Introduction
The inflammatory bowel diseases (IBD), ulcerative colitis (UC) and Crohn’s disease (CD), are chronic inflammatory disorders that primarily involve the luminal GI tract. The precise etiology for these disorders, while still undefined, involves a complex interaction between immune dysregulation, microbial dysbiosis, and environmental triggers in individuals with underlying genetic predisposition. These same factors also contribute to one of the most feared complications of chronic colonic inflammation—colorectal cancer (CRC). For decades, factors contributing to the development of colitis-associated colorectal cancer (CAC), and approaches to mitigate risk by endoscopic means and chemoprevention, have been the topic of investigation, and the reader is referred to previous reviews on the subject.1,2 This review is meant to provide a more up-to-date understanding of the epidemiology, pathogenesis, pathology, risk prediction and clinical management of CAC.
Epidemiology
Long-standing UC and Crohn’s colitis (except limited proctitis) have an approximate 2–3 fold increased risk of CRC, with estimates varying depending on the study, time period, and individual risk factors.3,4 Fortunately, it appears that rates of CAC are decreasing over time, probably reflecting improved medical therapies and colonoscopic screening/surveillance.5,6 Regardless, CAC is still a leading cause of mortality and reason for colectomy in this population.7 An older meta-analysis from 2001 of studies prior to the era of improved medical therapies and improved endoscopic imaging/management, reported an overall prevalence of CRC in any UC patient to be 3.2%, with a cumulative 2%, 8%, and 18% risk of CRC after 10, 20, and 30 years of disease, respectively. 8 More recent studies suggest this risk is lower, on the order of 1%, 3%, and 7% at 10-, 20- and 30-years.9 Data from Asian-Pacific populations, where the increase in IBD incidence is more recent and sharper compared to Western industrialized populations, demonstrate similarly increased risk of CRC in patients with IBD colitis. A meta-analysis of 31,287 patients with UC from 44 studies conducted in Asian countries reported a pooled prevalence of CRC of 0.85%, with 0.02%, 4.8%, and 13.9% cumulative risk at 10, 20, and 30 years.10 Whether CRC risk in IBD differs based on ethnic origin or geographic location remains to be clarified, but it is important to consider these estimates in the context of differential surveillance practices, access to GI specialty care, use of IBD therapies, possibly diet, and other non-biological factors.11
Pathogenesis of Colitis-Associated CRC
CRC arising in IBD is often considered the prototype of inflammation-induced carcinogenesis. Chronic inflammation generates oxidative stress-induced damage to DNA that results in activating tumor-promoting genes, and inactivating tumor suppressor genes.12 Markers of oxidative damage and DNA double strand breaks increase progressively in the inflammation-dysplasia-carcinoma sequence.13 Factors generated by the host immune response, with contributions from the gut microbiome and its products, contribute to the inflammatory and carcinogenic process. The net result is a sequence of events that causes genetic (eg. mutations) and epigenetic (eg. methylation) alterations, followed by clonal expansion of somatic epithelial cells, with influences coming from surrounding stromal and immune cells.
Unlike sporadic dysplastic polyps (adenomas, sessile serrated polyps), which develop as discrete, typically visible lesions that are few in number, in the case of IBD, large swaths of chronically inflamed mucosa are prone to neoplastic transformation in a process termed “field cancerization”. In studies that followed UC patients with repeated colonoscopies, cell populations that demonstrated aneuploidy as a marker of genomic instability remained in the same colonic location but spread over time to occupy larger areas of mucosa.14 Even before histological evidence of dysplasia, mutant cell clones demonstrate genomic and epigenomic alterations that confer a survival advantage, allowing them to spread over large areas of colonic mucosa, resulting in the clinically well-described high rates of synchronous and metachronous neoplasms.15
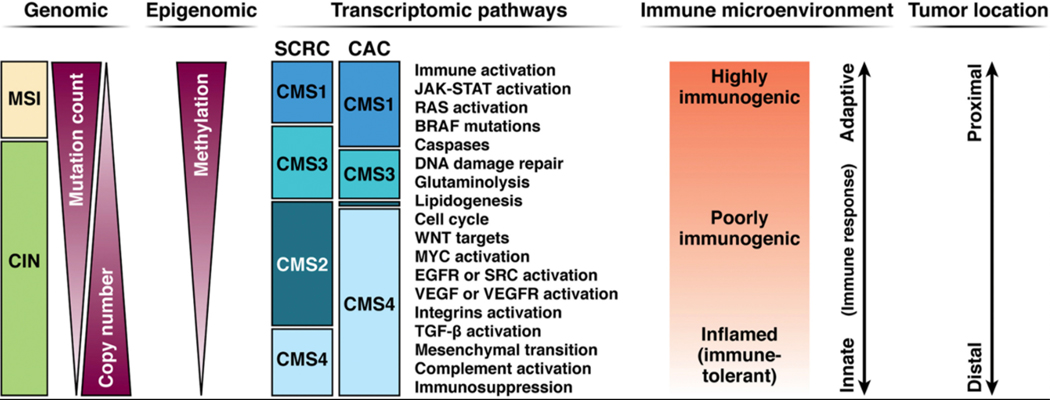
The same major molecular pathways that give rise to sporadic CRC (sCRC), namely chromosomal instability (CIN), microsatellite instability (MSI), and CpG island methylator phenotype (CIMP), contribute to the development of CAC.12 (Figure 1) Not surprisingly, therefore, many of the same driver genes, such as APC, KRAS, P53, PIK3CA, SMAD4, ARID1A, MYC and others, are operative in both types of cancers.16–18 Some gene mutations have been detected more frequently in CAC than in sCRC, but studies differ with respect to those individual genes and their frequency.16–19 A reproducible observation with respect to CAC pathogenesis is that the timing and frequency of some of the common gene alterations are different from sCRC. In the CAC process, APC gene mutation/loss occur less often and later in the dysplasia-carcinoma sequence, and p53 mutation/loss occur frequently and very early in the process, even prior to dysplasia.20 (Figure 2) Mutations in conventional driver genes tend to be clonal within a carcinoma, and the clonal frequency, even of the genes that are more commonly mutated in CACs, are often shared by the surrounding non-dysplastic mucosa, indicating common early events.19 Studies employing mutational analysis and chromosomal copy number alterations demonstrated similarity between high-grade dysplasia (HGD) and CAC, with a rather marked transition of molecular changes between low-grade dysplasia (LGD) and HGD. 19 This supports the so-called “Big Bang” model of CRC evolution21, whereby chronic inflammation induces tumor-promoting molecular alterations in preexisting clonal cell populations that occur rather abruptly, rather than a gradual accumulation driven by pressures from the microenvironment. Underlying all of the above observations is the stochastic model of sCRC carcinogenesis which posits that the colorectum is an organ with an inherently high rate of stem cell divisions which, over the course of a lifetime, translates into a high cancer risk, influenced by environmental and inherited factors.22
Figure 1.
Molecular pathogenesis of colitis-associated colorectal cancer (CRC) compared with sporadic CRC. (A) Sporadic CRC arises from adenoma (and sessile serrated polyp) precursors that progress through various stages until carcinoma. Loss of APC function is considered an early event and P53 mutations/loss are late. (B) In contrast, colitis-associated CRC also progresses through dysplastic precursor lesions, which tend to have a flatter morphology and demonstrate reversal of the APC and P53 sequence of molecular alterations, with P53 changes being very early, even before dysplasia. The molecular alterations associated with both sporadic and colitis-associated carcinogenesis are influenced by environmental factors, including general and specific components of the microbiome. COX-2, cyclooxygenase-2; DCC, deleted in colon cancer; DPC4, deleted in pancreatic cancer; Kras, Kirsten rat sarcoma viral oncogene homolog; LOH, loss of heterozygosity; miRNA, microRNA. Adapted from: Beaugerie and Itzkowitz20 with permission.
Figure 2.
Molecular and immunologic classification of colorectal cancers (CRCs). Categorization is predominantly based on observations from sporadic CRC (sCRC). Microsatellite instability (MSI) is highly correlated with hypermutation, hypermethylation, adaptive immune cell infiltration, activation of Kras and Braf, and proximal colon location. Tumors manifesting chromosomal instability (CIN) are more heterogeneous but are typically microsatellite stable. In general, sCRCs manifest epithelial canonic genetic pathways involving Wnt and Myc activation. In CAC, the proportion of MSI-positive tumors is approximately similar to that in sCRC. However, CACs demonstrate a shift from the epithelial consensus molecular subtype (CMS) 2 toward the more mesenchymal CMS4 phenotype (epithelial-mesenchymal transition), with dysregulation of Wnt signaling in favor of TGF-β activation, and an “immune-inflamed” immuosuppressive micro-environment enriched in CD4+ cells.17 EGFR, epidermal growth factor receptor; JAK, Janus kinase; STAT, signal transducer and activator of transcription; TGF, transforming growth factor; VEGF, vascular endothelial growth factor. Adapted from: Nature Reviews Cancer 17:79, 2017, with permission.
Transcriptomic analysis has classified sCRCs into four consensus molecular subtypes (CMS), permitting a more accurate prediction of CRC behavior, such as metastatic potential, prognosis, and response to therapy.23 (Figure 1) Most MSI-positive sCRCs cluster in the CMS1 group (“MSI immune subtype”; 14% of total), and are characterized by hypermutation, hypermethylation, BRAF V600E mutations, strong infiltration of immune cells and proximal colon predominance. These tumors are highly immunogenic due to neoantigens generated from the hypermutability that attract an infiltrate of adaptive cytotoxic cells and activated Th1 cells which is counterbalanced by upregulation of immune checkpoints. The remaining 86% of sCRCs demonstrate CIN, and are classified into three groups: CMS2 (“canonical subtype”; 37% of total) which demonstrate epithelial cell differentiation with upregulation of WNT and MYC downstream targets, and higher expression of EGFR and HFN4A; CMS3 (“metabolic subtype”; 13%) which are enriched in KRAS mutations and demonstrate reprogramming of metabolic pathways (eg. glutaminolysis and lipidogenesis); and CMS4 (“mesenchymal subtype”; 23%) which have activated pathways related to epithelial-mesenchymal transition and stemness, such as TGFbeta and integrins, and demonstrate a prominent stromal cell infiltration. Sporadic CMS4 tumors are considered the “inflamed” subtype, with infiltration by Treg, Th17, and innate immune cells, along with cytokines such as IL-23 and IL-17, and are associated with location in the distal colon, and higher rates of distant relapse and death.
Little is known about the CMS classification of CAC, but one comprehensive study of a limited number of CACs demonstrated a complete lack of CMS2 tumors, and a skewing towards the CMS4-associated epithelial-mesenchymal transition pathway and an increase in CD4+ T cells and monocytes compared to sCRC.17 (Figure 1). CACs also demonstrated a relative loss of HNF4 expression, and overexpression of OSMR which may contribute to a more mesenchymal phenotype. It is tempting to speculate that the remodeling of the mesenchyme surrounding the colonic crypts that is seen in patients with IBD, likely contributes to the carcinogenesis process.24 The shift towards a CMS-4 phenotype in IBD is also consistent with the observation that dysplasia is more frequent and progresses more rapidly to HGD and CRC in the distal colon.25
The role of bacteria in the carcinogenic process comes from studies employing a variety of experimental techniques such as in vitro cell culture and intestinal organoid models, and mouse models of inflammation. Translational studies using human colorectal biopsies and feces have been performed, but mostly on individuals with sporadic colorectal neoplasms. It is unrealistic to expect that a single organism, or two, will be identified as the carcinogenic culprit in CAC (or sCRC). More likely, CAC arises from dysbiosis among a community of commensals, involving a restriction of microbial diversity, as seen with colitis itself. And while studies have disclosed an association between certain tumor-promoting bacterial species and CRC, it is conceivable that an absence of protective strains might be important, albeit, more difficult to investigate.
Three bacterial species in particular have been linked to the process of human colorectal carcinogenesis: Fusobaterium nucleatum (Fn), Escherichia coli containing pathogenic polyketide synthetase (pks) islands, and Bacteroides fragilis expressing B. fragilis toxin (BFT).26 A role for Fn in human colon carcinogenesis is supported by clinical and preclinical studies demonstrating that Fn levels were higher in sporadic CRC and HGD tissues, and was associated with metastasis and worse prognosis.27,28 In some mouse models, introduction of Fn resulted in an acceleration of colorectal tumorigenesis, infiltration of myeloid cells into tumors, and an NF-kB pro-inflammatory signature.29 But, Fn did not accelerate tumorigenesis in two mouse models of colitis-associated carcinogenesis29, and less is known about a possible role for Fn in human CAC.
The intestinal microbiota of patients with IBD demonstrates a greater abundance of Enterobacteriaceae/E.coli, and patients with IBD and CRC have increased prevalence of mucosa-associated E. coli compared with non-IBD and non-CRC patients.30 E. coli strains that harbor the pks gene cluster have been found more often in biopsies from CRC (67%) and IBD (40%) than in healthy controls (21%). Such strains have received considerable attention because of their ability to promote tumor formation in murine models of CAC.31 The exposure of colonic epithelial cells to pks+ E coli results in oncogenic phenotypes that manifest WNT independence and increased proliferation.31 Mouse models of CAC have demonstrated that the presence of pks+ E. coli promoted DNA damage and neoplastic transformation, but only under conditions with mucosal inflammation (eg. azoxymethane treated IL10−/− mice) and not when inflammation was abrogated (IL10−/−; Rag2−/− mice).30 And, inhibiting nitrate reductase activity to reduce E. coli colonization during inflammation abrogated pks-associated tumorigenesis.32
The pks island encodes colibactin, a genotoxin implicated in causing a unique mutational signature in target cells. This mutational signature, found in approximately 5% of primary sCRCs and up to 9% of metastatic sCRCs, is even thought to arise from a mutational event in early childhood in healthy human colonic crypts.33 But the fact that the prevalence of pks+ E. coli colonization is so much greater than the proportion of individuals carrying the colibactin mutational signature suggests that the physiologic environment contributes to any genotoxic activity. Indeed, E. coli Nissle 1917, despite carrying the pks island, has been used as a beneficial probiotic. Thus, in human CAC, the role of pks+ E. coli, and colibactin specifically, remains to be further elucidated.
Enterotoxigenic Bacteroides fragilis elaborates the pathogenic B. fragilis toxin (BFT), which binds to a specific colonic epithelial cell receptor, activating Wnt and NF-kB signaling pathways resulting in increased cell proliferation, epithelial release of proinflammatory mediators and DNA damage.34 The bft gene sequences have been found in the mucosa of 90% of patients with sporadic colorectal neoplasia, compared to 55% of controls34 and in the stool of approximately 14% of patients with IBD.35 BFT induces acute and chronic colitis in mice, and in the MinApc+/− mouse model, it promotes IL-17-dependent colon carcinogenesis.34 In the APCMin model, BFT induces IL-17 and Stat3 activation along the length of the colon, and enhances distal colon carcinogenesis through activation of NF-kB in distal colonic epithelial cells.36 Nonenterotoxigenic B. fragilis, considered a commensal, does not appear to carry the same carcinogenic potential as enterotoxigenic Bacteroides fragilis.
Bacterial communities, rather than individual organisms, have been associated with colorectal carcinogenesis. Bacterial biofilms that are mucus-invasive have been identified on the colonic mucosa of about 50% of patients with sporadic CRC and 13% of healthy controls.37 Others have reported bacteria biofilms in 90–95% of patients with IBD and self-limited colitis, 65% of IBS patients and 35% of healthy controls, with B. fragilis being the major component of IBD biofilms.38 As with sCRC, parsing out the interplay between host factors (genetics, immune response) and environmental factors (diet, microbes), is the subject of continuing investigation.
Types of Dysplasia
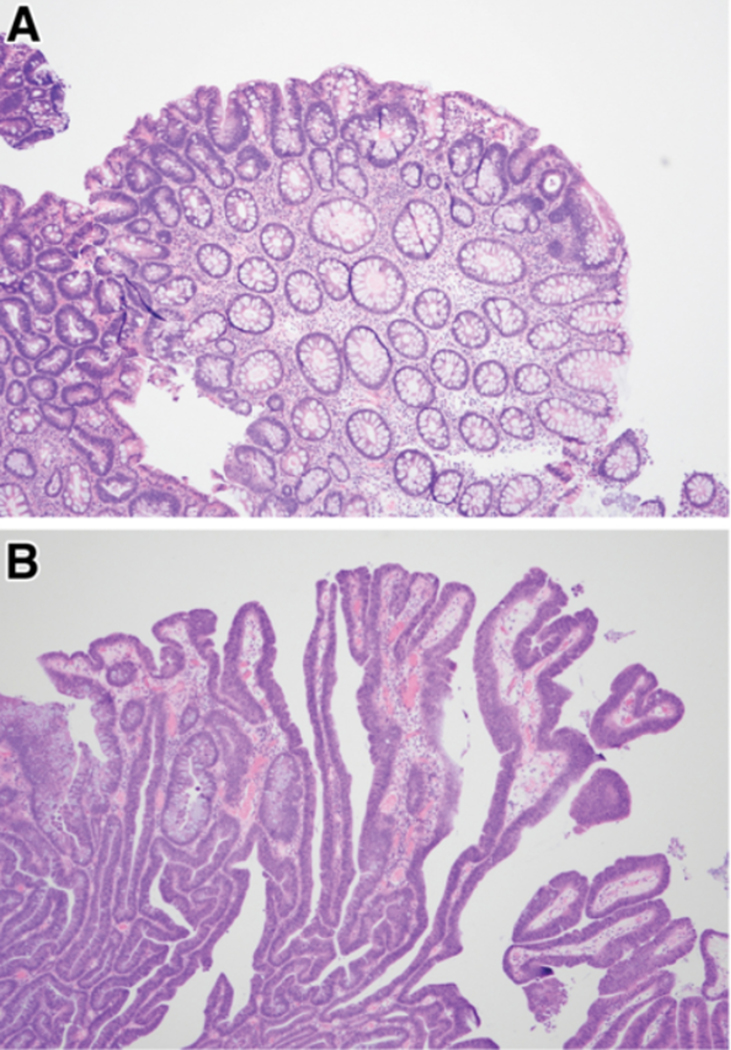
Histologically, dysplasia in IBD often looks like dysplasia in sporadic adenomas, resembling tubular, tubulovillous or villous adenomas. Although a clear distinction cannot be relied upon, dysplastic cells in sporadic adenomas often occupy the upper portion of neoplastic crypts (so-called, top-down dysplasia) whereas with colitis, the dysplastic cells tend to occupy the entire height of the crypts (Figure 3). To date, clinical algorithms for colonoscopic surveillance intervals are based on finding conventional dysplastic lesions. But, many types of non-conventional dysplasia are well described in IBD, even though strictly applied morphologic criteria are not yet universally agreed upon. These have been grouped into the following five categories: hypermucinous, goblet-cell deficient, crypt cell dysplasia/terminal epithelial differentiation, dysplasia with increased Paneth cell differentiation, and serrated type (including traditional serrate adenoma-like, sessile serrated lesion-like, serrated lesion not otherwise specified).39 Non-conventional dysplastic lesions may occur in up to one-third of patients with dysplasia, and are more likely than conventional types to be aneuploid, develop HGD or CRC in the same colonic segment on follow-up, and a sizable minority occur as endoscopically flat/invisible lesions, arguing in favor of performing random biopsies.40 Non-conventional types of dysplasia are often found in the same segment of colon as conventional dysplasia, and often adjacent to a CRC. When present without conventional dysplasia, non-conventional dysplasia has more often been found in the left colon and associated with CRC that is poorly differentiated.39
Figure 3.
Histologic examples of conventional dysplasia. (A) Sporadic adenoma demonstrating dysplastic crypts at the surface of the polyp (“top-down”). (B) Colitis-associated dysplastic lesion demonstrating dysplastic cells occupying the full height of the crypts. Both are examples of low-grade dysplasia. Courtesy of Noam Harpaz, MD, PhD.
While the serrated neoplasia pathway contributes to approximately 20% of sCRCs41 its contribution to IBD-associated carcinogenesis is less clear and more controversial. A series looking specifically at endoscopically visible serrated polyps (SPs) found them in 1–2% of patients with chronic IBD, usually manifesting as a protruding lesion in an area that had been involved with chronic inflammation.42 The authors subclassified these polyps according to the degree of cytologic dysplasia present, if any, and found that SPs negative for dysplasia histologically resembled sporadic sessile serrated lesions (SSLs) and likewise occurred mostly in women, in the proximal colon, and contained BRAF mutations. Conversely, IBD-associated SPs with LGD resembled sporadic traditional serrated adenomas (TSAs) and occurred mostly in men, in the distal colon and had KRAS mutations.42 Importantly, the actuarial 10-year rate of developing advanced neoplasia was 0% for IBD-associated SPs negative for dysplasia, similar to both a reference cohort population without dysplasia at baseline and to index hyperplastic polyps (HPs) in the study. In contrast, actuarial 10-year rate of advanced neoplasia was 17% for IBD-associated SPs with LGD, similar to the cohort population with LGD at baseline. These results suggest that for endoscopically visible, histologically confirmed SPs identified in IBD, the presence and degree of cytologic dysplasia dictates risk of neoplastic progression.
Serrated (or hyperplastic) epithelium is often seen in regenerating, post-inflammatory colonic epithelium and is therefore a normal component of mucosal healing in IBD. Thus, any neoplastic transformation assuming a serrated phenotype would have to be distinguished from a benign regenerating process, particularly since both are associated with longer disease and more severely active inflammation. In patients with UC, those with lesions that have been referred to as ‘serrated epithelial change’ overall had an approximate 4-fold greater risk of subsequent neoplasia compared to no SEC, and SEC was detected most often by random biopsy of flat mucosa (66%).43 Until more is known about the natural history of non-conventional dysplasia in IBD, it is not clear how to integrate these lesions into clinical surveillance and management decisions.
Clinical Approach for Preventing CAC
To attenuate CAC risk, the approach to management hinges on accurately assessing individual patient risk. Despite a substantial body of literature spanning decades, our understanding of the magnitude and significance of risk independently associated with putative determinants, along with their interaction, is still incomplete. Our inability to reliably predict which patients with IBD colitis will develop CAC, is reflected in the non-personalized approach to defining the timing of the index neoplasia screening exam as well as subsequent surveillance intervals. This section synthesizes 1) the current evidence for protective and risk factors, 2) current international guidelines for screening and surveillance, and 3) chemoprevention for CRC in patients with IBD colitis.
Identifying Risk factors
Our understanding of clinical risk factors associated with CAC is driven by observational cohort, population-based, and case-control studies, which are subject to inherent biases, confounding, competing risk of colectomy, and heterogeneous study designs. Most of these studies span time intervals prior to the current era of high-definition white light endoscopy (HD-WLE), enhanced imaging techniques for dysplasia detection (e.g. dye-based or virtual chromoendoscopy), and expanded use of very effective medical therapies for controlling inflammation. A recent comprehensive systematic review and meta-analysis of prognostic factors associated with advanced colorectal neoplasia (aCRN, defined as HGD or CRC) in IBD identified 164 studies (40 considered ‘good quality’), with only 8 studies from 6 distinct cohorts including follow-up data through at least 2015, and the majority incorporating intervals prior to 2000 with several prior to 1970s.44 Many of these earlier studies also provide risk estimates based on univariable or minimally adjusted models. Notwithstanding these limitations44,45, in the absence of accurate risk prediction models, having a systematic framework for evaluating CAC risk in any patient diagnosed with IBD colitis is key. One framework categorizes clinical risk factors as patient-related, disease-related, and pathology-related. On the whole, the risk factors that are most consistently associated with aCRN are those indicative of chronic inflammation (extensive disease, histological inflammation, cumulative inflammatory burden - which incorporates disease duration and inflammation severity, primary sclerosing cholangitis (PSC)), prior dysplasia, and family history of CRC in a first-degree relative. Determining disease extent based only on endoscopic appearance may underestimate the true histological involvement. Accordingly, because histological inflammation and extent are among the strongest drivers of CAC risk, disease extent should incorporate maximal histological extent for CAC risk stratification purposes.
Patient-related factors.
Data are mixed as to whether increasing age, by itself, is a risk factor for CAC, independent of the non-IBD-related background risk of sporadic CRC in older age groups.44,46–50 The increased risk associated with younger vs older age of IBD onset (defined variably in studies, but generally age cut-off of 30 years) reported in earlier studies, more likely reflects disease duration and cumulative inflammation over time, as opposed to accelerated carcinogenesis in younger-onset IBD.3,20,44,51 In one meta-analysis of 11 cohort studies using multivariable analyses, men demonstrated a significant 1.50–1.58-fold increased risk of aCRN compared to women.44 This same meta-analysis reported no significant association between race and risk of aCRN based on two cohort studies, both of which were US-based cohorts, one from 1981–1993 and the other 1998–2010.52,53 It is worth considering that these null findings might reflect a disproportionate lack of inclusion of traditionally underrepresented groups such as non-whites and ethnic minorities. While smoking is an established risk factor in sCRC, it is not consistently associated with increased risk of IBD-associated aCRN, although study biases are difficult to overcome given the divergent effects of tobacco smoking on UC versus Crohn’s disease. Family history of CRC (but not IBD per se) is an established risk factor for aCRN in patients with IBD based on multivariable analyses from case-control and cohort studies.44 This increased risk appears relevant irrespective of first- vs. second-degree, although having a first-degree relative with CRC diagnosed below age 50–55 years confers the greatest risk.2,54 Other risk factors such as obesity, low physical activity, red meat consumption and other dietary factors, have not been analyzed specifically in populations with IBD colitis, but may certainly be relevant.
Disease-related factors.
IBD-type (UC vs CD vs IBDU) has not been consistently associated with differential risk of aCRN. Disease-related factors that are most relevant include colitis extent, duration, and concomitant history of PSC. The risk of CAC in patients with IBD proctitis only is comparable to the general population and these patients should not be enrolled in surveillance in the absence of disease extension. Extensive disease (generally defined as >50% colonic involvement in CD, or inflammation extending proximal to the splenic flexure in UC at any time during the disease course) is associated with a significant 2–3-fold higher risk of IBD-associated neoplasia, compared to intermediate extent CD and left-sided UC—both of which are still associated with elevated risk of aCRN compared to IBD proctitis only.44 The basis for recommending against initiation of screening/surveillance prior to 8–10 years after disease onset comes from an older meta-analysis of 19 heterogeneously designed studies, generally spanning the 1950s-1990s, reported the cumulative risk of CRC at 10-, 20-, and 30-years after disease onset was 2%, 8%, and 18%.8 Another meta-analysis limited to population-based cohort studies reported a respective 2.6% and 6.6% cumulative risk of CRC at 10–20 years and >20 years of IBD duration, with 21% cumulative incidence after 20 years of extensive disease.3 While the majority of CRCs are diagnosed at least 8–10 years after onset of IBD symptoms in patients with non-limited IBD colitis, there is inherent bias since none of the current international society guidelines recommend screening/surveillance prior to this, unless there is concomitant PSC or a first-degree relative diagnosed with young-onset CRC. Indeed, a considerable proportion of CRCs occur in patients without longstanding colitis55–58, particularly in patients older than age 40 years at the time of colitis diagnosis.51 Indeed, cohort studies demonstrate that up to 22% of CACs are diagnosed prior to when the first screening exam is recommended based on current international guidelines. Cumulative inflammatory burden (CIB), discussed below, as opposed to absolute disease duration alone, may be a more reliable indicator for when patients should initiate screening/surveillance. CACs arising in symptomatic patients with IBD are typically diagnosed in more advanced stages compared to CACs diagnosed in patients already enrolled in surveillance.
Patients with IBD and concomitant PSC have an estimated 3- to 5-fold significantly higher independent risk of aCRN compared to patients without concomitant PSC.44,59,60 The reasons for this have not been elucidated but considerations include changes in bile acid metabolism, altered intestinal or biliary microbiome, and/or systemic immunologic alterations that predispose to cancers in the colon as well as the biliary tract in such patients. Compared to patients without PSC, CRN is more often right-sided and the risk of LGD progression to aCRN is higher in patients with PSC.60 Patients with concomitant IBD and PSC often have quiescent clinical and histological IBD, yet high prevalence of CRN at the time of PSC diagnosis. Accordingly, all international GI societies recommend that CRN surveillance start at the time of PSC diagnosis and with a more aggressive surveillance interval and lower threshold for total proctocolectomy if dysplasia is diagnosed. Anatomic structural alterations to the colon, including strictures and post-inflammatory polyps (‘pseudopolyps’), have also been associated with increased risk of aCRN in earlier studies, but these associations, especially pseudopolyps, have not been substantiated in recent, more robust analyses that control for histological inflammation and other relevant confounders. Instead, pseudopolyps (and strictures, at least in Crohn’s colitis) are more likely surrogate markers of CIB and, accordingly, their presence alone does not necessarily warrant heightened surveillance.44,61,62 While there are data demonstrating no significant increased risk of aCRN in patients with vs without colonic strictures, the majority of patients with strictures in these cohorts have Crohn’s colitis, with significantly less data regarding neoplastic risk in patients with UC who are diagnosed with colonic strictures.63,64 A stricture in UC should raise heightened concern for neoplasia, especially in the presence of obstructive symptoms and proximal colon location, and if at any time structural alterations impede a high-quality exam for neoplasia detection this can be an indication for surgery.
Pathology-related factors.
Histological inflammation, more so than gross endoscopic inflammation, is a major risk factor for CAC. Patients with concomitant PSC are an exception since they often have minimal histological colonic inflammation despite some of the highest rates of CAC risk. A factor that continues to be used in risk stratification algorithms for determining surveillance intervals is the severity of inflammation on the immediately preceding colonoscopy. However, this is actually a poor predictor of future CRN57,65 as it is the dynamic course of inflammation severity of over time that is most predictive. Robust data from established surveillance cohorts consistently demonstrate that CIB, but not inflammation on the immediately preceding colonoscopy, nor disease duration per se, is a strong independent predictor of CRN.57,65 It is possible that calculating CIB might help refine the decision for when to initiate screening in order to avoid “premature” cases of CAC that occur in the first 8 years of colitis.
History of dysplasia.
Due to the field cancerization effect in areas of the colon previously or currently inflamed, patients with IBD have a high risk of synchronous and metachronous neoplasia. Once pathologically confirmed dysplasia is detected, the patient should be considered at particularly high risk. Patients with HGD especially, are at high risk for prevalent or incident CRC within a short time interval, providing strong justification for total proctocolectomy as discussed further below. The magnitude and robustness of estimates for the association between LGD and incident aCRN vary widely in the literature—anywhere from 1.3–86-fold, reflecting the heterogeneity of the literature with respect to study population and design, certainty of the diagnosis (e.g. confirmation by an expert pathologist), characteristic of the LGD lesion(s) (e.g. size, shape, resection eligibility and outcome), and rigor of the analysis.44,66 According to the UC-CaRE risk prediction tool, validated using data from four United Kingdom centers, the following four variables accurately predicted progression of LGD to aCRN: large (>1cm) endoscopically visible LGD, LGD that was unresectable or incompletely resected, moderate/severe inflammation within 5 years of LGD diagnosis, and multifocal LGD.67 Concomitant PSC is also a significant independent risk factor for LGD progression.60 If any, or especially if several of these high-risk features are present, patient-provider multidisciplinary discussion is recommended, as colectomy should be strongly considered. There are mixed data regarding the association between indefinite dysplasia (IND) (in the absence of LGD) and risk of subsequent aCRN, again mostly related to study design.44 There is higher interobserver disagreement in the diagnosis of IND, especially in the presence of active inflammation. Based on a recent retrospective analysis of an established, well-annotated cohort of patients with confirmed colonic IBD undergoing surveillance, the presence of IND in the absence of concurrent LGD, with diagnostic confirmation by two expert GI pathologists, was associated with a 6.9-fold significantly higher independent risk of aCRN after adjusting for potential confounders, including histological inflammation.68 In this surveillance cohort, the incidence of aCRN was 0.4%, 3.1%, and 8.4% per patient-year after a diagnosis of no dysplasia, IND only, or LGD, respectively.
Other factors.
Clinical factors alone are insufficient for prediction models determining aCRN risk, and a paradigm shift toward a more personalized approach would seem warranted in this high-risk population. Tissue molecular factors, such as aneuploidy and p53 expression, have been suggested but more robust analyses with appropriate control populations are needed prior to determining how tissue molecular markers and/or noninvasive serological or stool-based biomarkers can be positioned for clinical practice. Patient genetics may also help refine prediction models in the future. Genomic and environmental factors have been used to develop personalized risk scores in sCRC.69 Very few genome-wide association studies have been conducted for CAC, which may reflect barriers such as insufficient power and difficulty in accurately identifying cases and appropriate controls.70 While over 200 IBD risk loci and over 40 CRC risk loci have been identified, no shared loci between IBD and CRC have been definitively established, except for at least one variant locus in STAT3.71–73 That individuals with essentially identical profiles of non-genetic risk factors and disease characteristics still differ with respect to CRN risk, further supports the influence of gene-environment interactions. Leveraging genetic susceptibility in predictive models for aCRN has the potential to substantially refine risk estimates and shift towards an individualized approach to screening and surveillance.
Endoscopic Management of CAC Risk
Endoscopic screening and surveillance approaches
In contrast to the various screening tests available for general population screening for CRC, there are no noninvasive biomarkers for aCRN screening or surveillance in patients with IBD colitis, although some stool-based tests appear promising.74,75 The mainstay of CAC risk attenuation remains colonoscopy, in parallel with a structured endoscopic surveillance program that includes ongoing IBD medical management for disease control. The first colonoscopy performed for CRN detection purposes is referred to as the ‘screening colonoscopy’, while subsequent exams are referred to as ‘surveillance’ exams. The screening colonoscopy also permits re-evaluation of histological extent of inflammation and structural abnormalities for risk stratification.63 Given the often subtle endoscopic appearance of IBD-associated CRN and the importance of accurately classifying the lesions to determine endoscopic resectability, attention to colonoscopic quality metrics is critical. As much as possible, the screening and surveillance exams should be performed with the patient in remission, should adhere to standard colonoscopic quality metrics, and should be performed by endoscopists with experience in IBD-associated neoplasia detection. Standard of care is to use HD-WLE, with or without additional image enhancing techniques (e.g. dye-based or virtual chromoendoscopy), as described below.63,76,77
There are no randomized controlled trials comparing surveillance to no surveillance, with respect to CRC incidence and related mortality.78 However, observational studies consistently demonstrate that patients with IBD colitis undergoing surveillance colonoscopies have less advanced stages of CRC than patients not undergoing surveillance, with stage of CRC at diagnosis a strong predictor of overall survival.51 The updated Cochrane Database Systematic Review demonstrated a higher overall frequency of CRC in the non-surveillance vs surveillance group (3.2% vs. 1.8%), with significantly lower CRC-related mortality in the surveillance vs. no surveillance group (8.5% vs. 22.3%; OR 0.36, 95% CI, 0.19–0.69), which was attributed to a higher rate of early-stage CRC in the surveillance group (15.5% vs. 7.7%; OR 5.40, 95% CI, 1.51–19.30).79,78 Of note, the majority of studies of neoplasia surveillance outcomes have mostly included patients with UC, and then generally extrapolated to patients with CD.
Several international societies provide guidance on both initiation of screening and subsequent surveillance intervals (Table 1). There are many overlaps, but also some slight nuances across these guidelines. In the absence of concomitant PSC, current guidelines recommend the timing of the screening colonoscopy based solely on duration of time after symptom onset—most often 8–10 years.11,63 For patients with PSC, screening colonoscopy should be performed at the time of PSC diagnosis with annual surveillance recommended thereafter, unless dysplasia is diagnosed. In patients without PSC, societies vary in their recommendation for subsequent surveillance based on different approaches to risk stratification, although some societies do not offer guidance past the first screening exam. All risk stratification algorithms rely on clinical factors, many of which have come under scrutiny based on more recent, robust data demonstrating null associations with aCRN risk (eg, inflammation on the immediately preceding colonoscopy, pseudopolyps, and strictures).45 The non-personalized approach for determining timing of screening initiation and subsequent surveillance intervals is increasingly under scrutiny as a suboptimal approach that is potentially missing a sizable proportion of people who develop CRC prior to screening initiation, and possibly over-surveilling individuals at lower risk than previously considered.45,56,80,81 One multi-center study demonstrated an extremely low risk of subsequent aCRN over median 6.1 years of follow-up in patients with IBD colitis undergoing surveillance who had at least two consecutive colonoscopies demonstrating histologically quiescent disease and in the absence of high-risk features (PSC; family history of CRC).82 In contrast to the non-IBD population, guidance as to when to lengthen surveillance intervals, or stop surveillance altogether, is lacking for patients with IBD.
Table 1.
US-based and International Guidance for Colonoscopic Surveillance in Inflammatory Bowel Disease
| Guidelines and Methodology | Screening initiation (yrs after symptom onset)* | Surveillance intervals | Risk Categories |
|---|---|---|---|
| US-Based Guidelines (ACG, ASGE) and Clinical Practice Update (AGA) | |||
| ACG 2019 GRADE |
8–10 yrs | 1–2 yrs | Annual surveillance in PSC, otherwise every 1–3 years based on the number of risk factors for CRC and findings from the previous colonoscopy, albeit with no discrete risk categorization groups |
| ASGE 2015 GRADE |
8 yrs | 1–3 yrs | Annual surveillance in PSC, ”active” inflammation*, anatomic abnormality (stricture, multiple pseudopolyps), history of dysplasia, CRC in FDR. Acknowledge optimal surveillance interval is otherwise not defined. |
| AGA 2021 (Clinical Practice Update) |
8–10 yrs | 1–5 yrs | Annual surveillance: PSC, moderate or severe inflammation (any extent), CRC in FDR<50, dense pseudopolyposis, history of invisible dysplasia or higher-risk visible dysplasia within the past 5 years Every 2–3 years: mild inflammation (any extent), strong family history of CRC (but not FDR<50), features of prior severe colitis (moderate pseudopolyps, extensive mucosal scarring), history of invisible dysplasia or higher-risk visible dysplasia >5 years ago, history of lower-risk visible dysplasia<5 years ago Every 5 years: continuous disease remission since last colonoscopy with mucosal healing on current exam, plus either: >/= 2 consecutive exams without dysplasia or minimal historical colitis extent |
| European-Based Guidelines | |||
| ECCO 2017 (UC only) Expert consensus agreement |
8 yrs | High: 1 yr Intermediate: 2–3 yrs Not intermediate or high: 5 yrs |
High-risk: extensive colitis with severe active inflammation; stricture or dysplasia detected within the past 5 years; PSC; or CRC in FDR <50 years Intermediate-risk: extensive colitis with mild-moderate active inflammation, pseudopolyps or CRC in FDR >50yrs |
| BSG 2019 GRADE |
8 yrs | High: 1 yr Intermediate: 3 yrs Low: 5 yrs |
High-risk: Same as ECCO except moderate-severe active endoscopic/histologic inflammation Intermediate-risk: Same as ECCO except mild active endoscopic/histologic inflammation Low-risk: extensive colitis with no active endoscopic/histologic inflammation, OR left-sided colitis, OR Crohn’s colitis <50% colon |
| NICE 2011 NICE guideline protocol |
10 yrs | High: 1 yr Intermediate: 3 yrs Low: 5 yrs |
High-, intermediate-, and low-risk: same as BSG |
| German 2019 (UC only) Expert consensus agreement |
8 yrs | High: 1 yr Intermediate: 2–3 yrs Low: 4 yrs |
High-risk: Same as ECCO Intermediate-risk: Same as ECCO Low-risk: No criteria for high- or intermediate-risk |
| Asian-Based Guidelines | |||
| AOCC and APAG, 2020 Expert consensus agreement; GRADE |
8 yrs | No guidance provided regarding routine surveillance | Patients with UC and LGD in flat mucosa should have repeat exam in 3–6 months. Otherwise, no statements regarding follow up surveillance are provided |
| JSG, 2020 GRADE; consensus |
8 yrs (UC only) |
No guidance provided regarding routine surveillance |
“A determination of the optimal surveillance interval incorporating both the CRC risk and progression speed is warranted”
|
| Other Geography-Specific Guidelines | |||
| Australian NHMRC 2018 NHMRC protocol |
8 yrs ^ | High: 1 yr Intermediate: 3 yrs Low: 5 yrs |
High-risk:
Any of: PSC, ongoing chronic active inflammation*, prior dysplasia, stricture, pseudopolyps, tubular colon, CRC in FDR ≤50 Intermediate-risk: All of: quiescent disease, no high-risk features, CRC in FDR >50yrs Low-risk: All of: no other risk factors and quiescent disease on consecutive exams |
| CAG-CDHF 2004 | No country specific consensus guidelines, but endorse both AGA and BSG guidelines | ||
Most guidelines distinctly state that these surveillance initiation intervals apply to patients without PSC. In patients with PSC, screening should occur at the time of PSC diagnosis.
or 10 yrs prior to the age of the youngest relative with CRC, whichever is first.
Abbreviations: ACG, American College of Gastroenterologists; AGA, American Gastroenterological Association; AOCC, Asian Organization for Crohn’s and Colitis; APAG, Asia Pacific Association of Gastroenterology; ASGE, American Society of Gastrointestinal Endoscopy; BSG, British Society of Gastroenterology; CAG-CDHF, Canadian Association of Gastroenterology and Canadian Digestive Health Foundation; CRC, colorectal cancer; CRN, colorectal neoplasia; ECCO, European Crohn’s and Colitis Organization; FDR, first-degree relative; JSG, Japanese Society of Gastroenterology; NHMRC, National Health and Medical Research Council; NICE, National Institute for Health and Care Excellence; PSC, primary sclerosing cholangitis
Note: The AGA retired the AGA guidelines published in 2010 (Farraye et al.). As such, these are not presented in the table above. A recent expert review was approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board on the topic of endoscopic surveillance and management of colorectal dysplasia in IBD. This document provides best practice advice statements which underwent internal and external peer review.
Endoscopic recognition of dysplasia
The vast majority of LGD and HGD is endoscopically visible in the current era where HD-WLE is typically standard of care, although chromoendoscopy may further improve rates of visible dysplasia.77,83 “Chromoendoscopy” refers to either dye-based (e.g. indigo carmine, methylene blue) or virtual (e.g. narrow band imaging and other image-enhancing techniques). Based on a pooled analysis, the prevalence of dysplasia identified on random biopsies only (“invisible dysplasia”) was 19.6% (11.5%−31.2%) with standard definition white light endoscopy (SD-WLE), 9.8% (6%−15%) with dye-based chromoendoscopy with indigo carmine (mix of SD-WLE and HD-WLE), and 9.4% (4.1%−19.9%) with HD-WLE, respectively. Based on the same pooled analysis, less than 1–1.5% of patients undergoing surveillance with HD-WLE or HD-chromoendoscopy would be misclassified as not having dysplasia if random biopsies were not obtained, although some studies report estimates as high as 6%.77,83 The RCT that compared HD-WLE vs. HD-chromoendoscopy reported invisible dysplasia in only 3.9% vs 2.0% of patients, respectively; the vast majority of invisible dysplasia was LGD (89%; only one patient with HGD) and one-third of the patients with invisible dysplasia had concomitant PSC. It is important to realize that the majority of IND is found on random biopsy and identifies the patient as higher-risk for subsequent aCRN compared to those with no dysplasia.68 As mentioned above, several types of non-conventional dysplasia do no alter crypt morphology and are therefore invisible, arguing in favor of taking random biopsies.40 There are no clear data to demonstrate that enhanced dysplasia detection (particularly IND or LGD when referring to invisible dysplasia) is associated with decreased CAC-related incidence and mortality. There remains a lack of consensus regarding HD-WLE vs. HD-chromoendoscopy, and whether to perform random biopsies vs. targeted biopsies during endoscopic surveillance.2,63,76,77,84–86
Our practice is to routinely perform HD-WLE with random biopsies while interrogating any visible lesions with dye-based or virtual chromoendoscopy, and reserve pan-colonic HD-chromoendoscopy for patients with PSC or prior history of invisible or visible dysplasia. With respect to selecting dye-based vs virtual chromoendoscopy (sometimes referred to as “surface” vs. “optical” chromoendoscopy), more recent studies comparing HD-virtual chromoendoscopy (specifically, narrow band imaging and i-scan) with dye-based chromoendoscopy demonstrate more rapid withdrawal times associated with virtual vs dye-based chromoendoscopy, along with overall comparable dysplasia detection rates87–89, findings which were also confirmed in a meta-analysis of 11 randomized controlled trials published between 2010–2018.90 Earlier studies had failed to demonstrate improved dysplasia detection with virtual chromoendoscopy, which underlied the ASGE’s recommendation against its routine use.91 Based on more recent data, the AGA Clinical Practice Updates committee stated that virtual chromoendoscopy “is a suitable alternative to dye spray chromoendoscopy for dysplasia detection […] when using HD-WLE”.92
All dysplasia diagnoses should be confirmed by an expert GI pathologist. In contrast to LGD and HGD, IND is subject to significant diagnostic uncertainty in the presence of background inflammation. For this reason, ideally, medical management should be optimized prior to the surveillance colonoscopy exam. In cases of invisible dysplasia that is histologically confirmed, a repeat examination with chromoendoscopy (CE) should be performed to re-evaluate for visible lesions; referral to an endoscopist with experience in IBD-associated CRN detection should be strongly considered.93
Endoscopic removal of dysplasia
The key to successful endoscopic management of dysplastic lesions in IBD is to first identify them, then assure complete endoscopic resection. Endoscopic resectability becomes the cornerstone of deciding whether or not to defer surgery and continue with surveillance. Of course, only visible dysplasia can be endoscopically removed. The SCENIC group proposed nomenclature for characterizing visible dysplasia that is adapted from the Paris classification and guides determination of whether a lesion is appropriate for endoscopic resection. For all lesions, providers should describe the characteristics of any lesions as well as the surrounding mucosa.77 Among eligible patients, all lesions deemed endoscopically resectable should be removed. Criteria for determining candidacy for endoscopic resection of visible IBD-associated dysplasia are not strictly defined, but generally overlap with considerations for endoscopic resection of colorectal lesions in the non-IBD population. The comfort level and expertise of the individual endoscopist are key factors in determining endoscopic resectability and likelihood of success. As such, referral to an endoscopist with appropriate expertise should be considered in any patient with IBD-associated dysplasia.
Endoscopically visible lesions with distinct borders should be considered for endoscopic resection as long as there are no findings concerning for submucosal invasion and the endoscopist is appropriately skilled.77 Options for endoscopic resection include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), or a hybrid ESD-EMR approach. Again, prior to endoscopic resection, patients should ideally be in endoscopic remission, and resection should only be attempted after a transparent discussion with the patient regarding expected outcome, risks, including complications and also the potential risk of incomplete resection and need for surgery, as well as the high probability of metachronous lesions and need for ongoing heightened surveillance. The IBD surgeon should also be engaged and be available for consultation pre-endoscopic resection. An in-depth discussion regarding the endoscopic management of IBD-associated dysplasia and, relatedly, patient selection for surgery is beyond the scope of this article, but clinical guidance is provided elsewhere.76,93,94 Additional details regarding the endoscopic management of IBD associated dysplasia, including the positioning of ESD, is provided in the Supplemental Material.
If dysplasia is identified on random biopsy only (invisible dysplasia), the management algorithm depends primarily on: patient factors (e.g. concomitant PSC); histological factors (e.g. the degree of dysplasia, active background inflammation); and multifocality.
Indications for colectomy in patients with IBD-associated CRN
The decision of endoscopic versus surgical resection (total or partial) should be based on a multidisciplinary discussion between the IBD provider and endoscopist, the patient, and in consultation with an experienced IBD surgeon and pathologist.93 Surgery should be strongly considered for: endoscopically non-resectable dysplasia (including invisible dysplasia) confirmed on histology regardless of grade; inability to perform adequate surveillance (eg, a nontraversable stricture or dense fields of pseudopolyps that preclude visualization); HGD in patients with PSC; and patient preference or lack of adherence to surveillance. Total proctocolectomy in patients with PSC and any grade dysplasia, even if not HGD, should be carefully considered given the high rate of short-interval and subsequent aCRN.60 Patients with IBD-associated dysplasia in other high risk groups, including family history of CRC in a first-degree relative < 55 years old, should also be considered. Previously, multifocal dysplasia and recurrent dysplasia were considered indications for colectomy, but given the safety and efficacy of endoscopic resection, heightened surveillance in cases of multifocal or recurrent endoscopically, resectable dysplasia can be considered based on patient preference in individual cases if appropriate.
When colectomy is performed for a neoplastic indication in patients with IBD colitis, the recommended procedure is most often total proctocolectomy with ileostomy or continent reservoir creation (e.g. ileal pouch-anal anastomosis (IPAA)), based on the increased risk of synchronous and metachronous aCRN due to field cancerization. However, there are certainly circumstances where partial or subtotal colectomy may be reasonable for individual patients. These include: significant comorbid conditions, strong patient preference, non-endoscopically resectable dysplasia that is unifocal in a patient without other high-risk features, proximal dysplasia that does not involve the rectum, and possibly segmental Crohn’s colitis. Except perhaps for a patient with segmental Crohn’s colitis where the retained colon/rectum has no histopathological evidence of current or prior involvement, the concept of field cancerization dictates that the in situ colon/rectum (including the rectal cuff) remains at risk for subsequent neoplastic progression. Accordingly, this retained risk provides rationale for ongoing colonoscopic surveillance and other risk attenuating measures (e.g. ongoing IBD therapy for mucosal healing). Notwithstanding, there is no evidence-based guidance to inform surveillance intervals or surveillance techniques, such as the yield of dye- or image-enhancing techniques for enhanced visualization. As such, the approach should be individualized based on the patient’s history and pre-colectomy characteristics. Postoperatively, heightened surveillance intervals in accordance with those recommended following complete endoscopic resection of dysplasia, is reasonable (see above). Similarly, it is likely safe to extend the duration between subsequent interval exams over time if consecutive exams are endoscopically and histologically normal. In patients who do undergo IPAA, pouch surveillance with annual pouchoscopy and biopsies of the pouch and any rectal cuff is recommended for patients at high risk for subsequent neoplasia, including those with a history of prior neoplasia, PSC, persistent pouchitis, or atrophic mucosa, based on limited data.63,85,91,92,95,96 In the absence of high-risk features, the risk of pouch neoplasia is very low and pouch surveillance is not routinely recommended.
Chemoprevention for CAC
Assuming that the vast majority of CRCs in patients with IBD arise as a consequence of chronic inflammation, sustained, complete control of inflammation is probably the most effective form of chemoprevention against CAC. However, because CAC still occurs, even in the absence of ongoing histological inflammation, the potential for adjunctive chemoprevention agents that would prevent or interrupt carcinogenesis, is very appealing. There are no RCTs with chemoprevention in IBD colitis as the primary outcome, and such studies are not feasible because of the very large number of patients and long study duration needed. Thus, the evidence for chemopreventive agents against CAC come from retrospective observational studies of variable quality, and from experience in the non-IBD population.
The updated 2017 ECCO guidelines recommend the use of mesalamine compounds in patients with UC extending past the rectum for chemoprevention based on the majority of evidence—including multiple case-control, cohort, and meta-analyses—supporting a reduced risk of CRC incidence in patients with UC (no comment on Crohn’s colitis).63 The most recent meta-analysis reported that 5-ASA vs no use was associated with a 49% reduced risk of aCRN (pooled multivariable OR 0.51, 95% CI 0.39–0.66).44 The exact dose (higher may be better97), the timing of initiation, the formulation/route of administration, and the minimum duration needed for benefit remain to be clarified. To this end, results from at least two large cohort studies suggest that 5-ASA’s are of limited utility in reducing progression once dysplasia develops98,99; it may be that 5-ASA’s exert their effect on early stages of inflammation-associated carcinogenesis and must therefore be given from disease onset and continued for prolonged periods to achieve benefit. In addition to the non-specific anti-inflammatory effect of 5-ASA’s, mechanistic studies from animal and cell-based models suggest 5-ASA’s also exert their anti-carcinogenic effect through other distinct pathways, several of which overlap with non-IBD CRC pathways, providing further support for their use.20,100
The data for thiopurines as CRC chemopreventive agents in patients with IBD are conflicting, including conclusions from meta-analyses.44,101,102 As such, ECCO guidelines state there is insufficient evidence to recommend for or against chemoprevention with thiopurines.63 Notwithstanding the mixed data, the realistic use of thiopurines for chemoprevention is further diminished by their safety profile, given the increased risk of certain non-CRC cancers with prolonged use, especially in the older population.
A discussion of other therapies which have been investigated as chemopreventive agents, such as biologics and ursodeoxycholic acid (UDCA) is provided in the Supplemental Material.
Conclusion
Our understanding of the pathogenesis and management of colorectal neoplasia in IBD has greatly advanced in the last decade. Although patients with IBD can develop sporadic neoplasia, the underlying culprit is chronic inflammation, which factors heavily into risk stratification. Years ago when endoscopic imaging, skill with endoscopic resection techniques, and use of biologics to control inflammation were quite limited, the finding of dysplasia prompted great angst, and tendency towards recommending colectomy for cancer prevention. Recent elucidation of the recognition and management of dysplasia and the molecular/immunological contributors to neoplasia, has ushered in a more sophisticated approach to the patient with longstanding IBD. It is hoped that advances in the genetic, immunologic, and microbiologic mechanisms of inflammation-induced colon carcinogenesis can be harnessed to further lower CRC risk, and provide patients and physicians with greater reassurance about how to manage the neoplastic complications of IBD.
Supplementary Material
Abbreviations
- aCRN
advanced colorectal neoplasia (high-grade dysplasia or colorectal cancer)
- BFT
Bacteroides fragilis toxin
- CAC
colitis-associated colorectal cancer
- CD
Crohns disease
- CIB
cumulative inflammatory burden
- CIMP
CpG island methylator phenotype
- CIN
chromosomal instability
- CPT
Current Procedural Terminology
- CRC
colorectal cancer
- CRN
colorectal neoplasia
- EMR
endoscopic mucosal resection
- ESD
endoscopic submucosal dissection
- Fn
Fusobacterium nucleatum
- HD-WLE
high definition white light endoscopy
- HGD
high-grade dysplasia
- IBD
inflammatory bowel disease
- IND
indefinite dysplasia
- IPAA
ileal pouch-anal anastomosis (surgery)
- LGD
low-grade dysplasia
- MSI
microsatellite instability
- NBI
narrow-band imaging
- PSC
primary sclerosing cholangitis
- RCT
randomized controlled trial
- sCRC
sporadic CRC
- SD-WLE
standard definition white light endoscopy
- UC
ulcerative colitis
- UDCA
ursodeoxycholic acid
Contributor Information
Shailja C. Shah, Division of Gastroenterology, University of California San Diego, GI Section, VA San Diego Healthcare Center, San Diego, CA.
Steven H. Itzkowitz, The Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York City, NY.
References
Bibliography
- 1.Lopez A, Pouillon L, Beaugerie L, Danese S. & Peyrin-Biroulet L. Colorectal cancer prevention in patients with ulcerative colitis. Best Pract. Res. Clin. Gastroenterol. 32–33, 103–109 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Farraye FA, Odze RD, Eaden J. & Itzkowitz SH AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology 138, 746–74, 774.e1 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Lutgens MWMD et al. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm. Bowel Dis. 19, 789–799 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Jess T, Gamborg M, Matzen P, Munkholm P. & Sørensen TIA Increased risk of intestinal cancer in Crohn’s disease: a meta-analysis of population-based cohort studies. Am. J. Gastroenterol. 100, 2724–2729 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Jess T, Horváth-Puhó E, Fallingborg J, Rasmussen HH & Jacobsen BA Cancer risk in inflammatory bowel disease according to patient phenotype and treatment: a Danish population-based cohort study. Am. J. Gastroenterol. 108, 1869–1876 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Choi C-HR et al. Forty-Year Analysis of Colonoscopic Surveillance Program for Neoplasia in Ulcerative Colitis: An Updated Overview. Am. J. Gastroenterol. 110, 1022–1034 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattar MC, Lough D, Pishvaian MJ & Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest. Cancer Res. 4, 53–61 (2011). [PMC free article] [PubMed] [Google Scholar]
- 8.Eaden JA, Abrams KR & Mayberry JF The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 48, 526–535 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selinger CP et al. Long-term follow-up reveals low incidence of colorectal cancer, but frequent need for resection, among Australian patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 12, 644–650 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Bopanna S, Ananthakrishnan AN, Kedia S, Yajnik V. & Ahuja V. Risk of colorectal cancer in Asian patients with ulcerative colitis: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2, 269–276 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ran Z. et al. Asian Organization for Crohn’s and Colitis and Asia Pacific Association of Gastroenterology practice recommendations for medical management and monitoring of inflammatory bowel disease in Asia. J. Gastroenterol. Hepatol. 36, 637–645 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Itzkowitz SH & Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G7–17 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Frick A. et al. Overt Increase of Oxidative Stress and DNA Damage in Murine and Human Colitis and Colitis-Associated Neoplasia. Mol. Cancer Res. 16, 634–642 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Rubin CE et al. DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology 103, 1611–1620 (1992). [DOI] [PubMed] [Google Scholar]
- 15.Choi C-HR, Bakir IA, Hart AL & Graham TA Clonal evolution of colorectal cancer in IBD. Nat. Rev. Gastroenterol. Hepatol. 14, 218–229 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Yaeger R. et al. Genomic Alterations Observed in Colitis-Associated Cancers Are Distinct From Those Found in Sporadic Colorectal Cancers and Vary by Type of Inflammatory Bowel Disease. Gastroenterology 151, 278–287.e6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajamäki K. et al. Genetic and Epigenetic Characteristics of Inflammatory Bowel Disease-Associated Colorectal Cancer. Gastroenterology (2021) doi: 10.1053/j.gastro.2021.04.042. [DOI] [PubMed] [Google Scholar]
- 18.Robles AI et al. Whole-Exome Sequencing Analyses of Inflammatory Bowel Disease-Associated Colorectal Cancers. Gastroenterology 150, 931–943 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker A-M et al. Evolutionary history of human colitis-associated colorectal cancer. Gut 68, 985–995 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beaugerie L. & Itzkowitz SH Cancers complicating inflammatory bowel disease. N. Engl. J. Med. 372, 1441–1452 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Sottoriva A. et al. A Big Bang model of human colorectal tumor growth. Nat. Genet. 47, 209–216 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomasetti C. & Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 347, 78–81 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dienstmann R. et al. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat. Rev. Cancer 17, 268 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Kinchen J. et al. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell 175, 372–386.e17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstone R, Itzkowitz S, Harpaz N. & Ullman T. Progression of low-grade dysplasia in ulcerative colitis: effect of colonic location. Gastrointest. Endosc. 74, 1087–1093 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Tilg H, Adolph TE, Gerner RR & Moschen AR The intestinal microbiota in colorectal cancer. Cancer Cell 33, 954–964 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Janati AI, Karp I, Laprise C, Sabri H. & Emami E. Detection of Fusobaterium nucleatum in feces and colorectal mucosa as a risk factor for colorectal cancer: a systematic review and meta-analysis. Syst. Rev. 9, 276 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shang F-M & Liu H-L Fusobacterium nucleatum and colorectal cancer: A review. World J. Gastrointest. Oncol. 10, 71–81 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kostic AD et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14, 207–215 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arthur JC et al. Microbial genomic analysis reveals the essential role of inflammation in bacteria-induced colorectal cancer. Nat. Commun. 5, 4724 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dougherty MW & Jobin C. Shining a light on colibactin biology. Toxins (Basel) 13, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu W. et al. Editing of the gut microbiota reduces carcinogenesis in mouse models of colitis-associated colorectal cancer. J. Exp. Med. 216, 2378–2393 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y. & Jobin C. Mutations in colon cancer match bacterial signature. Nature 580, (2020). [DOI] [PubMed] [Google Scholar]
- 34.Boleij A. et al. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin. Infect. Dis. 60, 208–215 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prindiville TP et al. Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerging Infect. Dis. 6, 171–174 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung L. et al. Bacteroides fragilis Toxin Coordinates a Pro-carcinogenic Inflammatory Cascade via Targeting of Colonic Epithelial Cells. Cell Host Microbe 23, 421 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomkovich S. et al. Human colon mucosal biofilms from healthy or colon cancer hosts are carcinogenic. J. Clin. Invest. 129, 1699–1712 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swidsinski A, Weber J, Loening-Baucke V, Hale LP & Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J. Clin. Microbiol. 43, 3380–3389 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi W-T et al. Nonconventional dysplasia in patients with inflammatory bowel disease and colorectal carcinoma: a multicenter clinicopathologic study. Mod. Pathol. 33, 933–943 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Lee H, Rabinovitch PS, Mattis AN, Lauwers GY & Choi W-T Non-conventional dysplasia in inflammatory bowel disease is more frequently associated with advanced neoplasia and aneuploidy than conventional dysplasia. Histopathology 78, 814–830 (2021). [DOI] [PubMed] [Google Scholar]
- 41.Rashtak S, Rego R, Sweetser SR & Sinicrope FA Sessile serrated polyps and colon cancer prevention. Cancer Prev Res (Phila Pa) 10, 270–278 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Ko HM et al. Serrated colorectal polyps in inflammatory bowel disease. Mod. Pathol. 28, 1584–1593 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Parian AM et al. Serrated Epithelial Change Is Associated With High Rates of Neoplasia in Ulcerative Colitis Patients: A Case-controlled Study and Systematic Review With Meta-analysis. Inflamm. Bowel Dis. (2020) doi: 10.1093/ibd/izaa312. [DOI] [PubMed] [Google Scholar]
- 44.Wijnands AM et al. Prognostic Factors for Advanced Colorectal Neoplasia in Inflammatory Bowel Disease: Systematic Review and Meta-analysis. Gastroenterology 160, 1584–1598 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Shah SC & Itzkowitz SH Reappraising Risk Factors for Inflammatory Bowel Disease-associated Neoplasia: Implications for Colonoscopic Surveillance in IBD. J Crohns Colitis 14, 1172–1177 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Friedman S, Rubin PH, Bodian C, Harpaz N. & Present DH Screening and surveillance colonoscopy in chronic Crohn’s colitis: results of a surveillance program spanning 25 years. Clin. Gastroenterol. Hepatol. 6, 993–8; quiz 953 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Greenstein AJ et al. Cancer in universal and left-sided ulcerative colitis: factors determining risk. Gastroenterology 77, 290–294 (1979). [PubMed] [Google Scholar]
- 48.Markowitz J. et al. Endoscopic screening for dysplasia and mucosal aneuploidy in adolescents and young adults with childhood onset colitis. Am. J. Gastroenterol. 92, 2001–2006 (1997). [PubMed] [Google Scholar]
- 49.Gyde SN et al. Colorectal cancer in ulcerative colitis: a cohort study of primary referrals from three centres. Gut 29, 206–217 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ekbom A, Helmick C, Zack M. & Adami HO Ulcerative colitis and colorectal cancer. A population-based study. N. Engl. J. Med. 323, 1228–1233 (1990). [DOI] [PubMed] [Google Scholar]
- 51.Hata K. et al. Surveillance Colonoscopy for Ulcerative Colitis-Associated Colorectal Cancer Offers Better Overall Survival in Real-World Surgically Resected Cases. Am. J. Gastroenterol. 114, 483–489 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Ananthakrishnan AN et al. Colonoscopy is associated with a reduced risk for colon cancer and mortality in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 13, 322–329.e1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bansal P. & Sonnenberg A. Risk factors of colorectal cancer in inflammatory bowel disease. Am. J. Gastroenterol. 91, 44–48 (1996). [PubMed] [Google Scholar]
- 54.Askling J. et al. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology 120, 1356–1362 (2001). [DOI] [PubMed] [Google Scholar]
- 55.Lutgens MWMD et al. High frequency of early colorectal cancer in inflammatory bowel disease. Gut 57, 1246–1251 (2008). [DOI] [PubMed] [Google Scholar]
- 56.Brackmann S. et al. Relationship between clinical parameters and the colitis-colorectal cancer interval in a cohort of patients with colorectal cancer in inflammatory bowel disease. Scand. J. Gastroenterol. 44, 46–55 (2009). [DOI] [PubMed] [Google Scholar]
- 57.Choi C-HR et al. Cumulative burden of inflammation predicts colorectal neoplasia risk in ulcerative colitis: a large single-centre study. Gut 68, 414–422 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baars JE et al. Age at diagnosis of inflammatory bowel disease influences early development of colorectal cancer in inflammatory bowel disease patients: a nationwide, long-term survey. J. Gastroenterol. 47, 1308–1322 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pekow JR et al. Outcome after surveillance of low-grade and indefinite dysplasia in patients with ulcerative colitis. Inflamm. Bowel Dis. 16, 1352–1356 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shah SC et al. High risk of advanced colorectal neoplasia in patients with primary sclerosing cholangitis associated with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 16, 1106–1113.e3 (2018). [DOI] [PubMed] [Google Scholar]
- 61.Mahmoud R. et al. No association between pseudopolyps and colorectal neoplasia in patients with inflammatory bowel diseases. Gastroenterology 156, 1333–1344.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Jong ME, Gillis VELM, Derikx LAAP & Hoentjen F. No increased risk of colorectal neoplasia in patients with inflammatory bowel disease and postinflammatory polyps. Inflamm. Bowel Dis. 26, 1383–1389 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Magro F. et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis 11, 649–670 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Axelrad JE et al. Colorectal strictures in patients with inflammatory bowel disease do not independently predict colorectal neoplasia. Inflamm. Bowel Dis. (2021) doi: 10.1093/ibd/izab177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gupta RB et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology 133, 1099–105; quiz 1340 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomas T, Abrams KA, Robinson RJ & Mayberry JF Meta-analysis: cancer risk of low-grade dysplasia in chronic ulcerative colitis. Aliment. Pharmacol. Ther. 25, 657–668 (2007). [DOI] [PubMed] [Google Scholar]
- 67.Curtius K. et al. Multicentre derivation and validation of a colitis-associated colorectal cancer risk prediction web tool. Gut (2021) doi: 10.1136/gutjnl-2020-323546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mahmoud R. et al. Association between indefinite dysplasia and advanced neoplasia in patients with inflammatory bowel diseases undergoing surveillance. Clin. Gastroenterol. Hepatol. (2019) doi: 10.1016/j.cgh.2019.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jeon J. et al. Determining risk of colorectal cancer and starting age of screening based on lifestyle, environmental, and genetic factors. Gastroenterology 154, 2152–2164.e19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Der Kraak L, Gros P. & Beauchemin N. Colitis-associated colon cancer: Is it in your genes? World J. Gastroenterol. 21, 11688–11699 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ryan BM et al. An analysis of genetic factors related to risk of inflammatory bowel disease and colon cancer. Cancer Epidemiol. 38, 583–590 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abraham C. & Cho JH Inflammatory bowel disease. N. Engl. J. Med. 361, 2066–2078 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ye BD & McGovern DPB Genetic variation in IBD: progress, clues to pathogenesis and possible clinical utility. Expert Rev. Clin. Immunol. 12, 1091–1107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kisiel JB & Ahlquist DA Stool DNA testing for cancer surveillance in inflammatory bowel disease: an early view. Therap. Adv. Gastroenterol. 6, 371–380 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Azuara D. et al. Novel methylation panel for the early detection of neoplasia in high-risk ulcerative colitis and Crohn’s colitis patients. Inflamm. Bowel Dis. 19, 165–173 (2013). [DOI] [PubMed] [Google Scholar]
- 76.Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG & Long MD ACG clinical guideline: ulcerative colitis in adults. Am. J. Gastroenterol. 114, 384–413 (2019). [DOI] [PubMed] [Google Scholar]
- 77.Laine L. et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest. Endosc. 81, 489–501.e26 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Bye WA et al. Strategies for Detecting Colorectal Cancer in Patients with Inflammatory Bowel Disease: A Cochrane Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 113, 1801–1809 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bye WA, Nguyen TM, Parker CE, Jairath V. & East JE Strategies for detecting colon cancer in patients with inflammatory bowel disease. Cochrane Database Syst. Rev. 9, CD000279 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang YR, Cangemi JR, Loftus EV & Picco MF Rate of early/missed colorectal cancers after colonoscopy in older patients with or without inflammatory bowel disease in the United States. Am. J. Gastroenterol. 108, 444–449 (2013). [DOI] [PubMed] [Google Scholar]
- 81.Mooiweer E. et al. Incidence of interval colorectal cancer among inflammatory bowel disease patients undergoing regular colonoscopic surveillance. Clin. Gastroenterol. Hepatol. 13, 1656–1661 (2015). [DOI] [PubMed] [Google Scholar]
- 82.Ten Hove JR et al. Consecutive negative findings on colonoscopy during surveillance predict a low risk of advanced neoplasia in patients with inflammatory bowel disease with long-standing colitis: results of a 15-year multicentre, multinational cohort study. Gut 68, 615–622 (2019). [DOI] [PubMed] [Google Scholar]
- 83.Alexandersson B. et al. High-Definition Chromoendoscopy Superior to High-Definition White-Light Endoscopy in Surveillance of Inflammatory Bowel Diseases in a Randomized Trial. Clin. Gastroenterol. Hepatol. 18, 2101–2107 (2020). [DOI] [PubMed] [Google Scholar]
- 84.Moussata D. et al. Are random biopsies still useful for the detection of neoplasia in patients with IBD undergoing surveillance colonoscopy with chromoendoscopy? Gut 67, 616–624 (2018). [DOI] [PubMed] [Google Scholar]
- 85.Lamb CA et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 68, s1–s106 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bessissow T. et al. Comparison of Endoscopic Dysplasia Detection Techniques in Patients With Ulcerative Colitis: A Systematic Review and Network Meta-analysis. Inflamm. Bowel Dis. 24, 2518–2526 (2018). [DOI] [PubMed] [Google Scholar]
- 87.Bisschops R. et al. Chromoendoscopy versus narrow band imaging in UC: a prospective randomised controlled trial. Gut 67, 1087–1094 (2018). [DOI] [PubMed] [Google Scholar]
- 88.Watanabe K. et al. 722 Comparison Between Newly-Developed Narrow Band Imaging and Panchromoendoscopy for Surveillance Colonoscopy in Patients With Longstanding Ulcerative Colitis: A Prospective Multicenter Randomized Controlled Trial, Navigator Study. Gastrointest. Endosc. 83, AB172 (2016). [Google Scholar]
- 89.Iacucci M. et al. A randomized trial comparing high definition colonoscopy alone with high definition dye spraying and electronic virtual chromoendoscopy for detection of colonic neoplastic lesions during IBD surveillance colonoscopy. Am. J. Gastroenterol. 113, 225–234 (2018). [DOI] [PubMed] [Google Scholar]
- 90.El-Dallal M. et al. Meta-analysis of Virtual-based Chromoendoscopy Compared With Dye-spraying Chromoendoscopy Standard and High-definition White Light Endoscopy in Patients With Inflammatory Bowel Disease at Increased Risk of Colon Cancer. Inflamm. Bowel Dis. 26, 1319–1329 (2020). [DOI] [PubMed] [Google Scholar]
- 91.American Society for Gastrointestinal Endoscopy Standards of Practice Committee et al. The role of endoscopy in inflammatory bowel disease. Gastrointest. Endosc. 81, 1101–21.e1 (2015). [DOI] [PubMed] [Google Scholar]
- 92.Murthy SK, Feuerstein JD, Nguyen GC & Velayos FS AGA clinical practice update on endoscopic surveillance and management of colorectal dysplasia in inflammatory bowel diseases: expert review. Gastroenterology 161, 1043–1051.e4 (2021). [DOI] [PubMed] [Google Scholar]
- 93.Shah SC & Itzkowitz SH Management of Inflammatory Bowel Disease-Associated Dysplasia in the Modern Era. Gastrointest. Endosc. Clin. N. Am. 29, 531–548 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shen B. et al. Role of interventional inflammatory bowel disease in the era of biologic therapy: a position statement from the Global Interventional IBD Group. Gastrointest. Endosc. 89, 215–237 (2019). [DOI] [PubMed] [Google Scholar]
- 95.Derikx LAAP, Nissen LHC, Smits LJT, Shen B. & Hoentjen F. Risk of Neoplasia After Colectomy in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 14, 798–806.e20 (2016). [DOI] [PubMed] [Google Scholar]
- 96.Das P, Johnson MW, Tekkis PP & Nicholls RJ Risk of dysplasia and adenocarcinoma following restorative proctocolectomy for ulcerative colitis. Colorectal Dis. 9, 15–27 (2007). [DOI] [PubMed] [Google Scholar]
- 97.Zhao L-N et al. 5-Aminosalicylates reduce the risk of colorectal neoplasia in patients with ulcerative colitis: an updated meta-analysis. PLoS ONE 9, e94208 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ullman T. et al. Progression to colorectal neoplasia in ulcerative colitis: effect of mesalamine. Clin. Gastroenterol. Hepatol. 6, 1225–30; quiz 1177 (2008). [DOI] [PubMed] [Google Scholar]
- 99.Choi CR et al. Low-grade dysplasia in ulcerative colitis: risk factors for developing high-grade dysplasia or colorectal cancer. Am. J. Gastroenterol. 110, 1461–71; quiz 1472 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lyakhovich A. & Gasche C. Systematic review: molecular chemoprevention of colorectal malignancy by mesalazine. Aliment. Pharmacol. Ther. 31, 202–209 (2010). [DOI] [PubMed] [Google Scholar]
- 101.Jess T, Lopez A, Andersson M, Beaugerie L. & Peyrin-Biroulet L. Thiopurines and risk of colorectal neoplasia in patients with inflammatory bowel disease: a meta-analysis. Clin. Gastroenterol. Hepatol. 12, 1793–1800.e1 (2014). [DOI] [PubMed] [Google Scholar]
- 102.Gong J. et al. Use of thiopurines and risk of colorectal neoplasia in patients with inflammatory bowel diseases: a meta-analysis. PLoS ONE 8, e81487 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.