Abstract
Imidazoquinoline derivatives (IMDs) and related compounds function as synthetic agonists of Toll-like receptors 7 and 8 (TLR7/8) and one is FDA approved for topical antiviral and skin cancer treatments. Nevertheless, these innate immune system-activating drugs have potentially much broader therapeutic utility; they have been pursued as antitumor immunomodulatory agents and more recently as candidate vaccine adjuvants for cancer and infectious disease. The broad expression profiles of TLR7/8, poor pharmacokinetic properties of IMDs, and toxicities associated with systemic administration, however, are formidable barriers to successful clinical translation. Herein, we review IMD formulations that have advanced to the clinic and discuss issues related to biodistribution and toxicity that have hampered the further development of these compounds. Recent strategies aimed at enhancing safety and efficacy, particularly through the use of bioconjugates and nanoparticle formulations that alter pharmacokinetics, biodistribution, and cellular targeting, are described. Finally, key aspects of the biology of TLR7 signaling, such as TLR7 tolerance, that may need to be considered in the development of new IMD therapeutics are discussed.
Keywords: Toll-like receptors, TLR7 agonists, immunomodulators, cancer, infectious disease, bioconjugation, vaccine adjuvants, nanoparticles
1. Introduction
In the mid-1980s, 3M Pharmaceuticals discovered that imidazoquinoline derivatives (IMDs) could possess antiviral properties [1]. At that time, the functions of Toll-like receptors (TLRs) were not defined and the antiviral feature of IMDs was not known to be related to TLR7/8 activation [2]. The first patent filing on this class of compounds focused on topical administration and detailed their efficacy in a guinea pig model of herpes simplex virus [3]. One of the first IMDs reported, imiquimod (also known as R837; Fig. 1A), progressed through clinical trials and was approved by the United States Food and Drug Administration (FDA) in 1997 for the topical treatment of genital and perianal warts and actinic keratosis (Aldara, 5%). It has since been approved for topical treatment of basal cell carcinoma [4]. Following the success of R837, a library of more potent and selective IMDs was developed and tested in preclinical studies and clinical trials [5,6]. A prominent compound in this class is resiquimod (R848; Fig. 1A), which was shown to be 100 times more potent than R837 in stimulating TLR7 [7]. Nevertheless, R848 has followed a more difficult path to the clinic, with multiple human trials in genital herpes and hepatitis C yielding no positive outcomes [8]. These disappointing results led 3M to discontinue development of R848 and license it to Spirig Pharma, which conducted clinical trials testing R848 on basal cell carcinoma and actinic keratosis without success [9]. Spirig Pharma was acquired by Galderma, and R848 has since received orphan status from the European Medicines Agency and is in continued clinical development for the treatment of cutaneous T cell lymphoma [10].
Figure 1. Representative structures of widely used synthetic TLR7/8 agonists.
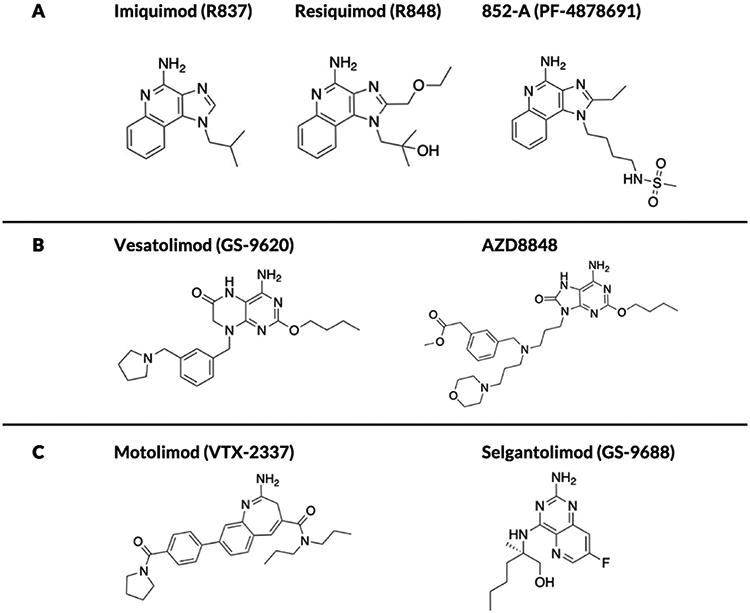
(A) Imidazoquinoline derivatives (IMDs) (B) pteridinone-based (GS-9620) and 8-oxoadenine (AZD-8848) derivatives (C) TLR8-specific benzazepine (VTX-2337) and pyrimidine (GS-9688) analogues.
The transition from treating viral infections to malignant tumors using IMDs was bolstered by several mechanistic insights into the biology of these molecules as activators of TLR7/8 [11]. In addition to inducing an antiviral state through secretion of type I interferons (IFNs), synthetic TLR7/8 agonists trigger acute inflammation and stimulate adaptive immunity by activating the nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) pathway [12]. This mechanistic rationale led compounds such as 852-A (Fig. 1A) to be tested for treatment of hematological malignancies (e.g., chronic lymphocytic leukemia) and solid tumors (e.g., ovarian, cervical, and breast cancers) [13]. In fact, 852-A and R848 generated sufficient excitement that, in 2008, they were both featured on the National Cancer Institute’s list of 20 agents with the highest potential for treating cancer [14]. Nevertheless, while potent immune activation was a consistent feature of the 852-A and R848 trials, severe adverse events associated with on-target, off-tumor activity hindered attempts to take these compounds from bench to bedside [15]. In parallel, mechanistic insights into the impact of TLR signaling on T cell immunity prompted studies focused on the use of IMDs as allergy and asthma treatments with the goal of skewing pathological Th2 immune responses toward a non-pathologic Th1 functional profile [16]. Promising results were obtained in large animal models and preliminary clinical work, but the toxicity of IMDs once again dampened success and forced the field to address systemic side effects using approaches rooted in medicinal chemistry, formulation, prodrug design, and drug delivery [17].
Despite the challenges described above, there is maintained clinical interest in the development of IMDs and related synthetic immune agonists, which is driven by significant progress in understanding innate immune sensors and their link to the adaptive immune system [18]. Crystal structures of TLR8 and TLR7 both with and without ligands have recently been elucidated, enabling structure-based compound development aimed toward enhanced receptor specificity [19]. These advances are especially important because R837 has been shown to have immune effects independent of TLR7/8, which could hamper attempts to reduce its off-target toxicity [20]. Today, in response to the multitude of toxicity-induced failures, research focus is moving toward localized delivery approaches, especially in the context of cancer, and prodrug or delivery formulations aiming to target IMDs to desired tissues and/or cells [21]. Here, we review preclinical and clinical studies of IMDs and related compounds, highlighting new strategies to overcome the safety challenges and address early clinical failures.
2. TLR7/8 biology and cellular implications of downstream effects
Pioneering work by Akira and others laid the foundation of TLR7/8 biology, and demonstrated the structural basis for recognition of guanosine- and uridine-rich viral single-stranded RNA (the likely natural ligands for these TLRs) [22-24]. We provide here a brief overview of the currently known signaling pathways and immune effector modules downstream of TLR7/8 agonists that will allow for an appreciation of the complexities involved and highlight the important players—the cells and proteins in the immune cascade—that will be discussed in later sections focused on preclinical and clinical studies.
2.1. TLR7/8 signaling pathways & downstream effects
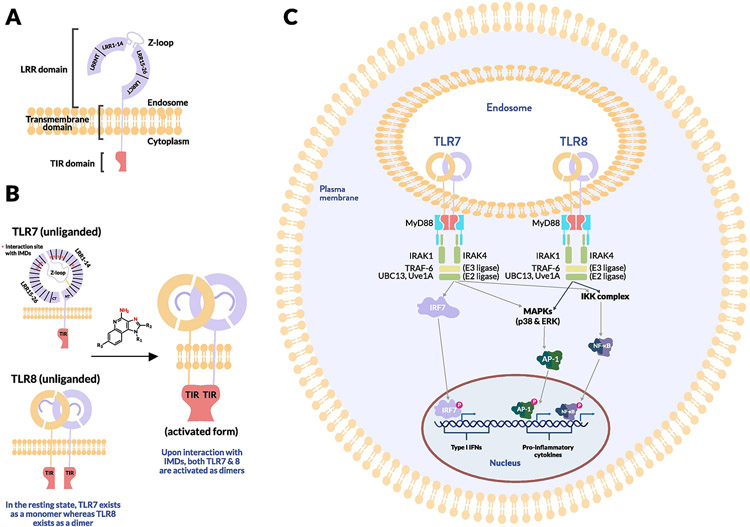
The TLRs are a family of 13 type 1 transmembrane proteins expressed both by tissue cells and immune cells that function as pattern recognition receptors (PRRs); that is, they identify conserved molecular structures on microbes or molecules released by dying cells and thereby comprise a major form of innate immune sensing [25]. TLRs share a common general structure: The N-terminal ectodomain consists of leucine-rich repeats (LRRs), formed in a horseshoe shape, followed by a single transmembrane domain and a cytosolic Toll-interleukin-1 receptor (TIR) domain [26] (Fig. 2A). TLRs dimerize upon binding their cognate ligand (Fig. 2B), leading to conformational changes that allow for the recruitment of adapter molecules and initiating signaling cascades that ultimately induce transcription of inflammatory mediators [27]. In the case of TLR7/8, dimerization initiates the TIR signaling cascade, which results in association with the adaptor protein myeloid differentiation primary response 88 (MyD88) at its carboxy terminus [28]. MyD88 also has an N-terminal death domain that recruits and associates with similar death domains present on two serine-threonine protein kinases: IL-1-receptor associated kinase (IRAK4) and IRAK1 [29] (Fig. 2C).
Figure 2. TLR7/8 signaling pathways and downstream immune-effector modules.
(A) Representative structure of TLR7/8 receptor. (B) Schematic of TLR7/8 receptor in the resting and activated states. (C) TLR7/8 signaling pathways from dimerization of the receptors to activation of the 3 transcriptional factors i.e., AP-1, NF-κB and IRF7.
The above complex consisting of MyD88, IRAK4, and IRAK1 recruits the enzyme tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6) [30]. TRAF-6 is an E3 ubiquitin ligase that, in combination with UBC13 (an E2 ubiquitin ligase) and Uve1A (a cofactor), initiates polymerization of ubiquitin via K63 linkages [31]. This polymerized scaffold recruits a signaling complex, resulting in activation of the AP-1 family transcription factors that transcribe cytokine genes such as TNF-α. The same scaffold also leads to the phosphorylation of the inhibitor of κB (IκB), the protein responsible for keeping NF-κB in the cytoplasm. This action results in the degradation of IκB, which releases NF-κB into the nucleus where it drives transcription of genes encoding proinflammatory cytokines such as IL-6 and TNF-α, resulting in a state of acute inflammation [32] (Fig. 2C).
In addition, IRAK1, as part of the IRAK complex, can physically associate with interferon regulatory factor 7 (IRF7), which is highly expressed in specific cell subsets such as plasmacytoid dendritic cells [33]. Phosphorylated IRF7 enters the nucleus to induce expression of type I IFNs, inducing an antiviral state (Fig. 2C).
2.2. Cell types responding to TLR7/8 ligands
Although the immune-effector modules of TLR7/8 activation can be broadly classified into “acute inflammation” and “antiviral state,” the downstream effects of TLR7/8 activation vary by cell type. Table 1 summarizes the current understanding of TLR7/8 expression in different cells across species [34-37]. Defining TLR expression patterns has been confounded by a lack of reliable antibodies for TLRs, conflicting results with different primer sequences used in reverse transcription (RT)-PCR measurements, and species-specific differences in TLR7/8 expression across cell types. Additionally, similar cell types might express different levels of TLR7/8 in different tissues, and this pattern of expression can be further altered by the activation, maturation or differentiation of the cells [38]. Under certain circumstances, expression may be induced even in cells with low basal TLR7 levels, in particular immune cells such as macrophages and monocytes [39]. Most studies have focused on quantifying TLR7/8 expression in immune cells and less is known about expression levels in other cell subsets, such as epithelial, endothelial or hematopoietic cells.
Table 1:
TLR7/8 expression levels and downstream effects
| Species | Sub-classification | TLR7/8 expression | Downstream effects (autocrine) |
Response | References | |
|---|---|---|---|---|---|---|

|
mouse | CD11c− Ly6c+ | 7++ : 8− | type I IFNs | anti-viral state | [37] [39] [43] [50] [70] |
| macaque | CD123+ | |||||
| human | CD123+ | |||||

|
mouse | DN | 7++ : 8++ | pro-inflammatory cytokines and chemokines including TNF-α, IL-6, IL-12 and MCP-1 | acute inflammation, maturation of DCs and expression of costimulatory molecules for priming T cells | [36] [37] [39] [44] [45] |
| CD4+ | ||||||
| CD8α+ | 7+/− : 8++ | |||||
| human | CD11c+x | 7+ : 8+ | ||||

|
mouse | GR1+Ly6chigh | 7++ : 8++ | activation of FcγR, differentiation into Mφ and DCs, secretion of pro-inflammatory cytokines such as IL-12 | acute inflammation | [36] [37] [39] [52] [61] |
| human | CD14+ | 7+/− : 8++ | ||||
|
mouse | RAW264.7 | 7++ : 8++ | pro-inflammatory cytokines including TNF-α and IL-12 | M1 type polarization effects | [37] [39] [62] |
| Bone-marrow derived macrophages | ||||||
|
mouse | Ly6G+ CD11b+ | 7+ : 8? | including IL-8, release of reactive oxygen species | acute inflammation | [56] [57] [63] [64] |
| human | CD62L+ CD16+ | 7+/− : 8++ | ||||

|
mouse | CD4+ | 7+: 8+* | pro-inflammatory cytokine secretion, suppression of T reg activity | Proliferation and IL-2 production, Shift toward Th1 response | [53] [54] |
| human | ||||||
|
mouse | CD19+ or CD20+ | 7++ : 8− | increased proliferation, antibody secretion, cytokine production such as TNF-α and IL-6 | humoral response | [69] [70] [72] [73] [74] |
| macaque | ||||||
| human |
a particular subset of CD4 T cells which also express CD25 referred to as regulatory T cells (Tregs) express TLR8
Dendritic cells (DCs) are broadly classified into conventional/myeloid DCs (cDCs), which express CD11c, or plasmacytoid DCs (pDCs), which do not express CD11c [40]. In humans, cDCs express TLR8 mRNA, but their expression of TLR7 is still under debate [41]. In mice, cDCs can be further subdivided into CD4+, CD8+, or double negative cells, of which only CD8α+ DCs lack TLR7 expression and fail to respond to synthetic TLR7 ligands [42]. pDCs, unlike cDCs, express high levels of TLR7 mRNA but lack TLR8 expression [43].
Upon TLR7/8 ligation in the presence of antigens, cDCs undergo multiple functional changes, including maturation and expression of co-stimulatory markers, such as CD80 and CD86, and secretion of proinflammatory cytokines, including IL-12, TNF-α, and IL-6 [44]. The migration of DCs to draining lymph nodes (dLNs) to stimulate naïve T cells is triggered by TLR7/8-induced downregulation of inflammatory chemokine receptors such as CCR6 in concert with upregulation of receptors for lymphoid chemokines such as CCR7 [45]. Activation of naïve T cells by DCs is contingent on receiving three signals: the first is provided by binding of the T cell receptor (TCR) to antigenic peptide presented by the major histocompatibility complexes (MHCs) present on DCs; the second signal is provided by co-stimulatory molecules such as CD80, CD86, or CD40, which trigger CD28 expression on naïve T cells; and the third signal is provided by cytokines, especially IL-12, which have further downstream effects on multiple immune subsets [46]. For example, increased IL-12 levels lead to IFN-γ secretion by T cells and natural killer (NK) cells, further amplifying the immunological cascade [47-49]. Thus, T cell activation depends upon the class of MHC that presents the antigenic peptide, the co-stimulatory environment, and the cytokine milieu [50].
Type 1 IFNs are one of the key products of TLR7/8 activation. In both humans and mice, upon TLR7 ligation, pDCs secrete large amounts of type I IFNs (IFN-α and IFN-β) [51]. Although ligands for other innate immune receptors such as TLR9 and more recently the STimulator of Interferon Genes (STING) have been explored for their capacity to induce type I IFNs [52,53], targeting TLR7/8 with synthetic agonist drugs may have some advantages over these other innate immune receptors. For example, the substantial difference in cellular distribution of TLR9 between rodents and humans and the rapid degradation of the most common cyclic-dinucleotide STING agonists by circulating and cell-bound enzymes are but a few of the limitations that widen the appeal of TLR7/8 agonists as potent immune modulators. The downstream effects of this type 1 IFN production are discussed in disease-specific contexts in Section 3.
Beyond DCs (arguably the most important cell type for TLR7/8 agonists), other innate immune cells such as monocytes, macrophages, neutrophils, mast cells, and eosinophils also express TLR7/8 and secrete a host of cytokines and chemokines upon ligation by synthetic agonists, as shown in Table 1 [54-63].
One thought-provoking aspect of the cellular effects of TLR7/8 ligation is the duality of direct and indirect immune processes [64]. Jakubzick and coworkers demonstrated that, in the mouse lung microenvironment, CD11b+ DCs need to be directly activated by TLR7 to efficiently cross-present antigens, whereas CD103+ (CD8α+) DCs require TLR3 activation [65]. This observation further supports theories by Reis e Sousa and colleagues on the requirement for direct DC activation to efficiently prime T cell activity [66]. Multiple studies, however, have indicated a role for indirect DC activation in priming the adaptive immune system. For example, type I IFN secreted from pDCs has been shown to activate cross-presenting CD8α+ DCs [64,67]. Apart from cellular immunity, B cells also express TLR7; ligation by synthetic TLR7 agonists has been shown to enhance activation of multiple B cell subsets [68-73].
2.3. Structure-activity relationships of IMD TLR7/8 agonists
The biological effects of these TLR7/8 agonists are heavily dependent on their chemical structures. Most of the structure–activity relationship (SAR) studies on IMDs were performed before the crystal structures of liganded TLR8 and TLR7 were elucidated in 2013 and 2016, respectively [74,75]. Moreover, in early SAR studies, Gerster and colleagues measured in vitro production of IFN-α in human peripheral blood mononuclear cells (hPBMCs) as a surrogate biomarker for potency (TLR7-specific bioassays were not available at the time) [76]. Nevertheless, important insights were gained from these earlier studies. As shown in Fig. 3A, the major sites for derivatization of the IMD core have been the N-1, C-2, C-4 amine, and the C-7 position. These studies showed that attachment of long alkyl chains or bulky substituents at N-1 or C-2 reduced IFN production, whereas short-chain alkyl substituents at C-2 and short hydroxyl chains at N-1 enhanced IFN production. Most importantly, the C-4 amine was shown to be a key requirement for activity; all substitutions at this position completely abrogated IFN-α production. Moreover, most substitutions on the aryl ring were not tolerated except at the C-7 position. David and colleagues synthesized a library of more than 50 compounds to further demonstrate the increased potency obtained via substituents at N-1 and C-2 using both IFN-α production from hPBMCs and the current standard HEK-TLR7/8 reporter gene assay [77]. The lead compounds were shown to be exceptionally potent inducers of cytokine production in human newborn and adult leukocytes, and the crystal structures generated for the liganded TLRs validated the positive effects of the N-1 and C-2 substituents [78]. Ferguson and colleagues showed improved potency with C-7 methoxy carbonyl derivatives, hypothesizing that this result could be due to inflammasome activation [79]. The same group further studied the effects of C-2 and N-1 substituents on the C-7 substituted scaffold and showed that TLR8 activity correlated with a hydrogen bond donor at the N-1 position, with compounds that contained a terminal amino group showing the highest potency [80,81]. These studies followed work by David and colleagues that showed quinoline amine-based derivatives lacking the imidazole groups were more potent and selective TLR8 agonists, with the terminal amino substituent extending into a pocket within the TLR8 ectodomain, forming hydrogen bonds with glycine 351 [82]. The same group explored IMD-based dimers (linked at N-1), trimers, and hexamers with improved potency that were validated in vivo in vaccine studies [83,84]. Extensive SAR studies on imidazopyridines, thiazoquinolines, furopyridines, and furoquinolines have been conducted by the David group in search of potent and specific TLR7 and TLR8 agonists [85-88].
Figure 3. SAR studies on synthetic TLR7/8 agonists.
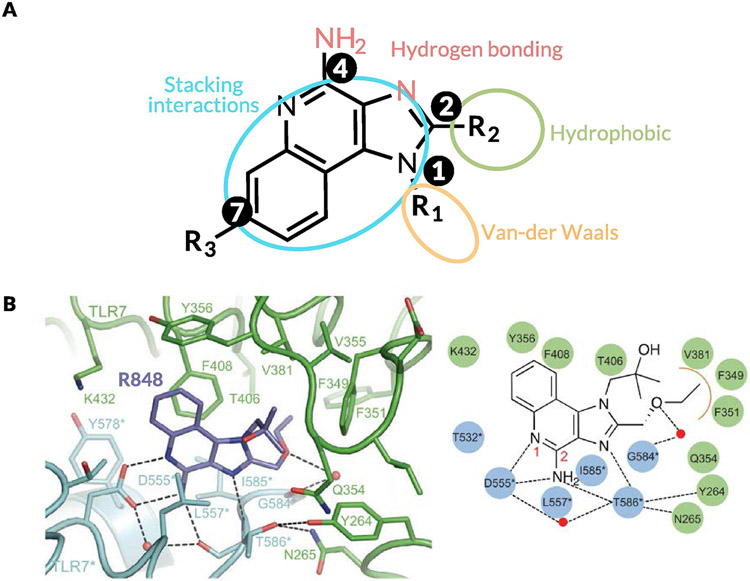
(A) Structure of IMDs indicating the sites at which substitutions were studied for improving potency. (B) Crystal structure of TLR7 bound to synthetic TLR7/8 agonist R848 highlighting the important interactions between the receptor and synthetic ligand.
The elucidation of the crystal structures of liganded TLR8 and TLR7 by Shimizu and colleagues, and the resulting identification of key interactions between the ligands and the receptors, has paved the way for the more targeted design of potent TLR7/8 agonists (Fig. 3B) [89]. For TLR7, the effects of N-1 substituents depended on van der Waals contacts with the loop region of LRR11 [90]. The pocket formed by phenylalanine 349, phenylalanine 351, valine 381, and phenylalanine 408 interacts with C-2 substituents via hydrophobic interactions (Fig. 3B). This interaction was particularly essential for TLR7 activity because R837 lacks the C-2 substituent and demonstrates weak TLR7 agonism. Similarly, for TLR8 agonism, stacking interactions or hydrogen bonds with phenylalanine 405 and aspartic acid 543 are critical components that need to be conserved in the ligand structure [91]. These insights have led to the development of synthetic ligands with higher potency and represent a key milestone on the path to TLR7/8 agonism for improved therapeutic outcomes [92,93].
Although we focus here on SAR studies of IMDs because they are the most studied, other classes of synthetic TLR7/8 agonists that expand upon nucleobase and ribonucleoside analogs include adenine-, guanosine-, and pteridinone-based derivatives in addition to benzazepines, benzonaphthyridines, and pyrrolopyrimidines [94-98]. Each of these series of agonists, particularly 8-oxo-adenine derivatives, have been expanded significantly via SAR studies to generate lead compounds that are in clinical trials [99-105].
3. Preclinical and Clinical Development of TLR7/8 agonists
In this section, we review preclinical and clinical studies using synthetic TLR7/8 agonists and focus on the clinical results, exploring in detail some of the unexpected outcomes and the hypotheses underlying these responses. Summaries of all of the compounds discussed, organized in order of progress toward clinical approval, are provided in Tables 2 and 3. Additionally, the compounds are classified by application into four areas: (1) skin conditions, the most widespread, with the FDA-approved imiquimod (R837) now having multiple generic formulations [106]; (2) advanced cancers, wherein these molecules serve as tumor immunomodulators and adjuvants; (3) infectious diseases, as antiviral agents, and vaccine adjuvants; and (4) respiratory ailments, as bronchodilators and anti-inflammatories.
Table 2:
List of synthetic TLR7/8 agonists in different stages of clinical development
| Active | Drug Name | Company Name | Therapy Area | Development Stage | Route of Administration | Target |
|---|---|---|---|---|---|---|
| 1 | Imiquimod | 3M Pharnaceuticals | Skin conditions, Infectious Diseases, Advanced Cancers | Marketed | Topical | TLR7 |
| 2 | Resiquimod (R-848) | Galderma (Originally 3M Pharmaceuticals) | Skin conditions, Infectious Diseases, Advanced Cancers | Phase II | Topical, Oral, Intra-tumoral | TLR7/8 |
| 3 | Motolimod (VTX-2337) | Bristol-Myers Squibb Co (previously VentiRx Pharmaceuticals) | Advanced Cancers | Phase II | Subcutaneous, Intra-tumoral | TLR8 |
| 4 | Selgantolimod (GS-9688) | Gilead Sciences Inc | Infectious Diseases | Phase II | Oral | TLR8 |
| 5 | NKTR-262 | Nektar Therapeutics | Advanced Cancers | Phase II | Intra-tumoral | TLR7/8 |
| 6 | RG-7854 (RO 7020531) | F. Hoffmann-La Roche Ltd | Infectious Diseases | Phase II | Oral | TLR7 |
| 7 | DSP-0509 | Sumitomo Dainippon Pharma Co Ltd | Advanced Cancers | Phase II | Intravenous | TLR7 |
| 8 | BDB-001 | Seven and Eight Biopharmaceuticals Corp | Advanced Cancers | Phase I | Intravenous | TLR7/8 |
| 9 | BDC-1001 | Bolt Biotherapeutics Inc | Advanced Cancers | Phase I | Intravenous | TLR7/8 (HER2+ cells) |
| 10 | LHC-165 | Novartis AG | Advanced Cancers | Phase I | Intra-tumoral | TLR7 |
| 11 | SHR-2150 | Jiangsu Hengrui Medicine Co Ltd | Advanced Cancers | Phase I | Oral | TLR7 |
| 12 | JNJ-4964 (TQ-A3334) | Johnson & Johnson, Chia Tai Tianqing Pharmaceutical Group Co Ltd | Infectious Diseases, Advanced Cancers | Phase I | Oral | Not specified |
| 13 | Vesatolimod (GS-9620) | Gilead Sciences Inc | Infectious Diseases | Phase I | Oral | TLR7 |
| 14 | RO-7119929 | F. Hoffmann-La Roche Ltd | Advanced Cancers | Phase I | Oral | TLR7 |
| 15 | DN-1508052 | Shanghai De Novo Pharmatech Co Ltd | Advanced Cancers | Phase I | Subcutaneous | TLR8 |
| 16 | VTX-1463 | Bristol-Myers Squibb Co (previously VentiRx Pharmaceuticals) | Respiratory Ailments | Phase I | Intra-nasal | TLR8 |
| 17 | BNT-411 (SC1) | BioNTech SE | Advanced Cancers | IND/CTA Filed | Intravenous | TLR7 |
| 18 | APR-003 | Apros Therapeutics | Advanced Cancers | IND/CTA Filed | Oral | TLR7 |
| Inactive/Discontinued | ||||||
| 1 | Bropirimine | Pfizer Inc | Advanced Cancers | Discontinued/Inactive (Phase III) | Oral | TLR7 |
| 2 | PF-4878691 (852-A) | Pfizer | Infectious Diseases, Advanced Cancers | Discontinued/Inactive (Phase II) | Intravenous, subcutaneous, Oral | TLR7 |
| 3 | GSK-2245035 | GlaxoSmithKline Plc | Respiratory Ailments | Discontinued/Inactive (Phase II) | Intra-nasal | TLR7 |
| 4 | RG-7795 (ANA 773, RO 6864018) | F. Hoffmann-La Roche Ltd | Infectious Diseases, Advanced Cancers | Discontinued/Inactive (Phase II) | Oral | TLR7 |
| 5 | Epitirimod (R-851) | Takeda (Originally 3M Pharnaceuticals) | Skin conditions | Discontinued/Inactive (Phase II) | Topical | TLR7 |
| 6 | DSP-3025 (AZD-8848) | AstraZeneca Plc, Sumitomo Dainippon Pharma Co Ltd | Respiratory Ailments | Discontinued/Inactive (Phase II) | Intra-nasal | TLR7 |
| 7 | Sotirimod (R-850, S-30594) | Meda AB | Skin conditions | Discontinued/Inactive (Phase II) | Topical | TLR7 |
| 8 | Telratolimod (3M-052, MEDI-9197) | MedImmune, AstraZeneca Plc | Advanced Cancers | Discontinued/Inactive (Phase I) | Intra-tumoral | TLR7/8 |
| 9 | Isatoribine (ANA-245) | F. Hoffmann-La Roche Ltd | Infectious Diseases | Discontinued/Inactive (Phase I) | Intravenous, Oral | TLR7 |
| 10 | Loxoribine | Johnson & Johnson | Advanced Cancers | Discontinued/Inactive (Phase I) | intramuscular | TLR7 |
| 11 | ANA-971 | F. Hoffmann-La Roche Ltd | Infectious Diseases | Discontinued/Inactive (Phase I) | Oral | TLR7 |
| 12 | ANA-975 | Novartis AG | Infectious Diseases | Discontinued/Inactive (Phase I) | Oral | TLR7 |
| 13 | RG-7863 (RO6870868) | F. Hoffmann-La Roche Ltd | Infectious Diseases | Discontinued/Inactive (Phase I) | Not specified | TLR7 |
Table 3 :
List of synthetic TLR7/8 agonists at the pre-clinical stage
| Drug Name | Formulation Approach | Company Name | Therapy Area | Route of Administration | Target |
|---|---|---|---|---|---|
| ALT-702 | depot-forming peptide | Altimmune Inc | Advanced Cancers | Intra-tumoral | TLR7/8 |
| GS-986 | orally bioavailable small molecule | Gilead Sciences Inc | Infectious Diseases | Oral | TLR7 |
| KUP-101 | liposomal formulation | Kupando GmbH | Advanced Cancers | Intravenous | TLR4/7 |
| PRTX-007 | orally bioavailable small molecule | Primmune Therapeutics Inc | Infectious Diseases, Advanced Cancers | Oral | TLR7 |
| PRX-034 | orally bioavailable small molecule | Primmune Therapeutics Inc | Advanced Cancers | Oral | TLR7 |
| S-34240 | cream | Pfizer Inc (previously 3M Pharmaceuticals) | Skin Conditions | Topical | TLR7 |
| TRANSCON | sustained release via cleavable linker | Ascendis Pharma | Advanced Cancers | Intra-tumoral | TLR7/8 |
| SBT-6050 | antibody-drug conjugate (IIER2) | Silverback Therapeutics Inc | Advanced Cancers | Intravenous | TLR8 (HER2+ cells) |
| SBT-6290 | antibody-drug conjugate (Nektin4) | Silverback Therapeutics Inc | Advanced Cancers | Intravenous | TLR8 |
| ZM-TLR8 agonist | small molecule with liver-targeting moiety | Zheming Biopharma | Infectious Diseases | Not specified | TLR8 |
| VX-001 | sustained release platform | Vaccex | Advanced Cancers | Intra-tumoral | TLR7/8 |
| MBS-8 | micellar formulation | MonTa Biosciences ApS | Advanced Cancers | Intravenous | TLR7 |
| APR-002 | small molecule with liver-targeting moiety | Apros Therapeutics | Infectious Diseases | Oral | TLR7 |
| SNAPvax | self-assembling peptide nanoparticle | Avidea Technologies | Advanced Cancers | Intravenous | TLR7/8 |
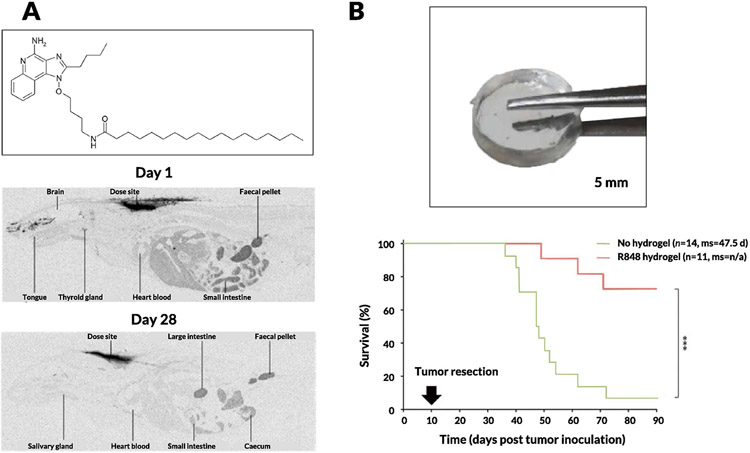
| R848-HA | hyaluronic acid (HA) hydrogel scaffold | STIMIT Technologies | Advanced Cancers | Intra-tumoral (intra-operative) | TLR7/8 |
3.1. Topical therapies for skin conditions
Topical administration of small molecule TLR7/8 agonists formulated as creams or gels overcomes the issues of poor circulation half-life and systemic toxicity that limit the systemic administration of these compounds [107]. Upon topical administration, TLR7/8 agonists induce significant accumulation of pDCs at the treatment site [108]. As discussed previously, pDCs secrete large amounts of type I IFNs in response to TLR7 activation, setting in motion a process that creates an antiviral state in the tissue. The levels of type I IFN-inducible products such as 2'-5'-oligoadenylate synthase (2'-5'-AS) and Mx proteins are increased in the affected area [109]. These products play a crucial role in inhibiting viral replication and survival. For example, 2'-5'-AS is an antiviral enzyme that counteracts viral attack by degrading viral RNA [110]. In addition, TLR7/8 activation causes Langerhans cells (LCs), a type of DCs resident in the epidermis, to differentiate into mature APCs, resulting in the generation of antigen-specific T cells [111]. Finally, the release of proinflammatory cytokines, such as IL-6 and TNF-α, upon NF-κB activation lead to increased NK cell activity and macrophage activation that furthers cytokine release, nitric oxide secretion, and B cell proliferation [112].
The first clinical application of synthetic TLR7/8 agonists was for the treatment of human papillomavirus (HPV)-associated genital and perianal warts. The standard treatment—injection of IFN-α—was expensive but also short-lived, associated with multiple side effects, and mostly unable to prevent recurrence [113]. These warts result from proliferation of keratinocytes infected with HPV and are difficult to treat because they activate basal keratinocytes without activating LCs, the latter of which are necessary for the generation of virus-specific T cells [114]. Topical administration of R837 (imiquimod) was shown to activate LCs, which migrate to the lymph nodes and prime HPV-specific T cells with both cytotoxic and memory phenotypes [115]. Two pivotal phase III studies showed that patients treated with R837 had over 75% reduction in wart area [116,117]. Side effects were minimal, with the most common being erythema at the application site, which correlated with mRNA expression of TNF-α, IFN-γ, and MCP-1. Skin biopsies showed decreases in CD1a mRNA in LCs, indicating activation and migration, as well as upregulation of IFN-γ, IL-2, IL-12p40, CD4, and CD8 mRNA in R837-treated patients. Wart regression was correlated with a decrease in viral load, as shown by decreases in HPV DNA and HPV E7 and L1 mRNA. Based on these results, R837 received FDA approval in 1997 for the treatment of genital and perianal warts.
Another promising, but ultimately unsuccessful, clinical application of R837 and R848 was in the treatment of herpes simplex virus (HSV). There are multiple reports of anecdotal evidence for the successful treatment of both acute and chronic HSV lesions that have resulted in off-label usage of these IMDs [118,119]; however, clinical trials failed due to insufficient efficacy at safe doses (i.e., narrow therapeutic indexes) [120]. Another off-label usage of these synthetic TLR7/8 agonists was for treatment of vaginal or cervical HPV lesions. Multiple clinical trials demonstrated the efficacy of R837 combined with photodynamic therapy or HPV therapeutic vaccines for treatment of vulvar intraepithelial neoplasia (lesions caused by HPV-16) [121,122]. Side effects were significant, however, with one patient needing hospitalization for severe vulval erythema. Moreover, clinical trials in patients with cervical intraepithelial neoplasia have been unsuccessful due to side effects and insufficient enrollment [123].
Beyond virus-induced topical lesions, R837 and R848 have shown promising results in topical treatment of precancerous and cancerous lesions. R837 is FDA-approved for treatment of actinic keratosis, a precancerous skin condition that can develop into squamous cell carcinoma or basal cell carcinoma [124]. In clinical trials, R837 had to compete with photodynamic therapies using 5-aminolevulinic acid as the standard of care [125]. In two phase III trials, R837 showed disease clearance rates greater than 50% (vs. 3% for the control arm) [126]. When the lesions were biopsied, significantly increased levels of CD11c+ DCs and CD4+ and CD8+ T cells were detected.
The third and final indication for which R837 received FDA approval, in 2004, was for basal cell carcinoma, with disease clearance rates of 75% in two phase III trials [127]. Skin biopsies revealed Th1 polarization, significantly increased type I IFN signaling, and recruitment of CXCR3+ lymphocytes. Mild erythema, edema, erosion, and ulceration were observed in 50% of patients. Recent clinical results using R848 for treatment of cutaneous T cell lymphoma have also been promising [128,129]. In a phase I trial, 9 of 12 patients showed significant improvement, with 4 experiencing complete clearance of all lesions [130]. The patients who responded to treatment showed significantly increased production of IFN-γ and TNF-α by CD4+ T cells, granzyme B by CD8+ T cells, and IFN-γ, perforin, and granzyme by CD56+ NK cells in skin biopsies of lesions.
For treatment of melanoma, some anecdotal successes using topical administration of IMDs have been reported, but no clinical approvals have been achieved [131]. R837 was studied in clinical trials as the primary treatment for an early form of melanoma called lentigo maligna, but the pathological complete regression rate did not reach the predefined end points needed to replace surgery as the standard treatment [132]. There is anecdotal evidence of topical R837 displaying efficacy in primary and metastatic melanoma with increased levels of CD4+ and CD8+ T cells in treated skin and sentinel lymph nodes (LNs), as well as regression of skin metastases in breast cancers, but large-scale randomized clinical trials have yet to demonstrate efficacy [133].
Recent clinical studies of IMD topical formulations have been focused on their use as vaccine adjuvants. Over 100 clinical trials using topical imiquimod as a vaccine adjuvant for cancer (mostly DC vaccinations), infectious diseases (hepatitis B vaccination), and respiratory ailments (influenza vaccines) are currently listed on clinicaltrials.gov. These clinical applications are discussed in detail below; it is important to note that despite the success of topical administration of synthetic TLR7/8 agonists, this route of administration has been associated with dose-limiting side effects, such as fever and pemphigus-like lesions, in some cases. These side effects need to be addressed through further molecular design to advance the use of IMDs as vaccine adjuvants [134-136].
3.2. IMDs as immunomodulators for Advanced Cancers
In the late 1990s, synthetic TLR7/8 agonists were touted as an alternative to FDA-approved high-dose exogenous IFN-α; widespread use of the latter in treating cancer was limited by significant toxicity and exorbitant cost [137]. Type I IFNs suppress tumor cell proliferation, upregulate expression of MHCs, and increase levels of IFN-γ in synergy with IL-12, thus improving cross-priming by DCs [138]. In certain cancers, synthetic TLR7/8 agonists have been shown to cause apoptosis of tumor cells [139,140]. Other studies, however, show they can promote tumor growth in the lung; thus, the effects of TLR7/8 ligands on different types of tumor cells are cancer and tissue context-dependent [141]. By contrast, the direct and indirect effects of TLR7/8 agonism on immune cells are well defined, with increased infiltration of cytotoxic T cells (via DCs) and NK cells, macrophage polarization, and decreased levels of myeloid-derived suppressor cells combining to yield potent antitumor efficacy [142,143].
The first attempts to demonstrate antitumor efficacy of IMDs studied parenteral administration of 852-A and progressed to phase II clinical trials before discontinuation due to considerable side effects [144,145]. Urosevic and colleagues tested intravenous administration of 852-A in patients with metastatic melanoma [146]. With thrice-weekly dosing, only 4 out of 21 patients showed disease stabilization and 1 patient had a partial response. This lack of efficacy was particularly puzzling given the pharmacologic activity measured; that is, significantly increased levels of type I IFN and activation markers such as CD86 on monocytes in peripheral blood. The authors hypothesized that immune activation might have been decoupled from tumor antigens and that the level of immune activation was insufficient for treating metastatic disease. Other failed attempts at parenteral delivery of TLR agonists for cancer therapy include research on loxoribine, a guanosine analog-based TLR7 agonist. Loxoribine significantly augmented NK cell activity of murine splenocytes, increased cytolytic activity when combined with IL-2 (the synergy was NK cell dependent), and prevented lung metastases in the B16 melanoma mouse model. [147,148]. Nevertheless, when loxoribine was evaluated in a phase I trial in 20 patients with advanced cancers, the disease was stabilized in 6 patients but progressed in 14 patients [149]. Moreover, one patient experienced a 46% decrease in absolute lymphocyte count on day 2 (which returned to baseline by day 8); the trial was discontinued following numerous other immune-related adverse events (irAEs). Preclinical work by Stratford and colleagues on DSR-6434 (a compound from the 8-oxoadenine library discussed in Section 2.3), showed synergy with ionizing radiation in mice bearing CT-26 tumors, with increased levels of IFN-α, IFN-γ, and TNF-α [150]. As a monotherapy, however, DSR-6434 could not elicit effective CD8+ T cell responses in animal models. Each of these compounds, although having ultimately failed, provided important insights that have guided current preclinical and clinical developments of synthetic TLR7/8 agonists as antitumor therapies.
Lessons learned from these unsuccessful attempts at parenteral delivery paved the way for next-generation TLR agonists, with a focus on supplementing immune checkpoint inhibitors rather than testing the TLR agonists as monotherapies. Activation of innate immune cells such as DCs by TLR agonists assists in generating potent, tumor-specific immune responses capable of significant tumor regression and long-lasting memory against tumor recurrence [151,152]. Moreover, TLR agonists can directly influence the immunological status of the tumor microenvironment by generating pro-inflammatory cytokines, thus complementing checkpoint inhibitors in overcoming immunosuppressive pathways in tumors [153]. The most clinically advanced TLR agonist in this context is DSP-0509, which is currently in a phase I/II trial in combination with pembrolizumab, the latter of which targets programmed cell death protein 1 (PD-1). Preclinical studies have shown DSP-0509 to have high water solubility, which allows for intravenous administration, resulting in increased serum levels of IFN-α. Antitumor efficacy was demonstrated in CT26 and 4T1 murine tumor models concomitant with upregulation of IFN-γ signature genes, CD8+ and effector memory tumor-infiltrating lymphocytes, and MHC class I expression on tumor cells, in addition to decreased levels of myeloid-derived suppressor cells [154]. Systemically administered liposomal formulations of IMDs have also been used in combination with checkpoint blockade therapy. For example, BDB-001, a TLR7/8 agonist formulated in liposomes, is in phase I clinical trials in combination with pembrolizumab. Antibody conjugation has also been explored to enable tumor-targeted IMD delivery. BDC-1001, a TLR7/8 agonist conjugated to the anti-HER2 IgG1 monoclonal antibody trastuzumab, is currently in phase I trials in combination with pembrolizumab [155,156]. In the same vein, SBT-6050 is an antibody conjugated TLR8-specific agonist that is advancing toward the clinic based on strong preclinical results. Potent antitumor activity was observed upon intravenous administration in CT26 Her2+ tumors, with minimal production of serum cytokines such as IL-6, IL-10, TNF-α, and MCP-1 [157,158]. Thus, parenteral administration of synthetic TLR7/8 agonists continues to receive significant clinical attention, despite failures, because of the potential to treat metastatic disease.
Oral delivery of synthetic TLR7/8 agonists has also been pursued for cancer treatments with the goal of increasing widespread use due to ease of administration and patient compliance. Initial clinical attempts have failed, however, due to insufficient efficacy and unacceptable toxicity. ANA773 (RG-7795) is an orally available TLR7 agonist that was developed by Anadys Pharmaceuticals. Preclinical studies showed promising NK cell-mediated antitumor activity with increased levels of cytokine secretion, cytolysis of tumor cells via increased levels of IFN-γ, and antibody-dependent tumor cytotoxicity mediated by increased levels of IFN-α [159]. Nevertheless, in a phase I clinical trial, only 1 of 20 patients had a partial response and significant irAEs were reported, including grade 3 neutropenia, grade 3 fatigue, nausea, diarrhea, headaches, vomiting, and weight loss [160]. Another orally administered TLR7 agonist called bropirimine was compared with bacillus Calmette-Guérin (BCG) intravesical immunotherapy (standard treatment for carcinoma in situ bladder cancer) in a pivotal phase III trial and showed lower levels of toxicity and treatment discontinuation (4% of patients in the bropirimine group withdrew vs. 14% in the BCG group). Unfortunately, antitumor efficacy was not sufficient to merit FDA approval [161].
Roche is currently developing orally available synthetic TLR7/8 agonists for advanced cancers. For example, the compound RO-7119929 is being tested in a phase I clinical trial in patients with metastatic hepatocellular carcinoma with the IL-6 inhibitor tocilizumab, the latter of which is to be administered in case of severe steroid refractory cytokine release syndrome. Additionally, Primmune Therapeutics is continuing where Anadys left off with two orally available TLR7/8 agonist prodrugs, PRTX-007 and PRX-034. Preclinical data show increased levels of type I IFNs, IL-6, and IL-1Ra upon incubating the prodrugs with human PBMCs, with no increases in IFN-γ, IL-2, or IL-12p70; these results indicate minimal engagement of the NF-κB proinflammatory pathway, which might minimize systemic toxicities [162].
Given the history of failed attempts at systemic delivery of synthetic TRL7/8 agonists, considerable effort has also focused on localized administration of these compounds to circumvent systemic toxicity [163]. Early work on local delivery evaluated a liquid formulation of R837, based on excipients such as poloxamers and β-cyclodextrin, for the treatment of non-muscle invasive bladder cancer [164]. A formulation called TMX-101 or Vesimune progressed through phase I and II trials, with promising results in patients with carcinoma in situ bladder cancer [165,166]. Among compounds that are currently in trials, VTX-2337, a benzazepine-based TLR8 agonist, was shown to be well tolerated in phase I and is currently in phase II trials in combination with Doxil (doxorubicin) for ovarian cancer treatment and with cetuximab for squamous cell carcinoma of the head and neck [167-169]. Intratumoral administration of MEDI-9197 (or 3M-052) was evaluated in a phase I clinical trial in combination with durvalumab, another anti-PD-1 antibody. Although the preclinical data were very promising, further development of this compound has been discontinued by AstraZeneca [170]. NKTR-262 is another TLR7/8 agonist being evaluated in the context of intratumoral administration in advanced cancers in combination with the CD122 agonist NKTR-214. Similarly, LHC165, a benzonaphthyridine TLR7 agonist adsorbed to aluminum hydroxide, is being tested in a phase I clinical trial with the anti-PD-1 antibody spartalizumab. Preclinical compounds that have shown promise in mitigating systemic toxicity by localized retention include ALT-702, TransCon-R848, and VX-001; ALT-702 is based on a depot-forming peptide technology and the latter two are described as “sustained-release R848 formulations.” These systems are discussed in greater detail in Section 4, where we focus on formulation and carrier-based approaches.
3.3. Systemic delivery of IMDs as Antivirals for Infectious Diseases
In addition to treatment of skin conditions as discussed in section 3.1, IMDs are being explored as systemic treatments for chronic infectious diseases such as hepatitis C (HCV), hepatitis B (HBV), and HIV. The development of orally available prodrug forms of TLR7/8 agonists has been extensively evaluated in the treatment of chronic infectious disease, with the goal of improving patient compliance and minimizing side effects [171]. As in the application to cancer, however, the transition from treating topical infectious conditions to chronic systemic infections has also been limited by narrow therapeutic indexes of systemically/orally administered TLR7/8 agonists. Low doses have insufficient therapeutic effects and higher doses are associated with vomiting, fatigue, lymphopenia, and fever, in addition to hepatic and renal impairment [172]. Although toxicity remains a concern, the antiviral state generated by type I IFN induction upon TLR7 ligation makes these agonists promising antiviral agents for chronic infectious diseases [173]. For example, type I interferons elicited by TLR7/8 activation can induce viral RNA transcript degradation and activation of protein kinase R, which blocks translation of viral mRNAs [174].
The first clinical attempts at treating chronic infectious diseases with synthetic TLR7/8 agonists focused on chronic HCV. Oral administration of R848 was extensively evaluated but failed in two phase IIa clinical trials because of toxicity at higher doses, similar to issues with PEGylated IFN-α injections, the standard treatment for chronic HCV [175]. Oral administration of PF-4878691 (852-A) was tested in a phase I trial in patients with chronic HCV but showed low bioavailability and highly variable absorption compared with parenteral administration. This drug was therefore discontinued for treatment of HCV but further evaluated in the context of advanced cancers [176]. ANA975 and ANA971 are oral prodrug formulations that are converted to active compound by first-pass metabolism [177]. They were also tested in phase I clinical trials in patients with chronic HCV but failed due to inadequate therapeutic indexes.
Although synthetic TLR7/8 agonists are no longer, to our knowledge, being tested for chronic HCV, treatments for chronic HBV have shown promising results. HBV infects hepatocytes in the liver without activating hepatic non-parenchymal cells such as Küpffer cells and liver sinusoidal endothelial cells or surrounding immune cells such as myeloid DCs (mDCs), pDCs, and macrophages [178]. TLR7/8 agonists play a role in preventing viral transmission and replication by mechanisms similar to those discussed earlier, including upregulation of IFN-stimulated genes (ISGs) and the generation of an antiviral state by type I IFN signaling [179]. One of the many lead compounds in the clinic, RO-7020531, has completed a phase I trial and is currently in phase II trials in combination with direct-acting antivirals [180-182]. This compound is a double prodrug of a TLR7 agonist that has shown promising preclinical results. In an AAV-HBV mouse model, RO-7020531 induced both innate pharmacodynamic responses and adaptive immune responses [183]. GS-9620 is another oral TLR7 agonist that was tested clinically for treatment of chronic hepatitis B. This compound exhibited high first-pass hepatic clearance and increased levels of type 1 IFN upon oral administration compared with intravenous administration, with similar systemic exposure in nonhuman primates (NHPs) [184]. At low doses, GS-9620 was shown to activate ISGs without inducing systemic interferons, which suggested the presence of a therapeutic window for inducing an anti-HBV response [185]. Ferrari and colleagues demonstrated sustained antiviral effects against woodchuck hepatitis virus (WHV) in a woodchuck model of HBV and against HBV in a chimpanzee model, with significantly increased production of IFN from monocytes rather than direct activation of antiviral pathways in hepatocytes. These findings indicated that GS-9620 could be used in concert with other standard antiviral treatments [186,187]. The same researchers observed significantly increased levels of antigen presentation in hepatocytes, which led to improved HBV-specific immune responses. In a phase 1b trial with 1 or 2 doses of oral GS-9620 administered 7 days apart, no change in serum HbsAg or HBV DNA levels were detected, despite induction of interferon-stimulated genes [185]. Clinical development of GS-9620 for chronic HBV has since been discontinued, but is still being examined for treatment of HIV (vide infra). APR002, a liver-targeted TLR7 agonist designed to act locally in the liver and gastrointestinal tract, is being developed by Apros Therapeutics. Preclinical studies in NHPs showed that retention in the liver is partly mediated by organic anion transporting polypeptide transporters [188]. These studies demonstrated sustained suppression of serum WHV DNA, lowered levels of serum WHsAg, and durable antibody responses in a woodchuck model of chronic WHV.
Bertoletti and colleagues probed purified hepatic immune cells from healthy and HBV-infected human livers with numerous TLR agonists and demonstrated that only TLR8 agonists were able to selectively activate liver-derived cells, resulting in substantial production of IFN-γ, which has been shown to mediate clearance of HBV in infected chimpanzees [189,190]. GS-9688, a selective TLR8 agonist currently in phase II clinical trials for treatment of chronic HBV, can activate TLR8-expressing gut and hepatic immune cells, leading to secretion of immune mediators in the portal vein [191]. An effective dose of 3 mg/kg was defined in WHV-infected woodchucks and 3 mg in virally suppressed and viremic patients with chronic HBV [192].
Beyond treatment of HCV and HBV, recent work has shown the potential of TLR7/8 agonists in reversing HIV latency (a state where cells are infected and contain an integrated and functional HIV viral genome, but where active viral transcription is silent). GS-9620, discussed earlier in the treatment of chronic HBV, is currently in a phase I trial for reversing HIV latency. Preclinical data indicated that TLR7/8 agonist-induced increases of IFN-α effectively inhibited HIV-1 replication in vitro in activated lymphocytes and macrophages [193]. Work done by Barouch and colleagues combining Ad26/MVA vaccination with oral TLR7 agonists GS-9620 or GS-986 (a GS-9620 analog) in an NHP model showed significantly reduced viral DNA in lymph nodes and peripheral blood [194]. They also observed improved virologic control and delayed viral rebound following discontinuation of antiretroviral therapy [195]. Whitney and colleagues demonstrated the ability of these oral TLR7 agonists to induce transient viremia in rhesus macaques (RMs) [196]. Thus, TLR7/8 agonists may have a role to play in HIV cure strategies.
Although we have focused here on viral infections, it is important to note the role that synthetic TLR7/8 agonists play as adjuvants in vaccines against bacterial infections. The mechanisms underlying TLR7/8 sensing of bacterial RNA are gradually being established [197-199]. Preclinical studies of these agonists in vaccine formulations against bacterial pathogens demonstrate the ability of these compounds to activate both neonatal and adult DCs alike thus also highlighting their potential in boosting neonatal immunizations [200-202].
3.4. Anti-inflammatories and bronchodilators for Respiratory Ailments
In addition to their utility as potential anti-viral compounds, synthetic TLR7/8 agonists are being explored as agents to shift the polarization of immune response and as bronchodilators for treatment of allergic conditions such as asthma and rhinitis. Allergic reactions are characterized by highly Th2-polarized immune responses and the production of allergen-specific IgE antibodies. One approach to treatment of such disorders has been to therapeutically shift the polarization of the immune response from pathology-causing Th2 to a non-pathologic Th1 response. Preclinical studies using R848 as a model compound have demonstrated its ability to activate DCs, airway epithelial cells, and Th1 cells, leading to Th1 immune polarization [203]. In animal models of allergy, R848 treatment decreased airway hyperreactivity, airway remodeling, and activation of airway nerves, leading to increased nitric oxide production [204]. R848 was also able to reverse airway reactivity to allergen challenge, thus preventing airway smooth muscle proliferation and goblet cell hyperplasia [205]. Initial use of synthetic TLR7/8 agonists in allergic asthma was motivated by work done on administration of CpG oligodeoxynucleotides (CpG ODN; TLR9 agonists) to airways, which were shown to induce a redirection of the immune response polarization from Th2 to Th1. Inappropriate Th2 responses to harmless environmental antigens need to be counterbalanced by a strong Th1 cytokine response to reduce the downstream effects of the allergy [206]. Asthma therapies have focused on addressing airway inflammation and excessive bronchoconstriction [207]. Preclinical work using murine models of asthma has shown the potential of synthetic TLR7/8 agonists to significantly reduce ovalbumin (OVA)-induced airway hyperreactivity and eosinophilic inflammation [208]. TLR7/8 agonists inhibit IgE synthesis in favor of IgA synthesis in human B lymphocytes and can reverse airway hyperresponsiveness by IFN-γ-mediated effects. Intracellular signaling through NF-κB and the AP-1 family of transcription factors is implicated as the mediator of this response in vitro, whereas acute bronchodilation effects are shown to be mediated via production of nitric oxide upon TLR7 activation.
Synthetic TLR agonists have also been evaluated for the treatment of allergic asthma and rhinitis clinically. GSK-2245035 was investigated in clinical trials for the treatment of allergic asthma and allergic rhinitis [209]. This compound was designed to preferentially upregulate IFN-α secretion without significant changes in TNF-α as a means to alter the immune microenvironment of the airway and thus to modify the Th2 response to aero-allergens [210]. Preclinical work on the development of the 8-oxo-adenine antedrug series, possessing an ester moiety that cleaves to an inactive acid in the presence of plasma esterases, allowed for lowered systemic toxicities [211]. AZD-8848 was the result of this selective antedrug TLR7 agonist library optimized for airway treatment [212]. However, AstraZeneca has since discontinued development of TLR7 agonists. VTX-1463, a selective TLR8 agonist, is currently undergoing phase I trials for treatment of allergic rhinitis. Preclinical data have shown significant upregulation of Th1 inflammatory mediators, such as IL-12, IFN-γ, and MCP-1, that shift the balance of the Th1:Th2 ratio toward lowering allergic effects [213].This compound was well tolerated in a phase I clinical trial for grass pollen allergy, with improved levels of total nasal symptom scores, the primary clinical endpoint [214].
4. Bioconjugation and other delivery strategies
The discussion above demonstrates the breadth of preclinical and clinical applications of synthetic TLR7/8 agonists, but also highlights the myriad unsuccessful attempts at clinical translation. Overall, two main problems have dominated failures in clinical trials: insufficient therapeutic efficacy and unacceptable toxicity (i.e., narrow therapeutic index). Therefore, significant research has focused on bioconjugation and other delivery strategies to overcome these limitations and realize the full potential of these synthetic small-molecule immunomodulators.
4.1. TLR7/8a conjugates to enhance vaccine efficacy
4.1.1. Antigen-TLR7/8 conjugates
Some of the early research on improving the potency of vaccine formulations involved conjugating synthetic TLR7/8 agonists to vaccine antigens as a means to prevent the former from entering the bloodstream and to focus their effects precisely on antigen-specific B cells and those DCs that acquire antigen and are most relevant for T cell activation. The hypothesis was that covalent conjugation would allow for colocalization of antigen and adjuvant, thus greatly increasing the likelihood that the antigen and adjuvant are taken up by the same APC, which in turn could enhance antigen presentation to CD4+ and CD8+ T cells [215].
Work done by Seder and colleagues comparing the efficacy of R848 to that of the TLR9 agonist CpG ODN as vaccine adjuvants showed that R848 induced lower levels of antigen-specific CD4+ and CD8+ T cells and reduced IgG1 and IgG2a responses to the HIV-1 Gag protein [216]. Using a reactive derivative of R848, the TLR agonist was conjugated to HIV-1 Gag protein, and the IMD-conjugated protein elicited elevated cytokine production by antigen-specific CD4+ and CD8+ T cells to the antigen. This study indicated that the discrepancy between innate activation of DCs and lack of T cell response from free R848 could be a delivery problem, wherein the timing of antigen presentation was not optimized with the activating effects of R848. Follow-up studies in rhesus macaques (RMs) comparing the TLR7/8 agonist-Gag protein conjugate with the Gag protein itself or with CpG ODN showed increased Th1 responses and Gag-specific CD8+ T cell responses for the conjugate [217]. The mechanism of action of the TLR7/8 bioconjugate was hypothesized to be due to enhanced migration of 6 distinct DC populations to dLNs, thus significantly increasing the total number of specific DC subsets in the dLNs. Increased uptake of OVA, the model antigen, was shown in the conjugate system compared with a mixture of unconjugated OVA and TLR7/8 agonist (37.8% vs. 8.8%, respectively), validating the above hypothesis [218]. This work supported that of Kedl et al., who showed that TLR7/8-antigen bioconjugates increased the sensitivity of responding T cells to low amounts of antigen [219].
An important issue in preparing antigen-IMD conjugates is ensuring that the IMD is not linked to the antigen at an important site for neutralizing antibody recognition. To this end, Hedestam and colleagues showed that attaching a TLR7/8 agonist onto surface-exposed lysine residues on the external subunit of the HIV-1 envelope glycoprotein gp120 led to reduced antibody recognition of the CD4 binding site, depending on the concentration of TLR7/8 agonist conjugated to gp120 [220]. Work by O’Hagan and colleagues also highlighted the potential loss of conformation that can occur with bioconjugation reactions in this context and noted that a disperse mixture of conjugated species can form due to the high number of equivalent surface-exposed amino acids available for conjugation [221]. These authors used an ortho-activated benzaldehyde instead of a non-substituted derivative to overcome the need to increase the reactivity of lysine amino groups, thus reducing the complexity of the reaction and purification steps and increasing the product homogeneity.
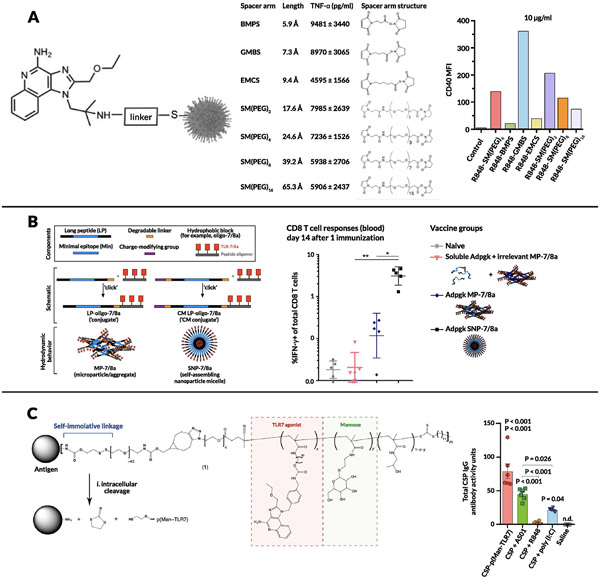
The above bioconjugate studies all focused on linkage of IMDs to single protein antigens. Work done by Alexander-Miller and colleagues allowed for multiple antigenic targets (i.e., incorporating all viral proteins) by conjugating an amine-modified R848 onto reduced inactivated influenza virus A particles (IPR8) using an amine to sulfhydryl crosslinker featuring N-hydroxysuccinimidyl ester and maleimide groups that connect the amine of the drug and the thiols on the virus particles respectively (SM(PEG)4; Fig. 4A) [222]. This system was tested in the African Green Monkey (AGM) model of neonatal vaccination and resulted in a 10-fold increase in anti-influenza virus IgG levels, increased levels of influenza-specific IgM and IFN-γ+ influenza-specific T cells, and decreased viral load in the trachea. This improved response was again tied to an increase in the total number of DCs in the dLNs and increased DC expression of maturation markers such as CD80 and CD86 [223]. The R848-IPR8 conjugate was further tested for long-term responses in the AGM model and showed significantly increased levels of long-lived IgG antibody response 6 months after dosing [224]. Recent work from the same group explored the development of second-generation R848-IPR8 conjugates prepared via a two-step synthesis process [225]. First, R848 was modified with different crosslinker moieties to introduce maleimide groups that were then conjugated to the reduced virus containing thiol groups. These systems were tested in RAW264.7 cells for TNF-α production with the N-γ-maleimidobutyl-oxysuccinimide ester (GMBS, ThermoFisher) linker showing higher CD40 expression (by an order of magnitude) compared with both the unconjugated control and the first-generation SM(PEG)4 linker (Fig. 4A). These conjugate vaccine strategies highlight the necessity for improved formulation approaches in order to maximize the potential of TLR7/8 adjuvantation.
Figure 4. Bioconjugation approaches to enhance vaccine potency.
(A) Dependence of R848-influenza conjugate particles on choice of linker strategy. (B) Charge-modified peptide strategy that results in particulate bioconjugates for personalized cancer vaccines. (C) Self-immolative linker approach for enhancing potency of malaria antigen-TLR7 conjugate.
4.1.2. Polymer and particulate formulations of TLR7/8 agonists
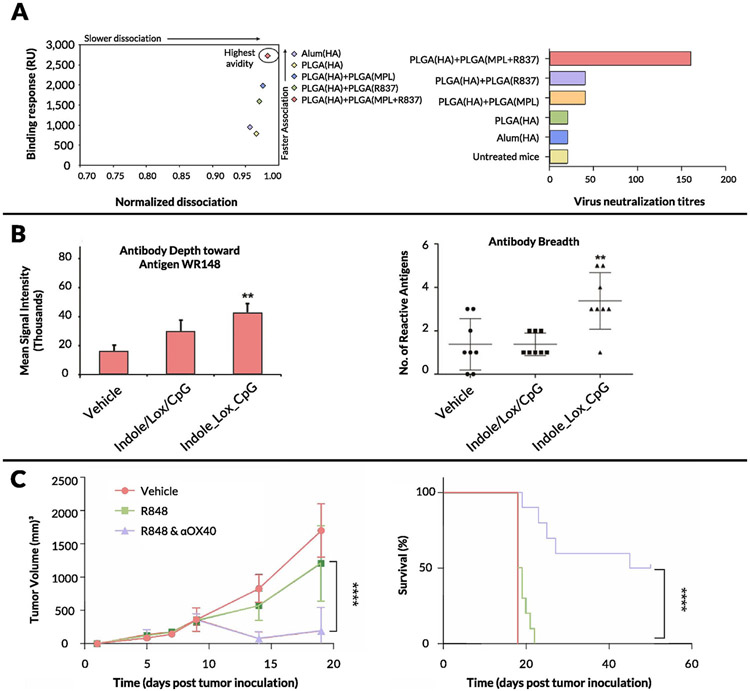
While the above examples demonstrate bioconjugation of TLR7/8 agonists onto soluble proteins, another approach pioneered by Seder and colleagues involves the intentional design of conjugate formulations that self-aggregate into larger particulates [226,227]. They attached a number of antigens or neoantigens using a charge modification conjugation approach that comprised peptide antigens linked to both a charge-modifying polypeptide and a hydrophobic polypeptide block through enzyme-degradable linkers at the N and C termini, respectively. Then, the oligopeptide-based hydrophobic blocks were linked to a precise number of TLR7/8 agonists. This work further strengthens the hypothesis that antigen form (soluble vs. particulate) is a critical aspect of CD8+ T cell immunogenicity. Vaccination with particle antigens, either covalently attached or mixed with adjuvant, led to 20-fold-higher CD8 T cell responses compared to soluble antigen, with the particle conjugates being retained longer in dLNs and showing higher uptake by CD11c+ DCs (Fig. 4B). Another example of this approach was demonstrated by Hubbell and colleagues using a reversible linker strategy [228]. In this system, protein antigens were conjugated via a cleavable linkage to a statistical copolymer containing mannose-binding receptors and a TLR7 agonist called P(Man-TLR7). Antigens were modified with an amine-reactive heterobifunctional bicyclononyne-decorated linker, which is sensitive to disulfide reduction (stable in serum and cleaves on endocytosis). Vaccination with a P(Man-TLR7) conjugate linked to CSP (circumsporozoite protein from Plasmodium falciparum malaria) induced a significant increase in CSP-specific CD8+ T cell response (TNF-α+ and IFN-γ+ T cells) compared with controls, including the adjuvant currently used in the malaria vaccine being studied in clinical trials (Fig. 4C).
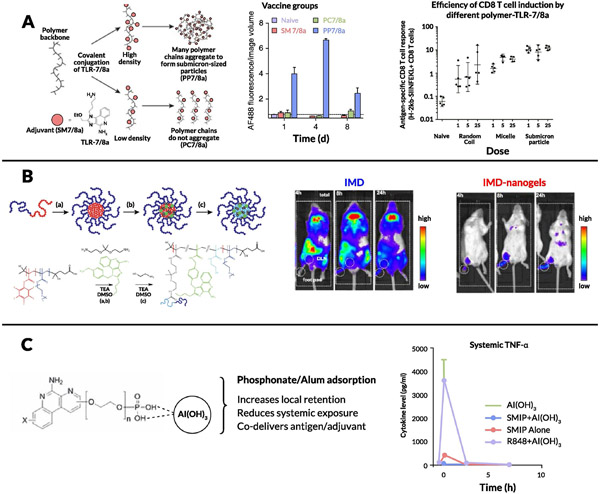
The rationale for particulate formulations has been bolstered by extensive work by Seder and colleagues, wherein TLR7/8 agonists were covalently conjugated onto synthetic polymer scaffolds to further explore the mechanistic effects of different linker structures and TLR7/8 agonist densities on the physicochemical properties of the prodrug particles [229]. Particulate TLR7/8 conjugates showed enhanced lymph node cytokine production and uptake by migratory APCs as well as an order of magnitude increase in the influx of CD11c+ DCs and monocytes in the dLN compared with soluble forms (Fig. 5A). These authors probed further into how the carrier size and morphology of these conjugates correlate with immune activation by comparing 3 types of carriers: a random coil polymer (~4 nm), a micelle nanoparticle (~10 nm), and a sub-micrometer particle (~300 nm) [230]. A significantly increased CD8+ response for the sub-micrometer particles as well as direct correlations between hydrodynamic radii and magnitude of lymph node IL-12 production as well as uptake by APCs were observed (Fig. 5A). Interestingly, the uptake of the sub-micrometer particles was 5-fold higher for monocytes and macrophages than for DCs, suggesting that monocytes and/or macrophages play a crucial role in adjuvant activity in this size range. Particulate polymer or peptide formulations thus represent a promising approach to improve LN accumulation and retention of these agonists.
Figure 5. Polymer and particulate approaches to improve adjuvant potency.
(A) Effect of varying TLR7 agonist density on lymph node residence time of the particle and varying size and architecture of the polymer on antigen-specific T cell response. (B) polymeric nanogel approach toward enhanced lymph node delivery and retention. (C) Adsorption to alum resulting in ‘depot’ effect which minimizes conc of TNF-α in the blood upon administration.
In a similar vein, De Geest and colleagues used bis-amino-ketal 2,2-bis(aminoethoxy)propane cross-linkers to create amphiphilic copolymer nanogels that were attached to IMD TLR7/8 agonists [231,232]. Compared to free TLR7/8a, immunization with TLR7/8a-conjugated nanogels led to increased internalization by multiple subsets of immune cells in LNs including DCs, B cells, macrophages, and monocytes, with the vast majority of the nanogel localized to the subcapsular and medullary sinuses of the lymph node (Fig. 5B). The same group developed lipid-polymer amphiphiles consisting of cholesterol as a lipid motif and a hydrophilic polymer conjugated to a TLR7/8 agonist [233]. They demonstrated that this polymer binds to albumin with high affinity (as assessed using bio-layer interferometry), allowing for highly efficient passive translocation to lymphoid tissue via “albumin hitchhiking” and leading to a significant increase in DCs in the dLN along with high expression of CD80 and CD86. This lymph node targeting approach was verified using IFN-β reporter mice, with bioluminescent imaging showing minimal systemic activation with a strong signal in the dLN. David and colleagues conjugated a TLR7/8 agonist onto hyaluronic acid via a 2-chloro-4,6-dimethoxy-1,3,5-triazine-activated amidation strategy, which resulted in an immunologically “silent” conjugate that was activated via proteolysis or enzymatic cleavage of the amide bond [234]. Upon co-administration of the conjugate with diphtheria toxoid (CRM197) as a model antigen, they observed a significant increase in antigen-specific IgG titers after a single boost. Kishimoto and colleagues showed that conjugating R848 onto polymer particles via an acid-labile bond resulted in an order of magnitude increase in antibody titer (Th1 focused; i.e., higher IgG2c/IgG1 ratio), increased antigen-specific T cells, and a significant influx of myeloid DCs, granulocytes, and macrophages into the dLN [235]. Another synthetic conjugate approach toward increasing DC activation and internalization in dLNs was shown by Hong and colleagues, wherein a TLR7/8 agonist with a terminal alkyne moiety was conjugated onto azide-coated iron oxide nanoparticles (NPs) through copper-catalyzed azide-alkyne cycloaddition and co-administered with OVA [236]. The resultant increased antigen-specific T cell activity reinforced the use of the synthetic conjugation approach as a method to improve vaccine potency. Likewise, Appel et al. conjugated a TLR7/8 agonist onto a copolymer of dimethyl acrylamide and neopentyl glycol diacrylate formulated with beta-cyclodextrin [237]. Upon co-injection with either OVA or gp120, the adjuvant formulation exhibited significantly improved IgG2c antibody titers.
An alternate approach to promote lymph node accumulation and reduce systemic dissemination of TLR7/8a adjuvants is to engineer their binding to existing clinical adjuvants such as alum. A team at Novartis pursued this approach by screening TLR7 agonists functionalized with polyethylene glycol (PEG) linkers and terminal phosphonate groups [238]. The PEG linkers allowed for increased solubility at neutral pH while the anionic phosphonate functional groups facilitated efficient adsorption to alum (Fig. 5C). Alum-bound TLR7 agonist adjuvants were tested in multiple vaccination mouse models; they demonstrated enhanced activation of APCs, priming of IFN-γ+CD4+ T cells, and, most importantly, antigen-specific B cell responses (both antibody-secreting and memory phenotypes) [239-243]. Alum-TLR7 conjugates were subsequently evaluated in a phase I clinical trial with the antigen present in the Menjugate (meningococcal group C–CRM197 conjugate) vaccine. In this study, the highest dose (100 μg) caused severe irAEs in one-fifth of patients, which correlated with high plasma levels of TLR7 agonist, while lower doses were deemed safe and effective in eliciting antibody responses similar to Menjugate (which contains alum as an adjuvant), though a larger-scale clinical trial was deemed necessary for verification [244]. TLR7 agonists have also been adsorbed to alum through mixing lipid tail-modified 3M-052 with phospholipids to generate very small lipid nanoparticles, which subsequently adsorb to alum via phosphate groups [245]. Upon co-injection of these alum/lipid nanoparticle-TLR7a complexes with a tuberculosis vaccine antigen, improved Th1 responses were observed relative to free 3M-052, with increased levels of antigen-specific CD4+ T cells and high levels of serum antibodies, which raises the question of whether there is potential synergy between TLR7/8 agonists and alum beyond simply a depot effect. This concept would be interesting to explore further, given that Seder and colleagues noted that adjuvant efficacy is lowered when TLR7 is co-formulated with an MF59-based nano-emulsion instead of alum, despite prior studies showing that MF59 induces higher binding titers than alum alone [246-248]. More recently, an Alum/TLR7a-adjuvanted whole-virion inactivated SARS-Cov-2 vaccine received emergency approval by India’s Central Drugs Standard Control Organization at the end of 2020 [249-254]. At the time of writing this review, this vaccine has been administered to over ten million people, further underscoring the importance of formulated TLR7/8 agonists as vaccine adjuvants.
In addition to alum adsorption, a commonly studied approach for formulating IMDs has been through their encapsulation with or without vaccine antigens in biodegradable poly(lactic-co-glycolic acid) (PLGA) nanoparticles or other polymeric NPs. Pulendran and colleagues encapsulated soluble gp140 Env and lipophilic TLR7/8 agonist 3M-052 in PLGA NPs and showed an adjuvant effect comparable to that of alum-TLR7 and significantly higher than that of alum alone in RMs [255,256]. They demonstrated increased levels of Env-specific IgG antibodies in serum and vaginal secretions, high levels of neutralizing antibodies, and upregulated expression of CD86 on monocyte subsets 2 weeks after vaccination. Another interesting finding was the enhanced protection observed in young and adolescent RMs compared with older RMs, which could tie in to work done by Levy and colleagues that showed potent activity of TLR7/8 agonists on neonatal macaque blood cells [257]. TLR7/8 PLGA-based vaccine formulations have also been studied in the context of mucosal and tumor immunizations [258,259]. Other polymeric systems include block copolymers, developed by Wilson and colleagues, decorated with pyridyl disulfide ethyl methacrylate moieties for conjugation of thiol-containing antigen and a fatty acid-mimetic core for encapsulation of TLR7 agonist [260]. Vaccination with these block copolymer nanoparticles carrying antigen (OVA) and TLR7 agonist elicited increased levels of antigen-specific CD8+ T cells in bronchoalveolar lavage fluid, lung vasculature, and lung interstitium in addition to enhanced antigen-specific IgG antibody titers upon intranasal administration compared to free protein/TLR7a immunization. In a similar approach, Levy and colleagues formulated a block copolymer system based on PEG-block-polypropylene-sulfide (PPS) polymersomes, encapsulating a TLR8 agonist that served as a potent adjuvant system when co-loaded with antigen [261]. Encapsulation in polymers has also been used to generate novel “needle-free” vaccination strategies, which represent potentially promising avenues for vaccine formulations based on TLR7 agonists [262-264].
4.2. Toward safe, systemic delivery via drug carrier approaches
As noted above, systemic toxicities associated with synthetic TLR7/8 agonists are a major roadblock to the clinical success of these compounds. Several drug carriers have been used in attempts to solve these issues, including PEGylation and other conjugation approaches, nano- and micro-particles, stimuli-responsive release, and molecular targeting moieties.
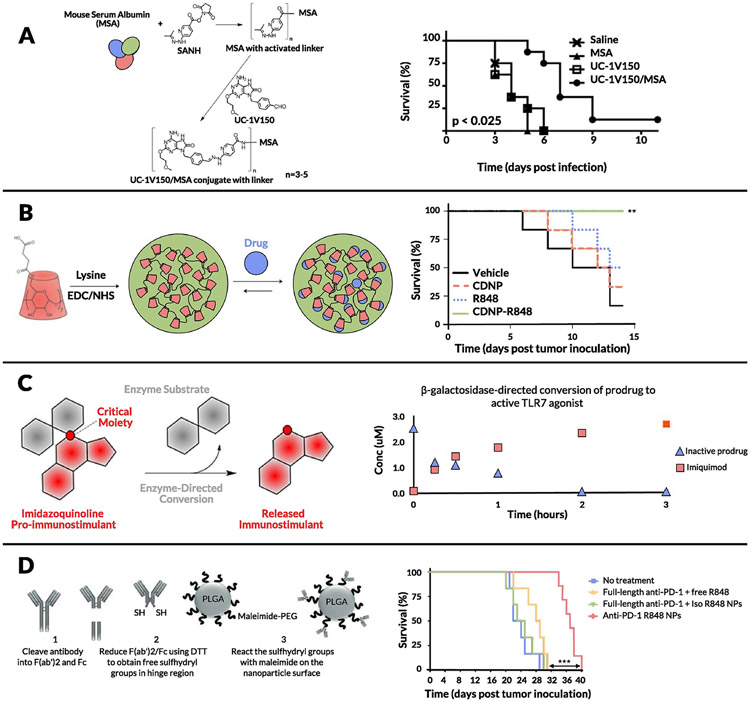
Carson and colleagues made a surprising observation when investigating PEGylation of TLR7 agonists. Specifically, while PEGylation improved solubility of these compounds, cytokine production in vitro was significantly lowered [265]. This loss of activity upon PEGylation was intriguing and the influences of PEG chain length (from 6 to 470 repeat units) on in vitro and in vivo effects of TLR7 ligands were examined. Short-chain PEGs displayed low potency to stimulate bone marrow-derived macrophages; however, when chain length exceeded 47, potency was restored, suggesting that spatially constrained conjugate ligands are limited in their ability to activate TLR7. Furthermore, conjugates with an amine end group were more potent than those ending with carboxyl. These data showing that conjugates using longer PEGs have improved solubility, circulation time, and plasma concentration, as well as increased IL-6 and TNF-α production in vivo, tie in nicely with previous observations that innate immune activity of PEG-TLR7 conjugates is affected by steric hindrance of the PEG linker [266]. The same group has shown that upon conjugation of a TLR7/8 agonist onto a carrier protein (mouse serum albumin) via a succinimidyl 6-hydrazone nicotinamide acetone hydrazone linker, the immunostimulatory potential of the agonist was significantly enhanced (Fig. 6A) [267]. Further work on TLR7 conjugation onto primary amine-functionalized Ficoll (400 kDa) or dextran (70, 500, and 2,000 kDa) using benzoic acid functional groups showed higher potency (by an order of magnitude) of TNF-α and IL-6 induction in vitro [268]. It is interesting to note that 12 nm dextran conjugates with similar conjugation ratios showed 10-fold higher potency than 37 nm dextran conjugates, whereas 29 nm linear dextran showed 10-fold higher potency than 14 nm spherical Ficoll conjugates. These observations were validated in vivo along with admixed controls, wherein the synthetic conjugates were at least 500-fold more potent at inducing production of cytokines such as IL-6, TNF-α, and IFN-γ than the unconjugated TLR7 ligand. These studies reinforce the idea that size, composition, and molecular architecture of synthetic TLR7 conjugates significantly affect immune potencies due to efficiency of uptake by APCs which is required for the generation of an adaptive immune response. These studies culminated in the development of 1V270, a phospholipid-conjugated TLR7 agonist that spontaneously self-assembles into 110–120 nm liposomes [269]. This liposomal formulation was tested in multiple murine tumor models, where NK cells were responsible for early efficacy and CD8+ T cells were critical for sustained inhibition of lung metastasis.
Figure 6. Toward systemic delivery of synthetic TLR7/8 agonists.
(A) TLR7/8 conjugation onto carrier protein (mouse serum albumin) significantly improves survival in a pulmonary infectious disease model of B anthracis. (B) Cyclodextrin nanoparticle (CDNP) formulation of R848 significantly improves survival in the MC38 colon cancer mouse model. (C) β-galactosidase enzyme-mediated tuned release of imiquimod (R837). (D) PD-1 targeting approach localizes effect of R848 NPs upon i.v. administration and significantly improves survival in mice bearing MC38 tumors.
Beyond molecular bioconjugates, numerous nano- and microparticle-based encapsulation approaches have been developed to enable intravenous delivery of TLR7 agonists. Ainslie and colleagues encapsulated R848 in acetalated dextran microparticles and showed efficacy in treating visceral leishmaniasis, a systemic parasitic disease [270]. Another significant stride toward systemic administration of TLR7/8 agonists was made by Weissleder and colleagues [271-273]. They developed a single-cell high-content screening approach to identify compounds that could alter the polarization of macrophages from an M2 (wound-healing phenotype) to M1 (inflammatory) state in vitro [271]. Among the compounds tested, R848 was the most potent driver of macrophage re-education and they subsequently designed cyclodextrin NPs to encapsulate R848 based via host–guest interactions. Cyclodextrin NPs carrying R848 exhibited efficacy in both the MC38 colon cancer and B16.F10 melanoma models upon intravenous administration (Fig. 6B). Notably, toxicity was not addressed in this initial study; in a follow-up study, the same group attached adamantane to R848 via the tertiary alcohol group thus increasing the host-guest binding affinity and improving the stability of the drug-loaded supramolecular complex. They obtained antitumor efficacy with lower weight loss than with R848-cyclodextrin NPs [272]. Another recent attempt at intravenous administration of TLR7/8 agonists was reported by Kabanov and coworkers; they encapsulated R848 in poly(2-oxazoline) particles and showed improved survival rates with low toxicity in a metastatic, orthotopic lung adenocarcinoma model [274]. They attributed the antitumor effects to higher levels of Ly6C+ monocytes and CD8+ T cells in the tumor microenvironment. Moving beyond polymeric systems, Bourquin and colleagues demonstrated that gold NPs coated with a mixture of 1-octanethiol and 11-mercaptoundecanesulfonic acid encapsulating R848 via hydrophobic interactions was a potent delivery vehicle, showing improved lymphatic accumulation and antitumor effects in the CT26 colon cancer mouse model [275].
Stimuli-responsive nanomaterials are ubiquitous in the field of drug delivery and attempts to use this paradigm to minimize toxicity of TLR7/8 agonists have yielded fruitful results in preclinical studies. Hubbell and colleagues formulated TLR7 agonists in oxidation-sensitive polymersomes based on PEG-b-PPS, which would specifically release TLR7a on exposure to the oxidative environment within antigen presenting cell endosomes [276]. They observed enhanced DC uptake and activation and proinflammatory cytokine secretion by these polymersomes compared to free TLR7a cultured with DCs in vitro. They hypothesized that this enhanced response was due to NOX-2-dependent reactive oxygen species in DC early endosomes and lysosomes triggering burst release of the encapsulated TLR7 agonist. Other oxidation-sensitive systems have been reported, including one developed by Broaders and colleagues that is based on a dextran polymer with stable boronic ester groups [277]. Light is another stimulus that has been extensively studied in the context of responsive biomaterial systems. Esser-Kahn and colleagues reported the use of 2-(2-nitrophenyl)-propyloxycarbonyl, a photocleavable protecting group, to form a carbamate linkage with the C4-amines in R837 and R848, demonstrating release of the free drugs in response to 360 nm light [278]. Mancini et al. attached a β-galactopyranoside to R837 covalently at the C-4 amine and reported β-galactosidase-mediated immune cell activation (Fig. 6C) [279]. This enzyme-responsive activity represents another promising step toward synthetic TLR7/8 agonist-based formulations that can preferentially release active cargo based on specific cues in the tumor microenvironment. The same group furthered this work by developing glycosidase-directed R848 prodrugs, which release free R848 based on cancer cell metabolism [280]. Another recent attempt at enzyme-responsive release was demonstrated by Shi and colleagues, wherein a TLR7/8 agonist was conjugated to PEG via benzyl carbamate residues capped by a β-glucuronidase (β-GUS)-sensitive glucuronide. The conjugates were then systematically studied by varying the number of benzyl repeat units (GL1, 2, or 3) and the molecular weight of PEG (0.75, 2, and 5 kDa) [281]. They found that PEG5k-GL2-IMD self-assembled into vesicular NPs and demonstrated native drug release in response to esterase and β-GUS.
Particulate formulations of TLR agonists favor uptake by APCs due to their natural propensity to phagocytose foreign material; however, enhancing delivery to these key cells and/or directing uptake by specific immune cell subsets is another strategy under investigation. One approach to enhance the concentration of IMDs in tumors is to target TLR7/8a-loaded nanoparticles to T cells circulating in the blood, which subsequently hone into tumors: Schmid et al. demonstrated this strategy by functionalizing TLR agonist-loaded PLGA NPs with anti-PD-1 antibody fragments (Fig. 6D) [282]. These fragments allow for preferential targeting of activated PD-1+ T cells in the circulation and tumors, leading to modestly enhanced antitumor efficacy of R848 in multiple mouse models. To more directly target DCs, Figdor and colleagues developed PLGA NPs coated with PEG-lipids displaying antibodies targeting the DC marker DC-SIGN [283]. They observed a significant increase in binding and uptake by human monocyte-derived DCs and increased expression of CD80 and TNF-α in vitro. DEC-205 is another marker of cDC1 dendritic cells; i.v. infusion of DEC-205-targeted NPs in mice elicited significantly lower levels of systemic type I IFNs compared with soluble TLR7 agonists, while still inducing potent cytotoxic T cell responses following OVA immunization. Edwards and colleagues conjugated a TLR7 agonist (UC-1V50) to an anti-hCD20 antibody, rituximab, through the use of a bifunctional N-hydroxysuccinimide (NHS) linker. They demonstrated a significant increase in IL-12p40 secretion from RAW264.7 macrophages and specific binding to CD20+CD19+ B cells in vitro [284]. Andresen and colleagues used maleimide functionalized liposomes attached to murine anti-human DCIR (dendritic cell immunoreceptor) monoclonal antibodies (IgG1 specific for a nonhuman epitope) via Traut’s reagent (i.e., 2-iminothiolane) [285]. They observed preferential uptake of the nanoparticles by monocytes and mDCs when evaluated in vitro in PBMCs and significantly enhanced secretion of inflammatory cytokines such as IL-6 and TNF-α. Beyond active targeting moieties, some promising attempts have been made to bias uptake toward specific immune cell subsets or tissues by modifying the physicochemical properties of the carrier. Jensen and colleagues tested a TLR7 agonist encapsulated in liposomes composed of neutral, negatively charged, or positively charged phospholipids and found that positively charged formulations effectively biased the NPs to CD14+ monocytes in human whole blood, demonstrating a higher level of IL-12p40 secretion in these monocyte subsets and differentiation into CD14/DC-SIGN+ DCs, which are potent APCs that can stimulate both CD4 and CD8 T cell responses [286]. Neither anionic nor neutral liposomes could produce these effects, demonstrating potential for charge-based cellular targeting, which has been highlighted recently in several other studies [287,288]. These immune-cell biasing approaches in tandem with the other strategies described above could play a key role in making systemic administration of synthetic TLR7/8 agonists a clinical reality.
4.3. Localized (in situ) delivery approaches to improve intratumoral efficacy
Although systemic administration remains the most common approach for delivery of anticancer therapies, significant advances in interventional radiology that allow for minimally invasive access to almost every organ in the body such that several malignant lesions can be treated simultaneously or sequentially have shifted clinical trends toward local administration [289,290]. Following the FDA approval in 2015 of intratumoral delivery of talimogene laherparepvec (T-VEC), an oncolytic viral therapy [291], key discoveries have been made in intratumoral delivery that have encouraged further preclinical and clinical development of this route of administration. Such local therapy is capable of eliciting systemic anti-tumor immunity, as tumor cell killing stimulated in the treated tumor leads to antigen presentation in tumor-draining lymph nodes, and priming of new T cell responses against the tumor, a concept now referred to as “in situ vaccination.” These tumor-specific T cells traffic to the treated tumor site as well as to distal, untreated tumors [292], enabling systemic tumor regression following a localized treatment [293,294].
In Section 3.2, we briefly discussed localized delivery approaches for TLR7/8 agonists that have already made their way to the clinic. In particular, 3M-052/MEDI-9197, an imidazoquinoline bearing a C18 lipid moiety, has been extensively evaluated preclinically for antitumor efficacy as a monotherapy and in combination with checkpoint blockade, OX-40 agonist antibodies, and other anticancer therapies (Fig. 7A). Despite the minimal systemic immune activation observed in mouse models, however, the phase I clinical trial showed systemic toxicities following intratumoral administration, with most patients experiencing pyrexia, fatigue, chills, decreased lymphocyte count, nausea, injection site pain, and cytokine release syndrome [295]. Thus, although the C18 lipid moiety did lower systemic toxicity, it was not enough to justify phase II trials. Schwarz and colleagues demonstrated that a Poloxamer 407 thermogel formulation of 3M-052 showed a significant improvement in tumor pharmacokinetics, with 20% of the drug still present 2 weeks after intratumoral injection, as well as decreased tumor burden and improved survival in mice bearing melanoma tumors [296]. An initial spike in serum drug concentration still occurred at 6 h post injection, however, indicating that further optimization was necessary to localize the drug in the tumor microenvironment. Wightman et al. showed an improved localized response when 3M-052 was formulated in liposomes, indicating potential for nano-formulations to localize the effect of synthetic TLR7/8 agonists in the tumor microenvironment [297]. Other localized TLR7/8 agonists discussed in Section 3.2 include NKTR-262, which is based on a 4-arm PEG star polymer to which 4 TLR7/8 agonists are attached via a hydrolysable glycine linker; this construct showed improved intratumoral retention and lowered plasma concentration compared to free drug. Alum-TLR7 was further optimized by Wu and colleagues via a medicinal chemistry approach to allow for phosphonate modification and maximum alum adsorption to minimize systemic dissemination [298]. One of these compounds, LHC165, is currently in phase I trials in combination with anti-PD-1 spartalizumab, administered intratumorally bound to alum [299]. In terms of preclinical formulations in industry, ALT-702 uses a depot-forming peptide with a fluorocarbon tail conjugated to a TLR7/8 agonist; it showed improved intratumoral retention and minimal systemic levels of inflammatory cytokines [300]. Finally, Ascendis Pharma is developing a long-acting prodrug of R848 formed by conjugation of R848 onto hydrogel microbeads via proprietary linkers [301].
Figure 7. Localized approaches to maximize intra-tumoral efficacy.
(A) C-18 lipid moiety of 3M-052 improves retention at injection site for up to 28 days. (B) HA-R848 scaffold, administered immediately post tumor resection, significantly improves survival in 4T1 mouse model of metastatic breast cancer.
Beyond these formulations advancing to the clinic via industry, some promising preclinical platforms have emerged from academic research that have also been able to localize the effect of TLR7/8 agonists using biomaterial-based strategies. De Geest and colleagues developed amphiphilic di-block copolymers that self-assembled into NPs [302] that were bound to a TLR7/8 agonist and cross-linked with pH-sensitive bis-amino-ketals via amide bond formation between reactive PFP esters and primary amines of the TLR7/8 agonist and crosslinker. Upon peritumoral administration, they observed lower tumor burden in the B16 tumor model at levels similar to that of free TLR7/8 agonist but without the systemic side effects. Lim and colleagues developed squalene emulsions encapsulating R848 in oleic acid [303]. Upon intratumoral administration, they observed lowered systemic IL-6 and significantly improved local IL-6, MCP-1, and MIP-1α kinetics. Forrest et al. formulated α-tocopherol-modified R848 with tocopherol-modified hyaluronic acid using an emulsification-solvent evaporation method [304]. Upon intratumoral administration in 6 canines with mast cell cancer, 4 demonstrated a reduction in tumor burden, and lesions disappeared in 1 animal. Recent work by Johnson et al utilized ring opening metathesis polymerization to synthesize injectable polynorbornene (pNb)-based triblock bottlebrush copolymer hydrogels that localize the effect of R848 therapy upon intra-tumoral administration to minimize systemic side effects [305]. Here, it was shown that co-delivery of R848 with paclitaxel in optimized multicompartment hydrogels led to enhanced cure rates and reduced toxicity compared to free drugs and nanoparticle formulations in mice bearing CT26 tumors. Nuhn and colleagues also utilized ring opening metathesis polymerization to synthesize (pNb-PEG)-(pNb-pentafluorophenyl) micelles which were covalently conjugated to a TLR7 agonist and cross-linked with pH-responsive ketal bisamines to obtain nanogels [306]. Interestingly, the nanogels showed dose-dependent activation of RAW 264.7 cells, whereas the soluble chains obtained upon degradation of the nanogel were immunologically silent; that is, they did not show TLR7 activity. Finally, Zhang and coworkers demonstrated that platelet-membrane coated NPs carrying R848 improved therapeutic efficacy in the MC38 colon cancer model upon intra-tumoral administration as a result of enhanced bioavailability in the tumor [307].
In addition to these particulate formulations of TLR agonists, another paradigm is to implant drug-releasing biomaterial matrices into a tumor resection site to achieve sustained and localized TLR signaling at the site of surgical resection. For example, Goldberg et al. developed a biodegradable hydrogel scaffold prepared from crosslinked hyaluronic acid (HA) that entrapped R848 [308]. In murine models of surgical implantation of TLR agonist-loaded gels into tumor resection sites, they observed no local tumor recurrence for at least one month and significantly improved survival, with the efficacy dependent on type I IFN signaling, NK cells, and CD4 and CD8 T cells (Fig. 7B). Interestingly, the authors showed that these antitumor effects were observed only upon local administration of the scaffold, with minimal survival following intravenous injections or a local bolus dose of the free drug.
4.4. In search of synergy: combination delivery approaches
The combination of synthetic TLR7/8 agonists with other therapies offers significant potential to further enhance their potency. Combination immunotherapies are particularly ubiquitous in the context of cancer, with countless preclinical investigations and more than 1800 ongoing clinical trials in the United States alone combining immune-checkpoint blockade with other immunotherapies. TLR7/8 agonists are showing promising preclinical results in overcoming tumor resistance to checkpoint blockade [309-311]. In the vaccine realm, work by Pulendran and coworkers demonstrated that the live attenuated yellow fever vaccine, one of the most effective immunological interventions worldwide, administered to over 400 million people, in fact activates multiple TLRs on DCs to elicit an immune response [312]. It has long been known that TLR agonists can exhibit synergistic activation of DCs; for example, R848 combined with either a TLR4 agonist or a TLR3 agonist allowed synergistic stimulation of inflammatory cytokines in multiple human DC subsets [313]. It is likely that TLR7/8 activation may synergize with multiple innate immune sensors or other immunostimulatory pathways beyond just TLRs to potentiate vaccine or immunotherapy responses [314,315].
Drug carriers may have an important role to play in maximizing the impact of synergistic immune agonist drugs. Pulendran and coworkers utilized NPs similar to those described above in section 4.1.2. and demonstrated that R837 and monophosphoryl lipid A (a synthetic TLR4 agonist) encapsulated in PLGA NPs functioned synergistically when tested in multiple antigen models [316]. They observed a significant increase in antigen-specific neutralizing antibodies and enhanced persistence of germinal centers and plasma cell responses (Fig. 8A). These antibody responses were dependent on direct triggering of both TLRs on B cells and DCs, in addition to T cell help. The system was tested in other vaccination models of RMs and showed significantly improved levels of antigen-specific B cell responses as well as durable protection against rechallenge [317]. Toxicity issues—a key concern when combining therapies—limited the dose of the TLR agonists in these NPs [318]. This combination of TLR7/8 agonists with TLR4 agonists has been verified in numerous other studies [319-321]. For example, Carter and colleagues tested this combination encapsulated in anionic liposomes with a recombinant malaria antigen [319]. They observed increased levels of antigen-specific IFN-γ production and IgG2:IgG1 levels indicative of an improved Th1 response. Hook and colleagues looked beyond liposomal structures toward cubosomes (i.e., cubic phase liquid crystalline nanostructures formed in this case from lipids), achieving similar responses [320]. Additionally, Carson and colleagues observed rapid seroconversion, antigen sparing, and protective efficacy using their TLR4-TLR7 agonist combination [321]. Shattock and colleagues demonstrated dose- and administration route-dependence of the synergy between TLR4 and TLR7/8 agonists in a Gottingen minipig model [322].
Figure 8. Combination delivery approaches resulting in synergic efficacy.
(A) Combination of TLR7 agonist (R837) and TLR4 agonist (MPLA) encapsulated in PLGA particles synergistically improves antibody responses against H5N1-influenza-derived HA. (B) A single molecule containing TLR7 agonist (loxoribine), TLR4 agonist (pyrimido-indole) and TLR9 agonist (CpG ODN) improves antibody responses compared to the admixed formulation of the three agonists. (C) Combination of TLR7/8 agonist (R848) and anti-OX40 antibody demonstrates synergistic anti-tumor effects in A20 B cell lymphoma mouse model.
Taking TLR combinations a step further, Haynes and colleagues added a TLR9 agonist into the mix and observed a durable antigen-specific antibody response [323]. Moreover, Roy and colleagues loaded this combination onto PLGA NPs and demonstrated synergistic activity in the context of antigen cross-presentation in vitro as well as lymph node germinal center and T helper cell responses in vivo [324]. Linking IMDs with other TLR agonists in a single molecule has also been shown to enhance activation of NF-κB and inflammatory cytokines in vitro and improved antibody response in vivo (Fig. 8B) [325]. Analysis of APC activation by multifunctional conjugates linking ligands for multiple distinct TLRs into the same molecule indicates that synergy between TLR agonists depends upon the physical length of the linker bridging innate immune ligands, the choice of ligand combination, and the dose [266,326]. These studies represent important early steps toward elucidating structure–activity dependence of TLR synergies, and complement work aiming to define rational TLR combinations based on the interplay between different intracellular signaling pathways [327-333].
In addition to studies of TLR agonist combinations as vaccine adjuvants, other groups have focused on the potential of TLR7/8 ligands to synergize with other immunotherapy modalities for cancer treatment. Illidge and colleagues combined radiation therapy with systemic R848 and observed improved antitumor efficacy in multiple tumor models [334]. Levy et al. demonstrated that the synergistic effect of TLR7/8 agonists with anti-OX40 antibodies was explained by the fact that TLR7/8 agonists such as R848 induce expression of the OX40 target on CD4 T cells in the tumor microenvironment (Fig. 8C) [335]. Although TLR7/8 agonists could be substituted by TLR9 agonists, surprisingly, checkpoint inhibitors against PD-1, PD-L1, or CTLA-4 could not substitute for anti-OX40 antibodies. A number of other studies, however, have reported synergy between TLR7/8 agonists and checkpoint inhibitors in various other murine tumor models [336-339]. Other attempts to augment the efficacy of synthetic TLR7/8 agonists have included combinations with antibodies such as anti-EGFR and anti-HER2/neu, cytokines such as IL-2, and photothermal therapies [340-345].
5. Conclusion and Future Outlook
Substantial evidence from preclinical studies and clinical trials suggests that synthetic TLR7/8 ligands have the potential to be powerful immunomodulators, vaccine adjuvants, and cancer therapeutics, but challenges in achieving suitable efficacy while avoiding toxicities remain a significant barrier. Recent elucidations of crystal structures, SAR analyses, and delivery approaches have led to steady progress, though systemic administration of TLR7/8 agonists still faces challenges [346]. Moreover, defining optimal dosing thresholds and timing intervals will be critical given that research on these compounds has followed closely on the heels of progress made on synthetic TLR4 and TLR9 agonists wherein significant efforts toward understanding tolerability and circadian effects have been pursued [347-350]. TLR tolerance, which is defined as a transient state of refractoriness of TLRs to subsequent activation post initial dosing, has evolved to avoid the induction of auto-immunity through repeated TLR agonism; however, it has important implications for dosing schedules involving synthetic TLR7/8 agonists that aim to generate pro-inflammatory immune responses [351]. Although TLR7 tolerance has been observed in multiple studies, the mechanism of induction is still an active area of research [352-356]. Initial studies provide confidence that dosing schemes can be devised that avoid TLR7 tolerance though this phenomenon must be studied in further detail to ensure maximum efficacy of these compounds [357,358]. Moreover, the dependence of TLR expression and activity of agonists on circadian biology has yielded promising results in other agonist systems but is yet to be explored in detail for TLR7/8 agonism [359,360]. Finally, as touched upon briefly in the previous sub-section, defining the synergistic role of synthetic TLR7/8 agonists in combination with established as well as emerging treatments is of utmost importance in our endeavor to achieve long-lasting cures to cancer, infectious diseases, and allergic and autoimmune conditions.
Acknowledgments
This work was supported in part by the NIH (awards UM1 AI144462 and 1R01CA22046801), the Ragon Institute of MGH, MIT, and Harvard, MIT Koch Institute Frontier Research Program and the Marble Center for Nanomedicine. SB is a fellow of the Ludwig Center at MIT Koch Institute. DJI is an investigator of the Howard Hughes Medical Institute.
References
- [1].Miller RL, Gerster JF, Owens ML, Slade HB, a Tomai M, Review Article Imiquimod applied topically: a novel immune response modifier and new class of drug, Int J Immunopharmaco. 21 (1999) 1–14. 10.1016/s0192-0561(98)00068-x. [DOI] [PubMed] [Google Scholar]
- [2].O’Neill LAJ, Golenbock D, Bowie AG, The history of Toll-like receptors — redefining innate immunity, Nat Rev Immunol. 13 (2013) 453–460. 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- [3].Gerster JF, 1H-imidazo[4,5-c]quinolin-4-amines and antiviral use, 4,689,338, 1987. https://patentimages.storage.googleapis.com/f3/d6/dd/f2177153a97ae4/US4689338.pdf. [Google Scholar]
- [4].Ryu J, Yang FC, A Review of Topical Imiquimod in the Management of Basal Cell Carcinoma, Actinic Keratoses, and Other Skin Lesions, Clin Medicine Ther. 1 (2009) CMT.S1969. 10.4137/cmt.s1969. [DOI] [Google Scholar]
- [5].Tomai MA, Gibson SJ, Imbertson LM, Miller RL, Myhre PE, Reiter MJ, Wagner TL, Tamulinas CB, Beaurline JM, Gerster JF, Horton VL, Immunomodulating and antiviral activities of the imidazoquinoline S-28463, Antivir Res. 28 (1995) 253–264. 10.1016/0166-3542(95)00054-p. [DOI] [PubMed] [Google Scholar]
- [6].Hirota K, Kazaoka K, Niimoto I, Kumihara H, Sajiki H, Isobe Y, Takaku H, Tobe M, Ogita H, Ogino T, Ichii S, Kurimoto A, Kawakami H, Discovery of 8-hydroxyadenines as a novel type of interferon inducer., Journal of Medicinal Chemistry. 45 (2002) 5419–22. 10.1021/jm0203581. [DOI] [PubMed] [Google Scholar]
- [7].Dockrell DH, Kinghorn GR, Imiquimod and resiquimod as novel immunomodulators, J Antimicrob Chemoth. 48 (2001) 751–755. 10.1093/jac/48.6.751. [DOI] [PubMed] [Google Scholar]
- [8].Wu J, Huang D, Tyring S, Resiquimod: a new immune response modifier with potential as a vaccine adjuvant for Th1 immune responses, Antivir Res. 64 (2004) 79–83. 10.1016/j.antiviral.2004.07.002. [DOI] [PubMed] [Google Scholar]
- [9].Szeimies RM, Bichel J, Ortonne JP, Stockfleth E, Lee J, Meng T, A phase II dose-ranging study of topical resiquimod to treat actinic keratosis, Brit J Dermatol. 159 (2008) 205–210. 10.1111/j.1365-2133.2008.08615.x. [DOI] [PubMed] [Google Scholar]
- [10].Tarabadkar ES, Shinohara MM, Skin Directed Therapy in Cutaneous T-Cell Lymphoma, Frontiers Oncol. 9 (2019) 260. 10.3389/fonc.2019.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S, Small anti-viral compounds activate immune cells via the TLR7 MyD88–dependent signaling pathway, Nature Immunology. 3 (2002). 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- [12].Kawai T, Akira S, TLR signaling, Semin Immunol. 19 (2007) 24–32. 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- [13].Inglefield JR, Dumitru CD, Alkan SS, Gibson SJ, Lipson KE, Tomai MA, Larson CJ, Vasilakos JP, TLR7 agonist 852A inhibition of tumor cell proliferation is dependent on plasmacytoid dendritic cells and type I IFN., J Interf Cytokine Res Official J Int Soc Interf Cytokine Res. 28 (2008) 253–63. 10.1089/jir.2007.0097. [DOI] [PubMed] [Google Scholar]
- [14].Cheever MA, Twelve immunotherapy drugs that could cure cancers., Immunol Rev. 222 (2008) 357–68. 10.1111/j.1600-065x.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- [15].Engel AL, Holt GE, Lu H, The pharmacokinetics of Toll-like receptor agonists and the impact on the immune system, Expert Rev Clin Phar. 4 (2014) 275–289. 10.1586/ecp.11.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dong Z, Xiong L, Zhang W, Gibson PG, Wang T, Lu Y, Wang G, Li H, Wang F, Holding the Inflammatory System in Check: TLRs and Their Targeted Therapy in Asthma, Mediat Inflamm. 2016 (2016) 1–8. 10.1155/2016/2180417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Anwar MA, Shah M, Kim J, Choi S, Recent clinical trends in Toll-like receptor targeting therapeutics, Med Res Rev. 39 (2018) 1053–1090. 10.1002/med.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Medzhitov R, Toll-like receptors and innate immunity, Nat Rev Immunol. 1 (2001) 135–145. 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- [19].Shimizu T, Structural insights into ligand recognition and regulation of nucleic acid-sensing Toll-like receptors, Curr Opin Struc Biol. 47 (2017) 52–59. 10.1016/j.sbi.2017.05.010. [DOI] [PubMed] [Google Scholar]
- [20].Groß CJ, Mishra R, Schneider KS, Médard G, Wettmarshausen J, Dittlein DC, Shi H, Gorka O, Koenig P-A, Fromm S, Magnani G, Ćiković T, Hartjes L, Smollich J, Robertson AAB, Cooper MA, Schmidt-Supprian M, Schuster M, Schroder K, Broz P, Traidl-Hoffmann C, Beutler B, Kuster B, Ruland J, Schneider S, Perocchi F, Groß O, K + Efflux-Independent NLRP3 Inflammasome Activation by Small Molecules Targeting Mitochondria, Immunity. 45 (2016) 761–773. 10.1016/j.immuni.2016.08.010. [DOI] [PubMed] [Google Scholar]
- [21].Milling L, Zhang Y, Irvine DJ, Delivering safer immunotherapies for cancer, Adv Drug Deliver Rev. 114 (2017) 79–101. 10.1016/j.addr.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Takeda K, Kaisho T, Akira S, Toll-like receptors., Annual Review of Immunology. 21 (2003) 335–76. 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- [23].Diebold SS, Kaisho T, Hemmi H, Akira S, e Sousa CR, Innate Antiviral Responses by Means of TLR7-Mediated Recognition of Single-Stranded RNA, Science. 303 (2004) 1529–1531. 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- [24].Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S, Species-Specific Recognition of Single-Stranded RNA via Toll-like Receptor 7 and 8, Science. 303 (2004) 1526–1529. 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- [25].Takeuchi O, Akira S, Pattern Recognition Receptors and Inflammation, Cell. 140 (2010) 805–820. 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- [26].Leulier F, Lemaitre B, Toll-like receptors — taking an evolutionary approach, Nat Rev Genet. 9 (2008) 165–178. 10.1038/nrg2303. [DOI] [PubMed] [Google Scholar]
- [27].Akira S, Takeda K, Kaisho T, Toll-like receptors: critical proteins linking innate and acquired immunity, Nature Immunology. 2 (2001). 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- [28].Janssens S, Beyaert R, A universal role for MyD88 in TLR/IL-1R-mediated signaling, Trends Biochem Sci. 27 (2002) 474–482. 10.1016/s0968-0004(02)02145-x. [DOI] [PubMed] [Google Scholar]
- [29].Kawagoe T, Sato S, Jung A, Yamamoto M, Matsui K, Kato H, Uematsu S, Takeuchi O, Akira S, Essential role of IRAK-4 protein and its kinase activity in Toll-like receptor–mediated immune responses but not in TCR signaling, J Exp Medicine. 204 (2007) 1013–1024. 10.1084/jem.20061523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kawai T, Akira S, TLR signaling, Cell Death Differ. 13 (2006) 816–825. 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- [31].Gay NJ, Symmons MF, Gangloff M, Bryant CE, Assembly and localization of Toll-like receptor signalling complexes, Nat Rev Immunol. 14 (2014) 546–558. 10.1038/nri3713. [DOI] [PubMed] [Google Scholar]
- [32].Kawai T, Akira S, The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors, Nat Immunol. 11 (2010) 373–84. 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- [33].Bell E, Intracellular trafficking, IRF7 and type-I-IFN responses, Nat Rev Immunol. 5 (2005) 361–361. 10.1038/nri1625. [DOI] [Google Scholar]
- [34].Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdörfer B, Giese T, Endres S, Hartmann G, Quantitative Expression of Toll-Like Receptor 1–10 mRNA in Cellular Subsets of Human Peripheral Blood Mononuclear Cells and Sensitivity to CpG Oligodeoxynucleotides, J Immunol. 168 (2002) 4531–4537. 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- [35].Iwasaki A, Medzhitov R, Toll-like receptor control of the adaptive immune responses, Nat Immunol. 5 (2004) 987–995. 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- [36].e Sousa CR, Toll-like receptors and dendritic cells: for whom the bug tolls., Seminars in Immunology. 16 (2004) 27–34. 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- [37].Iwasaki A, Medzhitov R, Control of adaptive immunity by the innate immune system, Nat Immunol. 16 (2015) 343–353. 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Trinchieri G, Sher A, Cooperation of Toll-like receptor signals in innate immune defence, Nat Rev Immunol. 7 (2007) 179–190. 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- [39].Petes C, Odoardi N, Gee K, The Toll for Trafficking: Toll-Like Receptor 7 Delivery to the Endosome, Front Immunol. 8 (2017) 1075. 10.3389/fimmu.2017.01075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Merad M, Sathe P, Helft J, Miller J, Mortha A, The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting., Annu Rev Immunol. 31 (2013) 563–604. 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Larangé A, Antonios D, Pallardy M, Kerdine-Römer S, TLR7 and TLR8 agonists trigger different signaling pathways for human dendritic cell maturation, J Leukocyte Biol. 85 (2009) 673–83. 10.1189/jlb.0808504. [DOI] [PubMed] [Google Scholar]
- [42].Edwards AD, Diebold SS, Slack EMC, Tomizawa H, Hemmi H, Kaisho T, Akira S, e Sousa CR, Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8α+ DC correlates with unresponsiveness to imidazoquinolines, European Journal of Immunology. 33 (2003) 827–833. 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- [43].Ketloy C, Engering A, Srichairatanakul U, Limsalakpetch A, Yongvanitchit K, Pichyangkul S, Ruxrungtham K, Expression and function of Toll-like receptors on dendritic cells and other antigen presenting cells from non-human primates, Vet Immunol Immunop. 125 (2008) 18–30. 10.1016/j.vetimm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- [44].Doxsee CL, Riter TR, Reiter MJ, Gibson SJ, Vasilakos JP, Kedl RM, The Immune Response Modifier and Toll-Like Receptor 7 Agonist S-27609 Selectively Induces IL-12 and TNF-α Production in CD11c+CD11b+CD8− Dendritic Cells, The Journal of Immunology. 171 (2003) 1156–1163. 10.4049/jimmunol.171.3.1156. [DOI] [PubMed] [Google Scholar]
- [45].Worbs T, Hammerschmidt SI, Förster R, Dendritic cell migration in health and disease, Nat Rev Immunol. 17 (2017) 30–48. 10.1038/nri.2016.116. [DOI] [PubMed] [Google Scholar]
- [46].Kapsenberg ML, Dendritic-cell control of pathogen-driven T-cell polarization, Nat Rev Immunol. 3 (2003) 984–993. 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- [47].Wagner TL, Ahonen CL, Couture AM, Gibson SJ, Miller RL, Smith RM, Reiter MJ, Vasilakos JP, Tomai MA, Modulation of TH1 and TH2 Cytokine Production with the Immune Response Modifiers, R-848 and Imiquimod, Cell Immunol. 191 (1999) 10–19. 10.1006/cimm.1998.1406. [DOI] [PubMed] [Google Scholar]
- [48].Peng G, Guo Z, Kiniwa Y, shin Voo K, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang R-F, Toll-Like Receptor 8-Mediated Reversal of CD4+ Regulatory T Cell Function, Science. 309 (2005) 1380–1384. 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- [49].Kabelitz D, Expression and function of Toll-like receptors in T lymphocytes, Curr Opin Immunol. 19 (2007) 39–45. 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- [50].Slifka MK, Whitton JL, Antigen-Specific Regulation of T Cell–Mediated Cytokine Production, Immunity. 12 (2000) 451–457. 10.1016/s1074-7613(00)80197-1. [DOI] [PubMed] [Google Scholar]
- [51].Guiducci C, Coffman RL, Barrat FJ, Signalling pathways leading to IFN-α production in human plasmacytoid dendritic cell and the possible use of agonists or antagonists of TLR7 and TLR9 in clinical indications, J Intern Med. 265 (2009) 43–57. 10.1111/j.1365-2796.2008.02050.x. [DOI] [PubMed] [Google Scholar]
- [52].Krieg AM, Toll-like receptor 9 (TLR9) agonists in the treatment of cancer, Oncogene. 27 (2008) 161–167. 10.1038/sj.onc.1210911. [DOI] [PubMed] [Google Scholar]
- [53].Ergun SL, Li L, Structural Insights into STING Signaling, Trends Cell Biol. 30 (2020) 399–407. 10.1016/j.tcb.2020.01.010. [DOI] [PubMed] [Google Scholar]
- [54].Larson SR, Atif SM, Gibbings SL, Thomas SM, Prabagar MG, Danhorn T, Leach SM, Henson PM, Jakubzick CV, Ly6C+ monocyte efferocytosis and cross-presentation of cell-associated antigens, Cell Death Differ. 23 (2016) 997–1003. 10.1038/cdd.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Janke M, Poth J, Wimmenauer V, Giese T, Coch C, Barchet W, Schlee M, Hartmann G, Selective and direct activation of human neutrophils but not eosinophils by Toll-like receptor 8, J Allergy Clin Immun. 123 (2009) 1026–1033. 10.1016/j.jaci.2009.02.015. [DOI] [PubMed] [Google Scholar]
- [56].Regli IB, Passelli K, Martínez-Salazar B, Amore J, Hurrell BP, Müller AJ, Tacchini-Cottier F, TLR7 Sensing by Neutrophils Is Critical for the Control of Cutaneous Leishmaniasis, Cell Reports. 31 (2020) 107746. 10.1016/j.celrep.2020.107746. [DOI] [PubMed] [Google Scholar]
- [57].Matsushima H, Yamada N, Matsue H, Shimada S, TLR3-, TLR7-, and TLR9-Mediated Production of Proinflammatory Cytokines and Chemokines from Murine Connective Tissue Type Skin-Derived Mast Cells but Not from Bone Marrow-Derived Mast Cells, J Immunol. 173 (2004) 531–541. 10.4049/jimmunol.173.1.531. [DOI] [PubMed] [Google Scholar]
- [58].Hayashi T, Cottam HB, Chan M, Jin G, Tawatao RI, Crain B, Ronacher L, Messer K, Carson DA, Corr M, Mast cell-dependent anorexia and hypothermia induced by mucosal activation of Toll-like receptor 7, American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 295 (2008). 10.1152/ajpregu.00527.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nagase H, Okugawa S, Ota Y, Yamaguchi M, Tomizawa H, Matsushima K, Ohta K, Yamamoto K, Hirai K, Expression and Function of Toll-Like Receptors in Eosinophils: Activation by Toll-Like Receptor 7 Ligand, The Journal of Immunology. 171 (2003) 3977–3982. 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- [60].Butchar JP, Mehta P, Justiniano SE, Guenterberg KD, Kondadasula S-V, Mo X, Chemudupati M, Kanneganti T-D, Amer A, Muthusamy N, Jarjoura D, Marsh CB, Carson WE, Byrd JC, Tridandapani S, Reciprocal Regulation of Activating and Inhibitory Fcγ Receptors by TLR7/8 Activation: Implications for Tumor Immunotherapy, Clin Cancer Res. 16 (2010) 2065–2075. 10.1158/1078-0432.ccr-09-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Applequist SE, Wallin RPA, Ljunggren H, Variable expression of Toll-like receptor in murine innate and adaptive immune cell lines, Int Immunol. 14 (2002) 1065–1074. 10.1093/intimm/dxf069. [DOI] [PubMed] [Google Scholar]
- [62].Hayashi F, Means TK, Luster AD, Toll-like receptors stimulate human neutrophil function, Blood. 102 (2003) 2660–2669. 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- [63].Wang JP, Bowen GN, Padden C, Cerny A, Finberg RW, Newburger PE, Kurt-Jones EA, Toll-like receptor–mediated activation of neutrophils by influenza A virus, Blood. 112 (2008) 2028–2034. 10.1182/blood-2008-01-132860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hou B, Reizis B, DeFranco AL, Toll-like Receptors Activate Innate and Adaptive Immunity by using Dendritic Cell-Intrinsic and -Extrinsic Mechanisms, Immunity. 29 (2008) 272–282. 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Desch AN, Gibbings SL, Clambey ET, Janssen WJ, Slansky JE, Kedl RM, Henson PM, Jakubzick C, Dendritic cell subsets require cis-activation for cytotoxic CD8 T-cell induction, Nature Communications. 5 (2014). 10.1038/ncomms5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Spörri R, e Sousa CR, Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function, Nat Immunol. 6 (2005) 163–170. 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- [67].Mouriès J, Moron G, Schlecht G, Escriou N, Dadaglio G, Leclerc C, Plasmacytoid dendritic cells efficiently cross-prime naive T cells in vivo after TLR activation, Blood. 112 (2008) 3713–3722. 10.1182/blood-2008-03-146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bishop GA, Hsing Y, Hostager BS, Jalukar SV, Ramirez LM, Tomai MA, Molecular Mechanisms of B Lymphocyte Activation by the Immune Response Modifier R-848, J Immunol. 165 (2000) 5552–5557. 10.4049/jimmunol.165.10.5552. [DOI] [PubMed] [Google Scholar]
- [69].Gujer C, Sundling C, Seder RA, Hedestam GBK, Loré K, Human and rhesus plasmacytoid dendritic cell and B-cell responses to Toll-like receptor stimulation, Immunology. 134 (2011) 257–269. 10.1111/j.1365-2567.2011.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Pettengill MA, van Haren SD, Li N, Dowling DJ, Bergelson I, Jans J, Ferwerda G, Levy O, Distinct TLR-mediated cytokine production and immunoglobulin secretion in human newborn naïve B cells, Innate Immun. 22 (2016) 433–443. 10.1177/1753425916651985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gururajan M, Jacob J, Pulendran B, Toll-Like Receptor Expression and Responsiveness of Distinct Murine Splenic and Mucosal B-Cell Subsets, PLoS ONE. 2 (2007). 10.1371/journal.pone.0000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bishop GA, Ramirez LM, Baccam M, Busch LK, Pederson LK, Tomai MA, The Immune Response Modifier Resiquimod Mimics CD40-Induced B Cell Activation, Cell Immunol. 208 (2001) 9–17. 10.1006/cimm.2001.1769. [DOI] [PubMed] [Google Scholar]
- [73].Rubtsova K, Rubtsov AV, Halemano K, Li SX, Kappler JW, Santiago ML, Marrack P, T Cell Production of IFNγ in Response to TLR7/IL-12 Stimulates Optimal B Cell Responses to Viruses, Plos One. 11 (2016) e0166322. 10.1371/journal.pone.0166322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Tanji H, Ohto U, Shibata T, Miyake K, Shimizu T, Structural Reorganization of the Toll-Like Receptor 8 Dimer Induced by Agonistic Ligands, Science. 339 (2013) 1426–1429. 10.1126/science.1229159. [DOI] [PubMed] [Google Scholar]
- [75].Zhang Z, Ohto U, Shibata T, Krayukhina E, Taoka M, Yamauchi Y, Tanji H, Isobe T, Uchiyama S, Miyake K, Shimizu T, Structural Analysis Reveals that Toll-like Receptor 7 Is a Dual Receptor for Guanosine and Single-Stranded RNA, Immunity. 45 (2016) 737–748. 10.1016/j.immuni.2016.09.011. [DOI] [PubMed] [Google Scholar]
- [76].Gerster JF, Lindstrom KJ, Miller RL, Tomai MA, Birmachu W, Bomersine SN, Gibson SJ, Imbertson LM, Jacobson JR, Knafla RT, Maye PV, Nikolaides N, Oneyemi FY, Parkhurst GJ, Pecore SE, Reiter MJ, Scribner LS, Testerman TL, Thompson NJ, Wagner TL, Weeks CE, Andre J-D, Lagain D, Bastard Y, Lupu M, Synthesis and Structure–Activity-Relationships of 1H-Imidazo[4,5-c]quinolines That Induce Interferon Production, J Med Chem. 48 (2005) 3481–3491. 10.1021/jm049211v. [DOI] [PubMed] [Google Scholar]
- [77].Shukla NM, Malladi SS, Mutz CA, Balakrishna R, David SA, Structure–Activity Relationships in Human Toll-Like Receptor 7-Active Imidazoquinoline Analogues, J Med Chem. 53 (2010) 4450–65. 10.1021/jm100358c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ganapathi L, Haren SV, Dowling DJ, Bergelson I, Shukla NM, Malladi SS, Balakrishna R, Tanji H, Ohto U, Shimizu T, David SA, Levy O, The Imidazoquinoline Toll-Like Receptor-7/8 Agonist Hybrid-2 Potently Induces Cytokine Production by Human Newborn and Adult Leukocytes, Plos One. 10 (2015) e0134640. 10.1371/journal.pone.0134640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Shi C, Xiong Z, Chittepu P, Aldrich CC, Ohlfest JR, Ferguson DM, Discovery of Imidazoquinolines with Toll-Like Receptor 7/8 Independent Cytokine Induction, Acs Med Chem Lett. 3 (2012) 501–504. 10.1021/ml300079e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Schiaffo CE, Shi C, Xiong Z, Olin M, Ohlfest JR, Aldrich CC, Ferguson DM, Structure–Activity Relationship Analysis of Imidazoquinolines with Toll-like Receptors 7 and 8 Selectivity and Enhanced Cytokine Induction, J Med Chem. 57 (2014) 339–347. 10.1021/jm4004957. [DOI] [PubMed] [Google Scholar]
- [81].Larson P, Kucaba TA, Xiong Z, Olin M, Griffith TS, Ferguson DM, Design and Synthesis of N1-Modified Imidazoquinoline Agonists for Selective Activation of Toll-like Receptors 7 and 8, Acs Med Chem Lett. 8 (2017) 1148–1152. 10.1021/acsmedchemlett.7b00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Beesu M, Caruso G, Salyer ACD, Khetani KK, Sil D, Weerasinghe M, Tanji H, Ohto U, Shimizu T, David SA, Structure-Based Design of Human TLR8-Specific Agonists with Augmented Potency and Adjuvanticity, J Med Chem. 58 (2015) 7833–7849. 10.1021/acs.jmedchem.5b01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Shukla NM, Mutz CA, Malladi SS, Warshakoon HJ, Balakrishna R, David SA, Toll-Like Receptor (TLR)-7 and -8 Modulatory Activities of Dimeric Imidazoquinolines, J Med Chem. 55 (2012) 1106–16. 10.1021/jm2010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Shukla NM, Salunke DB, Balakrishna R, Mutz CA, Malladi SS, David SA, Potent Adjuvanticity of a Pure TLR7-Agonistic Imidazoquinoline Dendrimer, Plos One. 7 (2012) e43612. 10.1371/journal.pone.0043612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Yoo E, Crall BM, Balakrishna R, Malladi SS, Fox LM, Hermanson AR, David SA, Structure–activity relationships in Toll-like receptor 7 agonistic 1H-imidazo[4,5-c]pyridines, Org Biomol Chem. 11 (2013) 6526–6545. 10.1039/c3ob40816g. [DOI] [PubMed] [Google Scholar]
- [86].Yoo E, Salunke DB, Sil D, Guo X, Salyer ACD, Hermanson AR, Kumar M, Malladi SS, Balakrishna R, Thompson WH, Tanji H, Ohto U, Shimizu T, David SA, Determinants of Activity at Human Toll-like Receptors 7 and 8: Quantitative Structure–Activity Relationship (QSAR) of Diverse Heterocyclic Scaffolds, J Med Chem. 57 (2014) 7955–7970. 10.1021/jm500744f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kokatla HP, Yoo E, Salunke DB, Sil D, Ng CF, Balakrishna R, Malladi SS, Fox LM, David SA, Toll-like receptor-8 agonistic activities in C2, C4, and C8 modified thiazolo[4,5-c]quinolines, Org Biomol Chem. 11 (2013) 1179. 10.1039/c2ob26705e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Beesu M, Salyer ACD, Brush MJH, Trautman KL, Hill JK, David SA, Identification of High-Potency Human TLR8 and Dual TLR7/TLR8 Agonists in Pyrimidine-2,4-diamines, J Med Chem. 60 (2017) 2084–2098. 10.1021/acs.jmedchem.6b01860. [DOI] [PubMed] [Google Scholar]
- [89].Asami J, Shimizu T, Structural and functional understanding of the Toll-like receptors, Protein Sci. (2021). 10.1002/pro.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zhang Z, Ohto U, Shibata T, Taoka M, Yamauchi Y, Sato R, Shukla NM, David SA, Isobe T, Miyake K, Shimizu T, Structural Analyses of Toll-like Receptor 7 Reveal Detailed RNA Sequence Specificity and Recognition Mechanism of Agonistic Ligands, Cell Reports. 25 (2018) 3371–3381.e5. 10.1016/j.celrep.2018.11.081. [DOI] [PubMed] [Google Scholar]
- [91].Miyake K, Shibata T, Ohto U, Shimizu T, Emerging roles of the processing of nucleic acids and Toll-like receptors in innate immune responses to nucleic acids, J Leukocyte Biol. 101 (2017) 135–142. 10.1189/jlb.4mr0316-108r. [DOI] [PubMed] [Google Scholar]
- [92].Maeda K, Akira S, TLR7 Structure: Cut in Z-Loop, Immunity. 45 (2016) 705–707. 10.1016/j.immuni.2016.10.003. [DOI] [PubMed] [Google Scholar]
- [93].Sakaniwa K, Shimizu T, Targeting the innate immune receptor TLR8 using small-molecule agents, Acta Crystallogr Sect D Struct Biology. 76 (2020) 621–629. 10.1107/s2059798320006518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Isobe Y, Kurimoto A, Tobe M, Hashimoto K, Nakamura T, Norimura K, Ogita H, Takaku H, Synthesis and Biological Evaluation of Novel 9-Substituted-8-Hydroxyadenine Derivatives as Potent Interferon Inducers, J Med Chem. 49 (2006) 2088–2095. 10.1021/jm051089s. [DOI] [PubMed] [Google Scholar]
- [95].Roethle PA, McFadden RM, Yang H, Hrvatin P, Hui H, Graupe M, Gallagher B, Chao J, Hesselgesser J, Duatschek P, Zheng J, Lu B, Tumas DB, Perry J, Halcomb RL, Identification and Optimization of Pteridinone Toll-like Receptor 7 (TLR7) Agonists for the Oral Treatment of Viral Hepatitis, J Med Chem. 56 (2013) 7324–7333. 10.1021/jm400815m. [DOI] [PubMed] [Google Scholar]
- [96].McGowan DC, Latest Advances in Small Molecule TLR 7/8 Agonist Drug Research, Curr Top Med Chem. 19 (2019) 2228–2238. 10.2174/1568026619666191009165418. [DOI] [PubMed] [Google Scholar]
- [97].Patinote C, Karroum NB, Moarbess G, Cirnat N, Kassab I, Bonnet P-A, Deleuze-Masquéfa C, Agonist and antagonist ligands of toll-like receptors 7 and 8: Ingenious tools for therapeutic purposes, Eur J Med Chem. 193 (2020) 112238. 10.1016/j.ejmech.2020.112238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Wang Y, Zhang S, Li H, Wang H, Zhang T, Hutchinson MR, Yin H, Wang X, Small-Molecule Modulators of Toll-like Receptors, Accounts Chem Res. 53 (2020) 1046–1055. 10.1021/acs.accounts.9b00631. [DOI] [PubMed] [Google Scholar]
- [99].Kurimoto A, Tobe M, Ogita H, Ogino T, Takaku H, Ichii S, Kawakami H, Isobe Y, Prodrugs of 9-Benzyl-8-hydroxy-2-(2-hydroxyethylthio)adenine: Potent Interferon Inducing Agents in Monkeys, Chem Pharm Bull. 52 (2004) 466–469. 10.1248/cpb.52.466. [DOI] [PubMed] [Google Scholar]
- [100].Nakamura T, Wada H, Kurebayashi H, McInally T, Bonnert R, Isobe Y, Synthesis and evaluation of 8-oxoadenine derivatives as potent Toll-like receptor 7 agonists with high water solubility, Bioorg Med Chem Lett. 23 (2013) 669–672. 10.1016/j.bmcl.2012.11.114. [DOI] [PubMed] [Google Scholar]
- [101].Heil F, Ahmad-Nejad P, Hemmi H, Hochrein H, Ampenberger F, Gellert T, Dietrich H, Lipford G, Takeda K, Akira S, Wagner H, Bauer S, The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily, Eur J Immunol. 33 (2003) 2987–2997. 10.1002/eji.200324238. [DOI] [PubMed] [Google Scholar]
- [102].Evans JT, Bess LS, Mwakwari SC, Livesay MT, Li Y, Cybulski V, Johnson DA, Bazin HG, Synthetic Toll-like Receptors 7 and 8 Agonists: Structure–Activity Relationship in the Oxoadenine Series, Acs Omega. 4 (2019) 15665–15677. 10.1021/acsomega.9b02138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Bazin HG, Li Y, Khalaf JK, Mwakwari S, Livesay MT, Evans JT, Johnson DA, Structural requirements for TLR7-selective signaling by 9-(4-piperidinylalkyl)-8-oxoadenine derivatives., Bioorg Med Chem Lett. 25 (2015) 1318–23. 10.1016/j.bmcl.2015.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Weterings JJ, Khan S, van Noort G.J. van der H., Melief CJM, Overkleeft HS, van der Burg SH, Ossendorp F, van der Marel GA, Filippov DV, 2-Azidoalkoxy-7-hydro-8-oxoadenine derivatives as TLR7 agonists inducing dendritic cell maturation, Bioorg Med Chem Lett. 19 (2009) 2249–2251. 10.1016/j.bmcl.2009.02.095. [DOI] [PubMed] [Google Scholar]
- [105].Yu H, Jin H, Sun L, Zhang L, Sun G, Wang Z, Yu Y, Toll-Like Receptor 7 Agonists: Chemical Feature Based Pharmacophore Identification and Molecular Docking Studies, Plos One. 8 (2013) e56514. 10.1371/journal.pone.0056514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Harrison LI, Stoesz JD, Battiste JL, Nelson RJ, Zarraga IE, A pharmaceutical comparison of different commercially available imiquimod 5% cream products, J Dermatol Treat. 20 (2009) 160–164. 10.1080/09546630802513693. [DOI] [PubMed] [Google Scholar]
- [107].Chollet JL, Jozwiakowski MJ, Phares KR, Reiter MJ, Roddy PJ, Schultz HJ, Ta QV, Tomai MA, Development of a Topically Active Imiquimod Formulation, Pharm Dev Technol. 4 (1999) 35–43. 10.1080/10837459908984222. [DOI] [PubMed] [Google Scholar]
- [108].Sauder DN, Imiquimod: modes of action, Brit J Dermatol. 149 (2003) 5–8. 10.1046/j.0366-077x.2003.05628.x. [DOI] [PubMed] [Google Scholar]
- [109].Samuel CE, Antiviral Actions of Interferons, Clin Microbiol Rev. 14 (2001) 778–809. 10.1128/cmr.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Silverman RH, Viral Encounters with 2′,5′-Oligoadenylate Synthetase and RNase L during the Interferon Antiviral Response, J Virol. 81 (2007) 12720–12729. 10.1128/jvi.01471-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Palamara F, Meindl S, Holcmann M, Lührs P, Stingl G, Sibilia M, Identification and Characterization of pDC-Like Cells in Normal Mouse Skin and Melanomas Treated with Imiquimod, J Immunol. 173 (2004) 3051–3061. 10.4049/jimmunol.173.5.3051. [DOI] [PubMed] [Google Scholar]
- [112].Vidal D, Topical Imiquimod: Mechanism of Action and Clinical Applications, Mini-Rev Med Chem. 6 (2006) 499–503. 10.2174/138955706776876131. [DOI] [PubMed] [Google Scholar]
- [113].Beglin M, Melar-New M, Laimins L, Human papillomaviruses and the interferon response., J Interf Cytokine Res Official J Int Soc Interf Cytokine Res. 29 (2009) 629–35. 10.1089/jir.2009.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Dahl MV, Imiquimod: A cytokine inducer, J Am Acad Dermatol. 47 (2002) S205–S208. 10.1067/mjd.2002.126586. [DOI] [PubMed] [Google Scholar]
- [115].Suzuki H, Wang B, Shivji GM, Toto P, Amerio P, Sauder DN, Tomai MA, Miller RL, Imiquimod, a Topical Immune Response Modifier, Induces Migration of Langerhans Cells 1 1 The authors have declared a conflict of interest., J Invest Dermatol. 114 (2000) 135–141. 10.1046/j.1523-1747.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- [116].Perry CM, Lamb HM, Topical Imiquimod: A Review of its Use in Genital Warts, Drugs. 58 (1999) 375–390. 10.2165/00003495-199958020-00017. [DOI] [PubMed] [Google Scholar]
- [117].Edwards L, Ferenczy A, Eron L, Baker D, Owens ML, Fox TL, Hougham AJ, Schmitt KA, Self-administered Topical 5% Imiquimod Cream for External Anogenital Warts, Arch Dermatol. 134 (1998) 25. 10.1001/archderm.134.1.25. [DOI] [PubMed] [Google Scholar]
- [118].Deza G, Martin-Ezquerra G, Curto-Barredo L, García JV, Pujol RM, Successful treatment of hypertrophic herpes simplex genitalis in HIV-infected patient with topical imiquimod, J Dermatology. 42 (2015) 1176–1178. 10.1111/1346-8138.12969. [DOI] [PubMed] [Google Scholar]
- [119].Miller RL, Imbertson LM, Reiter MJ, Gerster JF, Treatment of primary herpes simplex virus infection in guinea pigs by imiquimod, Antivir Res. 44 (1999) 31–42. 10.1016/s0166-3542(99)00052-2. [DOI] [PubMed] [Google Scholar]
- [120].Schacker TW, Conant M, Thoming C, Stanczak T, Wang Z, Smith M, Imiquimod 5-Percent Cream Does Not Alter the Natural History of Recurrent Herpes Genitalis: a Phase II, Randomized, Double-Blind, Placebo-Controlled Study, Antimicrob Agents Ch. 46 (2002) 3243–3248. 10.1128/aac.46.10.3243-3248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Winters U, Daayana S, Lear JT, Tomlinson AE, Elkord E, Stern PL, Kitchener HC, Clinical and Immunologic Results of a Phase II Trial of Sequential Imiquimod and Photodynamic Therapy for Vulval Intraepithelial Neoplasia, Clin Cancer Res. 14 (2008) 5292–5299. 10.1158/1078-0432.ccr-07-4760. [DOI] [PubMed] [Google Scholar]
- [122].Daayana S, Elkord E, Winters U, Pawlita M, Roden R, Stern PL, Kitchener HC, Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia, Brit J Cancer. 102 (2010) 1129–1136. 10.1038/sj.bjc.6605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Wouters T, Hendriks N, Koeneman M, Kruse A-J, van de Sande A, van Beekhuizen HJ, Gerestein KG, Bekkers RLM, Piek JMJ, Systemic adverse events in imiquimod use for cervical intraepithelial neoplasia – A case series, Case Reports Women’s Heal. 21 (2019) e00105. 10.1016/j.crwh.2019.e00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Hadley G, Derry S, Moore RA, Imiquimod for Actinic Keratosis: Systematic Review and Meta-Analysis, J Invest Dermatol. 126 (2006) 1251–1255. 10.1038/sj.jid.5700264. [DOI] [PubMed] [Google Scholar]
- [125].Uhlenhake EE, Optimal treatment of actinic keratoses., Clin Interv Aging. 8 (2013) 29–35. 10.2147/cia.s31930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Lebwohl M, Dinehart S, Whiting D, Lee PK, Tawfik N, Jorizzo J, Lee JH, Fox TL, Imiquimod 5% cream for the treatment of actinic keratosis: results from two phase III, randomized, double-blind, parallel group, vehicle-controlled trials, J Am Acad Dermatol. 50 (2004) 714–721. 10.1016/j.jaad.2003.12.010. [DOI] [PubMed] [Google Scholar]
- [127].Geisse J, Caro I, Lindholm J, Golitz L, Stampone P, Owens M, Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from two phase III, randomized, vehicle-controlled studies, J Am Acad Dermatol. 50 (2004) 722–733. 10.1016/j.jaad.2003.11.066. [DOI] [PubMed] [Google Scholar]
- [128].Wysocka M, Newton S, Benoit BM, Introcaso C, Hancock AS, Chehimi J, Richardson SK, Gelfand JM, Montaner LJ, Rook AH, Synthetic Imidazoquinolines Potently and Broadly Activate the Cellular Immune Response of Patients with Cutaneous T-Cell Lymphoma: Synergy with Interferon-γ Enhances Production of Interleukin-12, Clin Lymphoma Myeloma. 7 (2007) 524–534. 10.3816/clm.2007.n.037. [DOI] [PubMed] [Google Scholar]
- [129].Suchin KR, Junkins-Hopkins JM, Rook AH, Treatment of Stage IA Cutaneous T-Cell Lymphoma With Topical Application of the Immune Response Modifier Imiquimod, Arch Dermatol. 138 (2002) 1137. 10.1001/archderm.138.9.1137. [DOI] [PubMed] [Google Scholar]
- [130].Rook AH, Gelfand JM, Wysocka M, Troxel AB, Benoit B, Surber C, Elenitsas R, Buchanan MA, Leahy DS, Watanabe R, Kirsch IR, Kim EJ, Clark RA, Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma, Blood. 126 (2015) 1452–61. 10.1182/blood-2015-02-630335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Wolf IH, Smolle J, Binder B, Cerroni L, Richtig E, Kerl H, Topical Imiquimod in the Treatment of Metastatic Melanoma to Skin, Arch Dermatol. 139 (2003) 273. 10.1001/archderm.139.3.273. [DOI] [PubMed] [Google Scholar]
- [132].Narayan R, Nguyen H, Bentow JJ, Moy L, Lee DK, Greger S, Haskell J, Vanchinathan V, Chang P-L, Tsui S, Konishi T, Comin-Anduix B, Dauphine C, Vargas HI, Economou JS, Ribas A, Bruhn KW, Craft N, Immunomodulation by imiquimod in patients with high-risk primary melanoma., J Investigative Dermatology. 132 (2011) 163–9. 10.1038/jid.2011.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Adams S, Kozhaya L, Martiniuk F, Meng T-C, Chiriboga L, Liebes L, Hochman T, Shuman N, Axelrod D, Speyer J, Novik Y, Tiersten A, Goldberg JD, Formenti SC, Bhardwaj N, Unutmaz D, Demaria S, Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer., Clin Cancer Res Official J Am Assoc Cancer Res. 18 (2012) 6748–57. 10.1158/1078-0432.ccr-12-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Campanelli A, Krischer J, Saurat J-H, Topical application of imiquimod and associated fever in children, J Am Acad Dermatol. 52 (2005) E1. 10.1016/j.jaad.2004.10.858. [DOI] [PubMed] [Google Scholar]
- [135].Bauza A, Pozo LJD, Saus C, Martin A, Pemphigus-like lesions induced by imiquimod, Clin Exp Dermatol. 34 (2009) e60–e62. 10.1111/j.1365-2230.2008.03181.x. [DOI] [PubMed] [Google Scholar]
- [136].Kumar B, Narang T, Local and systemic adverse effects to topical imiquimod due to systemic immune stimulation, Sex Transm Infect. 87 (2011) 432–432. 10.1136/sextrans-2011-050025. [DOI] [PubMed] [Google Scholar]
- [137].Borden EC, Interferons α and β in cancer: therapeutic opportunities from new insights, Nat Rev Drug Discov. 18 (2019) 219–234. 10.1038/s41573-018-0011-2. [DOI] [PubMed] [Google Scholar]
- [138].Chi H, Li C, Zhao FS, Zhang L, Ng TB, Jin G, Sha O, Anti-tumor Activity of Toll-Like Receptor 7 Agonists, Front Pharmacol. 8 (2017) 304. 10.3389/fphar.2017.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Schon M, Bong AB, Drewniok C, Herz J, Geilen CC, Reifenberger J, Benninghoff B, Slade HB, Gollnick H, Schon MP, Tumor-Selective Induction of Apoptosis and the Small-Molecule Immune Response Modifier Imiquimod, Jnci J National Cancer Inst. 95 (2003) 1138–1149. 10.1093/jnci/djg016. [DOI] [PubMed] [Google Scholar]
- [140].Huang S-W, Chang S-H, Mu S-W, Jiang H-Y, Wang S-T, Kao J-K, Huang J-L, Wu C-Y, Chen Y-J, Shieh J-J, Imiquimod activates p53-dependent apoptosis in a human basal cell carcinoma cell line., J Dermatol Sci. 81 (2015) 182–91. 10.1016/j.jdermsci.2015.12.011. [DOI] [PubMed] [Google Scholar]
- [141].Dajon M, Iribarren K, Cremer I, Dual roles of TLR7 in the lung cancer microenvironment, Oncoimmunology. 4 (2015) e991615. 10.4161/2162402x.2014.991615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Schön MP, Schön M, TLR7 and TLR8 as targets in cancer therapy, Oncogene. 27 (2008) 190–199. 10.1038/sj.onc.1210913. [DOI] [PubMed] [Google Scholar]
- [143].Smits ELJM, Ponsaerts P, Berneman ZN, Tendeloo VFIV, The Use of TLR7 and TLR8 Ligands for the Enhancement of Cancer Immunotherapy, Oncol. 13 (2008) 859–875. 10.1634/theoncologist.2008-0097. [DOI] [PubMed] [Google Scholar]
- [144].Harrison LI, Astry C, Kumar S, Yunis C, Pharmacokinetics of 852A, an Imidazoquinoline Toll-Like Receptor 7-Specific Agonist, Following Intravenous, Subcutaneous, and Oral Administrations in Humans, J Clin Pharmacol. 47 (2007) 962–969. 10.1177/0091270007303766. [DOI] [PubMed] [Google Scholar]
- [145].Dudek AZ, Yunis C, Harrison LI, Kumar S, Hawkinson R, Cooley S, Vasilakos JP, Gorski KS, Miller JS, First in Human Phase I Trial of 852A, a Novel Systemic Toll-like Receptor 7 Agonist, to Activate Innate Immune Responses in Patients with Advanced Cancer, Clin Cancer Res. 13 (2007) 7119–7125. 10.1158/1078-0432.ccr-07-1443. [DOI] [PubMed] [Google Scholar]
- [146].Dummer R, Hauschild A, Becker JC, Grob J-J, Schadendorf D, Tebbs V, Skalsky J, Kaehler KC, Moosbauer S, Clark R, Meng T-C, Urosevic M, An Exploratory Study of Systemic Administration of the Toll-like Receptor-7 Agonist 852A in Patients with Refractory Metastatic Melanoma, Clin Cancer Res. 14 (2008) 856–864. 10.1158/1078-0432.ccr-07-1938. [DOI] [PubMed] [Google Scholar]
- [147].Pope BL, Chourmouzis E, Victorino L, MacIntyre JP, Capetola RJ, Lau CY, Loxoribine (7-allyl-8-oxoguanosine) activates natural killer cells and primes cytolytic precursor cells for activation by IL-2., J Immunol Baltim Md 1950. 151 (1993) 3007–17. [PubMed] [Google Scholar]
- [148].Pope BL, Sigindere J, Chourmouzis E, MacIntyre P, Goodman MG, 7-Allyl-8-oxoguanosine (loxoribine) inhibits the metastasis of B16 melanoma cells and has adjuvant activity in mice immunized with a B16 tumor vaccine, Cancer Immunol Immunother. 38 (1994) 83–91. 10.1007/bf01526202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Agarwala SS, Kirkwood JM, Bryant J, Phase I, Randomized, Double-blind Trial of 7-allyl-8-oxoguanosine (Loxoribine) in Advanced Cancer, Cytokines Cell Mol Ther. 6 (2009) 171–176. 10.1080/mccm.6.4.171.176. [DOI] [PubMed] [Google Scholar]
- [150].Adlard AL, Dovedi SJ, Telfer BA, Koga-Yamakawa E, Pollard C, Honeychurch J, Illidge TM, Murata M, Robinson DT, Jewsbury PJ, Wilkinson RW, Stratford IJ, A novel systemically administered toll-like receptor 7 agonist potentiates the effect of ionizing radiation in murine solid tumor models, Int J Cancer. 135 (2014) 820–9. 10.1002/ijc.28711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Disis ML, Bernhard H, Jaffee EM, Use of tumour-responsive T cells as cancer treatment., Lancet Lond Engl. 373 (2009) 673–83. 10.1016/s0140-6736(09)60404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Durgeau A, Virk Y, Corgnac S, Mami-Chouaib F, Recent Advances in Targeting CD8 T-Cell Immunity for More Effective Cancer Immunotherapy, Front Immunol. 9 (2018) 14. 10.3389/fimmu.2018.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Lu H, TLR Agonists for Cancer Immunotherapy: Tipping the Balance between the Immune Stimulatory and Inhibitory Effects, Front Immunol. 5 (2014) 83. 10.3389/fimmu.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Ota Y, Otsubo T, Koroki J, Hirose Y, Koga-Yamakawa E, Murata M, Goto M, Matsuki Y, Abstract 4726: Novel intravenous injectable TLR7 agonist, DSP-0509, synergistically enhanced antitumor immune responses in combination with anti-PD-1 antibody, (2018) 4726–4726. 10.1158/1538-7445.am2018-4726. [DOI] [Google Scholar]
- [155].Ackerman SE, Gonzalez JC, Gregorio JD, Paik JC, Hartmann FJ, Kenkel JA, Lee A, Luo A, Pearson CI, Nguyen ML, Ackerman B, Sheu LY, Laura RP, Chapin SJ, Safina BS, Bendall SC, Dornan D, Engleman EG, Alonso MN, Abstract 1559: TLR7/8 immune-stimulating antibody conjugates elicit robust myeloid activation leading to enhanced effector function and anti-tumor immunity in pre-clinical models, (2019) 1559–1559. 10.1158/1538-7445.am2019-1559. [DOI] [Google Scholar]
- [156].Ackerman SE, Pearson CI, Gregorio JD, Gonzalez JC, Kenkel JA, Hartmann FJ, Luo A, Ho PY, LeBlanc H, Blum LK, Kimmey SC, Luo A, Nguyen ML, Paik JC, Sheu LY, Ackerman B, Lee A, Li H, Melrose J, Laura RP, Ramani VC, Henning KA, Jackson DY, Safina BS, Yonehiro G, Devens BH, Carmi Y, Chapin SJ, Bendall SC, Kowanetz M, Dornan D, Engleman EG, Alonso MN, Immune-stimulating antibody conjugates elicit robust myeloid activation and durable antitumor immunity, Nat Cancer. (2020) 1–16. 10.1038/s43018-020-00136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Metz H, Childs M, Brevik J, Winship D, Brender T, Comeau M, Moyes K, Chang J, Adamo J, Setter B, Xu H, Fan L-Q, Smith S, Tan P, DuBose R, Latchman Y, Baum P, Odegard V, SBT6050, a HER2-directed TLR8 therapeutic, as a systemically administered, tumor-targeted human myeloid cell agonist., J Clin Oncol. 38 (2020) 3110–3110. 10.1200/jco.2020.38.15_suppl.3110. [DOI] [Google Scholar]
- [158].Moyes K, Brender T, Smith SW, Xu H, Setter B, Fan L-Q, Brunette R, Killebrew J, Tan P, Coburn C, Baum P, Odegard V, Abstract 3271: A systemically administered, conditionally active TLR8 agonist for the treatment of HER2-expressing tumors, (2019) 3271–3271. 10.1158/1538-7445.am2019-3271. [DOI] [Google Scholar]
- [159].Tan J, Harding T, Fletcher S, Fish S, Haley G, Lennox J, Rueden E, D.A. and Appleman J, The active metabolite of the TLR7 agonist prodrug ANA773 activates anti-tumor activity by natural killer cells, Cancer Res. 67 (9 Supplement) (1AD) 3556. https://cancerres-aacrjournals-org.libproxy.mit.edu/content/67/9_Supplement/3556. [Google Scholar]
- [160].Daniels GA, Carson WE, Mita AC, Kendra KL, Rahimy MH, Inman DW, Appleman JR, Freddo JL, McNeel DG, Phase I open-label, dose-escalation study of ANA773 tosylate, an oral prodrug of a toll-like receptor-7 agonist, in patients with advanced solid tumors., J Clin Oncol. 29 (2011) 2524–2524. 10.1200/jco.2011.29.15_suppl.2524. [DOI] [Google Scholar]
- [161].Witjes WPJ, König M, Boeminghaus FP, Hall RR, Schulman CC, Zurlo M, Fittipaldo A, Riggi M, Debruyne FMJ, Results of a European Comparative Randomized Study Comparing Oral Bropirimine versus Intravesical BCG Treatment in BCG-Naive Patients with Carcinoma in situ of the Urinary Bladder, Eur Urol. 36 (1999) 576–581. 10.1159/000020051. [DOI] [PubMed] [Google Scholar]
- [162].Appleman JR, Webber SE, Pharmacodynamic response in vitro and in vivo of novel orally administered Toll-like Receptor 7 agonists for systemic immunotherapy of cancer, in: Pharmacodynamic Response in Vitro and in Vivo of Novel Orally Administered Toll-like Receptor 7 Agonists for Systemic Immunotherapy of Cancer, 2019. [Google Scholar]
- [163].Wu TY-H, Strategies for designing synthetic immune agonists, Immunology. 148 (2016) 315–325. 10.1111/imm.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Hayashi T, Crain B, Corr M, Chan M, Cottam HB, Maj R, Barberis A, Leoni L, Carson DA, Intravesical Toll-like receptor 7 agonist R-837: Optimization of its formulation in an orthotopic mouse model of bladder cancer, Int J Urol. 17 (2010) 483–490. 10.1111/j.1442-2042.2010.02503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Arends TJH, Lammers RJM, Falke J, van der Heijden AG, Rustighini I, Pozzi R, Ravic M, Eisenhardt A, Vergunst H, Witjes JA, Pharmacokinetic, Pharmacodynamic, and Activity Evaluation of TMX-101 in a Multicenter Phase 1 Study in Patients With Papillary Non-Muscle-Invasive Bladder Cancer, Clin Genitourin Canc. 13 (2015) 204–209.e2. 10.1016/j.clgc.2014.12.010. [DOI] [PubMed] [Google Scholar]
- [166].Donin NM, Chamie K, Lenis AT, Pantuck AJ, Reddy M, Kivlin D, Holldack J, Pozzi R, Hakim G, Karsh LI, Lamm DL, Belkoff LH, Belldegrun AS, Holden S, Shore N, A phase 2 study of TMX-101, intravesical imiquimod, for the treatment of carcinoma in situ bladder cancer, Urologic Oncol Seminars Orig Investigations. 35 (2017) 39.e1–39.e7. 10.1016/j.urolonc.2016.09.006. [DOI] [PubMed] [Google Scholar]
- [167].Northfelt DW, Ramanathan RK, Cohen PA, Hoff DDV, Weiss GJ, Dietsch GN, Manjarrez KL, Randall TD, Hershberg RM, A Phase I Dose-Finding Study of the Novel Toll-like Receptor 8 Agonist VTX-2337 in Adult Subjects with Advanced Solid Tumors or Lymphoma, Clin Cancer Res. 20 (2014) 3683–3691. 10.1158/1078-0432.ccr-14-0392. [DOI] [PubMed] [Google Scholar]
- [168].Facciabene A, Malykhin A, Mathews M, Fang C, Dietsch G, Hershberg R, Coukos G, Abstract 4745: TLR8 agonist and Doxil chemotherapy potently activate human antitumor immune response in a human immune system mouse model, (2011) 4745–4745. 10.1158/1538-7445.am2011-4745. [DOI] [Google Scholar]
- [169].Dietsch GN, Randall TD, Gottardo R, Northfelt DW, Ramanathan RK, Cohen PA, Manjarrez KL, Newkirk M, Bryan JK, Hershberg RM, Late-Stage Cancer Patients Remain Highly Responsive to Immune Activation by the Selective TLR8 Agonist Motolimod (VTX-2337), Clin Cancer Res. 21 (2015) 5445–5452. 10.1158/1078-0432.ccr-15-0578. [DOI] [PubMed] [Google Scholar]
- [170].Singh M, Khong H, Dai Z, Huang X-F, Wargo JA, Cooper ZA, Vasilakos JP, Hwu P, Overwijk WW, Effective innate and adaptive antimelanoma immunity through localized TLR7/8 activation., J Immunol Baltim Md 1950. 193 (2014) 4722–31. 10.4049/jimmunol.1401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Brown TD, Whitehead KA, Mitragotri S, Materials for oral delivery of proteins and peptides, Nat Rev Mater. 5 (2019) 127–148. 10.1038/s41578-019-0156-6. [DOI] [Google Scholar]
- [172].Patel MC, Shirey KA, Pletneva LM, Boukhvalova MS, Garzino-Demo A, Vogel SN, Blanco JC, Novel drugs targeting Toll-like receptors for antiviral therapy, Future Virol. 9 (2014) 811–829. 10.2217/fvl.14.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Horscroft NJ, Pryde DC, Bright H, Antiviral applications of Toll-like receptor agonists, J Antimicrob Chemoth. 67 (2012) 789–801. 10.1093/jac/dkr588. [DOI] [PubMed] [Google Scholar]
- [174].Melchjorsen J, Learning from the messengers: innate sensing of viruses and cytokine regulation of immunity - clues for treatments and vaccines., Viruses. 5 (2013) 470–527. 10.3390/v5020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Pockros PJ, Guyader D, Patton H, Tong MJ, Wright T, McHutchison JG, Meng T-C, Oral resiquimod in chronic HCV infection: Safety and efficacy in 2 placebo-controlled, double-blind phase IIa studies, Journal of Hepatology. 47 (2007) 174–82. 10.1016/j.jhep.2007.02.025. [DOI] [PubMed] [Google Scholar]
- [176].Fidock MD, Souberbielle BE, Laxton C, Rawal J, Delpuech-Adams O, Corey TP, Colman P, Kumar V, Cheng JB, Wright K, Srinivasan S, Rana K, Craig C, Horscroft N, Perros M, Westby M, Webster R, van der Ryst E, The innate immune response, clinical outcomes, and ex vivo HCV antiviral efficacy of a TLR7 agonist (PF-4878691)., Clin Pharmacol Ther. 89 (2011) 821–9. 10.1038/clpt.2011.60. [DOI] [PubMed] [Google Scholar]
- [177].Xiang AX, Webber SE, Kerr BM, Rueden EJ, Lennox JR, Haley GJ, Wang T, Ng JS, Herbert MR, Clark DL, Banh VN, Li W, Fletcher SP, Steffy KR, Bartkowski DM, Kirkovsky LI, Bauman LA, Averett DR, Discovery of ANA975: An Oral Prodrug of the TLR-7 Agonist Isatoribine, Nucleosides Nucleotides Nucleic Acids. 26 (2007) 635–640. 10.1080/15257770701490472. [DOI] [PubMed] [Google Scholar]
- [178].Suslov A, Wieland S, Menne S, Modulators of innate immunity as novel therapeutics for treatment of chronic hepatitis B, Curr Opin Virol. 30 (2018) 9–17. 10.1016/j.coviro.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Miller RL, Meng T-C, Tomai MA, The antiviral activity of toll-like receptor 7 and 7/8 agonists, Drug News Perspect. 21 (2008) 69. 10.1358/dnp.2008.21.2.1188193. [DOI] [PubMed] [Google Scholar]
- [180].Luk A, Jiang Q, Glavini K, Triyatni M, Zhao N, Racek T, Zhu Y, Grippo JF, A Single and Multiple Ascending Dose Study of Toll-Like Receptor 7 Agonist (RO7020531) in Chinese Healthy Volunteers, Clin Transl Sci. (2020). 10.1111/cts.12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Grippo JF, Folitar I, Passe S, Jiang Q, Rodriguez I, Fettner SH, Calleja E, Safety, tolerability, pharmacokinetics and pharmacodynamics of a TLR7 agonist prodrug RO6870868 in healthy volunteers, Clin Transl Sci. (2021). 10.1111/cts.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Zhang H, Hu Y, Wu M, Liu J, Li X, Zhu X, Li C, Chen H, Liu C, Niu J, Ding Y, Safety, Pharmacokinetics, and Pharmacodynamics of TQ-A3334, an Oral Toll-Like Receptor 7 Agonist, in Healthy Individuals, Expert Opin Inv Drug. (2021). 10.1080/13543784.2021.1873275. [DOI] [PubMed] [Google Scholar]
- [183].Gao L, Dai L, Yu Y, Gu L, Zhao J, Ji Y, Yun H, Zhu W, Young J, O-015: Combination treatment of a TLR7 agonist RO7020531 and a capsid assembly modulator RO7049389 achieved sustainable viral load suppression and HBsAg loss in an AAV-HBV mouse model, J Viral Hepatitis. 25 (2018) 12–12. 10.1111/jvh.12_12922. [DOI] [Google Scholar]
- [184].Fosdick A, Zheng J, Pflanz S, Frey CR, Hesselgesser J, Halcomb RL, Wolfgang G, Tumas DB, Pharmacokinetic and Pharmacodynamic Properties of GS-9620, a Novel Toll-Like Receptor 7 Agonist, Demonstrate Interferon-Stimulated Gene Induction without Detectable Serum Interferon at Low Oral Doses, J Pharmacol Exp Ther. 348 (2014) 96–105. 10.1124/jpet.113.207878. [DOI] [PubMed] [Google Scholar]
- [185].Gane EJ, Lim Y-S, Gordon SC, Visvanathan K, Sicard E, Fedorak RN, Roberts S, Massetto B, Ye Z, Pflanz S, Garrison KL, Gaggar A, Subramanian GM, McHutchison JG, Kottilil S, Freilich B, Coffin CS, Cheng W, Kim YJ, The oral toll-like receptor-7 agonist GS-9620 in patients with chronic hepatitis B virus infection, J Hepatol. 63 (2015) 320–328. 10.1016/j.jhep.2015.02.037. [DOI] [PubMed] [Google Scholar]
- [186].Menne S, Tumas DB, Liu KH, Thampi L, AlDeghaither D, Baldwin BH, Bellezza CA, Cote PJ, Zheng J, Halcomb R, Fosdick A, Fletcher SP, Daffis S, Li L, Yue P, Wolfgang GHI, Tennant BC, Sustained efficacy and seroconversion with the Toll-like receptor 7 agonist GS-9620 in the Woodchuck model of chronic hepatitis B., J Hepatol. 62 (2015) 1237–45. 10.1016/j.jhep.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, Fosdick A, Frey CR, Zheng J, Wolfgang G, Halcomb RL, Tumas DB, GS-9620, an Oral Agonist of Toll-Like Receptor-7, Induces Prolonged Suppression of Hepatitis B Virus in Chronically Infected Chimpanzees, Gastroenterology. 144 (2013) 1508–1517.e10. 10.1053/j.gastro.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Korolowizc KE, Li B, Huang X, Yon C, Rodrigo E, Corpuz M, Plouffe DM, Kallakury BV, Suresh M, Wu TY-H, Miller AT, Menne S, Liver-Targeted Toll-Like Receptor 7 Agonist Combined With Entecavir Promotes a Functional Cure in the Woodchuck Model of Hepatitis B Virus, Hepatology Commun. 3 (2019) 1296–1310. 10.1002/hep4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Jo J, Tan AT, Ussher JE, Sandalova E, Tang X-Z, Tan-Garcia A, To N, Hong M, Chia A, Gill US, Kennedy PT, Tan KC, Lee KH, Libero GD, Gehring AJ, Willberg CB, Klenerman P, Bertoletti A, Toll-Like Receptor 8 Agonist and Bacteria Trigger Potent Activation of Innate Immune Cells in Human Liver, Plos Pathog. 10 (2014) e1004210. 10.1371/journal.ppat.1004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV, Viral Clearance Without Destruction of Infected Cells During Acute HBV Infection, Science. 284 (1999) 825–829. 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- [191].Mackman RL, Mish M, Chin G, Perry JK, Appleby T, Aktoudianakis V, Metobo S, Pyun P, Niu C, Daffis S, Yu H, Zheng J, Villasenor AG, Zablocki J, Chamberlain J, Jin H, Lee G, Suekawa-Pirrone K, Santos R, Delaney WE, Fletcher SP, Discovery of GS-9688 (Selgantolimod) as a Potent and Selective Oral Toll-Like Receptor 8 Agonist for the Treatment of Chronic Hepatitis B, J Med Chem. (2020). 10.1021/acs.jmedchem.0c00100. [DOI] [PubMed] [Google Scholar]
- [192].Daffis S, Balsitis S, Chamberlain J, Zheng J, Santos R, Rowe W, Ramakrishnan D, Pattabiraman D, Spurlock S, Chu R, Kang D, Mish M, Ramirez R, Li L, Li B, Ma S, Hung M, Voitenleitner C, Yon C, Suresh M, Menne S, Cote P, Delaney WE, Mackman R, Fletcher SP, Toll-Like Receptor 8 Agonist GS-9688 Induces Sustained Efficacy in the Woodchuck Model of Chronic Hepatitis B, Hepatology. (2020). 10.1002/hep.31255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Bam RA, Hansen D, Irrinki A, Mulato A, Jones GS, Hesselgesser J, Frey CR, Cihlar T, Yant SR, TLR7 Agonist GS-9620 Is a Potent Inhibitor of Acute HIV-1 Infection in Human Peripheral Blood Mononuclear Cells, Antimicrob Agents Ch. 61 (2017) e01369–16. 10.1128/aac.01369-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Borducchi EN, Cabral C, Stephenson KE, Liu J, Abbink P, Ng’ang’a D, Nkolola JP, Brinkman AL, Peter L, Lee BC, Jimenez J, Jetton D, Mondesir J, Mojta S, Chandrashekar A, Molloy K, Alter G, Gerold JM, Hill AL, Lewis MG, Pau MG, Schuitemaker H, Hesselgesser J, Geleziunas R, Kim JH, Robb ML, Michael NL, Barouch DH, Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys, Nature. 540 (2016) 284–287. 10.1038/nature20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Borducchi EN, Liu J, Nkolola JP, Cadena AM, Yu W-H, Fischinger S, Broge T, Abbink P, Mercado NB, Chandrashekar A, Jetton D, Peter L, McMahan K, Moseley ET, Bekerman E, Hesselgesser J, Li W, Lewis MG, Alter G, Geleziunas R, Barouch DH, Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys, Nature. 563 (2018) 360–364. 10.1038/s41586-018-0600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Lim S-Y, Osuna CE, Hraber PT, Hesselgesser J, Gerold JM, Barnes TL, Sanisetty S, Seaman MS, Lewis MG, Geleziunas R, Miller MD, Cihlar T, Lee WA, Hill AL, Whitney JB, TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy, Sci Transl Med. 10 (2018) eaao4521. 10.1126/scitranslmed.aao4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Eigenbrod T, Dalpke AH, Bacterial RNA: An Underestimated Stimulus for Innate Immune Responses, J Immunol. 195 (2015) 411–418. 10.4049/jimmunol.1500530. [DOI] [PubMed] [Google Scholar]
- [198].Moen SH, Ehrnström B, Kojen JF, Yurchenko M, Beckwith KS, Afset JE, Damås JK, Hu Z, Yin H, Espevik T, Stenvik J, Human Toll-like Receptor 8 (TLR8) Is an Important Sensor of Pyogenic Bacteria, and Is Attenuated by Cell Surface TLR Signaling, Front Immunol. 10 (2019) 1209. 10.3389/fimmu.2019.01209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Cervantes JL, Weinerman B, Basole C, Salazar JC, TLR8: the forgotten relative revindicated, Cell Mol Immunol. 9 (2012) 434–438. 10.1038/cmi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Dowling DJ, van Haren SD, Scheid A, Bergelson I, Kim D, Mancuso CJ, Foppen W, Ozonoff A, Fresh L, Theriot TB, Lackner AA, Fichorova RN, Smirnov D, Vasilakos JP, Beaurline JM, Tomai MA, Midkiff CC, Alvarez X, Blanchard JL, Gilbert MH, Aye PP, Levy O, TLR7/8 adjuvant overcomes newborn hyporesponsiveness to pneumococcal conjugate vaccine at birth., Jci Insight. 2 (2017) e91020. 10.1172/jci.insight.91020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Dowling DJ, Recent Advances in the Discovery and Delivery of TLR7/8 Agonists as Vaccine Adjuvants, Immunohorizons. 2 (2018) 185–197. 10.4049/immunohorizons.1700063. [DOI] [PubMed] [Google Scholar]
- [202].Abt MC, Buffie CG, Sušac B, Becattini S, Carter RA, Leiner I, Keith JW, Artis D, Osborne LC, Pamer EG, TLR-7 activation enhances IL-22–mediated colonization resistance against vancomycin-resistant enterococcus, Sci Transl Med. 8 (2016) 327ra25–327ra25. 10.1126/scitranslmed.aad6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Drake MG, Kaufman EH, Fryer AD, Jacoby DB, The Therapeutic Potential of Toll-Like Receptor 7 Stimulation in Asthma, Inflamm Allergy-Drug Targets. 11 (2012) 484–491. 10.2174/187152812803589967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Lebold KM, Jacoby DB, Drake MG, Toll-Like Receptor 7-Targeted Therapy in Respiratory Disease, Transfus Med Hemoth. 43 (2016) 114–119. 10.1159/000445324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Adner M, Starkhammar M, Georén SK, Dahlén S-E, Cardell L-O, Toll-like receptor (TLR) 7 decreases and TLR9 increases the airway responses in mice with established allergic inflammation, Eur J Pharmacol. 718 (2013) 544–551. 10.1016/j.ejphar.2013.09.004. [DOI] [PubMed] [Google Scholar]
- [206].Kline JN, Immunotherapy of asthma using CpG oligodeoxynucleotides, Immunol Res. 39 (2007) 279–286. 10.1007/s12026-007-0083-2. [DOI] [PubMed] [Google Scholar]
- [207].Barnes PJ, New drugs for asthma, Nat Rev Drug Discov. 3 (2004) 831–844. 10.1038/nrd1524. [DOI] [PubMed] [Google Scholar]
- [208].Drake MG, Scott GD, Proskocil BJ, Fryer AD, Jacoby DB, Kaufman EH, Toll-like Receptor 7 Rapidly Relaxes Human Airways, Am J Resp Crit Care. 188 (2013) 664–672. 10.1164/rccm.201303-0442oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Ellis AK, Tsitoura DC, Quint D, Powley W, Lee LA, Safety and pharmacodynamics of intranasal GSK2245035, a TLR7 agonist for allergic rhinitis: A randomized trial, Clin Exp Allergy. 47 (2017) 1193–1203. 10.1111/cea.12974. [DOI] [PubMed] [Google Scholar]
- [210].Biggadike K, Ahmed M, Ball DI, Coe DM, Wilk DAD, Edwards CD, Gibbon BH, Hardy CJ, Hermitage SA, Hessey JO, Hillegas AE, Hughes SC, Lazarides L, Lewell XQ, Lucas A, Mallett DN, Price MA, Priest FM, Quint DJ, Shah P, Sitaram A, Smith SA, Stocker R, Trivedi NA, Tsitoura DC, Weller V, Discovery of 6-Amino-2-{[(1S)-1-methylbutyl]oxy}-9-[5-(1-piperidinyl)pentyl]-7,9-dihydro-8H-purin-8-one (GSK2245035), a Highly Potent and Selective Intranasal Toll-Like Receptor 7 Agonist for the Treatment of Asthma., J Med Chem. 59 (2016) 1711–26. 10.1021/acs.jmedchem.5b01647. [DOI] [PubMed] [Google Scholar]
- [211].Biffen M, Matsui H, Edwards S, Leishman A, Eiho K, Holness E, Satterthwaite G, Doyle I, Wada H, Fraser N, Hawkins S, Aoki M, Tomizawa H, Benjamin A, Takaku H, McInally T, Murray C, Biological characterization of a novel class of toll-like receptor 7 agonists designed to have reduced systemic activity, Brit J Pharmacol. 166 (2012) 573–586. 10.1111/j.1476-5381.2011.01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Leaker BR, Singh D, Lindgren S, Almqvist G, Eriksson L, Young B, O’Connor B, Effects of the Toll-like receptor 7 (TLR7) agonist, AZD8848, on allergen-induced responses in patients with mild asthma: a double-blind, randomised, parallel-group study, Respir Res. 20 (2019) 288. 10.1186/s12931-019-1252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Royer CM, Rudolph K, Dietsch GN, Hershberg RM, Barrett EG, VTX-1463, a novel TLR-8 agonist, attenuates nasal congestion after ragweed challenge in sensitized beagle dogs., Immun Inflamm Dis. 4 (2015) 45–51. 10.1002/iid3.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Horak F, VTX-1463, a novel TLR8 agonist for the treatment of allergic rhinitis., Expert Opin Inv Drug. 20 (2011) 981–6. 10.1517/13543784.2011.583237. [DOI] [PubMed] [Google Scholar]
- [215].Blander J, Medzhitov R, On regulation of phagosome maturation and antigen presentation, Nat Immunol. 7 (2006) 1029–1035. 10.1038/ni1006-1029. [DOI] [PubMed] [Google Scholar]
- [216].Wille-Reece U, Wu C, Flynn BJ, Kedl RM, Seder RA, Immunization with HIV-1 Gag Protein Conjugated to a TLR7/8 Agonist Results in the Generation of HIV-1 Gag-Specific Th1 and CD8+ T Cell Responses, J Immunol. 174 (2005) 7676–7683. 10.4049/jimmunol.174.12.7676. [DOI] [PubMed] [Google Scholar]
- [217].Wille-Reece U, Flynn BJ, Loré K, Koup RA, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA, HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates, Proc National Acad Sci. 102 (2005) 15190–15194. 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Kastenmüller K, Wille-Reece U, Lindsay RWB, Trager LR, Darrah PA, Flynn BJ, Becker MR, Udey MC, Clausen BE, Igyarto BZ, Kaplan DH, Kastenmüller W, Germain RN, Seder RA, Protective T cell immunity in mice following protein-TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets, J Clin Investigation. 121 (2011) 1782–96. 10.1172/jci45416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Oh JZ, Kedl RM, The Capacity To Induce Cross-Presentation Dictates the Success of a TLR7 Agonist-Conjugate Vaccine for Eliciting Cellular Immunity, J Immunol Baltim Md 1950. 185 (2010) 4602–8. 10.4049/jimmunol.1001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Feng Y, Forsell MNE, Flynn B, Adams W, Loré K, Seder R, Wyatt RT, Hedestam GBK, Chemical cross-linking of HIV-1 Env for direct TLR7/8 ligand conjugation compromises recognition of conserved antigenic determinants, Virology. 446 (2013) 56–65. 10.1016/j.virol.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Vecchi S, Bufali S, Uno T, Wu T, Arcidiacono L, Filippini S, Rigat F, O’Hagan D, Conjugation of a TLR7 agonist and antigen enhances protection in the S. pneumoniae murine infection model., European J Pharm Biopharm Official J Arbeitsgemeinschaft Für Pharmazeutische Verfahrenstechnik E V. 87 (2014) 310–7. 10.1016/j.ejpb.2014.01.002. [DOI] [PubMed] [Google Scholar]
- [222].Holbrook BC, Kim JR, Blevins LK, Jorgensen MJ, Kock ND, D’Agostino RB, Aycock ST, Hadimani MB, King SB, Parks GD, Alexander-Miller MA, A Novel R848-Conjugated Inactivated Influenza Virus Vaccine Is Efficacious and Safe in a Neonate Nonhuman Primate Model, J Immunol Baltim Md 1950. 197 (2016) 555–64. 10.4049/jimmunol.1600497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Holbrook BC, Aycock ST, Machiele E, Clemens E, Gries D, Jorgensen MJ, Hadimani MB, King SB, Alexander-Miller MA, An R848 adjuvanted influenza vaccine promotes early activation of B cells in the draining lymph nodes of non-human primate neonates, Immunology. 153 (2018) 357–367. 10.1111/imm.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Holbrook BC, D’Agostino RB, Aycock ST, Jorgensen MJ, Hadimani MB, King SB, Alexander-Miller MA, Adjuvanting an inactivated influenza vaccine with conjugated R848 improves the level of antibody present at 6months in a nonhuman primate neonate model, Vaccine. 35 (2017) 6137–6142. 10.1016/j.vaccine.2017.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Westcott MM, Clemens EA, Holbrook BC, King SB, Alexander-Miller MA, The choice of linker for conjugating R848 to inactivated influenza virus determines the stimulatory capacity for innate immune cells, Vaccine. 36 (2018) 1174–1182. 10.1016/j.vaccine.2018.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Lynn GM, Sedlik C, Baharom F, Zhu Y, Ramirez-Valdez RA, Coble VL, Tobin K, Nichols SR, Itzkowitz Y, Zaidi N, Gammon JM, Blobel NJ, Denizeau J, de la Rochere P, Francica BJ, Decker B, Maciejewski M, Cheung J, Yamane H, Smelkinson MG, Francica JR, Laga R, Bernstock JD, Seymour LW, Drake CG, Jewell CM, Lantz O, Piaggio E, Ishizuka AS, Seder RA, Peptide–TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens, Nat Biotechnol. (2020) 1–13. 10.1038/s41587-019-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Baharom F, Ramirez-Valdez RA, Tobin KKS, Yamane H, Dutertre C-A, Khalilnezhad A, Reynoso GV, Coble VL, Lynn GM, Mulè MP, Martins AJ, Finnigan JP, Zhang XM, Hamerman JA, Bhardwaj N, Tsang JS, Hickman HD, Ginhoux F, Ishizuka AS, Seder RA, Intravenous nanoparticle vaccination generates stem-like TCF1+ neoantigen-specific CD8+ T cells, Nat Immunol. (2020) 1–12. 10.1038/s41590-020-00810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Wilson DS, Hirosue S, Raczy MM, Bonilla-Ramirez L, Jeanbart L, Wang R, Kwissa M, Franetich J-F, Broggi MAS, Diaceri G, Quaglia-Thermes X, Mazier D, Swartz MA, Hubbell JA, Antigens reversibly conjugated to a polymeric glyco-adjuvant induce protective humoral and cellular immunity, Nat Mater. 18 (2019) 175–185. 10.1038/s41563-018-0256-5. [DOI] [PubMed] [Google Scholar]
- [229].Lynn GM, Laga R, Darrah PA, Ishizuka AS, Balaci AJ, Dulcey AE, Pechar M, Pola R, Gerner MY, Yamamoto A, Buechler CR, Quinn KM, Smelkinson MG, Vanek O, Cawood R, Hills T, Vasalatiy O, Kastenmüller K, Francica JR, Stutts L, Tom JK, Ryu KA, Esser-Kahn AP, Etrych T, Fisher KD, Seymour LW, Seder RA, In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity, Nature Biotechnology. 33 (2015) 1201–1210. 10.1038/nbt.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Lynn GM, Chytil P, Francica JR, Lagová A, Kueberuwa G, Ishizuka AS, Zaidi N, Ramirez-Valdez RA, Blobel NJ, Baharom F, Leal J, Wang AQ, Gerner MY, Etrych T, Ulbrich K, Seymour LW, Seder RA, Laga R, Impact of Polymer-TLR-7/8 Agonist (Adjuvant) Morphology on the Potency and Mechanism of CD8 T Cell Induction, Biomacromolecules. 20 (2019) 854–870. 10.1021/acs.biomac.8b01473. [DOI] [PubMed] [Google Scholar]
- [231].Nuhn L, Vanparijs N, Beuckelaer AD, Lybaert L, Verstraete G, Deswarte K, Lienenklaus S, Shukla NM, Salyer ACD, Lambrecht BN, Grooten J, David SA, Koker SD, Geest BGD, pH-degradable imidazoquinoline-ligated nanogels for lymph node-focused immune activation, Proceedings of the National Academy of Sciences. 113 (2016). 10.1073/pnas.1600816113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Nuhn L, Hoecke LV, Deswarte K, Schepens B, Li Y, Lambrecht BN, Koker SD, David SA, Saelens X, Geest BGD, Potent anti-viral vaccine adjuvant based on pH-degradable nanogels with covalently linked small molecule imidazoquinoline TLR7/8 agonist, Biomaterials. 178 (2018) 643–651. 10.1016/j.biomaterials.2018.03.026. [DOI] [PubMed] [Google Scholar]
- [233].Vrieze JD, Louage B, Deswarte K, Zhong Z, Coen RD, Herck SV, Nuhn L, Frich CK, Zelikin AN, Lienenklaus S, Sanders NN, Lambrecht BN, David SA, Geest BGD, Potent Lymphatic Translocation and Spatial Control Over Innate Immune Activation by Polymer–Lipid Amphiphile Conjugates of Small-Molecule TLR7/8 Agonists, Angewandte Chemie Int Ed. 58 (2019) 15390–15395. 10.1002/anie.201905687. [DOI] [PubMed] [Google Scholar]
- [234].Yoo E, Salyer ACD, Brush MJH, Li Y, Trautman KL, Shukla NM, Beuckelaer AD, Lienenklaus S, Deswarte K, Lambrecht BN, Geest BGD, David SA, Hyaluronic Acid Conjugates of TLR7/8 Agonists for Targeted Delivery to Secondary Lymphoid Tissue, Bioconjugate Chem. 29 (2018) 2741–2754. 10.1021/acs.bioconjchem.8b00386. [DOI] [PubMed] [Google Scholar]
- [235].Ilyinskii PO, Roy CJ, O’Neil CP, Browning EA, Pittet LA, Altreuter DH, Alexis F, Tonti E, Shi J, Basto PA, Iannacone M, Radovic-Moreno AF, Langer RS, Farokhzad OC, von Andrian UH, Johnston LPM, Kishimoto TK, Adjuvant-carrying synthetic vaccine particles augment the immune response to encapsulated antigen and exhibit strong local immune activation without inducing systemic cytokine release, Vaccine. 32 (2014) 2882–95. 10.1016/j.vaccine.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Kim WG, Choi B, Yang H-J, Han J-A, Jung H, Cho H, Kang S, Hong SY, Covalent Conjugation of Small-Molecule Adjuvants to Nanoparticles Induces Robust Cytotoxic T Cell Responses via DC Activation, Bioconjugate Chemistry. 27 (2016) 2007–13. 10.1021/acs.bioconjchem.6b00277. [DOI] [PubMed] [Google Scholar]
- [237].Liong CS, Smith AAA, Mann JL, Roth GA, Gale EC, Maikawa CL, Ou BS, Appel Eric.A., Enhanced Humoral Immune Response by High Density TLR Agonist Presentation on Hyperbranched Polymers, Adv Ther. (2021) 2000081. 10.1002/adtp.202000081. [DOI] [Google Scholar]
- [238].Wu TY-H, Singh M, Miller AT, Gregorio ED, Doro F, D’Oro U, Skibinski DAG, Mbow ML, Bufali S, Herman AE, Cortez A, Li Y, Nayak BP, Tritto E, Filippi CM, Otten GR, Brito LA, Monaci E, Li C, Aprea S, Valentini S, Calabró S, Laera D, Brunelli B, Caproni E, Malyala P, Panchal RG, Warren TK, Bavari S, O’Hagan DT, Cooke MP, Valiante NM, Rational design of small molecules as vaccine adjuvants, Sci Transl Med. 6 (2014) 263ra160–263ra160. 10.1126/scitranslmed.3009980. [DOI] [PubMed] [Google Scholar]
- [239].Buonsanti C, Balocchi C, Harfouche C, Corrente F, Stampino LG, Mancini F, Tontini M, Malyala P, Bufali S, Baudner B, Gregorio ED, Valiante NM, O’Hagan DT, Rappuoli R, D’Oro U, Novel adjuvant Alum-TLR7 significantly potentiates immune response to glycoconjugate vaccines, Sci Rep-Uk. 6 (2016) 29063. 10.1038/srep29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Bagnoli F, Fontana MR, Soldaini E, Mishra RPN, Fiaschi L, Cartocci E, Nardi-Dei V, Ruggiero P, Nosari S, Falco MGD, Lofano G, Marchi S, Galletti B, Mariotti P, Bacconi M, Torre A, Maccari S, Scarselli M, Rinaudo CD, Inoshima N, Savino S, Mori E, Rossi-Paccani S, Baudner B, Pallaoro M, Swennen E, Petracca R, Brettoni C, Liberatori S, Norais N, Monaci E, Wardenburg JB, Schneewind O, O’Hagan DT, Valiante NM, Bensi G, Bertholet S, Gregorio ED, Rappuoli R, Grandi G, Vaccine composition formulated with a novel TLR7-dependent adjuvant induces high and broad protection against Staphylococcus aureus, P Natl Acad Sci Usa. 112 (2015) 3680–5. 10.1073/pnas.1424924112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Mancini F, Monaci E, Lofano G, Torre A, Bacconi M, Tavarini S, Sammicheli C, Arcidiacono L, Galletti B, Laera D, Pallaoro M, Tuscano G, Fontana MR, Bensi G, Grandi G, Rossi-Paccani S, Nuti S, Rappuoli R, Gregorio ED, Bagnoli F, Soldaini E, Bertholet S, One Dose of Staphylococcus aureus 4C-Staph Vaccine Formulated with a Novel TLR7-Dependent Adjuvant Rapidly Protects Mice through Antibodies, Effector CD4+ T Cells, and IL-17A, Plos One. 11 (2016) e0147767. 10.1371/journal.pone.0147767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Misiak A, Leuzzi R, Allen AC, Galletti B, Baudner BC, D’Oro U, O’Hagan DT, Pizza M, Seubert A, Mills KHG, Addition of a TLR7 agonist to an acellular pertussis vaccine enhances Th1 and Th17 responses and protective immunity in a mouse model, Vaccine. 35 (2017) 5256–5263. 10.1016/j.vaccine.2017.08.009. [DOI] [PubMed] [Google Scholar]
- [243].Malyala P, Laera D, Cianetti S, Bufali S, Aggravi M, Ianni E, Judge C, Otten G, Singh M, Hagan DTO, The preparation and physico-chemical characterization of aluminum hydroxide/TLR7a, a novel vaccine adjuvant comprising a small molecule adsorbed to aluminum hydroxide, J Pharm Sci. 107 (2018) 1577–1585. 10.1016/j.xphs.2018.01.024. [DOI] [PubMed] [Google Scholar]
- [244].Gonzalez-Lopez A, Oostendorp J, Koernicke T, Fadini T, D’Oro U, Baker S, O’Hagan DT, Giudice GD, Siena E, Finco O, Medini D, Adjuvant effect of TLR7 agonist adsorbed on aluminum hydroxide (AS37): A phase I randomized, dose escalation study of an AS37-adjuvanted meningococcal C conjugated vaccine, Clin Immunol. 209 (2019) 108275. 10.1016/j.clim.2019.108275. [DOI] [PubMed] [Google Scholar]
- [245].Fox CB, Orr MT, Hoeven NV, Parker SC, Mikasa TJT, Phan T, Beebe EA, Nana GI, Joshi SW, Tomai MA, Elvecrog J, Fouts TR, Reed SG, Adsorption of a synthetic TLR7/8 ligand to aluminum oxyhydroxide for enhanced vaccine adjuvant activity: A formulation approach, J Control Release. 244 (2016) 98–107. 10.1016/j.jconrel.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Francica JR, Zak DE, Linde C, Siena E, Johnson C, Juraska M, Yates NL, Gunn B, Gregorio ED, Flynn BJ, Valiante NM, Malyala P, Barnett SW, Sarkar P, Singh M, Jain S, Ackerman M, Alam M, Ferrari G, Tomaras GD, O’Hagan DT, Aderem A, Alter G, Seder RA, Innate transcriptional effects by adjuvants on the magnitude, quality, and durability of HIV envelope responses in NHPs, Blood Adv. 1 (2017) 2329–2342. 10.1182/bloodadvances.2017011411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Vaccari M, Gordon S, Fourati S, Schifanella L, Liyanage N, Cameron M, Keele B, Shen X, Tomaras G, Billings E, Rao M, Chung A, Dowell K, Bailey-Kellogg C, Brown E, Ackerman M, Vargas-Inchaustegui D, Whitney S, Doster M, Binello N, Pegu P, Montefiori D, Foulds K, Quinn D, Donaldson M, Liang F, Loré K, Roederer M, Koup R, McDermott A, Ma Z, Miller C, Phan T, Forthal D, Blackburn M, Caccuri F, Bissa M, Ferrari G, Kalyanaraman V, Ferrari M, Thompson D, Robert-Guroff M, Ratto-Kim S, Kim J, Michael N, Phogat S, Barnett S, Tartaglia J, Venzon D, Stablein D, Alter G, Sekaly R, Franchini G, Adjuvant-dependent innate and adaptive immune signatures of risk of SIVmac251 acquisition, Nat Med. 22 (2016) 762–770. 10.1038/nm.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Singh M, Ugozzoli M, Kazzaz J, Chesko J, Soenawan E, Mannucci D, Titta F, Contorni M, Volpini G, Guidice G, O’Hagan D, A preliminary evaluation of alternative adjuvants to alum using a range of established and new generation vaccine antigens, Vaccine. 24 (2006) 1680–1686. 10.1016/j.vaccine.2005.09.046. [DOI] [PubMed] [Google Scholar]
- [249].Yadav PD, Ella R, Kumar S, Patil DR, Mohandas S, Shete AM, Vadrevu KM, Bhati G, Sapkal G, Kaushal H, Patil S, Jain R, Deshpande G, Gupta N, Agarwal K, Gokhale M, Mathapati B, Metkari S, Mote C, Nyayanit D, Patil DY, Prasad BSS, Suryawanshi A, Kadam M, Kumar A, Daigude S, Gopale S, Majumdar T, Mali D, Sarkale P, Baradkar S, Gawande P, Joshi Y, Fulari S, Dighe H, Sharma S, Gunjikar R, Kumar A, Kalele K, Srinivas VK, Gangakhedkar RR, Ella KM, Abraham P, Panda S, Bhargava B, Immunogenicity and protective efficacy of inactivated SARS-CoV-2 vaccine candidate, BBV152 in rhesus macaques, Nat Commun. 12 (2021) 1386. 10.1038/s41467-021-21639-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Ella R, Vadrevu KM, Jogdand H, Prasad S, Reddy S, Sarangi V, Ganneru B, Sapkal G, Yadav P, Abraham P, Panda S, Gupta N, Reddy P, Verma S, Rai SK, Singh C, Redkar SV, Gillurkar CS, Kushwaha JS, Mohapatra S, Rao V, Guleria R, Ella K, Bhargava B, Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial, Lancet Infect Dis. (2021). 10.1016/s1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Ganneru B, Jogdand H, Daram VK, Das D, Molugu NR, Prasad SD, Kannappa SV, Ella KM, Ravikrishnan R, Awasthi A, Jose J, Rao P, Kumar D, Ella R, Abraham P, Yadav PD, Sapkal GN, Shete-Aich A, Desphande G, Mohandas S, Basu A, Gupta N, Vadrevu KM, Th1 skewed immune response of whole virion inactivated SARS CoV 2 vaccine and its safety evaluation, Iscience. 24 (2021) 102298. 10.1016/j.isci.2021.102298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Ella R, Reddy S, Jogdand H, Sarangi V, Ganneru B, Prasad S, Das D, Raju D, Praturi U, Sapkal G, Yadav P, Reddy P, Verma S, Singh C, Redkar SV, Gillurkar CS, Kushwaha JS, Mohapatra S, Bhate A, Rai S, Panda S, Abraham P, Gupta N, Ella K, Bhargava B, Vadrevu KM, Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial, Lancet Infect Dis. (2021). 10.1016/s1473-3099(21)00070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Li J-X, Zhu F-C, Adjuvantation helps to optimise COVID-19 vaccine candidate, Lancet Infect Dis. (2021). 10.1016/s1473-3099(21)00094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Jangra S, Vrieze JD, Choi A, Rathnasinghe R, Laghlali G, Uvyn A, Herck SV, Nuhn L, Deswarte K, Zhong Z, Sanders N, Lienenklaus S, David S, Strohmeier S, Amanat F, Krammer F, Hammad H, Lambrecht BN, Coughlan L, García-Sastre A, de Geest B, Schotsaert M, Sterilizing Immunity against SARS-CoV-2 Infection in Mice by a Single-Shot and Lipid Amphiphile Imidazoquinoline TLR7/8 Agonist-Adjuvanted Recombinant Spike Protein Vaccine, Angewandte Chemie Int Ed. (2021). 10.1002/anie.202015362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Petitdemange C, Kasturi SP, Kozlowski PA, Nabi R, Quarnstrom CF, Reddy PBJ, Derdeyn CA, Spicer LM, Patel P, Legere T, Kovalenkov YO, Labranche CC, Villinger F, Tomai M, Vasilakos J, Haynes B, Kang CY, Gibbs JS, Yewdell JW, Barouch D, Wrammert J, Montefiori D, Hunter E, Amara RR, Masopust D, Pulendran B, Vaccine induction of antibodies and tissue-resident CD8+ T cells enhances protection against mucosal SHIV-infection in young macaques, Jci Insight. 4 (2019) e126047. 10.1172/jci.insight.126047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Arunachalam PS, Charles TP, Joag V, Bollimpelli VS, Scott MKD, Wimmers F, Burton SL, Labranche CC, Petitdemange C, Gangadhara S, Styles TM, Quarnstrom CF, Walter KA, Ketas TJ, Legere T, Reddy PBJ, Kasturi SP, Tsai A, Yeung BZ, Gupta S, Tomai M, Vasilakos J, Shaw GM, Kang C-Y, Moore JP, Subramaniam S, Khatri P, Montefiori D, Kozlowski PA, Derdeyn CA, Hunter E, Masopust D, Amara RR, Pulendran B, T cell-inducing vaccine durably prevents mucosal SHIV infection even with lower neutralizing antibody titers, Nat Med. (2020) 1–9. 10.1038/s41591-020-0858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Philbin VJ, Dowling DJ, Gallington LC, Cortés G, Tan Z, Suter EE, Chi KW, Shuckett A, Stoler-Barak L, Tomai M, Miller RL, Mansfield K, Levy O, Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways., J Allergy Clin Immunol. 130 (2012) 195–204.e9. 10.1016/j.jaci.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Primard C, Poecheim J, Heuking S, Sublet E, Esmaeili F, Borchard G, Multifunctional PLGA-Based Nanoparticles Encapsulating Simultaneously Hydrophilic Antigen and Hydrophobic Immunomodulator for Mucosal Immunization, Mol Pharmaceut. 10 (2013) 2996–3004. 10.1021/mp400092y. [DOI] [PubMed] [Google Scholar]
- [259].Kim H, Niu L, Larson P, Kucaba TA, Murphy KA, James BR, Ferguson DM, Griffith TS, Panyam J, Polymeric nanoparticles encapsulating novel TLR7/8 agonists as immunostimulatory adjuvants for enhanced cancer immunotherapy, Biomaterials. 164 (2018) 38–53. 10.1016/j.biomaterials.2018.02.034. [DOI] [PubMed] [Google Scholar]
- [260].Sevimli S, Knight FC, Gilchuk P, Joyce S, Wilson JT, Fatty Acid-Mimetic Micelles for Dual Delivery of Antigens and Imidazoquinoline Adjuvants, Acs Biomater Sci Eng. 3 (2017) 179–194. 10.1021/acsbiomaterials.6b00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Dowling DJ, Scott EA, Scheid A, Bergelson I, Joshi S, Pietrasanta C, Brightman S, Sanchez-Schmitz G, Haren SDV, Ninković J, Kats D, Guiducci C, de Titta A, Bonner DK, Hirosue S, Swartz MA, Hubbell JA, Levy O, Toll-like receptor 8 agonist nanoparticles mimic immunomodulating effects of the live BCG vaccine and enhance neonatal innate and adaptive immune responses, J Allergy Clin Immun. 140 (2017) 1339–1350. 10.1016/j.jaci.2016.12.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Vicente S, Peleteiro M, Díaz-Freitas B, Sanchez A, González-Fernández Á, Alonso MJ, Co-delivery of viral proteins and a TLR7 agonist from polysaccharide nanocapsules: A needle-free vaccination strategy, J Control Release. 172 (2013) 773–781. 10.1016/j.jconrel.2013.09.012. [DOI] [PubMed] [Google Scholar]
- [263].Kim NW, Kim S-Y, Lee JE, Yin Y, Lee JH, Lim SY, Kim ES, Duong HTT, Kim HK, Kim S, Kim J-E, Lee DS, Kim J, Lee MS, Lim YT, Jeong JH, Enhanced Cancer Vaccination by In Situ Nanomicelle-Generating Dissolving Microneedles, Acs Nano. 12 (2018) 9702–9713. 10.1021/acsnano.8b04146. [DOI] [PubMed] [Google Scholar]
- [264].Park W, Seong KY, Han HH, Yang SY, Hahn SK, Dissolving microneedles delivering cancer cell membrane coated nanoparticles for cancer immunotherapy, Rsc Adv. 11 (2021) 10393–10399. 10.1039/d1ra00747e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Chan M, Hayashi T, Mathewson RD, Yao S, Gray C, Tawatao RI, Kalenian K, Zhang Y, Hayashi Y, Lao FS, Cottam HB, Carson DA, Synthesis and Characterization of PEGylated Toll Like Receptor 7 Ligands, Bioconjugate Chem. 22 (2011) 445–454. 10.1021/bc1004813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Ryu KA, Slowinska K, Moore T, Esser-Kahn A, Immune Response Modulation of Conjugated Agonists with Changing Linker Length, Acs Chem Biol. 11 (2016) 3347–3352. 10.1021/acschembio.6b00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Wu CCN, Hayashi T, Takabayashi K, Sabet M, Smee DF, Guiney DD, Cottam HB, Carson DA, Immunotherapeutic activity of a conjugate of a Toll-like receptor 7 ligand, Proc National Acad Sci. 104 (2007) 3990–3995. 10.1073/pnas.0611624104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Shinchi H, Crain B, Yao S, Chan M, Zhang SS, Ahmadiiveli A, Suda Y, Hayashi T, Cottam HB, Carson DA, Enhancement of the Immunostimulatory Activity of a TLR7 Ligand by Conjugation to Polysaccharides, Bioconjugate Chemistry. 26 (2015) 1713–23. 10.1021/acs.bioconjchem.5b00285. [DOI] [PubMed] [Google Scholar]
- [269].Hosoya T, Sato-Kaneko F, Ahmadi A, Yao S, Lao F, Kitaura K, Matsutani T, Carson DA, Hayashi T, Induction of oligoclonal CD8 T cell responses against pulmonary metastatic cancer by a phospholipid-conjugated TLR7 agonist, Proc National Acad Sci. 115 (2018) 201803281. 10.1073/pnas.1803281115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Duong AD, Sharma S, Peine KJ, Gupta G, Satoskar AR, Bachelder EM, Wyslouzil BE, Ainslie KM, Electrospray Encapsulation of Toll-Like Receptor Agonist Resiquimod in Polymer Microparticles for the Treatment of Visceral Leishmaniasis, Mol Pharmaceut. 10 (2013) 1045–1055. 10.1021/mp3005098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, Kohler RH, Pittet MJ, Weissleder R, TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy, Nature Biomedical Engineering. 2 (2018) 578–588. 10.1038/s41551-018-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Rodell CB, Ahmed MS, Garris CS, Pittet MJ, Weissleder R, Development of Adamantane-Conjugated TLR7/8 Agonists for Supramolecular Delivery and Cancer Immunotherapy, Theranostics. 9 (2019) 8426–8436. 10.7150/thno.35434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Koch PD, Rodell CB, Kohler RH, Pittet MJ, Weissleder R, Myeloid Cell-Targeted Nanocarriers Efficiently Inhibit Cellular Inhibitor of Apoptosis for Cancer Immunotherapy, Cell Chem Biol. 27 (2020). 10.1016/j.chembiol.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Vinod N, Hwang D, Azam S, Swearingen A, Wayne E, Fussell S, Sokolsky-Papkov M, Pecot C, Kabanov A, High-capacity poly(2-oxazoline) formulation of TLR 7/8 agonist extends survival in a chemo-insensitive, metastatic model of lung adenocarcinoma., Sci Adv. 6 (2020) eaba5542. 10.1126/sciadv.aba5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Mottas I, Bekdemir A, Cereghetti A, Spagnuolo L, Yang Y-SS, Müller M, Irvine DJ, Stellacci F, Bourquin C, Amphiphilic nanoparticle delivery enhances the anticancer efficacy of a TLR7 ligand via local immune activation, Biomaterials. 190 (2019) 111–120. 10.1016/j.biomaterials.2018.10.031. [DOI] [PubMed] [Google Scholar]
- [276].Scott EA, Stano A, Gillard M, Maio-Liu AC, Swartz MA, Hubbell JA, Dendritic cell activation and T cell priming with adjuvant- and antigen-loaded oxidation-sensitive polymersomes, Biomaterials. 33 (2012) 6211–6219. 10.1016/j.biomaterials.2012.04.060. [DOI] [PubMed] [Google Scholar]
- [277].Manaster AJ, Batty C, Tiet P, Ooi A, Bachelder EM, Ainslie KM, Broaders KE, Oxidation-Sensitive Dextran-Based Polymer with Improved Processability through Stable Boronic Ester Groups, Acs Appl Bio Mater. 2 (2019) 3755–3762. 10.1021/acsabm.9b00399. [DOI] [PubMed] [Google Scholar]
- [278].Ryu KA, Stutts L, Tom JK, Mancini RJ, Esser-Kahn AP, Stimulation of Innate Immune Cells by Light-Activated TLR7/8 Agonists, J Am Chem Soc. 136 (2014) 10823–10825. 10.1021/ja412314j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Hantho JD, Strayer TA, Nielsen AE, Mancini RJ, An Enzyme-Directed Imidazoquinoline for Cancer Immunotherapy, ChemMedChem. 11 (2016) 2496–2500. 10.1002/cmdc.201600443. [DOI] [PubMed] [Google Scholar]
- [280].Ryan AT, Pulukuri AJ, Davaritouchaee M, Abbasi A, Hendricksen AT, Opp LK, Burt AJ, Nielsen AE, Mancini RJ, Comparing the immunogenicity of glycosidase-directed resiquimod prodrugs mediated by cancer cell metabolism, Acta Pharmacol Sin. 41 (2020) 995–1004. 10.1038/s41401-020-0432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Wang B, Herck SV, Chen Y, Bai X, Zhong Z, Deswarte K, Lambrecht BN, Sanders NN, Lienenklaus S, Scheeren HW, David SA, Kiessling F, Lammers T, Geest BGD, Shi Y, Potent and Prolonged Innate Immune Activation by Enzyme-Responsive Imidazoquinoline TLR7/8 Agonist Prodrug Vesicles, J Am Chem Soc. (2020). 10.1021/jacs.0c01928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Schmid D, Park CG, Hartl CA, Subedi N, Cartwright AN, Puerto RB, Zheng Y, Maiarana J, Freeman GJ, Wucherpfennig KW, Irvine DJ, Goldberg MS, T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity, Nature Communications. 8 (2017) 1747. 10.1038/s41467-017-01830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Tacken PJ, Zeelenberg IS, Cruz LJ, van Hout-Kuijer MA, van de Glind G, Fokkink RG, Lambeck AJA, Figdor CG, Targeted delivery of TLR ligands to human and mouse dendritic cells strongly enhances adjuvanticity, Blood. 118 (2011) 6836–6844. 10.1182/blood-2011-07-367615. [DOI] [PubMed] [Google Scholar]
- [284].Gadd AJR, Greco F, Cobb AJA, Edwards AD, Targeted Activation of Toll-Like Receptors: Conjugation of a Toll-Like Receptor 7 Agonist to a Monoclonal Antibody Maintains Antigen Binding and Specificity, Bioconjugate Chem. 26 (2015) 1743–1752. 10.1021/acs.bioconjchem.5b00302. [DOI] [PubMed] [Google Scholar]
- [285].Klauber TCB, Laursen JM, Zucker D, Brix S, Jensen SS, Andresen TL, Delivery of TLR7 agonist to monocytes and dendritic cells by DCIR targeted liposomes induces robust production of anti-cancer cytokines, Acta Biomater. 53 (2017) 367–377. 10.1016/j.actbio.2017.01.072. [DOI] [PubMed] [Google Scholar]
- [286].Johansen PT, Zucker D, Parhamifar L, Pourhassan H, Madsen DV, Henriksen JR, Gad M, Barberis A, Maj R, Andresen TL, Jensen SS, Monocyte targeting and activation by cationic liposomes formulated with a TLR7 agonist, Expert Opin Drug Del. 12 (2015) 1045–58. 10.1517/17425247.2015.1009444. [DOI] [PubMed] [Google Scholar]
- [287].Miller JB, Zhang S, Kos P, Xiong H, Zhou K, Perelman SS, Zhu H, Siegwart DJ, Non-Viral CRISPR/Cas Gene Editing In Vitro and In Vivo Enabled by Synthetic Nanoparticle Co-Delivery of Cas9 mRNA and sgRNA., Angewandte Chemie Int Ed Engl. 56 (2016) 1059–1063. 10.1002/anie.201610209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ, Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing, Nat Nanotechnol. 15 (2020) 313–320. 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Aznar MA, Tinari N, Rullán AJ, Sánchez-Paulete AR, Rodriguez-Ruiz ME, Melero I, Intratumoral Delivery of Immunotherapy—Act Locally, Think Globally, J Immunol. 198 (2017) 31–39. 10.4049/jimmunol.1601145. [DOI] [PubMed] [Google Scholar]
- [290].Sloot S, Rashid OM, Zager JS, Intralesional therapy for metastatic melanoma, Expert Opin Pharmaco. 15 (2014) 2629–2639. 10.1517/14656566.2014.967682. [DOI] [PubMed] [Google Scholar]
- [291].Rehman H, Silk AW, Kane MP, Kaufman HL, Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy, J Immunother Cancer. 4 (2016) 53. 10.1186/s40425-016-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Hammerich L, Marron TU, Upadhyay R, Svensson-Arvelund J, Dhainaut M, Hussein S, Zhan Y, Ostrowski D, Yellin M, Marsh H, Salazar AM, Rahman AH, Brown BD, Merad M, Brody JD, Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination, Nature Medicine. 25 (2019) 814–824. 10.1038/s41591-019-0410-x. [DOI] [PubMed] [Google Scholar]
- [293].Daud A, Algazi AP, Ashworth MT, Fong L, Lewis J, Chan SE, Buljan M, Molina MA, Takamura KT, Diep TT, Heller R, Pierce RH, Bhatia S, Systemic antitumor effect and clinical response in a phase 2 trial of intratumoral electroporation of plasmid interleukin-12 in patients with advanced melanoma., J Clin Oncol. 32 (2014) 9025–9025. 10.1200/jco.2014.32.15_suppl.9025. [DOI] [Google Scholar]
- [294].Hong WX, Haebe S, Lee AS, Westphalen CB, Norton JA, Jiang W, Levy R, Intratumoral Immunotherapy for Early-stage Solid Tumors, Clin Cancer Res. (2020) clincanres.3642.2019. 10.1158/1078-0432.ccr-19-3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Gupta S, Grilley-Olson J, Hong D, Marabelle A, Munster P, Aggarwal R, Aspeslagh S, Dixon RG, Patel M, Subbiah V, Morehouse C, Wu Y, Zha J, Tseng L, Cooper ZA, Morris S, Brody J, Abstract CT091: Safety and pharmacodynamic activity of MEDI9197, a TLR 7/8 agonist, administered intratumorally in subjects with solid tumors, (2017) CT091–CT091. 10.1158/1538-7445.am2017-ct091. [DOI] [Google Scholar]
- [296].Fakhari A, Nugent S, Elvecrog J, Vasilakos J, Corcoran M, Tilahun A, Siebenaler K, Sun J, Subramony JA, Schwarz A, Thermosensitive Gel–Based Formulation for Intratumoral Delivery of Toll-Like Receptor 7/8 Dual Agonist, MEDI9197, J Pharm Sci. 106 (2017) 2037–2045. 10.1016/j.xphs.2017.04.041. [DOI] [PubMed] [Google Scholar]
- [297].Smirnov D, Schmidt JJ, Capecchi JT, Wightman PD, Vaccine adjuvant activity of 3M-052: An imidazoquinoline designed for local activity without systemic cytokine induction, Vaccine. 29 (2011) 5434–5442. 10.1016/j.vaccine.2011.05.061. [DOI] [PubMed] [Google Scholar]
- [298].Cortez A, Li Y, Miller AT, Zhang X, Yue K, Maginnis J, Hampton J, Hall DS, Shapiro M, Nayak B, D’Oro U, Li C, Skibinski D, Mbow ML, Singh M, O’Hagan DT, Cooke MP, Valiante NM, Wu TY-H, Incorporation of Phosphonate into Benzonaphthyridine Toll-like Receptor 7 Agonists for Adsorption to Aluminum Hydroxide, J Med Chem. 59 (2016) 5868–5878. 10.1021/acs.jmedchem.6b00489. [DOI] [PubMed] [Google Scholar]
- [299].Study of the Safety and Efficacy of LHC165 Single Agent and in Combination With PDR001 in Patients With Advanced Malignancies, https://clinicaltrials.gov/ct2/show/NCT03301896. [Google Scholar]
- [300].Georges B, Immunogenic Compound, US10,434,183 B2, 2019. https://patentimages.storage.googleapis.com/72/16/72/6d37b4c964d73a/US10434183.pdf. [Google Scholar]
- [301].Zuniga LA, Leßmann T, Holten-Andersen L, Bisek N, Zettler J, Stark S, Faltinger F, Kracker O, Weisbrod S, Müller R, Voigt T, Bigott K, Tabrizifard M, Breinholt V, Sprogöe K, Punnonen J, Intratumoral Delivery of TransCon™ TLR7/8 Agonist Provides Potent Anti-tumor Activity as a Monotherapy and in Combination With IL-2 While Minimizing Systemic Cytokine Induction, J Immunother Cancer. 7 (2019) 283. 10.1186/s40425-019-0764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Nuhn L, Koker SD, Lint SV, Zhong Z, Catani JP, Combes F, Deswarte K, Li Y, Lambrecht BN, Lienenklaus S, Sanders NN, David SA, Tavernier J, Geest BGD, Nanoparticle-Conjugate TLR7/8 Agonist Localized Immunotherapy Provokes Safe Antitumoral Responses, Adv Mater. 30 (2018) 1803397. 10.1002/adma.201803397. [DOI] [PubMed] [Google Scholar]
- [303].Kim S-Y, Kim S, Kim J-E, Lee SN, Shin IW, Shin HS, Jin SM, Noh Y-W, Kang YJ, Kim YS, Kang TH, Park Y-M, Lim YT, Lyophilizable and Multifaceted Toll-like Receptor 7/8 Agonist-Loaded Nanoemulsion for the Reprogramming of Tumor Microenvironments and Enhanced Cancer Immunotherapy, Acs Nano. 13 (2019) 12671–12686. 10.1021/acsnano.9b04207. [DOI] [PubMed] [Google Scholar]
- [304].Lu R, Groer C, Kleindl PA, Moulder KR, Huang A, Hunt JR, Cai S, Aires DJ, Berkland C, Forrest L, Formulation and preclinical evaluation of a toll-like receptor 7/8 agonist as an anti-tumoral immunomodulator, Journal of Controlled Release. 306 (2019) 165–176. 10.1016/j.jconrel.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Vohidov F, Milling LE, Chen Q, Zhang W, Bhagchandani S, Nguyen HV-T, Irvine DJ, Johnson JA, ABC triblock bottlebrush copolymer-based injectable hydrogels: design, synthesis, and application to expanding the therapeutic index of cancer immunochemotherapy, Chem Sci. (2020). 10.1039/d0sc02611e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Kockelmann J, Stickdorn J, Kasmi S, Vrieze JD, Pieszka M, Ng DYW, David SA, Geest BGD, Nuhn L, Control over Imidazoquinoline Immune Stimulation by pH-Degradable Poly(norbornene) Nanogels, Biomacromolecules. 21 (2020). 10.1021/acs.biomac.0c00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Bahmani B, Gong H, Luk BT, Haushalter KJ, DeTeresa E, Previti M, Zhou J, Gao W, Bui JD, Zhang L, Fang RH, Zhang J, Intratumoral immunotherapy using platelet-cloaked nanoparticles enhances antitumor immunity in solid tumors, Nat Commun. 12 (2021) 1999. 10.1038/s41467-021-22311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Park CG, Hartl CA, Schmid D, Carmona EM, Kim H-J, Goldberg MS, Extended release of perioperative immunotherapy prevents tumor recurrence and eliminates metastases, Sci Transl Med. 10 (2018) eaar1916. 10.1126/scitranslmed.aar1916. [DOI] [PubMed] [Google Scholar]
- [309].Melero I, Berman D, Aznar M, Korman A, Gracia J, Haanen J, Evolving synergistic combinations of targeted immunotherapies to combat cancer, Nat Rev Cancer. 15 (2015) 457–472. 10.1038/nrc3973. [DOI] [PubMed] [Google Scholar]
- [310].Lo JA, Kawakubo M, Juneja VR, Su MY, Erlich TH, LaFleur MW, Kemeny LV, Rashid M, Malehmir M, Rabi SA, Raghavan R, Allouche J, Kasumova G, Frederick DT, Pauken KE, Weng QY, da Silva MP, Xu Y, van der Sande AAJ, Silkworth W, Roider E, Browne EP, Lieb DJ, Wang B, Garraway LA, Wu CJ, Flaherty KT, Brinckerhoff CE, Mullins DW, Adams DJ, Hacohen N, Hoang MP, Boland GM, Freeman GJ, Sharpe AH, Manstein D, Fisher DE, Epitope spreading toward wild-type melanocyte-lineage antigens rescues suboptimal immune checkpoint blockade responses, Sci Transl Med. 13 (2021) eabd8636. 10.1126/scitranslmed.abd8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Wang L, Gao Y, Zhang G, Li D, Wang Z, Zhang J, Hermida LC, He L, Wang Z, Si J, Geng S, Ai R, Ning F, Cheng C, Deng H, Dimitrov DS, Sun Y, Huang Y, Wang D, Hu X, Wei Z, Wang W, Liao X, Enhancing KDM5A and TLR activity improves the response to immune checkpoint blockade, Sci Transl Med. 12 (2020) eaax2282. 10.1126/scitranslmed.aax2282. [DOI] [PubMed] [Google Scholar]
- [312].Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, Akira S, Ahmed R, Pulendran B, Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity, J Exp Medicine. 203 (2006) 413–424. 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A, Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1–polarizing program in dendritic cells, Nat Immunol. 6 (2005) 769–776. 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [314].Maisonneuve C, Bertholet S, Philpott DJ, Gregorio ED, Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants, Proceedings of the National Academy of Sciences. 111 (2014). 10.1073/pnas.1400478111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Shekarian T, Valsesia-Wittmann S, Brody J, Michallet MC, Depil S, Caux C, Marabelle A, Pattern recognition receptors: immune targets to enhance cancer immunotherapy, (2017). 10.1093/annonc/mdx179. [DOI] [PubMed] [Google Scholar]
- [316].Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, García-Sastre A, Compans R, Pulendran B, Programming the magnitude and persistence of antibody responses with innate immunity, Nature. 470 (2011) 543. 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Kasturi SP, Kozlowski PA, Nakaya HI, Burger MC, Russo P, Pham M, Kovalenkov Y, Silveira ELV, Havenar-Daughton C, Burton SL, Kilgore KM, Johnson MJ, Nabi R, Legere T, Sher ZJ, Chen X, Amara RR, Hunter E, Bosinger SE, Spearman P, Crotty S, Villinger F, Derdeyn CA, Wrammert J, Pulendran B, Adjuvanting a Simian Immunodeficiency Virus Vaccine with Toll-Like Receptor Ligands Encapsulated in Nanoparticles Induces Persistent Antibody Responses and Enhanced Protection in TRIM5α Restrictive Macaques, J Virol. 91 (2017) e01844–16. 10.1128/jvi.01844-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Kasturi SP, Rasheed MAU, Havenar-Daughton C, Pham M, Legere T, Sher ZJ, Kovalenkov Y, Gumber S, Huang JY, Gottardo R, Fulp W, Sato A, Sawant S, Stanfield-Oakley S, Yates N, LaBranche C, Alam SM, Tomaras G, Ferrari G, Montefiori D, Wrammert J, Villinger F, Tomai M, Vasilakos J, Fox CB, Reed SG, Haynes BF, Crotty S, Ahmed R, Pulendran B, 3M-052, a synthetic TLR-7/8 agonist, induces durable HIV-1 envelope–specific plasma cells and humoral immunity in nonhuman primates, Sci Immunol. 5 (2020) eabb1025. 10.1126/sciimmunol.abb1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Fox CB, Sivananthan SJ, Duthie MS, Vergara J, Guderian JA, Moon E, Coblentz D, Reed SG, Carter D, A nanoliposome delivery system to synergistically trigger TLR4 AND TLR7, J Nanobiotechnol. 12 (2014) 17. 10.1186/1477-3155-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Rizwan SB, McBurney WT, Young K, Hanley T, Boyd BJ, Rades T, Hook S, Cubosomes containing the adjuvants imiquimod and monophosphoryl lipid A stimulate robust cellular and humoral immune responses, J Control Release. 165 (2013) 16–21. 10.1016/j.jconrel.2012.10.020. [DOI] [PubMed] [Google Scholar]
- [321].Goff PH, Hayashi T, Martínez-Gil L, Corr M, Crain B, Yao S, Cottam HB, Chan M, Ramos I, Eggink D, Heshmati M, Krammer F, Messer K, Pu M, Fernandez-Sesma A, Palese P, Carson DA, Synthetic Toll-Like Receptor 4 (TLR4) and TLR7 Ligands as Influenza Virus Vaccine Adjuvants Induce Rapid, Sustained, and Broadly Protective Responses, Journal of Virology. 89 (2015). 10.1128/JVI.03337-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].McKay PF, King DFL, Mann JFS, Barinaga G, Carter D, Shattock RJ, TLR4 and TLR7/8 Adjuvant Combinations Generate Different Vaccine Antigen-Specific Immune Outcomes in Minipigs when Administered via the ID or IN Routes, Plos One. 11 (2016) e0148984. 10.1371/journal.pone.0148984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Moody MA, Santra S, Vandergrift NA, Sutherland LL, Gurley TC, Drinker MS, Allen AA, Xia S-M, Meyerhoff RR, Parks R, Lloyd KE, Easterhoff D, Alam SM, Liao H-X, Ward BM, Ferrari G, Montefiori DC, Tomaras GD, Seder RA, Letvin NL, Haynes BF, Toll-Like Receptor 7/8 (TLR7/8) and TLR9 Agonists Cooperate To Enhance HIV-1 Envelope Antibody Responses in Rhesus Macaques, J Virol. 88 (2014) 3329–3339. 10.1128/jvi.03309-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [324].Madan-Lala R, Pradhan P, Roy K, Combinatorial Delivery of Dual and Triple TLR Agonists via Polymeric Pathogen-like Particles Synergistically Enhances Innate and Adaptive Immune Responses, Scientific Reports. 7 (2017) 2530. 10.1038/s41598-017-02804-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Tom JK, Dotsey EY, Wong HY, Stutts L, Moore T, Davies DH, Felgner PL, Esser-Kahn AP, Modulation of Innate Immune Responses via Covalently Linked TLR Agonists, Acs Central Sci. 1 (2015) 439–448. 10.1021/acscentsci.5b00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326].Kimani FW, Ajit J, Galluppi A, Manna S, Howitz WJ, Tang S, Esser-Kahn AP, Receptor–Ligand Kinetics Influence the Mechanism of Action of Covalently Linked TLR Ligands, Acs Chem Biol. (2021). 10.1021/acschembio.0c00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin H-S, Valentine C, Hellman J, MyD88-Dependent and MyD88-Independent Pathways in Synergy, Priming, and Tolerance between TLR Agonists, J Immunol. 178 (2007) 1164–1171. 10.4049/jimmunol.178.2.1164. [DOI] [PubMed] [Google Scholar]
- [328].Whitmore MM, DeVeer MJ, Edling A, Oates RK, Simons B, Lindner D, Williams BRG, Synergistic Activation of Innate Immunity by Double-Stranded RNA and CpG DNA Promotes Enhanced Antitumor Activity, Cancer Res. 64 (2004) 5850–5860. 10.1158/0008-5472.can-04-0063. [DOI] [PubMed] [Google Scholar]
- [329].Albin TJ, Tom JK, Manna S, Gilkes AP, Stetkevich SA, Katz BB, Supnet M, Felgner J, Jain A, Nakajima R, Jasinskas A, Zlotnik A, Pearlman E, Davies DH, Felgner PL, Burkhardt AM, Esser-Kahn AP, Linked Toll-Like Receptor Triagonists Stimulate Distinct, Combination-Dependent Innate Immune Responses, Acs Central Sci. 5 (2019) 1137–1145. 10.1021/acscentsci.8b00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Manna S, Maiti S, Shen J, Du W, Esser-Kahn AP, Pathogen-like Nanoassemblies of Covalently Linked TLR Agonists Enhance CD8 and NK Cell-Mediated Antitumor Immunity, Acs Central Sci. 6 (2020) 2071–2078. 10.1021/acscentsci.0c01001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Pradhan P, Toy R, Jhita N, Atalis A, Pandey B, Beach A, Blanchard EL, Moore SG, Gaul DA, Santangelo PJ, Shayakhmetov DM, Roy K, TRAF6-IRF5 kinetics, TRIF, and biophysical factors drive synergistic innate responses to particle-mediated MPLA-CpG co-presentation, Sci Adv. 7 (2021) eabd4235. 10.1126/sciadv.abd4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Ni Q, Zhang F, Liu Y, Wang Z, Yu G, Liang B, Niu G, Su T, Zhu G, Lu G, Zhang L, Chen X, A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer, Sci Adv. 6 (2020) eaaw6071. 10.1126/sciadv.aaw6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333].Toy R, Keenum MC, Pradhan P, Phang K, Chen P, Chukwu C, Nguyen LAH, Liu J, Jain S, Kozlowski G, Hosten J, Suthar MS, Roy K, TLR7 and RIG-I dual-adjuvant loaded nanoparticles drive broadened and synergistic responses in dendritic cells in vitro and generate unique cellular immune responses in influenza vaccination, J Control Release. 330 (2021) 866–877. 10.1016/j.jconrel.2020.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Dovedi SJ, Melis MHM, Wilkinson RW, Adlard AL, Stratford IJ, Honeychurch J, Illidge TM, Systemic delivery of a TLR7 agonist in combination with radiation primes durable antitumor immune responses in mouse models of lymphoma, Blood. 121 (2013) 251–259. 10.1182/blood-2012-05-432393. [DOI] [PubMed] [Google Scholar]
- [335].Sagiv-Barfi I, Czerwinski DK, Levy S, Alam IS, Mayer AT, Gambhir SS, Levy R, Eradication of spontaneous malignancy by local immunotherapy, Science Translational Medicine. 10 (2018) eaan4488. 10.1126/scitranslmed.aan4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Huang C, Mendez N, Echeagaray OH, Weeks J, Wang J, Yao S, Blair SL, Gude N, Trogler WC, Carson DA, Hayashi T, Kummel AC, Immunostimulatory TLR7 Agonist-Nanoparticles Together with Checkpoint Blockade for Effective Cancer Immunotherapy, Adv Ther. 3 (2020) 1900200. 10.1002/adtp.201900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Sato-Kaneko F, Yao S, Ahmadi A, Zhang SS, Hosoya T, Kaneda MM, Varner JA, Pu M, Messer KS, Guiducci C, Coffman RL, Kitaura K, Matsutani T, Suzuki R, Carson DA, Hayashi T, Cohen EEW, Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer, JCI Insight. 2 (2017). 10.1172/jci.insight.93397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [338].Nishii N, Tachinami H, Kondo Y, Xia Y, Kashima Y, Ohno T, Nagai S, Li L, Lau W, Harada H, Azuma M, Systemic administration of a TLR7 agonist attenuates regulatory T cells by dendritic cell modification and overcomes resistance to PD-L1 blockade therapy, Oncotarget. 9 (2018) 13301–13312. 10.18632/oncotarget.24327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [339].Smith AAA, Gale EC, Roth GA, Maikawa CL, Correa S, Yu AC, Appel EA, Nanoparticles Presenting Potent TLR7/8 Agonists Enhance Anti-PD-L1 Immunotherapy in Cancer Treatment, Biomacromolecules. 21 (2020) 3704–3712. 10.1021/acs.biomac.0c00812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340].Kim H, Khanna V, Kucaba TA, Zhang W, Sehgal D, Ferguson DM, Griffith TS, Panyam J, TLR7/8 Agonist-Loaded Nanoparticles Augment NK Cell-Mediated Antibody-Based Cancer Immunotherapy, Mol Pharmaceut. 17 (2020). 10.1021/acs.molpharmaceut.0c00271. [DOI] [PubMed] [Google Scholar]
- [341].Hayashi T, Chan M, Norton JT, Wu CCN, Yao S, Cottam HB, Tawatao RI, Corr M, Carson DA, Daniels GA, Additive melanoma suppression with intralesional phospholipid-conjugated TLR7 agonists and systemic IL-2, Melanoma Res. 21 (2011) 66–75. 10.1097/cmr.0b013e328340ce6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342].Heo MB, Lim YT, Programmed nanoparticles for combined immunomodulation, antigen presentation and tracking of immunotherapeutic cells, Biomaterials. 35 (2014) 590–600. 10.1016/j.biomaterials.2013.10.009. [DOI] [PubMed] [Google Scholar]
- [343].Silva CGD, Camps MGM, Li TMWY, Chan AB, Ossendorp F, Cruz LJ, Co-delivery of immunomodulators in biodegradable nanoparticles improves therapeutic efficacy of cancer vaccines, Biomaterials. 220 (2019) 119417. 10.1016/j.biomaterials.2019.119417. [DOI] [PubMed] [Google Scholar]
- [344].Khanna V, Kim H, Zhang W, Larson P, Shah M, Griffith TS, Ferguson D, Panyam J, Novel TLR 7/8 agonists for improving NK cell mediated antibody-dependent cellular cytotoxicity (ADCC), Sci Rep-Uk. 11 (2021) 3346. 10.1038/s41598-021-83005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345].Díaz-Carballo D, Saka S, Acikelli AH, Homp E, Erwes J, Demmig R, Klein J, Schröer K, Malak S, D’Souza F, Noa-Bolaño A, Menze S, Pano E, Andrioff S, Teipel M, Dammann P, Klein D, Nasreen A, Tannapfel A, Grandi N, Tramontano E, Ochsenfarth C, Strumberg D, Enhanced antitumoral activity of TLR7 agonists via activation of human endogenous retroviruses by HDAC inhibitors, Commun Biology. 4 (2021) 276. 10.1038/s42003-021-01800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [346].Smith M, García-Martínez E, Pitter MR, Fucikova J, Spisek R, Zitvogel L, Kroemer G, Galluzzi L, Trial Watch: Toll-like receptor agonists in cancer immunotherapy, Oncoimmunology. 7 (2018) 1–15. 10.1080/2162402x.2018.1526250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347].Biswas SK, Lopez-Collazo E, Endotoxin tolerance: new mechanisms, molecules and clinical significance, Trends Immunol. 30 (2009) 475–487. 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- [348].Chen J, Ivashkiv LB, IFN-γ abrogates endotoxin tolerance by facilitating Toll-like receptor-induced chromatin remodeling, Proc National Acad Sci. 107 (2010) 19438–19443. 10.1073/pnas.1007816107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [349].Miles K, Heaney J, Sibinska Z, Salter D, Savill J, Gray D, Gray M, A tolerogenic role for Toll-like receptor 9 is revealed by B-cell interaction with DNA complexes expressed on apoptotic cells, Proc National Acad Sci. 109 (2012) 887–892. 10.1073/pnas.1109173109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [350].Silver AC, Arjona A, Walker WE, Fikrig E, The Circadian Clock Controls Toll-like Receptor 9-Mediated Innate and Adaptive Immunity, Immunity. 36 (2012) 251–261. 10.1016/j.immuni.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [351].Kondo T, Kawai T, Akira S, Dissecting negative regulation of Toll-like receptor signaling, Trends Immunol. 33 (2012) 449–458. 10.1016/j.it.2012.05.002. [DOI] [PubMed] [Google Scholar]
- [352].Shi Y, White D, He L, Miller RL, Spaner DE, Toll-like Receptor-7 Tolerizes Malignant B Cells and Enhances Killing by Cytotoxic Agents, Cancer Res. 67 (2007) 1823–1831. 10.1158/0008-5472.can-06-2381. [DOI] [PubMed] [Google Scholar]
- [353].Koga-Yamakawa E, Murata M, Dovedi SJ, Wilkinson RW, Ota Y, Umehara H, Sugaru E, Hirose Y, Harada H, Jewsbury PJ, Yamamoto S, Robinson DT, Li CJ, TLR7 tolerance is independent of the type I IFN pathway and leads to loss of anti-tumor efficacy in mice, Cancer Immunol Immunother. 64 (2015) 1229–1239. 10.1007/s00262-015-1730-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [354].Hayashi T, Gray CS, Chan M, Tawatao RI, Ronacher L, McGargill MA, Datta SK, Carson DA, Corr M, Prevention of autoimmune disease by induction of tolerance to Toll-like receptor 7, P Natl Acad Sci Usa. 106 (2009) 2764–9. 10.1073/pnas.0813037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [355].Poovassery JS, Bishop GA, Type I IFN Receptor and the B Cell Antigen Receptor Regulate TLR7 Responses via Distinct Molecular Mechanisms, The Journal of Immunology. 189 (2012) 1757–1764. 10.4049/jimmunol.1200624. [DOI] [PubMed] [Google Scholar]
- [356].Michaelis KA, Norgard MA, Levasseur PR, Olson B, Burfeind KG, Buenafe AC, Zhu X, Jeng S, McWeeney SK, Marks DL, Persistent Toll-like receptor 7 stimulation induces behavioral and molecular innate immune tolerance, Brain Behav Immun. 82 (2019) 338–353. 10.1016/j.bbi.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [357].Bourquin C, Hotz C, Noerenberg D, Voelkl A, Heidegger S, Roetzer LC, Storch B, Sandholzer N, Wurzenberger C, Anz D, Endres S, Systemic Cancer Therapy with a Small Molecule Agonist of Toll-like Receptor 7 Can Be Improved by Circumventing TLR Tolerance, Cancer Research. 71 (2011) 5123–5133. 10.1158/0008-5472.CAN-10-3903. [DOI] [PubMed] [Google Scholar]
- [358].Tsitoura D, Ambery C, Price M, Powley W, Garthside S, Biggadike K, Quint D, Early clinical evaluation of the intranasal TLR7 agonist GSK2245035: Use of translational biomarkers to guide dosing and confirm target engagement, Clin Pharmacol Ther. 98 (2015) 369–380. 10.1002/cpt.157. [DOI] [PubMed] [Google Scholar]
- [359].Silver AC, Buckley SM, Hughes ME, Hastings AK, Nitabach MN, Fikrig E, Daily oscillations in expression and responsiveness of Toll-like receptors in splenic immune cells, Heliyon. 4 (2018) e00579. 10.1016/j.heliyon.2018.e00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [360].Greenberg EN, Marshall ME, Jin S, Venkatesh S, Dragan M, Tsoi LC, Gudjonsson JE, Nie Q, Takahashi JS, Andersen B, Circadian control of interferon-sensitive gene expression in murine skin, Proc National Acad Sci. 117 (2020) 5761–5771. 10.1073/pnas.1915773117. [DOI] [PMC free article] [PubMed] [Google Scholar]