Abstract
Objectives:
In 2015, the Republic of Georgia initiated a National Hepatitis C Elimination Program, with a goal of 90% reduction in prevalence of chronic hepatitis C virus (HCV) infections by 2020. In this article, we explore the impact of the COVID-19 pandemic on the 2020 hepatitis C cascade of care in Georgia.
Study design:
Retrospective analytic study.
Methods:
We used a national screening registry that includes hospitals, blood banks, antenatal clinics, harm reduction sites, and other programs and services to collect data on hepatitis C screening. A separate national treatment database was used to collect data on viremia and diagnostic testing, treatment initiation, and outcome including testing for and achieving sustained virologic response (SVR). We used these databases to create hepatitis C care cascades for 2020 and 2019. Bivariate associations for demographic characteristics and screening locations per year and care cascade comparisons were assessed using a chi-squared test.
Results:
In 2020 compared to 2019, the total number of persons screened for HCV antibodies decreased by 25% (from 975,416 to 726,735), 59% fewer people with viremic infection were treated for HCV infection (3188 vs. 7868), 46% fewer achieved SVR (1345 vs. 2495), a significantly smaller percentage of persons with viremic infection initiated treatment for HCV (59% vs. 62%), while the percentage of persons who achieved SVR (99.2% vs. 99.3%) remained stable.
Conclusions:
The COVID-19 pandemic had a negative impact on the hepatitis C elimination program in Georgia. To ensure Georgia reaches its elimination goals, mitigating unintended consequences of delayed diagnosis and treatment of hepatitis C due to the COVID-19 pandemic are paramount.
Keywords: Hepatitis C (HCV), HCV elimination, COVID-19, Cascade of care, Georgia
Introduction
Georgia is a small country in the South Caucasus with a population of 3.7 million and a high prevalence of chronic hepatitis C virus (HCV) infection among the adult population. The national serosurvey in 2015 estimated that HCV viremic prevalence was 5.4%, and more than 150,000 Georgians were infected with HCV.1 In April of 2015, Georgia initiated a National Hepatitis C Elimination Program, which provides free treatment with direct-acting antivirals (DAAs) for all citizens, and set the ambitious target of a 90% reduction in the prevalence of chronic HCV infection by 2020.1 As of October 2019, prior to the start of the Coronavirus disease (COVID-19) pandemic, 53% of the estimated number of adults with chronic HCV infection had been identified as part of the elimination program and 78% of them initiated treatment.2 On average, 1000 persons were initiating treatment each month, which would have reduced HCV prevalence by 51% and incidence by 51% by the end of 2020.3 The progress toward the elimination was substantial, but continued scale-up is needed to reach elimination targets.4,5
The first severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) case in Georgia was reported at the end of February 2020.6 In response to the emerging pandemic, the Government of Georgia declared a state of emergency on March 21st, which progressed into a full national lockdown on March 30th. The lockdown measures included quarantining all international arrivals, closing borders and airports, restricting movement inside the country, banning mass gatherings, and maintaining closure of all schools, preschools, and universities. These measures were effective at controlling SARS-CoV-2 community spread, with only 1510 cumulative cases reported through September 1, 2020.6 Cases started to increase again in late September, and the number of COVID-19 cases across the country reached nearly 228,000 by the end of 2020. From November 2020 through January 2021, new restrictions and lockdowns were imposed.
Mitigation strategies deployed to reduce SARS-CoV-2 transmission in Georgia created new challenges for the hepatitis C elimination program. Travel restrictions in Georgia coupled with a suspension of most in-person healthcare delivery further reduced screening efforts and patients’ ability to seek care. Despite continued efforts to adapt (e.g., health service providers in Georgia increased the number of DAA pills per prescription, organized delivery of the prescribed medications including for those in quarantine or isolation, implemented distance-based provision of medical care where possible and patients with chronic conditions who were enrolled in the elimination program were asked to visit healthcare facilities every 28 days instead of 14), the monthly number of people tested and treated declined. In this study, we compare the HCV care cascades for 2019 and 2020 to determine the impact of the COVID-19 pandemic on a well-established hepatitis C elimination program in Georgia.
Methods
In 2017, a comprehensive national screening registry was created in Georgia to collect data on hepatitis C screening, including hospitals, blood banks, antenatal clinics, harm reduction sites, and other programs and services.2 Data from 2015 onward are available in the registry, including date and HCV antibody (anti-HCV) test results, age, sex, and location. A separate treatment database was also created for program monitoring and evaluation, which collects data on demographics, viremia and diagnostic testing, treatment initiation, and outcome including testing for sustained virologic response (SVR), and achieving SVR. In Supplementary Tables 1 and 2, we present a list of different methods used to detect HCV antibodies and HCV viremic infection in Georgia. SVR is always determined by polymerase chain reaction (PCR). All national data from both the screening registry and the treatment database are included in the analysis, linked by patients’ unique national ID. All data were deidentified, and national IDs were encrypted prior to analysis.
Care cascades were created and compared for 2020 and 2019 (using data from January 1 to December 31) to evaluate the potential impact of COVID-19 pandemic on the hepatitis C cascades of care in Georgia’s hepatitis C elimination program. No major programmatic changes were implemented during 2020 that would have otherwise substantially affected rates of screening or linkage to care. Monthly screening rates were computed, and demographic characteristics and location of screening were compared. For monthly screening rates, those screened multiple times were counted once for each month in which they were screened. For annual comparisons, repeat screeners were counted once per year, using data from the first time an individual was screened in a calendar year. To compare monthly screening for HCV prepandemic and during the pandemic, we calculated the percentage of persons screened in each month of 2020 compared to the same month of 2019.
To analyze linkage to care and treatment outcomes, separate care cascades were created for 2019 and 2020 based on the year in which a person first tested anti-HCV positive. Treatment data were included through February of the year following initial positive anti-HCV result (e.g., February 2020 for those screened positive in 2019) to better capture treatment initiation and SVR testing, which is performed 12–24 weeks after treatment completion. Bivariate associations for demographic characteristics and screening locations per year and care cascade comparisons were assessed using a chi-squared test. We considered findings to be statistically significant if the two-sided P-value was <.05. All analyses were performed in SAS, version 9.4 (Cary, North Carolina, USA).
Results
In 2019, 975,416 people were screened for anti-HCV and 21,405 tested positive. Of these, 12,627 were viremic and 7868 were treated for HCV infection. In 2020, 726,735 people were screened, 10,899 tested positive for anti-HCV, 5433 were viremic, and 3188 were treated. The total number of persons who were screened for anti-HCV decreased by 25.5% in 2020 compared to 2019, 59% fewer people were treated (3188 vs. 7868), and 46% fewer achieved SVR (1345 vs. 2495). Compared to the number of persons screened for HCV in 2019, the largest reduction occurred in October of 2020 (46% of 2019 levels), followed by April and November (both 47% of 2019 levels) (Fig. 1). Persons screened for anti-HCV in 2019 and 2020 were similar in age (median age: 41 years vs. 42, respectively) and sex (56.4% vs. 55.4% female). In 2020, we observed an increase in the percentage of persons screened for anti-HCV at blood banks (4.9% vs. 7.1%), antenatal clinics (3.1% vs. 5.3%), and inpatient settings (32.5% vs. 33.4%) and a decrease in the percentage tested in outpatient clinics (57.7% vs. 53.2%) and harm reduction programs (0.6% vs. 0.3%) (P-values all <.001; Table 1).
Fig. 1.
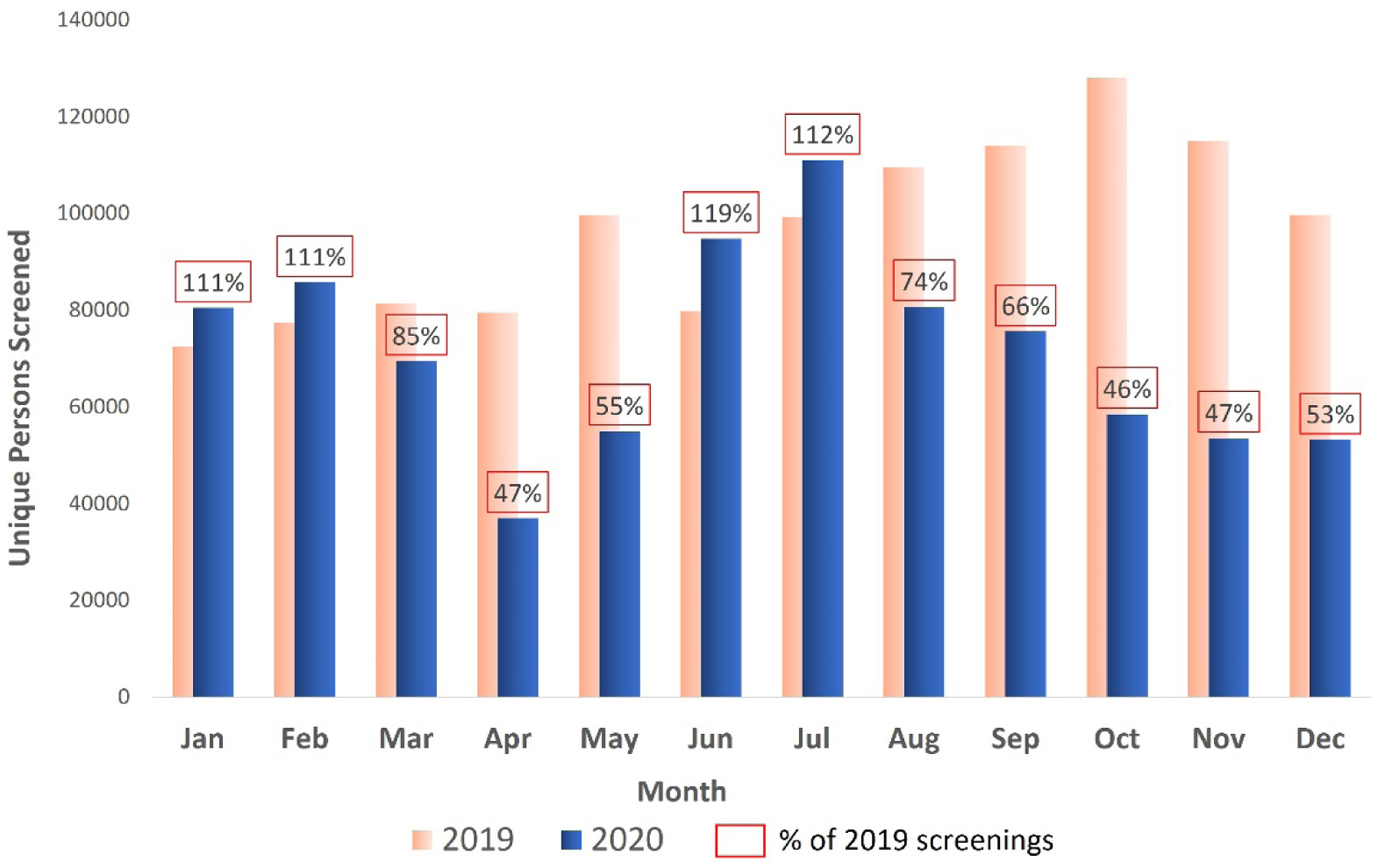
Monthly hepatitis C antibody screening rates by year, Georgia, 2019–2020. To compare monthly screening for HCV prepandemic and during the pandemic, we calculated the percentage of persons screened in each month of 2020 compared to the same month of 2019.
Table 1.
Demographic characteristics and hepatitis C screening settings by year, Georgia, 2019–2020
| 2019 (n = 975,416) |
2020 (n = 726,735) |
P-value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age Group | |||||
| < 18 | 122,130 | 12.5 | 77,554 | 10.7 | <.001 |
| 18–29 | 180,744 | 18.5 | 138,914 | 19.1 | |
| 30–39 | 159,241 | 16.3 | 124,844 | 17.2 | |
| 40–49 | 128,174 | 13.2 | 94,467 | 13.0 | |
| 50–59 | 135,263 | 13.9 | 96,116 | 13.2 | |
| ≥ 60 | 248,851 | 25.5 | 194,305 | 26.8 | |
| Median age, years (IQR) | 41 (26, 60) | 42 (27, 61) | <.001 | ||
| Sex | |||||
| Female | 550,160 | 56.4 | 402,489 | 55.4 | <.001 |
| Male | 425,256 | 43.6 | 324,246 | 44.6 | |
| Screening Setting * | |||||
| Outpatient | 563,000 | 57.7 | 387,000 | 53.2 | <.001 |
| Inpatient | 317,000 | 32.5 | 243,000 | 33.4 | |
| Harm Reduction | 5499 | 0.6 | 2076 | 0.3 | |
| Blood Bank | 48,034 | 4.9 | 51,765 | 7.1 | |
| Antenatal Clinic | 30,067 | 3.1 | 38,231 | 5.3 | |
| Other | 12,033 | 1.2 | 5233 | 0.7 | |
| Region * | |||||
| Tbilisi | 331,094 | 34.0 | 238,860 | 34.3 | <.001 |
| Adjara | 122,733 | 12.6 | 72,844 | 10.5 | |
| Guria | 50,573 | 5.2 | 33,310 | 4.8 | |
| Imereti | 143,314 | 14.7 | 113,058 | 16.2 | |
| Kakheti | 87,750 | 9.0 | 46,533 | 6.7 | |
| Kvemo Kartli | 54,195 | 5.6 | 37,124 | 5.3 | |
| Mtskheta-Mtianeti | 14,975 | 1.5 | 11,416 | 1.6 | |
| Racha-Lechkhumi-Kvemo Svaneti | 10,848 | 1.1 | 2680 | 0.4 | |
| Samegrelo-Zemo Svaneti | 101,022 | 10.4 | 66,149 | 9.5 | |
| Samtskhe-Javakheti | 20,598 | 2.1 | 38,072 | 5.5 | |
| Shida Kartli | 37,523 | 3.8 | 35,723 | 5.1 | |
Location of earliest screening in time period; IQR = interquartile range
In 2020, among all persons screened for HCV, 1.5% (n = 10,899) were anti-HCV positive, 70.8% of them were tested for viremia, and 70.4% of those tested had HCV infection. Among persons with viremia, 58.7% initiated treatment, 84.6% of whom completed treatment, and 76.5% of them were eligible to be tested for SVR. Among those eligible for SVR, 65.7% were tested and 99.2% achieved SVR (Fig. 2).
Fig 2.
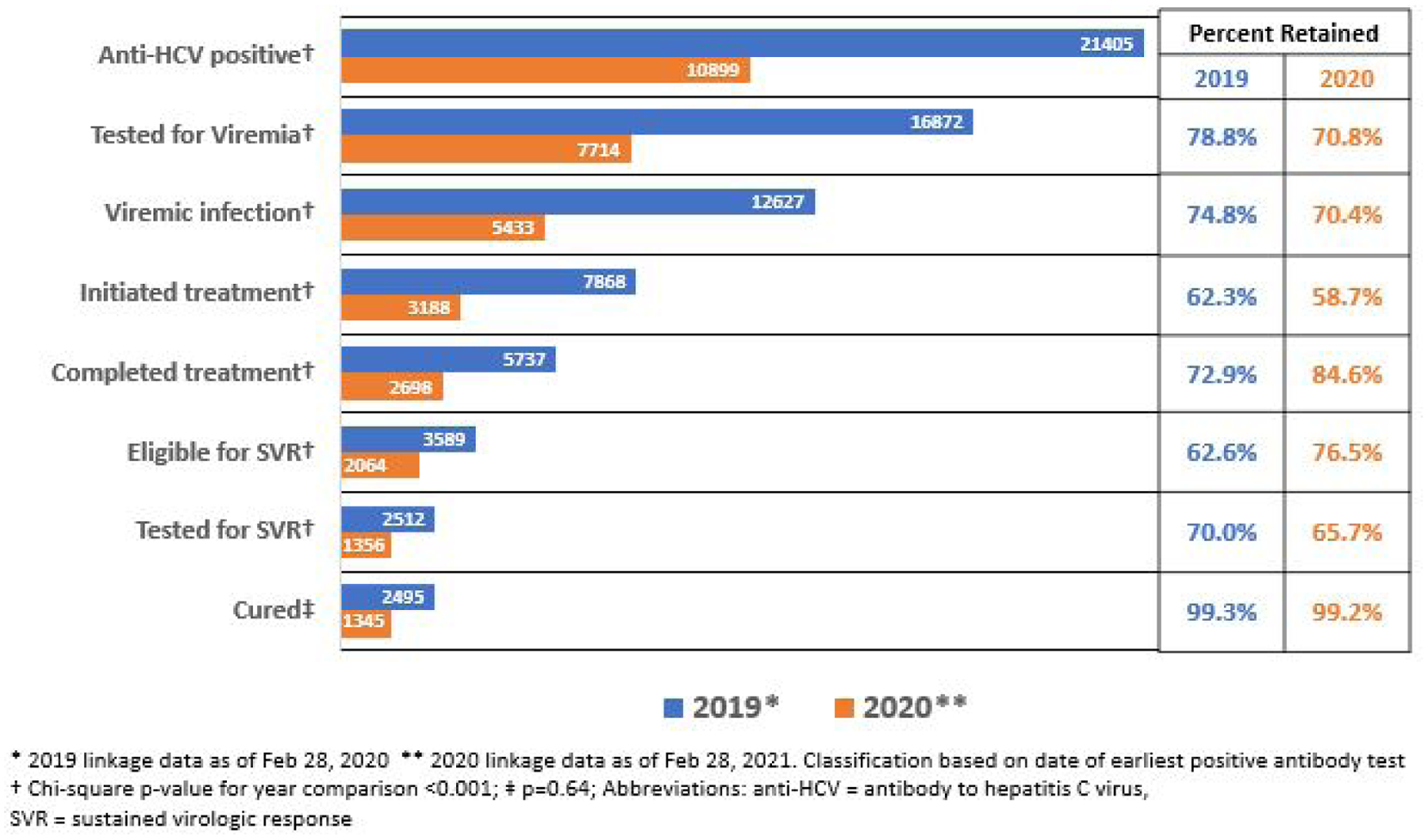
Comparison of hepatitis C care cascade by year of earliest positive antibody test, Georgia, 2019–2020.
Compared to 2019, there were fewer people at each step of HCV cascade of care in 2020. However, in 2020, the percentage of people who completed treatment (84.6% vs. 72.9%) and who were eligible for SVR testing (76.5% vs. 62.6%) was significantly higher than in 2019 (P-values all <.001). Conversely, there was a significantly smaller percentage of people who were anti-HCV positive (1.5% vs. 2.2%), tested for HCV viremia (70.8% vs. 78.8%), confirmed to have viremic infection (70.4% vs. 74.8%), initiated treatment for HCV (58.7% vs. 62.3%), and tested for SVR (65.7% vs. 70.0%) in 2020 than in 2019 (P-values all <.001). The percentage of persons who achieved SVR (99.2% vs. 99.3%; P-value = .64) or discontinued treatment (3.2% vs. 3.3%; P-value = .72) was similar in 2020 and 2019, respectively.
Discussion
COVID-19 significantly impacted many aspects of health policy, programs, and healthcare delivery throughout 2020. The Georgian Government acted swiftly in March 2020 to impose restrictions that proved effective at reducing transmission of SARS-CoV-2.5 While restrictions on population movement and mitigation measures were effective in reducing SARS-CoV-2 transmission, they led to challenges for the hepatitis C elimination program. A similar reduction in access and use of different healthcare services was observed across the Europe.7,8 Overall, there was a reduction in the number of individuals engaging in and benefiting from the program, hampering progress toward elimination targets. To adapt, the hepatitis C elimination program increased pill counts dispensed, adopted medication delivery systems, and utilized distance-based care (e.g., telemedicine). To continue progress toward hepatitis elimination, the hepatitis C elimination program must further adapt and find strategies to increase the number of people being screened, tested, and treated for HCV infection.
Our analysis showed that in Georgia, screening for anti-HCV was one of the areas most affected by COVID-19 related restrictions. The number of persons screened in April of 2020 was approximately half of what it was the same month of 2019. Restrictions were gradually lifted in late April 2020, and the state of emergency ended in May 2020. Shortly afterward, in June and July, there was a rebound in the number of screening tests conducted. When a second wave of SARS-CoV-2 cases occurred in September of 2020 and new restrictions were imposed, the number of persons screened dropped to approximately 50% of what it had been the year before.6,9 This effect is likely multifactorial; during times of widespread community COVID-19 transmission and restrictions on movement, people are less likely to access in-person screening services and preventive services. The similar pattern of reduction in testing for HCV at the onset of COVID-19 pandemic and rebound in spring and summer of 2020 was observed in other countries.10,11 At the same time, the healthcare system diverted attention to the treatment of COVID-19 and away from screening for hepatitis and other conditions. The shared needs of COVID-19 and the hepatitis C elimination program highlight the importance of mitigation measures for SARS-CoV-2 (e.g., vaccination, testing, isolation of cases) to allow recuperation of other healthcare services. It also presents the opportunity to consider alternatives to in-person screening (e.g., at-home testing) and additional outreach to populations disproportionally impacted by restrictions due to the COVID-19 pandemic.
In addition to an overall reduction in the absolute number of persons enrolled in each step of the HCV care cascade, a smaller percentage of people were 1) tested for HCV viremia, 2) treated for HCV, and 3) tested for SVR in 2020 than in 2019. The reduction in viremia and SVR testing could be either consequent to individuals being less likely to seek care in-person from the sites able to conduct this advanced laboratory testing or because diagnostic testing was less readily available at decentralized locations during the COVID-19 pandemic. The existing infrastructure for HCV testing provided a foundation for SARS-CoV-2 assessment in local and regional settings, but resources may have been diverted to focus on SARS-CoV-2 diagnostics. During 2020, there was a shift in the venues where people were screened, with fewer people screened in outpatient settings. While testing in inpatient settings leads to reflex confirmatory testing, the proportion of people linked to treatment is often less than in outpatient settings.4 Care provided at outpatient primary care sites in Georgia resulted in higher rates of retention in the care cascade.12 Since identification of people with viremia and subsequent treatment ultimately reduces the rate of further transmission in the population, measures to address deficiencies in these steps in the cascade should be considered.13
In 2020, among those screened and tested for viremia, we observed a lower percentage positive for both compared to 2019. This finding could be the result of the advances of hepatitis C elimination program and the fact that a large number of persons are already diagnosed and treated for HCV infection in Georgia. In addition, during the pandemic, population groups with a higher prevalence of HCV viremic infection, such as persons who inject drugs (PWIDs),14 experienced additional challenges in accessing healthcare services and harm reduction services (HRS), including hepatitis C screening and treatment. In 2020, substantially fewer people were screened for anti-HCV at HRS than in 2019. Lower participation in HCV testing by persons who are at a higher risk for HCV (e.g., PWID) in 2020 could have caused lower HCV viremia positivity than in prepandemic time. Decreases in hepatitis C testing and treatment among PWIDs, in addition to less frequent use of prevention interventions such as needle and syringe programs (NSPs) and opioid substitution treatment (OST), could lead to increases in HCV transmission among PWID, further increasing hepatitis C incidence and prevalence and making it more challenging for Georgia to reach HCV elimination goals.15,16 It is important to ensure that despite the COVID-19 pandemic, PWID and persons with substance use disorder continue to have low barriers to access HCV treatment and prevention services such as NSPs and OST that are shown to reduce the risk of HCV acquisition.17
Our analysis is subject to limitations. The national treatment database, which contains information on all diagnosed persons enrolled in the hepatitis C elimination program, provides accurate treatment-related information on a national level. However, this database has limited ability to explain why persons are lost to follow-up or what are the main reasons for such large reductions in the number of persons in each step of the cascade of care in 2020 compared to 2019. There may have been other factors contributing to a decrease in screening for hepatitis C between 2019 and 2020 unrelated to the COVID-19 pandemic which could not be assessed in this analysis.
Conclusions
In this article, we present the impact that the COVID-19 pandemic has had on reductions in hepatitis C testing and treatment in the hepatitis C elimination program in Georgia. These reductions could lead to an increase in HCV transmission and HCV-related morbidity and mortality and could threaten Georgian progress toward HCV elimination goals. Georgia has committed to eliminate hepatitis C, and efforts aimed at mitigating unintended consequences of delayed diagnosis and treatment of hepatitis C due to the COVID-19 pandemic are paramount to ensuring Georgia can reach its national hepatitis C elimination goals.
Supplementary Material
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Ethical approval
Data for this analysis come from Georgia’s National Hepatitis C Elimination Program, which was deemed by the Institutional Review Board of Georgia’s National Center for Disease Control (NCDC) to be a public health program activity. The United States CDC determined this activity was not research involving human subject. Some data from this study were presented as the poster presentation at the EASL conference held virtually in June of 2021.
Competing interests
The authors have no commercial associations or sources of support that may pose a conflict of interest.
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
References
- 1.Nasrullah M, Sergeenko D, Gvinjilia L, Gamkrelidze A, Tsertsvadze T, Butsashvili M, et al. The role of screening and treatment in national progress toward hepatitis C elimination d Georgia, 2015–2016. MMWR Morb Mortal Wkly Rep 2017. Jul 28;66(29):773e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsertsvadze T, Gamkrelidze A, Chkhartishvili N, Abutidze A, Sharvadze L, Kerashvili V, et al. Progress towards achieving hepatitis C elimination in the country of Georgia, April 2015-October 2019. J Hepatol 2020. Aug 1;73:S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker JG, Kuchuloria T, Sergeenko D, Fraser H, Lim AG, Shadaker S, et al. Interim effect evaluation of the hepatitis C elimination programme in Georgia: a modelling study. Lancet Global Health 2020. Feb;8(2):e244e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Averhoff F, Shadaker S, Gamkrelidze A, Kuchuloria T, Gvinjilia L, Getia V, et al. Progress and challenges of a pioneering hepatitis C elimination program in the country of Georgia. J Hepatol 2020. Apr 1;72(4):680e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsertsvadze T, Gamkrelidze A, Chkhartishvili N, Abutidze A, Sharvadze L, Kerashvili V, et al. Three years of progress toward achieving hepatitis c elimination in the country of Georgia, April 2015-March 2018. Clin Infect Dis 2020. Aug 22;71(5):1263e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker JG, Tskhomelidze I, Trickey A, Getia V, Gvinjilia L, Imnadze P, et al. Epidemiology and transmission of COVID-19 in cases and close contacts in Georgia in the first four months of the epidemic. Pre-print. 2021. [Google Scholar]
- 7.Mansfield KE, Mathur R, Tazare J, Henderson AD, Mulick AR, Carreira H, et al. Indirect acute effects of the COVID-19 pandemic on physical and mental health in the UK: a population-based study. Lancet Digit Health 2021. Apr 1;3(4):e217e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smolić Ŝ, Čipin I, MeCimurec P. Access to healthcare for people aged 50+ in Europe during the COVID-19 outbreak. Eur J Ageing 2021;2020(March 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Center for Disease Control and Public Health. COVID-19 in Georgia. The Fifth Revision. Tbilisi, Georgia. 2020. [Google Scholar]
- 10.Simões D, Stengaard AR, Combs L, Raben D. Impact of the COVID-19 pandemic on testing services for HIV, viral hepatitis and sexually transmitted infections in the WHO European Region, March to August 2020, vol. 25. Eurosurveillance. European Centre for Disease Prevention and Control (ECDC); 2020. p. 2001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufman HW, Bull-Otterson L, Meyer WA, Huang X, Doshani M, Thompson WW, et al. Decreases in hepatitis C testing and treatment during the COVID-19 pandemic. Am J Prev Med 2021. Sep 10;61(3):369e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abutidze A Assessment of HCV screening and linkage to care modalities within the national hepatitis C elimination program and designing the most optimal models for reaching the elimination targets. In: 6th hepatitis C Technical Advisory Group (TAG) meeting. Tbilisi, Georgia; 2021. [Google Scholar]
- 13.Fu B, Wang W, Shi X. Impact of delayed diagnosis time in estimating progression rates to hepatitis C virus-related cirrhosis and death. Stat Methods Med Res 2015. Dec 18;24(6):693e710. [DOI] [PubMed] [Google Scholar]
- 14.Hagan LM, Kasradze A, Salyer SJ, Gamkrelidze A, Alkhazashvili M, Chanturia G, et al. Hepatitis C prevalence and risk factors in Georgia, 2015: setting a baseline for elimination. BMC Public Health 2019. May 10;19(S3):480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). EMCDDA trendspotter briefing: impact of COVID-19 on drug markets, drug use, drug-related harms and responses in east European Neighbourhood Policy countries. Lisabon. 2020. [Google Scholar]
- 16.Radfar SR, De Jong CAJ, Farhoudian A, Ebrahimi M, Rafei P, Vahidi M, et al. Reorganization of substance use treatment and harm reduction services during the COVID-19 pandemic: a global survey. Front Psychiatry 2021. Apr 29;12: 639393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Platt L, Minozzi S, Reed J, Vickerman P, Hagan H, French C, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev 2017. Sep 18;2017(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


