Abstract
Objectives. This study was carried out to delineate the patients’ characteristics and the imaging findings and their relation to some biochemical markers of 31 critically ill patients with MIS-C. Design. A retrospective cross-sectional study including all critically ill MIS-C patients admitted to the PICU from June 23rd to July 22nd, 2020. Results. Eighteen males and 13 females, with a median age of 9 years (interquartile range 6-11) presented mainly with fever (100%) and hypotension (100%). Abnormalities in the chest computed tomography were detected in 22 cases (71%). Consolidation and architecture distortion were detected in 58.1% of patients; bilateral lesions and lower lobe infiltrates, each, was evident in 64.5% of patients, while the peripheral distribution of lesions was seen in 71% of the cases. Pleural thickening and effusion, each, was found in 51.6% of the patients. In this small case series, the presence of high ferritin was significantly associated with the bilaterality of the lesions. Elevated C-reactive protein was associated with the peripheral distribution of the lesions. Thrombocytopenia and hypoalbuminemia were significantly correlated with the CT disease stage and CT severity score respectively. Conclusions. Although a few children in this group of MIS-C patients presented with respiratory manifestations, yet, most of them demonstrated significant radiological lung involvement, which necessitates a longer-term follow-up.
Keywords: COVID-19, Pulmonary computed tomography, CT, MIS-C, PICU, characteristics, Egyptian
Introduction
Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19) is characterized by persistent fever, variable clinical manifestations and organs dysfunction, for example, abdominal pain, vomiting, diarrhea, rash, conjunctivitis, mucocutaneous manifestations, cardiac affection, etc. Some MIS-C cases may rapidly progress to hypotension and shock with cardiac and other end-organ injury.1,2
Although many radiologic findings in patients with MIS-C were reported, a scarce data of the spectrum of the pulmonary CT radiologic features and their relation with the biochemical markers were described.
The aim of this study is to describe the patients’ clinical characteristics, laboratory markers, echocardiographic changes, and the pulmonary computerized imaging findings (CT). The association of the CT findings with the laboratory markers is studied in critically ill MIS-C patients who presented with or without respiratory manifestations to the pediatric intensive care unit (PICU) of the Children’s Hospital, Ain Shams University, a referral and a tertiary care center for MIS-C in Cairo, Egypt.
Materials and Methods
This cross-sectional study included all critically ill patients with MIS-C admitted to the PICU of Ain shams university hospitals with a capacity of 32 non COVID-19 isolation beds and 6 COVID-19 isolation beds from June 23rd to July 22nd, 2020. All Patients with MIS-C had fever, multisystem involvement, and positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reverse transcriptase-polymerase chain reaction (RT-PCR) or antibody test results or recent exposure to SARS-Cov-2, with no alternate diagnosis, as defined by the CDC.2,3
The electronic medical records of the identified patients were accessed for the demographic data, medical history, vital signs, laboratory results, echocardiographic findings, CT pulmonary findings, hospital course, and disposition.
In addition to the standard PICU care measures (eg, vasopressors, intravenous fluids and antibiotics), all studied MIS-C cases were managed as per our institutional MIS-C protocol. 4 A single dose of intravenous immunoglobulins (IVIG) 2 g/kg was given, and all cases were started on a 2 to 3-day 30 mg/kg methylprednisolone 5 (maximum dose of 1 g) followed by gradual tapering as dictated by the clinical response. In addition to other therapeutics, a prophylactic dose of low molecular weight heparin was used for all patients. Interleukin-1receptor-antagonists (IL-1Ra) were used when indicated. 5
Laboratory workup included SARS-CoV-2 testing (RT- PCR on nasopharyngeal and oral swabs; serology for IgG and IgM [Epitope Diagnostics Inc, San Diego, California], tier 1 and 2 laboratory investigations performed on admission to PICU included, but not limited to, complete blood count (CBC), C-reactive protein (CRP), serum ferritin, D-dimer, and troponin were performed on admission and repeated as per PICU protocol, and published practice guidelines recommendations. 6
Echocardiography was done after clinical stabilization of patients according to the standards of American Society of Echocardiography 7 using Vivid 7 GE machine (Norway) with a suitable transducer frequency according to the patient build.
Due to the severity of the admitted MIS-C cases, the potential benefits of imaging outweighed its risks (eg, radiation exposure, equipment disinfection requirements, and staff exposure to COVID-19). In addition, because the syndrome was a novel diagnosis with constellation of a multitude of clinical findings and nonspecific radiologic findings, 8 we planned to perform pulmonary CT to all severe MIS-C patients at our PICU within 48 hours of admission to PICU. A multidetector computed tomography technology was used for the diagnostic imaging. The patients were scanned on supine position, with the field of view extends from the lung apices down to the diaphragm, sedation was needed for children below the age of 6-years. Non contrast CT of the chest was performed for 27 patients. For 4 patients with clinically suspected pulmonary embolism a CT pulmonary angiography was performed.
Two board certified radiologists with 20 and 31 years of experience reviewed the CT images simultaneously. The pattern of the lesions was determined according to Fleischner society glossary of terms for thoracic imaging. 9 The distribution of the lesions was further analyzed according to its laterality (bilateral or unilateral), multiple versus single lobe affection, the predominantly affected lobe, and the distribution of the lesions (peripheral or diffuse). The predominant feature present in each patient was determined (ground glass opacities, consolidation, or architecture distortion). Disease stage was determined as previously described with modification; stage 0: normal study; stage1: ground glass opacities; stage 2: presence of crazy paving and consolidation; stage 3: presence of consolidation as a predominant feature; stage 4: appearance of subpleural parenchymal bands. 10 Patients were categorized into patients with features of early disease stage if only ground glass appearance or consolidation was present ( stage 1 or 2 or 3), or patients with features of late stage if signs of architecture distortion were present ( stage 4). 11 The presence of mediastinal or axillary lymph nodes or pleural effusion was also documented. The CT severity score was also calculated according to the extent of lobar involvement (the percentage of the affected lung areas) as previously described. 12
A score ranging from 0 to 5 was determined for each lobe with 0 indicating no involvement, 1 indicating less than 5% involvement, 2 indicating 5% to 25% involvement, 3 indicating 26% to 49% involvement, 4 indicating 50% to 75% involvement, and 5 indicating more than 75% involvement. The total CT severity score was calculated as the sum of the individual scores of each lobe. It ranged from 0 to 25. In this study, stages 1 or 2 or 3 were considered early disease stages, while stage 4 is considered a late stage of the disease.
Statistical Analysis
Data was analyzed using the IBM statistical package for social science (SPSS) for windows, version 22(IBM Corp, Armonk, NY, USA). Patient laboratory and echocardiographic characteristics were expressed in numbers and percentages for categorical variables, and median with IQR for quantitative ones. The association between these laboratory and echocardiographic characteristics and pulmonary CT findings was performed using the Fisher’s exact test for categorical variables, and the Mann-Whitney U test for quantitative variables. To assess the correlation between initial inflammatory markers, echocardiographic changes, and the pulmonary CT findings, Spearman rank correlation coefficients test were used. Logistic regression analysis was performed to determine which variables were predictive for those changes. P-value ≤.05 was considered significant. For the radiologic findings’ description, the absolute interobserver agreement and 95% CIs of the imaging findings were calculated using VassarStats.net (R. Lowry). Imaging findings were summarized descriptively using absolute counts and percentages based on the final consensus interpretations.
The institutional research ethics committee approved the review process of the anonymized medical records and imaging studies; the written informed consent was waived because of the retrospective nature of the study.
Results
Thirty-one children with MIS-C, 18 males and 13 females, having a median age of 9 years (interquartile range of 6-11) with 16 patients in the age group between 5 and 10 years (51.6%) were admitted to the PICU due severe hypotension and shock. Rash was evident in 21 patients (67.7%), conjunctivitis was observed in 18 patients (58.1%), and seizures were recorded in one patient in (3.2%). Gastrointestinal manifestation was present in 19 patients ( 61.2%) and respiratory distress was evident in only 3 patients (9.7%).
Covid-19 PCR was positive in 11 patients (35.5%), IgM was positive in 5 patients (16.1%), while the majority of cases 26 (83.9%) had a positive IgG. The median total leukocytic count was 11.2 (IQR 6.5-15.3) ×103/µL, leukocytosis was found in 13 cases (41.9%), and lymphopenia (<1000 cell/µL) was detected in 9 patients (29%).
The median platelets count was 194 × 103/µL (IQR 95-270). The median CRP was 163.4 (IQR 80.1-298) mg/L, while highly elevated CRP >150 mg/L 13 was detected in 18 patients (58.1%). The median serum albumin concentration was 2.8 g/dL (IQR 2.5-3.5), the median LDH was 411 U/L (IQR 311-513), and the median D-dimer was 3 µg/mL (IQR 1.97-4.3). A D-dimer at or above 5 times the normal value was seen in 22 patients (71%), and the median ferritin level was 490 µg/L (IQR 314-619).
The echocardiographic findings included: left ventricular dysfunction (ejection fraction ≤50%) was diagnosed in 16 patients (51.6%), valvulitis in 23 patients (74.2%), coronary artery changes in 11patients (35.5%), while pericardial effusion was detected in 3 patients (9.7%). The echocardiographic findings were unremarkable in 8 patients (25.8%).
Pulmonary CT Data: Out of 31 patients, 22 (71%) had pulmonary CT findings. Architecture distortion found in 8 patients (25.8%) and consolidation in 10 patients (32.3%) were the most predominant features, while ground glass opacities were found in 6 (19.4%) patients. Bilaterally distributed lesions were evident in 20 patients (64.5%), and 20 patients (64.5%) had lesions involving multiple lobes. Peripheral lung zones involvement was seen in 22 patients (71%). The lower lobes were the most frequently affected lobes detected in 20 patients (64.5%). Pleural effusion and/or thickening was found in 16 cases (51.6%), and axillary, mediastinal, or both axillary and mediastinal lymphadenopathy were present in 7 (22.6%), 1 (3.2%), and 2 (65%) of the patients respectively.
Early disease stages (stages 1 and 3 only as none of the patients had stage 2 disease features) were detected in 12 patients (38.7%). The late stage of the disease (stage 4) was also similarly described in 12 patients (38.7%) patients. The median CT severity score was 3 (IQR = 1-5). Computed tomography pulmonary angiography scans were normal in the 4 patients with suspected pulmonary embolism. The clinical, laboratory, echocardiographic, and radiological findings of the MIS-C patients are summarized in Table 1. Figures 1 to 4 illustrate some of reported CT changes in selected patients. While the echocardiographic findings in the current study were not statically associated with either CT severity score or CT disease stage, a significant association of the inflammatory markers with certain patterns of pulmonary CT findings was detected.
Table 1.
Clinical, Laboratory, Echocardiographic and Radiologic Characteristics of 31 Patients with MIS-C. a
| No. (%) | |
|---|---|
| Clinical and laboratory features | |
| Fever | 31 (100) |
| Hypotension | 31 (100) |
| Rash | 21 (67.7) |
| GIT symptoms | 19 (61.2) |
| Vomiting | 8 (26.7) |
| Diarrhea | 6 (19.6) |
| Abdominal pain | 5 (16.1) |
| Conjunctivitis | 18 (58.1) |
| Respiratory distress | 3 (9.7) |
| Neurological symptoms (seizures) | 1 (3.2) |
| Comorbid conditions | 9 (29.7) |
| COVID-19 test results | |
| Positive RT -PCR | 11 (35.5) |
| Positive IgM | 5 (16.1) |
| Positive IgG | 26 (83.9) |
| Total leucocytic count | |
| Leukocytosis | 9 (29) |
| Leucopenia | 3 (9.7) |
| Lymphopenia (<1000/µL) | 9 (29) |
| Platelets’ count | |
| Thrombocytosis | 1 (3) |
| Thrombocytopenia | 14 (45.2) |
| CRP (mg/L) | |
| >20 | 28 (87.5) |
| >150 | 18 (58.1) |
| Normal | 3 (9.1) |
| Ferritin (ug/L) | |
| 500-1400 | 11 (35.5) |
| >1400 | 3 (9.7) |
| Hypoalbuminemia (<3.5 g/dL) | 22 (71) |
| D dimer (≥ 5 times ULN) | 22 (71) |
| Echocardiographic findings | |
| Left ventricular dysfunction | 16 (51.6) |
| Valvulitis | 23 (74.2) |
| Coronary artery changes | 11 (35.5) |
| Pericardial effusion | 3 (9.7) |
| Need for MV | 8 (25.8) |
| Outcome | |
| Discharged home | 28 (90.3) |
| Died | 3 (9.7) |
| Pulmonary CT characteristics | |
| Positive findings | 22 (71) |
| Negative findings | 9 (29) |
| Predominant feature | |
| Architecture distortion | 8 (25.8) |
| Consolidation | 10 (32.3) |
| Ground glass opacity | 6 (19.4) |
| Lesion distribution | |
| Unilateral | 4 (12.9) |
| Bilateral | 20 (64.5) |
| No. (%) | |
| Lobar distribution | |
| Single lobe | 4 (12.9) |
| Multiple lobes | 20 (64.5) |
| Predominant lobe affection | |
| Upper | 2 (6.5) |
| Middle | 1 (3.2) |
| Lower | 20 (64.5) |
| Diffuse | 1 (3.2) |
| Distribution of lesions | |
| Central | 1 (3.2) |
| Diffuse | 1 (3.2) |
| Peripheral | 22 (71) |
| Disease stage | |
| 0 | 7 (22.6) |
| 1 | 4 (12.9) |
| 3 | 8 (25.8) |
| 4 | 12 (38.7) |
| Lymph nodes | |
| Axillary | 7 (22.6) |
| Mediastinal | 1 (3.2) |
| Axillary and Mediastinal | 2 (6.5) |
| Pleural effusion/thickening | 16 (51.6) |
Abbreviations: COVID-19, coronavirus disease 2019; RT- PCR, reverse transcriptase polymerase chain reaction; CRP, C-reactive protein; CT, computed tomography scan; d, days; IQR, interquartile range; MV, mechanical ventilation; LOS, length of stay; PICU, pediatric intensive care unit; ULN, upper limit normal.
Data are given as numbers and percentage.
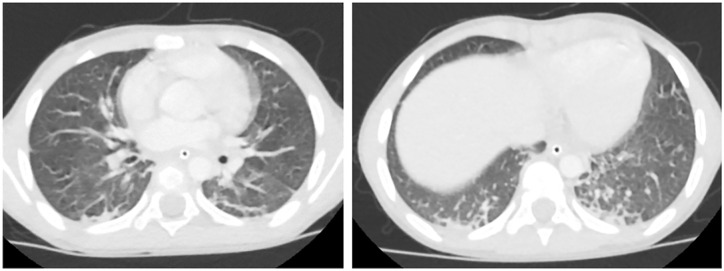
Figure 1.
A 6-year old girl with a 2-day history of fever, maculopapular rash, conjunctivitis, hypotension, and cardiogenic shock. COVID-19 PCR was negative , COVID-19 IgM was negative and COVID-19 IgG was positive. Chest CT axial images show bilateral basal subpleural arcades, peri-lobular thickening (Right), and mild architecture distortion (Left). CT severity score = 3.
Figure 4.
A 10-year-old boy with a 7-day history of fever, maculopapular rash, hypotension and cardiogenic shock. COVID-19 PCR was negative, COVID-19 IgM was negative and COVID-19 IgG was positive. Chest CT axial images shows left subpleural ground glass opacity. CT severity score = 1.
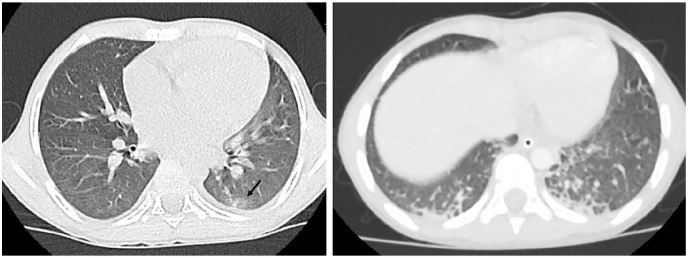
Figure 2.
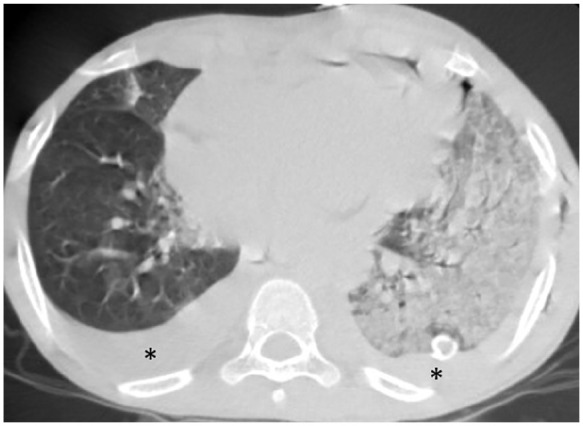
12-year-old boy with known case of acute lymphoblastic leukemia, presented with fever for 4 days, conjunctivitis, maculopapular rash, hypotension and cardiogenic shock he was ventilated due respiratory distress, his COVID status was PCR swab positive , COVID IgM negative COVID IgG positive , ; axial chest CT shows extensive consolidation implicating the left lung (CT severity score= 13). Note the associated pleural effusion on both sides (asterisk). The patient was on ventilatory support.
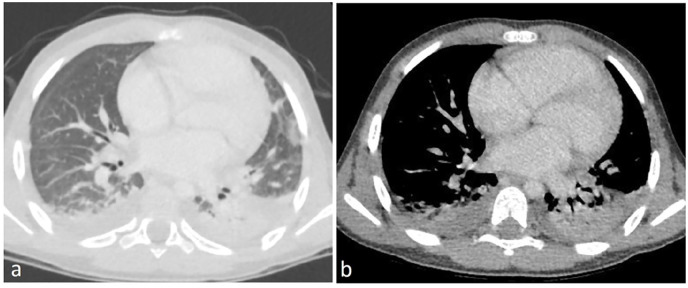
Figure 3.
A 7-year old boy with a 6-day history of fever, hypotension, cardiogenic shock, and respiratory distress; mechanically ventilated. COVID-19 PCR was negative, COVID-19 IgM was negative and COVID-19 IgG was positive. CT chest axial view (a) lung window (b) mediastinal window showing bilateral subpleural consolidation associated with bilateral pleural effusion. CT severity score = 9.
While reviewing patients’ data, we noticed that the majority of patients (87.5%) had an elevated CRP above 20 and that elevated CRP was associated with the distribution of lesions in peripheral lung zones (P = .032, Fisher’s exact). Hyper-ferritinemia was associated with the bilateral distribution of pulmonary lesions (Mann-Whitney z = 2.092 and P = .036). Three patients (9.7%) had very highly elevated ferritin (>1400 µg/L), 14 all of them had stage 4 CT changes.
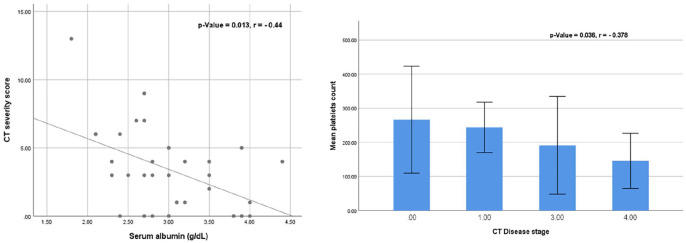
Thrombocytopenia was significantly correlated with CT disease stage (Mann-Whitney z = 2.092 and P = .036). In the correlation analysis of the CT severity score with the biochemical markers, there was a significant negative correlation between the serum albumin level and the CT severity score (Spearman’s rho: −0.44, P = .013) (Figure 5).
Figure 5.
Correlation analysis of serum albumin and CT severity score: the serum albumin was negatively correlated with the CT severity score (Left). The correlation analysis of the mean platelets count and the CT disease stage: the mean platelets count is negatively correlated with CT disease stage (Right).
Twenty-three patients (74.2%) were on supplemental oxygen, low flow nasal cannula, and only 8 patients (25.8%) required conventional mechanical ventilation. The duration of mechanical ventilation was 5 days (IQR 3-5) and the median total length of patients’ stay in the PICU was 5.5 days (IQR 5-8).
Among the studied cases, 9 patients (30%) had underlying comorbidities: 2 cases of Pre-B acute lymphocytic leukemia (ALL), and one case of each of the following: Diabetes Mellitus (DM), medulloblastoma, Hemophagocytic lymphohistiocytosis (HLH), Down Syndrome, seizure disorder, open heart surgery, and an autistic spectrum disorder.
All patients without comorbid conditions were discharged home while 3 patients out of the 9 cases with comorbidities died: 2 cases with pre-B acute lymphoblastic leukemia, and the one case with medulloblastoma, accounting for the mortality rate of 9.7%.
Discussion
Despite its rarity, MIS-C temporally related to SARS-Cov-2 exposure and or infection is of a world-wide significant concern. The characteristics of a considerable number of cases with severe illness and cardiovascular dysfunction requiring intensive care management were extensively published.
The frequency of clinical features (eg, fever, gastrointestinal symptoms, dermatological manifestations, etc.,), biochemical findings, and echocardiographic changes recognized in our patients follow the same pattern of frequency as described in most of the early published data with fewer numbers of studied cases as well as the recent meta-analyses with pooled data and hundreds of studied patients.1,14-16
The relatively lower frequency of respiratory symptoms reported world-wide is clearly noticed in our case series and affirm the previously reported results. It is clearly evident that less than 50% of MIS-C patients endure symptoms suggestive of respiratory system affection. 17
The unique thoracic imaging abnormalities reported in MIS-C resembling the late-stage of adult COVID-19 infection and reflect the consequences of the post-viral hyperinflammatory cytokine storm that is characteristically different from the thoracic imaging findings of acute pediatric COVID-19 infection.18,19 The main radiologic difference between typical pediatric COVID-19 and MIS-C resides in the location of imaging abnormalities. Typical pediatric COVID-19 infection predominantly affects the pulmonary parenchyma leading to lung opacities, 3 that are sometimes described as bilateral peripheral and subpleural airspace opacities, 9 a picture similar to the reported findings of consolidation and pneumonia in adult patients with COVID-19.20,21 In contrast, pediatric MIS-C, often presents with prominent cardiovascular abnormalities, such as heart failure manifesting with cardiomegaly, pulmonary edema, and pleural effusions. Nevertheless, it is reported that MIS-C and COVID-19 characteristic radiographic findings can simultaneously occur in some cases. 22 To our knowledge, this is the first study describing the pulmonary CT changes in hospitalized severe cases of MIS-C managed in the PICU. The association between those changes and the biochemical markers were carefully studied for possible associations. Our results demonstrated that some inflammatory markers changes are associated with certain CT characteristics. Although the inference from the statistical analysis is relatively weak due to the small sample studied, our data reveals intriguing associations between (1) hyper-ferritinemia and the bilateral distribution of pulmonary lesions, (2) elevated CRP and the peripheral distribution of the lesions, (3) hypo-albuminemia and a more severe pulmonary CT score, and (4) thrombocytopenia and a more severe CT disease stage.
The finding of pleural effusion was noticed in slightly more than 50% of our studied patients essentially in accordance with a similarly reported frequency in other studies. 23
Since all our described patients were admitted to the PICU, we anticipate that the interplay of the overwhelming systemic inflammatory response, depressed myocardial function, and possible volume overload could have contributed to the third space loss, pulmonary edema and conceptually to the pathogenesis of the radiologic features detected in more than two thirds of our patients (71%), a frequency higher than that reported in previous studies on different small case series of 50%, 24 52%, 25 and 41%. 26
Likewise, the patten of radiologic findings, specifically the bilaterality of the lesions and the lower incidence of ground glass opacities noticed in our case series, was similar to previously reported findings.20-22
The involvement of the lower lobes described in our study is consistent with the previously noticed description in other MIS-C studies. 9 In addition, our study points toward the predilection of a more peripheral distribution of the lesions (66.7%), and the multiple lobar affection (63.6%).
Unlike late stages of adults with COVID-19 infection, and in agreement with previously published pediatric data, we did not encounter the features of acute respiratory distress syndrome (ARDS).21-27 Other findings in chest radiography in MIS-C patients, not detected in our case series, were cardiomegaly, congestive heart failure, cardiogenic pulmonary edema, atelectasis, or segmental pulmonary embolism. 28
We have also noticed that none of our patients was considered stage 2 in CT staging, although crazy paving which distinguish stage 2 has been reported in some studies, yet it was found to be a rare finding. 25
The presence of lymphadenopathy, whether mediastinal or axillary or both in our patients, was reported by other investigators 23 who stated that the hyperinflammatory state of MIS-C associated with COVID-19 is often associated with adenopathy, which is rare and unusual in the typical pediatric COVID-19 infection.
The overall relatively good prognosis and survival (90.3%) with absence of short-term complications despite critical care interventions in our case series is similar to that reported in other studies. 4 The mortality rate (9.7%) we are reporting is higher than previously reported rate of 1.9% reported from pooled data of 27 16 and 68 studies. 29 We speculated the reason of our higher mortality rate as compared to previous studies resides in the nature of the comorbid conditions we encountered and the late presentation of cases before meeting the final diagnosis and referral to our center. It is noteworthy that 2 of the 3 patients who died in our study were diagnosed with pre-B acute lymphoblastic leukemia and the third case with the diagnosis of medulloblastoma.
In conclusion, the current data indicate that critically ill children with MIS-C might incur pulmonary architectural changes without significant symptomatology; these changes may provide an important helpful tool in evaluating the severity and consequences of the disease, and guide clinicians to a personalized treatment approach. Computerized pulmonary tomography may be considered specifically when inflammatory markers are considerably elevated and when there is a clinical concern for lung injury. Our observations provide an important platform for further detailed studies of pulmonary CT changes in patients with severe MIS-C in larger cohorts and for longer periods of follow-up. The current significant association between some inflammatory markers and certain characteristics of the pulmonary CT changes requires further studies in a larger cohort of patients.
Our study is limited by its retrospective nature and including only a small number of the severe cases of MIS-C who required PICU admission from a single tertiary care center.
Footnotes
Author Contributions: Hanan M. Ibrahim: conception and drafting of the study
Shaimaa Abdelsattar Mohammad: data analysis and interpretation
Eman Fouda: conception and drafting of the study
Khaled Abouelfotouh: data analysis and interpretation.
Neveen M. Habeeb: Echocardiographis data analysis
Ahmed Rezk Rezk: Data collection
Sondos Magdy: data collection, analysis, and critical review of the study.
Ahmad M. Allam: Critical revision of the article.
Sanaa A. Mahmoud: data analysis, final drafting and critical review of the artcile
All authors approved the version to be published
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Hanan M Ibrahim  https://orcid.org/0000-0002-9813-2508
https://orcid.org/0000-0002-9813-2508
Khaled Abouelfotouh  https://orcid.org/0000-0001-7297-4371
https://orcid.org/0000-0001-7297-4371
Ahmed Rezk Rezk  https://orcid.org/0000-0002-6591-9734
https://orcid.org/0000-0002-6591-9734
Sanaa A Mahmoud  https://orcid.org/0000-0002-9667-6763
https://orcid.org/0000-0002-9667-6763
References
- 1. Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607-1608. doi: 10.1016/S0140-6736(20)31094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Emergency preparedness and response: multisystem Inflammatory Syndrome (MIS-C) associated with coronavirus disease 2019 (COVID-19). Health advisory; (https://emergency.cdc.gov/han/2020/han0043.asp) [Google Scholar]
- 3. Godfred-Cato S, Bryant B, Leung J, et al. COVID-19-associated multisystem inflammatory syndrome in children – United States, March-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1074-1080. doi: 10.15585/mmwr.mm6932e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mahmoud S, Fouda EM, Kotby A, et al. The Golden Hours Algorithm for the management of the multisystem inflammatory syndrome in children (MIS-C). Glob Pediatr Health. 2021;8:2333794X21990339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hennon TR, Penque MD, Abdul-Aziz R, et al. COVID-19 associated multisystem inflammatory syndrome in children (MIS-C) guidelines; a western New York approach. Prog Pediatr Cardiol. 2020;57:101232. doi: 10.1016/j.ppedcard.2020.101232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: Version 2. Arthritis Rheumatol. 2021;73(4):e13-e29. doi: 10.1002/art.41616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lai WW, Geva T, Shirali GS, et al. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr. 2006;19(12):1413-1430. doi: 10.1016/j.echo.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 8. Palabiyik F, Akcay N, Sevketoglu E, Hatipoglu N, Sari EE, Inci E. Imaging of multisystem inflammatory disease in children (MIS-C) associated with COVID-19. Acad Radiol. 2021;28(9):1200-1208. doi: 10.1016/j.acra.2021.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697-722. doi: 10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 10. Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from Coronavirus disease 2019 (COVID-19). Radiology. 2020;295(3):715-721. doi: 10.1148/radiol.2020200370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Y, Dong C, Hu Y, et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020;296(2):E55-E64. doi: 10.1148/radiol.2020200843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Francone M, Iafrate F, Masci GM, et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020;30:6808-6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ouldali N, Toubiana J, Antona D, et al. Association of intravenous immunoglobulins plus methylprednisolone vs Immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA. 2021;325(9):855-864. doi: 10.1001/jama.2021.0694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pouletty M, Borocco C, Ouldali N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79(8):999-1006. doi: 10.1136/annrheumdis-2020-217960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 2020;383:347-358. doi: 10.1056/NEJMoa2021756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahmed M, Advani S, Moreira A, et al. Multisystem inflammatory syndrome in children: a systematic review. E Clinical Medicine. 2020;26:100527. doi: 10.1016/j.eclinm.2020.100527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yasuhara J, Watanabe K, Takagi H, Sumitomo N, Kuno T. COVID-19 and multisystem inflammatory syndrome in children: a systematic review and meta-analysis. Pediatr Pulmonol. 2021;56(5):837-848. doi: 10.1002/ppul.25245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Winant AJ, Blumfield E, Liszewski MC, Kurian J, Foust AM, Lee EY. Thoracic imaging findings of multisystem inflammatory syndrome in children associated with COVID-19: what radiologists need to know now. Radiol Cardiothorac Imaging. 2020;2(4):e200346. doi: 10.1148/ryct.2020200346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chao JY, Derespina KR, Herold BC, et al. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 at a tertiary care medical center in New York City. J Pediatr. 2020;223(14-19):14-19.e2 doi: 10.1016/j.jpeds.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24(1):54. doi: 10.1186/s13054-020-02880-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Belhadjer Z, Méot M, Bajolle F, et al. Heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context acute of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi: 10.1161/CIRCULATIONAHA.120.048360 [DOI] [PubMed] [Google Scholar]
- 23. Rostad BS, Shah JH, Rostad CA, et al. Chest radiograph features of multisystem inflammatory syndrome in children (MIS-C) compared to pediatric COVID-19. Pediatr Radiol. 2021;51(2):231-238. doi: 10.1007/s00247-020-04921-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hameed S, Elbaaly H, Reid CEL, et al. Spectrum of imaging findings at chest radiography, US, CT, and MRI in multisystem inflammatory syndrome in children associated with COVID-19. Radiology. 2021;298(1):E1-E10. doi: 10.1148/radiol.2020202543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lima-Setta F, Magalhães-Barbosa MCD, Rodrigues-Santos G, et al. Multisystem inflammatory syndrome in children (MIS-C) during SARS-CoV-2 pandemic in Brazil: a multicenter, prospective cohort study. J Pediatr. 2021;97(3):354-361. doi: 10.1016/j.jped.2020.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Radia T, Williams N, Agrawal P, et al. Multi-system inflammatory syndrome in children & adolescents (MIS-C): A systematic review of clinical features and presentation. Paediatr Respir Rev. 2021;38:51-57. doi: 10.1016/j.prrv.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Foust AM, Phillips GS, Chu WC, et al. International expert consensus statement on chest imaging in pediatric COVID-19 patient management: Imaging findings, imaging study reporting, and imaging study recommendations. Radiol Cardiothorac Imaging. 2020;2(2):e200214. doi: 10.1148/ryct.2020200214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blumfield E, Levin TL, Kurian J, Lee EY, Liszewski MC. Imaging findings in multisystem inflammatory syndrome in children (MIS-C) associated with Coronavirus disease (COVID-19). AJR Am J Roentgenol. 2021;216(2):507-517. doi: 10.2214/AJR.20.24032 [DOI] [PubMed] [Google Scholar]
- 29. Hoste L, Van Paemel R, Haerynck F. Multisystem inflammatory syndrome in children related to COVID-19: a systematic review. Eur J Pediatr. 2021;180(7):2019-2034. doi: 10.1007/s00431-021-03993-5 [DOI] [PMC free article] [PubMed] [Google Scholar]