Abstract
Metastatic tumours to the ovary comprise 10–25% of ovarian malignancies and may originate from various primary sites. Here, the case of a 49-year-old female patient who presented with periumbilical nodules and abdominal bloating is reported. She was found to have bilateral ovarian tumours with peritoneal carcinomatosis and ascites. Primary ovarian cancer was suspected while no contributory gastrointestinal lesion was detected by imaging studies and endoscopic examinations. Three cycles of neoadjuvant chemotherapy were administered, followed by interval debulking surgery. Appendiceal cancer was highly suspected based on analysis of a frozen section obtained during surgical debulking. Following the pathology investigation, the patient was finally diagnosed with primary appendiceal adenocarcinoma. She underwent chemotherapy comprising irinotecan and fluorouracil. Due to disease progression despite several chemotherapy regimens, the patient declined further treatment and was lost to follow-up 1 year after the debulking surgery. Metastatic tumours to the ovary may mimic primary ovarian cancers and often present with nonspecific manifestations. Therefore, meticulous exploration of the primary site is warranted if the diagnosis is clinically suspicious.
Keywords: Ovarian neoplasms, appendiceal neoplasms, neoplasm metastasis, case report, metastatic tumour, primary cancer
Introduction
Metastatic tumours to the ovary comprise 10–25% of ovarian malignancies. 1 The most common origin of such metastases varies among studies, and includes cancer of the breast, large intestine, stomach, and endometrium. Other less common sites reported in the literature include the small intestine, appendix, pancreas, biliary tract, and lung. 2 An ovarian tumour may often be detected before the primary cancer diagnosis, as symptoms attributable to ovarian lesions or peritoneal seeding may be present in advance of clinical manifestations associated with the primary tumours. 3 This condition makes identifying the primary cancer site initially quite difficult, and an inaccurately assigned diagnosis may result in suboptimal treatment.
Here, an appendiceal cancer case initially presenting with bilateral ovarian tumours combined with peritoneal carcinomatosis and massive ascites is presented. The appendiceal cancer was not diagnosed after surgical exploration. Issues concerning metastasis to the ovaries mimicking primary ovarian cancer are thus addressed.
Case report
The study was approved by the institutional review board of the National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University. Written informed consent to publish this case was obtained from the patient and the reporting of this study conforms to CARE guidelines. 4 A 49-year-old female patient, without known medical conditions or contributory family history, presented at the gastroenterology outpatient clinic of National Cheng Kung University Hospital in February 2020, due to noting the presence of a periumbilical nodule for 2 months. Associative symptoms included abdominal bloating, decreased urine output, and mild epigastralgia. Upon physical examination, a 2-cm nodule at the umbilical area was palpated. In an attempt to elucidate the cause of her discomfort, oesophagogastroduodenoscopy and colonfibroscopy were performed, but no contributory lesions were detected. Abdominal computed tomography (CT) revealed peritoneal carcinomatosis, massive ascites, and bilateral ovarian tumours (Figure 1). The appendix appeared to be normal in diameter without apparent imaging evidence of tumours (Figure 2). An ascitic tap was performed, and malignancy was detected through a cytology study, after which, the patient was referred to the Gynaecology Department of National Cheng Kung University Hospital (in March 2020).
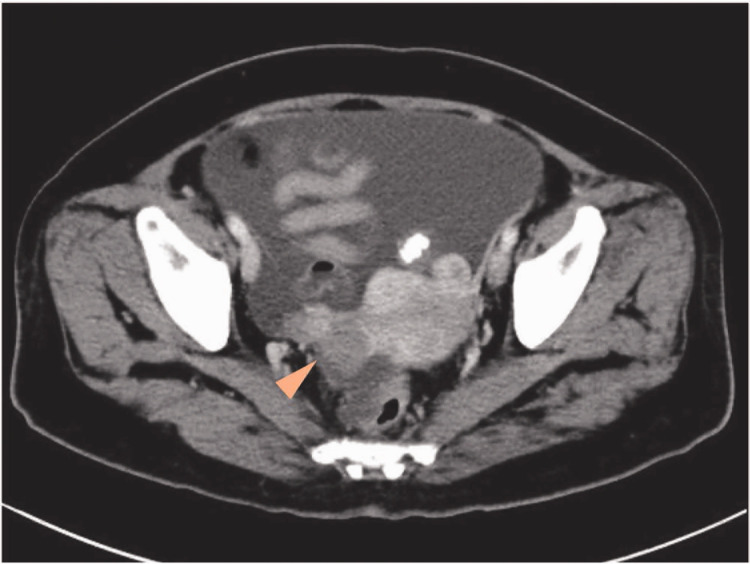
Figure 1.
Abdominal computed tomography image from a 49-year-old female patient, showing bilateral irregularly enhanced adnexal masses of 4.1 cm and 2.5 cm, respectively (arrow head).
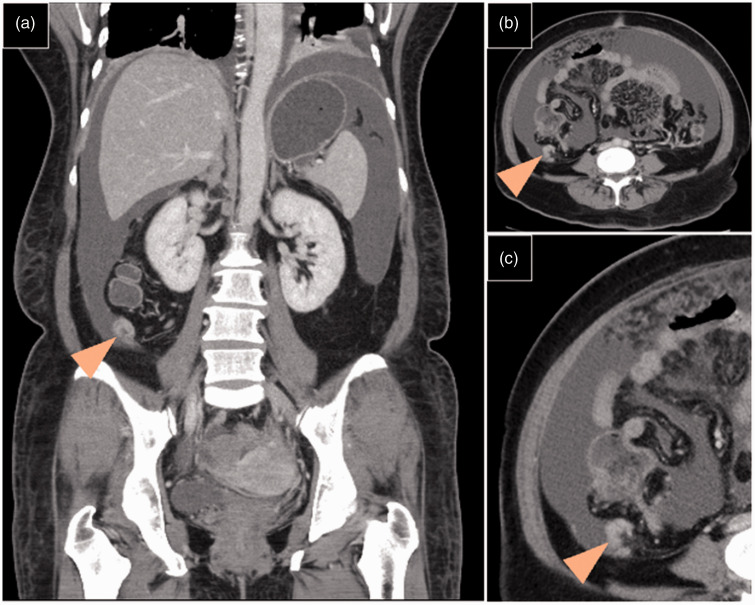
Figure 2.
Abdominal computed tomography images from a 49-year-old female patient, showing a normal sized appendix without obvious visible tumours (arrow heads): (a) coronal view; and (b and c) axial view images obtained during the same period.
Raised serum cancer antigen (CA) 125 (355 U/mL) was noted, while levels of other tumour markers, including CA 19-9 (5.6 U/mL) and carcinoembryonic antigen (CEA; 1.2 ng/mL), were within normal range, giving a tumour marker profile that was more compatible with a primary epithelial ovarian cancer. As the patient was experiencing dyspnoea and bilateral pleural effusion was detected by chest X-ray, thoracentesis was performed, which confirmed the presence of malignant cells. Consequently, ovarian cancer, clinical-stage cT3aN0M1, was presumptively diagnosed. Given the extent of the disease and relatively poor general performance status of the patient (Eastern Cooperative Oncology Group/World Health Organization [ECOG/WHO] performance status 2, corresponding to the capability of ambulation and selfcare but not working), 5 neoadjuvant chemotherapy was administered, comprising paclitaxel (175 mg/m2, intravenous [i.v.] infusion in 500 ml of 5% glucose over 180 min, day 2) and carboplatin (area under the free carboplatin plasma concentration versus time curve [AUC] 5, i.v. infusion in 250 ml of 5% glucose over 120 min, day 2) following concurrent bevacizumab (600 mg, i.v. infusion in 100 ml of 0.9% NaCl over 90 min, day 1). After three cycles of chemotherapy, the third of which was administered without concurrent bevacizumab, follow-up CT showed that the bilateral ovarian tumours were stable in size, and there was slight shrinkage of the peritoneal tumours, however, the ascites had recurred. As a result, interval debulking surgery was performed.
Intraoperatively, upon entering the intra-abdominal cavity, massive ascites was encountered and drained. Extensive omental caking was found, and severe adhesion between the colon and small bowel was noted. There was diffuse tumour seeding over the liver surface, gallbladder, mesentery, and diaphragm. The right ovarian tumour, 2.3 × 2.2 × 1.5 cm in size and tan-grey in colour, was resected, followed by a subtotal hysterectomy and bilateral salpingo-oophorectomy. A frozen section of the right ovarian tumour showed adenocarcinoma, suspected to have metastasized from the gastrointestinal tract, which was meticulously explored. The swollen appendix, with a spreading tumour on the surface, was excised, and biopsies of the liver tumour and omentum were performed (Figure 3). Permanent sections of formalin-fixed paraffin embedded specimens confirmed appendiceal adenocarcinoma with bilateral ovarian, uterine, and peritoneal metastases. Invasive non-mucinous adenocarcinoma with in situ lesion was revealed in the appendix, while both ovaries showed involvement with adenocarcinoma of similar morphology. Immunohistochemical analyses of ovarian tissue sections showed that the tumour cells were positive for DNA-binding protein SATB2 and homeobox protein CDX-2, but negative for paired box protein Pax-8 and oestrogen receptor, suggesting metastatic adenocarcinoma of appendiceal origin (Figure 4). At 2 weeks following debulking surgery, and after referral to an oncologist, the patient underwent chemotherapy with irinotecan (150 mg/m2, i.v. infusion in 250 ml of 0.9% NaCl over 90 min) and fluorouracil (2400 mg/m2, i.v. infusion in 500 ml of 0.9% NaCl over 46 h) for six cycles. Due to disease progression, a subsequent regimen was administered, including capecitabine (1500 mg orally, twice daily) for 6 months and then combined oxaliplatin (85 mg/m2, i.v. infusion in 250 ml of 5% glucose over 120 min) and fluorouracil (2400 mg/m2, i.v. infusion in 500 ml of 0.9% NaCl over 46 h) for three cycles. The patient then declined further treatment due to disease progression, and was lost to follow-up 1 year after surgery.
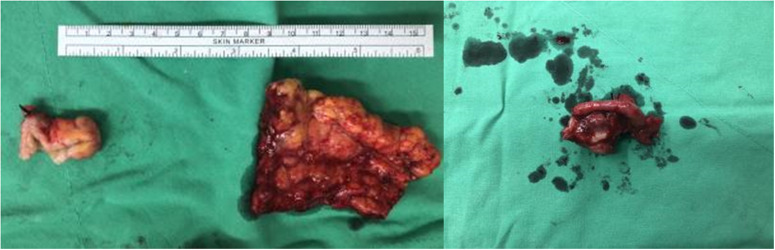
Figure 3.
Images of excised tissue from a 49-year-old female patient, showing an appendiceal tumour of approximately 2 cm in the largest diameter, and multiple omental seeding nodules on gross inspection (left); and the 2.3 × 2.2 × 1.5-cm right ovary, of tan-grey appearance and solid consistency, with a mild granular surface (right).
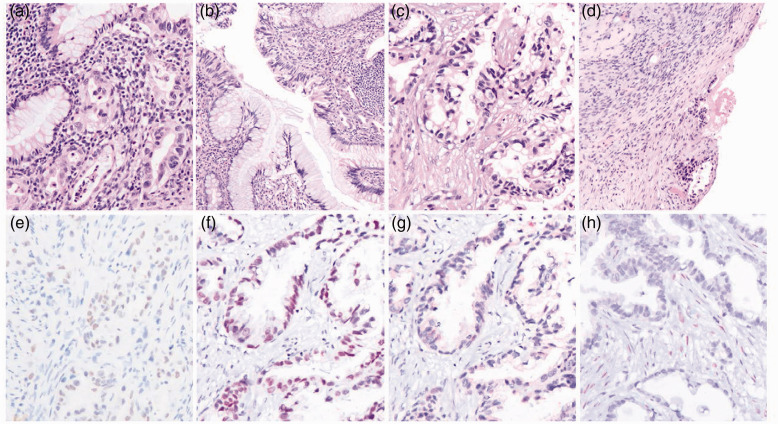
Figure 4.
Pathologic features of appendiceal and right ovarian tumours from a 49-year-old female patient: (a and b) haematoxylin and eosin (H&E)-stained appendix tissue section showing (a) invasive non-mucinous adenocarcinoma, composed of glands lined with columnar cells containing irregular nuclei infiltrating the wall (original magnification, ×200) and (b) a focus of in situ lesion in the appendix (original magnification, ×100); (c and d) H&E-stained right ovary tissue showing (c) adenocarcinoma with morphology similar to the appendix (original magnification, ×200) and (d) ovarian surface involvement (original magnification, ×100); and (e–h) immuno-stained right ovary tumour cells with focal positivity for (e) DNA-binding protein SATB2 and (f) homeobox protein CDX-2, but negativity for (g) paired box protein Pax-8 and (h) oestrogen receptor, suggestive of a metastatic adenocarcinoma of appendiceal origin (e–g original magnification, ×200).
Discussion
The initial presentation of a metastatic malignancy to the ovaries arises from the ovarian lesions themselves, that may have developed from gastrointestinal and breast cancer. Symptoms caused by metastatic tumours to the ovaries often mimic primary ovarian cancer, including abdominal pain (42% of cases), postmenopausal bleeding (18% of cases), and bloating (15% of cases). 6 Only 34% of patients with metastatic ovarian cancer have symptoms related to the primary sites. 7 The imaging features of metastatic ovarian cancers may resemble primary ovarian cancers, making diagnosis difficult without histological investigation. 3 Even though the presence of bilateral ovarian tumours raises the suspicion of secondary malignancies, it is also a common feature of primary undifferentiated and serous carcinomas. 8 A previous study of serum CA 125 to CEA ratio in differentially diagnosing between ovarian and colorectal adenocarcinoma showed that a ratio greater than 25 was highly specific and sensitive, and was associated with an accuracy rate of about 94% in terms of distinguishing between ovarian and colorectal origins of adenocarcinomas. 9 In a case series of 18 patients with nongenital metastatic ovarian tumours compared with 25 cases of primary ovarian cancers the sonographic features of a complex adnexal tumour with a raised serum CA 125 level (>170 U/mL) were found to be predictive of primary ovarian cancer. 10 However, in the present case, the CA 125 to CEA ratio was 355 U/ml to 1.2 ng/ml, and the high CA 125 level mimicked primary ovarian cancer at the initial diagnosis. 9
A treatment strategy of neoadjuvant chemotherapy with interval debulking surgery may be applied to patients with an advanced-stage ovarian tumour. 11 The present patient, with an ECOG/WHO performance status of 2, underwent colonfibroscopy and panendoscopy that revealed a normal mucosa layer, and abdominal CT showed only an adnexal mass without an enlarged gastrointestinal tract tumour. Pleural effusion and ascites cytology resulted in a diagnosis of malignant adenocarcinoma. In the CHORUS and EORTC 55971 clinical trials,11,12 neoadjuvant therapy led to similar progression-free survival and overall survival compared with primary debulking surgery. Therefore, the therapeutic strategy for the present case was neoadjuvant therapy followed by interval debulking surgery.11,12 According to case findings, a preoperative evaluation is vital. Fine-needle aspiration cytologic cells combined with immunochemistry with cytokeratin 7 and Pax-8 has been suggested as a tool that may be used to distinguish the primary site. 13
Neoplasms of the appendix are rare, occurring in approximately one or two people per million per year. Based on the Surveillance, Epidemiology, and End Results database, the most common cell type is epithelial adenocarcinoma, while mucinous adenocarcinoma (37%) is the most common epithelial subtype.14,15 In an investigation of presenting symptoms, up to 21% of patients were asymptomatic, while only two of those undergoing colonoscopy were diagnosed with appendiceal adenocarcinoma preoperatively. 16 In two retrospective reviews of 74 cases of appendiceal tumours among 7 970 patients and 31 cases among 5 307 patients undergoing appendectomies, the most common presentation was shown to be appendicitis.17,18 The remainder of the sample included incidental detection surgery, pelvic abscesses, gastrointestinal symptoms, and bowel obstructions.17,18
In conclusion, metastatic tumours to the ovary often present with nonspecific symptoms and may mimic primary ovarian cancers. Preoperative imaging studies and tumour markers may provide clues to the presence of secondary ovarian cancers, for which the primary sites should be explored as management of the cases may vary significantly. The present case displays a clinical scenario in which the primary site of metastatic ovarian tumours was not identified until interval debulking surgery, highlighting the importance of pretreatment evaluation.
Acknowledgements
We thank the Skeleton Materials and Bio-compatibility Core Lab, Research Centre of Clinical Medicine, and National Cheng Kung University Hospital (NCKUH-11002039) for their assistance with this project.
Footnotes
Declaration of conflicting interest: The Authors declare that there is no conflict of interest
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Chih-Wei Lin https://orcid.org/0000-0002-5353-639X
Pei-Ying Wu https://orcid.org/0000-0002-8168-6125
References
- 1.Kubecek O, Laco J, Spacek J, et al. The pathogenesis, diagnosis, and management of metastatic tumors to the ovary: a comprehensive review. Clin Exp Metastasis 2017; 34: 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang JJ, Cao DY, Yang JX, et al. Ovarian metastasis from nongynecologic primary sites: a retrospective analysis of 177 cases and 13-year experience. J Ovarian Res 2020; 13: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Waal YR, Thomas CM, Oei AL, et al. Secondary ovarian malignancies: frequency, origin, and characteristics. Int J Gynecol Cancer 2009; 19: 1160–1165. [DOI] [PubMed] [Google Scholar]
- 4.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 5.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5: 649–655. [PubMed] [Google Scholar]
- 6.Moore RG, Chung M, Granai CO, et al. Incidence of metastasis to the ovaries from nongenital tract primary tumors. Gynecol Oncol 2004; 93: 87–91. [DOI] [PubMed] [Google Scholar]
- 7.Lobo J, Machado B, Vieira R, et al. The challenge of diagnosing a malignancy metastatic to the ovary: clinicopathological characteristics vary and morphology can be different from that of the corresponding primary tumor. Virchows Arch 2017; 470: 69–80. [DOI] [PubMed] [Google Scholar]
- 8.Willmott F, Allouni KA, Rockall A. Radiological manifestations of metastasis to the ovary. J Clin Pathol 2012; 65: 585–590. [DOI] [PubMed] [Google Scholar]
- 9.Yedema CA, Kenemans P, Wobbes T, et al. Use of serum tumor markers in the differential diagnosis between ovarian and colorectal adenocarcinomas. Tumour Biol 1992; 13: 18–26. [DOI] [PubMed] [Google Scholar]
- 10.Bruchim I, Ben-Harim Z, Piura E, et al. Preoperative clinical and radiological features of metastatic ovarian tumors. Arch Gynecol Obstet 2013; 288: 615–619. [DOI] [PubMed] [Google Scholar]
- 11.Vergote I, Coens C, Nankivell M, et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol 2018; 19: 1680–1687. [DOI] [PubMed] [Google Scholar]
- 12.Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 2015; 386: 249–257. [DOI] [PubMed] [Google Scholar]
- 13.Park CK, Malinowski DP, Cho NH. Diagnostic algorithm for determining primary tumor sites using peritoneal fluid. PLoS One 2018; 13: e0199715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leonards LM, Pahwa A, Patel MK, et al. Neoplasms of the appendix: pictorial review with clinical and pathologic correlation. Radiographics 2017; 37: 1059–1083. [DOI] [PubMed] [Google Scholar]
- 15.Turaga KK, Pappas SG, Gamblin T. Importance of histologic subtype in the staging of appendiceal tumors. Ann Surg Oncol 2012; 19: 1379–1385. [DOI] [PubMed] [Google Scholar]
- 16.Dietrich CS, 3rd, Desimone CP, Modesitt SC, et al. Primary appendiceal cancer: gynecologic manifestations and treatment options. Gynecol Oncol 2007; 104: 602–606. [DOI] [PubMed] [Google Scholar]
- 17.Connor SJ, Hanna GB, Frizelle FA. Appendiceal tumors: retrospective clinicopathologic analysis of appendiceal tumors from 7,970 appendectomies. Dis Colon Rectum 1998; 41: 75–80. [DOI] [PubMed] [Google Scholar]
- 18.Esmer-Sanchez DD, Martinez-Ordaz JL, Roman-Zepeda P, et al. Appendiceal tumors. Clinicopathologic review of 5,307 appendectomies. Cir Cir 2004; 72: 375–378 [In Spanish, English abstract]. [PubMed] [Google Scholar]