Abstract
Background
Oropharyngeal dysphagia (OD) refers to any abnormality in the physiology of swallowing in the upper gastrointestinal tract, which leads to the related clinical complications, such as malnutrition, dehydration, and sever complication, such as aspiration pneumonia, suffocation, and eventually, premature death. The previous studies indicated a various range of prevalence of OD. The present systematic review and meta-analysis aimed to standardize the global prevalence of OD in different populations.
Methods
A systematic literature review was conducted using Embase, Scopus, PubMed, Web of Science (WoS) databases, and Google Scholar motor engine using related MeSH/Emtree and Free Text words, with no time limitation until November 2021. The heterogeneity among studies was quantified using I2 index and the random effects model was used, due to the high heterogeneity among the results of studies included in the meta-analysis.
Results
The systematic literature search retrieved 2092 studies. After excluding the irrelevant studies, ultimately 27 articles with a sample size of 9841 were included in the meta-analysis. After combining the studies, the overall estimate of the global prevalence rate of OD was 43.8% (95% CI 33.3–54.9%) and the highest prevalence rate was estimated in Africa with 64.2% (95% CI 53.2–73.9%). Given the subgroup analysis based on the study population, the highest prevalence of OD was related to Dementia with 72.4% (95% CI 26.7–95.0%). The results of meta-regression indicated that the prevalence of OD has an increasing trend with the enhancement of year of publication and mean age.
Conclusion
The results of the present systematic review and meta-analysis revealed that the prevalence of OD is high in different populations and its trend has been increasing in recent years. Therefore, the appropriate strategies should be applied to reduce the prevalence of OD by finding its causation and monitoring at all levels, as well as providing feedback to hospitals.
Keywords: Prevalence, Oropharyngeal dysphagia, Systematic review, Meta-analysis
Introduction
Swallowing is a process requiring the coordination of a complex series of motor, sensory, and psychological activities that are voluntary and involuntary, and most changes in its function occur with aging [1, 2]. Eating and drinking are essential for humans and dysphagia refers to swallowing difficulties [3]. There are different definitions for dysphagia. Given that the International Classification of Functioning, Disability, and Health (ICF) classifies swallowing as “the function of clearing food and drink through the oral cavity, pharynx, and oesophagus (gullet) with an appropriate rate”, dysphagia is defined as: the difficulty in transferring food from the mouth to the stomach [2, 3].
Dysphagia is classified into esophageal dysphagia and oropharyngeal dysphagia [4]. Oropharyngeal dysphagia refers to any abnormality in the physiology of swallowing in the upper gastrointestinal tract [5], including an imbalance in the coordination between the respiratory and nutritional functions [6], and leading to related clinical complications, such as malnutrition, dehydration, and some of the risk factors, such as aspiration pneumonia, asphyxiation, and eventually, premature death [7–9]. Some difficulties, such as loss of muscle mass, changes of the cervical spine, impaired dental status, and reduction of saliva production affect swallowing function. Thus, the risk of OD increases with age and the natural aging processes [10–12].
OD has a variety of causes, including aging, neurological diseases, such as Parkinson’s, dementia, multiple sclerosis, stroke, head and neck cancer, neck surgery, traumatic brain injury, and chronic obstructive pulmonary disease (COPD) [11, 13–15].
OD is associated with symptoms, such as painful swallowing (odynophagia), inability to swallow, sensation of food stuck in the throat or chest or behind the chest, saliva, sniff, reflux, frequent heartburn, acid or food reflux to the throat, unexpected weight loss, coughing or nausea when swallowing, and shrinking food or not eating certain foods, due to swallowing disorders [4–6, 11].
Initial assessments, including video fluoroscopy (VFS) and Fiberoptic Endoscopic Evaluation of Swallowing (FEES) are essential to minimize OD risks [16].
International data reported the prevalence of OD in the general populations between 2.3 and 16.0% [11]. Further, the prevalence of OD is high with predisposing conditions, such as aging and stroke. Its prevalence is reported 26.19% in the elderly [11], 8.1–80% in stroke patients [17], and 21.9–69.5% in patients taking antipsychotic drugs [18].
There are several preliminary studies on the prevalence of OD in different populations in different parts of the world, but these studies examine the prevalence in a small environment and have a smaller sample size. Also, the results of studies showed the different values of the prevalence of this disorder in different populations. None of these studies investigated the effect of potential factors, such as age and prevalence over time, so the present study aimed to standardizing the prevalence of OD in different populations by systematic review and meta-analysis.
Methods
The present study was conducted according to PRISMA guidelines, including identification, screening, eligibility, and included [19]. The searches, study selection, and data extraction were done independently by two researchers (Z.N. and M.K.) to minimize publication bias and error. Any conflict or disagreement between the two researchers was resolved by the consensus and consultation with a third researcher (F.R.) and the opinion of the third researcher was final.
Identification of studies
A systematic literature review was conducted using PubMed, Embase, Scopus, and Web of Science (WoS) databases, and Google Scholar motor engine to find out the relevant studies assessing the global prevalence rate of OD in different populations. The searches included the combinations of the following MeSH/Emtree and Free Text words: “Prevalen*”, “Oropharyngeal Dysphagia”, and “Dysphagia Oropharyngeal”. No time limitation was considered for the search to retrieve as comprehensive as possible related studies by November 2021. The references of all included articles and also the studies that cited to the included articles were manually reviewed to maximize the comprehensiveness of the search. Table 1 represents the search strategy of different databases.
Table 1.
Search strategies
| Database | Search strategy | Date | Number |
|---|---|---|---|
| PubMed | ((((Prevalence [MeSH Terms]) OR (Prevalen* [Title/Abstract])) OR (Prevalence* [Title/Abstract])) OR (Prevalent [Title/Abstract])) AND (("Oropharyngeal Dysphagia") OR ("Dysphagia, Oropharyngeal")) | 14 November 2021 | 171 |
| Scopus | (TITLE-ABS-KEY (Prevalence*) OR TITLE-ABS-KEY (Prevalence*) OR TITLE-ABS-KEY (Prevalent)) AND (ALL ("Dysphagia, Oropharyngeal") OR ALL ("Oropharyngeal Dysphagia")) | 16 November 2021 | 839 |
| WoS | TS=(Prevalence* OR Prevalence OR Prevalent) AND ALL=(“Oropharyngeal Dysphagia” OR “Dysphagia, Oropharyngeal”) | 16 November 2021 | 462 |
| Embase |
#1: 'prevalence*':ab,ti OR 'Prevalence*':ab,ti OR 'prevalent':ab,ti OR 'prevalence'/exp/mj #2: 'oropharyngeal dysphagia' #3: #1 AND #2 |
17 November 2021 | 370 |
| Google scholar | (Prevalence* OR Prevalence OR Prevalent) AND (“Oropharyngeal Dysphagia” OR “Dysphagia, Oropharyngeal”) | 18 November 2021 | 250 |
Inclusion criteria
The inclusion criteria were original scientific-research articles, observational studies, access to the full text of the article, and studies reported the prevalence rate of OD.
Exclusion criteria
The exclusion criteria included the irrelevant studies, cross-sectional studies, case reports, case series, papers presented at conferences, letter to the editor, qualitative studies, dissertations, systematic review and meta-analysis, animal studies, and lack of access to the full text of the articles.
Selection process of studies
All articles derived from various databases were imported into EndNote X8 software. After eliminating the duplicates, the title and abstract of the studies were thoroughly screened to excluded the irrelevant studies. The full text of remaining articles was carefully assessed for eligibility and irrelevant studies were removed. Finally, the quality assessment of the studies met inclusion criteria was done. Researchers extracted the articles without knowing the name of authors, institutes, and journals.
Qualitative evaluation of the studies
The quality assessment of studies was done using the Joanna Briggs Institute (JBI) checklist for prevalence studies [20], which consists of 9 different items, including sample frame, participants, sample size, study subjects and the setting described in detail, data analysis, valid methods for identifying conditions, measuring the situation, statistical analysis, and response rate adequate. The sources of bias were identified using the criteria that the reviewers qualified with answers, including yes, no, unclear, or not applicable. The sum of “yes” scores was calculated to evaluate each study. Therefore, the total score range based on the number of “yes” is between 0 and 9.
Data extraction
A pre-prepared electronic checklist was employed to extract the data. The items of this checklist included first author, year of publication, country, sample size, age, study design, diagnostic tools, prevalence rate, and quality assessment score.
Statistical analysis
The prevalence rate of OD was reviewed in this study and the frequency rate of OD, i.e., the frequency of patients suffered from OD was divided by the total number of subjects in each study to combine the results of different studies. The heterogeneity of studies was checked using I2 index and due to the high heterogeneity between the results of the studies included in the meta-analysis (I2 ˃ 75%), the random effects model was applied, which calculates the parameter changes between studies. Thus, the results of random effects model in heterogeneous conditions are more generalizable than those of fixed effect model. Funnel plot and Begg and Mazumdar rank correlation were used to assess the publication bias. In addition, meta-regression was used to examine the relationship between the global prevalence rate of OD and the year of publication, sample size, and mean age. The subgroup analysis was performed according to different continents (Asia, Europe, USA, Africa, and Australia), study population, and type of diagnostic tool. The comprehensive meta-analysis software (version 2) was applied for meta-analysis and P-value less than 0.05 was regarded as statistically significant.
Results
The summary of how studies included in the meta-analysis
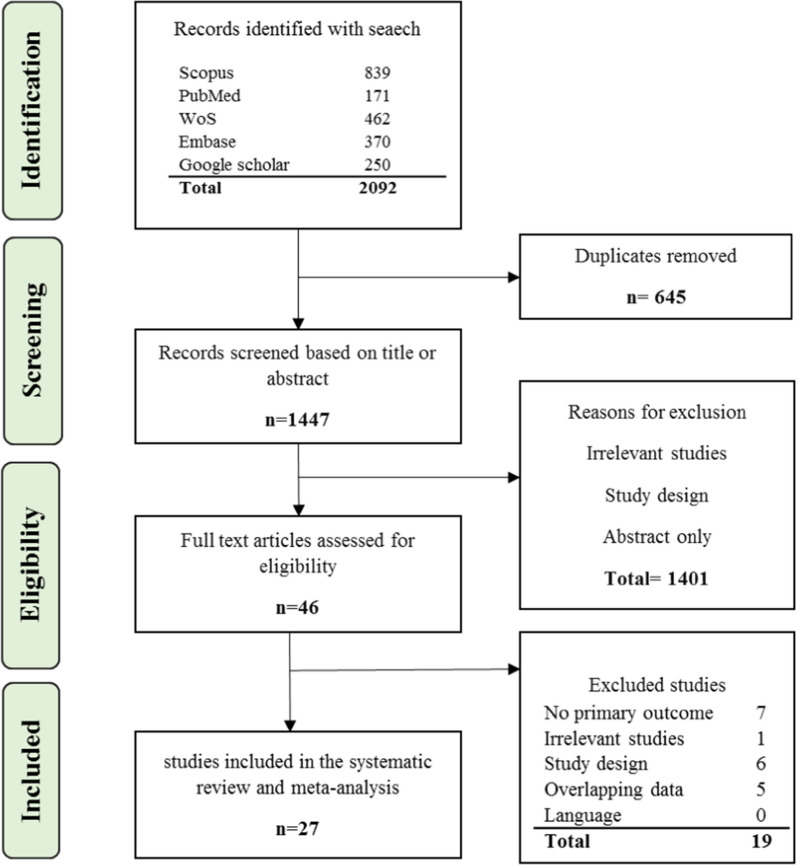
In the initial search, 2092 studies were identified. After eliminating 645 duplicates and studies with overlapping data, 1401 irrelevant studies were removed by screening the title and abstract. Then, full text of the remaining 46 studies were inspected carefully and 19 articles were excluded due to not meeting eligibility criteria. Finally, 27 articles met inclusion criteria were included in the meta-analysis. Figure 1 displays the PRISMA 2020 flow diagram.
Fig. 1.
PRISMA 2020 flow diagram for article selection
General characteristics of the studies
The total sample size was 9841. The oldest study was performed in 1991 and the most recent study in 2021. The highest number of studies was conducted in Spain with 7 articles. The maximum and minimum sample size was related to the study of David et al. [21] with 2973 subjects and the study of Almeida et al. [22] with 25, respectively. The diagnostic tool for OD in most studies was physical examination (12 articles) or volume–viscosity swallow test (10 articles). The highest quality assessment score based on the JBI checklist was related to the study of Wolf et al. [23] with a score of 9. Table 2 represents the characteristics of studies included in the systematic review and meta-analysis.
Table 2.
The characteristics of the studies included in the systematic review and meta-analysis
| First author, year, (References) | Country (continent) | Sample size (n) | Age (year) | Type of study | Diagnostic tool | Prevalence (%) | Population | Quality score | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | |||||||
| Wolf, 2020, [23] | Germany (Europe) | 200 | 69 | 131 | 84 ± 6.5 | Cross-sectional retrospective | Physical examination | 29.0 | – | – | Elderly | 9 |
| Ruth, 1991, [24] | Sweden (Europe) | 337 | – | – | 20–79 | Cross-sectional | Physical examination | 10.0 | – | – | General population | 4 |
| Cabre, 2010, [25] | Spain (Europe) | 134 | 80 | 54 | 84.51 ± 6.8 | Prospective cohort | Physical examination | 55.0 | – | – | Elderly patients with pneumonia | 5 |
| Melgaard, 2017, [26] | Denmark (Europe) | 154 | 84 | 70 | 80.90 ± 10.58 | Cross-sectional observational | Volume–viscosity swallow test | 34.42 | 47.61 | 17.14 | Elderly patients with community-acquired pneumonia | 7 |
| Michel, 2018, [27] | France (Europe) | 117 | 40 | 77 | 84.5 ± 5.1 | Prospective study | Volume–viscosity swallow test | 86.6 | 95.0 | 81.81 | Older patients with dementia | 7 |
| Elvira, 2020, [28] | Spain (Europe) | 255 | 98 | 157 | 83.5 ± 8 | Prospective longitudinal quasi-experimental | Volume–viscosity swallow test | 85.9 | 85.35 | 61.2 | Older patients with dementia | 8 |
| Holland, 2011, [29] | UK (Europe) | 637 | 149 | 488 | 89 (69–98) | Longitudinal study | Swallow questionnaire | 11.4 | – | – | Elderly | 5 |
| Garcı´a-Peris, 2007, [30] | Spain (Europe) | 87 | – | – | 58.2 ± 13.5 | Cross-sectional retrospective | Physical examination | 50.6 | – | – | Head and neck cancer | 8 |
| Rofes, 2018, [31] | Spain (Europe) | 395 | 211 | 184 | 73.2 ± 13.3 | Cohort | Volume–viscosity swallow test | 45.06 | 38.86 | 52.2 | Stroke | 7 |
| Mateos-Nozal, 2020, [32] | Spain (Europe) | 329 | 104 | 225 | 93.5 (81–106) | Observational prospective | Volume–viscosity swallow test | 82.4 | 87.5 | 70.8 | Elderly | 8 |
| Falsetti, 2009, [33] | Italy (Europe) | 151 | 77 | 74 | 79.4 ± 6.2 | Cross-sectional retrospective | Volume–viscosity swallow test | 26.5 | 40.25 | 12.16 | Stroke | 4 |
| Lendinez-Mesa, 2017, [34] | Spain (Europe) | 124 | 88 | 36 | 56.45 ± 12.35 | Cross-sectional | Physical examination | 79.3 | – | – | Stroke | 6 |
| Serra-Prat, 2012, [35] | Spain (Europe) | 254 | 136 | 118 | 77.4 ± 5.0 | Population-based prospective study | Volume–viscosity swallow test | 18.6 | 9.5 | 28.8 | Elderly | 6 |
| Hamdy, 2014, [36] | UK (Europe) | 180 | 93 | 87 | 74.2 ± 11.5 | Cross-sectional | Volume–viscosity swallow test | 41.7 | – | – | Stroke | 4 |
| Stefano, 2020, [37] | Italy (Europe) | 708 | 367 | 341 | 75.9 ± 8.6 | Cross-sectional retrospective | Physical examination | 32.7 | – | – | Older patients with dementia | 7 |
| Melgaard, 2018, [38] | Denmark (Europe) | 313 | 138 | 175 | 83.1 ± 7.8 | Cross-sectional observational | Volume–viscosity swallow test | 50.0 | 52.17 | 48.0 | Acute Geriatric Patients | 8 |
| Lindh, 2017, [39] | Sweden (Europe) | 51 | – | – | 48.3 ± 6.3 | Observational prospective | Physical examination | 49.0 | – | – | COPD | 7 |
| David, 2008, [21] | Australia (Australia) | 2973 | – | – | 49.4 (15–95) | Population-based prospective study | Physical examination | 7.3 | – | – | General population | 6 |
| Yang, 2013, [40] | Korea (Asia) | 415 | 195 | 220 | 77.3 ± 8.7 | Cohort | Swallow questionnaire | 33.7 | 39.5 | 28.6 | Elderly | 7 |
| Biglary, 2019, [41] | Iran (Asia) | 500 | – | – | 48.1 ± 7.5 | Cross-sectional study was a descriptive-analytic study | Physical examination | 17.39 | – | – | Neurological diseases and head and neck surgery | 5 |
| Costa, 2019, [42] | South African (African) | 81 | – | – | 11.7 ± 15.6 day | Prospective cross-sectional observational | Clinical feeding assessments | 64.2 | – | – | Neonates | 7 |
| Chiocca, 2005, [43] | Argentina (America) | 839 | 373 | 466 | 39.9 ± 15.4 | Cross-Sectional Observational | Physical examination | 29.6 | – | – | General population | 7 |
| Jacinto-Scudeiro, 2019, [44] | Brasil (America) | 36 | 6 | 30 | 34.7 ± 16.8 | Cross-sectional | Swallow questionnaire | 33.0 | – | – | Paraplegia | 5 |
| Delevatti, 2020, [45] | Brasil (America) | 229 | 49 | 180 | 77.90 ± 8.21 | Cross-sectional | Volume–viscosity swallow test | 58.0 | 49.0 | 60.5 | Older adults with orthopedic fractures | 6 |
| Almeida, 2015, [22] | Brasil (America) | 25 | – | – | 62 (44–80) | Descriptive retrospective | Physical examination | 96.0 | – | – | Stroke | 4 |
| Samantha, 2015, [46] | Colorado (America) | 206 | 9 | 187 | 32 (23–47) | Large retrospective review | Swallow questionnaire | 20.38 | 44.44 | 20.32 | Patients with severe anorexia nervosa | 5 |
| Benfer, 2018, [47] | USA (America) | 111 | 82 | 29 | 34.1 ± 11.9 month | Longitudinal population-based cohort | Physical examination | 79.7 | – | – | Children with cerebral palsy | 5 |
Meta-analysis of the global prevalence of OD
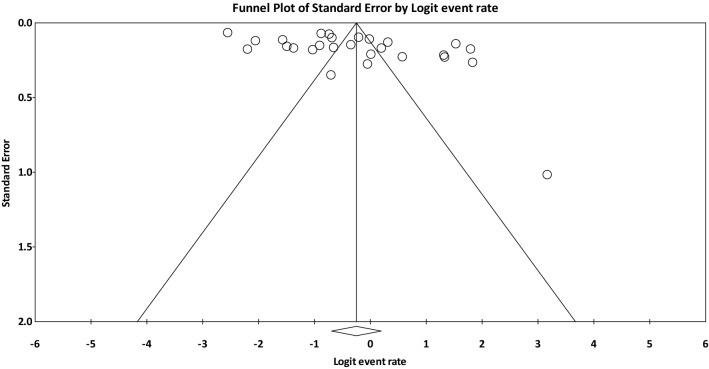
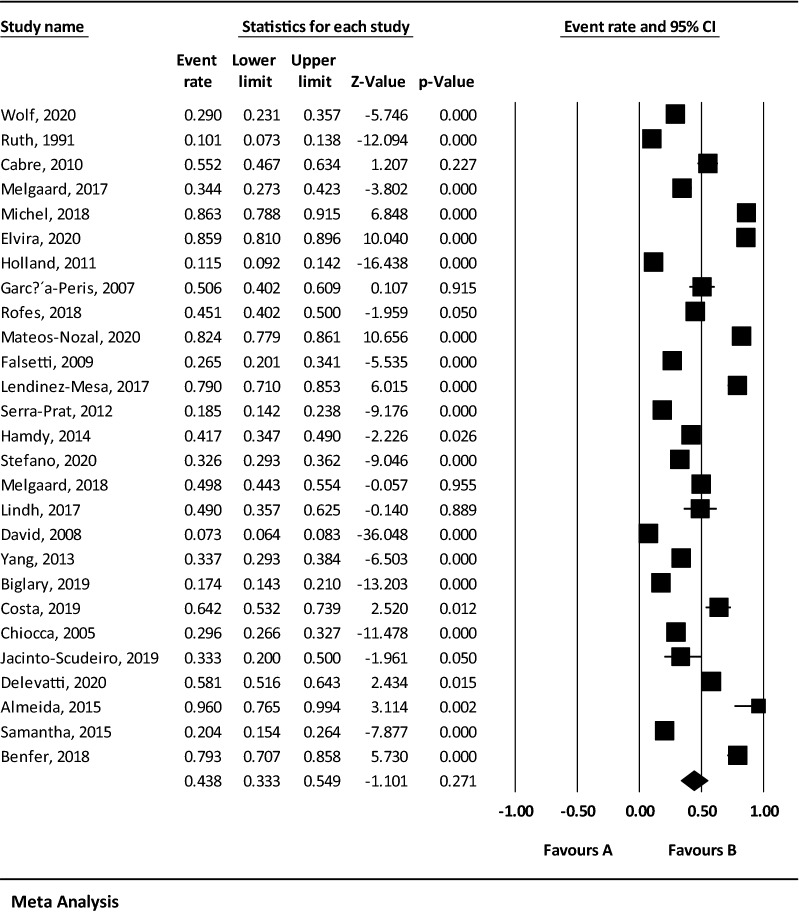
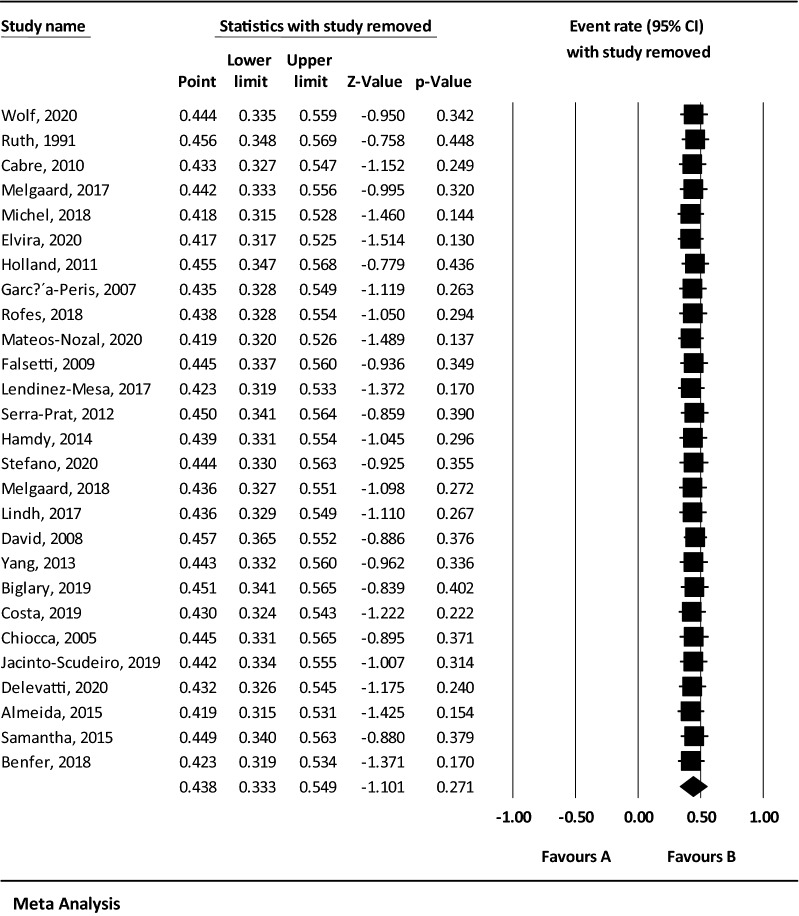
Considering that the result of I2 test for the global prevalence of OD indicated a significant heterogeneity among included studies (I2 = 98.60), the data were analyzed using a random effects model (Table 3). Based on the results of Begg and Mazumdar rank correlation, there was no publication bias at the level of 0.05 in the studies (P-valve = 0.103) (Fig. 2). As a result of combining the results of studies, the overall estimate of the global prevalence of OD was 43.8% (95% CI 33.3–54.9%) based on the random effects model. As shown in the Fig. 3, the black square represents the prevalence rate, the length of the line segment displays the 95% CI in each study, and the rhombus symbol illustrates the global prevalence rate of OD for all studies. The results of sensitivity analysis demonstrated that the pooled estimation did not change significantly by removing any of the studies (Fig. 4).
Table 3.
Reporting the results of fixed and random effects model on meta-analysis
| Model | Number studies | Point estimate | Lower limit | Upper limit | Z-value | P-value | Q-value | Df (Q) | P-value | I-squared | Tau squared | Standard error | Variance | Tau |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fixed | 27 | 0.316 | 0.305 | 0.327 | − 29.611 | 0.000 | 1859.987 | 26 | 0.000 | 98.602 | 1.339 | 0.501 | 0.251 | 1.157 |
| Random | 27 | 0.438 | 0.333 | 0.549 | − 1.101 | 0.271 |
Fig. 2.
The Funnel plot of the results of the overall estimation of the global prevalence of OD
Fig. 3.
The forest plot of the overall estimation of the global prevalence of OD based on the random effects model
Fig. 4.
The sensitivity analysis chart of the global prevalence of OD based on the random effects model
The meta-regression of the global prevalence of OD
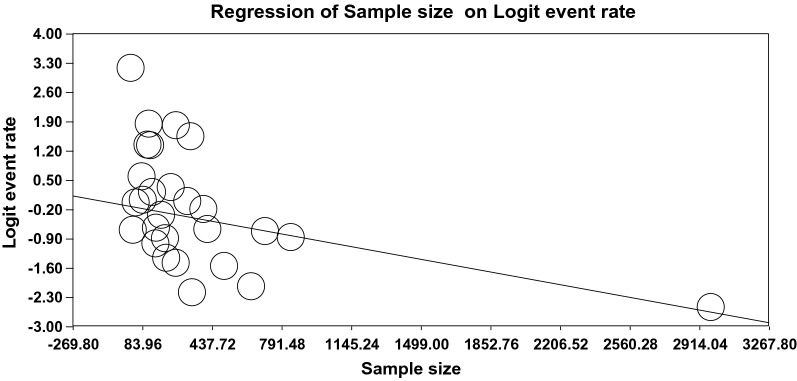
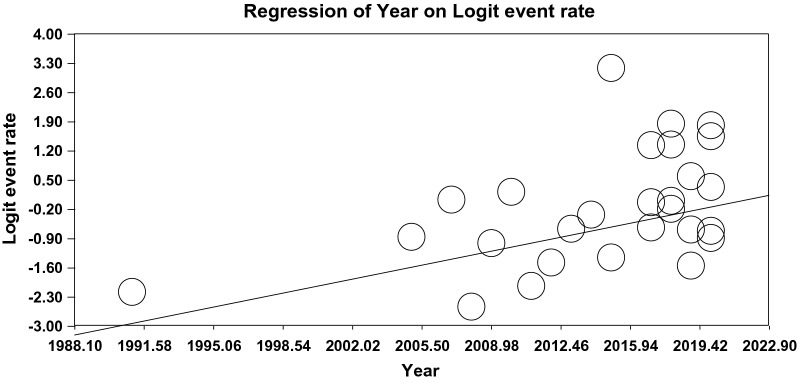
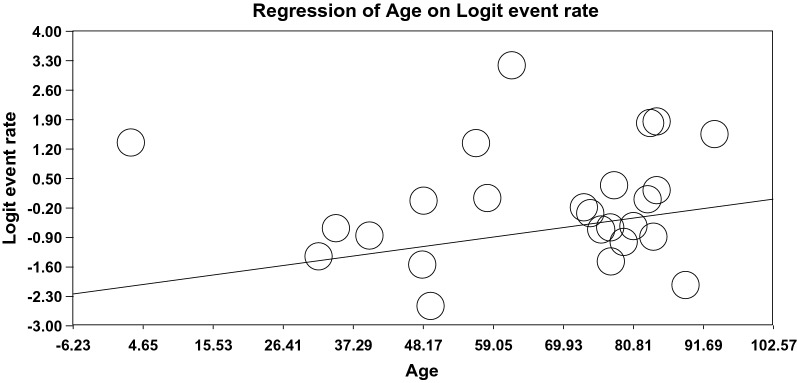
The relationship between the sample size (Fig. 5), year of the publication (Fig. 6), and mean age (Fig. 7) and the global prevalence of OD was assessed using meta-regression. The results indicated a significant difference between the global prevalence of OD and these potential factors (P < 0.001). Since the global prevalence of OD decreased by increasing sample size and this prevalence enhanced by increasing the year of the publication and mean age (Figs. 5, 6, 7).
Fig. 5.
The meta-regression of the relationship between sample size and the global prevalence of OD
Fig. 6.
The meta-regression of the relationship between the year of the publication and the global prevalence of OD
Fig. 7.
The meta-regression of the relationship between the mean age and the global prevalence of OD
Subgroup analysis
Given the high heterogeneity among the studies (I2 = 98.60), subgroup analysis was employed based on the continent, diagnostic tool, study population, and gender (Table 4). The results of the subgroup analysis illustrated that the highest prevalence rate of OD was related to the African continent with 64.2% (95% CI 53.2–73.9%), diagnostic tool of volume–viscosity swallow test with 54.4% (95% CI 39.2–68.8%), patients suffering from dementia with 72.4% (95% CI 26.7–95.0%), and men with 54.7% (95% CI 40.1–68.6%) (Table 4).
Table 4.
The subgroup analysis of estimating the prevalence rate of OD based on the continents, diagnostic tool, and study population
| Subgroups | Number of articles | Sample size | I2 | Begg and Mazumdar | Prevalence % (95% CI) |
|---|---|---|---|---|---|
| Continents | |||||
| Asia | 2 | 227 | 96.82 | – | 24.7 (95% CI 12.1–43.7) |
| Europe | 17 | 1777 | 98.12 | 0.433 | 45.7 (95% CI 33.3–58.5) |
| America | 6 | 1446 | 96.90 | 0.452 | 51.3 (95% CI 31.7–70.6) |
| African | 1 | 81 | 0.000 | – | 64.2 (95% CI 53.2–73.9) |
| Australia | 1 | 2973 | 0.000 | – | 7.3 (95% CI 6.4–8.3) |
| Diagnostic tools | |||||
| Physical examination | 12 | 6089 | 98.69 | 0.243 | 40.9 (95% CI 26.3–57.3) |
| Volume–viscosity swallow test | 10 | 1273 | 97.76 | 1.000 | 54.4 (95% CI 39.2–68.8) |
| Swallow questionnaire | 3 | 255 | 97.21 | 1.000 | 20.4 (95% CI 9.6–38.4) |
| Population | |||||
| Children | 2 | 192 | 81.10 | – | 72.3 (95% CI 55.5–84.6) |
| Adults | 8 | 4816 | 98.69 | 0.386 | 32.6 (95% CI 17.7–52.0) |
| Elderly | 11 | 1400 | 98.56 | 0.119 | 48.1 (95% CI 31.9–64.7) |
| General population | 3 | 4149 | 99.25 | 1.000 | 13.4 (95% CI 4.4–34.5) |
| Pneumonia | 2 | 288 | 91.92 | – | 44.6 (95% CI 25.8–65.0) |
| Dementia | 3 | 1080 | 99.10 | 1.000 | 72.4 (95% CI 26.7–95.0) |
| Head and neck cancer | 2 | 587 | 97.60 | – | 31.5 (95% CI 8.9–68.4) |
| Stroke | 5 | 875 | 94.96 | 0.806 | 55.4 (95% CI 37.2–72.2) |
| Gender | |||||
| Male | 11 | 1141 | 94.36 | 0.161 | 54.7 (95% CI 40.1–68.6) |
| Female | 11 | 1667 | 97.00 | 0.876 | 46.5 (95% CI 31.3–62.5) |
Discussion
The present systematic review and meta-analysis study aimed to estimate the global prevalence of OD in different populations. After combining the data from 27 articles, the global prevalence of OD was estimated to be 43.8%. The highest prevalence rate of OD (96%) was reported in the study of Almeida et al. [22] and the lowest rate (7.3%) in the study of Watson and Lally [21]. The highest quality assessment score based on JBI checklist criteria was related to the study of Wolf et al. [23], which reported the prevalence rate of OD as 29%.
Kertscher et al. reported the prevalence of OD in the Netherlands between 2.3 and 16% [11]. Further, the prevalence of OD was estimated between 8.1 and 80% in stroke patients, 11–81% in the Parkinson’s disease, 27–30% in the traumatic brain injury patients, and 91.7% in the community-acquired pneumonia in the systematic review study of Takizawa et al. [17]. The findings of the present study are not consistent with the results of the afore-mentioned systematic review or meta-analysis studies, which can be attributed to the high number of articles included in the present study (27 articles versus 6 articles in the study of Kertscher et al.). Further, the study of Kertscher et al. examined the studies conducted in the Netherlands while the present study included people with different races and geographies around the world, and the present study was conducted as a systematic review and meta-analysis, while the study of Takizawa et al. was done only systematically and they did not perform statistical analysis.
Considering the results of the meta-regression, the prevalence of OD showed an increasing trend by increasing the mean age. Additionally, the results of subgroup analysis demonstrated that the prevalence of OD is high in the elderly population. Kertscher et al. reported that the prevalence of OD in the population over 75 years old is more than other age groups [11], which is consistent with the results of the present study. Many physiological changes occur in body tissue with aging, such as muscle wasting, reduced endurance capacity, and muscle weakness [48, 49], hormonal changes and decreased ratio of anabolic to catabolic hormones [50], increased rates of neurological diseases [51–53], cardiovascular diseases [54], atrophy of the pharyngeal and laryngeal muscles [55], and many other chronic diseases. Considering these conditions in the treatment process and the improvement of the clinical outcomes of the elderly population can be helpful.
The results of the present study also showed that the prevalence of OD in the pediatric population is high. Although the number of studies investigated in the pediatric population was small (2 articles), the reasons for this could be abnormalities or dental problems, large tongue and tonsils, problems with prenatal development of cranial bones and structures of the mouth and throat (known as Craniofacial abnormalities), prenatal abnormalities of the gastrointestinal tract, such as esophageal atresia (esophageal obstruction) or tracheovasophageal fistula after prolonged exposure to a ventilator (which may occur in premature infants or very sick children), vocal cord paralysis, tracheostomy surgery, esophageal stimulation or ulceration due to gastric acid in gastroesophageal reflux disease. Esophageal obstruction by other body structures, such as enlarged heart, thyroid gland, blood vessels or lymph nodes, growth retardation, and prematurity of the baby [42, 47].
The results of subgroup analysis revealed that the prevalence of OD in patients with dementia is higher than that in other study population. Dementia is a chronic disease with a set of symptoms, such as memory impairment, language impairment, psychological changes, and behavioral disorders [56]. When dementia reaches its advanced stages, brain changes lead to the dysfunction of organs and physical activities, such as swallowing disorder, dysphagia, loss of balance, and incontinence [57, 58]. Dementia is a global challenge that directly affects 47.5 million people worldwide and 7.7 million people each year [59]. Taking into account these conditions and complications can assist the treatment process and improve the quality of life of patients with dementia.
Based on the results of the present study, the prevalence of OD is high in stroke, which is in line with the results of the systematic review study of Takizawa et al. [17]. Meng et al. reported that the prevalence of swallowing disorders was 36.3% (95% CI 33.3–39.3%) in patients with stroke [60]. Stroke is a sudden neurological disorder, resulting in impaired blood flow to the area affected by the stroke. In other words, when blood flow to a part of the brain is disrupted and stopped, that part can no longer function normally [61, 62]. The post-stroke complications, depending on the location of the stroke and the extent of brain tissue affected [61], can be vision problems, memory problems, dysphagia (paralysis of the muscles of the pharynx, tongue or mouth), lack of coordination between the eyes and hands, difficulty in decision making, lack of body temperature control, difficulty breathing, urinary and fecal incontinence, nervous system problems, tromboemboli, heart failure, depression, etc. [63–66]. Therefore, it is recommended that health care providers and policy makers pay more attention to the stroke prevention and post-stroke complications, especially OD.
Due to the variation of the population structure in different countries of the world, it was necessary to carefully study the prevalence of OD in different continents in order for planners to pay more attention to the process and its consequences. Therefore, according to the subgroup analyses based on the different continents, the highest prevalence of OD was related to the African continent with 64.2% and the lowest was related to Australia with 7.3%.
The high prevalence of OD in different populations, especially in the elderly and patients with dementia and stroke in the present systematic review and meta-analysis study reveals the need for the investigation and follow-up of OD disorder. Due to the complications of OD and its significant impact on various aspects of life, health care providers and policy makers should pay special attention to the prevalence of OD. Accordingly, we should be aware of OD, find and implement suitable solutions, and follow the results of the measures at the individual, group, and organizational levels to reduce its prevalence.
One of the strengths of this study was estimating the global prevalence of OD for the first time in different populations with a sample size above 9000 people and estimating prevalence of OD in continents and various diagnostic tools. In addition, high heterogeneity among studies (more than 95%) led us to perform subgroup analysis, which reduced a small amount of heterogeneity. However, there is still a lot of heterogeneity in all subgroups, which may be due to the sample size, demographic characteristics, and method.
The present study comes with some limitations, including the lack of uniform reporting of articles, non-random selection of some samples, non-uniform study design, and the lack of access to the full text of articles presented at conferences. Furthermore, the number of studies performed on some populations was limited, therefore, it is suggested to conduct further studies on some patients, such as patients with pneumonia, head and neck cancer, paraplegia, children, etc.
Conclusion
The results of the present study indicated that the prevalence of OD is high in different populations and its trend has been increasing in recent years. Therefore, the appropriate strategies should be employed to decrease the prevalence of OD by finding its causation and monitoring at all levels, as well as providing feedback to hospitals.
Acknowledgements
This study is the result of research project No. 50000785 approved by the Student Research Committee of Kermanshah University of Medical Sciences. We would like to thank the esteemed officials of that center for accepting the financial expenses of this study. We also thank the officials of the Systematic Review and Meta-Analysis Center (SYRMAN) for their guidance and advice in conducting this research.
Abbreviations
- OD
Oropharyngeal dysphagia
- WoS
Web of Science
- MeSH
Medical subject headings
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
Author contributions
MK and FR contributed to the design, MK and ZN participated in most of the study steps. MK and FR prepared the manuscript. FR, ZN and MK assisted in designing the study, conducting analysis, and interpreting the study. All authors read and approved the final manuscript.
Funding
By Deputy for Research and Technology, Kermanshah University of Medical Sciences (IR) (50000785). This deputy has no role in the study process.
Availability of data and materials
Datasets are available through the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Ethics approval was received from the ethics committee of deputy of research and technology, Kermanshah University of Medical Sciences (IR.KUMS.REC.1400.624).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fatemeh Rajati, Email: f.rajati@kums.ac.ir.
Nassim Ahmadi, Email: nassim.slp@gmail.com.
Zahra Al-sadat Naghibzadeh, Email: zahrasnaghibzadeh@gmail.com.
Mohsen Kazeminia, Email: mkazeminia69@gmail.com.
References
- 1.Matsuyama S, Nakauma M, Funami T, Hori K, Ono T. Human physiological responses during swallowing of gel-type foods and its correlation with textural perception. Food Hydrocoll. 2021;111:106353. doi: 10.1016/j.foodhyd.2020.106353. [DOI] [Google Scholar]
- 2.Suntrup-Krueger S, Muhle P, Kampe I, Egidi P, Ruck T, Lenze F, et al. Effect of capsaicinoids on neurophysiological, biochemical, and mechanical parameters of swallowing function. Neurotherapeutics. 2021 doi: 10.1007/s13311-020-00996-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon JH, Lee H-J. Perceptions on evaluation and treatment of swallowing disorders in speech-language pathologists. Phon Speech Sci. 2013;5(4):43–51. doi: 10.13064/KSSS.2013.5.4.043. [DOI] [Google Scholar]
- 4.Ortega O, Martín A, Clavé P. Diagnosis and management of oropharyngeal dysphagia among older persons, state of the art. J Am Med Dir Assoc. 2017;18(7):576–582. doi: 10.1016/j.jamda.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Cichero JA, Altman KW. Stepping stones to living well with dysphagia. Basel: Karger Publishers; 2012. Definition, prevalence and burden of oropharyngeal dysphagia: a serious problem among older adults worldwide and the impact on prognosis and hospital resources. [DOI] [PubMed] [Google Scholar]
- 6.Audag N, Liistro G, Goubau C, Vandervelde L, Poncin W, Toussaint M, et al. Screening for oropharyngeal dysphagia in adult patients with neuromuscular diseases using the Sydney swallow questionnaire. Muscle Nerve. 2021 doi: 10.1002/mus.27254. [DOI] [PubMed] [Google Scholar]
- 7.Cichero JA, Steele C, Duivestein J, Clavé P, Chen J, Kayashita J, et al. The need for international terminology and definitions for texture-modified foods and thickened liquids used in dysphagia management: foundations of a global initiative. Curr Phys Med Rehabil Rep. 2013;1(4):280–291. doi: 10.1007/s40141-013-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roldan-Vasco S, Orozco-Duque A, Suarez-Escudero JC, Orozco-Arroyave JR. Machine learning based analysis of speech dimensions in functional oropharyngeal dysphagia. Comput Methods Programs Biomed. 2021;208:106248. doi: 10.1016/j.cmpb.2021.106248. [DOI] [PubMed] [Google Scholar]
- 9.van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Meta-analysis of dysphagia and aspiration pneumonia in frail elders. J Dent Res. 2011;90(12):1398–1404. doi: 10.1177/0022034511422909. [DOI] [PubMed] [Google Scholar]
- 10.Baijens LW, Clavé P, Cras P, Ekberg O, Forster A, Kolb GF, et al. European Society for swallowing disorders–European Union geriatric medicine society white paper: oropharyngeal dysphagia as a geriatric syndrome. Clin Interv Aging. 2016;11:1403. doi: 10.2147/CIA.S107750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kertscher B, Speyer R, Fong E, Georgiou AM, Smith M. Prevalence of oropharyngeal dysphagia in the Netherlands: a telephone survey. Dysphagia. 2015;30(2):114–120. doi: 10.1007/s00455-014-9584-z. [DOI] [PubMed] [Google Scholar]
- 12.Roden DF, Altman KW. Causes of dysphagia among different age groups: a systematic review of the literature. Otolaryngol Clin North Am. 2013;46(6):965–987. doi: 10.1016/j.otc.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Airoldi M, Garzaro M, Raimondo L, Pecorari G, Giordano C, Varetto A, et al. Functional and psychological evaluation after flap reconstruction plus radiotherapy in oral cancer. Head Neck. 2011;33(4):458–468. doi: 10.1002/hed.21471. [DOI] [PubMed] [Google Scholar]
- 14.Marinho D, Brandão S, Lopes J, Nascimento S, Vianna LG. Functional capacity and quality of life among elderly patients with or without dysphagia after an ischemic stroke. Rev Assoc Med Bras. 2010;56(6):738–43. doi: 10.1590/s0104-42302009000600020. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues B, Nóbrega AC, Sampaio M, Argolo N, Melo A. Silent saliva aspiration in Parkinson’s disease. Mov Disord. 2011;26(1):138–141. doi: 10.1002/mds.23301. [DOI] [PubMed] [Google Scholar]
- 16.Jones E, Speyer R, Kertscher B, Denman D, Swan K, Cordier R. Health-related quality of life and oropharyngeal dysphagia: a systematic review. Dysphagia. 2018;33(2):141–172. doi: 10.1007/s00455-017-9844-9. [DOI] [PubMed] [Google Scholar]
- 17.Takizawa C, Gemmell E, Kenworthy J, Speyer R. A systematic review of the prevalence of oropharyngeal dysphagia in stroke, Parkinson’s disease, Alzheimer’s disease, head injury, and pneumonia. Dysphagia. 2016;31(3):434–441. doi: 10.1007/s00455-016-9695-9. [DOI] [PubMed] [Google Scholar]
- 18.Miarons Font M, Rofes SL. Antipsychotic medication and oropharyngeal dysphagia: systematic review. Eur J Gastroenterol Hepatol. 2017;29(12):1332–1339. doi: 10.1097/MEG.0000000000000983. [DOI] [PubMed] [Google Scholar]
- 19.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147–153. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 21.Watson DI, Lally CJ. Prevalence of symptoms and use of medication for gastroesophageal reflux in an Australian community. World J Surg. 2009;33(1):88–94. doi: 10.1007/s00268-008-9780-9. [DOI] [PubMed] [Google Scholar]
- 22.Almeida TMd, Cola PC, Magnoni D, França JÍD, Silva RGd. Prevalence of oropharyngeal dysphagia in stroke after cardiac surgery. Revista CEFAC. 2015;17:1415–9. doi: 10.1590/1982-0216201517520914. [DOI] [Google Scholar]
- 23.Wolf U, Eckert S, Walter G, Wienke A, Bartel S, Plontke SK, et al. Prevalence of oropharyngeal dysphagia in geriatric patients and real-life associations with diseases and drugs. Sci Rep. 2021;11(1):1–14. doi: 10.1038/s41598-020-79139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruth M, Månsson I, Sandberg N. The prevalence of symptoms suggestive of esophageal disorders. Scand J Gastroenterol. 1991;26(1):73–81. doi: 10.3109/00365529108996486. [DOI] [PubMed] [Google Scholar]
- 25.Cabre M, Serra-Prat M, Palomera E, Almirall J, Pallares R, Clavé P. Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing. 2010;39(1):39–45. doi: 10.1093/ageing/afp100. [DOI] [PubMed] [Google Scholar]
- 26.Melgaard D, Baandrup U, Bøgsted M, Bendtsen MD, Hansen T. The prevalence of oropharyngeal dysphagia in Danish patients hospitalised with community-acquired pneumonia. Dysphagia. 2017;32(3):383–392. doi: 10.1007/s00455-016-9765-z. [DOI] [PubMed] [Google Scholar]
- 27.Michel A, Vérin E, Gbaguidi X, Druesne L, Roca F, Chassagne P. Oropharyngeal dysphagia in community-dwelling older patients with dementia: prevalence and relationship with geriatric parameters. J Am Med Dir Assoc. 2018;19(9):770–774. doi: 10.1016/j.jamda.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Espinosa-Val M, Martín-Martínez A, Graupera M, Arias O, Elvira A, Cabré M, et al. Prevalence, risk factors, and complications of oropharyngeal dysphagia in older patients with dementia. Nutrients. 2020;12(3):863. doi: 10.3390/nu12030863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holland G, Jayasekeran V, Pendleton N, Horan M, Jones M, Hamdy S. Prevalence and symptom profiling of oropharyngeal dysphagia in a community dwelling of an elderly population: a self-reporting questionnaire survey. Dis Esophagus. 2011;24(7):476–480. doi: 10.1111/j.1442-2050.2011.01182.x. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Peris P, Paron L, Velasco C, De la Cuerda C, Camblor M, Bretón I, et al. Long-term prevalence of oropharyngeal dysphagia in head and neck cancer patients: impact on quality of life. Clin Nutr. 2007;26(6):710–717. doi: 10.1016/j.clnu.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Rofes L, Muriana D, Palomeras E, Vilardell N, Palomera E, Alvarez-Berdugo D, et al. Prevalence, risk factors and complications of oropharyngeal dysphagia in stroke patients: a cohort study. Neurogastroenterol Motil. 2018;30(8):e13338. doi: 10.1111/nmo.13338. [DOI] [PubMed] [Google Scholar]
- 32.Mateos-Nozal J, Montero-Errasquín B, García ES, Rodríguez ER, Cruz-Jentoft AJ. High prevalence of oropharyngeal dysphagia in acutely hospitalized patients aged 80 years and older. J Am Med Dir Assoc. 2020;21(12):2008–2011. doi: 10.1016/j.jamda.2020.04.032. [DOI] [PubMed] [Google Scholar]
- 33.Falsetti P, Acciai C, Palilla R, Bosi M, Carpinteri F, Zingarelli A, et al. Oropharyngeal dysphagia after stroke: incidence, diagnosis, and clinical predictors in patients admitted to a neurorehabilitation unit. J Stroke Cerebrovasc Dis. 2009;18(5):329–335. doi: 10.1016/j.jstrokecerebrovasdis.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Lendinez-Mesa A, del Carmen D-G, Casero-Alcázar M, Grantham SJ, de la Torre-Montero JC, Fernandes-Ribeiro AS. Prevalence of oropharyngeal dysphagia in patients related with cerebrovascular disease at a neurorehabilitation unit. Revista Científica de la Sociedad de Enfermería Neurológica. 2017;45:3–8. [Google Scholar]
- 35.Serra-Prat M, Palomera M, Gomez C, Sar-Shalom D, Saiz A, Montoya JG, et al. Oropharyngeal dysphagia as a risk factor for malnutrition and lower respiratory tract infection in independently living older persons: a population-based prospective study. Age Ageing. 2012;41(3):376–381. doi: 10.1093/ageing/afs006. [DOI] [PubMed] [Google Scholar]
- 36.Clavé P. Prevalence and risk factors of oropharyngeal dysphagia in stroke patients. Ann Phys Rehabil Med. 2014;57:e263. doi: 10.1016/j.rehab.2014.03.956. [DOI] [Google Scholar]
- 37.De Stefano A, Di Giovanni P, Kulamarva G, Gennachi S, Di Fonzo F, Sallustio V, et al. Oropharyngeal dysphagia in elderly population suffering from mild cognitive impairment and mild dementia: understanding the link. Am J Otolaryngol. 2020;41(4):102501. doi: 10.1016/j.amjoto.2020.102501. [DOI] [PubMed] [Google Scholar]
- 38.Melgaard D, Rodrigo-Domingo M, Mørch MM. The prevalence of oropharyngeal dysphagia in acute geriatric patients. Geriatrics. 2018;3(2):15. doi: 10.3390/geriatrics3020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez Lindh M, Larsson L, Koyi H. Prevalence of oropharyngeal dysphagia in patients with stable COPD. J Oral Health Dent. 2017;1(S1):A002. [Google Scholar]
- 40.Yang EJ, Kim MH, Lim J-Y, Paik N-J. Oropharyngeal dysphagia in a community-based elderly cohort: the Korean longitudinal study on health and aging. J Korean Med Sci. 2013;28(10):1534–1549. doi: 10.3346/jkms.2013.28.10.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biglary M, Ghelichi L, Kamali M. Prevalence of oropharyngeal dysphagia and its predictive factors in patients with neurological diseases and head and neck surgery. Middle Eastern J Disabil Stud. 2019;9:113. doi: 10.29252/mejds.0.0.40. [DOI] [Google Scholar]
- 42.Kritzinger A, Da Costa MA, Graham MA, Krüger E. Prevalence and associated prenatal and perinatal risk factors for oropharyngeal dysphagia in high-risk neonates in a South African hospital. S Afr J Commun Disord. 2019;66(1):1–8. doi: 10.4102/sajcd.v66i1.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiocca J, Olmos J, Salis G, Soifer L, Higa R, Marcolongo M, et al. Prevalence, clinical spectrum and atypical symptoms of gastro-oesophageal reflux in Argentina: a nationwide population-based study. Aliment Pharmacol Ther. 2005;22(4):331–342. doi: 10.1111/j.1365-2036.2005.02565.x. [DOI] [PubMed] [Google Scholar]
- 44.Jacinto-Scudeiro LA, Machado GD, Ayres A, Burguêz D, Polese-Bonatto M, González-Salazar C, et al. Prevalence of oropharyngeal dysphagia in hereditary spastic paraplegias. Arq Neuropsiquiatr. 2020;77:843–847. doi: 10.1590/0004-282x20190180. [DOI] [PubMed] [Google Scholar]
- 45.Delevatti C, Rodrigues EdC, Almeida STd, Santos KWd. Prevalence and risk factors for oropharyngeal dysphagia in fragile older adults with orthopedic fractures. Audiol-Commun Res. 2020 doi: 10.1590/2317-6431-2020-2388. [DOI] [Google Scholar]
- 46.Holmes SR, Sabel AL, Gaudiani JL, Gudridge T, Brinton JT, Mehler PS. Prevalence and management of oropharyngeal dysphagia in patients with severe anorexia nervosa: a large retrospective review. Int J Eat Disord. 2016;49(2):159–166. doi: 10.1002/eat.22441. [DOI] [PubMed] [Google Scholar]
- 47.Benfer KA, Weir KA, Bell KL, Ware RS, Davies PS, Boyd RN. Oropharyngeal dysphagia and cerebral palsy. Pediatrics. 2017 doi: 10.1542/peds.2017-0731. [DOI] [PubMed] [Google Scholar]
- 48.Camacho M, Certal V, Abdullatif J, Zaghi S, Ruoff CM, Capasso R, et al. Myofunctional therapy to treat obstructive sleep apnea: a systematic review and meta-analysis. Sleep. 2015;38(5):669–675. doi: 10.5665/sleep.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jun H-J, Kim K-J, Nam K-W, Kim C-H. Effects of breathing exercises on lung capacity and muscle activities of elderly smokers. J Phys Ther Sci. 2016;28(6):1681–1685. doi: 10.1589/jpts.28.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jablu DS, Hosseini RA. Effects of resistance and endurance exercises on serum androgens, cortisol and lactate in menopause women. Iran J Health Phys Act. 2012;3(1):21–29. [Google Scholar]
- 51.Chahine LM, Amara AW, Videnovic A. A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep Med Rev. 2017;35:33–50. doi: 10.1016/j.smrv.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lajoie AC, Lafontaine A-L, Kimoff RJ, Kaminska M. Obstructive sleep apnea in neurodegenerative disorders: current evidence in support of benefit from sleep apnea treatment. J Clin Med. 2020;9(2):297. doi: 10.3390/jcm9020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun A-P, Liu N, Zhang Y-S, Zhao H-Y, Liu X-L. The relationship between obstructive sleep apnea and Parkinson’s disease: a systematic review and meta-analysis. Neurol Sci. 2020;41(5):1153–1162. doi: 10.1007/s10072-019-04211-9. [DOI] [PubMed] [Google Scholar]
- 54.Loke YK, Brown JWL, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5(5):720–8. doi: 10.1161/CIRCOUTCOMES.111.964783. [DOI] [PubMed] [Google Scholar]
- 55.Tawfik GM, Alshareef A, Mostafa EM, Khaled S, Hmeda AB, Abdelwahed KA, et al. Association between radiotherapy and obstructive sleep apnea in cancer patients: a systematic review and meta-analysis. Am Soc Clin Oncol. 2019 doi: 10.1200/JCO.2019.37.15. [DOI] [PubMed] [Google Scholar]
- 56.Grande G, Haaksma ML, Rizzuto D, Melis RJ, Marengoni A, Onder G, et al. Co-occurrence of cognitive impairment and physical frailty, and incidence of dementia: systematic review and meta-analysis. Neurosci Biobehav Rev. 2019;107:96–103. doi: 10.1016/j.neubiorev.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Abdollahpour I, Nedjat S, Noroozian M, Golestan B, Majdzadeh R. Development of a caregiver burden questionnaire for the patients with dementia in Iran. Int J Prev Med. 2010;1(4):233. [PMC free article] [PubMed] [Google Scholar]
- 58.Liu S, Li C, Shi Z, Wang X, Zhou Y, Liu S, et al. Caregiver burden and prevalence of depression, anxiety and sleep disturbances in Alzheimer’s disease caregivers in China. J Clin Nurs. 2017;26(9–10):1291–1300. doi: 10.1111/jocn.13601. [DOI] [PubMed] [Google Scholar]
- 59.Milen MT, Nicholas DB. Examining transitions from youth to adult services for young persons with autism. Soc Work Health Care. 2017;56(7):636–648. doi: 10.1080/00981389.2017.1318800. [DOI] [PubMed] [Google Scholar]
- 60.Meng P-p, Zhang S-c, Han C, Wang Q, Bai G-t, Yue S-w. The occurrence rate of swallowing disorders after stroke patients in Asia: a PRISMA-compliant systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2020;29(10):105113. doi: 10.1016/j.jstrokecerebrovasdis.2020.105113. [DOI] [PubMed] [Google Scholar]
- 61.Kaddumukasa M, Mugenyi L, Kaddumukasa MN, Ddumba E, Devereaux M, Furlan A, et al. Prevalence and incidence of neurological disorders among adult Ugandans in rural and urban Mukono district; a cross-sectional study. BMC Neurol. 2016;16(1):1–9. doi: 10.1186/s12883-016-0732-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors J, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American heart association/American stroke association. Stroke. 2013;44(7):2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Z, Venkat P, Seyfried D, Chopp M, Yan T, Chen J. Brain–heart interaction: cardiac complications after stroke. Circ Res. 2017;121(4):451–468. doi: 10.1161/CIRCRESAHA.117.311170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim B-R, Lee J, Sohn MK, Kim DY, Lee S-G, Shin Y-I, et al. Risk factors and functional impact of medical complications in stroke. Ann Rehabil Med. 2017;41(5):753. doi: 10.5535/arm.2017.41.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol. 2010;9(1):105–118. doi: 10.1016/S1474-4422(09)70266-2. [DOI] [PubMed] [Google Scholar]
- 66.Menezes KK, Nascimento LR, Ada L, Polese JC, Avelino PR, Teixeira-Salmela LF. Respiratory muscle training increases respiratory muscle strength and reduces respiratory complications after stroke: a systematic review. J Physiother. 2016;62(3):138–144. doi: 10.1016/j.jphys.2016.05.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets are available through the corresponding author upon reasonable request.