Abstract
A micellar formulation of amphotericin B (AmB) solubilized with poloxamer 188 was evaluated against an AmB Leishmania donovani-resistant line. A concave isobologram showed a synergistic effect of this association against promastigotes. This result was confirmed with amastigotes since the 50% effective concentration of the new formulation was 100 times less than that of the control AmB formulation.
The use of amphotericin B (AmB) for the treatment of visceral leishmaniasis (VL) is increasing as a consequence of the worldwide spread of resistance to the first-line pentavalent antimonials (7) and the improvement of the therapeutic index of AmB in the commercialized lipidic formulations (AmBisome, Amphocil, and Albecet). Clinical trials have indicated that these formulations decrease the toxicity of the drug and that they are also more active against this parasite (3, 4, 5, 8, 9, 10, 17, 22, 24).
Since AmB is increasingly used, the risk of the appearance of clinical resistance could increase. In anticipation of this fact, a line of AmB-resistant (AmBr) Leishmania donovani promastigotes was established by stepwise drug pressure (16), and their biological properties were compared with those of the wild-type (WT) parent strain. Ergosterol, the main target of AmB in fungi, was present in the membranes of WT strains but was not found in the membranes of the AmB-resistant line of isolates (16). This modification was accompanied by an increase in membrane fragility and fluidity. The AmB-resistant promastigotes were infective for macrophages in vitro, but their virulence was considerably decreased in vivo. Strategies that can be used to overcome the resistance should be investigated.
In a previous work (11), we studied the physicochemical properties of a formulation of AmB solubilized with poloxamer 188. Poloxamers are water-soluble, nonionic, triblock copolymeric surfactants of poly(ethylene oxide) and poly(propylene oxide) (20). Among them, poloxamer 188 was approved by the Food and Drug Administration as a safe ingredient for injections (1), and it is reported in the National Formulary as a pharmaceutical ingredient (23). These formulations were less toxic to red blood cells, macrophages, and renal cells and were also less toxic in vivo in noninfected mice (M. S. Espuelas, P. Legrand, P. Loiseau, C. Bories, C. Gamazo, J.-P. Devissaguet, M. J. Renedo, and J. M. Irache, Proc. 1st World Meet. APGI/APV on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technol. p. 569–570, 1998).
The sizes of AmB aggregates decreased and the degree of AmB self-aggregation increased with the poloxamer concentration used to solubilize the drug. As Mullen et al. (19) reported a correlation between the degree of AmB aggregation in the lipidic formulations and their antileishmanial activities, in the present study, the antileishmanial activity of the micellar formulation of AmB solubilized with poloxamer 188 at two different concentrations was assessed in vitro against both WT and AmBr L. donovani DD8 (strain MHOM/IN/80/DD8) promastigotes and intramacrophagic amastigotes. Amastigotes were maintained in golden hamsters. The parasites were harvested from an infected spleen for the assays.
A stock solution of AmB aqueous dispersion used as a control was prepared by solubilization of AmB powder (Bristol-Myers Squibb, Barcelona, Spain) in dimethyl sulfoxide (DMSO) at a concentration of 10 mg/ml followed by dispersion of this organic stock solution in water to obtain a concentration of 1 mg/ml (AmB-DMSO). The micellar formulation of the drug with a low poloxamer 188 concentration (12.5 μg/ml; AmB-MM[12.5]) and a high poloxamer 188 concentration (125 μg/ml; AmB-MM[125]) was prepared as described previously (11).
Peritoneal macrophages were harvested from female CD1 mice (Charles River) 3 days after injection of sodium thioglycolate (Biomérieux) and were dispensed into eight-well chamber slides (LabTek Ltd.) at a concentration of 5 × 104/well (400 μl/well) in RPMI 1640 medium (Gibco BRL) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Gibco BRL) and 2 mM l-glutamine. After 24 h, the macrophages were infected with WT amastigotes at a ratio of 10 parasites per macrophage. In the case of AmBr amastigotes, a ratio of 20 parasites per macrophage was required to obtain similar percentages of infected macrophages (about 80%) and similar mean numbers of amastigote per macrophage (10 amastigotes per macrophage). After 24 h of incubation, the infected macrophages were exposed to different AmB formulations at concentrations that ranged from 0.001 to 1 μg/ml for WT parasites and 0.001 to 10 μg/ml for AmBr parasites. After 2 days, the percentage of infected macrophages was evaluated microscopically after Giemsa staining (Table 1). The 50% effective doses (EC50s) were determined by linear regression analysis with 95% confidence limits. As shown in Table 1, when the highest poloxamer concentration was used to solubilize AmB (AmB-MM[125]), the activity against AmBr parasites was increased 100 times compared to that of AmB-DMSO, whereas poloxamer 188 alone had no significant effect.
TABLE 1.
In vitro activities of AmB-DMSO and a micellar formulation of AmB solubilized with poloxamer 188 against WT and AmBr intramacrophagic amastigotes of L. donovani DD8
| Formulation | EC50 (μg/ml)a
|
|
|---|---|---|
| WT | AmBr | |
| AmB-DMSO | 0.083 ± 0.009 | 1.07 ± 0.08 |
| AmB-MM[12.5] | 0.085 ± 0.011 | 1.01 ± 0.13 |
| AmB-MM[125] | 0.032 ± 0.004b | 0.012 ± 0.001b |
| Poloxamer 188 | >1,250 | >1,250 |
Data are the means ± standard deviations (n = 3).
Significant difference (P < 0.05).
Two main hypotheses were tested to explain this reversion of resistance. The first one relies on the fact that poloxamers are described to have direct effects on macrophage activation (20), and this possibility may be involved in the increase in the antileishmanial activity (6). The level of nitrous oxide (NO) production and the tumor necrosis factor alpha (TNF-α) activity in the medium were determined. The amount of NO produced was measured spectrophotometrically at 540 nm (Labsystem microplate reader), and TNF-α activity was determined by a cytotoxicity assay with L929 cells as described previously (12). Table 2 shows that the formulation of AmB solubilized with poloxamer 188 did not stimulate macrophages. Furthermore, these formulations seemed to inhibit NO production caused by dependent effector mechanisms (TNF-α production) induced by administration of free drug in the presence of gamma interferon (20 IU/ml; Genzyme) as a costimulus (18).
TABLE 2.
NO and TNF-α production induced by AmB-DMSO and AmB-MM with AmB at 1 μg/ml combined with gamma interferon (20 IU/ml) after a 48-h treatment of macrophages infected with WT and AmBr promastigotes of L. donovani
| Formulation | WT
|
AmBr
|
||
|---|---|---|---|---|
| NO production (μM)a | TNF-α production (pg/ml)a | NO production (μM)a | TNF-α production (pg/ml)a | |
| Nontreated control | 8.50 ± 0.18 | 6.90 | 7.55 ± 0.84 | NDb |
| AmB-DMSO | 16.06 ± 2.97 | 10.61 | 10.25 ± 0.10 | 10.76 |
| Poloxamer 188 | 8.50 ± 0.26 | 6.99 | ND | 5.81 |
| AmB-MM[12.5] | 11.41 ± 1.71 | 7.57 | 6.84 ± 0.15 | 7.20 |
| AmB-MM[125] | 10.07 ± 0.21 | 6.26 | 6.89 ± 0.43 | 6.90 |
Data are the means ± standard deviations of two measurements.
ND, not determined.
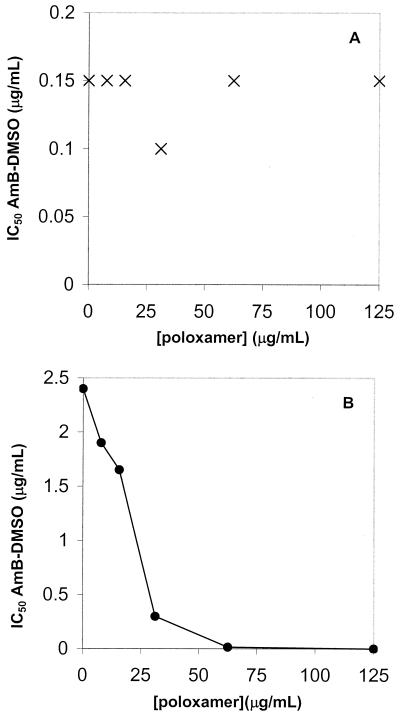
As a consequence, the antileishmanial activity of the micellar formulation of AmB solubilized with poloxamer 188 would principally be the result of a direct action of the drug and/or the poloxamer on the parasite membrane. To check this hypothesis, the possibility of synergy between AmB and poloxamer 188 was studied with the promastigote form of the parasite. Synergism between nonionic surfactants (i.e., Triton WR139 or poloxamers CRL8131 and CRL8142) and antibiotics (isoniazide or clindamycin) against other intramacrophage pathogens such as Mycobacterium (14) or Toxoplasma gondii has already been reported (2). However, in all cases, these surfactants were more hydrophobic than the poloxamer 188 used in the present study. On the other hand, only one example of synergism between AmB and a surfactant has been reported in vitro with alkyl glycerol ethers against fungi (13). In this work, promastigotes from a logarithmic-phase culture were used at 2 × 105 cells per well in 96-well microplates (Nunclon) in RPMI 1640 medium supplemented with 20% FCS. Different concentrations of AmB and/or poloxamer 188 were added to the same medium. After a 48-h incubation period for WT parasites and a 72-h incubation period for AmBr parasites, cell viability was evaluated by a colorimetric assay for mitochondrial oxidative activity with 3-(4,5-dimethylthiazol-2-yl)2,5- diphenyltetrazolium bromide. An isobologram prepared from the 50% inhibitory concentrations (IC50s) allowed assessment of the interactions between AmB and poloxamer 188. For both lines (WT and AmBr), the activity of poloxamer 188 alone increased with the concentration (0 to 125 μg/ml). The surfactant at 125 μg/ml killed 30% of the WT promastigotes and 50% of the AmBr promastigotes (data not shown). Poloxamer 188 did not modify the activity of AmB against WT parasites, as indicated by the planar form of the isobologram (Fig. 1A). However, against the AmBr line, a concave isobologram indicated synergistic action between AmB and poloxamer 188 (Fig. 1B). We suggest that poloxamer 188 is able to interact only within the fragile AmBr parasite membranes and promote the insertion of AmB within them. This could be the reason for the increase in toxicity exerted by AmB against the AmBr parasite, even though ergosterol, the main target of AmB, does not exist in AmBr parasites (21). We must also keep in mind the fact that if the AmB resistance is due to an overexpression of P glycoprotein, the poloxamer could inhibit the protein and increase the global absorption of the drug, as reviewed by Kwon and Okano (15).
FIG. 1.
Isobolograms of the IC50s obtained with combinations of AmB and poloxamer 188 against WT (A) and AmBr (B) L. donovani promastigotes.
The synergistic activity of AmB and poloxamer 188 against extracellular AmB-resistant promastigotes correlated with the strickingly increased activity and the reversion of resistance observed for amastigotes treated with poloxamer 188 and AmB at a high ratio (AmB-MM[12.5]). The increase in the activity of AmB against the WT amastigotes, even when poloxamer 188 does not modify the activity of free drug at the membrane level, could be a consequence of the surfactant's effect on the AmB aggregation. This fact may improve drug uptake by macrophages and drug availability for the parasite (19).
As no correlation has been found between the antileishmanial activity of AmB-lipid preparations in vitro (free AmB was four times more active than AmBisome against promastigotes and amastigotes) and in vivo (AmBisome was more active than conventional AmB) (24), further in vivo experiments must be carried out to fully evaluate our formulations. Nevertheless, this study suggests that the micellar formulation of AmB solubilized with poloxamer 188 could provide a simple and inexpensive way to increase the therapeutic index of AmB in the treatment of VL. It would also be able to reverse the resistance of the parasite to this drug if this problem begins to appear in clinical practice in the future.
Acknowledgments
This investigation received financial support from SIDACTION (France) and Gobierno de Navarra (Spain; resolucion 20/1998).
Special thanks go to Carlos Gamazo (Departamento de Microbiología, Universidad de Navarra) for review of the manuscript.
REFERENCES
- 1.Alléman E, Gurny R, Doelker E. Drug-loaded nanoparticles—preparation methods and drug targeting issues. Eur J Pharm Biopharm. 1993;39:173–191. [Google Scholar]
- 2.Araujo F G, Slifer T. Nonionic block copolymers potentiate activities of drugs for treatment of infections with Toxoplasma gondii. Antimicrob Agents Chemother. 1995;39:2696–2701. doi: 10.1128/aac.39.12.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berman J. Human leishmaniasis: clinical, diagnostic and chemotherapic developments in the last 10 years. Clin Infect Dis. 1997;24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 4.Berman J, Dietze R. Treatment of visceral leishmaniasis with amphotericin B colloidal dispersion. Chemotherapy (Basel) 1999;45(Suppl. 1):54–66. doi: 10.1159/000048471. [DOI] [PubMed] [Google Scholar]
- 5.Berman J D, Badaro R, Thakur C P, Wasunna K M, Behbehani K, Davidson R, Kuzoe F, Pang L, Weerasuriya K, Bryceson A D. Efficacy and safety of liposomal amphotericin B (AmBisome) for visceral leishmaniasis in endemic developing countries. Bull W H O. 1998;76:25–32. [PMC free article] [PubMed] [Google Scholar]
- 6.Bogdan C, Gessner A, Solbach W, Röllinghoff M. Invasion, control and persistence of Leishmania parasites. Curr Opin Immunol. 1996;8:517–525. doi: 10.1016/s0952-7915(96)80040-9. [DOI] [PubMed] [Google Scholar]
- 7.Borst P, Ouellette M. New mechanism of drug resistance in parasitic protozoa. Annu Rev Microbiol. 1995;49:427–460. doi: 10.1146/annurev.mi.49.100195.002235. [DOI] [PubMed] [Google Scholar]
- 8.Brajtburg J, Bolard J. Carrier effects on biological activity of amphotericin B. Clin Microbiol Rev. 1996;9:512–531. doi: 10.1128/cmr.9.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croft S L, Urbina J A, Brun R. Chemotherapy of human leishmaniasis and trypanosomiasis. In: Hide G, Mottram J C, Coombsand G H, Holmes P H, editors. Trypanosomiasis and leishmaniasis. Oxon, United Kingdom: CAB International; 1997. pp. 245–257. [Google Scholar]
- 10.Davidson R N, Di Martino L, Gradoni L, Giacchino R, Russo R, Gaeta G B, Pempinello R, Scotti S, Cascio A, Castagnola E, Maisto A, Gramiccia M, Di Caprio D, Wilkinson R J, Bryceson A D. Short-course treatment of visceral leishmaniasis with liposomal amphotericin B (AmBisome) Clin Infect Dis. 1996;22:938–943. doi: 10.1093/clinids/22.6.938. [DOI] [PubMed] [Google Scholar]
- 11.Espuelas M S, Legrand P, Chéron M, Barrat G, Devissaguet J-P, Irache J M. Interaction of amphotericin B with polymeric colloids. 1. A spectroscopic study. Colloids Surf B. 1998;11:141–151. [Google Scholar]
- 12.Flick P A, Gifford G E. Comparison of in vitro cell cytotoxicity assays for tumor necrosis factor. J Immunol Methods. 1984;68:167–173. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- 13.Haynes M P, Buckley H R, Higgins M L, Pieringer R A. Synergism between antifungal agents amphotericin B and alkyl glycerol ethers. Antimicrob Agents Chemother. 1994;38:1523–1529. doi: 10.1128/aac.38.7.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jagganath C, Allaudeen H S, Hunter R L. Activities of poloxamer CRL8131 against Mycobacterium tuberculosis in vitro and in vivo. Antimicrob Agents Chemother. 1995;39:1349–1354. doi: 10.1128/aac.39.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon G S, Okano T. Soluble self assembled block copolymers for drug delivery. Pharm Res. 1999;16:597–600. doi: 10.1023/a:1011991617857. [DOI] [PubMed] [Google Scholar]
- 16.Mbongo N, Loiseau P M, Billion M A, Robert-Gero M. Mechanism of amphotericin B resistance in Leishmania donovani promastigotes. Antimicrob Agents Chemother. 1998;42:352–357. doi: 10.1128/aac.42.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyerhoff A. U.S. Food and Drug administration approval of AmBisome (liposomal amphotericin B) for treatment of visceral leishmaniasis. Clin Infect Dis. 1999;28:42–48. doi: 10.1086/515085. [DOI] [PubMed] [Google Scholar]
- 18.Mozaffarian N, Berman J W, Casadevall A. Enhacement of nitric oxide synthesis by macrophages represents an additional mechanism of action by AmB. Antimicrob Agents Chemother. 1997;41:1825–1829. doi: 10.1128/aac.41.8.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullen A B, Carter K C, Baillie A J. Comparison of the efficacies of various formulations of amphotericin B against murine visceral leishmaniasis. Antimicrob Agents Chemother. 1997;41:2089–2092. doi: 10.1128/aac.41.10.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman M J, Actor J K, Balussubramanian M, Jagannath C. Use of noionic block copolymers in vaccines and therapeutics. Crit Rev Ther Drug Carrier Syst. 1998;15:89–142. doi: 10.1615/critrevtherdrugcarriersyst.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- 21.Ramos H, Milhaud J, Eleazar Cohen B, Bolard J. Enhanced action of amphotericin B on Leishmania mexicana resulting from heat transformation. Antimicrob Agents Chemother. 1990;34:1584–1589. doi: 10.1128/aac.34.8.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundar S, Agrawal N K, Sinha P R, Horwith G S, Murray H W. Short-course, low dose amphotericin B lipid complex therapy for visceral leishmaniasis unresponsive to antimony. Ann Intern Med. 1997;127:133–137. doi: 10.7326/0003-4819-127-2-199707150-00007. [DOI] [PubMed] [Google Scholar]
- 23.United States Pharmacopeial Convention, Inc. USP 24-NF 19. Poloxamer. Rockville, Md: United States Pharmacopeial Convention, Inc.; 2000. pp. 2492–2493. [Google Scholar]
- 24.Yardley V, Croft S L. A comparison of the activities of three amphotericin B lipid formulations against experimental and cutaneous leishmaniasis. Int J Antimicrob Agents. 2000;13:243–248. doi: 10.1016/s0924-8579(99)00133-8. [DOI] [PubMed] [Google Scholar]