020.12.046. [DOI] [PubMed] [Google Scholar]
- 106.Wang H., Ding F., Ma L., Zhang Y. Edible films from chitosan-gelatin: Physical properties and food packaging application. Food Biosci. 2021;40:100871. doi: 10.1016/j.fbio.2020.100871. [DOI] [Google Scholar]
- 107.Fasciglione G., Goñi M.G., Yommi A.K., Perez-Bravo J.J., Ortueta R., Scampini A., Buffa L., Andreu A.B., Creus C.M. Revaluation of waste from fishing industry through generation of chitosan coatings to improve quality and extend shelf-life of minimally processed lettuce. Postharvest Biol. Technol. 2020;170:111310. doi: 10.1016/j.postharvbio.2020.111310. [DOI] [Google Scholar]
- 108.Panda P.K., Yang J.-M., Chang Y.-H. Preparation and characterization of ferulic acid-modified water soluble chitosan and poly (γ-glutamic acid) polyelectrolyte films through layer-by-layer assembly towards protein adsorption. Int. J. Biol. Macromol. 2021;171:457–464. doi: 10.1016/j.ijbiomac.2020.12.226. [DOI] [PubMed] [Google Scholar]
- 109.Ngo T.M.P., Nguyen T.H., Dang T.M.Q., Tran T.X., Rachtanapun P. Characteristics and Antimicrobial Properties of Active Edible Films Based on Pectin and Nanochitosan. Int. J. Mol. Sci. 2020;21:2224. doi: 10.3390/ijms21062224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baron R.D., Perez L.L., Salcedo J.M., Cordoba L.P., Sobral P.J. Production and characterization of films based on blends of chitosan from blue crab (Callinectes sapidus) waste and pectin from Orange (Citrus sinensis Osbeck) peel. Int. J. Biol. Macromol. 2017;98:676–683. doi: 10.1016/j.ijbiomac.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 111.Valizadeh S., Naseri M., Babaei S., Hosseini S.M.H., Imani A. Development of bioactive composite films from chitosan and carboxymethyl cellulose using glutaraldehyde, cinnamon essential oil and oleic acid. Int. J. Biol. Macromol. 2019;134:604–612. doi: 10.1016/j.ijbiomac.2019.05.071. [DOI] [PubMed] [Google Scholar]
- 112.Youssef A.M., El-Sayed S.M., El-Sayed H.S., Salama H.H., Dufresne A. Enhancement of Egyptian soft white cheese shelf life using a novel chitosan/carboxymethyl cellulose/zinc oxide bionanocomposite film. Carbohydr. Polym. 2016;151:9–19. doi: 10.1016/j.carbpol.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 113.Hu D., Wang H., Wang L. Physical properties and antibacterial activity of quaternized chitosan/carboxymethyl cellulose blend films. LWT Food Sci. Technol. 2016;65:398–405. doi: 10.1016/j.lwt.2015.08.033. [DOI] [Google Scholar]
- 114.Xu T., Gao C., Feng X., Yang Y., Shen X., Tang X. Structure, physical and antioxidant properties of chitosan-gum arabic edible films incorporated with cinnamon essential oil. Int. J. Biol. Macromol. 2019;134:230–236. doi: 10.1016/j.ijbiomac.2019.04.189. [DOI] [PubMed] [Google Scholar]
- 115.Xu T., Gao C., Feng X., Huang M., Yang Y., Shen X., Tang X. Cinnamon and clove essential oils to improve physical, thermal and antimicrobial properties of chitosan-gum arabic polyelectrolyte complexed films. Carbohydr. Polym. 2019;217:116–125. doi: 10.1016/j.carbpol.2019.03.084. [DOI] [PubMed] [Google Scholar]
- 116.Luchese C.L., Pavoni J.M.F., Dos Santos N.Z., Quines L.K., Pollo L.D., Spada J.C., Tessaro I.C. Effect of chitosan addition on the properties of films prepared with corn and cassava starches. J. Food Sci. Technol. 2018;55:2963–2973. doi: 10.1007/s13197-018-3214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ren L., Yan X., Zhou J., Tong J., Su X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017;105:1636–1643. doi: 10.1016/j.ijbiomac.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 118.Lozano-Navarro J.I., Diaz-Zavala N.P., Velasco-Santos C., Martinez-Hernandez A.L., Tijerina-Ramos B.I., Garcia-Hernandez M., Rivera-Armenta J.L., Paramo-Garcia U., Reyes-de la Torre A.I. Antimicrobial, Optical and Mechanical Properties of Chitosan-Starch Films with Natural Extracts. Int. J. Mol. Sci. 2017;18:997. doi: 10.3390/ijms18050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu Y., Yuan Y., Duan S., Li C., Hu B., Liu A., Wu D., Cui H., Lin L., He J., et al. Preparation and characterization of chitosan films with three kinds of molecular weight for food packaging. Int. J. Biol. Macromol. 2020;155:249–259. doi: 10.1016/j.ijbiomac.2020.03.217. [DOI] [PubMed] [Google Scholar]
- 120.Zhao J., Wei F., Xu W., Han X. Enhanced antibacterial performance of gelatin/chitosan film containing capsaicin loaded MOFs for food packaging. Appl. Surf. Sci. 2020;510:145418. doi: 10.1016/j.apsusc.2020.145418. [DOI] [Google Scholar]
- 121.Rambabu K., Bharath G., Banat F., Show P.L., Cocoletzi H.H. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int. J. Biol. Macromol. 2019;126:1234–1243. doi: 10.1016/j.ijbiomac.2018.12.196. [DOI] [PubMed] [Google Scholar]
- 122.Kumar S., Shukla A., Baul P.P., Mitra A., Halder D. Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packag. Shelf Life. 2018;16:178–184. doi: 10.1016/j.fpsl.2018.03.008. [DOI] [Google Scholar]
- 123.Zhang L., Huang C., Zhao H. Application of Pullulan and Chitosan Multilayer Coatings in Fresh Papayas. Coatings. 2019;9:745. doi: 10.3390/coatings9110745. [DOI] [Google Scholar]
- 124.Kaewklin P., Siripatrawan U., Suwanagul A., Lee Y.S. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 2018;112:523–529. doi: 10.1016/j.ijbiomac.2018.01.124. [DOI] [PubMed] [Google Scholar]
- 125.Gao Q., Lei M., Zhou K., Liu X., Wang S., Li H. Preparation of a microfibrillated cellulose/chitosan/polypyrrole film for Active Food Packaging. Prog. Org. Coat. 2020;149:105907. doi: 10.1016/j.porgcoat.2020.105907. [DOI] [Google Scholar]
- 126.Lu S., Tao J., Liu X., Wen Z. Baicalin-liposomes loaded polyvinyl alcohol-chitosan electrospinning nanofibrous films: Characterization, antibacterial properties and preservation effects on mushrooms. Food Chem. 2022;371:131372. doi: 10.1016/j.foodchem.2021.131372. [DOI] [PubMed] [Google Scholar]
- 127.Azaza Y.B., Hamdi M., Charmette C., Jridi M., Li S., Nasri M., Nasri R. Development and characterization of active packaging films based on chitosan and sardinella protein isolate: Effects on the quality and the shelf life of shrimps. Food Packag. Shelf Life. 2022;31:100796. doi: 10.1016/j.fpsl.2021.100796. [DOI] [Google Scholar]
- 128.Lin L., Xue L., Duraiarasan S., Haiying C. Preparation of ε-polylysine/chitosan nanofibers for food packaging against Salmonella on chicken. Food Packag. Shelf Life. 2018;17:134–141. doi: 10.1016/j.fpsl.2018.06.013. [DOI] [Google Scholar]
- 129.Priyadarshi R., Kumar B., Deeba F., Kulshreshtha A., Negi Y.S. Chitosan films incorporated with Apricot (Prunus armeniaca) kernel essential oil as active food packaging material. Food Hydrocoll. 2018;85:158–166. doi: 10.1016/j.foodhyd.2018.07.003. [DOI] [Google Scholar]
- 130.Cui H., Surendhiran D., Li C., Lin L. Biodegradable zein active film containing chitosan nanoparticle encapsulated with pomegranate peel extract for food packaging. Food Packag. Shelf Life. 2020;24:100511. doi: 10.1016/j.fpsl.2020.100511. [DOI] [Google Scholar]
- 131.Indumathi M.P., Rajarajeswari G.R. Mahua oil-based polyurethane/chitosan/nano ZnO composite films for biodegradable food packaging applications. Int. J. Biol. Macromol. 2019;124:163–174. doi: 10.1016/j.ijbiomac.2018.11.195. [DOI] [PubMed] [Google Scholar]
- 132.Liu X., Xue F., Li C., Adhikari B. Physicochemical properties of films produced using nanoemulsions stabilized by carboxymethyl chitosan-peptide conjugates and application in blueberry preservation. Int. J. Biol. Macromol. 2022;202:26–36. doi: 10.1016/j.ijbiomac.2021.12.186. [DOI] [PubMed] [Google Scholar]
- 133.Vilvert J.C., de Freitas S.T., Ferreira M.A.R., Leite R.H.d.L., dos Santos F.K.G., Costa C.d.S.R., Aroucha E.M.M. Chitosan and graphene oxide-based biodegradable bags: An eco-friendly and effective packaging alternative to maintain postharvest quality of ‘Palmer’ mango. LWT. 2022;154:112741. doi: 10.1016/j.lwt.2021.112741. [DOI] [Google Scholar]
- 134.Yang K., Dang H., Liu L., Hu X., Li X., Ma Z., Wang X., Ren T. Effect of syringic acid incorporation on the physical, mechanical, structural and antibacterial properties of chitosan film for quail eggs preservation. Int. J. Biol. Macromol. 2019;141:876–884. doi: 10.1016/j.ijbiomac.2019.08.045. [DOI] [PubMed] [Google Scholar]
- 135.Yao X., Hu H., Qin Y., Liu J. Development of antioxidant, antimicrobial and ammonia-sensitive films based on quaternary ammonium chitosan, polyvinyl alcohol and betalains-rich cactus pears (Opuntia ficus-indica) extract. Food Hydrocoll. 2020;106:105896. doi: 10.1016/j.foodhyd.2020.105896. [DOI] [Google Scholar]
- 136.Duran A., Kahve H.I. The effect of chitosan coating and vacuum packaging on the microbiological and chemical properties of beef. Meat Sci. 2020;162:107961. doi: 10.1016/j.meatsci.2019.107961. [DOI] [PubMed] [Google Scholar]
- 137.Liu Z., Du M., Liu H., Zhang K., Xu X., Liu K., Tu J., Liu Q. Chitosan films incorporating litchi peel extract and titanium dioxide nanoparticles and their application as coatings on watercored apples. Prog. Org. Coat. 2021;151:106103. doi: 10.1016/j.porgcoat.2020.106103. [DOI] [Google Scholar]
- 138.Fathima P.E., Panda S.K., Ashraf P.M., Varghese T.O., Bindu J. Polylactic acid/chitosan films for packaging of Indian white prawn (Fenneropenaeus indicus) Int. J. Biol. Macromol. 2018;117:1002–1010. doi: 10.1016/j.ijbiomac.2018.05.214. [DOI] [PubMed] [Google Scholar]
- 139.Nagarajan M., Rajasekaran B., Benjakul S., Venkatachalam K. Influence of chitosan-gelatin edible coating incorporated with longkong pericarp extract on refrigerated black tiger Shrimp (Penaeus monodon) Curr. Res. Food Sci. 2021;4:345–353. doi: 10.1016/j.crfs.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Merlo T.C., Contreras-Castillo C.J., Saldaña E., Barancelli G.V., Dargelio M.D.B., Yoshida C.M.P., Ribeiro Junior E.E., Massarioli A., Venturini A.C. Incorporation of pink pepper residue extract into chitosan film combined with a modified atmosphere packaging: Effects on the shelf life of salmon fillets. Food Res. Int. 2019;125:108633. doi: 10.1016/j.foodres.2019.108633. [DOI] [PubMed] [Google Scholar]
- 141.Priyadarshi R., Kumar B., Negi Y.S. Chitosan film incorporated with citric acid and glycerol as an active packaging material for extension of green chilli shelf life. Carbohydr. Polym. 2018;195:329–338. doi: 10.1016/j.carbpol.2018.04.089. [DOI] [PubMed] [Google Scholar]
- 142.Orzali L., Corsi B., Forni C., Riccioni L. Biological Activities and Application of Marine Polysaccharides. BoD–Books on Demand; Norderstedt, Germany: 2017. Chitosan in Agriculture: A New Challenge for Managing Plant Disease. [Google Scholar]
- 143.Kulikov S.N., Chirkov S.N., Il’ina A.V., Lopatin S.A., Varlamov V.P. Effect of the molecular weight of chitosan on its antiviral activity in plants. Prikl. Biokhim. Mikrobiol. 2006;42:224–228. doi: 10.1134/S0003683806020165. [DOI] [PubMed] [Google Scholar]
- 144.Hadwiger L.A. Multiple effects of chitosan on plant systems: Solid science or hype. Plant Sci. 2013;208:42–49. doi: 10.1016/j.plantsci.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 145.Adamuchio-Oliveira L.G., Mazaro S.M., Mógor G., Sant’Anna-Santos B.F., Mógor Á.F. Chitosan associated with chelated copper applied on tomatoes: Enzymatic and anatomical changes related to plant defense responses. Sci. Hortic. 2020;271:109431. doi: 10.1016/j.scienta.2020.109431. [DOI] [Google Scholar]
- 146.Jogaiah S., Satapute P., De Britto S., Konappa N., Udayashankar A.C. Exogenous priming of chitosan induces upregulation of phytohormones and resistance against cucumber powdery mildew disease is correlated with localized biosynthesis of defense enzymes. Int. J. Biol. Macromol. 2020;162:1825–1838. doi: 10.1016/j.ijbiomac.2020.08.124. [DOI] [PubMed] [Google Scholar]
- 147.Alkahtani M.D.F., Attia K.A., Hafez Y.M., Khan N., Eid A.M., Ali M.A.M., Abdelaal K.A.A. Chlorophyll Fluorescence Parameters and Antioxidant Defense System Can Display Salt Tolerance of Salt Acclimated Sweet Pepper Plants Treated with Chitosan and Plant Growth Promoting Rhizobacteria. Agronomy. 2020;10:1180. doi: 10.3390/agronomy10081180. [DOI] [Google Scholar]
- 148.Kahromi S., Khara J. Chitosan stimulates secondary metabolite production and nutrient uptake in medicinal plant Dracocephalum kotschyi. J. Sci. Food Agric. 2021;101:3898–3907. doi: 10.1002/jsfa.11030. [DOI] [PubMed] [Google Scholar]
- 149.Rasheed R., Ashraf M.A., Arshad A., Iqbal M., Hussain I. Interactive effects of chitosan and cadmium on growth, secondary metabolism, oxidative defense, and element uptake in pea (Pisum sativum L.) Arabian J. Geosci. 2020;13:847. doi: 10.1007/s12517-020-05871-0. [DOI] [Google Scholar]
- 150.Kumari S., Singh A.K., Kumar A., Singh K.P., Bains G. Evaluating the efficacy of chitosan and salicylic acid on photosynthetic pigments and antioxidant enzymes towards resistance of mango malformation. Sci. Hortic. 2021;285:110160. doi: 10.1016/j.scienta.2021.110160. [DOI] [Google Scholar]
- 151.Picchi V., Gobbi S., Fattizzo M., Zefelippo M., Faoro F. Chitosan Nanoparticles Loaded with N-Acetyl Cysteine to Mitigate Ozone and Other Possible Oxidative Stresses in Durum Wheat. Plants. 2021;10:691. doi: 10.3390/plants10040691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li Y., Zhang Q., Ou L., Ji D., Liu T., Lan R., Li X., Jin L. Response to the Cold Stress Signaling of the Tea Plant (Camellia sinensis) Elicited by Chitosan Oligosaccharide. Agronomy. 2020;10:915. doi: 10.3390/agronomy10060915. [DOI] [Google Scholar]
- 153.Chaudhari A.K., Singh V.K., Das S., Dubey N.K. Fabrication, characterization, and bioactivity assessment of chitosan nanoemulsion containing allspice essential oil to mitigate Aspergillus flavus contamination and aflatoxin B1 production in maize. Food Chem. 2022;372:131221. doi: 10.1016/j.foodchem.2021.131221. [DOI] [PubMed] [Google Scholar]
- 154.Mondéjar-López M., Rubio-Moraga A., López-Jimenez A.J., García Martínez J.C., Ahrazem O., Gómez-Gómez L., Niza E. Chitosan nanoparticles loaded with garlic essential oil: A new alternative to tebuconazole as seed dressing agent. Carbohydr. Polym. 2022;277:118815. doi: 10.1016/j.carbpol.2021.118815. [DOI] [PubMed] [Google Scholar]
- 155.Wu P., Xin F., Xu H., Chu Y., Du Y., Tian H., Zhu B. Chitosan inhibits postharvest berry abscission of ‘Kyoho’ table grapes by affecting the structure of abscission zone, cell wall degrading enzymes and SO2 permeation. Postharvest Biol. Technol. 2021;176:111507. doi: 10.1016/j.postharvbio.2021.111507. [DOI] [Google Scholar]
- 156.Li Z., Xue S., Xu X., Wang B., Zheng X., Li B., Xie P., Bi Y., Prusky D. Preharvest multiple sprays with chitosan accelerate the deposition of suberin poly phenolic at wound sites of harvested muskmelons. Postharvest Biol. Technol. 2021;179:111565. doi: 10.1016/j.postharvbio.2021.111565. [DOI] [Google Scholar]
- 157.Jiang Y., Yin H., Zhou X., Wang D., Zhong Y., Xia Q., Deng Y., Zhao Y. Antimicrobial, antioxidant and physical properties of chitosan film containing Akebia trifoliata (Thunb.) Koidz. peel extract/montmorillonite and its application. Food Chem. 2021;361:130111. doi: 10.1016/j.foodchem.2021.130111. [DOI] [PubMed] [Google Scholar]
- 158.Kumar A., Pratap Singh P., Prakash B. Unravelling the antifungal and anti-aflatoxin B1 mechanism of chitosan nanocomposite incorporated with Foeniculum vulgare essential oil. Carbohydr. Polym. 2020;236:116050. doi: 10.1016/j.carbpol.2020.116050. [DOI] [PubMed] [Google Scholar]
- 159.Rajkumar V., Gunasekaran C., Paul C.A., Dharmaraj J. Development of encapsulated peppermint essential oil in chitosan nanoparticles: Characterization and biological efficacy against stored-grain pest control. Pestic. Biochem. Physiol. 2020;170:104679. doi: 10.1016/j.pestbp.2020.104679. [DOI] [PubMed] [Google Scholar]
- 160.Kangama A., Zeng D., Tian X., Fang J. Application of Chitosan Composite Flocculant in Tap Water Treatment. J. Chem. 2018;2018:2768474. doi: 10.1155/2018/2768474. [DOI] [Google Scholar]
- 161.Abhinaya M., Parthiban R., Kumar P.S., Vo D.-V.N. A review on cleaner strategies for extraction of chitosan and its application in toxic pollutant removal. Environ. Res. 2021;196:110996. doi: 10.1016/j.envres.2021.110996. [DOI] [PubMed] [Google Scholar]
- 162.Sadiq A.C., Olasupo A., Ngah W.S.W., Rahim N.Y., Suah F.B.M. A decade development in the application of chitosan-based materials for dye adsorption: A short review. Int. J. Biol. Macromol. 2021;191:1151–1163. doi: 10.1016/j.ijbiomac.2021.09.179. [DOI] [PubMed] [Google Scholar]
- 163.Saheed I.O., Oh W.D., Suah F.B.M. Chitosan modifications for adsorption of pollutants—A review. J. Hazard. Mater. 2021;408:124889. doi: 10.1016/j.jhazmat.2020.124889. [DOI] [PubMed] [Google Scholar]
- 164.Li Y., Liang Y.-Q., Mao X.-M., Li H. Efficient removal of Cu(II) from an aqueous solution using a novel chitosan assisted EDTA-intercalated hydrotalcite-like compound composite: Preparation, characterization, and adsorption mechanism. Chem. Eng. J. 2022;438:135531. doi: 10.1016/j.cej.2022.135531. [DOI] [Google Scholar]
- 165.Alsamman M.T., Sánchez J. Recent advances on hydrogels based on chitosan and alginate for the adsorption of dyes and metal ions from water. Arabian J. Chem. 2021;14:103455. doi: 10.1016/j.arabjc.2021.103455. [DOI] [Google Scholar]
- 166.Abdolmaleki A.Y., Zilouei H., Khorasani S.N., Zargoosh K. Adsorption of tetracycline from water using glutaraldehyde-crosslinked electrospun nanofibers of chitosan/poly(vinyl alcohol) Water Sci. Technol. 2018;77:1324–1335. doi: 10.2166/wst.2018.010. [DOI] [PubMed] [Google Scholar]
- 167.Afzal M.Z., Sun X.F., Liu J., Song C., Wang S.G., Javed A. Enhancement of ciprofloxacin sorption on chitosan/biochar hydrogel beads. Sci. Total Environ. 2018;639:560–569. doi: 10.1016/j.scitotenv.2018.05.129. [DOI] [PubMed] [Google Scholar]
- 168.Ahamad T., Chaudhary A.A., Naushad M., Alshehri S.M. Fabrication of MnFe2O4 nanoparticles embedded chitosan-diphenylureaformaldehyde resin for the removal of tetracycline from aqueous solution. Int. J. Biol. Macromol. 2019;134:180–188. doi: 10.1016/j.ijbiomac.2019.04.204. [DOI] [PubMed] [Google Scholar]
- 169.Ahamad T., Naushad M., Al-Shahrani T., Al-Hokbany N., Alshehri S.M. Preparation of chitosan based magnetic nanocomposite for tetracycline adsorption: Kinetic and thermodynamic studies. Int. J. Biol. Macromol. 2020;147:258–267. doi: 10.1016/j.ijbiomac.2020.01.025. [DOI] [PubMed] [Google Scholar]
- 170.Danalioglu S.T., Kerkez Kuyumcu O., Abdel Salam M., Bayazit S.S. Chitosan grafted SiO2-Fe3O4 nanoparticles for removal of antibiotics from water. Environ. Sci. Pollut. Res. 2018;25:36661–36670. doi: 10.1007/s11356-018-3573-y. [DOI] [PubMed] [Google Scholar]
- 171.Chen J., Ouyang J., Chen W., Zheng Z., Yang Z., Liu Z., Zhou L. Fabrication and adsorption mechanism of chitosan/Zr-MOF (UiO-66) composite foams for efficient removal of ketoprofen from aqueous solution. Chem. Eng. J. 2022;431:134045. doi: 10.1016/j.cej.2021.134045. [DOI] [Google Scholar]
- 172.Tang X., Huang Y., He Q., Wang Y., Zheng H., Hu Y. Adsorption of tetracycline antibiotics by nitrilotriacetic acid modified magnetic chitosan-based microspheres from aqueous solutions. Environ. Technol. Innov. 2021;24:101895. doi: 10.1016/j.eti.2021.101895. [DOI] [Google Scholar]
- 173.Rezaei H., Razavi A., Shahbazi A. Removal of Congo red from aqueous solutions using nano-Chitosan. Environ. Resour. Res. 2017;5:25–34. [Google Scholar]
- 174.Ulu A., Alpaslan M., Gultek A., Ates B. Eco-friendly chitosan/κ-carrageenan membranes reinforced with activated bentonite for adsorption of methylene blue. Mater. Chem. Phys. 2022;278:125611. doi: 10.1016/j.matchemphys.2021.125611. [DOI] [Google Scholar]
- 175.Vieira M.L.G., Esquerdo V.M., Nobre L.R., Dotto G.L., Pinto L.A.A. Glass beads coated with chitosan for the food azo dyes adsorption in a fixed bed column. J. Ind. Eng. Chem. 2014;20:3387–3393. doi: 10.1016/j.jiec.2013.12.024. [DOI] [Google Scholar]
- 176.Rathinam K., Singh S.P., Arnusch C.J., Kasher R. An environmentally-friendly chitosan-lysozyme biocomposite for the effective removal of dyes and heavy metals from aqueous solutions. Carbohydr. Polym. 2018;199:506–515. doi: 10.1016/j.carbpol.2018.07.055. [DOI] [PubMed] [Google Scholar]
- 177.Liu X., Zhang Y., Ju H., Yang F., Luo X., Zhang L. Uptake of methylene blue on divinylbenzene cross-linked chitosan/maleic anhydride polymer by adsorption process. Colloids Surf. A. 2021;629:127424. doi: 10.1016/j.colsurfa.2021.127424. [DOI] [Google Scholar]
- 178.Kalidason A., Kuroiwa T. Synthesis of chitosan–magnetite gel microparticles with improved stability and magnetic properties: A study on their adsorption, recoverability, and reusability in the removal of monovalent and multivalent azo dyes. React. Funct. Polym. 2022;173:105220. doi: 10.1016/j.reactfunctpolym.2022.105220. [DOI] [Google Scholar]
- 179.Abdul Mubarak N.S., Chuan T.W., Khor H.P., Jawad A.H., Wilson L.D., Sabar S. Immobilized Fe-Loaded Chitosan Film for Methyl Orange Dye Removal: Competitive Ions, Reusability, and Mechanism. J. Polym. Environ. 2021;29:1050–1062. doi: 10.1007/s10924-020-01949-8. [DOI] [Google Scholar]
- 180.Tanhaei B., Ayati A., Iakovleva E., Sillanpää M. Efficient carbon interlayed magnetic chitosan adsorbent for anionic dye removal: Synthesis, characterization and adsorption study. Int. J. Biol. Macromol. 2020;164:3621–3631. doi: 10.1016/j.ijbiomac.2020.08.207. [DOI] [PubMed] [Google Scholar]
- 181.Taher F.A., Kamal F.H., Badawy N.A., Shrshr A.E. Hierarchical magnetic/chitosan/graphene oxide 3D nanostructure as highly effective adsorbent. Mater. Res. Bull. 2018;97:361–368. doi: 10.1016/j.materresbull.2017.09.023. [DOI] [Google Scholar]
- 182.Abdulhameed A.S., Mohammad A.-T., Jawad A.H. Application of response surface methodology for enhanced synthesis of chitosan tripolyphosphate/TiO2 nanocomposite and adsorption of reactive orange 16 dye. J. Cleaner Prod. 2019;232:43–56. doi: 10.1016/j.jclepro.2019.05.291. [DOI] [Google Scholar]
- 183.Subramaniam S., Foo K.Y., Md Yusof E.N., Jawad A.H., Wilson L.D., Sabar S. Hydrothermal synthesis of phosphorylated chitosan and its adsorption performance towards Acid Red 88 dye. Int. J. Biol. Macromol. 2021;193:1716–1726. doi: 10.1016/j.ijbiomac.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 184.Rostamian M., Hosseini H., Fakhri V., Talouki P.Y., Farahani M., Gharehtzpeh A.J., Goodarzi V., Su C.-H. Introducing a bio sorbent for removal of methylene blue dye based on flexible poly(glycerol sebacate)/chitosan/graphene oxide ecofriendly nanocomposites. Chemosphere. 2022;289:133219. doi: 10.1016/j.chemosphere.2021.133219. [DOI] [PubMed] [Google Scholar]
- 185.Chen M., Bai Y., Liu J., Liu Y., Wang Z., Feng X. Adsorption properties of magnetic sodium ferrosilicate/carboxymethyl chitosan composite with more functional groups and surface negative potential. Sustain. Chem. Pharm. 2021;24:100519. doi: 10.1016/j.scp.2021.100519. [DOI] [Google Scholar]
- 186.Subramani S.E., Thinakaran N. Isotherm, kinetic and thermodynamic studies on the adsorption behaviour of textile dyes onto chitosan. Process Saf. Environ. Prot. 2017;106:1–10. [Google Scholar]
- 187.Zhang W., Huang T., Ren Y., Wang Y., Yu R., Wang J., Tu Q. Preparation of chitosan crosslinked with metal-organic framework (MOF-199)@aminated graphene oxide aerogel for the adsorption of formaldehyde gas and methyl orange. Int. J. Biol. Macromol. 2021;193:2243–2251. doi: 10.1016/j.ijbiomac.2021.11.056. [DOI] [PubMed] [Google Scholar]
- 188.Malek N.N.A., Jawad A.H., Ismail K., Razuan R., Alothman Z.A. Fly ash modified magnetic chitosan-polyvinyl alcohol blend for reactive orange 16 dye removal: Adsorption parametric optimization. Int. J. Biol. Macromol. 2021;189:464–476. doi: 10.1016/j.ijbiomac.2021.08.160. [DOI] [PubMed] [Google Scholar]
- 189.Shi Y., Song G., Li A., Wang J., Wang H., Sun Y., Ding G. Graphene oxide-chitosan composite aerogel for adsorption of methyl orange and methylene blue: Effect of pH in single and binary systems. Colloids Surf. A. 2022;641:128595. doi: 10.1016/j.colsurfa.2022.128595. [DOI] [Google Scholar]
- 190.Liu Y., Li L., Duan Z., You Q., Liao G., Wang D. Chitosan modified nitrogen-doped porous carbon composite as a highly-efficient adsorbent for phenolic pollutants removal. Colloids Surf. A. 2021;610:125728. doi: 10.1016/j.colsurfa.2020.125728. [DOI] [Google Scholar]
- 191.Zhang L., Sellaoui L., Franco D., Dotto G.L., Bajahzar A., Belmabrouk H., Bonilla-Petriciolet A., Oliveira M.L.S., Li Z. Adsorption of dyes brilliant blue, sunset yellow and tartrazine from aqueous solution on chitosan: Analytical interpretation via multilayer statistical physics model. Chem. Eng. J. 2020;382:122952. doi: 10.1016/j.cej.2019.122952. [DOI] [Google Scholar]
- 192.Schio R.R., Gonçalves J.O., Mallmann E.S., Pinto D., Dotto G.L. Development of a biosponge based on Luffa cylindrica and crosslinked chitosan for Allura red AC adsorption. Int. J. Biol. Macromol. 2021;192:1117–1122. doi: 10.1016/j.ijbiomac.2021.10.096. [DOI] [PubMed] [Google Scholar]
- 193.Saheed I.O., Oh W.-D., Suah F.B.M. Enhanced adsorption of acid Blue-25 dye onto chitosan/porous carbon composite modified in 1-allyl-3-methyl imidazolium bromide ionic liquid. Int. J. Biol. Macromol. 2021;183:1026–1033. doi: 10.1016/j.ijbiomac.2021.05.042. [DOI] [PubMed] [Google Scholar]
- 194.Tang T., Cao S., Xi C., Chen Z. Multifunctional magnetic chitosan-graphene oxide-ionic liquid ternary nanohybrid: An efficient adsorbent of alkaloids. Carbohydr. Polym. 2021;255:117338. doi: 10.1016/j.carbpol.2020.117338. [DOI] [PubMed] [Google Scholar]
- 195.Sahnoun S., Boutahala M. Adsorption removal of tartrazine by chitosan/polyaniline composite: Kinetics and equilibrium studies. Int. J. Biol. Macromol. 2018;114:1345–1353. doi: 10.1016/j.ijbiomac.2018.02.146. [DOI] [PubMed] [Google Scholar]
- 196.Daikh S., Ouis D., Benyoucef A., Mouffok B. Equilibrium, kinetic and thermodynamic studies for evaluation of adsorption capacity of a new potential hybrid adsorbent based on polyaniline and chitosan for Acetaminophen. Chem. Phys. Lett. 2022:139565. doi: 10.1016/j.cplett.2022.139565. in press. [DOI] [Google Scholar]
- 197.Pinheiro C.P., Moreira L.M.K., Alves S.S., Cadaval T.R.S., Jr., Pinto L.A.A. Anthocyanins concentration by adsorption onto chitosan and alginate beads: Isotherms, kinetics and thermodynamics parameters. Int. J. Biol. Macromol. 2021;166:934–939. doi: 10.1016/j.ijbiomac.2020.10.250. [DOI] [PubMed] [Google Scholar]
- 198.Turan B., Sarigol G., Demircivi P. Adsorption of tetracycline antibiotics using metal and clay embedded cross-linked chitosan. Mater. Chem. Phys. 2022;279:125781. doi: 10.1016/j.matchemphys.2022.125781. [DOI] [Google Scholar]
- 199.Jiang Q., Han Z., Li W., Ji T., Yuan Y., Zhang J., Zhao C., Cheng Z., Wang S. Adsorption properties of heavy metals and antibiotics by chitosan from larvae and adult Trypoxylus dichotomus. Carbohydr. Polym. 2022;276:118735. doi: 10.1016/j.carbpol.2021.118735. [DOI] [PubMed] [Google Scholar]
- 200.Almeida A.d.S.V.d., Mastelaro V.R., da Silva M.G.C., Prediger P., Vieira M.G.A. Adsorption of 17α-ethinylestradiol onto a novel nanocomposite based on graphene oxide, magnetic chitosan and organoclay (GO/mCS/OC): Kinetics, equilibrium, thermodynamics and selectivity studies. J. Water Process Eng. 2022;47:102729. doi: 10.1016/j.jwpe.2022.102729. [DOI] [Google Scholar]
- 201.Ranjbari S., Ayati A., Tanhaei B., Al-Othman A., Karimi F. The surfactant-ionic liquid bi-functionalization of chitosan beads for their adsorption performance improvement toward Tartrazine. Environ. Res. 2022;204:111961. doi: 10.1016/j.envres.2021.111961. [DOI] [PubMed] [Google Scholar]
- 202.Yang D., Li L., Chen B., Shi S., Nie J., Ma G. Functionalized chitosan electrospun nanofiber membranes for heavy-metal removal. Polymer. 2019;163:74–85. doi: 10.1016/j.polymer.2018.12.046. [DOI] [Google Scholar]
- 203.Li M., Zhao H., Lu Z.-Y. Porphyrin-based porous organic polymer, Py-POP, as a multifunctional platform for efficient selective adsorption and photocatalytic degradation of cationic dyes. Microporous Mesoporous Mater. 2020;292:109774. doi: 10.1016/j.micromeso.2019.109774. [DOI] [Google Scholar]
- 204.Wang B., Bai Z., Jiang H., Prinsen P., Luque R., Zhao S., Xuan J. Selective heavy metal removal and water purification by microfluidically-generated chitosan microspheres: Characteristics, modeling and application. J. Hazard. Mater. 2019;364:192–205. doi: 10.1016/j.jhazmat.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 205.Tang S., Yang J., Lin L., Peng K., Chen Y., Jin S., Yao W. Construction of physically crosslinked chitosan/sodium alginate/calcium ion double-network hydrogel and its application to heavy metal ions removal. Chem. Eng. J. 2020;393:124728. doi: 10.1016/j.cej.2020.124728. [DOI] [Google Scholar]
- 206.Zhang Z., He S., Zhang Y., Zhang K., Wang J., Jing R., Yang X., Hu Z., Lin X., Li Y. Spectroscopic investigation of Cu2+, Pb2+ and Cd2+ adsorption behaviors by chitosan-coated argillaceous limestone: Competition and mechanisms. Environ. Pollut. 2019;254:112938. doi: 10.1016/j.envpol.2019.07.106. [DOI] [PubMed] [Google Scholar]
- 207.Hu T., Zeng L., Li Y., Wu Y., Zhu Z., Zhang Y., Tian D., Gao C., Li W. Multifunctional chitosan non-woven fabrics modified with terylene carbon dots for selective detection and efficient adsorption of Cr(VI) Chem. Eng. J. 2022;432:134202. doi: 10.1016/j.cej.2021.134202. [DOI] [Google Scholar]
- 208.Zhu X., Tong J., Zhu L., Pan D. In situ growth of ZIF-8 on carboxymethyl chitosan beads for improved adsorption of lead ion from aqueous solutions. Int. J. Biol. Macromol. 2022;205:473–482. doi: 10.1016/j.ijbiomac.2022.02.120. [DOI] [PubMed] [Google Scholar]
- 209.Li B., Li M., Zhang P., Pan Y., Huang Z., Xiao H. Remediation of Cd (II) ions in aqueous and soil phases using novel porous cellulose/chitosan composite spheres loaded with zero-valent iron nanoparticles. React. Funct. Polym. 2022;173:105210. doi: 10.1016/j.reactfunctpolym.2022.105210. [DOI] [Google Scholar]
- 210.Kameda T., Honda R., Kumagai S., Saito Y., Yoshioka T. Adsorption of Cu2+ and Ni2+ by tripolyphosphate-crosslinked chitosan-modified montmorillonite. J. Solid State Chem. 2019;277:143–148. doi: 10.1016/j.jssc.2019.06.002. [DOI] [Google Scholar]
- 211.Lyu F., Yu H., Hou T., Yan L., Zhang X., Du B. Efficient and fast removal of Pb2+ and Cd2+ from an aqueous solution using a chitosan/Mg-Al-layered double hydroxide nanocomposite. J. Colloid Interface Sci. 2019;539:184–193. doi: 10.1016/j.jcis.2018.12.049. [DOI] [PubMed] [Google Scholar]
- 212.Malwal D., Gopinath P. Silica Stabilized Magnetic-Chitosan Beads for Removal of Arsenic from Water. Colloid Interface Sci. Commun. 2017;19:14–19. doi: 10.1016/j.colcom.2017.06.003. [DOI] [Google Scholar]
- 213.Marques Neto J.d.O., Bellato C.R., Silva D. Iron oxide/carbon nanotubes/chitosan magnetic composite film for chromium species removal. Chemosphere. 2019;218:391–401. doi: 10.1016/j.chemosphere.2018.11.080. [DOI] [PubMed] [Google Scholar]
- 214.Neeraj G., Krishnan S., Senthil Kumar P., Shriaishvarya K.R., Vinoth Kumar V. Performance study on sequestration of copper ions from contaminated water using newly synthesized high effective chitosan coated magnetic nanoparticles. J. Mol. Liq. 2016;214:335–346. doi: 10.1016/j.molliq.2015.11.051. [DOI] [Google Scholar]
- 215.Periyasamy S., Manivasakan P., Jeyaprabha C., Meenakshi S., Viswanathan N. Fabrication of nano-graphene oxide assisted hydrotalcite/chitosan biocomposite: An efficient adsorbent for chromium removal from water. Int. J. Biol. Macromol. 2019;132:1068–1078. doi: 10.1016/j.ijbiomac.2019.03.232. [DOI] [PubMed] [Google Scholar]
- 216.Nematidil N., Sadeghi M., Nezami S., Sadeghi H. Synthesis and characterization of Schiff-base based chitosan-g-glutaraldehyde/NaMMTNPs-APTES for removal Pb2+ and Hg2+ ions. Carbohydr. Polym. 2019;222:114971. doi: 10.1016/j.carbpol.2019.114971. [DOI] [PubMed] [Google Scholar]
- 217.Xiong Y., Xie L., Zhu L., Wang Y., Shan W., Lou Z., Cui J., Yu H. Superior adsorption of Re(VII) by anionic imprinted chitosan-silica composite: Adsorption performance, selectivity and mechanism study. J. Ind. Eng. Chem. 2022;108:344–355. doi: 10.1016/j.jiec.2022.01.013. [DOI] [Google Scholar]
- 218.Yang L., Luo X., Yan L., Zhou Y., Yu S., Ju H., Wang Y., Zhang L. Efficient selective adsorption of uranium using a novel eco-friendly chitosan-grafted adenosine 5′-monophosphate foam. Carbohydr. Polym. 2022;285:119157. doi: 10.1016/j.carbpol.2022.119157. [DOI] [PubMed] [Google Scholar]
- 219.Ding W., Zhang J., Liu Y., Guo Y., Deng T., Yu X. Synthesis of granulated H4Mn5O12/chitosan with improved stability by a novel cross-linking strategy for lithium adsorption from aqueous solutions. Chem. Eng. J. 2021;426:131689. doi: 10.1016/j.cej.2021.131689. [DOI] [Google Scholar]
- 220.Liu M., Zang Z., Zhang S., Ouyang G., Han R. Enhanced fluoride adsorption from aqueous solution by zirconium (IV)-impregnated magnetic chitosan graphene oxide. Int. J. Biol. Macromol. 2021;182:1759–1768. doi: 10.1016/j.ijbiomac.2021.05.116. [DOI] [PubMed] [Google Scholar]
- 221.Yang L., Huang C., Luo X., Zhang L., Ye Y., Jun H., Wang Y. Chitosan-based aerogel with anti-swelling for U(VI) adsorption from aqueous solution. Colloids Surf. A. 2021;630:127527. doi: 10.1016/j.colsurfa.2021.127527. [DOI] [Google Scholar]
- 222.Wang S., Wang H., Tang J., Chen Y., Wang S., Zhang L. Chitosan functionalized with N,N-(2-aminoethyl)pyridinedicarboxamide for selective adsorption of gold ions from wastewater. Int. J. Biol. Macromol. 2022;194:781–789. doi: 10.1016/j.ijbiomac.2021.11.125. [DOI] [PubMed] [Google Scholar]
- 223.Laureano-Anzaldo C.M., González-López M.E., Pérez-Fonseca A.A., Cruz-Barba L.E., Robledo-Ortíz J.R. Synthesis of silanized chitosan anchored onto porous composite and its performance in fixed-bed adsorption of Cr(VI) J. Environ. Chem. Eng. 2021;9:106353. doi: 10.1016/j.jece.2021.106353. [DOI] [Google Scholar]
- 224.Gamal A., Ibrahim A.G., Eliwa E.M., El-Zomrawy A.H., El-Bahy S.M. Synthesis and characterization of a novel benzothiazole functionalized chitosan and its use for effective adsorption of Cu(II) Int. J. Biol. Macromol. 2021;183:1283–1292. doi: 10.1016/j.ijbiomac.2021.05.080. [DOI] [PubMed] [Google Scholar]
- 225.Sharma G., Naushad M., Ala’a H., Kumar A., Khan M.R., Kalia S., Bala M., Sharma A. Fabrication and characterization of chitosan-crosslinked-poly(alginic acid) nanohydrogel for adsorptive removal of Cr(VI) metal ion from aqueous medium. Int. J. Biol. Macromol. 2017;95:484–493. doi: 10.1016/j.ijbiomac.2016.11.072. [DOI] [PubMed] [Google Scholar]
- 226.Chen Y., Tang J., Wang S., Zhang L. Ninhydrin-functionalized chitosan for selective removal of Pb(II) ions: Characterization and adsorption performance. Int. J. Biol. Macromol. 2021;177:29–39. doi: 10.1016/j.ijbiomac.2021.02.110. [DOI] [PubMed] [Google Scholar]
- 227.Zhuang S., Zhu K., Xu L., Hu J., Wang J. Adsorption of Co2+ and Sr2+ in aqueous solution by a novel fibrous chitosan biosorbent. Sci. Total Environ. 2022;825:153998. doi: 10.1016/j.scitotenv.2022.153998. [DOI] [PubMed] [Google Scholar]
- 228.Chen L., Tang J., Zhang X., Wang S., Ren Z. A novel benzothiazole modified chitosan with excellent adsorption capacity for Au(III) in aqueous solutions. Int. J. Biol. Macromol. 2021;193:1918–1926. doi: 10.1016/j.ijbiomac.2021.11.023. [DOI] [PubMed] [Google Scholar]
- 229.Huang H., Yang Q., Zhang L., Huang C., Liang Y. Polyacrylamide modified kaolin enhances adsorption of sodium alginate/carboxymethyl chitosan hydrogel beads for copper ions. Chem. Eng. Res. Des. 2022;180:296–305. doi: 10.1016/j.cherd.2022.02.030. [DOI] [Google Scholar]
- 230.Huang Y., Wu Y., Ding W., Sun Q., Hu C., Liu B., Liu H., Zheng H. Anion-synergistic adsorption enhances the selective removal of silver ions from complex wastewater by chitosan-coated magnetic silica core-shell nanoparticles. J. Cleaner Prod. 2022;339:130777. doi: 10.1016/j.jclepro.2022.130777. [DOI] [Google Scholar]
- 231.Ding Y., Liu D., Luo D., Sun X., Mei J., Wang S., Li Z. Rapid one-step preparation of a carboxymethyl chitosan gel with a novel crosslinker for efficient adsorption of Sr2+ Colloids Surf. A. 2022;641:128576. doi: 10.1016/j.colsurfa.2022.128576. [DOI] [Google Scholar]
- 232.Zeng H., Xu K., Wang F., Sun S., Li D., Zhang J. Adsorption of As(III) from aqueous solutions using MnO2 strengthened WTRs-chitosan beads made by homogenous method with freeze-drying. React. Funct. Polym. 2021;167:105016. doi: 10.1016/j.reactfunctpolym.2021.105016. [DOI] [Google Scholar]
- 233.Li H., Ji H., Cui X., Che X., Zhang Q., Zhong J., Jin R., Wang L., Luo Y. Kinetics, thermodynamics, and equilibrium of As(III), Cd(II), Cu(II) and Pb(II) adsorption using porous chitosan bead-supported MnFe2O4 nanoparticles. Int. J. Min. Sci. Technol. 2021;31:1107–1115. doi: 10.1016/j.ijmst.2021.10.004. [DOI] [Google Scholar]
- 234.Abugoch L.E., Tapia C., Villamán M.C., Yazdani-Pedram M., Díaz-Dosque M. Characterization of quinoa protein–chitosan blend edible films. Food Hydrocoll. 2011;25:879–886. doi: 10.1016/j.foodhyd.2010.08.008. [DOI] [Google Scholar]
- 235.Salam A., Lucia L.A., Jameel H. Synthesis, characterization, and evaluation of chitosan-complexed starch nanoparticles on the physical properties of recycled paper furnish. ACS Appl. Mater. Interfaces. 2013;5:11029–11037. doi: 10.1021/am403261d. [DOI] [PubMed] [Google Scholar]
- 236.Balea A., Monte M.C., Fuente E., Sanchez-Salvador J.L., Blanco A., Negro C. Cellulose nanofibers and chitosan to remove flexographic inks from wastewaters. Environ. Sci. Water Res. Technol. 2019;5:1558–1567. doi: 10.1039/C9EW00434C. [DOI] [Google Scholar]
- 237.Rahmaninia M., Rohi M., Hubbe M.A., Zabihzadeh S.M., Ramezani O. The performance of chitosan with bentonite microparticles as wet-end additive system for paper reinforcement. Carbohydr. Polym. 2018;179:328–332. doi: 10.1016/j.carbpol.2017.09.036. [DOI] [PubMed] [Google Scholar]
- 238.Todorova D., Lasheva V. Effect of Chitosan Addition during Paper-Making on Ageing Stability of Document Paper. Cellul. Chem. Technol. 2021;55:1083–1094. doi: 10.35812/CelluloseChemTechnol.2021.55.93. [DOI] [Google Scholar]
- 239.Ashori A., Harun J., Zin W.M., Yusoff M.N.M. Enhancing Dry-Strength Properties of Kenaf (Hibiscus cannabinus) Paper Through Chitosan. Polym. Plast. Technol. Eng. 2006;45:125–129. doi: 10.1080/03602550500373709. [DOI] [Google Scholar]
- 240.Kasim N.F.A., WIdris W.F., Abdullah A.H., Yusoh K., Ismail Z. The preparation of graphene ink from the exfoliation of graphite in pullulan, chitosan and alginate for strain-sensitive paper. Int. J. Biol. Macromol. 2020;153:1211–1219. doi: 10.1016/j.ijbiomac.2019.10.251. [DOI] [PubMed] [Google Scholar]
- 241.Khoushab F., Yamabhai M. Chitin research revisited. Mar. Drugs. 2010;8:1988–2012. doi: 10.3390/md8071988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Azharul Islam M., Tan Y.L., Atikul Islam M., Romić M., Hameed B.H. Chitosan–bleaching earth clay composite as an efficient adsorbent for carbon dioxide adsorption: Process optimization. Colloids Surf. A. 2018;554:9–15. doi: 10.1016/j.colsurfa.2018.06.021. [DOI] [Google Scholar]
- 243.Hsan N., Dutta P.K., Kumar S., Koh J. Arginine containing chitosan-graphene oxide aerogels for highly efficient carbon capture and fixation. J. CO2 Util. 2022;59:101958. doi: 10.1016/j.jcou.2022.101958. [DOI] [Google Scholar]
- 244.Mincke S., Asere T.G., Verheye I., Folens K., Vanden Bussche F., Lapeire L., Verbeken K., Van Der Voort P., Tessema D.A., Fufa F., et al. Functionalized chitosan adsorbents allow recovery of palladium and platinum from acidic aqueous solutions. Green Chem. 2019;21:2295–2306. doi: 10.1039/C9GC00166B. [DOI] [Google Scholar]
- 245.Borgohain R., Jain N., Prasad B., Mandal B., Su B. Carboxymethyl chitosan/carbon nanotubes mixed matrix membranes for CO2 separation. React. Funct. Polym. 2019;143:104331. doi: 10.1016/j.reactfunctpolym.2019.104331. [DOI] [Google Scholar]
- 246.Kamran U., Park S.-J. Tuning ratios of KOH and NaOH on acetic acid-mediated chitosan-based porous carbons for improving their textural features and CO2 uptakes. J. CO2 Util. 2020;40:101212. doi: 10.1016/j.jcou.2020.101212. [DOI] [Google Scholar]
- 247.Rehman A., Park S.-J. From chitosan to urea-modified carbons: Tailoring the ultra-microporosity for enhanced CO2 adsorption. Carbon. 2020;159:625–637. doi: 10.1016/j.carbon.2019.12.068. [DOI] [Google Scholar]
- 248.Hsan N., Dutta P.K., Kumar S., Das N., Koh J. Capture and chemical fixation of carbon dioxide by chitosan grafted multi-walled carbon nanotubes. J. CO2 Util. 2020;41:101237. doi: 10.1016/j.jcou.2020.101237. [DOI] [Google Scholar]