Abstract
Rifaximin showed moderately high MICs (the MIC at which 90% of the isolates tested were inhibited = 50 μg/ml) for 145 bacterial enteropathogens from patients with traveler's diarrhea acquired in Mexico during the summers of 1997 and 1998. Rifaximin concentrations in stool the day after oral administration (800 mg daily for 3 days) were high (average, 7,961 μg/g), proving the value of the drug.
Bacterial agents are known to be the major cause of traveler's diarrhea. It has been shown that antibacterial agents are effective in the treatment and prevention of diarrhea among persons traveling from developed to developing countries (3, 4). Rifaximin is poorly absorbed after single or repeated oral administrations to humans, even in patients with a damaged colonic mucosa (2, 7, 11). It is a rifamycin derivative with activity against gram-positive, gram-negative, and anaerobic bacteria (8). Recently, we evaluated rifaximin in the treatment of traveler's diarrhea. It was shown to be superior to trimethoprim-sulfamethoxazole (5) and equivalent to ciprofloxacin in terms of clinical response (H. L. DuPont, C. D. Ericsson, J. J. Mathewson, Z. D. Jiang, F. Martinez-Sandoval, E. Palazzini, and L. M. Riopel, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2227, 1999).
The objectives of this study were to determine the in vitro susceptibility to rifaximin of bacterial enteropathogens isolated from cases of traveler's diarrhea and to measure rifaximin concentrations in fecal samples after oral administration.
The bacterial strains used were isolates from adults who contracted traveler's diarrhea during the summers of 1997 and 1998 in Guadalajara, Mexico. The strains were identified by standard procedures and were stored at −70°C. The following numbers of strains were tested: 120 enterotoxigenic strains of Escherichia coli (ETEC), 17 Shigella strains, and 8 Salmonella strains.
MICs of rifaximin for each bacterial strain were determined by agar dilution testing according to the methods of the National Committee for Clinical Laboratory Standards (10). Agar dilution plates were prepared by making a stock solution of rifaximin with acetone and preparing twofold dilutions from 200 to 0.1 μg/ml. Plates with rifaximin were inoculated with 104 bacteria and incubated at 37°C overnight.
For quality control of antimicrobial potency, the rifaximin MICs for the recommended control strains (E. coli ATCC 25922, Staphylococcus aureus ATCC 29213, and Pseudomonas aeruginosa ATCC 27853) were determined daily with the test strains. MICs were defined as the lowest concentration of rifaximin that completely inhibited visible growth. A fine, barely visible haze or a single colony was disregarded.
This study was part of a large clinical trial where rifaximin was compared with ciprofloxacin in the treatment of traveler's diarrhea (DuPont et al., 39th ICAAC). Thirty-nine adult patients were included in this part of the study. All patients (≥18 years of age) had acute diarrhea but were otherwise in good health. The patients received 400-mg tablets of rifaximin every 12 h for 3 days consecutively. They were instructed to provide the next stool passed after the 3-day treatment period.
One gram of each stool sample collected posttherapy was diluted with 1 ml of solution in acetone. The mixture was vortexed and centrifuged for 10 min at 1,500 × g. The supernatant was filtered through 0.22-μm-pore-size filters (Nalge, Rochester, N.Y.). High-performance liquid chromatography was done with a model 717 autosampler and a 510 pump (Waters Corp., Milford, Mass.). Detection was done using a Waters 486 tunable absorbance detector. The mobile phase (made with 50 ml of phosphate [0.5 M, pH 7.2], 480 ml of water, and 470 ml of acetonitrile buffer) was filtered through a Millipore 0.2-μm-pore-size membrane. Under these conditions, the rifaximin standard retention time was 7.5 min. Quantitations of rifaximin concentration were based on the relative peak highest response ratio of each compound and the internal standard.
Rifaximin was shown to have an MIC at which 50% of the isolates tested were inhibited of 12.5 μg/ml and an MIC at which 90% of the isolates tested were inhibited of 50 μg/ml for the 145 bacterial isolates tested. The MICs for two ETEC and one Salmonella isolates were ≤0.098 μg/ml; overall, the MIC ranges were ≤0.098 to 200 μg/ml for the Salmonella and ETEC isolates and 1.25 to 200 μg/ml for the Shigella isolates.
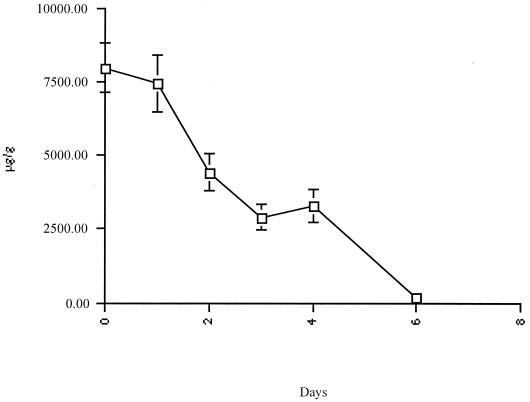
In Fig. 1, posttherapy stool rifaximin levels are graphically illustrated for the 39 patients studied. The concentrations of rifaximin in stool in each interval of collection were high, decreasing gradually over a 5-day period after therapy was completed. The posttreatment fecal concentrations of rifaximin exceeded the MICs for the bacterial isolates obtained in the study, with average drug levels of 7,961 μg/g on the first day posttreatment, 7,425 μg/g on the second, 4,405 μg/g on the third, 2,891 μg/g on the fourth, 3,266 μg/g on the fifth, and 154 μg/g on the sixth.
FIG. 1.
Stool concentrations of rifaximin in 39 patients with traveler's diarrhea tested after treatment with 800 mg of rifaximin/day. Bars, standard errors of the means.
Rifaximin is currently used in Italy to treat enteric bacterial infection (6), small intestinal bacterial overgrowth (1), and portosystemic encephalopathy (9). Rifaximin has a broad antimicrobial spectrum that includes gram-positive and gram-negative aerobic and anaerobic bacteria (11). As shown in the present study, rifaximin has inhibitory activity against bacterial enteropathogens identified in patients with traveler's diarrhea, although its MICs are high. Ordinarily these strains would be considered resistant to the antimicrobial agent. Rifaximin is largely unabsorbed, since less than 0.01% of the oral dose is detectable in plasma (2). It attains very high intestinal levels. In the present study, fecal levels reached 4,000 to 8,000 μg/g after 3 days of therapy with the drug. If the average daily stool weight passed per day is 150 g, up to 600 to 1,200 mg of rifaximin is eliminated in feces. The patients in the present study took a total of 400 mg of rifaximin twice a day for 3 days. This gives a total of 2,400 mg taken in the 3 days. If one looks at the fecal levels measured, it appears that most of the administered drug appeared in feces. These data agree with previous pharmacokinetic studies conducted with patients and adult volunteers (2, 7, 11).
Based on the results of two clinical trials (7, 8) in which rifaximin successfully shortened the course of traveler's diarrhea, we feel that it is a promising drug for the condition. Currently there is concern that fluoroquinolones should not be used broadly for common infections, to delay the spread of antimicrobial resistance which is already occurring (12). A concern with rifaximin is the emergence of rifampin resistance among mycobacteria, Neisseria meningitidis, and Enterococcus.
Acknowledgments
This project was supported by Alfa Wassermann, S.p.A., Bologna, Italy.
REFERENCES
- 1.Corazza G R, Ventrucci M, Strocchi A, Sorge M, Pranzo L, Pezzilli R, Gasbarrini G. Treatment of small intestine bacterial overgrowth with rifaximin, a non-absorbable rifamycin. J Int Med Res. 1988;16:312–316. doi: 10.1177/030006058801600410. [DOI] [PubMed] [Google Scholar]
- 2.Descombe J J, Dubourg D, Picard M, Palazzini E. Pharmacokinetic study of rifaximin after oral administration in healthy volunteers. Int J Clin Pharmacol Res. 1994;16:51–56. [PubMed] [Google Scholar]
- 3.DuPont H L, Reves R R, Galindo E, Sullivan P S, Wood L V, Mendiola J G. Treatment of travelers' diarrhea with trimethoprim/sulfamethoxazole and with trimethoprim alone. N Engl J Med. 1982;307:841–844. doi: 10.1056/NEJM198209303071401. [DOI] [PubMed] [Google Scholar]
- 4.DuPont H L, Ericsson C D. Prevention and treatment of traveler's diarrhea. N Engl J Med. 1993;328:1821–1827. doi: 10.1056/NEJM199306243282507. [DOI] [PubMed] [Google Scholar]
- 5.DuPont H L, Ericsson C D, Mathewson J J, Palazzini E, DuPont M W, Jiang Z D, Mosavi A, de la Cabada F J. Rifaximin: a nonabsorbed antimicrobial in the therapy of travelers' diarrhea. Digestion. 1998;59:708–714. doi: 10.1159/000007580. [DOI] [PubMed] [Google Scholar]
- 6.Gillis J, Brogden R. Rifaximin: a review of its antibacterial activity, pharmacokinetic properties and therapeutic potential in conditions mediated by gastrointestinal bacteria. Drugs. 1995;49:467–484. doi: 10.2165/00003495-199549030-00009. [DOI] [PubMed] [Google Scholar]
- 7.Gionchetti P, Rizzello F. Rifaximin in patients with moderate or severe ulcerative colitis refractory to steroid-treatment: a double-blind, placebo-controlled trial. Dig Dis Sci. 1999;44:1220–1221. doi: 10.1023/a:1026648812439. [DOI] [PubMed] [Google Scholar]
- 8.Hoover W, Gerlach E, Hoban D, Eliopoulos G, Pfaller M, Jones R. Antimicrobial activity and spectrum of rifaximin, a new tropical rifamycin derivative. Diagn Microbiol Infect Dis. 1993;16:111–118. doi: 10.1016/0732-8893(93)90004-q. [DOI] [PubMed] [Google Scholar]
- 9.Miglio F, Valpiani D, Ricca Rossellini S, Ferrieri A. Rifaximin, a non-absorbable rifamycin, for the treatment of hepatic encephalopathy. A double blind randomized trial. Curr Med Res Opin. 1997;13:593–601. doi: 10.1185/03007999709113333. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed. 1997. Approved standard. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 11.Rizzello F, Gionchetti P, Venturi A, Ferretti M, Peruzzo S, Raspanti X, Picard M, Canova N, Palazzini E, Campieri M. Rifaximin systemic absorption in patients with ulcerative colitis. Eur J Clin Pharmacol. 1998;54:91–93. doi: 10.1007/s002280050426. [DOI] [PubMed] [Google Scholar]
- 12.Segreti J, Gootz T D, Goodman L J, Parkhurst G W, Quinn J P, Martin B A, Trenholme G M. High-level quinolone resistance in clinical isolates of Campylobacter jejuni. J Infect Dis. 1992;165:667–670. doi: 10.1093/infdis/165.4.667. [DOI] [PubMed] [Google Scholar]
- 13.Verardi S, Verardi V. Bile rifaximin concentration after oral administration in patients undergoing cholecystectomy. Farmaco. 1990;45:131–135. [PubMed] [Google Scholar]