Abstract
Favorable clinical outcomes of intra-articular injection of adipose-derived stromal vascular fraction (SVF) cells for knee osteoarthritis (OA) have been reported, but the effects of different doses of SVF cells have not been examined. This study aimed to compare the short-term clinical and imaging outcomes of different doses of SVF cells for knee OA treatment. This study included 60 patients with knee OA who underwent intra-articular injection of SVF cells. The follow-up period was at least 12 months. Thirty patients received an intra-articular injection of 2.5×107 SVF cells (low-dose group), and the remaining 30 patients received an intra-articular injection of 5.0×107 SVF cells (high-dose group). Clinical evaluations were performed for the Knee injury and Osteoarthritis Outcome Score (KOOS). Imaging evaluations, including the magnetic resonance imaging Osteoarthritis Knee Score (MOAKS) features (bone marrow lesions, cartilage defects, osteophytes, Hoffa’s synovitis, and effusion synovitis), were also performed. All clinical and imaging evaluations were performed preoperatively and 12 months postoperatively and compared between the groups. In demographic data, no significant differences were found between the two groups. The total score of KOOS at 12 months postoperatively was significantly more favorable than the preoperative score in the high-dose groups. Pain and symptoms subscale scores of KOOS at 12 months postoperatively were significantly better in the high-dose group than in the low-dose group. The bone marrow lesions, Hoffa’s synovitis, and effusion synovitis improved approximately 30–40% at 12 months postoperatively compared to baseline in both groups. However, there were no significant differences in imaging evaluations between the two groups. In conclusion, the pain and symptoms subscale scores of KOOS from baseline to 12 months postoperatively improved better in the high-dose group than in the low-dose group. Our findings suggest that intra-articular injection of SVF cells for knee OA is an innovative approach.
Keywords: adipose-derived stromal vascular fraction cells, stem cell, osteoarthritis, magnetic resonance imaging osteoarthritis knee score, dose-effect
Introduction
Osteoarthritis (OA) is a chronic progressive disease characterized by cartilage degeneration, osteophyte formation, bone reorganization, and loss of joint function 1 . The knee is the most frequently involved weight-bearing joint, and OA is associated with significant morbidity and healthcare expenditures 2 . Knee OA leads to changes in the cartilage, tendons, ligaments, and muscles of the joint, resulting in poor psychosocial outcomes, imbalance, increased risk of falls, and limited physical activities 3 –5 . Treatments for knee OA include conservative methods and surgical therapies and depend on patients’ age, the severity of symptoms, and the type of lesion. Conservative treatments range from nonpharmacological (e.g., weight loss, physical therapy, and exercise) and pharmacological (e.g., nonsteroidal anti-inflammatory drugs or glucocorticoid injections) to surgical treatments (e.g., arthroscopic debridement with bone marrow stimulation, osteochondral grafts, or microfracture), with total knee arthroplasty as the last option for most patients 6 –8 .
In recent years, cell therapy using adipose tissue-derived mesenchymal stem cells (ADSCs) has attracted attention as a new potential treatment for knee OA 9,10 . ADSCs, which share similar properties with bone marrow-derived mesenchymal stem cells (BMSCs), have the potential to differentiate into adipogenic, osteogenic, chondrogenic, and other mesenchymal lineages and have been widely applied in knee OA studies 11,12 . However, ADSCs require culturing, which requires a few weeks between isolation and application and is expensive.
Adipose-derived stromal vascular fraction (SVF) cells are a heterogeneous cell population that contains regenerative cells, such as ADSCs, macrophages, pericytes, fibroblasts, blood cells, vessel-forming cells including endothelial and smooth muscle cells, and their progenitors 13,14 . These heterogeneous cell populations include cells with stem cell elements, in addition to ADSCs, and are thought to have a synergistic effect with ADSCs 15 –17 . Unlike ADSCs, SVF cells can be readily obtained from liposuction samples without the need for cell separation or culturing, which renders them more cost-efficient and convenient 18,19 . Furthermore, SVF cells are considered as effective as or more effective than ADSCs 20,21 . Semon et al. reported that SVF cells are easier and safer to access, further delay, and reduce experimental autoimmune encephalomyelitis disease course and pathology compared to ADSCs in mice 20 . You et al. also reported that intracavernous injection of SVF cells or ADSCs resulted in the recovery of penile erection in a rat model of cavernous nerve injury. Furthermore, it was reported that SVF cells were superior to ADSCs in terms of changes in tissue morphometry; that is, they showed enhancement of corpus cavernosum smooth muscle cells and might allow structural support 21 . It has also been demonstrated that there was no significant difference in pain, activity of life, sports, or quality of life subscales of Knee injury and Osteoarthritis Outcome Score (KOOS) between SVF cells and ADSCs treatments for knee OA 22 . We previously reported excellent short-term clinical effects of intra-articular injection of 2.5×107 SVF cells on knee OA, but the effects of different doses of SVF cells were not examined 23 . Furthermore, promising results from several reports of intra-articular injections of SVF cells alone for knee OA were published, but their average doses varied from 1.4×107 to 5.0×107 cells 24 .
Maijub et al. investigated vasculogenesis in vivo using human adipose-derived SVF cells. Dose-dependent vasculogenesis of SVF cells was observed in vivo and suggested that reconstitution of a microcirculation through cell transplantation was dose-dependent 25 . Jo et al. also reported that high-dose intra-articular injection of ADSCs into the knee OA tended to improve function and knee pain and reduced cartilage defects than low-dose injection 9 . These reports supported that SVF cells might exert therapeutic effects on knee OA in a dose-dependent manner.
The purpose of this study was to compare the short-term clinical and radiographic outcomes of different doses of intra-articular injections of SVF cells used to treat knee OA in a single institution. We hypothesized that clinical and radiographic results would be significantly different depending on the dose of SVF cells administered. Compared to our previous study 23 , the follow-up period and clinical evaluations were the same in this study. However, our previous study used T2 mapping values for magnetic resonance imaging (MRI) evaluations, whereas this study used MRI Osteoarthritis Knee Score (MOAKS). Our previous research and the low-dose group in this study had the exact dosage in terms of dosage.
Materials and Methods
Patient Selection
This study included 60 patients with knee OA who received unilateral treatment with intra-articular injection of SVF cells between February 2017 and January 2018. The minimum clinical and radiographic follow-up was 12 months (mean, 17.2 months; range, 12–21 months). The knee OA grade was evaluated using the Kellgren-Lawrence classification, and patients with grade I to IV OA participated in this study.
The inclusion criteria were as follows: (a) patients of any sex or age diagnosed with knee OA; (b) patients with any OA grade; (c) complaints of substantial knee pain and loss of function; (d) ineffectiveness of conservative treatments including rehabilitation, medication, and intra-articular injection of hyaluronic acid or steroids; and (e) written informed consent. The exclusion criteria were as follows: (a) severe bony defects on preoperative radiographs; (b) previous knee injury requiring operation; (c) active or previous knee joint infection; and (d) history of serious, related conditions, including systemic inflammatory diseases and vascular changes. The envelope method was used to prospectively quasi-randomize the patients to undergo treatment with different doses of intra-articular injection of SVF cells. Thirty patients (19 women and 11 men) received an intra-articular injection of 2.5×107 SVF cells (low-dose group), and 30 patients (24 women and 6 men) received an intra-articular injection of 5.0×107 SVF cells (high-dose group). After the treatment, the patients were asked to perform daily home exercises by themselves according to a standardized rehabilitation protocol, in addition to rehabilitation with a physical therapist.
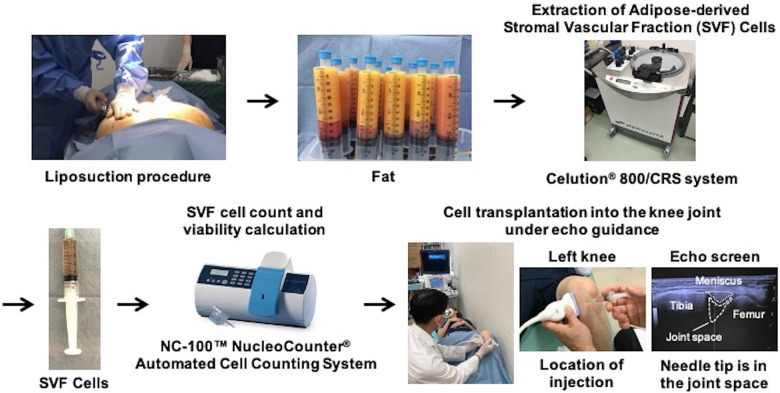
Treatment Procedures (Fig. 1)
Figure 1.
Schema of treatment procedures.
Treatment procedures were performed in the same way as in a previous study 23 . SVF cells were extracted from the patient’s abdominal or breech subcutaneous fat using the Celution® 800/CRS system (Cytori Therapeutics Inc., San Diego, CA, USA). This system consists of two parts: one for tissue washing and digestion and one for cell concentration. All patients underwent a liposuction procedure under general anesthesia to obtain 290–440 mL of adipose tissue. The extracted tissue was processed using the Celution® 800/CRS System according to the manufacturer’s instructions. Briefly, the tissue was washed to remove blood and debris, and Celase® GMP, a mixture of highly purified collagenase and neutral protease enzyme, was then added. The tissue was incubated at 37°C for 20 minutes with continuous mixing to assist in digestion. After digestion of the tissue, SVF cells were concentrated by centrifugation and washed to remove the Celase® reagent. The SVF cells were then extracted and counted to prepare the specified dose in 5 mL of lactated Ringer’s solution. The entire procedure was performed aseptically using clinical-grade solutions, such as saline and lactated Ringer’s solutions and the single-use Celution™ consumable sets. The SVF cell count and viability calculation were performed at each investigational site using the NC-100™ NucleoCounter® Automated Cell Counting System (Chemometec, Allerod, Denmark).
The mean volume of liposuction, number of purified SVF cells, and viability of SVF cells are listed in Table 1. The intra-articular injection of 2.5×107 or 5.0×107 SVF cells to each patient was administered depending on the number of purified SVF cells. SVF cells in each group were dissolved in 5 mL of lactated Ringer’s solution. The intra-articular injection was performed from the anterior medial side of the knee joint, with an echo-guided needle tip in the joint space (Fig. 1). Pain from the injection itself was not a problem. If the joint fluid level was excessive, aspiration was performed prior to cell transplantation. Cell transplantation into the knee joint was performed without anesthetic and under echo guidance. Patients were required to rest for one hour after the injection at the clinic. The exercise was also restricted for one week following the injection.
Table 1.
Stromal Vascular Fraction cell Characteristics; Number of Purified SVF Cells and SVF Cell Viability.
| Characteristics | Low-dose group | High-dose group | P value |
|---|---|---|---|
| Volume of liposuction; ml | 327.3 ± 51.2 | 352.8 ± 29.3 | 0.02※ |
| Number of purified SVF cells | 4.2 ± 1.8×107 | 8.5 ± 3.1×107 | <0.01※ |
| SVF cell viability; % | 90.0 ± 2.5 | 91.3 ± 3.1 | 0.08 |
SVF, Stromal vascular fraction.
Mean value ± Standard deviation.
※Statistically significant.
Clinical Evaluations and Scores
Clinical evaluation and scoring were performed at 1, 3, 6, and 12 months after treatment with SVF cells. Clinical evaluations included knee range of motion and muscle force of knee extension and flexion using a hand-held dynamometer. Clinical scores included the visual analog scale (VAS) for pain (0-100) and KOOS. To measure the muscle force of knee extension and flexion, patients were in a prone position with their knees at 45° flexion. A hand-held dynamometer was placed at the center of the lower leg, and patients were instructed to bend the knee and hold for 3 seconds to measure hamstring strength and to straighten the knee and hold for 3 seconds to measure quadriceps strength. The examiner added resistance to maintain the knee at 45° and measured the displayed value as muscle strength. These tests were performed three times, and the average value was recorded. Clinical evaluations were performed in a blinded manner by an independent, experienced physiotherapist.
Imaging Evaluations
Imaging evaluations included the hip-knee-ankle (HKA) angle assessed via radiography 26 and the MOAKS using a 1.5-T MRI unit (Sigma Excite HDx; GE Healthcare, Waukesha, WI, USA). MOAKS is a semi-quantitative tool 27 ; therefore, changes in MRI features are an important tool for monitoring knee OA 28 . The HKA angle was assessed at 1, 3, 6, and 12 months after treatment with SVF cells. The main MOAKS features (bone marrow lesions (BMLs), cartilage defects, osteophytes, Hoffa’s synovitis, and effusion synovitis) were assessed at 12 months after treatment with SVF cells. Scores of each evaluation item at 12 months postoperatively were compared with those at baseline and classified into three categories: improvement, no change, and progression. BMLs, cartilage defects, and osteophytes were evaluated in each of the three anatomical areas: the medial tibiofemoral (TF) joint, lateral TF joint, and patellofemoral (PF) joint. MRI evaluations were mainly performed twice in a blinded manner by an independent orthopaedic surgeon (SS) and performed once by another orthopaedic surgeon ([TM]1) with 15 years of experience analyzing MRI features of knee OA. The intra-rater and inter-rater reliabilities (Cohen’s Kappa) for reading baseline prevalence of the BMLs, cartilage, osteophytes, Hoffa’s synovitis, and effusion synovitis were calculated (Table S1). These intra-rater and inter-rater reliabilities were substantial agreement (0.61–0.80) or almost perfect agreement (0.81–1.0) 29 .
Statistical Analysis
All values are expressed as mean ± standard deviation, and error bars in the figure of clinical evaluation results show standard errors. Data analyses were performed using IBM SPSS statistical software (version 21; IBM Corp., Armonk, NY, USA). The Shapiro-Wilk test was used to analyze normally distributed data. Clinical evaluations and the HKA angle were compared among the five time periods using repeated measures analysis of variance in the low-dose or high-dose group and between the two groups using an unpaired t-test. Furthermore, we evaluated the main MOAKS features (BMLs, cartilage defects, osteophytes, Hoffa’s synovitis, and effusion synovitis) preoperatively and at 12 months postoperatively and investigated the number of patients who showed improvement, no change, and progression in each group using Pearson’s chi-square test. P < 0.05 was considered statistically significant. A statistical power analysis using G*Power 3 was performed before the study to compare the two groups with an unpaired t-test. This analysis indicated that a sample size of 26 was needed for a power of 0.8 based on a significance level of <0.05 and an assumed effect size of 0.80 30 .
Results
Demographic Data and Adverse Events
No significant differences were found in the demographic data between the two groups (Table 2). Neither death nor life-threatening adverse events were observed during the 12-month follow-up after cell therapy in either group. There were no moderate adverse events such as infections during follow-up either. However, mild adverse events such as swelling and pain of the knee were observed in 10.0% and 6.7% of the low-dose and high-dose groups, respectively. Symptoms disappeared within 3 days in all cases.
Table 2.
Patient Characteristics.
| Characteristics | Low-dose group | High-dose group | P value | |
|---|---|---|---|---|
| Sex (M/F); n (%) | 19/11 (63%/37%) | 24/6 (80%/20%) | 0.15 | |
| Age; yrs | 69.0 ± 8.3 | 70.7 ± 5.3 | 0.14 | |
| Body mass index; kg/m2 | 24.9 ± 3.2 | 25.8 ± 2.6 | 0.24 | |
| Duration of follow-up; months | 16.4 ± 3.8 | 15.3 ± 2.3 | 0.18 | |
| Hip-knee-ankle angle at baseline; degree | 6.5 ± 7.4 | 9.1 ± 5.9 | 0.14 | |
| Knee extension angle; degree | -8.2 ± 7.1 | -6.5 ± 6.0 | 0.33 | |
| Knee flexion angle; degree | 127.7 ± 16.0 | 134.3 ± 11.7 | 0.07 | |
| Kellgren-Lawrence classification; n (%) | I | 0 (0%) | 0 (0%) | 0.70 |
| II | 4 (13.3%) | 5 (16.7%) | ||
| III | 15 (50.0%) | 17 (56.6%) | ||
| IV | 11 (36.7%) | 8 (26.7%) | ||
Mean value ± Standard deviation
Clinical Evaluations
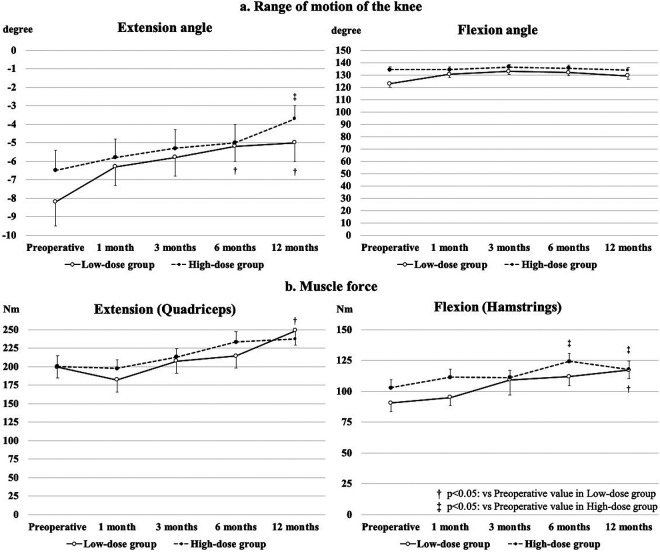
The improvements in the mean extension angle from baseline to 6 and 12 months in the low-dose group and from baseline to 12 months in the high-dose group were statistically significant. The mean flexion angle did not significantly improve from baseline in either group (Fig. 2a). The mean muscle force of knee extension was significantly higher at 12 months postoperatively than preoperatively in the low-dose group. The improvements in the mean muscle force of knee flexion from baseline to 6 months in the low-dose group and from baseline to 6 and 12 months in the high-dose group were statistically significant (Fig. 2b). However, there was no significant difference in knee range of motion and muscle force of knee between the two groups at any time point (Fig. 2).
Figure 2.
Clinical evaluation results of range of motion and muscle force.
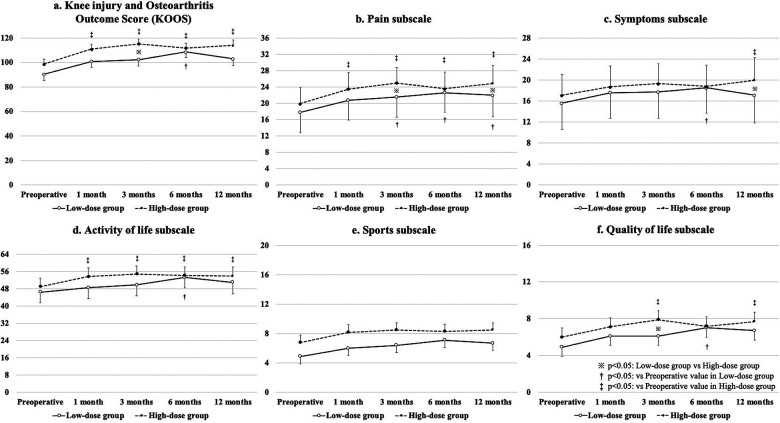
The mean VAS scores from baseline to 12 months postoperatively were significantly improved in both groups, without a significant difference between the groups (Fig. 3). A significant improvement in the total scores of KOOS from baseline to 12 months postoperatively was found in the high-dose group (Fig. 4a). There was a significant difference in the pain subscale scores from baseline to 12 months postoperatively in both groups, and the score at 12 months postoperatively in the high-dose group was significantly better than that in the low-dose group (Fig. 4b). The symptoms subscale score from baseline to 12 months postoperatively was significantly improved in the high-dose group, and it was also significantly better in the high-dose group than in the low-dose group (Fig. 4c). In addition, the activity of life subscale and quality of life subscale scores from baseline to 12 months postoperatively were significantly improved in the high-dose group (Fig. 4d and f).
Figure 3.
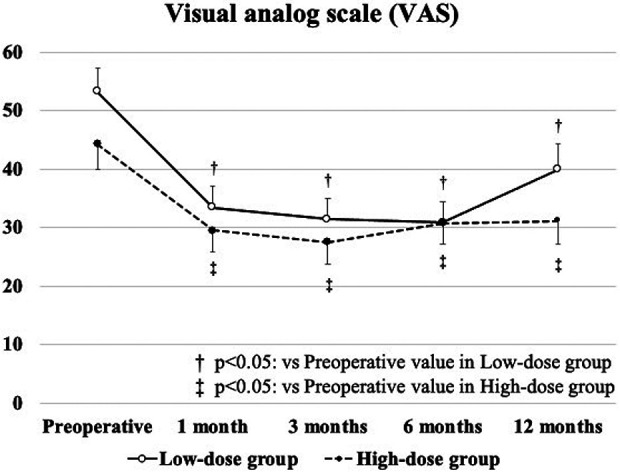
Results of visual analog scale for pain.
Figure 4.
Results of Knee injury and Osteoarthritis Outcome Score.
Imaging Evaluations
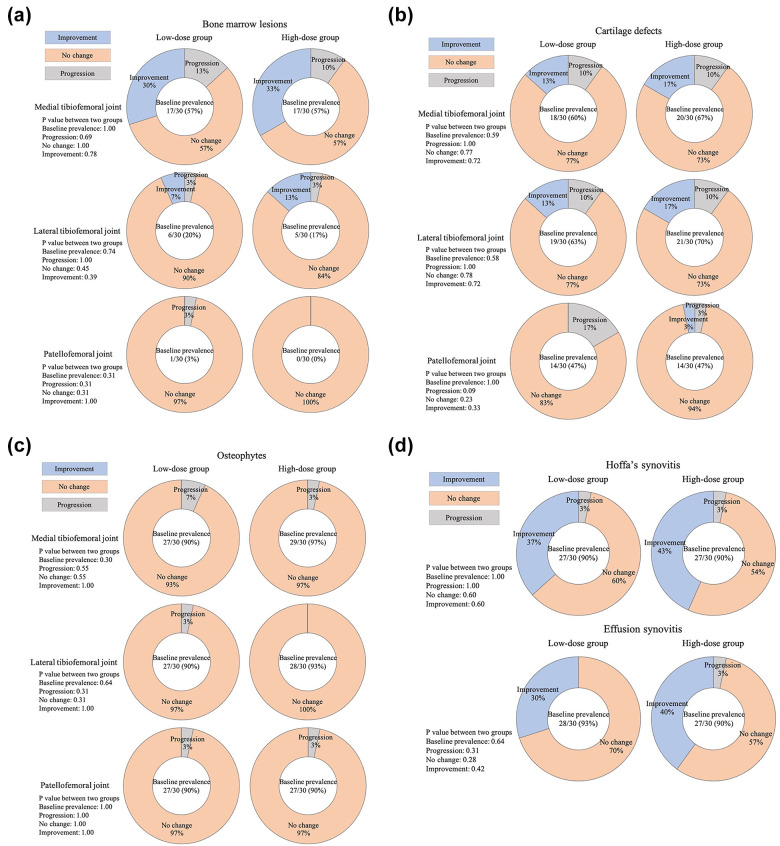
There was no significant improvement in the HKA angle from baseline to any time point in either group, and there was no significant difference in the HKA angle between the two groups at any time point (Table 3). The improvement rate of BMLs in the medial TF joint from baseline to 12 months postoperatively was 30.0% in the low-dose group and 33.3% in the high-dose group (Fig. 5a and 6a and Tables S2a and S3a). The improvement rates of cartilage defects in both the medial and lateral TF joints from baseline to 12 months postoperatively were 13.3% in the low-dose group and 16.7% in the high-dose group (Fig. 5b and 6b and Tables S2b and S3b). No patients showed improvement in the osteophytes subscale from baseline to 12 months (Fig. 5c and Tables S2c and S3c). The improvement rates of Hoffa’s synovitis from baseline to 12 months postoperatively were 36.7% in the low-dose group and 43.3% in the high-dose group (Fig. 5d and 6c and Tables S2d and S3d). Furthermore, the improvement rates of effusion synovitis from baseline to 12 months postoperatively were 30.0% in the low-dose group and 40.0% in the high-dose group (Fig. 5d and 6c Tables S2d and S3d). There was no significant difference in all imaging evaluations between the two groups.
Table 3.
Imaging Evaluation Results of Hip-Knee-Ankle Angle.
| Hip-knee-ankle angle | |||||
|---|---|---|---|---|---|
| Low-dose group | P value | High-dose group | P value | P value between two groups | |
| Preoperative | 6.5 ± 7.4 | 9.1 ± 5.9 | 0.14 | ||
| 1 month | 6.8 ± 6.8 | 0.86 | 8.6 ± 5.7 | 0.71 | 0.28 |
| 3 months | 6.7 ± 7.0 | 0.89 | 9.0 ± 5.6 | 0.97 | 0.16 |
| 6 months | 6.6 ± 7.1 | 0.95 | 8.8 ± 5.3 | 0.86 | 0.17 |
| 12 months | 6.8 ± 7.2 | 0.86 | 9.5 ± 5.5 | 0.77 | 0.10 |
Figure 5.
(a) Imaging evaluation results of bone marrow lesions by magnetic resonance imaging Osteoarthritis Knee Score system. (b) Imaging evaluation results of cartilage defects by magnetic resonance imaging Osteoarthritis Knee Score system. (c) Imaging evaluation results of osteophytes by magnetic resonance imaging Osteoarthritis Knee Score system. (d) Imaging evaluation results of Hoffa’s synovitis and effusion synovitis by magnetic resonance imaging Osteoarthritis Knee Score system.
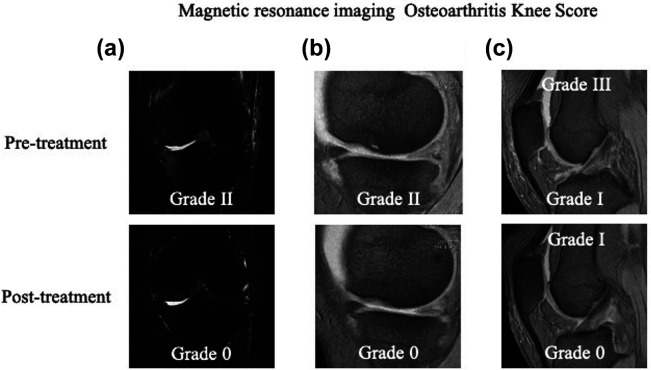
Figure 6.
Representative cases of imaging evaluations. (a) Bone marrow lesion improved from Grade II to 0 before and after treatment. (b) Cartilage defects improved from Grade II to Grade 0 before and after treatment. (c) Hoffa’s synovitis improved from Grade I to 0 before and after treatment, and effusion synovitis also improved from Grade III to I before and after treatment.
Discussion
Clinical and radiographic results of the patients in this study improved from baseline to 12 months postoperatively in both the low-dose and high-dose groups. There was no significant difference in imaging evaluations between the two groups, but clinical evaluations, especially KOOS, were better in the high-dose group. The findings partially support our hypothesis.
Favorable clinical outcomes of ADSC cell therapy for knee OA have been reported 10,31,32 . ADSCs have properties similar to those of BMSCs but require several weeks to isolate, culture, and amplify in specialized laboratories. In contrast, SVF cells are not cultured and can be harvested, prepared, and re-injected in a single procedure. Similar to BMSCs, SVF cells include cells with multilineage potential, can be easily isolated in large quantities from autologous adipose tissues, and can be used without culturing 18,19 . Several studies have reported the use of autologous SVF cells alone for the treatment of knee OA 23,33,34 . For example, Michialek et al. showed that the intra-articular injection of SVF cells was a safe and clinically effective strategy for improving the quality of life; however, detailed clinical evaluations were not conducted in their clinical trial 33 . Fodor et al. found that autologous SVF cells were safe and were a new potential treatment to reduce pain in patients with knee OA; however, their sample size was small 34 . In our previous study, we found that the short-term clinical effects of intra-articular injection of 2.5×107 SVF cells on knee OA were excellent, and our study had an effective sample size. However, the effects of different doses of SVF cells were not examined 23 . Furthermore, promising results from several studies of intra-articular injections of SVF cells alone for knee OA were demonstrated, but their average doses varied from 1.4×107 to 5.0×107 cells 24 . Thus, we compared the short-term clinical and radiographic outcomes of different doses of intra-articular injections of SVF cells used to treat knee OA in a single institution.
Recently, new therapies other than ADSCs and SVF cells have become widespread as potential treatment options for knee OA. Anz et al. reported that WOMAC scores improved from 35.3 to 19.4 points 12 months after administration of autologous bone marrow aspirate concentrate for knee OA 35 . Bansal et al. showed that administration of autologous platelet-rich plasma for knee OA significantly improved WOMAC scores during 12 months of follow-ups 36 . Additionally, Kon et al. found that WOMAC pain scores improved from 11.5 to 4.3 points, and the VAS score improved from 55 to 26 points after 1 year of administration of an autologous protein solution for knee OA 37 . However, presently, these new therapies cannot be compared with SVF cell therapy. Therefore, further studies need to be performed.
The mean extension angle from baseline to 12 months in both groups was significantly improved as the muscle force of knee extension gradually improved postoperatively. However, the mean flexion angle did not significantly improve from baseline in either group. The mean total VAS scores at 1, 3, 6, and 12 months postoperatively were significantly better than the preoperative VAS score. Notably, Luc-Harkey et al. reported that greater quadriceps and hamstring muscle strength was associated with less pain 38 . Therefore, the improvement in knee pain may influence extension muscle strength more than flexion muscle strength.
In this study, the significant improvement in the total scores of KOOS from baseline to 12 months postoperatively was found in the high-dose group. The pain subscale, symptoms subscale, activity of life subscale, and quality of life scores from baseline to 12 months postoperatively were also significantly improved in the high-dose group. Meanwhile, these scores were significantly improved from baseline to 6 months postoperatively in the low-dose group, but these improvements were decreased from 6 to 12 months, indicating the possibility to re-inject SVF cells into the knee joint from 6 to 12 months around the first intra-articular injection in the low-dose group. Notably, Minonzio et al. reported that freeze adipose-derived SVF cells maintained their growth and differential potential 39 ; Kaita et al. also showed that frozen SVF cells comprised a heterogeneous cell population, including stem cells and leukocytes, and expressed high levels of mesenchymal stem cell markers, similar to fresh SFV cells 40 . These results indicate that treatment options for knee OA can include administration of initial doses of 2.5×107 SVF cells and cryopreservation of the remaining cells for subsequent re-injection. Further studies are needed to investigate whether it is better to inject single high-dose SVF cells or two low-dose SVF cells with an interval.
In comparing the low-dose and high-dose groups, the pain and symptom subscale scores in KOOS of the high-dose group were significantly better than those of the low-dose group at 12 months postoperatively. SVF cells are a heterogeneous cell population containing a variety of regenerative cells, including cells with stem cell elements in addition to ADSCs, which may have synergistic effects with ADSCs 15 –17 . In addition, SVF cells contain a significant proportion of cells involved in the immunoregulatory function and cells of hematopoietic origin involved in vascular remodeling 41 . Macrophages present in rodent adipose tissue constitute 20% of SVF cells, 70% of which are positive for the anti-inflammatory M2 macrophage marker CD301 42,43 . This anti-inflammatory effect of M2 macrophages was thought to improve pain and symptom subscale scores. Furthermore, Jo et al. reported that treatment of knee OA using the injection of ADSCs was also dose-dependent 9 . The dose-dependent effect of SVF cells on knee OA in the current study was thought to be due to the enhanced anti-inflammatory effect of heterogeneous SVF cells by increasing the dose of SVF cells, the dose-dependent effect of ADSCs, and the more substantial synergistic effect of SVF cells and ADSCs. As a result, the high-dose group’s pain and symptom subscale scores were thought to be significantly better than those of the low-dose group at 12 months postoperatively.
A larger baseline BML size is associated with greater baseline knee pain and structural damage as well as disease progression, and baseline BML size may be particularly important when assessing the associations between changes in BML size and disease progression 44,45 . In the present study, approximately 30% of BMLs located in the medial TF joint improved at 12 months postoperatively compared to baseline in both the low-dose and high-dose groups. Interestingly, mediating cytokines and their signaling pathways are upregulated in OA joints and most often have catabolic effects; these cytokines include interleukin-1 beta and tumor necrosis factor-alpha, and their levels are elevated in the synovial fluid, synovium, cartilage, and subchondral bone of OA patients. The synergistic effects of these cytokines on signaling pathways result in an increase in inflammation and cartilage degradation during the OA process 46 . Approximately 30–40% of Hoffa’s synovitis and effusion synovitis improved at 12 months postoperatively compared to baseline in both the low-dose and high-dose groups in this study. As mentioned above, SVF cells obtained from adipose tissue contain a large number of M2 macrophages 42,43 . The anti-inflammatory effect of M2 macrophages is thought to contribute to the improvement of BMLs, Hoffa’s synovitis, and effusion synovitis in knee OA, thereby resulting in postoperative functional and pain improvements.
On the other hand, the cartilage defect’s improvement rate was lower than the improvement rate of BMLs, Hoffa’s synovitis, and effusion synovitis. This fact suggests that SVF cells may have had little effect on structural support. You et al. previously reported that intracavernous injection of SVF cells might enhance corpus cavernosum smooth muscle cells and enable structural support 21 . The difference between these results could be attributed to that SVF cells were injected directly into the subject in the previous report, whereas in our study, SVF cells were injected indirectly into the knee joint if the cartilage defects were set as the subject. The scaffold may be required when treating cartilage defects, primarily with SVF cells 47 .
The patients followed a standardized rehabilitation protocol after the procedure that required them to perform daily exercises at home by themselves in addition to being treated by a physical therapist. Notably, Sun et al. demonstrated that moderate physical exercise decreased the risk of severe osteoarthritis of the knee, and exercise had a protective effect against cartilage degradation 48 ; Hawker et al. also reported that an exercise program that combined endurance and strength training increased functional capacity and reduced pain in patients with osteoarthritis 49 . Indeed, rehabilitation has been recommended in addition to regenerative medicine 50 . Therefore, rehabilitation might have contributed to the improvement in the clinical score and the effect of SVF cell treatment in the current study.
The present study has some limitations. First, this study only compared two groups and did not include a control group that underwent other intra-articular interventions. Furthermore, the estimated sample size was calculated with a large effect size. The association between SVF cells and other intra-articular interventions should be investigated in future studies with a larger sample size. Second, the clinical and imaging evaluations were only performed preoperatively and at 1, 3, 6, and 12 months after the intra-articular injection of SVF cells. A long-term investigation of the clinical and structural changes is warranted. Third, some clinical evaluations are subjective data, and MOAKS is also a semi-quantitative tool. Objective data should be used to evaluate clinical outcomes and imaging evaluation in future research. On the other hand, it is also true that there is currently no objective quantitative evaluation method for image analysis in knee OA. In recent years, several studies have attempted to evaluate knee cartilage quantitatively. Schaefer et al. demonstrated and validated a novel modified version of the LocalArea Cartilage Segmentation method to provide quantitative measurements of cartilage volume on 2D MRI images 51 . Wei et al. also concluded that the quantitative susceptibility mapping allows in vivo imaging and quantification of the magnetic susceptibility of knee OA has good scan-rescan reproducibility 52 . In addition, Hou et al. developed a new software-based automatic cartilage segmentation method, investigated its reproducibility, and attempted to evaluate cartilage volume by MRI in knee OA quantitatively 53 . However, there is still no system to assess items such as BMLs and synovitis quantitatively, so it is desirable to establish an objective system to evaluate knee OA comprehensively. Fourth, we did not evaluate the relationship between clinical and imaging results. Fifth, the minimum dose required for a sufficient effect was not examined, and this study focused on patients who underwent a single injection of SVF cells. In order to determine the optimal treatment for OA, multiple injections and a single injection with different numbers of SVF cells should be evaluated in these patients.
In conclusion, the extension angle, flexion muscle force, VAS, and pain subscale score of KOOS from baseline to 12 months postoperatively with knee OA were well improved in both the low-dose and high-dose groups of SVF cell injections, with better pain and symptoms subscale scores in KOOS in the high-dose group. Our findings suggest that intra-articular injection of SVF cells into the knee joint can be considered an innovative approach to treat patients with knee OA.
Supplemental Material
Supplemental Material, sj-docx-1-cll-10.1177_09636897211067454 for Comparison of Clinical and Imaging Outcomes of Different Doses of Adipose-Derived Stromal Vascular Fraction Cell Treatment for Knee Osteoarthritis by Masanori Tsubosaka, Tomoyuki Matsumoto, Satoshi Sobajima, Takehiko Matsushita, Hideki Iwaguro and Ryosuke Kuroda in Cell Transplantation
Acknowledgments
We wish to thank Hitoshi Yamauchi and Yusuke Harada for collecting data.
Footnotes
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions: All authors have made substantial contributions to (1) the conception and design of the study, acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; and (3) final approval of the version to be submitted.
- Conception and design of the study: [TM]1, SS, and RK.
- Analysis and interpretation of the data: all authors; Drafting of the article: [TM]2, HI, and MT.
- Final approval: all authors.
Ethical Approval: The treatment using adipose-derived SVF cells for knee OA was approved by the Independent administrative institution advanced medical promotion organization specified regenerative medicine committee Nagoya under review number 145. Ethical approval to report this study was obtained from the Ethics Committee of Sobajima Clinic (identification number: SC002-1 M, 2 M) and Kobe University Graduate School of Medicine (identification number: 170181).
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the the Ethics Committee of Sobajima Clinic (identification number: SC002-1 M, 2 M) and Kobe University Graduate School of Medicine (identification number: 170181) approved protocols.
Statement of Informed Consent: Verbal informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Satoshi Sobajima  https://orcid.org/0000-0001-6702-1242
https://orcid.org/0000-0001-6702-1242
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis - an untreatable disease? Nat Rev Drug Discov. 2005;4(4):331–344. [DOI] [PubMed] [Google Scholar]
- 2. Leardini G, Salaffi F, Caporali R, Canesi B, Rovati L, Montanelli R. Direct and indirect costs of osteoarthritis of the knee. Clin Exp Rheumatol. 2004;22(6):699–706. [PubMed] [Google Scholar]
- 3. Mündermann A, Nigg BM, Humble RN, Stefanyshyn DJ. Orthotic comfort is related to kinematics, kinetics, and EMG in recreational runners. Med Sci Sports Exerc. 2003;35(10):1710–1719. [DOI] [PubMed] [Google Scholar]
- 4. Messier SP, Loeser RF, Hoover JL, Semble EL, Wise CM. Osteoarthritis of the knee: effects on gait, strength, and flexibility. Arch Phys Med Rehabil. 1992;73(1):29–36. [PubMed] [Google Scholar]
- 5. Nguyen U-SDT, Felson DT, Niu J, White DK, Segal NA, Lewis CE, Rasmussen M, Nevitt MC. The impact of knee instability with and without buckling on balance confidence, fear of falling and physical function: the Multicenter Osteoarthritis Study. Osteoarthr Cartil. 2014;22(4):527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthr Cartil. 2010;18(4):476–499. [DOI] [PubMed] [Google Scholar]
- 7. Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, Kraus VB, Lohmander LS, Abbott JH, Bhandari M, Blanco FJ, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. 2019;27(11):1578–1589. [DOI] [PubMed] [Google Scholar]
- 8. Desando G, Bartolotti I, Vannini F, Cavallo C, Castagnini F, Buda R, Giannini S, Mosca M, Mariani E, Grigolo B. Repair potential of matrix-induced bone marrow aspirate concentrate and matrix-induced autologous chondrocyte implantation for talar osteochondral repair: patterns of some catabolic, inflammatory, and pain mediators. Cartilage. 2017;8(1):50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, Ra JC, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254–1266. [DOI] [PubMed] [Google Scholar]
- 10. Pers Y-M, Rackwitz L, Ferreira R, Pullig O, Delfour C, Barry F, Sensebe L, Casteilla L, Fleury S, Bourin P, Noël D, et al. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase i dose-escalation trial. Stem Cells Transl Med. 2016;5(7):847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5(5):362–369. [DOI] [PubMed] [Google Scholar]
- 12. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. [DOI] [PubMed] [Google Scholar]
- 13. Han J, Koh YJ, Moon HR, Ryoo HG, Cho C-H, Kim I, Koh GY. Adipose tissue is an extramedullary reservoir for functional hematopoietic stem and progenitor cells. Blood. 2010;115(5):957–964. [DOI] [PubMed] [Google Scholar]
- 14. McIntosh K, Zvonic S, Garrett S, Mitchell JB, Floyd ZE, Hammill L, Kloster A, Di Halvorsen Y, Ting JP, Storms RW, Goh B, et al. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells. 2006;24(5):1246–1253. [DOI] [PubMed] [Google Scholar]
- 15. Lin K, Matsubara Y, Masuda Y, Togashi K, Ohno T, Tamura T, Toyoshima Y, Sugimachi K, Toyoda M, Marc H, Douglas A. Characterization of adipose tissue-derived cells isolated with the CelutionTM system. Cytotherapy. 2008;10(4):417–426. [DOI] [PubMed] [Google Scholar]
- 16. Zimmerlin L, Donnenberg VS, Rubin JP, Donnenberg AD. Mesenchymal markers on human adipose stem/progenitor cells. Cytom Part A. 2013;83(1):134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Traktuev DO, Prater DN, Merfeld-Clauss S, Sanjeevaiah AR, Saadatzadeh MR, Murphy M, Johnstone BH, Ingram DA, March KL. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ Res. 2009;104(12):1410–1420. [DOI] [PubMed] [Google Scholar]
- 18. De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174(3):101–109. [DOI] [PubMed] [Google Scholar]
- 19. Feng Z, Ting J, Alfonso Z, Strem BM, Fraser JK, Rutenberg J, Kuo H-C, Pinkernell K. Fresh and cryopreserved, uncultured adipose tissue-derived stem and regenerative cells ameliorate ischemia-reperfusion-induced acute kidney injury. Nephrol Dial Transplant. 2010;25(12):3874–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Semon JA, Zhang X, Pandey AC, Alandete SM, Maness C, Zhang S, Scruggs BA, Strong AL, Sharkey SA, Beuttler MM, Gimble JM, et al. Administration of murine stromal vascular fraction ameliorates chronic experimental autoimmune encephalomyelitis. Stem Cells Transl. Med. 2013;2(10):789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. You D, Jang MJ, Kim BH, Song G, Lee C, Suh N, Jeong IG, Ahn TY, Kim C-S. Comparative study of autologous stromal vascular fraction and adipose-derived stem cells for erectile function recovery in a rat model of cavernous nerve injury. Stem Cells Transl Med. 2015;4(4):351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yokota N, Hattori M, Ohtsuru T, Otsuji M, Lyman S, Shimomura K, Nakamura N. Comparative clinical outcomes after intra-articular injection with adipose-derived cultured stem cells or Noncultured stromal vascular fraction for the treatment of knee osteoarthritis. Am J Sports Med. 2019;47(11):2577–2583. [DOI] [PubMed] [Google Scholar]
- 23. Tsubosaka M, Matsumoto T, Sobajima S, Matsushita T, Iwaguro H, Kuroda R. The influence of adipose-derived stromal vascular fraction cells on the treatment of knee osteoarthritis. BMC Musculoskelet Disord. 2020;21(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shanmugasundaram S, Vaish A, Chavada V, Murrell WD, Vaishya R. Assessment of safety and efficacy of intra-articular injection of stromal vascular fraction for the treatment of knee osteoarthritis—a systematic review. Int Orthop. 2021;45(3):615–625. [DOI] [PubMed] [Google Scholar]
- 25. Maijub JG, Boyd NL, Dale JR, Hoying JB, Morris ME, Williams SK. Concentration-dependent vascularization of adipose stromal vascular fraction cells. Cell Transplant. 2015;24(10):2029–2039. [DOI] [PubMed] [Google Scholar]
- 26. Cooke D, Scudamore A, Li J, Wyss U, Bryant T, Costigan P. Axial lower-limb alignment: comparison of knee geometry in normal volunteers and osteoarthritis patients. Osteoarthr Cartil. 1997;5(1):39–47. [DOI] [PubMed] [Google Scholar]
- 27. Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, Roemer FW. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthr Cartil. 2011;19(8):990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Runhaar J, van Middelkoop M, Reijman M, Willemsen S, Oei EH, Vroegindeweij D, van Osch G, Koes B, Bierma-Zeinstra SMA. Prevention of knee osteoarthritis in overweight females: the first preventive randomized controlled trial in osteoarthritis. Am J Med. 2015;128(8):888–895.e4. [DOI] [PubMed] [Google Scholar]
- 29. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 30. Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. [DOI] [PubMed] [Google Scholar]
- 31. Russo A, Condello V, Madonna V, Guerriero M, Zorzi C. Autologous and micro-fragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis. J Exp Orthop. 2017;4(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hudetz D, Borić I, Rod E, Jeleč Ž, Radić A, Vrdoljak T, Skelin A, Lauc G, Trbojević-Akmačić I, Plečko M, Polašek O, et al. The effect of intra-articular injection of autologous microfragmented fat tissue on proteoglycan synthesis in patients with knee osteoarthritis. Genes (Basel). 2017;8(10):270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Michalek J, Moster R, Lukac L, Proefrock K, Petrasovic M, Rybar J, Capkova M, Chaloupka A, Darinskas A, Michalek JS, Kristek J, et al. WITHDRAWN: autologous adipose tissue-derived stromal vascular fraction cells application in patients with osteoarthritis. Cell Transplant. 2015. Jan. [DOI] [PubMed]
- 34. Fodor PB, Paulseth SG. Adipose derived stromal Cell (ADSC) injections for pain management of osteoarthritis in the human knee joint. Aesthetic Surg J. 2016;36(2):229–236. [DOI] [PubMed] [Google Scholar]
- 35. Anz AW, Hubbard R, Rendos NK, Everts PA, Andrews JR, Hackel JG. Bone marrow aspirate concentrate is equivalent to platelet-rich plasma for the treatment of knee osteoarthritis at 1 year: a prospective, randomized trial. Orthop J Sport Med. 2020;8(2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bansal H, Leon J, Pont JL, Wilson DA, Bansal A, Agarwal D, Preoteasa I. Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: correct dose critical for long term clinical efficacy. Sci Rep. 2021;11(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kon E, Engebretsen L, Verdonk P, Nehrer S, Filardo G. Autologous protein solution injections for the treatment of knee osteoarthritis: 3-year results. Am J Sports Med. 2020;48(11):2703–2710. [DOI] [PubMed] [Google Scholar]
- 38. Luc-Harkey BA, Safran-Norton CE, Mandl LA, Katz JN, Losina E. Associations among knee muscle strength, structural damage, and pain and mobility in individuals with osteoarthritis and symptomatic meniscal tear. BMC Musculoskelet Disord. 2018;19(1):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Minonzio G, Corazza M, Mariotta L, Gola M, Zanzi M, Gandolfi E, De Fazio D, Soldati G. Frozen adipose-derived mesenchymal stem cells maintain high capability to grow and differentiate. Cryobiology. 2014;69(2):211–216. [DOI] [PubMed] [Google Scholar]
- 40. Kaita Y, Tarui T, Yoshino H, Matsuda T, Yamaguchi Y, Nakagawa T, Asahi M, Ii M. Sufficient therapeutic effect of cryopreserved frozen adipose-derived regenerative cells on burn wounds. Regen Ther. 2019;10:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lapuente JP, Dos-Anjos S, Blázquez-Martínez A. Intra-articular infiltration of adipose-derived stromal vascular fraction cells slows the clinical progression of moderate-severe knee osteoarthritis: hypothesis on the regulatory role of intra-articular adipose tissue. J Orthop Surg Res. 2020;15(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morris DL, Oatmen KE, Wang T, DelProposto JL, Lumeng CN. CX3CR1 deficiency does not influence trafficking of adipose tissue macrophages in mice with diet-induced obesity. Obesity (Silver Spring). 2012;20(6):1189–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–887. [DOI] [PubMed] [Google Scholar]
- 44. Hunter DJ, Zhang W, Conaghan PG, Hirko K, Menashe L, Li L, Reichmann WM, Losina E. Systematic review of the concurrent and predictive validity of MRI biomarkers in OA. Osteoarthr Cartil. 2011;19(5):557–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Felson DT, McLaughlin S, Goggins J, LaValley MP, Gale ME, Totterman S, Li W, Hill C, Gale D. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139(5 Pt 1):330–336. [DOI] [PubMed] [Google Scholar]
- 46. Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ba K, Wei X, Ni D, Li N, Du T, Wang X, Pan W. Chondrocyte Co-cultures with the Stromal Vascular Fraction of Adipose Tissue in Polyhydroxybutyrate/Poly-(hydroxybutyrate-co-hydroxyhexanoate) scaffolds: evaluation of cartilage repair in rabbit. Cell Transplant. 2019;28(11):1432–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sun HB. Mechanical loading, cartilage degradation, and arthritis. Ann N Y Acad Sci. 2010;1211:37–50. [DOI] [PubMed] [Google Scholar]
- 49. Hawker GA, Mian S, Bednis K, Stanaitis I. Osteoarthritis year 2010 in review: Non-pharmacologic therapy. Osteoarthr Cartil. 2011;19(4):366–374. [DOI] [PubMed] [Google Scholar]
- 50. McKay J, Frantzen K, Vercruyssen N, Hafsi K, Opitz T, Davis A, Murrell W. Rehabilitation following regenerative medicine treatment for knee osteoarthritis-current concept review. J Clin Orthop Trauma. 2019;10(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schaefer LF, Nikac V, Lynch JA, Duryea J. Quantitative measurement of cartilage volume is possible using two-dimensional magnetic resonance imaging data sets. Osteoarthr Cartil. 2018;26(7):920–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wei H, Lin H, Qin L, Cao S, Zhang Y, He N, Chen W, Yan F, Liu C. Quantitative susceptibility mapping of articular cartilage in patients with osteoarthritis at 3T. J Magn Reson Imaging. 2019;49(6):1665–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hou W, Zhao J, He R, Li J, Ou Y, Du M, Xiong X, Xie B, Li L, Zhou X, Zuo P, et al. Quantitative measurement of cartilage volume with automatic cartilage segmentation in knee osteoarthritis. Clin Rheumatol. 2021;40(5):1997–2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-docx-1-cll-10.1177_09636897211067454 for Comparison of Clinical and Imaging Outcomes of Different Doses of Adipose-Derived Stromal Vascular Fraction Cell Treatment for Knee Osteoarthritis by Masanori Tsubosaka, Tomoyuki Matsumoto, Satoshi Sobajima, Takehiko Matsushita, Hideki Iwaguro and Ryosuke Kuroda in Cell Transplantation