Abstract
The rdxA gene of 30 independently isolated Helicobacter pylori strains was sequenced. A comparison of the rdxA sequences revealed a higher percentage of amino acid substitutions in the corresponding protein than in other housekeeping genes. Out of 122 point mutations, 41 were missense and 4 were nonsense. A resistant strain with a nucleotide insertion in the rdxA sequence was also found. With the exception of the point mutations and the insertion generating a stop signal, no particular nucleotide mutation or amino acid substitution could be associated to metronidazole resistance. Moreover, phylogenetic analysis of the 30 nucleotide sequences did not demonstrate specific clusters associated with the resistance phenotype.
Helicobacter pylori is a human bacterial pathogen whose ecological niche is the gastric mucosa. Once H. pylori has colonized the human stomach, the infection persists, usually for life. Although this bacterium infects up to 50% of the world's population, only a minority of patients will develop peptic ulcer disease or gastric cancer induced by the H. pylori infection. Metronidazole (MTZ), in combination with either amoxicillin or clarithromycin and a proton pump inhibitor (in a combination known as triple therapy), is the most effective H. pylori eradication treatment. Thus, the antibiotic has been widely used to treat H. pylori infections (9). This fact, together with the use of MTZ to treat other diseases such as gynecological infections, has generated in recent years an increase of H. pylori strains resistant to this drug (5, 9). This resistance is one of the major causes of treatment failure (13). In Europe, the prevalence of resistant strains is close to 30% (28% in the southern part of Switzerland), a value which raises great concern (3, 10).
For the laboratory, growing H. pylori isolates is a long and fastidious procedure. Thus, susceptibility testing is rarely performed. Understanding the molecular mechanisms of resistance is essential for the development of new tools for the rapid detection of resistance. Goodwin et al. have recently identified a genetic locus associated with the resistance phenotype (5). The gene, named rdxA, encodes an oxygen-insensitive NADPH nitroreductase and is 630 bp long. They found that the resistance to MTZ in H. pylori may result from nonsense mutations in the rdxA gene. These genetic alterations generated a premature stop in the translated protein, thereby inactivating the nitroreductase (MTZ has to be reduced for its antimicrobial action to be elicited). In order to verify the importance of this observation, we sequenced the rdxA gene from 30 strains (resistant or susceptible to MTZ) selected from our collection of H. pylori strains.
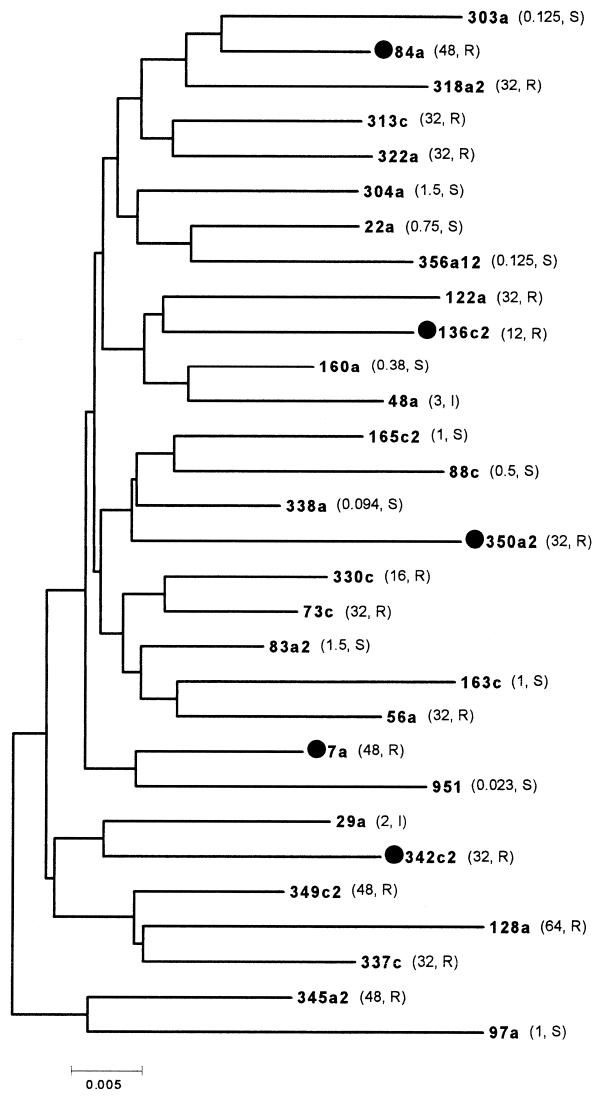
All the strains were isolated from patients living in the southern part of Switzerland who underwent a gastroduodenoscopy because of gastric complaints. MTZ susceptibility was determined by both the E-test method (according to the manufacturer's instructions [10]) and the agar dilution method (12). The quality control strain used was ATTC 43504. No major discrepancies were found between the two methods, contrary to what was reported by others (11). Strains for which the MIC was ≥8 μg/ml were classified as resistant (10, 11). A total of 30 isolates composed of 16 strains resistant to MTZ, two strains classified as intermediate (MIC, 2 to 4 μg/ml), and 12 strains susceptible to MTZ (MIC, <2 μg/ml) were used for this study. The MICs for the strains are included in the dendrogram of Fig. 1.
FIG. 1.
Neighbor-joining tree generated a Jukes-Cantor model distance matrix of the rdxA gene nucleotide sequences for 30 H. pylori strains. The MICs of MTZ (in micrograms per milliliter) are in parentheses. R, resistant; S, sensitive; I, intermediate. Strains marked with a filled circle show a premature stop signal.
DNA extraction and purification were performed in a single step using a commercial ion-exchange resin (InstaGene matrix; Bio-Rad Laboratories, Richmond, Calif.), according to the manufacturer's instructions. We designed the PCR primers RDXA-L (5′-AGGGATTTTATTGTATGCTACAA-3′) and RDXA-R (5′-AGGAGCATCAGATAGTTCTGA-3′) on the flanking regions of the rdxA gene. The amplified fragment was 886 bp long. The thermal profile used for the amplification was 2 min at 94°C, followed by 35 cycles consisting of 94°C for 1 min, 52°C for 1 min, and 72°C for 1.5 min. Prior to sequencing, PCR products were purified with the Qiaquick PCR purification kit (Qiagen GmbH, Hilden, Germany). Both primers were used for cycle sequencing with the ABI PRISM dRhodamine-dye terminator kit, and the DNA was analyzed by capillary electrophoresis on an ABI PRISM 310 automated sequencer (Perkin-Elmer Applied Biosystems, International Inc., Branchburg, N.J.). The DNA nucleotide sequences were stored and handled with the Lasergene program Editseq (1994 release; DNAstar, Madison, Wis.). Sequence data were analyzed by pairwise sequence alignment with the Lasergene program Megalign (1994 release; DNAstar). Phylogenetic analysis was performed using molecular evolutionary genetics analysis (8). A neighbor-joining tree using a Jukes-Cantor model distance matrix was obtained.
The PCR experiments generated an amplification product for each of the strains tested, which demonstrated the presence, in each, of the rdxA gene. The comparison of the rdxA gene sequences of all 30 strains revealed a nucleotide insertion in strain 350a2 at position 141, where an adenine was incorporated: the consequent frameshift caused a stop in the encoded peptide (Table 1). Also, in four other strains the inferred protein sequences showed stop codons which, in this case, were caused by nonsense substitutions; all these isolates were resistant to MTZ (Table 1). These nucleotide substitutions were in positions 148 (strains 7a and 84a), 223 (strain 342c2), and 523 (strain 136c2). The remaining 11 resistant isolates did not show stop codons in the rdxA gene. Further analyses of the nonsilent nucleotide point mutations present in the 29 strains which did not exhibit a frameshift (i.e., all strains except 350a2) revealed 12 nonsilent point mutations exclusively in one or more resistant strains, 9 mutations in sensitive strains, and 15 mutations shared by both resistant and sensitive isolates (Table 2). Analyses of the distribution of the mutations highlighted no hot-spot position: no close relationship between a particular amino acid substitution and MTZ resistance could be identified (Table 2).
TABLE 1.
Mutational events causing a stop codon in MTZ-resistant strains
| Strain | Type of mutation | Effect | MTZ MIC (μg/ml) |
|---|---|---|---|
| 350a2 | Insertion of A in position 141 | Frameshift: TAA stop codon in position 175 | 32 |
| 7a | Substitution of T for C in position 148 | TAG stop codon in position 148 | 48 |
| 84a | Substitution of T for C in position 148 | TAG stop codon in position 148 | 48 |
| 342c2 | Substitution of T for G in position 223 | TAA stop codon in position 223 | 32 |
| 136c2 | Substitution of T for G in position 523 | TAG stop codon in position 523 | 12 |
TABLE 2.
Amino acid changes in the rdxA gene in MTZ-susceptible and -resistant H. pylori isolates
| Strain | MIC (μg/ml) | Amino acid at position:
|
|||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 6 | 10 | 16 | 25 | 31 | 50 | 51 | 53 | 56 | 59 | 62 | 64 | 68 | 75 | 84 | 88 | 90 | 97 | 98 | 106 | 111 | 118 | 122 | 131 | 140 | 150 | 163 | 172 | 175 | 183 | 184 | 196 | 198 | 199 | 200 | 204 | 207 | ||
| Susceptible strains | |||||||||||||||||||||||||||||||||||||||
| 22aa | 0.75 | D | Q | R | R | H | T | Q | P | H | M | N | L | K | V | E | M | S | K | H | G | P | V | T | G | R | C | G | G | V | E | A | C | S | K | S | R | V | I |
| 160a | 0.38 | .b | H | . | . | . | E | . | . | . | . | . | . | . | . | . | . | . | R | Y | . | . | . | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 163c | 1 | . | H | . | . | . | . | . | . | . | . | . | . | . | A | . | . | . | . | . | . | . | . | . | . | K | . | . | . | I | . | V | . | . | . | . | . | . | . |
| 165c2 | 1 | N | . | . | . | . | . | . | . | . | . | . | . | . | A | . | . | . | R | Y | . | . | . | A | S | . | . | . | . | I | Q | . | . | . | . | . | . | . | . |
| 29a | 2 | . | H | . | . | . | E | . | . | . | . | . | . | . | A | . | . | . | . | T | . | . | . | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 303a | 0.125 | . | . | . | . | . | E | . | . | . | . | . | . | N | A | . | . | . | . | . | . | S | . | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 304a | 1.5 | . | H | . | . | . | . | . | . | . | I | . | . | . | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | K | . | . | . | . | . |
| 338a | 0.094 | . | . | . | . | . | . | . | . | . | . | . | . | . | A | . | . | . | R | . | . | . | . | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 356a12 | 0.125 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | R | . | . | . | . | A | . | K | . | . | . | . | . | . | . | . | . | . | . | . | V |
| 48a | 3 | . | H | . | . | . | E | . | . | . | . | . | . | . | A | . | . | . | . | . | . | . | . | A | . | . | . | . | . | . | Q | . | . | N | . | . | . | . | . |
| 83a2 | 1.5 | . | . | . | . | . | . | . | . | . | . | . | . | . | A | . | . | . | R | . | . | . | . | A | . | K | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 88c | 0.5 | N | . | . | . | . | . | . | . | . | . | . | V | . | A | . | . | . | R | Y | . | . | . | . | . | K | . | . | . | . | . | . | . | . | N | Q | G | . | . |
| 951 | 0.023 | . | . | . | . | . | E | . | . | . | I | . | . | . | . | . | . | . | R | . | S | . | . | A | . | . | . | . | . | . | . | V | . | . | . | . | . | . | . |
| 97a | 1 | . | . | . | . | . | E | . | . | R | . | . | . | N | A | . | . | . | . | T | . | S | A | A | . | . | . | . | . | I | Q | V | . | . | . | . | . | . | . |
| Resistant strains | |||||||||||||||||||||||||||||||||||||||
| 56a | 32 | . | . | . | . | . | E | . | . | . | . | . | . | . | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | I | Q | . | . | . | . | . | . | . | . |
| 84a | 48 | . | . | . | . | . | E | ∗c | . | . | . | . | . | . | A | . | . | . | . | . | . | S | A | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 122a | 32 | . | H | . | . | . | E | . | S | . | . | . | . | . | A | . | . | . | . | . | . | S | A | . | . | K | . | . | . | . | . | . | . | . | . | . | . | I | . |
| 128a | 64 | . | H | . | H | . | . | . | . | . | I | . | . | . | A | . | . | . | . | T | . | S | . | . | . | K | . | . | . | . | . | . | . | . | N | H | E | . | . |
| 136c2 | 12 | . | . | . | . | . | E | . | . | . | . | . | . | . | A | . | . | . | . | . | S | . | . | . | . | K | . | . | . | . | ∗ | . | . | . | . | . | . | . | . |
| 313c | 32 | . | . | . | . | . | . | . | . | . | I | . | . | . | A | . | I | . | R | . | . | . | . | A | . | . | Y | . | . | . | . | . | . | . | . | . | . | . | . |
| 318a2 | 32 | . | H | . | H | . | . | . | . | . | . | . | . | . | A | . | . | P | . | . | . | S | A | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 330c | 16 | . | . | T | C | . | . | . | . | . | . | . | . | . | A | . | . | . | . | . | . | . | . | A | . | K | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 322a | 32 | . | H | . | . | . | . | . | . | . | . | . | . | . | A | . | . | . | R | . | . | . | . | A | . | . | . | . | D | . | . | . | . | . | . | . | . | . | . |
| 337c | 32 | . | H | . | . | R | . | . | . | . | . | . | . | . | A | . | . | . | . | T | . | . | . | A | . | K | . | . | . | . | . | . | Y | . | . | . | . | . | . |
| 342c2 | 32 | . | . | . | . | . | . | . | . | . | I | . | . | . | A | ∗ | . | . | R | T | . | . | . | A | . | . | . | . | . | . | Q | . | . | . | . | . | . | . | . |
| 345a2 | 48 | . | . | . | . | R | . | . | . | . | . | . | . | . | A | . | . | . | R | T | . | S | A | A | . | K | . | D | . | . | . | . | . | . | . | . | . | I | . |
| 349c2 | 48 | . | . | . | H | . | . | . | . | . | I | . | . | . | A | . | . | . | . | T | . | . | . | A | . | K | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 73c | 32 | . | . | . | C | . | . | . | . | . | . | D | . | . | A | . | . | . | . | . | . | . | . | A | . | K | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 7a | 48 | . | . | . | . | . | . | ∗ | . | . | . | . | . | . | A | . | . | . | R | Y | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
22a was arbitrarily chosen as reference strain.
., same as for reference strain.
∗, stop codon.
By comparing matched pairs of MTZ-resistant and -sensitive strains isolated from the same patient, Goodwin et al. (5) also found MTZ-resistant strains that did not show stop mutations but, indeed, showed various nonsilent point mutations. They transformed Escherichia coli cells, which are naturally resistant to MTZ (because they lack the drug-inactivating nitroreductase), with specific sequences of the rdxA gene isolated from the MTZ-resistant H. pylori isolates: the E. coli cells retained the resistance phenotype. Conversely, in the control experiment, the transformation of resistant E. coli cells with the rdxA gene originating from MTZ-sensitive H. pylori strains conferred on them a sensitive phenotype: the wild-type rdxA gene of H. pylori was thus responsible for MTZ antimicrobial activity. With these experiments they concluded that the missense mutations found in MTZ-resistant H. pylori were involved in the MTZ resistance. Nevertheless, these observations could not be supported by an enzyme assay (5).
The strains used in our study were all independently isolated and corresponded to 30 different patients. Amino acid substitutions were found among both susceptible and resistant strains, and, as stated above, we could not ascribe particular missense mutations to the resistance phenotype. Only the four mutations causing a premature stop signal could be related to MTZ resistance. Interestingly, the mean number of amino acid changes for each RdxA protein sequence found among the resistant strains was not significantly higher than the value found in the susceptible strains (5 versus 6 changes). Cases in which a particular amino acid substitution was found only among resistant strains were rare, but the number of such strains was too small to draw any conclusion. Whether a number of the amino acid changes detected in the resistant strains induced a structural change in the RdxA protein, thus inactivating it, remains to be clarified. The value of 95.7, measured for the percentage of nucleotide similarity (Table 3), corresponded to that found by Goodwin et al. (5) and to those determined for other H. pylori housekeeping genes in our laboratory (N. Maggi Solcà, M. V. Bernasconi, C. Valsangiacomo, L.-J. Van Doorn, and J.-C. Piffaretti, submitted for publication). The number of polymorphic nucleotide sites is also within the range observed for other housekeeping genes (Table 3). However, in contrast to the other housekeeping genes sequenced in our laboratory, the deduced RdxA proteins showed a considerably higher percentage of amino acid substitutions (21.4% versus a mean of 9.3%). This is caused by the fact that, compared to the other genes analyzed, the rdxA gene has a higher percentage of nucleotide mutations in the first two codon positions (which affect the amino acid identity) and not in the third codon position (the wobble position). This observation suggests that this gene is still evolving under a selective pressure, possibly because of the frequent presence of MTZ in the environment of this microorganism.
TABLE 3.
Levels of H. pylori DNA polymorphism found in five housekeeping genes
| Gene | No. of polymorphic nucleotide sites (%) | No. of amino acid mutations (%) | % Nucleotide similarity (range) |
|---|---|---|---|
| atpDa | 88 (19.8) | 11 (7.4) | 96.4 (93–100) |
| scoB | 119 (21.3) | 20 (10.7) | 96.7 (94.1–99.8) |
| glnA | 121 (23.7) | 23 (13.5) | 95.5 (91.8–99.6) |
| recA | 106 (20.3) | 10 (5.7) | 95.3 (92.1–100) |
| rdxA | 122 (19.3) | 45 (21.4) | 95.7 (98.3–92.4) |
The values reported for the genes atpD, scoB, glnA, and recA are from N. Maggi Solcà et al. (submitted).
In order to ascertain whether particular strain genotypes could be associated to the resistant phenotype and/or to particular rdxA mutations, we generated a phylogenetic tree with a multiple alignment of the 30 rdxA nucleotide sequences (Fig. 1). No specific cluster could be evidenced; the resistant strains were randomly distributed throughout the dendrogram and not grouped together. This is in agreement with the observations that H. pylori has a recombinant population structure (1; N. Maggi Solcà et al., submitted) and that no particular strain characteristic (like the vacA genotype, the presence of cagA, or the clinical manifestation of the infection) could be associated with the phylogenetic tree topologies (N. Maggi Solcà et al., submitted). This could also suggest that the mutations in the rdxA gene are not always involved in the MTZ resistance, although the percentage of amino acid substitution was considerably higher than among other housekeeping genes. Other resistance mechanisms should be taken into account. In a previous study, we investigated the importance of MTZ resistance in H. pylori in our region (10). We analyzed 142 strains and found a prevalence of 28%. The MICs showed a bimodal distribution, with two distinct populations: the first one included strains for which the MIC levels were intermediate (MIC range, 0.125 to 6 μg/ml), and the second one comprised all the resistant strains (MIC, ≥32 μg/ml). The first peak suggested the presence of a still unexplained mechanism which decreases the susceptibility of H. pylori to MTZ, although not sufficiently to confer full resistance. The second peak suggested acquisition of a high-level resistance genetic mechanism. In H. pylori, complete resistance may be triggered by mutational events leading to variation in the activity of the enzyme(s) responsible for the reduction of MTZ to its toxic form (2, 6). The rdxA gene could be one of the genes involved in this process, but other genes or mechanisms, for instance, transcriptional regulatory control mechanisms, MTZ influx or efflux mechanisms, or genes encoding other enzymes involved in the drug activity, might be implicated in the generation of resistance (2, 6, 7).
In conclusion, the presence of mutations (substitution and insertion) causing a premature stop in the inferred RdxA protein was associated with the resistance phenotype. We could not conclude, however, that the amino acid substitutions found in the resistant strains were the only cause of the MTZ resistance, and further investigations are needed.
Nucleotide sequence accession numbers.
The nucleotide sequences referred to in this paper are available in GenBank under accession numbers AF180395 to AF180424.
Acknowledgments
We thank Claudio Valsangiacomo for helpful discussions and Claudia Ferrara and Romina Marone for technical help.
This research was supported by grant 31-45914.95 from the Swiss National Science Foundation, by the Helmut Horten Foundation, and by Astra Pharmaceutica (Dietikon, Switzerland).
REFERENCES
- 1.Achtman M, Azuma T, Berg D E, Ito Y, Morelli G, Pan Z J, Suerbaum S, Thompson S A, Van der Ende A, Van Doorn L J. Recombination and clonal groupings within Helicobacter pylori from different geographical regions. Mol Microbiol. 1999;32:459–470. doi: 10.1046/j.1365-2958.1999.01382.x. [DOI] [PubMed] [Google Scholar]
- 2.Alarcon T, Domingo D, Lopez-Brea M. Antibiotic resistance problems with Helicobacter pylori. Int J Antimicrob Agents. 1999;12:19–26. doi: 10.1016/s0924-8579(99)00051-5. [DOI] [PubMed] [Google Scholar]
- 3.European Helicobacter pylori Study Group. Current European concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report. Gut. 1997;41:8–13. doi: 10.1136/gut.41.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Study Group on Antibiotic Susceptibility of Helicobacter pylori. Results of a multicentre European survey in 1991 of metronidazole resistance in Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1992;11:777–781. [PubMed] [Google Scholar]
- 5.Goodwin A, Kersulyte D, Sisson G, Veldhuyzen van Zanten S J, Berg D E, Hoffman P S. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol Microbiol. 1998;28:383–393. doi: 10.1046/j.1365-2958.1998.00806.x. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman P S, Goodwin A, Johnsen J, Magee K, Veldhuyzen van Zanten S J. Metabolic activities of metronidazole-sensitive and -resistant strains of Helicobacter pylori: repression of pyruvate oxidoreductase and expression of isocitrate lyase activity correlate with resistance. J Bacteriol. 1996;178:4822–4829. doi: 10.1128/jb.178.16.4822-4829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenks P J, Ferrero R L, Labigne A. The role of the rdxA gene in the evolution of metronidazole resistance in Helicobacter pylori. J Antimicrob Chemother. 1999;43:753–758. doi: 10.1093/jac/43.6.753. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Tamura K, Nei M. Molecular evolutionary genetics analysis, version 1.01. University Park, Pa: The Pennsylvania State University; 1993. [Google Scholar]
- 9.Ling T K, Cheng A F, Sung J J, Yiu P Y, Chung S S. An increase in Helicobacter pylori strains resistant to metronidazole: a five-year study. Helicobacter. 1996;1:57–61. doi: 10.1111/j.1523-5378.1996.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 10.Maggi-Solcà, N., C. Valsangiacomo, and J.-C. Piffaretti. Prevalence of Helicobacter pylori resistant strains in the Southern part of Switzerland. Clin. Microbiol. Infect. 6:38–40. [DOI] [PubMed]
- 11.Mégraud F, Lehn N, Lind T, Bayerdörffer E, O'Morain C, Spiller R, Unge P, Veldhuyzen van Zanten S, Wrangstadh M, Burman C F. Antimicrobial susceptibility testing of Helicobacter pylori in a large multicenter trial: the MACH 2 study. Antimicrob Agents Chemother. 1999;43:2747–2752. doi: 10.1128/aac.43.11.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. 7th ed. 2000. pp. M100–S10. . National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 13.Rautelin H, Seppala K, Renkonen O V, Vainio U, Kosunen T U. Role of metronidazole resistance in therapy of Helicobacter pylori infections. Antimicrob Agents Chemother. 1992;36:163–166. doi: 10.1128/aac.36.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]