Abstract
Background:
The relative cardiovascular safety of gonadotropin-releasing hormone (GnRH) antagonists compared with GnRH agonists in men with prostate cancer and known atherosclerotic cardiovascular disease (ASCVD) remains controversial.
Methods:
In this international, multicenter, prospective, randomized, open-label trial, men with prostate cancer and concomitant ASCVD were randomized 1:1 to receive the GnRH antagonist degarelix or the GnRH agonist leuprolide for 12 months. The primary outcome was the time to first adjudicated major adverse cardiovascular event (MACE) (composite of death, myocardial infarction, or stroke) through 12 months.
Results:
Due to slower than projected enrollment and fewer than projected primary outcome events, enrollment was stopped before the 900 planned participants were accrued. From 3 May 2016 to 16 April 2020, a total of 545 patients from 113 sites across 12 countries were randomized. Baseline characteristics were balanced between study groups. The median age was 73 years, 49.8% had localized prostate cancer; 26.3% had locally advanced disease and 20.4% had metastatic disease. MACE occurred in 15 (5.5%) patients assigned to degarelix and 11 (4.1%) assigned to leuprolide (hazard ratio [HR] 1.28, 95% confidence interval [CI] 0.59–2.79; p=0.53).
Conclusions:
PRONOUNCE is the first, international, randomized clinical trial to prospectively compare the cardiovascular safety of a GnRH antagonist and a GnRH agonist in patients with prostate cancer. The study was terminated prematurely due to smaller than planned number of participants and events and no difference in MACE at 1 year between patients assigned to degarelix or leuprolide was observed. The relative cardiovascular safety of GnRH antagonists and agonists remains unresolved.
Clinical Trial Registration:
Keywords: prostate cancer, atherosclerotic cardiovascular disease, cardiovascular events, GnRH antagonist, GnRH agonist
INTRODUCTION
Atherosclerotic cardiovascular disease (ASCVD) is the leading non-cancer cause of death in patients with prostate cancer.1,2 In relation to common risk factors,3 contemporary data suggest that two-thirds of patients with prostate cancer have high cardiovascular risk and almost a quarter have established ASCVD.4,5 Androgen deprivation therapy (ADT), defined as lowering testosterone to castrate levels with orchiectomy or medical treatment, has been the cornerstone of advanced prostate cancer treatment for decades. There are over 3.1 million prostate cancer survivors in the United States,6 with approximately 50% receiving ADT at some point during their lifetime.7 Through different pituitary gonadotropin-releasing hormone (GnRH)-receptor mediated mechanisms, both GnRH agonists and antagonists indirectly or directly inhibit luteinizing hormone secretion, consequently inhibiting testosterone production.8 GnRH agonists are the most commonly prescribed form of ADT, with only 3–4% patients treated with a GnRH antagonist.9
Use of ADT has been associated with higher cardiovascular morbidity and mortality in prior studies, particularly in men with cardiovascular risk factors and pre-existing ASCVD.10–12 In the context of these observational studies and others linking ADT with higher rates of diabetes,13 lower insulin sensitivity,14,15 higher low-density lipoprotein- and high-density lipoprotein-cholesterol and triglycerides,15–17 sarcopenia and higher fat mass,18,19 and more thromboembolic events,20,21 the U.S. Food and Drug Administration (FDA) mandated in 2010 that manufacturers of GnRH agonists include the potential increased risk of ASCVD in their product safety information. The European Medicines Agency (EMA) had a similar recommendation. Less is known, however, about whether the mode of testosterone suppression (GnRH agonist or GnRH antagonist) differentially impacts cardiovascular risk, particularly among those with pre-existing ASCVD. Specifically, there are conflicting data about the cardiovascular safety of GnRH agonists compared with GnRH antagonists.8,22–24 Recently, a new oral GnRH antagonist, relugolix, was compared with the GnRH agonist, leuprolide, in a prospective head-to-head efficacy trial. Cardiovascular adverse events were lower with relugolix than with leuprolide.25 However, like all other previous trials evaluating ADT,26 cardiovascular events were extracted from analyses of adverse event data rather than being collected as prespecified, centrally adjudicated study endpoints. The PRONOUNCE trial was performed to compare the effect of a GnRH antagonist, degarelix, and an agonist, leuprolide, on the occurrence of adjudicated major adverse cardiovascular events (MACEs) over 12 months in patients with prostate cancer and pre-existing ASCVD.
METHODS
Trial Design and Oversight
PRONOUNCE (NCT02663908) was an international, multicenter, prospective, randomized, open-label trial with blinded endpoint adjudication comparing the effect of the GnRH antagonist, degarelix, with the GnRH agonist, leuprolide, on adjudicated MACE in patients with prostate cancer and established ASCVD.27 The trial was designed and led by an academic steering committee comprised of cardiologists, oncologists, and urologists who were responsible for its conduct and reporting. The Duke Clinical Research Institute (DCRI, Durham, NC) was the academic coordinating center and the trial was sponsored by Ferring Pharmaceuticals A/S. The data used to conduct this research will not be made available.
At 113 sites in 12 countries, eligible patients were randomized 1:1 to either a 240 mg subcutaneous starting dose of degarelix followed by 11 maintenance doses of 80 mg injections every 28 days or a 22.5 mg intramuscular injection of leuprolide followed by 3 similar injections every 84 days. Randomization was stratified by baseline age (<75 or ≥75 years) and region (North America or other) in fixed blocks of 4. Investigators were required to ensure that a cardiologist was treating enrolled participants during the trial to ensure optimization of secondary prevention medications for ASCVD.
As the dose schedule (once a month vs. once every 3 months), mode of administration (subcutaneous vs. intramuscular), and frequency of injection site reactions differed between the study drugs, a double-blind, multiple sham injection placebo-controlled design was deemed impractical. The nurse administering the study drug, who was unblinded, was kept separate from the study team and had no role in assessing the occurrence of potential cardiovascular events. All patients had monthly study visits regardless of treatment assignment. Sites completed a specific clinical events page, inquiring about potential cardiovascular events, to ensure a systematic and consistent assessment of possible cardiovascular events in each arm.
A clinical event classification (CEC) committee was established to provide independent, blinded, adjudication of cardiovascular events throughout the trial. The CEC committee consisted of cardiologists, neurologists, and an oncologist not otherwise involved with recruiting patients in the trial. Adjudicated primary endpoint data were transferred directly from the CEC to an independent data safety monitoring board (DSMB) to keep those data blinded to the sponsor and investigators.
Appropriate national and institutional regulatory and ethics boards approved the protocol, and the trial was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent prior to participation. An independent DSMB reviewed unblinded patient-level data at regular intervals during the trial.
All authors had full access to the data and assume responsibility for the completeness and accuracy of the data. Committee members and all participating investigators are listed in the Supplementary Appendix.
Study Population
Patients with histologically confirmed adenocarcinoma of the prostate and a history of ASCVD scheduled to receive at least 12 months of ADT were eligible for enrollment. Patients with prostate cancer were enrolled with localized disease, locally advanced disease, biochemical recurrence after definitive therapy, or metastatic disease. Included within the patient cohort were patients with very high-risk, high-risk, or intermediate-risk disease with features of unfavorable prognosis who would receive standard of care definitive radiation therapy in combination with at least 12 months of neoadjuvant/adjuvant ADT, patients with biochemical recurrence after local therapy who had a prostate-specific antigen (PSA) doubling time <12 months, or those eligible for salvage radiation therapy in combination with ADT. Patients had to be ADT naïve at the time of randomization with a serum testosterone of at least 150 ng/dL (5.2 nmol/L). Exceptions included those with a prior history of neoadjuvant/adjuvant ADT with definitive therapy for which the last injection of a depot ADT formulation was at least 12 months before randomization who were required to have a serum testosterone >50 ng/dL (≥1.73 nmol/L).
ASCVD was defined as prior history of myocardial infarction (MI); previous percutaneous or surgical revascularization of the carotid, coronary, iliac, femoral, or popliteal arteries; previous documentation of a stenosis of >50% in these vessels by angiography or carotid ultrasound; or peripheral artery disease with a diminished ankle-brachial pressure index less than 0.9. To assist sites in confirming the ASCVD inclusion criteria, supporting documentation for the first 3 patients enrolled by each site were reviewed centrally for eligibility by a cardiologist at the DCRI. Complete inclusion and exclusion criteria are available in the Supplementary Appendix.
Endpoints
The primary outcome was the time from randomization to first occurrence of centrally adjudicated MACE, a composite of all-cause death, MI, or stroke through 12 months. Sensitivity analyses for the primary outcome included time from randomization to a 4-point MACE outcome (all-cause death, MI, stroke, or unstable angina requiring hospitalization); time from randomization to MACE-related adverse event (Standardized MedDRA Queries [SMQ] definitions for MI [broad SMQ], central nervous system hemorrhages and cerebrovascular conditions [broad SMQ], and all-cause death); and the total occurrence, including first and recurrent events, of each of the components of the primary outcome. Key secondary outcomes were the time to first occurrence of cardiovascular-related death, nonfatal MI, or nonfatal stroke; time to first occurrence of cardiovascular-related death; and time to first occurrence of MI. A post-hoc analysis using adjudicated MACE plus MedDRA SMQ definitions that were used in the HERO trial was also performed.25 Prostate cancer-related outcomes included testosterone levels at days 28, 168, and 336, progression-free survival (PFS) (defined as time to either death, radiographic disease progression, introduction of additional prostate cancer therapies for progression, or PSA failure), and lower urinary tract symptoms using the International Prostate Symptom Score (IPSS) questionnaire.
Statistical Analysis
Over 12 months, primary outcome event rates were projected to be 5.1% for degarelix and 10.2% for leuprolide.22 Under this assumption, an estimated 876 patients would be required to yield 66 primary outcome events to provide the trial with 80% power to detect a hazard ratio of 0.49 for degarelix versus leuprolide for the composite outcome using a 2-sided alpha level of 0.05. An interim analysis was planned after 33 adjudicated MACE events. Recruitment into the trial was slower than anticipated and the aggregated primary outcome rate was lower than initially projected. After discussion with the steering committee, the sponsor closed enrollment in March 2020 with 545 of the planned 900 patients. The planned 12 months of follow-up for all enrolled patients was completed.
Analyses were based on the full analysis set, which included all patients who were randomized and received at least 1 dose of study drug. Time-to-event endpoints were censored at the time when a patient started a new treatment or a different ADT, was lost to follow-up or withdrew from the study, or at day 336, whichever occurred first. Kaplan-Meier time-to-event curves were constructed and hazard rates within treatment groups were compared using log-rank tests and Cox regressions stratified by age group and geographic region. Endpoints for counts of total number of events were analyzed using a negative binomial regression adjusted for the logarithm of the duration of exposure to estimate the occurrence rate ratio (ORR) between treatment groups, along with a 95 percent confidence interval and a p-value for a hypothesis of ORR=1.
RESULTS
Trial conduct
From 3 May 2016 to 16 April 2020, a total of 545 patients from 113 sites in 12 countries were randomly assigned to receive open-label degarelix or leuprolide. Incomplete follow-up occurred in 32 patients in the degarelix arm (11.6%) and 24 in the leuprolide arm (8.9%) with the most common reasons being an adverse event (4.4%) and withdrawal of consent (2.4%). Loss to follow-up occurred in 1 patient in the degarelix group (0.4%) and 5 patients in the leuprolide group (1.9%). Data on vital status was missing for 1 (0.2%) patient at the end of the trial (Figure 1).
Figure 1.
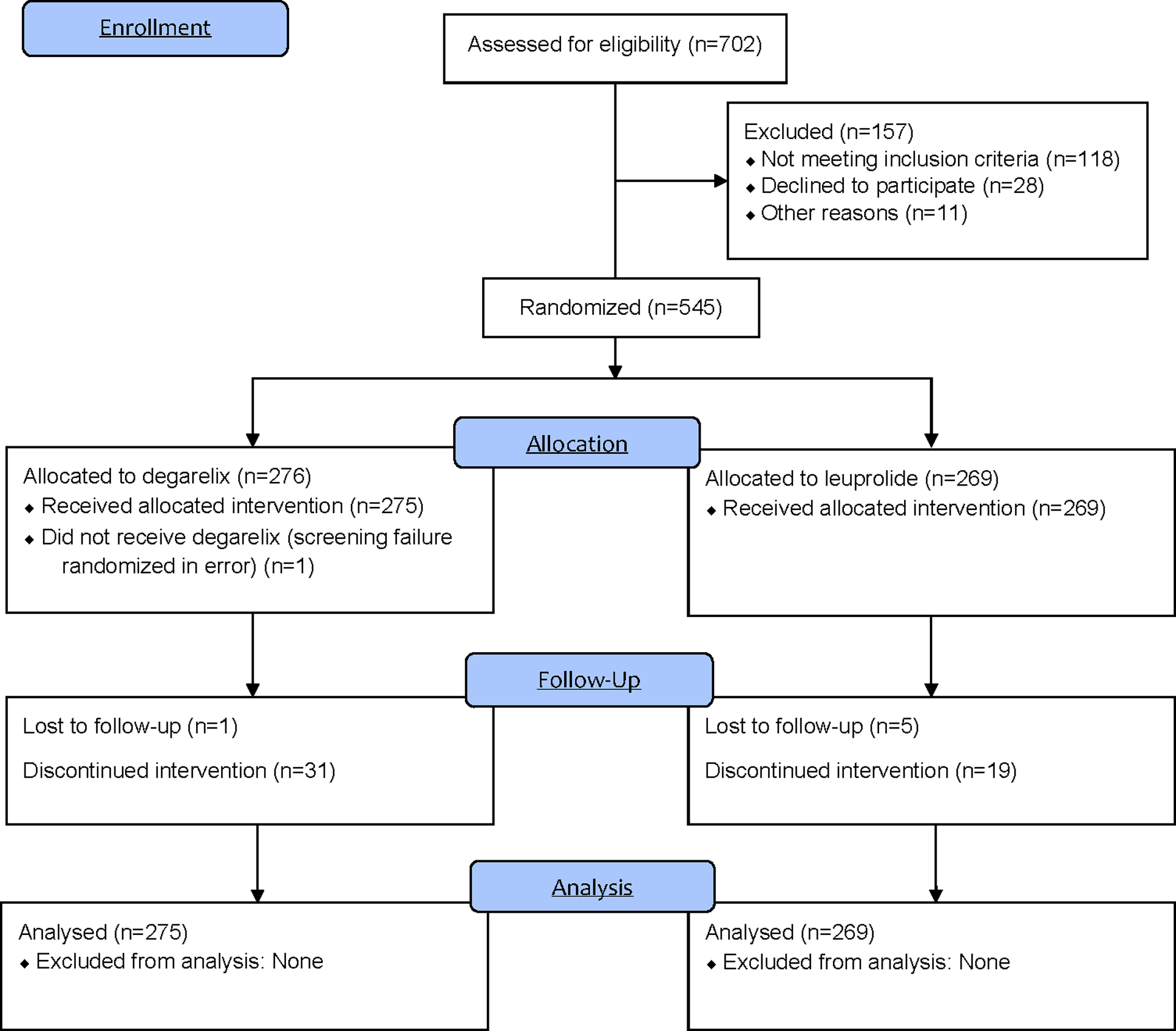
CONSORT diagram
Patients
The two groups were balanced with respect to baseline characteristics and cardiovascular secondary prevention medications (Table 1). The median age was 73 years, 44% were above 75 years of age. Enrollment from North America was 48.3% and 51.7% from Europe with 1 site in South Africa. Roughly half (49.8%) of patients had localized prostate cancer; a quarter (26.3%) had locally advanced disease, and one-fifth (20.4%) had metastatic disease. Baseline median testosterone level was 330 ng/dL and baseline median PSA level was 12.83 ng/dL.
Table 1.
Demographics and baseline characteristics
| Degarelix (n=275) |
Leuprolide (n=269) |
Total (N=544) |
|
|---|---|---|---|
| Age, mean (SD), yrs | 73.3 (7.28) | 73.1 (7.16) | 73.2 (7.22) |
| Race, no. (%) | |||
| American Indian or Alaska Native | 2 (0.7) | 2 (0.4) | |
| Asian | 3 (1.1) | 5 (1.9) | 8 (1.5) |
| Black or African American | 16 (5.9) | 12 (4.5) | 28 (5.2) |
| White | 252 (91.6) | 251 (93.3) | 503 (93.0) |
| All | 273 (100.0) | 268 (100.0) | 541 (100.0) |
| Ethnicity, no. (%) | |||
| Hispanic or Latino | 16 (5.9) | 14 (5.2) | 30 (5.6) |
| Not Hispanic or Latino | 256 (94.1) | 254 (94.8) | 510 (94.4) |
| All | 272 (100.0) | 268 (100.0) | 540 (100.0) |
| Weight, mean, kg | 86.01 | 87.10 | 86.55 |
| BMI, no./No. | 273/275 | 268/269 | 541/544 |
| Mean, kg/m2 | 28.38 | 28.58 | 28.48 |
| Smoking status, no. (%) | |||
| Current | 34 (12.4) | 48 (17.8) | 82 (15.1) |
| Former | 104 (37.8) | 107 (39.8) | 211 (38.8) |
| Never | 73 (26.5) | 68 (25.3) | 141 (25.9) |
| Baseline BP (diastolic >90 or systolic >140 mm Hg), no. (%) | 94 (34.2) | 90 (33.5) | 184 (33.8) |
| Total serum cholesterol, mean (SD), mmol/L | 4.1 (1.09) | 4.2 (1.09) | 4.1 (1.09) |
| Type 2 diabetes mellitus, no. (%) | 88 (32.0) | 87 (32.3) | 175 (32.2) |
| NT-proBNP, no./No. | 266/275 | 263/269 | 529/544 |
| Mean (SD), pg/mL | 665.4 (1552) | 675.0 (3154) | 670.2 (2479) |
| High sensitivity C-reactive protein, no./No. | 270/275 | 264/269 | 534/544 |
| Mean (SD), mg/dL | 0.6896 (1.825) | 0.5707 (1.747) | 0.6308 (1.786) |
| Troponin T, no./No. | 267/275 | 257/269 | 524/544 |
| Mean (SD), pg/mL | 18.79 (17.78) | 17.62 (17.66) | 18.21 (17.71) |
| Prostate cancer therapy history, no. (%) | |||
| Radiotherapy | 42 (15.3) | 31 (11.5) | 73 (13.4) |
| Radical prostatectomy | 37 (13.5) | 27 (10.0) | 64 (11.8) |
| Hormonal therapy | 21 (7.6) | 23 (8.6) | 44 (8.1) |
| Other | 13 (4.7) | 11 (4.1) | 24 (4.4) |
| Gleason score | |||
| 2–4 | 1 (0.4) | 2 (0.7) | 3 (0.6) |
| 5–6 | 34 (12.4) | 33 (12.3) | 67 (12.3) |
| 7–10 | 238 (86.5) | 234 (87.0) | 472 (86.8) |
| Stage of prostate cancer, no. (%) | |||
| Localised | 138 (50.2) | 133 (49.4) | 271 (49.8) |
| Locally advanced | 63 (22.9) | 80 (29.7) | 143 (26.3) |
| Metastatic | 63 (22.9) | 48 (17.8) | 111 (20.4) |
| Not classifiable | 11 (4.0) | 8 (3.0) | 19 (3.5) |
| Testosterone, no./No. | 274/275 | 269/269 | 543/544 |
| Median (25th, 75th), ng/dL | 325.5 (252, 416) | 338.0 (249, 415) | 330.0 (250, 416) |
| Prostate specific antigen, no./No. | 275/275 | 268/269 | 543/544 |
| Median (25th, 75th), ng/mL | 13.4 (5.9, 34.5) | 12.7 (5.8, 29.8) | 12.8 (5.8, 32.7) |
| Myocardial infarction | 127 (46.2%) | 125 (46.5%) | 252 (46.3%) |
| Coronary carotid, or iliofemoral revascularization | 199 (72.4%) | 194 (72.1%) | 393 (72.2%) |
| Coronary, carotid, or iliofemoral stenosis >50% by angiography | 108 (39.3%) | 120 (44.6%) | 228 (41.9%) |
| Carotid stenosis >50% by ultrasound | 19 (6.9%) | 16 (5.9%) | 35 (6.4%) |
| Ankle-brachial index <0.9 | 34 (12.4%) | 41 (15.2%) | 75 (13.8 %) |
| Atrial fibrillation | 55 (20.0) | 47 (17.5) | 102 (18.8) |
| Dyslipidemia, no. (%) | 106 (38.5) | 91 (33.8) | 197 (36.2) |
| Hypertension, no. (%) | 232 (84.4) | 235 (87.4) | 467 (85.8) |
| Concomitant medications, no. (%) | |||
| Cardiovascular medications | 268 (97.5) | 262 (97.4) | 530 (97.4) |
| Lipid modifying agents | 234 (85.1) | 224 (83.3) | 458 (84.2) |
| Agents acting on the renin-angiotensin system | 202 (73.5) | 194 (72.1) | 396 (72.8) |
| Beta blockers | 194 (70.5) | 180 (66.9) | 374 (68.8) |
BMI indicates body mass index; NT-proBNP, N-terminal pro-hormone B-type natriuretic peptide; SD, standard deviation.
Cardiovascular Outcomes
The primary outcome of all cause-death, MI, or stroke occurred in 15 patients in the degarelix group (5.5%) and 11 in the leuprolide group (4.1%) (hazard ratio [HR], 1.28; 95% confidence interval [CI] 0.59–2.79; p=0.53). The cumulative incidence of primary outcome events over the 12-month treatment period is shown in Figure 2. Details around the timing of MACE events are shown in Figure 3.
Figure 2.
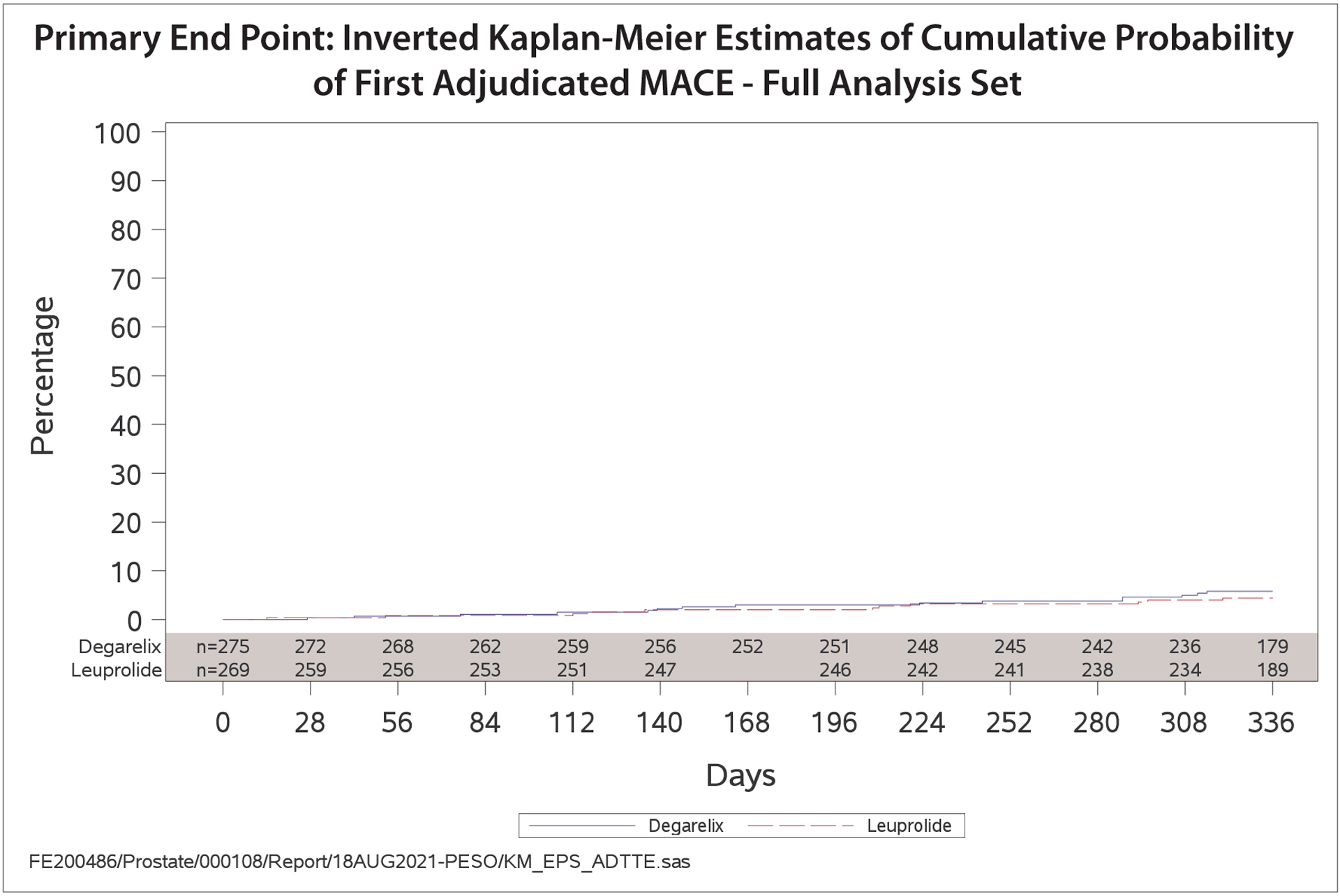
Primary endpoint: Kaplan-Meier plot of time from randomization to first adjudicated MACE, by treatment group
Figure 3.
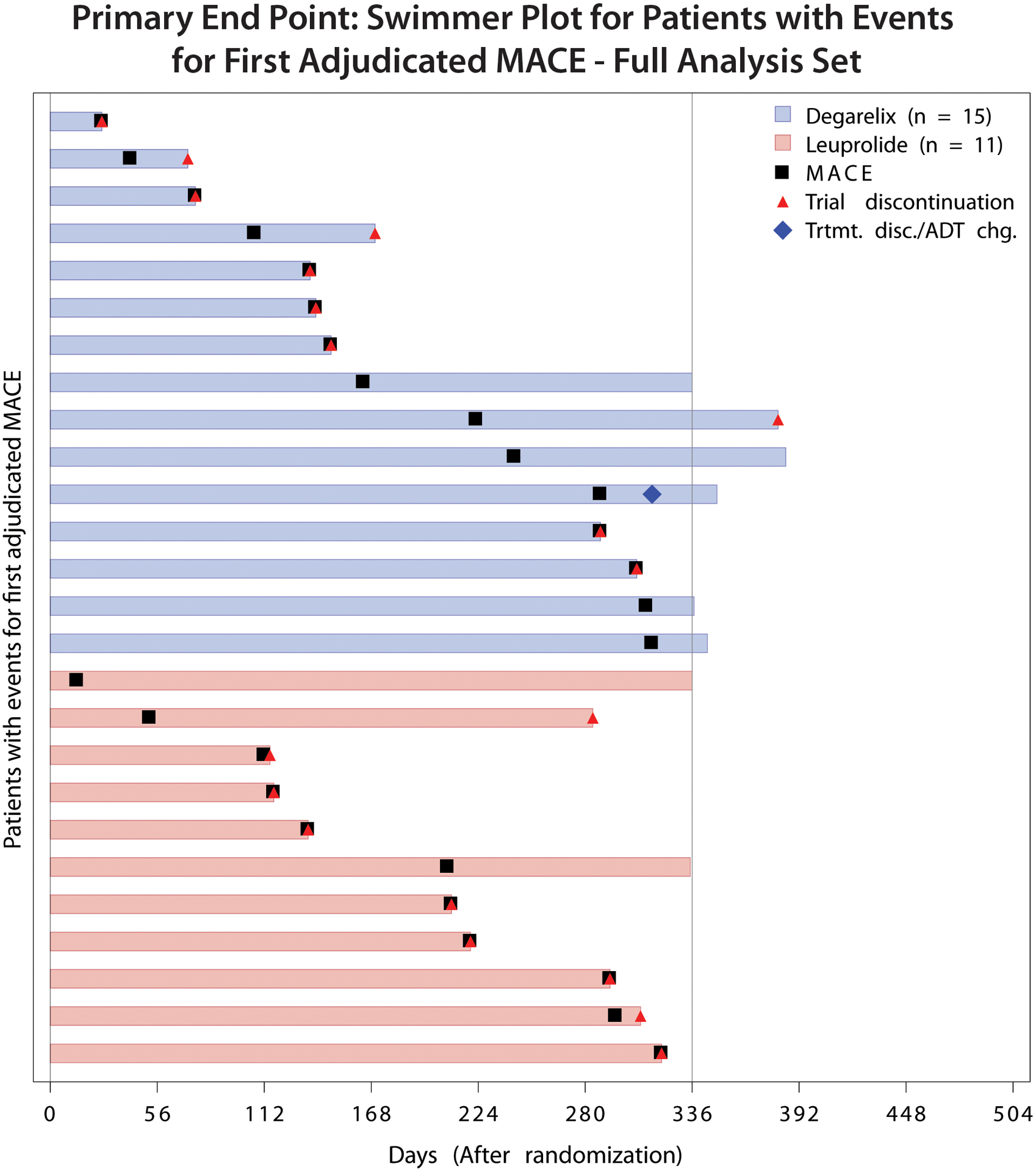
Timing of MACE by treatment group
Pre-specified sensitivity analyses of the primary endpoint using adjudicated data revealed similar results (Table 2). An expanded 4-point MACE endpoint that included unstable angina requiring hospitalization occurred in 17 (6.2%) patients assigned degarelix and 15 (5.6%) assigned leuprolide (HR 1.07, 95% CI 0.53–2.13). Analysis of total events revealed 21 MACE in patients assigned degarelix and 17 in patients assigned leuprolide (ORR 1.27, 95% CI 0.52–3.11). A final prespecified sensitivity analysis using investigator-reported adverse events with the application of a standardized MedDRA MACE definition by broad SMQ search used in the HERO study, occurred in 14 patients assigned degarelix and 20 patients assigned leuprolide (HR 0.67, 95% CI 0.34–1.32).
Table 2.
Clinical outcomes
| Endpoint | Patients with Events, No. (%) Degarelix vs. Leuprolide |
HR (95% CI)* | P-value† |
|---|---|---|---|
| Primary efficacy | |||
| Time from randomization to first adjudicated MACE | 15 (5.5%) vs. 11 (4.1%) | 1.283 (0.589–2.794) | 0.5294 |
| Sensitivity of primary efficacy | |||
| Time from randomization to first adjudicated MI, stroke, unstable angina requiring hospitalization, or all-cause death | 17 (6.2%) vs. 15 (5.6%) | 1.065 (0.532–2.134) | 0.8580 |
| Total occurrences of adjudicated MI, stroke, and all-cause death‡ | 21 vs. 17‡ | 1.265 (0.515–3.107) | 0.6076 |
| Time from randomization to first MACE-related AE according to broad SMQ | 14 (5.1%) vs. 20 (7.4%) | 0.665 (0.336–1.317) | 0.2389 |
| Time from randomization to first adjudicated occurrence of MACE (not censored at treatment discontinuation or change of ADT regimen) | 15 (5.5%) vs. 11 (4.1%) | 1.319 (0.606–2.873) | 0.4835 |
| Time from randomization to first adjudicated MACE over full trial duration# | 17 (6.2%) vs. 12 (4.5%) | 1.446 (0.677–3.088) | 0.3382 |
| Time from randomization to the first adjudicated MACE (using all CEC adjudicated events) | 18 (6.5%) vs. 13 (4.8%) | 1.468 (0.707–3.051) | 0.3003 |
| Key secondary efficacy§ | |||
| Time from randomization to first adjudicated occurrence of CV-related death, non-fatal MI, or non-fatal stroke | 9 (3.3%) vs. 7 (2.6%) | 1.204 (0.448–3.234) | 0.7126 |
| Time from randomization to first adjudicated CV-related death | 1 (0.4%) vs. 5 (1.9%) | 0.186 (0.022–1.595) | 0.0853 |
| Endpoints for MACE components | |||
| Time from randomization to first adjudicated MI | 5 (1.8%) vs. 3 (1.1%) | 1.594 (0.381–6.673) | 0.5196 |
| Time from randomization to first adjudicated stroke | 3 (1.1%) vs. 3 (1.1%) | 0.899 (0.181–4.457) | 0.8964 |
| Time from randomization to all-cause death | 8 (2.9%) vs. 9 (3.3%) | 0.839 (0.324–2.176) | 0.7184 |
Hazard ratio and 95 percent confidence interval for degarelix versus leuprolide are estimated using a Cox regression stratified for age group and region.
p-value of the log-rank test is based on comparison of the treatment groups stratified for age group and region.
For the total number of MACE, the occurrence rate ratio is presented along with a 95% confidence interval and the corresponding p-value derived from a negative binomial regression.
Endpoints controlled for multiplicity by a closed testing sequence. Formally, by this procedure and endpoint is only statistically significant if all previous endpoints are having a p-value below 0.05, ordered from top to bottom.
Over full trial duration indicates that this endpoint was not censored at day 336 and events occurring after 336 and before the end-of-study visit were included.
ADT indicates androgen deprivation therapy; CEC, clinical events committee; CI, confidence interval; CV, cardiovascular; HR, hazard ratio; MACE, major adverse cardiovascular events; MI, myocardial infarction; SMQ, Standardized MedDRA Queries.
Overall there were very few secondary outcome events. The composite endpoint of cardiovascular death, nonfatal MI, or nonfatal stroke occurred in 9 patients in the degarelix group and 7 in the leuprolide group (HR 1.20, 95% CI 0.45–3.23). There were a total of 8 events for the endpoint of MI (HR 1.59, 95% CI 0.38–6.67), and 6 events for the endpoint of cardiovascular death (HR 0.19, 95% CI 0.02–1.60). Finally, in the post-hoc analysis using adjudicated MACE plus MedDRA (version 22.0) SMQ definitions that were used in the HERO trial, there were 18 events in patients assigned to degarelix and 21 in patients assigned to leuprolide (HR 0.81, 95% CI 0.43–1.53).
Oncological Outcomes
Disease progression occurred in 24 patients assigned degarelix and 27 patients assigned leuprolide (HR 0.89, 95% CI 0.51–1.54) (Figure 4). Testosterone suppression to castration levels (<50 ng/dL or <1.73 nmol/L) at day 28 occurred in 96.6% of patients assigned degarelix and 96.5% assigned leuprolide (Figure 5). The rates of sustained castration at day 336 were similar between groups; 93.6% for degarelix and 94.8% for leuprolide, whereas profound castration (< 20 ng/dL) through day 336 was observed in 80% assigned to degarelix and 67.8% assigned to leuprolide (p=0.0003 for a log-rank test).
Figure 4.
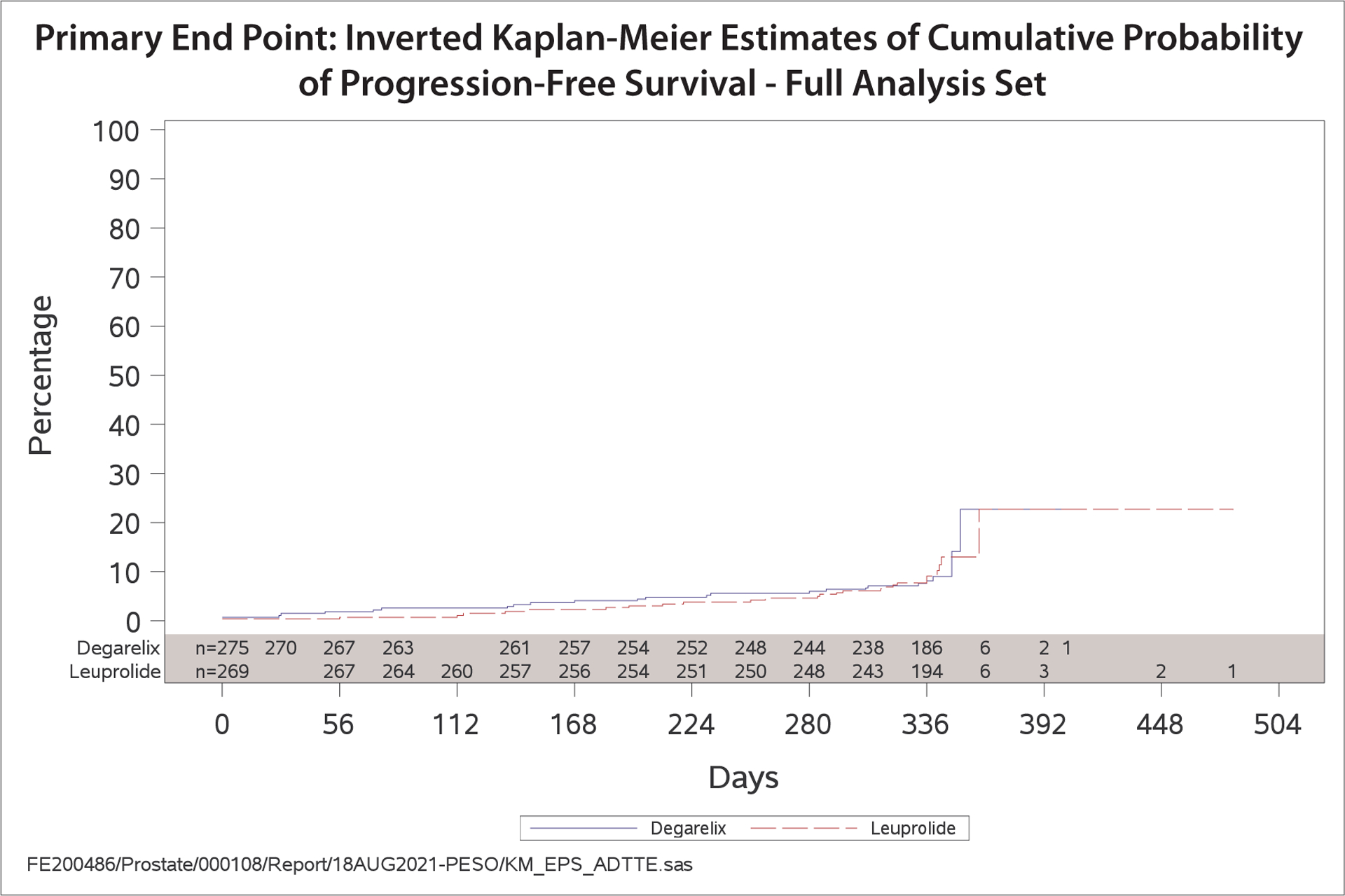
Kaplan-Meier plot of progression-free survival (PFS), time from randomization to PFS failure, by treatment group
Figure 5.
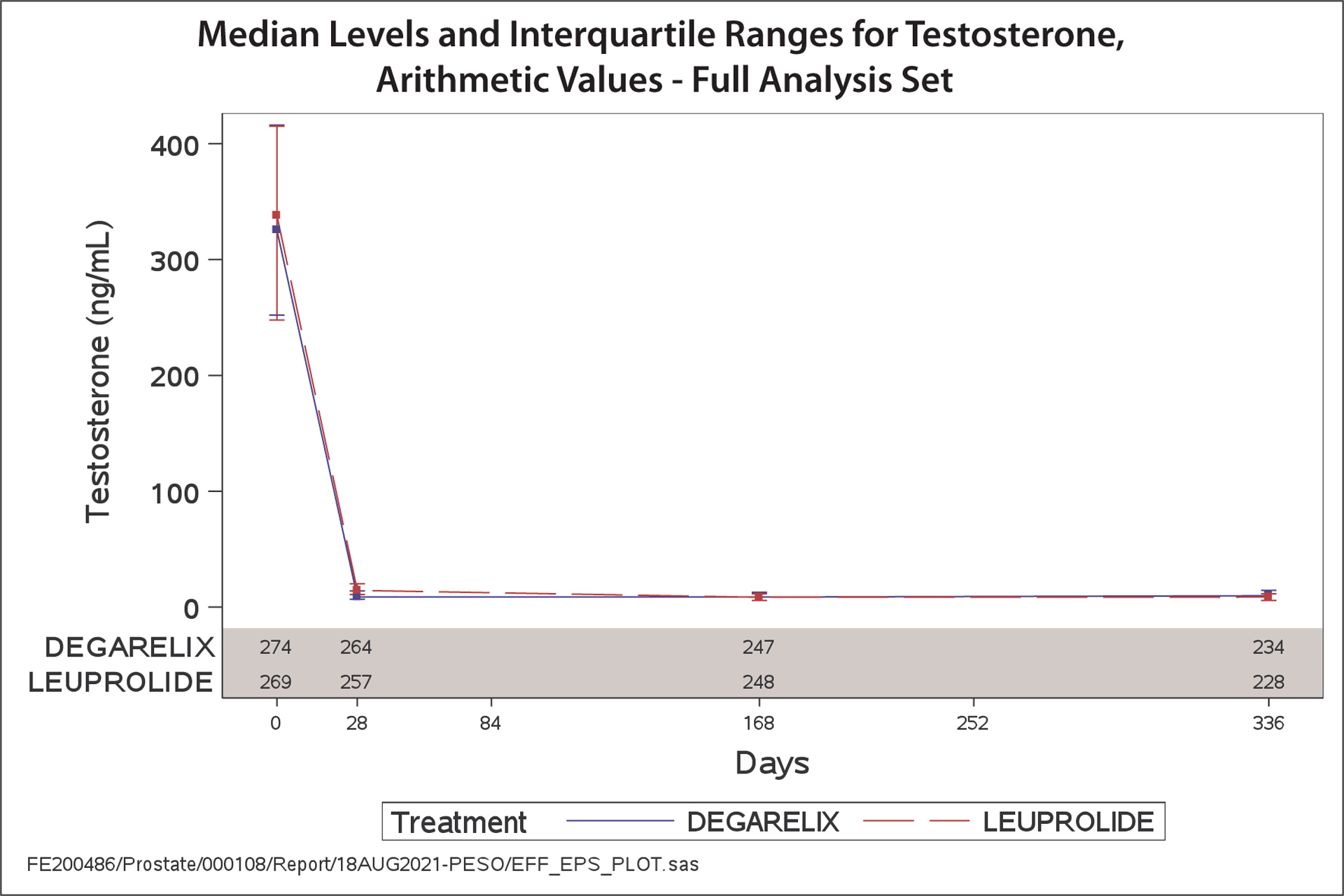
Median measured values and interquartile ranges for testosterone
Other Adverse Events
The incidence of severe adverse events was similar between groups (21.5% in degarelix group; 20.4% in leuoprolide group). Injection site reactions were more common among those assigned degarelix than those assigned leuprolide (60.4% vs. 26.8%). Fatigue was more common among those in the degarelix group than the leuprolide group (18.2% vs. 12.6%), while hot flashes were less common (38.9% vs. 44.6%). Adverse events leading to drug discontinuation were similar between groups and occurred in 13 patients assigned degarelix (4.7%) and 11assigned leuprolide (4.1%).
DISCUSSION
In this international, multicenter, prospective, randomized, open-label trial with blinded endpoint adjudication in patients with prostate cancer and pre-existing ASCVD, no difference was observed in the rate of cardiovascular events with the GnRH antagonist degarelix compared with the GnRH agonist leuprolide. However, accrual to the trial was stopped early and there were fewer than the planned participants and number of MACE, resulting in wide confidence intervals and low statistical power, and therefore, the relative cardiovascular safety of GnRH antagonists and agonists remains unresolved. As expected, testosterone levels, rates of progressive disease, and urinary symptoms were similar between the 2 agents.
Cardiovascular risk is a significant factor to consider when managing patients with prostate cancer with ADT alone or in combination with novel hormonal agents.28,29 The Role of Androgen Deprivation Therapy in Cardiovascular Disease – A Longitudinal Prostate Cancer (RADICAL PC) study reported that two-thirds of a cohort of 2492 men with prostate cancer had high cardiovascular risk with 22% having established cardiovascular disease, 16% having diabetes, 45% having hypertension, and 31% having obesity.1 ASCVD is the leading cause of non-cancer deaths in men with prostate cancer. ADT is the pillar of treatment for patients with prostate cancer. GnRH agonists (leuprolide, goserelin, triptorelin, histrelin) and GnRH antagonists (degarelix, relugolix) decrease luteinizing hormone levels, leading to testosterone suppression to castrate levels. GnRH agonists and antagonists are both effective for the treatment of prostate cancer and are used in men with intermediate- to high-risk localized disease in combination with radiation therapy, in selected patients with biochemical relapse, and as standard therapy in men with metastatic disease. Understanding the impact of ADT therapy on cardiovascular risk is critical because many of the risk factors associated with prostate cancer are also associated with cardiovascular disease. Novel hormonal agents such as abiraterone, enzalutamide, apalutamide, or darolutamide may also adversely affect cardiovascular risk in men already on ADT, as demonstrated by retrospective analyses of the phase III trials of each drug.29,30
The known unfavorable effects of ADT on cardiovascular risk factors including increasing lipids, blood pressure, and blood glucose and changing body composition, means that focusing on risk factor control is an important aspect of care for individuals on ADT.3 A cross-sectional analysis of more than 90,000 U.S. veterans with prostate cancer showed that cardiovascular burden is high, under-assessed, and likely undertreated.31 In a retrospective study of 616 patients undergoing exercise treadmill testing after a diagnosis of prostate cancer, prolonged ADT was associated with higher cardiovascular mortality in patients with high cardiovascular risk at baseline as well as with lower cardiorespiratory capacity.4 These findings suggest the need for close attention and education, as well as innovative tools and interventions, to improve the identification and facilitate the earlier treatment of cardiovascular risk in patients with prostate cancer. In our study, all patients were followed by a cardiologist and the use of evidence-based cardiovascular therapies was high, and higher than that reported in prior studies.8,32–35 Thus, a careful evaluation by cardiologists focusing on cardiovascular risk factors control may have a considerable impact on clinical outcomes of these patients. Additionally, changes in cardiovascular care since the Albertsen22 data were generated, which are reflected in a higher use of cardiovascular therapies in PRONOUNCE, may also have impacted the overall rate of cardiovascular events in our trial. However, this suggests that cardiovascular events can be reduced in patients with prostate cancer through better awareness and attention to cardiovascular interventions, resulting in potential improved overall survival time.
There are conflicting data from both observational and randomized studies comparing GnRH antagonists with GnRH agonists. Numerous observational studies demonstrate an association between GnRH antagonist use and a lower risk of cardiovascular events and both cardiovascular and all-cause mortality, particularly in those with high cardiovascular risk or established ASCVD, compared with GnRH agonists.22,23,32,34–36 Conversely, a more recent observational study using real-world data from Europe showed that men treated with GnRH antagonists with a history of ASCVD had a 30% higher risk of a cardiovascular event, 63% higher risk of an acute MI, and 74% higher risk of an arrhythmia compared with men treated with GnRH agonists.32 Important limitations of all these observational studies include lack of randomization, differences in baseline cardiovascular risk, short duration of follow-up, and the absence of rigorous ascertainment or adjudication of cardiovascular outcomes.37–39
A meta-analysis of adverse event data from phase 2, randomized trials reported fewer cardiovascular events among patients treated with the GnRH antagonist, degarelix, compared with GnRH agonists.40 However, another meta-analysis with slightly different inclusion criteria found no significant difference in cardiovascular outcomes between the two.32 Variation in the baseline cardiovascular risk of the studied populations has been hypothesized as an explanation for the conflicting results as these data were not ascertained in a consistent way. A recently reported randomized open-label trial in patients with prostate cancer comparing the GnRH antagonist relugolix with the GnRH agonist leuprolide showed that the incidence of major adverse cardiac events, defined based on MedDRA coded adverse events, was 2.9% in the relugolix group and 6.2% in the leuprolide group.25 The cardiac adverse events were not independently confirmed or adjudicated. Based on medical history, 90% of patients in HERO had at least 1 cardiovascular risk factor (lifestyle, tobacco use, diabetes, hypertension, and obesity) or a history of a cardiovascular event whereas patients in PRONOUNCE had to have had a history of ASCVD as defined above. In PRONOUNCE, using a similar MedDRA adverse event definition, we also noted numerically fewer major adverse cardiovascular events in the degarelix group than in the leuprolide group, though the difference was also not statistically significant. In many of these studies, analyses of coded investigator-reported adverse event data are challenged by the open label design and lack of blinded event adjudication, the variability of the adverse events included, and the fact that these analyses, with the exception of HERO, are often not prespecified.
Mechanistically, the association between GnRH agonists and cardiovascular events has been hypothesized to relate to the destabilization of existing vascular plaques seen in animal models.41,42 CD3 positive T cells embedded in atherosclerotic plaque express GnRH receptors. Treatment with a GnRH agonist may result in destabilization of a vulnerable thin cap. Activation of T cells can cause release of cytokines and stimulation of macrophages to secrete collagenases leading to rupture of an atherosclerotic plaque causing an acute cardiovascular event.41 In animals, treatment with a GnRH antagonist did not have this effect on plaque stability.41
PRONOUNCE has major limitations. The premature termination of enrollment and a lower than projected aggregate event rate resulted in wide confidence intervals and the inability to conclude on cardiovascular safety between degarelix and leuprolide. The lower than expected enrollment was in part due to changes in the standard of care during the years of enrollment including the addition of docetaxel or abiraterone to ADT for men with metastatic hormone sensitive disease.43–45 The trial was open-label, which could have led to differential treatment or event ascertainment; however, primary outcome events were adjudicated in a blinded fashion without knowledge of treatment assignment. Finally, a unique aspect of PRONOUNCE was that participants were required to have ongoing care of a cardiologist. This requirement, and the predominantly white population, may limit the external validity of these findings to other populations.
Conclusions
PRONOUNCE is the first, international, randomized clinical trial to prospectively compare the cardiovascular safety of a GnRH antagonist and a GnRH agonist in patients with prostate cancer. The study was terminated prematurely due to smaller than planned number of participants and events, and no difference in MACE at 1 year between patients assigned to degarelix or leuprolide was observed. The relative cardiovascular safety of GnRH antagonists and agonists remains unresolved. Nonetheless, PRONOUNCE provides a model for the interdisciplinary collaboration between urologists, oncologists and cardiologists with a shared goal of evaluating the impact of cancer therapies on cardiovascular outcomes.
Supplementary Material
Clinical Perspective.
What is new?
The relative cardiovascular safety of gonadotropin-releasing hormone (GnRH) antagonists compared with GnRH agonists in men with prostate cancer and known atherosclerotic cardiovascular disease (ASCVD) remains controversial.
PRONOUNCE is the first, international, randomized clinical trial to prospectively compare the cardiovascular safety of a GnRH antagonist and a GnRH agonist in patients with prostate cancer.
The study was terminated prematurely with smaller than the planned number of participants and events, and no difference in MACE was observed at 1 year between patients assigned to degarelix and leuprolide.
What are the clinical implications?
The relative cardiovascular safety of GnRH antagonists and agonists remains unresolved.
Cardiovascular events might be lower in patients with prostate cancer through better awareness and attention to cardiovascular risk factor control.
In the light of improved cancer survivorship and the competing risk of cardiovascular disease, there is an ongoing need for rigorous cardio-oncology clinical trials.
PRONOUNCE provides a model for interdisciplinary collaboration between urologists, oncologists and cardiologists with a shared goal of evaluating the impact of cancer therapies on cardiovascular outcomes.
ACKNOWLEDGEMENTS
We thank the patients who participated in the trial and their families, as well as all the investigators and site staff who made the trial possible. In addition, we thank the Ferring and IQVIA employees who contributed to the conduct of the trial. We thank the following past Ferring employees: Samreen Manson-Lefebvre, MD, Konstantin Zubovskiy, MD, and Allan Blemings, PhD.
FUNDING
This work was supported by Ferring Pharmaceuticals.
DISCLOSURES
RDL: Grant support, paid to his institution, and consulting fees from Bayer, Bristol Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi and consulting fees from Boehringer Ingelheim, Daiichi Sankyo, Merck, and Portola.
CSH: Grants from Aragon, Astellas, AstraZeneca, Clovis, Dendreon, eFFECTOR Therapeutics, Emergent, Ferring, Genentech, Hoffman-Laroche, Medivation, Pfizer, Bayer. Personal fees from Astellas, Clovis, Genentech, Pfizer, Bayer, Blue Earth Diagnostics, Dendreon, Hinova, Janssen, Merck, Orion, Tolmar, Carrick Theapeutics and Novartis. DSMB membership fees from Advantagene, Alliance Foundaation, AstraZeneca, Exelixis, Sotio,
AJN: Nothing to report.
CM: Nothing to report.
RB: Stock holdings in Merck, Johnson & Johnson, Covidien, Pfizer, Sanofi, and McKesson. Consulting fees from Rafael and Elixir.
PS: Employee of Ferring Phaemaceuticals A/S.
SGG: Research grant support (e.g., steering committee or data and safety monitoring committee) and/or speaker/consulting honoraria (e.g., advisory boards) from: Ferring Pharmaceuticals, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, Daiichi-Sankyo/American Regent, Eli Lilly, Esperion, GlaxoSmithKline, HLS Therapeutics, JAMP Pharma, Janssen/Johnson & Johnson, Merck, Novartis, Novo Nordisk A/C, Pendopharm, Pfizer, Regeneron, Sanofi, Servier, Valeo Pharma; and salary support/honoraria from the Heart and Stroke Foundation of Ontario/University of Toronto (Polo) Chair, Canadian Heart Research Centre and MD Primer, Canadian VIGOUR Centre, Cleveland Clinic Coordinating Centre for Clinical Research, Duke Clinical Research Institute, New York University Clinical Coordinating Centre, and PERFUSE Research Institute.
CPE: Research grants from Ferring, Astellas, Pfizer and Janssen, honoraria for speaking and consulting from Pfizer, Janssen, Astellas, and Myovant.
JN: Research grant support (e.g., steering committee or data and safety monitoring committee) and/or speaker/consulting honoraria (e.g., advisory boards) from: Ferring Pharmaceuticals, AstraZeneca and Follicum AB.
DLB: Grants from Ferring Pharmaceuticals, personal fees from Duke Clinical Research Institute, during the conduct of the study; grants from Amarin, grants from AstraZeneca, grants from Bristol-Myers Squibb, grants from Eisai, grants from Ethicon, grants from Medtronic, grants from sanofi aventis, grants from The Medicines Company, other from FlowCo, grants and other from PLx Pharma, other from Takeda, personal fees from Duke Clinical Research Institute, personal fees from Mayo Clinic, personal fees from Population Health Research Institute, personal fees, non-financial support and other from American College of Cardiology, personal fees from Belvoir Publications, personal fees from Slack Publications, personal fees from WebMD, personal fees from Elsevier, other from Medscape Cardiology, other from Regado Biosciences, other from Boston VA Research Institute, personal fees and non-financial support from Society of Cardiovascular Patient Care, non-financial support from American Heart Association, personal fees from HMP Global, grants from Roche, personal fees from Harvard Clinical Research Institute (now Baim Institute for Clinical Research), other from Clinical Cardiology, personal fees from Journal of the American College of Cardiology, other from VA, grants from Pfizer, grants from Forest Laboratories/AstraZeneca, grants from Ischemix, other from St. Jude Medical (now Abbott), other from Biotronik, grants and other from Cardax, other from Boston Scientific, grants from Amgen, grants from Lilly, grants from Chiesi, grants from Ironwood, personal fees from Cleveland Clinic, personal fees from Mount Sinai School of Medicine, other from Merck, grants from Abbott, grants from Regeneron, other from Svelte, grants and other from PhaseBio, grants from Idorsia, grants from Synaptic, personal fees from TobeSoft, grants, personal fees and other from Boehringer Ingelheim, personal fees from Bayer, grants and other from Novo Nordisk, grants from Fractyl, personal fees from Medtelligence/ReachMD, personal fees from CSL Behring, grants and other from Cereno Scientific, grants from Afimmune, grants from Ferring Pharmaceuticals, other from CSI, grants from Lexicon, personal fees from MJH Life Sciences, personal fees from Level Ex, grants from Contego Medical, grants and other from CellProthera, personal fees from K2P, personal fees from Canadian Medical and Surgical Knowledge Translation Research Group, grants and other from MyoKardia/BMS, grants from Owkin, grants from HLS Therapeutics, grants and other from Janssen, grants from 89Bio, grants and other from Novo Nordisk, grants from Garmin, grants and other from Novartis, grants and other from NirvaMed, outside the submitted work.
TKO: Previous employee of Ferring Pharmaceuticals Inc.
BDO: Employee of Ferring Pharmaceuticals A/S.
HK: Employee of Ferring Pharmaceuticals A/S.
LA: Nothing to report.
NWC: Personal fees from Janssen Pharmaceuticals.
SFS: Honoraria for advisory Boards/educational programs: Novartis, Janssen, Pfizer, Merck, Sanofi-Aventis, Clovis and Physician Education Resource; Research Funding: Sanofi-Aventis, Novartis, Poseida Pharma, Immunomedex.
JHA: Institutional grant support from Boehringer Ingelheim, Bayer, Bristol-Myers Squibb, CryoLife, CSL Behring, Ferring, Glaxosmithkline, Humacyte. Consultant to / honoraria from AbbVie, Bayer, Bristol-Myers Squibb, CryoLife, CSL Behring, Pfizer, Portola.
Non-standard Abbreviations and Acronyms
- ADT
androgen deprivation therapy
- ASCVD
atherosclerotic cardiovascular disease
- CEC
clinical events classification
- DCRI
Duke Clinical Research Institute
- DSMB
data safety monitoring board
- GnRH
gonadotropin-releasing hormone
- HERO
A Study to Evaluate the Safety and Efficacy of Relugolix in Men With Advanced Prostate Cancer
- IPSS
International Prostate Symptom Score
- MACE
major adverse cardiovascular events
- ORR
occurrence rate ratio
- PFS
progression-free survival
- PRONOUNCE
A Trial Comparing Cardiovascular Safety of Degarelix Versus Leuprolide in Patients With Advanced Prostate Cancer and Cardiovascular Disease
- PSA
prostate-specific antigen
- RADICAL PC
The Role of Androgen Deprivation Therapy in Cardiovascular Disease – A Longitudinal Prostate Cancer
- SMQ
standardized MedDRA queries
Footnotes
SUPPLEMENTAL MATERIALS
Steering Committee, Operations Committee, Clinical Events Committee, and Data Safety Monitoring Board Members
Investigators
Criteria for Inclusion/Exclusion
REFERENCES
- 1.Leong DP, Fradet V, Shayegan B, Duceppe E, Siemens R, Niazi T, Klotz L, Brown I, Chin J, Lavallee L, Mousavi N, Luke P, Lukka H, Gopaul D, Violette P, Hamilton RJ, Davis MK, Karampatos S, Mian R, Delouya G, Fradet Y, Mukherjee S, Conen D, Chen-Tournoux A, Johnson C, Bessissow A, Dresser G, Hameed AK, Abdel-Qadir H, Sener A, Pal R, Devereaux PJ, Pinthus J. Cardiovascular risk in men with prostate cancer: insights from the RADICAL PC Study. J Urol 2020;203:1109–1116. [DOI] [PubMed] [Google Scholar]
- 2.Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, Kelly SP, Zaorsky NG. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J 2019;40:3889–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine GN, D’Amico AV, Berger P, Clark PE, Eckel RH, Keating NL, Milani RV, Sagalowsky AI, Smith MR, Zakai N. Androgen-deprivation therapy in prostate cancer and cardiovascular risk. A science advisory from the American Heart Association, American Cancer Society, and American Urological Association endorsed by the American Society for Radiation Oncology. Circulation 2010;121:833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong J, Payne D, Caron J, Bay CP, McGregor BA, Hainer J, Partridge A, Neilan TG, Di Carli M, Nohria A, Groarke J. Reduced cardiorespiratory fitness and increased cardiovascular mortality after prolonged androgen deprivation therapy for prostate cancer. JACC CardioOnc 2020;2:553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith MR, Klotz L, van der Meulen E, Colli E, Tankó LB. Gonadotropin releasing hormone blockers and cardiovascular disease risk: analysis of prospective clinical trials of degarelix. J Urol 2011;186:1835–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin 2021;71:7–33. [DOI] [PubMed] [Google Scholar]
- 7.Gunner C, Gulamhusein A, Rosario DJ. The modern role of androgen deprivation therapy in the management of localised and locally advanced prostate cancer. J Clin Urol 2016;9(2Suppl):24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scailteux LM, Vincendeau S, Balusson F, Leclercq C, Happe A, Le Nautout B, Polard E, Nowak E, Oger E. Androgen deprivation therapy and cardiovascular risk: No meaningful difference between GnRH antagonist and agonists—a nationwide population-based cohort study based on 2010–2013 French Health Insurance data. Eur J Cancer 2017;77:99–108. [DOI] [PubMed] [Google Scholar]
- 9.Percentage of men with prostate cancer using select treatments in the U.S. as of 2019. https://www.statista.com/statistics/1061373/share-of-men-and-treatments-used-for-prostrate-cancer-by-men-in-the-us/ (12 July 2021).
- 10.O’Farrell S, Garmo H, Holmberg L, Adolfsson J, Stattin P, Van Hemelrijck M. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J Clin Oncol 2015;33:1243–1251. [DOI] [PubMed] [Google Scholar]
- 11.Bosco C, Bosnyak Z, Malmberg A, Adolfson J, Keating NC, Van Hemelrijck M. Quantifying observational evidence for risk of fatal and nonfatal cardiovascular disease following androgen deprivation therapy for prostate cancer: a meta-analysis. Eur Urol 2015;68:386–396. [DOI] [PubMed] [Google Scholar]
- 12.Zhao J, Zhu S, Sun L, Meng F, Zhao L, Zhao Y, Tian H, Li P, Niu Y. Androgen deprivation therapy for prostate cancer is associated with cardiovascular morbidity and mortality: a meta-analysis of population-based observational studies. PLoS One 2014;9:e107516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 2006;24:4448–4456. [DOI] [PubMed] [Google Scholar]
- 14.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab 2006;91:1305–1308. [DOI] [PubMed] [Google Scholar]
- 15.Braga-Basaria M, Dobs AS, Muller DC, Carducci MA, John M, Egan J, Basaria S. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol 2006;24:3979–3983. [DOI] [PubMed] [Google Scholar]
- 16.Smith MR, Finkelstein JS, McGovern FJ, Zietman AL, Fallon MA, Schoenfeld DA, Kantoff DW. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab 2002;87:599–603. [DOI] [PubMed] [Google Scholar]
- 17.Braga-Basaria M, Muller DC, Carducci MA, Dobs AS, Basaria S. Lipoprotein profile in men with prostate cancer undergoing androgen deprivation therapy. Int J Impot Res 2006;18:494–498. [DOI] [PubMed] [Google Scholar]
- 18.Alibhai SM, Breunis H, Timilshina N, Johnston C, Tomlinson G, Tannock I, Krahn M, Fleshner NE, Warde P, Canning SD, Klotz L. Impact of androgen-deprivation therapy on physical function and quality of life in men with nonmetastatic prostate cancer. J Clin Oncol 2010;28:5038–5045. [DOI] [PubMed] [Google Scholar]
- 19.Smith TJ, Temin S, Alesi ER, Abernethy AP, Balboni TA, Basch EM, Ferrell BR, Loscalzo M, Meier DE, Paice JA, Peppercorn JM. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol 2012;30:880–887. [DOI] [PubMed] [Google Scholar]
- 20.Nead KT, Boldbaatar N, Yang DD, Sinha S, Nguyen PL. Association of androgen deprivation therapy and thromboembolic events: a systematic review and meta-analysis. Urology 2018;114:155–162. [DOI] [PubMed] [Google Scholar]
- 21.Ehdaie B, Atoria CL, Gupta A, Feifer A, Lowrance WT, Morris MJ, Scardino PT, Eastham JA, Elkin EB. Androgen deprivation therapy and thromboemboic events in men with prostate cancer. Cancer 2012;118:3397–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albertsen PC, Klotz L, Tombal B, Grady J, Olesen TK, Nilsson J. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and antagonist. Eur Urol 2014;65:565–573. [DOI] [PubMed] [Google Scholar]
- 23.Margel D, Peer A, Ber Y. Cardiovascular morbidity in a randomized trial comparing GnRH agonist and GnRH antagonist among patients with advanced prostate cancer and preexisting cardiovascular disease. J Urol 2019;202:1199–1208. [DOI] [PubMed] [Google Scholar]
- 24.Sciarra A, Fasulo A, Ciardi A, Petrangeli E, Gentilucci A, Maggi M, Innocenzi M, Pierella F, Gentile V, Salciccia S, Cattarino S. A meta-analysis and systematic review of randomized controlled trials with degarelix versus gonadotropin-releasing hormone agonists for advanced prostate cancer. Medicine (Baltimore) 2016;95:e384521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shore ND, Saad F, Cookson MS, George DJ, Saltzstein DR, Tutrone R, Akaza H, Bossi A, van Veenhuyzen DF, Selby B, Fan X, Kang V, Walling J, Tombal B. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med 2020;382:2187–2196. [DOI] [PubMed] [Google Scholar]
- 26.Higano CS. Cardiovascular Disease and Androgen Axis–Targeted Drugs for Prostate Cancer. N Engl J Med 2020;382:2257–2259. [DOI] [PubMed] [Google Scholar]
- 27.Melloni C, Slovin SF, Blemings A, Goodman SG, Evans CP, Nilsson J, Bhatt DL, Zubovskiy K, Olesen TK, Dugi K, Clarke NW, Higano CS, Roe MT. Cardiovascular Safety of Degarelix Versus Leuprolide for Advanced Prostate Cancer: The PRONOUNCE Trial Study Design. JACC CardioOnc 2020;2:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgans AK, Shore N, Cope D, McNatty A, Moslehi J, Gomella L, Sartor O. Androgen receptor inhibitor treatments: cardiovascular adverse events and comorbidity considerations in patients with nonmetastatic prostate cancer. Urol Oncol 2021;39:52–62. [DOI] [PubMed] [Google Scholar]
- 29.Di Nunno V, Mollica V, Santoni M, Gatto L, Schiavina R, Fiorentino M, Brunocilla E, Ardizzoni A, Massari F. New hormonal agents in patients with nonmetastatic castration-resistant prostate cancer: meta-analysis of efficacy and safety outcomes. Clin Genitourin Cancer 2019;17:e871–e877. [DOI] [PubMed] [Google Scholar]
- 30.Iacovelli R, Ciccarese C, Bria E, Romano M, Fantinel E, Bimbatti D, Muraglia A, Porcaro AB, Siracusano S, Brunelli M, Mazzarotto R, Artibani W, Tortora G. The Cardiovascular Toxicity of Abiraterone and Enzalutamide in Prostate Cancer. Clin Genitourinary Cancer 2018;16:e645–e653. [DOI] [PubMed] [Google Scholar]
- 31.Sun L, Parikh RB, Hubbard RA, Cashy J, Takvorian SU, Vaughn DJ, Robinson KW, Narayan V, Ky B. Assessment and management of cardiovascular risk factors among US veterans with prostate cancer. JAMA Netw Open 2021;4:e210070–e210070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.George G, Garmo H, Scailteux L-M, Balusson F, De Coster G, De Schutter H, Kuiper JG, Oger E, Verbeeck J, Van Hemelrijck M. Risk of cardiovascular disease following gonadotropin-releasing hormone agonists vs antagonists in prostate cancer: Real-world evidence from five databases. Int J Cancer 2021;148:2203–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davey P, Kirby MG. Cardiovascular risk profiles of GnRH agonists and antagonists: real-world analysis from UK general practice. World J Urol 2021;39:307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hupe MC, Hammerer P, Ketz M, Kossack N, Colling C, Merseburger AS. Retrospective Analysis of Patients With Prostate Cancer Initiating GnRH Agonists/Antagonists Therapy Using a German Claims Database: Epidemiological and Patient Outcomes. Front Oncol 2018;8:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perrone V, Esposti LD, Giacomini E, Veronesi C, Blini V, Oderda M. Cardiovascular Risk Profile in Prostate Cancer Patients Treated with GnRH Agonists versus Antagonists: An Italian Real-World Analysis. Ther Clin Risk Manag 2020;16:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higano CS, Crawford ED, Shore ND, Bosnyak Z, Malmberg A, Klotz ANL. Risk of cardiovascular events with degarelix versus leuprolide after biochemical relapse of prostate cancer: Exploratory analysis of a randomized controlled trial. J Clin Oncol 2015;33(Suppl7):151. [Google Scholar]
- 37.Bhatt DL. Birth and maturation of cardio-oncology. JACC: CardioOnc 2019;1:114–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fanaroff AC, Califf RM, Harrington RA, Granger CB, McMurray JJV, Patel MR, Bhatt DL, Windecker S, Hernandez AF, Gibson CM, Alexander JH, Lopes RD. Randomized Trials Versus Common Sense and Clinical Observation: JACC Review Topic of the Week. J Am Coll Cardiol 2020;76:580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fanaroff AC, Califf RM, Lopes RD. New Approaches to Conducting Randomized Controlled Trials. J Am Coll Cardiol 2020;75:556–559. [DOI] [PubMed] [Google Scholar]
- 40.Cirne F, Aghel N, Petropoulos J-A, Klotz L, Lenihan DJ, Saad F, Pinthus J, Leong DP. The cardiovascular effects of GnRH antagonists in men with prostate cancer. Eur Heart J Cardiovasc Pharmacother 2021; doi: 10.1093/ehjcvp/pvab005. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 41.Knutsson A, Hsiung S, Celik S, Rattik S, Mattisson IY, Wigren M, Scher HI, Nilsson J, Hultgårdh-Nilsson A. Treatment with a GnRH receptor agonist, but not the GnRH receptor antagonist degarelix, induces atherosclerotic plaque instability in ApoE(−/−) mice. Sci Rep 2016;6:26220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tivesten A, Pinthus JH, Clarke N, Duivenvoorden W, Nilsson J. Cardiovascular risk with androgen deprivation therapy for prostate cancer: potential mechanisms. Urol Oncol 2015;33:464–475. [DOI] [PubMed] [Google Scholar]
- 43.Sweeney CJ, Chen Y-H, Carducci M, Liu G, Jarrard DF, Eisenberger M, Wong Y-N, Hahn N, Kohli M, Cooney MM, Dreicer R, Vogelzang NJ, Picus J, Shevrin D, Hussain M, Garcia JA, DiPaola RS. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med 2015;373:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, Ritchie AWS, Parker CC, Russell JM, Attard G, de Bono J, Cross W, Jones RJ, Thalmann G, Amos C, Matheson D, Millman R, Alzouebi M, Beesley S, Birtle AJ, Brock S, Cathomas R, Chakraborti P, Chowdhury S, Cook A, Elliott T, Gale J, Gibbs S, Graham JD, Hetherington J, Hughes R, Laing R, McKinna F, McLaren DB, O’Sullivan JM, Parikh O, Peedell C, Protheroe A, Robinson AJ, Srihari N, Srinivasan R, Staffurth J, Sundar S, Tolan S, Tsang D, Wagstaff J, Parmar MKB. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016;387:1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fizazi K, Tran NP, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S, Protheroe A, De Porre P, Kheoh T, Park YC, Todd MB, Chi KN. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2017;377:352–360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


