Abstract
Introduction
Lung cancer is a major challenge facing modern medicine. It is the leading cause of cancer-related death in the USA. Little is known of the incidence, prevalence and disease characteristics in lung transplant recipients, a population unique in its vulnerability and exposure to carcinogenic risk factors. We aimed to elaborate these characteristics of lung cancer in our population through a retrospective cohort study.
Methods
We retrospectively reviewed our institution’s 8-year experience with lung transplantation and searched for patients with a post-transplant diagnosis of lung cancer, neoplasia or mass. We focused on patient demographics, indication for transplant, smoking history, stage at diagnosis, location of the tumour, length of time between transplant and diagnosis, the treatment offered and length of time from diagnosis to death or last follow-up. Descriptive statistics and survival analysis standard Kaplan-Meier method was conducted from the date of cancer diagnosis to death from all-cause mortality or last follow-up as of August 2021.
Results
We identified 24 patients with de novo lung cancer postlung transplant in 905 recipients. More patients with an underlying diagnosis of idiopathic pulmonary fibrosis developed lung cancer. Twenty-one patients were diagnosed with non-small cell lung cancer and three had small cell lung cancer. The remaining native lung was involved most in single lung recipients with 17 patients. Patients with a diagnosis of lung cancer had a mean survival of 17.6 months after diagnosis.
Discussion
The incidence rate of lung cancer in our cohort was higher than reported for smokers from the general population in previous studies. In this study, we compare our findings with available literature. We also explore screening strategies, treatment modalities, survival and postulated mechanisms for the development of lung cancer in lung transplant recipients.
Keywords: lung transplantation, lung cancer, non-small cell lung cancer, small cell lung cancer
Key messages.
What is already known on this topic
Lung cancer affects recipients of lung transplant at an increased frequency when compared with the general population.
What this study adds
This study adds to the current published literature and describes the incidence rates.
It also examines the utility of current screening guidelines, and mortality in our lung transplant population.
How this study might affect research, practice and/or policy
This study advocates for heightened suspicion for lung cancer in the transplant recipient.
Our findings may encourage transplant pulmonologists to screen patients routinely.
Introduction
Lung cancer (LC) is a major clinical and public health challenge facing modern medicine. It remains a leading cause of death in the USA and comprises 23% of cancer deaths.1 2 While many risk factors are known for LC development, there are cohorts where the description of occult risks may be complex. Lung transplant recipients (LTRs) are one such group of patients. Lung transplantation (LT) is the ultimate treatment for advanced lung diseases. This therapeutic option has matured into a life-altering intervention, with >60 000 patients have received LT since its inception in 1963.3 Survival has improved from 18 days in the first patient to a median 1-year survival of 88% in North America. As most LTRs have predisposing conditions or exposure to carcinogens such as tobacco smoke, in addition to immunosuppressive therapy, it is essential to be wary of LC in these patients. Herein, we aim to explore LC in the LTR population at our institution. We also review current literature and discuss postulated mechanisms underlying the development of LC in LTR.
Materials and methods
After obtaining IRB approval (IRB # 28862), we retrospectively reviewed our institution’s 8-year experience with LT. We then searched for patients with a post-transplant diagnosis of LC, neoplasia or mass. We undertook a retrospective chart review of Temple University Hospital’s electronic medical record. We focused on patient demographics, indication for transplant, smoking history, stage at diagnosis, location of the tumour, length of time between transplant and diagnosis, the treatment offered and length of time from diagnosis to death or last follow-up.
Transplant candidates at our institution undergo a thorough preoperative evaluation. This includes a comprehensive laboratory workup, CT of the chest, abdomen and pelvis as well as pulmonary function testing and immunotyping.
Post-transplant, all patients follow-up a minimum of weekly in the first month, every other week over the next 5 months, then monthly until the first 12 months post-transplant. Patients receive a spirometry the day of an outpatient visit, and a surveillance bronchoscopy with bronchoalveolar lavage and surveillance transbronchial biopsies at 1, 3, 6 and 12 months post-transplant. However, this is at the discretion of the transplant pulmonologist and any diagnostic procedure is usually ordered in response to symptoms or changes in spirometry. Annually, all transplant recipients receive an echocardiogram and non-contrast chest CT. In the event of a nodule or lung mass being identified, a tailored approach to diagnosis is usually employed. The use of navigational bronchoscopy, endobronchial ultrasound with transbronchial biopsy, transthoracic biopsy or excisional biopsy have been used depending on the clinical stage.
Following the transplant, all patients receive standard immunosuppression per protocol. Intraoperatively, induction therapy with either basiliximab or alemtuzumab is initiated along with methylprednisolone. Immunosuppressive treatment is maintained postoperatively with tacrolimus (TAC), mycophenolate mofetil (MFM) and prednisone. MFM is usually discontinued if cancer is diagnosed, and the TAC dose is lowered.
If a malignancy is diagnosed, a multidisciplinary approach to treatment is usually taken through discussion with the thoracic tumour board which comprises the patient’s pulmonologist, thoracic surgery, medical and radiation oncology and radiology.
Statistical analysis
Descriptive statistics were reported as mean or median, with SD and IQR. Survival was calculated from the date of cancer diagnosis to death from all-cause mortality or last follow-up as of August 2021. We performed a survival analysis with the standard Kaplan-Meier method. All statistical analyses were performed using IBM SPSS V.25.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
Incidence of lung cancer post-transplant
From January 2013 to August 2021, our institution performed 905 lung transplants. Of these, 594 were single and 311 were double lung transplants (DLT). LTRs at our institution had a cumulative follow-up of 2298.67 patient-years to the analysis time (August 2021). Our search initially found 36 patients with a diagnosis of LC. We excluded two patients listed for transplant originally but were not transplanted because of a subsequent diagnosis of current LC on initial workup. After a thorough evaluation, we excluded three patients with lung lesions suspicious for LC but ultimately received alternate diagnoses. Of the 31 patients with LC, 7 had an in situ carcinoma on the explanted lung’s pathological evaluation, and thus were excluded from the analysis. Twenty-four patients were diagnosed with LC during follow-up after transplant and were the primary cohort of interest for this study (figure 1).
Figure 1.
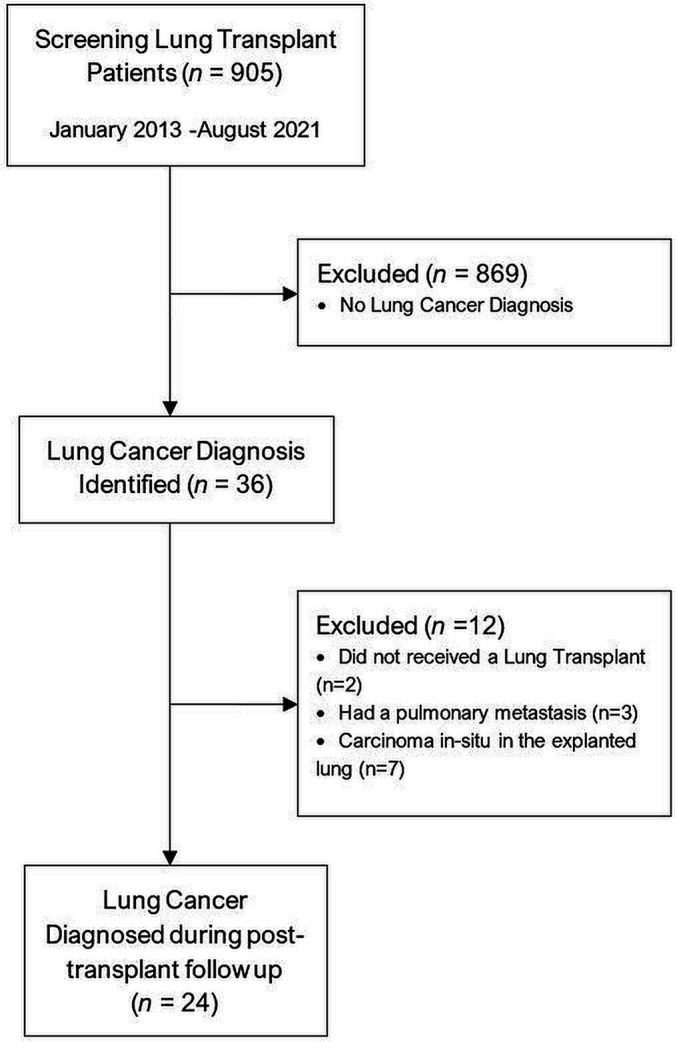
Patient selection.
The overall incidence of LC in our LTR population was 10.4 cases per 1000 patient-years. Of these, three (12.5%) patients received a DLT, with the incidence rate of LC in DLT 3.1 cases per 1000 patient-years. Twenty-one (87.5%) received a single lung transplant (SLT), with an incident rate of 15.76 per 1000 patient-years. The incidence of LC in patients with idiopathic pulmonary fibrosis (IPF) was 9.17 cases per 1000 patient-years, whereas in patients with chronic obstructive pulmonary disease (COPD), a rate of 21.1 per 1000 patient-years was observed.
Patient characteristics
Patient characteristics are described in table 1.
Table 1.
Recipient characteristics
| Gender | |
| Male | 20 (83.3 %) |
| Female | 4 (16.7 %) |
| Age | 69.5 (SD 6.6) |
| Diagnosis | |
| IPF | 11 (45.8 %) |
| COPD | 8 (33.3 %) |
| CPFE | 5 (20.8 %) |
| Smoking history | |
| Yes | 21 (87.5 %) |
| No | 3 (12.5 %) |
| Mean pack-years | 33.2 (SD 36.4) |
| Type of transplant | |
| Double lung | 3 (12.5 %) |
| Single lung | 21 (87.5 %) |
The mean age of patients was 69.5±6.6 years; there were 20 men and 4 women.
COPD, chronic obstructive pulmonary disease; CPFE, combined pulmonary fibrosis with emphysema; IPF, idiopathic pulmonary fibrosis.
IPF was the most common indication for transplant in this cohort at 45.8%. COPD was the second most common indication at 33.3%. Combined pulmonary fibrosis with emphysema indicated transplant in 20.8% of patients.
All patients had been on maintenance immunosuppression with at least tacrolimus and prednisone at the time of diagnosis, and none had received a mammalian target of rapamycin inhibitor. The use of mycophenolate was less uniform.
All but three patients had a history of smoking (87.5%). The mean pack-year history was 33.2 pack-years.
Screening, diagnosis and treatment
Tumour characteristics are described in table 2.
Table 2.
Lung cancer characteristics
| Pathology | |
| Adenocarcinoma | 8 (33.3%) |
| Squamous cell carcinoma | 10 (41.6%) |
| Small cell carcinoma | 3 (12.5%) |
| NSCLC NOS | 2 (8.3%) |
| Smooth muscle tumour | 1 (4.2%) |
| Involved lung | |
| Allograft | 7 (29.2%) |
| Native | 17 (70.8%) |
| Treatment | |
| Surgical | 9 (37.5%) |
| Chemotherapy | 7 (29.2%) |
| Radiation | 3 (12.5%) |
| None | 8 (33.3%) |
| Survival (in months) | 17.6 (95% CI 9.2 to 25.4) |
NOS, not otherwise specified; NSCLC, non-small cell lung cancer.
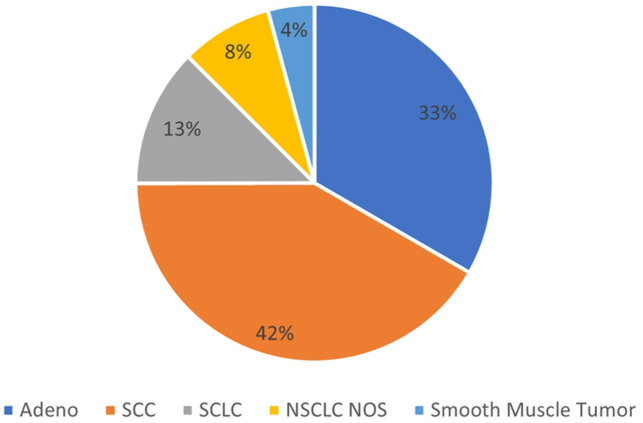
Patients were diagnosed with LC at variable times post-transplant. The mean time to diagnosis from transplant was 24.2 months (±20.3). The native lung was involved in 17 patients (70.8%), whereas the allograft was involved in 7 patients (29.2%). This included the DLT recipients (DLTRs). Squamous cell carcinoma (SCC) was the most common histopathological type, with 10 patients (41.6%), followed by adenocarcinoma in 8 patients (33.3%), small cell carcinoma (SCLC) in 3 patients. Two (8.3%) patients were diagnosed with non-small cell carcinoma not specified (NSCLC NOS), and one patient had an Epstein-Barr virus (EBV)-related smooth muscle tumour.
Fifteen patients had some symptoms suspicious of malignancy at diagnosis, with dyspnoea, cough and weight loss being the most common. Nine patients had no symptoms and were diagnosed on routine CT surveillance. Half (n=12) of the patients met the United States Preventive Services Task Force (USPSTF) criteria for lung cancer screening, whereas 67% met the more sensitive Pamplona International Early Lung Cancer Detection Program (P-IELCAP) criteria.
Eight patients had stage I disease at the time of diagnosis. Three patients had stage II and stage III each. At the same time, 10 patients had metastatic disease by the time of diagnosis.
The patients with metastatic disease chose no treatment and opted for hospice care. Three received either palliative chemotherapy or radiation.
Survival
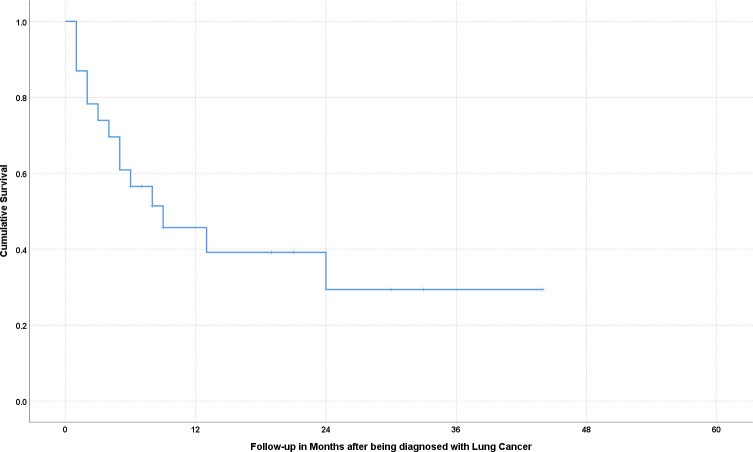
A survival analysis using the standard Kaplan-Meier method showed a mean survival of 17.6 months after diagnosis, with a SEM of 3.9. The cumulative survival fell below 50% less than a year after receiving a diagnosis of LC. A cumulative survival curve from the time of diagnosis for patients with LC is shown in figure 2.
Figure 2.
Survival curve for lung transplant recipients developing lung cancer post-transplant. Cumulative survival in months following the diagnosis is indicated by the blue line.
Nine patients had survived to follow-up at the analysis time (August 2021). Of these, all but two had a curative surgical resection. One patient received adjuvant chemotherapy, whereas one received chemotherapy alone for metastatic disease. The predominant histopathology remained SCC in six patients. The remaining three patients had been diagnosed with NSCLC NOS, adenocarcinoma and SCLC, respectively.
Six out of seven patients (85.7%) with an allograft-derived LC did not survive at the analysis time. These patients did not receive any cancer-directed therapy and opted for hospice care. Three patients had a diagnosis of adenocarcinoma. One had SCC with metastasis to the pericardium and survived less than a month after diagnosis. An EBV-associated smooth muscle tumour was diagnosed on excision from the right upper lobe of one patient; the patient survived recurrence-free for 24 months, ultimately expiring from respiratory complications 9 years post-transplant.
Patients with stage I disease had a median survival of 14 months postdiagnosis. Median survival for stage II and III disease was 21 and 5 months, respectively. Patients with stage IV disease at presentation were limited to a median survival of 3.5 months.
When surgical excision was performed, the median survival was 21 months. Patients who received chemotherapy alone had a median survival of 6 months, compared with 5 months in patients receiving chemoradiation and 1 month in patients receiving no treatment.
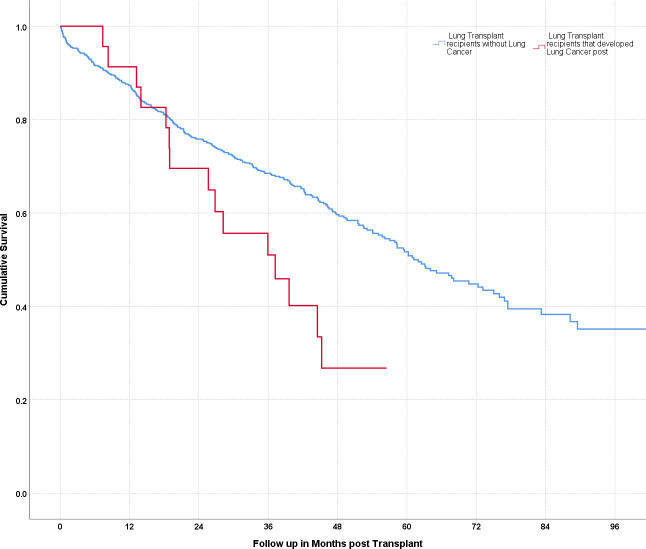
Survival post-transplant of patients with and without LC is shown in figure 3. Detailed characteristics of LTRs and LC are shown in tables 3 and 4. Relative frequencies of the malignancy are show in figure 4.
Figure 3.
Survival curves for lung transplant recipients with and without lung cancer. Cumulative survival in months following transplant. The red curve indicates patients with a diagnosis of cancer, the blue curve indicates patients without lung cancer.
Table 3.
Patient and transplant characteristics
| PATIENT characteristics | Transplant characteristics | ||||||||
| Immunosuppression | |||||||||
| Patient # | Age | Ethnicity | Indication | Single versus double | Induction agent | Calcineurin inhibitor maintenance | Antimetabolite maintenance | Steroid maintenance | mTOR inhibitor maintenance |
| 1 | 76 | Caucasian | COPD | D | N/A | TAC | None | Pred | N/A |
| 2 | 73 | Caucasian | IPF | D | BXB | TAC | None | Pred | N/A |
| 3 | 67 | Caucasian | IPF | R | BXB | CYC | None | Pred | N/A |
| 4 | 74 | Caucasian | IPF | L | AMB | TAC | MFM | Pred | N/A |
| 5 | 61 | Caucasian | IPF | R | BXB | TAC | None | Pred | N/A |
| 6 | 74 | Caucasian | COPD | D | BXB | TAC | None | Pred | N/A |
| 7 | 60 | Caucasian | CPFE | R | AMB | TAC | MFM | Pred | N/A |
| 8 | 63 | Caucasian | COPD | L | BXB | TAC | MFM | Pred | N/A |
| 9 | 75 | Caucasian | IPF | L | AMB | TAC | MFM | Pred | N/A |
| 10 | 75 | Caucasian | CPFE | R | BXB | TAC | MFM | Pred | N/A |
| 11 | 76 | Caucasian | IPF | L | BXB | TAC | MFM | Pred | N/A |
| 12 | 61 | Caucasian | ILD | L | AMB | TAC | MFM | Pred | N/A |
| 13 | 73 | Caucasian | COPD | L | BXB | TAC | AZA | Pred | N/A |
| 14 | 80 | Caucasian | CPFE | R | AMB | TAC | AZA | Pred | N/A |
| 15 | 73 | Caucasian | CPFE* | L | BXB | TAC | MFM | Pred | N/A |
| 16 | 82 | Caucasian | IPF | L | BXB | TAC | None | Pred | N/A |
| 17 | 61 | Caucasian | COPD | L | BXB | TAC | None | Pred | N/A |
| 18 | 76 | Caucasian | IPF | R | BXB | TAC | MFM | Pred | N/A |
| 19 | 70 | Caucasian | CPFE | R | AMB | TAC | AZA | Pred | N/A |
| 20 | 63 | Caucasian | IPF | R | AMB | TAC | MFM | Pred | N/A |
| 21 | 76 | African-American | COPD | L | BXB | TAC | MFM | Pred | N/A |
| 22 | 66 | Caucasian | COPD | L | BXB | TAC | MFM | Pred | N/A |
| 23 | 59 | Caucasian | COPD | R | AMB | TAC | None | Pred | N/A |
| 24 | 58 | Caucasian | IPF | L | BXB | TAC | MFM | Pred | N/A |
*Features of bronchiectasis present with underlying CPFE.
AMB, alemtuzumab; AZA, azathioprine; BXB, basiliximab; COPD, chronic obstructive pulmonary disease; CPFE, combined pulmonary fibrosis with emphysema; CYC, ciclosporin; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; MFM, mycophenolate mofetil; mTOR, mammalian target of rapamycin; Pred, prednisone; TAC, tacrolimus.
Table 4.
Lung cancer characteristics
| Patient # | Months since transplant | Location of tumour native versus allograft | Location of tumour | Smoking history in pack-years | Nodule size (mm) | Histology | TNM staging | Treatment | Mortality | Length of follow-up in months |
| 1 | 84 | Allograft | RUL | 18 | 14 | EBV smooth muscle tumour | T1N0M0 | Surgical | Yes | 24 |
| 2 | 0 | Allograft | RML, LUL | 0 | N/A | Adeno SCC |
N/A | None | Yes | 13 |
| 3 | 24 | Native | LLL | 90 | 10 | SCC | T1aN2M0 | Surgical, chemotherapy, radiation | Yes | 8 |
| 4 | 46 | Native | RUL | 24 | 29 | Adeno | T1cN0M1a | Chemotherapy | Yes | 2 |
| 5 | 48 | Allograft | RUL | 0 | N/A | Adeno | TxN2M1 | None | Yes | 1 |
| 6 | 13 | Allograft | R hilum | 180 | N/A | Adeno | TxN3M0 | None | Yes | 1 |
| 7 | 22 | Native | LLL | 25 | 20 | SCLC | T2N1M0 | Surgical, chemotherapy | No | 33 |
| 8 | 26 | Native | RML | 60 | 40 | SCC | T2N0M0 | Surgical | No | 21 |
| 9 | 48 | Native | RUL | 41 | 43 | SCC | T3N0M0 | Surgical | No | 9 |
| 10 | 44 | Native | LUL | 36 | 22 | SCC | T1cN0M0 | Surgical | No | 7 |
| 11 | 14 | Native | RUL | 6.6 | 45 | Adeno | T2aN3M1a | None | Yes | 4 |
| 12 | 12 | Native | R hilum | 30 | 52 | SCC | T4N2M1c | Chemotherapy, radiation | Yes | 5 |
| 13 | 26 | Allograft | L hilum and pericardium | 35 | 62 | SCC | T3N0M1c | None | Yes | <1 |
| 14 | 48 | Native | LUL | 57 | 23 | Adeno | T1cN0M0 | Radiation | Yes | 9 |
| 15 | 14 | Native | RUL | 13 | 14 | SCC | T1aN0M0 | Surgical | No | 30 |
| 16 | 24 | Native | RUL | 0 | 88 | SCC | T4N0M1a | Radiation | Yes | 2 |
| 17 | 6 | Allograft | LUL | 40 | 19 | NSCLC | T1bN3M1a | None | Yes | 3 |
| 18 | 37 | Allograft | Right lung | 25 | N/A | Adeno | TxN3M1c | None | Yes | 1 |
| 19 | 23 | Native | LUL | 40 | 79 | SCC | T4N0M0 | Chemotherapy, radiation | Yes | 5 |
| 20 | 19 | Native | LLL | 7 | 30 | SCC | T1cN0M0 | SRS SBRT | No | 8 |
| 21 | 30 | Allograft | L hilum | 80 | N/A | NSCLC | N/A | None | No | 19 |
| 22 | 0 | Native | LUL | 30 | 6 | Adeno | T1cN0M0 | Surgical resection | No | 44 |
| 23 | 5 | Native | LUL | 80 | 25 | SCLC | T3N1M1 | Chemotherapy | Yes | 6 |
| 24 | 40 | Native | RLL | 52 | 10 | SCLC | T1cN1M1a | Chemotherapy | No | 6 |
Adeno, adenocarcinoma; EBV, Epstein-Barr virus; N/A, not available; NSCLC, non-small cell lung cancer; SBRT, Stereotactic Body Radiotherapy; SCC, squamous cell carcinoma; SCLC, small cell lung cancer; SRS, Stereotactic Radiosurgery; TNM, tumour, node, metastases.
Figure 4.
Relative frequencies by pathology. Adeno, adenocarcinoma; NOS, not otherwise specified; NSCLC, non-small cell lung cancer; SCC, squamous cell carcinoma; SCLC, small cell lung cancer.
Discussion
Our findings add to the available data,4–20 summarised in table 5.
Table 5.
Summary of available literature
| Study | Patients with lung cancer/Total patients | Patients with native lung cancer | Patients with donor lung cancer | Indication for transplant | Documented pathology |
| Choi et al4 | 1/99 | 1 | N/A | IPF | SCC |
| Spiekerkoetter et al5 | 1/219 | 1 | N/A | N/A | SCC |
| de Perrot et al6 | 1/396 | 1 | N/A | COPD | BAC |
| Stagner et al7 | 2/46 | 2 | N/A | COPD | SCC ×1 Adeno ×1 |
| Schulman et al8 | 2/82 | 1 | N/A | COPD ×2 | SCC ×1 Adeno ×1 |
| Collins et al9 | 3/111 | N/A | N/A | COPD ×2 IPF ×1 |
SCC ×1 NSCLC NOS ×2 |
| Roithmaier et al10 | 5/200 | 4 | N/A | COPD ×4 | NOS* |
| Arcasoy et al11 | 6/251 | 6 | N/A | COPD ×5 IPF ×1 |
SCC ×2 Adeno ×3 Poorly diff ×1 |
| Raviv et al12 | 7/290 | 6 | 1 | COPD ×2 IPF ×4 SSc ×1 |
SCC ×3 Adeno ×3 Poorly diff ×1 |
| Dickson et al13 | 9/131 | 9 | N/A | COPD ×8 IPF ×1 |
SCC ×6 Adeno ×2 Sarcoma ×1 |
| Espinosa et al14 | 9/340 | 8 | N/A | COPD ×5 IPF ×3 CF ×1 |
SCC ×3 Adeno ×3 LCC ×2 SCLC ×1 |
| Gonzalez et al15 | 11/258 | 11 | N/A | COPD ×11 | N/A |
| Minai et al16 | 12/286 | 11 | 1 | COPD ×11 IPF ×1 |
SCC ×3 Adeno ×4 LCC ×3 SCLC ×1 |
| Belli et al17 | 13/335 | 6 | 1 | COPD ×7 IPF ×6 |
SCC ×5 Adeno ×4 Poorly diff ×2 SCLC ×1 Carcinoid ×1 |
| Yserbyt et al18 | 13/494 | 9 | 4 | COPD ×8 PF ×4 GIP ×1 |
SCC ×3 Adeno ×4 LCC ×3 SCLS ×1 NOS ×2 |
| Ekström et al19 | 18/331 | 11 | 7 | COPD ×18 | N/A |
| Pérez-Callejo et al20 | 23/633 | 12 | 6 | COPD ×10 IPF ×11 Primary pulmonary Hemosiderosis ×1 NSIP ×1 |
SCC ×7 Adeno ×13 LCC ×1 SCLC ×1 Poorly diff ×1 |
| Collins et al9 | 24/2168 | 24 | N/A | COPD ×18 IPF ×3 |
SCC ×8 Adeno ×4 Poorly diff ×1 Anaplastic ×1 BAC ×10 |
| This study | 24/905 | 17 | 7 | COPD ×8 IPF ×11 CPFE ×5 |
SCC ×10 Adeno ×8 Other NSCLC ×2 SCLC ×3 |
| Total | 184/7575 | 140 | 27 | COPD ×121 IPF ×43 Others ×20 |
SCC ×47 Adeno ×50 Other NSCLC ×36 SCLC ×8 |
Adeno, adenocarcinoma; BAC, Bronchoalveolar Carcinoma; COPD, chronic obstructive pulmonary disease; CPFE, chronic pulmonary fibrosis with emphysema; diff, differentiated; GIP, giant cell interstitial pneumonia; IPF, idiopathic pulmonary fibrosis; N/A, not available; NOS, not otherwise specified; NSIP, Non-specific Intersitial Pneumonia; PF, Pulmonary Fibrosis; SCC, squamous cell cancer; SCLC, small cell lung cancer; SSc, systemic sclerosis.
Incidences
The development of LC in LTR is a complex clinical problem, and data are limited to case series owing to the small patient population.
Our study demonstrates an overall incidence of LC at 10.4 cases per 1000 patient-years in LTRs. In perspective, smokers recruited in the NELSON study (Dutch-Belgian Randomised Lung Cancer Screening Trial (Dutch acronym: NELSON study)) had an incidence rate of 5.58 per 1000 patient-years.1 The nearly double incidence indicates the magnitude of the problem, limited only by the number of LTRs.
Non-transplant patients with COPD have an incidence rate of 16.7 cases per 1000 patient-years21; this was higher at 21.1 per 1000 patient-years in LTRs with COPD in our study. Whereas in IPF incidence of LC ranges between 11.2 and 36 cases per 1000 patient-years.22 Patients with IPF who received a LT at our centre had a LC incidence of 9.17 cases per 1000 patient-years.
DLTs appear to be at a lower risk of developing LC, with 3.1 cases per 1000 patient-years identified in our cohort. Single Lung Recipients (SLRs) have a higher incidence; 15.76 per 1000 patient-years.
Single lung transplant versus double lung transplant
Although scant, there is literature describing LC development in the explanted, allograft and remaining native lung.17 A recent review spanning two decades found 24 cases of native LC after a SLT in 2168 patients.9 Smaller series have reported similar findings.11 Across the literature, it appears that single lung transplant recipients (SLTRs) have a higher incidence of LC than DLTRs. Notably, in a study comparing 131 consecutive SLTRs with 131 successive DLTRs matched by native disease, 9 SLTRs developed primary LC in the native lung, whereas none of the matched bilateral lung transplant patients developed LC.13 Our results, highlighted above, mirror this finding, indicating a prevalence of LC in Double Lung Recipients (DLRs) of 0.96%, compared with 3.5% in SLRs.
Screening and diagnosis
The diagnosis of LC in the early stages is important to offer curative treatment and has indeed informed the lung cancer screening guidelines. However, no such guidelines exist for LTRs. The use of USPSTF to offer low-dose CT (LDCT) screening may not be clinically appropriate in these patients. In our cohort, if the USPSTF criteria were to be applied at the time of diagnosis, half of all cases would not have qualified for an LDCT. This signifies the lack of sensitivity of these criteria when applied to LTR populations. The P-IELCAP criteria, noted to be more sensitive for patients with underlying COPD in detecting LC,23 performed better in our population. If these were used for screening purposes, 67% of our patients would have qualified for an LDCT.
While patients at our institution usually get a CT chest at least once a year, a remarkable finding was the number of patients that were diagnosed with advanced disease. Diagnosis of LC at advanced stage has also been reported in a previous study.16 While the precise cause is unknown, the presence of occult malignancy aided in metastasis by immunosuppression may be one potential explanation.24 There appears to be a need for developing highly sensitive screening criteria and protocol in this population, and specifically designed clinical studies are required.
Treatment and survival
The biased difference in median survival heavily in favour of surgical resection likely reflects survival of early stage disease and not treatment modality. However, it does augment the argument for early detection. Due to the limited number of cases of stage II and III disease, and overall poor survival of LC, it is difficult to comment on the efficacy of systemic chemotherapy or locoregional radiation therapy. It is also important to note that the longer median survival noted in stage II disease when compared with stage I is likely due to the small number of patients with stage II disease. It is also interesting to note that among all fatalities that we observed in patients with LC, only one was unrelated to the underlying malignancy.
Postulated mechanisms
The mechanisms behind LC in LTRs are not clearly defined; however, many potential explanations for this phenomenon exist. Intuitively, the underlying diagnosis of lung disease in LTRs may be contributing factor. Interstitial lung disease is the underlying diagnosis in 34% of all LTRs.3 Among fibrotic lung disease, IPF has been described to have a fivefold increased risk of LC when compared with the general population.25 26 There are varied reports of prevalence ranging between 13.5% and 31%.26 27 An interesting finding is the predilection of LC in the lower lobes and peripheral lung zones.28 29 This anatomic distribution mimics the fibro-inflammatory changes in IPF. This obvolute distribution may be related to persistent inflammation. Myofibroblast activation proliferation, cellular stress, alterations of growth factors expression, oxidative injury and genetic factors are implicated as common denominators in both conditions.30 Genetic associations are involved in the pathogenesis of both IPF and LC, and some may be shared. There is increasing evidence for the importance of functional mutations of SFTPA 1 and SFTPA2 surfactant protein genes that lead to endoplasmic reticulum stress in the development of adenocarcinoma as well as IPF. Telomere instability caused by impaired enzymatic activity from mutations of the telomerase reverse transcriptase mutation is similarly shared by both processes. In addition, mutations of p53, p21, p16 and KRAS genes are also implicated in both processes.31 It is important to note, however, the frequency of KRAS mutation is lower in IPF associated adenocarcinoma when compared with smokers, indicating perhaps more endogenous carcinogenesis in IPF related to lung inflammation.32 Ongoing research into specific synergism between anticancer and antifibrotic agents may lead to the evolution of therapies for IPF and a better understanding of LC development in IPF.
COPD is the second leading indication of LT and is the underlying diagnosis in 21.4% of all LTRs.3 Abundant epidemiological evidence links the presence of COPD to the development of LC and remains a risk for all SLRs with native lung COPD. The connection between COPD and LC appears to be a complex interaction of multiple factors. When considering COPD, differentiating airflow obstruction and emphysema may be helpful. Chronic airway inflammation and adaptation to chronic irritant exposure is the hallmark of airflow limitation. Repeated injury and repair with metaplastic changes are one explanation for the increased frequency of LC in these patients. However, emphysematous destruction of the lung parenchyma appears to confer an increased risk of LC when compared with airflow obstruction.33–37 These findings suggest that the relationship between COPD and LC may be more profound than smoking alone. Emphysema is characterised by an imbalance of proteinases and inhibitors, an abundance of oxidative stress and an excessive infiltration of inflammatory cells leading to permanent airspace destruction. Chronic inflammation is also a common occurrence in LC. CD4+, CD8+ and macrophage recruitment and infiltration are shared among both disorders.38 Oxidative stress from tobacco smoke is abundantly linked to the pathogenesis of both COPD and LC. The xenobiotic metabolic pathway is known to metabolise many tobacco smoke constituents. Carbon monoxide, N-nitroso derivatives and polycyclic aromatic hydrocarbons are converted into carcinogens, generating oxygen free radicals as a by-product, leading to damaged cellular organelles and genetic material.39
SLRs with emphysema may be at an increased risk of LC when compared with those without. This can be attributed to the persistence of inflammation and maladaptive response in the remaining native lung.
Beyond underlying pathophysiology, there are other recipient and donor risk factors that could be associated with the development of LC in LTRs. The risk of LC in the general population increases with advancing age. The rate of LC in individuals aged 60 years or older is 284 per 100 000 individuals when compared with 32 per 100 000 in those aged <60 years. Simply crossing the age of 60 years appears to increase the risk of LC drastically; the rate of LC between ages 55 and 59 years is 92 per 100 000, whereas this increases to 151 per 100 000 for individuals aged 60–64 years in the USA.40 Over the past two decades, the proportion of LTRs aged >60 years has continued to increase and now forms almost a quarter of all recipients.3 Similarly, the pool of donors has also increased over this period, and organs from older individuals are more frequently acceptable. According to the International Society for Heart and Lung Transplantation (ISHLT) registry, up to 5% of organs are derived from donors aged 60 years or above. Additionally, as post-transplant outcomes and survival improve, current data suggest that many middle-aged recipients will live into high-risk decades.41 The age of donors and recipients can thus increase the individual risk of LC in LTRs.
LTRs are uniquely exposed to long-term immunosuppression. Unlike other solid organ transplant patients, therapeutic targets for immunosuppression in lung transplants are usually higher, owing to the higher risk of rejection. Calcineurin inhibitors (CNI) form the backbone of immunosuppression therapy. In addition to inhibiting calcineurin, tacrolimus and ciclosporin enhance the production and secretion of transforming growth factor β1 (TGFβ1).42 43 TGFβ1 has been known to increase tumour invasiveness and spread through angiogenesis, extracellular matrix production and suppression of host antitumour immune response.44 Suppression of the antitumour immune system is augmented by the inherent effects of CNI.
Immunosuppression also exposes the patient to infections with oncogenic viruses, which have been linked to bronchogenic carcinomas in mammalian models. Examples include Jaagsiekte sheep retrovirus causing pulmonary adenocarcinoma in sheep and Simian virus 40-related DNA sequences isolates in NSCLC.45 An association between oncogenic viruses and LC in humans is much less clear, and further work is required in this field.
Conclusion
LTRs represent a particularly vulnerable population in terms of LC development. The combination of underlying risk factors, exposures, immunosuppression and age all factor into an increased risk of LC development. The lack of data on the underlying mechanisms of development, and consensus screening and treatment guidelines add to the complexity of this clinical problem. The development of consensus statements and further study on this subject are required to inform clinical decision-making.
Footnotes
Contributors: BHL is the guarantor for this work and accepts full responsibility for the work and the conduct of the study. BHL contributed to the generation of the clinical question, design of the study, data review, statistical analysis, and manuscript writing. RJV contributed to the design of the study, data review and manuscript writing. DCF-S contributed to the literature review and manuscript writing. TS contributed to the data review, literature review and manuscript writing. GJC provided supervision for the entire project, was directly involved with the review of data, literature and provided oversight of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 2020;382:503–13. 10.1056/NEJMoa1911793 [DOI] [PubMed] [Google Scholar]
- 2.U.S. Cancer Statistics Working Group . U.S. cancer statistics data visualizations tool, based on 2020 submission data (1999-2018): U.S. department of health and human services, centers for disease control and prevention and National cancer Institute, 2021. Available: www.cdc.gov/cancer/dataviz [Accessed 19 Sep 2021].
- 3.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report--2010. J Heart Lung Transplant 2010;29:1104–18. 10.1016/j.healun.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 4.Choi IH, Song DH, Han KM, et al. Incidence of pulmonary non-epithelial tumors: 18 years' experience at a single Institute. Pathol Res Pract 2014;210:210–6. 10.1016/j.prp.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 5.Spiekerkoetter E, Krug N, Hoeper M, et al. Prevalence of malignancies after lung transplantation. Transplant Proc 1998;30:1523–4. 10.1016/s0041-1345(98)00343-1 [DOI] [PubMed] [Google Scholar]
- 6.de Perrot M, Fischer S, Waddell TK, et al. Management of lung transplant recipients with bronchogenic carcinoma in the native lung. J Heart Lung Transplant 2003;22:87–9. 10.1016/s1053-2498(02)00446-1 [DOI] [PubMed] [Google Scholar]
- 7.Stagner LD, Allenspach LL, Hogan KK, et al. Bronchogenic carcinoma in lung transplant recipients. J Heart Lung Transplant 2001;20:908–11. 10.1016/s1053-2498(01)00271-6 [DOI] [PubMed] [Google Scholar]
- 8.Schulman LL, Htun T, Staniloae C, et al. Pulmonary nodules and masses after lung and heart-lung transplantation. J Thorac Imaging 2000;15:173–9. 10.1097/00005382-200007000-00004 [DOI] [PubMed] [Google Scholar]
- 9.Collins J, Kazerooni EA, Lacomis J, et al. Bronchogenic carcinoma after lung transplantation: frequency, clinical characteristics, and imaging findings. Radiology 2002;224:131–8. 10.1148/radiol.2241011189 [DOI] [PubMed] [Google Scholar]
- 10.Roithmaier S, Haydon AM, Loi S, et al. Incidence of malignancies in heart and/or lung transplant recipients: a single-institution experience. J Heart Lung Transplant 2007;26:845–9. 10.1016/j.healun.2007.05.019 [DOI] [PubMed] [Google Scholar]
- 11.Arcasoy SM, Hersh C, Christie JD, et al. Bronchogenic carcinoma complicating lung transplantation. J Heart Lung Transplant 2001;20:1044–53. 10.1016/s1053-2498(01)00301-1 [DOI] [PubMed] [Google Scholar]
- 12.Raviv Y, Shitrit D, Amital A, et al. Lung cancer in lung transplant recipients: experience of a tertiary hospital and literature review. Lung Cancer 2011;74:280–3. 10.1016/j.lungcan.2011.02.012 [DOI] [PubMed] [Google Scholar]
- 13.Dickson RP, Davis RD, Rea JB, et al. High frequency of bronchogenic carcinoma after single-lung transplantation. J Heart Lung Transplant 2006;25:1297–301. 10.1016/j.healun.2006.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espinosa D, Baamonde C, Illana J, et al. Lung cancer in patients with lung transplants. Transplant Proc 2012;44:2118–9. 10.1016/j.transproceed.2012.07.067 [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez FJ, Alvarez E, Moreno P, et al. The influence of the native lung on early outcomes and survival after single lung transplantation. PLoS One 2021;16:e0249758. 10.1371/journal.pone.0249758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minai OA, Shah S, Mazzone P, et al. Bronchogenic carcinoma after lung transplantation: characteristics and outcomes. J Thorac Oncol 2008;3:1404–9. 10.1097/JTO.0b013e31818e1259 [DOI] [PubMed] [Google Scholar]
- 17.Belli EV, Landolfo K, Keller C, et al. Lung cancer following lung transplant: single institution 10 year experience. Lung Cancer 2013;81:451–4. 10.1016/j.lungcan.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 18.Yserbyt J, Verleden GM, Dupont LJ, et al. Bronchial carcinoma after lung transplantation: a single-center experience. J Heart Lung Transplant 2012;31:585–90. 10.1016/j.healun.2012.02.022 [DOI] [PubMed] [Google Scholar]
- 19.Ekström M, Riise GC, Tanash HA. Risk of cancer after lung transplantation for COPD. Int J Chron Obstruct Pulmon Dis 2017;12:2841–7. 10.2147/COPD.S147065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pérez-Callejo D, Torrente M, Parejo C, et al. Lung cancer in lung transplantation: incidence and outcome. Postgrad Med J 2018;94:15–19. 10.1136/postgradmedj-2017-134868 [DOI] [PubMed] [Google Scholar]
- 21.de Torres JP, Marín JM, Casanova C, et al. Lung cancer in patients with chronic obstructive pulmonary disease-- incidence and predicting factors. Am J Respir Crit Care Med 2011;184:913–9. 10.1164/rccm.201103-0430OC [DOI] [PubMed] [Google Scholar]
- 22.Kato E, Takayanagi N, Takaku Y, et al. Incidence and predictive factors of lung cancer in patients with idiopathic pulmonary fibrosis. ERJ Open Res 2018;4. 10.1183/23120541.00111-2016. [Epub ahead of print: 02 02 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez-Salcedo P, Wilson DO, de-Torres JP, et al. Improving selection criteria for lung cancer screening. The potential role of emphysema. Am J Respir Crit Care Med 2015;191:924–31. 10.1164/rccm.201410-1848OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev 2018;32:1267–84. 10.1101/gad.314617.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon JH, Nouraie M, Chen X, et al. Characteristics of lung cancer among patients with idiopathic pulmonary fibrosis and interstitial lung disease - analysis of institutional and population data. Respir Res 2018;19:195. 10.1186/s12931-018-0899-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.JafariNezhad A, YektaKooshali MH. Lung cancer in idiopathic pulmonary fibrosis: a systematic review and meta-analysis. PLoS One 2018;13:e0202360. 10.1371/journal.pone.0202360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park J, Kim DS, Shim TS, et al. Lung cancer in patients with idiopathic pulmonary fibrosis. Eur Respir J 2001;17:1216–9. 10.1183/09031936.01.99055301 [DOI] [PubMed] [Google Scholar]
- 28.Archontogeorgis K, Steiropoulos P, Tzouvelekis A, et al. Lung cancer and interstitial lung diseases: a systematic review. Pulm Med 2012;2012:315918. 10.1155/2012/315918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aubry M-C, Myers JL, Douglas WW, et al. Primary pulmonary carcinoma in patients with idiopathic pulmonary fibrosis. Mayo Clin Proc 2002;77:763–70. 10.4065/77.8.763 [DOI] [PubMed] [Google Scholar]
- 30.Ballester B, Milara J, Cortijo J. Idiopathic pulmonary fibrosis and lung cancer: mechanisms and molecular targets. Int J Mol Sci 2019;20. 10.3390/ijms20030593. [Epub ahead of print: 30 Jan 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzouvelekis A, Gomatou G, Bouros E, et al. Common pathogenic mechanisms between idiopathic pulmonary fibrosis and lung cancer. Chest 2019;156:383–91. 10.1016/j.chest.2019.04.114 [DOI] [PubMed] [Google Scholar]
- 32.Guyard A, Danel C, Théou-Anton N, et al. Morphologic and molecular study of lung cancers associated with idiopathic pulmonary fibrosis and other pulmonary fibroses. Respir Res 2017;18:120. 10.1186/s12931-017-0605-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson DO, Weissfeld JL, Balkan A, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med 2008;178:738–44. 10.1164/rccm.200803-435OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueda K, Jinbo M, Li T-S, et al. Computed tomography-diagnosed emphysema, not airway obstruction, is associated with the prognostic outcome of early-stage lung cancer. Clin Cancer Res 2006;12:6730–6. 10.1158/1078-0432.CCR-06-1196 [DOI] [PubMed] [Google Scholar]
- 35.de Torres JP, Bastarrika G, Wisnivesky JP, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest 2007;132:1932–8. 10.1378/chest.07-1490 [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Swensen SJ, Karabekmez LG, et al. Effect of emphysema on lung cancer risk in smokers: a computed tomography-based assessment. Cancer Prev Res 2011;4:43–50. 10.1158/1940-6207.CAPR-10-0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zulueta JJ, Wisnivesky JP, Henschke CI, et al. Emphysema scores predict death from COPD and lung cancer. Chest 2012;141:1216–23. 10.1378/chest.11-0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer 2013;13:233–45. 10.1038/nrc3477 [DOI] [PubMed] [Google Scholar]
- 39.Zhang JY, Wang Y, Prakash C. Xenobiotic-Metabolizing enzymes in human lung. Curr Drug Metab 2006;7:939–48. 10.2174/138920006779010575 [DOI] [PubMed] [Google Scholar]
- 40.USCS . Data Visualizations. Available: https://gis.cdc.gov/grasp/USCS/DataViz.html [Accessed 19 Sep 2021].
- 41.Costa J, Benvenuto LJ, Sonett JR. Long-Term outcomes and management of lung transplant recipients. Best Pract Res Clin Anaesthesiol 2017;31:285–97. 10.1016/j.bpa.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 42.Prashar Y, Khanna A, Sehajpal P, et al. Stimulation of transforming growth factor-beta 1 transcription by cyclosporine. FEBS Lett 1995;358:109–12. 10.1016/0014-5793(94)01382-b [DOI] [PubMed] [Google Scholar]
- 43.Maluccio M, Sharma V, Lagman M, et al. Tacrolimus enhances transforming growth factor-beta1 expression and promotes tumor progression. Transplantation 2003;76:597–602. 10.1097/01.TP.0000081399.75231.3B [DOI] [PubMed] [Google Scholar]
- 44.Teicher BA. Malignant cells, directors of the malignant process: role of transforming growth factor-beta. Cancer Metastasis Rev 2001;20:133–43. 10.1023/A:1013177011767 [DOI] [PubMed] [Google Scholar]
- 45.Mathew J, Kratzke RA. Lung cancer and lung transplantation: a review. J Thorac Oncol 2009;4:753–60. 10.1097/JTO.0b013e31819afdd9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data are available.