Abstract
Introduction
The goal of this project was to create an up‐to‐date joint European clinical practice guideline for the diagnosis and treatment of faecal incontinence (FI), using the best available evidence. These guidelines are intended to help guide all medical professionals treating adult patients with FI (e.g., general practitioners, surgeons, gastroenterologists, other healthcare workers) and any patients who are interested in information regarding the diagnosis and management of FI.
Methods
These guidelines have been created in cooperation with members from the United European Gastroenterology (UEG), European Society of Coloproctology (ESCP), European Society of Neurogastroenterology and Motility (ESNM) and the European Society for Primary Care Gastroenterology (ESPCG). These members made up the guideline development group (GDG). Additionally, a patient advisory board (PAB) was created to reflect and comment on the draft guidelines from a patient perspective. Relevant review questions were established by the GDG along with a set of outcomes most important for decision making. A systematic literature search was performed using these review questions and outcomes as a framework. For each predefined review question, the study or studies with the highest level of study design were included. If evidence of a higher‐level study design was available, no lower level of evidence was sought or included. Data from the studies were extracted by two reviewers for each predefined important outcome within each review question. Where possible, forest plots were created. After summarising the results for each review question, a systematic quality assessment using the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) approach was performed. For each review question, we assessed the quality of evidence for every predetermined important outcome. After evidence review and quality assessment were completed, recommendations could be formulated. The wording used for each recommendation was dependent on the level of quality of evidence. Lower levels of evidence resulted in weaker recommendations and higher levels of evidence resulted in stronger recommendations. Recommendations were discussed within the GDG to reach consensus.
Results
These guidelines contain 45 recommendations on the classification, diagnosis and management of FI in adult patients.
Conclusion
These multidisciplinary European guidelines provide an up‐to‐date comprehensive evidence‐based framework with recommendations on the diagnosis and management of adult patients who suffer from FI.
Keywords: clinical guidelines, diagnosis, faecal incontinence, fecal incontinence, GRADE, guidelines, ptns, treatment, Sacral neuromodulation, unwanted loss of feces
RECOMMENDATIONS

Key summary.
Current knowledge
Faecal incontinence is debilitating anorectal problem with an estimated prevalence of 7.7%.
There is a lack of up‐to‐date, evidence based guidance according to international standards for clinicians for the diagnosis and treatment of adult patients with faecal incontinence.
What is new here
These guidelines contain 45 up‐to‐date recommendations on the classification, diagnosis and management of faecal incontinence in adult patients.
A patient advisory board was involved in this project from start to finish to provide their invaluable perspectives and to ensure issues important to this patient group were covered.
General recommendation for all chapters

Evaluation of symptoms, diagnosis and classification

First line treatment

Diagnostic tests prior to second line treatment

Second line: non‐surgical interventions

Second line: surgical interventions

Special situations


Developing and other treatments
![]()

INTRODUCTION
The most recent guideline for management of faecal incontinence in adults was published in 2007. 3 Since then, a large number of studies have been published related to the treatment and diagnosis of faecal incontinence. As such, the goal of this project was to create an up‐to‐date joint European clinical practice guideline for the diagnosis and treatment of faecal incontinence (FI), using the best available evidence. This guideline has been created in cooperation with members from United European Gastroenterology (UEG), European Society of Coloproctology (ESCP), European Society of Neurogastroenterology and Motility (ESNM) and the European Society for Primary Care Gastroenterology (ESPCG).
This guideline provides guidance on the added value of diagnostic tests and the effectiveness of management options for FI. The guideline contains the following chapters:
-
‐
Evaluation of symptoms, diagnosis and classification
-
‐
First line treatment
-
‐
Diagnostic tests prior to second line treatment
-
‐
Second line: non‐surgical interventions
-
‐
Second line: surgical interventions
-
‐
Special situations
-
‐
Other and developing treatments
The management options have been grouped into chapters to improve readability. There is a variation between countries and sometimes even hospitals in which treatment options are available at which point in the treatment pathway of a patient suffering from FI. Some treatment options may be available to you or your patient at an earlier or later time point and may not correspond exactly to the order in which they have been presented within these guidelines.
This guideline is intended for use for all healthcare professionals treating patients with FI (e.g., general practitioners, gastroenterologists, colorectal surgeons, nurses etc.) and for any patients with FI who are interested in further information regarding the diagnosis and management of FI.
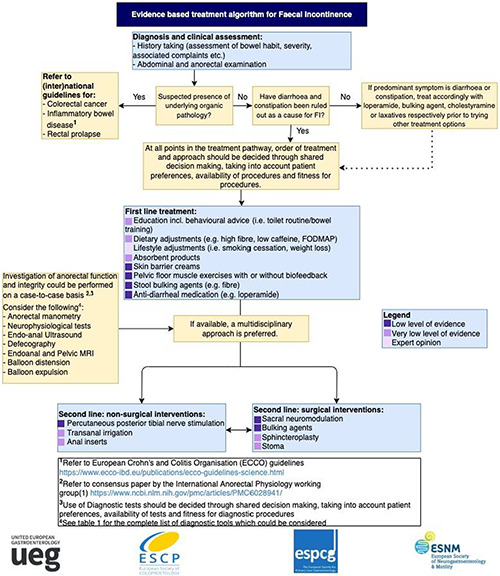
To summarise the results of this current guideline, an evidence‐based treatment algorithm (Figure 1) has been created which presents the most important recommendations formulated in this guideline. We suggest using this algorithm as a guide in combination with the main body of text to determine which possible steps can be taken when treating a patient with FI.
FIGURE 1.
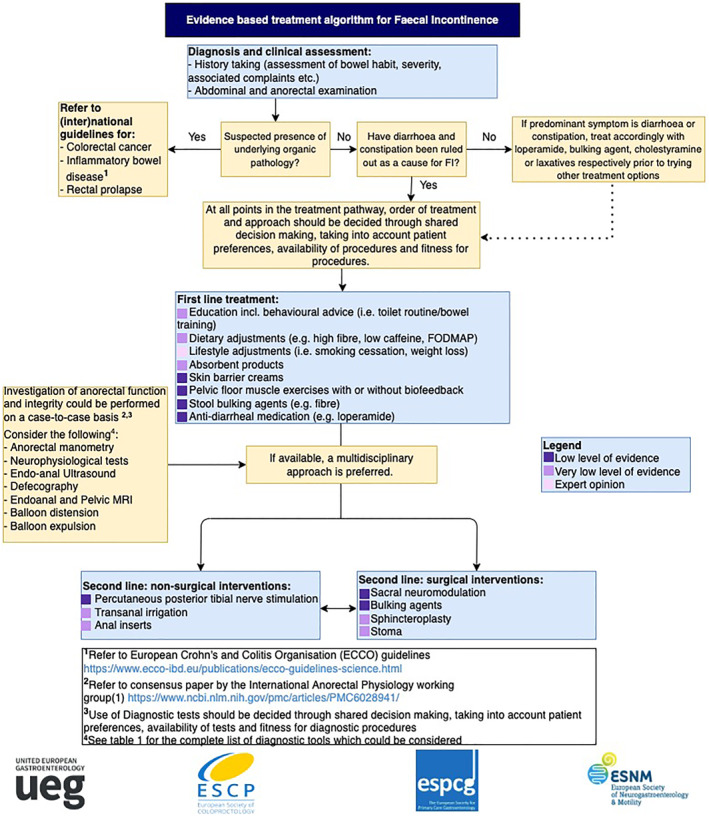
Treatment algorithm Faecal Incontinence
This guideline was funded by the UEG and the ESCP.
The Guideline Development Group had full control over the wording of the guideline without any influence from the funding body. The full method can be found in Appendix 1.
EVALUATION OF SYMPTOMS, DIAGNOSIS AND CLASSIFICATION
Introduction
When a patient with complaints related to faecal incontinence (FI) visits a health care professional for the first time, the health care professional should establish clarity of the exact complaint the patient has, including any associated symptoms. After making a diagnosis, the physician should aim to manage the patient's expectations regarding FI and regarding the different available treatment options. This chapter discusses definition, classification/subdivision, diagnosis and patients' expectations of FI prognosis.
Review questions
We considered the following questions for our evidence review:
What is the current definition of FI?
Which classification should be used for FI?
Which factors should be assessed during history taking?
-
Which physical examination should be performed prior to treatment?
-
‐
Value of digital rectal examination
-
‐
In which position should physical examination be performed to exclude local malignancies and to assess quality of pelvic floor function?
Definition
Faecal Incontinence can be caused by multiple factors and is usually accompanied by numerous associated complaints which affect the patients' lives. The health aspects related to faecal incontinence are complex and multi‐layered. Predisposing factors, related complaints, pathophysiological mechanisms and the psychological and social impact faecal incontinence can have on a patient should all be given consideration when diagnosing and/or treating a patient with FI.
For the purpose of these guidelines, the following definition for FI as defined by the Rome IV criteria will be utilised: “The recurrent uncontrolled passage of faecal material for at least 3 months”. 4 This definition excludes secretion of clear mucus and flatus incontinence.
Classification/subdivision of FI
Although classification systems for FI based on underlying cause, on severity scales or on character of leakage (i.e., urge, passive, combined) do currently exist, there is no established and universally accepted classification system. We aimed to find literature which examines whether classifying/subdividing FI into any of these systems could be clinically meaningful.
Analysis of the literature revealed one study by Pahwa et al. from 2020 which assessed whether categorizing FI into urgency or passive FI is clinically meaningful. 5 Patients with urgency FI are able to feel the presence of faeces in the rectum but are not able to hold the faeces in for a prolonged period of time, resulting in unwanted loss of faeces. Patients with passive FI are not able to feel the presences of faeces in the rectum, resulting in unwanted loss of faeces which can leak out of the rectum without the patient's knowledge. 4
The study by Pahwa et al. demonstrated functional differences between women with urgeny FI compared to women with passive FI. Whilst in women with urgency FI, a weaker squeeze pressure was found during digital rectal examination (DRE) and an earlier rectal sensation during anorectal manometry compared to patients with passive FI, patients with passive FI had a lower resting tone during DRE and a lower resting pressure on anorectal manometry compared to women with urge FI. 5
Previous studies have shown that certain treatments such as percutaneous tibial nerve stimulation (PTNS) and biofeedback are more effective in patients with urge incontinence than in patients with passive incontinence. 6 , 7 These findings may help physicians to make a treatment decision based on FI subdivision into type of leakage.
We did not find any evidence favouring one classification system over another.
Diagnosis
History taking
Patients who suffer from FI often feel ashamed to talk about their problem and may find it difficult to describe their complaints. Many people who suffer from FI delay seeking help or never discuss their problem with a healthcare professional at all. 8
A patient may come in with a complaint related to FI (e.g., abdominal pain), rather than telling the health care professional they suffer from FI in a straightforward fashion due to shame surrounding the subject. When FI is suspected, health care professionals should take it upon themselves to ask the patient whether they suffer from FI, rather than waiting for the patient to mention this problem. Direct questioning rather than talking around the issue should be aimed for when talking about FI. Asking the patient about their unwanted loss of faeces should be done in a sensitive manner, cultural preferences for wording should be taken into account (e.g. ‘accidents’, ‘unwanted loss of faeces’, etc.).
Diagnosis should start with medical history taking to identify the patient's complaints suggestive of faecal incontinence. Bowel habit should be thoroughly assessed to ensure diarrhoea and overflow constipation are not the cause for the unwanted loss of faeces. When it has been established that the patient suffers from FI, the severity and characteristics of the problem should be established. The patient should be asked about any possible underlying reason for the presence of FI and about their obstetric history (in women). The presence of other diseases generally causing diarrhoea such as colorectal cancer, inflammatory bowel disease (IBD), coeliac disease, diabetes and prolapse should be ruled out, considering factors such as age at presentation and red flag features (weight loss, rectal blood loss, sudden change in stool consistency etc.). If during history taking it becomes evident that any of these other diseases are present, the relevant (inter)national guidelines should be consulted (e.g., the European Crohn's and Colitis Organisation (ECCO) guideline for IBD: https://www.ecco‐ibd.eu/publications/ecco‐guidelines‐science.html).
Any associated FI complaints should be identified, including the effects on social and psychological well‐being. It should be determined whether the patient has tried any treatment options before and if so, whether any of these options were successful. The patient's preferences and daily schedule as well as their ability or their carers ability to administer a certain treatment should be determined. Appendix 2 can be consulted for guiding questions.
Physical examination
Physical examination of a patient with FI starts with the general appearance of the patient, including any signs of mental distress. A general abdominal examination should be conducted, taking into account any signs or characteristics of possible gastrointestinal disease. Inspection and physical examination of the anorectal region should be performed to exclude other anorectal pathology, to assess for previous perianal surgery/trauma and faecal impaction and to evaluate anal state/tone at rest and during voluntary effort. It should also be observed whether the patient is using any incontinence material. 9
Digital rectal examination (DRE) should be performed in a position that facilitates reliable diagnosis and comfort for the patient and physician, for example, the left lateral position. Lithotomy and knee‐chest position may be alternatives.
Patient expectations
When a patient with faecal incontinence visits a healthcare professional for their FI complaints for the first time, the healthcare professional should aim to talk to the patient about their expectations regarding symptom control and prognosis and should try to adjust these expectations where necessary. The impact of having FI on the patient's life and the possible time lag between symptom presentation and receiving a treatment should be discussed. The healthcare professional should also aim to provide the patient with information concerning the different types of treatment options available for FI. The possible treatment pathway and possible outcomes, along with any advantages and disadvantages of treatments should be discussed. Additionally, patients with FI may like to hear messages of hope and encouragement from their healthcare provider regarding living with FI. 10
Conclusion
Due to lack of evidence, the recommendations in this chapter are predominantly based on expert opinion and the strongest wording used in the recommendations are ‘should’.
Recommendations for evaluation of symptoms, diagnosis and classification

FIRST LINE TREATMENT FOR FAECAL INCONTINENCE
Introduction
After a diagnosis of faecal incontinence is made, the first management steps which should be considered, are compiled as “first line treatment”. First line treatment options aim to reduce FI complaints and to give patients a greater sense of security. First line treatment includes lifestyle adjustments, dietary advice (fibre and water intake, possibly reduction of caffeine and FODMAP intake), basic behavioural advice (toilet routine/bowel training), stool bulking agents and/or anti‐diarrheal medication, pelvic floor muscle exercises, absorbent products for containment and possibly skin care products to treat irritation of the skin around the anus.
In addition, it is important to manage the patients' expectations regarding symptom reduction.
Review questions
We considered the following questions for our evidence review plus the comparison between any two of the below mentioned treatment options:
-
What are the effects of basic behavioural advice/education versus no behavioural advice/education on episodes and symptoms in patients with FI?
-
‐
Toilet routine
-
‐
Bowel training
-
‐
-
What are the effects of dietary adjustments versus no dietary adjustments on episodes and symptoms in patients with FI?
-
‐
Sufficient water intake
-
‐
Sufficient fibre intake
-
‐
Decreased caffeine intake
-
‐
Decreased FODMAP intake
-
‐
-
What are the effects of lifestyle adjustments versus no lifestyle adjustments on frequency of episodes and symptoms in patients with FI?
-
‐
Smoking cessation in smokers
-
‐
Weight loss in overweight patients
-
‐
What are the effects of skin care products versus no skin care products on preventing and treating incontinence associated dermatitis and on patient satisfaction and quality of life in patients with FI?
What are the effects of absorbent products versus no absorbent products on leakage, patient satisfaction and quality of life?
-
What are the effects of pelvic floor physiotherapy versus no pelvic floor physiotherapy on frequency of episodes and symptoms in patients with FI?
-
‐
Muscle training only
-
‐
Muscle training with digital guidance
-
‐
(home) Biofeedback training
-
‐
What are the effects of stool bulking agents versus no stool bulking agents on frequency of episodes and symptoms in patients with FI?
What are the effects of anti‐diarrheal medication versus no anti‐diarrheal medication on frequency of episodes and symptoms in patients with FI?
Evidence in the literature
Basic behavioural advice
No systematic reviews, RCT's or observational studies with a control group were found regarding the effects of basic behavioural advice versus no basic behavioural advice on FI.
Analysis of the literature did however reveal two randomised factorial trial by Jelovsek et al. from 2019 and Norton et al. from 2003 which aimed to investigate the effects of loperamide and pelvic floor physiotherapy and biofeedback respectively, but also included education as one of the four different treatment groups. 11 The study by Jelovsek et al. compared four different treatment groups to one another after a period of 24 weeks in 300 patients: education plus oral placebo (group 1), anorectal manometry‐assisted biofeedback plus oral placebo (group 2), education plus loperamide (group 3) and loperamide plus anorectal manometry‐assisted biofeedback (group 4). The study showed that there was no significant difference in FI severity (measured using the St. Mark's score) between the education plus loperamide group (group 3) versus the education plus placebo group (group 1) MD −1.5 (95% CI −3.4, 0.4), between the biofeedback plus placebo (group 2) versus the education plus placebo group (group 1) MD −0.7 (95% CI −2.6, 1.2) or loperamide plus biofeedback (group 4) versus education plus loperamide (group 3) MD −1.1 (95% CI −3.4, 1.1). Differences in St. Mark's score between the education plus placebo group (group 1) after treatment compared to baseline were significant MD −5.1 (95% CI −6.6, −3.6). 11
The RCT by Norton et al. from 2003 which included a total 171 participants and compared four different groups: education (group 1), education plus sphincter exercises (group 2), education plus sphincter exercises plus clinic biofeedback (group 3) and education plus sphincter exercises plus clinic biofeedback plus home biofeedback (group 4). 12
No significant differences were found between any of the groups in mean number of FI episodes per week (p = 0.51 KW), severity of FI (St. Mark's) (p = 0.54 KW) or quality of life (QoL) (numerical data not available) after 1 year follow‐up, however both groups improved in all outcomes compared to baseline (numerical data not available). 12
These results may suggest that an educational pamphlet alone would be effective in treating FI. Results should be interpreted with caution due to lack of control group.
Conclusion: An education pamphlet regarding FI may be effective in treatment of FI. Results should be interpreted with caution.
Dietary adjustments
Analysis of the literature revealed one systematic review which examined the effects of dietary adjustment on FI. 13 This systematic review focused on fibre supplementation and as such will be discussed under Section: 4.3.7 ‘stool bulking agents’.
Analysis of the literature also revealed a retrospective chart review which examined the effects of a low FODMAP (fermentable oligo‐, di‐, mono‐saccharides and polyols) diet on FI complaints. This study by Menees et al. from 2019 included 65 patients with FI who underwent formal dietary education. 14 This study did not report on any of our predefined outcomes. However, the study did report that 42 (64.6%) subjects reported a reduction in their FI symptoms whilst on a low FODMAP diet. No serious adverse events were reported. 14
Conclusion: A low FODMAP diet may help in reducing mean number of FI symptoms, however, evidence is limited.
Lifestyle adjustments
No studies were found regarding the effects of lifestyle adjustments (i.e., weight‐loss and smoking cessation) on FI.
Skin care products
Analysis of the literature revealed two systematic reviews which examined the effects of skin care products to prevent and/or reduce incontinence associated dermatitis (IAD). 15 , 16 The first review by Beeckman et al. from 2016 included a total of 6 (quasi) RCT's with a total of 450 patients which answered our research question. 15 Seven of the studies from this review were excluded due to assessing effect of timing of application or due to lack of data in combination with a lack of up‐to‐date contact details. The second review by Pather et al. from 2017 did not include any additional studies which were not already included in the first review to answer our research question. Both reviews included patients with urinary incontinence, faecal incontinence, or both and did not differentiate between the types of incontinence within their analysis.
Both reviews were narrative synthesis. Meta‐analyses of the results was not possible for either review due to heterogeneity of the interventions, measurement outcomes and tools.
Skin care products versus control (water and soap)
Prevention of Incontinence associated dermatitis (IAD).
One of the RCT's which compared no rinse skin cleanser (Clinisan™) showed that significantly less patients in the no rinse skin cleanser group (6/33 [18.2%]) developed IAD over the 14‐day study period compared to the patients in the control (soap and water) group (15/32 [46.9%]) (RR 0.39, 95% CI 0.17, 0.87). 17
Reduction of number of patients with Incontinence associated dermatitis (IAD).
Another RCT which compared a 3‐in‐1 washcloth impregnated with 3% dimethicone (n = 73) to soap and water (n = 68) showed that significantly less participants in the washcloth group had IAD compared to the soap and water group after 120 days (7.9% vs. 25.9%) (RR 0.31, 95% CI 0.12, 0.79). 18
Skin care products versus other skin care products
Prevention of Incontinence associated dermatitis (IAD).
One RCT compared zinc oxide cream to Sudocrem® and showed no significant difference between the two groups after 14 days. Out of the 11 participants in the zinc oxide group, 2 (18.2%) developed IAD compared to 3 out of 16 (18.8%) participants in the Sudocrem® group (RR 1.03, 95% 0.20, 5.19). 19
Another RCT compared Cavilon™ skin cleanser, Cavilon™ no sting barrier film and Comfort shield perineal care washcloth Dimethicone 3% to each other and found no significant difference between groups. Seven out of 31 (22.6%) participants using the Cavilon products developed IAD compared to 9 out of 33 (27.3%) participants who used the washcloth (RR 0.83, 95% CI 0.35, 1.95) (length of treatment not reported). 20
Reduction of Incontinence associated dermatitis (IAD) severity.
One RCT compared antiseptic skin cleanser (n = 18) to durable barrier cream (n = 13) and showed no significant difference between the two groups in severity of IAD after 6 weeks of treatment MD −0.49 (95% CI −1.29, 0.31). 21
Reduction of number of patients with Incontinence associated dermatitis (IAD).
One RCT which compared two zinc oxide creams to eachother (Calmoseptine® vs. Desitin®) showed that 15 out of 69 (21.7%) participants in the Calmoseptine® group no longer had IAD after 6 days, compared to 7 out of 73 (9.6%) of the participants in the Desitin group. No significant difference was found between the two groups (RR 0.44, 95% CI 0.19, 1.02). 22
For a further breakdown of the results of these reviews, see Appendices: Appraisal Beeckman et al. and appraisal Pather et al.
Conclusion: Skin care products such as moisturisers, skin protectant or a combination of the two have beneficial effects on preventing and treating incontinence associated dermatitis compared to soap and water. No significant difference was found between different types of skin care products. A definitive conclusion cannot be drawn on which product may be the best option.
Absorbent products
No systematic reviews, RCT's or observational studies with control groups were found which answered our PICO question comparing absorbent products to a control group. However, a systematic review which examined the effects of different types of absorbent products to each other was available. This systematic review by Fader et al. from 2008 concluded that there was not enough evidence on use of absorbent products in faecal incontinence to draw definitive conclusions on which products are superior. 23 The results from this review should be interpreted with caution due to indirectness, as the included studies assess patients with urinary incontinence and/or faecal incontinence. 23 As this systematic review did not answer our predefined question, the results will not be discussed any further and we would like to refer you to the systematic review for further information. 23
Conclusion: Absorbent products have a beneficial effect on reducing leakage of faeces, however, not enough evidence on use of absorbent products in faecal incontinence is available to draw definitive conclusions on which products are superior.
Pelvic floor physiotherapy with or without biofeedback
Analysis of the literature revealed five systematic reviews which examined the effects of pelvic floor physiotherapy (PFPT) and/or biofeedback on FI. 24 , 25 , 26 , 27 , 28 One of the RCT's included in the reviews answered our PICO question comparing PFPT to education only. 12 Two RCT's answered our PICO question regarding the effects of pelvic floor physiotherapy with biofeedback compared to the effects of pelvic floor physiotherapy without biofeedback. 12 , 29
Pelvic floor physiotherapy versus education
See Section Basic behavioural advice for effects of PFPT versus education (Norton et al. 2003). 12
Pelvic floor physiotherapy versus pelvic floor physiotherapy + biofeedback
Group 2 and 3 and group 2 and 4 from the above‐mentioned study by Norton et al. were included for our comparison, as well as an RCT by Heymen et al. from 2009 which examined 108 patients who underwent either pelvic floor physiotherapy (PFPT) or PFPT with biofeedback. 12 , 29 In the RCT by Norton et al. no significant differences were found between any of the groups in mean number of FI episodes per week, continence score and quality of life, however all groups improved in all outcomes compared to baseline at 1 year follow‐up. 12 In the RCT by Heymen et al. mean number of FI episodes per week was not measured, instead number of days per week with FI was assessed. 29 Mean number of days with FI per week was significantly lower after 3 months in the biofeedback group compared to the PFPT group MD 0.77 (95% CI 0.11, 1.43). Participants in the biofeedback group had significantly greater reductions in severity of FI (measured using the FISI score) compared to participants in the PFPT group after 3 months (estimated MD from baseline to 3‐month follow‐up from graph: 35 to 22.5 vs. 37 to 32, p = 0.01). No difference in QoL was found between groups (p = 0.64). It should be noted that in the RCT by Heymen et al. one of the inclusion criteria for participation was that the patient had to be a non‐responder to conservative measures (i.e., medical management instructions for the use of fibre supplements and/or anti‐diarrheal medication as well as education on anatomy and physiology of the pelvic floor muscles) during the 4‐week run‐in period.
Biofeedback + education versus education alone
Analysis of the literature revealed one RCT which examined the effects of biofeedback + education versus education alone. 30 This RCT by Ilnyckyj et al. from 2005 did not report on any of our exact predefined outcomes, but instead examined whether participants had a complete response (defined as no FI episodes during the last week of treatment). No significant difference was found between two groups (p = 0.15). 30
Conclusion: The study which compared pelvic floor physiotherapy to education showed no significant difference between the groups in any FI complaints. PFPT combined with biofeedback resulted in less days with FI episodes per week and a reduction in FI severity compared to PFPT without biofeedback. No difference in QoL was found. Biofeedback may not be superior to education only.
Stool bulking agents
Analysis of the literature revealed one systematic review which examined the effects of fibre on FI complaints. 13 Only two of the included RCT's examined the effects of dietary fibre compared to placebo. The RCT's by Bliss et al. from 2001 to 2014 include 42 (of which three dropped out during the baseline period) and 189 participants respectively. 31 , 32 In the 2001 study, patients were assigned to one of three groups for 31 days; 25 g of Metamucil supplementation per day (which includes 7.1 g of psyllium), 25 g of Gum Arabic supplementation per day and 0.25 g of pectin (negligible amount) supplementation per day given as a placebo. FI episodes per week were not recorded, instead proportion of incontinent stools was assessed. A significantly lower proportion of incontinent stool was seen in the Metamucil and Gum Arabic groups compared to the placebo group MD −0.33 (95% CI −0.38, −0.28) and MD −0.32 (95% CI −0.37, −0.27) respectively. Quality of life, severity of FI and adverse events were not assessed in this study. 31
In the 2014 study, patients were assigned to one of four groups; carboxymethylcellulose (CMC), Gum Arabic, psyllium or placebo. The length of the study was 52 days; 14 days baseline period, 6 days incremental dosing period and 32 days steady amount period. Amount of fibre given was dependent on solubility of fibre. An average amount of 16.6, 14.6 and 16.2 g/day were given for GA, psyllium and CMC respectively. Mean number of FI episodes after treatment period were significantly less in the psyllium group compared to placebo (p = 0.003), significantly more in the CMC group compared to placebo (p < 0.001) and no significant difference was seen between the GA group and the placebo group (p = 0.92). FI severity (calculated using number of FI episodes, consistency and amount of stool) was significantly better in the psyllium group (p = 0.03), significantly worse in the CMC group (p < 0.01) and no significant difference was seen in the GA group compared to placebo group (p = 0.81). No significant difference was found in quality of life within any of the four FIQoL domains (numerical data no longer available). Adverse events were not assessed. 32 For a further breakdown of the results of this review see Appendix: Appraisal Colavita et al.
Fibre versus loperamide
Analysis of the literature revealed one randomised cross‐over trial by Markland et al. from 2015 which examined the effects of psyllium fibre versus loperamide in 80 patients with FI. In this study participants received daily loperamide (2 mg) plus placebo psyllium powder for 4 weeks, followed by a 2‐week wash‐out period followed by a 4‐week period of daily psyllium powder (3.4 mg) plus placebo loperamide (L1P2) or vice versa (P1L2). 33 No significant difference was seen between loperamide and psyllium groups in mean number of FI episodes after the end of first treatment: L1P2 mean 4.1 ± 5.1 (SD) versus P1L2 mean 4.8 ± 4.8, MD −0.7 (95% CI −2.97, 1.57) or at the end of second treatment L1P2 mean 4.7 ± 5.7 versus P1L2 mean 3.5 ± 6.6, MD 1.2 (95% CI −1.87, 4.27). No significant difference in FI severity (measured using the FISI score) was seen at the end of first treatment: L1P2 mean 24.9 ± 13.3 versus P1L2 mean 24.9 ± 12.2, MD 0 (95% CI −5.86, 5.86) or at the end of second treatment: L1P2 mean 23.2 ± 12.7 versus P1L2 22 ± 17.1, MD 1.2 (95% CI −6.4, 8.8). No significant difference was seen between groups in QoL (measured using MMHQ) at the end of the first treatment period: L1P2 41.7 ± 25.9 versus P1L2 35.3 ± 24.2, MD −6.4 (−18.01, 5.21) or at the end of the second treatment period: L1P2 40.6 ± 29.6 versus P1L2 31.2 ± 25.6, MD −9.4 (−23.39, 4.59). Adverse events were similar between groups (p = 0.59). One serious adverse event occurred, a participant in the P1L2 group died during the loperamide phase. Mild to moderate adverse events were common in both treatment groups, occurring in 43 patients in the L1P2 group and in 37 patients in the P1L2 group. Constipation was the most common adverse event for loperamide use (29.1%) and diarrhoea was the most common adverse event for psyllium use (23.3%). Number of adverse events was similar between groups.
Conclusion: Fibre supplementation, especially Psyllium helps reduce FI episodes. Gum Arabic helped reduce FI episodes in one study, but not in the other and Carboxymethylcellulose resulted in increased episodes of FI compared to placebo.
Psyllium is not significantly better in reducing FI complaints compared to loperamide.
Anti‐diarrheal medication
Analysis of the literature revealed one systematic review which examined the effects of anti‐diarrheal medication on FI. The review by Omar et al. from 2013 included two RCT's which compared the effects of loperamide to placebo on FI, one RCT which compared the effects of loperamide oxide to placebo on FI and one RCT which compared the effects of Diphenoxylate plus Atropine (co‐phenotrope) to placebo on FI. The review concluded that all three treatments had a positive impact on diarrhoea and/FI symptoms compared to placebo. 34 However, these study populations consisted of patients who suffered from ulcerative colitis or chronic diarrhoea with or without FI, rather than the general FI population we intended to assess. 34 As this systematic review did not assess our predefined population, the results will not be discussed any further and we would like to refer you to the systematic review for further information. 34
Loperamide versus fibre
See Section Fibre versus loperamide for effects of loperamide versus psyllium.
Conclusion: Anti‐diarrheal medication has a positive impact on diarrheal and/or FI symptoms. Loperamide is not significantly better in reducing FI complaints compared to Psyllium.
Recommendations for first line treatment


DIAGNOSTIC TESTS PRIOR TO SECOND LINE TREATMENT
Introduction
In patients where initial first line treatment has not resulted in acceptable symptom reduction additional diagnostic test could be considered prior to starting second line treatment. This chapter focuses on whether the outcomes of any of the additional diagnostic tests could have an impact on treatment decisions and whether these diagnostic tests have any added value.
Review questions
We considered the following questions for our evidence review:
What are the effects of using an additional diagnostic tool compared to using no additional diagnostic tool on therapeutic decisions and in improving outcomes (decreased frequency and symptoms) in patients with FI?
What are the effects of using endoscopy compared to not using endoscopy on therapeutic decisions and in improving outcomes (decreased frequency and symptoms) in patients with FI?
What are the effects of using anorectal manometry compared to not using anorectal manometry on therapeutic decisions and in improving outcomes (decreased frequency and symptoms) in patients with FI?
What are the effects of using pudendal nerve terminal motor latency (PNTML) to not using PNTML on therapeutic decisions and in improving outcomes (decreased frequency and symptoms) in patients with FI?
What are the effects of using defecography compared to not using defecography on therapeutic decisions and in improving outcomes (decreased frequency and symptoms) in patients with FI?
What are the effects of using endoanal ultrasound compared to not using endoanal ultrasound on therapeutic decisions and in improving outcomes (decreased frequency and symptoms) in patients with FI?
What are the effects of using rectal balloon expulsion compared to not using rectal balloon expulsion on therapeutic decisions and in improving outcomes (decreased frequency and symptoms) in patients with FI?
What are the effects of using rectal barostat compared to not using rectal barostat on therapeutic decisions and in improving outcomes (decreased frequency and symptoms) in patients with FI?
Evidence in the literature
Evidence in the literature regarding the effects of additional diagnostic tests on therapeutic decision making and in improving outcomes in patients with FI is low. Our literature search aimed to find articles which assessed the added value with regard to treatment decisions for the following diagnostic tools: Endoscopy, anorectal manometry, pudendal nerve terminal motor latency (PNTML), defecography, endoanal ultrasound, rectal balloon expulsion and rectal barostat. No systematic reviews, randomized controlled trials (RCT) or observational studies with a control group were found regarding the added value of any of these diagnostic tools with regards to affecting treatment decisions.
A consensus paper by Carrington et al. from 2018 which provides the reader with indications, study performance, clinical utility and strengths and weaknesses of common anorectal structure‐ and function tests has been created on behalf of the International Anorectal Physiology Working Group and the International Working Group for Disorders of Gastrointestinal Motility and Function. 2
We would like to refer you to this consensus paper as a useful guide in determining which additional diagnostic test (including anorectal manometry, neurophysiological tests, endo‐anal ultrasound, defecography, endoanal or pelvic MRI, balloon distention and balloon expulsion) may be of use (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6028941/). 2
The table below has been extracted from this article and shows a concise summary of the clinical utility of each diagnostic test (Table 1).
TABLE 1.
Clinical utility of investigations of anorectal physiological function
| Function | Investigation | Clinical utility |
|---|---|---|
| Anus | ||
| Motor | Anorectal manometry (conventional) | ++++ |
| Anorectal manometry (high resolution) | ++++ | |
| Anorectal manometry (3D) | +++ | |
| Electromyography | +++ | |
| Pudendal nerve terminal motor latencies | + | |
| Structure | Endoanal ultrasonography | ++++ |
| Transperineal ultrasonography | +++ | |
| Endoanal or pelvic MRI | +++ | |
| MRI muscle fibre tracking | + | |
| Electrostimulation | + | |
| Sensory | Light‐touch stimulation | + |
| Anal evoked potentials | ++ | |
| Rectum | ||
| Sensory | Balloon distension | ++++ |
| Rectal barostat | +++ | |
| Rectal evoked potentials | ++ | |
| Motor | Distal colonic manometry | ++ |
| Rectal barostat | +++ | |
| Rectal motor evoked potentials | + | |
| Anorectal unit | ||
| Motor | Anorectal manometry (conventional, high resolution or 3D) | ++++ |
| Balloon expulsion | ++++ | |
| Motor, sensory and structure | Barium defecography | ++++ |
| Magnetic resonance defecography | +++ | |
| Functional lumen imaging probe | + | |
Note: + limited clinical utility or of research interest only; ++, emerging technology with limited data of clinical utility; +++, recognized clinical utility but less commonly performed; ++++, good clinical utility and commonly performed.
Table extracted from: Carrington, E. V. et al. (2018) Advances in the evaluation of anorectal function Nat. Rev. Gastroenterol. Hepatol. doi:10.1038/nrgastro.2018.27. This work is licensed under a Creative Commons Attribution 4.0 International License: http://creativecommons.org/licenses/by/4.0/.
Recommendations for diagnostic tests prior to second line treatment

SECOND LINE: NON‐SURGICAL INTERVENTIONS FOR FAECAL INCONTINENCE
Introduction
In patients where first line treatment has not resulted in acceptable symptom reduction, further procedures should be considered. We propose that in general, health care professionals should first consider second line non‐surgical procedures prior to moving on to more invasive and/or more expensive treatments and/or treatments with a higher risk of (serious) complications such as surgical procedures. However, depending on patient preferences, physician preferences and availability, going from first line treatment options straight to second line surgical intervention options can be considered. Second line non‐surgical intervention options include posterior tibial nerve stimulation (PTNS), either percutaneous or transcutaneous, transanal irrigation and anal inserts for containment purposes.
Review questions
We considered the following questions for our evidence review and the comparison between any two of the below mentioned treatment options:
What are the effects of percutaneous posterior tibial nerve stimulation (PPTNS) versus no PPTNS on frequency of episodes and symptoms in patients with FI?
What are the effects of transcutaneous tibial nerve stimulation (TPTNS) versus no TPTNS on frequency of episodes and symptoms in patients with FI?
What are the effects of transanal irrigation versus no transanal irrigation on frequency of episodes and symptoms in patients with FI?
What are the effects of the use of anal inserts versus no anal inserts on patient satisfaction and quality of life in patients with FI?
Evidence in the literature
Posterior tibial nerve stimulation (PTNS)
Analysis of the literature revealed six systematic reviews which examined the effects of PTNS on FI complaints. 35 , 36 , 37 , 38 , 39 , 40 The four most recent reviews which included additional RCT's not included in the older systematic reviews, examined the effectiveness of tibial nerve stimulation on FI without distinguishing between trans‐ and percutaneous stimulation. 35 , 36 , 37 , 40 Therefore, we performed our own meta‐analyses for percutaneous and transcutaneous PTNS separately to allow us to compare effects of transcutaneous PTNS (TPTNS) versus control group (sham) and percutaneous PTNS (PPTNS) versus control group (sham).
For a further breakdown of the results of these reviews see Appendices: Appraisal Edenfield et al., appraisal Arroyo et al. appraisal Sarveazad et al.
Transcutaneous posterior tibial nerve stimulation (TPTNS) versus no TPTNS
Our meta‐analysis includes two RCT's by Leroi et al. from 2012 and George et al. from 2013, which examined the effects of transcutaneous PTNS (TPTNS) compared to sham. 41 , 42 The meta‐analyses comparing mean number of FI episodes per week, severity of FI (measured using the CCF‐FI scale), and QoL (measured using the FIQoL questionnaire) between TPTNS and sham group found no significant difference in any of these items after a treatment period ranging from 6 to 12 weeks: Mean number of FI episodes per week MD −0.51 (95% CI −1.55, 0.54), FI severity MD −0.91 (−1.91, 0.09), FIQoL Lifestyle domain MD 0.07 (95% CI −0.21, 0.35), Coping behaviour domain MD 0.16 (95% CI −0.36, 0.69), Depression and self‐perception domain MD 0.05 (−0.41, 0.51) and the Embarrassment domain MD −0.06 (−0.29, 0.17). Adverse events were only reported in the RCT by Leroi et al. A total of two treatment related mild to moderate adverse events were reported, one participant in the TPTNS group reported burning and itching in their leg and one participants in the sham group reported constipation. 42
Percutaneous posterior tibial nerve stimulation (PPTNS) versus no (PPTNS)
Analysis of the literature revealed a total of three RCT's, two of which were also included in the systematic reviews which examined the effects of percutaneous PTNS (PPTNS) on mean number of FI episodes, FI severity and QoL.
Our meta‐analysis which includes three RCT's from 2013, 2015 and 2017 by George et al. 41 Knowles et al. 43 and van der Wilt et al. 44 respectively, examined the effects of PPTNS compared to sham in a total of 305 patients of which 155 were randomized into a PPTNS group and 150 were randomized into a sham group. 41 , 43 , 44 The meta‐analysis shows that participants in the PPTNS group had a significantly lower mean number of FI episodes per week compared to the sham group MD −3.01 (95% CI −4.52, −1.5) with a follow‐up duration ranging from 3 to 12 months. It should be noted that the outcome assessed in the study by Knowles et al. was ‘a 50% or greater reduction in number of FI episodes’ rather than FI episodes per week. No significant difference was seen between groups when using ‘a 50% or greater reduction’ as outcome. 43 In the meta‐analysis which compared severity of FI (measured using the CCF‐FI scale) between PPTNS and sham group, no significant difference was found between the two groups after treatment, MD −0.82 (−1.68, 0.04). No significant difference was found for QoL on any of the four FIQoL domains: Lifestyle MD −0.08 (95% CI −0.29, 0.12), Coping behaviour MD 0.01 (95% CI −0.18, 0.19), Depression and self‐perception MD 0.04 (−0.16, 0.24), Embarrassment MD 0.04 (−0.14, 0.22). When interpreting the results, it should be noted that treatment time was different in each RCT, but were all short‐term, ranging between 6 and 12 weeks. It should also be taken into consideration that values were not normally distributed in the study by van der Wilt et al.
Adverse events were reported in two of the RCT's, a total of seven mild adverse events (pain/bruising near insertion point) were reported in the PTNS group and four in the sham group. 43 , 44
Percutaneous posterior tibial nerve stimulation (PPTNS) versus sacral neuromodulation (SNM)
Analysis of the literature revealed two systematic reviews which examined the effects of PTNS versus SNM on FI complaints. 45 , 46 The most up‐to‐date review by Simillis et al. from 2018 included four studies, of which one RCT and three non‐randomized prospective studies with a total of 302 patients who were evaluated. 45 Follow‐up period ranged between 3 and 12 months. Mean number of FI episodes per week was assessed in two studies (n = 214) and was significantly lower in the SNM group compared to the PTNS group WMD 8.11 (95% CI 4.13, 12.09). FI severity (measured using the CCF‐FI scale) was assessed in all four studies (n = 293) and was significantly better in the SNM group compared to the PTNS group: WMD 2.27 (95% CI 1.12, 3.42). QoL (measured using the FIQoL questionnaire) was assessed in two studies (n = 91) and was significantly better in the SNM group compared to the PTNS group in the coping and depression and self‐perception domains: WMD 0.51 (95% CI 0.16, 0.86) and WMD 0.4, (95% CI 0.11, 0.69) respectively. No significant difference was found between groups on the Lifestyle domain: WMD 0.13 (95%CI −0.95, 1.21) or the Embarrassment domain: WMD 0.5 (95% CI −0.38, 1.38). Adverse events were reported in two studies included in the review. In total, two patients in the PTNS group suffered mild discomfort along with paraesthesia in the foot, while four patients in the SNM group experienced a device related adverse event; two patients suffered from stimulator site pain and two patients suffered from a wound infection which resulted in reimplementation of the electrode. 45
Cost: One of the studies reported on cost of the two treatment options. The cost of 1 year treatment was £11,374 (€13122) per patient for SNM compared to £1740 (€2007) per patient for PTNS. 47
Conclusion: Percutaneous posterior tibial nerve stimulation (PPTNS) is effective in reducing mean number of FI episodes per week, however, severity and QoL were not significantly different between PPTNS and sham groups. Additionally, although the studies showed a significant reduction in FI episodes per week, the largest included study found no significant difference between groups in ‘a 50% reduction in FI episodes per week’, which was their primary outcome, but not one of our predefined outcomes. This should be taken into consideration when deciding on the use of PPTNS.
When comparing results of Transcutaneous posterior tibial nerve stimulation (TPTNS) and sham, no significant difference was found between mean number of FI episodes, FI severity or QoL.
SNM is superior to PTNS in terms of a lower mean number of FI episodes per week, a lower severity of FI and better QoL in the coping and depression domains of the FIQoL scale. However, adverse events seem to be slightly more frequent and slightly more severe in treatment with SNM compared to PTNS.
Transanal irrigation
No systematic reviews, randomized controlled trials (RCT) or observational studies with a control group were found regarding transanal irrigation.
Analysis of the literature revealed one non‐controlled prospective cohort study which evaluated the effects of transanal irrigation (TAI) in patients with FI. The study from 2017 by Juul et al. intended to examine the effects of TAI on FI severity in 238 (ITT) patients over a period of 12 months, however only 103 (43%) completed the 12‐month study and were included in the analysis. Both FI severity measured on the CCF‐FI scale as well as on the St. Marks scale improved after treatment with TAI compared to baseline MD −2.2 (95% CI −1.6, −2.8) MD −2.2 (95% CI −1.5, −2.9) respectively. When interpreting the results, it should be taken into account that analysis was only performed in participants who completed the whole study period (PP). QoL and adverse events were not assessed for patients with FI separately (patients with constipation were also included in analysis), and therefore were not included in our analysis, however, no serious adverse events were reported. 48
Conclusion: Treatment with TAI improved FI severity in a prospective cohort study compared to baseline.
Anal inserts
Analysis of the literature revealed three systematic reviews which assessed the effectiveness of anal inserts on FI complaints. 49 , 50 , 51
The most up‐to‐date systematic review by How et al. from 2020 included an additional two studies which assessed the effectiveness of anal inserts in adult patients with FI, not assessed in the previous two reviews. 51
The descriptive review from 2020 by How et al. included six observational studies without control group which assessed the effectiveness of anal inserts in the general adult FI population (i.e., does not belong to a ‘special situations’ population). Three different types of anal inserts were assessed, namely, the Coloplast® plug, the Renew® anal insert and the Procon/ProTect® incontinence device.
Coloplast plug
Three observational studies by Mortensen et al. from 1991, by Christiansen et al. from 1993 and by Norton and Kamm from 2001 assessed the effectiveness of the Coloplast® plug in 10, 15 and 20 patients respectively. 52 , 53 , 54
One of 10 participants dropped out due to discomfort of the plug in the first study, 11 of 15 patients withdrew after a median of 7 days due to discomfort in the second study and 9 out of 20 patients dropped out due to discomfort in the third study.
The first study reported that no FI episodes took place 82% of the time the plug was in use, the second study reported that 64% of participants were continent with the plug in place and the third study reported that 50% of the participants were continent with the plug in place. It should be noted that these results include the PP‐ rather than the ITT population.
Only mild adverse events were reported but were frequent. In the first study, slippage of the plug was reported in 19%–20% of participants and discomfort in 10%–19% of participants. In the second study, occasional slippage of the plug was reported by 43% of participants and discomfort by 71% of participants. The third study reported discomfort in 70% of patients.
Renew anal insert
Two observational studies by Lukacz et al. from 2015 and by Leo et al. from 2019 assessed the effectiveness of the Renew® anal insert in 91 and 30 patients respectively. 55 , 56 In the first study 18 out of 91 patients did not complete the total 12‐week treatment period due to various reasons (e.g., protocol too demanding, loss of insert during urination, flatus or exercise, unrelated medical conditions, etc.). In the second study, 4 out of 20 patients stopped using the insert after a few days due to mild pain/discomfort, while the other 16 participants continued for a median time of 11 weeks.
In the first study 62% (95% CI 51%–71%) of participants in the ITT cohort had a ≥50% reduction in frequency of FI episodes and 77% (95% CI 66%–85%) of the participants in the PP cohort had a reduction of ≥50%. In the second study, the authors reported on whether leakage increased, decreased or stayed the same for each patient. In 20/30 leakage decreased, in 3/30 leakage increased and in 7/30 leakage stayed the same.
FI severity decreased in both studies after treatment compared to baseline. In the study by Lukacz et al. a reduction in FI severity (measured using the CCF‐FI scale) was seen MD −5.3 (95% CI −4.18, −6.42). In the study by Leo et al. a reduction in FI severity (measured using the St Mark's scale) from a mean of 15 (range 7–18) at baseline to a mean of 10 (range 2–18) after treatment was reported. It should be noted that the results for FI severity include the PP‐ rather than the ITT population. Seventy‐eight percent of participants who completed the treatment period were very or extremely satisfied with the anal inserts (63% in ITT cohort) in the study by Lukacz et al. In the study by Leo et al. 80% of participants were satisfied with the use of the anal insert.
No serious adverse events were reported in either study. A total of 4 mild to moderate adverse events were reported (faecal urgency, soreness, bleeding of haemorrhoids, rectal bleeding).
Procon/ProTect incontinence device
Two observational studies both by Giamundo et al. from 2002 to 2007 assessed the effectiveness of the Procon/ProTect® incontinence device in 18 and 17 patients respectively. 57 , 58 In the first study 11 out of 18 participants dropped out before the end of the 14‐day trial due to the device being too difficult to operate (n = 8) and frequent alarm activation of device due to sensitivity (n = 3). In the second study, 9 out of 17 patients dropped out before the end of the 14‐day trial due to the frequent alarm activation of device due to sensitivity (n = 3), non‐compliance and lack of motivation (n = 2), inability to follow study protocol (n = 1) and 3 unknown reasons.
In the first study, five out of seven participants (71.4%) of the PP population were satisfied with the device. It should be noted that this is only 27.8% of the ITT cohort. In the second study, 7 out of 11 of the PP participants (63.6%) were satisfied with the device, however satisfaction in the ITT cohort was only 41.2%. In the second study, 7 out of 11 (63.6%) of the PP participants were satisfied with the device. It should be noted that this is only 41.2% of the ITT cohort.
The second study reported that mean number of FI episodes decreased from a number of times a day (exact amount not reported) to one or zero weekly episode(s) in the seven participants who continued with the study. A reduction in FI severity (measured using the CCF‐FI scale) from a mean of 15.3 (range, 13–20) at baseline to 7.2 (range, 2–12) after treatment (p < 0.001) was reported. A significant improvement in quality of life (measured using the FIQoL questionnaire) compared to baseline was reported in all but the ‘depression and self‐perception’ domain MD 4.7 (95% CI −9.81, 0.41). Mean difference between baseline and after treatment period was MD −8.6 (95% CI −16.2, −1) for the Lifestyle domain, MD −9.4 (95% CI −15.64, −3.16) for the Coping behaviour domain and MD −4.3 (95% CI −6.56, −2.04) for the Embarrassment domain. Again, it should be noted that QoL was only measured in the seven participants who completed the entire study period.
This device achieved better results in patients with (semi) formed stool, due to the sensor in the device setting off an alarm too frequently when watery diarrhoea is present. No adverse events were reported in either study.
For a further breakdown of the results of these reviews see Appendix: Appraisal Deutekom et al., appraisal Buono et al., appraisal How et al.
Conclusion: Anal inserts are intolerable for a large part of patients with FI. The renew® anal device had higher tolerability rates than the Conseal® and Procon/ProTect® devices. In patients who are able to tolerate the device, patient satisfaction and continence are generally high. The Procon/ProTect® device should not be used in patients with continuous liquid stool due to frequent activation of the alarm on the device when it senses faeces in the rectum.
Recommendations for second line: Non‐surgical interventions

SECOND LINE: SURGICAL INTERVENTIONS FOR FAECAL INCONTINENCE
Introduction
In patients where first line treatment and/or second line non‐surgical procedures have not resulted in acceptable outcomes, or where second line non‐surgical procedures are not preferred, surgical procedures could be considered. Augmentation of a defective anal sphincter such as through a Dynamic Graciloplasty (DGP) or the Artificial Bowel Sphincter (ABS) were common treatment options for faecal incontinence in the past. However, with the introduction of sacral neuromodulation (SNM), these treatment options have mostly discontinued. Any treatments which have been discontinued were not included in these guidelines. The surgical procedures which are still commonly used and included in these guidelines are sacral neuromodulation (SNM), injectable bulking agents, sphincteroplasty and colostomy. For evidence regarding rechargeable SNM we would like to refer you to the supplementary material regarding ‘sub questions PICO's’.
Review questions
When should surgical treatment options be considered for FI treatment?
What is the most appropriate surgical procedure when conservative treatments fail?
What are the effects of Sacral neuromodulation (SNM) versus no SNM in patients with FI on frequency of episodes, symptoms and severe complications*?
What are the effects of injectable bulking agents versus no injectable bulking agents on frequency of episodes, symptoms and severe complications in patients with FI?
What are the effects of sphincteroplasty versus no sphincteroplasty in patients with FI with a damaged sphincter on frequency of episodes, symptoms and severe complications?
What are the effects of a stoma versus no stoma in patients with FI in whom previous surgical or interventional treatment options have failed on patient satisfaction, quality of life and severe complications?
*severe complications defined as ≥Grade 3 CTCAE classification.
Evidence in the literature
Sacral neuromodulation (SNM)
The analysis of the literature revealed six systematic reviews which examined the effects of SNM versus no SNM (either SNM switched off or conservative treatment) on FI complaints in the general FI population. 46 , 59 , 60 , 61 , 62 , 63
The most up to date review by Thaha et al. from 2015 included five randomized controlled‐ and cross‐over trials which examined the effects of SNM versus no SNM on FI. 46 Due to heterogeneity in intervention, trial design and reported outcomes, meta‐analyses of the outcomes were not possible.
Four of the five included studies are cross‐over designs in which participants received staged implantation of the SNM. 64 , 65 , 66 , 67 The studies by Vaizey et al., Leroi et al., Sorenson et al. and Kahlke et al. included 2, 34, 7 and 16 patients each respectively. Participants in all four RCT's were fitted with a sacral neuromodulator ≥3 months ago. Participants were randomized into an ON or OFF group for a period of 2 weeks, 1 month, 1 week and 3 weeks respectively followed by the other setting (ON or OFF) for the same period of time. None of the studies had a wash‐out period.
In the two participants in the Vaizey et al. trial, FI episodes per week were significantly less during the ON period with a mean of 6 in the OFF period compared to 0.5 in the ON period MD −6 (95% CI −11.59, −0.41). 67 In the trial by Leroi et al. in which only 27 out of 34 participants completed the cross‐over period, median number of episodes in the ON group was roughly 0.71 (range 0–11) compared to 1.7 (range 0–11) in the OFF group (data estimated from graph, no significance stated). No significant difference was seen in severity of FI (measured using the CCF‐FI score) in the ON group with a median score of 8.5 (range 3–18) compared to the OFF group with a median score of 10.5 (range 4–17) (p = 0.2). QoL was only compared between baseline and a follow‐up period in which participants chose their preferred stimulation mode and was therefore not included. 65 In the trial by Sorenson et al. no significant difference in FI episodes was reported as no FI episodes occurred in either the ON or OFF week. 66 The cross‐over trial by Kahlke et al. showed a significantly lower mean number of FI episodes per week in the ON period of 1 compared to the OFF period of 8.4, MD −7.4 (95% CI −12.04, −2.76). The mean severity of FI (measured using the CCF‐FI scale) was significantly less in the ON period mean 8.7 ± 3.6 compared to the OFF period mean 14.6 ± 4.6, MD −5.9 (95% CI −8.96, −2.84). 64
One of the five RCT's compared the effectiveness of SNM to optimal conservative treatment (pelvic floor exercises, bulking agents). This study by Tjandra et al. from 2008 included a total of 120 participants, of which seven participants in the SNM group did not make it past the test phase. 68 Mean number of FI episodes were significantly less in the SNM‐compared to the conservative treatment group at both 3 (2.9 vs. 8.1) and 12 months (3.1 vs. 9.4), MD −5.2 (95% CI −9.15, −1.25) and MD −6.3 (95% CI −10.34, −2.26) respectively. Severity of FI (measured using the CCF‐FI scale) was significantly better in the SNM‐compared to the conservative treatment group at both 3 and 12 months, MD −11 (95% CI −11.60, −10.40) and MD −12.90 (95% CI −13.85, −12.22). QoL (measured using the FIQoL scale) was significantly better for the SNM group compared to the conservative treatment group on all four domains at both 3 months: lifestyle MD 1.22 (95% CI 0.92, 1.52), Coping behaviour MD 1.02 (95% CI 0.70, 1.34), Depression and self‐perception MD 0.63 (95% CI 0.37, 0.89), Embarrassment MD 1.19 (95% CI 0.91, 1.47) and 12 months: Lifestyle MD 1.00 (95% CI 0.70, 1.30), Coping behaviour MD 0.82 (95% CI 0.50, 1.14), Depression and self‐perception MD 0.61 (95% CI 0.31, 0.91), Embarrassment MD 0.98 (95% CI 0.68, 1.28).
Adverse events were reported in 3 out of 5 studies included in this review, which assessed a total of 96 patients. 64 , 65 , 68 Implementation site pain was reported in four of these patients, excessive tingling in the vaginal region in five patients, haematoma formation in three patients, misplacement of tined lead in one patient, seroma which required percutaneous aspiration in one patient, unresolved pain leading to explantation in three patients and a recurrent infection leading to explantation in one patient. 64 , 65 , 68 When interpreting the results, it should be taken into account that only participants who had a successful test phase moved on to a permanent implant and were included in any of the studies. Additionally, the studies included only a small number of participants and none of the studies included a true sham group.
For a further breakdown of the results of this review, see Appendix: Appraisal Thaha et al.
Furthermore, analysis of the literature revealed one systematic review which reports on the complications associated with sacral neuromodulation. 69 This study by Maeda et al. from 2011 included 48 cohort studies reporting on post‐operative adverse events in a total of 1661 patients who underwent percutaneous nerve evaluation (PNE) of which 1600 proceeded to receive a sacral neuromodulator. Pain after implementation was the most common reported adverse event, reported in 13.0% of patients. The second most common adverse event was infection which was reported in 3.9% of patients. The authors note that there is an underreporting of adverse events in these studies, suggesting the incidence of adverse events is likely to be higher. 69
Cost‐effectiveness: a cost‐effectiveness study by Leroi et al. from 2011 reports that the cost effectiveness expressed as incremental costs per 50% of improved severity score (incremental cost‐effectiveness ratio) was €185,160 for SNM at 24 months follow‐up. 70 Another cost‐effectiveness study by Brosa et al. from 2008 reported that the incremental cost‐effectiveness ratio for SNM was €22,195 per QALY gained for patients with a structurally deficient anal sphincter and €16,181 for patients with a structurally intact anal sphincter. 71 While a study by Indinnimeo et al. from 2010 reported that the incremental cost‐effectiveness ratio for SNM was €28,285 per QALY gained for patients with a structurally deficient anal sphincter and €38,662 for patients with a structurally intact anal sphincter. 72 All three studies reported the cost to be acceptable.
SNM versus injectable bulking agents
Analysis of the literature revealed one RCT which examined the effects of SNM versus injectable bulking agents on FI complaints. The RCT by Rydningen et al. from 2017 specifically looks at women with FI following obstetric anal sphincter injury (OASIS) and included 56 women of which 30 were randomized to SNM and 26 to Permacol bulking agent. 73 A reduction of ≥50% in weekly FI episodes after 6 months occurred in 93% of participants in the SNM group compared to 32% of the Permacol group (p = 0.001). Improvement in severity of FI (measured using the St. Mark's scale) between baseline and 6 months were significantly greater in the SNM versus the Permacol group MD 8.9 (95% CI 6.20, 11.60). Improvement in disease specific QoL (measured using the FIQoL questionnaire) between baseline and 6 months was significantly greater in the SNM versus the Permacol group on all 4 domains: Lifestyle MD 0.90 (95% CI 0.52, 1.28), Coping behaviour MD 1.05 (95% CI 0.64, 1.46), Depression and self‐perception MD 0.51 (95% CI 0.17, 0.85) and Embarrassment MD 0.92 (95% CI 0.50, 1.34). However, no significant difference was seen in the generic QoL (measured using the EQ−5D questionnaire) between the SNM and injectable bulking agent group (p = 0.55). Adverse events were minor. Nine patients in the SNM group reported an adverse event, whereas seven patients in the Permacol group reported an adverse event. There was no significant difference in frequency of adverse events between groups (p = 0.77). Adverse events in the SNM group were related to pain and deterioration of urinary function which in most cases were resolved after adjusting the implantable pulse generator (IPG). Adverse events in the Permacol group included anal pain after the first injection and obstructed defecation. 73
Cost‐effectiveness: A cost‐effectiveness study by Bernstein et al. from 2014 reports that the incremental costs per QALY gained was $244,509 (€205,192) for SNM versus injectable bulking agents (NASHA/Dx). 74 Although this study was industry funded, we suspect this did not result in bias as the study results were negative for the funding industry.
SNM versus sphincteroplasty
No systematic reviews, RCT's or observational studies with a control group were found which assessed the effects of SNM versus sphincteroplasty in patients with FI.
Analysis of the literature revealed one retrospective analysis which assessed the effects of SNM versus sphincteroplasty. This study by Ratto et al. from 2010 included 24 women with FI and anal sphincter lesions of which 14 underwent sphincteroplasty and 10 underwent SNM. 75 The follow‐up period for the sphincteroplasty group ranged from 6 to 96 months (median 60 months) and ranged from 6 to 84 months in the SNM group (median 33 months). When comparing the SNM‐ and sphincteroplasty groups, no significant difference was seen in mean number of FI episodes: SNM baseline 25.6, follow‐up 0.8, Sphincteroplasty baseline 21.4, follow‐up 4.1 (significance levels for difference between the two groups not reported). No significant difference was seen between groups in the severity of FI (measured using the CCF‐FI scale) MD 2 (95% CI −1.21, 5.21). 75
Conclusion: SNM ON generally results in fewer mean number of weekly FI episodes compared to SNM OFF and compared to conservative treatment (significantly in 3/5 studies, no significance reported in one study and no difference in one study). Severity of FI was also generally less in the SNM group (Significantly less in two‐fifths studies, significance not reported in one study, no difference in one study and severity not assessed in one study). QoL was significantly better in the SNM group compared to conservative treatment. Adverse events such as pain and infection are common.
SNM results in greater reduction of FI episodes, FI severity and greater improvement in QoL compared to injectable bulking agents (NASHA Dx) in women with OASIS. In the small study comparing SNM to sphincteroplasty, no significant differences in efficacy were seen between treatment options.
In a small non‐controlled study, an improvement in mean number of FI episodes and QoL were seen after a period of 6 months with a rechargeable SNM compared to baseline.
Injectable bulking agents (silicone elastomer, polysaccharide gel)
Analysis of the literature revealed five systematic reviews which assessed the effects of injectable bulking agents versus no injectable bulking agents on FI complaints in the general FI population. 76 , 77 , 78 , 79 , 80 In total two of the RCTs included in the systematic reviews answer the PICO question by assessing the effects of injectable bulking agents compared to a control (sham). The first RCT by Siproudhis et al. from 2007 assessed the effects of polydimethylsiloxane (silicone) elastomer (PTQ)‐ compared to a saline injection in patients with FI and included 44 patients. 81 The second RCT by Graf et al. from 2011 assessed the effects of Solesta® (polysaccharide) gel ‐ compared to a sham injection (no substance) and included 206 patients. 82
No significant difference was seen in the median decrease in number of FI episodes per fortnight between the injectable bulking agent group compared to the sham group in the study by Graf et al. at both 3 months, 4.8 (IQR 0.96–10.0) versus 3.0 (0.0–7.0) p = 0.14 and 6 months follow‐up, 6.0 (0.0–12.5) versus 3.0 (0.0–8.9) p = 0.09. FI episodes per week were not reported in the study by Siproudhis et al. No significant difference was seen between groups in severity of FI (measured using the CCF‐FI scale) in either of the two studies. In the study by Graf et al. the difference in mean reduction in FI severity was −2.6 versus −2.0 at 3 months and mean −2.5 versus mean −1.7 at 6 months (no SD's reported). In the study by Siproudhis et al. difference between the two groups in reduction in FI severity was not significant, MD 0.3 (95% CI −2.53, 3.13). In the study by Siproudhis et al. QoL (measured using the FIQoL questionnaire) had improved significantly less in the injectable bulking agent group compared to sham from baseline to 3 months in all four domains: Lifestyle MD 0.5 (95% CI 0.40, 0.60), Coping behaviour MD 0.3 (95% CI 0.20, 0.40), Depression and self‐perception MD 0.2 (0.11, 0.29), Embarrassment MD 0.05 (95% CI 0.02, 0.08). In the study by Graf et al. a significantly greater improvement in QoL in the injectable bulk versus sham group was only seen in the domain coping behaviour 27.3% versus 10.9% improvement (p = 0.0016). No significant difference was seen in any of the other domains.
Siproudhis et al. reported adverse events in 7/22 (31.8%) of participants in the intervention group and in 2/22 (9.1%) participants in the control group. However, adverse events which were unlikely related to the treatment were also included in the results (angina pectoris, arm fracture and dizziness). No serious adverse events which were likely related to the treatment were reported in either group. The most common adverse event was pain at implant site. 81 In the study by Graf et al. 128 adverse events were reported in the intervention group of which two were serious (rectal and prostate abscesses) and 29 adverse events were reported in the control group, of which none were serious. Proctalgia was the most common adverse event in the injectable bulking agent group (14%) and injection site bleeding the most common one in the sham group (17%). 82
Injectable bulking agents versus pelvic floor physiotherapy with biofeedback
Analysis of the literature revealed one RCT by Dehli et al. which examined the effects of injectable bulking agents (Solesta® gel) versus pelvic floor physiotherapy with biofeedback. 83 The study included 126 patients which were randomly assigned to an injectable bulking agent or a biofeedback group. No significant difference was seen in severity of FI (measured using the St. Mark's scale) or QoL (measured using the EQ−5D and FIQoL questionnaires) between the two groups after 6 months of treatment (no significance level reported for difference between groups). 83
Conclusion: No significant difference in mean number of FI episodes in the injectable bulking agent group versus sham was seen in one study. No significant difference in FI severity improvement was seen between injectable bulking agents and sham in either study. Inconsistency between results regarding QoL are seen between studies but results for QoL tend to be worse in the injectable bulking agent group. QoL and severity of FI did not differ between treatment with injectable bulking agents and pelvic floor exercises with biofeedback. Minor adverse events are common with the use of injectable bulking agents but mainly consisted of injection site pain or bleeding.
Sphincteroplasty (secondary)
Analysis of the literature revealed one systematic review which examined different types of surgical interventions, including sphincteroplasty for the treatment of FI.
However, this systematic review by Forte et al. from 2016 which included two RCT's and five observational studies reported that insufficient evidence was available to determine the effectiveness of sphincteroplasty. 84 Furthermore, the available studies assessed the effectiveness of different techniques and different combinations of treatments rather than comparing sphincteroplasty to a control group.
Analysis of the literature revealed one retrospective study which examined the effect of sphincteroplasty on FI complaints. The study by Malouf et al. from 2000 included 55 patients who had undergone overlapping anal sphincter repair a minimum of 5 years (range 60–96 months) prior to answering the questionnaire and interview. 85 Our predefined outcomes were not answered in this study. This study reported that after a median of 15 months (range 6–36) 2 women did not yet have their stomas closed. Of the remaining 53 women, 42 (79%) reported to be continent for stool. After a minimum of 5 years post sphincter repair, participants were contacted. Eight participants were lost to follow‐up and one patient had undergone an ileostomy for Crohn's disease 3 years after sphincteroplasty and was also excluded from analysis. Of the remaining 46 participants, 7 had to undergo further surgery for their FI (1 colostomy, 6 postanal repair). Furthermore, one patient never had her stoma closed after the original repair due to poor treatment results. In 38 patients, the long‐term effects of the sphincter repair alone could be examined. Twenty‐seven out of the 38 patients reported and improvement in symptoms, with 23 reporting an improvement of ≥50%, 5 reported no improvement and 6 reported deterioration of FI symptoms. Only 4 patients were continent to solid and liquid stool. 85
Conclusion: In the short term, sphincteroplasty generally results in a reduction in FI complaints, but the effects seem to deteriorate over time. Sphincteroplasty seems to be effective in a small group of patients in the long term.
Stoma
No systematic reviews, RCT's or observational studies with a control group were found which assessed the effects of stoma versus no stoma in patients with FI.
Analysis of the literature revealed one retrospective study which assessed whether QoL is better in patients who received a colostomy who suffered from FI compared to patients without a colostomy who still suffer from FI. This study by Colquhoun et al. included 39 patients in the colostomy group and 71 patients in the FI group. 86 QoL (measured using the FIQoL questionnaire) was significantly better in the colostomy group compared to the FI group in the coping behaviour (2.7 vs. 2.0, p = 0.005), and embarrassment domains (2.7 vs. 2.2, p = 0.014). No significant difference was seen in the lifestyle (3.2 vs. 2.7, p = 0.14) and self‐perception and depression domains (3.1 vs. 2.9, p = 0.62).
Conclusion: QoL was significantly better in two of the FIQoL domains (coping behaviour and embarrassment) in the colostomy group compared to the FI group.
Recommendations for second line: Surgical interventions

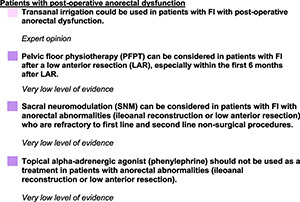
SPECIAL SITUATIONS
Introduction
For a subset of patients with FI, a different treatment approach may need to be considered to that of the general FI population. For this chapter three ‘special situation’ populations were defined: patients in nursing homes, patients with a history of neurological and/or cognitive impairment and patients with postoperative anorectal dysfunction.
This chapter describes the available evidence and recommendations for patients in these populations. Patients with FI due to congenital anorectal abnormalities are outside of the scope of these guidelines as symptom onset usually starts in childhood and these guidelines focus on adult patients with FI.
Review questions
What is the clinical effectiveness of interventions to manage faecal incontinence in nursing home patients?
What is the clinical effectiveness of interventions to manage faecal incontinence in patients with cognitive impairment?
What is the clinical effectiveness of interventions to manage faecal incontinence in patients with a history of neurological disease?
What is the clinical effectiveness of interventions to manage faecal incontinence in patients with postoperative anorectal dysfunction?
Populations
Patients in nursing homes
Any patient group with FI who reside in a nursing home were included in this section. Patients with and without cognitive impairment (e.g., dementia) were included in this group.
Patients with history of neurological disease and/or cognitive impairment
Any patient group with FI with any type of neurological disease and/or cognitive impairment, whether mild or severe (e.g., Dementia, Parkinson, Spinal Cord Injury, Stroke etc.) were included in this section. Neurological disease and cognitive impairment frequently overlap, therefore these populations were combined for the purpose of these guidelines.
Patients with postoperative anorectal dysfunction
Any patient group with anorectal dysfunction after surgery to the anorectal region (e.g., ileoanal reconstruction and low anterior resection) were included in this section.
Evidence in the literature
Patients in nursing homes
Analysis of the literature revealed three RCT's which examined treatment options for faecal incontinence in patients who reside in nursing homes. 87 , 88 , 89
Toilet assistance + exercise + frequent food and drink offers
Analysis of the literature revealed one RCT by Schnelle et al. from 2010 which assessed the effectiveness of an intervention which combines toilet assistance, exercise and offering food and fluid more frequently than usual (every 2 h for a period of 8 h), while the control group continued to receive care as usual. 89 The study included 125 nursing home patients with FI of which 112 completed the 12‐week treatment period and were included for analysis. Drop‐out was seven in the intervention group and 6 in the control group. The study reported that the intervention did not have a significant effect on FI episodes (p = 0.53), however appropriate faecal toileting (calculated by dividing number of defaecation episodes in the toilet by total number of defaecation episodes) did significantly improve (p < 0.001) (numerical data for differences between groups not reported for episodes or appropriate toileting). 89
Skin barrier cream with moisturising and skin‐protectant properties
Analysis of the literature revealed one RCT by Kon et al. from 2017 which assessed the effectiveness of a skin barrier cream with moisturising and skin‐protectant properties. 87 Thirty‐three elderly women in nursing homes who suffer from incontinence (urinary, faecal, or both) and mild incontinence associated dermatitis (IAD) of the buttocks or inner thigh were included in the study and randomized into the intervention or control group. All participants received daily cleansing with skin cleanser and application of a moisturizer. In addition, the intervention group received a skin barrier cream 3 times daily. Erythema was significantly less after 14 days in the intervention group compared to the control group (p = 0.018) and hydration of the stratum corneum was significantly better in the intervention group after 14 days (p = 0.031). Adverse events were not reported.
Glycerine suppository + enema
Analysis of the literature revealed one RCT by Chassagne et al. from 2000 which assessed the effectiveness of an osmotic laxative (30 g lactulose) compared to an osmotic laxative (30 g lactulose) + daily glycerine suppository + weekly enemas. 88 This RCT included 206 participants of which 101 completed the 8 weeks study period and were included for analysis. 88 A similar drop‐out rate was seen in both groups. No significant difference was seen in the number of FI episodes between groups (p = 0.9). 88
Conclusion: Proportion of faecal voiding in the toilet out of total faecal voiding was greater in the group who received an intervention which combined toilet assistance, exercise and offering food and fluid more frequently (every 2 h for a period of 8 h) compared to the care as usual group. No significant difference was seen in the number of FI episodes between groups.
Skin barrier cream with moisturising and skin‐protectant properties reduces incontinence associated dermatitis (IAD) in women in nursing homes with mild IAD.
No additional value was seen in adding a glycerine suppository and weekly enema to nursing home patients who were using an oral laxative.
Patients with a history of neurological disease and/or cognitive impairment
Analysis of the literature revealed one systematic review and two RCT's which assessed treatment options for faecal incontinence in patients with a neurological disease and/or cognitive impairment.
Transanal irrigation (TAI)
Analysis of the literature revealed one RCT by Christensen et al. from 2006 which assessed the effects of Transanal irrigation versus conservative treatment in patients who suffer from either FI, constipation or a combination of both with spinal cord injury (SCI). The RCT includes a total of 87 patients of which 17 patients had faecal incontinence as their predominant symptom. 90 Forty‐two patients (of which 9 with predominant FI) were randomized to TAI and 45 patients (of which 8 with predominant FI) were randomized to conservative treatment for a period of 10 weeks. Severity of FI (measured using the St. Mark's scale was significantly less in the TAI group compared to the conservative treatment group after treatment MD −2.3 (95% CI −4.12, −0.48). For QoL a significantly better score was seen in the TAI group for the coping behaviour and embarrassment domains after treatment: MD 0.4 (95% CI 0.08, 0.72) and MD 0.4 (95% CI 0.04, 0.76) respectively. No significant differences were seen between groups in the lifestyle and depression and self‐perception domains: MD 0.2 (95% CI −0.12, 0.52) and MD 0.3 (95% CI −0.04, 0.64). Adverse events related to bowel management during the study period were reported in 3 patients in the TAI group. One patient reported acute abdominal distention in the pre‐treatment period after taking the capsule to determine colonic transit time and two patients reported severe abdominal pain which improved after disimpaction of constipated stool in the hospital. 90
Oxymetazoline (α‐agonist)
Analysis of the literature revealed one cross‐over trial by Barak et al. from 2019 which assessed the effects of topical Oxymetazoline (α‐agonist) (1%) versus topical placebo gel in patients who suffer from FI with spinal cord injury. 91 In this RCT, 19 patients were randomly assigned to 4 weeks of treatment with Oxymetazoline, followed by a 2‐week wash‐out period, followed by 4 weeks of treatment with placebo or vice versa. Patients experienced significantly less FI episodes with a mean of 6.3 ± 2.1 (SD) episodes during the 4‐week treatment period compared to a mean of 10.1 ± 4.3 (SD) episodes during the 4‐week placebo period, MD −3.8 (95% CI −5.95, −1.65). It should be noted that this is only a reduction of roughly one episode per week and that only participants with >4 incontinence episodes during the placebo period were included in this analysis. No significant difference was seen between treatment and placebo period in severity of FI (measured using the FISI scale) MD −0.11 ± 1.69 (p = 0.79). QoL (measured using the FIQoL questionnaire) did not improve in any of the 4 domains (significance levels not presented for all domains). No serious adverse events were reported. Two minor adverse events were reported; muscle spasms and constipation. 91
Any therapeutic option
Analysis of the literature revealed one systematic review which assessed the effects of any treatment for FI in patients with central neurological diseases or injury. This review by Coggrave et al. from 2014 examined the effects of different types of treatment options on both faecal incontinence and/or constipation. 92 Only one study included in this review reported on our predefined outcomes, this study by Christensen et al. has been described in the TAI paragraph above. We therefore did not include this review in the guideline and would like to refer you to this systematic review by Coggrave et al. from 2014 for further information. 92
Conclusion: Severity of FI and QoL in two out of the four FIQoL domains were significantly better in the TAI group compared to the conservative treatment group in patients with spinal cord injury and FI.
Treatment with Oxymetazoline resulted in a significant reduction in mean number of FI episodes per 4‐weeks. No significant differences were seen in FI severity or QoL.
Patients with post‐operative anorectal dysfunction
Analysis of the literature revealed five systematic reviews and three RCT's which assessed treatment options for faecal incontinence in different subgroups of patients with postoperative anorectal dysfunction. Evidence was found for patients who underwent ileoanal reconstruction and patients who underwent low anterior resection.
Patients who underwent ileoanal reconstruction with a pouch
Evidence regarding patients who underwent ileoanal reconstruction with a pouch included one systematic review which assessed the effects of SNM on FI complaints and one RCT which assessed the effects phenylephrine on FI complaints. 93 , 94
SNM
The systematic review by Kong et al. from 2017 included three uncontrolled studies (case report, cohort study, retrospective study) which examined the effects of SNM on FI in patients who underwent ileoanal reconstruction with a pouch. 93 The three studies consist of a total of 12 participants of which 10 received a definitive implant and were included in the analysis. Follow‐up of these studies was 3, 6 and 24 months. Two of the studies reported on changes in median number of FI episodes per week between baseline and follow‐up. In one study a reduction from a median number of 4 (range 4–25) episodes per week at baseline to 1.1 (0–4) at follow‐up was seen (no significance reported). In the other study a significant reduction from 4 (2–9) at baseline to 1.8 (0–3.5) episodes per week (p = 0.03) at follow‐up was seen. All three studies reported on FI severity (measured using the CCF‐FI scale). A significant reduction between baseline from a score of 15 (7–19) to a score of 1.5 (0–14) at follow‐up (p = 0.01) was seen in one study. A reduction was also seen in the other two studies from baseline 14.5 (13–25) to follow‐up 5.7 (0–10) and from 16 at baseline to <4 at follow‐up (significance not reported). QoL was reported in two studies, of which only one performed statistical analysis. This study showed no significant improvement in any of the four subdomains of the FIQoL questionnaire (lifestyle p = 0.06, coping behaviour p = 0.09, self‐perception and depression p = 0.14, Embarrassment p = 0.05) (no numerical values for baseline and follow‐up scores). 93 , 95
Phenylephrine
The RCT by Lumi et al. from 2009 assessed the effects of topical phenylephrine (10%) to placebo on FI complaints in patients who underwent ileoanal reconstruction with a pouch. 94 The study included 19 patients who predominantly suffer from nocturnal FI. Participants were randomized into a phenylephrine (10%) group (n = 7) or placebo group (n = 5) and received treatment for 1 month. Mean number of FI episodes were recorded for 1 month prior to treatment and for 1 month during treatment. In the treatment group the mean number of FI episodes per month went from 7 prior to treatment to 9 during treatment compared to a mean of 3–5.4 episodes per month in the placebo group. No significant difference was seen between groups (significance level not reported). No adverse events were reported. 94
Conclusion: Treatments in patients with an ileoanal reconstruction and FI: A general improvement in FI complaints was seen between baseline and follow‐up after implementation of the SNM, significance was not reported for all outcomes in each study. Topical phenylephrine had no significant effect on mean number of FI episodes.
It should be taken into account that the quality of evidence was very low for both treatment options.
Patients who underwent low anterior resection (LAR)
Analysis of the literature revealed two systematic reviews which asses the effects of SNM on FI in patients with lower anterior resection syndrome (LARS) one systematic review and one RCT which assessed the effects of pelvic floor muscle training (PFMT) on FI in patients who underwent stoma closure after a low anterior resection, one RCT which assessed the effects of phenylephrine on FI in patients who underwent lower anterior resection and one systematic review which assessed the effects of any therapeutic option on anorectal function in patients who underwent rectal resection. 96 , 97 , 98 , 99 , 100 , 101
SNM
Analysis of the literature revealed two systematic reviews which asses the effectiveness of SNM on FI complaints in patients after lower anterior resection (LAR). 96 , 97 The most up‐to‐date systematic review and meta‐analysis by Ram et al. from 2020 included 13 studies with a total of 114 patients. 97 Follow‐up in this review ranged from 9 to 50 months. Overall success rate (defined as marked improvement in FI complaints, a ≥50% reduction in FI episodes or complaints or a reduction of LARS score from major to minor) was 83.3% (95% CI 71.33%–95.25%). Severity of FI (measured using the CCF‐FI) decreased significantly from baseline to follow‐up, MD 10.79 (95% CI 8.55, 13.02). QoL (measured using the SF−36 questionnaire) was only reported in 2 out of 13 studies in a total of 6 patients and data was lacking for statistical analysis. Differences between post‐ and pre‐treatment QoL reported per domain and study: Physical function 5.71% and 22.63%, Social function 56.32% and 39.23%, Role‐physical 60% and 49.7%, Role‐emotional 100% and 49.5%, Mental health 44.44% and 55.22%, Vitality 31.82% and 18.87%, Body pain 0% and 23.32%, and general health 84.44% and 55.75%. Adverse events were reported in 10 out of the 13 studies. Five studies reported that no adverse events occurred, and five studies reported that several adverse events did occur. In total, 13 out of 95 patients had the implant removed prior to end of study period. Post‐operative wound infection was recorded in 6 patients, stimulation site pain was recorded in 4 patients and implantation site pain was recorded in 2 patients. 97
It should be noted that the studies included in this meta‐analysis were heterogeneous and changes in FI outcomes were measured by comparison of pre‐ and post‐treatment rather than comparison to a control group.
PFPT
Analysis of the literature revealed a systematic review including one RCT and several uncontrolled studies which assessed the effects of pelvic floor physiotherapy (PFPT) on FI in patients after LAR. 98 , 99 Due to the availability of higher evidence, the uncontrolled studies will not be discussed for purposes of these guidelines. The RCT by Lin et al. from 2016 included 53 patients of which 27 were randomized into a PFPT group and 26 were randomized into the no PFPT group. 99 Follow‐up was measured at different times points up to 9 months after the start of the treatment period and discharge. Severity of FI (measured using the CCF‐FI scale) was significantly better in the PFPT group compared to the control group at 1 month MD −2.79 (95% CI −5.3, −0.28), 2 months MD −2.52 (95% CI −4.46, −0.58), 3 months MD −2.89 (95% CI −4.68, −1.1) and 6 months MD −1.87 (95% CI −3.42, −0.32). No significant difference was seen at 9 months MD −0.31 (95% CI −3.8, 3.18). This may suggest that PFPT is only effective in the short term after LAR. Mean number of FI episodes and QoL were not assessed. No adverse events were reported. 99
Phenylephrine
Analysis of the literature revealed one RCT by Park et al. which assessed the effects of topical phenylephrine (30%) versus placebo gel twice daily for 4 weeks on FI complaints in patients after LAR. 100 Nineteen patients were randomized into the topical phenylephrine group and 16 into the placebo group. Two participants in the treatment group and four in the placebo group were not included in the analysis due to poor compliance. Mean number of FI episodes was not reported. There was no significant difference in FI severity (measured using the FISI scale) between two groups after treatment MD −0.1 (95% CI −10.83, 10.63). No significant difference was seen in QoL in any of the four domains between the two groups after treatment: Lifestyle MD −0.1 (95% CI −0.76, 0.56), Coping behaviour MD 0 (95% CI −0.51, 0.51), Depression and self‐perception MD 0 (95% CI −0.47, 0.47), Embarrassment 0.4 (95% CI −0.16, 0.96). No serious adverse events were reported. Minor adverse events occurred in 7/17 participants in the Phenylephrine group (dermatitis reaction, headache) and in 2/16 participants in the placebo group (dermatitis reaction, palpitations). 100
Any therapeutic option
Analysis of the literature revealed one systematic review which assessed the effects of any treatment for FI in patients who have undergone LAR. This study examined the effects of SNM, PFPT and transanal irrigation (TAI). For SNM and PFPT no additional controlled studies were included in this review that were not included in the previously mentioned paragraphs and TAI was assessed in two studies, but did not assess any of our predefined outcomes. We therefore did not include this review in the guideline and would like to refer you to this systematic review by Maris et al. from 2013 for further information. 101
Conclusion: The included studies in this section were of low quality so should be interpreted with caution. SNM generally resulted in improvement in FI complaints in patients with FI who have undergone LAR. PFPT resulted in improvement of FI severity compared to the control group at 1, 2, 3 and 6 month(s) follow‐up, but not at 9 months follow‐up. This could suggest PFPT is only effective in the short term after LAR.
Phenylephrine did not result in improvement of FI severity or QoL compared to placebo in patients with FI who have undergone LAR.
Recommendations for special situations


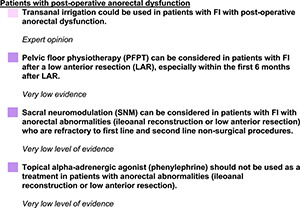
DEVELOPING AND OTHER TREATMENTS
Introduction
In patients where first line and second line (surgical or non‐surgical) treatment has not resulted in acceptable symptom reduction, or where any of these treatment options are not preferred, other treatments may be considered. The treatments described in this chapter are novel treatment options or treatment options which do not belong within any of the previous chapters.
Review questions
What are the effects of a vaginal insert device versus no vaginal insert device in patients with FI on frequency of episodes, symptoms and severe complications?
What are the effects of alpha‐adrenergic agonist versus no alpha‐adrenergic agonist in patients with FI on frequency of episodes, symptoms and severe complications?
What are the effects of oestrogen therapy versus no oestrogen therapy in patients with FI on frequency of episodes, symptoms and severe complications?
What are the effects of cell therapy/regenerative medicine versus no cell therapy/regenerative medicine in patients with FI on frequency of episodes, symptoms and severe complications?
What are the effects of acupuncture versus no acupuncture in patients with FI on frequency of episodes, symptoms and severe complications?
What are the effects of electroacupuncture versus standard acupuncture in patients with FI on frequency of episodes, symptoms and severe complications?
What are the effects of bulking implants versus no bulking implants on frequency of episodes, symptoms and severe complications in patients with FI?
Evidence in the literature
Vaginal insert device
Analysis of the literature revealed one systematic review which assessed the effectiveness of the eclipse vaginal insert device on FI complaints. 49 This systematic review by Buono et al. from 2019 included a prospective non‐controlled study which included 110 patients with FI and a secondary analysis of this same study. Because the secondary analysis only included patients who wanted to continue using the plug because they found the product useful, this analysis was excluded for purposes of this guideline due to bias. Of the 110 patients included in the first study, 49 dropped‐out due to an unsuccessful fit or due to incompletion of the fitting assessment prior to study start. 102 An additional five women dropped out during the treatment period. FI episodes per 2 weeks reduced from a mean of 11.6 ± 9.5 (SD) at baseline to a mean of 2.1 ± 2.9 (SD) after 1 month of treatment (p < 0.001). Quality of life (measured using the FIQoL scale) improved on all four domains between baseline and treatment after 1 month: Lifestyle 2.63 ± 0.92 versus 3.06 ± 0.91, Coping behaviour 1.82 ± 0.71 versus 2.28 ± 0.95, Depression and self‐perception 2.89 ± 0.84 versus 3.3 + 0.74 and Embarrassment 1.71 ± 0.63 versus 2.25 ± 1 (all p < 0.001). FI severity was not assessed.
Adverse events occurred frequently. Out of the 110 participants who participated in the fitting assessment, 47 reported a (mild) adverse event. During the 1‐month treatment period 14 out of 61 participants reported a (mild) adverse event. Adverse events consisted of pelvic cramping or discomfort, urinary incontinence, pelvic pain, vaginal spotting, vaginal irritation, among others. No serious adverse events were reported. 102 Results should be interpreted with cautions as the analyses of the above‐mentioned outcomes were performed in the per protocol‐rather than the intention to treat population, results are expected to be much less favourable if an ITT analysis had been performed.
For a further breakdown of the results of this review, see Appendix: Appraisal Buono et al.
Conclusion: Use of a vaginal insert device resulted in a reduction of number of fortnightly FI episodes and resulted in improved quality of life in all four domains in patients who finished the treatment period. It should be noted that nearly half of the participants dropped out, mainly related to the device being uncomfortable. In patients who were able to tolerate the device, the device improves QoL and reduces mean number of FI episodes. Results should be interpreted with caution due to lack of control group and analysis being performed in the per protocol population instead of intention to treat.
Alpha‐adrenergic agonist
Analysis of the literature revealed two RCT's which assessed the effectiveness of an α‐adrenergic agonist on symptoms in women with FI. 103 , 104 The first cross‐over trial by Carapeti et al. from 2000 included 36 patients who were randomized into a 4 week topical phenylephrine (10%) treatment, followed by a 1 week washout period, followed by a 4 week topical placebo treatment or vice versa. 103 No significant difference in FI severity (measured using the St. Mark's scale) between the two groups was found. Difference in severity between treatment versus placebo in period 1 was: mean 12.5 ± 3.4 versus 12.6 ± 4.2 and was 13.4 ± 4.7 versus 13.0 ± 4.7 in period 2 (p = 0.7). In the second study by Bharucha et al. from 2014, 43 women with urge predominant FI were randomized into an oral clonidine (0.1 mg) or an oral placebo twice daily group for a study period of 4 weeks. 104 Differences between the clonidine group and the placebo group after treatment were calculated taking into account baseline differences. A significantly greater improvement in mean number of FI episodes per week was seen in the placebo group (reduction from 31 ± 5–19 ± 4 episodes) compared to the clonidine group (reduction from 20 ± 3–12 ± 3) MD −4 (95% CI 3.32, 4.68). It should be noted that this study also included staining as an FI episode. A significantly greater improvement in FI severity (measured using the FISI scale) was seen in the placebo group (reduction from 37.3 ± 2.5 to 31.2 ± 2.5) compared to the clonidine group (reduction from 36.2 ± 2.7 to 29.3 ± 2.8) MD −0.8 (95% CI −1.28, −0.32). Improvement in QoL (measured using the FIQoL) differed per domain. A significantly larger improvement in the ‘lifestyle’ domain was seen in the placebo group compared to the clonidine group MD −0.1 (95% CI −0.14, −0.06). However, a significantly larger improvement in the ‘embarrassment’ domain was seen in the clonidine group MD 0.4 (95% CI 0.36, 0.44). No significant difference was seen between the two groups in the ‘coping behaviour’ or the ‘depression and self‐perception’ domains MD 0 (95% CI −0.02, 0.02) and MD 0 (95% CI −0.03, 0.03) respectively. Only mild adverse events were reported in both studies. In the study by Carapeti et al. 3 of 36 patients developed a mild dermatitis reaction to the phenylephrine. The study by Bharucha et al. also only reported mild adverse events such as dry mouth (1 in placebo group, 16 in treatment group), drowsiness (3 in placebo group, 5 in treatment group), light‐headedness (2 in placebo group, 6 in treatment group) and fatigue (5 in placebo group, 8 in treatment group).
Conclusion: Alpha‐adrenergic agonist treatment did not result in a greater reduction of mean number of FI episodes per week in one study (not measured in other study). A significantly greater improvement in severity score was seen in one study, but not in the other study. QoL was measured in one study, showing inconsistent results between domains.
Oestrogen therapy
Analysis of the literature revealed one systematic review which assessed the effectiveness of both local and systemic hormone therapy on FI in postmenopausal women. 105 In this systematic review by Bach et al. from 2019, 8 studies were assessed, of which only two studies determined the effects of hormone therapy treatment on FI complaints in patients with FI, including one RCT and one observational study. The aim of the other six studies was to assess the correlation between prevalence of FI and use of hormone therapy and were therefore excluded. The observational study included a total of 20 post‐menopausal women with FI. 106 Four of these women had a hysterectomy in the past and received an oestrogen patch for 6 months during the treatment period, the other 16 women with intact uteruses received the oestrogen patch for 6 months as well as oral progesterone (50 μgm oestradiol every 24 h) and oral progestogen (norethisterone acetate 1 mg daily for 12 days per cycle). 106 No significant difference was seen between baseline and after treatment in the occurrence of liquid and solid stool (no significance level reported). 106 The RCT which included 36 patients assessed the effects of a topical estriol compared to a placebo. 107 Both groups were asked to apply the ointment three times daily for a period of 6 weeks. Severity of FI (measured using the CCF‐FI scale) did not significantly differ between the two groups MD −2 (−4.62, 0.62). Quality of life also did not significantly differ between the two groups on any of the four domains included in the Spanish quality of life questionnaire (ECIF): style of life MD 0 (95% CI 0.66, 0.66), Conduct MD −0.02 (95% CI −0.54, 0.50), Depression MD −0.01 (95% CI −0.60, 0.58), Embarrassment MD −0.03 (95% CI −0.63, 0.57). Mean number of FI episodes per week were not measured. A total of five patients, all in the oestrogen group experienced pruritus ani, no other adverse events were reported. 107
Conclusion: The limited evidence regarding the effects of hormone therapy treatment on FI symptoms suggests that the effects are not greater than the effects of a placebo treatment.
Cell therapy/regenerative medicine
The analysis of literature reveals two systematic reviews and three additional RCT's which assessed the effects of cell therapy/regenerative medicine in patients with sphincter defects who suffer from FI. 108 , 109 , 110 , 111 , 112 Both of the systematic reviews included animal studies and no RCT's and will therefore not be included in this guideline. 108 , 109
The first RCT by Sarveazad et al. from 2017 compared the effects of human adipose‐derived stromal/stem cells (hADSCs)‐ to a placebo injection. The study included 18 patients and had a follow‐up of 2 months. 111 The second RCT by Boyer et al. from 2018 compared the effects of injections of Autologous myoblasts (AM) to placebo on FI complaints in 24 patients and had a follow‐up of 12 months. 112 The third RCT by de la Portilla et al. from 2020 compared the effects of autologous expanded mesenchymal stem cells derived from adipose tissue (AdMSCs) to a placebo on FI complaints and had a total follow‐up of 48 weeks. 110
Mean number of FI episodes per week was only determined in the study by de la Portilla et al. Significantly less FI episodes were seen in the AdMSCs group compared to the placebo group after 4 weeks but not after 48 weeks of treatment MD −10.10 (95% CI −16.51, −3.69) and MD 2.24 (95% CI −9.61, 14.09) respectively.
Severity of FI (measured using the CCF‐FI scale) was measured in all three studies. The studies by Sarveazad et al. and de la Portilla et al. could be combined for a meta‐analysis looking at short term effects on FI severity. A significantly greater reduction of FI severity was seen in the treatment group compared to the placebo group MD −0.74 (95% CI −1.10, −0.39) in the short term (4 and 8 weeks). A significantly greater reduction in FI severity was seen in the study by de la Portilla et al. MD −4.12 (95% CI −6.11, −2.13) in the longer term (48 weeks), but not in the study by Boyer et al. No significant difference in FI severity between the two groups was seen at 12‐month follow‐up: −4.5 (range −12 to 2) in the myoblast group versus −2 (range −8 to 6) in the placebo group (p = 0.08). QoL (measured using the FIQoL) was reported in the study by de la Portilla et al. and the study by Boyer et al. No significant difference was seen on any of the four quality of life domains in the study by de la Portilla et al.: Lifestyle 4 weeks MD 0.03 (95% CI −0.94, 1.00), Coping behaviour 4 weeks MD 0.23 (95% CI −0.79, 1.25), Depression and self‐perception 4 weeks MD −0.17 (95% CI −1.23, 0.89), Embarrassment 4 weeks MD 0.64 (95% CI −0.23, 1.51), Lifestyle 48 weeks MD −0.45 (95% CI −1.22, 0.32), Coping behaviour 48 weeks MD −0.73 (95% CI −1.47, 0.01), Depression and self‐perception 48 weeks MD −0.06 (95% CI −0.74, 0.62), Embarrassment 48 weeks MD 0 (95% CI −0.65, 0.65). In the study by Boyer et al. a significantly better QoL was seen in the lifestyle domain in the treatment group compared to the placebo group: median 0.7 (−0.3 to 2.0) versus −0.3 (−1.2 to 2.2) (p = 0.03). No significant difference was seen between groups in any of the other three domains: Coping and behaviour, median 0.5 (−0.1 to 2.0) versus 0 (−1.4 to 2.2) (p = 0.1), Depression and self‐perception, median 0.4 (−0.3 to 1.5) versus −0.2 (−1.3 to 1) (p = 0.11), Embarrassment, median 0 (−1 to 1.7) versus 0.3 (−0.4 to 2.7) (p = 0.82).
Adverse events were assessed in all three studies. In the study by Sarveazad et al. no adverse events were reported. In the study by Boyer et al. mild adverse events were reported in 3 out of 24 participants of transient pain at biopsy site (n = 2) and infection at biopsy site (n = 1). In the study by de la Portilla et al. one out of 18 participants reported a (mild) intervention‐related adverse event of a haematoma formation during tissue extraction. No serious adverse events were reported.
Conclusion: Mean number of FI episodes per week was measured in one study, significantly less episodes were seen in the cell treatment group compared to the placebo group at 4 weeks, but no significant difference was seen at 48 weeks. A significantly greater reduction in FI severity was seen in the cell treatment groups in two studies in the short term, but only in one out of two studies in the longer term.
QoL was measured in two studies, a significantly better QoL on the lifestyle domain was seen in one study, but no difference was seen between groups in any of the other domains in either study.
Acupuncture
No systematic reviews, RCT's or observational studies with a control group were found regarding the effects of acupuncture on FI.
Two non‐controlled observational studies which assessed the effects of acupuncture on FI complaints in the general FI population were found.
These studies by Scaglia et al. from 2009 and Franco et al. from 2016 included 15 and 18 patients respectively. 113 , 114 Severity and QoL improved after 10 weekly acupuncture sessions compared to baseline. Due to methodological shortcomings, these studies were not discussed in any further detail.
Conclusion: FI severity and QoL significantly improved on the short‐term. Findings should be interpreted with caution due significant methodological shortcomings.
Electroacupuncture versus regular acupuncture
No studies were found which compare the effects of electroacupuncture to regular acupuncture in patients with FI.
Bulking implants (implantable polyacrylonitrile)
No systematic reviews, RCT's or observational studies with a control group were found which assessed the effects of bulking implants versus no bulking implants in patients with FI.
Analysis of the literature revealed two uncontrolled prospective studies which assessed the effects of the Gatekeeper implant. The first study by Ratto et al. from 2016 included 54 patients who received the Gatekeeper implant and had a follow‐up period of 12 months. 115 The second study by Brusciano et al. from 2020 included 20 women who received the Gatekeeper implant and had a follow‐up period of 36 months. 116 In the study by Ratto et al. a significant reduction in both liquid (p < 0.001) and solid stool (p = 0.010) episodes between baseline and 12‐month follow‐up were reported. FI severity measured using both the CCF‐FI scale and St. Mark's scale decreased significantly from a median CCF‐FI score of 12 (range 3–20) at baseline to 5 (0–16) (p < 0.001) and a median St. Mark's score of 14 (3–24) to 6.5 (0–17) (p < 0.001) at 12 months post implantation in the study by Ratto et al. FI severity (measured using the CCF‐FI scale) decreased significantly from a mean of 12.4 ± 1.8 at baseline to 4.9 ± 1.7, MD 7.5 (95% CI 6.41, 8.59) at 36 months in the study by Brusciano et al. In the study by Ratto et al. QoL (measured using the FIQoL questionnaire) improved significantly on all four domains when comparing baseline to 1 year follow‐up: Lifestyle 3 (1.2–4) versus 3.7 (1.1–4.4) (p = 0.01), Coping behaviour 2.4 (1–4) versus 3 (1–4) (p = 0.001), Depression and self‐perception 3 (1–4.2) versus 3 (1.25–4.3) (p = 0.029), Embarrassment 2 (1–4) versus 2.9 (1–4.7) (p = 0.001) (data presented as median and ranges, data estimated from graph). Mean number of FI episodes per week and QoL were not measured in the study by Brusciano et al.
Adverse events did not occur in the study by Brusciano et al. and adverse events in the study by Ratto et al. were all minor and consisted mainly of anal discomfort or pain (13%). The pain resolved in all patients. 115 , 116
Conclusion: Where measured, FI episodes, FI severity and QoL all improved after implantation of the Gatekeeper compared to baseline. Results should be interpreted with caution due to lack of control group.
Recommendations for developing and other treatments


DISCUSSION
This is an up‐to‐date, multidisciplinary, European clinical practice guideline for the diagnosis and treatment of faecal incontinence (FI) in adult patients. The most recent guideline for management of FI prior to this project was published in 2007. 3 A more up‐to‐date guideline was necessary as a large number of studies have been published related to the treatment and diagnosis of faecal incontinence since then. Another strength of this guideline is that patients with FI were involved in this project from start to finish to provide their invaluable perspectives and to ensure issues important to this patient group were covered.
This guideline contains 45 recommendations on the classification, diagnosis and management of FI in adult patients. An evidence‐based treatment algorithm (Figure 1) has been created to summarise the most important recommendations. We suggest using this algorithm as a guide in combination with the main body of text to determine which possible steps can be taken when treating a patient with FI.
The strength of the evidence found, assessed using the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) approach, was either low, very low or expert opinion for all PICO questions. It is a well‐known problem that level of evidence in the faecal incontinence field is fairly low despite the high prevalence of patients suffering from FI. 117 To improve strength of evidence, and thus strength of recommendations, more high quality randomized controlled trials should be performed which all should assess the same outcomes, facilitating comparison and evidence synthesis. A Core Outcome Set (COS) could help ensure uniformity in outcome measures being used among different trials in the future. A COS for faecal incontinence is currently being developed which also prioritises patient involvement. 118
A limitation of this guideline is that recommendations could have been biased towards surgical treatments considering the fact that the majority of GDG members were colorectal surgeons. Nevertheless, we have attempted to assemble a diverse group of healthcare professionals across Europe with proper representations of scientific and professional background, age, gender, and geography.
CONFLICT OF INTEREST
The authors would like to report the following potential conflict(s) of interest:
D. Keszthelyi, ZonMw (Dutch government), Foundation for Gastroenterology (MLDS), Allergan, Grunenthal GmbH, Will Pharma SA research supports, Bayer GmbH, Biocodex Benelux consulatation fees; J. Kleijnen, European Society of Coloproctology consultation fees; E. Bradshaw, Medtronic honoraria; E.V. Carrington, MMS/Sandhill scientific research suppor, Laborie consultations fees and speaking at event; G. Chiarioni, Takeda, Alpha sigma consulation fees, Kyowa‐Kirin speaking at event, member of the Anorectal Committee of the Rome Foundation and of the International Consultation on Continence; Y. Maeda, Medtronic research support, Creo medical consultation fee and Atellas speaking at event; D. Pohl, Vifor, Alfa Sigma research supports, Sanofi, Allergan, Medtronic consultation fee, Sanofi speaking at event; M. Rydningen, Medtronic consultation fees; C.J. Vaizey, Renew medical stock shareholder and United Kingdom continence society research support; S.O. Breukink, Medtronic research support; M. Gladman, Medtronic consultation fees.
AUTHOR CONTRIBUTION
Stephanie O. Breukink and Daniel Keszthelyi were the lead authors responsible for assembly of the guideline development group (GDG) and drafting the guidelines protocol. The initial list of research questions to be covered by the guidelines were drafted by Stephanie O. Breukink, Daniel Keszthelyi, Sadé L. Assmann, the methodologist Jos Kleijnen and revised by all GDG members (Sadé L. Assmann, Daniel Keszthelyi, Jos Kleijnen, Foteini Anastasiou, Elissa Bradshaw, Ann E. Brannigan, Emma V. Carrington, Giuseppe Chiarioni, Yasuko Maeda, Jarno Melenhorst, Giovanni Milito, Jean W. M. Muris, Julius Orhalmi, Daniel Pohl, Yvonne Tillotson, Mona Rydningen, Saulius Svagzdys, Carolynne J. Vaizey, Stephanie O. Breukink). The literature search was conducted by the methodologist Jos Kleijnen and Sadé L. Assmann. Systematic selection of articles and data extraction were performed independently by Sadé L. Assmann and Liora D. A. Ebben. Sadé L. Assmann systematically appraised the literature and assessed the evidence according to GRADE with the methodological support from Jos Kleijnen. All GDG members and the external advisor (Marc A. Gladman) discussed the results and agreed on the recommendations. The lead authors Stephanie O. Breukink, Daniel Keszthelyi and Sadé L. Assmann drafted the manuscript, which was reviewed, revised and approved by all GDG members.
DISCLAIMER
These guidelines have been developed with reasonable care and with the best of knowledge available to the authors at the time of preparation. They are intended to assist healthcare professionals and allied healthcare professionals as an educational tool to provide information that may support them in providing care to patients. Patients or other community members using these guidelines shall do so only after consultation with a health professional and shall not mistake these guidelines as professional medical advice. These guidelines must not substitute seeking professional medical and health advice from a health professional.
These guidelines may not apply to all situations and should be interpreted in the light of specific clinical situations and resource availability. It is up to every clinician to adapt these guidelines to local regulations and to each patient's individual circumstances and needs. The information in these guidelines shall not be relied upon as being complete, current or accurate, nor shall it be considered as inclusive of all proper treatments or methods of care or as a legal standard of care. The development of this guideline was made possible by a grant from UEG (‘UEG Activity Grant 2019’) which was supplemented by the ESCP.
UEG makes no warranty, express or implied, in respect of these guidelines and cannot be held liable for any damages resulting from the application of these guidelines, in particular for any loss or damage (whether direct or indirect) resulting from a treatment based on the guidance given herein.
UEG shall not be held liable to the utmost extent permissible according to the applicable laws for any content available on such external websites, which can be accessed by using the links included herein.
Supporting information
Supporting Information S1
Supporting Information S2
Supporting Information S3
Supporting Information S4
Supporting Information S5
Supporting Information S6
Supporting Information S7
Supporting Information S8
Assmann SL, Keszthelyi D, Kleijnen J, Anastasiou F, Bradshaw E, Brannigan AE, et al. Guideline for the diagnosis and treatment of Faecal Incontinence—A UEG/ESCP/ESNM/ESPCG collaboration. United European Gastroenterol J. 2022;10(3):251–86. 10.1002/ueg2.12213
[Correction added on 02 April 2021, after first online publication: Text citation of Appendices 1 and 2 are revised].
DATA AVAILABILITY STATEMENT
Data sharing is not applicable for most part of this study as little new data were created or analysed in this study. All results presented in this manuscript were directly taken from‐ or derived from data presented in the articles included in the reference list.
REFERENCES
- 1. GRADE WG. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carrington E, Scott S, Bharucha A, Bharucha A, Mion F, Remes‐Troche JM, et al. International Anorectal Physiology Working Group and the International Working Group for Disorders of Gastrointestinal Motility and Function . Expert consensus document: Advances in the evaluation of anorectal function. Nat Rev Gastroenterol Hepatol. 2018;15:309–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Institute for Health and Clinical Excellence . Faecal incontinence: the management of faecal incontinence in adults NICE; 2007. [Google Scholar]
- 4. Rao SS, Bharucha AE, Chiarioni G, Felt‐Bersma R, Knowles C, Malcolm A, et al. Anorectal disorders. Gastroenterology. 2016;150(6):1430–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pahwa AK, Khanijow KD, Harvie HS, Arya LA, Andy UU. Comparison of patient impact and clinical characteristics between urgency and passive fecal incontinence phenotypes. Female Pelvic Med Reconstr Surg. 2020;26(9):570–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Norton C, Kamm M. Outcome of biofeedback for faecal incontinence. Br J Surg. 1999;86(9):1159–63. [DOI] [PubMed] [Google Scholar]
- 7. Hotouras A, Thaha MA, Boyle DJ, Allison ME, Currie A, Knowles CH, et al. Short‐term outcome following percutaneous tibial nerve stimulation for faecal incontinence: a single‐centre prospective study. Colorectal Dis. 2012;14(9):1101–5. [DOI] [PubMed] [Google Scholar]
- 8. Brown HW, Guan W, Schmuhl NB, Smith PD, Whitehead WE, Rogers RG. If we don't ask, they won't tell: screening for urinary and fecal incontinence by primary care providers. J Am Board Fam Med. 2018;31(5):774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keighley MR, Williams NS. Keighley & Williams' surgery of the anus, rectum and colon: two‐volume set CRC Press; 2018. [Google Scholar]
- 10. Cichowski SB, Dunivan GC, Rogers RG, Komesu YM. Patients' experience compared with physicians' recommendations for treating fecal incontinence: a qualitative approach. Int Urogynecol J. 2014;25(7):935–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jelovsek JE, Markland AD, Whitehead WE, Barber MD, Newman DK, Rogers RG, et al. Controlling faecal incontinence in women by performing anal exercises with biofeedback or loperamide: a randomised clinical trial. Lancet Gastroenterol Hepatol. 2019;4(9):698–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Norton C, Chelvanayagam S, Wilson‐Barnett J, Redfern S, Kamm MA. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenterology. 2003;125(5):1320–9. [DOI] [PubMed] [Google Scholar]
- 13. Colavita K, Andy UU. Role of diet in fecal incontinence: a systematic review of the literature. Int Urogynecol J. 2016;27(12):1805–10. [DOI] [PubMed] [Google Scholar]
- 14. Menees SB, Chandhrasekhar D, Liew EL, Chey WD. A low FODMAP diet may reduce symptoms in patients with fecal incontinence. Clin Transl Gastroenterol. 2019;10(7):e00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beeckman D, Van Damme N, Schoonhoven L, Van Lancker A, Kottner J, Beele H, et al. Interventions for preventing and treating incontinence‐associated dermatitis in adults. Cochrane Database Syst Rev. 2016;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pather P, Hines S, Kynoch K, Coyer F. Effectiveness of topical skin products in the treatment and prevention of incontinence‐associated dermatitis: a systematic review. JBI Database Syst Rev Implement Rep. 2017;15(5):1473–96. [DOI] [PubMed] [Google Scholar]
- 17. Cooper P, Gray D. Comparison of two skin care regimes for incontinence. Br J Nurs. 2001;10(6 Suppl):S6, S8, S10 passim. [DOI] [PubMed] [Google Scholar]
- 18. Beeckman D, Verhaeghe S, Defloor T, Schoonhoven L, Vanderwee K. A 3‐in‐1 perineal care washcloth impregnated with dimethicone 3% versus water and pH neutral soap to prevent and treat incontinence‐associated dermatitis: a randomized, controlled clinical trial. J Wound Ostomy Continence Nurs. 2011;38(6):627–34. [DOI] [PubMed] [Google Scholar]
- 19. Anthony D, Barnes E, Malone‐Lee J, Pluck R. A clinical study of Sudocrem in the management of dermatitis due to the physical stress of incontinence in a geriatric population. J Adv Nurs. 1987;12(5):599–603. [DOI] [PubMed] [Google Scholar]
- 20. Brunner M, Droegemueller C, Rivers S, Deuser WE. Prevention of incontinence‐related skin breakdown for acute and critical care patients: comparison of two products. Urol Nurs. 2012;32(4):214‐9. [PubMed] [Google Scholar]
- 21. Lewis‐Byers K, Thayer D. An evaluation of two incontinence skin care protocols in a long‐term care setting. Ostomy Wound Manage. 2002;48(12):44. [PubMed] [Google Scholar]
- 22. Buckley B, Dofitas R, Baltazar W, Quiambao P, Razor B. Topical zinc oxide based creams in a structured care regimen for the treatment of incontinence associated dermatitis in hospitalized adults and older children: a randomized, controlled trial. Neurourol Urodyn. 2014;33(6):760–1. [Google Scholar]
- 23. Fader M, Cottenden A, Getliffe K, Gage H, Clarke‐O'Neill S, Jamieson K, et al. Absorbent products for urinary/faecal incontinence: a comparative evaluation of key product designs. Health Technol Assess. 2008;12(29). iii‐iv, ix‐185. [DOI] [PubMed] [Google Scholar]
- 24. Norton C, Cody JD. Biofeedback and/or sphincter exercises for the treatment of faecal incontinence in adults. Cochrane Database Syst Rev. 2012;7:CD002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vonthein R, Heimerl T, Schwandner T, Ziegler A. Electrical stimulation and biofeedback for the treatment of fecal incontinence: a systematic review. Int J Colorectal Dis. 2013;28(11):1567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Enck P, Van der Voort IR, Klosterhalfen S. Biofeedback therapy in fecal incontinence and constipation. Neuro Gastroenterol Motil. 2009;21(11):1133–41. [DOI] [PubMed] [Google Scholar]
- 27. Heymen S, Jones KR, Ringel Y, Scarlett Y, Whitehead WE. Biofeedback treatment of fecal incontinence: a critical review. Dis Colon Rectum. 2001;44(5):728–36. [DOI] [PubMed] [Google Scholar]
- 28. Palsson OS, Heymen S, Whitehead WE. Biofeedback treatment for functional anorectal disorders: a comprehensive efficacy review. Appl Psychophysiol Biofeedback. 2004;29(3):153–74. [DOI] [PubMed] [Google Scholar]
- 29. Heymen S, Scarlett Y, Jones K, Ringel Y, Drossman D, Whitehead WE. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52(10):1730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ilnyckyj A, Fachnie E, Tougas G. A randomized‐controlled trial comparing an educational intervention alone vs education and biofeedback in the management of faecal incontinence in women. Neuro Gastroenterol Motil. 2005;17(1):58–63. [DOI] [PubMed] [Google Scholar]
- 31. Bliss DZ, Jung HJ, Savik K, Lowry A, LeMoine M, Jensen L, et al. Supplementation with dietary fiber improves fecal incontinence. Nurs Res. 2001;50(4):203–13. [DOI] [PubMed] [Google Scholar]
- 32. Bliss DZ, Savik K, Jung HJ, Whitebird R, Lowry A, Sheng X. Dietary fiber supplementation for fecal incontinence: a randomized clinical trial. Res Nurs Health. 2014;37(5):367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Markland AD, Burgio KL, Whitehead WE, Richter HE, Wilcox CM, Redden DT, et al. Loperamide versus psyllium fiber for treatment of fecal incontinence: the fecal incontinence prescription (Rx) management (FIRM) randomized clinical trial. Dis Colon Rectum. 2015;58(10):983–93. [DOI] [PubMed] [Google Scholar]
- 34. Omar MI, Alexander CE. Drug treatment for faecal incontinence in adults. Cochrane Database Syst Rev. 2013;6:CD002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Edenfield AL, Amundsen CL, Wu JM, Levin PJ, Siddiqui NY. Posterior tibial nerve stimulation for the treatment of fecal incontinence: a systematic evidence review. Obstet Gynecol Surv. 2015;70(5):329–41. [DOI] [PubMed] [Google Scholar]
- 36. Arroyo‐Fernández R, Avendaño‐Coy J, Ando‐Lafuente S, Martín‐Correa T, Ferri‐Morales A. Posterior tibial nerve stimulation in the treatment of fecal incontinence: a systematic review. Rev Esp Enferm Dig. 2018;110(9):577–88. [DOI] [PubMed] [Google Scholar]
- 37. Sarveazad A, Babahajian A, Amini N, Shamseddin J, Yousefifard M. Posterior tibial nerve stimulation in fecal incontinence: a systematic review and meta‐analysis. Basic Clin Neurosci. 2019;10(5):419–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Horrocks E, Thin N, Thaha M, Taylor S, Norton C, Knowles C. Systematic review of tibial nerve stimulation to treat faecal incontinence. Br J Surg. 2014;101(5):457–68. [DOI] [PubMed] [Google Scholar]
- 39. Findlay JM, Maxwell‐Armstrong C. Posterior tibial nerve stimulation and faecal incontinence: a review. Int J Colorectal Dis. 2011;26(3):265–73. [DOI] [PubMed] [Google Scholar]
- 40. Tan K, Wells CI, Dinning P, Bissett IP, O'Grady G. Placebo response rates in electrical nerve stimulation trials for fecal incontinence and constipation: a systematic review and meta‐analysis. Neuromodulation. 2020. Dec 1;23(8):1108–16. [DOI] [PubMed] [Google Scholar]
- 41. George A, Kalmar K, Sala S, Kopanakis K, Panarese A, Dudding T, et al. Randomized controlled trial of percutaneous versus transcutaneous posterior tibial nerve stimulation in faecal incontinence. Br J Surg. 2013;100(3):330–8. [DOI] [PubMed] [Google Scholar]
- 42. Leroi A, Siproudhis L, Etienney I, Damon H, Zerbib F, Amarenco G, et al. Transcutaneous electrical tibial nerve stimulation in the treatment of fecal incontinence: a randomized trial (CONSORT 1a). Am J Gastroenterol. 2012;107(12):1888–96. [DOI] [PubMed] [Google Scholar]
- 43. Knowles CH, Horrocks EJ, Bremner SA, Stevens N, Norton C, O'Connell PR, et al. Percutaneous tibial nerve stimulation versus sham electrical stimulation for the treatment of faecal incontinence in adults (CONFIDeNT): a double‐blind, multicentre, pragmatic, parallel‐group, randomised controlled trial. Lancet (London, England). 2015;386(10004):1640–8. [DOI] [PubMed] [Google Scholar]
- 44. van der Wilt AA, Giuliani G, Kubis C, van Wunnik BPW, Ferreira I, Breukink SO, et al. Randomized clinical trial of percutaneous tibial nerve stimulation versus sham electrical stimulation in patients with faecal incontinence. Br J Surg. 2017;104(9):1167–76. [DOI] [PubMed] [Google Scholar]
- 45. Simillis C, Lal N, Qiu S, Kontovounisios C, Rasheed S, Tan E, et al. Sacral nerve stimulation versus percutaneous tibial nerve stimulation for faecal incontinence: a systematic review and meta‐analysis. Int J Colorectal Dis. 2018;33(5):645–8. [DOI] [PubMed] [Google Scholar]
- 46. Thaha MA, Abukar AA, Thin NN, Ramsanahie A, Knowles CH. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hotouras A, Murphy J, Allison M, Curry A, Williams NS, Knowles CH, et al. Prospective clinical audit of two neuromodulatory treatments for fecal incontinence: sacral nerve stimulation (SNS) and percutaneous tibial nerve stimulation (PTNS). Surg Today. 2014;44(11):2124–30. [DOI] [PubMed] [Google Scholar]
- 48. Juul T, Christensen P. Prospective evaluation of transanal irrigation for fecal incontinence and constipation. Tech Coloproctol. 2017;21(5):363–71. [DOI] [PubMed] [Google Scholar]
- 49. Buono K, Davé‐Heliker B. Mechanical inserts for the treatment of faecal incontinence: a systematic review. Arab J Urol. 2019;17(1):69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Deutekom M, Dobben AC. Plugs for containing faecal incontinence. Cochrane Database Syst Rev. 2015(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. How P, Trivedi P, Bearn P, Thomas G. Insert devices for faecal incontinence. Tech Coloproctol. 2020:1–11. [DOI] [PubMed] [Google Scholar]
- 52. Mortensen N, Humphreys MS. The anal continence plug: a disposable device for patients with anorectal incontinence. Lancet. 1991;338(8762):295–7. [DOI] [PubMed] [Google Scholar]
- 53. Christiansen J, Roed‐Petersen K. Clinical assessment of the anal continence plug. Dis Colon Rectum. 1993;36(8):740–2. [DOI] [PubMed] [Google Scholar]
- 54. Norton C, Kamm M. Anal plug for faecal incontinence. Colorectal Dis. 2001;3(5):323–7. [DOI] [PubMed] [Google Scholar]
- 55. Lukacz ES, Segall MM, Wexner SD. Evaluation of an anal insert device for the conservative management of fecal incontinence. Dis Colon Rectum. 2015;58(9):892–8. [DOI] [PubMed] [Google Scholar]
- 56. Leo CA, Thomas GP, Hodgkinson JD, Segal JP, Maeda Y, Murphy J, et al. The Renew R anal insert for passive faecal incontinence: a retrospective audit of our use of a novel device. Colorectal Dis. 2019;21(6):684–8. [DOI] [PubMed] [Google Scholar]
- 57. Giamundo P, Altomare D, De Nardi P, D’Onofrio V, Infantino A, Pucciani F, et al. The ProTect device in the treatment of severe fecal incontinence: preliminary results of a multicenter trial. Tech Coloproctol. 2007;11(4):310–14. [DOI] [PubMed] [Google Scholar]
- 58. Giamundo P, Welber A, Weiss EG, Vernava AM III, Nogueras JJ, Wexner SD. The procon incontinence device: a new nonsurgical approach to preventing episodes of fecal incontinence. Am J Gastroenterol. 2002;97(9):2328–32. [DOI] [PubMed] [Google Scholar]
- 59. Jarrett MED, Mowatt G, Glazener CMA, Fraser C, Nicholls RJ, Grant AM, et al. Systematic review of sacral nerve stimulation for faecal incontinence and constipation. Br J Surg. 2004;91(12):1559–69. [DOI] [PubMed] [Google Scholar]
- 60. Tan E, Ngo N‐T, Darzi A, Shenouda M, Tekkis PP. Meta‐analysis: sacral nerve stimulation versus conservative therapy in the treatment of faecal incontinence. Int J Colorectal Dis. 2011;26(3):275–94. [DOI] [PubMed] [Google Scholar]
- 61. Ratto C, Litta F, Parello A, Donisi L, De Simone V, Zaccone G. Sacral nerve stimulation in faecal incontinence associated with an anal sphincter lesion: a systematic review. Colorectal Dis. 2012;14(6):e297–304. [DOI] [PubMed] [Google Scholar]
- 62. Mirbagheri N, Sivakumaran Y, Nassar N, Gladman MA. Systematic review of the impact of sacral neuromodulation on clinical symptoms and gastrointestinal physiology. ANZ J Surg. 2016;86(4):232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhu Y, Wu G, Zhang J, Yan W, Han M, Zhang H, et al. [Meta‐analysis of sacral nerve stimulation for fecal incontinence]. Zhonghua Wei Chang Wai Ke Za Zhi [Chinese Journal of Gastrointestinal Surgery]. 2017;20(12):1417–21. [PubMed] [Google Scholar]
- 64. Kahlke V, Topic H, Peleikis HG, Jongen J. Sacral nerve modulation for fecal incontinence: results of a prospective single‐center randomized crossover study. Dis Colon Rectum. 2015;58(2):235–40. [DOI] [PubMed] [Google Scholar]
- 65. Leroi A‐M, Parc Y, Lehur P‐A, Mion F, Barth X, Rullier E, et al. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double‐blind crossover study. Ann Surg. 2005;242(5):662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sorensen M, Thomsen F. Sacral nerve stimulation increases rectal sensitivity in patients with faecal incontinence: results of a randomised double‐blinded crossover study (Abstract number 437). In: Proceedings of the joint meeting of the International Continence Society (ICS) and the International Urogynecological Association, 2010, Aug 23‐27, Toronto, Canada [Internet]; 2010. Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN‐00766350/full [Google Scholar]
- 67. Vaizey CJ, Kamm MA, Roy AJ, Nicholls RJ. Double‐blind crossover study of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2000;43(3):298–302. [DOI] [PubMed] [Google Scholar]
- 68. Tjandra JJ, Chan MKY, Yeh CH, Murray‐Green C. Sacral nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum. 2008;51(5):494–502. [DOI] [PubMed] [Google Scholar]
- 69. Maeda Y, Matzel K, Lundby L, Buntzen S, Laurberg S. Postoperative issues of sacral nerve stimulation for fecal incontinence and constipation: a systematic literature review and treatment guideline. Dis Colon Rectum. 2011;54(11):1443–60. [DOI] [PubMed] [Google Scholar]
- 70. Leroi A‐M, Lenne X, Dervaux B, Chartier‐Kastler E, Mauroy B, Normand LL, et al. Outcome and cost analysis of sacral nerve modulation for treating urinary and/or fecal incontinence. Ann Surg. 2011;253(4):720–32. [DOI] [PubMed] [Google Scholar]
- 71. Brosa M, Munoz‐Duyos A, Navarro‐Luna A, Rodriguez JM, Serrano D, Gisbert R, et al. Cost‐effectiveness analysis of sacral neuromodulation (SNM) with Interstim for fecal incontinence patients in Spain. Curr Med Res Opin. 2008;24(3):907–18. [DOI] [PubMed] [Google Scholar]
- 72. Indinnimeo M, Ratto C, Moschella CM, Fiore A, Brosa M, Giardina S. Sacral neuromodulation for the treatment of fecal incontinence: analysis of cost‐effectiveness. Dis Colon Rectum. 2010;53(12):1661–9. [DOI] [PubMed] [Google Scholar]
- 73. Rydningen M, Dehli T, Wilsgaard T, Rydning A, Kumle M, Lindsetmo RO, et al. Sacral neuromodulation compared with injection of bulking agents for faecal incontinence following obstetric anal sphincter injury – a randomized controlled trial. Colorectal Dis. 2017;19(5):O134–44. [DOI] [PubMed] [Google Scholar]
- 74. Bernstein MA, Purdy CH, Becker A, Magar R. Three‐year cost‐effectiveness model for non‐animal stabilized hyaluronic acid and dextranomer copolymer compared with sacral nerve stimulation after conservative therapy for the management of fecal incontinence. Clin Therapeut. 2014;36(6):890–905. [DOI] [PubMed] [Google Scholar]
- 75. Ratto C, Litta F, Parello A, Donisi L, Doglietto GB. Sacral nerve stimulation is a valid approach in fecal incontinence due to sphincter lesions when compared to sphincter repair. Dis Colon Rectum. 2010;53(3):264–72. [DOI] [PubMed] [Google Scholar]
- 76. Hong KD, Kim J, Ji WB, Um JW. Midterm outcomes of injectable bulking agents for fecal incontinence: a systematic review and meta‐analysis. Tech Coloproctol. 2017;21(3):203–10. [DOI] [PubMed] [Google Scholar]
- 77. Winkler R, Wild C. [Injectable bulking agents for faecal incontinence: systematic review]. Health Technol Assess Decis Supp Doc. 2015;87:1–41. [Google Scholar]
- 78. Maeda Y, Laurberg S, Norton C. Perianal injectable bulking agents as treatment for faecal incontinence in adults. Cochrane Database Syst Rev. 2013(2):CD007959. [DOI] [PubMed] [Google Scholar]
- 79. Leung FW. Treatment of fecal incontinence (FI) by injection of bulking agent into peri‐anal tissue‐review of observational studies (OS) and randomized controlled trials (RCT). Neuro Gastroenterol Motil. 2011;1:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Luo C, Samaranayake CB, Plank LD, Bissett IP. Systematic review on the efficacy and safety of injectable bulking agents for passive faecal incontinence. Colorectal Dis. 2010;12(4):296–303. [DOI] [PubMed] [Google Scholar]
- 81. Siproudhis L, Morcet J, Laine F. Elastomer implants in faecal incontinence: a blind, randomized placebo‐controlled study. Aliment Pharmacol Ther. 2007;25(9):1125–32. [DOI] [PubMed] [Google Scholar]
- 82. Graf W, Mellgren A, Matzel KE, Hull T, Johansson C, Bernstein M, et al. Efficacy of dextranomer in stabilised hyaluronic acid for treatment of faecal incontinence: a randomised, sham‐controlled trial. Lancet. 2011;377(9770):997–1003. [DOI] [PubMed] [Google Scholar]
- 83. Dehli T, Stordahl A, Vatten LJ, Romundstad PR, Mevik K, Sahlin Y, et al. Sphincter training or anal injections of dextranomer for treatment of anal incontinence: a randomized trial. Scand J Gastroenterol. 2013;48(3):302–10. [DOI] [PubMed] [Google Scholar]
- 84. Forte ML, Andrade KE, Lowry AC, Butler M, Bliss DZ, Kane RL. Systematic review of surgical treatments for fecal incontinence. Dis Colon Rectum. 2016;59(5):443–69. [DOI] [PubMed] [Google Scholar]
- 85. Malouf AJ, Norton CS, Engel AF, Nicholls RJ, Kamm MA. Long‐term results of overlapping anterior anal‐sphincter repair for obstetric trauma. Lancet. 2000;355(9200):260–5. [DOI] [PubMed] [Google Scholar]
- 86. Colquhoun P, Kaiser R Jr., Efron J, Weiss EG, Nogueras JJ, Vernava AM 3rd, et al. Is the quality of life better in patients with colostomy than patients with fecal incontience? World J Surg. 2006;30(10):1925–8. [DOI] [PubMed] [Google Scholar]
- 87. Kon Y, Ichikawa‐Shigeta Y, Iuchi T, Nakajima Y, Nakagami G, Tabata K, et al. Effects of a skin barrier cream on management of incontinence‐associated dermatitis in older women: a cluster randomized controlled trial. J Wound Ostomy Continence Nurs. 2017;44(5):481–6. [DOI] [PubMed] [Google Scholar]
- 88. Chassagne P, Jego A, Gloc P, Capet C, Trivalle C, Doucet J, et al. Does treatment of constipation improve faecal incontinence in institutionalized elderly patients? Age Ageing. 2000;29(2):159–64. [DOI] [PubMed] [Google Scholar]
- 89. Schnelle JF, Leung FW, Rao SSC, Beuscher L, Keeler E, Clift JW, et al. A controlled trial of an intervention to improve urinary and fecal incontinence and constipation. J Am Geriatr Soc. 2010;58((8):1504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Christensen P, Bazzocchi G, Coggrave M, Abel R, Hultling C, Krogh K, et al. A randomized, controlled trial of transanal irrigation versus conservative bowel management in spinal cord‐injured patients. Gastroenterology. 2006;131(3):738–47. [DOI] [PubMed] [Google Scholar]
- 91. Barak N, Gecse KB, Takacs I. Topical oxymetazoline for fecal incontinence in patients with spinal cord injury: a double‐blind randomized controlled crossover study. Dis Colon Rectum. 2019;62(2):234–40. [DOI] [PubMed] [Google Scholar]
- 92. Coggrave M, Norton C, Cody JD. Management of faecal incontinence and constipation in adults with central neurological diseases. Cochrane Database Syst Rev. 2014(1):CD002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kong E, Nikolaou S, Qiu S, Pellino G, Tekkis P, Kontovounisios C. A systematic review of sacral nerve stimulation for faecal incontinence following ileal pouch anal anastomosis. Updates Surg. 2018;70(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lumi CM, La Rosa L, Coraglio MF, Munoz JP, Gualdrini UA, Masciangioli G. [Prospective, double‐blind study of topical phenylephrine treatment for nocturnal fecal incontinence in patients after ileoanal pouch construction]. Acta Gastroenterol Latinoam. 2009;39(3):179–83. [PubMed] [Google Scholar]
- 95. Mege D, Meurette G, Vitton V, Leroi AM, Bridoux V, Zerbib P, et al. Sacral nerve stimulation can alleviate symptoms of bowel dysfunction after colorectal resections. Colorectal Dis. 2017;19(8):756–63. [DOI] [PubMed] [Google Scholar]
- 96. Ramage L, Qiu S, Kontovounisios C, Tekkis P, Rasheed S, Tan E. A systematic review of sacral nerve stimulation for low anterior resection syndrome. Colorectal Dis. 2015;17(9):762–71. [DOI] [PubMed] [Google Scholar]
- 97. Ram E, Meyer R, Carter D, Gutman M, Rosin D, Horesh N. The efficacy of sacral neuromodulation in the treatment of low anterior resection syndrome: a systematic review and meta‐analysis. Tech Coloproctol. 2020;24(8):803–15. [DOI] [PubMed] [Google Scholar]
- 98. Lin K‐Y, Granger CL, Denehy L, Frawley HC. Pelvic floor muscle training for bowel dysfunction following colorectal cancer surgery: a systematic review. Neurourol Urodyn. 2015;34(8):703–12. [DOI] [PubMed] [Google Scholar]
- 99. Lin YH, Yang HY, Hung SL, Chen HP, Liu KW, Chen TB, et al. Effects of pelvic floor muscle exercise on faecal incontinence in rectal cancer patients after stoma closure. Eur J Cancer Care. 2016;25(3):449–57. [DOI] [PubMed] [Google Scholar]
- 100. Park J‐S, Kang S‐B, Kim D‐W, Namgung H‐W, Kim H‐L. The efficacy and adverse effects of topical phenylephrine for anal incontinence after low anterior resection in patients with rectal cancer. Int J Colorectal Dis. 2007;22(11):1319–24. [DOI] [PubMed] [Google Scholar]
- 101. Maris A, Devreese AM, D'Hoore A, Penninckx F, Staes F. Treatment options to improve anorectal function following rectal resection: a systematic review. Colorectal Dis. 2013;15(2):e67. [DOI] [PubMed] [Google Scholar]
- 102. Richter HE, Matthews CA, Muir T, Takase‐Sanchez MM, Hale DS, Van Drie D, et al. A vaginal bowel‐control system for the treatment of fecal incontinence. Obstet Gynecol Surv. 2015;70(6):387–8. [DOI] [PubMed] [Google Scholar]
- 103. Carapeti EA, Kamm MA, Phillips RK. Randomized controlled trial of topical phenylephrine in the treatment of faecal incontinence. Br J Surg. 2000;87(1):38–42. [DOI] [PubMed] [Google Scholar]
- 104. Bharucha AE, Fletcher JG, Camilleri M, Edge J, Carlson P, Zinsmeister AR. Effects of clonidine in women with fecal incontinence. Clin Gastroenterol Hepatol. 2014;12(5):843–51. quiz e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bach FL, Sairally BZF, Latthe P. Effect of oestrogen therapy on faecal incontinence in postmenopausal women: a systematic review. Int Urogynecol J. 2020;31(7):1289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Donnelly V, O'Connell PR, O'Herlihy C. The influence of oestrogen replacement on faecal incontinence in postmenopausal women. BJOG. 1997;104(3):311–15. [DOI] [PubMed] [Google Scholar]
- 107. Pinedo G, Garcia E, Zarate A, León F, Bellolio F, Molina M, et al. Are topical oestrogens useful in faecal incontinence? Double‐blind randomized trial. Colorectal Dis. 2009;11(4):390–3. [DOI] [PubMed] [Google Scholar]
- 108. De Ligny WR, Kerkhof MH, Ruiz‐Zapata AM. Regenerative medicine as a therapeutic option for fecal incontinence: a systematic review of preclinical and clinical studies. Am J Obstet Gynecol. 2019;220(2):142–54. [DOI] [PubMed] [Google Scholar]
- 109. Gras S, Tolstrup CK, Lose G. Regenerative medicine provides alternative strategies for the treatment of anal incontinence. Int Urogynecol J. 2017;28(3):341–50. [DOI] [PubMed] [Google Scholar]
- 110. de la Portilla F, Guerrero JL, Maestre MV, Leyva L, Mera S, Garcia‐Olmo D, et al. Treatment of fecal incontinence with autologous expanded mesenchymal stem cells: results of a pilot Study. Colorectal Dis. 2020;28:28. [DOI] [PubMed] [Google Scholar]
- 111. Sarveazad A, Newstead GL, Mirzaei R, Joghataei MT, Bakhtiari M, Babahajian A, et al. A new method for treating fecal incontinence by implanting stem cells derived from human adipose tissue: preliminary findings of a randomized double‐blind clinical trial. Stem Cell Res Ther. 2017;8(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Boyer O, Bridoux V, Giverne C, Bisson A, Koning E, Leroi A‐M, et al. Autologous myoblasts for the treatment of fecal incontinence: results of a phase 2 randomized placebo‐controlled study (MIAS). Ann Surg. 2018;267(3):443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Scaglia M, Delaini G, Destefano I, Hultén L. Fecal incontinence treated with acupuncture‐a pilot study. Auton Neurosci. 2009;145(1‐2):89–92. [DOI] [PubMed] [Google Scholar]
- 114. Franco J, Agulhon A, Viani F, Viebig R. Systemic acupuncture in patients with faecal incontinence. Compl Ther Clin Pract. 2016;24:162–6. [DOI] [PubMed] [Google Scholar]
- 115. Ratto C, Buntzen S, Aigner F, Altomare DF, Heydari A, Donisi L, et al. Multicentre observational study of the Gatekeeper for faecal incontinence. Br J Surg. 2016;103(3):290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Brusciano L, Tolone S, Del Genio G, Grossi U, Schiattarella A, Piccolo FP, et al. Medium‐term outcomes of gatekeeper implantation for fecal incontinence. Tech Coloproctol. 2020;24(4):367. [DOI] [PubMed] [Google Scholar]
- 117. Kuiper SZ, Dirksen CD, Breukink SO. Proctological oblivion. Colorectal Dis: Off J Association Coloproctol Great Br Irel. 2021;23(3):573. [DOI] [PubMed] [Google Scholar]
- 118. Assmann S, Keszthelyi D, Kleijnen J, Kimman M, Anastasiou F, Bradshaw E, et al. The development of a faecal incontinence core outcome set: an international Delphi study protocol. Int J Colorectal Dis. 2021;36(3):617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Supporting Information S2
Supporting Information S3
Supporting Information S4
Supporting Information S5
Supporting Information S6
Supporting Information S7
Supporting Information S8
Data Availability Statement
Data sharing is not applicable for most part of this study as little new data were created or analysed in this study. All results presented in this manuscript were directly taken from‐ or derived from data presented in the articles included in the reference list.


