Abstract
The Notch signaling pathway, which is highly conserved from sea urchins to humans, plays an important role in cell-differentiation, survival, proliferation, stem-cell renewal, and determining cell fate during development and morphogenesis. It is well established that signaling pathways are dysregulated in a wide-range of diseases, including human malignancies. Studies suggest that the dysregulation of the Notch pathway contributes to carcinogenesis, cancer stem cell renewal, angiogenesis, and chemo-resistance. Elevated levels of Notch receptors and ligands have been associated with cancer-progression and poor survival. Furthermore, the Notch signaling pathway regulates the transcriptional activity of key target genes through crosstalk with several other signaling pathways. Indeed, increasing evidence suggests that the Notch signaling pathway may serve as a therapeutic target for the treatment of several cancers, including breast cancer. Researchers have demonstrated the anti-tumor properties of Notch inhibitors in various cancer types. Currently, Notch inhibitors are being evaluated for anticancer efficacy in a number of clinical-trials. However, because there are multiple Notch receptors that can exhibit either oncogenic or tumor-suppressing roles in various cells, it is important that the Notch inhibitors are specific to particular receptors that are tumorigenic in nature. This review critically evaluates existing Notch inhibitory drugs and strategies and summarizes the previous discoveries, current understandings, and recent developments in support of Notch receptors as therapeutic targets in breast cancer.
Keywords: Breast cancer, Notch receptor, γ-secretase inhibitors, Monoclonal antibodies, Chemo-resistance
1. Introduction
Breast cancer (BC) is the second leading cause of cancer deaths among women worldwide. In 2019, approximately 268,600 new cases of invasive BC and 62,930 new cases of in situ BC are estimated to be diagnosed, along with 41,760 BC-related deaths, in the U.S. alone. The majority of BCs are estrogen receptor-positive (ER+ve) and can be treated using anti-hormonal therapy; however, recurrence is frequently observed in BC patients after five years of endocrine therapy. The dysregulation of several signaling pathways, including Notch, contributes to cancer progression and recurrence. Cross-talk between estradiol and Notch signaling has a major role in human breast carcinogenesis and angiogenesis [1–4]. In fact, recent studies have established that Notch signaling is dysregulated in multiple cancer types. Notch signaling contributes significantly to cell survival, proliferation, differentiation, apoptosis, tissue patterning, cell-fate decision, and morphogenesis [2]. Therefore, the Notch pathway might serve as a promising target for the treatment of BC. For example, cleavage of Notch receptors in the cytoplasm by γ-secretase is a major step in their activation, and inhibition of γ-secretase arrests the signaling pathway [3, 4]. Recent studies suggest that γ-secretase inhibitors (GSIs) could be promising therapeutic agents for the treatment of cancers [5]. However, Notch receptors can act as either tumor suppressors or oncogenes, depending upon the cell context. Therefore, Notch inhibitors must be context-specific. In the present review, we summarize the established knowledge, as well as recent advancements, regarding the Notch signaling pathway in BC and evaluate the potential of its inhibition as a therapeutic approach for BC treatment.
2. Structure of Notch Receptors
Notch genes, which are highly conserved from sea urchins to humans, encode transmembrane receptors. Initially, Notch receptors were identified as responsible for a specific “notch” shaped phenotype on the wings of Drosophila melanogaster [1, 2]. In mammals, there is one ortholog (Notch 1) of the single Notch receptor in Drosophila; however, there are three additional mammalian Notch receptors (Notch 2–4), as well. Notch receptors consist of three domains: an extracellular domain (NECD), a transmembrane domain (NTM), and an intracellular domain (NICD) [3]. The NECDs of Notch 1 and Notch 2 consist of 36 repeats of epidermal growth factor (EGF)-like repeats, which are required for ligand interactions, whereas the NECDs of Notch 3 and Notch 4 contain 34 and 29 EGF-like repeats, respectively [4, 6–8]. The EGF- like repeats are followed by a negative regulatory region (NRR), which consists of cysteine-rich Lin12 (N/Lin12) repeats that modulate the interactions between the NECD and the membrane-bound NICD [9, 10]. The Lin12 repeats prevent metalloprotease-driven, ligand-independent cleavage to stabilize the interactions between the subunits [11, 12]. The NICD also includes an RBP-jk association molecule (RAM) domain, followed by seven ankyrin (ANK) repeats, two nuclear localization signals (NLSs), a trans-activation domain (TAD), which ends with a polyglutamine region (OPA), and a PEST sequence rich in proline (P), glutamic acid (E), serine (S), and threonine (T) residues [13–16]. The multiple phosphorylation sites present in the C-terminal region of the PEST sequence are responsible for the stability of NIC and consecutively trigger its ubiquitination [16–18] (Fig. 1). The NTM, which consists of a short extracellular region with a pair of highly conserved cysteine residues, mainly participates in heterodimerization [13, 14].
Figure 1: Structure of Notch receptors and ligands.
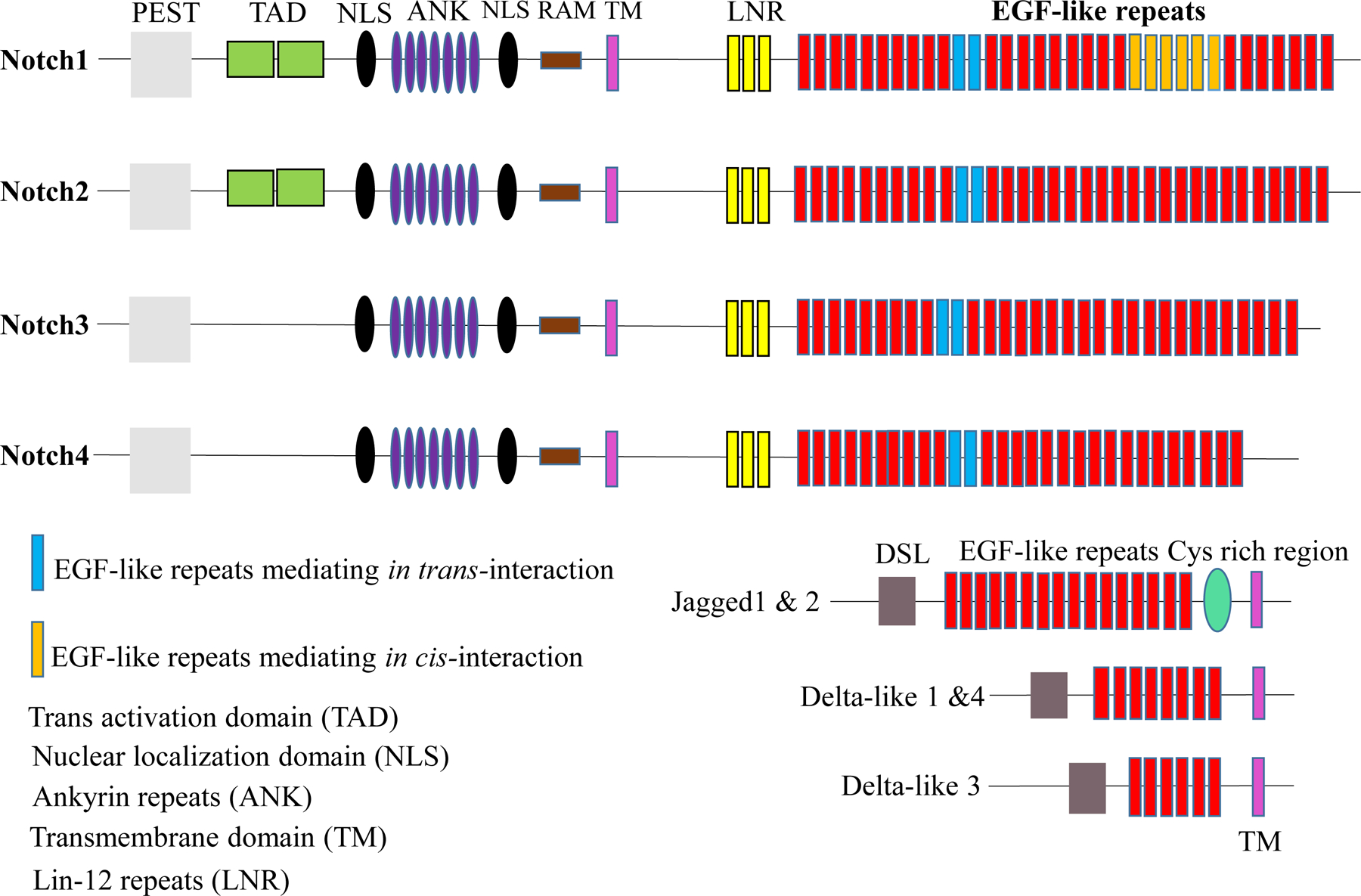
Notch proteins are a highly conserved family of transmembrane receptors. Notch receptors and ligands contain multiple domains. The extracellular domains (NECDs) of Notch receptors 1–4 and their ligands (Jagged 1, Jagged 2, Dll1, Dll3, and Dll4) contain EGF-like repeats. Notch 1 and Notch 2 contain 36 EGF-like repeats, whereas Notch 3 and Notch 4 contain 34 and 29, respectively. The intracellular domains (NICDs) of Notch 1 and 2 contains a RAM (RBP-jk association molecule) domain, NLSs (Nuclear localization signals), an ANK (Ankyrin repeat) domain, a TAD (Trans-activation domain), and a PEST domain. The NICDs of Notch 3 and Notch 4 are similar, but the TAD is absent in both. The extracellular domain of Serrate-like ligands Jagged 1 and Jagged 2 consists of a DSL domain, EGF-like repeats, and a Cys-rich region. The extracellular domain of the Delta-like ligands (Dll1, Dll3, and Dll4) is similar, but the Cys-rich region is absent.
3. Maturation of Notch Receptors
The Notch precursor protein is fucosylated through its interaction with O- fucosyltransferase 1 (POFUT1 in mammals) in the endoplasmic reticulum [19–21]. The fucosylated protein is then transported and subjected to proteolytic cleavage by a Furin-like convertase at site 1 (S1) in the Golgi complex [22]. Finally, the Notch precursor is glycosylated by the Fringe family of N-acetyl-glucosaminidyl transferases, which add N-acetylglucosamine to O-linked fucose on the EGF-like repeats [23]. This matured Notch receptor gets transported on to the cell surface as a heterodimer (Fig. 2).
Figure 2: Maturation of Notch receptors.
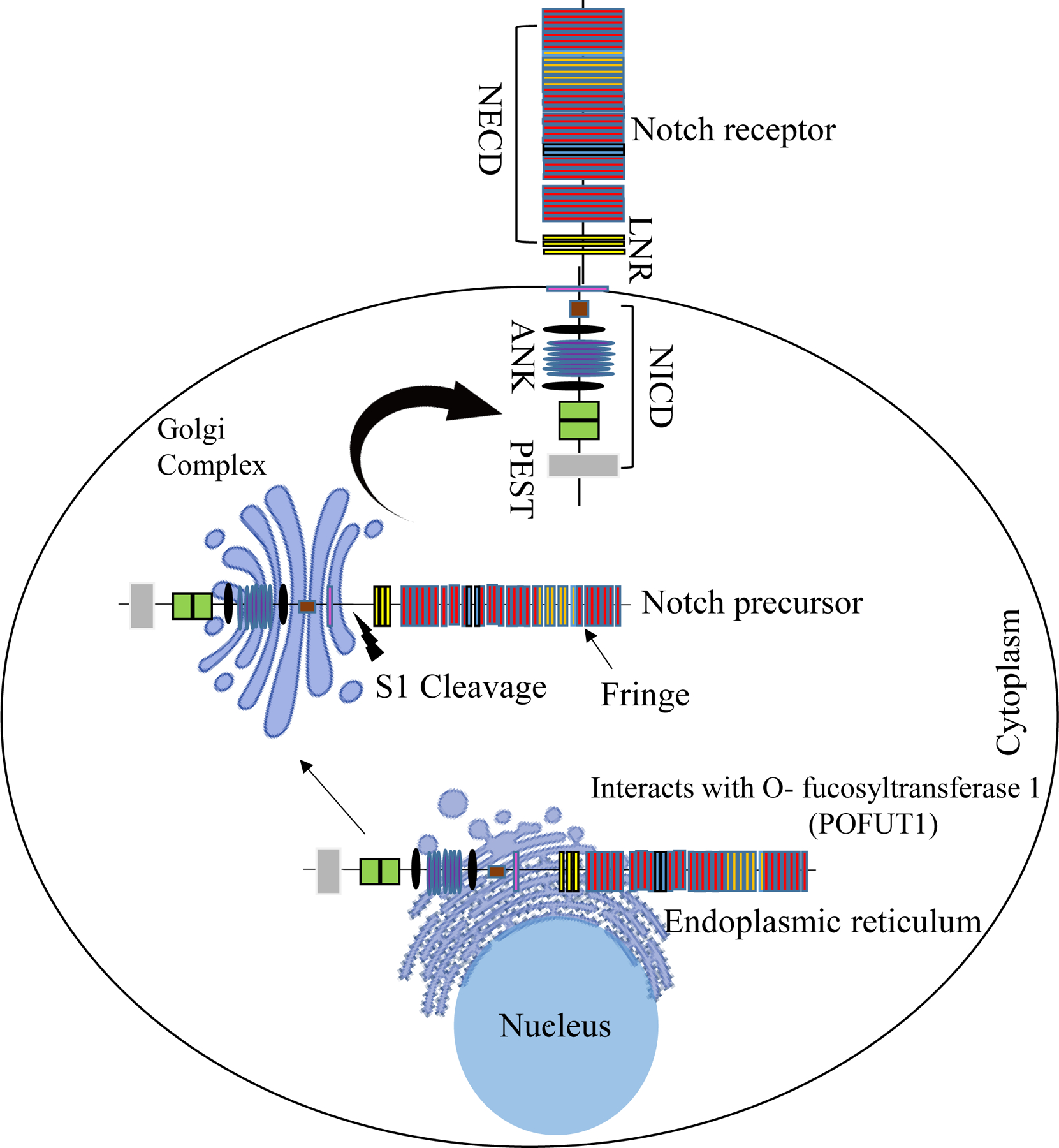
Notch receptors mature in the endoplasmic reticulum (ER) and Golgi complex. Fucosylation is essential and occurs through the interaction between the Notch precursor protein and O-fucosyltransferase 1 (OFUT1 in Drosophila, POFUT1 in mammals) in the ER. The fucosylated Notch precursor is then transported to the Golgi complex, where proteolytic cleavage by Furin-like convertase at site 1 (S1) occurs. Finally, the matured Notch is transported to the cell surface.
4. Activation of Notch Receptors
4.1. Canonical Pathway
In addition to the four Notch receptors, five canonical ligands have been identified in mammals, including humans: Delta-like ligand 1 (Dll1), Dll3, Dll4, and Serrate-like ligands jagged 1 and 2 [2]. The Notch receptors and ligands are type I cell surface proteins, and cell-cell interactions are instrumental for the activation of the Notch signaling pathway [24, 25]. Activation of Notch receptor is mediated by a sequence of proteolytic events. A trans-interaction between a Notch receptor and the Delta/Serrate/Lag-2 (DSL) ligand of an adjacent cell initiates the Notch signaling pathway in the receptor-bearing cell [26]. Contrarily, cis-interactions between the receptors and ligands on a single cell lead to pathway suppression [27]. Upon the successive trans-interaction between Notch receptor and ligand, conformational change in the receptor occurs, allowing metalloprotease 10 (ADAM10) or 17 (ADAM17)/ TACE (TNFα converting enzyme) mediated proteolytic cleavage at NECD site 2 (S2) [28]. This proteolytic cleavage produces the membrane-bound Notch extracellular truncation (NEXT) protein, which is further subjected to a second proteolytic cleavage at the NTM site 3 (S3) by γ-secretase [29, 30]. Γ-secretase consists of five subunits: presenilin 1, presenilin 2, nicastrin, presenilin enhancer 2 (Pen-2), and anterior pharynx-defective 1 (Aph1) [31–33]. Presenilin, an aspartyl protease, forms the catalytic subunit of the γ-secretase complex [34, 35]. Nicastrin is required to maintain the stability of presenilin and helps regulate the intracellular trafficking of the complex [31, 36, 37]. Aph1 is required to support the proteolytic activities of the complex, and Pen2 is responsible for stabilizing the complex after proteolysis [38, 39]. γ-secretase cleaves the Notch receptor in the plasma membrane or in endosomal compartments of the cell and releases the NICD into the cytoplasm. It has been reported that NICD produced from cleavage in the plasma membrane is more stable than that produced in the endosomal compartments [40, 41]. The NICD translocates from the cytoplasm to the nucleus, where it binds to and activates the transcription factor CSL (also termed CBF1 or RBP-Jk), through which it transcribes Notch target genes.
CSL represses the transcriptional activity of its target genes by binding to the DNA as part of a larger co-repressor complex consisting of HDACs, N-CoR, CSL interacting repressor (CIR) and SMRT/MINT/SPEN [42]. NICD interacts with CSL through its RAM domain and replaces the co-repressors in the CSL complex [42, 43]. The interaction between the ANK domain of the NICD with CSL facilitates the recruitment of the co-activator Mastermind-like 1 (MAML1) [44]. This ternary complex then recruits other co-activators, such as p300 [45] and PCAF/GCN5 [46], through the C-terminal region of MAML1. These co-activators, in turn, convert CSL from a transcriptional repressor to a transcriptional activator. The NICD-CSL-MAML1-P300 complex mediates the transcription of Hes 1 (hairy/enhancer-of-split), Hey 1 (Hes-related with YRPW motif), Cyclin D1, p21, p27cip1/waf1, cMyc, Survivin, Slug, pre-Ta (pre-T cell receptor alpha chain), GATA3 and Nanog. The Notch signaling pathway also activates the nuclear factor-kappa B (NFκB) pathway [47] (Fig. 3).
Figure 3: Schematic representation of Notch receptor activation.
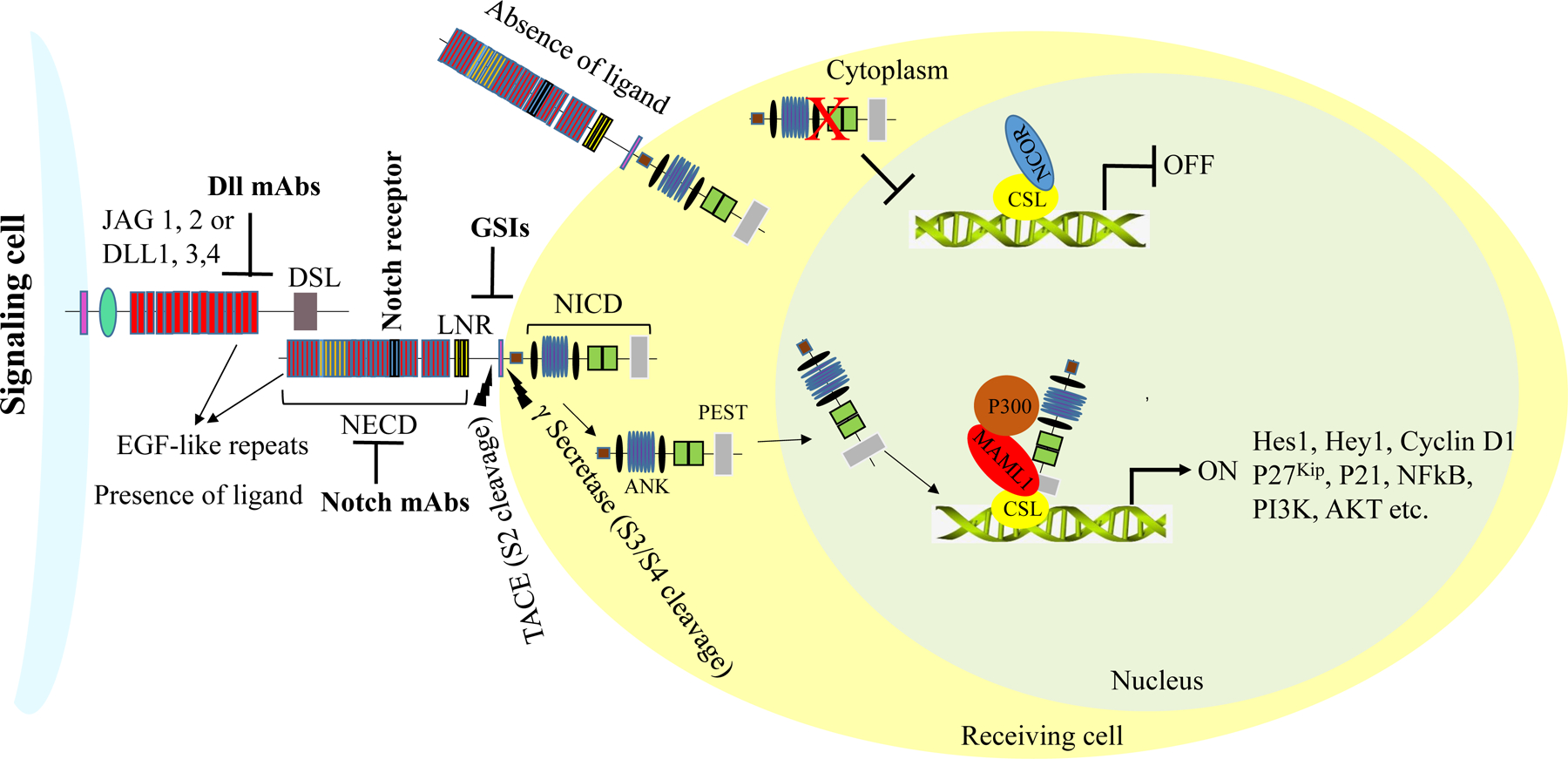
Notch receptors are activated upon binding to Serrate- and Delta-like ligands present on the cell membranes of adjacent cells. Following successful activation, Notch receptors undergo a series of proteolytic cleavages at site 2 (S2), mediated by metalloprotease 10 (ADAM10) and TACE (TNFα converting enzyme). Additional proteolytic cleavages at the transmembrane domain (NTD) are carried out by a multi-subunit complex, γ-secretase, at site 3 (S3). The Notch intracellular domains (NICDs) are then released into the cytoplasm. The NICDs further translocate into the nucleus, where they displace histone deacetylase and co-repressors in CSL repressor complexes and recruit MAML1 and histone acetyltransferase p300 to form active transcriptional complexes, which regulate the transcriptional activity of Notch target genes.
4.2. Non-canonical Pathway
Notch signaling can also be activated through non-canonical, ligand-independent pathways. Three types of non-canonical Notch pathways have been characterized: CSL-independent (Type I); S3 cleavage-independent (Type II); and Notch cleavage- and NICD release-independent (Type III) [48]. The functions of these non-canonical pathways have been identified predominantly in undifferentiated cell populations, such as stem/progenitor and embryonic/primordial cells, and they have been found to contribute to the maturation of both CD4+ and CD8+ single-positive thymocytes [49].
5. Role of the Notch Signaling Pathway in Breast Cancer
Cellular functions are precisely monitored and tightly controlled in normal cells but not in cancerous cells. The dysregulation of developmental pathways has been correlated with several diseases, including cancer [50, 51]. It has been established that organ development and tumorigenesis share similar mechanisms and that the Notch signaling pathway is crucial for embryonic development [52]. Studies suggest that developmental pathways such as Wnt, Hedgehog, and Notch were engaged in tumor cell development, progression, and survival [47, 53, 54]. Notch signaling plays an import role in the progression of several cancers, including BC [47, 51, 55]. The expression of the Notch receptors and their ligands was found to be highly elevated in BC tissues and correlated with poor survival of human BC patients [55–57]. In 1987, it was discovered that the Notch 4 locus is a common integration site for mouse mammary tumor virus (MMTV). This integration results in the constitutive ligand-independent activation of Notch 4, leading to the release of the NICD and thus increased activation of its target genes. These events facilitate the development of mammary adenocarcinoma [58]. Notch 1 was also found to be involved in the development of murine mammary tumors. Upon MMTV insertion, Notch 1 is truncated and can act as an oncogene [59]. Notch 1 is highly expressed in poorly differentiated breast tumors and it is associated with poor overall survival [55]. Interestingly, elevated levels of Notch 2 have been correlated with high rates of disease-free survival [60]. These observations suggest antagonistic functions of Notch 1 and Notch 2. The tumor-suppressing function of Notch 3 has also been suggested, as it upregulates Cyclin D1 in human BC cells and causes the accumulation of p27Kip, which leads to cell cycle arrest at the G0/G1 phase [61]. In contrast, triple-negative BC (TNBC) cells ectopically expressing Notch 4 showed increased proliferation and invasiveness, whereas inhibition/knockdown of Notch4 decreased the cell proliferation, invasion, tumor volume and tumorigenicity. Several studies have established that Notch signaling exhibits its oncogenic properties through its interactions with other signaling pathways, such as Ras, TGFβ, and Wnt in the mammary gland tumorigenesis [47]. Weijzen et al. demonstrated a significant correlation between the expression of Notch1 and H-Ras (a known oncogene) in human primary breast ductal carcinoma cases [62, 63]. Notch signaling also regulates cellular processes, including apoptosis [51, 64], angiogenesis [65, 66], and the epithelial-to-mesenchymal transition (EMT) [67]. Notch prevents apoptosis in breast epithelial cells by inducing Akt signaling through the secretion of an autocrine signaling protein or the downregulation of PTEN expression [51, 64, 68].
Cancer stem-like cells/cancer-initiating cells (CSC/CIC) also play an important role in the initiation and metastasis of BC [69, 70]. It is well known that Notch1 influences the self-renewal of breast CSCs/CICs by increasing ErbB2 transcription [71, 72]. A comparison of activated Notch receptors in breast CSCs/CICs versus luminally differentiated CD24+ cells indicated that Notch4 is highly activated in breast CSC-enriched cells [73]. These suggest that Notch pathway has a major participation and multiple roles during breast tumor progression.
6. Role of Notch Receptor Ligands in Breast Cancer
Expression of the Serrate-like ligand Jagged 1 in cancer cells promotes angiogenesis in neighboring endothelial cells, and elevated levels of Jagged 1 have been associated with poor overall survival in human BCs [55]. Jagged 1-mediated Notch 1 activation inhibits E-cadherin expression through the induction of slug, thus promoting EMT in human breast epithelial cells [67]. Aberrant expression of Jagged 1 also induces bone metastasis of BC cells [74]. Dll1 is significantly up-regulated in ER+ve luminal breast neoplasms, and its expression has been associated with poor prognosis of the same subtype. Intriguingly, Dll1 expression has shown no such effect in other BC subtypes [75]. Joana Sales-Dias et al. demonstrated the oncogenic properties of Dll1 in hormone positive BC cells [76]. Specifically, RNA interference-mediated downregulation of Dll1 in ER+ve MCF7 BC cells resulted in reduced cell proliferation, migration, and colony formation. Kontomanolis et al. 2014 observed that the expression of Dll4 is highly correlated with metastasis in BCs. The authors investigated Dll4 levels in the plasma and neoplastic tissues of BC patients and found that patients with highly metastatic BC exhibited elevated levels of Dll4 in both [56]. This observation suggests that Dll4 plays a pivotal role in BC metastasis. Altogether, this mounting evidence clearly demonstrates that activation of the Notch pathway plays a key role in BC and is therefore a promising potential therapeutic target.
7. Notch Signaling as a Therapeutic Target for Cancer
7.1. γ-Secretase Inhibitors
The aberrant activation of Notch signaling is highly correlated with carcinogenesis. The comprehensive study of the Notch pathway and its crosstalk with other oncogenic signaling pathways has provided enough evidence to identify potential therapeutic targets and to design effective strategies for the treatment of various cancers. The binding of ligands to NECDs triggers transmembrane cleavage of Notch receptors, which allows the release of NICDs into the cytoplasm. This proteolytic cleavage is carried out by γ-secretase. γ-secretase, a large, multi-subunit integral membrane protein complex, is important for the activation of Notch receptors and the transcriptional regulation of its target genes [15, 29, 30]. Thus, blocking transmembrane proteolytic cleavage using γ-secretase inhibitors (GSIs) could be a promising therapeutic approach (Table 1). GSIs prevent the generation of NICDs and thus inhibit Notch activity and its downstream events [77]. Most synthetic γ-secretase inhibitors have been developed to competitively inhibit presenilins. Z-Ile-Leu-CHO, popularly known as GSI-I, is a dipeptide that showed anticancer properties in Ras-transformed fibroblasts [61]. GSI-I was found to promote cell cycle arrest at the G2/M phase and to suppress BC cell survival, which further triggered apoptosis [78]. Recent reports suggest that GSI-I decreases cell proliferation by reducing the expression of Ki67 and glucose transporter 1 (Glut1), as well as by inhibiting the Notch and mTOR/Akt pathways [79]. Interestingly, the effects of GSI-I are greater in HER2+ve cell lines than in HER2−ve BC cell lines [78, 80, 81]. Because trastuzumab, an inhibitor of HER2, can activate the Notch pathway, it would be interesting to investigate the combinatorial effects of trastuzumab and GSI-I in HER2+ve BC patients. LY411575 is a GSI that binds to presenilin 1 (PS1), induces apoptosis in HER2+ve BC cells, and re-sensitizes resistant HER2+ve cells to herceptin [82]. Several GSIs, including LY450139, MK-0752, PF-03084014, and RO4929097, have been or are currently being evaluated in phase I clinical trials [83–86]. RO4929097 has high selectivity and efficacy; however, for unknown reasons, it induces a “less transformed” and slower-growing tumor phenotype, rather than inhibiting cell proliferation or inducing apoptosis [85, 86]. Despite progress in the field, poor pharmacokinetics and off-target effects present major drawbacks to the widespread use of these peptides in the clinic.
Table 1:
List of Notch inhibitors
| Inhibitor name | Cancer type | Molecular target | Functions | Clinical Studies/Significance | Refs. |
|---|---|---|---|---|---|
| γ-secretase inhibitors (GSIs) | |||||
| Z-Ile-Leu-CHO | BC | Notch1, Bcl2, Bax and Bcl-XL | Arrest cells at G2/M phase leading to apoptosis | NA | 62 |
| LY411,575 | BC | Notch1 | Increase number of cells in G2/M and G0 phase | Phase I clinical trials for Alzheimer disease | 82 |
| LY450139 | BC | Notch1 | Reduction of NICD and HES1 | Phase I clinical trials for Alzheimer disease | 83 |
| MK-0752 | BC and Solid tumors | γ-secretase | Reduce BCSCs | Phase Ib | 84 |
| RO4929097 | BC | γ-secretase | Decreases NICD, HES1 expression | Phase I | 85 |
| PF-03084014 | BC and T-ALL | γ-secretase | Reduction of NICD, HES1 and cMyc | Phase I | 86 |
| Monoclonal antibodies | |||||
| Anti-Dll4 mAbs (OMP-21M18) | Solid tumors | Dll4 | Inhibits growth, antiangiogenic | Phase II | 89, 90 |
| Notch mAbs | T-cell leukemia cells | Notch 1 | Reduction of NICD and HES1 | NA | 93, 94 |
| Natural Compounds | |||||
| Sulforaphane | BC and PC | Notch1 | Increases chemo-sensitivity | NA | 97, 98 |
| Genistein | BC | Notch1, NFκB and Capase3 | Induces apoptosis | NA | 99, 100 |
| Curcumin | BC and PaC | Notch1, NFκB | Induces apoptosis | NA | 101 |
| Quercetin | BC and PaC | Notch1 | Arrest cell cycle at G0/G1 phase and induces apoptosis | NA | 102, 103 |
BC, Breast cancer; PC, Prostrate cancer; PaC, pancreatic cancer; T-ALL, T-cell acute lymphoblastic leukemia;
7.2. Monoclonal Antibodies
Although GSIs have demonstrated strong potential in clinical trials, they fail to distinguish Notch paralogs. They inhibit all Notch receptors, which could be a disadvantage because some receptors may play tumor-suppressing roles that should not be inhibited. Furthermore, γ-secretase affects additional targets beyond the Notch pathways. For instance, γ-secretase cleaves β-amyloid precursor protein (APP), resulting in the accumulation of β-amyloid (Aβ) peptides that form plaques in the brain. GSIs might inhibit several such signaling pathways indiscriminately [87]. Indeed, the administration of GSIs has been found to cause intestinal toxicity in several other cancer types [88]. It will be important to discover new drugs with high specificity and affinity that can efficiently discriminate Notch receptor paralogs. In addition to GSIs, researchers have recently proposed a new therapeutic strategy to inhibit Notch signaling using monoclonal antibodies (mAbs) highly specific for Notch receptors and ligands (Table 1). Anti-Dll4 mAbs have been demonstrated to dysregulate tumor angiogenesis and growth by inhibiting the Notch signaling pathway in endothelial cells [89]. The humanized anti-Dll4 mAb (OMP-21M18) inhibits Notch signaling by blocking the interactions of Dll4 with Notch 1 and Notch 4 and was evaluated in clinical trials in patients with solid tumors. OMP-21M18 also showed anti-tumorigenic activity in patient-derived xenografts [90]. In contrast, some mAbs induce proteolytic cleavage of Notch 3 by binding to overlapping epitopes and mimicking ligand-induced Notch signaling activation [91]. mAbs against specific Notch receptors have also been developed and are under investigation [92]. Notch receptor-specific antibodies bind to the NECD and prevent ADAM10-mediated proteolytic cleavage [91, 93, 94]. These mAbs (OMP-59R5) have also shown promising anti-tumorigenic activity and are being tested in clinical trials [91]. Nicastrin mAbs were found to be efficient in the inhibition of γ-secretase and had anti-CSC and therapeutic activity in BC. However, these mAbs are also not specific to an individual Notch receptor [95].
7.3. Natural Compounds
Natural compounds have gradually been gaining attention due to their anticancer activity. Consumption of citrus fruits, soybeans, and green cruciferous vegetables has been associated with reduced risk of cancer [96, 97]. Natural compounds have shown promising results as chemopreventive agents in various cancer types, and their pleiotropic effects against cancer are under investigation. Several natural compounds, such as flavonoids and polyphenols, have demonstrated anticancer properties by inducing apoptosis and reducing the proliferation of various cancer types. Recent studies suggest that a few flavonoids also target the Notch signaling pathway (Table 1). The natural compound sulforaphane, derived from cruciferous vegetables, inhibits BC stem cell growth by down-regulating the Wnt/β-catenin self-renewal pathway in vitro and in vivo. Sulforaphane also inhibits the Notch 1 receptor. Moreover, it has been found to increase the sensitivity of pancreatic cancer cells to chemotherapeutic agents such as gemcitabine, cisplatin, doxorubicin, and 5-fluorouracil [98, 99]. The isoflavonoid genistein, derived from soy products, has exhibited anti-tumorigenic activity in pancreatic cancer and BC [99]. It was determined that genistein induced apoptosis in both ER+ve and ER−ve BC cells through caspase3 activation. In MDA-MB231 cells, genistein induced apoptosis by inhibiting NFκB via the Notch 1 receptor. Genistein-treated MDA-MB231 cells accumulated at the G2/M phase in a dose-dependent manner [100]. The well-known natural compound curcumin, derived from the roots of the Zingiberaceae family plant (i.e., Curcuma long), is a constituent of turmeric and is widely used as a flavoring agent in food. Curcumin inactivates NFκB by down-regulating Notch 1, inducing apoptosis in pancreatic cancer cells [101]. Quercetin is a polyphenol and flavonoid widely distributed in red grapes, apples, raspberries, citrus fruits, and green leafy vegetables. Quercetin decreased the expression of Notch 1 in a leukemia cell line and targeted pancreatic CSCs. Quercetin arrests the cell cycle at the G0/G1 phase and induces apoptosis in BC cells [102, 103].
7.4. Notch receptors in tumor immune response
Although several studies suggested the tumorigenic properties of Notch receptors, there are few reports which also demonstrated the role of these receptors in the anti-tumor immune response. It has been reported that Notch receptors favor the differentiation of T-cell lineage over B cell development from the common lymphoid progenitor cells in the bone marrow [104, 105]. CD4 T-helper 1 (TH1) cells and CD8 cytotoxic T-lymphocytes (CTL) play an important role in mediating anti-tumor immune response and Notch is found to be required for the activation and effector function of these cells [106]. Conditional activation of Notch 2 in CD8 T-cells induced an anti-tumor immune response and reduced the tumor burden in mice [107, 108]. This suggests that, Notch signaling pathway is crucial for the activation and effector function of T-cells.
Tumor cells adopt several defensive mechanisms such as producing immunosuppressive cytokines, expressing inhibitory ligands and recruiting immunosuppressive myeloid and lymphoid cells into the microenvironment to evade the anti-tumor immune response [109]. To overcome this, researchers either isolated tumor antigen-specific T-cells from the tumor site or engineered using chimeric antigen receptors (CARs) specific for tumor antigens [110, 111]. Recently, synthetic Notch receptors (synNotch) have been engineered to improve the generation and enhance the specificity of CAR T-cells [112–114]. These studies emphasize the importance of understating the role of Notch signaling pathway in T-cell-mediated anti-tumor immune response in order to design more effective T-cell-based immunotherapies.
8. Significance
Targeted therapies have emerged over the last decade as a new strategy for cancer treatment. The Notch signaling pathway, is one of the most commonly activated signaling pathways in cancer, plays an important role in cell differentiation, proliferation, angiogenesis, survival, and chemo-resistance, acting as an oncogene or tumor suppressor, depending on cellular context. Notch receptors bind to ligands present on adjacent cells, facilitate proteolytic cleavage by γ-secretase, and are released into the cytoplasm as NICDs, which translocate into the nucleus and regulate the transcriptional activity of target genes. The expression of several Notch receptors and ligands has been associated with the progression of several cancers, including BC, and correlated with poor prognosis.
Inhibition of the Notch signaling pathway using a number of promising approaches may provide a significant contribution to therapeutic strategies to treat BC. A few recent agents targeting Notch signaling are GSIs that inhibit all Notch receptors and have delivered promising results. Notch antibodies (currently under clinical trials) were developed to improve specificity and have exhibited successful tumor suppression. Some natural products have also been found to inhibit the Notch signaling pathway. In addition to GSIs, mAbs, and natural compounds, one of the most important Notch inhibition methods involves blocking peptides, which were also under clinical trials for the treatment of human malignancies. Interestingly, Notch receptors are found to be playing an important role in anti-tumor immune response [106]. Over expression of Notch receptors induce anti-tumor immune response by activating T cells and also reduce tumor burden in mice [107, 108]. The present review suggests that Notch signaling pathway may be a promising therapeutic target for the treatment of BC.
9. Concluding Remarks and Future Perspectives
Since recurrent BC is typically incurable, the propensity of BCs to recur following surgery, chemotherapy, and hormonal therapy is the most important determinant of clinical outcome. A role for Notch signaling in cancer progression and survival suggests that targeting this pathway alone or in combination with other pathways represents a promising therapeutic strategy. BC is a heterogeneous disease. Although 60% of BCs are hormone receptor-positive and receive anti-hormone therapy, they often develop resistance over time. On the other hand, hormone receptor-negative BCs are highly aggressive with minimal treatment options. There is an urgent need to understand the heterogeneity and complex molecular biology of BC in order to discover and develop the new therapeutic drugs to treat it. It is well known that the Notch signaling pathway plays an important role in BC survival, progression, cell growth, migration, invasion, and metastasis. Accumulating evidence and recent advancements in our understanding of Notch signaling indicate that it is a promising therapeutic target for the treatment of BC. To this end, it is important to understand the structure, function, and regulation of the Notch pathway, as well as its complex crosstalk with other signaling pathways.
Individual Notch family members may have opposing roles in cancer, depending on the cellular context and tumor type. For instance, highly elevated levels of Notch 1 and Notch 4 have been observed in BCs, and both have been categorized as oncogenes in several cancers. Surprisingly, Notch 3 was found to be a tumor suppressor. In addition to the Notch receptors, Notch receptor ligands Jagged 1 and Dll4 have been significantly associated with tumor angiogenesis. Following the successful interaction between a Notch receptor and a ligand on an adjacent cell, a series of proteolytic cleavages by TACE and γ-secretase are important for Notch pathway activation. Therefore, it would be wise to design GSIs as therapeutic drugs for the treatment of cancer. GSIs are novel compounds that can inhibit an important component of the Notch signaling pathway. The anticancer properties of GSIs in several cancer types are quite promising. However, GSIs fail to efficiently discriminate between Notch receptor, which is a major drawback. To address this specificity issue, mAbs have been developed to target specific Notch receptors or ligands and have been tested for their anti-tumorigenic effects in various cancer types. Anti-Notch1 and anti-Dll4 mAbs have strongly proven their efficiency and are under clinical trials. However, the Notch pathway interacts with several other oncogenic pathways, including PI3K/Akt, NFκB, and STAT3. Moreover, Notch receptors can have oncogenic or tumor-suppressing properties in various cancer types. Surprisingly, Notch receptors are shown to be effective in differentiation, activation of T cells in order to anti-tumor immune response. Synthetic Notch receptors were used to enhance the specificity of CAR T cells. This suggests that it will be impossible to achieve satisfactory therapeutic endpoints using Notch targeted monotherapy alone. Combinatorial treatments that include Notch inhibitors/mAbs in addition to traditional individual medicines may produce synergistically beneficial results in the clinical setting. Natural compounds, such as sulforaphane, genistein, curcumin, and quercetin, have also gained attention due to their anti-tumorigenic properties and bioavailability and have shown promising results in several cancer types, including BC. Natural compounds showed anti-tumorigenic properties through modulating several oncogenic pathways including Notch signaling pathway, whereas Notch inhibitors will be able to inhibit single pathway. The bio-availability and stereospecificity of natural compounds is high comparing with the synthetic drugs. Natural compounds tend to show lesser side-effects than the synthetic drugs, which is an advantage. However, we need to study the possible roles of natural compounds thoroughly. Cumulatively, the past and ongoing research suggests that Notch signaling pathway may be a promising therapeutic target for the treatment of BC. However, it is important to consider the following aspects to successfully design a therapeutic Notch-targeting drug for the treatment of cancer: (i) specificity, (ii) affinity for a particular receptor or ligand, (iii) minimal efficacy:toxicity ratio, (iv) pharmacokinetics, (v) bioavailability, and (vi) inhibition of other oncogenic signaling pathways. Overall, here we summarize the current knowledge about the impact of the Notch signaling pathway in BC progression and the therapeutic role of Notch’s inhibition.
Highlights.
The salient features of this review article with potential clinical relevance include:
Notch receptors can behaves as either oncogene or tumor suppressor depending on cellular context.
Elevated levels of Notch1, Notch4 and Dll4 are observed in several cancers and are correlated with poor prognosis.
Inhibition of Notch signaling pathway using γ-secretase inhibitors (GSIs) can deliver promising tumor suppressor results.
Natural products are also found to be playing an important role in the inhibition of Notch signaling pathway.
This review suggests that Notch signaling pathway can be therapeutic target for the treatment of breast cancer.
Acknowledgements
This work was supported in part by grants from the United States Department of Defense (W81XWH-16–1-0641) and the National Cancer Institute of the National Institutes of Health (P30CA33572). Funding from the Beckman Research Institute of City of Hope is also acknowledged. We apologize to all colleagues whose work we could not cite due to space constraints.
The abbreviations used are:
- ANK
ankyrin repeats
- BC
breast cancer
- CSC
cancer stem-like cells
- DLL
Delta-like ligand
- EGF
epidermal growth factor
- EMT
epithelial-to-mesenchymal transition
- GSI
γ-secretase inhibitors
- JAG
jagged
- mAbs
monoclonal antibodies
- MMTV
mouse mammary tumor virus
- NECD
Notch extracellular domain
- NICD
Notch intracellular domain
- NLS
nuclear localization signal
- NTM
Notch transmembrane domain
- PEST
proline glutamic acid serine and threonine
- RAM
RBP-jk association molecule
- TAD
trans-activation domain
Footnotes
Conflict of interest: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Theodosiou A, Arhondakis S, Baumann M, Kossida S. Evolutionary scenarios of Notch proteins. Mol Biol Evol 2009; 26: 1631–40. [DOI] [PubMed] [Google Scholar]
- 2.Xu J, Gridley T. Notch Signaling during Oogenesis in Drosophila melanogaster. Gene Res Intl 2012; 2012: 648207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rebay I, Fleming RJ, Fehon RG, Cherbas L, Cherbas P, Artavanis-Tsakonas S. Specific EGF repeats of Notch mediate interactions with Delta and Serrate: implications for Notch as a multifunctional receptor. Cell 1991; 67: 687–99. [DOI] [PubMed] [Google Scholar]
- 4.Weinmaster G, Roberts VJ, Lemke G. Notch2: a second mammalian Notch gene. Development 1992; 116: 931–41. [DOI] [PubMed] [Google Scholar]
- 5.Ran Y, Hossain F, Pannuti A, Lessard CB, Ladd GZ, Jung JI, Minter LM, Osborne BA, Miele L, Golde TE. Gamma-secretase inhibitors in cancer clinical trials are pharmacologically and functionally distinct. EMBO Molecular Medicine 2017; 9: 950–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.del Amo FF, Gendron-Maguire M, Swiatek PJ, Jenkins NA, Copeland NG, Gridley T. Cloning, analysis, and chromosomal localization of Notch-1, a mouse homolog of Drosophila Notch. Genomics 1993; 15: 259–64. [DOI] [PubMed] [Google Scholar]
- 7.Lardelli M, Williams R, Lendahl U. Notch-related genes in animal development. The Intl J Develop Biol 1995; 39: 769–80. [PubMed] [Google Scholar]
- 8.Uyttendaele H, Marazzi G, Wu G, Yan Q, Sassoon D, Kitajewski J. Notch4/int-3, a mammary proto-oncogene, is an endothelial cell-specific mammalian Notch gene. Development 1996; 122: 2251–9. [DOI] [PubMed] [Google Scholar]
- 9.Aster JC, Simms WB, Zavala-Ruiz Z, Patriub V, North CL, Blacklow SC. The folding and structural integrity of the first LIN-12 module of human Notch1 are calcium-dependent. Biochemistry 1999; 38: 4736–42. [DOI] [PubMed] [Google Scholar]
- 10.Gordon WR, Vardar-Ulu D, Histen G, Sanchez-Irizarry C, Aster JC, Blacklow SC. Structural basis for autoinhibition of Notch. Nature Structural & Mol Biol 2007; 14: 295–300. [DOI] [PubMed] [Google Scholar]
- 11.Kopan R, Schroeter EH, Weintraub H, Nye JS. Signal transduction by activated mNotch: importance of proteolytic processing and its regulation by the extracellular domain. Proc Natl Acad Sci 1996; 93: 1683–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez-Irizarry C, Carpenter AC, Weng AP, Pear WS, Aster JC, Blacklow SC. Notch subunit heterodimerization and prevention of ligand-independent proteolytic activation depend, respectively, on a novel domain and the LNR repeats. Mol Cell Biol 2004; 24: 9265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lieber T, Kidd S, Alcamo E, Corbin V, Young MW. Antineurogenic phenotypes induced by truncated Notch proteins indicate a role in signal transduction and may point to a novel function for Notch in nuclei. Genes & development 1993; 7: 1949–65. [DOI] [PubMed] [Google Scholar]
- 14.Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ, Ray WJ, Kopan R. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol cell 2000; 5: 197–206. [DOI] [PubMed] [Google Scholar]
- 15.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 2009; 137: 216–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchal C, Haguenauer-Tsapis R, Urban-Grimal D. A PEST-like sequence mediates phosphorylation and efficient ubiquitination of yeast uracil permease. Mol Cell Biol 1998; 18: 314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer RD, Srinivasan S, Singh AJ, Mahoney JE, Gharahassanlou KR, Rahimi N. PEST motif serine and tyrosine phosphorylation controls vascular endothelial growth factor receptor 2 stability and downregulation. Mol Cell Biol 2011; 31: 2010–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Bioche Sci 1996; 21: 267–71. [PubMed] [Google Scholar]
- 19.Stahl M, Uemura K, Ge C, Shi S, Tashima Y, Stanley P. Roles of Pofut1 and O-fucose in mammalian Notch signaling. J Biol Chem 2008; 283: 13638–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi S, Stanley P. Protein O-fucosyltransferase 1 is an essential component of Notch signaling pathways. Proc Natl Acad Sci 2003; 100: 5234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okajima T, Xu A, Lei L, Irvine KD. Chaperone activity of protein O-fucosyltransferase 1 promotes notch receptor folding. Science 2005; 307: 1599–603. [DOI] [PubMed] [Google Scholar]
- 22.Logeat F, Bessia C, Brou C, LeBail O, Jarriault S, Seidah NG, Israel A. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci 1998; 95: 8108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haines N, Irvine KD. Glycosylation regulates Notch signalling. Nature Rev Mol Cell Biol 2003; 4: 786–97. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto S, Charng WL, Bellen HJ. Endocytosis and intracellular trafficking of Notch and its ligands. Current topics Develop Biol 2010; 92: 165–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovall RA, Blacklow SC. Mechanistic insights into Notch receptor signaling from structural and biochemical studies. Current topics Develop Biol 2010; 92: 31–71. [DOI] [PubMed] [Google Scholar]
- 26.de Celis JF, Bray S. Feed-back mechanisms affecting Notch activation at the dorsoventral boundary in the Drosophila wing. Development 1997; 124: 3241–51. [DOI] [PubMed] [Google Scholar]
- 27.Micchelli CA, Rulifson EJ, Blair SS. The function and regulation of cut expression on the wing margin of Drosophila: Notch, Wingless and a dominant negative role for Delta and Serrate. Development 1997; 124: 1485–95. [DOI] [PubMed] [Google Scholar]
- 28.Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, Cumano A, Roux P, Black RA, Israel A. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol cell 2000; 5: 207–16. [DOI] [PubMed] [Google Scholar]
- 29.Borggrefe T, Oswald F. The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell Molec Life Sci: CMLS 2009; 66: 1631–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Struhl G, Adachi A. Nuclear access and action of notch in vivo. Cell 1998; 93: 649–60. [DOI] [PubMed] [Google Scholar]
- 31.Gertsik N, Chiu D, Li YM. Complex regulation of gamma-secretase: from obligatory to modulatory subunits. Front Aging Neurosci 2014; 6: 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao G, Liu Z, Ilagan MX, Kopan R. Gamma-secretase composed of PS1/Pen2/Aph1a can cleave notch and amyloid precursor protein in the absence of nicastrin. J Neurosci 2010; 30: 1648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Placanica L, Tarassishin L, Yang G, Peethumnongsin E, Kim SH, Zheng H, Sisodia SS, Li YM. Pen2 and presenilin-1 modulate the dynamic equilibrium of presenilin-1 and presenilin-2 gamma-secretase complexes. J Biol Chem 2009; 284: 2967–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steiner H, Duff K, Capell A, Romig H, Grim MG, Lincoln S, Hardy J, Yu X, Picciano M, Fechteler K, Citron M, Kopan R, et al. A loss of function mutation of presenilin-2 interferes with amyloid beta-peptide production and notch signaling. J Biol Chem 1999; 274: 28669–73. [DOI] [PubMed] [Google Scholar]
- 35.Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature 1999; 398: 513–7. [DOI] [PubMed] [Google Scholar]
- 36.Hu Y, Ye Y, Fortini ME. Nicastrin is required for gamma-secretase cleavage of the Drosophila Notch receptor. Developmental cell 2002; 2: 69–78. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Schier H, St Johnston D. Drosophila nicastrin is essential for the intramembranous cleavage of notch. Developmental cell 2002; 2: 79–89. [DOI] [PubMed] [Google Scholar]
- 38.Lee SF, Shah S, Yu C, Wigley WC, Li H, Lim M, Pedersen K, Han W, Thomas P, Lundkvist J, Hao YH, Yu G. A conserved GXXXG motif in APH-1 is critical for assembly and activity of the gamma-secretase complex. J Biol Chem 2004; 279: 4144–52. [DOI] [PubMed] [Google Scholar]
- 39.Hansson EM, Stromberg K, Bergstedt S, Yu G, Naslund J, Lundkvist J, Lendahl U. Aph-1 interacts at the cell surface with proteins in the active gamma-secretase complex and membrane-tethered Notch. J Neurochem 2005; 92: 1010–20. [DOI] [PubMed] [Google Scholar]
- 40.Tagami S, Okochi M, Yanagida K, Ikuta A, Fukumori A, Matsumoto N, Ishizuka-Katsura Y, Nakayama T, Itoh N, Jiang J, Nishitomi K, Kamino K, et al. Regulation of Notch signaling by dynamic changes in the precision of S3 cleavage of Notch-1. Mol Cell Biol 2008; 28: 165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pratt EB, Wentzell JS, Maxson JE, Courter L, Hazelett D, Christian JL. The cell giveth and the cell taketh away: an overview of Notch pathway activation by endocytic trafficking of ligands and receptors. Acta Histochemica 2011; 113: 248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miele L, Osborne B. Arbiter of differentiation and death: Notch signaling meets apoptosis. J Cell Physiol 1999; 181: 393–409. [DOI] [PubMed] [Google Scholar]
- 43.Nam Y, Sliz P, Song L, Aster JC, Blacklow SC. Structural basis for cooperativity in recruitment of MAML coactivators to Notch transcription complexes. Cell 2006;124: 973–83. [DOI] [PubMed] [Google Scholar]
- 44.Wu L, Aster JC, Blacklow SC, Lake R, Artavanis-Tsakonas S, Griffin JD. MAML1, a human homologue of Drosophila mastermind, is a transcriptional co-activator for NOTCH receptors. Nature Genetics 2000; 26: 484–9. [DOI] [PubMed] [Google Scholar]
- 45.Wallberg AE, Pedersen K, Lendahl U, Roeder RG. p300 and PCAF act cooperatively to mediate transcriptional activation from chromatin templates by notch intracellular domains in vitro. Mol Cell Biol 2002; 22: 7812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurooka H, Honjo T. Functional interaction between the mouse notch1 intracellular region and histone acetyltransferases PCAF and GCN5. J Biol Chem 2000; 275: 17211–20. [DOI] [PubMed] [Google Scholar]
- 47.Guo S, Liu M, Gonzalez-Perez RR. Role of Notch and its oncogenic signaling crosstalk in breast cancer. Biochim Biophys Acta 2011; 1815: 197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanalkumar R, Dhanesh SB, James J. Non-canonical activation of Notch signaling/target genes in vertebrates. Cell Molec Life Sci: CMLS 2010; 67: 2957–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deftos ML, Huang E, Ojala EW, Forbush KA, Bevan MJ. Notch1 signaling promotes the maturation of CD4 and CD8 SP thymocytes. Immunity 2000; 13: 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Z, Zhang Y, Li Y, Banerjee S, Liao J, Sarkar FH. Down-regulation of Notch-1 contributes to cell growth inhibition and apoptosis in pancreatic cancer cells. Mol Cancer Ther 2006; 5: 483–93. [DOI] [PubMed] [Google Scholar]
- 51.Stylianou S, Clarke RB, Brennan K. Aberrant activation of notch signaling in human breast cancer. Cancer Res 2006; 66: 1517–25. [DOI] [PubMed] [Google Scholar]
- 52.Souilhol C, Cormier S, Tanigaki K, Babinet C, Cohen-Tannoudji M. RBP-Jkappa-dependent notch signaling is dispensable for mouse early embryonic development. Mol Cell Biol 2006; 26: 4769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nature Rev Cancer 2003; 3: 903–11. [DOI] [PubMed] [Google Scholar]
- 54.Croce JC, McClay DR. Evolution of the Wnt pathways. Methods Molec Biol 2008; 469: 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reedijk M, Odorcic S, Chang L, Zhang H, Miller N, McCready DR, Lockwood G, Egan SE. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res 2005; 65: 8530–7. [DOI] [PubMed] [Google Scholar]
- 56.Kontomanolis E, Panteliadou M, Giatromanolaki A, Pouliliou S, Efremidou E, Limberis V, Galazios G, Sivridis E, Koukourakis MI. Delta-like ligand 4 (DLL4) in the plasma and neoplastic tissues from breast cancer patients: correlation with metastasis. Med Oncol 2014; 31: 945. [DOI] [PubMed] [Google Scholar]
- 57.Xing F, Okuda H, Watabe M, Kobayashi A, Pai SK, Liu W, Pandey PR, Fukuda K, Hirota S, Sugai T, Wakabayshi G, Koeda K, et al. Hypoxia-induced Jagged2 promotes breast cancer metastasis and self-renewal of cancer stem-like cells. Oncogene 2011; 30: 4075–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gallahan D, Callahan R. Mammary tumorigenesis in feral mice: identification of a new int locus in mouse mammary tumor virus (Czech II)-induced mammary tumors. J Virol 1987; 61: 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dievart A, Beaulieu N, Jolicoeur P. Involvement of Notch1 in the development of mouse mammary tumors. Oncogene 1999; 18: 5973–81. [DOI] [PubMed] [Google Scholar]
- 60.Parr C, Watkins G, Jiang WG. The possible correlation of Notch-1 and Notch-2 with clinical outcome and tumor clinico-pathological parameters in human breast cancer. Intl J Mol Med 2004; 14: 779–86. [DOI] [PubMed] [Google Scholar]
- 61.Chen CF, Dou XW, Liang YK, Lin HY, Bai JW, Zhang XX, Wei XL, Li YC, Zhang GJ. Notch3 overexpression causes arrest of cell cycle progression by inducing Cdh1 expression in human breast cancer cells. Cell cycle 2016; 15: 432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weijzen S, Rizzo P, Braid M, Vaishnav R, Jonkheer SM, Zlobin A, Osborne BA, Gottipati S, Aster JC, Hahn WC, Rudolf M, Siziopikou K. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nature Medicine 2002; 8: 979–86. [DOI] [PubMed] [Google Scholar]
- 63.Mittal S, Sharma A, Balaji SA, Gowda MC, Dighe RR, Kumar RV, Rangarajan A. Coordinate hyperactivation of Notch1 and Ras/MAPK pathways correlates with poor patient survival: novel therapeutic strategy for aggressive breast cancers. Mol Cancer Ther 2014; 13: 3198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meurette O, Stylianou S, Rock R, Collu GM, Gilmore AP, Brennan K. Notch activation induces Akt signaling via an autocrine loop to prevent apoptosis in breast epithelial cells. Cancer Res 2009; 69: 5015–22. [DOI] [PubMed] [Google Scholar]
- 65.Zeng Q, Li S, Chepeha DB, Giordano TJ, Li J, Zhang H, Polverini PJ, Nor J, Kitajewski J, Wang CY. Crosstalk between tumor and endothelial cells promotes tumor angiogenesis by MAPK activation of Notch signaling. Cancer cell 2005; 8: 13–23. [DOI] [PubMed] [Google Scholar]
- 66.Dufraine J, Funahashi Y, Kitajewski J. Notch signaling regulates tumor angiogenesis by diverse mechanisms. Oncogene 2008; 27: 5132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leong KG, Niessen K, Kulic I, Raouf A, Eaves C, Pollet I, Karsan A. Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherin. J Expl Med 2007; 204: 2935–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dang TP. Notch, apoptosis and cancer. Adv Expl Med Biol 2012; 727: 199–209. [DOI] [PubMed] [Google Scholar]
- 69.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci 2003; 100: 3983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCubrey JA, Davis NM, Abrams SL, Montalto G, Cervello M, Libra M, Nicoletti F, D’Assoro AB, Cocco L, Martelli AM, Steelman LS. Targeting breast cancer initiating cells: advances in breast cancer research and therapy. Adv Biol Regul 2014; 56: 81–107. [DOI] [PubMed] [Google Scholar]
- 71.Ju JH, Yang W, Oh S, Nam K, Lee KM, Noh DY, Shin I. HER2 stabilizes survivin while concomitantly down-regulating survivin gene transcription by suppressing Notch cleavage. Biochem J 2013; 451: 123–34. [DOI] [PubMed] [Google Scholar]
- 72.Suman S, Das TP, Damodaran C. Silencing NOTCH signaling causes growth arrest in both breast cancer stem cells and breast cancer cells. Br J Cancer 2013; 109: 2587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nagamatsu I, Onishi H, Matsushita S, Kubo M, Kai M, Imaizumi A, Nakano K, Hattori M, Oda Y, Tanaka M, Katano M. NOTCH4 is a potential therapeutic target for triple-negative breast cancer. Anticancer Res 2014; 34: 69–80. [PubMed] [Google Scholar]
- 74.Sethi N, Dai X, Winter CG, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 2011; 19: 192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kumar S, Srivastav RK, Wilkes DW, Ross T, Kim S, Kowalski J, Chatla S, Zhang Q, Nayak A, Guha M, Fuchs SY, Thomas C. Estrogen-dependent DLL1-mediated Notch signaling promotes luminal breast cancer. Oncogene 2019; 38: 2092–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sales-Dias J, Silva G, Lamy M, Ferreira A, Barbas A. The Notch ligand DLL1 exerts carcinogenic features in human breast cancer cells. PloS one 2019; 14: e0217002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shih Ie M, Wang TL. Notch signaling, gamma-secretase inhibitors and cancer therapy. Cancer Res 2007; 67: 1879–82. [DOI] [PubMed] [Google Scholar]
- 78.Rasul S, Balasubramanian R, Filipovic A, Slade MJ, Yague E, Coombes RC. Inhibition of gamma-secretase induces G2/M arrest and triggers apoptosis in breast cancer cells. Br J Cancer 2009; 100: 1879–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Efferson CL, Winkelmann CT, Ware C, Sullivan T, Giampaoli S, Tammam J, Patel S, Mesiti G, Reilly JF, Gibson RE, Buser C, Yeatman T. Downregulation of Notch pathway by a gamma-secretase inhibitor attenuates AKT/mammalian target of rapamycin signaling and glucose uptake in an ERBB2 transgenic breast cancer model. Cancer Res 2010; 70: 2476–84. [DOI] [PubMed] [Google Scholar]
- 80.Lee CW, Raskett CM, Prudovsky I, Altieri DC. Molecular dependence of estrogen receptor-negative breast cancer on a notch-survivin signaling axis. Cancer Res 2008; 68: 5273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rizzo P, Miao H, D’Souza G, Osipo C, Song LL, Yun J, Zhao H, Mascarenhas J, Wyatt D, Antico G, Hao L, Yao K. Cross-talk between notch and the estrogen receptor in breast cancer suggests novel therapeutic approaches. Cancer Res 2008; 68: 5226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Osipo C, Patel P, Rizzo P, Clementz AG, Hao L, Golde TE, Miele L. ErbB-2 inhibition activates Notch-1 and sensitizes breast cancer cells to a gamma-secretase inhibitor. Oncogene 2008; 27: 5019–32. [DOI] [PubMed] [Google Scholar]
- 83.Fleisher AS, Raman R, Siemers ER, Becerra L, Clark CM, Dean RA, Farlow MR, Galvin JE, Peskind ER, Quinn JF, Sherzai A, Sowell BB, et al. Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in alzheimer disease. Arch Neurol 2008; 65: 1031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krop I, Demuth T, Guthrie T, Wen PY, Mason WP, Chinnaiyan P, Butowski N, Groves MD, Kesari S, Freedman SJ, Blackman S, Watters J. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J Clin Oncol 2012; 30: 2307–13. [DOI] [PubMed] [Google Scholar]
- 85.Luistro L, He W, Smith M, Packman K, Vilenchik M, Carvajal D, Roberts J, Cai J, Berkofsky-Fessler W, Hilton H, Linn M, Flohr A. Preclinical profile of a potent gamma-secretase inhibitor targeting notch signaling with in vivo efficacy and pharmacodynamic properties. Cancer Res 2009; 69: 7672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei P, Walls M, Qiu M, Ding R, Denlinger RH, Wong A, Tsaparikos K, Jani JP, Hosea N, Sands M, Randolph S, Smeal T. Evaluation of selective gamma-secretase inhibitor PF-03084014 for its antitumor efficacy and gastrointestinal safety to guide optimal clinical trial design. Mol Cancer Ther 2010; 9: 1618–28. [DOI] [PubMed] [Google Scholar]
- 87.Beel AJ, Sanders CR. Substrate specificity of gamma-secretase and other intra-membrane proteases. Cellular Molec Life Sci: CMLS 2008; 65: 1311–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 2005; 435: 959–63. [DOI] [PubMed] [Google Scholar]
- 89.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I, Singh M, Chien M. Inhibition of Dll4 signalling inhibits tumor growth by deregulating angiogenesis. Nature 2006; 444: 1083–7. [DOI] [PubMed] [Google Scholar]
- 90.Brunner A, Cattaruzza F, Yen WC, Yeung P, Fischer M, Cancilla B, O’Young G, Tam R, Liu YW, Gurney. Abstract 4652: Effects of anti-dll4 treatment on non-small cell lung cancer (nsclc) human xenograft tumors. Cancer Res 2016; 76: 4652. [Google Scholar]
- 91.Li K, Li Y, Wu W, Gordon WR, Chang DW, Lu M, Scoggin S, Fu T, Vien L, Histen G, Zheng J, Martin-Hollister R. Modulation of Notch signaling by antibodies specific for the extracellular negative regulatory region of NOTCH3. J Biol Chem 2008; 283: 8046–54. [DOI] [PubMed] [Google Scholar]
- 92.Yan M, Plowman GD. Delta-like 4/Notch signaling and its therapeutic implications. Clin Cancer Res 2007; 13: 7243–6. [DOI] [PubMed] [Google Scholar]
- 93.Aste-Amezaga M, Zhang N, Lineberger JE, Arnold BA, Toner TJ, Gu M, Huang L, Vitelli S, Vo KT, Haytko P, Zhao JZ, Baleydier F. Characterization of Notch1 antibodies that inhibit signaling of both normal and mutated Notch1 receptors. PloS one 2010; 5: e9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, Finkle D, Venook R, Wu X, Ridgway J, Schahin-Reed D, Dow GJ. Therapeutic antibody targeting of individual Notch receptors. Nature 2010; 464: 1052–7. [DOI] [PubMed] [Google Scholar]
- 95.Lombardo Y, Filipovic A, Molyneux G, Periyasamy M, Giamas G, Hu Y, Trivedi PS, Wang J, Yague E, Michel L, Coombes RC. Nicastrin regulates breast cancer stem cell properties and tumor growth in vitro and in vivo. Proc Natl Acad Sci 2012; 109: 16558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee MM, Gomez SL, Chang JS, Wey M, Wang RT, Hsing AW. Soy and isoflavone consumption in relation to prostate cancer risk in China. Cancer Epidemiol Biomarkers Prev 2003; 12: 665–8. [PubMed] [Google Scholar]
- 97.Smith-Warner SA, Spiegelman D, Yaun SS, Albanes D, Beeson WL, van den Brandt PA, Feskanich D, Folsom AR, Fraser GE, Freudenheim JL, Giovannucci E, Goldbohm RA. Fruits, vegetables and lung cancer: a pooled analysis of cohort studies. Int J Cancer 2003; 107: 1001–11. [DOI] [PubMed] [Google Scholar]
- 98.Kallifatidis G, Labsch S, Rausch V, Mattern J, Gladkich J, Moldenhauer G, Buchler MW, Salnikov AV, Herr I. Sulforaphane increases drug-mediated cytotoxicity toward cancer stem-like cells of pancreas and prostate. Molecular Therapy 2011; 19: 188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bi YL, Min M, Shen W, Liu Y. Genistein induced anticancer effects on pancreatic cancer cell lines involves mitochondrial apoptosis, G0/G1cell cycle arrest and regulation of STAT3 signalling pathway. Phytomedicine 2018; 39: 10–6. [DOI] [PubMed] [Google Scholar]
- 100.Pan H, Zhou W, He W, Liu X, Ding Q, Ling L, Zha X, Wang S. Genistein inhibits MDA-MB-231 triple-negative breast cancer cell growth by inhibiting NF-kappaB activity via the Notch-1 pathway. Intl J Mol Med 2012; 30: 337–43. [DOI] [PubMed] [Google Scholar]
- 101.Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer 2006; 106: 2503–13. [DOI] [PubMed] [Google Scholar]
- 102.Kawahara T, Kawaguchi-Ihara N, Okuhashi Y, Itoh M, Nara N, Tohda S. Cyclopamine and quercetin suppress the growth of leukemia and lymphoma cells. Anticancer Res 2009; 29: 4629–32. [PubMed] [Google Scholar]
- 103.Zhou W, Kallifatidis G, Baumann B, Rausch V, Mattern J, Gladkich J, Giese N, Moldenhauer G, Wirth T, Buchler MW, Salnikov AV, Herr I. Dietary polyphenol quercetin targets pancreatic cancer stem cells. Int J Oncol 2010; 37: 551–61. [DOI] [PubMed] [Google Scholar]
- 104.Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity 1999; 10: 547–58. [DOI] [PubMed] [Google Scholar]
- 105.Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, Ikuta K, Honjo T. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Intl Immunol 2002; 14: 637–45. [DOI] [PubMed] [Google Scholar]
- 106.Amsen D, Helbig C, Backer RA. Notch in T cell differentiation: all things considered. Trends in Immunol 2015; 36: 802–14. [DOI] [PubMed] [Google Scholar]
- 107.Sierra RA, Thevenot P, Raber PL, Cui Y, Parsons C, Ochoa AC, Trillo-Tinoco J, Del Valle L, Rodriguez PC. Rescue of notch-1 signaling in antigen-specific CD8+ T cells overcomes tumor-induced T-cell suppression and enhances immunotherapy in cancer. Cancer Immunol Res 2014; 2: 800–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sugimoto K, Maekawa Y, Kitamura A, Nishida J, Koyanagi A, Yagita H, Kojima H, Chiba S, Shimada M, Yasutomo K. Notch2 signaling is required for potent antitumor immunity in vivo. J Immunol 2010; 184: 4673–8. [DOI] [PubMed] [Google Scholar]
- 109.Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara H, Signori E, Honoki K, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Sem Cancer Biol 2015; 35 Suppl: S185–S98. [DOI] [PubMed] [Google Scholar]
- 110.Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nature Rev Clin Oncol 2016; 13: 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Themeli M, Riviere I, Sadelain M. New cell sources for T cell engineering and adoptive immunotherapy. Cell Stem Cell 2015; 16: 357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gordon WR, Zimmerman B, He L, Miles LJ, Huang J, Tiyanont K, McArthur DG, Aster JC, Perrimon N, Loparo JJ, Blacklow SC. Mechanical Allostery: Evidence for a force requirement in the proteolytic activation of Notch. Developmental Cell 2015; 33: 729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morsut L, Roybal KT, Xiong X, Gordley RM, Coyle SM, Thomson M, Lim WA. Engineering customized cell sensing and response behaviors using synthetic Notch receptors. Cell 2016; 164: 780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roybal KT, Rupp LJ, Morsut L, Walker WJ, McNally KA, Park JS, Lim WA. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell 2016; 164: 770–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


