Abstract
Background & objective
Contralesional 1-Hz repetitive transcranial magnetic stimulation (rTMS) over the right pars triangularis combined with speech-language therapy (SLT) has shown positive results on the recovery of naming in subacute (5–45 days) post-stroke aphasia. NORTHSTAR-CA is an extension of the previously reported NORTHSTAR trial to chronic aphasia (>6 months post-stroke) designed to compare the effectiveness of the same rTMS protocol in both phases.
Methods
Sixty-seven patients with left middle cerebral artery infarcts (28 chronic, 39 subacute) were recruited (01-2014 to 07-2019) and randomized to receive rTMS (N = 34) or sham stimulation (N = 33) with SLT for 10 days. Primary outcome variables were Z-score changes in naming, semantic fluency and comprehension tests and adverse event frequency. Intention-to-treat analyses tested between-group effects at days 1 and 30 post-treatment. Chronic and subacute results were compared.
Results
Adverse events were rare, mild, and did not differ between groups. Language outcomes improved significantly in all groups irrespective of treatment and recovery phase. At 30-day follow-up, there was a significant interaction of stimulation and recovery phase on naming recovery (P <.001). Naming recovery with rTMS was larger in subacute (Mdn = 1.91/IQR = .77) than chronic patients (Mdn = .15/IQR = 1.68/P = .015). There was no significant rTMS effect in the chronic aphasia group.
Conclusions
The addition of rTMS to SLT led to significant supplemental gains in naming recovery in the subacute phase only. While this needs confirmation in larger studies, our results clarify neuromodulatory vs training-induced effects and indicate a possible window of opportunity for contralesional inhibitory stimulation interventions in post-stroke aphasia.
NORTHSTAR trial registration
Keywords: aphasia, stroke, transcranial magnetic stimulation, speech therapy, language therapy, randomized controlled trial
Introduction
About one-third of all patients with first ever stroke suffer from a language disorder, or aphasia.1–8 About half of these patients will remain aphasic in the chronic phase after stroke, especially those with ischemic stroke, who have a low likelihood of recovery.1,3,9 Speech and language therapy (SLT) is the recommended therapeutic approach to aphasia, 10 since it is effective for language and functional communication improvement. 11 However, the affordable intensity of SLT in usual care settings is likely insufficient to achieve significant treatment effects on the language deficit. 12
In this context, repetitive transcranial magnetic stimulation (rTMS) has emerged as a potential supplementary treatment to potentiate the effectiveness and possibly reduce the duration of SLT. 13 rTMS induces cortical currents of short duration through rapidly changing magnetic fields, which can modulate the excitability and activity of targeted cortical regions. Low-frequency (1-Hz) rTMS has been associated with downregulation of cortical activity. 14 This modality has been used in post-stroke aphasia to limit the recruitment of compensatory cerebral networks of the right (unaffected) hemisphere in order to favor intrahemispheric compensation, which usually is associated with better recovery of certain language functions.15,16 Low-frequency rTMS may be most effective in the subacute phase when there is a chance to prevent the formation of maladaptive networks. However, modulating those networks in the chronic phase may also be possible and beneficial.
Meta-analyses of a few small randomized controlled trials (RCTs) have reported significant effects of rTMS on aphasia both in subacute and chronic stroke.17,18 As recently pointed out, 17 there is a disproportion between the few original experimental studies on rTMS for aphasia compared to the number of reviews of the literature, which all conclude that more multicenter RCTs, with larger populations and homogenous intervention protocols are required.
We recently published the results of an international and multilingual sham-controlled blinded prospective proof-of-concept trial called NORTHSTAR (NOn-invasive Repeated Therapeutic STimulation for Aphasia Recovery), designed to directly compare the effectiveness of rTMS vs tDCS and sham stimulation in subacute post-stroke aphasia (<45 days). We confirmed a medium to large positive effect of 1-Hz rTMS targeting the right pars triangularis on the recovery of naming performance compared to sham stimulation. 19 Here, we report the results of NORTHSTAR in chronic aphasia (NORTHSTAR-CA), where the same rTMS protocol was tested in patients at 6 months or more after a first ever ischemic stroke. This extension of the NORTHSTAR study protocol to patients with chronic aphasia allows for the first direct comparison of the same treatment regime in different phases of aphasia recovery and thus disentangles timing-specific training related effects of SLT from neuromodulatory effects of rTMS.
Methods
Trial Design
This extension of the NORTHSTAR trial in chronic aphasia was a two-armed sham-controlled blinded prospective proof-of-concept study, where patients were randomized to sham or rTMS treatment (allocation ratio 1:1). For 10 sessions over 2 weeks, patients received 45 minutes of individualized SLT by a certified therapist according to best-practice guidelines. 10 SLT started immediately following real/sham rTMS. Outcome measures were assessed one and 30 days following the last therapy session.
Study Settings
NORTHSTAR in subacute aphasia took place from January 2014 to March 2018 in Canada at the Jewish General Hospital (JGH, Montreal), CHUM-Hôpital Notre-Dame (Montreal) and Toronto Rehabilitation Hospital (Toronto); in the United States at Burke Rehabilitation Hospital (White Plains); and in Germany at the Rehabilitation Hospital RehaNova (Cologne). Subacute participants were recruited in the stroke units at each site. The extension to chronic aphasia started in September 2014 and lasted until July 2019 in Canada at the JGH. Chronic participants were recruited from the neurology outpatient clinic of the JGH and from associations of people with aphasia in Montreal. The trial protocol was approved by the Research Ethics Boards both at the central coordinating center (JGH) and each participating site. Institutional Review Board of the Faculty of Medicine, McGill University (Study approval ID A05-M47-13A). Patients and their relatives received written and conversational information about the study. Supplementary pictographic material was used if needed. Procedures were in accordance with institutional guidelines. Written informed consent was obtained from all participants before the study.
Patient Population
Eligible participants for NORTHSTAR were right-handed adults aged 18–90 years, with English, French, or German as language of daily use, having sustained a single left middle cerebral artery ischemic stroke resulting in aphasia, and whose performance was below the lower limit of the norm on at least one of the primary outcome measures (see below) at baseline. Exclusion criteria included a previous stroke, a new stroke since the initial event causing the aphasia, severe comprehension deficit that might compromise informed consent or understanding of instructions, contraindications to MRI and/or TMS, neurodegenerative or psychiatric disease, epilepsy or EEG-documented epileptic discharges, severe chronic renal or liver failure, life-threatening diseases, auditory or visual deficits that could not be corrected and might have impaired testing.
In this study, post-stroke stages were defined as follows: acute (<5 days), subacute (5 days–4 mo), and chronic (>4 mo). Chronic patients were recruited 6 months or more after aphasia onset, whereas subacute patients were included 5 to 45 days after the stroke. Recruitment age-range and subacute time-window was extended from the initial protocol (age 50–85, 5–30 days post-stroke) in February 2015 to accelerate recruitment. Patients with chronic aphasia were recruited at the JGH, and through advertisements in associations of people with aphasia in the province of Quebec.
Included patients underwent a T1-weighted MRI. Prior to analysis and unblinding, 2 raters (AT and AZ) independently stratified patients’ scans by infarct location: as affecting the extended Broca’s area (i.e. the inferior or middle frontal gyri, anterior insula, premotor cortex and underlying white matter, and basal ganglia 20 ) or not. For divergent classification, consensus was reached by joint review.
Intervention
rTMS was applied over the non-affected right hemisphere (pars triangularis of the right inferior frontal gyrus) using a 70 mm figure-of-eight coil at 1 Hz for 900 pulses (15 minutes) at 90% resting motor threshold (RMT). RMT was determined prior to each treatment session over the right primary motor area (amplitude of motor evoked potentials >50 μV in 5/10 pulses). 21 The coil was oriented to direct the current perpendicular to the target gyrus. For sham stimulation, the coil was placed over the inter-hemispheric fissure at the vertex and stimulation was applied with 10% RMT. Stimulation devices used were the eXimia NBS4, Nexstim Ltd. (JGH, Burke Rehabilitation Hospital), Magstim R2, Magstim Company Ltd. (RehaNova) and MagPro X100, MagVenture A/S (Sunnybrook Hospital and Hôpital Notre-Dame). The stimulation target (right pars triangularis) was localized using the patient’s T1-weighted MRI and transferred to the patient’s head using the neuronavigation system of the TMS device or a modification of the surface distance measurements method. 22 SLT sessions were given immediately following the rTMS procedure to ensure treatment within the period of maximum rTMS after-effect (about 45 minutes). 23
Randomization and Blinding
Participants with chronic aphasia were assigned to study arms by means of a computer-generated list of random numbers (non-restricted randomization), concealed from investigators and research personnel in sequentially numbered, opaque and sealed envelopes. The technician performing the stimulation opened a participant’s envelope on the first day of treatment, after the participant had completed all baseline assessments.
For participants with subacute aphasia, computer-generated, non-restricted randomization by site was performed through an online system located at the Department of Clinical Epidemiology at the JGH (Montreal). Only the technician performing the stimulation had access to the randomization information when logging into the study platform, on the first day of treatment.19,24 All patients, therapists, principal investigators and research personnel assessing clinical outcomes were blinded to the treatment assignment. Therapists did not attend rTMS sessions.
Primary Outcome Measures and Variables
Primary outcomes were lexical access (in naming and verbal fluency) and auditory sentence comprehension, core language functions measured by 3 tests commonly used in English, French, and German, and comparable across languages: The Boston Naming Test (BNT, total correct score), 25 the semantic verbal fluency task (SF1min),26–28 and the 36-item Token Test (TT). 29 We derived standardized Z-scores based on the normative data of each test.
The primary outcome variables were the difference in Z-scores at days 1 and 30 post-treatment, relative to baseline on each of these tests.
Secondary Outcome Measures and Variable
Secondary outcome measures were integral measures of aphasic impairment. In the absence of a single test for aphasic impairment normalized in all 3 languages, we used approved language specific batteries: the Protocole Montréal-Toulouse-86 in French, 30 the Western Aphasia Battery (WAB) 31 in English, and the Aachener Aphasie Test in German. 32 We derived a standardized T-score, the Unified Aphasia Score (UnAS) based on the normative data available for each battery. 19
The secondary outcome variable was the percent difference of UnAS at days 1 and 30 post-treatment, relative to baseline.
Adverse Events and Serious Adverse Event Outcomes
AE during or ≤1h after session (headache, scalp dysesthesia/paresthesia at stimulation site, and muscle pain of temporal or neck muscles) and SAE during or following session (seizures) were documented after each therapy session. Cumulative AE and SAE during 10 days of therapy are reported.
Sample Size Determination
The sample size of the NORTHSTAR chronic extension was determined similarly to the subacute study. 24 In order to detect a 5% difference between treatment groups at P = .01 with a power of .95 in an ANOVA, assuming a standard deviation of 4%, 12 patients per group (sham and TMS) were necessary. Based on our previous stroke recovery studies, we expected a subject attrition rate of 10%. We thus planned to recruit 30 patients in total. The study was terminated after enrollment of 28 subjects because lower than expected recruitment rates would have resulted in further study extension, and we already had more than 12 chronic patients per group having completed the study.
Analyses of Chronic Data
Data from participants with chronic aphasia were analyzed similarly to the study in subacute aphasia. 19 Only data entry errors were corrected. Distributions were assessed for normality with Shapiro-Wilks tests. We used parametric tests in the absence of outliers and when data (or square-root transformed data) were normally distributed. Means (M) and standard deviations (SD) are reported. If criteria were not met for parametric tests, related non-parametric tests were used, and we report median (Mdn) and interquartile ranges (IQR). Analyses were performed on SPSS-24.0 (IBM Corp.) and RStudio. 33 The significance threshold was P < .05.
Pre-planned primary analyses in chronic patients were between-group (rTMS vs. Sham) comparisons according to the intention-to-treat principle for the primary and secondary variables (i.e. Z-score changes on the BNT, SF1min, and TT, and percent difference of UnAS at days 1 and 30 post-treatment, relative to baseline). Missing data were replaced by the mean of each corresponding randomization group.
Secondary analyses in chronic patients were performed on the whole chronic sample (N = 28), independent of treatment allocation. We used repeated-measures analyses to identify significant changes in all measures between the 3 different observation time points: baseline, immediately after treatment (1 day post-treatment), and at follow-up (30 days post-treatment). Missing data of the patient who declined follow-up evaluation (see Figure 1) were replaced using the Last-Observation-Carried-Forward (LOCF) method for this analysis.
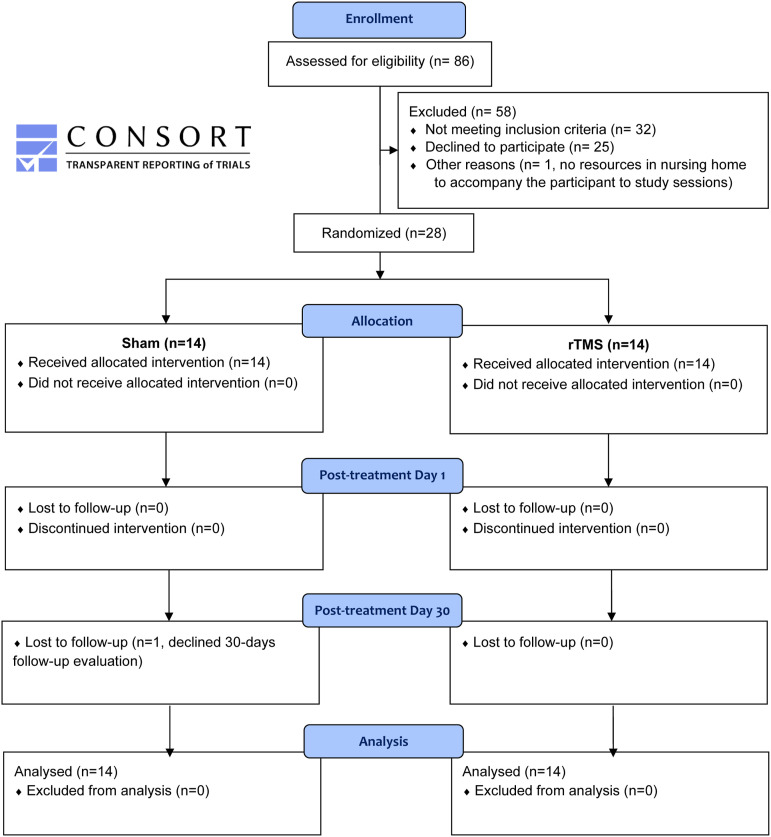
Figure 1.
NORTHSTAR-CA participant flow diagram.
Analyses of Chronic Versus Subacute Data
We compared the effect of rTMS in the subacute and chronic phases post-stroke with 2-way ANOVAs (rTMS/sham; subacute/chronic) for medians, a robust alternative to the traditional 2-way ANOVA, which was not suited for our data distributions. We performed the analysis in RStudio 33 using the med2way function available in the WRS2 package. 34 The test statistics for the main effects in med2way are F-distributed; the (heteroscedastic) test for the interaction is chi-square distributed.
Results
Chronic Aphasia Results
Participant Flow
A total of 86 chronic patients were assessed for eligibility to participate in the study. Thirty-two did not meet the inclusion criteria. Out of 54 eligible patients, 25 declined to participate and 1 patient living in a nursing home had no resources to be accompanied to study sessions. The remaining 28 patients were randomized in the 3 study arms. Figure 1 displays the participant flow.
Baseline Characteristics
Baseline characteristics were similar across intervention groups (Table 1). In each group, every patient but one had a lesion affecting Broca’s complex. 20 Individual baseline data are presented in Supplementary Table 1.
Table 1.
Demographic & Baseline Clinical Characteristics of Patients with Chronic Aphasia (NORTHSTAR-CA) Per Intervention Group.
| Sham | rTMS | Between-group comparison (P-value of t-tests or median tests, where appropriate) | |
|---|---|---|---|
| N | 14 | 14 | |
| Male; Female | 9; 5 | 10; 4 | |
| English; French | 4; 10 | 5; 9 | |
| Lesion in Broca’s complex, N (% participants) | 13 (93%) | 13 (93%) | |
| Age, mean (SD) | 66 (9) | 65 (11) | .761 |
| Months post-stroke at recruitment, median (IQR) | 33 (84) | 33 (82) | 1.000 |
| Naming (BNT total correct Z-score), mean (SD) | −7.34 (3.77) | −6.34 (3.14) | .449 |
| Verbal fluency (SF1min Z-score), median (IQR) | −2.54 (2.47) | −2.44 (2.30) | 1.000 |
| Comprehension (TT Z-score), mean (SD) | −9.65 (5.36) | −8.14 (3.88) | .400 |
| UnAS (0-100), mean (SD) | 21.7 (18.8) | 34.9 (27.0) | .145 |
Means (and standard deviations) or medians (and interquartile ranges) are displayed according to the normality of the data distribution.
BNT: Boston Naming Test; SF1min: semantic fluency test (animals in 1 minute); TT: 36-item Token Test; UnAS: unified aphasia score.
rTMS Versus Sham Comparisons
As shown in Table 2, changes relative to baseline in primary and secondary outcomes did not differ between treatment groups in patients with chronic aphasia, neither immediately after treatment (day 1) nor at follow-up (day 30).
Table 2.
Comparison of Language Recovery in Patients with Subacute (NORTHSTAR) and Chronic (NORTHSTAR-CA) Post-Stroke Aphasia in the rTMS and Sham Stimulation Groups.
| Change Relative to Baseline | Subacute Phase Post-stroke (NORTHSTAR) | Chronic Phase Post-stroke (NORTHSTAR-CA) | Sham Chronic/Subacute Comparison | rTMS Chronic/Subacute Comparison | Stimulation x Phase (P values of Robust Median ANOVAs) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham N = 19 | rTMS N = 20 | Post-hoc P value | Sham N = 14 | rTMS N = 14 | P value | P value | P value | Stimulation Main Effect | Phase Main Effect | Interaction Effect | |
| Naming (BNT total correct Z-score) | |||||||||||
| day 1 | .73 (.94) | .90 (1.19) | − | .21 (1.08) | .56 (1.69) | .706 | .839 | .727 | .406 | .170 | .455 |
| day 30 | 1.02 (1.71) | 1.91 (.77) | rTMS>Sham (P = .046)* | .61 (1.00) | .15 (1.68) | .706 | .839 | .015* | .571 | .004* | <.001* |
| Verbal fluency (SF1min Z-score) | |||||||||||
| day 1 | .00 (.20) | .00 (.46) | − | .17 (1.95) | .26 (.71) | 1.000 | .839 | .103 | .845 | .320 | .634 |
| day 30 | .73 (1.14) | .48 (.75) | − | .17 (.49) | .36 (.71) | .706 | .107 | .727 | .857 | .060 | .144 |
| Comprehension (TT Z-score) | |||||||||||
| day 1 | 1.12 (1.87) | .87 (1.99) | − | .35 (1.75) | .82 (2.10) | .706 | .02* | .727 | .836 | .423 | .430 |
| day 30 | 2.07 (2.57) | 1.54 (2.22) | − | .93 (2.22) | .47 (2.16) | .706 | .364 | .145 | .351 | .036* | .735 |
| UnAS (percent change) | |||||||||||
| day 1 | 9.00 (19.9) | 6.85 (17.7) | − | 13.64 (46.60) | 9.39 (25.48) | .706 | .616 | .727 | .788 | .762 | .886 |
| day 30 | 15.40 (63.5) | 26.25 (28.3) | − | 6.51 (56.44) | 12.53 (37.41) | .257 | .107 | .296 | .487 | .352 | .368 |
Post-treatment changes relative to baseline immediately after treatment (day 1), and at follow-up (day 30).
Medians (and interquartile ranges) are displayed for each subgroup as well as P values of median tests.
Significance level =.05.
BNT: Boston Naming Test; SF1min: semantic fluency test (animals in 1 minute); TT: 36-item Token Test; UnAS: unified aphasia score.
*Identifies P values <.05.
Safety
There was no SAE. AEs were rare and their cumulative numbers did not differ between groups: one patient in the sham group reported a headache after sessions #1 and #6, and one patient in the rTMS group reported dysesthesia (tension in the right shoulder) after session #8.
Main Effect of Time
Table 3 displays the results of the secondary analyses on the whole chronic participant sample, irrespective of the treatment (rTMS/sham) group. Repeated-measure comparisons showed significant differences between the 3 observation time points in all language recovery outcomes. Post-hoc tests revealed that patients improved significantly on most measures during the intervention phase (i.e. from baseline to immediately post-treatment), where SLT was provided, and remained stable during the 1-month follow-up period (day 1 to day 30 post-treatment).
Table 3.
Whole Sample (N = 28) Data Across Intervention and Follow-Up Phases in Chronic Patients (NORTHSTAR-CA).
| Measures | Pre-treatment | Post-treatment day 1 | Post-treatment day 30 | Repeated measure comparison (P value of ANOVAs or equivalent non-parametric tests) | Post-hoc tests: mean difference and [95% CI], or median and (IQR) where appropriate, and P-value with Bonferroni correction | |
|---|---|---|---|---|---|---|
| Intervention Phase (Pre- to Post-treatment day 1) | Follow-up Phase (Post-treatment day 1 to 30) | |||||
| Naming (BNT total correct Z-score), mean (SD) | −6.84 (3.44) | −6.22 (3.54) | −6.16 (3.62) | .004* | .62 (.13, 1.11) .010* | .06 (−.24, .35) 1.000 |
| Verbal fluency (SF1min Z-score), median (IQR) | −2.44 (2.36) | −1.68 (2.90) | −2.16 (2.68) | .014* | .26 (.84) 048* | .00 (.56) 1.000 |
| Comprehension (TT Z-score), mean (SD) | −8.90 (4.66) | −8.27 (5.09) | −8.03 (5.08) | .017* | .63 (−.10, 1.35) .110 | .24 (−.45, .94) 1.000 |
| UnAS, sqrt-transformed (0-10), mean (SD) | 4.77 (2.40) | 5.07 (2.33) | 5.10 (2.31) | <.001* | .30 (.13, .47) <.001* | .03 (−.15, .21) 1.000 |
BNT: Boston Naming Test; SF1min: semantic fluency test (animals in 1 minute); TT: 36-item Token Test; UnAS: unified aphasia score; sqrt: square root; IQR: interquartile range.
*Identifies P values <.05.
Subacute Aphasia Results
We have reported the detailed results of NORTHSTAR in subacute aphasia elsewhere. 19 Thirty-nine participants received either rTMS (N = 20) or sham stimulation (N = 19). Naming was significantly improved by rTMS at 30 days vs sham stimulation (χ2 [1] = 5.867; P = .046; φ = .39, medium–large effect, Figure 2; Table 2). All other primary results were non-significant. The rTMS effect was driven by the patient subgroup with intact Broca’s area. 19
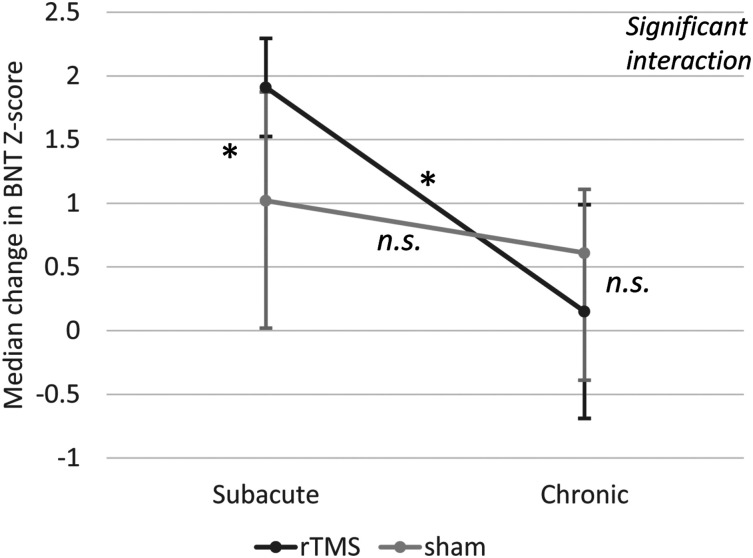
Figure 2.
Improvement in naming at follow-up (day 30) after speech-language therapy combined with rTMS or sham stimulation in patients in the subacute or chronic phase. Error bars represent Interquartile ranges (IQR); * indicates significant differences; n.s., non-significant; BNT, Boston Naming Test. There was a significant interaction between stimulation and phase (P < .001). In subacute patients, naming was significantly improved by rTMS (Mdn = 1.91, IQR = .77) compared to sham stimulation (Mdn = 1.02, IQR = 1.71, P = .046 φ = .39, medium–large effect). Chronic patients, however, did not differ between treatment groups. Naming recovery with rTMS was larger in subacute (Mdn=1.91/IQR=.77) than chronic patients (Mdn = .15, IQR = 1.68, P = .015, φ = .48, medium–large effect). There was similar improvement in chronic and subacute patients allocated to sham stimulation (i.e.,, speech-language therapy only).
Comparison of Sham Stimulation in Chronic Versus Subacute Aphasia
In patients allocated to the sham protocol (receiving only SLT), there was a significantly higher improvement in comprehension (TT) in subacute compared to chronic patients immediately after the treatment (χ2 [1] = 7.127; P = .020; φ=.47, medium–large effect, Table 2). The remaining language changes did not differ significantly between the 2 groups of patients.
Contralesional rTMS Effects in Chronic Versus Subacute Aphasia
As shown in Table 2, the median 2-way ANOVAs did not reveal any significant effect immediately after the treatment. However, there was a significant main effect of time at follow-up (day 30) on the recovery of naming and comprehension performance favoring patients in the subacute post-stroke phase. There was no main effect of stimulation, but there was a significant interaction effect of stimulation and phase on the changes in naming ability (Figure 2). The effect of rTMS on naming recovery was significantly larger in subacute than chronic patients (χ2 [1] = 7.771; P = .015; φ = .48, medium-large effect).
Discussion
This randomized sham-controlled blinded study is the first to compare the effectiveness of 1 Hz subthreshold rTMS over the right pars triangularis in chronic vs subacute aphasia. In line with the literature, 11 we found improvements on language measures during SLT and maintenance of gains at 1-month follow-up, with similar to larger effects being observed in the subacute (vs. chronic) phase. An add-on effect of rTMS, however, was only present in the subacute phase, with effect sizes exceeding that of SLT alone. There was no significant rTMS effect in the chronic aphasia group.
This direct comparison may support the hypothesis of a window of neuroplasticity which renders the brain specifically susceptible to neuromodulatory intervention relatively early during the course of recovery. 35 Most imaging studies in post-stroke aphasia concur that a shift of task-induced brain activation is observed in the first weeks after the stroke and that a normalization of activity patterns occurs after several months.36–38 It has been shown in combined imaging and brain stimulation studies, that this natural occurring process can be modulated with rTMS in the first 2–12 weeks and that such successful modulation correlates with therapy response.14,39 Our results in subacute aphasia show a medium to large effect of rTMS, which almost doubles naming recovery compared to SLT alone. 19 This effect size is in line with recent meta-analyses17,18 and supports the potential clinical relevance of rTMS in the early subacute post-stroke phase.
In contrast, our results in chronic aphasia differ from the conclusions of meta-analyses17,18 and some previous RCTs using contralesional 1-Hz rTMS in this post-stroke phase.40–43 Methodological differences point to possible explanations and open avenues for future research. Some “distributed” intervention protocols (applying brain stimulation 10 to 30 times per day) or delivering therapy during stimulation40,42 have resulted in improvements in this population, but it may be very challenging to implement such high-frequency treatments in most public health systems. Our protocol was designed to fit into subacute in-patient rehabilitation care pathways in the participating countries and was readily transferable to an outpatient setting for chronic participants.
Another possibility is that a significant effect of rTMS in chronic patients may only appear at later follow-up. In subacute patients the rTMS effect on naming recovery reached significance 1 month after the end of the treatment. This delay might be even longer in patients at the chronic stage, where improvement can also occur, but at a lower rate. Results of Barwood et al 43 are consistent with this idea in chronic aphasia. They reported an absence of significant results 1 week after 10 days of rTMS in daily blocks of 20 min (without SLT) but a significant beneficial effect at 2-month follow-up.
Among studies on chronic aphasia, the closest RCTs to our design are those of Tsai et al. 41 , where 33 participants received 10 daily stimulation blocks of 10 min followed by 60 min of SLT, and Wang et al., 42 where a group of 15 patients received 10 daily stimulation blocks of 20 min followed by 20 min of SLT. Both studies were led by the same laboratory and run in the same clinical settings, but results were inconsistent. In line with our results, there was no significant effect with the stimulation protocol in Wang et al, whereas beneficial effects were reported in Tsai et al where participants received half the dose of rTMS. Of note is the larger sample size in Tsai et al.’s RCT and absence of improvement in the control group despite the provision of SLT.
These inconsistent results in chronic aphasia trials indicate the need for larger RCTs. While for example, contralesional rTMS for motor recovery has shown evidence of effectiveness in chronic stroke patients in smaller RCTs, a recent well designed RCT with approximately 200 participants recruited 3 to 12 month after stroke failed to demonstrate a therapeutic effect on recovery of arm function, and led to revision of the levels of evidence of rTMS for chronic motor recovery.44,45
These inconsistencies must also draw our attention to individual factors that could influence response to rTMS, such as lesion location and cortical excitability. In the NORTHSTAR (subacute) trial the positive effect of rTMS on naming recovery was driven by the patient subgroup whose lesion had spared Broca’s complex. In NORTHSTAR-CA, all but two participants had a lesion in Broca’s complex (Table 1). If this lesion location mitigates the response to rTMS on the contralesional pars triangularis, our chronic sample was at considerable disadvantage for showing benefits from this stimulation protocol. Tsai et al did not examine their data according to lesion location, but they found that the initial resting motor threshold (RMT i.e. the level of cortical excitability for the FDI of the left hand, which is tested to calibrate the stimulation intensity before each stimulation session), was significantly associated with the response to rTMS. We performed this complementary analysis but found no such significant association (see Supplemental material).
Limitations
Patients in the chronic phase had significantly larger lesions than patients in the subacute phase (Figure 3) and a high percentage had lesions in Broca’s complex. This difference, which might have masked a potential effect of our protocol in chronic aphasia, might be related to recruitment procedures. In the subacute group, every potential patient with speech or language impairment was approached in the stroke units, whereas in the chronic group, the study was advertised through posters and patients or their caregiver had to contact the study coordinator to express their desire to participate to the study. This could have introduced a recruitment bias.
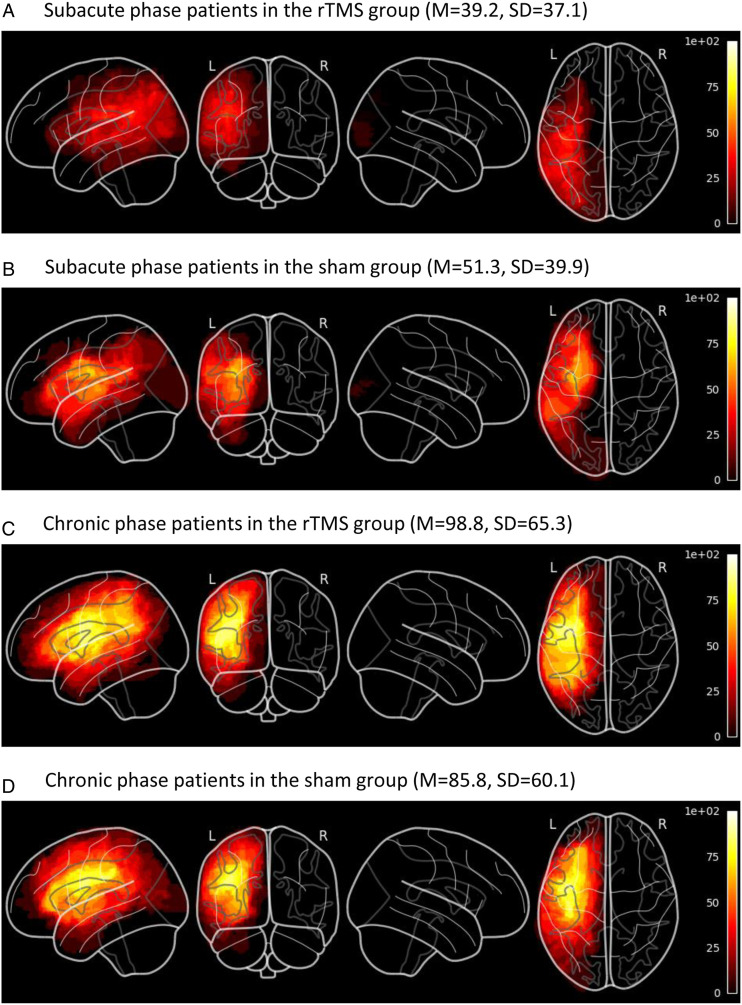
Figure 3.
Lesion overlap maps spatially normalized to MNI-stereotaxic space for subgroups of patients, and lesion volumes in mL (means and standard deviations). Lesion volumes were not significantly different in rTMS and sham groups (main effect: F [1, 54] < .01, P = .99, partial η2 = .00) and there was no statistically significant interaction between phases and treatment groups (F [1, 54] = .86, P = .36, partial η2 = .02). Patients in the chronic phase had significantly larger lesions than patients in the subacute phase (main effect: F [1, 54] = 11.97, P < .01, partial η2 = .18). (a) Subacute phase patients in the rTMS group (M = 39.2, SD = 37.1). (b) Subacute phase patients in the sham group (M = 51.3, SD = 39.9). (c) Chronic phase patients in the rTMS group (M = 98.8, SD = 65.3). (d) Chronic phase patients in the sham group (M = 85.8, SD = 60.1).
Beyond statistical significance, it is difficult to state with certainty the clinical relevance of the results of the language measures since there is no consensus yet on clinically significant changes in most of the tests. This is because they have typically been constructed to diagnose disorders rather than to document changes. The absence of parallel versions in most tests also limits the possibility of ruling out some learning effect between two closely spaced administrations. This limitation applies to our secondary results on changes with speech therapy, since we did not have a comparison group without speech therapy. However, we think the possibility of a practice effect is probably low given the large number of items in the test we have used, and our results are consistent with current data from RCTs showing the effectiveness of SLT in aphasia. Psychometric advances regarding language measurement tools may be incorporated into future studies on rTMS for aphasia and include measurements of connected speech to probe more closely to everyday language skills.
Note also that there is no consensus on the definition of exact time windows for acute, subacute, and chronic post-stroke phases. Our subacute participants were recruited between 5 and 45 days after stroke, which can be considered an early period of the subacute phase.
Conclusion and Perspectives
Contralesional 1-Hz rTMS over the right pars triangularis combined with SLT is a safe add-on therapy for post-stroke aphasia, independent of the post-stroke phase. The addition of rTMS to SLT led to significant supplemental gains in naming recovery in the early subacute phase only. While there remains anecdotal evidence for the efficacy of rTMS in chronic aphasia, larger trials with longer follow-up periods are needed to identify potentially smaller and possibly delayed effects on top of effective SLT. Further investigation of this rTMS protocol should also include patients with different lesion types (e.g.,, hemorrhagic strokes) and determine neuroimaging or other biomarker factors for its efficacy.
Supplemental Material
Supplemental Material – for Differential Effects of Speech and Language Therapy and rTMS in Chronic Versus Subacute Post-stroke Aphasia: Results of the NORTHSTAR-CA Trial by Anna Zumbansen, Heike Kneifel, Latifa Lazzouni, Anja Ophey, Sandra E. Black, Joyce L. Chen, Dylan Edwards, Thomas Funck, Alexander Erich Hartmann, Wolf-Dieter Heiss, Franziska Hildesheim, Sylvain Lanthier, Paul Lespérance, George Mochizuki, Caroline Paquette, Elizabet Rochon, Ilona Rubi-Fessen, Jennie Valles, Susan Wortman-Jutt and Alexander Thiel; on behalf of the NORTHSTAR-study group in Neurorehabilitation and Neural Repair
Acknowledgments
We thank Sharon Shapiro (research coordinator), Stephanie Houston, Mica Vincent, Dominique Gillis, Mélissa Bouchard, Sophie Audy, Susan Martin, Lauren Tittley, Michèle Masson-Trottier, and Édith Durand (speech-language pathologists) and all patients who volunteered to participate in this study.
Declaration of Conflicting Interests: The authors declare that there is no conflict of interest.
Funding: This trial was supported by research grants from the Canadian Institutes for Health Research (CIHR) [MOP#125954]; the W.-D. Heiss Foundation; and the Lady Davis Institute at the Jewish General Hospital [CLIPP#2014]. A.Z. was funded by a CIHR postdoctoral fellowship.
NORTHSTAR Study Group: Alexander Thiel, Anna Zumbansen, Latifa Lazzouni, Sharon Shapiro, Anja Ophey, Stephanie Houston, Mica Vincent, Dominique Gillis, Mélissa Bouchard, Caroline Paquette, Franziska Hildesheim and Thomas Funck (Jewish General Hospital, Montreal);
Alexander Hartmann, Ilona Rubi-Fessen, Heike Kneifel and Thomas Rommel (RehaNova, Cologne)
Wolf-Dieter Heiss (Max Planck Institute für Stoffwechsel Forschung—MPI for Metabolism Research & Universität zu Köln, Cologne)
Josef Kessler and Anja Ophey (Universität zu Köln, Cologne)
Dylan Edwards, Jennie Valles, and Susan Wortman-Jutt (Burke Neurological Institute, White Plains)
Sylvain Lanthier, Marlène Lapierre, Diana Mina, Liliana Jastrzabek, Paul Lespérance, and Walid El-Abyad (CHUM, Montreal)
Elizabeth Rochon, Laura Laird, Fiona Höbler, Lisa McQueen, Ruth Tannenbaum, Joanna Wong, Amy Lewis, and Alyssa Bobkin (Toronto Rehabilitation Institute, Toronto)
Sandra E. Black, George Mochizuki, Joyce Chen, Valerie Closson, Andrew Centen, and Tyler Saumur (Sunnybrook Hospital, Toronto).
Supplementary material for this article is available on the Neurorehabilitation & Neural Repair website at https://journals.sagepub.com/home/nnr.
ORCID iDs
Anna Zumbansen https://orcid.org/0000-0003-2332-8263
Heike Kneifel https://orcid.org/0000-0003-0524-3143
Anja Ophey https://orcid.org/0000-0001-5858-7762
Sandra E. Black https://orcid.org/0000-0001-7093-8289
Dylan Edwards https://orcid.org/0000-0001-7346-3100
Caroline Paquette https://orcid.org/0000-0001-8109-5301
Ilona Rubi-Fessen https://orcid.org/0000-0002-9775-3812
References
- 1.Croquelois A., Bogousslavsky J. Stroke aphasia: 1,500 consecutive cases. Cerebrovasc Dis. 2011;31(4):392-399. [DOI] [PubMed] [Google Scholar]
- 2.Engelter S. T., Gostynski M., Papa S., et al. Epidemiology of aphasia attributable to first ischemic stroke. Stroke. 2006;37(6):1379-1384. [DOI] [PubMed] [Google Scholar]
- 3.Godefroy O., Dubois C., Debachy B., Leclerc M., Kreisler A. Vascular aphasias. Stroke. 2002;33(3):702-705. [DOI] [PubMed] [Google Scholar]
- 4.Inatomi Y., Yonehara T., Omiya S., Hashimoto Y., Hirano T., Uchino M. Aphasia during the acute phase in ischemic stroke. Cerebrovasc Dis. 2008;25(4):316-323. [DOI] [PubMed] [Google Scholar]
- 5.Laska A. C., Hellblom A., Murray V., Kahan T., Von Arbin M. Aphasia in acute stroke and relation to outcome. J Intern Med. 2001;249(5):413-422. [DOI] [PubMed] [Google Scholar]
- 6.Maas M. B., Lev M. H., Ay H., et al. The prognosis for aphasia in stroke. J Stroke Cerebrovasc Dis. 2012;21(5):350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedersen P. M., Stig Jørgensen H., Nakayama H., Raaschou H. O., Olsen T. S. Aphasia in acute stroke: incidence, determinants, and recovery. Ann Neurol. 1995;38(4):659-666. [DOI] [PubMed] [Google Scholar]
- 8.Ellis C., Hardy R. Y., Lindrooth R. C., Peach R. K. Rate of aphasia among stroke patients discharged from hospitals in the United States. Aphasiology. 2018;32(9):1075-1086. [Google Scholar]
- 9.Pedersen P. M., Vinter K., Olsen T. S. Aphasia after stroke: Type, severity and prognosis. Cerebrovasc Dis. 2004;17(1):35-43. [DOI] [PubMed] [Google Scholar]
- 10.Hebert D., Lindsay M. P., McIntyre A., et al. Canadian stroke best practice recommendations: Stroke rehabilitation practice guidelines, update 2015. Int J Stroke. 2016;11(4):459-484. [DOI] [PubMed] [Google Scholar]
- 11.Brady MC, Kelly H, Godwin J, Enderby P, Campbell P. Speech and language therapy for aphasia following stroke. Cochrane Database Syst Rev. 2010;(5):CD000425. [DOI] [PubMed] [Google Scholar]
- 12.Code C., Petheram B. Delivering for aphasia. Int J Speech Lang Pathol. 2011;13(1):3-10. [DOI] [PubMed] [Google Scholar]
- 13.Zumbansen A, Thiel A. Recent advances in the treatment of post-stroke aphasia. Neural Regen Res. 2014;9(7):703-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiel A., Hartmann A., Rubi-Fessen I., et al. Effects of noninvasive brain stimulation on language networks and recovery in early poststroke aphasia. Stroke. 2013;44(8):2240-2246. [DOI] [PubMed] [Google Scholar]
- 15.Heiss W.-D., Kessler J., Thiel A., Ghaemi M., Karbe H. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol. 1999;45(4):430-438. [DOI] [PubMed] [Google Scholar]
- 16.Rosen H. J., Petersen S. E., Linenweber M. R., et al. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55(12):1883-1894. [DOI] [PubMed] [Google Scholar]
- 17.Bucur M., Papagno C. Are transcranial brain stimulation effects long-lasting in post-stroke aphasia? A comparative systematic review and meta-analysis on naming performance. Neurosci Biobehav Rev. 2019;102:264-289. [DOI] [PubMed] [Google Scholar]
- 18.Shah-Basak P. P., Wurzman R., Purcell J. B., Gervits F., Hamilton R., neuroscience. Fields or flows? A comparative metaanalysis of transcranial magnetic and direct current stimulation to treat post-stroke aphasia. Restor Neurol Neurosci. 2016;34(4):537-558. [DOI] [PubMed] [Google Scholar]
- 19.Zumbansen A., Black S. E., Chen J. L., et al. Non-invasive brain stimulation as add-on therapy for subacute post-stroke aphasia: A randomized trial (NORTHSTAR). Eur Stroke J. 2020;5(4):402-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aboitiz F., Garcia V. R. The evolutionary origin of the language areas in the human brain. A neuroanatomical perspective. Brain Res Rev. 1997;25(3):381-396. [DOI] [PubMed] [Google Scholar]
- 21.Mishory A., Molnar C., Koola J., et al. The maximum-likelihood strategy for determining transcranial magnetic stimulation motor threshold, using parameter estimation by sequential testing is faster than conventional methods with similar precision. J ECT. 2004;20(3):160-165. [DOI] [PubMed] [Google Scholar]
- 22.Weiduschat N., Habedank B., Lampe B., et al. Localizing broca's area for transcranial magnetic stimulation: Comparison of surface distance measurements and stereotaxic positioning. Brain stimul. 2009;2(2):93-102. [DOI] [PubMed] [Google Scholar]
- 23.Avanzino L., Bove M., Trompetto C., Tacchino A., Ogliastro C., Abbruzzese G.. 1-Hz repetitive TMS over ipsilateral motor cortex influences the performance of sequential finger movements of different complexity. Eur J Neurosci. 2008;27(5):1285-1291. [DOI] [PubMed] [Google Scholar]
- 24.Thiel A., Black S. E., Rochon E. A., et al. Non-invasive repeated therapeutic stimulation for aphasia recovery: a multilingual, multicenter aphasia trial. J Stroke Cerebrovasc Dis. 2015;24(4):751-758. [DOI] [PubMed] [Google Scholar]
- 25.Tombaugh T. N., Hubiey A. M. The 60-item boston naming test: Norms for cognitively intact adults aged 25 to 88 years. J Clin Exp Neuropsychol. 1997;19(6):922-932. [DOI] [PubMed] [Google Scholar]
- 26.Tombaugh T. N., Kozak J., Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14(2):167-177. [PubMed] [Google Scholar]
- 27.Cardebat D, Doyon B, Puel M, Goulet P, Joanette Y. [Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level]. Acta Neurol Belg. 1989;90(4):207-17. [PubMed] [Google Scholar]
- 28.Aschenbrenner S, Tucha O, Lange KW. Regensburger Wortflüssigkeits-Test. Hogrefe: Verlag für Psychologie; 2000. [Google Scholar]
- 29.De Renzi E., Faglioni P. Normative data and screening power of a shortened version of the Token Test. Cortex. 1978;14(1):41-49. [DOI] [PubMed] [Google Scholar]
- 30.Nespoulous J, Lecours A, Lafond D, Lemay A, Puel M, Joannette Y. Protocole Montréal-Toulouse d’examen linguistique de l’aphasie [Montreal-Toulouse Protocol of aphasia linguistic examination]. Isbergues, France: L’Ortho-Edition; 1992. [Google Scholar]
- 31.Kertesz A. Western Aphasia Battery–Revised. Austin, TX: Pro-Ed; 2006. [Google Scholar]
- 32.Huber W, Poeck K, Weniger D, Willmes K. Der Aachner Aphasie Test. Göttingen, Germany: Hogrefe; 1983. [Google Scholar]
- 33.RStudio. Integrated Development Environment For R [computer Program]. Boston, MA: RStudio, PBC.; 2020. [Google Scholar]
- 34.Mair P., Wilcox R. Robust statistical methods in R using the WRS2 package. Behav Res Methods. 2020;52(2):464-488. [DOI] [PubMed] [Google Scholar]
- 35.Hordacre B., Austin D., Brown K. E., et al. Evidence for a window of enhanced plasticity in the human motor cortex following ischemic stroke. Neurorehabil Neural Repair. 2021;35(4):307-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saur D., Lange R, Baumgaertner A, et al. Dynamics of language reorganization after stroke. Brain. 2006;129(6):1371-1384. [DOI] [PubMed] [Google Scholar]
- 37.Stockert A., Wawrzyniak M., Klingbeil J., et al. Dynamics of language reorganization after left temporo-parietal and frontal stroke. Brain. 2020;143(3):844-861. [DOI] [PubMed] [Google Scholar]
- 38.Anglade C., Thiel A., Ansaldo A. I. The complementary role of the cerebral hemispheres in recovery from aphasia after stroke: a critical review of literature. Brain Inj. 2014;28(2):138-145. [DOI] [PubMed] [Google Scholar]
- 39.Weiduschat N., Thiel A., Rubi-Fessen I., et al. Effects of repetitive transcranial magnetic stimulation in aphasic stroke. Stroke. 2011;42(2):409-415. [DOI] [PubMed] [Google Scholar]
- 40.Hu X.-Y., Zhang T., Rajah G. B., et al. Effects of different frequencies of repetitive transcranial magnetic stimulation in stroke patients with non-fluent aphasia: a randomized, sham-controlled study. Neurol Res. 2018;40(6):459-465. [DOI] [PubMed] [Google Scholar]
- 41.Tsai P.-Y., Wang C.-P., Ko J. S., Chung Y.-M., Chang Y.-W., Wang J.-X. The persistent and broadly modulating effect of inhibitory rTMS in nonfluent aphasic patients. Neurorehabil Neural Repair. 2014;28(8):779-787. [DOI] [PubMed] [Google Scholar]
- 42.Wang C.-P., Hsieh C.-Y., Tsai P.-Y., Wang C.-T., Lin F.-G., Chan R.-C. Efficacy of synchronous verbal training during repetitive transcranial magnetic stimulation in patients with chronic aphasia. Stroke. 2014;45(12):3656-3662. [DOI] [PubMed] [Google Scholar]
- 43.Barwood C. H. S., Murdoch B. E., Riek S., et al. Long term language recovery subsequent to low frequency rTMS in chronic non-fluent aphasia. Neurorehabil. 2013;32(4):915-928. [DOI] [PubMed] [Google Scholar]
- 44.Lefaucheur J.-P., Aleman A., Baeken C., et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014-2018). Clin Neurophysiol. 2020;131(2):474-528. [DOI] [PubMed] [Google Scholar]
- 45.Harvey R. L., Edwards D., Dunning K., et al. Randomized sham-controlled trial of navigated repetitive transcranial magnetic stimulation for motor recovery in stroke. Stroke. 2018;49(9):2138-2146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material – for Differential Effects of Speech and Language Therapy and rTMS in Chronic Versus Subacute Post-stroke Aphasia: Results of the NORTHSTAR-CA Trial by Anna Zumbansen, Heike Kneifel, Latifa Lazzouni, Anja Ophey, Sandra E. Black, Joyce L. Chen, Dylan Edwards, Thomas Funck, Alexander Erich Hartmann, Wolf-Dieter Heiss, Franziska Hildesheim, Sylvain Lanthier, Paul Lespérance, George Mochizuki, Caroline Paquette, Elizabet Rochon, Ilona Rubi-Fessen, Jennie Valles, Susan Wortman-Jutt and Alexander Thiel; on behalf of the NORTHSTAR-study group in Neurorehabilitation and Neural Repair