Abstract
Background:
Observational data suggest catheter ablation may be safe and effective to treat younger and older patients with atrial fibrillation (AF). No large randomized trial has examined this issue. This report describes outcomes according to age at entry in the Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation trial (CABANA).
Methods:
Patients with AF age ≥65, or <65 with ≥1 risk factor for stroke, were randomly assigned to catheter ablation versus drug therapy. The primary outcome was a composite of death, disabling stroke, serious bleeding, or cardiac arrest. Secondary outcomes included all-cause mortality, the composite of mortality or cardiovascular hospitalization, and recurrence of AF. Treatment effect estimates were adjusted for baseline covariables using proportional hazards regression models.
Results:
Of 2204 patients randomized in CABANA, 766 (34.8%) were age <65, 1130 (51.3%) were 65–74, and 308 (14.0%) were ≥75. Catheter ablation was associated with a 43% reduction in the primary outcome for age <65 patients (adjusted hazard ratio [aHR] 0.57, 95% confidence interval [CI] 0.30–1.09), a 21% reduction for age 65–74 (aHR 0.79; 95% CI 0.54–1.16), and an indeterminate effect for age ≥75 (aHR 1.39; 95% CI 0.75–2.58). Four year event rates for ablation versus drug therapy across age groups, respectively, were 3.2% versus 7.8%, 7.8% versus 9.6%, and 14.8% versus 9.0%. For every 10-year increase in age, the primary outcome aHR increased (i.e., less favorable to ablation) an average of 27% (interaction p value= 0.215). A similar pattern was seen with all-cause mortality: for every 10-year increase in age, the aHR increased an average of 46% (interaction p value= 0.111). AF recurrence rates were lower with ablation compared to drug therapy across age subgroups (aHR 0.47, 0.58, and 0.49, respectively). Treatment-related complications were infrequent for both arms (<3%) regardless of age.
Conclusions:
We found age-based variations in clinical outcomes for catheter ablation compared with drug therapy, with the largest relative and absolute benefits of catheter ablation in younger patients. No prognostic benefits for ablation were seen in the oldest patients. No differences were found by age in treatment-related complications or in the relative effectiveness of catheter ablation in preventing recurrent atrial arrhythmias.
Keywords: Atrial Fibrillation, Anti-Arrhythmic Drug Therapy, Catheter Ablation, Pulmonary Vein Isolation, Age
INTRODUCTION
Initial randomized trials of catheter ablation for atrial fibrillation (AF) focused on relatively young patients and reported that ablation was superior to drug therapy for reducing or eliminating AF and improving quality of life.1, 2 Subsequent observational reports of catheter ablation suggested that the relative benefits of catheter ablation to prevent AF recurrences extended to older age groups, in association with reasonably low complication rates.3–5 However, no large randomized controlled trial data have examined whether, and in what ways, the long-term clinical outcomes of catheter ablation, compared with medical therapy, vary as a function of patient age.
The Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial was the first large prospective randomized trial with extended follow-up to compare catheter ablation to drug therapy with regard to mortality-inclusive outcomes in a diverse patient population that included a broad spectrum of ages and all AF types.6 As previously reported, the trial showed that catheter ablation had an inconclusive effect on the primary composite study outcome (a composite of death, disabling stroke, serious bleeding, or cardiac arrest) and on all-cause mortality.7 The secondary outcomes of AF recurrence, quality of life, and the composite of mortality and cardiovascular hospitalization showed clinically consequential benefits of catheter ablation compared to drug therapy.8, 9
Prespecified subgroup analyses revealed about a 50% reduction in the primary outcome with catheter ablation relative to drug therapy in the patients who were <65 years old at enrollment, but showed a diminishing benefit in older patients.7 This report provides a more comprehensive description of the relationship of patient age with the benefits and risks of catheter ablation in CABANA.
METHODS
Overview
CABANA is an NIH/NHLBI sponsored trial and the trial datasets will be made public via the NIH website BioLINCC.7
CABANA enrolled 2,204 patients with untreated or undertreated AF (patients were excluded if they had failed >1 membrane-active anti-arrhythmic drug). Patients ≥18 years old were eligible regardless of the type of AF (paroxysmal, persistent, or long-standing persistent) so long as treatment of AF was clinically indicated in the judgment of the treating physician.6, 7 Eligibility further required that those <65 had additional comorbidities that conferred increased risk of stroke (hypertension, heart failure, history of stroke, diabetes, or other heart problems).6
Patients were randomized 1:1 to the treatment strategy of catheter ablation versus drug therapy. Catheter ablation included pulmonary vein isolation confirmed with a circular mapping catheter, and additional ancillary ablation was permitted at the discretion of operators.6 Patients randomized to drug therapy could undergo sequential antiarrhythmic drug or rate control therapies, directed by the judgment of the treating physicians, with the majority of patients receiving rhythm control therapy with antiarrhythmic drugs.7 Additional details about the randomized treatment strategies have been previously reported.6 Median follow-up in CABANA was 48.5 months. Each site’s institutional review board or ethics committee approved the study, and written informed consent was obtained from all patients.
CABANA Trial Outcomes
The CABANA primary outcome was a composite of death, disabling stroke, serious bleeding, or cardiac arrest.6 Secondary outcomes included all-cause mortality, the composite of death or cardiovascular hospitalization, and recurrent AF. Recurrent AF was recorded using a proprietary monitoring system (CABANA Box) available at 86% of enrolling sites.9 Recurrent AF was defined as an episode of atrial arrhythmia outside the 90-day blanking period lasting 30 seconds or longer, and AF recurrences were adjudicated by the CABANA ECG Core Laboratory.
Statistical Analysis
This age subgroup analysis of the CABANA population was pre-specified.6 Patients were aggregated into age groups for descriptive purposes using CHA2DS2-VASc cut-points of <65, 65–74, and ≥75 years of age.7 Primary analyses used age as a continuous variable in prognostic models.
For descriptive statistics, we employed medians (25th, 75th percentiles) for continuous variables and counts (percentages) for categorical variables. Treatment comparisons were performed using intention-to-treat (ITT) to define treatment assignment. Kaplan-Meier estimation was used to construct survival curves based on time-to-event analyis.10
Unadjusted and adjusted Cox proportional hazards models were used to estimate average relative treatment effects hazard ratios (HR) with associated 95% confidence intervals (CI).11 The effect of age on the ablation to drug therapy hazard ratio was estimated by including age and an age X treatment interaction term in the models, with age as a continuous variable. Adjusted Cox models included the following variables: treatment, age, age x treatment interaction, sex, race/ethnicity, AF type, years since onset of AF, history of heart failure, structural heart disease (mitral regurgitation, left ventricular hypertrophy, and increased left atrial diameter), CHA2DS2-VASc score, history of coronary artery disease, and hypertension. These models were used to produce graphical descriptive representations of the relationship between age and estimated treatment effect and to calculate the relative increase in the HR associated with a 10- year increase in age.
To avoid dropping the few patients who had one or more missing baseline covariates from the Cox model analyses, the main analyses employed a single-imputation method using either the median (continuous variables) or the mode (categorical variables). Estimates generated without any imputed data were almost identical (data not shown).
Recurrent AF (AF/atrial flutter/atrial tachycardia) cumulative incidence rates were estimated using a proportional hazards (Fine-Gray) model assuming death as a competing risk and adjusting for the covariables enumerated above.12 Only the 1,240 patients who used the proprietary CABANA-Box recorder and provided post-blanking period recordings were included in this portion of the analysis.9
P values, where provided, are intended as adjunctive interpretive aids reflecting the unexpectedness of the observed effects or differences under the assumption that the null hypothesis is true.13 Treatment x covariable interaction p values are commonly used to probe for the presence of consequential treatment interactions. However, given the poor statistical power these tests typically have in this context, we also examined the relative and absolute effects of age variations on treatment outcomes and used graphical displays to supplement numerical estimates in providing a comprehensive examination of the relevant relationships.14 No adjustments were made for multiple comparisons. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Baseline Characteristics
Age was equally distributed by treatment group in CABANA. Within each age subgroup, major demographic and clinical characteristics were reasonably well balanced by randomized treatment assignment (Table 1). However, a number of baseline factors varied as a function of age. Patients ≥75 had a higher proportion of females, lower proportions of racial or ethnic minorities and diabetes, and greater proportions had CHA2DS2-VASc >2 or prior revascularization compared to younger age groups (Supplemental Table 1). The proportion of patients with paroxysmal or persistent/long-standing persistent AF did not differ significantly between age groups (Supplemental Table 1). Median duration of AF prior to study enrollment was 1.2 years, 1.1 years, and 0.8 years for the <65 years, 65–74 years, and ≥75 years age group, respectively.
Table 1.
Baseline Characteristics by Age Group and Treatment
| Age: <65 Years (N=766) | Age: 65 to 74 (N=1130) | Age: ≥75 Years (N=308) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristics | Ablation (N=375) | Drug (N=391) | Ablation (N=577) | Drug (N=553) | Ablation (N=156) | Drug (N=152) |
| Age | ||||||
| N | 375 | 391 | 577 | 553 | 156 | 152 |
| Median (Q1, Q3) | 59.2 (54.3, 62.1) | 59.4 (55.7, 62.6) | 69.3 (67.3, 71.8) | 69.3 (67.2, 71.9) | 77.6 (76.5, 80.0) | 77.3 (76.0, 79.4) |
| Female Sex | 94/375 (25.1%) | 104/391 (26.6%) | 234/577 (40.6%) | 234/553 (42.3%) | 85/156 (54.5%) | 68/152 (44.7%) |
| Minorities | 54/375 (14.4%) | 51/389 (13.1%) | 47/574 (8.2%) | 49/553 (8.9%) | 12/155 (7.7%) | 12/152 (7.9%) |
| History of CVA or TIA | 48/375 (12.8%) | 32/390 (8.2%) | 50/577 (8.7%) | 52/553 (9.4%) | 19/156 (12.2%) | 19/152 (12.5%) |
| History of Heart Failure | 64/375 (17.1%) | 68/389 (17.5%) | 80/577 (13.9%) | 74/553 (13.4%) | 30/156 (19.2%) | 21/152 (13.8%) |
| NYHA Class II or Greater | 130/373 (34.9%) | 154/388 (39.7%) | 184/569 (32.3%) | 179/551 (32.5%) | 64/155 (41.3%) | 67/150 (44.7%) |
| CHA2DS2-VASc > 2 | 117/375 (31.2%) | 113/391 (28.9%) | 359/577 (62.2%) | 364/553 (65.8%) | 151/156 (96.8%) | 141/152 (92.8%) |
| Type of AF At Enrollment | ||||||
| Paroxysmal | 159/375 (42.4%) | 155/390 (39.7%) | 247/577 (42.8%) | 263/553 (47.6%) | 64/156 (41.0%) | 58/152 (38.2%) |
| Persistent and Longstanding Persistent | 216/375 (57.6%) | 235/390 (60.3%) | 330/577 (57.2%) | 290/553 (52.4%) | 92/156 (59.0%) | 94/152 (61.8%) |
| Mitral Valve Regurgitation | 129/304 (42.4%) | 127/296 (42.9%) | 210/426 (49.3%) | 191/362 (52.8%) | 66/109 (60.6%) | 53/99 (53.5%) |
| Diastolic Dysfunction | 30/243 (12.3%) | 41/255 (16.1%) | 43/308 (14.0%) | 39/308 (12.7%) | 15/84 (17.9%) | 21/80 (26.3%) |
| LVH | 145/314 (46.2%) | 166/304 (54.6%) | 143/436 (32.8%) | 121/374 (32.4%) | 46/114 (40.4%) | 41/102 (40.2%) |
| Prior use of anti-arrhythmic drug | 174/355 (49.0%) | 183/368 (49.7%) | 249/547 (45.5%) | 289/534 (54.1%) | 68/145 (46.9%) | 79/146 (54.1%) |
| Prior revascularization (PCI or CABG) | 29/375 (7.7%) | 41/390 (10.5%) | 65/577 (11.3%) | 69/553 (12.5%) | 37/156 (23.7%) | 23/152 (15.1%) |
| Diabetes | 111/375 (29.6%) | 117/390 (30.0%) | 139/577 (24.1%) | 131/553 (23.7%) | 30/156 (19.2%) | 33/152 (21.7%) |
| Prior Stroke | 26/375 (6.9%) | 17/390 (4.4%) | 33/577 (5.7%) | 33/553 (6.0%) | 9/156 (5.8%) | 8/152 (5.3%) |
| Prior use of DOAC | 231/375 (61.6%) | 259/391 (66.2%) | 364/577 (63.1%) | 325/553 (58.8%) | 94/156 (60.3%) | 104/152 (68.4%) |
| Hypertension | 305/375 (81.3%) | 344/390 (88.2%) | 440/577 (76.3%) | 434/553 (78.5%) | 131/156 (84.0%) | 122/152 (80.3%) |
| BMI | ||||||
| N | 374 | 384 | 560 | 548 | 152 | 152 |
| Median (Q1, Q3) | 31.9 (27.6, 36.4) | 31.7 (28.2, 36.6) | 29.7 (26.7, 33.7) | 30.0 (26.4, 34.6) | 27.6 (24.7, 31.3) | 27.6 (25.2, 31.1) |
| Left Atrial Diameter | ||||||
| N | 210 | 228 | 270 | 258 | 72 | 70 |
| Median (Q1, Q3) | 4.6 (4.1, 5.1) | 4.6 (4.2, 5.2) | 4.4 (4.0, 4.9) | 4.3 (3.9, 4.8) | 4.4 (3.8, 4.6) | 4.5 (4.0, 4.9) |
| Left Atrial Volume Index | ||||||
| N | 75 | 66 | 104 | 105 | 23 | 17 |
| Median (Q1, Q3) | 41.0 (32.0, 50.0) | 40.4 (35.3, 48.0) | 39.0 (29.9, 48.7) | 36.0 (27.7, 44.2) | 42.0 (30.7, 56.0) | 34.0 (33.0, 47.0) |
| Years since onset of AF | ||||||
| N | 373 | 383 | 576 | 551 | 151 | 151 |
| Median (Q1, Q3) | 1.3 (0.4, 4.3) | 1.1 (0.3, 3.7) | 1.0 (0.3, 3.9) | 1.1 (0.3, 3.8) | 0.8 (0.2, 4.1) | 0.8 (0.2, 3.5) |
AF=atrial fibrillation, BMI=body mass index, CABG=coronary artery bypass graft surgery, CVA=cerebrovascular accident, DOAC=direct oral anticoagulants, LVH=left ventricular hypertrophy, NYHA=New York Heart Association, PCI=percutaneous coronary intervention, Q1=1st quartile, Q3=3rd quartile, TIA=transient ischemic attack,
Treatment Data
Of the 766 patients who were age <65 at baseline, 375 (49.0%) were randomized to the ablation group and 391 (51.0%) to the drug therapy group (Table 1). In the 1,130 patients who were age 65–74, 577 (51.1%) were randomized to ablation and 553 (48.9%) to drug therapy. For the 308 patients who were ≥75, 156 (50.6%) were randomized to ablation and 152 (49.4%) to drug therapy.
Of the 375 ablation arm patients who were age <65, 345 (92.0%) had their assigned ablation procedure at a median of 32 days (25th-75th percentile, 10, 60). The corresponding values for the 577 patients age 65–74 were 522 (90.5%) at a median of 28 days (25th-75th percentile 14, 51) and for the 156 patients ≥75 were 139 (89.1%) at a median of 30 days (25th-75th percentile 17, 58). There were 145 (43.2%), 232 (45.8%), and 59 (43.7%) ablation-arm patients on a rhythm control drug at some point during the post-blanking period, and 78 (23.2%), 143 (28.3%), and 40 (29.6%) patients on a rhythm control drug at the last available follow-up contact.
Of the 391 drug therapy arm patients <65, 113 (28.9%) patients crossed over to ablation at a median of 381 days. The corresponding values for the 553 patients ages 65–74 were 157 (28.4%) at a median of 369 days and for the 152 patients ≥75 were 31 (20.4%) at a median of 282 days. A rhythm control drug was being used in 310 (80.7%), 468 (87.0%), and 118 (83.7%) patients at some point during the post-blanking period, and 201 (52.3%), 285 (53.0%), and 73 (51.8%) patients at the last available follow-up.
Treatment-Related Complications
Treatment-related adverse events were uncommon in both arms and showed no evident association with age (Supplemental Table 2). For patients in the ablation arm who received an ablation, hematoma and pericardial effusion not requiring intervention were the most common procedure-related adverse events and occurred in less than 3%. Among drug therapy patients, thyroid dysfunction was the most common adverse event and occurred in less than 2%.
Clinical Outcomes by Intention-to-Treat
CABANA Primary Outcome
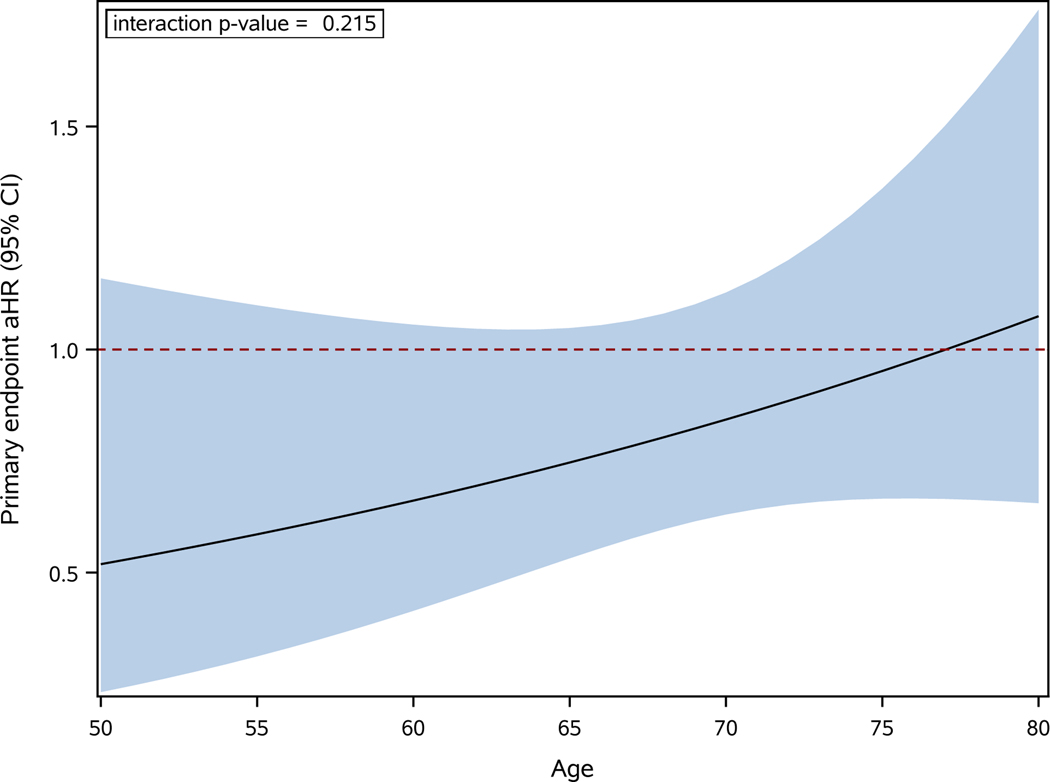
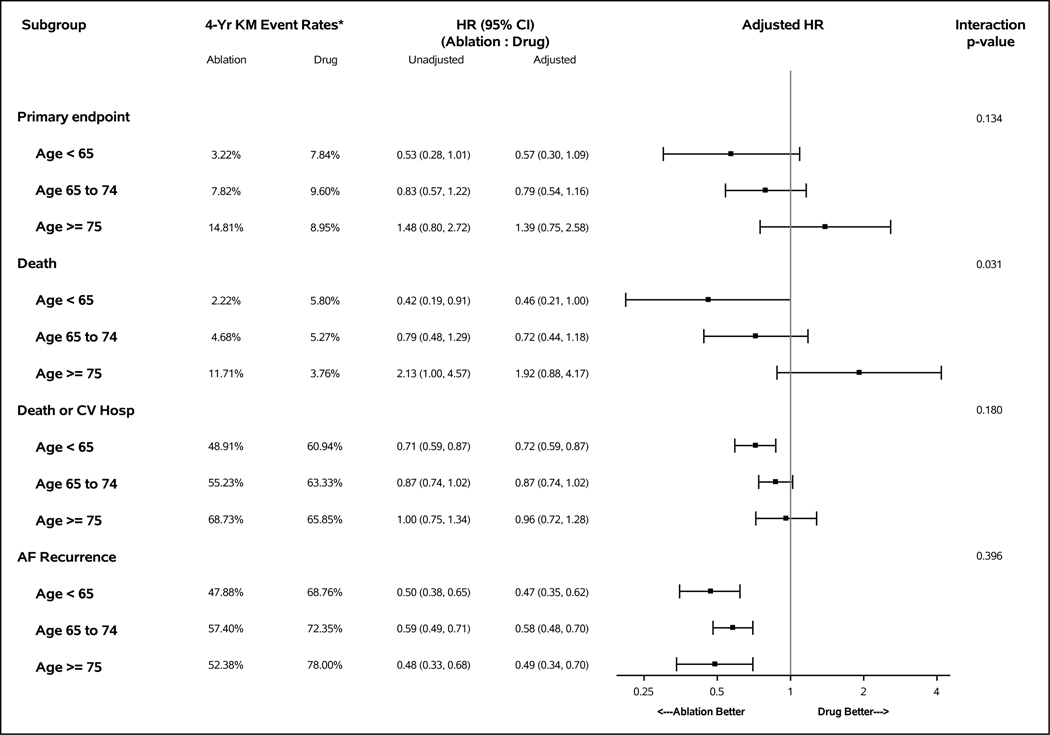
For the CABANA primary composite outcome, the ablation to drug therapy hazard ratio varied continuously with age, with the largest estimated relative benefits for ablation occurring in the youngest portion of the cohort and a HR of 1 (consistent with no difference in treatment effects) or above occurring at around age 78 (Figure 1). For every 10-year increase in age, the aHR increased (became less favorable for ablation) an average of 27% (interaction p value= 0.215). When patients were grouped into three prespecified age subgroups, the ablation to drug aHR for the primary outcome was 0.57 (95% CI 0.30–1.09) for age <65, 0.79 (95% CI 0.54–1.16) for ages 65–74, and 1.39 (95% CI 0.75–2.58) for age 75 and above (interaction p value= 0.134, Figure 2). Corresponding 4-year Kaplan-Meier primary composite event rates were (Figure 2): for age <65, ablation 3.2%, drug therapy 7.8%; for ages 65–74, ablation 7.8%, drug therapy 9.6%; for age ≥75, ablation 14.8%, drug therapy 9.0%. The Kaplan-Meier primary composite event rate age subgroup plots are shown in Supplemental Figure 1.
Figure 1:
Treatment Effect on Primary Outcome as a Function of Age as a Continuous Variable.
The relative risk reduction with catheter ablation vs drug therapy as a function of age as a continuous variable for the primary composite outcome of death, disabling stroke, serious bleeding, or cardiac arrest. The figure shows the adjusted hazard ratio as a solid black line with the 95% confidence intervals represented as the shaded area. The drug arm is used as reference group. aHR=adjusted hazard ratio, CI=confidence interval
Figure 2:
Four-Year Kaplan-Meier Event Rates and Unadjusted and Adjusted Hazard Ratios by Intention-to-Treat Age Subgroups. AF=atrial fibrillation, CI=confidence interval, CIF=cumulative incidence function, CV=cardiovascular, HR=hazard ratio, KM=Kaplan-Meier
Total Mortality
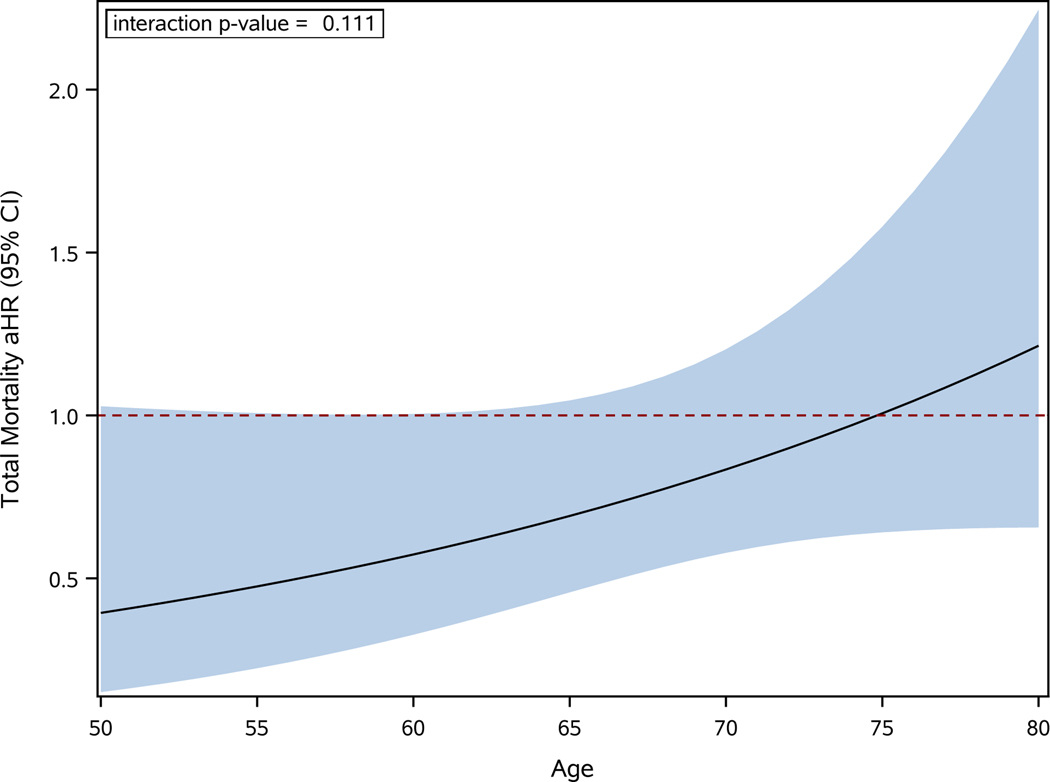
A similar pattern of results was obtained for the estimated treatment effect on total mortality alone as a function of age at enrollment (interaction p value= 0.111, Figure 3). For every 10-year increment in age, the ablation:drug therapy aHR increased an average of 46%. The ablation to drug therapy aHR was 0.46 (95% CI 0.21–1.00) for age <65, 0.72 (95% CI 0.44–1.18) for ages 65–74, and 1.92 (95% CI 0.88–4.17) for age 75 or older (interaction p value= 0.031, Figure 2). Kaplan-Meier total mortality age subgroup plots are shown in Supplemental Figure 2.
Figure 3:
Treatment Effect on Total Mortality as a Function of Age as a Continuous Variable.
The relative risk reduction with catheter ablation vs drug therapy as a function of age as a continuous variable for total mortality. The figure shows the adjusted hazard ratio as a solid black line with the 95% confidence intervals represented as the shaded area. The drug arm is used as reference group. aHR=adjusted hazard ratio, CI=confidence interval.
Patients in the ablation arm showed the expected monotonic increase in mortality as a function of increasing age (Figure 2). Four-year mortality was 2.2% in the patients <65, 4.7% in the 65–74 group, and 11.7% in the 75 and above group. In the ablation arm, no deaths occurred during the first 6 months of follow-up, regardless of age. In the drug therapy arm, the corresponding 4-year mortality estimates were 5.8%, 5.3%, and 3.8%, respectively.
This pattern of divergent relationship between age and total mortality by treatment group was also seen for the primary composite outcome (Figure 2) and for cardiovascular mortality (data not shown).
Mortality or Cardiovascular Hospitalization
For the composite outcome of death or cardiovascular hospitalization, a similar inverse age gradient of benefit of ablation was found, but the treatment effect size was substantially larger and had better precision (narrower confidence intervals) (interaction p value= 0.031, Figure 2, Supplemental Figure 3).
AF Recurrence
In the subset of patients who used the CABANA recording system, freedom from recurrent AF (AF/atrial flutter/atrial tachycardia) was consistently improved by catheter ablation relative to drug therapy across the age spectrum: aHR 0.47 (95% CI 0.35–0.62) for age <65, aHR 0.58 (95%CI 0.48–0.70) for ages 65–74, and aHR 0.49 (95% CI 0.34–0.70) for age ≥75 (Figure 2, Supplemental Figure 4).
In the ablation arm, patients age <65 had a 4-year AF recurrence rate of 48% versus 57% and 52% for patients ages 65–74 and age ≥75, respectively (Figure 2). In the drug therapy arm, the corresponding 4-year AF recurrence rates were 69%, 72%, and 78%, respectively (Figure 2).
DISCUSSION
Primary Findings
As part of the pre-specified subgroup analyses in the CABANA trial, we previously reported that the effect of ablation on the primary outcome varied by age. Specifically, ablated patients who were <65 years old showed benefit compared to drug therapy (HR 0.52, 95% CI 0.27–1.00), but the older age groups did not.7 The purpose of the present report is to examine this result in greater depth. The primary findings of this investigation confirm that treatment benefit assessed on both relative and absolute scales varied as a function of age, with older patients having progressively smaller incremental prognostic benefits from catheter ablation. Among the oldest patients (≥75 years), our analyses include a possibility of worse outcomes with ablation, although such inferences must be tempered by an appreciation of the increased uncertainty in this age range due to the smaller numbers of such patients (308 out of 2204, 14%) enrolled in the trial. Similar variations by age were seen for the primary outcome and for all-cause mortality. Although age was a prespecified subgroup in CABANA, the observed age-based variation in relative treatment effectiveness was not expected.
Age-related Variation in Outcomes with Ablation Versus Drug Therapy: Possible Explanations
Several explanations can be proposed for why the observed outcomes of catheter ablation relative to drug therapy in AF might vary as a function of age. First, older subjects might reasonably be expected to face higher short-term procedural risks, which could cancel out some longer-term benefits from more effective AF suppression. However, in CABANA, no procedural mortality occurred in any patients randomized to the ablation strategy, and significant non-fatal complications were quite infrequent in both arms. Second, older patients with AF could have more advanced, established disease, with more atrial myopathy and remodeling. The implications of this possibility are that AF in older patients would be harder to treat effectively to achieve sustained suppression of AF and thus the magnitude of any benefit associated with suppression of AF would be smaller. However, there is little evidence that older subjects in CABANA actually had more severe or advanced stage AF. No differences were seen across age subgroups in the proportion of subjects with persistent AF at baseline (Supplemental Table 1). The median time from AF onset to enrollment was not longer for older subjects. In the subset of subjects with baseline imaging data, left atrial diameter and volume did not consistently increase as a function of age (data not shown). Therefore, older age in CABANA was not clearly a marker for more advanced atrial myopathy, at least by these measures.
A third potential explanation for the age-related variation in the CABANA ablation treatment effect is the possibility that the crossover rate from drug therapy to ablation varied by age. If ablation actually lowers the risk of the primary outcome (mostly mortality), then more crossovers from the drug arm should narrow the difference in the primary event rates between the two arms and reduce the treatment effect size. Crossovers in the age ≥75 drug therapy subgroup were lower (20% crossover rate) than in the younger patients (28%−29% crossover rate), so crossover differences do not appear to be a sufficient explanation for the observed long term treatment differences.
A fourth possibility is that the observed age-related treatment effect variation is a marker for some important, but as yet unrecognized, variation in the causal relationships between AF and adverse clinical outcomes. Atrial fibrillation is well-known to be associated with increased mortality in both clinical cohorts and populations.15, 16 Whether the AF is a fully modifiable cause of that increased risk, an unmodifiable risk indicator (like age itself), or a combination of both is unsettled. In other words, it is possible that the modifiable risk associated with AF is more prevalent in younger Patients with AF while AF in older patients may be associated more often with unmodifiable risk. An example of this in a very different context can be found in the case of ventricular arrhythmias leading to sudden death. In the Sudden Death in Heart Failure (SCD-HeFT) trial, we observed that primary prevention ICD therapy was beneficial in reducing mortality in New York Heart Association (NYHA) Class II patients but not in NYHA Class III.17 The explanation appears to be that, in the context of more advanced heart failure, the proportion of arrhythmic deaths that are potentially preventable with appropriate ICD therapy is substantially less than in NYHA Class II (a competing risks problem) and that, even when the mechanism of death is a ventricular tachyarrhythmia, the ICD more often fails to restore a stable heart rhythm (possibly an effectiveness of therapy problem).18, 19 The competing risks possibility seems unlikely in CABANA given that the mortality rate we observed in the ≥75-year-old patients randomized to drug therapy was quite low, much lower in fact than the overall mortality rates from recent large observational studies of AF that included many subjects older than 75 years.20, 21 The dissociation in the oldest drug therapy arm patients between poor maintenance of sinus rhythm/AF recurrence (worse than in younger drug therapy patients and ablation arm patients at all ages) and their very low mortality favors a non-AF explanation.
A final possibility to consider, therefore, is that the absence of an age-related gradient in mortality in the drug therapy arm, and the resulting variation in the relative treatment benefits of ablation, reflects “the play of chance.” Randomization only guarantees treatment group balance, or exchangeability, in expectation, but does not guarantee that every potentially relevant characteristic is completely balanced in both arms in a specific trial cohort. When subgroups are examined, the possibilities for imbalances become greater and if these affect unmeasured factors with causal/prognostic importance, unexpected patterns in treatment-related outcomes may be created.
AF Ablation and Mortality Outcomes
The CABANA trial was originally designed to test the AF-mortality connection by hypothesizing that catheter ablation would reduce AF and thereby would reduce all-cause mortality relative to drug therapy.6 The primary mechanisms that are presumed to connect AF with mortality are large strokes and progressive heart failure. The stroke risk is mitigated primarily with effective oral anticoagulation. Whether rhythm control adds stroke protection to anticoagulation is still unsettled. Progressive heart failure now appears to be the greatest prognostic threat from AF, particularly among older patients.20 In a large cohort of older subjects with implanted cardiac devices and non-permanent AF, greater AF burden was associated with increased risk for new onset heart failure and for all-cause mortality.22 This is consistent with evolving thinking regarding the importance of atrial cardiomyopathies and inflammation in advancing AF.
The first trial-based evidence that more effective suppression of AF with catheter ablation relative to drug therapy produced a mortality benefit came from the CASTLE-AF trial, which enrolled patients with AF and systolic heart failure (NYHA class ≥II) with an ejection fraction ≤35%.23 In CASTLE-AF, the relative benefits of ablation versus medical therapy on mortality were somewhat larger in patients <65 years of age (HR 0.48, 95% CI 0.27–0.85) than in patients ≥65 (HR 0.79, 95% CI 0.50–1.23).23
Noseworthy et al. reported on 135,688 CABANA-eligible patients treated with catheter ablation or drug therapy in an administrative database of subjects with health insurance coverage and found that patients <65 years of age had the greatest relative benefit of catheter ablation on the primary CABANA outcome (HR 0.57, 95% CI 0.47–0.69), with somewhat less benefit in older patients (HR 0.77 for ages 65–74 years, 95% CI 0.66–0.90; HR 0.73 for ≥75, 95% CI 0.62–0.87).21 The discrepancies for the oldest subgroup (age ≥75) between randomized trial data (CABANA) and observational registry data together with the absence of any good causal explanation for the lack of treatment benefit for the oldest patients in CABANA suggest that older patients with AF who are otherwise appropriate candidates for ablation should not be denied the choice of ablation based on these results.
Limitations
Several important caveats should be considered in interpreting our results. First, single variable subgroup analyses of clinical trials, even when prespecified, should be interpreted with substantial caution. The CABANA trial results showed that, for the overall population ITT comparison, catheter ablation had an indeterminate effect on the primary outcome and on all-cause mortality, further reinforcing the need for caution in interpreting outcome variations in subgroup data. In CABANA, we had no reason based on experience and on the published literature available at the time our analyses plans were finalized to expect a major variation in the effects of the treatment assignment on the primary outcome of CABANA by age. Second, large efficacy trials are almost never powered for subgroup analyses, and that was clearly true in the case of CABANA. Finally, cutting the trial cohort into subgroups even when analysis is done by intention to treat creates the possibility for complex, difficult to detect biases to influence observed results. Such concerns may be particularly relevant in procedure-based trials when treatment assignment masking is infeasible. Nonetheless, subgroup analyses, when performed carefully and presented with appropriate caveats, can provide useful supplemental data in helping to understand the complex interplay between patients and the treatment being studied. If unusual patterns are found, independent replication is an important step in assessing their credibility.
Conclusions
In patients with AF enrolled in the CABANA trial, prespecified subgroup analyses showed an age-based variation in clinical outcomes for catheter ablation relative to drug therapy, such that younger patients had the largest relative and absolute clinical outcome benefits with ablation. For the oldest patients enrolled in CABANA, relative and absolute treatment estimates did not show any prognostic advantages of ablation. No differences were found by age in treatment safety or in the advantage of catheter ablation in preserving freedom from recurrent atrial arrhythmias.
Supplementary Material
Clinical Perspective.
- What is new?
- This is the first complete report of the age subgroup analysis from the CABANA trial.
- The relationships between key mortality inclusive study outcomes and atrial fibrillation recurrence outcomes and patient age are reported using age as a continuous variable.
- A clear relationship between age and select CABANA outcomes was identified whereby the relative benefit of catheter ablation compared to drug therapy was greatest for younger patients and declined with advancing age; catheter ablation was superior to drug therapy to reduce AF recurrence across age groups.
- Clinical Implications
- The evidence for a prognostic benefit from catheter ablation in AF was strongest in younger patients.
- Regardless of age, patients with symptomatic atrial fibrillation for whom a rhythm control strategy is preferred and who have drug intolerance or inefficacy, catheter ablation is a reasonable treatment strategy.
ACKNOWLEDGEMENTS
We are particularly indebted to the study coordinators at the CABANA sites and to the patients who agreed to participate in the trial.
SOURCES OF FUNDING
NIH, grants U01HL89709, U01HL089786, U01HL089907 and U01HL089645
The content of this article does not necessarily represent the views of the National Heart, Lung, and Blood Institute or the Department of Health and Human Services.
St Jude Drug Foundation and Corporation
Biosense Webster Inc,
Medtronic Inc, and
Boston Scientific Corporation
DISCLOSURES
Tristram D. Bahnson, MD reports grants from the NIH/NHLBI and Mayo Clinic during the conduct of the study; grants from St. Jude Medical, Abbott Medical, Medtronic, Biosense Webster, Johnson & Johnson, and Boston Scientific; consulting fees from Cardiofocus and Ventrix outside of the submitted work; patents pending for a catheter for intracardiac imaging and intracardiac electrogram signal analysis.
Anna Giczewska, MSc reports none.
Daniel B. Mark, MD reports grants from NIH/NHLBI, HeartFlow, Merck, and Mayo Clinic.
Andrea M. Russo, MD reports research funding from Boston Scientific, Kestra, Medilynx; consulting with compensation from Biosense Webster, Boston Scientific and Medtronic; research steering committee with compensation from Boston Scientific and Medtronic; and royalties from Up-To-Date.
Kristi H. Monahan, RN reports grants from NIH/NHLBI, St. Jude Foundation and Corporation, Biosense Webster, Inc., Medtronic, Inc., and Boston Scientific Corp., during the conduct of the study; consulting without compensation from Biosense Webster, Inc.; personal fees from Thermedical outside the submitted work.
Hussein R. Al-Khalidi, PhD reports grants from the NIH/NHLBI and Mayo.
Adam P. Silverstein, MS reports none.
Jeanne E. Poole, MD reports none.
Kerry L. Lee, PhD reports DSMB service for studies funded by Medtronic and the Cardiovascular Research Foundation.
Dr. D. Packer in the past 12 months has provided consulting services for Abbott, AtriFix, Biosense Webster, Inc., Cardio Syntax, EBAmed, Johnson & Johnson, MediaSphere Medical, LLC, MedLumics, Medtronic, NeuCures, St. Jude Medical, Siemens, Spectrum Dynamics, Centrix, and Thermedical. Dr. Packer received no personal compensation for these consulting activities, unless noted.
Dr. Packer receives research funding from the Abbott, Biosense Webster, Boston Scientific/EPT, CardioInsight, EBAmed, Medtronic, Inc, Siemens, St. Jude Medical, Inc, Thermedical, Inc., NIH, Robertson Foundation, Vital Project Funds, Inc., Mr. and Mrs. J. Michael Cook/Fund.
Mayo Clinic and Dr. Packer have a financial interest in Analyze-AVW technology that may have been used to analyze some of the heart images in this research. In accordance with the Bayh-Dole Act, this technology has been licensed to commercial entities, and both Mayo Clinic and Dr. Packer have received royalties greater than $10,000, the federal threshold for significant financial interest. In addition, Mayo Clinic holds an equity position in the company to which the AVW technology has been licensed.
Dr. Packer and Mayo Clinic jointly have equity in a privately held company, EBAmed. Royalties from Wiley & Sons, Oxford, and St. Jude Medical.
Non-standard Abbreviations and Acronyms
- AF
atrial fibrillation
- aHR
adjusted hazard ratio
- CABANA
the Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation trial
- CASTLE-AF
the Catheter Ablation vs. Standard Conventional Treatment in Patients with LV Dysfunction and AF trial
- CI
confidence interval
- HR
hazard ratio
- ITT
intention to treat
- NYHA
New York Heart Association
Footnotes
Clinical Trial Registration: ClinicalTrials.gov Identifier: NCT00911508
REFERENCES
- 1.Jais P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah R, Hocini M, Extramiana F, Sacher F, Bordachar P, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2498–505. [DOI] [PubMed] [Google Scholar]
- 2.Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A, Macle L, Daoud EG, Calkins H, Hall B, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–40. [DOI] [PubMed] [Google Scholar]
- 3.Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, Anderson JL, Lappe DL, Muhlestein JB, Nelson J, et al. Long-term clinical efficacy and risk of catheter ablation for atrial fibrillation in octogenarians. Pacing Clin Electrophysiol. 2010;33:146–52. [DOI] [PubMed] [Google Scholar]
- 4.Santangeli P, Di Biase L, Mohanty P, Burkhardt JD, Horton R, Bai R, Mohanty S, Pump A, Gibson D, Couts L, et al. Catheter ablation of atrial fibrillation in octogenarians: safety and outcomes. J Cardiovasc Electrophysiol. 2012;23:687–93. [DOI] [PubMed] [Google Scholar]
- 5.Zado E, Callans DJ, Riley M, Hutchinson M, Garcia F, Bala R, Lin D, Cooper J, Verdino R, et al. Long-term clinical efficacy and risk of catheter ablation for atrial fibrillation in the elderly. J Cardiovasc Electrophysiol. 2008;19:621–6. [DOI] [PubMed] [Google Scholar]
- 6.Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Moretz K, Poole JE, Mascette A, Rosenberg Y, Jeffries N, et al. Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial: Study rationale and design. Am Heart J. 2018;199:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: The CABANA randomized clinical trial. JAMA. 2019;321:1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, Daniels MR, Bahnson TD, Poole JE, Rosenberg Y, et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: The CABANA randomized clinical trial. JAMA. 2019;321:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poole JE, Bahnson TD, Monahan KH, Johnson G, Rostami H, Silverstein AP, Al-Khalidi HR, Rosenberg Y, Mark DB, Lee KL, et al. Recurrence of atrial fibrillation after catheter ablation or antiarrhythmic drug therapy in the CABANA trial. J Am Coll Cardiol. 2020;75:3105–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan EL and Meier P. Nonparametric estimation from incomplete observations. J Am Statistical Assoc. 1958:457–481. [Google Scholar]
- 11.Cox D. Regression models and life-tables (with discussion). J Royal Statist Soc B. 1972;34:187–220. [Google Scholar]
- 12.Fine JP and Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statistical Assoc. 1999;94:496–509. [Google Scholar]
- 13.Mark DB, Lee KL and Harrell FE Jr. Understanding the role of P values and hypothesis tests in clinical research. JAMA Cardiol. 2016;1:1048–1054. [DOI] [PubMed] [Google Scholar]
- 14.Gelman A. You need 16 times the sample size to estimate an interaction than to estimate a main effect. Statistical Modeling, Causal Inference, and Social Science. 2018;date accessed 08/26/2021. https://statmodeling.stat.columbia.edu/2018/03/15/need-16-times-sample-size-estimate-interaction-estimate-main-effect/ [Google Scholar]
- 15.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB and Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 16.Miyasaka Y, Barnes ME, Bailey KR, Cha SS, Gersh BJ, Seward JB and Tsang TS. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol. 2007;49:986–992. [DOI] [PubMed] [Google Scholar]
- 17.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. [DOI] [PubMed] [Google Scholar]
- 18.Levy WC, Hellkamp AS, Mark DB, Poole JE, Shadman R, Dardas TF, Anderson J, Johnson G, Fishbein DP, Lee KL, et al. Improving the use of primary prevention implantable cardioverter-defibrillators therapy with validated patient-centric risk estimates. JACC Clin Electrophysiol. 2018;4:1089–1102. [DOI] [PubMed] [Google Scholar]
- 19.Packer DL, Prutkin JM, Hellkamp AS, Mitchell LB, Bernstein RC, Wood F, Boehmer JP, Carlson MD, Frantz RP, McNulty SE, et al. Impact of implantable cardioverter-defibrillator, amiodarone, and placebo on the mode of death in stable patients with heart failure: analysis from the sudden cardiac death in heart failure trial. Circulation. 2009;120:2170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piccini JP, Hammill BG, Sinner MF, Hernandez AF, Walkey AJ, Benjamin EJ, Curtis LH and Heckbert SR. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J. 2014;35:250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noseworthy PA, Gersh BJ, Kent DM, Piccini JP, Packer DL, Shah ND and Yao X. Atrial fibrillation ablation in practice: assessing CABANA generalizability. Eur Heart J. 2019;40:1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinberg BA, Li Z, O’Brien E, Pritchard J, Chew DS, Bunch TJ, Mark DB, Nabutovsky Y, Greiner MA and Piccini JP. Atrial fibrillation burden and heart failure: Data from 39,710 individuals with cardiac implanted electronic devices. Heart Rhythm. 2021;18(5):709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.