Abstract
Background:
The effectiveness of platelet-rich plasma (PRP) injection in the treatment of lateral epicondylitis remains debatable.
Purpose:
To evaluate the effectiveness of PRP in lateral epicondylitis treatment using minimal clinically important difference (MCID) values as a reference and to investigate if leukocyte content can influence the effectiveness of the therapy.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
Following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, the authors searched the Medline and Scopus databases for studies on lateral epicondylitis and PRP therapy that used the following patient-reported outcome measures (PROMs): visual analog scale (VAS) for pain; Disabilities of the Arm, Shoulder and Hand (DASH); Patient-Rated Tennis Elbow Evaluation (PRTEE); and Mayo Clinic Performance Index (MAYO). The weighted arithmetic means for the PROMs were calculated at baseline (week 0) and follow-up weeks 4, 8, 12, 24, 52, and 104. The mean differences in outcomes (ΔVAS, ΔDASH, ΔPRTEE, and ΔMAYO) were compared with the MCID values at each follow-up point. In addition, the effectiveness of leukocyte-rich PRP (LR-PRP) versus leukocyte-poor PRP (LP-PRP) was also compared. The Student t test was used in all analyses.
Results:
A total of 26 studies were included in the analysis. After PRP injection, all PROM scores improved with time. The scores improved significantly from baseline to each follow-up time (P < .0001), with the exception of the PRTEE (no significant difference at follow-up weeks 12 and 52). The mean difference in scores from baseline exceeded the respective MCIDs from weeks 4 to 104 for the VAS and DASH, from weeks 4 to 52 for the MAYO, and from weeks 8 to 52 for the PRTEE. The MCID for each of the PROMs was exceeded at almost every observation period in both the LR-PRP and the LP-PRP systems.
Conclusion:
Based on comparisons with the MCID values of commonly used outcome scores, PRP seems to be an effective form of treatment for lateral epicondylitis. Both the LR- PRP and the LP- PRP systems were effective in the context of meeting the MCID.
Keywords: platelet-rich plasma, tennis elbow, lateral epicondylitis, minimal clinically important difference
Recovery from injuries within the human musculoskeletal system is a complex process that depends on many factors. At the cellular level, the regeneration process depends to a large extent on growth factors that are stored in platelets’ granules and secreted at the site of injury. Research on the biological potential of platelets has led to the development of techniques for the preparation of platelet-rich concentrates, which in turn has contributed to their use in regenerative medicine. 43 Currently, autologous preparations of platelet-rich plasma (PRP) are widely used in the treatment of complex and difficult-to-heal complications in bone union and in enthesopathies, including lateral epicondylitis (tennis elbow). 4,42
Many studies have been published on the use of PRP and its effectiveness in the treatment of lateral epicondylitis. 9,10 Interestingly, although recent systematic reviews and meta-analyses have been based on common or similar original papers, their conclusions have often differed significantly. 9,31 Generally, the authors have concluded that despite limited data and lack of method standardization, PRP therapy seems to be effective. In contrast, de Vos et al 8 concluded that the evidence against the use of PRP to treat lateral epicondylitis is strong. Although other authors have pointed out some methodological and interpretational errors in that study, 13 similar opinions are not uncommon in the literature. 10
Despite common use, the effectiveness of PRP for lateral epicondylitis remains debatable. 8 –10,13 Clinically, it remains uncertain when to expect the improvement in pain and function or for how long and to what extent. The primary aim of this study was to address these uncertainties by comparing outcome scores for lateral epicondylitis treatment as published in the available literature with the minimal clinically important difference (MCID) of these scores. Because therapeutic effect may depend on the composition of the PRP preparation, our secondary aim was to evaluate whether the leukocyte content in PRP may affect the effectiveness of the therapy as measured using the MCID. Our hypotheses were that PRP is an effective form of lateral epicondylitis treatment in terms of MCID and that the effectiveness of the PRP therapy depends on the PRP leukocyte content.
Methods
Search Strategy
The study was conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. Medline (PubMed) and Scopus databases were searched by 2 independent researchers (A.B. and P.N.) using the following terms: “lateral epicondylitis” or “tennis elbow” and “platelet-rich plasma.” Relevant studies published in English by November 2020 were screened and selected. Next, the eligibility assessment for inclusion was based on the title or abstract and on the full text if required. The inclusion criteria, necessary to qualify the study for the analysis, were evaluation of the effectiveness of lateral epicondylitis treated with PRP, raw results of at least 1 patient-reported outcome measure (PROM) or percentage of PROM improvement in relation to the baseline level, and the presence of outcome scores in at least 1 of the follow-up points selected for this analysis. The selection process and exclusion criteria are described in Figure 1. Ultimately, a total of 26 studies were qualified for the analysis. ∥ A summary of the study characteristics is available in Appendix Table A1.
Figure 1.
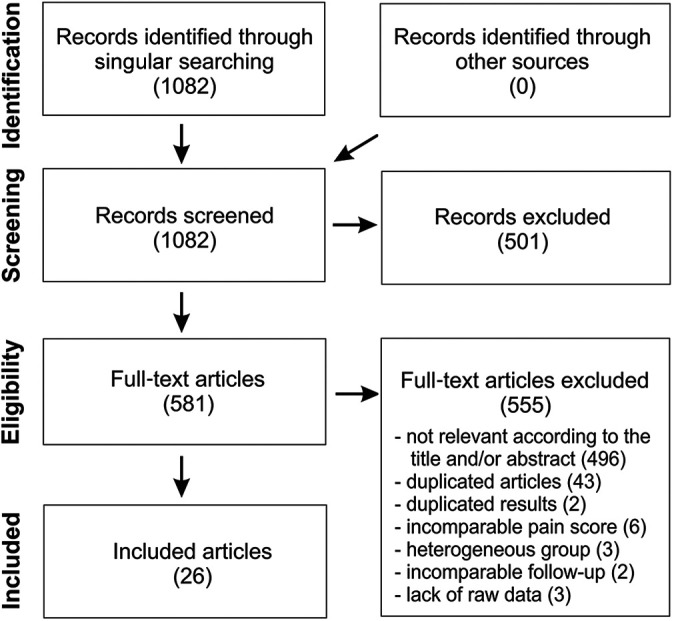
Flowchart of the study selection.
Two analyses were performed. The first included all studies that met the inclusion criteria to determine the overall effect. This analysis included both studies using commercial PRP separation kits and those using original manual protocols (ie, without the use of available kits). In the second analysis, we compared the effectiveness of PRP therapy in studies that used leukocyte-rich (LR-PRP) 14,18,19,23,24,30,38 versus leukocyte-poor (LP-PRP) systems. 1,3,12,21,22,25,37
Articles with duplicate results were rejected at the inclusion stage of the study. Studies with missing data were rejected from the respective comparisons.
Outcomes
We included the following frequently used PROMs for assessing lateral epicondylitis treatment: visual analog scale (VAS) for pain; Disabilities of the Arm, Shoulder and Hand score (DASH); Patient-Rated Tennis Elbow Evaluation (PRTEE); and Mayo Clinic Performance Index (MAYO). The following ranges were assumed: 0 minimum and 10 maximum pain for VAS (results given on the 100-point VAS scale were converted to a 10-point scale), 0 minimum and 100 maximum disability for DASH, 0 minimum and 100 maximum pain and disability for PRTEE, and 0 poor and 100 excellent performance for MAYO.
Follow-up
The effectiveness of PRP therapy was analyzed over time relative to the clinical condition before therapy onset (baseline, week 0). The outcome scores were analyzed at follow-up points that were quoted by the literature most frequently: weeks 4, 8, 12, 24, 52, and 104.
Measures of Effectiveness
Based on the results of each study, the weighted arithmetic means (±SDs) of the outcome scores were calculated. In addition, we calculated the mean differences in scores versus baseline (ΔVAS, ΔDASH, ΔPRTEE, and ΔMAYO) and the percentage improvement from baseline for each of the follow-up points. When studies lacked raw data for the outcome scores, 19,24 only the results of the outcome improvements were used for further analysis. Finally, for each outcome measure, we calculated the weighted arithmetic means of the percentage improvement in scores over time.
The MCID values were defined in accordance with the literature. The therapy for lateral epicondylitis was considered effective when the mean difference in outcome scores between baseline and the follow-up point exceeded the following MCIDs: 1.5 points for VAS, 17 15.8 points for DASH, 34 11 points for PRTEE, 29 and 15 points for MAYO. 33
Statistical Analysis
The analysis of the differences in weighted arithmetic means of the outcome scores was computed using the Student t test. The mean differences and percentage improvement of scores versus baseline were calculated using Excel 2016 (Microsoft Corp) and Corel Draw X7 (Corel Corp) software. Statistical analysis was performed using Statistica 13.3 software (Tibco). P < .05 was considered statistically significant.
Results
Overall Effect
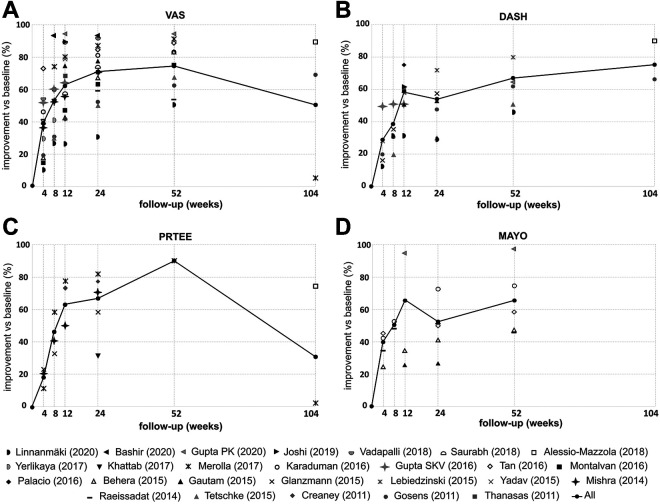
For each study analyzed, the PROM scores at baseline and follow-up, as well as the percentage change from baseline, are shown in Appendix Table A2. Table 1 shows the weighted arithmetic means of each PROM score, as well as mean difference and percentage improvement in scores versus baseline. For each outcome, the relevant studies and sample sizes for each follow-up time are given. Figure 2 presents the weighted arithmetic means of the percentage improvement in each PROM at each follow-up point. The results for each PROM are described separately.
Table 1.
Weighted Arithmetic Means and Change in Outcome Scores From 4 to 104 Weeks of Follow-up a
| VAS | DASH | PRTEE | MAYO | |||||
|---|---|---|---|---|---|---|---|---|
| Follow-up | Score, Mean ± SD | ΔVAS vs Baseline, Points (% Improvement) | Score, Mean ± SD | ΔDASH vs Baseline, Points (% Improvement) | Score, Mean ± SD | ΔPRTEE vs Baseline, Points (% Improvement) | Score, Mean ± SD | ΔMAYO vs Baseline, Points (% Improvement) |
| Baseline | 7.40 ± 1.30 | NA | 60.8 ± 12.5 | NA | 55.6 ± 14.7 | NA | 55.5 ± 6.1 | NA |
| n = 684 | n = 458 | n = 384 | n = 193 | |||||
| 4 wk | 4.43 ± 1.73 b | 2.97 c (39.1) | 41.6 ± 16.4 b | 19.2 c (27.0) | 47.3 ± 19.6 b | 8.3 (18.7) | 79.7 ± 8.8 b | 24.2 c (39.2) |
| n = 471 | n = 583 | n = 233 | n = 233 | n = 217 | n = 217 | n = 138 | n = 138 | |
| 8 wk | 3.53 ± 2.07 b | 3.87 c (54.6) | 32.2 ± 16.8 b | 28.6 c (37.5) | 31.4 ± 20.9 b | 24.2 c (45.2) | 79.5 ± 12.0 b | 24.0 (49.9) |
| n = 332 | n = 480 | n = 205 | n = 205 | n = 200 | n = 200 | n = 31 | n = 67 | |
| 12 wk | 2.41 ± 1.58 b | 4.99 c (63.2) | 28.5 ± 12.6 b | 32.2 c (56.8) | 25.4 ± NR | 30.2 c (63.2) | 88.7 ± 5.1 b | 33.2 c (67.7) |
| n = 410 | n = 501 | n = 276 | n = 276 | n = 234 | n = 234 | n = 70 | n = 70 | |
| 24 wk | 2.01 ± 1.61 b | 5.39 c (70.9) | 22.9 ± 18.0 b | 37.9 c (52.2) | 25.5 ± 20.6 b | 30.1 c (66.0) | 86.2 ± 5.6 b | 30.7 c (51.8) |
| n = 384 | n = 476 | n = 210 | n = 210 | n = 249 | n = 249 | n = 117 | n = 153 | |
| 52 wk | 1.57 ± 1.66 b | 5.83 c (76.8) | 19.3 ± 16.8 b | 41.5 c (63.9) | 9.0 ± NR | 46.6 c (87.2) | 93.0 ± 6.7 b | 37.5 c (67.0) |
| n = 325 | n = 361 | n = 201 | n = 201 | n = 50 | n = 50 | n = 142 | n = 178 | |
| 104 wk | 3.71 ± 2.35 b | 3.69 c (50.2) | 13.0 ± 18.5 b | 47.8 c (76.7) | 48.8 ± 4.1 b | 6.8 (28.9) | NR | NR |
| n = 132 | n = 132 | n = 82 | n = 82 | n = 81 | n = 81 | |||
a References: VAS pain score, 1-3, 11, 14, 15, 16, 18, 19, 22, 23, 25, 30, 32, 36-41; ΔVAS, 1-3, 11, 13, 15, 16, 18, 19, 22-25, 30, 32, 36-41; DASH score, 1, 11-13, 15, 16, 18, 21, 22, 28, 37, 40; ΔDASH, 1, 11, 12, 14, 15, 16, 18, 21, 22, 28, 37, 40; PRTEE score, 1, 5, 12, 20, 23, 24, 28; ΔPRTEE, 1, 5, 12, 20, 23, 24, 28; MAYO score, 3, 11, 16, 19, 30, 36; ΔMAYO, 3, 11, 16, 19, 30, 36. DASH, Disabilities of the Arm, Shoulder and Hand; MAYO, Mayo Clinic Performance Index; NA, not applicable; NR, not reported; PRTEE, Patient-Rated Tennis Elbow Evaluation; VAS, visual analog scale for pain.
b Statistically significant difference versus baseline (P < .0001).
c Difference in score is greater than minimal clinically important difference.
Figure 2.
Plotting of the weighted arithmetic means of the improvement in scores over time (% of improvement vs baseline [week 0]): (A) visual analog scale (VAS) for pain; (B) Disabilities of the Arm, Shoulder and Hand (DASH); (C) Patient-Rated Tennis Elbow Evaluation (PRTEE); and (D) Mayo Clinic Performance Index (MAYO) scores.
VAS Pain Score
VAS pain was the most frequently reported score. The number of patients varied between 684 (baseline) and 132 (week 104). In each of the studies, the VAS score was decreased (improved) after PRP therapy. The best effects were observed between weeks 8 and 52 after the PRP injection. There were few studies with >1 of follow-up, and the results were inconclusive. According to a study by Gosens et al, 14 the therapeutic effect not only persisted after 2 years but also further improved. The reduction in VAS in year 2 of follow-up was also confirmed by Alessio-Mazzola et al. 1 Yet, a shortcoming of this study is the absence of other follow-up points. In opposition to this stands the study of Merolla et al 23 reporting the recurrence of pain almost back to the baseline value at year 2 of follow-up. Regardless of this, the weighted arithmetic means of VAS were significantly decreased in relation to the baseline level at all follow-up points. Also, ΔVAS exceeded the MCID for VAS at each follow-up point. Weighted arithmetic means of VAS improvement (% vs baseline) increased between weeks 4 and 52 (from 39.1% to 76.8%) then decreased to 50.2% at 2 years of observation.
DASH Score
The studies included in this analysis were characterized by a high variability in the baseline DASH values, which ranged between 35.6 (Linnanmäki et al 22 ) and 88.0 (Yadav et al 40 ), with an average value of 60.8. The weighted arithmetic means of DASH decreased (improved) successively from week 4 to week 104 of follow-up, with differences in relation to the baseline being statistically significant. ΔDASH exceeded the MCID for DASH from week 4 to 2 years of observation. The weighted arithmetic means of DASH improvement varied between 27.0% at week 4 of follow-up and 76.7% at year 2 of follow-up. No decrease in the effectiveness of the therapy during the 2-year observation period was observed for DASH.
PRTEE Score
The baseline PRTEE values showed a large diversity across studies such as in the case of DASH. Similar to VAS scores, a decrease (improvement) in the weighted arithmetic means of PRTEE pain outcomes was observed in the period from weeks 4 to 52, with an increase again at year 2 back to the level from week 4. Differences between means of PRTEE at weeks 4, 8, 24, and 104 versus baseline value showed statistical significance. It was impossible to calculate the differences for the remaining follow-up points (weeks 12 and 52) because of the lack of standard deviations (SDs) of PRTEEs in the source publications. ΔPRTEE exceeded the MCID for PRTEE between weeks 8 and 52 of follow-up. The weighted arithmetic means of PRTEE improvement increased between weeks 4 and 52 (from 18.7% to 87.2%) and then decreased to 28.9% at year 2 of observation.
MAYO Score
MAYO was the least frequently used scale among the publications included in our analysis. The number of patient reports was 2.3 to 11.1 times smaller (depending on the time of follow-up) than that of VAS. Some of the follow-up scores were calculated from only 2 studies. Also, there has been no study using a MAYO score beyond a year of observation. This all resulted in a nonlinear increase (improvement) in MAYO weighted arithmetic means and fluctuations in the means of MAYO improvement. Nevertheless, the differences between the means for MAYO in relation to the baseline level showed statistical significance for all the follow-up points (weeks 4-52). Also, the ΔMAYO values exceeded the MCID for MAYO at each observation point.
LR-PRP Versus LP-PRP
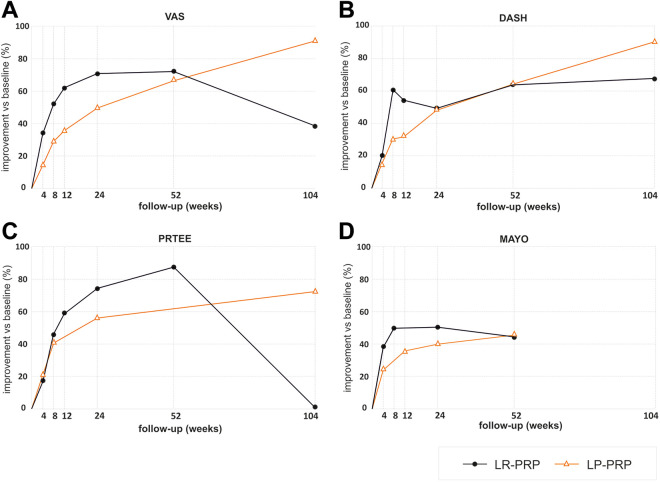
Weighted arithmetic means of PROMs as well as the data on MCID with regard to the leukocyte content of the PRP preparations are presented in Table 2. Figure 3 shows improvement of the PROMs in both studies that used LR-PRP 14,18,19,23,24,30,38 and those with LP-PRP. 1,3,12,21,22,25,37
Table 2.
Weighted Arithmetic Means of Outcome Scores at Weeks 4-104 of Follow-up, Based on the Results of Studies Using LR-PRP Versus LP-PRP a
| VAS | ΔVAS | DASH | ΔDASH | PRTEE | ΔPRTEE | MAYO | ΔMAYO | |
|---|---|---|---|---|---|---|---|---|
| Baseline | ||||||||
| LR-PRP | 7.28 ± 1.60 (181) b | — | 63.2 ± 19.5 (81) b | — | 59.0 ± NR (166) | — | 52.0 ± 16.0 (67) b | — |
| LP-PRP | 6.61 ± 1.94 (137) | — | 48.8 ± 15.0 (212) | — | 56.0 ± 5.6 (93) | — | 63.2 ± 10.2 (15) | — |
| 4 wk | ||||||||
| LR-PRP | 4.63 ± 2.32 (168) c | 2.65 d | 43.1 ± 21.6 (51) c | 20.1 d | 49.0 ± NR (162) | 10 | 71.7 ± 16.0 (67) c | 19.7 d |
| LP-PRP | 5.49 ± 1.75 (77) c | 1.12 | 38.0 ± 20.1 (88) c | 10.8 | 42.3 ± 19.6 (55) c | 13.7 d | 78.3 ± 10.4 (15) c | 15.1 d |
| 8 wk | ||||||||
| LR-PRP | 3.57 ± 2.35 (132) c | 3.71 d | 37.2 ± 24.7 (51) c | 26.0 d | 31.3 ± NR (162) | 27.7 d | 79.5 ± 12.0 (31) c | 27.5 d |
| LP-PRP | 3.97 ± 2.11 (56) c | 2.64 d | 28.9 ± 19.1 (94) c | 19.9 d | 31.8 ± 20.9 (38) c | 24.2 d | NR | NR |
| 12 wk | ||||||||
| LR-PRP | 2.27 ± 2.10 (145) c | 5.01 d | 24.4 ± 22.0 (81) c | 38.8 d | 24.0 ± NR (151) | 35 d | NR | NR |
| LP-PRP | 4.05 ± 2.14 (70) c | 2.56 d | 24.0 ± 19.0 (30) c | 24.8 d | NR | NR | 84.7 ± 9.2 (15) c | 21.5 d |
| 24 wk | ||||||||
| LR-PRP | 2.32 ± 2.87 (146) c | 4.96 d | 27.8 ± 24.7 (51) c | 35.4 d | 14.4 ± NR (106) | 44.6 d | 81.2 ± 16.0 (31) c | 29.2 d |
| LP-PRP | 2.96 ± 1.94 (93) c | 4.96 d | 20.2 ± 17.0 (144) c | 28.6 d | 23.2 ± 18.2 (38) c | 32.8 d | 88.8 ± 8.4 (15) c | 25.6 d |
| 52 wk | ||||||||
| LR-PRP | 1.98 ± 2.84 (132) c | 5.30 d | 20.0 ± 23.5 (51) c | 43.2 d | 9.0 ± NR (50) | 50.0 d | 78.2 ± 18.0 (31) c | 26.2 d |
| LP-PRP | 1.98 ± 1.91 (97) c | 4.63 d | 14.4 ± 18.5 (110) c | 34.4 d | NR | NR | 92.8 ± 6.0 (15) c | 29.6 d |
| 104 wk | ||||||||
| LR-PRP | 4.58 ± 2.80 (101) c | 2.57 d | 17.6 ± 24.0 (51) c | 45.6 d | 69.2 ± NR (50) | –10.2 | NR | NR |
| LP-PRP | 0.90 ± 1.60 (31) c | 5.71 d | 5.5 ± 9.5 (31) c | 43.3 d | 15.9 ± 4.1 (31) c | 40.1 d | NR | NR |
a Data are reported as weighted arithmetic mean ± SD (No. of patients) unless otherwise indicated. References: LR-PRP, 14, 18, 19, 23, 24, 30, 38; LP-PRP, 1, 3, 12, 21, 22, 25, 37. DASH, Disabilities of the Arm, Shoulder and Hand; LP, leukocyte-poor; LR, leukocyte-rich; MAYO, Mayo Clinic Performance Index; NR, not reported; PRP, platelet-rich plasma; PRTEE, Patient-Rated Tennis Elbow Evaluation; VAS, visual analog scale for pain. Dashes indicate no difference.
b Statistically significant difference between LR-PRP and LP-PRP (P < .008).
c Statistically significant difference versus baseline (P < .004).
d Difference in score is greater than minimal clinically important difference.
Figure 3.
Plotting of the weighted arithmetic means of the improvement in scores over time (% of improvement vs baseline [week 0]) in studies using leukocyte-rich platelet-rich plasma (LR-PRP) 14,18,19,23,24,30,38 and leukocyte-poor platelet-rich plasma (LP-PRP) 1,3,12,21,22,25,37 : (A) visual analog scale (VAS) for pain; (B) Disabilities of the Arm, Shoulder and Hand (DASH); (C) Patient-Rated Tennis Elbow Evaluation (PRTEE); and (D) Mayo Clinic Performance Index (MAYO) scores.
PRP therapy significantly decreased the VAS score in relation to the baseline level at all follow-up points, in both the LR-PRP and LP-PRP groups. As there was a difference in VAS levels between LR-PRP and LP-PRP systems at baseline, the means at individual follow-up points were not compared. ΔVAS exceeded the MCID for VAS at each follow-up point in the LR-PRP group and from week 8 to 2 years of observation in the LP-PRP group.
PRP therapy also significantly decreased the DASH score at all follow-up points versus baseline, in both the LR-PRP and the LP-PRP groups. Also, in the case of DASH, the baseline values in the LR-PRP and LP-PRP groups differed significantly; therefore, the means at individual observation points were not compared. ΔDASH exceeded the MCID for DASH at each follow-up point in the LR-PRP group and from week 8 to 2 years of observation in the LP-PRP group.
The lack of SDs of PRTEEs in the source studies made it impossible to calculate the differences between baseline level and the levels at follow-up points in the LR-PRP group. In the LP-PRP group, these differences were significant at all follow-up points. ΔPRTEE exceeded the MCID for PRTEE between weeks 8 and 52 in the LR-PRP group and at all follow-up points in the LP-PRP group.
The differences between the means of MAYO in relation to the baseline level were statistically significant for all observation points, in both the LR-PRP and LP-PRP groups. As there was a difference in MAYO levels between LR-PRP and LP-PRP at the baseline, the means at individual follow-up points were not compared. ΔMAYO also exceeded the MCID for MAYO at each point of observation.
Figure 3 shows that there is an advantage of LR-PRP preparations in short-term follow-up (greater improvement of all PROMs at weeks 4-24 vs baseline) and greater effectiveness of LP-PRP preparations in long-term follow-up (greater improvement of VAS, DASH, and PRTEE at week 104). However, inference on this basis should be approached with great caution because of the impossibility of statistical verification (ie, incomplete source data, significant differences in baseline PROM scores between LR-PRP and LP-PRP groups).
Discussion
The results of our comparisons indicated that after the injection of PRP, the VAS, DASH, PRTEE, and MAYO scores improved significantly with time, with statistically significant differences (P < .0001) between the baseline and the posttreatment values reported at almost each follow-up point for nearly all PROMs. The exception was the PRTEE, where the lack of SD values in the source studies made it impossible to calculate the significance of differences for some follow-up points.
Our comparison of ΔVAS, ΔDASH, ΔPRTEE, and ΔMAYO for each follow-up point with the MCID values 17,29,33,34 indicated that the MCID for the VAS, DASH, and MAYO was met throughout our observation period and the MCID for the PRTEE was met between 8 weeks and 1 year of follow-up. In the latter case, we found a reduction in the effectiveness of the therapy at the 2-year follow-up, characterized by an increase in PRTEE scores from 9.0 at 52 weeks to 48.8 at 104 weeks. This finding was determined by 2 factors: a small number of studies with a long observation period and the outlying values from the study by Merolla et al, 23 which alone made up >60% of the weighted arithmetic mean of PRTEE at the 2-year follow-up. Despite the fact that the difference in PRTEE scores from baseline to 2 years was statistically significant (P < .0001), the effectiveness of PRP therapy over a period of >1 year requires confirmation via additional long-term studies on a larger group of patients.
The results of the current study also indicated that both the LR-PRP and LP-PRP systems are effective tools in the treatment of lateral epicondylitis. Despite the fact that the comparison of weighted arithmetic means in individual follow-up points was impossible for several reasons (mean values of VAS, DASH, and MAYO in the LR-PRP and LP-PRP groups differed significantly at baseline, there was a lack of SDs of PRTEEs in source studies, etc), the MCID for each of the PROMs was exceeded at almost every observation period in both the LR-PRP and the LP-PRP groups. According to the obtained results, our second hypothesis regarding the influence of leukocyte content on the effectiveness of therapy was not confirmed because both LP-PRP and LR-PRP proved to be equally effective in the context of MCID.
The cellular composition of PRP, different in the preparations of individual producers, undoubtedly influences the differences in the concentration of growth factors. For example, it has been shown that the presence of leukocytes in PRP accounts for approximately 30% to 50% of the variability in the concentration of growth factors. 44 Preparations of LR-PRP systems (eg, GPS III) contain significantly more cytokines than do LP-PRP systems (eg, Arthrex). Growth factors secreted by white blood cells may be involved in the healing process by removing microorganisms, cleaning the wound from dead tissue fragments, and stimulating angiogenesis. 7 However, a high concentration of white blood cells, specifically neutrophils, means increased release of free radicals and intensification of the inflammatory process, which may adversely affect the therapeutic process at the injection site. The high concentration of neutrophils correlates not only with the concentration of free radicals but also with the amount of catabolic proinflammatory proteins, such as matrix metallopeptidase–9 and interleukin-1β, which are known predicators of poor healing. 35 Their presence may counteract the beneficial effects resulting from the concentration of growth factors promoting healing in the LR-PRP systems. The results of the current work indicate that there is a need for further research on the evaluation of PRP effectiveness, depending on the cellular composition of the preparations.
The individual studies included in the current analysis differed at the base level. Parameters such as study protocol, type of PRP preparation, preparation technique and administration, postinjection management including rehabilitation, patient characteristics, baseline clinical conditions, and many others may substantially influence the outcomes and therapy efficacy. 6,7,9,10 We were surprised with the fact that many source studies used in the present analysis did not include basic data such as age, sex distribution, PRP separation parameters, raw PROM values, and SDs of outcomes. The same applies to the randomized trials. All the factors listed above are potential study limitations, not only of the present work, but also of all the summaries published so far on the topic of PRP treatment of lateral epicondylitis. Also, earlier studies including the meta-analyses 9,10 showed a high index of heterogeneity among the compared clinical studies. This enforces caution in interpreting the results of direct meta-analyses and identifying the sources of potential discrepancies in such studies. A direct comparison and arrangement of existing knowledge in a transparent way can help to eliminate the difficulties in interpretation, which is particularly important for clinicians making treatment decisions; they still lack clear guidelines in lateral epicondylitis treatment. For the above reasons, we decided to use the current methodology and relate the data available in the literature on lateral epicondylitis treatment with PRP to the objective parameter, which appears to be MCID.
Treatment of lateral epicondylitis with PRP is a relatively inexpensive and safe method with a low recurrence rate. The procedure does not require hospitalization and is effective in terms of the MCID values of the commonly used outcome scores. There is, however, variability in the individual responses to PRP therapy reflected in the different rate and intensity of the regeneration process. Greater knowledge about the predictors of the regeneration process within the musculoskeletal system may enable more accurate selection of patients to maximize potential benefits of treatment with PRP. Such better understanding could also lead to therapy modifications that would improve PRP treatment efficacy in patients who respond insufficiently. In addition to differences in PRP preparation protocols and in PRP types used, it seems that the efficacy of PRP therapy may also depend on the age and sex of the patient, comorbidities, or pharmacotherapy. Also, little is known about the importance of genetic variability within genes encoding molecules involved in the regeneration process, wherein our recent study showed that PDGFB gene variants may influence the effectiveness of lateral epicondylitis treatment with PRP. 27 Full understanding of the role and effect of these factors on the rate and effectiveness of PRP in treating lateral epicondylitis remains a challenge for further research, the results of which should be reported in a transparent manner, enabling their later use, for example, in meta-analyses and other comparative works. Undoubtedly, the use of the Minimum Information for Studies Evaluating Biologics in Orthopaedics checklist should be the standard for reporting clinical trials assessing PRP. 26
Conclusion
Based on the MCID values, PRP seems to be an effective form of treatment for lateral epicondylitis. Both the LR-PRP and LP-PRP systems were effective in the context of MCID.
APPENDIX
Table A1.
Characteristics of the Analyzed Studies a
| First Author | Study Type (LOE) | Sample Size (F/M), n | Age, y, mean ± SD (Range) | Type of PRP; Product (Manufacturer) or Manual Protocol (No. of Spins) b | Platelet Concentration (× Whole Blood) | Injections; Interval weeks (n) | Side Effects, n (%) |
|---|---|---|---|---|---|---|---|
| Alessio-Mazzola 1 | RCS (3) | 31 (13/18) | 46.3 ± 10.1 (18-69) | LP-PRP; manual (3 spins) | 2-3 | 1 | 0 |
| Bashir 2 | PCT (2) | 24 (36/12) | 37.9 ± NR (20-58) | NR | NR | 1 | NR |
| Behera 3 | RCT (1) | 15 (NR) | NR (27-50) c | LP-PRP; Immuguard III-PL (Thirurananthapuram) | 2-3 | 1 | 0 |
| Creaney 5 | RCT (1) | 63 (27/36) | 53.0 ± NR (NR) | NR; manual (spins NR) | 2 | 2; 4 wk | 0 |
| Gautam 11 | RCT (1) | 15 (NR) | NR (18-60) d | NR; manual (1 spin) | NR | 1 | NR |
| Glanzmann 12 | PCT (2) | 62 e (35/27) | 48.2 ± NR (32-65) | LP-PRP; Arthrex | 2-3 | 1 2 (n = 26); 4 wk |
NR |
| Gosens 14 | RCT (1) | 51 (28/23) | 46.8 ± 8.5 (NR) | LR-PRP; Biomet Biologics GPS III | 5 | 1 (n = 48) 2 (n = 3); 12 wk |
NR |
| Gupta 16 | CS (4) | 60 (36/24) | 40.5 ± 10.1 (NR) | NR; manual (2 spins) | 2-5 | 1 (n = 58) 2 (n = 2); 4 wk |
0 |
| Gupta 15 | RCT (1) | 40 (46/34) | 40.8 ± NR (18-55) | NR; manual (2 spins) | NR | 1 | NR |
| Joshi 18 | PCT (2) | 30 (35/25) | 43.8 ± NR (29-55) | LR-PRP; Tricell (REV-MED) | NR | 1 | 0 |
| Karaduman 19 | RCS (3) | 36 (14/22) | 63.7 ± NR (58-72) | LR-PRP; Biomet Biologics GPS III | 5 | 1 | 0 |
| Khattab 20 | CS (4) | 42 (31/11) | 38.0 ± NR (30-50) | NR; manual (spins NR) | 3.3-6.7 | 1 | NR |
| Lebiedziński 21 | RCT (1) | 53 (25/28) | 47.0 ± NR (25-67) | LP-PRP; Arthrex | 2-3 | 1 | 11 (21) |
| Linnanmäki 22 | RCT (1) | 40 f (64/55) | 47.0 ± 7.7 (NR) | LP-PRP; Arthrex | 2 | 1 | NR |
| Merolla 23 | PCT (2) | 50 (21/29) | 47.0 ± 6.1 (NR) | LR-PRP; PRPS (Biomed Device) | NR | 2; 2 wk | 0 |
| Mishra 24 | RCT (2) | 116 g (NR) | 48.4 ± NR (NR) | LR-PRP; Biomet Biologics GPS | 5 | 1 | NR |
| Montalvan 25 | RCT (1) | 25 (8/17) | 47.0 ± 9.2 (NR) | LP-PRP; Arthrex | 2-3 | 2; 4 wk | 4 (16) |
| Palacio 28 | RCT (1) | 20 (NR) | 46.6 ± NR (26-61) | NR; manual (2 spins) | NR | 1 | NR |
| Raeissadat 30 | RCT (1) | 31 (23/8) | 43.0 ± 6.0 (NR) | LR-PRP; Rooyagen (Arya Mabna Tashkhis Corp) | 4.8 | 1 | NR |
| Saurabh 32 | CS (4) | 30 (22/8) | 39.3 ± NR (25-58) | NR; manual (2 spins) | 4-6 | 1 | NR |
| Tan 36 | CS (4) | 56 (35/21) | 45.0 ± NR (36-62) | NR; manual (1 spin) | 3 | 3; 1 wk | 0 |
| Tetschke 37 | PCT (2) | 26 (14/12) | 51.5 ± 10.4 (NR) | LP-PRP; Arthrex | 2-3 | 1 | 0 |
| Thanasas 38 | RCT (1) | 14 (11/3) | 43.6 ± NR (29-52) | LR-PRP; Biomet Biologics GPS III | 5 | 1 | NR |
| Vadapalli 39 | PCT (2) | 20 (25/15) | 44.0 ± NR (22-63) | NR; manual (1 spin) | NR | 1 | NR |
| Yadav 40 | RCT (1) | 30 (20/10) | 36.6 ± NR (NR) | NR; manual (spins NR) | 3.3-6.7 | 1 | 0 |
| Yerlikaya 41 | RCT (1) | 60 (64/26) | 38.6 ± NR (18-75) | LP-PRP and LR-PRP; manual (2/4 spins) | 2-8 | 1 | NR |
a CS, case series; F, female; LOE, level of evidence; LP, leukocyte-poor; LR, leukocyte-rich; M, male; NR, not reported; PCT, prospective comparative trial; PRP, platelet-rich plasma; RCS, retrospective comparative study; RCT, randomized controlled trial.
b Manual protocol = without the use of commercially available kits.
c One age range for both studied groups (PRP and bupivacaine injections as controls).
d One age range for both studied groups (PRP and corticosteroid groups).
e Sample size ranged from 62 (baseline) through 55 (week 4) and 38 (weeks 8 and 24).
f Sample size ranged from 40 (baseline) through 37 (week 4), 30 (weeks 8 and 12), 27 (week 26), and 31 (week 52).
g Sample size ranged from 116 (baseline) through 112 (weeks 4 and 8), 101 (week 12), and 56 (week 24).
Table A2.
Outcome Scores and Improvement From Baseline in the Analyzed Studies a
| Raw Score, Mean ± SD (% of Improvement vs Baseline) | |||||||
|---|---|---|---|---|---|---|---|
| First Author | Baseline | 4 wk | 8 wk | 12 wk | 24 wk | 52 wk | 104 wk |
| VAS (0 Min–10 Max Pain) | |||||||
| Alessio-Mazzola 1 | 8.40 ± 1.10 | NR | NR | NR | NR | NR | 0.90 ± 1.60 (89.3) b |
| Bashir 2 | 7.73 ± 0.81 | NR | 0.30 ± 0.55 (96.1) b | NR | 0.30 ± 0.70 (96.1) b | NR | NR |
| Behera 3 | 7.50 ± 6.40 | 6.20 ± 0.90 (17.7) | NR | 4.30 ± 1.60 (42.5) | 2.50 ± 2.10 (67.2) b | 1.30 ± 1.40 (83.1) b | NR |
| Gautam 11 | 7.10 ± 0.80 | NR | NR | 1.80 ± 0.60 (74.6) b | 1.60 ± 0.50 (77.5) b | NR | NR |
| Gosens 14 | 6.90 ± 1.60 | 5.60 ± 2.40 (19.3) | 4.80 ± 2.50 (30.9) b | 4.00 ± 2.10 (41.7) b | 3.30 ± 3.10 (52.3) b | 2.60 ± 3.10 (62.5) b | 2.10 ± 2.80 (69.1) b |
| Gupta 16 | 8.10 ± 0.77 | 3.80 ± 1.23 (53.1) b | 3.05 ± 1.30 (62.3) b | 2.90 ± 1.40 (64.2) b | NR | NR | NR |
| Gupta 15 | 8.10 ± 0.85 | NR | NR | 0.40 ± 0.60 (95.1) b | NR | 0.25 ± 0.55 (96.9) b | NR |
| Joshi 18 | 8.30 ± NR | NR | NR | NR | NR | NR | NR |
| Karaduman 19 | 7.03 ± NR | 3.79 ± NR (46.1) b | NR (60.0) b | NR | NR (81.0) b | NR (83.0) b | NR |
| Linnanmäki 22 | 5.70 ± 1.70 | 5.00 ± 2.00 (12.3) | 4.20 ± 2.20 (26.3) b | 4.30 ± 2.60 (24.6) b | 3.9 ± 2.50 (31.6) b | 2.70 ± 2.40 (52.6) b | NR |
| Merolla 23 | 7.60 ± NR | 4.50 ± NR (40.8) b | 2.50 ± NR (67.1) b | 1.50 ± NR (80.3) b | 1.10 ± NR (85.5) b | 0.60 ± NR (92.1) b | 7.10 (6.6) |
| Mishra 24 | NR | NR (38.4) | NR (53.9) b | NR (55.1) | NR (71.5) b | NR | NR |
| Montalvan 25 | 6.80 ± 0.80 | 5.80 ± 1.90 (14.7) | NR | 3.60 ± 1.90 (47.1) | 2.50 ± 1.60 (63.2) b | 1.70 ± 1.50 (75.0) b | NR |
| Raeissadat 30 | 7.10 ± 1.20 | 4.17 ± 2.20 (41.3) b | 3.29 ± 2.10 (53.7) b | NR | 2.91 ± 2.47 (59.0) b | 3.29 ± 2.41 (53.7) b | NR |
| Saurabh 32 | 7.70 ± NR | NR | NR | 3.20 ± NR (58.4) b | 1.80 ± NR (76.6) b | NR | NR |
| Tan 36 | 7.30 ± 0.40 | 2.00 ± 0.60 (72.8) b | NR | NR | 1.10 ± 0.20 (84.5) b | 0.80 ± 0.30 (88.7) b | NR |
| Tetschke 37 | 5.23 ± 1.84 | NR | 3.67 ± 2.04 (29.8) | NR | 2.67 ± 1.59 (48.9) b | 1.81 ± 2.02 (65.4) b | NR |
| Thanasas 38 | 6.10 ± NR | NR | NR | 1.92 ± NR (68.5) | 1.78 ± NR (70.8) | NR | NR |
| Vadapalli 39 | 7.37 ± 0.90 | 3.32 ± 1.06 (55.0) b | NR | 0.78 ± 1.00 (89.4) b | 0.56 ± 0.81 (92.4) b | NR | NR |
| Yadav 40 | 7.60 ± NR | 4.60 ± NR (39.5) b | NR | 1.60 ± NR (78.9) b | NR | NR | NR |
| Yerlikaya 41 | 8.30 ± 1.75 | 5.70 ± 2.65 (31.3) b | 4.75 ± 3.05 (42.8) b | NR | NR | NR | NR |
| DASH (0 Min–100 Max Disability) | |||||||
| Alessio-Mazzola 1 | 65.1 ± 10.5 | NR | NR | NR | NR | NR | 5.5 ± 9.5 (91.6) b |
| Gautam 11 | 69.7 ± 6.1 | NR | NR | 33.6 ± 5.1 (51.8) b | 32.0 ± 4.5 (54.1) b | NR | NR |
| Glanzmann 12 | 50.3 ± NR | 42.0 ± 18.0 (16.5) | 32.1 ± 17.7 (36.2) | NR | 20.7 ± 18.5 (58.8) b | NR | NR |
| Gosens 14 | 54.3 ± 19.5 | 43.1 ± 21.6 (20.6) b | 37.2 ± 24.7 (31.5) b | 21.3 ± 22.0 (60.8) b | 27.8 ± 24.7 (48.8) b | 20.0 ± 23.5 (63.2) b | 17.6 ± 24.0 (67.6) b |
| Gupta 16 | 72.0 ± 6.5 | 36.2 ± 9.4 (49.7) b | 33.3 ± 6.6 (53.8) b | 33.6 ± 9.5 (53.3) b | NR | NR | NR |
| Gupta 15 | 87.1 ± 5.7 | NR | NR | 35.1 ± 3.1 (59.7) | NR | 31.6 ± 3.9 (63.7) | NR |
| Joshi 18 | 78.3 ± NR | NR | NR | 29.6 ± NR (62.2) b | NR | NR | NR |
| Lebiedziński 21 | 53.2 ± 15.5 | NR | NR | NR | 14.2 ± 13.4 (73.3) b | 9.9 ± 17.1 (81.4) b | NR |
| Linnanmäki 22 | 35.6 ± 15.5 | 31.0 ± 18.0 (12.9) | 24.0 ± 19.0 (32.6) | 24.0 ± 19.0 (32.6) | 25.0 ± 18.0 (29.8) | 19.0 ± 20.0 (46.6) | NR |
| Palacio 28 | 45.7 ± NR | NR | NR | 10.7 ± NR (76.6) b | NR | NR | NR |
| Tetschke 37 | 37.0 ± 18.3 | NR | 29.8 ± 21.1 (19.5) | NR | 26.5 ± 21.1 (28.4) | 18.2 ± 19.5 (50.8) | NR |
| Yadav 40 | 88.0 ± NR | 62.5 ± NR (29.0) b | NR | 34.2 ± NR (61.2) b | NR | NR | NR |
| PRTEE (0 Min–100 Max Pain and Disability) | |||||||
| Alessio-Mazzola 1 | 60.1 ± 5.6 | NR | NR | NR | NR | NR | 15.9 ± 4.1 (73.5) b |
| Creaney 5 | 45.8 ± NR | NR | NR | 33.0 ± NR (72.1) b | 35.8 ± NR (76.4) b | NR | NR |
| Glanzmann 12 | 54.0 ± NR | 42.3 ± 19.6 (21.7) | 31.8 ± 20.9 (41.1) | NR | 23.2 ± 18.2 (57.0) b | NR | NR |
| Khattab 20 | 60.4 ± 21.4 | NR | NR | NR | 40.2 ± 22.8 (33.4) b | NR | NR |
| Merolla 23 | 70.1 ± NR | 63.0 ± NR (10.1) | 29.6 ± NR (57.8) b | 17.7 ± NR (74.8) b | 12.3 ± NR (82.4) b | 9 ± NR (87.2) b | 69.2 ± NR (1.3) |
| Mishra 24 | 54.2 ± NR | 42.8 ± NR (21.0) | 32.0 ± NR (41.0) | 27.1 ± NR (50.0) | 16.2 ± NR (70.1) | NR | NR |
| Palacio 28 | 47.1 ± NR | NR | NR | 13.0 ± NR (72.4) b | NR | NR | NR |
| MAYO (0 Poor–100 Excellent) | |||||||
| Behera 3 | 63.2 ± 10.2 | 78.3 ± 10.4 (23.9) | NR | 84.7 ± 9.2 (34.0) | 88.8 ± 8.4 (40.5) b | 92.8 ± 6.0 (46.8) b | NR |
| Gautam 11 | 56.1 ± 6.9 | NR | NR | 70.2 ± 2.2 (25.1) b | 70.7 ± 3.0 (26.0) b | NR | NR |
| Gupta 15 | 49.5 ± 0.8 | NR | NR | 97.2 ± 4.7 (96.4) b | NR | 98.2 ± 4.7 (98.4) b | NR |
| Karaduman 19 | 50.3 ± NR | 71.3 ± NR (41.7) b | NR (52.0) b | NR | NR (72.0) b | NR (74.0) b | NR |
| Raeissadat 30 | 53.9 ± 16.0 | 72.1 ± 16.0 (33.8) b | 79.5 ± 12.0 (47.5) b | NR | 81.2 ± 16.0 (50.6) b | 78.2 ± 18.0 (45.1) b | NR |
| Tan 36 | 61.9 ± 3.0 | 89.6 ± 4.3 (44.7) b | NR | NR | 92.5 ± 3.3 (49.4) b | 97.6 ± 2.1 (57.7) b | NR |
a DASH, Disabilities of the Arm, Shoulder and Hand; max, maximum; MAYO, Mayo Clinic Performance Index; min, minimum; NR, not reported; PRTEE, Patient-Rated Tennis Elbow Evaluation; VAS, visual analog scale for pain.
b Statistically significant difference versus baseline, as stated in source study (P < .05).
Final revision submitted December 1, 2021; accepted January 10, 2022.
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was funded by Medical University of Silesia in Katowice, Poland (grant No. KNW-1-008/N/7/K). AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Alessio-Mazzola M, Repetto I, Biti B, Trentini R, Formica M, Felli L. Autologous US-guided PRP injection versus US-guided focal extracorporeal shock wave therapy for chronic lateral epicondylitis: a minimum of 2-year follow-up retrospective comparative study. J Orthop Surg (Hong Kong). 2018;26:2309499017749986. [DOI] [PubMed] [Google Scholar]
- 2. Bashir SI, Lone F, Rameez R. Injection of platelet rich plasma versus corticosteroid injection in the treatment of tennis elbow: a prospective randomized comparative study. Int J Orthop Sci. 2020;6:1164–1167. [Google Scholar]
- 3. Behera P, Dhillon M, Aggarwal S, Marwaha N, Prakash M. Leukocyte-poor platelet-rich plasma versus bupivacaine for recalcitrant lateral epicondylar tendinopathy. J Orthop Surg (Hong Kong). 2015;23:6–10. [DOI] [PubMed] [Google Scholar]
- 4. Brkljac M, Conville J, Sonar U, Kumar S. Long-term follow-up of platelet-rich plasma injections for refractory lateral epicondylitis. J Orthop. 2019;16(6):496–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Creaney L, Wallace A, Curtis M, Connell D. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, single-blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. Br J Sports Med. 2011;45:966–971. [DOI] [PubMed] [Google Scholar]
- 6. Davis VL, Abukabda AB, Radio NM, et al. Platelet-rich preparations to improve healing, part I: workable options for every size practice. J Oral Implantol. 2014;40:500–510. [DOI] [PubMed] [Google Scholar]
- 7. Davis VL, Abukabda AB, Radio NM, et al. Platelet-rich preparations to improve healing, part II: platelet activation and enrichment, leukocyte inclusion, and other selection criteria. J Oral Implantol. 2014;40:511–521. [DOI] [PubMed] [Google Scholar]
- 8. de Vos RJ, Windt J, Weir A. Strong evidence against platelet-rich plasma injections for chronic lateral epicondylar tendinopathy: a systematic review. Br J Sports Med. 2014;48:952–956. [DOI] [PubMed] [Google Scholar]
- 9. Dong W, Goost H, Lin XB, et al. Injection therapies for lateral epicondylalgia: a systematic review and Bayesian network meta-analysis. Br J Sports Med. 2016;50:900–908. [DOI] [PubMed] [Google Scholar]
- 10. Franchini M, Cruciani M, Mengoli C, et al. Efficacy of platelet-rich plasma as conservative treatment in orthopaedics: a systematic review and meta-analysis. Blood Transfus. 2018;16:502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23:1–5. [DOI] [PubMed] [Google Scholar]
- 12. Glanzmann MC, Audigé L. Platelet-rich plasma for chronic lateral epicondylitis: is one injection sufficient? Arch Orthop Trauma Surg. 2015;135:1637–1645. [DOI] [PubMed] [Google Scholar]
- 13. doi: 10.1136/bjsports-2014-093704. Gosens T, Mishra AK. Editorial in response to the systematic review by de Vos: “Strong evidence against platelet-rich plasma injections for chronic lateral epicondylar tendinopathy: a systematic review.” Br J Sports Med. 2014;48:945–946. [DOI] [PubMed] [Google Scholar]
- 14. doi: 10.1177/0363546510397173. Gosens T, Peerbooms JC, van Laar W, den Oudsten BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39:1200–1208. [DOI] [PubMed] [Google Scholar]
- 15. Gupta PK, Acharya A, Khanna V, Roy S, Khillan K, Sambandam SN. PRP versus steroids in a deadlock for efficacy: long-term stability versus short-term intensity—results from a randomised trial. Musculoskelet Surg. 2020;104(3):285–294. [DOI] [PubMed] [Google Scholar]
- 16. Gupta SKV, Bandari D. Autologous platelet-rich plasma injection in tennis elbow and plantar fasciitis. Curr Orthop Pract. 2016;27:405–408. [Google Scholar]
- 17. Hao Q, Devji T, Zeraatkar D, et al. Minimal important differences for improvement in shoulder condition patient-reported outcomes: a systematic review to inform a BMJ Rapid Recommendation. BMJ Open. 2019;9:e028777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Joshi A, Arora K, Gotecha D, Giroti C. Comparison of steroid injection and platelet-rich plasma injection in the treatment of chronic lateral epicondylitis. Int J Orthop Sci. 2019;5:55–58. [Google Scholar]
- 19. Karaduman M, Okkaoglu MC, Sesen H, Taskesen A, Ozdemir M, Altay M. Platelet-rich plasma versus open surgical release in chronic tennis elbow: a retrospective comparative study. J Orthop. 2016;13:10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khattab EM, Abowarda MH. Role of ultrasound guided platelet-rich plasma (PRP) injection in treatment of lateral epicondylitis. Egypt J Radiol Nucl Med. 2017;48:403–413. [Google Scholar]
- 21. Lebiedziński R, Synder M, Buchcic P, Polguj M, Grzegorzewski A, Sibiński M. A randomized study of autologous conditioned plasma and steroid injections in the treatment of lateral epicondylitis. Int Orthop. 2015;39:2199–2203. [DOI] [PubMed] [Google Scholar]
- 22. Linnanmäki L, Kanto K, Karjalainen T, Leppänen OV, Lehtinen J. Platelet-rich plasma or autologous blood do not reduce pain or improve function in patients with lateral epicondylitis: a randomized controlled trial. Clin Orthop Relat Res. 2020;478:1892–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Merolla G, Dellabiancia F, Ricci A, et al. Arthroscopic debridement versus platelet-rich plasma injection: a prospective, randomized, comparative study of chronic lateral epicondylitis with a nearly 2-year follow-up. Arthroscopy. 2017;33:1320–1329. [DOI] [PubMed] [Google Scholar]
- 24. Mishra AK, Skrepnik NV, Edwards SG, et al. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014;42:463–471. [DOI] [PubMed] [Google Scholar]
- 25. Montalvan B, Le Goux P, Klouche S, Borgel D, Hardy P, Breban M. Inefficacy of ultrasound-guided local injections of autologous conditioned plasma for recent epicondylitis: results of a double-blind placebo-controlled randomized clinical trial with one-year follow-up. Rheumatology (Oxford). 2016;55:279–285. [DOI] [PubMed] [Google Scholar]
- 26. Murray IR, Geeslin AG, Goudie EB, Petrigliano FA, LaPrade RF. Minimum Information for Studies Evaluating Biologics in Orthopaedics (MIBO): platelet-rich plasma and mesenchymal stem cells. J Bone Joint Surg Am. 2017;99:809–819. [DOI] [PubMed] [Google Scholar]
- 27. Niemiec P, Szyluk K, Balcerzyk A, et al. Why PRP works only on certain patients with tennis elbow? Is PDGFB gene a key for PRP therapy effectiveness? A prospective cohort study. BMC Musculoskelet Disord. 2021;22:710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Palacio EP, Schiavetti RR, Kanematsu M, Ikeda TM, Mizobuchi RR, Galbiatti JA. Effects of platelet-rich plasma on lateral epicondylitis of the elbow: prospective randomized controlled trial. Rev Bras Ortop. 2016;51:90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poltawski L, Watson T. Measuring clinically important change with the Patient-rated Tennis Elbow Evaluation. Hand Ther. 2011;16:52–57. [Google Scholar]
- 30. Raeissadat SA, Rayegani SM, Hassanabadi H, Rahimi R, Sedighipour L, Rostami K. Is platelet-rich plasma superior to whole blood in the management of chronic tennis elbow: one year randomized clinical trial. BMC Sports Sci Med Rehabil. 2014;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saltychev M, Laimi K, Virolainen P, Fredericson M. Effectiveness of platelet-rich plasma in treatment of lateral epicondylitis—a systematic review and meta-analysis. PRM+ 2018;1(1):7–16. [Google Scholar]
- 32. Saurabh J, Rajeev K, Laxman B, Vaibhav G. Treatment of lateral epicondylitis with autologous platelet rich plasma injection. Int J Orthop Sci. 2018;4:437–441. [Google Scholar]
- 33. Smith MV, Calfee RP, Baumgarten KM, Brophy RH, Wright RW. Upper extremity-specific measures of disability and outcomes in orthopaedic surgery. J Bone Joint Surg Am. 2012;94:277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith-Forbes EV, Howell DM, Willoughby J, Pitts DG, Uhl TL. Specificity of the minimal clinically important difference of the quick Disabilities of the Arm Shoulder and Hand (QDASH) for distal upper extremity conditions. J Hand Ther. 2016;29:81–88. [DOI] [PubMed] [Google Scholar]
- 35. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39:2135–2140. [DOI] [PubMed] [Google Scholar]
- 36. Tan XX, Ju HY, Yan W, et al. Autologous platelet lysate local injections for the treatment of refractory lateral epicondylitis. J Orthop Surg Res. 2016;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tetschke E, Rudolf M, Lohmann CH, Stärke C. Autologous proliferative therapies in recalcitrant lateral epicondylitis. Am J Phys Med Rehabil. 2015;94:696–706. [DOI] [PubMed] [Google Scholar]
- 38. Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39:2130–2134. [DOI] [PubMed] [Google Scholar]
- 39. Vadapalli RC. A comparative study of efficacy between platelet rich plasma (PRP) versus local corticosteroid injection in the treatment of lateral epicondylitis (tennis elbow). Int J Integr Med Sci. 2018;5:721–724. [Google Scholar]
- 40. Yadav R, Kothari SY, Borah D. Comparison of local injection of platelet rich plasma and corticosteroids in the treatment of lateral epicondylitis of humerus. J Clin Diagn Res. 2015;9:RC05–RC07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yerlikaya M, Çaliş HT, Sütbeyaz ST, et al. Comparison of effects of leukocyte-rich and leukocyte-poor platelet-rich plasma on pain and functionality in patients with lateral epicondylitis. Arch Rheumatol. 2017;33:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang Y, Xing F, Luo R, Duan X. Platelet-rich plasma for bone fracture treatment: a systematic review of current evidence in preclinical and clinical studies. Front Med (Lausanne). 2021;8:676033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou Y, Wang JH. PRP treatment efficacy for tendinopathy: a review of basic science studies. Biomed Res Int. 2016;2016:9103792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zimmermann R, Jakubietz R, Jakubietz M, et al. Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion. 2001;41:1217–1224. [DOI] [PubMed] [Google Scholar]