Abstract
One hundred eighty Streptococcus pneumoniae strains isolated from children at a pediatric hospital in Singapore from 1997 to 1999 were serotyped and their antimicrobial susceptibility patterns were determined. Sixty-three percent of the isolates were resistant to penicillin. Significantly large numbers of the strains investigated were resistant to trimethoprim-sulfamethoxazole (87.8%), tetracycline (71.7%), erythromycin (67.8%), and chloramphenicol (40%). Penicillin and multidrug resistance was mostly associated with the frequently isolated S. pneumoniae isolates of serotypes (serotypes 19F, 23F, 6B, and 14). Isolates of serotype 19F, the serotype most commonly encountered in Singapore (41.1%), had the highest prevalence of penicillin (78.4%) and multidrug resistance (94.6%). Most of the invasive S. pneumoniae isolates (8 of 17; 47.1%) were of serotype 14.
Nasopharyngeal carriage of Streptococcus pneumoniae is common, but in some, the organism causes severe infections such as pneumonia, meningitis, and associated bacteremia (3, 4). In recent years, the emergence of penicillin- and multiple-drug-resistant strains and their increased occurrence worldwide have been of considerable concern. In France, the prevalence of penicillin-resistant S. pneumoniae (PRSP) was found to have increased from 13% in 1984 to 48% in 1990 (8). In the Far East, alarmingly high rates of penicillin resistance (55 and 79.7% for Hong Kong and South Korea, respectively) were reported (11, 16). Two earlier studies carried out in 1995 and 1997 with clinical isolates from the Singapore General Hospital and the National University Hospital reported rates of resistance to penicillin of 25 and 23.1%, respectively (13, 16). Children under 6 years of age are one of the groups with the highest rates of community carriage of PRSP, especially if they are attending day-care centers or have recently been given antimicrobial therapy (3, 5). Children who are less than 2 years old are the most susceptible to pneumococcal infections (3). At present, no epidemiological data on S. pneumoniae infections in the pediatric population in Singapore are available. Knowledge of the antibiotic susceptibility patterns and pneumococcal serotypes of the S. pneumoniae strains isolated from children may help in planning future treatment or preventive strategies. Therefore, an investigation was conducted to determine the serotype distribution and the antimicrobial susceptibility patterns of S. pneumoniae strains isolated from pediatric patients.
A total of 180 clinical isolates of S. pneumoniae were randomly collected over 3 years from July 1997 to August 1999 from patients in a Singapore pediatric hospital. The isolates were categorized as invasive (blood, cerebrospinal fluid, pleural fluid) and noninvasive (other nonsterile areas). Isolates were identified as S. pneumoniae by their colony morphology, their Gram staining and result, and the result of optochin susceptibility testing with a 5-μg optochin (ethyl hydrocuprein hydrochloride) disk for cultures grown on Mueller-Hinton agar supplemented with 5% sheep blood. The isolates were serotyped by the capsule swelling method with the Pneumotest kit (Statens Seruminstitut, Copenhagen, Denmark), which consisted of 12 pooled antisera (pools of serotypes A to H and P to T). Further typing of certain serogroups into serotypes was done with factor-specific sera.
Testing of susceptibility to erythromycin, trimethoprim-sulfamethoxazole, tetracycline, and chloramphenicol was done by the Kirby-Bauer disk diffusion method (BBL Sensi-Discs; Becton-Dickinson, Cockeysville, Md.), and the results were interpreted according to the guidelines of the National Committee for Clinical Laboratory Standards (NCCLS) (14). The MICs of penicillin G were determined by the E-test (AB Biodisk, Solna, Sweden) with an inoculum recommended by NCCLS for the disk diffusion method (14). With reference to the guidelines of NCCLS, penicillin G MICs by the E-test that fell between two marks on the E-test strip were rounded up to the next higher twofold dilution. Susceptibility to penicillin was categorized as susceptible if the MIC was <0.1 μg/ml, intermediate if the MIC was between 0.1 and 1.0 μg/ml, and resistant if the MIC was >1.0 μg/ml. An S. pneumoniae strain was defined as multidrug resistant if it was resistant to three or more different classes of antibiotics.
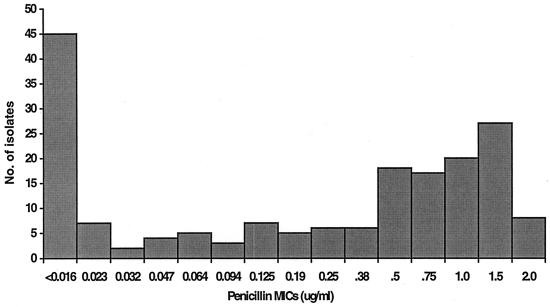
Of the 180 isolates investigated, 114 isolates (63.3%) were found to be resistant to penicillin. Seventy-nine (79 of 180; 43.9%) isolates were resistant to penicillin at levels from 0.125 to 1 μg/ml, and 35 isolates (35 of 180; 19.4%) were resistant to penicillin at levels from 1.5 to 2 μg/ml (Fig. 1). Among the strains that had intermediate resistance to penicillin, 17.8% (32 of 180 isolates) were resistant to all four antibiotics (trimethoprim-sulfamethoxazole, tetracycline, erythromycin, and chloramphenicol) tested. Twenty (11.1%) of the penicillin-resistant strains for which MICs were 1.5 to 2.0 μg/ml were also resistant to the four antibiotics. Resistance to erythromycin, tetracycline, chloramphenicol, and trimethoprim-sulfamethoxazole was observed at a remarkably high level (67.2%; 121 of 180 isolates). The highest prevalence of resistance was to trimethoprim-sulfamethoxazole (87.8%), followed by resistance to tetracycline (71.7%), erythromycin (67.8%), and chloramphenicol (40%) (Table 1).
FIG. 1.
Distribution of penicillin MICs for isolates from pediatric patients in Singapore.
TABLE 1.
Serotype distribution and antibiotic susceptibility patterns of S. pneumoniae isolates from pediatric patients in Singapore
| Resistance and susceptibility patterna | No. of isolates of serotype:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 19F | 23F | 6B | 14 | 6A | 15*b | 3 | 20 | 9* | NTc | |
| P, E, SXT, TE, C | 52 | 30 | 15 | 5 | 2 | ||||||
| P, E, SXT, TE | 38 | 24 | 4 | 3 | 5 | 2 | |||||
| P, SXT, TE, C | 10 | 2 | 6 | 1 | 1 | ||||||
| P, E, SXT | 3 | 1 | 1 | 1 | |||||||
| P, SXT, TE | 3 | 1 | 1 | 1 | |||||||
| P, E, TE | 1 | 1 | |||||||||
| P, SXT | 5 | 5 | |||||||||
| P | 2 | 1 | 1 | ||||||||
| E, SXT, TE, C | 4 | 3 | 1 | ||||||||
| E, SXT, TE | 15 | 9 | 2 | 2 | 1 | 1 | |||||
| E, SXT, C | 2 | 2 | |||||||||
| SXT, TE | 3 | 1 | 2 | ||||||||
| E, SXT | 6 | 1 | 1 | 1 | 1 | 2 | |||||
| TE, C | 1 | 1 | |||||||||
| SXT, C | 2 | 1 | 1 | ||||||||
| SXT | 15 | 2 | 8 | 2 | 1 | 2 | |||||
| TE | 2 | 1 | 1 | ||||||||
| C | 1 | 1 | |||||||||
| E | 1 | 1 | |||||||||
| Susceptible | 14 | 1 | 5 | 1 | 2 | 1 | 2 | 2 | |||
| Total | 180 | 74 | 46 | 18 | 17 | 7 | 4 | 2 | 1 | 1 | 10 |
Resistance to penicillin (P) was determined by the E-test. MICs ranged from 0.1 to 2.0 μg/ml. Resistance to erythromycin (E), trimethoprim-sulfamethoxazole (SXT), tetracycline (TE), and chloramphenicol (C) were determined by the Kirby-Bauer disk diffusion method and were interpreted according to NCCLS guidelines.
*, not serotyped.
NT, nontypeable.
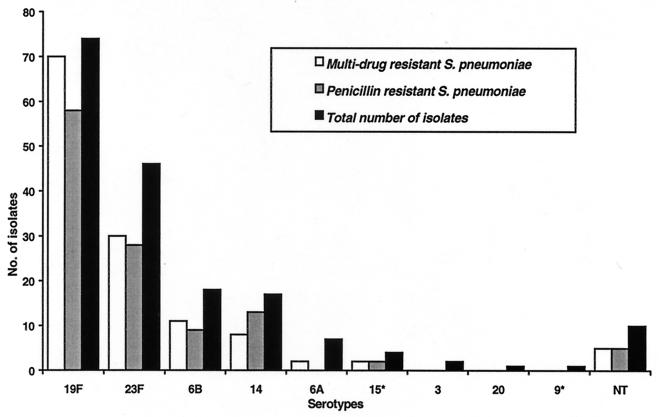
The most prevalent serotype encountered in the pediatric population is 19F, followed by serotypes 23F, 6B, 14, 6A, and 15. Strains of serotypes 3, 20, and 9* were less commonly encountered. Most of the penicillin-resistant as well as multidrug-resistant strains were of serotype 19F. Seventy-eight percent (58 of 74) of the serotype 19F strains were resistant to penicillin, and 94.6% exhibited multidrug resistance. Serotype 23F is the next most common group, with 58.7% of the isolates of serotype 23F being penicillin resistant and 57.4% showing multidrug resistance. Among the serotype 6B strains, 50% were penicillin resistant and 61.1% were multidrug resistant. The proportions of penicillin-resistant and multidrug-resistant strains among strains of serotype 14 were also high; 76.5% and 47.1%, respectively. Fifty percent of the isolates of serotype 15* were resistant to penicillin and the four antibiotics (Fig. 2). Significantly larger numbers of strains of serotype 19F than of other serotypes were resistant to penicillin (50 of 74; 67.6%). MICs were 0.5 to 2.0 μg/ml for more strains of serotype 19F than strains of serotypes 23F (24 of 46; 52.2%), 14 (8 of 17; 47.1%), and 6B (3 of 18; 16.7%). Of the eight strains for which penicillin MICs were 2.0 μg/ml, four were of serotype 19F, two were of serotype 23F, and the remaining two were of serotype 14.
FIG. 2.
Distribution of S. pneumoniae serotypes in Singapore's pediatric population with relationship to penicillin and multidrug resistance. NT, nontypeable.
Penicillin-resistant S. pneumoniae strains isolated from pediatric patients in Singapore accounted for 63.3% of the 180 isolates investigated in this study. The resistance level is significantly higher than those in two previous studies, which reported a 25% prevalence of resistance for S. pneumoniae strains isolated in 1995 and a 23.1% prevalence of resistance for strains isolated from September 1996 to June 1997 (13, 16). The increase in the prevalence of penicillin-resistant S. pneumoniae is not unexpected, as most countries have reported an alarming increase in the numbers of penicillin-resistant S. pneumoniae strains in recent years. From an Asian Network for Surveillance of Resistant Pathogens study of S. pneumoniae isolates collected from 11 Asian countries from September 1996 to June 1997, Korea was reported to have the highest prevalence of penicillin-resistant S. pneumoniae (79.7%), followed by Japan (65.3%). The prevalence rate for Taiwan reported in that study was 38.7%, but more recent studies with isolates from Taiwan have shown the prevalence of penicillin-resistant S. pneumoniae to be higher than 70% (4). In the present study, a larger proportion of the isolates (79 of 180) had intermediate resistance (MICs, 0.125 to 1 μg/ml) than (35 of 180) resistance (MICs, 1.5 to 2 μg/ml) to penicillin. Only 8 isolates (3.9%) were resistant to 2 μg of penicillin per ml, and the MICs did not exceed 2 μg/ml for any of the 180 isolates tested. This is in the contrast to the case for S. pneumoniae strains isolated from Korea, Taiwan, and Indonesia, for which penicillin MICs were 8 μg/ml or more (16). Despite a lower prevalence of penicillin-resistant S. pneumoniae in Malaysia (7 to 9%), resistant strains for which the MIC was an alarming >32 μg/ml were found (15). Since MICs for the penicillin-resistant S. pneumoniae strains were ≤2 μg/ml, intravenous administration of large doses of penicillin will still remain the treatment of choice, and all patients have responded and recovered.
In the present study, the prevalence of resistance to trimethoprim-sulfamethoxazole among the 180 isolates investigated was as high as 87.8%, that to tetracycline was 71.7%, that to erythromycin was 67.8%, and that to chloramphenicol was 40%. The extremely high level of resistance to trimethoprim-sulfamethoxazole among S. pneumoniae strains isolated from pediatric patients in Singapore is unexpected, as this antibiotic is not frequently used for empirical treatment. The incidence of resistance to trimethoprim-sulfamethoxazole in the United States was reported to vary from 18 to 60% (7, 9). The prevalence of resistance to chloramphenicol (40%) is also high, even though this antibiotic was hardly administered to pediatric patients in the hospital. High frequencies of resistance to tetracycline and erythromycin were observed among the isolates, and the figures were comparable to those reported for China and South Korea (12, 17). Of the 180 isolates, 28.9% were resistant to all four non-beta-lactam antibiotics tested, and the prevalence of drug-resistant S. pneumoniae in Singapore is higher than that determined from the results of a 30-center national surveillance study, which showed that the prevalence of drug-resistant S. pneumoniae was less than 18% in the United States (7). In the present study, among the group of 79 isolates which had intermediate levels of resistance to penicillin, 17.8% (32 of 180) were resistant to all four antibiotics. Most of the penicillin-resistant isolates were resistant to at least one non-beta-lactam antibiotics, with 11.1% (20 of 180) resistant to trimethoprim-sulfamethoxazole, tetracycline, erythromycin, and chloramphenicol. The S. pneumoniae strains that were isolated in Taiwan and that were resistant to high levels of penicillin were also found to be resistant to extended-spectrum cephalosporins and erythromycin (10).
The four most common serotypes encountered in Singapore's pediatric population over the last 3 years were 19F (74 of 180), 23F (46 of 180), 6B (18 of 180), and 14 (17 of 180). These four serotypes were also the serotypes most frequently encountered in Hong Kong (11). Large proportions of strains of each of these four serotypes were penicillin resistant. Similarly, three of the major serotypes (serotypes 6B, 14, and 23F) isolated from Brazil were also associated with penicillin resistance (1). Almost all of the serotype 19F (94.6%) isolates and at least half of the serotype 23F, 6B, and 14 isolates were multidrug resistant. Most of the invasive S. pneumoniae strains isolated in this study were of serotype 14 (7 of 17; 41.2%), followed by serotypes 23F and 19F, which constituted 13% (6 of 46) and 6.8% (5 of 74) of the isolates, respectively. A very high proportion of the invasive strains were also resistant to penicillin and multidrug resistant.
The increasing incidence in Singapore of S. pneumoniae strains that are resistant to penicillin as well as to trimethoprim-sulfamethoxazole, tetracycline, erythromycin, and chloramphenicol emphasizes the need to implement preventive measures. However, infection control measures such as hand washing, limited empirical use of antimicrobial agents, and isolation of children who are carriers of multidrug-resistant S. pneumoniae strains might not be practical or sufficient to stop the spread. In light of the increasing prevalence of S. pneumoniae strains resistant to multiple antibiotics, the need for an effective pneumococcal vaccine is becoming urgent. The current formulations of the vaccine contain a mixture of purified capsular polysaccharides derived from 23 different types of S. pneumoniae. However, the vaccine was found not to be effective for children <2 years old (2, 3, 6). In view of Food and Drug Administration approval of a new seven-valent pneumococcal conjugate vaccine by which covers the most common serotypes reported in this study, we hope to greatly reduce the emergence of penicillin-resistant and multidrug-resistant S. pneumoniae strains in Singapore through either a vaccination program or controlled use of antibiotics.
Acknowledgments
This work was supported by a grant (grant NMRC/0287/1998) from the National Medical Research Council, Singapore.
REFERENCES
- 1.Brandileone M C D C, Fabio J L D, Vieira V S D, Zanella R C, Casagrande S T, Pignatari A C, Tomasz A. Geographic distribution of penicillin resistance of Streptococcus pneumoniae in Brazil: genetic relatedness. Microb Drug Res. 1998;4:209–217. doi: 10.1089/mdr.1998.4.209. [DOI] [PubMed] [Google Scholar]
- 2.Briles D E, Tart R C, Swiatlo E, Dillard J P, Smith P, Benton K A, Ralph B A, Brooks-Walter A, Crain M J, Hollingshead S K, McDaniel L S. Pneumococcal diversity: considerations for new vaccine strategies with emphasis on pneumococcal surface protein A (PspA) Clin Microbiol Rev. 1998;11:645–657. doi: 10.1128/cmr.11.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell G D, Silberman R. Drug-resistant Streptococcus pneumoniae. Clin Infect Dis. 1998;26:1188–1195. doi: 10.1086/520286. [DOI] [PubMed] [Google Scholar]
- 4.Chiou C C C, Liu Y C, Huang T S, Hwang W K, Wang J H, Lin H H, Yen M Y, Hsieh K S. Extremely high prevalence of nasopharyngeal carriage of penicillin-resistant Streptococcus pneumoniae among children in Kaohsiung, Taiwan. J Clin Microbiol. 1998;36:1933–1937. doi: 10.1128/jcm.36.7.1933-1937.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christenson B, Sylvan S P E, Noreen B. Drug-resistant Streptococcus pneumoniae in day-care centres in Stockholm County. J Infect. 1998;37:9–14. doi: 10.1016/s0163-4453(98)90174-8. [DOI] [PubMed] [Google Scholar]
- 6.Collier J, Iheanacho I. The place of pneumococcal vaccination. Drug Ther Bull. 1998;36:73–76. doi: 10.1136/dtb.1998.361073. [DOI] [PubMed] [Google Scholar]
- 7.Doern G V, Brueggemann A, Holley H P, Rauch A M. Antimicrobial resistance of Streptococcus pneumoniae recovered from outpatients in the United States during the winter months of 1994 to 1995: results of a 30-center National Surveillance Study. Antimicrob Agents Chemother. 1996;40:1208–1213. doi: 10.1128/aac.40.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doit C, Loukil C, Fitoussi F, Geslin P, Bingen E. Emergence in France of multiple clones of clinical Streptococcus pneumoniae isolates with high-level resistance to amoxicillin. Antimicrob Agents Chemother. 1999;43:1480–1483. doi: 10.1128/aac.43.6.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fairchok M P, Ashton W S, Fisher G W. Carriage of penicillin-resistant pneumococci in a military population in Washington, DC: risk factors and correlation with clinical diseases. Clin Infect Dis. 1996;22:966–972. doi: 10.1093/clinids/22.6.966. [DOI] [PubMed] [Google Scholar]
- 10.Hsueh P R, Teng L J, Lee L N, Yang P C, Ho S W, Luh K T. Dissemination of high-level penicillin-, extended-spectrum cephalosporin-, and erythromycin-resistant Streptococcus pneumoniae clones in Taiwan. J Clin Microbiol. 1999;37:221–224. doi: 10.1128/jcm.37.1.221-224.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ip M J, Lyon D J, Yung R W H, Chan C, Cheng A F B. Evidence of clonal dissemination of multidrug-resistant Streptococcus pneumoniae in Hong Kong. J Clin Microbiol. 1999;37:2837–2839. doi: 10.1128/jcm.37.9.2834-2839.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S N, Kim S W, Choi I H, Pyo S N, Rhee D K. High incidence of multidrug-resistant Streptococcus pneumoniae in South Korea. Microb Drug Resist. 1996;2:401–406. doi: 10.1089/mdr.1996.2.401. [DOI] [PubMed] [Google Scholar]
- 13.Koh T H, Sng L H, Ngan C C L. Molecular typing of multiresistant Streptococcus pneumoniae serogroup 19 in Singapore. Pathology. 1998;30:395–398. doi: 10.1080/00313029800169696. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility test. Eighth informational supplement. NCCLS document M2-A6. Wayne, Pa: National Committee for Laboratory Standards; 1998. [Google Scholar]
- 15.Rohani M Y, Raudzah A, Ng A J, Ng P P, Zaidatul A A R, Asmah I, Murtaza M, Parasakthy N, Mohd Yasmin M Y, Cheong Y M. Epidemiology of Streptococcus pneumoniae infection in Malaysia. Epidemiol Infect. 1999;122:77–82. doi: 10.1017/s0950268898001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song J H, Lee N Y, Ichiyama S, Yoshida R, Hirakata Y, Fu W, Chongthaleong A, Aswapokee N, Chiu C H, Lalitha M K, Thomas K, Perera J, Yee T T, Jamal F, Warsa U C, Vinh B X, Jacobs M R, Appelbaum P C, Pai C H the ANSORP Study Group. Spread of drug-resistant Streptococcus pneumoniae in Asian countries: Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Clin Infect Dis. 1999;28:1206–1211. doi: 10.1086/514783. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Huebner R, Chen M, Klugman K. Antibiotic susceptibility patterns of Streptococcus pneumoniae in China and comparison of MICs by agar dilution and E-test methods. Antimicrob Agents Chemother. 1998;42:2633–2636. doi: 10.1128/aac.42.10.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]