Abstract
Background
Osteoporosis is a skeletal metabolic disease that constitutes a great threaten to human health. However, there is currently no gold standard for its treatment. High-mobility group box chromosomal protein-1 (HMGB-1) has been reported to play an important role in various orthopedic diseases. Till now, its role in osteoporosis remains elusive.
Methods
Rats underwent ovariectomy (OVX) were used to construct a postmenopausal model of osteoporosis. Then, rats were divided into sham groups without OVX surgery, OVX model group, HMGB-1 knockdown (HMGB-1 KD) OVX model groups. The expression of HMGB1 was evaluated by qRT-PCR and western blotting. Subsequently, the changes of trabeculae were evaluated by micro-computed tomography (CT) assay. Skeletal necrosis and metabolism were further analyzed by hematoxylin–eosin (HE) staining, Alcian blue staining and Masson’s trichrome staining. The contents of serum alkaline phosphatase (ALP) and osteocalcin were detected by ELISA assay. Expression of osteoclast-associated receptor (OSCAR) and tartrate-resistant acid phosphatase (TRAP) were determined to investigate the effects of HMGB-1 loss on osteoclastogenesis.
Results
Single HMGB-1 deletion exerted no significant effect on rat trabeculae, serum ALP and osteocalcin. Noticeably, HMGB1 knockdown dramatically ameliorated OVX-induced changes in above indexes. Trabeculae structures of OVX rats were sparse with disorder arrangement, which were greatly recovered after HMGB-1 deletion. Enhanced osteoclastogenesis was observed in OVX rats by increasing number of TRAP + cells and expression of TRAP and OSCAR, and loss of HMGB1 ameliorated osteoclastogenesis in OVA rats. Moreover, HMGB-1 deletion antagonized OVX-evoked downregulation of osteoblast activity markers osterix (OSX), collagen type I alpha 1(COL1A1) and distal-less homeobox 2 (DLX2) protein. Furthermore, loss of HMGB-1 attenuated fluctuation of inflammatory factors in OVX rats. Additionally, HMGB-1 deficiency inhibited OVX-evoked activation of the Toll-like receptor (TLR) 4/NF-κB signaling pathway. Moreover, reactivating the TLR4 signaling further aggravated OVX-induced osteoporosis, which was reversed by HMGB1 knockdown.
Conclusion
HMGB-1 deletion alleviated OVX-triggered osteoporosis by suppressing osteoclastogenesis and inflammatory disorder via the inhibition of the TLR4 signaling. Therefore, HMGB-1 may be a promising therapeutic target for osteoporosis.
Keywords: Targeted therapy, Osteogenesis, Signal pathway
Introduction
Osteoporosis is identified as a skeletal degenerative disease accompanied by low mineral density and deterioration of the bone microarchitecture and usually increases risk of fracture [1, 2]. Osteoporosis is proved to be more prevalent in middle-aged and elderly population, especially in postmenopausal women [1]. There are more than 8.9 million people suffering from fracture induced by osteoporosis, with a new case per 3 s around the world [1]. Currently, osteoporosis has constituted a great threat to the health of elderly people and a high burden of medical care [1, 3]. Although the treatments of osteoporosis have been optimized dramatically over past decades, the latest treatment still has many drawbacks. Bisphosphonate, a kind of chemically stable analogs of pyrophosphate compounds, is the most common therapeutic strategy for osteoporosis at present and can reduce bone loss and prevent fractures in postmenopausal women, men suffering from osteoporosis, and the patients with glucocorticoid therapy [4]. However, bisphosphonates have potential complications, such as osteonecrosis of the jaw [4] and atypical femoral fractures [5]. Therefore, new treatments for osteoporosis are urgently needed.
Nowadays, more and more researchers confirm a prevailing point that osteoporosis often develops from the imbalance between bone formation and resorption. In elderly and postmenopausal patients, osteoporosis is a common consequence that involves in osteoclast-regulated bone resorption exceeding osteoblast-mediated bone formation. Intriguingly, inflammatory response exerts a critical role in the process of bone remodeling in osteoporosis [6]. It is a fact that inflammatory reaction can regulate bone formation and resorption [7]. Besides, acute inflammation has been recognized as the first stage of fracture healing [8]. In postmenopausal women, estrogen deficiency will induce receptor activator of NF-κB to induce spontaneous elevation in pro-inflammatory and pro-osteoclastic cytokine such as tumor necrosis factor-α (TNF-α) [9, 10]. Acceptably, TNF-αplays a vital role in orthopedic diseases by regulating the release of pro-inflammatory cytokines, the activation of endothelial cell and osteoclast, and the accumulation of leukocyte [11]. Previous researches indicate that macrophages were converted to multinucleated giant cells by interleukin (IL)-4 during bone resorption [12, 13]. Therefore, anti-inflammation has been considered as a potential therapeutic approach against osteoporosis [6, 10, 12].
High-mobility group box chromosomal protein-1 (HMGB-1) is a DNA binding protein, which facilitates DNA combination, stabilizes nucleosome formation and modulates the interactions between regulatory molecules and their targets [14]. Previous researches revealed that HMGB-1 played an important role in many physiological processes, such as injury repair, immune response and tumor [15, 16]. Intriguingly, emerging evidence confirms the implication of HMGB1 in orthopedic disorders [17–19]. For instance, HMGB1 can target numerous immunological pathway like Toll-like receptor 4 (TLR4)-mediated NF-κB signaling to trigger inflammatory response, ultimately leading to the development of rheumatoid arthritis [17]. Moreover, targeting HMGB1 alleviates lipopolysaccharide-induced osteoarthritis progression [18]. Noticeably, serum HMGB1 levels can serve as a useful marker of inflammatory activity in postmenopausal women with rheumatoid arthritis [19]. However, there is no study looking for the relationship between HMGB-1 and osteoporosis.
In this research, we mainly investigated whether and how HMGB-1 deletion inhibited OVX-induced osteoporosis. Rat model was applied to osteoporosis establishment.
Materials and methods
Animal preparation
A total of 18 wild-type (WT) female rats (3-month-old) [20] and 12 HMGB-1 down (HMGB-1 KD) female rats were obtained from the Shanghai Model Organisms Center (China) using CRISPR/Cas9 technology. All the rats were fed with adequate food and water in the environment with humidity of 60%, temperature of 23℃ and a 12-h light/dark cycle for 7 days.
All experiments on animals in the study are consistent with the Animal Care and Use Committee of the Third Affiliated Hospital of Guangzhou Medical University, and we made our best to minimize suffering.
Construction of OVX model
All rats were divided into 3 groups: the WT sham group, the WT ovariectomy (OVX) group and the HMGB-1 knockdown (KD) OVX group. Before the surgery, rats were anesthetized with 80 mg/kg pentobarbital sodium. The WT sham group received a sham surgery and was maintained as the healthy control; rats in the WT OVX group and the HMGB-1 KD group experienced the OVX treatment under anesthesia to build the OVX model; no special postoperative care was required.
Quantitative real-time PCR
Bone tissues were homogenized with liquid nitrogen, and the homogenates were lysed. Total RNA was extracted using Trizol Reagent (Invitrogen, Waltham, MA, USA). The concentration of RNA was measured with a spectrophotometer (NanoDrop 2000, Thermo Scientific, Shanghai, China). RNA samples were reversed to obtain the cDNA sample using a reverse transcritptase kit (Fermentas, Waltham, MA, USA) according to the manufacturer’s instructions. The cDNA samples were used for the following quantitative real-time PCR (qRT-PCR), and the primer sequences of the target genes are shown in Table 1. Relative gene expression was normalized to the internal control actin, and data analysis was performed using the Bio-Rad CFX Manger software.
Table 1.
Primer sequences used in qRT-PCR
| β-Actin forward | 5′-CTTCCTTCCTGGGTATGGAATC-3′ |
|---|---|
| β-Actin reverse | 5′-CTGTGTTGGCATAGAGGTCTT-3′ |
| HMGB-1 forward | 5′-GCGAAGAAACTGGGAGAGATGTG-3′ |
| HMGB-1 reverse | 5′-GCATCAGGCTTTCCTTTAGCTCG-3′ |
| OSCAR forward | 5′-GTTCTGGCTCCTTGGACTATAC-3′ |
| OSCAR reverse | 5′-GCTGTGCCAATCGAAAGTAAC-3′ |
| TRAP forward | 5′-GGCTACCTACGCTTTCACTATG-3′ |
| TRAP reverse | 5′-TTTCCAGAGGCTTCCACATAC-3′ |
Micro-computed tomography
Trabeculae structures of the left femur were evaluated based on the parameters [21, 22] including bone surface/bone volume (BS/BV), trabecular bone surface/bone volume (BSA/BV), bone volume/tissue volume (BV/TV), trabeculae thickness (Tb. Th), trabeculae number (Tb. N) and trabeculae separation (Tb. Sp) using a SkyScan 1076 micro-computed tomography (CT) scanner (viva CT40, Belgium/Skyscan). The scanning was conducted from the femoral head to the middle of the femur, with a laminar thickness of 18 μm and rotation angle of 180°. Two images were taken at every 0.5° rotation. All images were analyzed on a SkyScan 1076 micro-CT scanner (viva CT40, Belgium/Skyscan).
Elisa assay
Blood of rats in each group was collected and was then centrifuged at 3500 × g for 15 min. Serum osteocalcin, TNF-α, IL-4 and alkaline phosphatase (ALP) concentrations were then examined using the Elisa kit (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer’s protocols. The absorbance value of the samples was read, and the concentration of each protein was calculated according to the standard curve.
Western blotting
Total proteins were extracted from fresh bone tissue with Radio Immunoprecipitation Assay (RIAP) (Beyotime, China) and separated with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The following specific primary antibodies are used: anti-osteoclast-associated receptor (OSCAR) (ab156742, Abcam, 1:1000), anti-tartrate-resistant acid phosphatase (TRAP) (ab2391, Abcam, 1:1000), anti-HMGB-1 (6893, CST, 1:1000), anti-OSTERIX (ab22552, Abcam, 1:1000), anti-COL1A1 (96321S, CST, 1:1000), anti-DLX2 (ab272902, Abcam, 1:1000), anti-IL-10 (ab34843, Abcam, 1:1000), anti-iNOS (ab213987, Abcam, 1:1000) and anti-β-actin (3700, CST, 1:1000). EnlightTM (Engreen, China) was used for protein band imaging.
Hematoxylin–eosin (H&E) staining
H&E staining was performed to examine the trabeculae of rats. Fresh bones were obtained from the rats euthanized with carbon dioxide. After fixed in formalin solution, bone pieces were soaked in serial ethanol solutions and then were steeped in 5% nitric acid solution. Next, the pieces were processed with dehydration and paraffin embedding and then were cross-sectioned into 5-µm-thick sections. Then, all specimens were stained with hematoxylin and eosin. Lastly, the slides were viewed with microscope.
Alcian blue staining
Alcian blue staining was performed to detect the change of chondrocytes. The slides were processed with 1%Alcian blue solution (3% acetic acid contained, pH 2.5) and 1% nuclear fast red solution (5% aluminum sulfate contained).
Masson’s trichrome staining and tartrate-resistant acid phosphatase (TRAP) staining
Masson’s trichrome staining was performed to detect the change of muscle fiber and collagenous fiber in rats. The slides were fixed in Bouin’s or Zenker’s liquor and then stained with Mayer’s hematoxylin, acid ponceau and aniline blue or brilliant green. At last, the slides were dehydrated with different concentration ethanols (95% and 100%) and sealed with resinous mounting medium. Besides, TRAP staining was performed to visualize osteoclast of rats in each group with acid phosphatase, leukocyte (TRAP) Kit (Sigma-Aldrich, Merck, Darmstadt, Germany) according to manufacturer’s protocol.
Fluorescence-activated cell sorting
Peripheral blood (1% EDTA contained) was used for fluorescence-activated cell sorting (FACS) to detect the expression of F4/80, iNOS and IL-10. The blood samples were processed with corresponding fluorescent antibody against F4/80-FITC (cat. no. 123107), iNOS-PE (cat. no. 696805) and IL-10 PE (cat. no. 505007) (BioLegend, Inc.). After centrifugation and resuspension, the blood cells were subjected to fluorescence-activated cell sorting.
Statistical analysis
All experiments in this study were independently repeated at least three independent times. Data were represented as the means ± standard deviation. Comparisons between two groups were analyzed by unpaired Student’s t test. Differences among multiple groups were compared by one-way analysis of variance (ANOVA) with Dunnett’s post hoc test using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA). *P < 0.05, #P < 0.05 and **P < 0.01.
Results
HMGB-1 deficiency protects against bone structural changes in OVX rat model
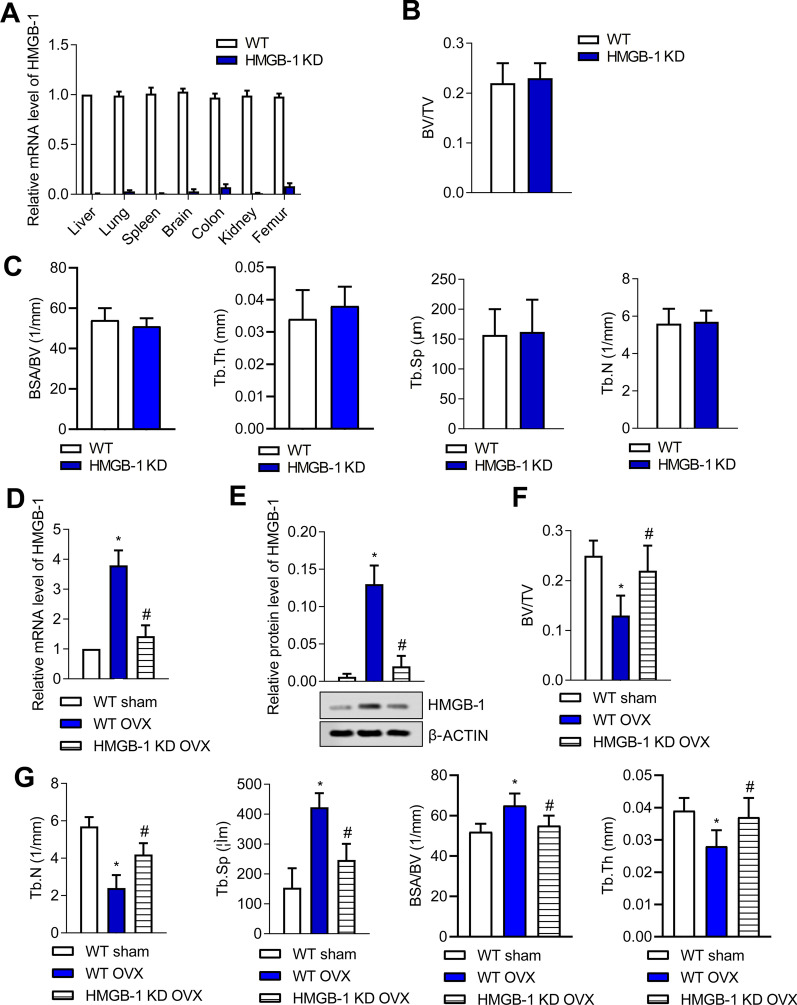
QRT-PCR and micro-CT were carried out to verify the effects of HMGB-1 deficiency on bone structural features. As shown in Fig. 1a, relative mRNA levels of HMGB-1 in liver, lung, spleen, brain, colon, kidney and femur in WT were much higher than those in HMGB-1 KD rats (P < 0.05). Contrarily, BV/TV, BSA/BV, Tb. Th, Tb. Sp and Tb. N between WT rats and HMGB-1 KD rats were similar (Fig. 1b, c) (P < 0.05), suggesting that HMGB-1 deficiency had little effect on trabeculae structures of rats.
Fig. 1.
HMGB-1 deficiency protects against bone structural changes in OVX rat model. a To confirm the success of HMGB-1 knockdown, HMGB-1 mRNA expression levels in main organs of wild-type (WT) and HMGB-1 KD rats were, respectively, determined by qRT-PCR. b, c The trabeculae structures in WT rats and HMGB-1 rats were identified by Micro-CT. *P < 0.05; **P < 0.01; vs WT group. n = 6. Graphs are representative of three biologically independent experiments. Then, all rats were divided into three groups: the WT sham group, the WT OVX group and the HMGB-1 KD OVX group. d, e The mRNA and protein levels of HMGB-1 were, respectively, detected by qRT-PCR and western blotting in each group. f, g The trabeculae structures of all rats were identified by Micro-CT. Error bars indicate SD. *P < 0.05, vs WT Sham group; #P < 0.05, vs WT OVX group. n = 6. Graphs are representative of three biologically independent experiments
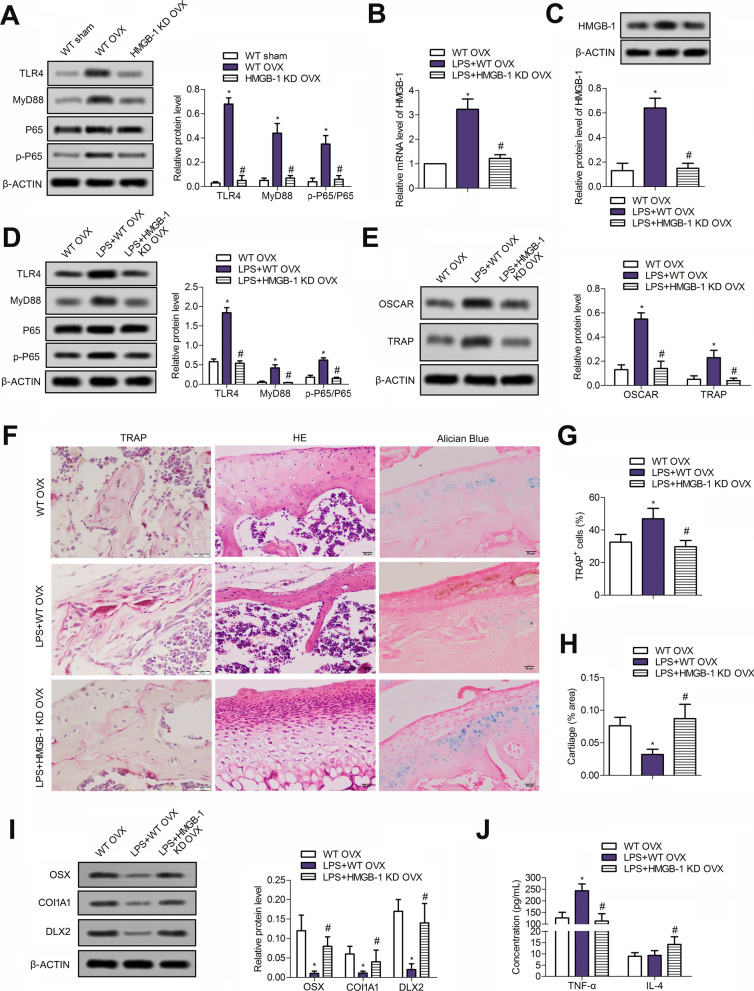
Then, the rats were divided as the following three groups: The WT sham group, WT OVX group and the HMGB-1 KD OVX group. As shown in Fig. 1d, e, mRNA and proteins levels of HMGB-1 in WT OVX group were both increased compared with that in the WT sham group; however, these increases were then suppressed in HMGB-1 KD OVX group (P < 0.05), indicating the successful construction of rats with HMGB1 loss. Micro-CT analysis showed lower values of BV/TV, Tb.Th, Tb.N and higher values of Tb. Sp and BSA/BV in trabecular of OVX groups compared to sham groups, indicating the obvious osteoporosis triggered by OVX (P < 0.05) (Fig. 1f, g). However, OVX-induced above changes were reversed in HMGB-1 KD OVX rats (P < 0.05) (Fig. 1f, g).
HMGB-1 knockdown restrains osteoclastogenesis in OVX rats
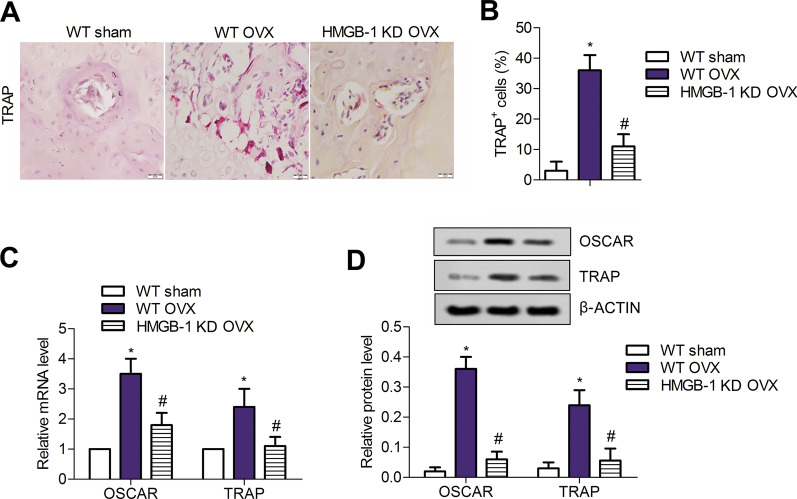
We next elaborated the effects of HGMB-1 loss on osteoclastogenesis in OVX rats and confirmed that the number of TRAP + cells was significantly increased post-OVX compared with the sham group, which was sharply reversed by HMGB-1 deletion (P < 0.05) (Fig. 2a, b). The mRNA and protein expressions of OSCAR and TRAP, as representative factors of RANK/RANKL/OPG system, were augmented significantly post-OVX compared with the sham group (P < 0.05) (Fig. 2c, d), and these increases were largely weakened in HMGB-1 KD OVX rats (P < 0.05). Thus, knockdown of HMGB-1 suppressed osteoclastogenesis in rats underwent OVX treatment.
Fig. 2.
HMGB-1 deficiency inhibits the osteoclastogenesis after OVX. a, b The numbers of TRAP-positive cells were detected with TRAP staining in all the rats. Data are from three independent experiments. c, d The expressions of OSCAR and TRAP are detected though qRT-PCR and western blotting. Error bars indicate SD. *P < 0.05 vs WT Sham group, #P < 0.05 vs WT OVX group. n = 6. Data are from four independent experiments
HMGB-1 suppresses osteoporosis of OVX rats
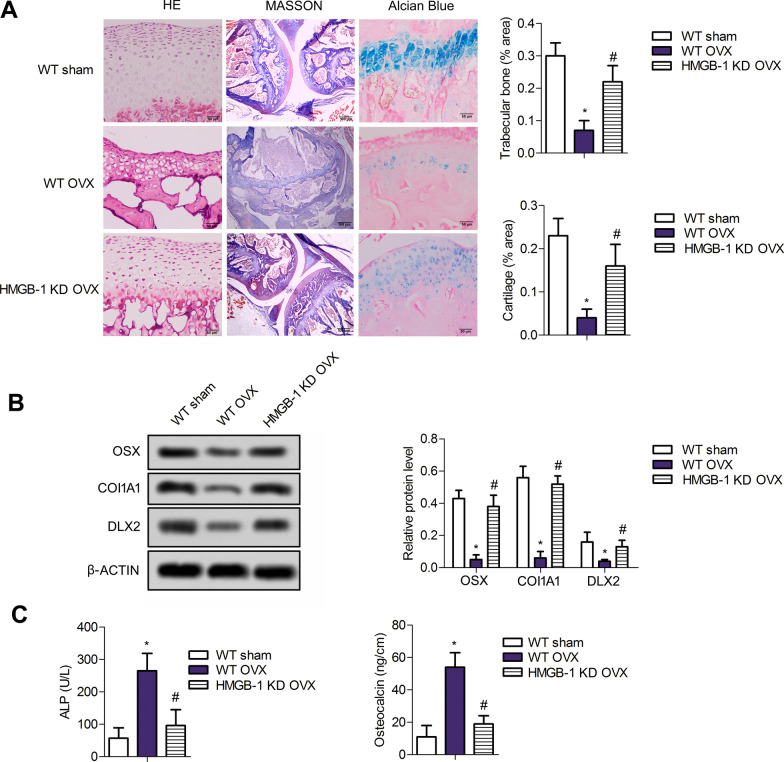
To further verify the effects of HMGB-1 on osteoporosis, H&E staining, Alcian staining, Masson’s trichrome staining and western blotting were used. Pathological results (Fig. 3a) indicated that the trabeculae in WT sham rats were abundant, continuous and dense, while those in WT OVX rats were significantly thinner, sparser, disordered arrangement and wider inter-trabeculae spaces (P < 0.05). The trabeculae in HMGB-1 KD OVX rats exhibited mild pathological changes including relatively neat arrangement and relatively complete net structure (P < 0.05). Moreover, OVX treatment induced loss of muscle and collagenous fiber relative to sham groups (P < 0.05). Thus, the significant osteoporosis was observed in OVX rats; nevertheless, the pathological changes were alleviated in HMGB-1 KD OVX group (P < 0.05). Similarly, Alcian blue staining corroborated that higher number of chondrocytes were observed in HMGB-1 KD groups relative to the OVX groups (P < 0.05). Furthermore, the expression of OSX, COL1A1 and DLX2 was notably downregulated in WT OVX rats (P < 0.05) compared with the sham group and was elevated in HMGB-1 KD group (P < 0.05) (Fig. 3b). Additionally, osteoporosis-related biochemical parameter analysis substantiated the higher levels of ALP and osteocalcin in serum of OVX rats than that in sham groups (Fig. 3c), which were reversed after HMGB-1 deletion. Thus, HMGB-1 may ameliorate OVX-induced osteoporosis.
Fig. 3.
HMGB-1 suppresses osteoporosisb of OVX rats. a The trabeculae in all rats were visualized via H&E staining, Masson’s trichrome staining and Alcian blue staining. b The expressions of OSX, COL1A1 and DLX2 were measured with western blotting. c The levels of ALP and osteocalcin in serum were determined by ELISA assay. Error bars indicate SD. *P < 0.05 vs WT Sham group, #P < 0.05, vs WT OVX group. n = 6. Graphs are representative of three biologically independent experiments
HMGB-1 deletion relieves OVX-induced inflammation
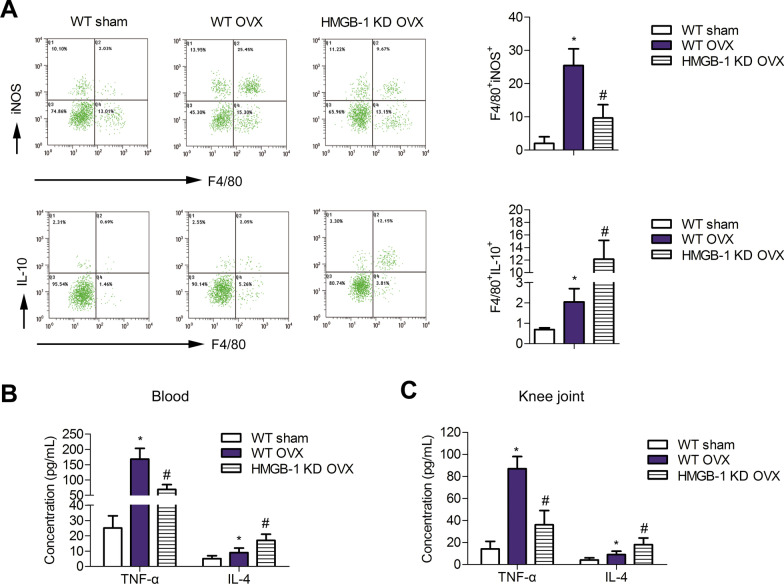
To clarify the effect of HMGB-1 deletion on OVX-induced inflammatory disorder, the expression levels of IL-10 and iNOS were detected by FACs. Compared to the WT sham group, the numbers of F4/80 + IL-10 + and F4/80 + iNOS + cells were strongly augmented in both WT OVX rats, which was decreased in HMGB-1 KD rats (Fig. 4a) (P < 0.05). Furthermore, the levels of TNF-ɑ in the blood and knee joint of OVX WT group were increased significantly compared to WT sham rats (P < 0.05) (Fig. 4b, c); however, these increases were overturned after HMGB-1 knockdown (P < 0.05). In addition, expression of anti-inflammatory factor IL-4 was elevated in the case of OVX as a normal immune response phenomenon, but more obvious increase in IL-4 was found in HMGB-1 deletion group. Thus, HMGB-1 deletion alleviates OVX-induced inflammatory disorder.
Fig. 4.
HMGB-1 deletion is a relief to OVX-induced inflammatory disorder. a The expression levels of IL-10 and iNOS were detected though FACs. b, c The levels of TNF-ɑ and IL-4 in blood and knee joint were measured via Elisa assays. Error bars indicate SD.*P < 0.05, vs WT Sham group. #P < 0.05, vs WT OVX group. n = 6. Data are from four independent experiments
HMGB-1 affects osteoporosis via the inhibition of TLR signaling pathway
As shown in Fig. 5a, OVX treatment activated the TLR4 signaling by increasing the expression of TLR signaling pathway-related TLR4, myeloid differentiation factor 88 (MyD88) and the rate of p-P65/ P65 compared with the sham group. Nevertheless, HMGB-1knockdown abrogated OVX-induced activation of TLR4/MyD88/p65 NF-κB pathway. Then, TLR signaling pathway activator lipopolysaccharide (LPS, 10 μg/mL) was used in the following experiments. As shown in Fig. 5b, c, the mRNA and protein levels of HMGB-1 were obviously elevated in the external force of LPS. Accordingly, the TLR signaling pathway was effectively activated by LPS (Fig. 5d). Subsequently, osteoporosis-related indexes were evaluated again. Expressions of osteoclast marker genes OSCAR and TRAP were increased in the presence of LPS, which were suppressed in the HMGB-1 KD group (Fig. 5e). Results of H&E staining, Alcian staining and Masson’s trichrome staining showed that reactivating the TLR4 signaling by LPS further aggravated osteoporosis; however, this progression was alleviated by HMGB-1 deficiency (Fig. 5f–h). The expression of osteoblast activity markers OSX, COL1A1 and DLX2 was inhibited by LPS, which was elevated by HMGB-1 deficiency (Fig. 5i, j). Thus, the above results further indicated that HMGB-1 deficiency relieved osteoporosis via suppressing the TLR signaling pathway.
Fig. 5.
HMGB-1 affects osteoporosis via the inhibition of TLR signaling pathway. a Expression of TLR signaling pathway-related proteins TLR4, MyD88 p-P65 and P65 was detected by western blot. *P < 0.05, vs WT Sham group; #P < 0.05, vs WT OVX group. n = 6. LPS (10 μg/mL) was used as an activator of TLR signaling pathway in the following experiments. b, c Relative mRNA expression of HMGB-1 and HMGB-1 protein were detected through qRT-PCR and western blotting, respectively. d Expression of TLR signaling pathway-related proteins TLR4, MyD88 p-P65 and P65 was detected by western blot. e Expressions of osteoclast marker genes OSCAR and TRAP were detected by western blot. f Trabeculae in all rats were visualized by H&E staining, Masson’s trichrome staining and Alcian blue staining. g The proportion of TRAP+ cells was shown. h The area ratio of cartilage was calculated. i The expressions levels of OSX, COL1A1 and DLX2 were measured with western blotting. j The levels of TNF-ɑ and IL-4 were measured via Elisa assays. *P < 0.05, vs WT OVX group; #P < 0.05, vs LPS + WT OVX group. n = 6. Data are from three biologically independent experiments
Discussion
Postmenopausal osteoporosis is known to be caused by osteoclast-induced bone resorption and has posed a big threat to global public health [1, 3]. In recent years, a lot of money has been applied to the treatment of osteoporosis [23]. Since the osteoporosis is mostly found in case of fracture [1], in-time diagnosis and proper treatments remain unmet. Therefore, new potential treatments are urgently needed. Intriguingly, the current study investigated the relationship between HMGB-1 deletion and osteoporosis and confirmed that HMGB-1 deletion alleviated OVX-induced osteoporosis, indicating a promising therapeutic option for osteoporosis.
As a multifunction factor, the role of HMGB-1 in physiological and pathological processed has been revealed gradually. Noticeably, increasing evidence substantiates the relationship between HMGB and inflammation-related diseases, such as rheumatoid arthritis [24, 25]. However, the relationship between HMGB-1 and osteoclastogenesis remains indistinct. OVX rat is a common and effective model to study postmenopausal osteoporosis, which causes impaired trabeculae [26]. Therefore, we constructed osteoporotic rats by OVX and revealed the high expression of HMGB1 in OVX rats, indicating a potential correlation between HMGB1 and osteoporosis.
The change of trabeculae structure is a vital indicator in the evaluation of osteoporosis [27]. Noticeably, trabeculae structures of OVX rats were sparse with disorder arrangement, which were greatly recovered after HMGB-1 deletion. It is generally believed that osteoporosis is majorly caused by the imbalance between osteoblast-mediated bone formation and osteoblast-evoked bone resorption [6–8]. ALP, a phosphomonoesterase secreted by osteoblasts, is always increased following bone fracture [28]. In this study, enhanced expression of ALP and another osteoblast-specific protein, osteocalcin [29], were observed in osteoporotic rats; however, these increases were reversed after HMGB-1 knockdown. Thus, these findings indicate that HMGB-1 may relieve OVX-induced osteoporosis and recover the bone formation to a certain extent.
The effects of HMGB-1 on bone formation were further evaluated by detecting the expression of osteoclast marker genes OSCAR and TRAP. The results showed that the induced expression of TRAP and OSCAR by OVX was suppressed by HMGB-1 deletion. Besides, pathological change of trabeculae induced by OVX was also effectively mitigated by HMGB-1 deficiency. Besides, detection about osteoblast activity markers OSX, COL1A1 and DLX2 showed that osteoblast activity was suppressed by OVX, which were recovered by HMGB-1 deletion. In this study by Qu et al. [30], the increase in DLX2 and OSX promotes osteogenic differentiation, which is consistence with the finding in the present study. The above results suggested that HMGB-1 deletion alleviated bone formation attenuated by OVX though balancing the osteogenic and osteoclastic processes.
Inflammation is proved to exert an important role in bone diseases. Without timely treatment, inflammation induced by bone diseases will transform into chronic conditions naturally, which is accompanied by increased bone resorption and decreased bone formation [31]. As anti-osteoclastogenesis factors, F4/80 + IL-10 + cells were increased in HMGB-1 KD OVX rats, while F4/80 + iNOS + cells, pro-osteoclastogenesis factors, were decreased in HMGB-1 KD OVX rats. This finding indicated that HMGB-1 deletion promoted bone formation by elevating the proportion of osteoblasts and suppressing the proportion of osteoclasts. TNF-α is an important factor to induce osteoclastogenesis and bone resorption [6]. Furthermore, IL-4 has been implicated in bone homeostasis by regulating osteoblast and osteoclast [32]. Both in blood and the knee joint, IL-4 expression was increased by HMGB-1 deletion, which is keeping with previous research by Chen [33]. The results mentioned above indicated that HMGB-1 deletion was capable of alleviating osteoclastogenesis via relieving the inflammation induced by OVX surgery.
Besides, emerging evidence supports the involvement of TLR4/MyD88/NF-kappaB in bone remodeling [34, 35]. For instance, inhibiting the TLR4/MyD88/NF-kappaB signaling in osteoclast precursors alleviates the progression of osteoporosis and subsequently bone loss [34]. Moreover, targeting TLR signaling improves cartilage repair and bone remodeling during osteoporosis [35]. Thus, the TLR signaling pathway is closely associated with osteoporosis. In our study, the TLR signaling pathway was activated by OVX surgery and was inactivated by HMGB-1 deletion. Additionally, the activation of TLR signaling pathway by LPS aggravated OVX-induced osteoporosis and HMGB-1 deficiency relieved osteoporosis. Thus, HMGB-1 deficiency may relieve osteoporosis via suppressing the TLR signaling pathway.
Conclusions
The present study reveals that knockdown of HMGB1 may attenuate OVX-induced osteoporosis by suppressing osteoclastogenesis and inflammatory response. Moreover, the blockage of TLR4 signaling was responsible for above process. Therefore, targeting HMGB-1 may represent a promising therapeutic approach against osteoporosis. However, there still remain some defects in this study. Of course, other possible pathway mechanisms associated with HMGB-1 may also affect osteoporosis process and will be further investigated in our next plan.
Acknowledgements
Thanks to the assistance of The Third Affiliated Hospital of Guangzhou Medical University.
Abbreviations
- HMGB-1
High-mobility group box chromosomal protein-1
- OVX
Ovariectomy
- CT
Computed tomography
- HE
Hematoxylin–eosin
- TRAP
Tartrate-resistant acid phosphatase
- ALP
Alkaline phosphatase
- TNF-α
Tumor necrosis factor-α
- IL-4
Interleukin
- TLR4
Toll-like receptor 4
- WT
Wild type
- KD
Knockdown
- BS/BV
Bone surface/bone volume
- BSA/BV
Trabecular bone surface/bone volume
- BV/TV
Bone volume/tissue volume
- Tb. Th
Trabeculae thickness
- Tb. N
Trabeculae number
- Tb. Sp
Trabeculae separation
- SDS-PAGE
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- OSCAR
Osteoclast-associated receptor
- TRAP
Tartrate resistant acid phosphatase
- OSX
Osterix
- COL1A1
Collagen, type I, alpha 1
Authors' contributions
HY and WZ conducted all the experiments and arranged the figures and the manuscript. ZZ and RQ assisted with animal preparation and data analyzing. GC revised the manuscript critically. PZ initiated and supervised the project, and formed the conclusion. All authors read and approved the final manuscript.
Funding
The present study was supported by a grant from the Science and technology development special fund of Guangdong Province (grant no. 2017ZC0255).
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
The present study was approved by the Ethics Review Board of The third Affiliated Hospital of Guangzhou Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1186/s13018-025-05623-4
Change history
3/4/2025
This article has been retracted. Please see the Retraction Notice for more detail: 10.1186/s13018-025-05623-4
References
- 1.Johnston CB, Dagar M. Osteoporosis in older adults. Med Clin N Am. 2020;104(5):873–84. 10.1016/j.mcna.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Bliuc D, Nguyen ND, Alarkawi D, Nguyen TV, Eisman JA, Center JR. Accelerated bone loss and increased post-fracture mortality in elderly women and men. Osteoporos Int. 2015;26(4):1331–9. 10.1007/s00198-014-3014-9. [DOI] [PubMed] [Google Scholar]
- 3.Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2013;8(1):136. 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S, McCauley LK, McGowan J, McKee MD, Mohla S, Pendrys DG, Raisz LG, Ruggiero SL, Shafer DM, Shum L, Silverman SL, Van Poznak CH, Watts N, Woo SB, Shane E. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22(10):1479–91. 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 5.Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster D, Einhorn TA, Genant HK, Geusens P, Klaushofer K, Koval K, Lane JM, McKiernan F, McKinney R, Ng A, Nieves J, O’Keefe R, Papapoulos S, Sen HT, van der Meulen MC, Weinstein RS, Whyte M. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25(11):2267–94. 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 6.Loi F, Córdova LA, Pajarinen J, Lin TH, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone. 2016;86:119–30. 10.1016/j.bone.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res. 1996;11(8):1043–51. 10.1002/jbmr.5650110802. [DOI] [PubMed] [Google Scholar]
- 8.Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42(6):551–5. 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Daghri NM, Aziz I, Yakout S, Aljohani NJ, Al-Saleh Y, Amer OE, Sheshah E, Younis GZ, Al-Badr FBM. Inflammation as a contributing factor among postmenopausal Saudi women with osteoporosis. Medicine (Baltimore). 2017;96(4): e5780. 10.1097/MD.0000000000005780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mundy GR. Osteoporosis and inflammation. Injury. 2007;65(12 Pt 2):S147-151. 10.1111/j.1753-4887.2007.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 11.Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Investig. 2008;118(11):3537–45. 10.1172/jci36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang DH, Yang MY. The role of macrophage in the pathogenesis of osteoporosis. Int J Mol Sci. 2019. 10.3390/ijms20092093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeganathan S, Fiorino C, Naik U, Sun HS, Harrison RE. Modulation of osteoclastogenesis with macrophage M1- and M2-inducing stimuli. PLoS One. 2014;9(8):e104498. 10.1371/journal.pone.0104498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ulloa L, Batliwalla FM, Andersson U, Gregersen PK, Tracey KJ. High mobility group box chromosomal protein 1 as a nuclear protein, cytokine, and potential therapeutic target in arthritis. Arthritis Rheum. 2003;48(4):876–81. 10.1002/art.10854. [DOI] [PubMed] [Google Scholar]
- 15.Mandke P, Vasquez KM. Interactions of high mobility group box protein 1 (HMGB1) with nucleic acids: implications in DNA repair and immune responses. DNA Repair. 2019;83:102701. 10.1016/j.dnarep.2019.102701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chirico V, Lacquaniti A, Salpietro V, Munafò C, Calabrò MP, Buemi M, Arrigo T, Salpietro C. High-mobility group box 1 (HMGB1) in childhood: from bench to bedside. Eur J Pediatr. 2014;173(9):1123–36. 10.1007/s00431-014-2327-1. [DOI] [PubMed] [Google Scholar]
- 17.Kaur I, Behl T, Bungau S, Kumar A, Mehta V, Setia D, Uddin MS, Zengin G, Aleya L, Arora S. Exploring the therapeutic promise of targeting HMGB1 in rheumatoid arthritis. Life Sci. 2020;258:118164. 10.1016/j.lfs.2020.118164. [DOI] [PubMed] [Google Scholar]
- 18.Meng Y, Qiu S, Sun L, Zuo J. Knockdown of exosome-mediated lnc-PVT1 alleviates lipopolysaccharide-induced osteoarthritis progression by mediating the HMGB1/TLR4/NF-kappaB pathway via miR-93-5p. Mol Med Rep. 2020;22(6):5313–25. 10.3892/mmr.2020.11594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pullerits R, Urbonaviciute V, Voll RE, Forsblad-D’Elia H, Carlsten H. Serum levels of HMGB1 in postmenopausal patients with rheumatoid arthritis: associations with proinflammatory cytokines, acute-phase reactants, and clinical disease characteristics. J Rheumatol. 2011;38(7):1523–5. 10.3899/jrheum.110091. [DOI] [PubMed] [Google Scholar]
- 20.Feng L, Xia B, Tian BF, Lu GB. MiR-152 influences osteoporosis through regulation of osteoblast differentiation by targeting RICTOR. Pharm Biol. 2019;57(1):586–94. 10.1080/13880209.2019.1657153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin Z, Zhu W, Wu Q, Zhang Q, Guo S, Liu T, Li S, Chen X, Peng D, Ouyang Z. Glycyrrhizic acid suppresses osteoclast differentiation and postmenopausal osteoporosis by modulating the NF-kappaB, ERK, and JNK signaling pathways. Eur J Pharmacol. 2019;859:172550. 10.1016/j.ejphar.2019.172550. [DOI] [PubMed] [Google Scholar]
- 22.Peng S, Zhang G, Zhang BT, Guo B, He Y, Bakker AJ, Pan X, Zhen W, Hung L, Qin L, Leung WN. The beneficial effect of icaritin on osteoporotic bone is dependent on the treatment initiation timing in adult ovariectomized rats. Bone. 2013;55(1):230–40. 10.1016/j.bone.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Sun W, Gao R, Su Y, Umehara H, Dong L, Gong F. The role of high mobility group box chromosomal protein 1 in rheumatoid arthritis. Rheumatology (Oxford). 2013;52(10):1739–47. 10.1093/rheumatology/ket134. [DOI] [PubMed] [Google Scholar]
- 24.Nogueira-Machado JA, de Oliveira Volpe CM. HMGB-1 as a target for inflammation controlling. Recent Patents Endocr Metab Immune Drug Discov. 2012;6(3):201–9. 10.2174/187221412802481784. [DOI] [PubMed] [Google Scholar]
- 25.Taniguchi N, Kawakami Y, Maruyama I, Lotz M. HMGB proteins and arthritis. Hum Cell. 2018;31(1):1–9. 10.1007/s13577-017-0182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69(3):990–1047. 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 27.Khairallah P, Nickolas TL. Management of osteoporosis in CKD. Clin J Am Soc Nephrol. 2018;13(6):962–9. 10.2215/cjn.11031017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martineau P, Silva BC, Leslie WD. Utility of trabecular bone score in the evaluation of osteoporosis. Curr Opin Endocrinol Diabetes Obes. 2017;24(6):402–10. 10.1097/med.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Gong Z. Clinical characteristics and prognostic factors in bone metastases from lung cancer. Med Sci Monit Int Med J Exp Clin Res. 2017;23:4087–94. 10.12659/msm.902971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Zhang W, Dai J, Wang X, Shen SG. Overexpression of Dlx2 enhances osteogenic differentiation of BMSCs and MC3T3-E1 cells via direct upregulation of Osteocalcin and Alp. Int J Oral Sci. 2019;11(2):12. 10.1038/s41368-019-0046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qu B, Liu O, Fang X, Zhang H, Wang Y, Quan H, Zhang J, Zhou J, Zuo J, Tang J, Tang Z. Distal-less homeobox 2 promotes the osteogenic differentiation potential of stem cells from apical papilla. Cell Tissue Res. 2014;357(1):133–43. 10.1007/s00441-014-1833-9. [DOI] [PubMed] [Google Scholar]
- 32.Miller CH, Smith SM. RBP-J-regulated miR-182 promotes TNF-α-induced osteoclastogenesis. J Immunol. 2016;196(12):4977–86. 10.4049/jimmunol.1502044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno JL, Kaczmarek M, Keegan AD, Tondravi M. IL-4 suppresses osteoclast development and mature osteoclast function by a STAT6-dependent mechanism: irreversible inhibition of the differentiation program activated by RANKL. Blood. 2003;102(3):1078–86. 10.1182/blood-2002-11-3437. [DOI] [PubMed] [Google Scholar]
- 34.Vijayan V, Khandelwal M, Manglani K, Gupta S, Surolia A. Methionine down-regulates TLR4/MyD88/NF-κB signalling in osteoclast precursors to reduce bone loss during osteoporosis. Br J Pharmacol. 2014;171(1):107–21. 10.1111/bph.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Zhao Q. AEG-1 deletion promotes cartilage repair and modulates bone remodeling-related cytokines via TLR4/MyD88/NF-kappaB inhibition in ovariectomized rats with osteoporosis. Ann Transl Med. 2020;8(20):1298. 10.21037/atm-20-5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.