Abstract
Bifidobacterium species are beneficial and dominant members of the breastfed infant gut microbiome; however, their health benefits are partially species-dependent. Here, we characterize the species and subspecies of Bifidobacterium in breastfed infants around the world to consider the potential impact of a historic dietary shift on the disappearance of B. longum subsp. infantis in some populations. Across populations, three distinct patterns of Bifidobacterium colonization emerged: (1) The dominance of Bifidobacterium longum subspecies infantis, (2) prevalent Bifidobacterium of multiple species, and (3) the frequent absence of any Bifidobacterium. These patterns appear related to a country’s history of breastfeeding, with infants in countries with historically high rates of long-duration breastfeeding more likely to be colonized by B. longum subspecies infantis compared with infants in countries with histories of shorter-duration breastfeeding. In addition, the timing of infant colonization with B. longum subsp. infantis is consistent with horizontal transmission of this subspecies, rather than the vertical transmission previously reported for other Bifidobacterium species. These findings highlight the need to consider historical and cultural influences on the prevalence of gut commensals and the need to understand epidemiological transmission patterns of Bifidobacterium and other major commensals.
Keywords: breastfeeding, Bifidobacterium, microbial extinction, infants
1. Introduction
Bifidobacterium colonization has a number of potential health benefits for infants, including a reduction of allergies [1,2], improved vaccine response [3], reduced carriage of antimicrobial resistance genes [4], reduced carriage of virulence factor genes [5], reduced enteric inflammation [6], and immunoregulation via microbial metabolites [7]. However, some of these health benefits are dependent on which species of Bifidobacterium are present in the gut microbiome [3,8].
Despite the importance of Bifidobacterium for infant health, there is evidence that at least one subspecies, Bifidobacterium longum subsp. infantis, is in danger of extinction [9,10]. The extinction of gut commensals is a growing concern, because of the potential for health and immunological disruptions associated with the loss of commensal microbes [11]. A number of factors contribute to the loss of microbial diversity, including disruptions to vertical transmission (for example, via C-section and intrapartum antibiotic use) and disruptions to horizontal transmission (notably, via dietary shifts) [12].
Changes to diet alone can drive extinctions in the gut microbiome over generations [13]. Such extinctions occur by removing microbial-accessible carbohydrates (MAC) from the diet [13]. MACs are carbohydrates that are available for fermentation by the microbiome but are not digested by the host. Loss of MACs from the mouse diet drive irreversible losses of commensal species as the microbes that would otherwise consume those MACs decline, and so are unable to return to populations even in subsequent generations again fed a high-MAC diet [13].
The concept of MAC-driven extinctions is potentially relevant to understanding the modern, breast-fed infant gut microbiome. Diet is a major driver of the infant gut microbiome [14]. The earliest studies of the infant gut microbiome were conducted a century ago and highlighted the difference between breastfed infants and formula-fed infants, with breastfed infants exhibiting near monocultures of Bifidobacterium and formula-fed infants exhibiting a more mixed microbiome without Bifidobacterium [15]. The ability of Bifidobacterium to dominate the breast-fed infant gut is driven, in part, by human milk oligosaccharides (HMOs) [16,17,18], the third most abundant solid component of human milk [19]. Despite the abundance of HMOs in human milk, these specialized carbohydrates are not digested by the infant [20]. Instead, HMOs act as MACs for beneficial bacteria, including Bifidobacterium, and as decoy receptors for pathogens [21]. Some Bifidobacterium species are efficient consumers of HMOs, to the point that HMOs are identified as the “bifidogenic factor” of human milk [16,22]. As a result, a breastfed infant exposed to an HMO-consuming species of Bifidobacterium from the mother or from other environmental sources will likely have very high levels of that species of Bifidobacterium.
Human milk substitutes have typically lacked HMOs, although a few formulas first introduced in 2018 do contain a single HMO. Despite this, HMOs are not typically included in infant formula, and when they are included, the diversity of HMO structures is far below that found in human milk. As a result, infants fed artificial formulas consume a diet low in HMOs, and therefore a diet that is low MAC.
Not all Bifidobacterium are equally efficient at consuming HMOs [23]. Bifidobacterium is a genus composed of 54 different species that had been identified as of January 2017 [24]. Some Bifidobacterium, such as B. longum subsp. infantis, are efficient consumers of HMOs but lack the ability to consume plant oligosaccharides, while others, such as B. longum subsp. longum, are better adapted for the consumption of plant oligosaccharides but some strains have the ability to also consume a subset of HMOs [23]. This creates the possibility that while some species of Bifidobacterium are likely to be members of the adult microbiome and to undergo vertical transmission from the mother [25], others such as B. longum subsp. infantis may rely on horizontal transmission between breastfed infants for ongoing persistence in populations. These species may be uniquely at risk of extinction should a period of disruption to breastfeeding occur in a population, as enough breastfed infants must come into contact with other breastfed infants already colonized by an HMO-dependent Bifidobacterium to maintain sustained transmission of the species. In fact, there is already some evidence that this extinction has occurred in Western populations as B. longum subsp. infantis has become increasingly difficult to detect in these populations, even in breastfed infants never exposed to antibiotics [10]. Therefore, historical breastfeeding patterns must be considered in addition to current breastfeeding in order to understand the relationship between breastfeeding and the infant gut microbiome in the present day. Infants from populations that experienced periods of lower breastfeeding initiation and shorter durations may be living in regions where HMO-consuming Bifidobacterium are near extinction due to the loss in prior generations. Should B. longum subsp. infantis be at risk of extinction due to low breastfeeding rates, we would expect to find all of the following to be true: (1) It would be found at lower rates in populations with historically lower breastfeeding rates, even if breastfeeding rates have rebounded in the present day; (2) in populations with lower breastfeeding rates, we would expect delayed colonization by B. longum subsp. infantis compared to other species of Bifidobacterium because it will take longer for infants to come into contact with the bacterium; and (3) we would expect that B. longum subsp. infantis would be lost from the gut microbiome as breastfeeding ends due to the loss of HMO access. Conversely, if the presence of B. longum subsp. infantis is not tightly tied to HMO consumption, it will remain in the adult population and will not be at risk of extinction due to infant diet changes. In brief, we hypothesize that commensals such as B. longum subsp. infantis dependent on HMO consumption may be lost from populations with a history of lower breastfeeding initiation and duration while Bifidobacterium species capable of consuming both HMOs and plant oligosaccharides may have a better chance at surviving in populations that have experienced low breastfeeding rates. Here we work to describe the species of Bifidobacterium found in global infant populations and consider how the population history of breastfeeding practices and longitudinal observations on infant Bifidobacterium colonization relate to the currently observed patterns.
2. Methods
2.1. Inclusion Criteria for Cohorts
For this analysis, published and unpublished cohorts of term infants where at least some infants were breastfed were selected for inclusion. Cohorts needed to have a minimum of 20 infants and have species-level data on the relative abundance of the Bifidobacterium as measured by 16S rRNA gene sequencing and Bifidobacterium-specific terminal restriction fragment length polymorphism (Bif-TRFLP) and Bifidobacterium Longum-Infantis Ratio (BLIR) analyses in infant stools between the ages of 1 or 2 months or have 16S rRNA gene sequencing data available with DNA available for species-level analysis of Bifidobacterium. Publicly available datasets were included from Gambia [26] and Bangladesh [3]. The PASTURE cohorts from Austria, Finland, Germany, and Switzerland are previously published [27,28], although this is the first publication to include species-level Bifidobacterium data from the PASTURE cohorts. Additionally, data from a subset of infants enrolled in the University of California Davis Lactation Study (Davis, CA, USA) [29,30] and a subset of infants enrolled in the Pediatric Respiratory and Enteric Virus Acquisition and Immunogenesis Longitudinal (PREVAIL) study (Cincinnati, OH, USA) [31] were included. Table 1 summarizes the cohorts and lists the total number of infants in each cohort, the number of infants who had a sample from the ages of 1 to 2 months for inclusion in this study, and the number of infants who were at least partially breastfed at the time of sample collection. Historical breastfeeding patterns were defined by searching the literature for references on breastfeeding rates published between 1900 and the present day. A high breastfeeding pattern was defined as one where breastfeeding initiation was nearly universal and breastfeeding duration was typically longer than 1 year. The medium breastfeeding pattern was defined as high current and past rates of breastfeeding initiation, but with evidence of historically short (median less than 6 months) duration. The low breastfeeding pattern was defined as occurring in the case of a documented period where at least half of infants were never breastfed (meaning breastfeeding was not initiated), regardless of the current population’s breastfeeding initiation and duration. The medium and low historical breastfeeding patterns are distinct because most infants in the medium breastfeeding pattern were consistently breastfed for at least brief periods of time, while there was a measurable and recorded period when the majority of infants in low historical breastfeeding pattern regions never received even a single feeding of breastmilk. While breastfeeding rates for each cohort are calculated using all infants with available breastfeeding data in each cohort (Table 1), only infants who were at least partially breastfed at the time of the sample taken at month 1 or 2 are included in all subsequent analyses in this paper. The UC Davis Lactation Study was approved by the UC Davis Institutional Review Board (Protocol ID 216198, first approved 22 February 2011), and all mothers provided written informed consent for their and their infant’s participation in this study. The PREVAIL cohort was approved by the Centers for Disease Control and Prevention (CDC) Institutional Review Board (Number 6952) and the Cincinnati Children’s Hospital Medical Center Institutional Review Board (Protocol Number 2016_9093, 21 February 2017), and all mothers provided written informed consent for their and their infant’s participation in this study. For the PASTURE cohorts, the study was approved by local research ethics committees, and written informed consent was obtained from the infant’s parents. The Bangladeshi cohort was approved by the Research Review Committee (RRC) and Ethical Review Committee (ERC) of the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b, Protocol #PR—13068, approved 2 December 2013). UC Davis IRB also approved the protocol (549272-6, 21 February 2014). Mothers provided written informed consent. For the Gambian cohort, all data used for this project were publicly available [26].
Table 1.
Source of included cohorts, and number of infants included in the present study from each cohort.
| Country of Origin | Published | Study | Enrollment Based on Intent to Breastfeed? | Total Number Infants in Cohort | Number of Infants with Stool Samples | Number of Breastfed Infants at Time of Sample Collection |
|---|---|---|---|---|---|---|
| Austria | Partially | PASTURE | No | 207 | 181 | 122 |
| Bangladesh | Yes | Efficacy of Newborn Vitamin A Supplementation in Improving Immune Function (clinicaltrials.gov NTC01583972) | No | 306 | 274 | 274 |
| Finland | Partially | PASTURE | No | 171 | 153 | 135 |
| Gambia | Yes | Sub-study in The Early Nutrition and Immune Development (ENID) Trial, ISRCTN49285450 | Yes | 33 | 24 | 24 |
| Germany | Partially | PASTURE | No | 237 | 198 | 149 |
| Switzerland | Partially | PASTURE | No | 231 | 227 | 189 |
| Davis, CA, USA | Partially | UC Davis Lactation Cohort | Yes | 95 | 60 | 60 |
| Cincinnati, OH, USA | No | PREVAIL | No | 245 | 45 | 26 |
For each cohort, data on the median duration and initiation rates of breastfeeding are reported if available for the cohort. In addition, historical breastfeeding practices of each country of origin of a cohort are described based on reviews of the published literature. In addition to the month 1–2 samples described above, six cohorts had longitudinal samples available. The Bangladesh cohort had additional samples from a subset of infants aged 2 years with 16S rRNA gene sequencing and Bif-TRFLP/BLIR. The four PASTURE cohorts had additional samples from the age of 1 year on a subset of infants. The Davis cohort had additional samples from a subset of 3-day-old and 1-month-old infants with 16S rRNA gene sequencing and Bif-TRFLP/BLIR analyses. These cohorts will permit a limited examination of Bifidobacterium species colonization over time.
2.2. 16S rRNA Gene Sequencing and Bif-TRFLP/BLIR
All infant stool samples were extracted and sequenced as described in Davis et al. [23] Analysis of the 16S rRNA gene sequencing results of all raw data files were completed using QIIME2 [32] (version qiime2-2017.8) and DADA2 [33]. The identification of Bifidobacterium species and B. longum subspecies were completed using Bifidobacterium-specific terminal restriction fragment length polymorphism (Bif-TRFLP) and the Bifidobacterium Longum-Infantis Ratio (BLIR) as described in Davis et al. [26,34] Bif-TRFLP and BLIR are well validated by culture for the identification of Bifidobacterium species and subspecies [26,34], and enable efficient and cost-effective identification of Bifidobacterium species in a large number of samples.
The relative abundance of total Bifidobacterium was compared by cohort and historical breastfeeding pattern using a Kruskal–Wallis test, followed by Dunn’s test with Bonferroni correction if the results were significant. The prevalence of Bifidobacterium species present in at least two cohorts was compared based on breastfeeding history in each cohort using generalized estimating equations (GEE) as implemented in the gee package in R version 3.6.3 [35] with a binomial family and logit linker, the cohort of origin was used as the clustering variable, and using an exchangeable correlation structure. A Bifidobacterium species was considered present in an infant if there was any detectable level of that species in the infant’s microbiome. Infants who receive a smaller portion of breastmilk in their diet are likely to have lower levels of Bifidobacterium, but any breastmilk in the diet will ensure that infants are consuming at least some amount of HMOs. By focusing on any breastfeeding and the presence or absence of Bifidobacterium species in infants fed at least some breastmilk, this analysis is focused on the prevalence of Bifidobacterium in populations where an infant exposed to a Bifidobacterium has a reasonable chance of having any detectable level of that Bifidobacterium. A Bifidobacterium species was considered present in a cohort if that species was present in at least one infant, as any detection of that species indicates it has not been eradicated. The species included for this analysis were B. adolescentis, B. animalis, B. bifidum, B. breve, B. longum subsp. infantis, B. longum subsp. longum, B. longum of unknown subsp., and B. pseudocatenulatum. This meant eight separate models were constructed, leading to a Bonferroni corrected p-value of less than 0.0062 to be considered significant after adjusting for multiple comparisons.
To understand the extent to which individual species of Bifidobacterium shape the microbiome, we sought to identify species that could dominate (>50% relative abundance) the infant gut microbiome. To do this, for each species of Bifidobacterium in each cohort, we sought to identify infants whose microbiome had >50% relative abundance of a single species or subspecies of Bifidobacterium.
Finally, in the cohorts with samples from infants collected at older ages, Chi-square tests were used to compare the chances of detecting B. longum subsp. infantis in infants who were still breastfed compared to those who were weaned.
3. Results
3.1. Study Cohorts and Breastfeeding Patterns
This study included eight cohorts with a total of 979 breastfed infants with stool samples collected at age 1 or 2 months. Two cohorts were conducted in two low-income countries—Bangladesh and the Gambia—and six cohorts were conducted in five high-income countries—Austria, Finland, Germany, Switzerland, and the United States (US). The countries included have three historically different breastfeeding patterns, described here as “high”, “medium”, and “low” breastfeeding.
The first breastfeeding pattern, classified as “high”, is characterized by consistent, high rates of breastfeeding initiation and long durations of breastfeeding without evidence of historical interruptions in either breastfeeding initiation or duration. This is the pattern observed in the Gambia and Bangladesh. In the 1980s, when breastfeeding was low in much of the world, 97.5% of Bangladeshi children were fed at least some breastmilk and the mean duration of breastfeeding was 26 months [36]. Furthermore, this pattern of high rates of breastfeeding and long duration remained consistent in studies of Bangladesh from the 1970s through to the 1990s [37]. More recently, the initiation of breastfeeding in Bangladesh remains high (98.3%) and the duration of breastfeeding has increased to a mean of 31.9 months [38]. The Gambia has a similar historical and modern pattern, where a report from 1979 points out that “all Gambian women breast feed their babies for up to two years” [39]. Despite a shorter-than-optimal duration of exclusive breastfeeding, breastfeeding rates remain high in the Gambia today, with a median duration of 20 months with over 95% of infants ever breastfed [40]. Therefore, in this study, infants in the Bangladesh and Gambian cohorts were therefore considered to have a “high” historical breastfeeding pattern. The median duration of breastfeeding is unknown for the Gambian cohort, but all infants in this group were breastfed for at least 5 months as inclusion criteria included the availability of week 20 milk samples [26]. The median duration of breastfeeding in the Bangladeshi cohort is also unknown as infants in this cohort were only followed until age 2 years, and more than half of the infants enrolled in the study were still breastfed at this time point (Table 2). This means that the high breastfeeding pattern is still observed in the present day in these countries.
Table 2.
Summary of Bifidobacterium prevalence in infants aged 1 to 2 months. Except for information on breastfeeding initiation and duration in a cohort, values are based solely on infants who were breastfed. The breastfeeding initiation (ever breastfed) rate and breastfeeding duration were calculated using data from all infants in a cohort with an available sample and a known breastfeeding status.
| Cohort Location | Ever Breastfed (Median Duration) | Historic Breastfeeding Pattern | Any Bifidobacterium | B. adolescentis | B. animalis | B. bifidum | B. breve | B. longum subsp. infantis | B. longum subsp. longum | B. longum subsp. Unknown | B. pseudocatenulatum |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bangladesh | 100% (>2 years) | High | 100% | 5.8% | 2.6% | 32.5% | 40.1% | 83.6% | 26.3% | 1.1% | 8.4% |
| Gambia | 100% (Unknown) | High | 100% | 0% | 0% | 0% | 25% | 91.7% | 54.2% | 12.5% | 0% |
| Austria | 91.8% (6.8 months) | Medium | 100% | 23.7% | 0.82% | 6.6% | 55.7% | 4.1% | 61.5% | 4.9% | 52.4% |
| Finland | 99.3% (8 months) | Medium | 100% | 15.5% | 0% | 8.8% | 40.0% | 0.74% | 56.3% | 3.7% | 51.1% |
| Germany | 91.1% (7.4 months) | Medium | 100% | 18.1% | 0.67% | 7.4% | 61.1% | 4.0% | 61.7% | 4.0% | 57.0% |
| Switzerland | 97.3% (8 months) | Medium | 100% | 4.8% | 0% | 11.1% | 58.7% | 14.8% | 41.8% | 6.3% | 49.7% |
| Davis, CA, USA | 100% (9.3 months) | Low | 65% | 8.3% | 0% | 13.3% | 36.7% | 8.3% | 36.7% | 1.7% | 15% |
| Cincinnati, OH, USA | 86.5% (3.1 months) | Low | 97% | 11.5% | 0% | 19.2% | 61.5% | 0% | 69.2% | 0% | 19.2% |
The second breastfeeding pattern, classified as “medium”, is characterized by high, consistent breastfeeding initiation but historical evidence of a short duration of breastfeeding (less than 6 months of any breastfeeding in the majority of infants). This pattern is observed in Austria, Finland, Germany, and Switzerland, with historical documentation of a median duration of breastfeeding of less than 6 months during the 1970s and 1980s. In European countries, there was a general decline in breastfeeding following World War II followed by increased rates of breastfeeding starting in the 1970s [41]. In Austria, Germany, and Finland, in the 1970s and 1980s, most women initiated breastfeeding, but the duration was short [42]. In Austria, only 5% of infants were breastfed at 3 months post-partum in 1980, which increased to 41% by 1984 [43], indicating that the majority of infants did not receive a full six months of breastfeeding during the early 1980s. In Finland in the early 1980s, most women initiated breastfeeding, but only one-third of infants were breastfed until 3 months [44], again indicating a historical period where the majority of infants were not at least partially breastfed for six months. In Germany, only 2% of infants were still breastfed at the age of 6 months in 1980 [45]. In Switzerland, in 1978, 92% of mothers initiated breastfeeding, but only 30% were still breastfeeding by 4 months [46]. These past patterns are no longer present in these countries. In Austria today, breastfeeding initiation rates are currently approximately 98% with a median duration of 27 weeks [47]. In Finland today, almost all mothers initiate breastfeeding, 60% are still breastfeeding at 6 months, and one-third are still breastfed at 11 months of age [48]. In Germany, 90% of women initiate breastfeeding and more than half of all infants are still breastfed at 6 months [49]. In Switzerland, breastfeeding initiation is 95% and the median duration of breastfeeding is 31 weeks [50]. These numbers are consistent with those observed for the study cohorts (Table 2). Therefore, even though present-day practices in these countries would qualify for the “high” category, historical evidence supports a period with low HMO consumption by infants creating the opportunity for shifts in infant Bifidobacterium populations.
The third pattern of breastfeeding, classified as “low”, is characterized by low historic rates of breastfeeding initiation. The two cohorts from the United States meet this pattern, as the United States experienced a low point in breastfeeding in 1971 when less than 1 in 4 women breastfed their infants even once [51]. Following sustained public health efforts, rates partially recovered to approximately 50–60% initiating breastfeeding in the 1980s [52]. Breastfeeding initiation remained at only 60% in 1995, with only 22% of mothers still breastfeeding at 6 months of age in 1995 [53]. As recently as 2004, the United States had lower breastfeeding initiation and duration rates than many continental European countries [54]. Since that time, breastfeeding in the US has increased so that in 2017, 84% of infants were ever breastfed and 58% were still breastfed at 6 months [55]. In the state of Ohio (where Cincinnati is located), in 2017 (the year that the Cincinnati PREVAIL cohort began enrollment), 80% of infants were ever breastfed and 51% were still breastfed at 6 months of age [55]. For the state of California (where Davis is located), in 2009 (the year the UC Davis Lactation Cohort began enrollment), 85% of infants were ever breastfed and 53% were still breastfed at 6 months of age [56]. Because the Davis Lactation Cohort enrolled based on the intention to breastfeed, this cohort has a longer median duration of breastfeeding than was observed in the general population (Table 2). The Cincinnati cohort had a lower duration of breastfeeding (Table 2) than that reported for Ohio, in part because this cohort is an urban, diverse cohort, which tends to mean a shorter duration of breastfeeding.
3.2. Bifidobacterium Species across Cohorts and Breastfeeding Patterns
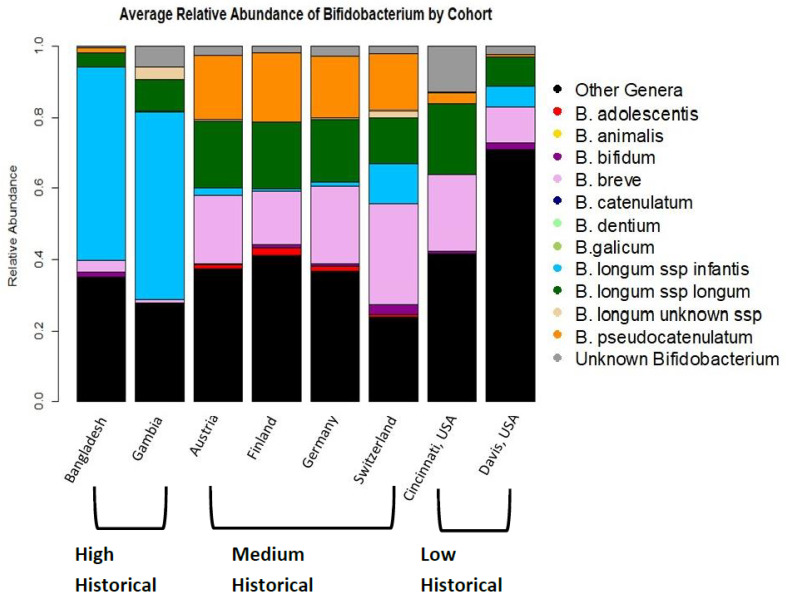
Average Bifidobacterium levels differed between cohorts (Figure 1). The prevalence of detection of any Bifidobacterium was 100% of infants in all cohorts except those in the United States. The total relative abundance of Bifidobacterium in individual infants also differed significantly by cohort (Kruskal–Wallis test, p < 0.0001) and by breastfeeding pattern (Kruskal–Wallis test, p < 0.0001). By cohort, Davis had a significantly lower total relative abundance of Bifidobacterium than all other cohorts (Dunn’s test, p < 0.0001 compared to all cohorts except Cincinnati where p = 0.03). Switzerland had a significantly higher relative abundance of Bifidobacterium than all other cohorts except for Gambia (Dunn’s test, p < 0.0001 for all cohorts except Gambia, no significant difference in total Bifidobacterium between Switzerland and Gambia, p = 1.0). There were no other significant differences in total Bifidobacterium relative abundance by cohort. By historical breastfeeding status, the low-breastfeeding-pattern infants had a lower relative abundance of Bifidobacterium than the high-breastfeeding-pattern infants (Dunn’s test, p < 0.0001) and the medium-breastfeeding-pattern infants (Dunn’s test, p < 0.0001). The medium-breastfeeding-pattern infants had higher total Bifidobacterium than the high-breastfeeding infants (Dunn’s test, p = 0.02).
Figure 1.
Average gut microbiome colonization patterns by country.
Importantly, the cohorts differed in the species of Bifidobacterium that colonized infants’ guts. The average infant gut microbiome in Bangladesh and Gambia is dominated (meaning greater than 50% relative abundance Bifidobacterium of a single subspecies) by B. longum subsp. infantis, with an average relative abundance of 54% B. longum subsp. infantis in Bangladesh and 53% in the Gambia. In the European and USA cohorts, the average infant gut microbiome was not dominated by any single species of Bifidobacterium (Figure 1).
The presence or absence of particular Bifidobacterium species in infants reflected historical breastfeeding practices among the cohorts (see Table 3 for the summary of findings from GEE models). Notably, B. longum subsp. infantis and B. bifidum were significantly less likely to be present in infants from medium- and low-breastfeeding-pattern cohorts compared to infants in high-breastfeeding-pattern cohorts. B. pseudocatenulatum was more likely to be present in infants from medium- or low-breastfeeding-pattern cohorts than in infants from high-breastfeeding-pattern cohorts. B. adolescentis and B. breve were detected more often in medium-breastfeeding-pattern cohorts than in high-breastfeeding-pattern cohorts, but there was no significant difference in the presence of these species between low-breastfeeding-pattern cohorts and high-breastfeeding-pattern cohorts. The prevalence of these species in each cohort is shown in Table 2. Because B. breve, B. pseudocatenulatum, and B. bifidum may be difficult to distinguish when using Bif-TRFLP, the values in Table 2 may underestimate the true prevalence of these taxa in all cohorts. All figures include the relative abundance of these mixed peaks as “Unknown Bifidobacterium”, but indistinguishable peaks do not count towards the estimates of relative abundance for these species or towards the presence or absence of these species in any infant.
Table 3.
Summary of GEE models comparing presence or absence of Bifidobacterium species in individual infants by cohort history of breastfeeding. Reference group was the high-breastfeeding group. To account for multiple comparisons, a p-value less than 0.0062 is considered significant. The model for B. animalis failed to run, because after excluding infants who were not breastfed at time of sample collection, there were no infants from a low historical breastfeeding pattern cohort colonized by B. animalis. Taxa that differed significantly in prevalence from the high historical breastfeeding pattern cohorts are in bold.
| Cohort Breast-Feeding Pattern |
B. adolescentis Odds Ratio (95% CI, p-Value) |
B. animalis Odds Ratio (95% CI, p-Value) |
B. bifidum Odds Ratio (95% CI, p-Value) |
B. breve Odds Ratio (95% CI, p-Value) |
B. longum Subspecies infantis
Odds Ratio (95% CI, p-Value) |
B. longum Subspecies longum Odds Ratio (95% CI, p-Value) |
B. longum Unknown Subspecies Odds Ratio (95% CI, p-Value) |
B. pseudocatenulatum Odds Ratio (95% CI, p-Value) |
|---|---|---|---|---|---|---|---|---|
| Medium | 4.1 (1.6–11, p = 0.0041) | NA | 0.22 (0.16–0.31, p < 0.0001) | 2.0 (1.3–3.1, p = 0.0019) | 0.010 (0.0037–0.029, p < 0.0001) | 2.2 (0.98–5.0, p = 0.055) | 2.8 (0.92–8.2, p = 0.069) | 12 (10–13, p < 0.0001) |
| Low | 2.4 (1.0–5.6, p = 0.041) | NA | 0.40 (0.28–0.58, p < 0.0001) | 1.4 (0.70–2.9, p = 0.32) | 0.0084 (0.0024–0.029, p < 0.0001) | 1.8 (0.57–5.7, p = 0.31) | 0.63 (0.16–2.4, p = 0.51) | 2.1 (1.8–2.5, p < 0.0001) |
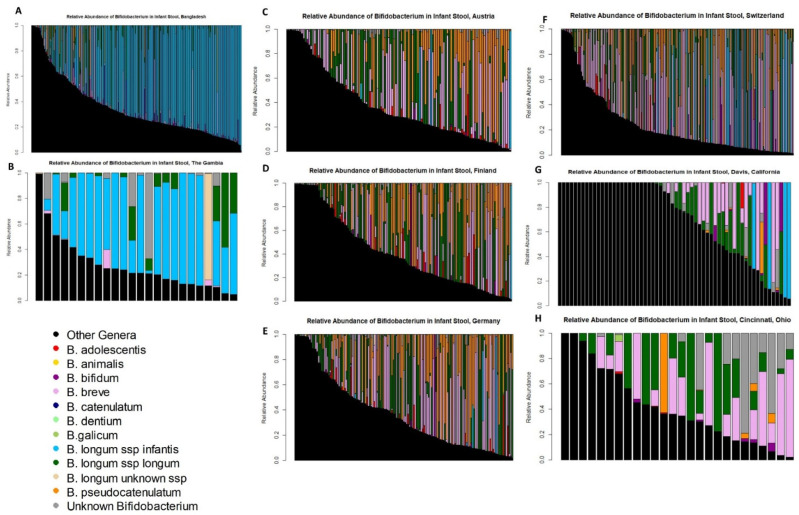
Detailed visualizations of Bifidobacterium colonization of individual infants in each cohort demonstrate the substantial concordance between historical breastfeeding patterns and infant gut colonization (Figure 2). The similarities of infant Bifidobacterium colonization patterns by historical breastfeeding are striking. The majority of infants in the high historical breastfeeding cohorts have gut microbiomes dominated by B. longum subsp. infantis with a lower relative abundance of other Bifidobacterium species (Figure 2A,B). In the medium historical breastfeeding cohorts, infants still have very high levels of total Bifidobacterium, but no single species dominates (Figure 2C–F). In the low historical breastfeeding cohorts, some breastfed infants completely lack Bifidobacterium, but those who are colonized by Bifidobacterium generally appear similar to the pattern observed in the medium historical breastfeeding cohorts. As medium-breastfeeding-pattern infants had higher total Bifidobacterium levels than high-breastfeeding-pattern infants and because there were significant differences in the presence and absence of specific Bifidobacterium species, we examined which Bifidobacterium may dominate the infant gut microbiome. In the high-historical-breastfeeding-pattern cohorts, approximately two-thirds of infants had a gut microbiome dominated (meaning greater than 50% relative abundance from a single source) by B. longum subsp. infantis. In addition, B. breve, B. longum subsp. longum, and B. pseudocatenulatum or a mixture of multiple Bifidobacterium species would occasionally result in an infant gut microbiome dominated by Bifidobacterium. In the medium-historical-breastfeeding-pattern cohorts, a microbiome dominated by B. longum subsp. infantis was rare, but roughly two-thirds of infants had a gut microbiome dominated by B. breve, B. longum subsp. longum, B. pseudocatenulatum, or a mixture of multiple Bifidobacterium species. In the low-historical-breastfeeding-pattern cohorts, some breastfed infants completely lacked any detectable level of Bifidobacterium. When infants did have gut microbiomes dominated by Bifidobacterium, it was typically the same mix of species as observed in the medium-historical-breastfeeding-pattern cohorts. On occasion, B. bifidum could also dominate an infant gut microbiome, but this was a rare occurrence with only one infant in the German cohort, one infant in the Finnish cohort, and one infant in the Davis, USA cohort.
Figure 2.
Relative abundance of different Bifidobacterium species in infant stools. Each bar represents the microbiome from an individual infant; cohorts with smaller numbers of infants may fail to identify the presence of rarer Bifidobacterium species in those cohorts by chance alone. B. longum subsp. infantis is in blue, non-Bifidobacterium taxa are black. (A) Bangladesh, (B) the Gambia, (C) Austria, (D) Finland, (E) Germany, (F) Switzerland, (G) Davis, CA, USA, (H) Cincinnati, OH, USA.
3.3. Timing of Bifidobacterium Species Colonization
Six cohorts, namely the Bangladesh cohort, the four PASTURE cohorts (from Austria, Finland, Germany, and Switzerland), and the UC Davis lactation cohort, had additional longitudinal data on infant Bifidobacterium colonization patterns, although the timing of the available data differed. We analyzed these additional time points to address questions of timing regarding the acquisition of Bifidobacterium species (in the UC Davis cohort) and the loss or consistency of colonization with Bifidobacterium species comparing early infancy to 1 or 2 years of age (Bangladesh cohort and PASTURE cohorts).
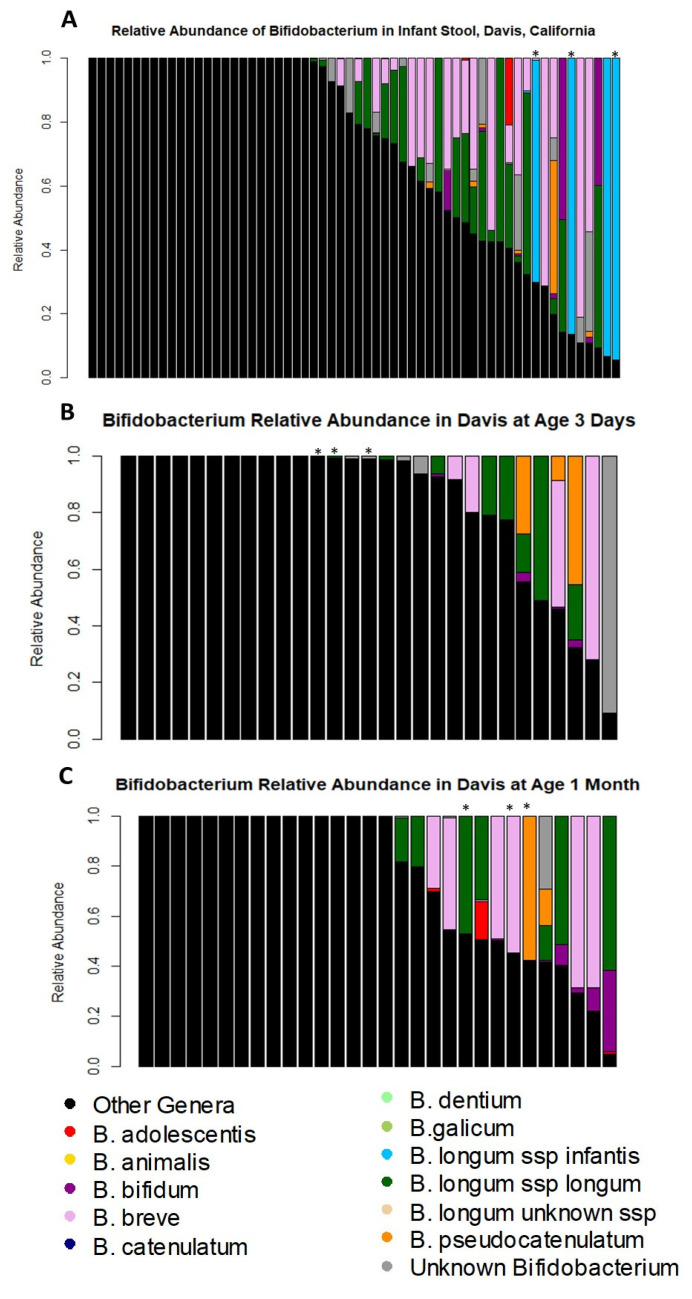
There were differences over time in the colonization of Davis infants with B. longum subsp. infantis (Figure 3). Figure 3A shows the 2-month Bifidobacterium colonization of the Davis infants, where the stars indicate the infants colonized by B. longum subsp. infantis who also had samples at an earlier time point. Regarding the timing of acquisition of Bifidobacterium species, the UC Davis lactation cohort had additional samples with known Bifidobacterium colonization patterns from day 3 of life (29 infants; Figure 3B) and month 1 of life (30 infants; Figure 3C) in addition to the month 2 samples described above. The median relative abundance of total Bifidobacterium was only 1% at day 3 (range 0–91%), with 38% of infants having no detectable level of Bifidobacterium present. The species of Bifidobacterium detected at day 3 of life were B. longum subsp. longum (detected in 28% of infants), B. bifidum (in 14% of infants), B. breve (in 14% of infants), B. pseudocatenulatum (in 14% of infants), and B. adolescentis (in 3% of infants) (Figure 3B). At the age of one month, 30 of the infants (including all 29 with day 3 of life samples) had additional Bif-TRFLP/BLIR results (Figure 3C). Forty-three percent of Davis infants had no detectable Bifidobacterium at month 1 of life, and a median relative abundance of total Bifidobacterium of 0.2% (range 0–95%). The species that were present were B. longum subsp. longum (detected in 23% of infants), B. bifidum (in 23% of infants), B. breve (in 23% of infants), B. adolescentis (in 10% of infants), and B. pseudocatenulatum (in 7% of infants). Notably, B. longum subsp. infantis was not found in any Davis infants prior to 2 months of age.
Figure 3.
Bifidobacterium colonization in Davis, CA infants over time. Each bar represents the microbiome of a single infant. Stars indicate samples from infants who were colonized by B. longum subsp. infantis at age 2 months with longitudinal samples. (A) 2 months of age samples (repeated from Figure 2); (B) 3 days of age; (C) 1 month of age.
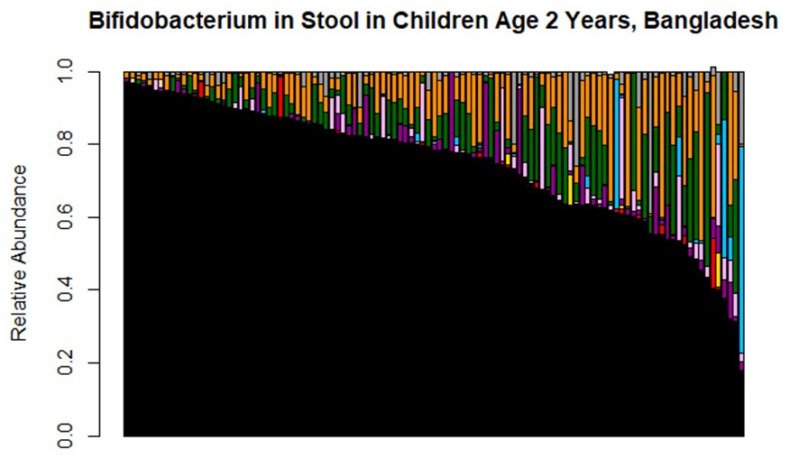
One hundred and nine of the 274 Bangladeshi infants had data on Bifidobacterium colonization at 2 years of age. At age 2, all of these infants were still colonized with at least some Bifidobacterium, but the median total relative abundance of Bifidobacterium was lower than in early life at 21% relative abundance of Bifidobacterium (range 3–82%; Figure 4). Furthermore, the prevalence of B. longum subsp. infantis had dropped considerably and was found in just 17% of infants overall, including 20% of still-breastfed infants and 11% of infants who had not received breastmilk in the past week (Table 4). In contrast to the dominance of B. longum subsp. infantis in early infancy, the species of Bifidobacterium found in the Bangladeshi infants aged 2 years, in order of decreasing prevalence, were B. pseudocatenulatum (detected in 95% of infants), B. longum subsp. longum (detected in 95% of infants), B. bifidum (detected in 78% of infants), B. breve (detected in 65% of infants), B. adolescentis (detected in 32% of infants), B. longum subsp. infantis (detected in 17% of infants), B. animalis (detected in 9% of infants), B. magnum (detected in 2% of infants), and an indistinguishable mix of B. choerinum and/or B. pseudolongum (1% of infants, and this indistinguishable mix was also plotted as part “Unknown Bifidobacterium”).
Figure 4.
Bifidobacterium colonization in Bangladeshi cohort in infants aged 2 years. Each bar represents the microbiome of an individual infant. Slight deviations from 100% are related to rounding errors in the Bif-TRFLP calculation of percentages. Note the reduced prevalence of B. longum subspecies infantis in blue; this is a subset of the same infants shown in Figure 2B.
Table 4.
Table of number of European infants colonized at any level by B. longum subsp. infantis at 1 year of age by breastfeeding status. Prevalence of B. longum subsp. infantis appears to decrease rapidly during the weaning period, supporting the assumption that vertical transmission will be rare. p-values are for the Chi-square tests comparing B. longum subsp. infantis detection in still breastfed vs. no longer breastfed infants.
| Country | Breast Fed at 1 Year (European Countries) or 2 Years (Bangladesh) | B. longum Subspecies infantis Detected | B. longum Subspecies infantis Not Detected | Percentage of Infants Colonized |
|---|---|---|---|---|
| Austria (p = 0.003) |
Yes | 5 | 14 | 36% |
| No | 2 | 70 | 2.8% | |
| Finland (p = 0.65) |
Yes | 1 | 37 | 2.6% |
| No | 0 | 91 | 0% | |
| Germany (p = 1) |
Yes | 1 | 18 | 5.3% |
| No | 2 | 89 | 2.2% | |
| Switzerland (p < 0.0001) |
Yes | 10 | 24 | 29% |
| No | 6 | 135 | 4.3% | |
| Bangladesh (p = 0.35) |
Yes | 13 | 51 | 20.3% |
| No | 5 | 40 | 11.1% |
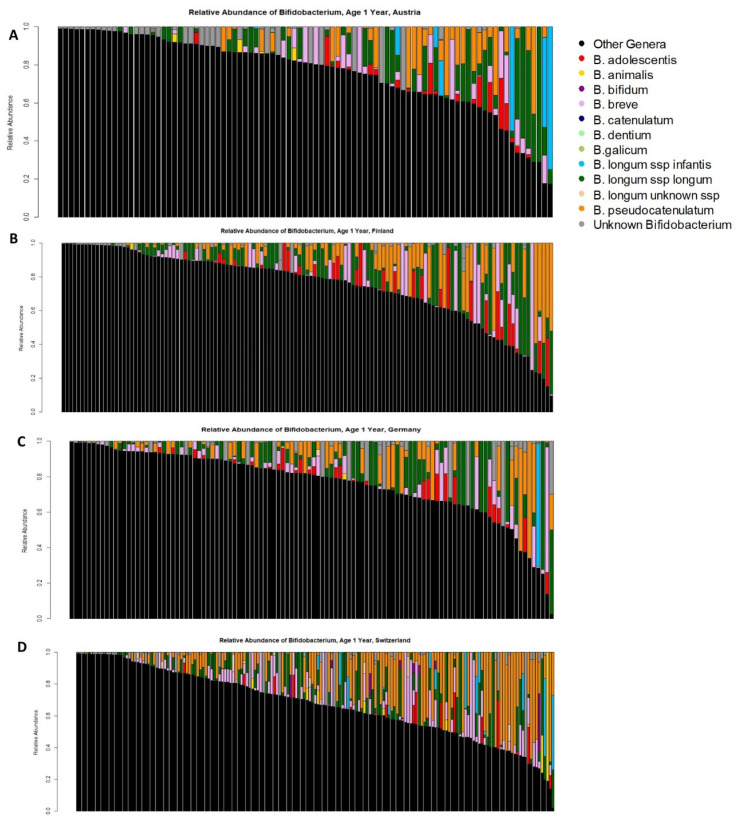
In the Austrian cohort, 91 of the infants breastfed at 2 months also had a 1-year sample. In the Finnish cohort, 129 of the infants breastfed at 2 months also had a 1-year sample. In the German cohort, 110 of the infants breastfed at 2 months also had a 1-year sample. In the Swiss cohort, 175 of the infants breastfed at 2 months also had a 1-year sample. All infants were still colonized with at least some Bifidobacterium (Figure 5), but usually at a lower relative abundance of total Bifidobacterium than was present at 2 months of age. Comparing colonization with B. longum subsp. infantis between infants who were or were not still breastfed at 1 year, infants who were breastfed always had a higher prevalence of B. longum subsp. infantis than those who were not, but this difference only reached statistical significance in the Austrian (Chi-square test, p = 0.003) and Swiss (Chi-square-test, p < 0.001) cohorts (Table 4). The greater prevalence of B. longum subsp. infantis in infants breastfed at 1 year compared to infants not breastfed at 1 year in the Austrian and Swiss cohorts is consistent with horizontal transmission of B. longum subsp. infantis, as this species does not remain at high rates in infants who are no longer breastfed. Furthermore, across all the European cohorts, there were 28 infants with detectable levels of B. longum subsp. infantis at 2 months but not at 1 year, 18 infants with detectable levels of B. longum subsp. infantis at 1 year but not at 2 months, and 9 infants with detectable levels at both time points. Of the 18 infants who acquired B. longum subsp. infantis between 2 months and 1 year, 13 (72%) were breastfed at 1 year of age while only 5 (28%) were not breastfed at 1 year of age. In contrast, of the 28 infants who lost B. longum subsp. infantis between 2 months and 1 year, 26 (93%) were not breastfed at 1 year and only 2 (7%) were breastfed at 1 year. This is consistent with the idea that infants who are breastfed are more likely to acquire B. longum subsp. infantis and that this subspecies is likely to be lost after the end of breastfeeding. The mix of Bifidobacterium species found in these infants at 1 year of age was generally similar to those observed at 2 months (Table 5).
Figure 5.
Bifidobacterium colonization in infants aged 1 year in (A) Austria, (B) Finland, (C) Germany, and (D) Switzerland.
Table 5.
Summary of Bifidobacterium prevalence in infants aged 1 year.
| Cohort Location | Any Bifidobacterium | B. adolescentis | B. animalis | B. bifidum | B. breve | B. longum subsp. infantis | B. longum subsp. longum | B. longum Unknown Subspecies | B. pseudocatenulatum |
|---|---|---|---|---|---|---|---|---|---|
| Austria | 100% | 23% | 5.5% | 1.1% | 35% | 7.7% | 53% | 0% | 36% |
| Finland | 100% | 25% | 2.3% | 0% | 38% | 0.8% | 70% | 0% | 47% |
| Germany | 100% | 27% | 3.6% | 0% | 44% | 2.7% | 67% | 0.9% | 52% |
| Switzerland | 100% | 9.1% | 6.8% | 4.6% | 51% | 9.1% | 62% | 7.4% | 61% |
4. Discussion
In our study of cohorts from across the world, we found that infants from countries with a high historical breastfeeding pattern are more likely to be colonized with B. longum subsp. infantis and B. bifidum than infants from other cohorts. The pattern of Bifidobacterium colonization by cohort is consistent with the apparent loss of B. longum subsp. infantis in locations of the world with historically lower breastfeeding rates and shorter breastfeeding durations. We also found that the timing of colonization by B. longum subsp. infantis is distinct from the timing of colonization of other Bifidobacterium species. Most species of Bifidobacterium appeared early in the Davis cohort, suggesting a pattern of vertical transmission consistent with prior work [57]. B. longum subsp. infantis, however, did not appear as a part of any infant’s gut microbiome until 2 months of age, suggesting that this subspecies may not transmit vertically from the mother. The longitudinal data from the Bangladeshi cohort further supports our hypothesis as described in the introduction, as B. longum subsp. infantis is found in 84% of infants at 6 weeks of age, but only 17% of infants aged 2 years. This suggests that even in populations where B. longum subsp. infantis is commonly found in infants, this subspecies is unlikely to persist at older ages. This is consistent with horizontal transmission of B. longum subsp. infantis between infants. The results from the European cohorts also support this trend, as a number of infants who were still breastfed at 1 year of age acquired B. longum subsp. infantis despite not having detectable levels at 2 months of age, while Bifidobacterium species likely to be vertically transmitted from the mother remained at similar prevalence between 2 months and 1 year of age.
When B. longum subsp. infantis is absent, several other Bifidobacterium can at least occasionally dominate the gut microbiome of individual infants. Three species are likely to contribute substantially to the dominance of total Bifidobacterium in the absence of B. longum subsp. infantis: B. breve, B. longum subsp. longum, and B. pseudocatenulatum. One species, B. pseudocatenulatum, is also more likely to colonize infants from medium- and low-breastfeeding countries. This suggests that this species may contribute more to the infant gut microbiome when species such as B. bifidum and B. longum subsp. infantis, who more frequently consume HMOs, are absent. Infants from countries with a medium breastfeeding pattern also had higher rates of colonization with B. adolescentis and B. breve than countries with a high breastfeeding pattern. In medium-breastfeeding cohorts, the open ecological niche created by the loss of B. longum subsp. infantis is filled by Bifidobacterium from multiple species rather than a single subspecies. The differences in species found may depend, in part, on population genetics, as, unlike B. longum subsp. infantis, other Bifidobacterium species are likely to consume only a subset of HMOs [58,59]. The infants from low-breastfeeding-pattern cohorts sometimes lack any detectable Bifidobacterium, but otherwise appear similar to the infants of medium-breastfeeding cohorts, suggesting that other species of Bifidobacterium may also be at risk of extinction in the absence of HMO (and therefore any microbiota-accessible carbohydrates) in the infant diet, or that other factors (for example, antibiotic use) have further challenged Bifidobacterium survival in these locations. Despite its rarity, when B. longum subsp. infantis is detected in infants from the medium- and low-breastfeeding-pattern cohorts, it typically dominates the infant gut microbiome of those infants, similar to the pattern observed in infants from high breastfeeding countries.
The pattern of increased prevalence of B. longum subsp. infantis in cohorts with an uninterrupted history of long-duration breastfeeding is consistent with other studies. Consider a population in the US that never widely adopted formula: Old-Order Mennonites. This group is unusual in the United States because they have never widely adopted formula use, and generally avoid antibiotic use [60]. Consistent with what is discussed in the cohorts included in this study, colonization by B. longum subsp. infantis or with any Bifidobacterium is more prevalent in the Old-Order Mennonites, with B. longum subsp. infantis detected by qPCR in 70% of Old-Order Mennonite infants [1]. Furthermore, a search of the literature found the decline in the prevalence of Bifidobacterium in US breastfed infants occurred during the period of the 1970s with the lowest breastfeeding rates, although no explanation of the absence of Bifidobacterium could be found at the time [61]. Importantly, this change predates the increase in C-section delivery rates to above 10% of all deliveries in the United States [62] and before the widespread use of intrapartum antibiotics to prevent group B Streptococcus-associated sepsis [63], further suggesting diet, rather than other factors influencing the infant microbiome, drove the disappearance of Bifidobacterium. Also supporting the concept that antibiotic use did not drive the disappearance of B. longum subsp. infantis, antibiotic use is prevalent in Bangladesh, where approximately 40% of children under the age of 5 with acute respiratory infection receive antibiotics [64]. Despite the high rate of antibiotic use, our work shows that colonization with B. longum subsp. infantis remains prevalent in this region.
This study does have limitations, including the small number of cohorts included. Importantly, the limited number of cohorts is due, in part, to only selecting cohorts of infants with species-level identification of Bifidobacterium completed using the same technique during a narrow infant age window. This careful limitation to a single method permits direct comparisons across cohorts that would not be possible if a broader range of time points or methods were included. The small number of included cohorts is balanced by the more than 900 breastfed infants included in this study. An additional strength of this study is the inclusion of cohorts from four different continents and a clear gradient in breastfeeding history across cohorts. Using Bif-TRFLP/BLIR also presents a challenge, where in some cases, species identification may be ambiguous. This imprecision in species identification is particularly common with B. bifidum, B. breve, and B. pseudocatenulatum as these three species produce peaks of very similar size. Most of the unknown Bifidobacterium reported in the plots is related to ambiguous peaks generated by some strains of B. bifidum, B. breve, and B. pseudocatenulatum. This suggests that abundance and prevalence estimates for these three species may be higher than what is reported here. B. bifidum occurred more frequently in Bangladesh than in any other cohort but was completely absent in the Gambia. As the number of infants in the Gambian cohort was small, this may represent a lower prevalence of B. bifidum, perhaps similar to what is seen in the European countries rather than a true absence. Another possibility is that B. bifidum is present in this cohort but goes undetected because of the similarity of B. bifidum to B. pseudocatenulatum and B. breve when using Bif-TRFLP. Because of these limitations in the data, it is difficult to draw conclusions about B. bifidum global colonization patterns from this work. The limitation in separating B. bifidum, B. breve, and B. pseudocatenulatum is counter-balanced by the improved ability to distinguish B. longum subsp. infantis from B. longum subsp. longum, even when both subspecies are found in the same infant with one at much lower levels than the other. As B. longum subsp. infantis has been proposed to be endangered in western countries [9], a method that could reliably and efficiently distinguish these two subspecies was critical to this work. Finally, there is a chance that subconscious bias influenced the classification in this work as the same scientists who analyzed the microbiome data completed the literature searches on the breastfeeding status of each country, after having seen the microbiome data. However, the differences between the groups both in breastfeeding history and in Bifidobacterium are substantial, and the key findings of this work, including the reduction in B. longum subsp. infantis and increases in B. pseudocatenulatum, are robust and detected even when the medium and low historical breastfeeding categories are combined.
Bifidobacterium species are important infant commensals, but the full health implications of colonization by different species of Bifidobacterium remains unclear. This is despite evidence that colonization by Bifidobacterium is important to health and evidence that some Bifidobacterium species may be more effective at supporting infant health than others. Some benefits of high levels of Bifidobacterium colonization do not appear to be species-dependent, such as the reduced carriage of antimicrobial resistance genes [4]. Other benefits of high levels of Bifidobacterium are species-dependent. For example, higher levels of B. longum subsp. infantis are associated with improved vaccine response, but the same association is not seen with higher levels of B. longum subsp. longum or B. breve [3]. B. longum subsp. longum is found more often in healthy infants than in those with allergic symptoms, but the same trend was not seen for other Bifidobacterium species [65]. B. longum subsp. infantis was found more frequently in Old-Order Mennonite infants with lower risk of atopic disease than in infants from a nearby Rochester (Rochester, NY, USA) cohort [1]. As such, understanding the differences in Bifidobacterium species colonization in infant populations is important when studying the health implications of the early life microbiome. The fact that the health implications of Bifidobacterium vary by species and that Bifidobacterium species colonization patterns vary by country and population history of breastfeeding means that care needs to be taken in interpreting health findings associated with high levels of Bifidobacterium without also identifying species if trying to apply the findings to other populations. This means it is critical to understand the epidemiological factors that support the transmission of different species of Bifidobacterium, including considering how historic formula-driven extinctions may have changed the colonization patterns observed in the present day.
Acknowledgments
Thank you to all the mother–infant dyads who participated in this research. Funding for this project includes US Department of Agriculture–Agricultural Research Service project 5306-51530-018-00, World Health Organization project 2010168947, Thrasher Research fund grant 11488. Funding for this project also includes National Institutes of Health award F32HD093185 (D.H.T.) and the Peter J. Shields Endowed Chair in Dairy Food Science (D.A.M.).
Author Contributions
Conceptualization, D.H.T., Z.T.L., K.H., A.L.M. and D.A.M.; methodology, formal analysis, and visualization, D.H.T., Z.T.L., D.J.T., A.L.M. and D.A.M.; data curation, D.H.T., Z.T.L., N.N., S.H., C.M., V.D.-C., M.N.H., C.B.S., E.v.M., P.V.K., J.-C.D., R.L., J.R., J.T.S., J.B.G., A.L.M. and D.A.M.; supervision, A.L.M. and D.A.M.; funding, D.H.T., C.B.S., E.v.M., A.L.M. and D.A.M.; writing—original draft preparation, D.H.T., Z.T.L., A.L.M. and D.A.M.; writing—review and editing, all authors except J.-C.D. who passed away before the manuscript draft was complete. All authors have read and agreed to the published version of the manuscript, except J.-C.D. who passed away before the manuscript draft was complete.
Funding
Funding for this project includes US Department of Agriculture–Agricultural Research Service project 5306-51530-018-00, World Health Organization project 2010168947, and Thrasher Research fund grant 11488. Additional funding was received from CDC–Division of Viral Diseases. Funding for this project also includes National Institutes of Health award F32HD093185 (D.H.T.) and the Peter J. Shields Endowed Chair in Dairy Food Science (D.A.M.).
Institutional Review Board Statement
The Gambian cohort included only publicly available deidentified data. The Bangladeshi cohort was approved by the Research Review Committee (RRC) and the Ethical Review Committee (ERC) of the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b, Protocol #PR—13068, approved 2 December 2013). UC Davis IRB also approved the protocol (549272-6, 21 February 2014). The UC Davis Lactation Study was approved by the UC Davis Institutional Review Board (Protocol ID 216198, 22 February 2011). The PREVAIL cohort was approved by the Centers for Disease Control and Prevention (CDC) Institutional Review Board and the Cincinnati Children’s Hospital Medical Center Institutional Review Board (Protocol Number 2016_9093, 21 February 2017).
Informed Consent Statement
The Bangladesh, Gambian, and PASTURE cohorts include already-published data. The UC Davis Lactation Study was approved by the UC Davis Institutional Review Board (Protocol ID 216198, initial approval 22 February 2011) and all mothers provided written informed consent for their and their infant’s participation in this study. The PREVAIL cohort was approved by the Centers for Disease Control and Prevention (CDC) Institutional Review Board and the Cincinnati Children’s Hospital Medical Center Institutional Review Board, and all mothers provided written informed consent for their and their infant’s participation in this study.
Data Availability Statement
Bangladesh cohort sequencing data can be found in SRA under accession IDs PRJNA636907 and PRJNA636905. The Gambia cohort sequencing data can be found in QIITA under study ID 10297 and the European Nucleotide Archive under accession number ERP017462. PASTURE cohort sequences can be found as part of the supplementary data of DOI 10.1038/s41591-020-1095-x without metadata; PASTURE is a cohort with ongoing field cohort. As long as the cohort is ongoing, European data protection prohibits sharing of individual data, even if pseudonymized. The Davis Lactation cohort data can be found in SRA under accession ID PRJNA820048. The PREVAIL (Cincinnati) cohort data can be found in SRA under accession ID PRJNA819967.
Conflicts of Interest
David A. Mills and J. Bruce German are co-founders of Evolve Biosystems and BCD Biosciences. Jennifer T. Smilowitz consults with Evolve Biosystems. Evolve Biosystems and BCD Biosciences had no role in the funding, design, data acquisition and analysis, or decision to publish this work. Ardythe L. Morrow is a co-founder of Glycosyn, LLC, which had no role in the funding, design, data acquisition and analysis, or decision to publish this work.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Seppo A.E., Bu K., Jumabaeva M., Thakar J., Choudhury R.A., Yonemitsu C., Bode L., Martina C.A., Allen M., Tamburini S., et al. Infant gut microbiome is enriched with Bifidobacterium longum ssp. infantis in Old Order Mennonites with traditional farming lifestyle. Allergy. 2021;76:3489–3503. doi: 10.1111/all.14877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cukrowska B., Bierła J.B., Zakrzewska M., Klukowski M., Maciorkowska E. The relationship between the infant gut microbiota and allergy. The role of Bifidobacterium breve and prebiotic oligosaccharides in the activation of anti-allergic mechanisms in early life. Nutrients. 2020;12:946. doi: 10.3390/nu12040946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huda M.N., Ahmad S.M., Alam M.J., Khanam A., Kalanetra K.M., Taft D.H., Raqib R., Underwood M.A., Mills D.A., Stephensen C.B. Bifidobacterium abundance in early infancy and vaccine response at 2 years of age. Pediatrics. 2019;143:e20181489. doi: 10.1542/peds.2018-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taft D.H., Liu J., Maldonado-Gomez M.X., Akre S., Huda M.N., Ahmad S.M., Stephensen C.B., Mills D.A. Bifidobacterial dominance of the gut in early life and acquisition of antimicrobial resistance. mSphere. 2018;3:e00441-18. doi: 10.1128/mSphere.00441-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casaburi G., Frese S.A. Colonization of breastfed infants by Bifidobacterium longum subsp. infantis EVC001 reduces virulence gene abundance. Hum. Microbiome J. 2018;9:7–10. doi: 10.1016/j.humic.2018.05.001. [DOI] [Google Scholar]
- 6.Henrick B.M., Chew S., Casaburi G., Brown H.K., Frese S.A., Zhou Y., Underwood M.A., Smilowitz J.T. Colonization by B. infantis EVC001 modulates enteric inflammation in exclusively breastfed infants. Pediatr. Res. 2019;86:749–757. doi: 10.1038/s41390-019-0533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henrick B.M., Rodriguez L., Lakshmikanth T., Pou C., Henckel E., Arzoomand A., Olin A., Wang J., Mikes J., Tan Z., et al. Bifidobacteria-mediated immune system imprinting early in life. Cell. 2021;184:3884–3898.e11. doi: 10.1016/j.cell.2021.05.030. [DOI] [PubMed] [Google Scholar]
- 8.He F., Ouwehand A.C., Isolauri E., Hashimoto H., Benno Y., Salminen S. Comparison of mucosal adhesion and species identification of bifidobacteria isolated from healthy and allergic infants. FEMS Immunol. Med. Microbiol. 2001;30:43–47. doi: 10.1111/j.1574-695X.2001.tb01548.x. [DOI] [PubMed] [Google Scholar]
- 9.Tannock G.W., Lawley B., Munro K., Gowri Pathmanathan S., Zhou S.J., Makrides M., Gibson R.A., Sullivan T., Prosser C.G., Lowry D., et al. Comparison of the compositions of the stool microbiotas of infants fed goat milk formula, cow milk-based formula, or breast milk. Appl. Environ. Microbiol. 2013;79:3040–3048. doi: 10.1128/AEM.03910-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tannock G.W., Lee P.S., Wong K.H., Lawley B. Why Don’t all infants have bifidobacteria in their stool? Front. Microbiol. 2016;7:834. doi: 10.3389/fmicb.2016.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaser M.J. The past and future biology of the human microbiome in an age of extinctions. Cell. 2018;172:1173–1177. doi: 10.1016/j.cell.2018.02.040. [DOI] [PubMed] [Google Scholar]
- 12.Blaser M.J. The theory of disappearing microbiota and the epidemics of chronic diseases. Nat. Rev. Immunol. 2017;17:461–463. doi: 10.1038/nri.2017.77. [DOI] [PubMed] [Google Scholar]
- 13.Sonnenburg E.D., Smits S.A., Tikhonov M., Higginbottom S.K., Wingreen N.S., Sonnenburg J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529:212–215. doi: 10.1038/nature16504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis E.C., Wang M., Donovan S.M. The role of early life nutrition in the establishment of gastrointestinal microbial composition and function. Gut Microbes. 2017;8:143–171. doi: 10.1080/19490976.2016.1278104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logan W.R. The intestinal flora of infants and young children. J. Pathol. Bacteriol. 1913;18:527–551. doi: 10.1002/path.1700180154. [DOI] [Google Scholar]
- 16.Ward R.E., Niñonuevo M., Mills D.A., Lebrilla C.B., German J.B. In vitro fermentability of human milk oligosaccharides by several strains of bifidobacteria. Mol. Nutr. Food Res. 2007;51:1398–1405. doi: 10.1002/mnfr.200700150. [DOI] [PubMed] [Google Scholar]
- 17.LoCascio R.G., Ninonuevo M.R., Freeman S.L., Sela D.A., Grimm R., Lebrilla C.B., Mills D.A., German J.B. Glycoprofiling of bifidobacterial consumption of human milk oligosaccharides demonstrates strain specific, preferential consumption of small chain glycans secreted in early human lactation. J. Agric. Food Chem. 2007;55:8914–8919. doi: 10.1021/jf0710480. [DOI] [PubMed] [Google Scholar]
- 18.Sela D.A., Chapman J., Adeuya A., Kim J.H., Chen F., Whitehead T.R., Lapidus A., Rokhsar D.S., Lebrilla C.B., German J.B., et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc. Natl. Acad. Sci. USA. 2008;105:18964. doi: 10.1073/pnas.0809584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newburg D., Neubauer S., Jensen R. In: Handbook of Milk Composition. Jensen R.G., editor. Academic Press; San Diego, CA, USA: 1995. pp. 273–349. [Google Scholar]
- 20.Petherick A. Development: Mother’s milk: A rich opportunity. Nature. 2010;468:S5. doi: 10.1038/468S5a. [DOI] [PubMed] [Google Scholar]
- 21.Ballard O., Morrow A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. 2013;60:49–74. doi: 10.1016/j.pcl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bode L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology. 2012;22:1147–1162. doi: 10.1093/glycob/cws074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sela D.A., Mills D.A. Nursing our microbiota: Molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol. 2010;18:298–307. doi: 10.1016/j.tim.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattarelli P., Biavati B. In: The Bifidobacteria and Related Organisms: Biology, Taxonomy, and Applications. Mattarelli P., Biavati B., Holzapfel W.H., Wood B.J.B., editors. Elsevier; Amsterdam, The Netherlands: 2017. [Google Scholar]
- 25.Milani C., Mancabelli L., Lugli G.A., Duranti S., Turroni F., Ferrario C., Mangifesta M., Viappiani A., Ferretti P., Gorfer V., et al. Exploring vertical transmission of bifidobacteria from mother to child. Appl. Environ. Microbiol. 2015;81:7078–7087. doi: 10.1128/AEM.02037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis J.C.C., Lewis Z.T., Krishnan S., Bernstein R.M., Moore S.E., Prentice A.M., Mills D.A., Lebrilla C.B., Zivkovic A.M. Growth and morbidity of gambian infants are influenced by maternal milk oligosaccharides and infant gut microbiota. Sci. Rep. 2017;7:40466. doi: 10.1038/srep40466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Mutius E., Schmid S., the PASTURE Study Group The PASTURE project: EU support for the improvement of knowledge about risk factors and preventive factors for atopy in Europe. Allergy. 2006;61:407–413. doi: 10.1111/j.1398-9995.2006.01009.x. [DOI] [PubMed] [Google Scholar]
- 28.Depner M., PASTURE Study Group. Taft D.H., Kirjavainen P.V., Kalanetra K.M., Karvonen A.M., Peschel S., Schmausser-Hechfellner E., Roduit C., Frei R., et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat. Med. 2020;26:1766–1775. doi: 10.1038/s41591-020-1095-x. [DOI] [PubMed] [Google Scholar]
- 29.Smilowitz J.T., O’Sullivan A., Barile D., German J.B., Lönnerdal B., Slupsky C.M. The human milk metabolome reveals diverse oligosaccharide profiles. J. Nutr. 2013;143:1709–1718. doi: 10.3945/jn.113.178772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis Z.T., Totten S.M., Smilowitz J.T., Popovic M., Parker E., Lemay D.G., Van Tassell M.L., Miller M.J., Jin Y.-S., German J.B., et al. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome. 2015;3:13. doi: 10.1186/s40168-015-0071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrow A.L., Staat M.A., DeFranco E.A., McNeal M.M., Cline A.R., Conrey S.C., Schlaudecker E.P., Piasecki A.M., Burke R.M., Niu L., et al. Pediatric respiratory and enteric virus acquisition and immunogenesis in US mothers and children aged 0–2: PREVAIL cohort study. JMIR Res. Protoc. 2021;10:e22222. doi: 10.2196/22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Gonzalez Peña A., Goodrich J.K., Gordon J.I., et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callahan B.J., Mcmurdie P.J., Rosen M.J., Han A.W., Johnson A.J.A., Holmes S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis Z.T., Bokulich N.A., Kalanetra K.M., Ruiz-Moyano S., Underwood M.A., Mills D.A. Use of bifidobacterial specific terminal restriction fragment length polymorphisms to complement next generation sequence profiling of infant gut communities. Anaerobe. 2012;19:62–69. doi: 10.1016/j.anaerobe.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The R Project for Statistical Computing. [(accessed on 22 February 2022)]. Available online: https://www.R-project.org/
- 36.Ferry B. Breastfeeding. International Statistical Institute World Fertility Survey; Voorburg, The Netherlands: London, UK: 1981. [Google Scholar]
- 37.Greiner T. Breastfeeding in Bangladesh: A review of the literature. Bangladesh J. Nutr. 1997;10 doi: 10.6084/m9.figshare.1331087. [DOI] [Google Scholar]
- 38.Akter S., Rahman M.M. Duration of breastfeeding and its correlates in Bangladesh. J. Health Popul. Nutr. 2010;28:595–601. doi: 10.3329/jhpn.v28i6.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitehead R. Nutritional requirements: Infant feeding practices and the development of malnutrition in rural Gambia. Food Nutr. Bull. 1979;1:1–6. doi: 10.1177/156482657900100412. [DOI] [Google Scholar]
- 40.UNICEF . The Gambia Multiple Indicator Cluster Survey 2010, Final Report. UNICEF; New York, NY, USA: 2011. [Google Scholar]
- 41.Thorvaldsen G. Was there a European breastfeeding pattern? Hist. Fam. 2008;13:283–295. doi: 10.1016/j.hisfam.2008.08.001. [DOI] [Google Scholar]
- 42.Kersting M., Wember T., Goddemeier T., Koester H., Wennemann J., Schöch G. Breast feeding studies 1981–1983 in 1500 mothers in Dortmund and Haltern. III. Rates of breast feeding and duration of breast feeding in the first half year. Mon. Kinderheilkd. 1987;135:314–319. [PubMed] [Google Scholar]
- 43.Helsing E. Supporting breastfeeding: What governments and health workers can do—European experiences. Int. J. Gynecol. Obstet. 1990;31:69–76. doi: 10.1016/0020-7292(90)90080-5. [DOI] [PubMed] [Google Scholar]
- 44.Verronen P. Imetys on muotia (breast-feeding is popular) Suom Lääkäril. 1984;39:1078–1079. [Google Scholar]
- 45.Brunn S. Grass-roots support for breast-feeding. World Health Forum. 1986;7:65–68. [Google Scholar]
- 46.Tönz O., Schwaninger U., Holzherr E., Schafroth M. Infant nutrition in Switzerland 1978. A prospective study on the nutritional habits during the first 6 months of life. I. Natural nutrition: Breast feeding. Schweiz. Med. Wochenschr. 1980;110:937–947. [PubMed] [Google Scholar]
- 47.Bürger B., Schindler K., Tripolt T., Stüger H., Wagner K.-H., Weber A., Wolf-Spitzer A. Breastfeeding prevalence in Austria according to the WHO IYCF Indicators—The SUKIE-study. Nutrients. 2021;13:2096. doi: 10.3390/nu13062096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasunen K. Imeväisikäisten Ruokinta Suomessa Vuonna. Ministry of Social Affairs and Health; Helsinki, Finland: 2005. [Google Scholar]
- 49.Kohlhuber M., Rebhan B., Schwegler U., Koletzko B., Fromme H. Breastfeeding rates and duration in Germany: A Bavarian cohort study. Br. J. Nutr. 2008;99:1127–1132. doi: 10.1017/S0007114508864835. [DOI] [PubMed] [Google Scholar]
- 50.Dratva J., Gross K., Späth A., Zemp E. SWIFS-Swiss Infant Feeding Study: A National Study on Infant Feeding and Health in the Child′s First Year: Executive Summary. Swiss Tropical and Public Health Institute; Basel, Switzerland: 2014. [Google Scholar]
- 51.Wolf J.H. Low breastfeeding rates and public health in the United States. Am. J. Public Health. 2003;93:2000–2010. doi: 10.2105/AJPH.93.12.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryan A.S., Rush D., Krieger F.W., Lewandowski G.E. Recent declines in breast-feeding in the United States, 1984 through 1989. Pediatrics. 1991;88:719–727. doi: 10.1542/peds.88.4.719. [DOI] [PubMed] [Google Scholar]
- 53.Ryan A.S. The resurgence of breastfeeding in the United States. Pediatrics. 1997;99:e12. doi: 10.1542/peds.99.4.e12. [DOI] [PubMed] [Google Scholar]
- 54.Callen J., Pinelli J. Incidence and duration of breastfeeding for term infants in Canada, United States, Europe, and Australia: A literature review. Birth. 2004;31:285–292. doi: 10.1111/j.0730-7659.2004.00321.x. [DOI] [PubMed] [Google Scholar]
- 55.Rupnicki S. Breastfeeding Report Card United States, 2020. CDC; Atlanta, GA, USA: 2020. [Google Scholar]
- 56.U.S. Department of Health & Human Services Breastfeeding Report Card. National Immunization Survey. [(accessed on 15 April 2009)]; Available online: http://www.cdc.gov/breastfeeding/data/report_card2.htm.
- 57.Duranti S., Lugli G.A., Mancabelli L., Armanini F., Turroni F., James K., Ferretti P., Gorfer V., Ferrario C., Milani C., et al. Maternal inheritance of bifidobacterial communities and bifidophages in infants through vertical transmission. Microbiome. 2017;5:66. doi: 10.1186/s40168-017-0282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asakuma S., Hatakeyama E., Urashima T., Yoshida E., Katayama T., Yamamoto K., Kumagai H., Ashida H., Hirose J., Kitaoka M. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J. Biol. Chem. 2011;286:34583–34592. doi: 10.1074/jbc.M111.248138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lefebvre G., Shevlyakova M., Charpagne A., Marquis J., Vogel M., Kirsten T., Kiess W., Austin S., Sprenger N., Binia A. Time of lactation and maternal fucosyltransferase genetic polymorphisms determine the variability in human milk oligosaccharides. Front. Nutr. 2020;7:574459. doi: 10.3389/fnut.2020.574459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Leary J. Do Old Order Mennonites Hold the Key to Understanding Food Allergies? [(accessed on 25 August 2021)]. Available online: https://www.urmc.rochester.edu/news/story/do-old-order-mennonites-hold-the-key-to-understanding-food-allergies.
- 61.Poupard J.A., Husain I., Norris R.F. Biology of the bifidobacteria. Bacteriol. Rev. 1973;37:136–165. doi: 10.1128/br.37.2.136-165.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Placek P.J., Taffel S.M. Trends in cesarean section rates for the United States, 1970–1978. Public Health Rep. 1980;95:540–548. [PMC free article] [PubMed] [Google Scholar]
- 63.Schuchat A. Group B streptococcus. Lancet. 1999;353:51–56. doi: 10.1016/S0140-6736(98)07128-1. [DOI] [PubMed] [Google Scholar]
- 64.Hassan M.Z., Monjur M.R., Biswas M.A.A.J., Chowdhury F., Kafi M.A.H., Braithwaite J., Jaffe A., Homaira N. Antibiotic use for acute respiratory infections among under-5 children in Bangladesh: A population-based survey. BMJ Glob. Health. 2021;6:e004010. doi: 10.1136/bmjgh-2020-004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akay H.K., Tokman H.B., Hatipoglu N., Hatipoglu H., Siraneci R., Demirci M., Borsa B.A., Yuksel P., Karakullukcu A., Kangaba A.A., et al. The relationship between bifidobacteria and allergic asthma and/or allergic dermatitis: A prospective study of 0–3 years-old children in Turkey. Anaerobe. 2014;28:98–103. doi: 10.1016/j.anaerobe.2014.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Bangladesh cohort sequencing data can be found in SRA under accession IDs PRJNA636907 and PRJNA636905. The Gambia cohort sequencing data can be found in QIITA under study ID 10297 and the European Nucleotide Archive under accession number ERP017462. PASTURE cohort sequences can be found as part of the supplementary data of DOI 10.1038/s41591-020-1095-x without metadata; PASTURE is a cohort with ongoing field cohort. As long as the cohort is ongoing, European data protection prohibits sharing of individual data, even if pseudonymized. The Davis Lactation cohort data can be found in SRA under accession ID PRJNA820048. The PREVAIL (Cincinnati) cohort data can be found in SRA under accession ID PRJNA819967.