Abstract
Background
Triple-negative breast cancer (TNBC) is the most severe type of breast cancer owing to its high heterogeneity, aggressiveness and lack of treatment. Studies have reported that uncarboxylated osteocalcin (GluOC) promotes the development of prostate and other cancers. Studies have also found elevated levels of serum osteocalcin in breast cancer patients with bone metastasis, and serum osteocalcin can be a marker of bone metastasis. However, whether GluOC promotes the development of TNBC and the related mechanisms need to be further clarified.
Results
Our results revealed that GluOC is associated with the proliferation and metastasis of MDA-MB-231 cells. GluOC increased the viability and proliferation of MDA-MB-231 cells. In addition, GluOC enhanced the metastatic ability of MDA-MB-231 cells by promoting the expression of matrix metalloproteinase-2 (MMP2), matrix metalloproteinase-13 (MMP13), and vascular endothelial growth factor (VEGF) and inducing epithelial-mesenchymal transition (EMT). We also found that GluOC upregulated the expression of interleukin-8 (IL-8) and parathyroid hormone-related protein (PTHrP) genes in MDA-MB-231 breast cancer cells. Moreover, the promoting effect of GluOC was reversed in MDA-MB-231 breast cancer cells treated with specific inhibitor of SMAD3 (SIS3), a SMAD3 phosphorylation inhibitor.
Conclusion
Our research proved for the first time that GluOC facilitates the proliferation and metastasis of MDA-MB-231 cells by accelerating the transforming growth factor-β (TGF-β)/SMAD3 signaling pathway. Moreover, GluOC also promotes the gene expression of IL-8 and PTHrP. Both IL-8 and PTHrP can act as osteolytic factors in breast cancer cells. This study indicates that GluOC may be a useful target for preventing TNBC bone metastasis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12860-022-00416-7.
Keywords: Breast cancer, Osteocalcin, TGF-β, Proliferation, Metastasis
Background
Breast cancer (BC) has become most common cancer among women [1]. The World Health Organization's International Agency for Research on Cancer (IARC) 2018 GLOBOCAN report ranked breast cancer as the most common cancer among females (representing 24% of female cancer cases). The occurrence of breast cancer among Chinese women is approximately 41/100,000, and the incidence is increasing year by year. Among all breast cancers, the occurrence of triple-negative breast cancer (TNBC) is approximately 15% ~ 20% [2]. TNBC is estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER2) negative breast cancer, is not sensitive to common endocrine therapy and targeted therapy, has a poor prognosis, and is prone to early recurrence and metastasis [3]. Patients with metastatic TNBC have a survival period of approximately 18 months, which is lower than that of patients with other types of breast cancer, for whom the survival may be longer than 5 years [4]. To date, the treatment of TNBC is still unsatisfactory; therefore, there is an urgent need to understand the mechanisms of TNBC invasion and metastasis, which can help identify new targets and screen drugs.
Tumor metastasis is a complex, multistage process. In this process, matrix metalloproteinases (MMPs), which participate in pathways leading to the development of metastasis, degrade and remodel the extracellular matrix. Vascular endothelial growth factor (VEGF) promotes the development of vascular endothelial cells to form new tumor blood vessels to provide nutrition and nutrients for their growth and metastasis and thus accelerates the occurrence and development of breast cancer [5]. Epithelial-mesenchymal transition (EMT) endows cancer cells with a mesenchymal phenotype to achieve mobility as well as additional properties and promotes invasion, migration and subsequent spread [6]. Breast cancer cells, by expressing parathyroid hormone-related protein (PTHrP) and interleukin-8 (IL-8), promote the activity of osteoclasts, which are responsible for perturbing bone homeostasis [7]. Jie Wang et al. found that the migration, invasion and bone metastasis of MDA-MB-231 cells can be prevented by silencing the bone sialoprotein (BSP) gene [8]. Shuang Qu et al. clarified that Osterix stimulates MDA-MB-231 migration and angiogenesis by upregulating S100A4 [9]. However, the mechanism of bone metastasis in TNBC needs to be further studied.
The regulatory cytokine transforming growth factor beta (TGF-β) is a factor of the cytokine network and has the function of regulating biological growth [10]. The receptor complex is activated, and TGF-β signaling is ultimately initiated through the specific C-terminal phosphorylation of SMADs (R-SMADs), SMAD2 and SMAD3 [11]. TNBC cells also produce SMAD2/3 and SMAD4, which lead to metastasis and angiogenesis [12]. TGF-β can not only play a role in local invasion, but increasing evidence has also shown that TGF-β has an essential effect in cancer cell metastasis by promoting the cancer cell proliferation, EMT and other processes. Hu Z et al. found that the TGF-β signaling pathway can be exploited to develop a PC-3 (human prostatic cancer cell line) bearing mouse model of bone metastasis of prostate cancer [13]. Kakonen et al. also discovered that TGF-β may stimulate osteolytic cytokines (PTHrP) released by cancer cells and induce osteolytic bone metastasis [14]. Brewster AM et al. reported that TNBC signaling pathway therapies include those targeting PARP, PI3K/mTOR, JAK/STAT, Notch, etc. [15]. However, the signaling pathways related to bone metastasis in TNBC still need to be further explored.
Osteocalcin is expressed and secreted by osteoblasts [16]. After protein synthesis, its three glutamic acid residues can be carboxylated to varying degrees [17, 18]. Osteocalcin can take on the forms of completely carboxylated osteocalcin and incompletely carboxylated osteocalcin. Incompletely carboxylated osteocalcin includes uncarboxylated osteocalcin (GluOC) [19]. Increasing evidence has suggested that GluOC regulates glucose metabolism, neural development, and the male reproductive track, and has a relationship with tumorigenesis [20]. Ye P [21] and Kayed H et al. [22] found that GluOC has a tumor-promoting effect on the human prostate cancer cell line PC-3 and pancreatic cancer cells. Kyung-Hun Lee et al. clarified that the level of circulating osteocalcin-positive cells can be used as a predictor of early bone metastasis in patients with breast cancer metastasis [23]. Salem AM et al. also suggested that serum osteocalcin content in breast cancer patients and bone metastasis patients was increased remarkably compared with that in the control group [24]. However, the mechanism by which GluOC affects the proliferation and bone metastasis of TNBC remains unclear.
Here, we selected MDA-MB-231 cells, which are highly metastatic, and MCF7 cells, which are nonmetastatic, as research objects to explore the impact of GluOC on TNBC metastasis. The results demonstrated that GluOC can significantly promote the proliferation and migration of MDA-MB-231 cells via the TGF-β/SMAD3 signaling pathway, but there was no obvious influence on MCF7 breast cancer cells. These research results showed that GluOC has an indispensable role in the proliferation and migration of MDA-MB-231 cells. In addition, our study also emphasizes that GluOC is a novel target for the prevention and/or treatment of TNBC bone metastasis.
Materials and methods
Reagents and antibodies
Antibodies against proliferating cell nuclear antigen (PCNA, cat. no. ab92552, Abcam), matrix metallopeptidase 2 (MMP2, cat. no. ab2536, Abcam), Vimentin (cat. no. ab92547, Abcam), N-cadherin (cat. no. ab76011, Abcam), Snail (cat. no. ab216347, Abcam), E-cadherin (cat. no. ab231303, Abcam), TGF-β (cat. no. ab215715, Abcam), SMAD2 + SMAD3 (SMAD2/3, cat. no. ab202445, Abcam), p-SMAD3 (cat. no. ab52903, Abcam), cyclin D1 (cat. no. CPA4263, Cohesion), and β-actin (cat. no. 13E5, Cell Signaling Technology, Inc.) and goat anti-rabbit IgG(H + L)-HRP (cat. no. S0101, Lablead Biotech, Co. Ltd), goat anti-mouse IgG(H + L)-HRP (cat. no. S0100, Lablead Biotech, Co. Ltd), 1% crystal purple solution (cat. no. G1064, Solarbio), Native Lysis Buffer (cat. no. R0030, Solarbio), a BCA protein concentration assay kit (cat. no. B5000; Beijing Lablead Biotech, Co., Ltd.), and trypsin cell digestive fluid (0.25% trypsin, cat. no. C0202, Beyotime) were used in this study.
Cell culture
MDA-MB-231 is a human TNBC cell line that is highly invasive and metastatic. MCF7 is a human ER-positive breast cancer cell line that is a nonmetastatic and has low invasiveness. Cells were purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) containing 10% fetal bovine serum (FBS, cat. no. 10270–106, Gibco). After that, the cells were cultured in a moist 37 °C incubator with 5% CO2. Thereafter, the medium was replenished every 24 h. Finally, the cells were cultured in serum-free medium for 12 h before treatment with GluOC.
Cell viability assay
A Cell Counting Kit-8 (CCK8, cat. no. CK04, Dojindo Molecular Technologies, Inc.) was used to quantify cell viability. Cells were inoculated at 1 × 104 cells/well into 96-well plates. After the number of cancer cells reached 60%, the cells were cultured in serum-free medium and GluOC at different doses (10, 20, 40, 100 and 160 ng/ml) for 4 h, 8 h, 12 h, 24 h, 48 h, 72 h, 96 h and 120 h. Room temperature phosphate buffer saline (PBS) was used to clean the cells, and CCK8 solution (10 μL) was added to 6-well plates, which were incubated in the incubator for 1 h. The absorbance was measured at 450 nm with an automated microplate reader (Synergy H1; Biotek Instruments, Inc.).
Wound healing assay
The wound healing assay was applied to calculate the migration capacity of cancer cells. The cells were placed in a 6-well plate and incubated until they were approximately 90% confluent. A 10 µl micropipette tip was used to scrape the cell monolayer, and the debris was rinsed four times with PBS. The cells were placed in culture medium containing different concentrations of GluOC (10, 20, 40, 100 and 160 ng/ml), incubated for 24 h, and then observed with an inverted microscope (cat. no. DP71, OLYMPUS). Cell images were taken at 100 × magnification. Three random areas with cells were selected, and the migration rate was calculated. Cells in culture medium without GluOC were used as vector controls. Wound area can be calculated by manually tracing the cell-free area in images using ImageJ software version 6 (National Institutes of Health). Under normal conditions, the wound area will decrease over the time. The migration rate can be expressed as the percentage of area reduction of wound closure, which increases as cells migrate over time. Wound closure %: (At=0 h – At=24 h) / At=oh × 100%. Where At = 0 h is the area of the wound measured immediately after scratching (time zero), and At=24 h is the area of the wound measured 24 h after the scratch is performed. All experimental data are from three independent experiments.
Cell migration assay
Migration was tested as previously described using a transwell chamber (6.5 mm in diameter, 8 μM pore-size, Corning Costar, Cambridge, MA) [25]. The cells were serum-free for 12 h, rinsed four times with PBS, and then recultivated in a serum-free medium. The cell density in the upper compartment was 1 × 104. Culture medium containing 10% FBS was added to the lower compartment. The 24-well plate was placed in the incubator for culture for 12 h. Different concentrations of GluOC were added for culture for 24 h, and the samples were washed 3 times with PBS, fixed with 10% formaldehyde solution for 30 min, dyed with 1% crystal violet solution and incubated for 30 min. After air drying, the cells above the chamber were wiped off with a cotton swab, and pictures were taken using an inverted microscope (cat. no. MK3, Multiskan). Cell images were taken at 100 × magnification (avoiding the edge of the chamber, to avoid distorting the results with edge effects).
SIS3 treatment
Specific inhibitor of SMAD3 (SIS3) is a specific inhibitor of SMAD3 phosphorylation [26]. SIS3 powder (cat. no. T3636, TargetMoL) was dissolved in 100% dimethyl sulfoxide (DMSO, cat. no. D6258, Macklin Biochemical Co., Ltd.) and prepared into a solution with a final concentration of 1 mM. MDA-MB-231 cells were grown to approximately 90% confluency in a 6-well plate, and gently washed with PBS four times. Subsequently, the cultures were grouped into three different culture conditions: (A) control; (B) SIS3 (10 μM); and (C) SIS3 (10 μM) + GluOC (40, 100, 160 ng/mL). After continuous culture for 24 h, the effect of SIS3 on the proliferation and migration of breast cancer cells was detected by western blotting.
Western blot analysis
The breast cancer cells were gently rinsed four times with 1 × PBS and harvested in cell lysis buffer containing 1 mM phenylmethanesulfonyl fluoride (PMSF, cat. no. P0100, Solarbio). The cells were lysed on ice for 10 min. The whole cell lysate was centrifuged at 1,2000 g for 10 min at 4 °C, and the supernatant was collected for subsequent applications. Equal amounts of protein were isolated by 12% SDS-PAGE, and the proteins were transferred to polyvinylidene fluoride (PVDF, cat. no. ISEQ00010, Merck Millipore, Co., Ltd.) membranes by membrane electrophoresis for 2 h. The membranes were then sealed with TBS‑5% Tween‑20 (TBST) containing 5% skim milk at room temperature for 2 h to prevent binding of nonspecific antibodies. The dilutions of various primary antibodies were applied, followed by overnight incubation at 4 °C at 1:1000 concentration as recommended by the supplier. Then, the PVDF membranes were washed three times with TBST (10 mM Tris, 10 mM NaCl, 0.1% Tween—20), and incubated for 2 h with the secondary antibody at a concentration of 1:10,000. The samples were tested with Millipore ECL (WBKLS0100, Millipore). An antibody against actin was used to control protein loading and transfer. ImageJ version 6 (National Institutes of Health) was used to measure the strip density.
Quantitative real-time PCR
The cells were treated with or without GluOC at various doses (40,100, and 160 ng/mL). Total RNA was extracted using an RNA Simple Total RNA Kit (cat. no. DP419, Tiangen Biotech, Co., Ltd.). The purity and quality of RNA were examined by a NanoDrop2000 (Thermo Scientific, Wilmington, DE). A total of 1 µg of RNA was reverse transcribed into cDNA using the TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (cat. no. A T311‑03, TransGen Biotech.). Then, quantitative real-time PCR (qRT–PCR) was performed using the SYBR Green qPCR kit (cat. no. AQ132‑24, TransGen Biotech.). The thermal cycle conditions were 94° 30 s, followed by 40 cycles of 94° for 5 s, 55° for 15 s, and 72° for 10 s. Quantification was based on the 2^(-△△Ct) method. β-Actin served as a control, and the primer sequences are shown in Table 1.
Table 1.
Primers for qRT–PCR
| Gene | Primer sequences (5’ → 3’) |
|---|---|
| MMP13 |
F: AAATTATGGAGGAGATGCCCATT R: TCCTTGGAGTGGTCAAGACCTAA |
| IL8 |
F: TTCACTGGCATCTTCACTGATTCTT R: CTGCGCCAACACAGAAATTAT |
| VEGF |
F: CAGAATCATCACGAAGTG R: TCTGCATGGTGATGTTGGAC |
| PTHrP |
F: CTGGTTCAGCAGTGGAGC R: TTCTGCGATCAGATGGTG |
| β-Actin |
F: AGATGTGGTCAGCAAGCAG R: GCGCAAGTTAGGTTTTGTCA |
Statistical analysis
Data are presented as the mean ± standard deviation of at least three independent experiments. The significance of differences between different groups was analyzed by one-way ANOVA with Tukey’s post-hoc test of variance using GraphPad Prism software (version 6.0, GraphPad software, Inc.). P < 0.05 was considered statistically significant.
Results
GluOC promotes the proliferation of MDA-MB-231 cells
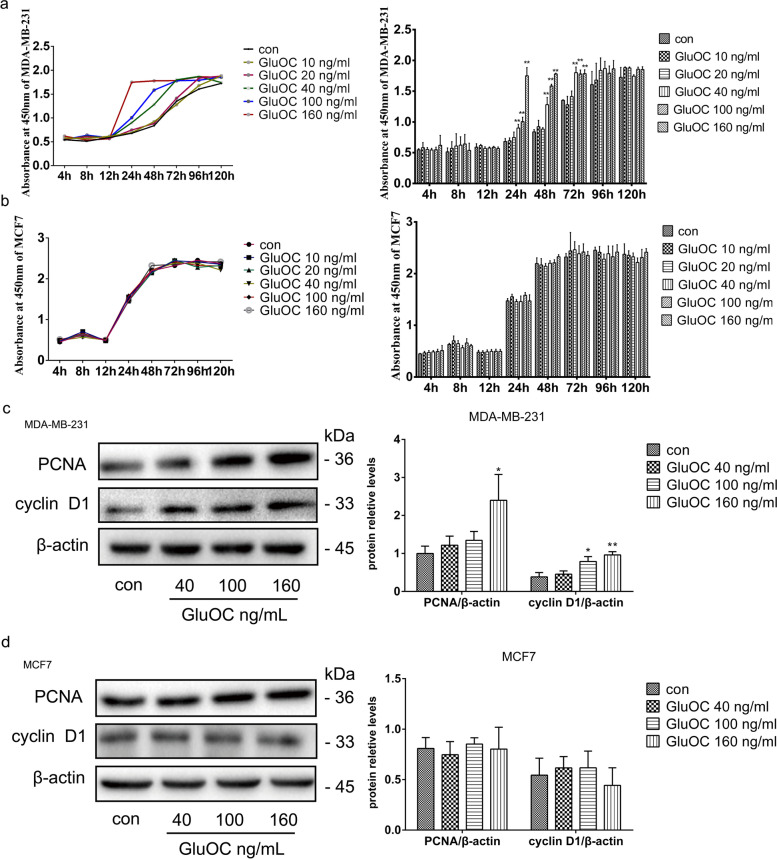
To clarify the effect of GluOC on the proliferation of breast cancer cells, MDA-MB-231 and MCF7 cells were treated with different doses of GluOC (10, 20, 40, 100, and 160 ng/mL) for different times (4, 8, 12, 24, 48, 72, 96, and 120 h). After 24 h of culture with GluOC, the proliferation of MDA-MB-231 cells treated with 40, 100, and 160 ng/mL GluOC was significantly improved (Fig. 1a). The proliferation of MCF7 cells treated with GluOC showed no significant change (Fig. 1b). Based on these results, the 40, 100, and 160 ng/mL concentrations of GluOC were selected for subsequent experiments. Similarly, GluOC (100 and 160 ng/mL) increased the PCNA and cyclin D1 content in MDA-MB-231 cells compared with that in the control group (Fig. 1c). However, GluOC had no obvious effect on the PCNA and cyclin D1 protein levels in MCF7 breast cancer cells (Fig. 1d). These results showed that GluOC accelerated the proliferation of MDA-MB-231 cells but did not influence MCF7 cells.
Fig. 1.
GluOC promotes MDA-MB-231 cell proliferation. a Growth curves of MDA-MB-231 cells treated with different doses of GluOC (10, 20, 40, 100, and 160 ng/ml) for different times (8, 12, 24, 48, 72, 96, and 120 h). b Growth curves of MCF7 cells treated with various doses of GluOC (10, 20, 40, 100 and 160 ng/ml) for different times (8, 12, 24, 48, 72, 96, and 120 h). c The protein levels of PCNA and cyclin D1 in MDA-MB-231 cells before and after GluOC stimulation were determined by western blotting and quantified densitometrically with ImageJ software. β-Actin was used as the control. d The protein levels of PCNA and cyclin D1 in MCF7 cells before and after GluOC stimulation were detected by western blotting. The results represent at least three independent experiments, and the data are presented as the mean ± SD (n = 3). *P < 0.05 and **P < 0.01, vs. the control group (con = control)
GluOC promotes the migration of MDA-MB-231 cells
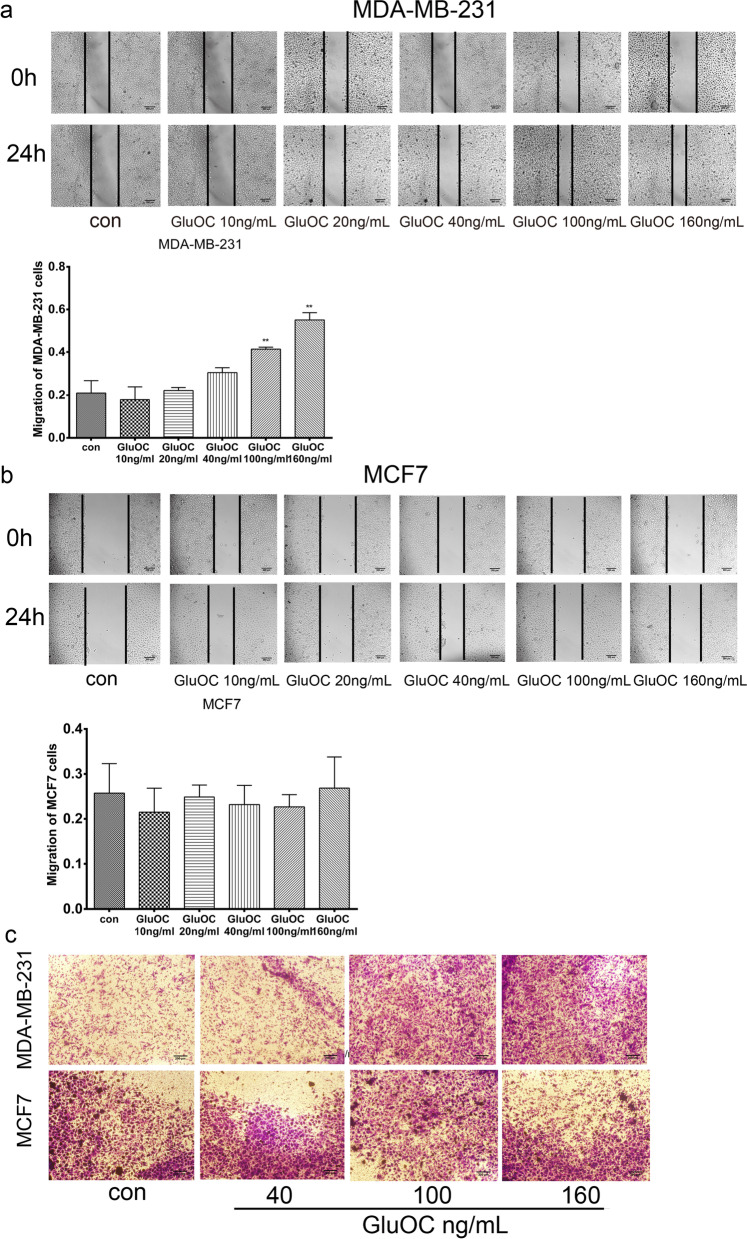
To determine whether GluOC might facilitate the migration of breast cancer cells, wound healing and transwell experiments were performed on MDA-MB-231 and MCF7 cells. The wound healing assay results are shown in Fig. 2a & b. After culture for 24 h with GluOC, the wound healing of MDA-MB-231 breast cancer cells was markedly increased compared with that of the control group, but migration was not obviously changed in MCF7 cells. The transwell assay results (Fig. 2c) showed that the GluOC treatment (100 and160 ng/mL) increased the capacity of MDA-MB-231 cells to penetrate the cellulose membrane in a manner depending on the GluOC concentration, while GluOC had no obvious effect on the migration of MCF7 cells. These results suggested that GluOC promotes the migration of MDA-MB-231 cells, but does not significantly influence the migration of MCF7 cells.
Fig. 2.
GluOC promotes MDA-MB-231 cell migration. a Wound healing assay of MDA-MB-231 cells. The bar chart shows the wound healing ability, magnification × 100. b Wound healing assay of MCF7 breast cancer cells. The bar chart shows the wound healing ability, magnification × 100. The results represent at least three independent experiments, and the data are presented as the mean ± SD (n = 3). c Transwell assay. MDA-MB-231 and MCF-7 cells were transferred onto the upper membrane of Transwell plates. Photographs were taken with a microscope for counting, with at least five fields photographed per treatment group, magnification × 100. *P < 0.05 and **P < 0.01, vs. the control group (con = control)
GluOC promotes the expression of MMP2, MMP13 and VEGF in MDA-MB-231 cells
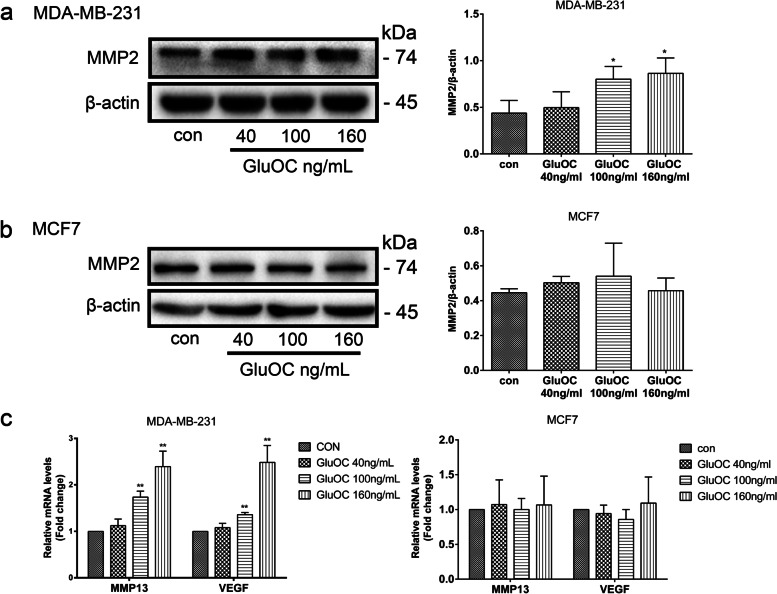
Matrix metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF) play vital roles in cancer migration, invasion and metastasis [27, 28]. To study the impact of GluOC on the degradation of extracellular matrix and induction of angiogenesis, the expression of MMPs and VEGF was analyzed. After GluOC treatment, the content of MMP2 protein in MDA-MB-231 cells increased significantly (Fig. 3a). GluOC increased the mRNA content of MMP13 and VEGF in MDA-MB-231 cells, while it did not obviously affect the expression levels of MMP2, MMP13 and VEGF in MCF7 cells (Fig. 3b & c). All the above results further confirmed that GluOC stimulates the migration of MDA-MB-231 cells.
Fig. 3.
GluOC promotes the expression of MMP2, MMP13 and VEGF in MDA-MB-231 cells. a The expression of MMP2 in MDA-MB-231 cells was analyzed by western blotting and quantified densitometrically with ImageJ software. b Western blot analysis of MMP2 expression in MCF7 cells. c mRNA expression levels of MMP13 and VEGF in MDA-MB-231 and MCF7 cells. The results represent at least three independent experiments, and the data are presented as the mean ± SD (n = 3). *P < 0.05 and **P < 0.01 vs. the control group (con = control). MMP2, matrix metalloproteinase 2; MMP13, matrix metalloproteinase 13; VEGF, vascular endothelial growth factor
GluOC promotes the EMT process in MDA-MB-231 cells
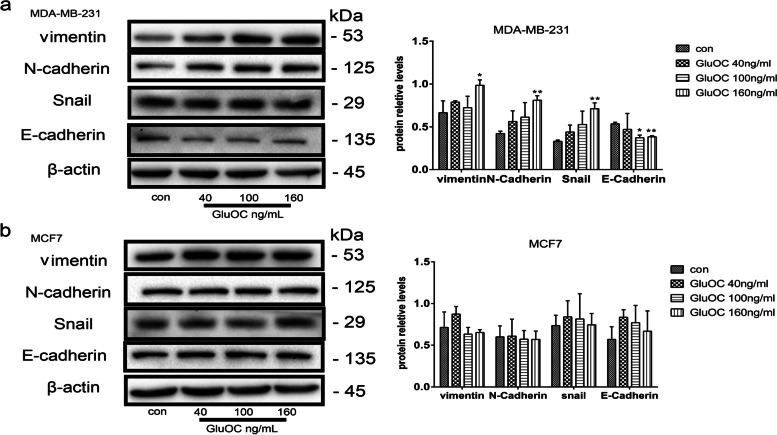
EMT is a pivotal process promoting cancer cell metastasis [29]. We explored the function of GluOC in the EMT of breast cancer cells by assessing the levels of vimentin, N-cadherin, Snail and E-cadherin. Our results showed that vimentin, Snail and N-cadherin protein levels in MDA-MB-231 cells treated with GluOC were significantly increased, while the E-cadherin levels were decreased (Fig. 4a). In MCF7 cells, GluOC treatment did not obviously affect the levels of vimentin, Snail, N-cadherin and E-cadherin (Fig. 4b). In summary, our study illustrated that GluOC promotes the EMT of MDA-MB-231 cells.
Fig. 4.
GluOC promotes EMT and metastasis of MDA-MB-231 cells. a Western blotting was performed to illustrate the influence of various doses of GluOC on the protein levels of vimentin, Snail, N-cadherin and E-cadherin in MDA-MB-231 cells. b Western blotting was used to detect the effect of different doses of GluOC on the protein levels of vimentin, Snail, N-cadherin and E-cadherin in MCF7 breast cancer cells. β-Actin was used as an internal reference. The expression level was quantified densitometrically with the ImageJ software. The results represent at least three independent experiments, and the data are presented as the mean ± SD (n = 3). *P < 0.05 and **P < 0.01, vs. the control group (con = control)
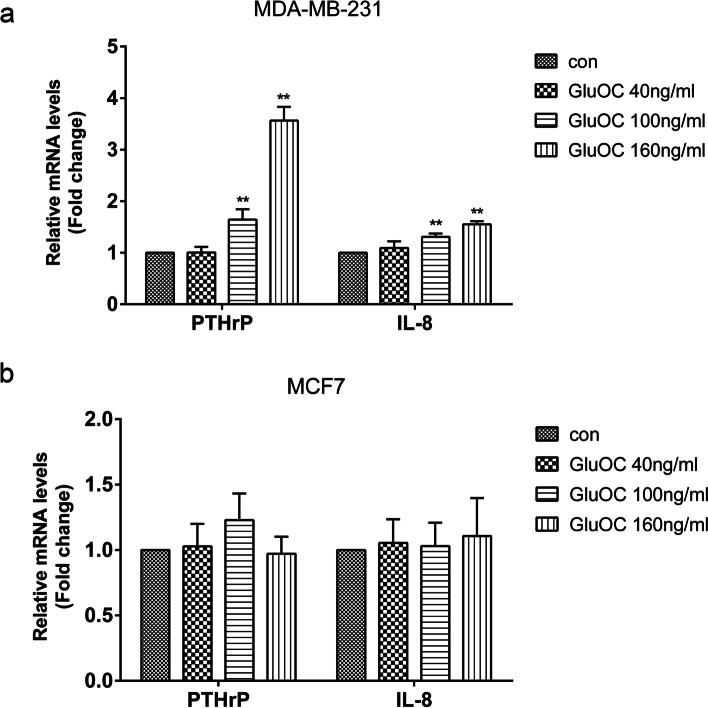
GluOC promotes the expression of PTHrP and IL-8 in MDA-MB-231 cells
Bone metastasis of breast cancer often causes osteolytic damage. Both IL-8 and PTHrP can act as osteolytic factors in breast cancer cells [30]. Therefore, we studied the effect of GluOC on the levels of PTHrP and IL-8. We found that after GluOC treatment, the expression of the PTHrP and IL-8 genes in MDA-MB-231 cells was increased (Fig. 5a). In MCF7 cells, the PTHrP and IL-8 gene expression levels were not obviously changed (Fig. 5b). These results showed that GluOC facilitates the osteolytic potency of MDA-MB-231 cells.
Fig. 5.
GluOC increases the levels of PTHrP and IL-8 in MDA-MB-231 cells. a qRT–PCR was used to detect the PTHrP and IL-8 gene levels in MDA-MB-231 cells. b qRT–PCR was used to detect the levels of PTHrP and IL-8 in MCF7 cells. The results represent at least three independent experiments, and the data are presented as the mean ± SD (n = 3). *P < 0.05 and **P < 0.01, vs. the control group (con = control)
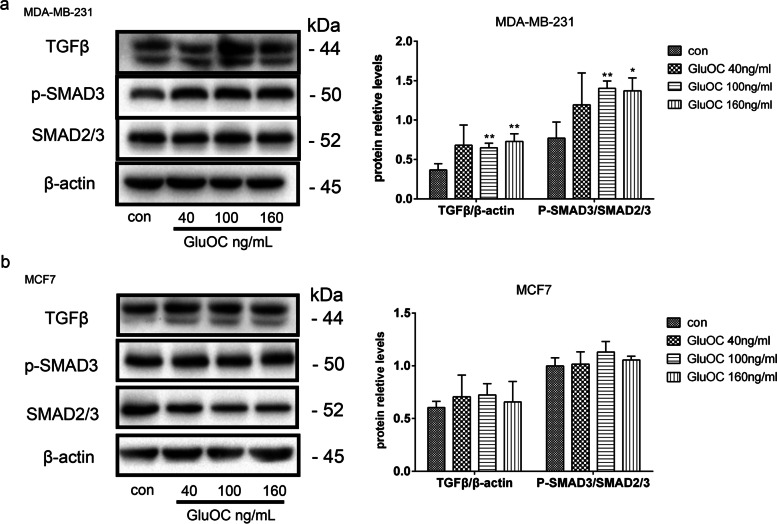
GluOC promotes the TGF-β/ SMAD3 signaling pathway
The TGF-β/SMAD3 pathway plays an indispensable role in tumor development [31]. Therefore, the effect of GluOC on the TGF-β/ SMAD3 pathway in MDA-MB-231 and MCF7 cells was detected by western blotting. When the GluOC concentrations were 100 ng/mL and 160 ng/mL, the total amount of SMAD2/3 in MDA-MB-231 cells was not affected, while the phosphorylation of SMAD3 was significantly increased (Fig. 6a). In addition, TGF-β protein levels after GluOC treatment were markedly increased compared with those in the control group (Fig. 6a). Conversely, in MCF7 cells, different concentrations of GluOC did not affect the phosphorylation of SMAD3, the total amount of SMAD2/3, or the protein level of TGF-β (Fig. 6b). Our study indicated that GluOC can effectively improve the expression of TGF-β/SMAD3 in MDA-MB-231 cells, while GluOC did not have effects on MCF7 breast cancer cells.
Fig. 6.
GluOC promotes the expression of TGF-β and p-SMAD3 in MDA-MB-231 cells. a Western blotting was used to analyze the levels of TGF-β, SMAD2/3, p-SMAD3 and β-actin in MDA-MB-231 cells. b Western blotting was used to analyze the levels of TGF-β, SMAD2/3, p-SMAD3 and β-actin in MCF7 breast cancer cells. The results represent at least three independent experiments, and the data are presented as the mean ± SD (n = 3). *P < 0.05 and **P < 0.01, vs. the control group (con = control)
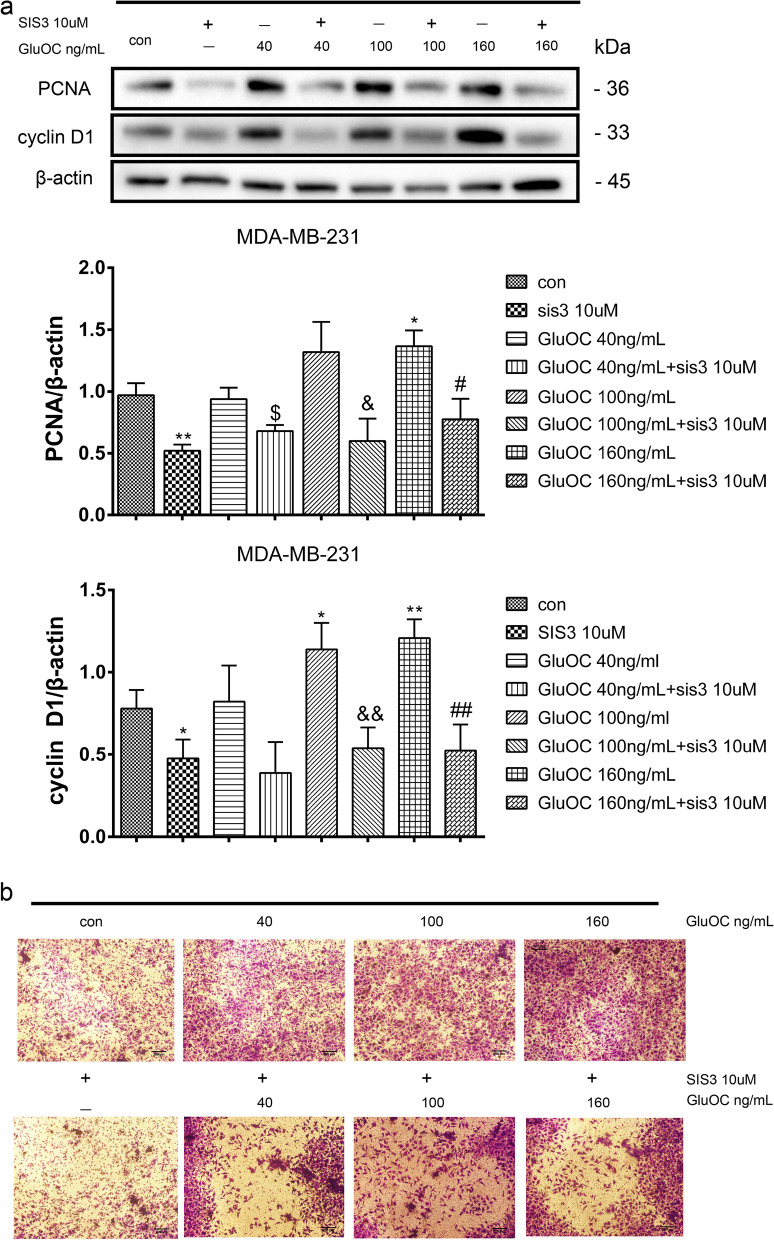
GluOC promotes the proliferation and migration of MDA-MB-231 cells through the TGF-β/ SMAD3 pathway
To further explore the function of SMAD3 in the promotion of the growth and migration of MDA-MB-231 cells by GluOC, the SMAD3 phosphorylation inhibitor SIS3 (10 μM) was used to treat MDA-MB-231 cells. Our western blot analysis revealed that SIS3 abrogated the GluOC-induced increase in PCNA and cyclin D1 in MDA-MB-231 cells (Fig. 7a). Additionally, the capacity of MDA-MB-231 cells to cross the cellulose membrane was reduced after the addition of SIS3 (Fig. 7b). These findings suggested that SIS3 inhibits the increased growth and migration of MDA-MB-231 cells induced by GluOC.
Fig. 7.
GluOC stimulates the growth and migration of MDA-MB-231 cells by activating the TGF-β/SMAD3 pathway. a After adding SIS3 and different concentrations of GluOC, we detected cyclin D1 and PCNA protein expression by western blotting. The results represent at least three independent experiments, and the data are presented as the mean ± SD (n = 3). *P < 0.05 and **P < 0.01, vs. the control group (con = control); $P < 0.05, $$P < 0.01 vs. the GluOC 40 ng/mL group; &P < 0.05, &&P < 0.01 vs. the GluOC 100 ng/mL group; #P < 0.05, ##P < 0.01 vs. the GluOC 160 ng/mL group. b After adding SIS3 and different concentrations of GluOC, we tested the migration capacity of MDA-MB-231 cells by Transwell assay. Photographs were taken with a microscope, with at least five field captured per treatment group, magnification × 100
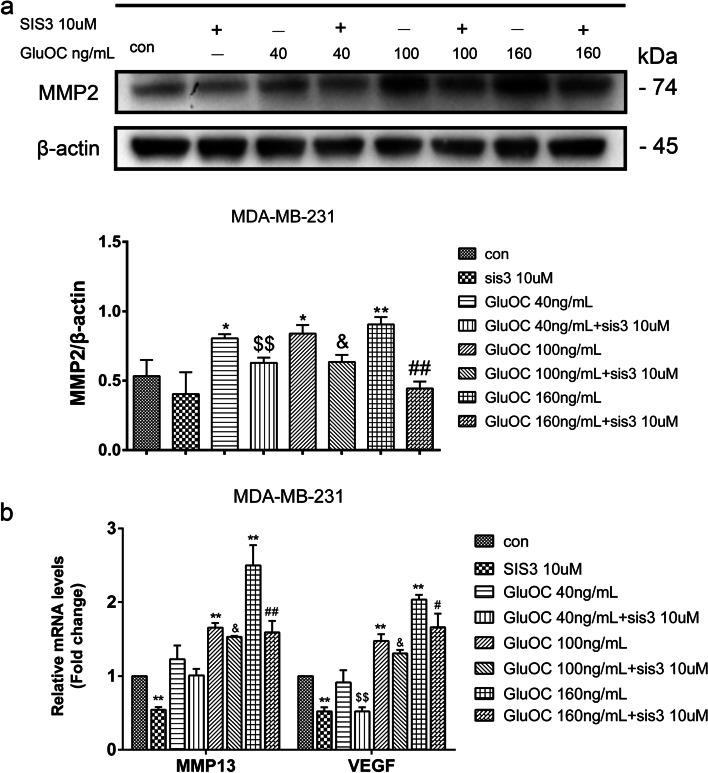
GluOC increases MMP2, MMP13 and VEGF in MDA-MB-231 cells through the TGF-β/SMAD3 pathway
Subsequently, the role of GluOC in promoting cancer cell migration via SMAD3 was further verified. After adding SIS3, the increased expression of MMP2 induced by GluOC was suppressed by SIS3. Additionally, SIS3 reversed the increase in the gene expression of MMP13 and VEGF induced by GluOC (Fig. 8a & b). These results further verified that GluOC increased the transfer of MDA-MB-231 cells via the TGF-β/SMAD3 pathway.
Fig. 8.
GluOC induces the expression of MMP2, MMP13 and VEGF in MDA-MB-231 cells by promoting phosphorylation of SMAD3. a Western blotting was used to detect the content of MMP2. The protein expression level was quantified densitometrically with ImageJ software. b GluOC promoted the expression of MMP13 and VEGF in MDA-MB-231 cells via the TGF-β/SMAD3 pathway. The results represent at least three independent experiments, and the data are presented as the mean ± SD (n = 3). *P < 0.05 and **P < 0.01, vs. the control group (con = control); $P < 0.05, $$P < 0.01 vs. the GluOC 40 ng/mL group; &P < 0.05, &&P < 0.01 vs. the GluOC 100 ng/mL group; #P < 0.05, ##P < 0.01 vs. the GluOC 160 ng/mL group
GluOC promotes EMT of MDA-MB-231 cells via phosphorylation of SMAD3
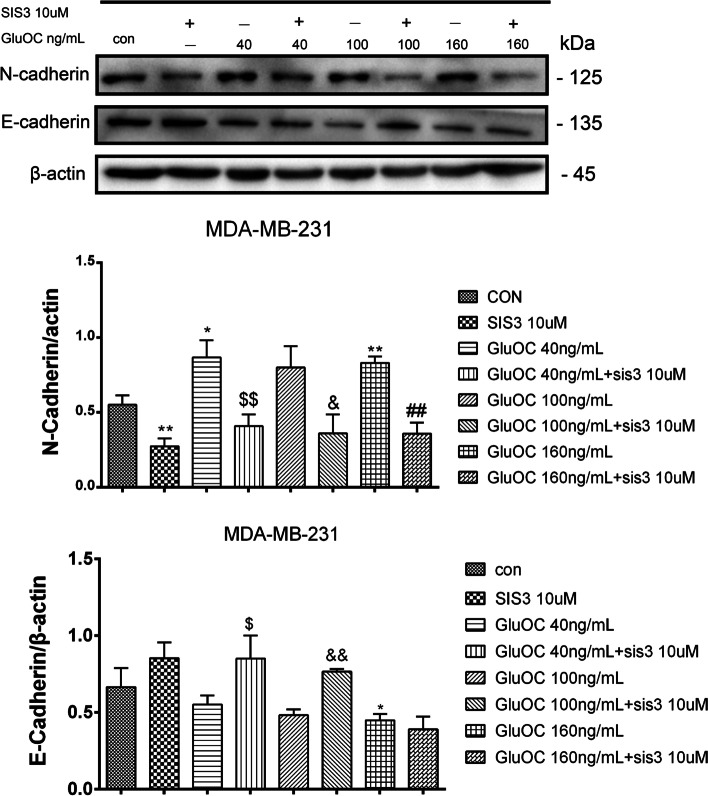
To further confirm whether GluOC stimulates EMT of MDA-MB-231 cells through the TGF-β/SMAD3 pathway, western blotting was used to detect related protein expression. The western blot results showed that SIS3 abrogated the GluOC-induced increase in the N-cadherin protein. Additionally, it was demonstrated that the addition of SIS3 reversed the inhibitory effects of GluOC on the E-cadherin protein (Fig. 9). These findings suggested that SIS3 inhibits the phosphorylation of SMAD3 and blocks the promoting effect of GluOC on MDA-MB-231 cell EMT.
Fig. 9.
GluOC promotes EMT of MDA-MB-231 cells via the TGF-β/SMAD3 pathway. Western blotting was used to analyze the levels of N-cadherin and E-cadherin. The results represent at least three independent experiments, and the data are presented as the mean ± SD (n = 3). *P < 0.05 and **P < 0.01, vs. the control group (con = control); $P < 0.05, $$P < 0.01 vs. the GluOC 40 ng/mL group; &P < 0.05, &&P < 0.01 vs. the GluOC 100 ng/mL group; #P < 0.05, ##P < 0.01 vs. the GluOC 160 ng/mL group
GluOC promotes the expression of PTHrP and IL-8 in MDA-MB-231 cells via phosphorylation of SMAD3
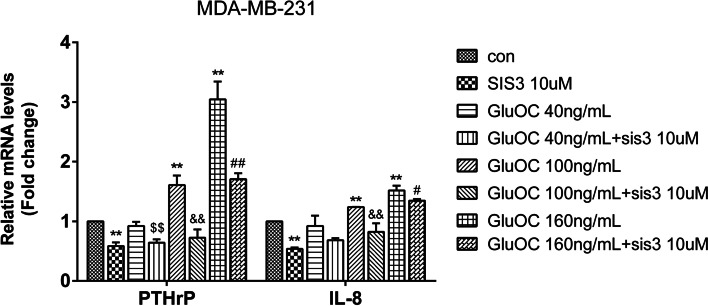
We next explored whether GluOC in MDA-MB-231 cells induces osteolytic destruction via the TGF-β/SMAD3 signaling pathway. The results showed that GluOC increased the mRNA expression of PTHrP and IL-8 in cells. However, the addition of SIS3 attenuated this effect and reversed the increase in PTHrP and IL-8 induced by GluOC (Fig. 10). The study showed that GluOC increases the osteolytic properties of MDA-MB-231 cells by activating the TGF-β/SMAD3 signaling pathway.
Fig. 10.
GluOC promotes the expression of PTHrP and IL-8 in MDA-MB-231 cells by activating the TGF-β/SMAD3 pathway. After SIS3 and GluOC were added, the expression of the PTHrP and IL-8 genes was detected by qRT–PCR. The results represent at least three independent experiments, and the data are presented as the mean ± SD (n = 3). *P < 0.05 and **P < 0.01, vs. the control group (con = control); $P < 0.05, $$P < 0.01 vs. the GluOC 40 ng/mL group; &P < 0.05, &&P < 0.01 vs. the GluOC 100 ng/mL group; #P < 0.05, ##P < 0.01 vs. the GluOC 160 ng/mL group. IL8: GluOC, uncarboxylated osteocalcin; IL-8: interleukin-8; PTHrP: parathyroid hormone related protein
Discussion
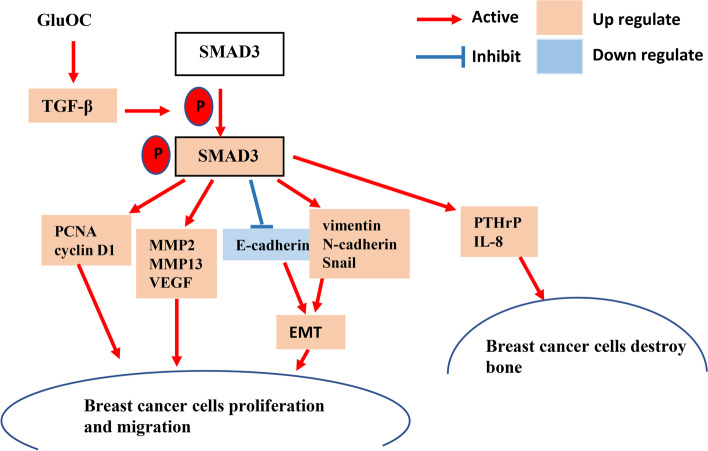
According to literature reports, serum osteocalcin levels in patients with breast cancer and breast cancer bone metastasis are significantly increased [24]. However, the mode of action is still unclear. Our study shows that GluOC facilitates the growth and migration of MDA-MB-231 cells through the TGF-β/SMAD3 pathway and promotes the expression of the PTHrP and IL8 genes, which suggests that GluOC may be related to TNBC osteolytic bone metastasis. (Fig. 11).
Fig. 11.
Model of GluOC functions in the growth and migration of MDA-MB-231 cells. By stimulating the TGF-β/SMAD3 pathway, GluOC accelerates the growth and migration of MDA-MB-231 cells
Breast cancer is the leading cause of death in females worldwide, and metastasis leads to a high mortality rate [32, 33]. Cancer tends to spread to the bone, and the most common form of metastasis in breast cancer is osteolytic metastasis [34]. TNBC is highly metastatic and is characterized by ER, PR and HER2 deficiency. TNBC accounts for nearly 20% of all breast cancers, carries a death rate of 83%, and is on the rise globally. TNBC is the most challenging subtype to treat due to its lack of a specific target [35]. MDA-MB-231 is a TNBC cell line that can cause lytic bone destruction [36]. MCF-7 is a noninvasive cell line that is ER-positive and normally characterized by low metastatic potential [37]. Therefore, in this study, MDA-MB-231 and MCF7 cells were employed.
Osteocalcin is a small noncollagen protein that is mainly produced by osteoblasts and plays a role in regulating glucose and lipid metabolism [38]. Recent research has found that GluOC is related to the occurrence and progression of cancer [20]. GluOC has been found to facilitate the growth and migration of lung tumors through the aggregation of neutrophils [39]. GluOC has also been found to induce the growth of prostate cancer cells through GPRC6A [40]. Studies have demonstrated that osteocalcin has a tumor-promoting effect in pancreatic ductal carcinoma and osteosarcoma [22, 23]. An increasing number of studies have confirmed that serum osteocalcin levels are significantly increased in breast cancer patients with bone metastasis [41, 42]. However, whether GluOC has an effect on breast cancer cells remains unclear. This research showed that GluOC promotes the viability and growth of MDA-MB-231cells. GluOC enhanced the viability of MDA-MB-231 cells and increased the levels of PCNA and cyclin D1. Our results suggest that GluOC can induce the proliferation of MDA-MB-231 cells but has no obvious influence on MCF7 cells.
The metastasis of breast cancer cells involves multiple steps: growth in situ, degradation of extracellular matrix, EMT, angiogenesis, and subsequent migration into the blood circulation to reach target organs. MMPs are zinc-dependent neutral endopeptidases that can selectively degrade ECM and are related to cancer invasion and metastasis [43]. MMP2 is elevated in many tumors, and its increase promotes the growth and migration of malignant cancer cells [44]. In breast cancer, increased MMP13 is related to bone metastasis [30]. Angiogenesis is another necessary factor for cancer metastasis. VEGF is responsible for regulating angiogenesis, and angiogenesis is a determinant of growth and metastasis in breast cancer [45]. The impact of GluOC on the metastasis of MDA-MB-231 and MCF7 cells was investigated. The results showed that GluOC improved the wound healing ability and ability to penetrate a cellulose membrane of MDA-MB-231 cells but had no obvious effect on MCF7 cells. Then, we detected the expression of MMP2 protein by western blot. In concordance with the cell migration results, GluOC promoted the expression of MMP2 in MDA-MB-231 cells but did not induce the expression of MMP2 in MCF7 cells. Subsequently, we detected the gene expression levels of MMP13 and VEGF by qRT–PCR. The results showed that GluOC enhanced the expression of the MMP13 and VEGF genes in MDA-MB-231 cells but did not influence them in MCF7 cells. Our results indicate that GluOC strengthens the migration ability of MDA-MB-231 cells but does not significantly affect MCF7 breast cancer cells.
During metastasis, breast cancer cells undergo EMT, which promotes invasion of surrounding tissues by cancer cells from the primary tumor site. A decrease in N-cadherin, vimentin and Snail expression and an increase in E-cadherin protein content promote the EMT process and increase the invasion and metastasis of cancer cells [46]. We also found that after adding GluOC, the N-cadherin, vimentin and Snail protein levels in MDA-MB-231 cells were significantly increased, and the E-cadherin level was significantly decreased. In summary, GluOC promotes EMT of MDA-MB-231 cells but does not significantly affect MCF7 breast cancer cells.
Osteolytic metastasis is the most common form of breast tumor metastasis. Breast tumors generate molecules that activate osteoclast bone absorption when they invade the microenvironment of bone tissue [47]. PTHrP is highly increased in breast cancer cells and is related to the growth and metastasis of cancer cells [48]. PTHrP also stimulates the differentiation and activation of osteoclasts [49]. IL-8 is increased in diverse cancer cells with different metastatic potentials and is related to the enhancement of breast cancer cell metastasis to bone [50]. Moreover, IL-8 facilitates osteoclastogenesis and injury of bone in metastatic bone diseases [51]. Based on these previous findings, the mRNA expression of PTHrP and IL-8 in MDA-MB-231 and MCF7 cells was detected. Our study showed that GluOC significantly increased the amount of PTHrP and IL-8 in MDA-MB-231 cells, but the content of PTHrP and IL-8 in MCF7 cells did not change significantly. This result suggests that GluOC may be related to the osteolytic properties of MDA-MB-231 cells.
Activation of diverse signaling pathways leads to breast cancer cell proliferation and metastasis. TGF-β is a secreted cytokine that can regulate tumor cell proliferation and migration. The TGF-β signaling network induces the key effector molecules SMAD2, SMAD3, and SMAD4. SMAD3, as an intermediate in the typical TGF-β signaling pathway, has an indispensable influence on TGF-β mediated transcriptional regulation [52]. SMAD3 can also regulate N-cadherin, E-cadherin, vimentin and Snail [53], thus affecting the EMT process. SMAD3 can also affect the proliferation and metastasis of cancer cells [54]. The results of Lian GY et al. showed that TGF-β can increase MMP2, MMP13, and VEGF to promote tumor invasion and metastasis [55–57]. There is also evidence that TGF-β/SMAD3 can regulate IL-8 and PTHrP, which can stimulate bone metastasis of breast cancer cells [58, 59]. Our results showed that GluOC stimulates TGF-β expression and SMAD3 phosphorylation in MDA-MB-231 cells. After adding SIS3, the increased proliferation of MDA-MB-231 cells induced by GluOC was abrogated. It appears that SIS3 blocks GluOC's promotion of the cyclin D1 and PCNA proteins. SIS3 also blocked the migration of MDA-MB-231 cells. First, SIS3 inhibited the promotion of the expression of MMP2, MMP13 and VEGF induced by GluOC in tumor cells. Second, SIS3 eliminated the increase in N-cadherin and the decrease in E-cadherin induced by GluOC in MDA-MB-231 cells. This result indicates that SIS3 weakened the promotion of MDA-MB-231 cell EMT induced by GluOC. Finally, the addition of SIS3 attenuated the increase in PTHrP and IL-8 mRNA expression induced by GluOC. GluOC may affect the osteolytic process of MDA-MB-231 cells via the TGF-β/SMAD3 pathway. These results further confirm that GluOC stimulates the growth and migration of MDA-MB-231 cells via the TGF-β/SMAD3 pathway.
Conclusions
In conclusion, our research clarified for the first time that GluOC induces the proliferation and metastasis of MDA-MB-231 cells through the TGF-β/SMAD3 signaling pathway. In addition, GluOC also stimulated the expression of the IL-8 and PTHrP genes in MDA-MB-231 breast cancer cells, both of which can act as osteolytic factors. The results indicate that GluOC may be a novel therapeutic target that can be used to prevent or treat TNBC bone metastasis in the clinic. All the results of the present study are from cell-level experiments. As such, the effect of GluOC in animals is still unclear, and further experimental verification in vivo is needed.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- GluOC
Uncarboxylated osteocalcin
- BM
Bone metastasis
- SRE
Skeletal -related events
- PCNA
Proliferating cell nuclear antigen
- TNBC
Triple-negative breast cancer
- ER
Estrogen receptor
- PR
Progesterone receptor
- HER2
Human epidermal growth factor receptor 2
- MMPs
Matrix metalloproteinases
- MMP2
Matrix metallopeptidase 2
- MMP13
Matrix metalloproteinase13
- IL8
Interleukin-8
- VEGF
Vascular endothelial growth factor
- PTHrP
Parathyroid hormone related protein
- ECM
Extracellular matrix
Authors’ contributions
JX, LM, DW, and JY provided ideas and the design for the study. LM and DW summarized the known functions of osteocalcin. JX performed the experiments and wrote the manuscript. JX and JY edited the manuscript. All authors have read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
All analyses and results we obtained from the experiments are included in this article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fahad Ullah M. breast cancer: current perspectives on the disease status. Adv Exp Med Biol. 2019;1152:51–64. doi: 10.1007/978-3-030-20301-6_4. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Vagia E, Mahalingam D, Cristofanilli M. The landscape of targeted therapies in TNBC. Cancers (Basel) 2020;12(4):916. doi: 10.3390/cancers12040916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Knott A, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med. 2013;19(11):1450–1464. doi: 10.1038/nm.3391. [DOI] [PubMed] [Google Scholar]
- 6.Demirkan B. The roles of Epithelial-to-Mesenchymal Transition (EMT) and Mesenchymal-to-Epithelial Transition (MET) in breast cancer bone metastasis: potential targets for prevention and treatment. J Clin Med. 2013;2(4):264–282. doi: 10.3390/jcm2040264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chowdhury K, Sharma A, Sharma T, Kumar S, Mandal CC. Simvastatin and MBCD inhibit breast cancer-induced osteoclast activity by targeting osteoclastogenic factors. Cancer Invest. 2017;35(6):403–413. doi: 10.1080/07357907.2017.1309548. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Wang L, Xia B, Yang C, Lai H, Chen X. BSP gene silencing inhibits migration, invasion, and bone metastasis of MDA-MB-231BO human breast cancer cells. PLoS One. 2013;8(5):e62936. doi: 10.1371/journal.pone.0062936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu S, Wu J, Bao Q, Yao B, Duan R, Chen X, Li L, Yuan H, Jin Y, Ma C. Osterix promotes the migration and angiogenesis of breast cancer by upregulation of S100A4 expression. J Cell Mol Med. 2019;23(2):1116–1127. doi: 10.1111/jcmm.14012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massague J. TGFbeta in Cancer. Cell. 2008;134(2):215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie F, Ling L, van Dam H, Zhou F, Zhang L. TGF-beta signaling in cancer metastasis. Acta Biochim Biophys Sin (Shanghai) 2018;50(1):121–132. doi: 10.1093/abbs/gmx123. [DOI] [PubMed] [Google Scholar]
- 12.Medina MA, Oza G, Sharma A, Arriaga LG, Hernandez Hernandez JM, Rotello VM, Ramirez JT. Triple-negative breast cancer: a review of conventional and advanced therapeutic strategies. Int J Environ Res Public Health. 2020;17(6):2078. doi: 10.3390/ijerph17062078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu Z, Gupta J, Zhang Z, Gerseny H, Berg A, Chen YJ, Zhang Z, Du H, Brendler CB, Xiao X, et al. Systemic delivery of oncolytic adenoviruses targeting transforming growth factor-beta inhibits established bone metastasis in a prostate cancer mouse model. Hum Gene Ther. 2012;23(8):871–882. doi: 10.1089/hum.2012.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakonen SM, Selander KS, Chirgwin JM, Yin JJ, Burns S, Rankin WA, Grubbs BG, Dallas M, Cui Y, Guise TA. Transforming growth factor-beta stimulates parathyroid hormone-related protein and osteolytic metastases via Smad and mitogen-activated protein kinase signaling pathways. J Biol Chem. 2002;277(27):24571–24578. doi: 10.1074/jbc.M202561200. [DOI] [PubMed] [Google Scholar]
- 15.Brewster AM, Chavez-MacGregor M, Brown P. Epidemiology, biology, and treatment of triple-negative breast cancer in women of African ancestry. Lancet Oncol. 2014;15(13):e625–e634. doi: 10.1016/S1470-2045(14)70364-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69(3):990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 17.Poser JW, Esch FS, Ling NC, Price PA. Isolation and sequence of the vitamin K-dependent protein from human bone. Undercarboxylation of the first glutamic acid residue. J Biol Chem. 1980;255(18):8685–8691. doi: 10.1016/S0021-9258(18)43554-5. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Li J, Liao J, Hu Y, Zhang H, Yang X, Wang Q, Mo Z, Cheng J. potential protective effect of Osteocalcin in middle-aged men with Erectile Dysfunction: evidence from the FAMHES project. Sci Rep. 2018;8(1):6721–6721. doi: 10.1038/s41598-018-25011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zoch ML, Clemens TL, Riddle RC. New insights into the biology of osteocalcin. Bone. 2016;82:42–49. doi: 10.1016/j.bone.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moser SC, van der Eerden BCJ. Osteocalcin-a versatile bone-derived hormone. Front Endocrinol (Lausanne) 2018;9:794. doi: 10.3389/fendo.2018.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye R, Pi M, Cox JV, Nishimoto SK, Quarles LD. CRISPR/Cas9 targeting of GPRC6A suppresses prostate cancer tumorigenesis in a human xenograft model. J Exp Clin Cancer Res. 2017;36(1):90–90. doi: 10.1186/s13046-017-0561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kayed H, Bekasi S, Keleg S, Michalski CW, Giese T, Friess H, Kleeff J. BGLAP is expressed in pancreatic cancer cells and increases their growth and invasion. Mol Cancer. 2007;6:83. doi: 10.1186/1476-4598-6-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KH, Lee KJ, Kim TY, Hutomo F, Sun HJ, Cheon GJ, Park SI, Cho SW, Im SA. Circulating Osteocalcin-positive cells as a novel diagnostic biomarker for bone metastasis in breast cancer patients. J Bone Miner Res. 2020;35(10):1838–1849. doi: 10.1002/jbmr.4041. [DOI] [PubMed] [Google Scholar]
- 24.Salem AM, Zohny SF, Abd El-Wahab MM, Hamdy R. Predictive value of osteocalcin and beta-CrossLaps in metastatic breast cancer. Clin Biochem. 2007;40(16–17):1201–1208. doi: 10.1016/j.clinbiochem.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 25.van de Merbel AF, van der Horst G, Buijs JT, van der Pluijm G. Protocols for migration and invasion studies in prostate cancer. Methods Mol Biol. 2018;1786:67–79. doi: 10.1007/978-1-4939-7845-8_4. [DOI] [PubMed] [Google Scholar]
- 26.Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expression. Mol Pharmacol. 2006;69(2):597–607. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- 27.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 28.Jiang X, Wang J, Deng X, Xiong F, Zhang S, Gong Z, Li X, Cao K, Deng H, He Y, et al. The role of microenvironment in tumor angiogenesis. J Exp Clin Cancer Res. 2020;39(1):204. doi: 10.1186/s13046-020-01709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao Y, Baker D, Ten Dijke P. TGF-beta-mediated Epithelial-Mesenchymal transition and cancer metastasis. Int J Mol Sci. 2019;20(11):2767. doi: 10.3390/ijms20112767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao B, Wang J, Qu S, Liu Y, Jin Y, Lu J, Bao Q, Li L, Yuan H, Ma C. Upregulated osterix promotes invasion and bone metastasis and predicts for a poor prognosis in breast cancer. Cell Death Dis. 2019;10(1):28. doi: 10.1038/s41419-018-1269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miret N, Pontillo C, Ventura C, Carozzo A, Chiappini F, de Pisarev DK, Fernández N, Cocca C, Randi A. Hexachlorobenzene modulates the crosstalk between the aryl hydrocarbon receptor and transforming growth factor-β1 signaling, enhancing human breast cancer cell migration and invasion. Toxicology. 2016;366–367:20–31. doi: 10.1016/j.tox.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Kang Y. Emerging therapeutic targets in metastatic progression: a focus on breast cancer. Pharmacol Ther. 2016;161:79–96. doi: 10.1016/j.pharmthera.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ibrahim MF, Mazzarello S, Shorr R, Vandermeer L, Jacobs C, Hilton J, Hutton B, Clemons M. Should de-escalation of bone-targeting agents be standard of care for patients with bone metastases from breast cancer? a systematic review and meta-analysis. Ann Oncol. 2015;26(11):2205–2213. doi: 10.1093/annonc/mdv284. [DOI] [PubMed] [Google Scholar]
- 35.Deepak KGK, Vempati R, Nagaraju GP, Dasari VR. S N, Rao DN, Malla RR: Tumor microenvironment: challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol Res. 2020;153:104683. doi: 10.1016/j.phrs.2020.104683. [DOI] [PubMed] [Google Scholar]
- 36.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3(6):537–549. doi: 10.1016/S1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 37.Maistrelli GL, Vaughan PA, Evans DC, Barrington TW. Lumbar disc herniation in the elderly. Spine (Phila Pa 1976) 1987;12(1):63–66. doi: 10.1097/00007632-198701000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Schatz M, Saravanan S, d’Adesky ND, Bramlett H, Perez-Pinzon MA, Raval AP. Osteocalcin, ovarian senescence, and brain health. Front Neuroendocrinol. 2020;59:100861. [DOI] [PubMed]
- 39.Engblom C, Pfirschke C, Zilionis R, Da Silva Martins J, Bos SA, Courties G, Rickelt S, Severe N, Baryawno N, Faget J, et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecF(high) neutrophils. Science. 2017;358(6367):eaal5081. doi: 10.1126/science.aal5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pi M, Quarles LD. GPRC6A regulates prostate cancer progression. Prostate. 2012;72(4):399–409. doi: 10.1002/pros.21442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pietschmann P, Zielinski C, Woloszczuk W. Serum osteocalcin levels in breast cancer patients. J Cancer Res Clin Oncol. 1989;115(5):456–458. doi: 10.1007/BF00393337. [DOI] [PubMed] [Google Scholar]
- 42.Shimozuma K, Sonoo H, Fukunaga M, Ichihara K, Aoyama T, Tanaka K. Biochemical markers of bone turnover in breast cancer patients with bone metastases: a preliminary report. Jpn J Clin Oncol. 1999;29(1):16–22. doi: 10.1093/jjco/29.1.16. [DOI] [PubMed] [Google Scholar]
- 43.Bogenrieder T, Herlyn M. Axis of evil: molecular mechanisms of cancer metastasis. Oncogene. 2003;22(42):6524–6536. doi: 10.1038/sj.onc.1206757. [DOI] [PubMed] [Google Scholar]
- 44.Ren F, Tang R, Zhang X, Madushi WM, Luo D, Dang Y, Li Z, Wei K, Chen G. Overexpression of MMP family members functions as prognostic biomarker for breast cancer patients: a systematic review and meta-analysis. PLoS One. 2015;10(8):e0135544. doi: 10.1371/journal.pone.0135544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bando H. Vascular endothelial growth factor and bevacitumab in breast cancer. Breast Cancer. 2007;14(2):163–173. doi: 10.2325/jbcs.968. [DOI] [PubMed] [Google Scholar]
- 46.Kar R, Jha NK, Jha SK, Sharma A, Dholpuria S, Asthana N, Chaurasiya K, Singh VK, Burgee S, Nand P. A “NOTCH” deeper into the Epithelial-To-Mesenchymal Transition (EMT) program in breast cancer. Genes (Basel). 2019;10(12):961. [DOI] [PMC free article] [PubMed]
- 47.Wang J, Rouse C, Jasper JS, Pendergast AM. ABL kinases promote breast cancer osteolytic metastasis by modulating tumor-bone interactions through TAZ and STAT5 signaling. Sci Signal. 2016;9(413):ra12. doi: 10.1126/scisignal.aad3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Assaker G, Camirand A, Abdulkarim B, Omeroglu A, Deschenes J, Joseph K, Noman ASM, Ramana Kumar AV, Kremer R, Sabri S. PTHrP, a biomarker for CNS metastasis in triple-negative breast cancer and selection for adjuvant chemotherapy in node-negative disease. JNCI Cancer Spectr. 2020;4(1):pkz063. doi: 10.1093/jncics/pkz063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamalakar A, Washam CL, Akel NS, Allen BJ, Williams DK, Swain FL, Leitzel K, Lipton A, Gaddy D, Suva LJ. PTHrP(12–48) modulates the bone marrow microenvironment and suppresses human osteoclast differentiation and lifespan. J Bone Miner Res. 2017;32(7):1421–1431. doi: 10.1002/jbmr.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bendre MS, Gaddy-Kurten D, Mon-Foote T, Akel NS, Skinner RA, Nicholas RW, Suva LJ. Expression of interleukin 8 and not parathyroid hormone-related protein by human breast cancer cells correlates with bone metastasis in vivo. Cancer Res. 2002;62(19):5571–5579. [PubMed] [Google Scholar]
- 51.Wang M, Xia F, Wei Y, Wei X. Molecular mechanisms and clinical management of cancer bone metastasis. Bone Res. 2020;8:30. doi: 10.1038/s41413-020-00105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Datto MB, Frederick JP, Pan L, Borton AJ, Zhuang Y, Wang XF. Targeted disruption of Smad3 reveals an essential role in transforming growth factor beta-mediated signal transduction. Mol Cell Biol. 1999;19(4):2495–2504. doi: 10.1128/MCB.19.4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo F, Parker Kerrigan BC, Yang D, Hu L, Shmulevich I, Sood AK, Xue F, Zhang W. Post-transcriptional regulatory network of epithelial-to-mesenchymal and mesenchymal-to-epithelial transitions. J Hematol Oncol. 2014;7:19. doi: 10.1186/1756-8722-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H, Yang K, Ren T, Huang Y, Tang X, Guo W. miR-16-5p inhibits chordoma cell proliferation, invasion and metastasis by targeting Smad3. Cell Death Dis. 2018;9(6):680. doi: 10.1038/s41419-018-0738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lian GY, Wang QM, Mak TS, Huang XR, Yu XQ, Lan HY. Inhibition of tumor invasion and metastasis by targeting TGF-beta-Smad-MMP2 pathway with Asiatic acid and Naringenin. Mol Ther Oncolytics. 2021;20:277–289. doi: 10.1016/j.omto.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simpson RW, Hirst GK. Temperature-sensitive mutants of influenza A virus: isolation of mutants and preliminary observations on genetic recombination and complementation. Virology. 1968;35(1):41–49. doi: 10.1016/0042-6822(68)90303-6. [DOI] [PubMed] [Google Scholar]
- 57.Seystahl K, Tritschler I, Szabo E, Tabatabai G, Weller M. Differential regulation of TGF-beta-induced, ALK-5-mediated VEGF release by SMAD2/3 versus SMAD1/5/8 signaling in glioblastoma. Neuro Oncol. 2015;17(2):254–265. doi: 10.1093/neuonc/nou218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta J, Robbins J, Jilling T, Seth P. TGFbeta-dependent induction of interleukin-11 and interleukin-8 involves SMAD and p38 MAPK pathways in breast tumor models with varied bone metastases potential. Cancer Biol Ther. 2011;11(3):311–316. doi: 10.4161/cbt.11.3.14096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhatia V, Cao Y, Ko TC, Falzon M. Parathyroid Hormone-Related Protein Interacts With the Transforming Growth Factor-beta/Bone Morphogenetic Protein-2/Gremlin Signaling Pathway to Regulate Proinflammatory and Profibrotic Mediators in Pancreatic Acinar and Stellate Cells. Pancreas. 2016;45(5):659–670. doi: 10.1097/MPA.0000000000000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All analyses and results we obtained from the experiments are included in this article.